Abstract
In eukaryotic cells, ankyrins serve as adaptor proteins that link membrane proteins to the underlying cytoskeleton. These adaptor proteins form protein complexes consisting of integral membrane proteins, signalling molecules and cytoskeletal components. With their modular architecture and ability to interact with many proteins, ankyrins organize and stabilize these protein networks, thereby establishing the infrastructure of membrane domains with specialized functions. To this end, ankyrin collaborates with a number of proteins including cytoskeletal proteins, cell adhesion molecules and large structural proteins. This review addresses the targeting and stabilization of protein networks related to ankyrin interactions with the cytoskeletal protein β-spectrin, L1-cell adhesion molecules and the large myofibrillar protein obscurin. The significance of these interactions for differential targeting of cardiac proteins and neuronal membrane formation is also presented. Finally, this review concludes with a discussion about ankyrin dysfunction in human diseases such as haemolytic anaemia, cardiac arrhythmia and neurological disorders.
Keywords: ankyrin, β-spectrin, obscurin, L1-CAM, cardiomyocyte, axon initial segment, node of Ranvier
- Introduction
- Ankyrin
- Ankyrin functional domains
- Ankyrin genes, alternative splicing and the diversity of ankyrin polypeptides
- Mechanisms that target and stabilize ankyrin
- β-spectrin
- L1-cell adhesion molecules
- Obscurin
- Ankyrins and disease
- Ankyrin-R
- Ankyrin-G
- Ankyrin-B
Conclusion
Introduction
Unique cellular functions are the result of cell-type specific proteins and the distinctive arrangement of these proteins in the context of organelles, the cytoskeleton and the plasma membrane. In eukaryotic cells, there are a number of adaptor proteins that serve as the interface between the plasma membrane and cytoskeleton including 4.1 proteins, proteins from the ezrin–radixin–moesin family and ankyrins. These adaptor proteins organize protein networks with particular structural, signalling and electrogenic properties. Accordingly, these adaptor proteins are integral for the formation of subcellular domains with specialized functions such as ionic movement across the plasma membrane or providing adherence between cell membranes. The prevalence of human disease associated with dysfunction in these adaptor proteins and associated molecules attests to their significance for normal cellular physiology. This review focuses on the adaptor protein ankyrin and its role in forming protein networks that constitute specialized membrane domains in cardiomyocytes and neurons. Specifically, the first section provides a general overview of ankyrins covering topics such as functional domains, genes and alternative isoforms. The second section is focused on proteins that contribute to ankyrin targeting and stabilization at specialized cardiac and neuronal membrane domains. Such proteins include the cytoskeletal protein β-spectrin, the L1-family of cell adhesion molecules and the large structural protein obscurin. The final section describes ankyrin dysfunction in human diseases including haemolytic anaemia, cardiac arrhythmias and neurological disorders.
Ankyrins
Ankyrins are a family of adaptor proteins that link integral membrane proteins with the submembranous actin/β-spectrin cytoskeleton. The first ankyrin was characterized over 30 years ago as an adaptor protein that tethered the anion exchanger to β-spectrin in red blood cells [1]. Ankyrins are now regarded as pivotal choreographers in the formation of protein complexes consisting of ion channels and transporters, cell adhesion molecules, signalling proteins and cytoskeletal elements. These ankyrin-associated protein complexes comprise specialized membrane domains with distinct electrogenic and/or structure properties in eukaryotic cells. In addition, new functions now ascribed to ankyrin include membrane biogenesis and the formation of diffusion barriers that maintain the subcellular polarity of migrating proteins.
Ankyrin functional domains
The prototypical ankyrin consists of three functional domains (Fig. 1). The membrane-binding domain, which mediates ankyrin binding to integral membrane proteins, contains 24 ANK repeats assembled as a superhelical spiral. An ANK repeat consists of 33 amino acids arranged as two anti-parallel α-helices followed by a long loop [2]. Adjacent ANK repeats are connected by a β-hairpin loop and these solvent-exposed domains mediate protein interactions. The binding sites are often spread across adjacent β-hairpin loop tips. For example, ankyrin interacts with the sodium/calcium exchanger (NCX) via ANK repeats 16–18 [3], the inositol(1,4,5)-triphosphate receptor (IP3 receptor) via ANK repeats 22–24 [4] and the voltage-gated sodium channel via ANK repeats 14 and 15 [5]. Additional integral membrane proteins that interact with the ankyrin membrane-binding domain include the anion exchanger [6–8], sodium/potassium ATPase (NKA) [9, 10], voltage-gated potassium channel subunits (KCNQ2 and KCNQ3) [11–14] and the L1 family of cell adhesion molecules (Fig. 1) [15, 16].
Figure 1.
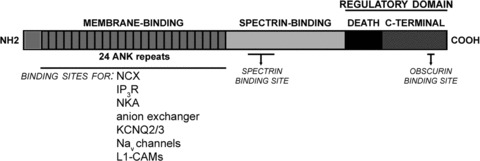
Ankyrin functional domains. The membrane binding domain consists of 24 ANK repeats that mediate interactions with a variety of ion channels, transporters and cell adhesion molecules. Ankyrin interacts with the cytoskeleton viaβ-spectrin binding to the spectrin binding domain. The death and C-terminal domains comprise the C-terminal regulatory domain that governs ankyrin inter- and intra-molecular interactions. NCX: sodium/calcium exchanger, NKA: sodium/potassium ATPase, IP3R: inositol(1,4,5) triphosphate receptor.
The ankyrin associated protein complex are tethered to the actin/spectrin cytoskeleton via its spectrin-binding domain. This domain is relatively large with a molecular weight of 62 kD, but the minimal spectrin-binding domain is contained within a 160 amino acid ZU-5 motif [17]. This motif has two conserved sites that are critical for spectrin-binding activity (ankyrin-B: DAR976, A1000 and ankyrin-G: DAR999, A1024) [17, 18]. For β-spectrin, the minimal ankyrin-binding domain is contained in spectrin repeats 14 and 15 [19–21]. The complementary electrostatic charges of ankyrin’s ZU-5 motif (positive) and β-spectrin’s repeat 14 (negative) suggest that the protein interactions are partially mediated by their oppositely charged domains [20]. With the exception of identifying the minimal ankyrin and spectrin binding sites, very little is known about the regulatory mechanisms of ankyrin/spectrin interactions. For example, it is not known whether different ankyrins preferentially associate with particular spectrins.
The C-terminal regulatory domain is comprised of a death domain and an unstructured stretch of 300 amino acids. This domain regulates protein interactions with ankyrin’s membrane-binding and spectrin-binding domains. For example, an alternative isoform of ankyrin-R lacking a portion of the C-terminal domain exhibits increased binding affinity for the anion exchanger and spectrin [22, 23]. This increase in binding affinity is reversed by co-expression of the C-terminal fragment, validating the auto-inhibitory functions of the C-terminal regulatory domain [22, 23]. The regulatory activity appears to be mediated by an intra-molecular interaction between this domain and the first ANK repeat of the membrane-binding domain [24]. Additional evidence for an intra-molecular interaction comes from studies of ankyrin-B mutations in cardiac disorders. In particular, the ankyrin-B loss-of-function mutation E1425G impairs ankyrin-B binding to NCX, NKA and the IP3 receptor [9]. Interestingly, this mutation is not found in the membrane-binding or the C-terminal regulatory domain, but at the junction of the spectrin-binding and C-terminal domains. This mutation may reside in a ‘hinge’ domain that allows the C-terminal domain to pivot over the membrane-binding domain. Currently, the mechanisms regulating these intra-molecular interactions are unknown. Ankyrin function is also indirectly regulated by the C-terminal domain through targeting motifs present in this domain that govern ankyrin recruitment and retention to different subcellular domains. As will be elaborated on in a subsequent section, a subpopulation of ankyrin-B is targeted to the M-line of ventricular cardiomyocytes through an interaction between the C-terminal regulatory domain of ankyrin-B and the large Rho-GEF obscurin (Fig. 1).
Ankyrin genes, alternative splicing and the diversity of ankyrin polypeptides
The diversity of ankyrin polypeptides is the product of unexpectedly complex alternative splicing of three genes (see Table 1). Located on human chromosome 8p11, ANK1 contains 42 exons that are alternatively spliced to encode a variety of ankyrin-R isoforms expressed in erythrocytes, cardiac and skeletal muscle, and neurons [23, 25–33]. Ankyrin-B isoforms are encoded by ANK2, which is located on human chromosome 4q25–27 and contains 53 exons spanning approximately 560 kb [34, 35]. Ankyrin-B isoforms have been found in a variety of tissues including brain, heart, skeletal muscle and thymus [36–40]. Finally, the gene for ankyrin-G (ANK3) located on human chromosome 10q21 encodes numerous isoforms broadly expressed in epithelial tissue, kidney, skeletal and cardiac muscle, and brain [41–49].
Table 1.
