Abstract
Background: Incisional hernia is a common and important complication of laparotomies. Epidemiological studies allude to an underlying biological cause, at least in a subset of population. Interest has mainly focused on abnormal collagen metabolism. However, the role played by other determinants of extracellular matrix (ECM) composition is unknown. To date, there are few laboratory studies investigating the importance of biological factors contributing to incisional hernia development. We performed a descriptive tissue-based analysis to elucidate the possible relevance of matrix metalloproteinases (MMPs) and their tissue inhibitors (TIMPs) in association with local cytokine induction in human incisional hernia tissues. The expression profiles of MMPs, TIMPs and pro-inflammatory cytokine signalling were investigated in aponeurosis and skeletal muscle specimens taken intraoperatively from incisional hernia (n= 10) and control (n= 10) patients. Semiquantitative RT-PCR, zymography and immunoblotting analyses were done. Incisional hernia samples displayed alterations in the microstructure and loss of ECM, as assessed by histological analyses. Moreover, incisional hernia tissues showed increased MMP/TIMP ratios and de-regulated inflammatory signalling (tumor necrosis factor [TNFA] and interleukin [IL]-6 tended to increase, whereas aponeurosis TNFA receptors decreased). The changes were tissue-specific and were detectable at the mRNA and/or protein level. Statistical analyses showed several associations between individual MMPs, TIMPs, interstitial collagens and inflammatory markers. The increment of MMPs in the absence of a counterbalance by TIMPs, together with an ongoing de-regulated inflammatory signalling, may contribute in inducing a functional defect of the ECM network by post-translational mechanisms, which may trigger abdominal wall tissue loss and eventual rupture. The notable TIMP3 protein down-regulation in incisional hernia fascia may be of pathophysiological significance. We conclude that this study may help to pinpoint novel hypotheses of pathogenesis that can lead to a better understanding of the disease and ultimately to improvement in current therapeutic approaches.
Keywords: incisional hernia, extracellular matrix, aponeurosis, skeletal muscle, MMP/TIMP, TNFA, TNFRSFs, IL-6
Introduction
Incisional hernia is the most common complication of laparotomies, with a cumulative incidence of about 20%[1–3]. It occurs when the abdominal wall heals incompletely and impedes the timely re-establishment of an efficient load-bearing scar at the myofascial layer. Patient- and technical-related risk factors have been identified [4]. However, the molecular mechanisms that control its formation remain ambiguous. The late appearance after laparotomy and the recurrence rate curves observed after operation strongly suggest a biological underlying dysfunction, at least in a subset of patients [5, 6]. Interest has mainly focused on abnormal collagen metabolism, and a number of studies have investigated changes in type I to type III collagen ratios [7, 8] and alterations in individual matrix metalloprotenaises (MMPs) [7–9] and collagen-interacting proteins [10]. Nevertheless, as the majority of hernias occur in patients that have no history of a wound-healing defect (making them safe surgical candidates) and also do not express a defect at the primary surgical site [6], other triggers are feasible. Some studies provide experimental evidence relating mechanical failure and incisional hernia development (reviewed in reference 7), possibly as a consequence of altered mechanotransduction and repair fibroblast biology [7, 11]. Other mechanisms have so far received little attention. It is possible that new descriptive tissue-based research may help to establish novel hypotheses of pathogenesis. For instance, the relevance of local factors intrinsic to the target tissue, like an ongoing induction of pro-inflammatory mediators or persistent extracellular matrix (ECM) turnover, is still unknown.
The balance between MMPs and tissue inhibitors of metalloproteinase (TIMPs) is a critical determinant of ECM integrity and function. Alterations in MMPs/TIMPs ratios are implicated in multiple diseases [12]. De-regulated specific MMPs expression has been previously reported in incisional hernia [7, 9]. Because studies have usually focused on a single MMP (MMP1, MMP13 [7] and MMP2 [9]) and there are no reports concerning TIMPs measurement, there is a need to perform a more comprehensive survey of MMPs and TIMPs to study them in a more integrated fashion, because it is the concerted action of the MMPs and TIMPs that determines their biological actions.
Also, cytokines are important regulators of MMPs/TIMPs systems. In the setting of surgical stress, cytokines and other factors are released with consequent autocrine/paracrine actions [13, 14]. Initially decisive for the host recovery, the persistence of de-regulated inflammatory signalling may be detrimental. ECM is critical in fine-tuning the spatial and temporal expression patterns of cytokines at sites of inflammation and healing [15, 16].
Finally, incisional hernia affects both the skeletal muscle and the fascial layers. To date, the fascia has been focused on in most of the investigations [17], but it is probable that insights into disease pathogenesis may be gained by simultaneously analysing both tissues. Skeletal muscle is a load-bearing structure that confers abdominal wall compliance. It is highly responsive to changes in functional demands, but it has been scarcely studied in relation to incisional hernia in human cases. Because the skeletal muscle is especially sensitive to pro-inflammatory stimuli, responding with tissue loss and failure [18], we hypothesized that it may effectively contribute to incisional hernia formation if persistently challenged by a continuous exposition to increased pro-inflammatory gene products, thus being a potential therapeutic target.
The aim of this study was basically exploratory to generate new biologically plausible hypotheses into how, in a pathophysiological sense, incisional hernia formation and local tissue microenvironment can be linked. In particular, we wanted to observe whether in incisional hernia patients: (1) there is a local tissue-specific pattern of changes in the ECM proteolytic system characterized by ongoing increased MMPs/TIMPs ratios, that is, a profile known to impair ECM turnover and favour ECM degradation; and (2) the abdominal myofascial tissues (fascia and skeletal muscle) participate in the overproduction of cytokines and/or in the de-regulation of the inflammatory response.
Patients and methods
Tissue sources
Samples included in this study were selected from our incisional hernia tissue bank. The tissue bank stores fascia and skeletal muscle biopsies obtained from the abdominal wall tissues of incisional hernia patients and non-incisional hernia controls and data associated with them, such as demographic features, co-morbidities, etc. Specimens are taken intraoperatively at the Department of Surgery of the Hospital Universitari Vall d'Hebron, by a specialized surgeon (incisional hernia samples are collected at repair, at >8 cm of the edge of the defect). The areas of visible necrosis or granulation tissue are intentionally avoided. The biopsies are immediately divided into multiple pieces and fixed in 10% formalin for histopathological analyses or snap-frozen in liquid nitrogen until analysis. Only in relation to specific research projects are DNA, RNA and proteins isolated from the solid tissue for downstream applications.
The patient characteristics are described in Table 1. Incisional hernias were diagnosed in the outpatient clinic by physical examination. Previous operations leading to primary incisional hernia formation included resection of colon or rectal tumour (n= 8) and cholecystectomy (n= 2). To our knowledge, the patients did not experience immediate major post-operative complications. The interval between prior laparotomy and repair was 69 ± 32 months (range 13–252 months; 13 months, n= 4; 14 months, n= 3; 96 months, n= 1; 252 months, n= 2). All the hernias were in the midline. Hernia repair was performed with an open onlay technique. The size of the defect is not available. Reference tissue samples were obtained from voluntary donors undergoing elective digestive abdominal surgery. Patients with previous wound infections or those with diabetes or connective or systemic inflammatory diseases were not included in this study. The research protocol was reviewed and approved by the institutional ethics committee. Written informed consent was obtained from all participants.
