Abstract
Endothelial progenitor cells (EPC) enhance endothelial cell repair, improve endothelial dysfunction and are a predictor for cardiovascular mortality. High-density lipoprotein (HDL) cholesterol levels inversely correlate with cardiovascular events and have vasculoprotective effects. Here we postulate that HDL influences EPC biology. HDL and EPC were isolated according to standard procedures. Differentiation of mononuclear cells into DiLDL/lectin positive cells was enhanced after HDL treatment compared to vehicle. HDL was able to inhibit apoptosis (TUNEL assay, annexin V staining) while proliferation (BrdU incorporation) of early outgrowth colonies after extended cell cultivation (14 days) was increased. Flow chamber experiments revealed an improved adhesion of HDL pre-incubated EPC on human coronary artery endothelial cells (HCAEC) compared to vehicle while HDL treatment of HCAEC prevented adhesion of inflammatory cells. Flow cytometry demonstrated an up-regulation of β2- and α4-integrins on HDL pre-incubated EPC. Blocking experiments revealed a unique role of β2-integrin in EPC adhesion. Treatment of wild-type mice with recombinant HDL after endothelial denudation resulted in enhanced re-endothelialization compared to vehicle. Finally, in patients with coronary artery disease a correlation between circulating EPC and HDL concentrations was demonstrated. We provide evidence that HDL mediates important vasculoprotective action via the improvement of function of circulating EPC.
Keywords: high-density lipoprotein, endothelium, endothelial progenitor cells
Introduction
Atherosclerosis is a systemic inflammatory disease characterized in the early stages by a disrupture of the endothelium’s integrity resulting in endothelial dysfunction and atherosclerotic lesion formation. The effective restoration of the endothelium after endothelial cell (EC) damage is pivotal in order to prevent atherosclerotic lesion formation. It has been previously demonstrated that apoptotic EC can be in part regenerated by the adjacent ECs but also by circulating bone marrow (BM)-derived endothelial progenitor cells (EPC) [1–6]. In a steady-state condition, equilibrium exists between EC apoptosis and EC regeneration. However, in conditions of severe EC damage, e.g. in the presence of cardiovascular risk factors, EC apoptosis overwhelms the regenerative potential and a disruptured endothelium is no longer restored [7, 8]. Furthermore, numerous studies have demonstrated that cardiovascular risk factors have detrimental effects on the regenerative system itself [9–12]. Functional properties and absolute numbers of EPC are severely impaired in patients suffering from cardiovascular diseases [12, 13].
Strengthening of the organisms regenerative potential, e.g. by enhancing the number and function of circulating EPC by physical activity, drug treatment or hormone replacement therapy, enhances EC repair after focal EC damage and improves endothelial dysfunction [14–17]. Interestingly, enhanced regenerative capacity translates into improved survival. In patients with coronary artery disease, the number and function of circulating EPC are an important cellular risk predictor for cardiovascular mortality and morbidity [18, 19].
High-density lipoprotein (HDL) levels inversely correlate with cardiovascular events and seem to have important vasculoprotective effects [20]. Individuals with elevated HDL plasma levels are less susceptible for the development of endothelial dysfunction and atherosclerosis [21]. In contrast, low HDL levels predict an increased incidence of myocardial infarction [22]. A recently published study demonstrates the highly predictive value of HDL-cholesterol independent of low-density lipoprotein (LDL) cholesterol for cardiovascular events [20]. However, the mechanisms by which HDL exerts atheroprotection are multiple but poorly understood [23]. HDL facilitates the reverse cholesterol transport and delivers cholesterol from the vasculature to the liver for excretion from the body. HDL leads to the reduction of vascular oxidative stress, which may contribute to the atheroprotective effects of HDL. In addition, anti-inflammatory and anti-apoptotic effects of HDL on EC have been attributed. First studies in mice and human beings indicate that HDL treatment also influences EPC numbers [24–27]. The accumulation of these cellular and molecular effects is probably responsible for the beneficial effects of HDL on the vascula-ture. However, clinical studies have shown that elevation of HDL via systemic infusion or pharmacological intervention is not necessarily associated with improved vessel function [28–30]. The underlying reasons remain unclear.
Because HDL has a beneficial effect on the endothelium, we reasoned that HDL mediates its atheroprotective effects in parts via modulation of EC and EPC function.