Ankyrin isoforms and tissue expression
| Tissue | Ankyrin-G | Ankyrin-B | Ankyrin-R |
|---|---|---|---|
| Brain | 270, 480 kD | 220, 440 kD | 186, 215 kD |
| Heart | 190 kD | 160, 220 kD | 210 kD |
| Skeletal muscle | 107–130, 119 kD | 220 kD | 20–30 kD |
| Lung | 190, 200–215 kD | 220 kD | |
| Kidney | 119, 190, 200--215 kD | 220 kD | |
| Erythrocyte | 186, 215 kD |
Alternative splicing of the ankyrin genes enables ankyrin polypeptides to associate with many different proteins and display differential subcellular localization. For example, the targeting of ankyrin-G to the axon initial segments (AIS) of peripheral neurons is partly dependent on a serine/threonine rich domain that is only present in neuronal isoforms of ankyrin-G [49]. In addition, alternative transcription of ANK1 results in small ankyrin-R isoforms that integrate into the sarcoplasmic reticulum (SR) of skeletal muscle via unique N-terminal transmembrane domains [25, 33, 50, 51]. With all ankyrin genes, the exons encoding the C-terminal regulatory domains are often extensively spliced. As mentioned previously, this domain regulates ankyrin’s association with integral membrane proteins and cytoskeletal elements. Altering the composition of this domain would partially explain both the functional diversity and differential localization of alternative ankyrin isoforms. Alternative splicing of exons encoding the membrane-binding domain would also contribute to ankyrin functional diversity. In heart, alternative splicing of the ANK2 gene removes key exons that encode known binding sites for ankyrin-B associated proteins NCX and IP3 receptor (reviewed in Van Oort, 2008) [34, 52]. Taken together, alternative splicing alters binding sites and targeting motifs in ankyrin, thereby enabling its selective association with particular proteins that ultimately culminate in the distinct subcellular localization of ankyrin-associated protein complexes. By some accounts, these mechanisms may also underlie a role for ankyrin in the formation of specialized membrane domains. The following section will discuss three proteins that contribute to the targeting and retention of ankyrin-associated protein complexes to specific membrane domains.
Mechanisms that target and stabilize ankyrin
β-spectrin
Spectrins are a family of filamentous proteins that assemble as heterotetramers of two α and two β subunits. In conjunction with actin filaments, these heterotetramers form the underlying cytoskeleton of plasma membranes in all metazoan cell types including erythrocytes, cardiomyocytes and neurons. While two genes encode the various isoforms of α-spectrin, five genes encode numerous isoforms of β-spectrin [53]. The prototypical β-spectrin contains an N-terminal actin-binding domain, 16 triple-helical spectrin repeats and a C-terminal pleckstrin homology domain. In addition to acting as molecular springs that ensure membrane resilience to mechanical stress, the spectrin repeats also mediate protein interactions. For example, ankyrin interacts with β-spectrin repeats 14 and 15 [19–21]. β-spectrin associates with the plasma membrane through adaptor proteins such as ankyrin and protein 4.1 [54], integral membrane proteins including the neuronal glutamate transporter [55] and phosphatidylinositol lipids [56]. In human, mutations to β-spectrin have been linked to hereditary elliptocytosis, a type of haemolytic anaemia, and spinocerebellar ataxia type 5, a progressive neurodegenerative disorder characterized by slurred speech and loss of coordination [57–59]. The association of spectrin mutations with these two seemingly disparate diseases underscores spectrin’s role as a multifunctional protein that not only insures membrane integrity in erythrocytes but also contributes to the stabilization of membrane proteins in the cerebellum.
Previous studies have focused on the relative significance of ankyrin and β-spectrin in the formation of specialized membrane domains. This analysis includes examining the respective roles for ankyrin and β-spectrin in the targeting and stabilization of each other. Some studies have concluded that β-spectrin is targeted and stabilized by ankyrin. For example, in ventricular cardiomyocytes the M-line localization of β2-spectrin is dependent on ankyrin-B [17]. Likewise, ankyrin-B is necessary for β2-spectrin localization at the inner segments of rod photoreceptors [60]. In cerebellar-specific ankyrin-G knockout mice, the loss of ankyrin-G disrupts β4-spectrin localization at AIS in Purkinje cells [61]. Contrary to these findings, some studies have concluded that ankyrin targeting and stabilization is dependent on β-spectrin. In Drosophila melanogaster, ankyrin is localized to the basolateral domains of midgut copper cells through its interaction with β-spectrin [62].
Recently, an emerging theme is that ankyrin and β-spectrin are interdependent and are mutually involved in the formation and maintenance of membrane domains. For example, evidence from co-localization experiments demonstrate that expression of ankyrin-G or β4-spectrin at AIS of Purkinje neurons is dependent on each other [61, 63]. In bronchial epithelial cells, both ankyrin-G and β2-spectrin cooperate in the formation of lateral membrane domains. Specifically, loss of lateral membrane domains following AnkG-siRNA treatment is not rescued by exogenous expression of an ankyrin-G construct that lacks β-spectrin binding activity [64].
The relative prominence of ankyrin or spectrin in membrane biogenesis most likely depends on additional factors in the microdomain including cell adhesion molecules, other cytoskeletal elements and regional phospholipids. In support of this hypothesis, it was shown that different domains are necessary for β-spectrin targeting in various Drosophila tissues. For example, β-spectrin targeting in midgut copper cells is dependent on the C-terminal PH-domain, while β-spectrin targeting to neuronal plasma membranes requires both the PH and ankyrin-binding domains [65]. Interestingly, neither the PH nor the ankyrin-binding domains are required for β-spectrin localization in salivary epithelial cells [65]. In fact, the novel targeting motif has yet to be identified. Ankyrin-independent β-spectrin targeting mechanisms may include phospholipids, integral membrane proteins (α-catenin) [66] and additional adaptor proteins (4.1 protein) [53]. Similar to β-spectrin, multiple ankyrin-G domains contribute to its retention at specific membrane domains. For example, axolemmal targeting information is contained in the ankyrin-G spectrin-binding and C-terminal regulatory domains [49]. Furthermore, additional targeting motifs that restrict ankyrin-G expression to AIS are present in the serine/threonine rich and C-terminal regulatory domains [49]. These findings suggest that ankyrin-G targeting to AIS is dependent on multiple protein interactions with different ankyrin-G domains. Apparent discrepancies in the targeting and retention of ankyrin/spectrin complexes to different membrane domains may be explained by the involvement of multiple proteins. For example, as will be described in greater detail below, the cell adhesion molecule neurofascin recruits ankyrin-G to the nodes of Ranvier, but not to AIS of peripheral neurons [67–69].
L1-cell adhesion molecules
Ankyrins bind to a variety of cell adhesion molecules including CD44 [70], E-cadherin [71], β-dystroglycan [72] and members of the L1 family [15, 16]. This association is important for the proper subcellular targeting of these cell adhesion molecules. For example, ankyrin-G recruits E-cadherin to the lateral membrane domains of bronchial epithelial cells where in concert with β2-spectrin it facilitates membrane biogenesis [18, 64, 71]. Recently, it was shown that ankyrin-B and ankyrin-G are involved in the targeting and retention of β-dystroglycan to the sarcolemma, neuromuscular junction and costameres of skeletal muscle [72].
Many studies have characterized the association of ankyrins with members of the L1 family of cell adhesion molecules that includes L1, close homologue of L1 (CHL1), neuron glia related CAM (NrCAM) and neurofascin. These proteins are predominantly expressed in the nervous system where they have been implicated in many aspects of neural differentiation including neurite outgrowth, axonal guidance and synaptogenesis [73]. The prototypical extracellular domain of L1 cell adhesion molecules has six immunoglobulin domains followed by four to five fibronectin type III domains. The cytoplasmic domain is relatively small ranging in size from 85 to 148 amino acids and contains the ankyrin-binding motif FIGQY that is highly conserved in L1 proteins [74–76]. Homophilic interactions between the extracellular domains of these proteins are stabilized by ankyrin binding to the FIGQY motif. Moreover, phosphorylation of the tyrosine residue in this motif disrupts ankyrin interaction with the C-terminal domain [74, 75]. For example, ankyrin/L1 interactions cause neuroblastoma cells to aggregate via homophilic interactions of the L1 extracellular domains [75]. These aggregates disperse following treatment with nerve growth factor because tyrosine phosphorylation of the FIGQY motif disrupts ankyrin binding to L1.
The phosphorylation-dependent regulation of ankyrin/L1 interaction allows L1 to function in two different capacities during neural development. In developmental events that favour static adhesion such as axon fasciculation or the formation of AIS, the tyrosine residue is not phosphorylated and ankyrin interacts with the L1 protein [73]. In contrast, for dynamic developmental processes such as neurite outgrowth and migration, L1 proteins are phosphorylated thereby inhibiting ankyrin interactions. Consistent with this hypothesis, neurite outgrowth is increased by 50% in cerebellar granular neurons following inhibition of L1/ankyrin interactions [77]. Interestingly, phosphorylated FIGQY preferentially interacts with the microtuble associated protein doublecortin that is linked to the neuronal migration disorder lissencephaly, which is characterized by thickening of the neocortex and reduced cortical gyrations [78, 79].
The relative contribution of ankyrin or L1 proteins to the targeting and retention of the other depends on the membrane domain. For example, ankyrin-G recruits neuronal isoforms of neurofascin (neurofascin-186) to AIS of cerebellar Purkinje neurons [80]. Similarly, in hippocampal neurons ankyrin-G depletion by RNA interference extinguishes AIS targeting of neurofascin-186, NaV 1.6, and β4-spectrin [81]. As in the central nervous system, ankyrin-G recruits neurofascin-186 to AIS of peripheral neurons, but interestingly their roles are reversed at the nodes of Ranvier (Fig. 2). Specifically, neurofascin-186 recruits ankyrin-G to the nodes of peripheral neurons [67–69]. Consistent with this finding, neurofascin-186 clusters at the nodes before ankyrin-G or voltage-gated sodium channels [82, 83]. The nodal clustering of neurofascin-186 is mediated by direct interactions between its extracellular domain and gliomedin, an extracellular matrix protein that accumulates in the perinodal region after being cleaved from the membrane surface of Schwann cell microvilli [84]. Decreasing gliomedin expression through RNA interference reduces the nodal expression of both neurofascin and NaV channels [84]. In summary, ankyrin interactions with L1 proteins demonstrate the relative contribution of intracellular and/or extracellular cues to the formation of specialized membrane domains. Namely, ankyrin-G clustering and the ensuing formation of nodes of Ranvier are dependent on extracellular cues from myelinating Schwann cells that are relayed via neurofascin-186.