Table 1.
Patient characteristics
| Variable | Incisional hernia | Non-incisional hernia controls | P-value* |
|---|---|---|---|
| (n= 10) | (n= 10) | ||
| Age ± SEM (range) | 61 ± 4 (42–72) | 55 ± 3 (43–69) | 0.19 |
| Male sex | 7 | 7 | 1.00 |
| Smokers | 3 | 2 | 1.00 |
| Alcoholics | 0 | 2 | 0.47 |
| Obesity (body mass index >29) | 2 | 5 | 0.35 |
| Diabetes | 0 | 0 | 1.00 |
| Hyperlipidaemia | 1 | 1 | 1.00 |
Student’s t-test or Fisher’s exact test
Histology and immunohistochemistry
Biopsies for histology and immunohistochemistry were immediately fixed in 10% neutral buffered formaldehyde and embedded in paraffin. Consecutive sections (4-μm-thick) were cut and stained with haematoxylin and eosin to visualize the morphology, Masson’s trichrome to determine collagenous components and Alcian blue (pH 2.5) to estimate glycosaminoglycans (GAGs) abundance, as performed in routine clinical practice. For immunohistochemistry, paraffin-embedded sections were deparaffinized and rehydrated with xylene and a graded alcohol series. Endogenous peroxidases were blocked by incubation with 2% H2O2 for 30 min. Antigen retrieval was performed in 0.01 mol/l sodium citrate buffer (pH 6.0) with an electric pressure cooker. The tissue sections were blocked in serum-blocking solution and staining was performed with anti-α-SMA (Clone 1A4, 1:100; Dako M0851, Dako Diagnósticos, Barcelona, Spain) as primary antibody [19]. Detection was completed with the avidin–biotin complex peroxidase method (ABC Elite kit; Vector, Burlingame, CA, USA) followed by 3,3′-diaminobenzidine tetrachloride staining (DAB; Sigma, Deisenhofen, Germany) and haematoxylin counterstaining. Negative controls consisted in the omission of the primary antibody.
An experienced soft-tissue pathologist from the Vall d’Hebron University Hospital Department of Pathology (P.H.), who was blinded to the clinical diagnosis, examined the biopsy specimens. The samples were characterized in detail based on haematoxylin and eosin- and Masson’s trichrome-stained slides. The staining intensities of Masson’s trichrome and Alcian blue stains preparations were assessed visually and graded semiquantitatively. A minimum of three sections (10 randomly chosen, non-contiguous and no overlapping high-power fields each) were examined per patient.
RNA extraction and RT-PCR
Total RNA was extracted with TRIzol reagent, using a single-step method (Invitrogen, Barcelona, Spain). The RNA quality and quantity were assessed with a 2100 Bioanalyzer (Agilent, Madrid, Spain). RNA was reverse transcribed with the use of Superscript II reverse transcriptase (Invitrogen) and random primers. The PCR protocols were performed with cDNA products, human-specific primers, and 1 U of TaqDNA polymerase (Invitrogen), as depicted in Table 2. The Multiplex PCR Kit for Human MMP Genes Set-1 (Maxim Biotech, Inc., Madrid, Spain) was used to amplify MMP1, MMP3, MMP7, MMP13 and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) cDNAs. The PCR products were separated on 2% agarose gels, visualized with ethidium bromide and quantified by densitometry (Quantity One Quantitation Software™; Bio-Rad, Barcelona, Spain). The results were expressed as a ratio between signals corresponding to the gene of interest and ribosomal protein L19 (RPL19; or GAPDH for Multiplex kit genes). All samples received pooled reagents and comparisons were made only with simultaneously performed experiments. Negative controls were done with omission of retrotranscription.
Table 2.
Sequences of PCR primers
| Group | Gene | Primer sequence forward 5′–3′ (exon) | Primer sequence reverse 5′–′3′ (exon) | Product size | Acc. number |
|---|---|---|---|---|---|
| Collagens | COL1A1 | GTCTTCCTGGCCCCTCTGGTG (40) | TCGCCCTGTTCGCCTGTCTCA (45) | 390 | NM_000088 |
| COL3A1 | CAGGGGCCCCAGGACTTAGAG (30) | GGGCCAGGAGGACCAATAGGT (33) | 249 | NM_000090 | |
| COL4A1 | CAATGCCCTTCCTGTTCTGC (51) | GTGGACGGCGTAGGCTTCTT (53∼54) | 451 | NM_001845 | |
| MMPs | MMP2 | ACATCAAGGGCATTCAGGAG (8) | TGAACCGGTCCTTGAAGAAG (9∼10) | 168 | NM_004530 |
| MMP9 | GGGAAGATGCTGCTGTTCA (11) | TCAACTCACTCCGGGAACTC (13) | 202 | NM_004994 | |
| MMP14 | GCAGAAGTTTTACGGCTTGC (2) | CTTGGGGGTGTAATTCTGGA (4) | 199 | NM_004995 | |
| TACE/ADAM-17 | TCATTGACCAGCTGAGCATC (17) | CGCAGGAAAGGGTTTGATAA (20) | 233 | NM_003183 | |
| TIMPs | TIMP1 | CTGCGGATACTTCCACAGGT (3) | GTTTGCAGGGGATGGATAAA (5) | 209 | NM_003254 |
| TIMP2 | GATGCACATCACCCTCTGTG (4) | GTGCCCGTTCATGTTCTTCT (5) | 196 | NM_003255 | |
| TIMP3 | CTGACAGGTCGCGTCTATGA (3∼4) | AGTCACAAAGCAAGGCAGGT (5) | 165 | NM_000362 | |
| TIMP4 | CTTGGTGCAGAGGGAAAGTC (4) | GGCTGAACGATGTTCAACAAA (5) | 249 | NM_003256 | |
| Cytokines and receptors | TNFA | CAGAGGGCCTGTACCTCATC (2) | GGAAGACCCCTCCCAGATAG (4) | 219 | NM_000594 |
| TNFSFR1A | GTGCCTACCCCAGATTGAGA (7) | TGTCGATTTCCCACAAACAA (8∼9) | 175 | NM_001065 | |
| TNFSFR1B | GGAAACTCAAGCCTGCACTC (4) | TGCAAATATCCGTGGATGAA (5) | 224 | NM_001066 | |
| IL-6 | CCACACAGACAGCCACTCAC (2) | TTTCACCAGGCAAGTCTCCT (3) | 217 | NM_000600 | |
| Ribosomal | RPL19 | CAATGCCAACTCCCGTCAGC (1∼2) | CTTGGTCTCTTCCTCCTTGG (6) | 487 | NM_000981 |
Primers were generated with the Primer 3 software (http://frodo.wi.mit.edu/cgi-bin/primer3/primer3_www.cgi) and were mRNA/cDNA-specific. Preliminary experiments were conducted to ensure that the number of cycles represented a linear portion for the PCR optical density (OD) curve. The specificity of each primer pair was verified by sequencing the amplified products. PCR cycles were as follows: 25 (TIMP2 aponeuroses), 27 (MMP2 and TIMP3), 28 (COL1A1 aponeuroses), 29 (RPL19), 30 (TIMP1 and COL4A1; and TIMP2 and TIMP3 muscles), 31 (TNFSFR1B and COL3A1; and COL1A1 muscles), 32 (MMP2 muscles), 35 (MMP14, TIMP4, TNFA and TNFSFR1A; and IL-6 aponeuroses), 36 (IL-6 muscles), 37 (MMP9 aponeuroses) or 40 (MMP9 muscles).