Materials and methods
Isolation of high-density lipoprotein
HDL (density, 1.063–1.21 g/ml) was isolated from the plasma of normo-cholesterolemic individuals (serum cholesterol < 6.2 mmol/l) or from samples of expired human plasma by ultracentrifugation accordingto the method described by Redgrave et al.[31] The HDL fraction was dialysed against 0.15 M NaCl, 0.34 mM ethylenediaminetetraacetic acid, pH 7.4. HDL was stored at 4°C and used within 1 week. No changes in activity were observed during this time period. Quantification of HDL was performed according to standard laboratory methods (automated HDL cholesterol flex method for the Dimension clinical chemistry system, AHDL Dimension, Sciences Healthcare Diagnostics, Hamburg, Germany).
Cell culture
Human coronary artery endothelial cells (HCAEC) were grown in a 5% CO2 atmosphere at 37°C and maintained in EGM 2 MV basal medium (PromoCell, Heidelberg, Germany) with EC growth supplements as recommended by the supplier. Growth factor deprivation was induced by changing the media to EGM 2 MV without supplements.
Preparation of mononuclear cells
Mononuclear cells (MNC) were isolated from 20 ml sodium citrate buffered blood or buffy coats from normocholesterolemic individuals using a Ficoll density gradient (Biocoll Separating Solution; Biochrom AG, Berlin, Germany) according to standard protocols. For mouse MNC, spleens were explanted, mechanically minced and MNC were isolated using a Ficoll gradient (Lympholite-M, Cedarlane, Burlington, Canada).
Early outgrowth EPC
A total of 2 × 106 human or spleen-derived MNC were seeded on fibronectin (Sigma, Steinheim, Germany) coated 24-well plates in 1.0 ml endothelial basal medium-2 (Lonza, Cologne, Germany) with supplements as previously described [3, 5, 15]. Cells were incubated with 100–1000 μg/ml HDL or vehicle for 7 days starting at day 0. After 7 days in culture, cells were extensively washed with normal saline and resuspended. Adherent cells were incubated with 2.4 μg/ml 1,1′-dioctadecyl-3,3,3′,3′-tertamethylindocarbocyanine-labeled acetylated LDL (DiI-Ac-LDL, CellSystems, St. Katharinen, Germany) and stained with FITC-labelled Ulex europaeus agglutinin I (lectin, 10 μg/ml; Sigma) for human early outgrowth EPC, and FITC-labelled Giffonia (bandeiraea) simpliciforia lectin I (lectin, 10 μg/ml; Vector Laboratories) for mouse EPC. Morphological characteristics of early outgrowth EPC were assessed using DiI-Ac-LDL positive cells. Cells were evaluated concerning their morphological phenotype (spindle shaped versus rounded cells) and were counted by a blinded observer.
Colony forming units-endothelial cells/hill
For colony forming units-endothelial cells (CFU-EC, also called CFU-Hill), 5 × 106 human MNC were sub-cultured for 48 hrs. A total of 1 × 106 cells derived from the supernatant were cultured for additional 7 days in endothelial basal medium with a change of medium every second day. Cells were incubated with 100–1000 μg/ml HDL or vehicle for 9 days starting at day 0. The numbers of colonies were manually counted by a blinded observer. The number of mouse CFU-EC was determined accordingly using 5 × 106 spleen-derived MNC.
RT-PCR
The mRNA of early outgrowth EPC was extracted using peqGOLD RNAPure Reagent (peqlab). RT was carried out for 1 hr at 42°C on 1 μg of RNA using p(dN6)-oligonucleotide random primers (Roche, Mannheim, Germany) and MMLV RT (Invitrogen, Karlsruhe, Germany). For PCR amplification, 2 μg of the produced single stranded cDNA was amplified by PCR with Taq DNA-polymerase (Boehringer, Mannheim, Germany) for 35 cycles, using the following cycles: 94°C for 3 min., 94°C for 1 min., 58°C for 1 min. and 72°C for 3 min. for 35 cycles. Human primers for endothelial nitric oxide synthase (eNOS) were 5′-GACATTTTCGGGCTCACGCTG-3′ (forward) and 5′-TTGGGTAGGCAGTTTAGTTCTC-3′ (reverse). For GAPDH the primers were 5′-CCT GGA CCA CCC AGC CAG CAA-3′ (forward) and 5′-TGT TAT GGG GTC TGG GAT A-3′ (reverse). For quantification, eNOS mRNA abundance was normalized to the abundance of the housekeeping gene GAPDH.
Western blot
Total cell lysates and proteins were prepared according to standard procedures. Immunoblotting was performed with a purified eNOS Type III monoclonal antibody (1:400 dilution; BD Biosciences, Heidelberg, Germany), goat antimouse secondary antibody (1:400 dilution Sigma), and the Western blotting detection system (Amersham, Freiburg, Germany).
