Abstract
Background
To examine the contemporary role of ureteroscopy in the diagnosis of upper urinary tract urothelial carcinoma.
Methods
We retrospectively evaluated 116 diagnostic ureteroscopies, performed in our institution to rule out primary UTUC. Demographics, cytological findings and interpretation of preoperative imaging were obtained. Ureteroscopic diagnosis and histological results were recorded and the predictive values of diagnostic studies were determined. Follow-up data was reviewed to evaluate the oncological outcomes in patients treated endoscopically.
Results
The pre-ureteroscopic evaluation included CTU in 91 (78 %) patients. Positive and Negative predictive values of CTU were 76 and 80 %, respectively. Typical filling defect on CTU was demonstrated in 38 of 89 patients. UTUC has been ruled out in 9 patients (24 %) with suspicious filling defect on CTU. Endoscopic approach was implemented in 7 patients (18 %). During a median follow up period of 17 months (IQR, 9–25) none of the followed patients experienced disease progression.
Conclusions
Nephroureterectomy was spared from 42 % of patients who underwent diagnostic ureteroscopy for suspected UTUC, demonstrated on CTU. In about half of those patients tumor has been ruled out and the others were managed endoscopically. Therefore, diagnostic ureteroscopy is advised as a crucial step in confirming UTUC and treatment planning.
Keywords: Upper urinary tract urothelial carcinoma, Ureteroscopy, Computed tomography urography
Background
Upper urinary tract urothelial carcinoma (UTUC) is an uncommon malignancy, accounting for ~5 % of urothelial tumors [1]. The diagnosis of UTUC can be challenging, requiring a combination of radiographic, cytologic and endoscopic means. Time honored radiological tools such as intravenous urography and retrograde uretropyelograpy are currently replaced by modern computerized tomography urography (CTU) [2]. Diagnostic ureteroscopy is often performed following CTU. Flexible ureteroscopy allows exploration of the upper urinary tract and is beneficial when diagnostic uncertainty exists. It has the advantages of offering direct view of the tumor, ruling out other pathologies and achieving tissue diagnosis. Although nephrouereterectomy is considered the gold standard treatment of UTUC, endoscopic ablation and resection of the tumor can be successfully utilized in selected cases based on tumor size and histology, as determined during ureteroscopoy. In low grade low volume tumors endoscopic management provides cancer related and overall survival equivalent to that of nephroureterectomy [3]. Despite the above, the routine use of ureterosocpy following CTU is controversial. Ureteroscopy is an invasive procedure that may be associated with morbidity as well as a potential risk of tumor seeding [4, 5]. In the last edition of “Campbell’s urology” the authors do not support a routine ureteroscopic confirmation of UTUC [6], while the European guidelines on UTUC (2015 version) advocate the use of diagnostic ureteroscopy with biopsy, especially in cases where additional information will impact treatment decisions. The purpose of this study was to evaluate the diagnostic value of ureteroscopy in patients who underwent workup for suspected UTUC and to assess the impact of ureteroscopy on the management of UTUC.
Methods
This study was approved by “Rabin Medical Center” ethics committee. Between 2003 and 2010, 1818 ureteroscopies, were performed at our institution, of which 116 (6.3 %) were diagnostic, aimed to rule out primary UTUC. The indications for diagnostic ureteroscopy included painless hematuria with positive urinary cytology and negative cystoscopy or imaging findings that may indicate UTUC. CTU was not performed in patients with chronic renal failure (eGFR < 30 mL/min/1.73 m2) or severe contrast allergy. Although ultrasound is not accepted as a standard modality for UTUC investigation, we reported on ultrasound results when it was available (either performed as first imaging modality in the community or in cases of chronic renal failure or severe contrast allergy). The medical chart of each patient was reviewed to obtain demographics, cytological findings and the interpretation of preoperative imaging. Because of the retrospective design of this study our institutional ethics committee waived the need for written informed consent from participants.
