Abstract
New therapeutic approaches aim to eradicate tumours by expression of tumouricidal proteins in the tumour stroma. One such anti-neoplastic protein is tumour necrosis factor-related apoptosis-inducing ligand (TRAIL) because it induces apoptosis in cancerous cells, but not in non-transformed cells. Stem cells can migrate to, survive and proliferate in tumours. We examined the suitability of bone marrow-derived adult mesenchymal stem cells (bmMSC), foetal-MSC and umbilical cord matrix stem cells (Wharton’s Jelly MSCs) as TRAIL-delivery vehicles. Although all MSC types expressed DR4 and/or DR5, none of them were sensitive to TRAIL-induced apoptosis. Selective activation of DR4 or DR5 with agonistic antibodies or DR5-selective TRAIL-mutant (D269H/E195R) revealed that the TRAIL receptors are inactive in MSCs. In fMSC DR5 was not fully inactivated, its activity however was minimal in comparison to the colon carcinoma cell, Colo205. The intracellular components of the TRAIL-apoptotic pathway, such as pro-caspase-8 and -9 were also expressed at very low; almost undetectable levels in all three MSC types. In conclusion, the MSC species examined are resistant to TRAIL and thus can be suitable tools for TRAIL delivery to tumours.
Keywords: apoptosis, Wharton’s Jelly stem cells, umbilical cord matrix stem cells (UCMS), foetal mesenchymal stem cells (fMSC), bone marrow-derived adult MSC, TRAIL, DR4, DR5, D269H/E195R, caspases
Introduction
The cytokine, tumour necrosis factor-related apoptosis-inducing ligand (TRAIL) is a member of the TNF death ligand family and is an attractive anti-neoplastic agent because it induces apoptosis in a wide range of cancerous cells, but not in normal, non-transformed cells [1]. TRAIL can interact with four distinct membrane-bound receptors (DR4/TRAIL-R1, DR5/TRAIL-R2, DcR1/TRAIL-R3 and DcR2/TRAIL-R4), all expressed ubiquitously in the body [2]. DR4 and DR5 contain a conserved cytoplasmic region called the death domain (DD) that is required for TRAIL-induced apoptosis. DcR1 and DcR2 do not have a functional DD and they act as decoy receptors [2]. Despite TRAIL’s significant potential as anti-cancer agent, several factors may limit its application. One of these is the ability to target the ligand to the tumour stroma.
The therapeutic approaches aimed at eradication of tumours through expression of anti-cancer/pro-apoptotic proteins or pro-drug activating enzymes in the tumour stroma have several advantages [3]. The major problem is how to deliver the anti-cancer gene or its product exclusively to the tumour. Adult bone marrow mesenchymal stem cells (bmMSC) can be isolated based on their ability to adhere to plastic and expand ex vivo. bmMSC are multipotent, unlikely to induce allogenic immune rejection and can be transduced with viral vectors [4]. Several studies demonstrated that bmMSC specifically migrate to tumours and metastases. More importantly, bmMSC could survive, proliferate and differentiate in the tumour and form a significant portion of the tumour stroma [5]. It was also shown that bmMSC engineered to secrete interferon β (IFN-β) prolonged survival of mice bearing a human breast tumour xenograft and pulmonary metastases compared to mice treated with recombinant IFN-β[6]. Similarly, intravenous injection of neural stem cells secreting rabbit carboxylesterase, an enzyme capable of activating the anti-cancer pro-drug CPT-11, completely eradicated a metastasing neuroblastoma and provided long-term (over 6 months) survival in 100% of the mice in the study [7]. Another study found that MSCs exert a tumouricidal effect on glioma cells in vitro and in vivo after transplantation into experimental tumours [8].
Mesenchymal stem cells (MSCs) can also be isolated by car-diocentesis from the human first-trimester foetal blood [9]. Similarly to adult bone marrow-derived MSCs, they express pro-lyl-4-hydroxylase (a marker of fibroblasts), CD73, vimentin, CD29, 44 and 106, and fibronectin and they are negative for haematopoietic lineage markers (CD14, 31, 34, 45 and 68) and collagen type 1 (another fibroblastic marker). fMSC have mesodermal (adipogenic, osteogenic and chondrogenic) differentiation potential. Their immunological properties have been found to be similar to bone marrow-derived MSCs [10].