COL1A1, collagen, type I, alpha-1;
COL3A1, collagen, type III, alpha-1;
COL4A1, collagen, type IV, alpha 1.
Tissue extraction and immunoblotting
The muscle tissues were homogenized in Tissue-PE LB™ lysis buffer (GenoTechnology, Inc., Barcelona, Spain) containing protease inhibitors (Set III, 1:100; Calbiochem, Barcelona, Spain) and centrifuged (12,000×g, 20 min.). The supernatants were collected, and the pellets were further dissolved in RIPA buffer (50mM Tris-HCl pH 7.6, 150 mM NaCl, 1 mM ethylenediaminetetraacetic acid [EDTA], 0.25% sodium deoxycolate, 1% Triton X-100, 2% SDS, plus protease inhibitors), centrifuged and the supernatants collected. Aponeuroses were homogenized in RIPA buffer and centrifuged (12,000×g, 20 min.). The supernatants were collected and concentrated with Ultrafree®-MC devices (Millipore, Madrid, Spain). Protein levels were quantified (BCA Protein Assay; Pierce, Madrid, Spain). Equal amounts of total protein were size-fractioned in 7.5, 10, 12 or 15% SDS-PAGE under reducing conditions (20 mA/gel; RT), transferred overnight to nitrocellulose membranes (60 V, 4°C), and stained with red Ponceau. The membranes were blocked in TBST buffer (0.9% NaCl, 0.02 M Tris, pH 7.5, 0.05% Tween 20) and 10% skimmed milk (1 hr, RT) before incubation with the respective anti-human primary antibodies: Clone I-8H5 (1:40, 1:20) for collagen I, Clone III-53 (1:100) for collagen III, Clone 41–1E5 (1:50) for MMP1 and Clone 136–13H4 (1:20) for TIMP3, from Calbiochem; tumor necrosis factor-alpha converting enzyme (TACE)/ADAMT17 (catalogue no. AB19027; 1:1000) and Clone loop no. 3pAb (1:200) for TIMP4, from Chemicon Int. (Madrid, Spain); Clone T3 (1:10) for TNFA, Clone H398 (1:10) for tumor necrosis factor receptor superfamily, member 1A (TNFRSF1A) and Clone 80M2 (1:10) for tumor necrosis factor receptor superfamily, member 1B (TNFRSF1B), from HyCult Biotech (Madrid, Spain); and Clone pAb 1:666 for interleukin (IL)-6, from R&D Systems (Madrid, Spain). Detection was performed with appropriate peroxidase-labelled secondary antibodies. An enhanced chemiluminescence kit (Amersham, Barcelona, Spain) was used to detect immunoreactive bands.
Zymography and reverse zymography analyses
Equal protein quantities diluted in denaturing non-reducing Laemmli buffer were resolved on SDS-PAGE gels co-polymerized with gelatin (7.5%, 1.5 mg/ml), casein (10%, 1.5 mg/ml) or carboxymethylated transferrin (10%, 1 mg/ml), as described previously [19]. The gels were washed, incubated (37°C, 22 hrs) in substrate buffer and stained. TIMPs activities were analysed by reverse zymography [20] under non-reducing conditions. For reverse zymography, equal protein quantities were loaded on 12% SDS-PAGE gels impregnated with both 2.5 mg/ml gelatin and gelatinase A (1.2 μg/10 ml of gel; Oncogene, Barcelona, Spain). The gels were run, washed and incubated likewise gelatin zymography, excepting incubation time (37°C, 15 hrs). Proteolytic activities were quantified by densitometry scanning (Quantity One Quantitation Software™).
Statistical analysis
Data were quantified as means ± standard error (SE). Differences between group means were analysed using the two-way unpaired Student’s t-test or the Mann–Whitney U-test, after checking the homogeneity of variances. Categorical data were analysed by Fisher’s exact test. Multi-variate data analyses (principal component analysis [PCA] and multiple regression analysis) were performed with data obtained from RT-PCR to unravel potential relationships between transcripts. We extracted principle components (PCs) separately from aponeurosis and muscle. PCA is a simple, non-parametric method, which was used as an exploratory tool to reduce the dimensionality of the data set [21]. Backward stepwise multiple regression analysis (with entry criteria of P < 0.10, adjusted by group) was performed to determine possible significant associations between individual genes. A P-value of less than 0.05 was considered significant. A P-value between 0.05 and 0.10 was considered to indicate a trend towards an association.
Results
Table 1 shows patient characteristics at biopsy collection. Of interest, age, sex and co-morbidities were not statistically different between the control and the incisional hernia groups. All patients in this study had developed a primary incisional hernia after a previous operation. Thus, samples were collected when the patients were treated for their first incisional hernia repair when meshes were not present.
Histological analysis
The fascial layer consisted of a small number of fibroblasts, vessels and a large ECM. Compared with controls (Fig. 1A), incisional hernia fascias (Fig. 1B) showed histoarchitecture damage and loss of ECM, together with a tendency towards more rounded fibroblasts and disruption of the cell–matrix contacts. Incisional hernia skeletal muscle was characterized by a higher deposition of connective tissue in the endomysium, together with a higher percentage of fibres with centrally located nuclei (Fig. 1D). No signs of infection were seen. Histological evidence of inflammatory infiltration or granulation tissue was negligible in all samples studied. Interestingly, Alcian blue staining (pH 2.5) was increased in hernia samples (data not shown).
Figure 1.
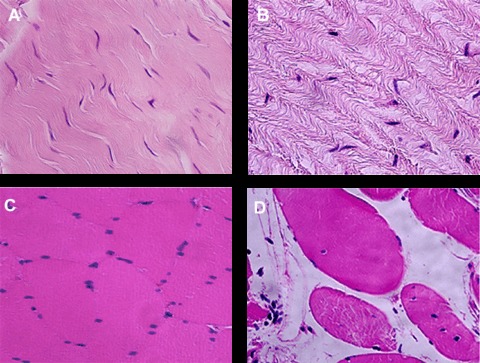
Representative haematoxylin and eosinstained sections. As compared with controls (A), incisional hernia aponeuroses (B) are characterized by a loss of ECM organization, tissue wasting and more rounded fibroblasts. Incisional hernia muscles (D) display higher endomysial connective tissue deposition and more fibres with centrally located nuclei than control muscles (C). Original magnification, ×400.