Figure 2.
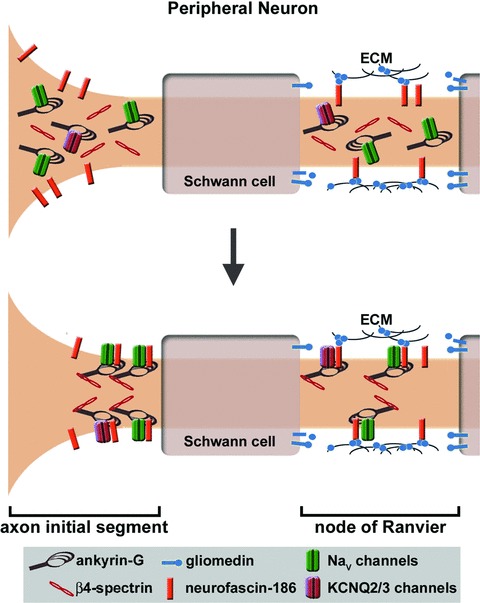
Ankyrin-G targeting to membrane domains in the peripheral neuron. Ankyrin-G is recruited to the nodes of Ranvier by gliomedin, which is produced by Schwann cells and accumulates in the perinodal extracellular matrix. As a ligand for neurofascin-186, gliomedin causes the nodal clustering of this cell adhesion molecule, which in turn recruits to the nodal plasma membrane an ankyrin-G protein network consisting of voltage-gated sodium or potassium channels (KCNQ2/3) and β4-spectrin. In contrast, ankyrin-G localization to the AIS is not dependent on an extracellular cue or neurofascin-186. The AIS targeting of ankyrin-G appears to be mediated by an intrinsic mechanism that has yet to be discovered, but AIS targeting of ankyrin-G associated proteins (i.e. neurofascin-186, sodium and potassium channels, β4-spectrin) is dependent on ankyrin-G.
Obscurin
In skeletal and cardiac tissue, large sarcomeric proteins coordinate the proper alignment of protein complexes to facilitate the integration of membrane structures including the SR and transverse tubules (T-tubules) with myofibrils. Obscurin is an 800 kD protein that regulates the formation and alignment of myofibrils. In skeletal muscle, obscurin is expressed early at the M-lines of developing sarcomeres in nascent myofibrils [85, 86]. Loss of obscurin activity causes the misalignment of M-lines in adjacent myofibrils and reduced myosin incorporation into maturing thick filaments [85, 87]. These observations are contradicted by the recent finding that there is no significant difference in skeletal muscle M-line architecture between obscurin knockout and wild-type mice [88]. It is possible that obscurin like 1 protein compensates for the loss of obscurin to preserve M-line architecture. Nevertheless, this knockout model demonstrates that obscurin plays a key role in establishing/maintaining the longitudinal SR that extends the length of a sarcomere and connects neighbouring junctional SR [88]. In zebrafish, obscurin deficiency due to antisense morpholino treatment results in ventricular hypoplasia and a reduced heart rate [89]. Obscurin mutations have been also associated with human cardiomyopathy [90].
While many models of obscurin deficiency demonstrate that this protein predominantly functions at the M-line, obscurin isoforms have also been detected at the M-line, Z-line, Z/I junctions and A/I junctions of skeletal muscle [91]. Multiple obscurin isoforms are produced by alternative splicing of the 117 exons that make up the obscurin gene OBSCN that is located on human chromosome 1q42.13 [92]. These isoforms contain variable combinations of modular domains including immunoglobulin (Ig) and fibronectin (Fn) repeats, a calcium/calmodulin binding domain, a Rho-GEF domain, and two serine/threonine kinase domains. Only the 800 kD obscurin isoform contains an ankyrin-binding site following the Rho-GEF domain in a unique C-terminus [92]. This isoform is predominantly localized to the M-line via the association of its N-terminal Ig domains with the M-line resident proteins myomesin and M-line titin (Fig. 3) [93]. Interestingly, titin mutations within the region of the obscurin-binding site are associated with tibial muscular dystrophy and limb girdle muscular dystrophy [94–96].
Figure 3.
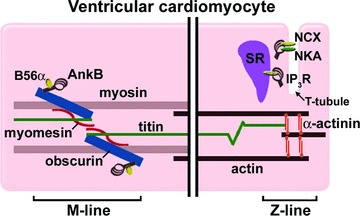
Ankyrin-B localization in ventricular cardiomyocytes. Ankyrin-B is targeted to the M-line via its interaction with the C-terminal domain of the large sarcomeric protein obscurin. Obscurin is targeted to the M-line via its N-terminal interactions with myomesin and titin. This population of ankyrin-B recruits B56α, a regulatory subunit of protein phosphatase 2A, to the M-line where the phosphatase may regulate the phosphorylation status of contractile and signalling proteins. Another population of ankyrin-B is located at the Z-lines where they interact with ion channels and transporters that regulate calcium efflux from the SR/T-tubule junction. The protein network associated with this ankyrin-B subpopulation includes NCX, NKA and the inositol(1,4,5) triphosphate receptor (IP3R).
In ventricular cardiomyocytes, ankyrin-B is localized at two distinct domains: overlying the structural and myofibrillar proteins of the M-line and at the SR/T-tubule junctions (Fig. 3). Recently, it was demonstrated that a subpopulation of ankyrin-B is targeted to the M-line via its interaction with the C-terminal domain of 800 kD obscurin [97]. This interaction is regulated by alternative splicing of an exon in the C-terminal regulatory domain that dramatically increases obscurin-binding activity of ankyrin. Moreover, it was demonstrated that a regulatory subunit of protein phosphatase 2A, a signalling molecule that controls the phosphorylation status of proteins such as myosin-binding protein C and troponin-I, is brought to the M-line via ankyrin-B/obscurin interactions [97].
In addition to ankyrin-B, small ankyrin-R isoforms 1.5 and 1.9 interact with the carboxyl-terminus of 800 kD obscurin [50, 51, 98]. These small ankyrins are unique because they lack many of the domains found in a prototypical ankyrin. In fact, they only retain a small portion of the C-terminal regulatory domain of ankyrin-R. The amino terminal domain contains a novel stretch of amino acids that forms a transmembrane domain that integrates into the membrane of the SR. Based on their interactions with obscurin, these small isoforms are targeted to the M-line where it is thought that they position the developing SR in reference to myofibrils [50, 51, 98].
Ankyrins and disease
As the field of ankyrin biology continues to advance, the clinical manifestations of ankyrin dysfunction will be recognized as complex and multi-systemic for a number of reasons. First, ankyrins (R, G, B) and their alternative isoforms are expressed in many tissues (see Table 1). Often they display overlapping expression patterns in the same tissues. For example, isoforms of all three ankyrins are expressed in brain, heart and skeletal muscle. Findings from ankyrin knockout mouse models and cell-based structure/function analysis suggest that ankyrins are not functionally redundant. For example, ankyrin-G does not compensate for ankyrin-B loss in ventricular cardiomyocytes [99]. Nevertheless, nothing is known about the relationship between ankyrins co-expressed in the same tissue, so the extent of functional redundancy between ankyrins has yet to be fully explored. Finally, owing to the structural and functional diversity of ankyrin isoforms across different tissues, an amino acid variation/mutation in ankyrin may be tolerated in a particular tissue background, all the while causing significant dysfunction in another tissue background. Moreover, the location of these variable residues may have a significant impact on ankyrin function. For example, 8 out of 9 loss-of-function mutations associated with ‘ankyrin-B syndrome’, which encompasses a wide range of cardiac disorders, reside within the C-terminal regulatory domain (see Table 2) [100–102]. The following sections will present diseases associated with ankyrin dysfunction in the erythrocyte, heart and brain.
Table 2.
Clinical phenotypes of ankyrin-B missense mutations
| ANK2 exon | Residue change | Protein domain | Clinical phenotype |
|---|---|---|---|
| Exon 37 | T1404I | Spectrin binding | Ankyrin-B syndrome |
| Exon 38 | E1425G | Spectrin binding | Ankyrin-B syndrome |
| Exon 43 | V1516D | Death | Ankyrin-B syndrome |
| Exon 43 | T1552N | Death | Ankyrin-B syndrome |
| Exon 44 | L1622I | C-terminal | Ankyrin-B syndrome |
| Exon 44 | T1626N | C-terminal | Ankyrin-B syndrome |
| Exon 47 | V1777M | C-terminal | Ankyrin-B syndrome |
| Exon 48 | R1788W | C-terminal | Ankyrin-B syndrome |
| Exon 48 | E1813K | C-terminal | Ankyrin-B syndrome |
Ankyrin-B syndrome may include a number of clinical phenotypes including sinus node dysfunction (bradycardia and heart rate variability), atrial fibrillation, polymorphic ventricular arrhythmia and risk of sudden cardiac death.
Ankyrin-R
While ankyrin-R is expressed in red blood cells, muscle tissue and neurons, the most prominent phenotype associated with ankyrin-R mutations is hereditary sphereocytosis. This haemolytic anaemia is characterized by increased haemolysis due to altered red blood cell morphology from the normal biconcave disk to a spherical conformation. Although it is rare, some neurological problems have been associated with hereditary sphereocytosis [103]. As a model of ankyrin-R deficiency, normoblastosis (nb/nb) mice display motor ataxia in addition to severe haemolytic anaemia [32, 104]. These deficiencies are the result of a hypomorphic mutation in ankyrin-R [105] and the movement disorder is consistent with the predominant expression of ankyrin-R in the cell bodies and dendrites of Purkinje and granule cells of the cerebellum [32, 104]. While multiple ankyrin-R isoforms have been characterized in skeletal muscle, no muscular dystrophies in human have been associated with ankyrin-R dysfunction.
