Abstract
Parkinson disease (PD) is a progressive neurodegenerative disease that is associated with substantial morbidity and early mortality. Disease-related costs exceed $10 billion, not including medications, out-of-pocket expenses, or societal costs. Symptomatic treatment with levodopa, which has been available for over 30 years, and advanced therapies such as deep brain stimulation improve outcomes. Yet most new medications for PD provide a therapeutic benefit that is relatively modest compared to the benefits from levodopa. Despite dozens of neuroprotective clinical trials, there are no medications proven to slow the progression of the disease. Given these limitations, we provide evidence of the potential public health impact of a research agenda that emphasizes identification of risk factors to reduce disease burden through exposure mitigation. In addition, we emphasize health care policy that focuses on increasing health care expenditures for neurologic evaluation and management services to increase access to specialists to improve disease outcomes and reduce costs through better disease management.
In the story “Elephant and the blind men,”1 6 blind men discover a creature (an elephant) in their village that they have never encountered. They touch the various parts of the elephant and describe the elephant as a pillar, rope, tree trunk, wall, and tree branch. They argue bitterly until a wise man tells them all of their analyses are accurate, but incomplete. A common interpretation of that story is that without the big picture, one can easily come to a false conclusion. In many ways, the current research approach to Parkinson disease (PD) is similar to the “Elephant and the blind men.” We have made substantial advances in understanding PD, but the big picture eludes us. In the following paragraphs, we argue that we have the potential to reduce PD burden and morbidity considerably by shifting to the clinical and research approach used in stroke, namely a public health approach that focuses providing access to the most effective treatments without disparity and prioritizing primary prevention and health outcomes research.
The National Institutes for Neurologic Disease and Stroke recently announced the failure of the >$60 million NIH Exploratory Trials in Parkinson's Disease trial of creatine to demonstrate efficacy in slowing PD progression. This joins a long list of failed disease-modifying PD trials, including selegiline,2,3 riluzole, CEP-1347,4 rasagiline,5 creatine,6 and cell-based therapies.7,8 Possible explanations for these disappointments include disease models that do not replicate neurodegeneration, incomplete understanding of disease pathogenesis, residual confounding, and suboptimal timing of disease modification (too late in the disease course to impact disease progression). The overwhelming research focus at the NIH and private foundations has been on what can broadly be considered the cure. The search for the cure has driven the current individual-centered (as opposed to public health) approach to PD.9 A well-treated patient with PD can function nearly normally for years, during which time, patients understandably lobby for treatments that can halt disease progression or improve symptom control. Countless studies have investigated new dopaminergic therapies or stepwise medication approaches to improve “on” time to reduce disability. While helpful for many individuals, the potential public health impact of most of the newer symptomatic therapies is relatively modest. The overwhelming research focus on improving symptom management or on curing PD is far too narrow and there are substantial public health benefits to understanding and mitigating disease risk and improving utilization of high-quality health care services without disparity at the population level.
THE PUBLIC HEALTH APPROACH TO STROKE MAY BENEFIT PD
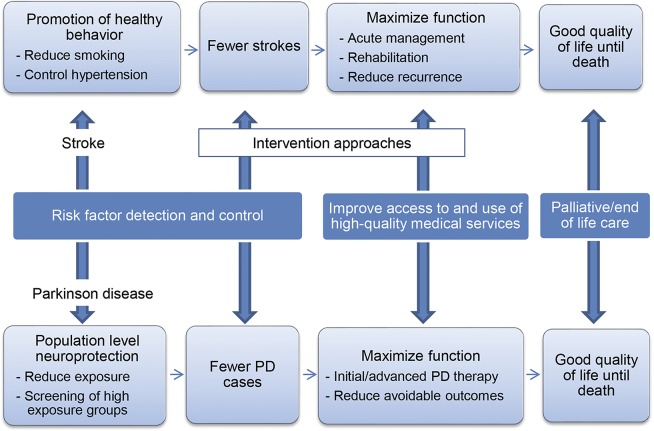
The approach taken to reduce stroke burden, disability, and mortality serves as a useful guide for the PD research agenda and clinical management of symptomatic disease, despite the fact that stroke is a disease with maximum disability at onset, and PD is a chronic neurodegenerative disease. In figure 1, we show how an action framework for PD research prevention and treatment can be developed, by modifying the current public action plan for stroke issued by the US Centers for Disease Control and Prevention.10
Figure 1. Public health approach to Parkinson disease (PD) and stroke.