Wharton's jelly (WJ) is the gelatinous connective tissue that constitutes the umbilical cord [11] and is abundant in myofibroblast-like stromal (Wharton's jelly) cells, collagen fibres and proteoglycans. Wharton's jelly cells express several stem cells markers, including c-kit and Oct-4, as well as telomerase. They also have osteogenic, chondrogenic, adipogenic and neurogenic potentials [12].
Foetal mesenchymal stem cells (fMSC) and Wharton's jelly stem cells (umbilical cord matrix stem cells, UCMS) provide additional advantages over bmMSC. Isolation and ex vivo expansion of primary, non-immortalized, adult mesenchymal stem cells is cumbersome that limits their therapeutic utility. UCMS on the other hand are abundant and easy to obtain. Both fMSC and UCMS are easy to expand ex vivo and the cells are able to retain multipoten-cy for at least 40 passages [13, 14].
Despite the potential of stem cells as TRAIL-delivery and targeting vehicle, neither TRAIL receptor expression nor the effect of TRAIL on these cells have been studied previously. Being non-transformed, these cells may be protected against TRAIL-induced apoptosis through high concentration of decoy receptors expressed on the cell surface or lack of expression of DR4 or DR5 receptors. We examined how bmMSC, fMSC and UCMS respond to TRAIL or selective-activation of the death-inducing TRAIL receptors in order to assess the suitability of these cells as TRAIL-delivery vehicles.
Materials and methods
Cell culture
Colo205 cells were maintained in RPMI with 10% foetal bovine serum (FBS), 2 mM glutamine, 1 mM sodium pyruvate, 50 U penicillin, 50 mg/ml streptomycin. Fibroblasts were a kind gift of Dr. Linda Howard (REMEDI, NUI Galway) and were cultured in low glucose-DMEM with 10% FBS, 2 mM glutamine, 50 U penicillin, 50 mg/ml streptomycin.
Isolation and culture of foetal human mesenchymal stem cells
Foetal blood collection was approved by the Research Ethics Committee of Hammersmith and Queen Charlotte’s National Health Service (NHS) Trust (99/5575; 2001/6194; 2001/6234). National guidelines (1988 Polkinghorne Guidelines on Foetal Tissues) were complied with in relation to the use of foetal tissues for research purposes. First trimester foetal blood samples (50–500 μl) were obtained, foetal MSCs were isolated and maintained as described before [9, 15].
Isolation and culture of human UCMS cells
Ten centimetres of umbilical cord was collected from consenting mothers post-partum (ethics code RREC 2758; R&D reference 02TE002). The tissue was dissected and the pieces of Wharton’s jelly were transferred to collagenase I and II (1 mg/ml, Gibco, Grand Island, NY, USA) and incubated for 2 hrs at 37°C before adding Ca2+ and Mg2+-free PBS, centrifuging at 2000 rpm for 10 min. and seeding into an uncoated tissue culture dish in DMEM + 10% FBS. After 2 to 5 days, the non-adherent cells were discarded. Adhered cells were grown to 80% confluence and immunophenotyped before expansion.
Immunostaining of TRAIL receptors
Cell surface expression of the TRAIL receptors on cells was carried out as described before [16].
Annexin V assay
Externalization of phosphatidylserine (PS) to the outer leaflet of the plasma membrane was detected by Annexin-V labelling followed by flow cytometry as described before [17]. All TRAIL receptor antibodies were selective for the target receptors, with maximum 5% cross-reactivity reported by the supplier (Alexis Corporation, Lausen, Switzerland for DR4 and DR5 and R&D Systems, Inc. Minneapolis, MN, USA for DcR1 and DcR2).
Western blotting
Western blotting was carried out as described before [18]. Detection of antigens was done by incubating the membrane for 1 hr at room temperature with antibody to actin (1:500; Sigma-Aldrich, St. Louis, MO, USA) or overnight at 4°C with antibodies to caspases-8, -9, c-FLIP (1:1000; Cell Signaling Technologies, Danvers, MA, USA), Bcl-2 (Santa Cruz Biotechnology Inc., Heidelberg, Germany) followed by 2 hrs incubation at room temperature with appropriate secondary antibodies (1:5000, Pierce Biotechnology, Rockford, IL, USA). Protein bands were visualized with Supersignal Ultra Chemiluminescent Substrate (Pierce) on X-ray film (Agfa, Mortsel Belgium).
