Mitochondrial encephalomyopathy, lactic acidosis, and stroke-like episodes (MELAS) syndrome (MT-TL1 gene) is a progressive neurologic disorder with stroke-like episodes (SLEs), which are recurrent neurologic deficits resembling vasoocclusive strokes.1 However, SLEs are not restricted to vascular territories and have a predilection for the occipital and posterior parietal and temporal cortices,2 may evolve subacutely over hours to days,3 and have greater potential for reversibility.4 Their pathophysiology is incompletely understood. Current literature suggests a combination of neuronal mitochondrial energy failure and cerebrovascular angiopathy with dysregulated perfusion.5
Recent work has demonstrated a beneficial effect of l-arginine in MELAS for acute treatment and prevention of SLEs,6 and a relative serum arginine deficiency in patients with MELAS at baseline with a further decrease during SLEs.6 The rationale was to utilize the vasodilatory properties of arginine through its conversion to nitric oxide; however, blood flow dysregulation in MELAS is complex involving both hypo- and hyperperfusion. Arginine is also a precursor for creatine, can be decarboxylated to agmatine (neurotransmitter), and can be converted to α-ketoglutarate, improving tricarboxylic acid cycle kinetics and cellular anaplerosis.7
Level of evidence.
This study provides Class IV level of evidence. It is a single observational study without controls.
Case report.
A right-handed boy aged 10 years, 9 months presented with fever and upper respiratory tract infection followed by acute onset of headache, vomiting, change in mental status, right-sided focal seizures, and hemiparesis, which developed over 3 hours.
He was born of normal pregnancy and term delivery with normal early development. Two years before presentation, he had an episode of fever and encephalopathy with left-sided focal seizures and left hemiparesis with right temporal lobe swelling on head CT and was treated for suspected herpes encephalitis with acyclovir (PCR was negative). He made a near-complete recovery with mild residual left leg weakness at discharge. At age 10 years, 2 months, he had a second episode of encephalopathy with right-sided focal seizures and negative virology and had a large area of abnormal high FLAIR (fluid-attenuated inversion recovery) signal in the left temporal lobe. At 3-month follow-up, he had memory, processing, and word-finding difficulties but no focal weakness or field defect.
At presentation, he appeared confused and Glasgow Coma Scale score was 13. He had a receptive aphasia and a right superior quadrantanopia. His bilateral asymmetric ptosis, which had developed over 2 years, was worse with fatigue or illness. He had asthenic muscle bulk and a right hemiparesis.
Brain MRI showed normal basal ganglia and high FLAIR signal in the left superior temporal gyrus (figure, A), and left parietal lobe with effacement of sulci (figure, B). Magnetic resonance spectroscopy showed a lactate peak at 1.33 ppm (figure, G). CSF lactate was elevated at 5.29 mM. Genetic testing confirmed the m.3243A>G tRNALeu(UUR) mutation with 32% mutant heteroplasmy in blood leukocytes.
Figure. Brain MRI at time of clinical presentations and after l-arginine treatment.
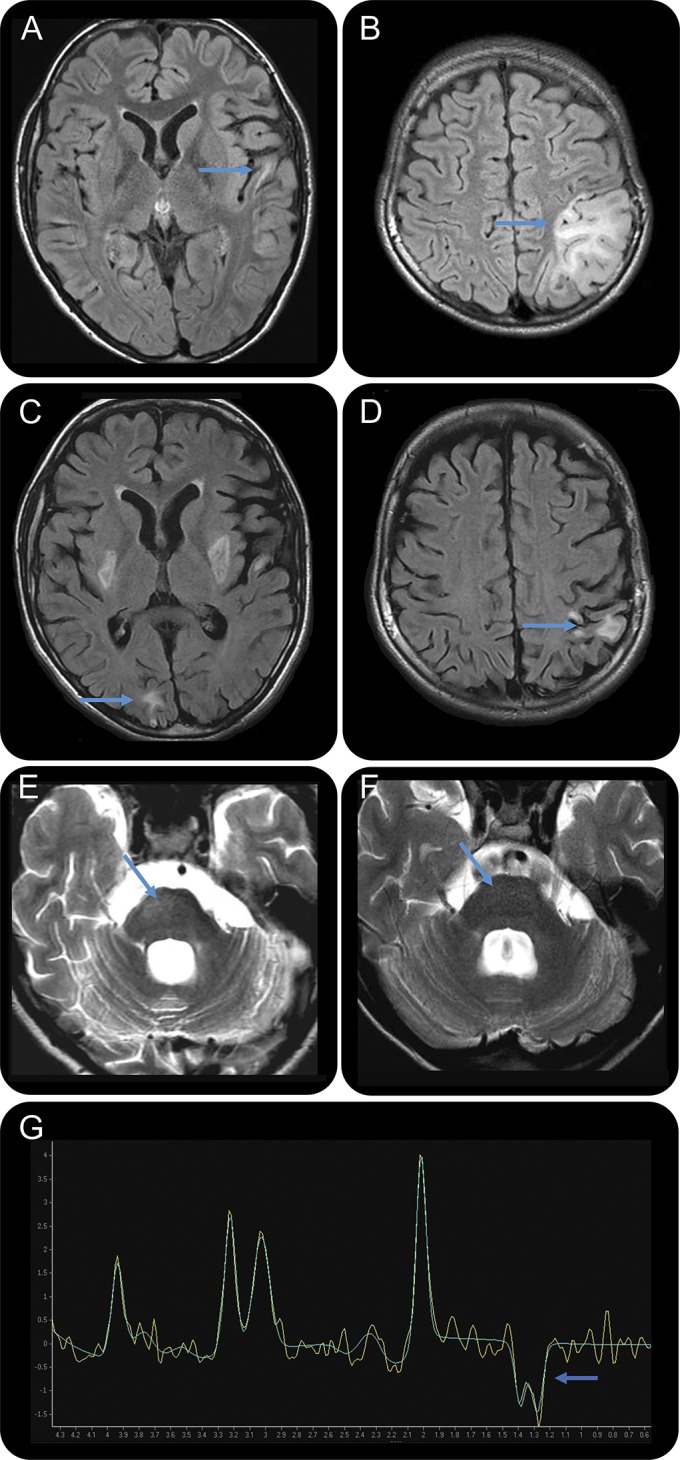
(A) MRI showing normal basal ganglia, left superior temporal gyrus high FLAIR signal, and (B) left parietal lobe high FLAIR signal with effacement of sulci. (C) New abnormal signal in bilateral basal ganglia, as well as right occipital pole, and (D) improvement of the left parietal lobe lesion. (E) New lesion of the right pons. (F) MRI repeated after l-arginine treatment showing resolution of the right pontine lesion, but the basal ganglia, right occipital pole, and left superior temporal gyrus lesions remained unchanged (not shown). (G) Magnetic resonance spectroscopy (baseline subtracted in light green and nonbaseline subtracted in yellow) shows high lactate doublet peak at a chemical shift of 1.33 ppm acquired from left basal ganglia. FLAIR = fluid-attenuated inversion recovery.