Ankyrin-G
Recently, multiple genome wide association studies have linked single nucleotide polymorphisms in the ANK3 locus to bipolar disorder, a mental illness characterized by recurring episodes of mania and depression [106–108]. Considering neurotransmitters commonly associated with mood disorders (i.e. serotonergic, dopaminergic and cholinergic neurotransmitters) are not preferentially affected in bipolar disorder, the molecular basis for bipolar disorder may reflect wholesale changes to synaptic connectivity and neural circuitry. This view is consistent with the loss of synaptic connectivity between basket interneurons and Purkinje neurons in ankyrin-G deficient cerebellums [109]. To regulate neuronal excitability, basket interneurons form GABAergic synapses with AIS of Purkinje neurons, a developmental process guided by an ankyrin-G dependent subcellular gradient of neurofascin in Purkinje neurons [109]. Additional support for ankyrin function in synaptogenesis comes from genetic screens that identified the large isoform of Drosophila ankyrin 2 as a critical regulator of synaptic stability and maintenance at the Drosophila neuromuscular junction [110, 111]. Specifically, the loss of this ankyrin precipitates the disassembly and retraction of presynaptic boutons, caused by cytoskeletal destabilization following the loss of an interaction between synaptic microtubules and the extended C-terminal domain of this ankyrin [110, 111].
Another mechanism by which ankyrin-G impacts overall neural circuitry is through its targeting of voltage-gated sodium and potassium channels to AIS and nodes of Ranvier in neurons of the central nervous system. For example, ankyrin-G directs the targeting of voltage-gated sodium channels to AIS of granule cells and Purkinje neurons [80]. In the absence of ankyrin-G, Purkinje neurons display impaired ability to initiate action potentials and the mice demonstrate neuronal degeneration of the cerebellum and progressive motor ataxia [80].
Many disease-related mutations have been characterized in ankyrin-G associated proteins. For example, a mutation in cardiac voltage-gated sodium channel NaV1.5 was shown to disrupt the channel’s association with ankyrin-G and to cause Brugada syndrome, a cardiac disorder characterized by precordial ST segment elevation, right bundle branch block, and fatal arrhythmias [5, 45]. The Brugada syndrome mutation (E1053K) resides within a 9 amino acid ankyrin-binding motif that is highly conserved in the DII-DIII loops of voltage-gated sodium channels [45, 112, 113]. By disrupting the association of ankyrin-G and NaV1.5, this mutation impairs ankyrin-G dependent targeting of the channel to the intercalated disc resulting in decreased sodium channel current density (INa) [5, 45]. Similar ankyrin-binding motifs are also present in the voltage-gated potassium channel subunits KCNQ2 and KCNQ3. These motifs are required for ankyrin-dependent targeting of these channel subunits to AIS where they control overall neuronal excitability [11, 13, 14]. Mutations in these subunits associated with benign familial neonatal convulsions (BFNC) cause epilepsy and myokymia [114–116]. Moreover, BFNC mutations in the KCNQ2 subunit that remove the ankyrin-binding site either through a frameshift or premature stop codon cause reduced axonal surface expression of potassium channels in hippocampal neurons [11]. Recently, the β1 subunit of cyclic nucleotide-gated channel (CNG-β1) was identified as a binding partner for ankyrin-G in the retina [117]. This interaction is necessary for ankyrin-dependent targeting of CNG-β1 to rod outer segments and domain formation [117]. Interestingly, the ankyrin-G binding site resides in the last twenty amino acids of CNG-β1 and a mutation that abolishes this domain is associated with retinitis pigmentosa, a progressive retinal dystrophy that causes gradual vision loss due to abnormalities in the photoreceptors or retinal pigment epithelium [117, 118]. Finally, both ankyrin-B and ankyrin-G were recently shown to interact with dystrophin, an essential component of the dystrophin glycoprotein complex that provides membrane stability by linking the actin-based cytoskeleton to integral membrane proteins connected to the extracellular matrix [72]. A dystrophin mutation linked to Becker muscular dystrophy was shown to reduce ankyrin binding thereby resulting in decreased sarcolemmal localization of dystrophin [72].
Ankyrin-B
While ankyrin-B is expressed in a variety of tissues, human mutations to ankyrin-B have almost exclusively been associated with cardiac disorders (see Table 2). The uniform expression of ankyrin-B polypeptides in different cardiac regions (i.e. ventricles, atria, sinoatrial node) accounts for the diversity of ankyrin-B associated cardiac disorders including bradycardia, sinus arrhythmia, delayed conduction/conduction block, idiopathic ventricular fibrillation and catecholaminergic polymorphic ventricular tachycardia [100–102]. In the ventricles, ankyrin-B is responsible for the proper targeting and retention of ion channels and transporters that regulate local calcium dynamics at the SR/T-tubule junction (Fig. 3). In ankyrin-B haploinsufficient mice, ventricular cardiomyocytes display reduced expression and proper SR/T-tubule localization of NCX, NKA and the IP3 receptor [9, 101, 102]. Furthermore, when given a catecholamine bolus during exercise, these mice exhibit ventricular tachycardia and often sudden death, thereby mimicking human cases of catecholaminergic polymorphic ventricular tachycardia that result from ankyrin-B dysfunction [101].
Recently, the molecular basis for ankyrin-B dysfunction was characterized in ‘sick sinus syndrome’ including bradycardia and variable heart rate. Specifically, ankyrin-B is necessary for the proper targeting of ion channels and transporters associated with calcium influx and efflux in the cardiac pacemaker, or sinoatrial node [119]. In addition to reduced expression of NCX, NKA and IP3 receptor, sinoatrial cells from ankyrin-B heterozygous mice display inappropriate targeting of CaV1.3, an important regulator of extracellular calcium entry that is necessary for normal cardiac pacemaker function [119]. Future research efforts to assess ankyrin-B activity in other cardiac regions (i.e. the atrium and other components of the conduction system) will advance our understanding of ankyrin-B function in normal cardiac physiology.
Studies of the ankyrin-B knockout mouse provide insight into potential disorders linked to human mutations in ankyrin-B. For example, ankyrin-B deficiency in mouse has been reported to be associated with mild skeletal muscular phenotypes including elevated creatine kinase levels and sporadically disorganized sarcomeres [40]. As mentioned above, ankyrin-B was recently found to interact with dystrophin consistent with its role in skeletal muscle organization and integrity. To date, no human skeletal muscle disorder has been associated with an ankyrin-B mutation, although co-expression of ankyrin-G and ankyrin-R isoforms may compensate for ankyrin-B dysfunction.
Ankyrin-B deficient mice also exhibit significant neurological pathology including hypoplasia of the corpus callosum and pyramidal tracts, dilated ventricles and severe postnatal degeneration of the optic nerve [39]. At the cellular level, many of these abnormalities are the result of ankyrin-B functions in neurite outgrowth, axonal guidance and axon fasciculation. At the molecular level, these functions are fulfilled by ankyrin-B interactions with the cell adhesion molecule L1. In fact, many of the neurological defects manifested in ankyrin-B deficient mice are mimicked in L1-deficient mice including axon guidance errors in pyramidal tracts and corpus callosum, degeneration of sensory axons and enlarged lateral ventricles [120–123]. The neuropathology manifested in ankyrin-B deficient mice may also arise in part from a loss of ankyrin-B dependent targeting of β-dystroglycan to astrocyte endfeet, which ensures the compact formation of the glia limitans that serves as an interface between cerebral spinal fluid and the brain [72].
In many animal models, both ankyrin and L1 are involved in axonal guidance and fasciculation. For example, mutations to the ankyrin-related gene unc-44 in C. elegans result in abnormal axonal guidance and fasciculation [124, 125]. Likewise, mutations to the Drosophila homologue of L1 neuroglian cause abnormal pathfinding of motorneuron projections and impaired contralateral projections of olfactory receptor neurons [126, 127]. Two different mechanisms have been shown to regulate L1-mediated axonal guidance and neurite outgrowth. First, L1 interacts with neuropilin-1, which is a receptor for the semaphorin family of chemo-attractants and repellents [128–130]. Second, the homophilic interactions between L1 proteins activate receptor tyrosine kinases (i.e. the epidermal and fibroblast growth factor receptors) and second messenger pathways (i.e. MAP kinase and phospholipase C) [131]. Tyrosine phosphorylation of the FIGQY motif abolishes ankyrin interaction with L1, and neurite outgrowth is positively regulated by untethered L1 molecules. Interestingly, mutations in the L1 FIGQY motif have been linked to human cases of CRASH syndrome that is characterized by corpus callosum hypoplasia, retardation, adducted thumbs, spastic paraplegia and hydrocephalus [132, 133].