Results and discussion
The TRAIL sensitivity of fMSC, UCMS and bmMSC was examined and compared to human primary fibroblasts (hFB), as a model of non-transformed, differentiated cells and Colo205, colon carcinoma cells as a model of cancerous, TRAIL-sensitive cells. All experiments were carried out on at least two different preparations of stem cells and fibroblasts to exclude donor-to-donor variation. As a first determinant of TRAIL sensitivity, cell surface expression of DR4, DR5, DcR1 and DcR2 of fMSC, UCMS cells and bmMSC was measured and compared to hFB and Colo205 cells. fMSC and UCMS cells expressed high levels of all four TRAIL receptors, similar to Colo205 cells. bmMSC expressed only DR5 at a low, but detectable level, which was most similar to hFB that expressed similar, low levels of DR5 and DcR1, but not DR4 or DcR2 (Fig. 1). Despite the presence of at least one of the death-inducing TRAIL receptors on the surface of all stem cell types, treatment with up to 500 ng/ml hrTRAIL for 24 hrs did not cause any significant reduction in cell viability in any of the stem cell types or in hFB measured by MTT assay. On the other hand, 50 times lower concentration (10 ng/ml) of TRAIL was sufficient to cause a 54 ± 3% reduction of cell viability in Colo205 cells (Fig. 2A). The kinase inhibitor, staurosporine (1 μM), was used as a positive control to induce cell death. TRAIL sensitivity of stem cells was also measured after 48 hrs treatment as well as after 24 hrs in the presence of low (0.5%) serum concentration. No reduction in cell viability was detectable in either case, confirming that fMSC, UCMS cells and bmMSC, similarly to hFB, are all resistant to TRAIL (data not shown).
Figure 1.
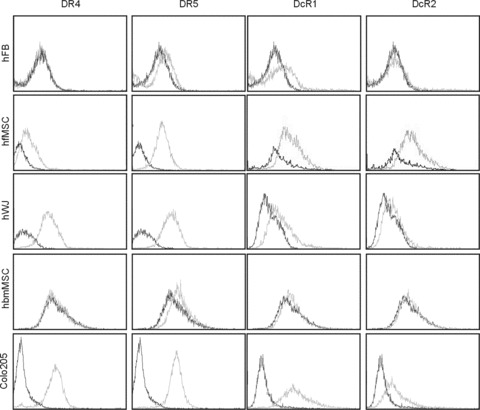
Expression of DR4, DR5, DcR1 and DcR1 on the surface of foetal MSC, WJ cells (UMCS), adult bone marrow MSC, primary fibroblasts and Colo205 colon carcinoma cells. Cells in the log phase of their growth were harvested by trypsinization and labelled with DR4, DR5, DcR1 and DcR2-specific antibodies as described in Materials and Methods. The level of receptor expression was analysed by flow cytometry. The figure shows overlaid histograms of isotype control antibody (black line) and TRAIL-receptor antibody (grey line) labelled samples.
Figure 2.
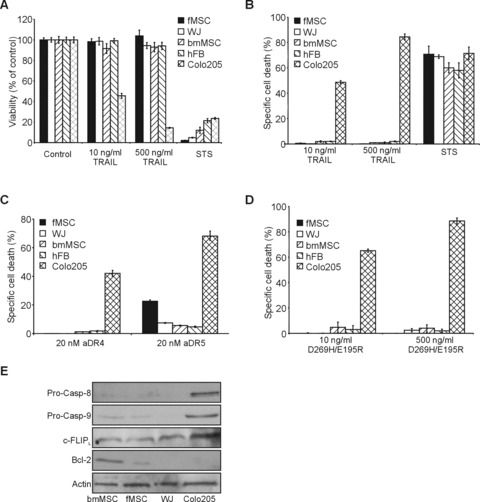
Stem cells are resistant to TRAIL-induced cell death. Foetal MSC, WJ cells (UMCS), adult bone marrow MSC were treated with low (10 ng/ml) or high (500 ng/ml) dose of TRAIL (A and B), 20 nM cross-linked anti-DR4 and anti-DR5 agonistic antibodies (C) or D269H/E195R (D) for 24 hrs. Primary fibroblasts and Colo205 colon carcinoma cells were also treated and used as models of TRAIL resistant non-transformed cells and TRAIL sensitive cancer cells, respectively. (A) Effect of TRAIL on stem cell viability. The graph shows the viability expressed as average percentage of the untreated control ± S.E.M. of three independent experiments (B) Induction of cell death by TRAIL. Average percentage of cell death induced ± S.E.M. of three independent experiments is plotted measured by flow cytometric analysis of Annexin V stained samples. (C) Cell death induced by selective activation of DR4 and DR5 with agonistic antibodies. The graph shows average percentage cell death induced ± S.E.M. of three independent experiments measured by Annexin V staining. (D) Cell death induced by selective activation of DR5 with the DR5-selective TRAIL mutant D269H/E195R. The graph shows average percentage cell death induced ± S.E.M. of three independent experiments measured by Annexin V staining. (E) Expression of intracellular determinants of TRAIL sensitivity. Expression of pro-caspase-8, -9, c-FLIP and Bcl-2 proteins was detected in whole cell lysatets of bmMSC, fMSC and WJ (UMCS) cells by Western blotting. Expression of actin was also determined as a loading control.