Complete endoscopic examination of the ureter, renal pelvis and calyx has been performed in all patients. Ureteroscopy was performed following a retrograde study, using 8FR rigid ureteroscope (Wolf), advancing the instrument as much as possible. Renal inspection was performed using flexible ureteroscope (DUR-8 (ACMI) or in later years the Flex- X™ (Stortz)). Biopsy forceps or stone collection basket were used to obtain tissue from suspected lesion. Tumor grade and stage were assigned using the world health organization classification [7] and the 6th edition of the AJCC/UICC TNM staging system [8] by specialized pathologists at our institution. Patients with histologically confirmed UTUC were referred to nephroureterectomy usually within one month following the diagnostic procedure or, in selected cases, managed conservatively with endoscopic resection. Patients with low grade, <1 cm UTUC were eligible for endoscopic treatment. Holmium laser and a bugbee electrode were utilized for tumor resection and ablation.
Intra- and peri-operative complications were reviewed and staged according to Clavien-Dindo classification [9]. The first follow-up ureteroscopy was preformed 3 month after endoscopic tumor resection. Thereafter, patients were followed with ureteroscopy or CTU, alternately, every 3 months during the first two years. Patients with chronic kidney disease were referred to a nephrologist for consultation. Follow-up data was reviewed to include the oncological outcomes (disease recurrence and progression) of patients managed with nephron sparing endoscopic approach.
The diagnostic value (positive and negative predictive values) of urine cytology, ultrasound and CTU was determined. Positive cytology was defined as malignant cells or atypical cells, highly suggestive of urothelial carcinoma. Intraluminal Soft-tissue mass, demonstrated by ultrasound, or filling defect in contrast opacified collecting system, demonstrated by CTU, were considered positive for UTUC. The endoscopic appearance, supported by histologic examination, when available, served as the standard of reference.
Results
Clinical characteristics and diagnostic studies
The study cohort included 116 patients who underwent diagnostic ureteroscopy between November 2003 and December 2010. Demographics and clinical characteristics of the study patients are summarized in Table 1. Half (51 %) of the patients reported at least one episode of gross hematuria and 35 % had a history of lower urinary tract urothelial carcinoma. The preureteroscopic evaluation included urine cytology, ultrasound and CTU in 91 (78 %), 84 (72 %) and 89 (77 %) patients, accordingly. Results of all three studies were available in 47 patients (40.5 %). CTU was not performed in 22 patients with chronic renal failure and 3 patients with severe contrast allergy. Detailed description of the findings, according to the performed study is presented in Table 2. Visual diagnosis of UTUC was made in 47 patients (40 %), supported by histology in 38 of them (80 %). A reliable histological report could not be obtained in 9 patients due to insufficient amount of tissue or technical artifacts.
Table 1.
Demographic and clinical patient characteristics
| Characteristic | N = 116 |
|---|---|
| Age, years | |
| Median (IQR) | 70.5 |
| Mean (range) | 68 (16–90) |
| Patient gender n, (%) | |
| Female | 38 (33) |
| Male | 78 (67) |
| Hematuria n, (%) | |
| Macroscopic | 59 (51) |
| Microscopica | 88 (76) |
| History of LUTUC n, (%) | |
| None | 75 (65) |
| Low grade | 24 (21) |
| High grade | 17 (14) |
LUTUC Lower urinary tract urothelial carcinoma
a Defined as three or more red blood cells per high power microscopic filed on urinary sediment
Table 2.
Results of preoperative evaluation - cytological, sonographic and radiographic findings
| Method | |
|---|---|
| Urine Cytology (n, %) | |
| Atypia | 26 (22) |
| Dysplasia | 10 (9) |
| Normal | 55 (48) |
| N/A | 25 (21) |
| Ultra-Sound (n, %) | |
| Intra-luminal mass | 9 (9) |
| Dilation | 43 (37) |
| No finding | 32 (27) |
| N/A | 32 (27) |
| CTU (n, %) | |
| Filling defect | 38 (33) |
| Wall thickening | 14 (12) |
| Dilation | 39 (33) |
| External mass | 5 (4) |
| No finding | 12 (10) |
| N/A | 27 (22) |
Of the 38 patients, 27 (71 %) had low grade disease and 11(29 %) had high grade disease on biopsy. In 24 cases tumor stage was determined. Ta, T1 and T2 were assigned in 21 (87 %), 2 (8 %) and 1 (5 %) cases, respectively.
