Abstract
Objective:
We studied the biomarker signatures and prognoses of 3 different subtle cognitive impairment (SCI) groups (executive, memory, and multidomain) as well as the subjective memory complaints (SMC) group.
Methods:
We studied 522 healthy controls in the Alzheimer's Disease Neuroimaging Initiative (ADNI). Cutoffs for executive, memory, and multidomain SCI were defined using participants who remained cognitively normal (CN) for 7 years. CSF Alzheimer disease (AD) biomarkers, composite and region-of-interest (ROI) MRI, and fluorodeoxyglucose-PET measures were compared in these participants.
Results:
Using a stringent cutoff (fifth percentile), 27.6% of the ADNI participants were classified as SCI. Most single ROI or global-based measures were not sensitive to detect differences between groups. Only MRI-SPARE-AD (Spatial Pattern of Abnormalities for Recognition of Early AD), a quantitative MRI pattern-based global index, showed differences between all groups, excluding the executive SCI group. Atrophy patterns differed in memory SCI and SMC. The CN and the SMC groups presented a similar distribution of preclinical dementia stages. Fifty percent of the participants with executive, memory, and multidomain SCI progressed to mild cognitive impairment or dementia at 7, 5, and 2 years, respectively.
Conclusions:
Our results indicate that (1) the different SCI categories have different clinical prognoses and biomarker signatures, (2) longitudinally followed CN subjects are needed to establish clinical cutoffs, (3) subjects with SMC show a frontal pattern of brain atrophy, and (4) pattern-based analyses outperform commonly used single ROI-based neuroimaging biomarkers and are needed to detect initial stages of cognitive impairment.
The development of different neuropsychological batteries and biomarkers and their use in different cohorts have led to the study of earlier stages of dementia. Therefore, mild cognitive impairment (MCI) was defined as a stage that may precede Alzheimer disease (AD) or other dementias. MCI is characterized by moderate cognitive impairment without impairment of daily living activities,1 that can be further categorized based on the cognitive profile into amnestic and nonamnestic MCI subtypes. Biomarkers correlate with neuropathologic hallmarks of AD2–4 and a model of biomarker and clinical changes extending from normal cognition to AD dementia has been proposed.5 Based on the recent recognition that β-amyloid (Aβ) deposition and biomarker changes reflecting this occur several years before the onset of cognitive symptoms, preclinical AD stages were recently proposed. The assumption that there are stereotypical sequential changes affecting Aβ and neurodegeneration biomarkers followed thereafter by the appearance of subtle cognitive impairment (SCI) led to a 3-stage pathologic model for the onset of cognitive impairment and progression to MCI and AD dementia.6 However, the study of cognitively normal (CN) individuals led to the definition of 2 new categories that did not fit this model, namely, those with suspected nonamyloid pathology (SNAP)7 and those with SCI who show normal neuronal injury biomarkers (SCINIB)8 (table e-1 on the Neurology® Web site at Neurology.org). Both categories might include non-AD causes of cognitive impairment. The distributions of the presumed non-AD preclinical dementia groups and the corresponding longitudinal outcomes have been studied in several different cohorts.7–13 However, none of the studies explored clinical implications and biomarker correlates of the different cognitive domains, which could have important clinical and diagnostic implications, as extensively as for MCI.14 To answer this, we compared the clinical, biomarker, and longitudinal outcomes of subjects with SCI due to executive, memory, or combined impairments.
METHODS
Participants.
Data used in the preparation of this article were obtained from the Alzheimer's Disease Neuroimaging Initiative (ADNI) database (adni.loni.usc.edu). The ADNI was launched in 2003 by the National Institute on Aging, the National Institute of Biomedical Imaging and Bioengineering, the Food and Drug Administration, private pharmaceutical companies, and nonprofit organizations15 as detailed in the ADNI Manuscript Citations protocol (http://adni.loni.usc.edu/wp-content/uploads/how_to_apply/ADNI_Manuscript_Citations.pdf and supplementary material) (see additional information in http://www.adni-info.org).
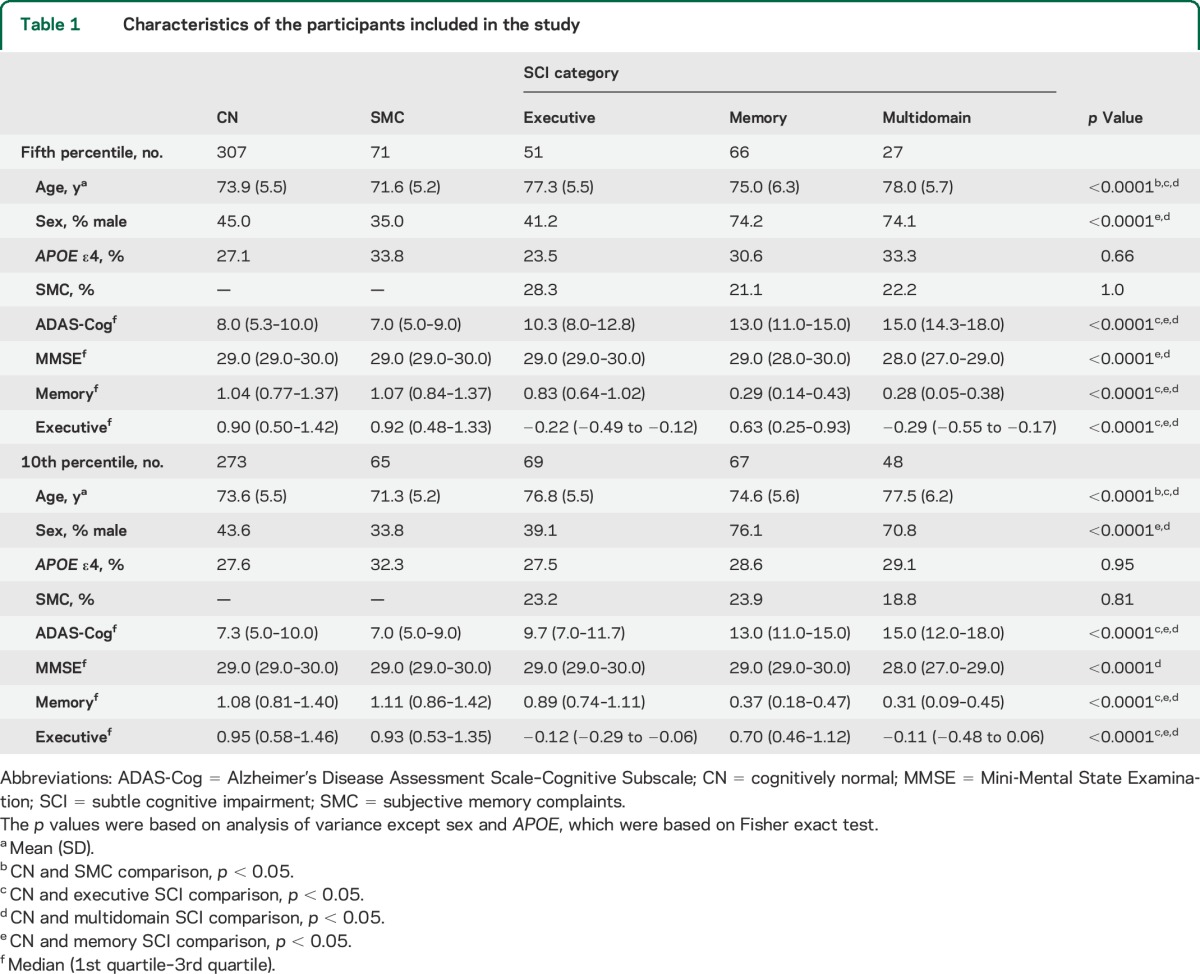
A total of 522 healthy controls (HCs), 106 of them with subjective memory complaints (SMC), were included in this study (table 1 and e-Methods). HCs without SMC are further referred in this report as CN participants. Data were downloaded November 2, 2014. Three hundred seventy-one participants had CSF Aβ1–42, total tau (t-tau), and tau phosphorylated at threonine 181 (p-tau181) data available, which were obtained from UPENNBIOMK.csv, UPENNBIOMK5-7.csv (all the measurements for each participant were selected from a single file). Four hundred thirty-eight and 460 participants had adjusted hippocampal volume (aHV) measures and MRI-SPARE-AD (Spatial Pattern of Abnormality for Recognition of Early AD)16,17 values, respectively. Finally, 306 and 301 participants had data on PET-fluorodeoxyglucose (FDG) hypometabolic convergence index (PET-HCI)18 and posterior cingulate cerebral metabolic rate for glucose (posterior cingulate PET) available, respectively. The diagnosis of AD dementia was established based on the National Institute of Neurological and Communicative Disorders and Stroke–Alzheimer's Disease and Related Disorders Association criteria for probable AD,19 whereas participants with MCI did not meet these AD criteria and had largely intact general cognition and functional performance and met predetermined criteria for MCI.1 ADNI-1, ADNI-GO, and ADNI-2 participants were recruited in different waves at different times so the available follow-up varies between cohorts. Median follow-up was 6 years (first and third quartiles: 3.0 and 7.4) and 1.9 years (first and third quartiles: 0.5 and 2.0) for ADNI-1 and ADNI-GO/2 participants, respectively (figure e-1). Percentages of participants lost to follow-up were 56.3% in ADNI-1 and 8.2% in ADNI-GO/2.