Conclusions
While ankyrins were initially characterized as anchors tethering membrane proteins to the underlying cytoskeleton, they are now thought to play a crucial role in the formation of specialized membrane domains. With their modular architecture and ability to interact with many proteins, ankyrins organize and stabilize protein networks in collaboration with other proteins including cytoskeletal proteins, cell adhesion molecules and large structural proteins. Cytoskeletal proteins such as β-spectrin contribute stability to the ankyrin protein network. This stability underlies the structural integrity of the plasma membrane such that cells remain resilient to mechanical stress associated with development and normal cellular functions. Furthermore, incorporation of β-spectrin allows the ankyrin protein network to remain static in the fluid lipid bilayer thereby accommodating the initiation, development and maintenance of membrane domains. Cell adhesion molecules enable extracellular signals from neighbouring cells or the extracellular matrix to interact with the ankyrin protein complex and to guide the formation of membrane domains such as the nodes of Ranvier in peripheral neurons. Ankyrin interactions with cell adhesion molecules are important for connecting adjacent cells and anchoring cellular projections that migrate through the extracellular matrix. Finally, ankyrin interactions with large structural proteins such as obscurin allow for the proper integration of ankyrin protein complexes into the larger cellular scheme, ensuring its correct placement in the context of other protein networks and neighbouring cellular structures. While the targeting and stabilization of ankyrin protein networks are facilitated by each of the aforementioned proteins, it is certain that many additional proteins will contribute to ankyrin-dependent membrane formation. The tailoring of ankyrin polypeptides through alternative splicing enables modified ankyrins to adapt to the variable conditions presented during the formation of different specialized domains. This versatility accounts for ankyrin’s multifunctional capabilities in diverse cellular backgrounds (i.e. erythrocytes versus cardiomyocytes) and their apt intercellular functioning within different organelles (i.e. lysosome, trans Golgi network and SR) [41, 47, 134, 135]. As the field of ankyrin biology continues to develop, so too will our appreciation of ankyrin’s pivotal role in normal and diseased cellular conditions.
Acknowledgments
We acknowledge financial support from the NIH (HL084583 and HL083422 to P.J.M.; HL092232 to S.R.C.) and the Pew Scholars Trust (P.J.M.).
References
- 1.Bennett V. Immunoreactive forms of human erythrocyte ankyrin are present in diverse cells and tissues. Nature. 1979;281:597–9. doi: 10.1038/281597a0. [DOI] [PubMed] [Google Scholar]
- 2.Michaely P, Tomchick DR, Machius M, et al. Crystal structure of a 12 ANK repeat stack from human ankyrinR. EMBO J. 2002;21:6387–96. doi: 10.1093/emboj/cdf651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cunha SR, Bhasin N, Mohler PJ. Targeting and stability of Na/Ca exchanger 1 in cardiomyocytes requires direct interaction with the membrane adaptor ankyrin-B. J Biol Chem. 2007;282:4875–83. doi: 10.1074/jbc.M607096200. [DOI] [PubMed] [Google Scholar]
- 4.Mohler PJ, Davis JQ, Davis LH, et al. Inositol 1,4,5-trisphosphate receptor localization and stability in neonatal cardiomyocytes requires interaction with ankyrin-B. J Biol Chem. 2004;279:12980–7. doi: 10.1074/jbc.M313979200. [DOI] [PubMed] [Google Scholar]
- 5.Lowe JS, Palygin O, Bhasin N, et al. Voltage-gated Nav channel targeting in the heart requires an ankyrin-G dependent cellular pathway. J Cell Biol. 2008;180:173–86. doi: 10.1083/jcb.200710107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Davis JQ, Bennett V. The anion exchanger and Na+K(+)-ATPase interact with distinct sites on ankyrin in in vitro assays. J Biol Chem. 1990;265:17252–6. [PubMed] [Google Scholar]
- 7.Davis LH, Otto E, Bennett V. Specific 33-residue repeat(s) of erythrocyte ankyrin associate with the anion exchanger. J Biol Chem. 1991;266:11163–9. [PubMed] [Google Scholar]
- 8.Michaely P, Bennett V. The ANK repeats of erythrocyte ankyrin form two distinct but cooperative binding sites for the erythrocyte anion exchanger. J Biol Chem. 1995;270:22050–7. doi: 10.1074/jbc.270.37.22050. [DOI] [PubMed] [Google Scholar]
- 9.Mohler PJ, Davis JQ, Bennett V. Ankyrin-B coordinates the Na/K ATPase, Na/Ca exchanger, and InsP(3) receptor in a cardiac T-tubule/SR microdomain. PLoS Biol. 2005;3:2158–67. doi: 10.1371/journal.pbio.0030423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nelson WJ, Veshnock PJ. Ankyrin binding to (Na++ K+)ATPase and implications for the organization of membrane domains in polarized cells. Nature. 1987;328:533–6. doi: 10.1038/328533a0. [DOI] [PubMed] [Google Scholar]
- 11.Chung HJ, Jan YN, Jan LY. Polarized axonal surface expression of neuronal KCNQ channels is mediated by multiple signals in the KCNQ2 and KCNQ3 C-terminal domains. Proc Natl Acad Sci USA. 2006;103:1–15. doi: 10.1073/pnas.0603376103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hill AS, Nishino A, Nakajo K, et al. Ion channel clustering at the axon initial segment and node of Ranvier evolved sequentially in early chordates. PLoS Genetics. 2008;4:e1000317. doi: 10.1371/journal.pgen.1000317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pan Z, Kao T, Horvath Z, et al. A common ankyrin-G-based mechanism retains KCNQ and NaV channels at electrically active domains of the axon. J Neurosci. 2006;26:2599–613. doi: 10.1523/JNEUROSCI.4314-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rasmussen HB, Frokjaer-Jensen C, Jensen CS, et al. Requirement of subunit co-assembly and ankyrin-G for M-channel localization at the axon initial segment. J Cell Sci. 2007;120:953–63. doi: 10.1242/jcs.03396. [DOI] [PubMed] [Google Scholar]
- 15.Davis JQ, Bennett V. Ankyrin binding activity shared by the neurofascin/L1/NrCAM family of nervous system cell adhesion molecules. J Biol Chem. 1994;269:27163–6. [PubMed] [Google Scholar]
- 16.Davis JQ, McLaughlin T, Bennett V. Ankyrin-binding proteins related to nervous system cell adhesion molecules: candidates to provide transmembrane and intercellular connections in adult brain. J Cell Biol. 1993;121:121–33. doi: 10.1083/jcb.121.1.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mohler PJ, Yoon W, Bennett V. Ankyrin-B targets beta2-spectrin to an intracellular compartment in neonatal cardiomyocytes. J Biol Chem. 2004;279:40185–93. doi: 10.1074/jbc.M406018200. [DOI] [PubMed] [Google Scholar]
- 18.Kizhatil K, Yoon W, Mohler PJ, et al. Ankyrin-G and beta2-spectrin collaborate in biogenesis of lateral membrane of human bronchial epithelial cells. J Biol Chem. 2007;282:2029–37. doi: 10.1074/jbc.M608921200. [DOI] [PubMed] [Google Scholar]
- 19.Davis L, Abdi K, Machius M, et al. Localization and structure of the ankyrin-binding site on beta2-spectrin. J Biol Chem. 2009;284:6982–7. doi: 10.1074/jbc.M809245200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ipsaro JJ, Huang L, Mondragon A. Structures of the spectrin-ankyrin interaction binding domains. Blood. 2009;113:5385–93. doi: 10.1182/blood-2008-10-184358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kennedy SP, Warren SL, Forget BG, et al. Ankyrin binds to the 15th repetitive unit of erythroid and nonerythroid beta-spectrin. J Cell Biol. 1991;115:267–77. doi: 10.1083/jcb.115.1.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Davis LH, Davis JQ, Bennett V. Ankyrin regulation: an alternatively spliced segment of the regulatory domain functions as an intramolecular modulator. J Biol Chem. 1992;267:18966–72. [PubMed] [Google Scholar]
- 23.Hall TG, Bennett V. Regulatory domains of erythrocyte ankyrin. J Biol Chem. 1987;262:10537–45. [PubMed] [Google Scholar]
- 24.Abdi KM, Mohler PJ, Davis JQ, et al. Isoform specificity of ankyrin-B: a site in the divergent C-terminal domain is required for intramolecular association. J Biol Chem. 2006;281:5741–9. doi: 10.1074/jbc.M506697200. [DOI] [PubMed] [Google Scholar]
- 25.Birkenmeier CS, Sharp JJ, Gifford EJ, et al. An alternative first exon in the distal end of the erythroid ankyrin gene leads to production of a small isoform containing an NH2-terminal membrane anchor. Genomics. 1998;50:79–88. doi: 10.1006/geno.1998.5305. [DOI] [PubMed] [Google Scholar]
- 26.Birkenmeier CS, White RA, Peters LL, et al. Complex patterns of sequence variation and multiple 5′ and 3′ ends are found among transcripts of the erythroid ankyrin gene. J Biol Chem. 1993;268:9533–40. [PubMed] [Google Scholar]
- 27.Gallagher PG, Forget BG. An alternate promoter directs expression of a truncated, muscle-specific isoform of the human ankyrin 1 gene. J Biol Chem. 1998;273:1339–48. doi: 10.1074/jbc.273.3.1339. [DOI] [PubMed] [Google Scholar]
