Abstract
Reactive oxygen species (ROS), particularly hydrogen peroxide, and the proteins that regulate them play important roles in the migration and adhesion of cells. Stimulation of cell surface receptors with growth factors and chemoattractants generates ROS, which relay signals from the cell surface to key signaling proteins inside the cell. ROS act within cells to promote migration and also in nonmigrating cells to influence the behavior of migrating cells. Hydrogen peroxide has also been suggested to act as a chemoattractant in its own right, drawing immune cells to wounds. We discuss recent progress made towards understanding how organisms use ROS, and to what degree they depend on them, during the related processes of cell migration and adhesion.
ROS in migration
The movement and migration of cells are crucial during the development of organisms as they transition from embryo to adult, and for the homeostasis of adult tissues. Cell migration and adhesion also play important roles in the pathology of diseases such as metastatic cancer, which can inappropriately reactivate developmental migratory programs. Although the cell movements that occur during both normal and pathological processes are remarkably diverse [1], studies of these movements have revealed several common features [1–7]. Migration often begins when a cell or group of cells receives a signal that triggers polarization and extension of cellular protrusions, such as lamellipodia, in the direction of movement. These protrusions then adhere to the substrate on which the cell is moving, providing traction for migration, while the lagging edge of the cell retracts. Gradients of attractive and repulsive cues are used to direct cell migration. Chemoattractants are received by transmembrane proteins on the surface of migrating cells and direct their migration by translating these cues into cytoskeletal and adhesive changes through effector molecules. Changes in the substrate on which cells move also influence cell migration. Immune cells, for instance, require changes in the endothelium to penetrate tissues and clear infection.
Mounting evidence suggests that ROS, and hydrogen peroxide in particular, are used to relay signals from activated cell surface receptors to direct changes necessary for cell movement. ROS act intrinsically within migrating cells to promote movement, and permissively in the surrounding stationary cells to influence migration. It has also been suggested that hydrogen peroxide acts as a primary chemoattractant produced upon injury to attract immune cells to wounds [8]. In this review, we discuss the latest progress made towards understanding the roles of ROS in cell migration and adhesion, paying particular attention to in vivo studies.
General principles of redox signaling
Initially thought to be entirely unwanted byproducts of oxidative respiration, ROS are now known to act beneficially as signaling molecules regulating various cellular functions, including cell proliferation, migration and adhesion [9,10]. The general mechanism by which ROS are thought to signal is as follows: (i) in response to stimuli, such as growth factors, ROS are generated at the surface of cells or within intracellular compartments, such as endosomes, by NADPH oxidases; (ii) ROS enter the cytoplasm, where they react with specific proteins to modulate protein function; (iii) the changes that ROS induce in protein activity, in part, drive cellular processes such as migration; and (iv) once the stimulus is no longer present, the ROS are degraded and the system returns to its original state. Below, we describe this process in greater detail. For the purposes of this review, the term ROS is defined as molecules containing oxygen-centered radicals such as the superoxide radical anion (O2•−), as well as reactive non-radical derivatives of molecular oxygen such as hydrogen peroxide (H2O2) [11]. Each ROS has distinct properties and activities, and where possible we avoid the term ROS and instead refer to the particular species involved. However, because of methodological difficulties in measuring ROS and distinguishing between species, often the precise identity of the particular ROS involved is unknown. In such cases the term ROS is used.
The binding of growth factors and chemoattractants to cell surface receptors triggers NADPH oxidases to generate ROS. NADPH oxidases are membrane protein complexes that generate ROS by transferring electrons from NADPH (or NADH) across membranes to molecular oxygen [12] (Figure 1, reaction 1). Defined by the specific NOX or DUOX catalytic subunit they contain, seven members of the NADPH oxidase family have been identified in mammalian tissues to date [12]. NADPH oxidases are activated during cell migration and adhesion by numerous growth factors such as tumor necrosis factor-α (TNF-α) [13,14], angiopoietin-1 [15], platelet-derived growth factor (PDGF) [16], vascular endothelial growth factor (VEGF) [17–19] and extracellular matrix components [20–22]. How activation of cell surface receptors triggers NADPH oxidases to generate ROS during cell migration is not entirely clear. In endothelial cells, however, it is thought to be mediated by p21-activated kinase-1 (PAK1), the small GTPase Rac1 and NADPH oxidase regulatory subunits, such as p47phox [23]. Because of the diffusibility of ROS, their short-lived nature, and the abundance of ROS-degrading enzymes, localized production of ROS by NADPH oxidases is probably necessary for efficient signal transduction. Although some reports have also implicated mitochondrially generated ROS in cell adhesion and migration [21,24], most evidence suggests that ROS produced for these purposes are generated by NADPH oxidases [8,25,26].
Figure 1. The generation of ROS, their reaction with protein thiols, and their breakdown by ROS-degrading enzymes.
The mechanisms by which Prx, catalase and glutathione peroxidase degrade hydrogen peroxide are distinct; for simplicity, these mechanisms have not been included in reaction 6. Pr, protein; X, protein or glutathione.
NADPH oxidases primarily generate superoxide, which itself may act as a redox signal [27], but in most cases is rapidly degraded either spontaneously or enzymatically by superoxide dismutases (SODs) to hydrogen peroxide (Figure 1, reaction 2), which then acts to direct cellular changes for migration. Evidence also suggests that some NADPH oxidase isoforms, such as NOX4, may also release hydrogen peroxide directly [28]. NADPH oxidases produce superoxide or hydrogen peroxide in the extracellular or luminal space [12]. With some exceptions [29,30], for ROS to transduce signals they must first be internalized into the cytoplasm of the cell. Several mechanisms have been proposed regarding how extracellularly or luminally generated superoxide or hydrogen peroxide enters cells; how this occurs during cell migration and adhesion is not clear, but it may occur by passive diffusion or regulated uptake [12].
Once inside the cell, the NADPH oxidase-generated hydrogen peroxide can act as a redox signal by reversibly modifying the activity of specific proteins, most often by oxidizing responsive thiols on cysteine residues [10,31,32]. These redox modifications may occur directly, through the reaction of hydrogen peroxide with a protein thiol to form a sulfenic acid (Figure 1, reaction 3). The sulfenic acid can in turn go on to form disulfides with other protein thiols or with glutathione (GSH) (Figure 1, reaction 4). Alternatively, hydrogen peroxide may modify target protein thiols indirectly, for example by oxidation of other thiol proteins such as peroxiredoxins (Prxs), which then act as a redox relay to modify the target proteins [10,32]. The accessibility of a protein thiol and its reactivity (the proportion of it in a deprotonated, thiolate state at physiological pH) will determine in part whether a protein is regulated by hydrogen peroxide. If proteins such as Prx are used to relay redox signals from NADPH oxidases to target proteins, further specificity can be obtained through protein– protein interactions between the relaying protein and its target. The proximity of target proteins to the site of hydrogen peroxide generation may be yet another determinant of specificity [33]. In most instances, the exact redox-regulated proteins oxidized during migration and adhesion are unknown; however, ROS can activate transcription factors [34] and influence protein phosphorylation [18,35] during cell migration. Either directly or indirectly, hydrogen peroxide causes selective post-translational modifications of proteins, modulating their function and influencing cell migration.