ECM synthesis and degradation
Some degree of up-regulated interstitial collagen mRNA expression was observed in incisional hernia samples, although, at the statistical level, only a trend towards increased skeletal muscle collagen, type III, alpha-1 (COL3A1) transcripts was detected (P= 0.093; Fig. 2). Discordant levels were observed at the protein level, which may be ascribable to partially inefficient protein extraction and solubilization.
Figure 2.
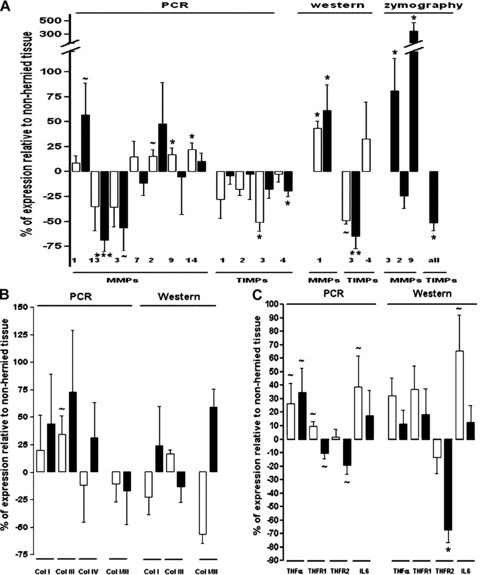
Representative RT-PCR, Western blot and zymography analyses obtained in incisional hernia and control tissues. (A) MMPs and TIMPs, (B) collagens (types I, III and IV) and type I to type III collagen ratios and (C) inflammatory mediators (TNFA, TNFRSF1A, TNFRSF1B and IL-6). Results are displayed graphically using percentages of change of each molecule (incisional hernia versus non-incisional hernia controls) to facilitate the interpretation of the results. Only detectable results are represented. ∼P value 0.05– 0.10, *P value 0.05–0.01, **P value 0.01–0.001, ***P value < 0.001. ▪, aponeurosis; □, skeletal muscle.
Conversely, changes in MMPs and TIMPs expression were identified in incisional hernia tissues both at the transcriptional and at the post-transcriptional levels (Figs. 2 and 3). These changes resulted in higher MMP/TIMP ratios (Fig. 4), which is consistent with a net MMP system activation. Specifically, incisional hernia specimens displayed increased RNA transcripts for MMP1 (P= 0.055) in aponeurosis, and for MMP2 (P= 0.074), MMP9 (P= 0.045) and MMP14 (P= 0.016) in skeletal muscle, and reduced RNA transcripts for MMP13 (P < 0.001), MMP3 (P= 0.060) and TIMP4 (P= 0.036) in aponeuroses, and for TIMP3 (P= 0.012) in skeletal muscle. Significant gene expression results were further corroborated by enzyme assays and immunoblotting to determine activities and amounts of MMPs and TIMPs. So, active MMP1 (43 kD) was overexpressed (muscle P= 0.014, aponeurosis P= 0.031), and TIMP3 (24 kD) was underexpressed (muscle P= 0.080, aponeurosis P= 0.008) in incisional hernia samples, as assessed by immunoblotting (Figs. 2 and 3). Zymography and reverse zymography (Figs. 2 and 3) revealed a strong induction of MMP9 (92 kD and 86 kD, P= 0.026) and MMP3 (P= 0.040) activities, together with largely decreased TIMPs activities in incisional hernia aponeuroses (P= 0.015). Under the conditions used (and probably due to the fact that muscle ECM protein is minimal as compared with total protein), MMPs and TIMPs activities were undetectable in skeletal muscle specimens.
Figure 3.
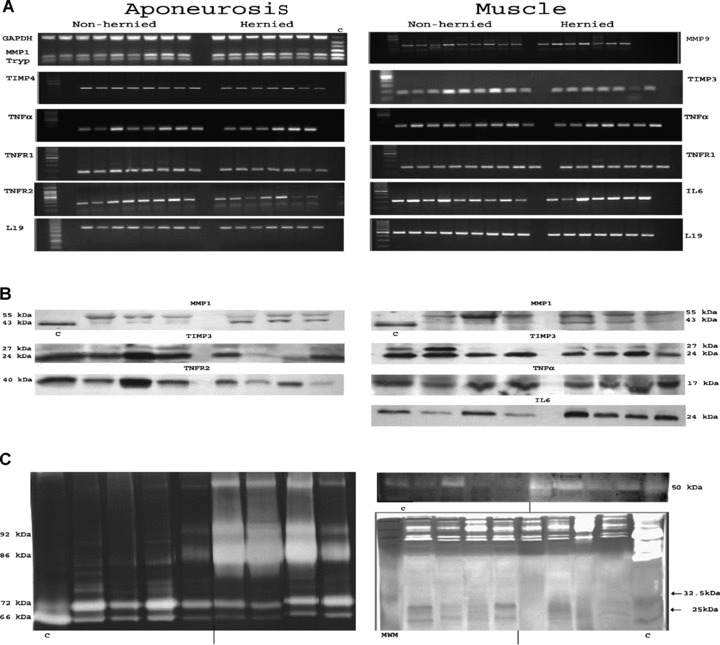
(A) Representative RT-PCR products obtained in incisional hernia and control tissues. Only statistically significant transcripts are displayed (aponeurosis: MMP1, TIMP4, TNFA, TNFRSF1A, TNFRSF1B and RPL19; skeletal muscle: MMP9, TIMP3, TNFA, TNFRSF1A, IL-6, RPL19), c = Multiplex® PCR control, provided by the manufacturer. (B) Representative immunoblotting analyses obtained in incisional hernia and control specimens. Only statistically significant blots are presented (aponeurosis: MMP1, TIMP3 and soluble TNFRSF1B; skeletal muscle: MMP1, TIMP3, TNFA and IL-6), c = MMP1 control provided by Sigma. (C) Representative gelatinolytic (left), caseinolytic activity (right, up) and reversed zymography (right, down) in aponeurosis tissues. A concentrate of serum-free-conditioned medium (SFCM) from human skin fibroblasts treated with TPA (Sigma-Aldrich, Barcelona, Spain) was added to each gel to serve as a positive control. Only results obtained in aponeurosis are presented.
Figure 4.
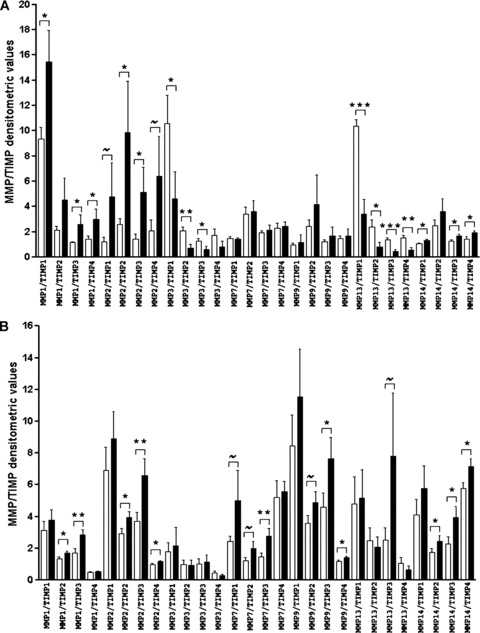
MMP/TIMP mRNA ratios obtained in incisional hernia and control tissues. (A) aponeurosis and (B) skeletal muscle. ∼P value 0.05–0.10, *P value 0.05–0.01, **P value 0.01–0.001, ***P value < 0.001 (incisional hernia versus control values). ▪, incisional hernia; □, non-incisional hernia.