- 28.Gallagher PG, Tse WT, Scarpa AL, et al. Structure and organization of the human ankyrin-1 gene. Basis for complexity of pre-mRNA processing. J Biol Chem. 1997;272:19220–8. doi: 10.1074/jbc.272.31.19220. [DOI] [PubMed] [Google Scholar]
- 29.Lambert S, Bennett V. Postmitotic expression of ankyrinR and beta R-spectrin in discrete neuronal populations of the rat brain. J Neurosci. 1993;13:3725–35. doi: 10.1523/JNEUROSCI.13-09-03725.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lambert S, Yu H, Prchal JT, et al. cDNA sequence for human erythrocyte ankyrin. Proc Natl Acad Sci USA. 1990;87:1730–4. doi: 10.1073/pnas.87.5.1730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lux SE, Tse WT, Menninger JC, et al. Hereditary spherocytosis associated with deletion of human erythrocyte ankyrin gene on chromosome 8. Nature. 1990;345:736–9. doi: 10.1038/345736a0. [DOI] [PubMed] [Google Scholar]
- 32.Peters LL, Birkenmeier CS, Bronson RT, et al. Purkinje cell degeneration associated with erythroid ankyrin deficiency in nb/nb mice. J Cell Biol. 1991;114:1233–41. doi: 10.1083/jcb.114.6.1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhou D, Birkenmeier CS, Williams MW, et al. Small, membrane-bound, alternatively spliced forms of ankyrin 1 associated with the sarcoplasmic reticulum of mammalian skeletal muscle. J Cell Biol. 1997;136:621–31. doi: 10.1083/jcb.136.3.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cunha SR, Le Scouarnec S, Schott JJ, et al. Exon organization and novel alternative splicing of the human ANK2 gene: implications for cardiac function and human cardiac disease. J Mol Cell Cardiol. 2008;45:724–34. doi: 10.1016/j.yjmcc.2008.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tse WT, Menninger JC, Yang-Feng TL, et al. Isolation and chromosomal localization of a novel nonerythroid ankyrin gene. Genomics. 1991;10:858–66. doi: 10.1016/0888-7543(91)90173-c. [DOI] [PubMed] [Google Scholar]
- 36.Kunimoto M. A neuron-specific isoform of brain ankyrin, 440-kD ankyrinB, is targeted to the axons of rat cerebellar neurons. J Cell Biol. 1995;131:1821–9. doi: 10.1083/jcb.131.6.1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kunimoto M, Otto E, Bennett V. A new 440-kD isoform is the major ankyrin in neonatal rat brain. J Cell Biol. 1991;115:1319–31. doi: 10.1083/jcb.115.5.1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Otto E, Kunimoto M, McLaughlin T, et al. Isolation and characterization of cDNAs encoding human brain ankyrins reveal a family of alternatively spliced genes. J Cell Biol. 1991;114:241–53. doi: 10.1083/jcb.114.2.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Scotland P, Zhou D, Benveniste H, et al. Nervous system defects of AnkyrinB (−/−) mice suggest functional overlap between the cell adhesion molecule L1 and 440-kD AnkyrinB in premyelinated axons. J Cell Biol. 1998;143:1305–15. doi: 10.1083/jcb.143.5.1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tuvia S, Buhusi M, Davis L, et al. Ankyrin-B is required for intracellular sorting of structurally diverse Ca2+ homeostasis proteins. J Cell Biol. 1999;147:995–1008. doi: 10.1083/jcb.147.5.995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Devarajan P, Stabach PR, Mann AS, et al. Identification of a small cytoplasmic ankyrin (AnkG119) in the kidney and muscle that binds beta I sigma spectrin and associates with the Golgi apparatus. J Cell Biol. 1996;133:819–30. doi: 10.1083/jcb.133.4.819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Doctor RB, Chen J, Peters LL, et al. Distribution of epithelial ankyrin (Ank3) spliceoforms in renal proximal and distal tubules. Am J Physiol. 1998;274:F129–38. doi: 10.1152/ajprenal.1998.274.1.F129. [DOI] [PubMed] [Google Scholar]
- 43.Hopitzan AA, Baines AJ, Ludosky MA, et al. Ankyrin-G in skeletal muscle: tissue-specific alternative splicing contributes to the complexity of the sarcolemmal cytoskeleton. Exp Cell Res. 2005;309:86–98. doi: 10.1016/j.yexcr.2005.04.013. [DOI] [PubMed] [Google Scholar]
- 44.Kordeli E, Lambert S, Bennett V. AnkyrinG. A new ankyrin gene with neural-specific isoforms localized at the axonal initial segment and node of Ranvier. J Biol Chem. 1995;270:2352–9. doi: 10.1074/jbc.270.5.2352. [DOI] [PubMed] [Google Scholar]
- 45.Mohler PJ, Rivolta I, Napolitano C, et al. Nav1.5 E1053K mutation causing Brugada syndrome blocks binding to ankyrin-G and expression of Nav1.5 on the surface of cardiomyocytes. Proc Natl Acad Sci USA. 2004;101:17533–8. doi: 10.1073/pnas.0403711101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Peters B, Kaiser HW, Magin TM. Skin-specific expression of ank-3(93), a novel ankyrin-3 splice variant. J Invest Dermatol. 2001;116:216–23. doi: 10.1046/j.1523-1747.2001.01210.x. [DOI] [PubMed] [Google Scholar]
- 47.Peters LL, John KM, Lu FM, et al. Ank3 (epithelial ankyrin), a widely distributed new member of the ankyrin gene family and the major ankyrin in kidney, is expressed in alternatively spliced forms, including forms that lack the repeat domain. J Cell Biol. 1995;130:313–30. doi: 10.1083/jcb.130.2.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Thevananther S, Kolli AH, Devarajan P. Identification of a novel ankyrin isoform (AnkG190) in kidney and lung that associates with the plasma membrane and binds alpha-Na, K-ATPase. J Biol Chem. 1998;273:23952–8. doi: 10.1074/jbc.273.37.23952. [DOI] [PubMed] [Google Scholar]
- 49.Zhang X, Bennett V. Restriction of 480/270-kD ankyrin G to axon proximal segments requires multiple ankyrin G-specific domains. J Cell Biol. 1998;142:1571–81. doi: 10.1083/jcb.142.6.1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bagnato P, Barone V, Giacomello E, et al. Binding of an ankyrin-1 isoform to obscurin suggests a molecular link between the sarcoplasmic reticulum and myofibrils in striated muscles. J Cell Biol. 2003;160:245–53. doi: 10.1083/jcb.200208109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kontrogianni-Konstantopoulos A, Jones EM, Van Rossum DB, et al. Obscurin is a ligand for small ankyrin 1 in skeletal muscle. Mol Biol Cell. 2003;14:1138–48. doi: 10.1091/mbc.E02-07-0411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Van Oort RJ, Altamirano J, Lederer WJ, et al. Alternative splicing: a key mechanism for ankyrin-B functional diversity. J Mol Cell Cardiol. 2008;45:709–11. doi: 10.1016/j.yjmcc.2008.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bennett V, Baines AJ. Spectrin and ankyrin-based pathways: metazoan inventions for integrating cells into tissues. Physiol Rev. 2001;81:1353–92. doi: 10.1152/physrev.2001.81.3.1353. [DOI] [PubMed] [Google Scholar]
- 54.An X, Debnath G, Guo X, et al. Identification and functional characterization of protein 4.1R and actin-binding sites in erythrocyte beta spectrin: regulation of the interactions by phosphatidylinositol-4, 5-bisphosphate. Biochemistry. 2005;44:10681–8. doi: 10.1021/bi047331z. [DOI] [PubMed] [Google Scholar]
- 55.Jackson M, Song W, Liu MY, et al. Modulation of the neuronal glutamate transporter EAAT4 by two interacting proteins. Nature. 2001;410:89–93. doi: 10.1038/35065091. [DOI] [PubMed] [Google Scholar]
- 56.Wang DS, Shaw G. The association of the C-terminal region of beta I sigma II spectrin to brain membranes is mediated by a PH domain, does not require membrane proteins, and coincides with a inositol-1,4,5 triphosphate binding site. Biochem Biophys Res Commun. 1995;217:608–15. doi: 10.1006/bbrc.1995.2818. [DOI] [PubMed] [Google Scholar]
- 57.Delaunay J, Alloisio N, Morle L, et al. Molecular genetics of hereditary elliptocytosis and hereditary spherocytosis. Ann Genet. 1996;39:209–21. [PubMed] [Google Scholar]
- 58.Tse WT, Lecomte MC, Costa FF, et al. Point mutation in the beta-spectrin gene associated with alpha I/74 hereditary elliptocytosis. Implications for the mechanism of spectrin dimer self-association. J Clin Invest. 1990;86:909–16. doi: 10.1172/JCI114792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Soong BW, Paulson HL. Spinocerebellar ataxias: an update. Curr Opin Neurol. 2007;20:438–46. doi: 10.1097/WCO.0b013e3281fbd3dd. [DOI] [PubMed] [Google Scholar]
- 60.Kizhatil K, Sandhu NK, Peachey NS, et al. Ankyrin-B is required for coordinated expression of beta2-spectrin, the Na/K-ATPase and the Na/Ca exchanger in the inner segment of rod photoreceptors. Exp Eye Res. 2009;88:57–64. doi: 10.1016/j.exer.2008.09.022. [DOI] [PubMed] [Google Scholar]
- 61.Jenkins SM, Bennett V. Ankyrin-G coordinates assembly of the spectrin-based membrane skeleton, voltage-gated sodium channels, and L1 CAMs at Purkinje neuron initial segments. J Cell Biol. 2001;155:739–46. doi: 10.1083/jcb.200109026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Das A, Base C, Dhulipala S, et al. Spectrin functions upstream of ankyrin in a spectrin cytoskeleton assembly pathway. J Cell Biol. 2006;175:325–35. doi: 10.1083/jcb.200602095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Komada M, Soriano P. [Beta]IV-spectrin regulates sodium channel clustering through ankyrin-G at axon initial segments and nodes of Ranvier. J Cell Biol. 2002;156:337–48. doi: 10.1083/jcb.200110003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kizhatil K, Bennett V. Lateral membrane biogenesis in human bronchial epithelial cells requires 190-kDa ankyrin-G. J Biol Chem. 2004;279:16706–14. doi: 10.1074/jbc.M314296200. [DOI] [PubMed] [Google Scholar]