Major components of this approach for stroke are risk factor prevention, screening, and management. Similar to PD, there are multiple (known and unknown) genetic, environmental, and behavioral risk factors for stroke. Investment in research to identify common stroke risk factors has produced data demonstrating the benefits on incident stroke risk associated with individual and population reductions in the prevalence and severity of hypertension, diabetes, smoking, and atrial fibrillation. Treatment of common risk factors, as well as health education, focused on prevention and lifestyle choices are aimed at reducing stroke incidence (figure 1). Conversely, PD research has centered on risk factors that are relevant for only a small portion of patients with PD (such as those who have specific predisposing genetic risk factors). A broader research agenda to identify new modifiable PD risk factors is needed to reduce PD incidence.
As shown in figure 1, another critical component of the public health approach is maximizing the utilization of medical services that provide the most effective treatments, without disparity, through end of life. To achieve this for stroke, the US health care system underwent structural and process changes in the 1990s. Initially this included widespread efforts to maximize aspirin use for those at risk of primary and recurrent stroke. Once tissue plasminogen activator (tPA)11 was demonstrated to improve outcomes in acute ischemic stroke, national implementation systems were developed to increase tPA use. These included the creation of comprehensive stroke centers of excellence, drip and ship protocols for tPA patients treated in outlying hospitals, national public awareness campaigns, mandatory training for medical center personnel, and reimbursed telemedicine services. Stroke, like PD, is chronic and can be disabling. The public health approach to stroke includes ensuring stroke clinical expertise and use of palliative care throughout the disease course to optimize quality of life and minimize suffering.12 The national research and clinical programs aimed at preventing stroke and maximizing poststroke recovery have collectively led to stroke declining from the third to the fourth leading cause of death.13 While there are clearly differences in disease onset and response to symptomatic therapy between stroke and PD, the general framework of risk factor identification, primary and secondary prevention, and broad access to the range of effective therapies across the population has the potential to inform similar efforts to improve the health of the US PD population.
PRIMARY PREVENTION (NEUROPREVENTION) OF PD
Despite much research into causes of PD, the strongest risk factors remain age, race, and sex. While these nonmodifiable risk factors may teach us about the disease pathogenesis, only sex differences have the potential to reduce disease burden, potentially through hormonal regulation. Other potential risk factors for PD, including pesticide application,14 urban residence,15 and industrial/urban metal exposure,16,17 provide an opportunity for primary prevention or neuroprevention. To understand the potential public health impact of primary prevention of PD, it is essential to know the measurable health costs of PD. A recent projection of the economic impact of PD suggested that the annual indirect costs of PD associated with reduced/lost employment are approximately $10,000 per patient, and the estimated PD-related medical expenses are $12,800 per patient.18 This translates to an estimated societal burden of over $14 billion USD per year. Given the effectiveness of our symptomatic therapies, incident PD patients have the potential to live with the disease for decades, resulting in an enormous societal burden and highlighting the need to reduce incident disease.