Once the redox signaling event ends, biological processes operate to reverse the effect of the signal and return the system to its starting state. In most cases, hydrogen peroxide-induced modifications are reversible through thiol–disulfide exchange with reduced GSH and thioredoxin (Figure 1, reaction 5). Several antioxidant enzymes, such as catalase, glutathione peroxidases and Prxs, are also present within cells to degrade hydrogen peroxide (Figure 1, reaction 6) and rapidly terminate the signal. Indeed, manipulation of the levels of these enzymes has been used extensively to infer the involvement of ROS in cell migration and adhesion. Given the abundant expression of enzymes such as Prxs, how do hydrogen peroxide signals accumulate in the first place? Recent evidence suggests that localized inactivation of antioxidant enzymes, such as PrxI, allows for transient accumulation of hydrogen peroxide around membranes, where signaling components are concentrated [36].
To summarize, in response to stimuli, such as growth factors and chemoattractants, receptors on the surface of proteins activate NADPH oxidases, in most instances to generate superoxide. This superoxide is then converted to hydrogen peroxide, which reacts locally with specific proteins to modulate their function. In the next section we discuss how migrating cells use this mode of signaling to facilitate movement and how ROS also act non-autonomously in non-migrating cells to influence cell migration.
ROS act within cells to promote their migration
Early indications that ROS might play a role within cells to promote their migration came from in vitro studies showing that treatment of endothelial-derived cells, neutrophils, fibroblasts and vascular smooth muscle cells (VSMCs) with ROS-degrading enzymes suppressed migration of these cells towards growth factors and chemoattractants [15,16,37–40]. The use of nonspecific ROS probes showed that ROS were produced in these cells upon stimulation with growth factors [15,16,37,39,40]. Both superoxide and hydrogen peroxide were suggested to promote cell migration because enhanced degradation of either species slowed chemoattractant-induced chemotaxis [15,16,37,39,40].
In endothelial-derived cells and leukocytes, the source of ROS was determined to be NADPH oxidase because inhibition of NADPH oxidase or reduction in the levels of NOX2 (also called gp91phox), a catalytic subunit of NADPH oxidase, significantly reduced ROS generation and impeded cell migration in vitro [15,17,19,41,42]. In vitro studies in VSMCs have also implicated NADPH oxidase-derived ROS in cell migration because reducing the levels of the NADPH oxidase catalytic subunits NOX1 or NOX4 impedes growth factor-induced VSMC migration, whereas overexpression of NOX1 increases it [43–45]. These results are further supported by in vivo studies of angiogenesis in mice, where the migration of vascular endothelial cells into subcutaneous implanted VEGF-treated sponges was significantly retarded in NOX2−/− mice in comparison to wild-type animals [19]. Further studies have suggested that NOX2-derived ROS also play an important role in cell migration during the formation of new blood vessels (neovascularization) after tissue ischemia. In NOX2−/− mice, both ischemia-induced ROS production and neovascularization were impaired [46]. However, this has been challenged by a study that found the opposite: that NOX2 deficiency improves ischemia-induced neovascularization in mice [47]. The reasons for the discrepancy remain obscure. NADPH oxidase and ROS are also involved in thickening of the blood vessel wall (neointimal lesion formation) in response to arterial injury, a process that involves the migration of VSMCs and myofibroblasts into the intima and their subsequent proliferation [48]. Deletion of either NOX1 or NOX2 causes a reduction in the neointimal lesion formation in response to arterial injury [45,49], whereas adenovirus-mediated overexpression of the cytosolic regulator subunit of NOX1, NOXA1, leads to a significant increase in neointimal lesion formation as well as ROS production in medial VSMCs [50,51]. Lastly, in vivo experiments also suggest a role for NOX2 during neutrophil migration because adoptively transferred NOX2-deficient neutrophils showed impaired recruitment to sites of inflammation in live mice [41]. Evidence from in vitro assays of chemoattractant-induced migration and in vivo models of cell migration supports the involvement of NADPH oxidase-generated ROS in migration.
Recent evidence suggests that NADPH oxidase might not be the only source of ROS in endothelial cells produced during cell migration. Treatment of endothelial-derived cells with VEGF caused an increase in hydrogen peroxide in both mitochondria and the cytoplasm, as measured using the hydrogen peroxide sensor HyPer [52,53]. Incubation with a mitochondrial-targeted version of vitamin E (mitoE) reduced hydrogen peroxide in both mitochondria and the cytoplasm, and impeded VEGF-induced endothelial cell migration in vitro and in vivo, suggesting that mitochondria are an important source of hydrogen peroxide during cell migration [53]. Because nonspecific effects of mitochondrial-targeted compounds have been reported previously [54], it remains unclear whether these could account for the decrease in hydrogen peroxide levels and the effects on cell migration. However, expression of a mitochondrial-targeted catalase also impeded migration, again suggesting that mitochondrial hydrogen peroxide may indeed play a role in endothelial cell migration [53].
Studies in mice that either lack or overexpress ROS-degrading enzymes also support a role for ROS in cell migration in vivo. Mice lacking extracellular SOD (SOD3) or intracellullar copper–zinc SOD (SOD1) showed impaired ischemia-induced blood flow recovery and angiogenesis in the hindlimb [55,56]. Conversely, overexpression of SOD3 increased extracellular hydrogen peroxide production and promoted angiogenesis after hindlimb ischemia [18], suggesting that superoxide does not promote migration directly, but rather through its conversion to hydrogen peroxide. The importance of hydrogen peroxide in cell migration is further supported by studies with mice lacking PrxII, where VSMC migration, as measured by neointimal lesion formation, was increased upon injury [37].
Taken together, numerous in vivo and in vitro studies support the involvement of NADPH oxidase-derived hydrogen peroxide in mediating migration from within endothelial cells. However, because of the nature of the various models used to study migration in vivo, there remains some doubt as to whether hydrogen peroxide is (i) acting autonomously within cells to influence their migration and/or (ii) influencing other processes, such as proliferation, which could provide an alternative explanation of the observed phenotypes. To address tissue dependence and, more specifically, assess the role of hydrogen peroxide in cell migration, tissue-specific manipulation of gene expression and live imaging of migrating cells in vivo will be necessary.
A much more detailed understanding of the coordination and production of ROS during cell migration has been gleaned from recent in vitro studies. These studies show that production of ROS in migrating endothelial-derived cells is probably spatially and functionally coordinated at the leading edge to promote directional cell migration (Figure 2). Both NOX2 and its regulatory subunit p47phox localize to the leading edge of motile endothelial-derived cells [35,57,58]. NOX2 colocalizes and associates there with actin and the scaffold protein IQ motif-containing GTPase activating protein 1 (IQGAP1) during migration [57]; disruption of either actin or IQGAP1 causes loss of NOX2 at the leading edge [57]. p47phox also associates with actin and the proteins TNF receptor-associated factor 4 (TRAF4) and Wiskott–Aldrich syndrome protein family verprolin homologous protein 1 (WAVE1), which possibly recruit it to focal complexes and membrane ruffles, respectively [35,58]. The production of ROS by NADPH oxidase also depends on its interaction with Rac1 and PAK1 because expression of dominant negative forms of Rac1 and PAK1 block ROS production, membrane ruffling and cell migration [15,35,58]. Owing to a lack of sensitive, specific probes for hydrogen peroxide that allow quantitative and dynamic assessment in live cells, accumulation of hydrogen peroxide at the leading edge or in membrane ruffles has not yet been observed directly in migrating cells. Application of recently developed hydrogen peroxide probes, such as the genetically encoded fluorescent probe HyPer and small-molecule boronate-based fluorophores, may overcome this limitation [52,59].