- 65.Das A, Base C, Manna D, et al. Unexpected complexity in the mechanisms that target assembly of the spectrin cytoskeleton. J Biol Chem. 2008;283:12643–53. doi: 10.1074/jbc.M800094200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Pradhan D, Lombardo CR, Roe S, et al. Alpha -catenin binds directly to spectrin and facilitates spectrin-membrane assembly in vivo. J Biol Chem. 2001;276:4175–81. doi: 10.1074/jbc.M009259200. [DOI] [PubMed] [Google Scholar]
- 67.Dzhashiashvili Y, Zhang Y, Galinska J, et al. Nodes of Ranvier and axon initial segments are ankyrin G-dependent domains that assemble by distinct mechanisms. J Cell Biol. 2007;177:857–70. doi: 10.1083/jcb.200612012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lustig M, Zanazzi G, Sakurai T, et al. Nr-CAM and neurofascin interactions regulate ankyrin G and sodium channel clustering at the node of Ranvier. Curr Biol. 2001;11:1864–9. doi: 10.1016/s0960-9822(01)00586-3. [DOI] [PubMed] [Google Scholar]
- 69.Sherman DL, Tait S, Melrose S, et al. Neurofascins are required to establish axonal domains for saltatory conduction. Neuron. 2005;48:737–42. doi: 10.1016/j.neuron.2005.10.019. [DOI] [PubMed] [Google Scholar]
- 70.Lokeshwar VB, Fregien N, Bourguignon LY. Ankyrin-binding domain of CD44(GP85) is required for the expression of hyaluronic acid-mediated adhesion function. J Cell Biol. 1994;126:1099–109. doi: 10.1083/jcb.126.4.1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kizhatil K, Davis JQ, Davis L, et al. Ankyrin-G is a molecular partner of E-cadherin in epithelial cells and early embryos. J Biol Chem. 2007;282:26552–61. doi: 10.1074/jbc.M703158200. [DOI] [PubMed] [Google Scholar]
- 72.Ayalon G, Davis JQ, Scotand PB, et al. An ankyrin-based mechanism for functional organization of dystrophin and dystroglycan. Cell. 2008;135:1189–200. doi: 10.1016/j.cell.2008.10.018. [DOI] [PubMed] [Google Scholar]
- 73.Hortsch M, Nagaraj K, Godenschwege TA. The interaction between L1-type proteins and ankyrins-a master switch for L1-type CAM function. Cell Mol Biol Lett. 2009;14:57–69. doi: 10.2478/s11658-008-0035-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Garver TD, Ren Q, Tuvia S, et al. Tyrosine phosphorylation at a site highly conserved in the L1 family of cell adhesion molecules abolishes ankyrin binding and increases lateral mobility of neurofascin. J Cell Biol. 1997;137:703–14. doi: 10.1083/jcb.137.3.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Tuvia S, Garver TD, Bennett V. The phosphorylation state of the FIGQY tyrosine of neurofascin determines ankyrin-binding activity and patterns of cell segregation. Proc Natl Acad Sci USA. 1997;94:12957–62. doi: 10.1073/pnas.94.24.12957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zhang X, Davis JQ, Carpenter S, et al. Structural requirements for association of neurofascin with ankyrin. J Biol Chem. 1998;273:30785–94. doi: 10.1074/jbc.273.46.30785. [DOI] [PubMed] [Google Scholar]
- 77.Gil OD, Sakurai T, Bradley AE, et al. Ankyrin binding mediates L1CAM interactions with static components of the cytoskeleton and inhibits retrograde movement of L1CAM on the cell surface. J Cell Biol. 2003;162:719–30. doi: 10.1083/jcb.200211011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kerjan G, Gleeson JG. Genetic mechanisms underlying abnormal neuronal migration in classical lissencephaly. Trends Genet. 2007;23:623–30. doi: 10.1016/j.tig.2007.09.003. [DOI] [PubMed] [Google Scholar]
- 79.Kizhatil K, Wu YX, Sen A, et al. A new activity of doublecortin in recognition of the phospho-FIGQY tyrosine in the cytoplasmic domain of neurofascin. J Neurosci. 2002;22:7948–58. doi: 10.1523/JNEUROSCI.22-18-07948.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zhou D, Lambert S, Malen PL, et al. AnkyrinG is required for clustering of voltage-gated Na channels at axon initial segments and for normal action potential firing. J Cell Biol. 1998;143:1295–304. doi: 10.1083/jcb.143.5.1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hedstrom KL, Xu X, Ogawa Y, et al. Neurofascin assembles a specialized extracellular matrix at the axon initial segment. J Cell Biol. 2007;178:875–86. doi: 10.1083/jcb.200705119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Custer AW, Kazarinova-Noyes K, Sakurai T, et al. The role of the ankyrin-binding protein NrCAM in node of Ranvier formation. J Neurosci. 2003;23:10032–9. doi: 10.1523/JNEUROSCI.23-31-10032.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lambert S, Davis JQ, Bennett V. Morphogenesis of the node of Ranvier: co-clusters of ankyrin and ankyrin-binding integral proteins define early developmental intermediates. J Neurosci. 1997;17:7025–36. doi: 10.1523/JNEUROSCI.17-18-07025.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Eshed Y, Feinberg K, Poliak S, et al. Gliomedin mediates Schwann cell-axon interaction and the molecular assembly of the nodes of Ranvier. Neuron. 2005;47:215–29. doi: 10.1016/j.neuron.2005.06.026. [DOI] [PubMed] [Google Scholar]
- 85.Kontrogianni-Konstantopoulos A, Catino DH, Strong JC, et al. Obscurin modulates the assembly and organization of sarcomeres and the sarcoplasmic reticulum. FASEB J. 2006;20:2102–11. doi: 10.1096/fj.06-5761com. [DOI] [PubMed] [Google Scholar]
- 86.Young P, Ehler E, Gautel M. Obscurin, a giant sarcomeric Rho guanine nucleotide exchange factor protein involved in sarcomere assembly. J Cell Biol. 2001;154:123–36. doi: 10.1083/jcb.200102110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Borisov AB, Sutter SB, Kontrogianni-Konstantopoulos A, et al. Essential role of obscurin in cardiac myofibrillogenesis and hypertrophic response: evidence from small interfering RNA-mediated gene silencing. Histochem Cell Biol. 2006;125:227–38. doi: 10.1007/s00418-005-0069-x. [DOI] [PubMed] [Google Scholar]
- 88.Lange S, Ouyang K, Meyer G, et al. Obscurin determines the architecture of the longitudinal sarcoplasmic reticulum. J Cell Sci. 2009;122:2640–50. doi: 10.1242/jcs.046193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Raeker MO, Su F, Geisler SB, et al. Obscurin is required for the lateral alignment of striated myofibrils in zebrafish. Dev Dyn. 2006;235:2018–29. doi: 10.1002/dvdy.20812. [DOI] [PubMed] [Google Scholar]
- 90.Arimura T, Matsumoto Y, Okazaki O, et al. Structural analysis of obscurin gene in hypertrophic cardiomyopathy. Biochem Biophy Res Commun. 2007;362:281–7. doi: 10.1016/j.bbrc.2007.07.183. [DOI] [PubMed] [Google Scholar]
- 91.Bowman AL, Kontrogianni-Konstantopoulos A, Hirsch SS, et al. Different obscurin isoforms localize to distinct sites at sarcomeres. FEBS Let. 2007;581:1549–54. doi: 10.1016/j.febslet.2007.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Fukuzawa A, Idowu S, Gautel M. Complete human gene structure of obscurin: implications for isoform generation by differential splicing. J Muscle Res Cell Motil. 2005;26:427–34. doi: 10.1007/s10974-005-9025-6. [DOI] [PubMed] [Google Scholar]
- 93.Fukuzawa A, Lange S, Holt M, et al. Interactions with titin and myomesin target obscurin and obscurin-like 1 to the M-band: implications for hereditary myopathies. J Cell Sci. 2008;121:1841–51. doi: 10.1242/jcs.028019. [DOI] [PubMed] [Google Scholar]
- 94.Hackman P, Vihola A, Haravuori H, et al. Tibial muscular dystrophy is a titinopathy caused by mutations in TTN, the gene encoding the giant skeletal-muscle protein titin. Am J Hum Genet. 2002;71:492–500. doi: 10.1086/342380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Udd B, Vihola A, Sarparanta J, et al. Titinopathies and extension of the M-line mutation phenotype beyond distal myopathy and LGMD2J. Neurology. 2005;64:636–42. doi: 10.1212/01.WNL.0000151853.50144.82. [DOI] [PubMed] [Google Scholar]
- 96.Van den Bergh PY, Bouquiaux O, Verellen C, et al. Tibial muscular dystrophy in a Belgian family. Ann Neurol. 2003;54:248–51. doi: 10.1002/ana.10647. [DOI] [PubMed] [Google Scholar]
- 97.Cunha SR, Mohler PJ. Obscurin targets ankyrin-B and protein phosphatase 2A to the cardiac M-line. J Biol Chem. 2008;283:31968–80. doi: 10.1074/jbc.M806050200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Armani A, Galli S, Giacomello E, et al. Molecular interactions with obscurin are involved in the localization of muscle-specific small ankyrin1 isoforms to subcompartments of the sarcoplasmic reticulum. Exp Cell Res. 2006;312:3546–58. doi: 10.1016/j.yexcr.2006.07.027. [DOI] [PubMed] [Google Scholar]
- 99.Mohler PJ, Hoffman JA, Davis JQ, et al. Isoform specificity among ankyrins: an amphipathic alpha-helix in the divergent regulatory domain of ankyrin-B interacts with the molecular co-chaperone Hdj1/Hsp40. J Biol Chem. 2004;279:25798–804. doi: 10.1074/jbc.M401296200. [DOI] [PubMed] [Google Scholar]