The potential public health impact gained from risk factor mitigation may exceed benefits from developing new adjunctive medications for PD motor symptoms. For example, a recent, delayed-start study of rasagiline as a disease-modifying treatment in newly diagnosed PD (ADAGIO) found that 1 mg of rasagiline resulted in a slower rate of clinical worsening (2.82 ± 0.53 points in the early-start group vs 4.52 ± 0.56 points in the delayed-start group) at 72 weeks.5 The higher dose of rasagiline used in the study did not meet the same efficacy endpoints, leading to controversy over interpretation of these results.19,20 ADAGIO was a well-designed study using methodologies that are considered state-of-the-art, but the impact of a 1.7-point difference in the total Unified Parkinson's Disease Rating Scale (UPDRS) over 72 weeks is small compared to the difference in UPDRS motor score seen with the use of levodopa.21 As a result, the actual impact on disease-related disability and quality of life is modest. Similarly, hundreds of millions of dollars have been spent investigating symptomatic therapies for PD that result in increases in motor “on” time within the range of 1–2 hours per day.22–24 While these treatments may result in health gains for some individual patients, the public health benefits of these treatments are small. Reducing disease burden through primary prevention has the potential to net sizable public health benefits, yet studies that address population disease burden represent the minority at NIH and other funding agencies.
There are multiple tangible examples of the potential impact of primary prevention of PD. Gorrell et al.25 examined occupational exposures and PD risk, and calculated a population attributable risk (PAR) of 8.1% for insecticide exposure and a PAR of 3.9% for copper + lead exposure. Using California Pesticide Use Reports and land use maps, Costello et al.26 estimated residential exposure to pesticides in rural California from 1974 to 1999. Residential exposure to the pesticide Maneb (which contains manganese) plus Paraquat (a pesticide that structurally resembles MPTP) was associated with increased PD risk (odds ratio [OR] 1.75, 1.13–2.73), which corresponds to an overall PAR of 10.1% (calculated using the adjusted OR [AOR]). We used a geographic information systems approach to study urban industrial metal emissions and PD, analyzing over 29 million Medicare beneficiaries from 2003 and comparing the age-, race-, and sex-standardized incidence of neurologist-diagnosed PD among nonmobile urban Medicare beneficiaries living in counties with high vs no reported manganese, copper, and lead industrial releases.16 We found that beneficiaries living in counties with high industrial manganese emission had a relative risk of 1.78 of being diagnosed with PD compared to those living in counties with no/low emission, which translates to PAR of 44.6% (confidence interval [CI] 33.6%–53.8%). These provide a useful demonstration of the potential impact of risk mitigation in rural, urban, and at-risk younger populations. Using our study as an example, 3,964 urban Medicare beneficiaries diagnosed with PD lived in a high industrial manganese emission area for 8 years prior to diagnosis, translating to 1,768 potential cases of parkinsonism that could be prevented by eliminating this exposure. Using the estimate of $22,800 per patient, the reduction in health care and societal costs would be over $40.3 million (in 2005 dollars). According to 2010 census data, 38% of the US adult population currently lives in a high manganese release county; similar proportions may have pesticide or other neurotoxicant exposures, underscoring the need for neuropreventive measures.
The public health impact from exposure mitigation may not be limited to reducing disease incidence. In another Medicare study, we investigated survival of 138,000 incident elderly PD patients in relation to industrial manganese release.27 Those beneficiaries living in counties with high manganese release, as reported by the Environmental Protection Agency, had higher 6-year mortality (OR 1.19, 95% CI 1.10–1.29) as compared to beneficiaries living in counties with low/no manganese release. If confirmed in other studies and expanded to include other potential basal ganglia toxicants, environmental exposure mitigation would provide a unique opportunity to modify disease outcomes.
Technological advances and government mandates have led to reductions in environmental release of industrial particulate matter and agricultural chemicals. This has occurred without any health outcome–driven governmental policy. Conversely, both federal and state laws regulate and monitor pesticide use by applicators, nonoccupational sources of pesticide exposure, exposure to pesticides with common mechanisms of toxicity, and pesticide-associated health outcomes in adults and children.28,29 Studies that provide evidence of dose-dependent health outcomes, like PD, are essential to determine the cost-benefit ratio of further reductions in neurotoxicants to the general population. Advances in agricultural and industrial technologies are evolving constantly and could be accelerated with clear evidence of adverse health outcomes.