Table 1.
Characteristics of the participants included in the study
Standard protocol approvals, registrations, and patient consents.
Protocols were submitted to institutional review boards for each participating location and their written unconditional approval was obtained and submitted to Regulatory Affairs at the ADNI Coordinating Center before commencement of the study. Written informed consent for the study was obtained from all participants and/or authorized representatives.
CSF collection and Aβ1–42 measurement.
After an overnight fast, lumbar puncture was performed in the morning. The multiplex xMAP Luminex platform was used to measure Aβ1–42, t-tau, and p-tau181 (Luminex Corp., Austin, TX) using INNO-BIA AlzBio3 immunoassay kit–based reagents (for research use–only reagents; Innogenetics, Ghent, Belgium). Information on the procedures and standard operating procedures was described previously20,21 (supplementary material and online at http://www.adni-info.org/).
MRI and FDG-PET acquisition and processing.
At each performance site, 1.5-tesla (T) (ADNI-1) and 3T (ADNI-GO/2) nonaccelerated sagittal volumetric, 3-dimensional magnetization-prepared rapid-acquisition gradient echo MRIs were acquired (http://adni.loni.ucla.edu). Only images that passed the quality-control evaluations were included. Cortical gray matter volumes were processed using FreeSurfer software package version 4.4 (for 1.5T MRI scans) and 5.1 (for 3T MRI scans) image processing framework (http://surfer.nmr.mgh.harvard.edu/).22,23 The aHV was calculated as previously described to account for differences between different field strengths and software packages.8 The MRI-SPARE-AD index captures brain atrophy related to AD.16,24 ODVBA (optimally discriminative voxel-based analysis) was applied for voxel-based analysis.25
A standardized protocol was followed to reconstruct the acquired FDG-PET data with the use of measured attenuation correction and the specified reconstruction algorithm for each scanner type (http://adni.loni.ucla.edu/). Images were downloaded and preprocessed using SPM5 by investigators at Banner Alzheimer's Institute (http://www.fil.ion.ucl.ac.uk/spm). We calculated the PET-HCI,18 a pattern-based summary score, and the cerebral metabolic rate for glucose for the posterior cingulate.
Definition of preclinical dementia stages and biomarker and cognitive cutoffs.
The CN participants were classified in SCI groups based on their composite memory26 and executive function scores.27 To define the cutoffs, we identified ADNI-1 CN participants with a follow-up of at least 7 years (n = 117); 81 remained clinically stable after at least 7 years of follow-up (70%), whereas 27 participants progressed to MCI (23%) and 9 participants progressed to AD (7%). Baseline age (p = 0.23), education (p = 0.46), and sex (p = 0.76) of these 81 participants did not differ from the remainder of the CN participants. We calculated cutoffs for the memory and executive performance scores in these 81 CN participants using the fifth (0.010 and 0.48, respectively) and 10th (0.19 and 0.54, respectively) percentiles. Finally, multidomain SCI was defined by the presence of abnormal values in memory and executive scores. Assessment of the visuospatial domain was not included because there were no detailed cognitive assessments for this domain. The language domain was assessed using the semantic fluency and Boston Naming tests as previously described.28 There was a small number of participants with language SCI (because of a large overlap with the executive SCI) and this group was not associated with longitudinal outcomes; therefore, results including this domain are presented in table e-2.