Apoptosis induction by TRAIL was also assessed by measuring phosphatidyl serine exposure. TRAIL (10 ng/ml and 500 ng/ml, 24 hrs) failed to induce a significant increase in Annexin V positivity in any of the stem cell populations or fibroblasts, whereas 10 ng/ml TRAIL was sufficient to induce 42 ± 3% Annexin V positivity in Colo205 cells (Fig. 2B).
To determine whether the DR4 and DR5 receptors are active, cells were treated with agonistic DR4- or DR5-specific antibodies or a DR5-selective TRAIL mutant (D269H/E195R [16]) and induction of cell death was measured after 24 hrs with Annexin V staining (Fig. 2C and D). Engagement of the DR4 receptor with cross-linked agonistic DR4 antibody did not induce apoptosis in any of the three stem cell types or in fibroblasts. Activation of the DR5 receptor with cross-linked, agonistic anti-DR5 antibody did not induce more than 4.1 ± 2.2% cell death in bmMSC and UCMS cells, even at a high concentration (20 nM), showing that in these cells the pro-apoptotic activity of DR5 is blocked intracellularly. In fMSC, the cross-linked agonistic anti-DR5 antibody (20 nM) induced a 20.9 ± 2.3% apoptosis after 24 hrs, indicating that in fMSC the DR5-mediated apoptotic pathway is not completely blocked (Fig. 2C). Increasing the antibody concentration to 50 nM did not increase cell death (data not shown). Colo205 cells showed much higher sensitivity to activation of DR4 and DR5 by agonistic antibodies, with 42 ± 2% and 66 ± 5% increase in Annexin V positivity after treatment with anti-DR4 and -DR5, respectively. The resistance of MSC populations to TRAIL probably lies with the very low expression of key apoptosis transducers, including pro-caspase-8 and pro-caspase-9 in combination with considerable expression of the caspase-8 inhibitor, c-FLIP long (c-FLIPL, Fig. 2E). Interestingly, bmMSCs and fMSCs also express high quantities of Bcl-2, which can provide further protection against apoptosis inducers in general, as well as TRAIL (Fig. 2E).
To further assess the sensitivity of fMSC to selective activation of DR5, fMSC and Colo205 cells were treated with 5–20 nM anti-DR5 antibody and cell viability was measured by MTT assay (Fig. S1A). In parallel, Colo205 cells were also treated for comparison. Colo205 cells demonstrated a profoundly higher sensitivity to cross-linked anti-DR5 antibody than fMSC already after 3 hrs exposure. IC50 for Colo205 cells was found to be 4.1 ± 0.2 nM, whereas the IC50 for fMSC was calculated as 39.8 ± 0.7 nM, which is 9.94-fold higher than the IC50 for Colo205. A concentration of 5 nM anti-DR5 antibody induced a 58 ± 6% reduction in Colo205 cell viability, whereas the same concentration caused 10 ± 2% reduction in fMSC viability. In addition to agonistic DR5 antibody, sensitivity to DR5 engagement was also measured using the DR5-selective TRAIL variant, D269H/E195R (Fig. 2D). D269H/E195R did not induce death in any of the stem cell types nor in hFB, although our previous studies showed that it is a more potent inducer of apoptosis than WT TRAIL in Colo205 cells [16].