APPROACHES TO MAXIMIZE FUNCTION
In addition to primary prevention, a public health approach to PD should focus on removing barriers to care and improving access to and use of high-quality medical services. Drastic changes in medical structure are not likely needed, as numerous effective treatments (both medical and surgical) for PD are already available. New therapies such as continuous duodenal levodopa infusion30 will further increase the complexity of PD management. This complexity is occurring in the setting of extensive changes in medical education, which may prove challenging for already overburdened primary care physicians. Neurology is the clinical area with which medical students and recent medical graduates feel the least comfortable.31 Resident work hour restrictions further limit neurology training in primary care specialties. Primary care specialties may not be able to provide adequate training in the management of chronic, progressive neurodegenerative diseases. It is doubtful, for example, that many primary care physicians are aware of or follow clinical guidelines for PD care published by the American Academy of Neurology.32
Studies suggest that neurologist care in the management of PD improves outcomes. Using Medicare data to identify patients with PD, we found that only 40% of prevalent PD patients saw a neurologist over a 4-year period.33 Neurologist care rates were lower in women and black, lower income, and rural patients, demonstrating sociodemographic and geographic disparities in specialty care. We hypothesized that this differential access would translate to worse outcomes in patients not seen by a neurologist. To test this hypothesis, we investigated PD-specific health outcomes in a cohort of Medicare beneficiaries followed from 2002 to 2006 and found that neurologist treatment reduced overall hospitalization rate, skilled nursing facility days, and health care costs.34 Moreover, there was a dose-dependent reduction in hospitalization for PD-related diagnostic-related group illnesses (psychosis, depression, urinary tract infection, traumatic injury)34 but no impact on general medical illnesses (angina, diabetes, hypertension, gastrointestinal obstruction, congestive heart failure), suggesting a specific impact of neurologist care on disease symptomatology.
There are several reasons why neurologist treatment may improve PD disease outcomes. A Veteran's Administration study demonstrated that adherence to quality care indicators for PD was greater in patients treated by a neurologist than those treated by a primary care physician,35 with the greatest impact on assessment of depression, falls, hallucinations, and wearing off, likely due to addressing these issues in the outpatient setting, prior to need for hospitalization. A second reason that specialist care may improve disease outcomes is greater, more appropriate use of advanced therapies. To investigate the utilization of DBS in advanced PD, we studied predictors of DBS use in a cohort of 657,000 Medicare beneficiaries with PD and found that beneficiaries not treated by neurologists were less likely to receive DBS. Patients with PD treated in minority-serving PD practices were also less likely to receive DBS, regardless of beneficiary race or physician specialty (AOR 0.76, 95% CI 0.66–0.87).36 Similarly, high neighborhood socioeconomic status was associated with higher odds of receiving DBS, even after adjusting for patient, clinical, and provider factors (AOR 1.42, 95% CI 1.33–1.53).36 These studies demonstrate the potential public health impact of improving specialty care access for patients with PD. Of course, some of the differential use of specialty services and advanced surgical therapies may be due to financial and social barriers that prevent following recommended referrals. Nevertheless, these studies highlight the difficulty in translating the latest advanced therapies into standard practice, when physicians do not have the practical ability or relevant expertise to choose or implement these therapies.
Given the expected rise in PD prevalence,37 providing high-quality neurologic care to these patients represents a substantial challenge. Proper use of medical and surgical therapies clearly improves patient quality of life and survival, but we do a poor job of distributing that expertise to the US population. There are 2 primary barriers in providing high-quality neurologic care to patients with PD: lack of clinical expertise of the primary care physician and difficulty accessing neurologists in some regions of the United States. The United States has more neurologists per capita than most other developed countries, but there is significant geographical concentration of these physicians. A recent study reported that only the Northeastern United States and Minnesota have an adequate neurology workforce,38 and over the next decade, the supply of patients will exceed demand by over 20% in most of the country. These numbers are likely an underestimate given the large number of neurologists employed in academic institutions who do not provide full-time neurology care.39 As neurologists, we must educate policy-makers on the fact that neurologist care is a bargain. Figure 2 shows the 2005 costs to Medicare of caring for patients with PD. The cost to Medicare of evaluation and management (E&M) services was over $115 million in 2005. To put this in context, a recent release of CMS data demonstrates that Medicare paid $5.6 billion to ophthalmologists, $2.2 billion to dermatologists, and $366 million to interventional pain management physicians in 2012. While we do not suggest that these other services are unnecessary, specialty care of patients with PD is associated with clear benefits in health outcome and survival, yet we are undertreating patients with this treatable condition.