Figure 2. The mechanism by which ROS are generated and act within endothelial-derived cells to promote migration.
During cell migration, NOX2, p47phox and probably other NADPH oxidase subunits are targeted to the leading edge of the cell, where they generate superoxide in a Rac1- and PAK1-dependent manner. Superoxide is produced into the extracellular or luminal space, where it is converted to hydrogen peroxide by SOD and enters into the cytoplasm of the cell. Intracellular hydrogen peroxide then oxidizes proteins such as PTPs (possibly directly or through other thiol proteins) to promote migration.
Exactly how oxidase activity and ROS production at the leading edge promote migration remains unclear. Several processes that occur during migration, such as actin polymerization and restructuring [42,57] and changes in gene expression and protein phosphorylation [15,17,19], are dependent on ROS in certain contexts; however, in most instances the direct targets of hydrogen peroxide oxidation are not known. Oxidation of protein tyrosine phosphatases (PTPs), PTP1B, density-enhanced phosphatase-1 (DEP-1), low molecular weight (LMW)-PTP and PTP-PEST were observed in cells under conditions that promote migration [18,21,35]. PTPs negatively regulate cell migration by dephosphorylating and inactivating proteins that promote migration, such as the focal adhesion kinase Pyk2 and p160Rho-GAP [21,35,60]. PTPs have an active-site cysteine residue that is susceptible to direct oxidation by hydrogen peroxide, causing reversible inhibition of their function [61]. Given their sluggish reaction rates with hydrogen peroxide, however, it remains unclear whether PTPs are reactive enough to appreciably compete for hydrogen peroxide in vivo [62]. Colocalization and compartmentalization of PTPs with NADPH oxidases might facilitate PTP oxidation and inactivation [33]. Alternatively, oxidation of PTPs may not be direct; instead, they may be mediated through another thiol protein such as a Prx [62]. Whether NADPH oxidase-generated ROS oxidize multiple targets or exert their function entirely through PTPs to influence cell migration also remains unclear. Very recently, the accumulation of protein sulfenates (Figure 1, reaction 3) was observed at the leading edges of migrating endothelial-derived cells using the biotin-conjugated dimedone analog DCP-Bio1 [63]. Further analysis revealed IQGAP1 to be one such protein, identifying it as a putative direct target of hydrogen peroxide oxidation [63]. Precisely which cysteine residues on IQGAP1 are modified and how this affects IQGAP1 function are unknown. Further experimentation using similar redox proteomic techniques [64–66] and unbiased genetic screening may lead to the identification of other direct targets of ROS during migration, clarifying the means by which hydrogen peroxide acts within cells to influence their migration.
Taken together, accumulating evidence supports a model whereby cells generate hydrogen peroxide by activating NADPH oxidases at their leading edges. In endothelial cells, this process depends on the localization and activation of NADPH oxidase, which is dependent on various scaffold proteins, Rac1 and PAK1. The hydrogen peroxide generated at the leading edge then oxidizes proteins, such as PTPs, to bring about changes that promote migration through incompletely understood mechanisms.
ROS act within non-migrating cells to influence cell migration and adhesion
In addition to acting within migrating cells, ROS mediate changes in non-migrating cells that permit motile cells to bind and penetrate tissues. The mechanisms by which ROS mediate these permissive changes has been most extensively studied in vascular mammalian endothelial cells with respect to how they influence the migration and shuttling of leukocytes between the bloodstream and interstitial tissues. Upon injury, free-flowing leukocytes are captured from the circulation by adhesion to the endothelium, leading to leukocyte rolling along the vasculature. This rolling stimulates firm attachment and the subsequent migration of leukocytes through the endothelium into the extravascular tissue. How ROS act within endothelial cells to facilitate the adhesion and penetration of leukocytes is discussed later (Figure 3a). Recent research suggests that some of these mechanisms may be conserved in other cell types.
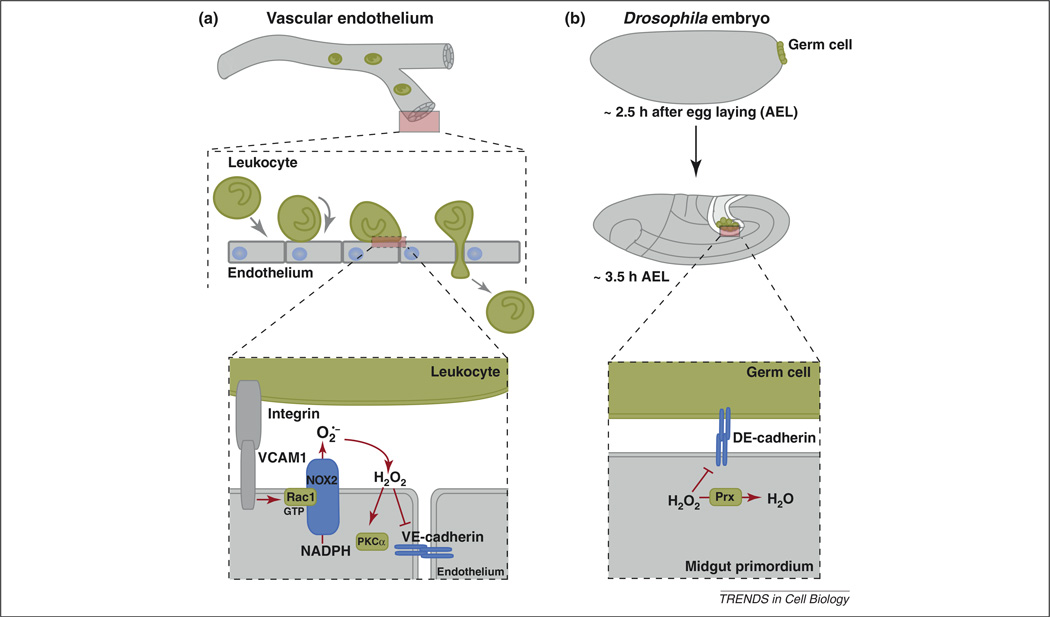
Figure 3. ROS influence cell–cell adhesion in mammals during transendothelial migration of leukocytes and in Drosophila embryos during germ cell migration.
(a) As leukocytes migrate across an endothelium toward a wound, NADPH oxidase is activated when integrins on leukocytes bind to endothelial VCAM1 through a process that requires Rac1. The hydrogen peroxide generated then oxidizes proteins such as PKC-α (possibly directly or through other thiol proteins) and decreases the surface expression of VE-cadherin, allowing gaps to form through which leukocytes pass. (b) As Drosophila embryos develop, germ cells (which form at the posterior of the embryo) are taken into the embryo during the process of gastrulation. The cell adhesion molecule E-cadherin is required for all germ cells to be taken into the embryo during this process. Loss of the Drosophila cytosolic Prx Jafrac1 causes a decrease in the levels of E-cadherin, resulting in a failure of germ cells to adhere properly to the embryo and to be internalized.