This study shows that although fMSC and UCMS cells have high surface expression of the death-inducing TRAIL receptors, DR4 and DR5, they are insensitive to apoptosis induced by either by TRAIL or by D269H/E195R. The current data indicate that intracellular processes are responsible for the resistance, rather than decoy receptors expressed on the cell surface, because selective ligation of DR4 or DR5 failed to kill bmMSC and UCMS. Indeed, the ratio of pro-caspase-8 to c-FLIP is very low in all three stem cell types, which can efficiently block DR4/DR5-downstream apoptosis signalling. Pro-caspase-9, the key caspase to amplify the death receptor signal, is also lowly expressed, which together with high Bcl-2 expression in bmMSCs and fMSCs represents another level of apoptosis inhibition.
fMSC displayed some sensitivity to anti-DR5 antibody when used in high concentration, but not by D269H/E195R. This was, however, at least a magnitude lower than the effect of the agonistic DR5 antibody in Colo205 cells. D269H/E195R also binds to DcR2, suggesting that decoy receptors may play a role in limiting DR5-mediated apoptosis in fMSC. Alternatively, the ability of the agonistic antibody and D269H/E195R to cross-link the DR5 receptor is different.
In conclusion, these results show that MSC derived from adult bone marrow, foetal blood or umbilical cord matrix are all resistant to apoptosis induced by human recombinant TRAIL, and the DR5-selective TRAIL variant due to inhibition of intracellular signal transduction, and thus they are suitable tools for TRAIL delivery to tumours.
Acknowledgments
The authors would like to thank Georgina Shaw for technical assistance and Linda Howard (Regenerative Medicine Institute) for the provision of primary human fibroblasts. This work was supported by the European Union, Framework 6 programme (LSHC-CT-2006-037686) and Cancer Research Ireland.
Supporting Information
Figure S1. Colo205 colon cancer cells are a magnitudemore sensitive to DR5-induced cell death than fMSC. (A) Theeffect of DR5 activation by agonistic anti-DR5 antibody in fMSC andColo205 cell viability. Cells were treated with increasingconcentration of cross-linked agonistic anti-DR5 antibody for 24hrs and cell viability was measured by MTT assay. The graph showsaverage viability ± S.E.M. expressed as percentage ofcontrol from three independent experiments. (B) Induction ofapoptosis by anti-DR5 antibody in Colo205 cells. Cells were treatedwith increasing concentration of cross-linked anti-DR5 antibody for3 hrs after which induction of cell death was measured with AnnexinV staining. The graph shows averaged cell death induced ± S.E.M. of three independent experiments.
Please note: Blackwell Publishing are not responsible for the content or functionality of any supporting materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
Supporting info item
References
- 1.Walczak H, Miller RE, Ariail K, et al. Tumoricidal activity of tumor necrosis factor-related apoptosis-inducing ligand in vivo. Nat Med. 1999;5:157–63. doi: 10.1038/5517. [DOI] [PubMed] [Google Scholar]
- 2.Ashkenazi A, Dixit VM. Apoptosis control by death and decoy receptors. Curr Opin Cell Biol. 1999;11:255–60. doi: 10.1016/s0955-0674(99)80034-9. [DOI] [PubMed] [Google Scholar]
- 3.Young LS, Searle PF, Onion D, et al. Viral gene therapy strategies: from basic science to clinical application. J Pathol. 2006;208:299–318. doi: 10.1002/path.1896. [DOI] [PubMed] [Google Scholar]
- 4.Barry FP, Murphy JM. Mesenchymal stem cells: clinical applications and biological characterization. Int J Biochem Cell Biol. 2004;36:568–84. doi: 10.1016/j.biocel.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 5.Studeny M, Marini FC, Champlin RE, et al. Bone marrow-derived mesenchymal stem cells as vehicles for interferon-beta delivery into tumors. Cancer Res. 2002;62:3603–8. [PubMed] [Google Scholar]