Figure 2. Medicare costs associated with Parkinson disease care in 2005.
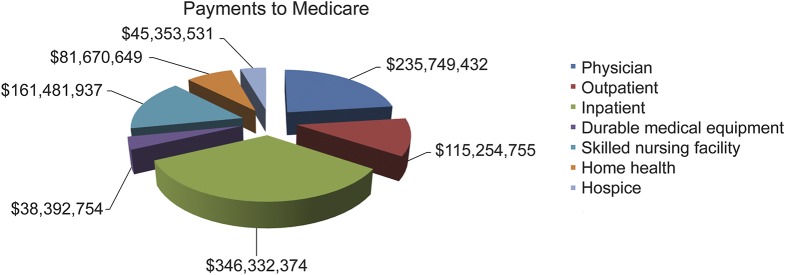
Graph indicates costs associated with care of patients with Parkinson disease in 2005. Carrier services were formerly referred to as part B or physician services and represent noninstitutional care. Of note, the cost to Medicare for outpatient visits (typically evaluation and management services) was $115 million.
SOLUTIONS
Primary prevention of PD will require considerable research effort to identify additional exposures and develop strategies to reduce disease risk. First, greater NIH and private funding directed at studying modifiable disease risk factors, including studies to test exposure mitigation/disease burden hypotheses (primary prevention), are needed. The removal of lead from gasoline, in response to clear and compelling evidence of an inverse relation between lead emissions due to gasoline combustion engines and children's IQ, is an example of public policy driven by robust environmental health data.40,41 These types of studies are lacking for PD. In addition, studies are needed to test hypotheses related to environmental exposure modification of disease progression, which would represent a novel approach to neuroprotection. Another easily implementable first step would be to require all NIH-funded clinical PD studies to contribute residential and occupational data to a common data element database to be used for large data studies. NET-PD did not collect this type of data, limiting the utility of this substantial NIH investment for environmental health research. Similarly, databases developed from public tax dollars should be freely available for researchers. Many of the studies cited in this article used Medicare data from the Centers for Medicare and Medicaid Services (CMS). These data were created through taxes that support Medicare and Medicaid. If a researcher wishes to use CMS data in an NIH study, the researcher must budget the cost of data into the grant application. In the end, the taxpayer pays for these data twice. Given the potential value of big data for research, we suggest that CMS data be available for free to NIH-supported studies. Finally, NIH has invested substantially in large, collaborative genome-wide association studies for PD. Similar investments in environmental-wide association studies42 may yield new clues to the etiology of PD and new targets for neuroprevention.