In response to infection or injury, locally presented stimulating factors, such as cytokines, induce endothelial cells that line blood vessel walls to establish adhesive contacts with immune cells (Figure 3a) [67,68]. ROS were first suggested to play a role in this process when it was noted that treatment of endothelial cell cultures with moderate to high concentrations of hydrogen peroxide (100 µM to 5 mM) induced cell surface expression of the adhesion molecules intercellular adhesion molecule 1 (ICAM-1) and P-selectin. This treatment also increased the binding of polymorphonuclear leukocytes (PMNs) to endothelial cells [69–71]. The magnitude of this hydrogen peroxide-induced, endothelial cell-dependent adhesion to PMNs was similar to that induced by the cytokine TNF-α, raising the possibility that TNF-α might act through hydrogen peroxide to increase cell surface expression of adhesion molecules [71]. Indeed, subsequent experiments demonstrated that TNF-α, as well as two other cytokines, TNF-related activation-induced cytokine (TRANCE) and visfatin, induced a transient increase in ROS levels in endothelial cells. Furthermore, inhibition of NADPH oxidase attenuated this increase in ROS levels, as well as expression of the cell surface adhesion molecules ICAM-1 and vascular cell adhesion molecule 1 (VCAM-1) [13,14,34,72]. In addition to acting through hydrogen peroxide, superoxide itself has been suggested to direct TNF-α-induced surface expression of adhesion molecules because overexpression of SOD1 suppresses this expression [13]. How precisely hydrogen peroxide and superoxide promote the expression of adhesion molecules is unclear, but several reports have provided evidence that this is achieved through activation of the transcription factor NF-κB [13,14,34].
In vivo studies further support a role for ROS in endothelial cells in promoting expression of adhesion molecules and the subsequent binding of leukocytes. In mice lacking either the p47phox subunit of NADPH oxidase or NOX2, induction of VCAM-1, ICAM-1, E-selectin and P-selectin expression in vascular wall cells was reduced [73,74]. Adhesion of leukocytes to these cells upon inflammation was also reduced [74,75]. Chimeric mice lacking p47phox in either their leukocytes or their vessel walls showed reduced leukocyte to endothelial cell adhesion upon inflammation [74,75]. However, only mice with nonfunctional vessel wall p47phox oxidase had decreased expression of cellular adhesion molecules, suggesting that NADPH oxidase is required in vascular endothelial cells to increase adhesion molecule expression upon inflammation [74]. Transgenic overexpression of SOD1 and SOD3 also reduced endothelial cell to leukocyte adhesion during inflammation and adhesion molecule expression, respectively, further suggesting that, in vivo, this process is mediated by superoxide [75,76]. How superoxide induces the expression of adhesion molecules in vivo and whether its effects are direct or secondary, for example through increased oxidative stress, remain obscure. If superoxide acts directly, how it is mechanistically sensed is also unknown. The bacterial transcription factor SoxR uses an iron sulfur [Fe–S] cluster to sense superoxide [77]. Whether endothelial cell proteins use a similar mechanism has not yet been determined; however, both in vitro and in vivo evidence supports a role for ROS in promoting the expression of adhesion molecules on endothelial cells to facilitate the binding of leukocytes.
Once leukocytes establish contact with the endothelium near the site of injury, they must migrate across the endothelium to reach the site of infection or injury. The binding of leukocytes to the endothelium induces substantial morphological changes in both leukocytes and endothelial cells [68]. These changes together support leukocyte migration across and through the endothelium. In addition to promoting the binding of immune cells to endothelia, ROS play an important role in mediating some of the changes induced in endothelial cells upon leukocyte binding. In vitro, leukocyte binding to VCAM-1 activates NADPH oxidase-dependent ROS production through a Rac1- and Ca2+-dependent mechanism [25,78,79]. In vivo studies in mice that lack NOX2 in all cells except for hematopoietic cells further support this [80]. They show that in response to inflammation caused by ovalbumin challenges, reduced numbers of eosinophils (a type of leukocyte) migrate through the endothelium into the lung tissue and bronchoalveolar lavage of these mice [80].
A more detailed mechanistic understanding of how ROS act within endothelial cells to promote endothelial permeability and leukocyte transendothelial cell migration has been ascertained from recent in vitro experiments. ROS produced by VCAM-1 stimulation promote transendothelial migration by activating endothelial cell-associated matrix metalloproteinases (MMPs) on the extracellular surface [29], and by inducing changes in protein phosphorylation and actin [79,81,82]. Of note, recent evidence suggests that ROS produced by NOX1 and NOX2 also promote MMP secretion in VSMCs and macrophages, respectively [44,83]. That many of these changes can be mimicked by treatment of endothelial cells with hydrogen peroxide and attenuated by overexpressing catalase suggests that they are, indeed, caused by hydrogen peroxide [29,81]. Although changes in the activity of protein kinase C alpha (PKC-α), p38 mitogen-activated protein kinase (p38 MAPK) and PTP1B all occur upon VCAM-1 stimulation, so far only PKC-α has been shown to be oxidized by VCAM-1 stimulation [79,81,84]. Whether PKC-α is oxidized directly by hydrogen peroxide or indirectly by some other thiol protein is unknown, but oxidation of PKC-α transiently increases its activity [81,85]. How precisely changes in protein phosphorylation promote leukocyte transendothelial migration is not completely understood. However, hydrogen peroxide increases tyrosine phosphorylation and turnover of key endothelial cell–cell junction proteins such as vascular-endothelial cell cadherin (VE-cadherin), β-catenin and α-catenin, causing the formation of gaps through which leukocytes are thought to pass [86,87].
Although many studies have investigated how ROS act within endothelial cells to influence the migratory behavior of immune cells, recent research suggests that ROS might also act within other types of non-migrating cells to influence cell migration. Using a transwell migration assay, it was recently shown that human mammary fibroblasts (RMF-EG) stimulate the migration of a weakly invasive epithelial cell line (MCF-7) [88]. Inhibition of NADPH oxidase with diphenyleneiodonium (DPI) or knockdown of NOX4 in the fibroblast cell line abolished their migratory stimulating properties. Precisely how fibroblast NOX4 promotes epithelial cell migration remains unclear, although NADPH oxidases have been implicated in chemokine secretion in other contexts [89,90]. Hydrogen peroxide is not thought in this case to act directly as a chemoattractant, however, because addition of catalase to the medium did not diminish stimulated epithelial cell migration [88].
Although some of the described functions of ROS may be specific to endothelial cells, several studies have shown that ROS induce loss of E-cadherin expressed on the outer surface of several cell types in vitro, suggesting this might be a more general phenomenon [24,91–93]. Recently, studies of germ cell migration in Drosophila have demonstrated that hydrogen peroxide regulates adherens junction components in vivo as well. Loss of the Drosophila cytosolic Prx Jafrac1 caused a decrease in the levels of adherens junction components and resulted in failure of germ cells to adhere properly to the embryo during development (Figure 3b) [94]. Treatment of wild-type embryos with hydrogen peroxide also caused a decrease in E-cadherin and β-catenin, suggesting that hydrogen peroxide acts within Drosophila embryos to decrease the levels of adherens junction components. How hydrogen peroxide effects this change is yet to be determined; however, because the levels of E-cadherin mRNA were not altered in these embryos, hydrogen peroxide probably acts post-transcriptionally on E-cadherin.