- 6.Studeny M, Marini FC, Dembinski JL, et al. Mesenchymal stem cells: potential precursors for tumor stroma and targeted-delivery vehicles for anticancer agents. J Natl Cancer Inst. 2004;96:1593–603. doi: 10.1093/jnci/djh299. [DOI] [PubMed] [Google Scholar]
- 7.Aboody KS, Bush RA, Garcia E, et al. Development of a tumor-selective approach to treat metastatic cancer. PLoS ONE. 2006;1:e23. doi: 10.1371/journal.pone.0000023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nakamura K, Ito Y, Kawano Y, Kurozumi K, et al. Antitumor effect of genetically engineered mesenchymal stem cells in a rat glioma model. Gene Ther. 2004;11:1155–64. doi: 10.1038/sj.gt.3302276. [DOI] [PubMed] [Google Scholar]
- 9.Campagnoli C, Roberts IA, Kumar S, et al. Identification of mesenchymal stem/progenitor cells in human first-trimester fetal blood, liver, and bone marrow. Blood. 2001;98:2396–402. doi: 10.1182/blood.v98.8.2396. [DOI] [PubMed] [Google Scholar]
- 10.Gotherstrom C, Ringden O, Tammik C, et al. Immunologic properties of human fetal mesenchymal stem cells. Am J Obstet Gynecol. 2004;190:239–45. doi: 10.1016/j.ajog.2003.07.022. [DOI] [PubMed] [Google Scholar]
- 11.Speert H. Obstetric-gynecologic eponyms; Thomas Wharton and the jelly of the umbilical cord. Obstet Gynecol. 1956;8:380–2. [PubMed] [Google Scholar]
- 12.Mitchell KE, Weiss ML, Mitchell BM, et al. Matrix cells from Wharton’s jelly form neurons and glia. Stem Cells. 2003;21:50–60. doi: 10.1634/stemcells.21-1-50. [DOI] [PubMed] [Google Scholar]
- 13.O’Donoghue K, Fisk NM. Fetal stem cells. Best Pract Res Clin Obstet Gynaecol. 2004;18:853–75. doi: 10.1016/j.bpobgyn.2004.06.010. [DOI] [PubMed] [Google Scholar]
- 14.Wang HS, Hung SC, Peng ST, et al. Mesenchymal stem cells in the Wharton’s jelly of the human umbilical cord. Stem Cells. 2004;22:1330–7. doi: 10.1634/stemcells.2004-0013. [DOI] [PubMed] [Google Scholar]
- 15.Kennea NL, Stratou C, Naparus A, et al. Functional intrinsic and extrinsic apoptotic pathways in human fetal mesenchymal stem cells. Cell Death Differ. 2005;12:1439–41. doi: 10.1038/sj.cdd.4401641. [DOI] [PubMed] [Google Scholar]
- 16.Van Der Sloot AM, Tur V, Szegezdi E, et al. Designed tumor necrosis factor-related apoptosis-inducing ligand variants initiating apoptosis exclusively via the DR5 receptor. Proc Natl Acad Sci USA. 2006;103:8634–9. doi: 10.1073/pnas.0510187103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Holohan C, Szegezdi E, Ritter T, et al. Cytokine-induced beta-cell apoptosis is NO-dependent, mitochondria-mediated and inhibited by BCL-X(L) J Cell Mol Med. 2008;12:591–606. doi: 10.1111/j.1582-4934.2007.00191.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Szegezdi E, Herbert KR, Kavanagh ET, et al. Nerve growth factor blocks thapsigargin-induced apoptosis at the level of the mitochondrion via regulation of Bim. J Cell Mol Med. 2008;12:2482–96. doi: 10.1111/j.1582-4934.2008.00268.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Colo205 colon cancer cells are a magnitudemore sensitive to DR5-induced cell death than fMSC. (A) Theeffect of DR5 activation by agonistic anti-DR5 antibody in fMSC andColo205 cell viability. Cells were treated with increasingconcentration of cross-linked agonistic anti-DR5 antibody for 24hrs and cell viability was measured by MTT assay. The graph showsaverage viability ± S.E.M. expressed as percentage ofcontrol from three independent experiments. (B) Induction ofapoptosis by anti-DR5 antibody in Colo205 cells. Cells were treatedwith increasing concentration of cross-linked anti-DR5 antibody for3 hrs after which induction of cell death was measured with AnnexinV staining. The graph shows averaged cell death induced ± S.E.M. of three independent experiments.
Please note: Blackwell Publishing are not responsible for the content or functionality of any supporting materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
Supporting info item