All complications observed in this study were Clavien grade I or II. Intraoperative complications included contrast extravasation, observed during ureteroscopy, in 4 patients (3 %). All patients were managed with ureteral stent for one week with no clinical sequel. Febrile UTI and renal colic were observed after ureteroscopy in 7 (6 %) and 4 (3 %) patients, respectively. Conservative treatment was successfully applied in all cases.
Correlation with ureteroscopy findings
Positive urinary cytology, reported in 36 of 91patients, included atypia in 26 and dysplasia in 10 patients. The calculated PPV and NPV for UTUC were 47 and 58 %, accordingly. Sonographic appearance of intraluminal mass in 9 patients (9 %), yielded PPV and NPV of 89 and 65 %. Typical filling defect, demonstrated during the excretory phase of CTU, was described in 38 of 89 patients. PPV and NPV of CTU were 76 and 80 %, respectively. Considering “wall thickening” as a positive result, increased the NPV to 90 % but decreased the PPV to 67 %. In 4/39 patients with confirmed UTUC no “filing defect” or “wall thickening” was demonstrated on CTU. Three of these patients had Ta low grade tumors in the ureter and one patient had carcinoma in situ of the renal pelvis. Table 3 summarizes the association between the results of preoperative studies and ureteroscopic diagnosis.
Table 3.
Correlation of preoperative studies with the ureteroscopic results
| Preoperative findings | Ureteroscopic results | ||
|---|---|---|---|
| Positive for UTUC | Negative for UTUC | Total | |
| CTU | |||
| Positive | 29 | 9 | 38 |
| Negative | 10 | 41 | 51 |
| Total | 39 | 50 | 89 |
| Ultrasound | |||
| Positive | 8 | 1 | 9 |
| Negative | 26 | 49 | 75 |
| Total | 34 | 50 | 84 |
| Urine cytology | |||
| Positive | 17 | 19 | 36 |
| Negative | 23 | 32 | 55 |
| Total | 40 | 51 | 91 |
Filling defect on CTU – ureteroscopic results and therapeutic outcomes
UTUC had been confirmed during URS in 29 of 38 (76 %) patients with characteristic filling defect on CTU. 7 patients had high grade disease on biopsy, including: stage T1 in 4, T2 in 1 and non- determined stage in 2 patients. 20 patients had Ta low grade and in 2 patients neither stage nor grade could be determined. UTUC has been ruled out in 9 patients (24 %). Mucosa fold, small extraluminal mass and parapelvic hyperdense cyst and bullous mucosal edema of the renal pelvis were found in 4 patients and no findings, explaining the filling defects, were reported in the remaining 5 patients. No additional intervention was required in these cases.
Patients with UTUC were subjected to nephroureterectomy or endoscopic resection according to tumor characteristics (see above). Two patients, found to have metastatic disease, were referred to cisplatin based chemotherapy.
Overall, 20 patients underwent nephroureterectomy while nephron sparing endoscopic approach was implemented in 7 patients who underwent tumor resection with continues surveillance. One of these patients was lost to follow-up. During a median follow up period of 17 months (IQR, 9–25) none of the 6 followed patients experienced disease progression. Tumor recurrence was diagnosed and treated successfully in 2 patients, 5 and 14 months after initial resection (Fig. 1).
Fig. 1.
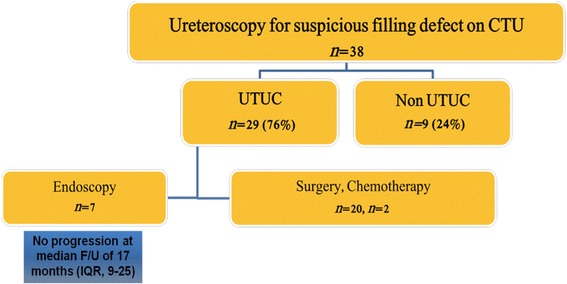
Diagnostic-Therapeutic Flowchart of patients with filling defect on pre-ureteroscopic CTU
Discussion
CTU is currently the recommended imaging modality for evaluation of the upper urinary tract. With ongoing radiological improvements, the added value of diagnostic ureteroscopy is not always clear. We examined the current role of ureteroscopy in the diagnosis of urothelial carcinoma. We found that nephroureterectomy was spared from 42 % (16/38) of patients who underwent ureteroscopy for presumptive UTUC, based on CTU. UTUC was ruled out in 9 patients and treated endoscopically in 7 patients. In the nephron sparing group we observed no disease progression during short term follow-up.