In addition to influencing cell–cell adhesion through adherens junctions, ROS also play a role in cell–matrix interactions. In vitro experiments with several different cell types have implicated ROS in the processes of integrin-mediated cell adhesion, spreading and migration. Upon binding to fibronectin-treated dishes, fibroblast cells transiently increased ROS levels, as measured using the non-specific ROS probe 2′,7′-dichlorofluorescein diacetate (DCF-DA) [20,95]. Inhibition of NAPDH oxidase with DPI and/or 5-lipoxygenase with nordihydroguaiaretic acid (NDGA) decreased ROS and the binding of fibroblasts to fibronectin-treated dishes, suggesting that ROS play a role in integrin-mediated adhesion [20]. Similarly, overexpression of catalase in HeLa cells impeded their ability to spread when plated on fibronectin, implicating hydrogen peroxide in this process [21]. Further experiments in both cell types demonstrated that ROS production required Rac1 and was correlated with oxidation of LMW-PTP [20,21]. Co-expression of catalytically inactive LMW-PTP with catalase restored the defect in cell spreading caused by catalase overexpression, indicating that downregulation of LMW-PTP, possibly by ROS, is a necessary step in integrin-mediated cell spreading [21]. The mechanism by which ROS and LMW-PTP downregulation facilitate cell spreading involves downregulation of RhoA activity through activation of p190Rho-GAP, a negative regulator of RhoA. More recently, a similar mechanism has been proposed for regulation of the spreading and movement of epithelial cells on collagen I [22]. Knockdown of NOX1 or inhibition with DPI in these cells also led to an increase in RhoA-GTP levels; however, in contrast to the results in the previous cell types, cell–matrix contact was increased by NADPH oxidase inhibition. This increase in cell–matrix contact was caused by an increase in the surface expression of α3 integrin; blocking of α3 integrin using an antibody reversed the increase in cell–matrix contact caused by NADPH oxidase inhibition [22].
In summary, abundant evidence suggests that ROS act within stationary cells to influence the behavior of migrating cells. ROS seem to affect cell–cell and cell–matrix adhesion by altering the cell surface expression of integrins and cell adhesion molecules such as VCAM-1, and decreasing adherens junction components.
Hydrogen peroxide might act between cells as a chemoattractant
Although many examples exist of ROS acting as an intracellular signaling molecule, there are far fewer cases of ROS acting as signals between cells. One possible example of this that was alluded to earlier is that ROS produced by endothelial cells induce changes in leukocyte-associated MMPs [29]. Apart from this, some early in vitro evidence also suggested that hydrogen peroxide could act as a chemoattractant [96]. More recently, in vivo evidence supporting the possibility that hydrogen peroxide acts as a chemoattractant has been found. In wounded tails of zebrafish, a gradient of hydrogen peroxide emanating approximately 100–200 µm from the wound margin was detected using HyPer [8]. Wound margin hydrogen peroxide production preceded recruitment of leukocytes, suggesting that tail-fin epithelial cells were the source of the hydrogen peroxide [8]. Using pharmacological and genetic inhibition, the NADPH oxidase isoform DUOX was found to be responsible for the hydrogen peroxide production and was required for rapid recruitment of leukocytes to the wound [8]. Because the hydrogen peroxide produced during wound healing was measured intracellularly, it remains unclear whether a gradient of hydrogen peroxide is formed in the extracellular space and whether leukocytes sense it directly.
Further evidence supporting a role for hydrogen peroxide as a chemoattractant was found in Drosophila. When Drosophila are wounded, they too generate ROS at the wound margin [97]. A decrease in the levels of DUOX using RNAi specifically in the epidermis significantly reduced the recruitment of Drosophila immune cells (hemocytes) to the wound relative to controls [97]. Taken together, experiments in both Drosophila and zebrafish support a putative conserved role for hydrogen peroxide in the process of attracting immune cells to wounds. Further experimentation should help to determine whether hydrogen peroxide acts directly to attract immune cells or is an intermediate in this process.
Of note, similar responses to injury are observed in mammalian endothelial cells in culture. When endothelial cell monolayers are scratched, ROS are generated by NADPH oxidases proximal to the scratch and sulfenic acid-modified proteins accumulate at the wound margin [42,57,63]. Hydrogen peroxide is thought to act within endothelial cells to facilitate wound closure by promoting their proliferation and migration [42,57]. Whether zebrafish and Drosophila epithelial cells in an intact organism also generate hydrogen peroxide for this purpose is unknown.
Concluding remarks
ROS are involved in many aspects of cell migration and adhesion. Most evidence of their involvement in these processes is inferred from studies in which NADPH oxidases and the ROS-degrading enzymes SOD and Prx are overexpressed, inhibited or removed. With few exceptions, measurement of the specific ROS involved in cell migration is still lacking. The application of new probes that allow quantitative and dynamic assessment of specific ROS in live cells would provide valuable support.
Although the generation of ROS is relatively well characterized during migration and adhesion, the precise proteins oxidized by hydrogen peroxide and ROS have, in most cases, not been determined. The application of proteomic methods to determine the proteins that are oxidized by ROS during migration should allow for a more detailed understanding of how ROS pass information from the cell surface to promote cell migration and adhesive changes.
Whether ROS act simply to modulate the amplitude of signaling or are integral components of signaling pathways remains an outstanding question. In many cases, knock out of NADPH oxidases or ROS-degrading enzymes causes changes in cell migration and adhesion in vivo, but does not completely abolish it. Whether this is simply because of redundancy or instead reveals a fundamental aspect of redox signaling remains to be determined.
Acknowledgments
We thank Daria Siekhaus, Allison Blum and Susan Schwab for helpful and insightful comments on this manuscript. T. Hurd is supported by the Canadian Institute of Health Research. Our work on primordial germ cell migration is supported by NIH grant RO1HD041900. R. Lehmann is a Howard Hughes Medical Institute investigator.