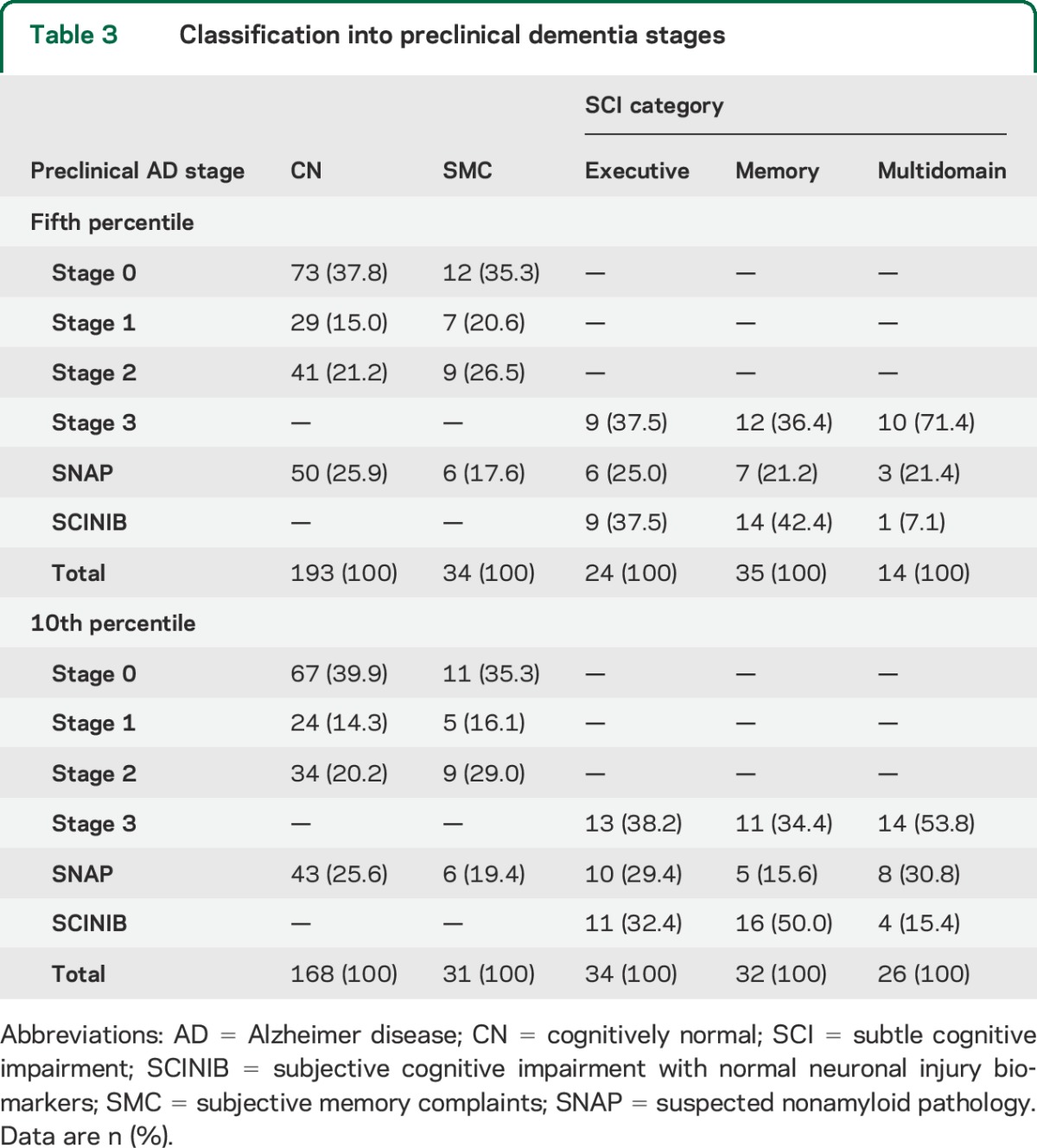
For the classification of HCs into preclinical stages (table e-1), we selected CSF Aβ1–42 as the Aβ deposition marker and t-tau and aHV as neuronal injury biomarkers and therefore only 300 participants who had all measures available were included in these analyses. We used CSF Aβ1–42 as the Aβ deposition marker because it was available for 366 HCs whereas baseline florbetapir-PET scans were only available for 265 HCs and 8 of these did not have CSF Aβ1–42 measurements. In addition, CSF Aβ1–42 and florbetapir-PET scans show high classification agreement29,30 although the association is limited to the middle range of values.30 Therefore, one of these measures of Aβ burden is reasonably sufficient to classify subjects. However, because there is a low agreement between the different neurodegeneration biomarkers,8 we selected these biomarkers based on their association with CN progression to MCI/dementia.8 CSF Aβ1–42 and t-tau cutoffs were selected based on cutoffs previously established and validated in a cohort including subjects with autopsy-confirmed AD.20 The aHV cutoff (404.5) was chosen based on values that would give 90% sensitivity using a group of 271 participants with AD dementia for this purpose.7,8 Participants were categorized as neurodegeneration-positive if any of the 2 included neurodegeneration biomarkers was abnormal. Therefore, CN participants were classified into the following categories: stage 0,7 stage 1, stage 2, and SNAP.7 The different SCI groups were classified into stage 3,6 SNAP, and SCINIB.8
Statistical analysis.
For group comparisons of quantitative variables, analysis of variance and linear regression analysis were applied, depending on the presence of covariates. To achieve a normal distribution of the residuals, a power transformation was applied when needed. Age and sex were included as covariates in the group comparison of biomarker values. Fisher exact test was applied for the analyses of qualitative variables. For the analysis of conversion from HC to MCI/dementia, a Cox proportional hazards model that included age, sex, APOE ε4 presence, and education as covariates was used. The proportional hazards assumption was tested analyzing the correlation between the Schoenfeld residuals and survival time.31 SCI groups did not meet the proportional hazards assumption; therefore, we applied a proportional hazards model with a heaviside function. No multiple comparisons adjustment was applied to p values among groups for the biomarkers studied here because biomarkers were selected a priori based on recommendations for the preclinical AD categories and we consider this an exploratory study to define the use of biomarker for preclinical dementia categories. Significance was defined as p = 0.05 type I error.
RESULTS
Demographic characteristics and cognitive scores.
As expected, the groups differed in cognitive impairment, with the 3 SCI groups showing worse performance than the CN group, but no differences for the cognitive scores were found between the SMC and the CN groups (tables 1 and e-2). Male participants were overrepresented in the memory SCI and multidomain SCI groups. Conversely, there were no differences in the percentage of SMC in the different groups.
CSF and neuroimaging biomarkers.
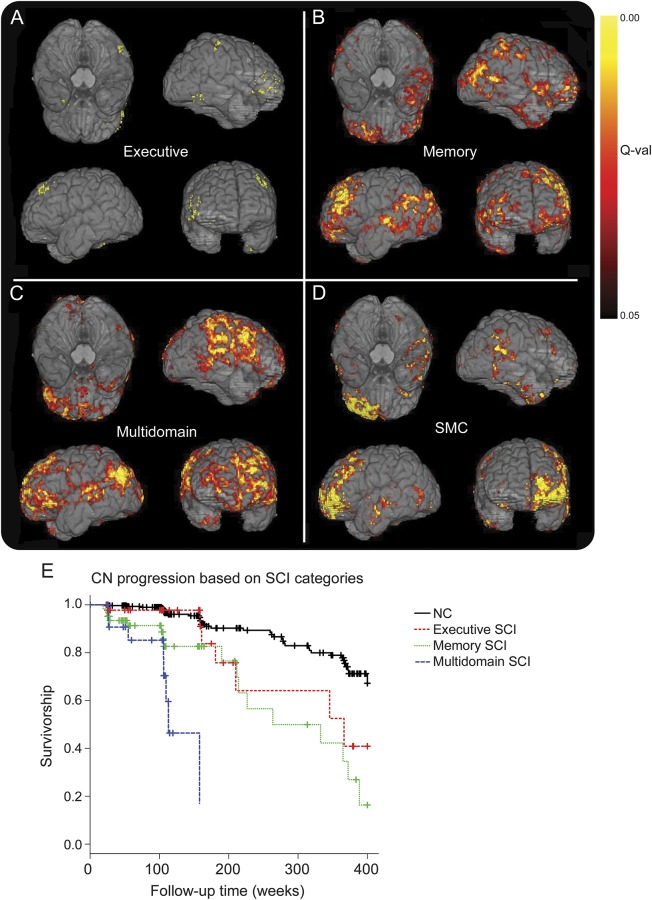
Several biomarkers were altered between groups (tables 2 and e-3). MRI-SPARE-AD showed the largest differences between groups, revealing increased brain atrophy in participants with SMC and memory and multidomain SCI compared with CN participants. In addition, the multidomain SCI group presented greater posterior cingulate FDG-PET hypometabolism and lower CSF Aβ1–42 values compared with the CN group. Finally, the memory SCI and the SMC groups showed lower CSF Aβ1–42 and higher p-tau181 levels than the CN group, respectively. Results using the same participants in all comparisons are presented in table e-4. Comparison of the ADNI-2 participants' baseline MRIs is presented in figure 1.
Table 2.