Although UTUC is relatively rare, its incidence has increased in the last decades [10, 11]. One possible explanation for this trend is increased use of CTU, a sensitive tool to detect upper urinary tract abnormalities [2]. The ability of CTU to demonstrate small tumors is well accepted but at the same time, growing use of CTU may increase the detection of benign findings. The positive predictive value (PPV) of CTU for the detection of UTUC is variable, depends on CTU technique and has been reported to be moderate (50-80 %) [12–15]. Intra- and extra-luminal conditions may simulate UTUC on CTU. Inflammatory changes, debris or blood clots may mimic intraluminal tumor. Indentation by extraluminal mass or vascular malformation may also cause false positive results [12]. In our study, the PPV of CTU was 80 %. Non-UTUC findings (mucosa fold, small extraluminal mass and parapelvic hyperdense cyst and bullous mucosal edema) were found during ureteroscopy in 4/9 cases and normal upper urinary tract was described in 5/9 cases. None of these patients had positive urinary cytology. Sadow et al. reported on extremely low PPV in small (≤5 mm) upper urinary tract filling defects on CTU [14]. All 17 cases of small filling defects in their study were found to be false positive. In accordance, the five completely normal ureteroscopies in our study were performed in patients with small filling defects on CTU.
The treatment options for confirmed UTUC include nephroureterectomy or, in selected patients, nephron sparing procedure. In the last two decades endoscopic management of UTUC has been extended from patients with imperative indications (chronic kidney disease, bilateral disease or solitary kidney) to selected patients with elective indications. In a review article, Cutress et al. reported that the long term renal preservation rate in patients treated endoscopically is 80 % [16]. This information is highly important when considering the potential cardiovascular and overall survival benefits of renal function preservation [17]. Recently published, long term retrospective studies showed that low grade UTUC can be managed endoscopically with cancer related and overall survival equivalent to that of nephroureterectomy [18–20]. Because tumor grade is a significant factor, determining the oncological outcomes of UTUC in patients treated endoscopically, ureteroscopic biopsy is of paramount importance. In our study, 7/38 patients with presumptive UTUC on CTU were treated endoscopically. All had biopsy confirming low grade UTUC less than 1 cm in size. Recurrence occurred in two patients and was amenable for repeat endoscopic resection. No progression was observed during a median follow-up time of 17 months. All complications observed in this study were Clavien grade I or II. Febrile UTI and renal colic were the only post operative complications, reported in 7 (6 %) patients and 4 (3 %) patients, respectively. All patients were successfully treated with antibiotics and analgesics. The potential adverse oncological effect that may be related to delayed nephroureterectomy was beyond the scope of our study, but was addressed in previous publications. No difference in 5 years metastasis-free, cancer specific or overall survival was found in patients who underwent diagnostic ureteroscopy prior to nephroureterectomy compared with patients who did not undergo ureteroscopy [21–23].
Our study is undoubtedly limited by its retrospective design and small size. By the reliance on imaging interpretation, CTU technique was not uniform and the results were subjected to inter observer bias. Despite that, the PPV of CTU in our study is in accordance with results of previous studies [12–15]. Another limitation is the relatively short follow up period of patients treated endoscopically. As mention above, high rate of renal preservation and favorable oncological outcomes were observed in long term studies when patients were carefully selected. This is also reflected by the European guidelines on UTUC, which recognize the option of endoscopic treatment in low grade low stage UTUC [24].
Conclusions
In this retrospective study, nephroureterectomy was spared from 42 % of patients with presumptive UTUC, demonstrated on CTU. In about half of those patients UTUC has been ruled out and the others were managed endoscopically. Although false positive seems to occur mainly in small filling defects on CTU, there is no room for diagnostic error when nephroureterectomy is being considered. Hence, our study favors ureteroscopy as a crucial step in confirming UTUC and treatment planning.
Acknowledgements
No financial support was received for this study.