References
- 1.Montell DJ. Morphogenetic cell movements: diversity from modular mechanical properties. Science. 2008;322:1502–1505. doi: 10.1126/science.1164073. [DOI] [PubMed] [Google Scholar]
- 2.Ridley AJ, et al. Cell migration: integrating signals from front to back. Science. 2003;302:1704–1709. doi: 10.1126/science.1092053. [DOI] [PubMed] [Google Scholar]
- 3.Charras G, Paluch E. Blebs lead the way: how to migrate without lamellipodia. Nat. Rev. Mol. Cell Biol. 2008;9:730–736. doi: 10.1038/nrm2453. [DOI] [PubMed] [Google Scholar]
- 4.Fackler OT, Grosse R. Cell motility through plasma membrane blebbing. J. Cell Biol. 2008;181:879–884. doi: 10.1083/jcb.200802081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Webb DJ, et al. Cell migration: an overview. Methods Mol. Biol. 2005;294:3–11. [PubMed] [Google Scholar]
- 6.Franz CM, et al. Cell migration in development and disease. Dev. Cell. 2002;2:153–158. doi: 10.1016/s1534-5807(02)00120-x. [DOI] [PubMed] [Google Scholar]
- 7.Richardson BE, Lehmann R. Mechanisms guiding primordial germ cell migration: strategies from different organisms. Nat. Rev. Mol. Cell Biol. 2010;11:37–49. doi: 10.1038/nrm2815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Niethammer P, et al. A tissue-scale gradient of hydrogen peroxide mediates rapid wound detection in zebrafish. Nature. 2009;459:996–999. doi: 10.1038/nature08119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Flohe L. Changing paradigms in thiology from antioxidant defense toward redox regulation. Methods Enzymol. 2010;473:1–39. doi: 10.1016/S0076-6879(10)73001-9. [DOI] [PubMed] [Google Scholar]
- 10.Janssen-Heininger YM, et al. Redox-based regulation of signal transduction: principles, pitfalls, and promises. Free Radic. Biol. Med. 2008;45:1–17. doi: 10.1016/j.freeradbiomed.2008.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Halliwell B, Gutteridge JMC. Free Radicals in Biology and Medicine. Oxford University Press; 2007. [Google Scholar]
- 12.Bedard K, Krause KH. The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol. Rev. 2007;87:245–313. doi: 10.1152/physrev.00044.2005. [DOI] [PubMed] [Google Scholar]
- 13.Chen XL, et al. Rac1 and superoxide are required for the expression of cell adhesion molecules induced by tumor necrosis factor-alpha in endothelial cells. J. Pharmacol. Exp. Ther. 2003;305:573–580. doi: 10.1124/jpet.102.047894. [DOI] [PubMed] [Google Scholar]
- 14.Min JK, et al. TNF-related activation-induced cytokine enhances leukocyte adhesiveness: induction of ICAM-1 and VCAM-1 via TNF receptor-associated factor and protein kinase C-dependent NF-kappaB activation in endothelial cells. J. Immunol. 2005;175:531–540. doi: 10.4049/jimmunol.175.1.531. [DOI] [PubMed] [Google Scholar]
- 15.Harfouche R, et al. Roles of reactive oxygen species in angiopoietin-1/tie-2 receptor signaling. FASEB J. 2005;19:1728–1730. doi: 10.1096/fj.04-3621fje. [DOI] [PubMed] [Google Scholar]
- 16.Sundaresan M, et al. Requirement for generation of H2O2 for platelet-derived growth factor signal transduction. Science. 1995;270:296–299. doi: 10.1126/science.270.5234.296. [DOI] [PubMed] [Google Scholar]
- 17.Abid MR, et al. NADPH oxidase activity is required for endothelial cell proliferation and migration. FEBS Lett. 2000;486:252–256. doi: 10.1016/s0014-5793(00)02305-x. [DOI] [PubMed] [Google Scholar]
- 18.Oshikawa J, et al. Extracellular SOD-derived H2O2 promotes VEGF signaling in caveolae/lipid rafts and post-ischemic angiogenesis in mice. PLoS ONE. 2010;5:e10189. doi: 10.1371/journal.pone.0010189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ushio-Fukai M, et al. Novel role of gp91(phox)-containing NAD(P)H oxidase in vascular endothelial growth factor-induced signaling and angiogenesis. Circ. Res. 2002;91:1160–1167. doi: 10.1161/01.res.0000046227.65158.f8. [DOI] [PubMed] [Google Scholar]
- 20.Chiarugi P, et al. Reactive oxygen species as essential mediators of cell adhesion: the oxidative inhibition of a FAK tyrosine phosphatase is required for cell adhesion. J. Cell Biol. 2003;161:933–944. doi: 10.1083/jcb.200211118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nimnual AS, et al. Redox-dependent downregulation of Rho by Rac. Nat. Cell Biol. 2003;5:236–241. doi: 10.1038/ncb938. [DOI] [PubMed] [Google Scholar]
- 22.Sadok A, et al. NADPH oxidase 1 controls the persistence of directed cell migration by a Rho-dependent switch of alpha2/alpha3 integrins. Mol. Cell Biol. 2009;29:3915–3928. doi: 10.1128/MCB.01199-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ushio-Fukai M. Localizing NADPH oxidase-derived ROS. Sci. STKE. 2006;2006:re8. doi: 10.1126/stke.3492006re8. [DOI] [PubMed] [Google Scholar]
- 24.Radisky DC, et al. Rac1b and reactive oxygen species mediate MMP-3-induced EMT and genomic instability. Nature. 2005;436:123–127. doi: 10.1038/nature03688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cook-Mills JM, et al. Vascular cell adhesion molecule-1 expression and signaling during disease: regulation by reactive oxygen species and antioxidants. Antioxid. Redox Signal. 2011;15:1607–1638. doi: 10.1089/ars.2010.3522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Frey RS, et al. NADPH oxidase-dependent signaling in endothelial cells: role in physiology and pathophysiology. Antioxid. Redox Signal. 2009;11:791–810. doi: 10.1089/ars.2008.2220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brigelius-Flohe R, Flohe L. Basic principles and emerging concepts in the redox control of transcription factors. Antioxid. Redox Signal. 2011;15:2335–2381. doi: 10.1089/ars.2010.3534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Takac I, et al. The E-loop is involved in hydrogen peroxide formation by the NADPH oxidase Nox4. J. Biol. Chem. 2011;286:13304–13313. doi: 10.1074/jbc.M110.192138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Deem TL, Cook-Mills JM. Vascular cell adhesion molecule 1 (VCAM-1) activation of endothelial cell matrix metalloproteinases: role of reactive oxygen species. Blood. 2004;104:2385–2393. doi: 10.1182/blood-2004-02-0665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kassim SY, et al. NADPH oxidase restrains the matrix metalloproteinase activity of macrophages. J. Biol. Chem. 2005;280:30201–30205. doi: 10.1074/jbc.M503292200. [DOI] [PubMed] [Google Scholar]
- 31.Rhee SG, et al. Controlled elimination of intracellular H2O2: regulation of peroxiredoxin, catalase, and glutathione peroxidase via post-translational modification. Antioxid. Redox Signal. 2005;7:619–626. doi: 10.1089/ars.2005.7.619. [DOI] [PubMed] [Google Scholar]
- 32.D’Autreaux B, Toledano MB. ROS as signalling molecules: mechanisms that generate specificity in ROS homeostasis. Nat. Rev. Mol. Cell Biol. 2007;8:813–824. doi: 10.1038/nrm2256. [DOI] [PubMed] [Google Scholar]
- 33.Chen K, et al. Regulation of ROS signal transduction by NADPH oxidase 4 localization. J. Cell Biol. 2008;181:1129–1139. doi: 10.1083/jcb.200709049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kim SR, et al. Visfatin enhances ICAM-1 and VCAM-1 expression through ROS-dependent NF-kappaB activation in endothelial cells. Biochim. Biophys. Acta. 2008;1783:886–895. doi: 10.1016/j.bbamcr.2008.01.004. [DOI] [PubMed] [Google Scholar]