Amyloid burden and neurodegeneration biomarkers in CN, SMC, and the different SCI categories
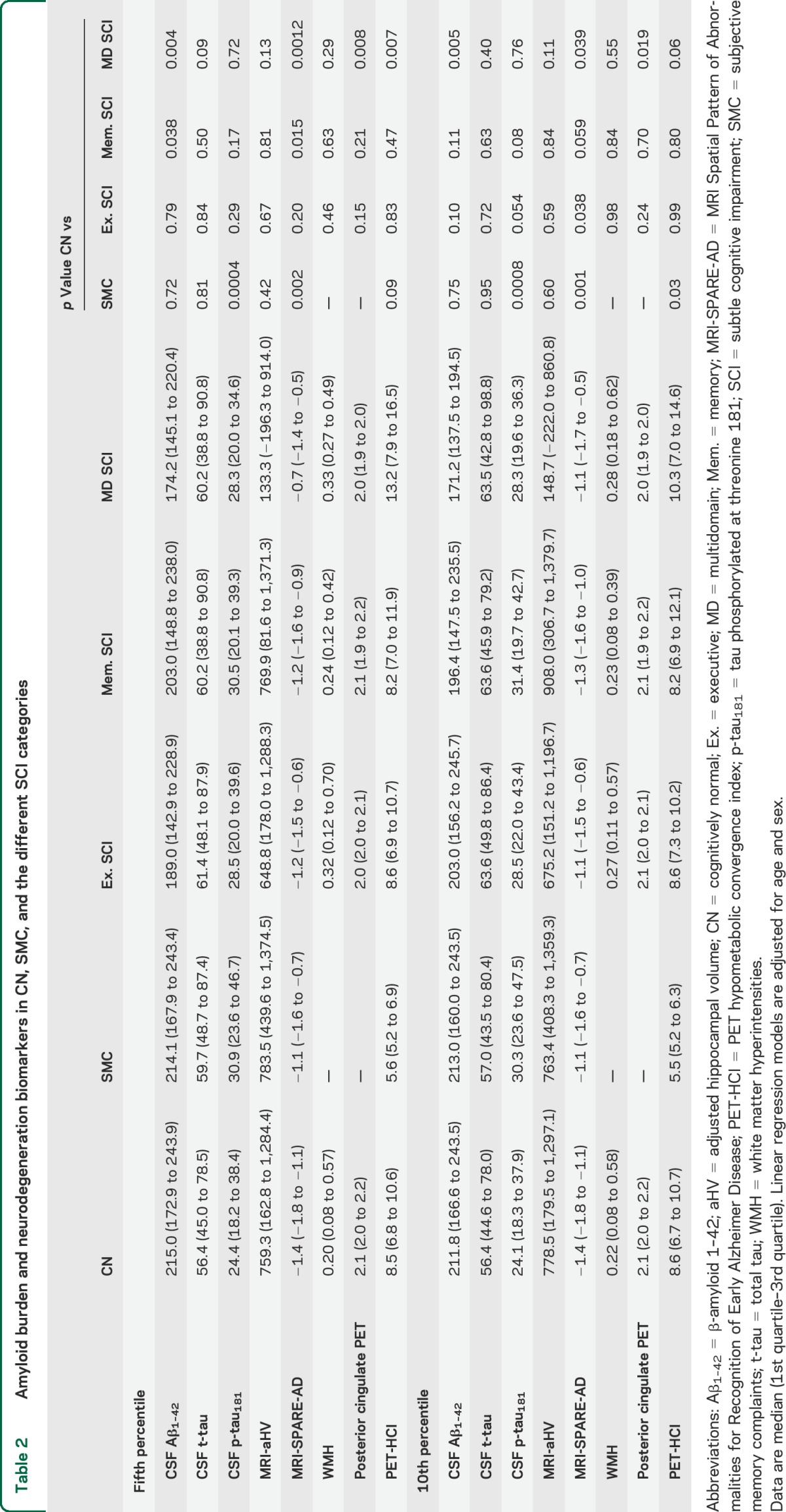
Figure 1. Atrophy in SCI categories and SMC, and CN progression to MCI/dementia.
Atrophy in executive SCI (A), memory SCI (B), multidomain SCI (C), and SMC (D) compared with CN participants in ADNI-2. (E) Conversion from CN to MCI/dementia stratified by SCI categories. ADNI = Alzheimer's Disease Neuroimaging Initiative; CN = cognitively normal; MCI = mild cognitive impairment; SCI = subtle cognitive impairment; SMC = subjective memory complaints.
Preclinical dementia stages and clinical progression.
Three different biomarkers (CSF Aβ1–42, t-tau, and aHV) were needed for the classification of presumed AD/non-AD pathology preclinical dementia stages, i.e., preclinical AD, SCINIB, and SNAP, in the different clinical groups (tables 3 and e-5). No differences were found between the different categories when the NC and the SMC groups were compared (p = 0.66). The multidomain SCI group showed a higher percentage of stage 3 participants compared with the memory SCI (p = 0.035) and there was a trend for the comparison with the executive SCI (p = 0.076).
Table 3.
Classification into preclinical dementia stages
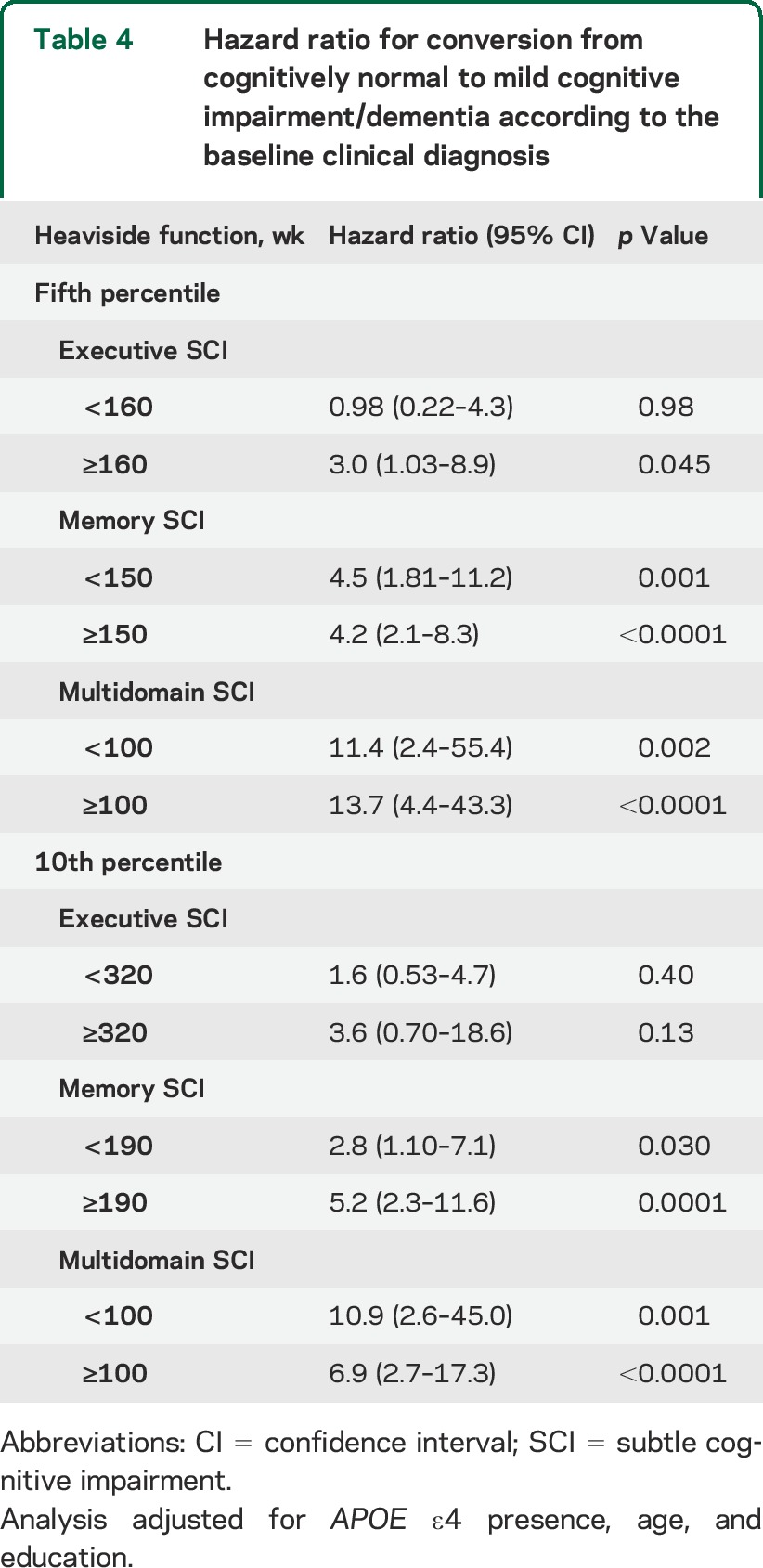
Overall, ADNI-1 CN participants showed a conversion rate to MCI/dementia of 16% by year 5 and 27.5% by year 7 (figure e-1). Conversion from memory SCI to MCI/dementia reached 50% at 5 years, whereas the same conversion rate was reached within 2 years for the multidomain SCI cases while taking 7 years for the executive MCI (figure 1) using the fifth percentile cutoff. The different SCIs did not fulfill the proportional hazards assumption, therefore we created 2 heaviside functions for each category; all the heaviside functions were associated with a greater conversion to MCI/dementia except the first heaviside function for executive SCI (table 4). The median time to conversion was 5, 6, and again 6 years for the multidomain, memory, and executive groups, respectively, using the 10th percentile cutoff; results of the survival model are summarized in table 4. The SMC was not included in the analyses because of short follow-up.
Table 4.
Hazard ratio for conversion from cognitively normal to mild cognitive impairment/dementia according to the baseline clinical diagnosis
DISCUSSION
In this large sample of ADNI CN participants studied here, 27.6% had SCI, with single-domain memory SCI being the most frequent. The 3 types of SCI groups showed differences in clinical prognoses and distribution of preclinical dementia stages, but only the cases with multidomain SCI showed distinct biomarker profiles when compared with the CN group because of the lack of sensitivity of several neurodegeneration biomarkers to detect changes in the executive and memory SCI groups. The multidomain SCI had the highest proportion of participants with stage 3 preclinical AD.
Previous studies had described cognitive and biomarker changes in subjects with pre-MCI,32,33 but it was only relatively recently that criteria for preclinical AD stages were established. These criteria provided a significant new framework for classifying individuals into 3 distinct categories,6 but neither was specific recommendation given for the selection of specific neurodegeneration biomarkers nor were any criteria defined for neuropsychological cutoffs to be used to identify the various preclinical stages that might lead to progression to MCI/dementia. One approach to the operational implementation of these criteria selected a cutoff for the neurodegeneration biomarkers that had 90% sensitivity for AD and a clinical cutoff for the composite cognitive score based on the 10th percentile of the baseline CN controls.7 Thereafter, 3 different studies confirmed that these categories were associated with the risk of progression to MCI/dementia.8,12,13 However, 2 of the studies used nonoverlapping neurodegeneration biomarkers,12,13 whereas only one study included all the different available neurodegeneration biomarkers.8 The latter found low agreement between the different neurodegeneration biomarkers, indicating that each study might classify subjects differently.