Pro-inflammatory signalling
As shown in Figs. 2 and 3, TNFA and IL-6 transcripts tended to increase in incisional hernia (TNFA: aponeurosis P= 0.064, skeletal muscle P= 0.077; IL-6: skeletal muscle P= 0.097). TNFA receptors mRNA expression tended to decrease in incisional hernia aponeuroses (TNFRSF1A P= 0.071, TNFRSF1B P= 0.063), whereas TNFRSF1A tended to increase in skeletal muscle (P= 0.089). At the protein level, IL-6 (P= 0.056) and TNFA (P= 0.099) tended to be up-regulated in incisional hernia skeletal muscle. A striking down-regulation of the 40 kD highly soluble TNFRSF1B protein was observed in incisional hernia aponeuroses (P= 0.022). Noteworthy, only the soluble 17 kD TNFA protein (sTNFA) was detected in muscle, whereas, in the aponeurosis, exclusively low levels of 26 kD mTNFA protein were obtained. Finally, IL-6 protein was highly embedded in the ECM, and it was only rescued by strong extraction procedures.
Statistical relationships between transcripts
Complex networks of reciprocal interactions are established between MMPs, TIMPs, cytokines and growth factors, which regulate a wide variety of inflammatory and tissue remodelling mediators [22]. As it is difficult to compare the expression pattern of the 17 molecules simultaneously, PCA was performed. PCA is a powerful, exploratory non-parametric tool that has found application in several fields to extract relevant information from multi-dimensional data sets, that is, it filters out the noise and reveals hidden structure, allowing the selection of the most useful variables [21, 23]. By observing the correlations between the indices and the original variables (factor loadings), associations between the original variables can be identified and summarized. Factor loadings revealed the genes that provided the largest positive and negative contribution to incisional hernia (loading > ±0.6). Each correlation or loading indicates how strongly the original variable ‘loads’ on it. As depicted in Table 3, the PCA included in this report identifies interstitial collagens, TIMPs, MMPs (MMP1, MMP9 and MMP14) and TNF receptors as closely associated to the incisional hernia phenotype in a tissue-specific manner. Finally, several predictive models were constructed using different combinations of variables by backward multiple regression analysis. The most effective ones are described in Table 4 and yield interesting insights into the co-regulation of gene sets within the TNF and MMP marker families.
Table 3.
Loading factors
| Non-incisional hernia | Incisional hernia | ||||||
|---|---|---|---|---|---|---|---|
| Aponeurosis | Aponeurosis | ||||||
| PC1 | PC2 | PC1 | PC2 | ||||
| MMP14 | 0.973 | TNFRSF1B | 0.966 | COL3A1 | −0.842 | COL1A1 | 0.880 |
| MMP1 | 0.934 | TIMP4 | 0.933 | TNFRSF1A | 0.835 | TIMP2 | 0.867 |
| TNF | 0.933 | MMP1 | −0.814 | MMP9 | −0.827 | ||
| TIMP3 | 0.926 | TIMP4 | 0.797 | TIMP1 | 0.754 | ||
| MMP7 | 0.920 | TIMP3 | 0.730 | MMP14 | 0.740 | ||
| MMP13 | 0.920 | MMP2 | 0.677 | ||||
| COL1A1 | 0.898 | ||||||
| TIMP1 | 0.889 | ||||||
| TIMP2 | 0.848 | ||||||
| MMP3 | 0.829 | ||||||
| COL3A1 | 0.796 | ||||||
| MMP2 | 0.764 | ||||||
| MMP9 | 0.691 | ||||||
| TNFRSF1B | 0.608 | ||||||
| Skeletal muscle | Skeletal muscle | ||||||
|---|---|---|---|---|---|---|---|
| PC1 | PC2 | PC1 | PC2 | ||||
| MMP3 | 0.900 | TIMP4 | 0.740 | MMP9 | −0.978 | TNF | 0.979 |
| TIMP2 | 0.896 | MMP2 | 0.666 | TIMP2 | 0.921 | COL1A1 | 0.871 |
| MMP7 | 0.893 | TIMP3 | 0.901 | COL3A1 | 0.846 | ||
| MMP14 | −0.886 | MMP14 | −0.871 | TIMP4 | 0.786 | ||
| TIMP1 | 0.869 | TNFRSF1B | 0.855 | MMP1 | 0.635 | ||
| MMP9 | −0.832 | ||||||
| TNFRSF1A | 0.830 | ||||||
| COL1A1 | 0.790 | ||||||
| TNFRSF1B | 0.706 | ||||||
| MMP13 | 0.678 | ||||||
| MMP1 | 0.666 | ||||||
| TIMP3 | 0.665 | ||||||
Representative loading factors (≥0.6) of the first two principal components (PCs) for non-incisional hernia and incisional hernia in aponeurosis and skeletal muscle.
Table 4.
Backward multiple regression analysis
| Aponeurosis | Skeletal muscle | ||||||
|---|---|---|---|---|---|---|---|
| TNF | B | P | % change R2 | TNFRSF1A | B | P | % change R2 |
| Intercept | 38.67 | 0.007 | Intercept | 192.46 | 0.000 | ||
| MMP9 | 0.21 | 0.002 | 38 | MMP9 | −0.93 | 0.001 | 31 |
| MMP1 | 0.19 | 0.018 | 17 | MMP7 | 7.06 | 0.011 | 14 |
| MMP3 | 2.27 | 0.026 | 15 | MMP2 | 0.51 | 0.015 | 12 |
| MMP13 | −4.82 | 0.074 | 8 | MMP3 | −46.98 | 0.046 | 8 |
| Group | 2.79 × G | 0.810 | 0 | Group | −12.30 × G | 0.089 | 5 |
| Adjusted R2= 0.78; P= 0.003 | Adjusted R2= 0.78; P= 0.003 | ||||||
| TNFRSF1A | |||||||
| Intercept | 54.42 | 0.007 | |||||
| MMP14 | 0.57 | 0.010 | 19 | ||||
| MMP1 | −0.12 | 0.080 | 7 | ||||
| MMP3 | −1.07 | 0.078 | 7 | ||||
| Group | 15.94 × G | 0.040 | 10 | ||||
| Adjusted R2= 0.43; P= 0.046 | |||||||
| TNFRSF1B | |||||||
| Intercept | 34.07 | 0.000 | |||||
| MMP13 | 3.44 | 0.028 | 26 | ||||
| MMP9 | 0.12 | 0.031 | 24 | ||||
| Group | −8.13 × G | 0.421 | 3 | ||||
| Adjusted R2= 0.43; P= 0.046 | |||||||
Stepwise backward multiple regression was performed with an F-ratio probability of 0.10 as the criterion for removal or inclusion in the model. G = 0, incisional hernia (IH) group; G = 1, non-IH group.