- 100.Mohler PJ, Le Scouarnec S, Denjoy I, et al. Defining the cellular phenotype of “ankyrin-B syndrome” variants: human ANK2 variants associated with clinical phenotypes display a spectrum of activities in cardiomyocytes. Circulation. 2007;115:432–41. doi: 10.1161/CIRCULATIONAHA.106.656512. [DOI] [PubMed] [Google Scholar]
- 101.Mohler PJ, Schott JJ, Gramolini AO, et al. Ankyrin-B mutation causes type 4 long-QT cardiac arrhythmia and sudden cardiac death. Nature. 2003;421:634–9. doi: 10.1038/nature01335. [DOI] [PubMed] [Google Scholar]
- 102.Mohler PJ, Splawski I, Napolitano C, et al. A cardiac arrhythmia syndrome caused by loss of ankyrin-B function. Proc Natl Acad Sci USA. 2004;101:9137–42. doi: 10.1073/pnas.0402546101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Birkenmeier CS, Barker JE. Hereditary haemolytic anaemias: unexpected sequelae of mutations in the genes for erythroid membrane skeletal proteins. J Path. 2004;204:450–9. doi: 10.1002/path.1636. [DOI] [PubMed] [Google Scholar]
- 104.Kordeli E, Bennett V. Distinct ankyrin isoforms at neuron cell bodies and nodes of Ranvier resolved using erythrocyte ankyrin-deficient mice. J Cell Biol. 1991;114:1243–59. doi: 10.1083/jcb.114.6.1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Birkenmeier CS, Gifford EJ, Barker JE. Normoblastosis, a murine model for ankyrin-deficient hemolytic anemia, is caused by a hypomorphic mutation in the erythroid ankyrin gene Ank1. Hematol J. 2003;4:445–9. doi: 10.1038/sj.thj.6200307. [DOI] [PubMed] [Google Scholar]
- 106.Ferreira MA, O’Donovan MC, Meng YA, et al. Collaborative genome-wide association analysis supports a role for ANK3 and CACNA1C in bipolar disorder. Nat Genet. 2008;40:1056–8. doi: 10.1038/ng.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Schulze TG, Detera-Wadleigh SD, Akula N, et al. Two variants in ankyrin 3 (ANK3) are independent genetic risk factors for bipolar disorder. Mol Psychiatry. 2009;14:487–91. doi: 10.1038/mp.2008.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Scott LJ, Muglia P, Kong XQ, et al. Genome-wide association and meta-analysis of bipolar disorder in individuals of European ancestry. Proc Natl Acad Sci USA. 2009;106:7501–6. doi: 10.1073/pnas.0813386106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Ango F, Di Cristo G, Higashiyama H, et al. Ankyrin-based subcellular gradient of neurofascin, an immunoglobulin family protein, directs GABAergic innervation at purkinje axon initial segment. Cell. 2004;119:257–72. doi: 10.1016/j.cell.2004.10.004. [DOI] [PubMed] [Google Scholar]
- 110.Koch I, Schwarz H, Beuchle D, et al. Drosophila ankyrin 2 is required for synaptic stability. Neuron. 2008;58:210–22. doi: 10.1016/j.neuron.2008.03.019. [DOI] [PubMed] [Google Scholar]
- 111.Pielage J, Cheng L, Fetter RD, et al. A presynaptic giant ankyrin stabilizes the NMJ through regulation of presynaptic microtubules and transsynaptic cell adhesion. Neuron. 2008;58:195–209. doi: 10.1016/j.neuron.2008.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Garrido JJ, Giraud P, Carlier E, et al. A targeting motif involved in sodium channel clustering at the axonal initial segment. Science. 2003;300:2091–4. doi: 10.1126/science.1085167. [DOI] [PubMed] [Google Scholar]
- 113.Lemaillet G, Walker B, Lambert S. Identification of a conserved ankyrin-binding motif in the family of sodium channel alpha subunits. J Biol Chem. 2003;278:27333–9. doi: 10.1074/jbc.M303327200. [DOI] [PubMed] [Google Scholar]
- 114.Biervert C, Schroeder BC, Kubisch C, et al. A potassium channel mutation in neonatal human epilepsy. Science. 1998;279:403–6. doi: 10.1126/science.279.5349.403. [DOI] [PubMed] [Google Scholar]
- 115.Dedek K, Kunath B, Kananura C, et al. Myokymia and neonatal epilepsy caused by a mutation in the voltage sensor of the KCNQ2 K+ channel. Proc Natl Acad Sci USA. 2001;98:12272–7. doi: 10.1073/pnas.211431298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Singh NA, Charlier C, Stauffer D, et al. A novel potassium channel gene, KCNQ2, is mutated in an inherited epilepsy of newborns. Nat Genet. 1998;18:25–9. doi: 10.1038/ng0198-25. [DOI] [PubMed] [Google Scholar]
- 117.Kizhatil K, Baker SA, Arshavsky VY, et al. Ankyrin-G promotes cyclic nucleotide-gated channel transport to rod photoreceptor sensory cilia. Science. 2009;323:1614–7. doi: 10.1126/science.1169789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Kondo H, Qin M, Mizota A, et al. A homozygosity-based search for mutations in patients with autosomal recessive retinitis pigmentosa, using microsatellite markers. Invest Ophthalmol Vis Sci. 2004;45:4433–9. doi: 10.1167/iovs.04-0544. [DOI] [PubMed] [Google Scholar]
- 119.Le Scouarnec S, Bhasin N, Vieyres C, et al. Dysfunction in ankyrin-B-dependent ion channel and transporter targeting causes human sinus node disease. Proc Natl Acad Sci USA. 2008;105:15617–22. doi: 10.1073/pnas.0805500105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Cohen NR, Taylor JS, Scott LB, et al. Errors in corticospinal axon guidance in mice lacking the neural cell adhesion molecule L1. Curr Biol. 1998;8:26–33. doi: 10.1016/s0960-9822(98)70017-x. [DOI] [PubMed] [Google Scholar]
- 121.Dahme M, Bartsch U, Martini R, et al. Disruption of the mouse L1 gene leads to malformations of the nervous system. Nat Genet. 1997;17:346–9. doi: 10.1038/ng1197-346. [DOI] [PubMed] [Google Scholar]
- 122.Demyanenko GP, Tsai AY, Maness PF. Abnormalities in neuronal process extension, hippocampal development, and the ventricular system of L1 knockout mice. J Neurosci. 1999;19:4907–20. doi: 10.1523/JNEUROSCI.19-12-04907.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Haney CA, Sahenk Z, Li C, et al. Heterophilic binding of L1 on unmyelinated sensory axons mediates Schwann cell adhesion and is required for axonal survival. J Cell Biol. 1999;146:1173–84. doi: 10.1083/jcb.146.5.1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Otsuka AJ, Boontrakulpoontawee P, Rebeiz N, et al. Novel UNC-44 AO13 ankyrin is required for axonal guidance in C. elegans, contains six highly repetitive STEP blocks separated by seven potential transmembrane domains, and is localized to neuronal processes and the periphery of neural cell bodies. J Neurobiol. 2002;50:333–49. doi: 10.1002/neu.10036. [DOI] [PubMed] [Google Scholar]
- 125.Otsuka AJ, Franco R, Yang B, et al. An ankyrin-related gene (unc-44) is necessary for proper axonal guidance in Caenorhabditis elegans. J Cell Biol. 1995;129:1081–92. doi: 10.1083/jcb.129.4.1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Hall SG, Bieber AJ. Mutations in the Drosophila neuroglian cell adhesion molecule affect motor neuron pathfinding and peripheral nervous system patterning. J Neurobiol. 1997;32:325–40. [PubMed] [Google Scholar]
- 127.Chen W, Hing H. The L1-CAM, Neuroglian, functions in glial cells for Drosophila antennal lobe development. Dev Neurobiol. 2008;68:1029–45. doi: 10.1002/dneu.20644. [DOI] [PubMed] [Google Scholar]
- 128.Castellani V, Falk J, Rougon G. Semaphorin3A-induced receptor endocytosis during axon guidance responses is mediated by L1 CAM. Mol Cell Neurosci. 2004;26:89–100. doi: 10.1016/j.mcn.2004.01.010. [DOI] [PubMed] [Google Scholar]
- 129.Castellani V, De Angelis E, Kenwrick S, et al. Cis and trans interactions of L1 with neuropilin-1 control axonal responses to semaphorin 3A. EMBO J. 2002;21:6348–57. doi: 10.1093/emboj/cdf645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Castellani V, Chedotal A, Schachner M, et al. Analysis of the L1-deficient mouse phenotype reveals cross-talk between Sema3A and L1 signaling pathways in axonal guidance. Neuron. 2000;27:237–49. doi: 10.1016/s0896-6273(00)00033-7. [DOI] [PubMed] [Google Scholar]
- 131.Chen L, Ong B, Bennett V. LAD-1, the Caenorhabditis elegans L1CAM homologue, participates in embryonic and gonadal morphogenesis and is a substrate for fibroblast growth factor receptor pathway-dependent phosphotyrosine-based signaling. J Cell Biol. 2001;154:841–56. doi: 10.1083/jcb.200009004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Needham LK, Thelen K, Maness PF. Cytoplasmic domain mutations of the L1 cell adhesion molecule reduce L1-ankyrin interactions. J Neurosci. 2001;21:1490–500. doi: 10.1523/JNEUROSCI.21-05-01490.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Fransen E, Van Camp G, D’Hooge R, et al. Genotype-phenotype correlation in L1 associated diseases. J Med Genet. 1998;35:399–404. doi: 10.1136/jmg.35.5.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Hoock TC, Peters LL, Lux SE. Isoforms of ankyrin-3 that lack the NH2-terminal repeats associate with mouse macrophage lysosomes. J Cell Biol. 1997;136:1059–70. doi: 10.1083/jcb.136.5.1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Beck KA, Buchanan JA, Nelson WJ. Golgi membrane skeleton: identification, localization and oligomerization of a 195 kDa ankyrin isoform associated with the Golgi complex. J Cell Sci. 1997;110:1239–49. doi: 10.1242/jcs.110.10.1239. [DOI] [PubMed] [Google Scholar]