Whereas the different MCI subtypes have been studied regarding progression to dementia,14 this has not been the case for the SCI groups. Therefore, we studied the clinical aspects, biomarker values, and prognosis of each of the different SCI groups. Previously, cognitive cutoffs have been established based on the 10th percentile of a composite score in subjects that were CN at baseline7 or on the 10th percentile of a composite memory score in subjects followed for 5 years.13 In the present study, the clinical cutoffs were based on the baseline scores of CN participants in ADNI who remained stable for at least 7 years. Although we used the fifth percentile, this corresponded to the 18th and 15th percentile of the memory and executive scores of all the CN participants at baseline, respectively. This emphasizes the need for long follow-up periods to establish clinical cutoffs based on subjects who remain stable longitudinally, resulting in a more stringent but more reliable selection of cases rather than criteria based on baseline scores of any cross-sectionally verified CN subjects, and the development of sensitive scores to detect the earliest cognitive changes that precede the onset of MCI and dementia.34,35
In this study, a wide range of biomarkers was examined (table 2) although only the multidomain SCI group showed consistent differences compared with the CN group. Of note, the MRI-SPARE-AD method was shown to be different in the SMC and memory and multidomain SCI groups. The MRI-SPARE-AD is a quantitative pattern-based classifier that was developed to classify CN individuals vs those with AD achieving a high accuracy and a good correlation with clinical measures,17,24 i.e., the higher the value, the higher the probability of having AD. Moreover, we also showed that MRI-SPARE-AD had a discriminating capability among CN participants. Patterns of neurodegeneration seem to be diffuse but subtle at early stages. Thus, it is possible that a more specific and sensitive SPARE algorithm focusing on the subtleties of these early stages36 might be needed to detect the different SCI categories, as opposed to SPARE-AD, which is constructed to quantify significant AD-like neurodegeneration. Whereas participants with multidomain SCI showed larger atrophy than those with memory SCI involving similar areas, the participants with SMC showed atrophy that mainly affected the frontal pole and orbitofrontal cortex, indicating that it might represent a different underlying process.
However, biomarkers based on a single region of interest (ROI) were less discriminating; the aHV showed no overall differences between groups, and the posterior cingulate FDG-PET only detected differences in the participants with multidomain SCI. Alternatively, the lack of discrimination might be attributable to a mixture of participants with preclinical AD and other comorbidities of preclinical dementia, related to other areas that might be better detected by other ROIs. White matter hyperintensity values did not reveal any differences between groups. However, it must be noted that similar to most randomized treatment trials of AD or MCI, a history of “stroke” or a Hachinski score above 4 is an exclusion criterion for ADNI. Because of this, ADNI sites do not recruit subjects known or expected to have large amounts of cerebrovascular pathology. Therefore, individuals with heavy cerebrovascular pathology are not enrolled in ADNI and vascular pathology is underrepresented, although it is a common finding in patients with dementia.37,38
An unexpected finding was that the percentage of participants with SMC did not vary between the different SCI and the CN groups, although it was 10% higher in the executive SCI group. Larger samples might be needed to assess whether individuals with SMC are at higher risk of having SCI. The participants with SMC showed higher p-tau and MRI-SPARE-AD values, both indicative of pathologic changes. However, no differences were found regarding the distribution of the different preclinical dementia categories (table 3). This contrasted with the SCI groups. The multidomain SCI group included a higher number of cases with preclinical AD stage 3 (vs SNAP and SCINIB) when compared with memory SCI; there was only a trend for the comparison with executive SCI possibly because of the small sample size. Thus, the multidomain SCI category has the strongest association with AD compared with the other categories. Of interest, the 3 SCI groups showed different risks of conversion to MC/dementia; 50% of the participants with executive, memory, and multidomain SCI progressed to MCI/dementia at 7, 5, and 2 years, respectively. It must be noted that the same cognitive scores that constitute the summary cognitive measures are used for the diagnosis of the patients. However, there are differences in biomarker signatures and prognoses, which indicate that refinements in categorization of these patients might be needed to capture the different combination of biomarkers that underlie the cognitive changes as well as longer follow-up depending on the involved pathologic process.