- 35.Wu RF, et al. Subcellular targeting of oxidants during endothelial cell migration. J. Cell Biol. 2005;171:893–904. doi: 10.1083/jcb.200507004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Woo HA, et al. Inactivation of peroxiredoxin I by phosphorylation allows localized H2O2 accumulation for cell signaling. Cell. 2010;140:517–528. doi: 10.1016/j.cell.2010.01.009. [DOI] [PubMed] [Google Scholar]
- 37.Choi MH, et al. Regulation of PDGF signalling and vascular remodelling by peroxiredoxin II. Nature. 2005;435:347–353. doi: 10.1038/nature03587. [DOI] [PubMed] [Google Scholar]
- 38.Haurani MJ, et al. Nox4 oxidase overexpression specifically decreases endogenous Nox4 mRNA and inhibits angiotensin II-induced adventitial myofibroblast migration. Hypertension. 2008;52:143–149. doi: 10.1161/HYPERTENSIONAHA.107.101667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kato T, et al. Lucigenin-induced chemiluminescence in human neutrophils in the process of chemotactic migration measured in a modified Boyden chamber. Dermatologica. 1989;179(Suppl. 1):113–115. doi: 10.1159/000248460. [DOI] [PubMed] [Google Scholar]
- 40.Wach F, et al. Inhibition of fibroblast chemotaxis by superoxide dismutase. Eur. J. Cell Biol. 1987;44:124–127. [PubMed] [Google Scholar]
- 41.Hattori H, et al. Small-molecule screen identifies reactive oxygen species as key regulators of neutrophil chemotaxis. Proc. Natl. Acad. Sci. U.S.A. 2010;107:3546–3551. doi: 10.1073/pnas.0914351107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Moldovan L, et al. Redox changes of cultured endothelial cells and actin dynamics. Circ. Res. 2000;86:549–557. doi: 10.1161/01.res.86.5.549. [DOI] [PubMed] [Google Scholar]
- 43.Lyle AN, et al. Poldip2, a novel regulator of Nox4 and cytoskeletal integrity in vascular smooth muscle cells. Circ. Res. 2009;105:249–259. doi: 10.1161/CIRCRESAHA.109.193722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schroder K, et al. Nox1 mediates basic fibroblast growth factor-induced migration of vascular smooth muscle cells. Arterioscler. Thromb. Vasc. Biol. 2007;27:1736–1743. doi: 10.1161/ATVBAHA.107.142117. [DOI] [PubMed] [Google Scholar]
- 45.Lee MY, et al. Mechanisms of vascular smooth muscle NADPH oxidase 1 (Nox1) contribution to injury-induced neointimal formation. Arterioscler. Thromb. Vasc. Biol. 2009;29:480–487. doi: 10.1161/ATVBAHA.108.181925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tojo T, et al. Role of gp91phox (Nox2)-containing NAD(P)H oxidase in angiogenesis in response to hindlimb ischemia. Circulation. 2005;111:2347–2355. doi: 10.1161/01.CIR.0000164261.62586.14. [DOI] [PubMed] [Google Scholar]
- 47.Haddad P, et al. Nox2-containing NADPH oxidase deficiency confers protection from hindlimb ischemia in conditions of increased oxidative stress. Arterioscler. Thromb. Vasc. Biol. 2009;29:1522–1528. doi: 10.1161/ATVBAHA.109.191437. [DOI] [PubMed] [Google Scholar]
- 48.Hui DY. Intimal hyperplasia in murine models. Curr. Drug Targets. 2008;9:251–260. doi: 10.2174/138945008783755601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chen Z, et al. Decreased neointimal formation in Nox2-deficient mice reveals a direct role for NADPH oxidase in the response to arterial injury. Proc. Natl. Acad. Sci. U.S.A. 2004;101:13014–13019. doi: 10.1073/pnas.0405389101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Niu XL, et al. Nox activator 1: a potential target for modulation of vascular reactive oxygen species in atherosclerotic arteries. Circulation. 2010;121:549–559. doi: 10.1161/CIRCULATIONAHA.109.908319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rivera J, et al. Nox isoforms in vascular pathophysiology: insights from transgenic and knockout mouse models. Redox Rep. 2010;15:50–63. doi: 10.1179/174329210X12650506623401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Belousov VV, et al. Genetically encoded fluorescent indicator for intracellular hydrogen peroxide. Nat. Methods. 2006;3:281–286. doi: 10.1038/nmeth866. [DOI] [PubMed] [Google Scholar]
- 53.Wang Y, et al. Regulation of VEGF-induced endothelial cell migration by mitochondrial reactive oxygen species. Am. J. Physiol. Cell Physiol. 2011;301:C695–C704. doi: 10.1152/ajpcell.00322.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Smith RA, et al. Mitochondria-targeted antioxidants in the treatment of disease. Ann. N. Y. Acad. Sci. 2008;1147:105–111. doi: 10.1196/annals.1427.003. [DOI] [PubMed] [Google Scholar]
- 55.Kim HW, et al. Essential role of extracellular SOD in reparative neovascularization induced by hindlimb ischemia. Circ. Res. 2007;101:409–419. doi: 10.1161/CIRCRESAHA.107.153791. [DOI] [PubMed] [Google Scholar]
- 56.Groleau J, et al. Essential role of copper–zinc superoxide dismutase for ischemia-induced neovascularization via modulation of bone marrow-derived endothelial progenitor cells. Arterioscler. Thromb. Vasc. Biol. 2010;30:2173–2181. doi: 10.1161/ATVBAHA.110.212530. [DOI] [PubMed] [Google Scholar]
- 57.Ikeda S, et al. IQGAP1 regulates reactive oxygen species-dependent endothelial cell migration through interacting with Nox2. Arterioscler. Thromb. Vasc. Biol. 2005;25:2295–2300. doi: 10.1161/01.ATV.0000187472.55437.af. [DOI] [PubMed] [Google Scholar]
- 58.Wu RF, et al. Vascular endothelial growth factor causes translocation of p47phox to membrane ruffles through WAVE1. J. Biol. Chem. 2003;278:36830–36840. doi: 10.1074/jbc.M302251200. [DOI] [PubMed] [Google Scholar]
- 59.Miller EW, et al. Molecular imaging of hydrogen peroxide produced for cell signaling. Nat. Chem. Biol. 2007;3:263–267. doi: 10.1038/nchembio871. [DOI] [PubMed] [Google Scholar]
- 60.Lyons PD, et al. Inhibition of the catalytic activity of cell adhesion kinase beta by protein-tyrosine phosphatase-PEST-mediated dephosphorylation. J. Biol. Chem. 2001;276:24422–24431. doi: 10.1074/jbc.M011080200. [DOI] [PubMed] [Google Scholar]
- 61.Tonks NK. Redox redux: revisiting PTPs and the control of cell signaling. Cell. 2005;121:667–670. doi: 10.1016/j.cell.2005.05.016. [DOI] [PubMed] [Google Scholar]
- 62.Winterbourn CC, Hampton MB. Thiol chemistry and specificity in redox signaling. Free Radic. Biol. Med. 2008;45:549–561. doi: 10.1016/j.freeradbiomed.2008.05.004. [DOI] [PubMed] [Google Scholar]
- 63.Kaplan N, et al. Localized cysteine sulfenic acid formation by vascular endothelial growth factor: role in endothelial cell migration and angiogenesis. Free Radic. Res. 2011;45:1124–1135. doi: 10.3109/10715762.2011.602073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chouchani ET, et al. Proteomic approaches to the characterization of protein thiol modification. Curr. Opin. Chem. Biol. 2011;15:120–128. doi: 10.1016/j.cbpa.2010.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hurd TR, et al. Measuring redox changes to mitochondrial protein thiols with redox difference gel electrophoresis (redox-DIGE) Methods Enzymol. 2009;456:343–361. doi: 10.1016/S0076-6879(08)04419-4. [DOI] [PubMed] [Google Scholar]
- 66.Thamsen M, Jakob U. The redoxome: proteomic analysis of cellular redox networks. Curr. Opin. Chem. Biol. 2011;15:113–119. doi: 10.1016/j.cbpa.2010.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ley K, et al. Getting to the site of inflammation: the leukocyte adhesion cascade updated. Nat. Rev. Immunol. 2007;7:678–689. doi: 10.1038/nri2156. [DOI] [PubMed] [Google Scholar]