Once patients have symptomatic PD, we need to support a research agenda that focuses on health outcomes and to improve access to appropriate clinical expertise. Health care equity and quality studies in PD should be prioritized by agencies such as the Agency for Healthcare Research and Quality and NIH and should be quickly translatable to clinical practice. Current research supports system modifications such as (1) better training of primary care physicians and medical students to recognize the need for neurologist referral; (2) mandates for specialist referral for neurologic care, similar to what occurs with oncology and cardiology; and (3) increasing the effective number of physicians who can and want to practice clinical neurology in the United States by improving reimbursement. Encouraging newly trained neurologists to pursue practices focusing on E&M work is becoming increasingly difficult, given poor reimbursement for E&M services, as a function of cost of living, education debt, and clinical practice administrative costs. Ultimately, reforming the relative value unit system to reward E&M work proportionately with procedural specialties would improve access to the medical expertise needed to care for these and similarly medically complex patients. An hour spent with a new patient with PD is as challenging as time spent on many procedures, is reimbursed at a fraction of most procedures, yet has a substantial associated impact on disease outcome. Providing neurologists reimbursement for cognitive medicine that is comparable to procedures of similar duration would improve physician willingness to enter into nonprocedural practices, reduce overall health care costs, and reduce disease morbidity through improved medical management. Reimbursement for new care models, such as telemedicine, may also allow neurologist access in less populated areas of the United States.43 Ultimately, focusing on primary prevention of PD and providing broad access to high-quality neurologic care to those with PD would represent a fundamental shift from our highly focused (i.e., the blind men and the elephant) approach to PD under which we operate.
GLOSSARY
- AOR
adjusted odds ratio
- CI
confidence interval
- CMS
Centers for Medicare and Medicaid Services
- E&M
evaluation and management
- OR
odds ratio
- PAR
population attributable risk
- PD
Parkinson disease
- tPA
tissue plasminogen activator
- UPDRS
Unified Parkinson's Disease Rating Scale
AUTHOR CONTRIBUTIONS
Brad A. Racette drafted the original draft of this manuscript. Allison W. Willis edited the original draft and performed statistical analyses.
STUDY FUNDING
Supported by the National Institute for Environmental Health Sciences (R01ES021488, K24ES017765, P42ES004696, R21ES024120, R01ES021488 to B. Racette), the National Institute of Neurological Disorders and Stroke (NINDS) (K23NS081087 to A. Willis), the Michael J. Fox Foundation, NINDS, National Center for Research Resources (NCRR0), NIH Roadmap for Medical Research grant UL1 RR024992, the American Parkinson Disease Association, and the St. Louis Chapter of the American Parkinson Disease Association.
DISCLOSURE
B. Racette received research support from Teva (PI), Adamas Pharmaceuticals (PI), Auspex Pharmaceuticals (PI), Eisai (PI), Allergan (PI), Merz Pharmaceuticals GmbH (PI), Pfizer (PI), Civitas Therapeutics (PI), Kyowa Hakko Kinn Pharma (PI), and AbbVie (PI); government research support from NIH (K24ES017765 [PI], R21ES024120 [PI], R01 ES021488 [PI], R01ES021488-02S1 [PI], P42 ES04696 [co-I]); and support from the Michael J. Fox Foundation. A. Willis receives government research support from the NIH (KL2 RR024994, K23NS081087) and the National Center for Research Resources (NCRR0) and private endowments to the University of Pennsylvania. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Elephant and the blind men. Available at: JainWorld.com. Accessed February 19, 2015.