Limitations of this study are the variable availability of biomarkers in the different participants, the shorter follow-up for ADNI-GO/2 participants, and the high rate of loss to follow-up in ADNI-1.
Our results indicate that the different SCI categories have different clinical prognoses and biomarkers signatures, with multidomain SCI being more related to AD, and that neuroimaging biomarkers based on single-area ROI might not be sensitive enough for initial stages of cognitive impairment. Further studies are needed to characterize what underlying pathologies are involved in each specific SCI group.
Supplementary Material
GLOSSARY
- Aβ
β-amyloid
- AD
Alzheimer disease
- ADNI
Alzheimer's Disease Neuroimaging Initiative
- aHV
adjusted hippocampal volume
- CN
cognitively normal
- FDG
fluorodeoxyglucose
- HC
healthy control
- MCI
mild cognitive impairment
- MRI-SPARE-AD
MRI Spatial Pattern of Abnormalities for Recognition of Early Alzheimer Disease
- PET-HCI
PET hypometabolic convergence index
- p-tau181
tau phosphorylated at threonine 181
- ROI
region of interest
- SCI
subtle cognitive impairment
- SCINIB
subtle cognitive impairment with normal neuronal injury biomarkers
- SMC
subjective memory complaints
- SNAP
suspected nonamyloid pathology
- t-tau
total tau
- WMI
white matter hyperintensity
Footnotes
Supplemental data at Neurology.org
Contributor Information
Collaborators: Michael Weiner, Paul Aisen, Michael Weiner, Paul Aisen, Ronald Petersen, Clifford R. Jack, Jr., William Jagust, John Q. Trojanowki, Arthur W. Toga, Laurel Beckett, Robert C. Green, Andrew J. Saykin, John Morris, Enchi Liu, Robert C. Green, Tom Montine, Ronald Petersen, Paul Aisen, Anthony Gamst, Ronald G. Thomas, Michael Donohue, Sarah Walter, Devon Gessert, Tamie Sather, Laurel Beckett, Danielle Harvey, Anthony Gamst, Michael Donohue, John Kornak, Clifford R. Jack, Jr., Anders Dale, Matthew Bernstein, Joel Felmlee, Nick Fox, Paul Thompson, Norbert Schuff, Gene Alexander, Charles DeCarli, William Jagust, Dan Bandy, Robert A. Koeppe, Norm Foster, Eric M. Reiman, Kewei Chen, Chet Mathis, John Morris, Nigel J. Cairns, Lisa Taylor-Reinwald, J.Q. Trojanowki, Les Shaw, Virginia M.Y. Lee, Magdalena Korecka, Arthur W. Toga, Karen Crawford, Scott Neu, Andrew J. Saykin, Tatiana M. Foroud, Steven Potkin, Li Shen, Zaven Kachaturian, Richard Frank, Peter J. Snyder, Susan Molchan, Jeffrey Kaye, Joseph Quinn, Betty Lind, Sara Dolen, Lon S. Schneider, Sonia Pawluczyk, Bryan M. Spann, James Brewer, Helen Vanderswag, Judith L. Heidebrink, Joanne L. Lord, Ronald Petersen, Kris Johnson, Rachelle S. Doody, Javier Villanueva-Meyer, Munir Chowdhury, Yaakov Stern, Lawrence S. Honig, Karen L. Bell, John C. Morris, Beau Ances, Sue Leon, Stacy Schneider, Daniel Marson, Randall Griffith, David Clark, Hillel Grossman, Effie Mitsis, Aliza Romirowsky, Leyla deToledo-Morrell, Raj C. Shah, Ranjan Duara, Daniel Varon, Peggy Roberts, Marilyn Albert, Chiadi Onyike, Stephanie Kielb, Henry Rusinek, Mony J de Leon, Lidia Glodzik, Susan De Santi, P. Murali Doraiswamy, Jeffrey R. Petrella, R. Edward Coleman, Steven E. Arnold, Jason H. Karlawish, David Wolk, Charles D. Smith, Greg Jicha, Peter Hardy, Oscar L. Lopez, MaryAnn Oakley, Donna M. Simpson, Anton P. Porsteinsson, Bonnie S. Goldstein, Kim Martin, Kelly M. Makino, M. Saleem Ismail, Connie Brand, Ruth A. Mulnard, Gaby Thai, Catherine Mc-Adams-Ortiz, Kyle Womack, Dana Mathews, Mary Quiceno, Ramon Diaz-Arrastia, Richard King, Myron Weiner, Kristen Martin-Cook, Michael DeVous, Allan I. Levey, James J. Lah, Janet S. Cellar, Jeffrey M. Burns, Heather S. Anderson, Russell H. Swerdlow, Liana Apostolova, Po H. Lu, George Bartzokis, Daniel H.S. Silverman, Neill R Graff-Radford, Francine Parfitt, Heather Johnson, Martin R. Farlow, Ann Marie Hake, Brandy R. Matthews, Scott Herring, Christopher H. van Dyck, Richard E. Carson, Martha G. MacAvoy, Howard Chertkow, Howard Bergman, Chris Hosein, Sandra Black, Bojana Stefanovic, Curtis Caldwell, Ging-Yuek Robin Hsiung, Howard Feldman, Benita Mudge, Michele Assaly, Andrew Kertesz, John Rogers, Dick Trost, Charles Bernick, Donna Munic, Diana Kerwin, Marek-Marsel Mesulam, Kristina Lipowski, Chuang-Kuo Wu, Nancy Johnson, Carl Sadowsky, Walter Martinez, Teresa Villena, Raymond Scott Turner, Kathleen Johnson, Brigid Reynolds, Reisa A. Sperling, Keith A. Johnson, Gad Marshall, Meghan Frey, Jerome Yesavage, Joy L. Taylor, Barton Lane, Allyson Rosen, Jared Tinklenberg, Marwan Sabbagh, Christine Belden, Sandra Jacobson, Neil Kowall, Ronald Killiany, Andrew E. Budson, Alexander Norbash, Patricia Lynn Johnson, Thomas O. Obisesan, Saba Wolday, Salome K. Bwayo, Alan Lerner, Leon Hudson, Paula Ogrocki, Evan Fletcher, Owen Carmichael, John Olichney, Charles DeCarli, Smita Kittur, Michael Borrie, T-Y Lee, Dr Rob Bartha, Sterling Johnson, Sanjay Asthana, Cynthia M. Carlsson, Steven G. Potkin, Adrian Preda, Dana Nguyen, Pierre Tariot, Adam Fleisher, Stephanie Reeder, Vernice Bates, Horacio Capote, Michelle Rainka, Douglas W. Scharre, Maria Kataki, Earl A. Zimmerman, Dzintra Celmins, Alice D. Brown, Godfrey D. Pearlson, Karen Blank, Karen Anderson, Andrew J. Saykin, Robert B. Santulli, Eben S. Schwartz, Kaycee M. Sink, Jeff D. Williamson, Pradeep Garg, Franklin Watkins, Brian R. Ott, Henry Querfurth, Geoffrey Tremont, Stephen Salloway, Paul Malloy, Stephen Correia, Howard J. Rosen, Bruce L. Miller, Jacobo Mintzer, Crystal Flynn Longmire, Kenneth Spicer, Elizabether Finger, Irina Rachinsky, John Rogers, Andrew Kertesz, Dick Drost, Nunzio Pomara, Raymundo Hernando, Antero Sarrael, Susan K. Schultz, Laura L. Boles Ponto, Hyungsub Shim, Karen Elizabeth Smith, Norman Relkin, Gloria Chaing, Lisa Raudin, Amanda Smith, Kristin Fargher, and Balebail Ashok Raj
AUTHOR CONTRIBUTIONS
J.B. Toledo: study concept and design, analysis and interpretation of the data, drafting the manuscript. J.Q. Trojanowski: acquisition of data, interpretation of the data, drafting the manuscript. K. Chen, C.R. Jack, Jr., M.W. Weiner, E.M. Reiman, and L.M. Shaw: acquisition of data, interpretation of the data, critical revision of the manuscript for important intellectual content. M. Bjerke and S.E. Arnold: interpretation of the data, critical revision of the manuscript for important intellectual content. C. Davatzikos and M. Rozycki: analysis and interpretation of the data, critical revision of the manuscript for important intellectual content.