- 68.Nourshargh S, et al. Breaching multiple barriers: leukocyte motility through venular walls and the interstitium. Nat. Rev. Mol. Cell Biol. 2010;11:366–378. doi: 10.1038/nrm2889. [DOI] [PubMed] [Google Scholar]
- 69.Lewis MS, et al. Hydrogen peroxide stimulates the synthesis of platelet-activating factor by endothelium and induces endothelial cell-dependent neutrophil adhesion. J. Clin. Invest. 1988;82:2045–2055. doi: 10.1172/JCI113825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lo SK, et al. Hydrogen peroxide-induced increase in endothelial adhesiveness is dependent on ICAM-1 activation. Am J. Physiol. 1993;264:L406–L412. doi: 10.1152/ajplung.1993.264.4.L406. [DOI] [PubMed] [Google Scholar]
- 71.Patel KD, et al. Oxygen radicals induce human endothelial cells to express GMP-140 and bind neutrophils. J. Cell Biol. 1991;112:749–759. doi: 10.1083/jcb.112.4.749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Tummala PE, et al. NF-kappa B independent suppression of endothelial vascular cell adhesion molecule-1 and intercellular adhesion molecule-1 gene expression by inhibition of flavin binding proteins and superoxide production. J. Mol. Cell. Cardiol. 2000;32:1499–1508. doi: 10.1006/jmcc.2000.1183. [DOI] [PubMed] [Google Scholar]
- 73.Chen H, et al. Inhibition of NADPH oxidase is neuroprotective after ischemia–reperfusion. J. Cereb. Blood Flow Metab. 2009;29:1262–1272. doi: 10.1038/jcbfm.2009.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Vendrov AE, et al. Atherosclerosis is attenuated by limiting superoxide generation in both macrophages and vessel wall cells. Arterioscler. Thromb. Vasc. Biol. 2007;27:2714–2721. doi: 10.1161/ATVBAHA.107.152629. [DOI] [PubMed] [Google Scholar]
- 75.Stokes KY, et al. NAD(P)H oxidase-derived superoxide mediates hypercholesterolemia-induced leukocyte-endothelial cell adhesion. Circ. Res. 2001;88:499–505. doi: 10.1161/01.res.88.5.499. [DOI] [PubMed] [Google Scholar]
- 76.Laurila JP, et al. SOD3 reduces inflammatory cell migration by regulating adhesion molecule and cytokine expression. PLoS ONE. 2009;4:e5786. doi: 10.1371/journal.pone.0005786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hidalgo E, Demple B. An iron–sulfur center essential for transcriptional activation by the redox-sensing SoxR protein. EMBO J. 1994;13:138–146. doi: 10.1002/j.1460-2075.1994.tb06243.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Cook-Mills JM, et al. Calcium mobilization and Rac1 activation are required for VCAM-1 (vascular cell adhesion molecule-1) stimulation of NADPH oxidase activity. Biochem. J. 2004;378:539–547. doi: 10.1042/BJ20030794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.van Wetering S, et al. VCAM-1-mediated Rac signaling controls endothelial cell–cell contacts and leukocyte transmigration. Am. J. Physiol. Cell Physiol. 2003;285:C343–C352. doi: 10.1152/ajpcell.00048.2003. [DOI] [PubMed] [Google Scholar]
- 80.Abdala-Valencia H, et al. Nonhematopoietic NADPH oxidase regulation of lung eosinophilia and airway hyperresponsiveness in experimentally induced asthma. Am. J. Physiol. Lung Cell. Mol. Physiol. 2007;292:L1111–L1125. doi: 10.1152/ajplung.00208.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Abdala-Valencia H, Cook-Mills JM. VCAM-1 signals activate endothelial cell protein kinase Calpha via oxidation. J. Immunol. 2006;177:6379–6387. doi: 10.4049/jimmunol.177.9.6379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Matheny HE, et al. Lymphocyte migration through monolayers of endothelial cell lines involves VCAM-1 signaling via endothelial cell NADPH oxidase. J. Immunol. 2000;164:6550–6559. doi: 10.4049/jimmunol.164.12.6550. [DOI] [PubMed] [Google Scholar]
- 83.Kim SY, et al. Role of NADPH oxidase-2 in lipopolysaccharide-induced matrix metalloproteinase expression and cell migration. Immunol. Cell Biol. 2010;88:197–204. doi: 10.1038/icb.2009.87. [DOI] [PubMed] [Google Scholar]
- 84.Deem TL, et al. VCAM-1 activation of endothelial cell protein tyrosine phosphatase 1B. J. Immunol. 2007;178:3865–3873. doi: 10.4049/jimmunol.178.6.3865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Gopalakrishna R, Jaken S. Protein kinase C signaling and oxidative stress. Free Radic. Biol. Med. 2000;28:1349–1361. doi: 10.1016/s0891-5849(00)00221-5. [DOI] [PubMed] [Google Scholar]
- 86.van Buul JD, et al. Proline-rich tyrosine kinase 2 (Pyk2) mediates vascular endothelial-cadherin-based cell–cell adhesion by regulating beta-catenin tyrosine phosphorylation. J. Biol. Chem. 2005;280:21129–21136. doi: 10.1074/jbc.M500898200. [DOI] [PubMed] [Google Scholar]
- 87.van Wetering S, et al. Reactive oxygen species mediate Rac-induced loss of cell–cell adhesion in primary human endothelial cells. J. Cell Sci. 2002;115:1837–1846. doi: 10.1242/jcs.115.9.1837. [DOI] [PubMed] [Google Scholar]
- 88.Tobar N, et al. NOX4-dependent ROS production by stromal mammary cells modulates epithelial MCF-7 cell migration. Br. J. Cancer. 2010;103:1040–1047. doi: 10.1038/sj.bjc.6605847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lee HM, et al. Roles of reactive oxygen species in CXCL8 and CCL2 expression in response to the 30-kDa antigen of Mycobacterium tuberculosis. J. Clin. Immunol. 2009;29:46–56. doi: 10.1007/s10875-008-9222-3. [DOI] [PubMed] [Google Scholar]
- 90.Heo SK, et al. NADPH oxidase activation is required for migration by LIGHT in human monocytes. Biochem. Biophys. Res. Commun. 2008;371:834–840. doi: 10.1016/j.bbrc.2008.04.184. [DOI] [PubMed] [Google Scholar]
- 91.Mori K, et al. Invasive potential induced under long-term oxidative stress in mammary epithelial cells. Cancer Res. 2004;64:7464–7472. doi: 10.1158/0008-5472.CAN-04-1725. [DOI] [PubMed] [Google Scholar]
- 92.Rao RK, et al. Tyrosine phosphorylation and dissociation of occludin–ZO-1 and E-cadherin–beta-catenin complexes from the cytoskeleton by oxidative stress. Biochem. J. 2002;368:471–481. doi: 10.1042/BJ20011804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Thews O, et al. Impact of reactive oxygen species on the expression of adhesion molecules in vivo. Adv. Exp. Med. Biol. 2009;645:95–100. doi: 10.1007/978-0-387-85998-9_15. [DOI] [PubMed] [Google Scholar]
- 94.DeGennaro M, et al. Peroxiredoxin stabilization of DE-cadherin promotes primordial germ cell adhesion. Dev. Cell. 2011;20:233–243. doi: 10.1016/j.devcel.2010.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Keller A, et al. Analysis of dichlorodihydrofluorescein and dihydrocalcein as probes for the detection of intracellular reactive oxygen species. Free Radic. Res. 2004;38:1257–1267. doi: 10.1080/10715760400022145. [DOI] [PubMed] [Google Scholar]
- 96.Klyubin IV, et al. Hydrogen peroxide-induced chemotaxis of mouse peritoneal neutrophils. Eur. J. Cell Biol. 1996;70:347–351. [PubMed] [Google Scholar]
- 97.Moreira S, et al. Prioritization of competing damage and developmental signals by migrating macrophages in the Drosophila embryo. Curr. Biol. 2010;20:464–470. doi: 10.1016/j.cub.2010.01.047. [DOI] [PubMed] [Google Scholar]