Abbreviations
- UTUC
Upper tract urothelial carcinoma
- CTU
Computerized tomography urography
- PPV
Positive predictive value
- NPV
Negative predictive value
Footnotes
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
SG has made substantial contributions to conception and design, acquisition of data and data analysis. He has drafted the manuscript and approved the submitted version. AN has made substantial contributions to study design and manuscript revising. He approved the submitted version. DL has made substantial contributions to conception and design, data analysis and interpretation. He has been involved in drafting and revising the manuscript and approved the submitted version. All authors read and approved the final manuscript.
Contributor Information
Shay Golan, Phone: 972-3-937-6553, Email: shaygo1@yahoo.com.
Andrei Nadu, Email: andrein2002@yahoo.com.
David Lifshitz, Email: davidlif@clalit.org.il.
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics. CA Cancer J Clin. 2012;62(1):10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 2.Wang LJ, Wong YC, Huang CC, Wu CH, Hung SC, Chen HW. Multidetector computerized tomography urography is more accurate than excretory urography for diagnosing transitional cell carcinoma of the upper urinary tract in adults with hematuria. J Urol. 2010;183(1):48–55. doi: 10.1016/j.juro.2009.08.144. [DOI] [PubMed] [Google Scholar]
- 3.Gadzinski AJ, Roberts WW, Faerber GJ, Wolf JS., Jr Long-term outcomes of nephroureterectomy versus endoscopic management for upper tract urothelial carcinoma. J Urol. 2010;183(6):2148–2153. doi: 10.1016/j.juro.2010.02.005. [DOI] [PubMed] [Google Scholar]
- 4.Grasso M, McCue P, Bagley DH. Multiple urothelial recurrences of renal cell carcinoma after initial diagnostic ureteroscopy. J Urol. 1992;147(5):1358–1360. doi: 10.1016/s0022-5347(17)37565-1. [DOI] [PubMed] [Google Scholar]
- 5.Lim DJ, Shattuck MC, Cook WA. Pyelovenous lymphatic migration of transitional cell carcinoma following flexible ureterorenoscopy. J Urol. 1993;149(1):109–111. doi: 10.1016/s0022-5347(17)36014-7. [DOI] [PubMed] [Google Scholar]
- 6.Wein AJ, Kavoussi LR, Novick AC, Partin AW, Peters CA. Campbell-Walsh Urology, Vol two. 10 2011. [Google Scholar]
- 7.Epstein JI, Amin MB, Reuter VR, Mostofi FK. The World Health Organization/International Society of Urological Pathology consensus classification of urothelial (transitional cell) neoplasms of the urinary bladder. Bladder Consensus Conference Committee. Am J Surg Pathol. 1998;22(12):1435–1448. doi: 10.1097/00000478-199812000-00001. [DOI] [PubMed] [Google Scholar]
- 8.Greene GL, Page D, Fleming ID. AJCC Cancer Staging Manual. Genitourinary Sites: Renal Pelvis and Ureter. New York: Springer Verlag; 2002. [Google Scholar]
- 9.Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240(2):205–213. doi: 10.1097/01.sla.0000133083.54934.ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eylert MF, Hounsome L, Verne J, Bahl A, Jefferies ER, Persad RA. Prognosis is deteriorating for upper tract urothelial cancer: data for England 1985–2010. BJU Int. 2013;112(2):E107–113. doi: 10.1111/bju.12025. [DOI] [PubMed] [Google Scholar]
- 11.Raman JD, Messer J, Sielatycki JA, Hollenbeak CS. Incidence and survival of patients with carcinoma of the ureter and renal pelvis in the USA, 1973–2005. BJU Int. 2011;107(7):1059–1064. doi: 10.1111/j.1464-410X.2010.09675.x. [DOI] [PubMed] [Google Scholar]
- 12.Cowan NC, Turney BW, Taylor NJ, McCarthy CL, Crew JP. Multidetector computed tomography urography for diagnosing upper urinary tract urothelial tumour. BJU Int. 2007;99(6):1363–1370. doi: 10.1111/j.1464-410X.2007.06766.x. [DOI] [PubMed] [Google Scholar]