Dependent variables: TNF, TNFRSF1A, TNFRSF1B (aponeurosis) and TNFRSF1A (skeletal muscle).
G, group; B, estimate of the change in the dependent variable attributable to a change of one unit in the independent variable; P, significance; R2, R-squared.
Discussion
We performed a tissue-based, exploratory molecular survey to gain a better understanding of the role of local tissue microenvironment in patients with a clinical history of incisional hernia. Our major novel findings were that in incisional hernia: (1) the constitutive baseline MMPs/TIMPs quotients are significantly higher, (2) there is a trend towards de-regulated pro-inflammatory cytokine content and signalling and (3) both the aponeurosis and the skeletal muscle are affected and exhibit multiple tissue-specific alterations. Because biopsies were obtained avoiding the hernia ring, the results point to the presence of subclinical events reflected systemically. Also, this study seems to be one of the first to explore human skeletal muscle samples in this disease.
Limited information is available on MMPs and TIMPs expression within human abdominal wall tissues. Here, we showed that, in hernia, the MMPs/TIMPs balance was altered in favour of net proteolytic activity, which may alter the ECM network by post-translational mechanisms and contribute to incisional hernia development. The apparent failure to replace connective tissue with newly synthesized material could be ascribed to inefficient collagen synthesis [24, 25]. Also, albeit not mechanistically investigated here, the lack of collagen deposition could be due to improper extracellular post-translational collagen maturation secondary to cytokine action [26].
Among the MMPs analysed, stands out the elevated expression of MMP1, MMP3 and MMP9 in incisional hernia fascia, especially at the activity level. Because tissue biopsies failed to show any inflammatory exudates, we speculate that they may be produced by native mesenchymal cells subtly stimulated by an unrelenting inflammatory signalling [27, 28]. MMP1 plays a unique role in eliminating defective pro-collagens during new collagen formation [29], and its anomalous activity has been associated with progression of several diseases including congenital diaphragmatic hernia lungs [30, 31]. MMP3 acts as an activator for several MMPs, and it can directly stimulate cellular transformation [32]. MMP9 is important in the inflammatory phase of wound healing [33, 34], and its excessive production relates to tissue damage and matricryptin generation, as observed in degenerative inflammatory and/or autoimmune disorders [35, 36].
Previous studies did not measure TIMPs expression in the herniated abdominal wall, excepting a sole report describing decreased TIMP2 levels within the fascia transversalis of inguinal hernia [37]. A unique finding of the present study was TIMP3 down-regulation. TIMP3 is single among TIMPs in that it binds firmly to and is localized in the ECM, where it influences matrix remodelling and cell survival and inhibits matrix MMPs, aggrecanases and TNFA-converting enzyme (TACE, ADAM17) [38, 39]. Mice deficient in TIMP3 have elevated levels of TACE/ADAM17 and TNFA [40–42]. Not initially investigated, during manuscript preparation, we measured TACE levels. TACE mRNA expression did not differ between both groups (data not shown), but immunoblotting revealed a tendency towards increased active TACE (80 kD) in incisional hernia skeletal muscle (≈50%, P= 0.075), suggestive of TACE post-transcriptional activation. Although it is not clear yet whether this TIMP3 down-regulation is a primary defect or the result of the loss of ECM content and structure, it could partially account for the transition to tissue rupture by providing a direct mechanistic link between inflammation and degradation. Future researches in this field may allow unravelling these questions.
Another interesting and novel finding reported here is the de-regulated expression of pro-inflammatory cytokines and receptors within incisional hernia tissues. Inflammation is a crucial component of the host response to tissue injuries, but if unnecessarily persistent, it may be detrimental [43]. TNFA is involved in the pathogenesis of chronic inflammation in vivo[44, 45] and is one of the most important inducers of MMP protein production [27]. The specific effects of TNFA are signalled via TNFRSF1A and TNFRSF1B. TNFRSF1B, which mediates TNFA effects in a paracrine/autocrine manner, was strongly down-regulated in incisional hernia fascia. The selective modulation of TNFRSF1B may represent a mechanism for protecting the host against the local deleterious effects of TNFA [46–48]. Interestingly, TNFA has also a role in mediating muscle loss in patients with chronic wasting conditions, inflammatory myopathies and insulin resistance [18, 49, 50]. The trend towards increased TNFA levels in hernia could also help to explain the more vigorous expression of IL-6 in incisional hernia skeletal muscle through a stimulatory mechanism for its secretion [51] (simple correlation analyses confirmed that TNFA and IL-6 expression is highly interrelated in skeletal muscle, r= 0.595, P= 0.020). IL-6 up-regulates the cathepsin and ubiquitin pathways of muscle proteolysis [52]. Collectively, these results raise the hypothesis that the tissue remodelling and destruction detected in incisional hernia may be related, at least in part, to the excessive cytokine production, which favours a state of chronic low-grade molecular inflammatory status, even in the absence of inflammatory cell exudates [43]. Also, it emphasizes the role of the skeletal muscles as a potential target in incisional hernia.
Concerning the limitations of this study, analyses were performed with an observational design and therefore inferences regarding causal pathways cannot be conclusively drawn. Investigating the aetiology of complex traits represents a major challenge, and observational research is a valuable investigative tool, providing rapid results at low cost. One major disadvantage is that descriptive studies do not give any indication of the absolute risk of the factor in question, but if the evidence found is convincing enough, resources can be then allocated to further more comprehensive studies. Also, although many hernias become manifest early, others may not be noted until many years after the index procedure. Thus, it is not easy to choose appropriate ‘matched’ controls. A group of patients who had not developed incisional hernia after a previous abdominal operation but were laparatomized for other medical indications could have been added as an additional control. However, even in this case, a hernia too small to be detected clinically may have been present. Also, we could not discard that these patients would develop an incisional defect in the (near) future. Finally, we cannot reject that the changes observed in gene expression are also due to a loss of mechanotransduction, secondary to connective tissue mechanical unloading [6], and/or to a loss of fibroblast–ECM attachment [24, 53].
In summary, the results of the present study are consistent with the hypothesis of a biology of hernia formation [5, 6]. Although studies with samples directly acquired from human patients usually do not achieve the level of mechanicistic depth and/or proof of causality than those using more tractable animal and in vitro systems, our findings indicate that, in addition to a fundamental biological disorder, such as abnormal collagen metabolism, other molecular mechanisms are implicated in the pathogenesis of incisional hernias and might be amenable to therapeutic modification (i.e. the control of collagen synthesis alone would not be sufficient to control progressive incisional hernia formation, if cytokine de-regulation persists beyond the short-term inflammatory host-response period). Thus, it seems likely that the ongoing changes in MMPs/TIMPs profiles and pro-inflammatory signalling seen in the present study are compartmentalized processes that occur concomitantly in vivo and probably play a role in the pathogenesis of the disease by jointly driving local ECM fibres disruption and mechanical rupture. In a clinical perspective, we conclude that the role of the local milieu in the development/progression of abdominal wall impairment warrants further investigation and will help in the development of more effective prevention and new treatment strategies.