Following informed consent, he was treated with oral l-arginine 500 mg/kg/d divided 3 times daily and within 24 hours showed rapid neurologic improvement. With subsequent emesis of his l-arginine, he experienced recrudescent encephalopathy, apraxia, and aphasia and was treated with IV l-arginine 500 mg/kg/d divided 3 times daily. After 24 hours, the l-arginine was reduced to 200 mg/kg/d divided 3 times daily for 48 hours and symptoms quickly resolved. l-Arginine was continued and after discharge very slowly tapered over 6 weeks to 1 g twice daily orally as maintenance. At 3-month follow-up, he had no field defect or focal weakness and had steady improvement in his speech, cognitive processing, and memory.
At age 13 years, he presented again with an upper respiratory tract infection and emesis and was hospitalized for hydration. Six days later, he experienced acute frontal headaches with transient diplopia, partial right VI nerve palsy, and vomiting. MRI demonstrated a new abnormal signal in the bilateral basal ganglia as well as the right occipital pole (figure, C) and the right pons (figure, E) and improvement of the previous left parietal lobe lesion (figure, D). He was treated with high-dose IV l-arginine 500 mg/kg/d divided 3 times daily followed by a gradual taper to maintenance. Within 24 hours, he had complete resolution of symptoms. Repeat MRI 3 days later showed complete resolution of the pontine lesion (figure, F), but the basal ganglia, right occipital pole, and left superior temporal gyrus remained unchanged.
Discussion.
This case highlights the rapid and consistent reversal of neurologic deficits and neuroradiologic lesions following high-dose l-arginine. Our proband had recurrence of SLEs during emesis of oral l-arginine, which quickly reversed with IV l-arginine suggesting an important role for IV arginine during acute SLEs. Given the accelerating frequency of SLEs between ages 8 and 10 years and the subsequent 2-year interval without SLEs during maintenance l-arginine, this may suggest a preventative role of l-arginine in MELAS.
Footnotes
Author contributions: Ishita Siddiq, MD, contributed to the drafting of the manuscript for intellectual content. Elysa Widjaja, MD, contributed to the drafting and revision of the manuscript for intellectual content. Ingrid Tein, MD, contributed to the drafting and revision of the manuscript for intellectual content.
Study funding: No targeted funding reported.
Disclosure: I. Siddiq and E. Widjaja report no disclosures relevant to the manuscript. I. Tein reports operating grants from the United Mitochondrial Disease Foundation, The Physician's Services Incorporated Foundation, and The Myositis Foundation, which did not support or influence this study. Go to Neurology.org for full disclosures.
References
- 1.Sproule DM, Kaufmann P. Mitochondrial encephalopathy, lactic acidosis, and strokelike episodes: basic concepts, clinical phenotypes, and therapeutic management of MELAS syndrome. Ann N Y Acad Sci 2008;1142:133–158. [DOI] [PubMed] [Google Scholar]
- 2.Ito H, Mori K, Kagami S. Neuroimaging of stroke-like episodes in MELAS. Brain Dev 2011;33:283–288. [DOI] [PubMed] [Google Scholar]
- 3.Iizuka T, Sakai F, Kan S, Suzuki N. Slowly progressive spread of stroke-like lesions in MELAS. Neurology 2003;61:1238–1244. [DOI] [PubMed] [Google Scholar]
- 4.Iizuka T, Sakai F, Suzuki N, et al. Neuronal hyperexcitability in stroke-like episodes of MELAS syndrome. Neurology 2002;59:816–824. [DOI] [PubMed] [Google Scholar]
- 5.Koga Y, Povalko N, Nishioka J, Katayama K, Yatsuga S, Matsuishi T. Molecular pathology of MELAS and L-arginine effects. Biochim Biophys Acta 2012;1820:608–614. [DOI] [PubMed] [Google Scholar]
- 6.Koga Y, Povalko N, Nishioka J, Katayama K, Kakimoto N, Matsuishi T. MELAS and L-arginine therapy: pathophysiology of stroke-like episodes. Ann N Y Acad Sci 2010;1201:104–110. [DOI] [PubMed] [Google Scholar]
- 7.Morris SM., Jr Arginine metabolism: boundaries of our knowledge. J Nutr 2007;137:1602S–1609S. [DOI] [PubMed] [Google Scholar]