STUDY FUNDING
Dr. Toledo is supported by P01 AG032953, PO1 AG017586, P30 AG010124, and P50 NS053488. Dr. Trojanowski is the William Maul Measey–Truman G. Schnabel Jr., Professor of Geriatric Medicine and Gerontology. Data collection and sharing for this project was funded by the ADNI (NIH grant U01 AG024904) and DOD ADNI (Department of Defense award W81XWH-12-2-0012). ADNI is funded by the National Institute on Aging, the National Institute of Biomedical Imaging and Bioengineering, and through generous contributions from the following: Alzheimer's Association; Alzheimer's Drug Discovery Foundation; Araclon Biotech; BioClinica, Inc.; Biogen Idec Inc.; Bristol-Myers Squibb Company; Eisai Inc.; Elan Pharmaceuticals, Inc.; Eli Lilly and Company; EuroImmun; F. Hoffmann-La Roche Ltd. and its affiliated company Genentech, Inc.; Fujirebio; GE Healthcare; IXICO Ltd.; Janssen Alzheimer Immunotherapy Research & Development, LLC.; Johnson & Johnson Pharmaceutical Research & Development LLC.; Medpace, Inc.; Merck & Co., Inc.; Meso Scale Diagnostics, LLC.; NeuroRx Research; Neurotrack Technologies; Novartis Pharmaceuticals Corporation; Pfizer Inc.; Piramal Imaging; Servier; Synarc Inc.; and Takeda Pharmaceutical Company. The Canadian Institutes Health Research is providing funds to support ADNI clinical sites in Canada. Private sector contributions are facilitated by the Foundation for the NIH (www.fnih.org). The grantee organization is the Northern California Institute for Research and Education, and the study is coordinated by the Alzheimer's Disease Cooperative Study at the University of California, San Diego. ADNI data are disseminated by the Laboratory for Neuro Imaging at the University of Southern California. Dr. Arnold receives research funding from NIH (R01AG039478, P30AG10124, R01DA025201, U01DK057135, U01AG024904), the BrightFocus Foundation (principal investigator), and the Marian S. Ware Charitable Giving Fund. Dr. Jack receives research funding from the NIH (R01-AG011378, U01-HL096917, U01-AG024904, RO1 AG041851, R01 AG37551, R01AG043392, U01-AG06786) and the Alexander Family Alzheimer's Disease Research Professorship of the Mayo Foundation. Dr. Reiman receives research funding from National Institute on Aging, Nomis Foundation, Banner Alzheimer's Foundation, and the state of Arizona.
DISCLOSURE
J.B. Toledo, M. Bjerke, K. Chen, and M. Rozycki report no disclosures relevant to the manuscript. C.R. Jack has provided consulting services for Janssen Research & Development, LLC, and Eli Lilly. M.W. Weiner reports stock/stock options from Elan, Synarc; travel expenses from Novartis, Tohoku University, Fundacio Ace, Travel eDreams, MCI Group, NSAS, Danone Trading, ANT Congress, NeuroVigil, CHRU-Hôpital Roger Salengro, Siemens, AstraZeneca, Geneva University Hospitals, Lilly, University of California, San Diego–ADNI, Paris University, Institut Catala de Neurociencies Aplicades, University of New Mexico School of Medicine, Ipsen, Clinical Trials on Alzheimer's Disease, Pfizer, AD PD meeting, Paul Sabatier University; board membership for Lilly, Araclon, Institut Catala de Neurociencies Aplicades, Gulf War Veterans Illnesses Advisory Committee, VACO, Biogen Idec, Pfizer; consultancy from AstraZeneca, Araclon, Medivation/Pfizer, Ipsen, TauRx Therapeutics, Bayer Healthcare, Biogen Idec, ExonHit Therapeutics, Servier, Synarc, Pfizer, Janssen; honoraria from NeuroVigil, Insitut Catala de Neurociencies Aplicades, PMDA/Japanese Ministry of Health, Labour, and Welfare, Tohoku University; commercial research support from Merck, Avid; government research support, DOD, VA, outside the submitted work. S.E. Arnold serves as an associate editor for Translational Neuroscience and Journal of Alzheimer's Disease, has been a consultant for Teva Pharmaceuticals, Inc., has received commercial research support from Johnson & Johnson and Pain Therapeutics, Inc., and served in a clinical trial site for Merck and Bristol-Myers Squibb. E.M. Reiman serves as scientific advisor AstraZeneca, CereSpir, Eisai, Eli Lilly, GlaxoSmithKline, Pfizer, Sanofi, has received commercial research support from Avid/Eli Lilly and Genentech, and has a pending patent: Biomarker strategy for the evaluation of presymptomatic AD treatments. C. Davatzikos reports no disclosures relevant to the manuscript. L.M. Shaw serves as consultant for Janssen AI R & D and Lilly, outside the submitted work. J.Q. Trojanowski may accrue revenue in the future on patents submitted by the University of Pennsylvania wherein he is coinventor and he received revenue from the sale of Avid to Eli Lilly as coinventor on imaging-related patents submitted by the University of Pennsylvania. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Petersen RC, Smith GE, Waring SC, Ivnik RJ, Tangalos EG, Kokmen E. Mild cognitive impairment: clinical characterization and outcome. Arch Neurol 1999;56:303–308. [DOI] [PubMed] [Google Scholar]
- 2.Toledo JB, Brettschneider J, Grossman M, et al. CSF biomarkers cutoffs: the importance of coincident neuropathological diseases. Acta Neuropathol 2012;124:23–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Strozyk D, Blennow K, White LR, Launer LJ. CSF Abeta 42 levels correlate with amyloid-neuropathology in a population-based autopsy study. Neurology 2003;60:652–656. [DOI] [PubMed] [Google Scholar]
- 4.Clark CM, Schneider JA, Bedell BJ, et al. Use of florbetapir-PET for imaging beta-amyloid pathology. JAMA 2011;305:275–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jack CR, Jr, Knopman DS, Jagust WJ, et al. Tracking pathophysiological processes in Alzheimer's disease: an updated hypothetical model of dynamic biomarkers. Lancet Neurol 2013;12:207–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sperling RA, Aisen PS, Beckett LA, et al. Toward defining the preclinical stages of Alzheimer's disease: recommendations from the National Institute on Aging–Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement 2011;7:280–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jack CR, Jr, Knopman DS, Weigand SD, et al. An operational approach to National Institute on Aging–Alzheimer's Association criteria for preclinical Alzheimer disease. Ann Neurol 2012;71:765–775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Toledo JB, Weiner MW, Wolk DA, et al. Neuronal injury biomarkers and prognosis in ADNI subjects with normal cognition. Acta Neuropathol Commun 2014;2:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Roe CM, Fagan AM, Grant EA, et al. Amyloid imaging and CSF biomarkers in predicting cognitive impairment up to 7.5 years later. Neurology 2013;80:1784–1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ivanoiu A, Dricot L, Gilis N, et al. Classification of non-demented patients attending a memory clinic using the new diagnostic criteria for Alzheimer's disease with disease-related biomarkers. J Alzheimers Dis 2015;43:835–847. [DOI] [PubMed] [Google Scholar]