Acknowledgments
This work was supported in part by grants from the Carlos III Spanish Institute of Health (projects PI030290 and PI070507). We thank E Espín, J Sánchez and R Lozoya who collected surgical tissue specimens.
References
- 1.Kingsnorth A, LeBlanc K. Hernias: inguinal and incisional. Lancet. 2003;362:1561–71. doi: 10.1016/S0140-6736(03)14746-0. [DOI] [PubMed] [Google Scholar]
- 2.Flum DR, Horvath K, Koepsell T. Have outcomes of incisional hernia repair improved with time? A population-based analysis. Ann Surg. 2003;237:129–35. doi: 10.1097/00000658-200301000-00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Klinge U, Conze J, Krones CJ, et al. Incisional hernia: open techniques. World J Surg. 2005;29:1066–72. doi: 10.1007/s00268-005-7970-2. [DOI] [PubMed] [Google Scholar]
- 4.Yahchouchy-Chouillard E, Aura T, Picone O, et al. Incisional hernias. I. Related risk factors. Dig Surg. 2003;20:3–9. doi: 10.1159/000068850. [DOI] [PubMed] [Google Scholar]
- 5.Jansen PL, Mertens PR, Klinge U, et al. The biology of hernia formation. Surgery. 2004;136:1–4. doi: 10.1016/j.surg.2004.01.004. [DOI] [PubMed] [Google Scholar]
- 6.Franz MG. The biology of hernia formation. Surg Clin North Am. 2008;88:1–15. doi: 10.1016/j.suc.2007.10.007. vii. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Klinge U, Si ZY, Zheng H, et al. Collagen I/III and matrix metalloproteinases (MMP) 1 and 13 in the fascia of patients with incisional hernias. J Invest Surg. 2001;14:47–54. doi: 10.1080/089419301750072202. [DOI] [PubMed] [Google Scholar]
- 8.Si Z, Bhardwaj R, Rosch R, et al. Impaired balance of type I and type III procollagen mRNA in cultured fibroblasts of patients with incisional hernia. Surgery. 2002;131:324–31. doi: 10.1067/msy.2002.121376. [DOI] [PubMed] [Google Scholar]
- 9.Salameh JR, Talbott LM, May W, et al. Role of biomarkers in incisional hernias. Am Surg. 2007;73:561–7. [PubMed] [Google Scholar]
- 10.Rosch R, Junge K, Knops M, et al. Analysis of collagen-interacting proteins in patients with incisional hernias. Langenbecks Arch Surg. 2003;387:427–32. doi: 10.1007/s00423-002-0345-3. [DOI] [PubMed] [Google Scholar]
- 11.Riley G. Tendinopathy – from basic science to treatment. Nat Clin Pract Rheumatol. 2008;4:82–9. doi: 10.1038/ncprheum0700. [DOI] [PubMed] [Google Scholar]
- 12.Woessner JF., Jr MMPs and TIMPs-an historical perspective. Mol Biotechnol. 2002;22:33–49. doi: 10.1385/MB:22:1:033. [DOI] [PubMed] [Google Scholar]
- 13.Giannoudis PV, Dinopoulos H, Chalidis B, et al. Surgical stress response. Injury. 2006;37:S3–9. doi: 10.1016/S0020-1383(07)70005-0. Suppl 5: [DOI] [PubMed] [Google Scholar]
- 14.Di Vita G, Patti R, D’Agostino P, et al. Cytokines and growth factors in wound drainage fluid from patients undergoing incisional hernia repair. Wound Repair Regen. 2006;14:259–64. doi: 10.1111/j.1743-6109.2006.00120.x. [DOI] [PubMed] [Google Scholar]
- 15.Schönherr E, Hausser HJ. Extracellular matrix and cytokines: a functional unit. Dev Immunol. 2000;7:89–101. doi: 10.1155/2000/31748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tran KT, Griffith L, Wells A. Extracellular matrix signalling through growth factor receptors during wound healing. Wound Repair Regen. 2004;12:262–8. doi: 10.1111/j.1067-1927.2004.012302.x. [DOI] [PubMed] [Google Scholar]
- 17.Rath AM, Chevrel JP. The healing of laparotomies: review of the literature. Hernia. 1998;2:145–9. [Google Scholar]
- 18.Demoule A, Divangahi M, Yahiaoui L, et al. Endotoxin triggers nuclear factor-kappaB-dependent up-regulation of multiple proinflammatory genes in the diaphragm. Am J Respir Crit Care Med. 2006;174:646–53. doi: 10.1164/rccm.200509-1511OC. [DOI] [PubMed] [Google Scholar]
- 19.Arbos MA, Ferrando JM, Quiles MT, et al. Improved surgical mesh integration into the rat abdominal wall with arginine administration. Biomaterials. 2006;27:758–68. doi: 10.1016/j.biomaterials.2005.06.027. [DOI] [PubMed] [Google Scholar]
- 20.Oliver GW, Leferson JD, Stetler-Stevenson WG, et al. Quantitative reverse zymography: analysis of picogram amounts of metalloproteinase inhibitors using gelatinase A and B reverse zymograms. Anal Biochem. 1997;244:161–6. doi: 10.1006/abio.1996.9895. [DOI] [PubMed] [Google Scholar]
- 21.Morrison DF. Multivariate statistical methods. 3rd ed. McGraw-Hill; 1990. New York: [Google Scholar]
- 22.Clark IM, Swingler TE, Sampieri CL, et al. The regulation of matrix metalloproteinases and their inhibitors. Int J Biochem Cell Biol. 2008;40:1362–78. doi: 10.1016/j.biocel.2007.12.006. [DOI] [PubMed] [Google Scholar]
- 23.Celli BR, Calverley PM, Rennard SI, et al. Proposal for a multidimensional staging system for chronic obstructive pulmonary disease. Respir Med. 2005;99:1546–54. doi: 10.1016/j.rmed.2005.03.019. [DOI] [PubMed] [Google Scholar]
- 24.Varani J, Dame MK, Rittie L, et al. Decreased collagen production in chronologically aged skin: roles of age-dependent alteration in fibroblast function and defective mechanical stimulation. Am J Pathol. 2006;168:1861–8. doi: 10.2353/ajpath.2006.051302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fligiel SE, Varani J, Datta SC, et al. Collagen degradation in aged/photodamaged skin in vivo and after exposure to matrix metalloproteinase-1 in vitro. J Invest Dermatol. 2003;120:842–8. doi: 10.1046/j.1523-1747.2003.12148.x. [DOI] [PubMed] [Google Scholar]
- 26.Pischon N, Darbois LM, Palamakumbura AH, et al. Regulation of collagen deposition and lysyl oxidase by tumor necrosis factor-alpha in osteoblasts. J Biol Chem. 2004;279:30060–5. doi: 10.1074/jbc.M404208200. [DOI] [PubMed] [Google Scholar]
- 27.Stamenkovic I. Extracellular matrix remodelling: the role of matrix metalloproteinases. J Pathol. 2003;200:448–64. doi: 10.1002/path.1400. [DOI] [PubMed] [Google Scholar]