- 13.Sudakoff GS, Dunn DP, Guralnick ML, Hellman RS, Eastwood D, See WA. Multidetector computerized tomography urography as the primary imaging modality for detecting urinary tract neoplasms in patients with asymptomatic hematuria. J Urol. 2008;179(3):862–867. doi: 10.1016/j.juro.2007.10.061. [DOI] [PubMed] [Google Scholar]
- 14.Sadow CA, Wheeler SC, Kim J, Ohno-Machado L, Silverman SG. Positive predictive value of CT urography in the evaluation of upper tract urothelial cancer. AJR Am J Roentgenol. 2010;195(5):W337–343. doi: 10.2214/AJR.09.4147. [DOI] [PubMed] [Google Scholar]
- 15.Xu AD, Ng CS, Kamat A, Grossman HB, Dinney C, Sandler CM. Significance of upper urinary tract urothelial thickening and filling defect seen on MDCT urography in patients with a history of urothelial neoplasms. AJR Am J Roentgenol. 2010;195(4):959–965. doi: 10.2214/AJR.09.4177. [DOI] [PubMed] [Google Scholar]
- 16.Cutress ML, Stewart GD, Zakikhani P, Phipps S, Thomas BG, Tolley DA. Ureteroscopic and percutaneous management of upper tract urothelial carcinoma (UTUC): systematic review. BJU Int. 2012;110(5):614–628. doi: 10.1111/j.1464-410X.2012.11068.x. [DOI] [PubMed] [Google Scholar]
- 17.Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351(13):1296–1305. doi: 10.1056/NEJMoa041031. [DOI] [PubMed] [Google Scholar]
- 18.Grasso M, Fishman AI, Cohen J, Alexander B. Ureteroscopic and extirpative treatment of upper urinary tract urothelial carcinoma: a 15-year comprehensive review of 160 consecutive patients. BJU Int. 2012;110(11):1618–1626. doi: 10.1111/j.1464-410X.2012.11066.x. [DOI] [PubMed] [Google Scholar]
- 19.Cutress ML, Stewart GD, Tudor EC, Egong EA, Wells-Cole S, Phipps S, et al. Endoscopic versus laparoscopic management of noninvasive upper tract urothelial carcinoma: 20-year single center experience. J Urol. 2013;189(6):2054–2060. doi: 10.1016/j.juro.2012.12.006. [DOI] [PubMed] [Google Scholar]
- 20.Simhan J, Smaldone MC, Egleston BL, Canter D, Sterious SN, Corcoran AT, et al. Nephron sparing management versus radical nephroureterectomy for low or moderate grade, low stage upper tract urothelial carcinoma. BJU Int. 2014;114(2):216–20. doi: 10.1111/bju.12341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hendin BN, Streem SB, Levin HS, Klein EA, Novick AC. Impact of diagnostic ureteroscopy on long-term survival in patients with upper tract transitional cell carcinoma. J Urol. 1999;161(3):783–785. doi: 10.1016/S0022-5347(01)61768-3. [DOI] [PubMed] [Google Scholar]
- 22.Boorjian S, Ng C, Munver R, Palese MA, Vaughan ED, Jr, Sosa RE, et al. Impact of delay to nephroureterectomy for patients undergoing ureteroscopic biopsy and laser tumor ablation of upper tract transitional cell carcinoma. Urology. 2005;66(2):283–287. doi: 10.1016/j.urology.2005.02.022. [DOI] [PubMed] [Google Scholar]
- 23.Nison L, Roupret M, Bozzini G, Ouzzane A, Audenet F, Pignot G, et al. The oncologic impact of a delay between diagnosis and radical nephroureterectomy due to diagnostic ureteroscopy in upper urinary tract urothelial carcinomas: results from a large collaborative database. World J Urol. 2013;31(1):69–76. doi: 10.1007/s00345-012-0959-1. [DOI] [PubMed] [Google Scholar]
- 24.Roupret M, Babjuk M, Comperat E, Zigeuner R, Sylvester R, Burger M, et al. European guidelines on upper tract urothelial carcinomas: 2013 update. Eur Urol. 2013;63(6):1059–1071. doi: 10.1016/j.eururo.2013.03.032. [DOI] [PubMed] [Google Scholar]