- 11.Mormino EC, Betensky RA, Hedden T, et al. Synergistic effect of beta-amyloid and neurodegeneration on cognitive decline in clinically normal individuals. JAMA Neurol 2014;71:1379–1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Knopman DS, Jack CR, Jr, Wiste HJ, et al. Short-term clinical outcomes for stages of NIA-AA preclinical Alzheimer disease. Neurology 2012;78:1576–1582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vos SJ, Xiong C, Visser PJ, et al. Preclinical Alzheimer's disease and its outcome: a longitudinal cohort study. Lancet Neurol 2013;12:957–965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Petersen RC, Roberts RO, Knopman DS, et al. Mild cognitive impairment: ten years later. Arch Neurol 2009;66:1447–1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Weiner MW, Veitch DP, Aisen PS, et al. The Alzheimer's Disease Neuroimaging Initiative: a review of papers published since its inception. Alzheimers Dement 2013;9:e111–e194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fan Y, Shen D, Gur RC, Gur RE, Davatzikos C. COMPARE: classification of morphological patterns using adaptive regional elements. IEEE Trans Med Imaging 2007;26:93–105. [DOI] [PubMed] [Google Scholar]
- 17.Da X, Toledo JB, Zee J, et al. Integration and relative value of biomarkers for prediction of MCI to AD progression: spatial patterns of brain atrophy, cognitive scores, APOE genotype and CSF biomarkers. Neuroimage Clin 2013;4:164–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen K, Ayutyanont N, Langbaum JB, et al. Characterizing Alzheimer's disease using a hypometabolic convergence index. Neuroimage 2011;56:52–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McKhann GM, Knopman DS, Chertkow H, et al. The diagnosis of dementia due to Alzheimer's disease: recommendations from the National Institute on Aging–Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement 2011;7:263–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shaw LM, Vanderstichele H, Knapik-Czajka M, et al. Cerebrospinal fluid biomarker signature in Alzheimer's Disease Neuroimaging Initiative subjects. Ann Neurol 2009;65:403–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shaw LM, Vanderstichele H, Knapik-Czajka M, et al. Qualification of the analytical and clinical performance of CSF biomarker analyses in ADNI. Acta Neuropathol 2011;121:597–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Reuter M, Rosas HD, Fischl B. Highly accurate inverse consistent registration: a robust approach. Neuroimage 2010;53:1181–1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Reuter M, Schmansky NJ, Rosas HD, Fischl B. Within-subject template estimation for unbiased longitudinal image analysis. Neuroimage 2012;61:1402–1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Toledo JB, Da X, Bhatt P, et al. Relationship between plasma analytes and SPARE-AD defined brain atrophy patterns in ADNI. PLoS One 2013;8:e55531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang T, Davatzikos C. Optimally-discriminative voxel-based morphometry significantly increases the ability to detect group differences in schizophrenia, mild cognitive impairment, and Alzheimer's disease. Neuroimage 2013;79:94–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Crane PK, Carle A, Gibbons LE, et al. Development and assessment of a composite score for memory in the Alzheimer's Disease Neuroimaging Initiative (ADNI). Brain Imaging Behav 2012;6:502–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gibbons LE, Carle AC, Mackin RS, et al. A composite score for executive functioning, validated in Alzheimer's Disease Neuroimaging Initiative (ADNI) participants with baseline mild cognitive impairment. Brain Imaging Behav 2012;6:517–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Toledo JB, Vanderstichele H, Figurski M, et al. Factors affecting Abeta plasma levels and their utility as biomarkers in ADNI. Acta Neuropathol 2011;122:401–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Landau SM, Lu M, Joshi AD, et al. Comparing positron emission tomography imaging and cerebrospinal fluid measurements of beta-amyloid. Ann Neurol 2013;74:826–836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Toledo JB, Bjerke M, Da D, et al. Non-linear association between CSF and florbetapir Aβ measures across the spectrum of Alzheimer's disease. JAMA Neurol 2015;72:571–581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kleinbaum DG, Klein M. Survival Analysis: A Self-learning Text. New York: Springer; 2012. [Google Scholar]
- 32.Caselli RJ, Chen K, Lee W, Alexander GE, Reiman EM. Correlating cerebral hypometabolism with future memory decline in subsequent converters to amnestic pre-mild cognitive impairment. Arch Neurol 2008;65:1231–1236. [DOI] [PubMed] [Google Scholar]
- 33.Caselli RJ, Reiman EM, Locke DE, et al. Cognitive domain decline in healthy apolipoprotein E epsilon4 homozygotes before the diagnosis of mild cognitive impairment. Arch Neurol 2007;64:1306–1311. [DOI] [PubMed] [Google Scholar]
- 34.Langbaum JB, Hendrix SB, Ayutyanont N, et al. An empirically derived composite cognitive test score with improved power to track and evaluate treatments for preclinical Alzheimer's disease. Alzheimers Dement 2014;10:666–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Donohue MC, Sperling RA, Salmon DP, et al. The preclinical Alzheimer cognitive composite: measuring amyloid-related decline. JAMA Neurol 2014;71:961–970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Clark VH, Resnick SM, Doshi J, et al. Longitudinal imaging pattern analysis (SPARE-CD index) detects early structural and functional changes before cognitive decline in healthy older adults. Neurobiol Aging 2012;33:2733–2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Toledo JB, Arnold SE, Raible K, et al. Contribution of cerebrovascular disease in autopsy confirmed neurodegenerative disease cases in the National Alzheimer's Coordinating Centre. Brain 2013;136:2697–2706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yarchoan M, Xie SX, Kling MA, et al. Cerebrovascular atherosclerosis correlates with Alzheimer pathology in neurodegenerative dementias. Brain 2012;135:3749–3756. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.