- 28.Han YP, Nien YD, Garner WL. Tumor necrosis factor-alpha-induced proteolytic activation of pro-matrix metalloproteinase-9 by human skin is controlled by down-regulating tissue inhibitor of metalloproteinase-1 and mediated by tissue-associated chymotrypsin-like proteinase. J Biol Chem. 2002;277:27319–27. doi: 10.1074/jbc.M202842200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sørensen LT. Effect of lifestyle, gender and age on collagen formation and degradation. Hernia. 2006;10:456–61. doi: 10.1007/s10029-006-0143-x. [DOI] [PubMed] [Google Scholar]
- 30.Brinckerhoff CE, Matrisian LM. Matrix metalloproteinases: a tail of a frog that became a prince. Nat Rev Mol Cell Biol. 2002;3:207–14. doi: 10.1038/nrm763. [DOI] [PubMed] [Google Scholar]
- 31.Masumoto K, De Rooij JD, Suita S, et al. The distribution of matrix metalloproteinases and tissue inhibitors of metalloproteinases in the lungs of congenital diaphragmatic hernia patients and age-matched controls. Histopathology. 2006;48:588–95. doi: 10.1111/j.1365-2559.2006.02379.x. [DOI] [PubMed] [Google Scholar]
- 32.Radisky DC, Levy DD, Littlepage LE, et al. Rac1b and reactive oxygen species mediate MMP-3-induced EMT and genomic instability. Nature. 2005;436:123–7. doi: 10.1038/nature03688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mirastschijski U, Impola U, Jahkola T, et al. Ectopic localization of matrix metalloproteinase-9 in chronic cutaneous wounds. Hum Pathol. 2002;33:355–64. doi: 10.1053/hupa.2002.32221. [DOI] [PubMed] [Google Scholar]
- 34.St-Pierre Y, Van Themsche C, Estève PO. Emerging features in the regulation of MMP-9 gene expression for the development of novel molecular targets and therapeutic strategies. Curr Drug Targets Inflamm Allergy. 2003;2:206–15. doi: 10.2174/1568010033484133. [DOI] [PubMed] [Google Scholar]
- 35.Tran KT, Lamb P, Deng JS. Matrikines and matricryptins: implications for cutaneous cancers and skin repair. J Dermatol Sci. 2005;40:11–20. doi: 10.1016/j.jdermsci.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 36.Opdenakker G, Dillen C, Fiten P, et al. Remnant epitopes, autoimmunity and glycosylation. Biochim Biophys Acta. 2006;1760:610–5. doi: 10.1016/j.bbagen.2005.12.014. [DOI] [PubMed] [Google Scholar]
- 37.Abci I, Bilgi S, Altan A. Role of TIMP-2 in fascia transversalis on development of inguinal hernias. J Invest Surg. 2005;18:123–8. doi: 10.1080/08941930590956147. [DOI] [PubMed] [Google Scholar]
- 38.Woessner JF., Jr That impish TIMP: the tissue inhibitor of metalloproteinases-3. J Clin Invest. 2001;108:799–800. doi: 10.1172/JCI13709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Amour A, Slocombe PM, Webster A, et al. TNF-alpha converting enzyme (TACE) is inhibited by TIMP-3. FEBS Lett. 1998;435:39–44. doi: 10.1016/s0014-5793(98)01031-x. [DOI] [PubMed] [Google Scholar]
- 40.Black RA. TIMP3 checks inflammation. Nat Genet. 2004;36:934–5. doi: 10.1038/ng0904-934. [DOI] [PubMed] [Google Scholar]
- 41.Leco KJ, Waterhouse P, Sanchez OH, et al. Spontaneous air space enlargement in the lungs of mice lacking tissue inhibitor of metalloproteinases-3 (TIMP-3) J Clin Invest. 2001;108:817–29. doi: 10.1172/JCI12067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fedak PW, Smookler DS, Kassiri Z, et al. TIMP-3 deficiency leads to dilated cardiomyopathy. Circulation. 2004;110:2401–9. doi: 10.1161/01.CIR.0000134959.83967.2D. [DOI] [PubMed] [Google Scholar]
- 43.Hotamisligil GS. Inflammation and metabolic disorders. Nature. 2006;444:860–7. doi: 10.1038/nature05485. [DOI] [PubMed] [Google Scholar]
- 44.Vassalli P. The pathophysiology of tumor necrosis factors. Annu Rev Immunol. 1997;10:411–52. doi: 10.1146/annurev.iy.10.040192.002211. [DOI] [PubMed] [Google Scholar]
- 45.MacEwan DJ. TNF ligands and receptors-a matter of life and death. Br J Pharmacol. 2002;135:855–75. doi: 10.1038/sj.bjp.0704549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Theiss AL, Simmons JG, Jobin C, et al. Tumor necrosis factor (TNF) alpha increases collagen accumulation and proliferation in intestinal myofibroblasts via TNF receptor 2. J Biol Chem. 2005;280:36099–109. doi: 10.1074/jbc.M505291200. [DOI] [PubMed] [Google Scholar]
- 47.Porteu F, Hieblot C. Tumour necrosis factor induces a selective shedding of its p75 receptor from human neutrophils. J Biol Chem. 1994;269:2834–40. [PubMed] [Google Scholar]
- 48.Higuchi Y, McTiernan CF, Frye CB, et al. Tumor necrosis factor receptors 1 and 2 differentially regulate survival, cardiac dysfunction, and remodeling in transgenic mice with tumor necrosis factor-alpha-induced cardiomyopathy. Circulation. 2004;109:1892–7. doi: 10.1161/01.CIR.0000124227.00670.AB. [DOI] [PubMed] [Google Scholar]
- 49.Zhan M, Jin B, Chen SE, et al. TACE release of TNF-alpha mediates mechanostransduction induced activation of p38 MAPK and myogenesis. J Cell Sci. 2006;120:692–701. doi: 10.1242/jcs.03372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chen S, Jin B, Li YP. TNF-alpha regulates myogenesis and muscle regeneration by activating p38 MAPK. Am J Physiol Cell Physiol. 2007;292:C1660–71. doi: 10.1152/ajpcell.00486.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kern PA, Ranganathan S, Li C, et al. Adipose tissue tumor necrosis factor and interleukin-6 expression in human obesity and insulin resistance. Am J Physiol Endocrinol Metab. 2001;280:E745–51. doi: 10.1152/ajpendo.2001.280.5.E745. [DOI] [PubMed] [Google Scholar]
- 52.Tsujinaka T, Fujita J, Ebisui C, et al. Interleukin 6 receptor antibody inhibits muscle atrophy and modulates proteolytic systems in interleukin 6 transgenic mice. J Clin Invest. 1996;97:244–9. doi: 10.1172/JCI118398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fisher GJ, Varani J, Voorhees JJ. Looking older: fibroblast collapse and therapeutic implications. Arch Dermatol. 2008;144:666–72. doi: 10.1001/archderm.144.5.666. [DOI] [PMC free article] [PubMed] [Google Scholar]