- 2.The Parkinson Study Group. Effect of deprenyl on the progression of disability in early Parkinson's disease. N Engl J Med 1989;321:1364–1371. [DOI] [PubMed] [Google Scholar]
- 3.Golbe LI, Langston JW, Shoulson I. Selegiline and Parkinson's disease: protective and symptomatic considerations. Drugs 1990;39:646–651. [DOI] [PubMed] [Google Scholar]
- 4.The Parkinson Study Group. Mixed lineage kinase inhibitor CEP-1347 fails to delay disability in early Parkinson disease. Neurology 2007;69:1480–1490. [DOI] [PubMed] [Google Scholar]
- 5.Olanow CW, Rascol O, Hauser R, et al. A double-blind, delayed-start trial of rasagiline in Parkinson's disease. N Engl J Med 2009;361:1268–1278. [DOI] [PubMed] [Google Scholar]
- 6.Kieburtz K, Tilley BC, Elm JJ, et al. Effect of creatine monohydrate on clinical progression in patients with Parkinson disease: a randomized clinical trial. JAMA 2015;313:584–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Olanow CW, Goetz CG, Kordower JH, et al. A double-blind controlled trial of bilateral fetal nigral transplantation in Parkinson's disease. Ann Neurol 2003;54:403–414. [DOI] [PubMed] [Google Scholar]
- 8.Kordower JH, Goetz CG, Freeman TB, Olanow CW. Dopaminergic transplants in patients with Parkinson's disease: neuroanatomical correlates of clinical recovery. Exp Neurol 1997;144:41–46. [DOI] [PubMed] [Google Scholar]
- 9.van der Eijk M, Nijhuis FA, Faber MJ, Bloem BR. Moving from physician-centered care towards patient-centered care for Parkinson's disease patients. Parkinsonism Relat Disord 2013;19:923–927. [DOI] [PubMed] [Google Scholar]
- 10.CDC. A Public Health Action Plan to Prevent Heart Disease and Stroke. Available at: http://www.cdc.gov/dhdsp/action_plan/pdfs/action_plan_full.pdf. Accessed February 19, 2015. [DOI] [PubMed]
- 11.An international randomized trial comparing four thrombolytic strategies for acute myocardial infarction: The GUSTO investigators. N Engl J Med 1993;329:673–682. [DOI] [PubMed] [Google Scholar]
- 12.Holloway RG, Arnold RM, Creutzfeldt CJ, et al. Palliative and end-of-life care in stroke: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2014;45:1887–1916. [DOI] [PubMed] [Google Scholar]
- 13.Stroke Mortality 1935–2010. Available at: http://www.cdc.gov/nchs/data/databriefs/db88.pdf. Accessed February 19, 2015.
- 14.Ritz BR, Manthripragada AD, Costello S, et al. Dopamine transporter genetic variants and pesticides in Parkinson's disease. Environ Health Perspect 2009;117:964–969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wright WA, Evanoff BA, Lian M, Criswell SR, Racette BA. Geographic and ethnic variation in Parkinson disease: a population-based study of US Medicare beneficiaries. Neuroepidemiology 2010;34:143–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Willis AW, Evanoff BA, Lian M, et al. Metal emissions and urban incident Parkinson disease: a community health study of Medicare beneficiaries by using geographic information systems. Am J Epidemiol 2010;172:1357–1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Finkelstein MM, Jerrett M. A study of the relationships between Parkinson's disease and markers of traffic-derived and environmental manganese air pollution in two Canadian cities. Environ Res 2007;104:420–432. [DOI] [PubMed] [Google Scholar]
- 18.Kowal SL, Dall TM, Chakrabarti R, Storm MV, Jain A. The current and projected economic burden of Parkinson's disease in the United States. Mov Disord 2013;28:311–318. [DOI] [PubMed] [Google Scholar]
- 19.Olanow CW, Rascol O. The delayed-start study in Parkinson disease: can't satisfy everyone. Neurology 2010;74:1149–1150. [DOI] [PubMed] [Google Scholar]
- 20.Ahlskog JE, Uitti RJ. Reply to Drs. Olanow and Rascol. Neurology 2010;74:1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fahn S, Oakes D, Shoulson I, et al. Levodopa and the progression of Parkinson's disease. N Engl J Med 2004;351:2498–2508. [DOI] [PubMed] [Google Scholar]
- 22.LeWitt PA, Lyons KE, Pahwa R. Advanced Parkinson disease treated with rotigotine transdermal system: PREFER Study. Neurology 2007;68:1262–1267. [DOI] [PubMed] [Google Scholar]
- 23.The Parkinson Study Group. Entacapone improves motor fluctuations in levodopa-treated Parkinson's disease patients. Ann Neurol 1997;42:747–755. [DOI] [PubMed] [Google Scholar]
- 24.Guttman M. Double-blind comparison of pramipexole and bromocriptine treatment with placebo in advanced Parkinson's disease: International Pramipexole-Bromocriptine Study Group. Neurology 1997;49:1060–1065. [DOI] [PubMed] [Google Scholar]
- 25.Gorell JM, Peterson EL, Rybicki BA, Johnson CC. Multiple risk factors for Parkinson's disease. J Neurol Sci 2004;217:169–174. [DOI] [PubMed] [Google Scholar]
- 26.Costello S, Cockburn M, Bronstein J, Zhang X, Ritz B. Parkinson's disease and residential exposure to maneb and paraquat from agricultural applications in the central valley of California. Am J Epidemiol 2009;169:919–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Willis AW, Schootman M, Kung N, Evanoff BA, Perlmutter JS, Racette BA. Predictors of survival in patients with Parkinson disease. Arch Neurol 2012;69:601–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Food Quality Protection Act (FQPA). Available at: http://www.epa.gov/pesticides/regulating/laws/fqpa/. Accessed February 19, 2015.
- 29.Federal Insecticide, Fungicide, and Rodenticide Act (FIFRA). Available at: http://www.epa.gov/agriculture/lfra.html. Accessed February 19, 2015.
- 30.Nyholm D, Jansson R, Willows T, Remahl IN. Long-term 24-hour duodenal infusion of levodopa: outcome and dose requirements. Neurology 2005;65:1506–1507. [DOI] [PubMed] [Google Scholar]
- 31.Jozefowicz RF. Neurophobia: the fear of neurology among medical students. Arch Neurol 1994;51:328–329. [DOI] [PubMed] [Google Scholar]
- 32.Cheng EM, Tonn S, Swain-Eng R, Factor SA, Weiner WJ, Bever CT., Jr Quality improvement in neurology: AAN Parkinson disease quality measures: report of the Quality Measurement and Reporting Subcommittee of the American Academy of Neurology. Neurology 2010;75:2021–2027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Willis AW, Schootman M, Evanoff BA, Perlmutter JS, Racette BA. Neurologist care in Parkinson disease: a utilization, outcomes, and survival study. Neurology 2011;77:851–857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Willis AW, Schootman M, Tran R, et al. Neurologist-associated reduction in PD-related hospitalizations and health care expenditures. Neurology 2012;79:1774–1780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cheng EM, Swarztrauber K, Siderowf AD, et al. Association of specialist involvement and quality of care for Parkinson's disease. Mov Disord 2007;22:515–522. [DOI] [PubMed] [Google Scholar]
- 36.Willis AW, Schootman M, Kung N, Wang XY, Perlmutter JS, Racette BA. Disparities in deep brain stimulation surgery among insured elders with Parkinson disease. Neurology 2014;82:163–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dorsey ER, Constantinescu R, Thompson JP, et al. Projected number of people with Parkinson disease in the most populous nations, 2005 through 2030. Neurology 2007;68:384–386. [DOI] [PubMed] [Google Scholar]
- 38.Dall TM, Storm MV, Chakrabarti R, et al. Supply and demand analysis of the current and future US neurology workforce. Neurology 2013;81:470–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Racette BA, Holtzman DM, Dall TM, Drogan O. Supply and demand analysis of the current and future US neurology workforce. Neurology 2014;82:2254–2255. [DOI] [PubMed] [Google Scholar]
- 40.Needleman HL, Tuncay OC, Shapiro IM. Lead levels in deciduous teeth of urban and suburban American children. Nature 1972;235:111–112. [DOI] [PubMed] [Google Scholar]
- 41.Needleman HL, Gunnoe C, Leviton A, et al. Deficits in psychologic and classroom performance of children with elevated dentine lead levels. N Engl J Med 1979;300:689–695. [DOI] [PubMed] [Google Scholar]
- 42.Patel CJ, Bhattacharya J, Butte AJ. An environment-wide association study (EWAS) on type 2 diabetes mellitus. PLoS One 2010;5:e10746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dorsey ER, Venkataraman V, Grana MJ, et al. Randomized controlled clinical trial of “virtual house calls” for Parkinson disease. JAMA Neurol 2013;70:565–570. [DOI] [PMC free article] [PubMed] [Google Scholar]