Proliferation
Proliferation was measured using cell counting (DiLDL/lectin positive cells) and BrdU incorporation as previously described [32]. Briefly, EPC were incubated with 100–1000 μg/ml HDL or vehicle for 24 hrs. At days 7 and 14, cells were stained for Di-Ac-LDL and lectin for cell counting or incubated with 10 μM BrdU (Beckton Dickinson, Heidelberg, Germany). Cells were fixed and stained with anti-BrdU monoclonal antibody (Beckton Dickinson). Cells were stained with 20 μg/ml propidium iodide (PI) in the presence of 100 μg/ml DNase-free RNase A (Roche). Measurements were performed with an FACS Calibur and analysed with CellQuestPro software (Becton Dickinson). Cell doublets were discriminated from G2 cells based on the difference in pulse shape. At least 20,000 cells were analysed per sample.
Apoptosis
Apoptosis was induced using 500 U/ml tumour necrosis factor (TNF)-α and incubated with 500 μg/ml HDL or vehicle for 7 days. Cells were cytocentrifuged (100 ×g, 8 min.) and fixed in 1% paraformaldehyde in phosphate-buffered salt solution. Cells were incubated in 50 μl of terminal of deoxynucleotidyl transferase reaction buffer (Roche) and 8 μl 5-Bromo-2-deoxyuridine-5-triphosphate (Sigma-Aldrich, Steinheim, Germany). Cells were incubated for 60 min. at 37°C and post-incubated with the anti-BrdU mononuclear antibody for 60 min. at 37°C. DNA was counterstained with 25 μg/ml PI (Sigma-Aldrich) in the presence of 100 μg/ml DNase-free RNase (Roche). The BrdU-labelled apoptotic cells were identified on the two-parameter histogram (anti-BrdU versus PI). At least 20,000 cells were analysed per sample. As a confirmatory method to measure apoptosis, cultured cells were centrifuged and resuspended in binding buffer, pH 7.4, containing 10 mM Hepes (Sigma), 140 mM NaCl and 2.5 mM CaCl2. Five microlitres FITC-annexin V (BD Biosciences) was added to 1 × 105 cells. The cells were placed at room temperature in the dark for 20 min., rinsed in binding buffer, resuspended in 1 ml of 1% formaldehyde binding buffer for 30 min. on ice, rinsed twice, resuspended in 0.5 ml of PI solution containing 50 μg/ml PI, placed at room temperature for 45 min. in the dark, and analysed by flow cytometry.
Matrigel pseudo-tube formation assay
Ten microlitres Matrigel (BD Biosciences) was applied to a 4-mm culture dish (Ibidi, Martinsreid, Germany) and incubated at 37°C to induce gelling. Four-day cultured EPC were seeded at a density of 4 × 105 cells/ml/well in EMB-2 medium plus supplements in the presence of HDL or vehicle. The morphology and reorganization of EPC were monitored using a phase-contrast optical microscope (Zeiss, Jena, Germany). Within 1 day of culture, elongated processes were observed and after 2 days the cell cultures showed networks of branching and anastomosing cord of cells. Analyses evaluating pseudo-tube formation length was performed on day 2 with the use of AxioVision version 4.5.0 software.
Static adhesion
A total of 2 × 105 HCAEC per millilitre were cultured for 3 days in 96-well tissue culture plates. Cultured EPC were added to the wells and co-cultured for 2 hrs. Unattached cells were gently washed away with phosphate-buffered solution. The attached cells were then fixed for 30 min. with 1% formaldehyde at room temperature and stained with methylene blue according standard protocols. The relative number of adherent cells was calculated by lysing the stained cells with 50% ethanol and 50% hydrochloric acid, and then reading the absorbance at 620 nm on a microplate autoreader. The percentage of cells adhering to the plate was determined based on the linear relationship between the absorbance reading and the number of cells counted in a haemocytometer.
Flow-mediated adhesion/transmigration
EPC adhesion experiments were performed in a parallel-plate flow chamber (μ-slide VI ibitreat, Ibidi) mounted on the stage of a phase-contrast optical microscope (Zeiss) with a 10× objective. A syringe pump (TSE) was used to simulate a uniform laminar flow field in the flow chamber. Confluent HCAEC monolayers grown on 17-mm length, 3.8-mm width flow chambers were stimulated with IFN-γ 10 ng/ml (final concentration) for 72 hrs. TNF-α (100 U/ml) was added to the culture medium 16 hrs before the experiments. The culture medium was changed daily. Treatment with interferon-γ (IFN-γ) caused a characteristic elongation of the ECs and with the addition of TNF-α resulted in an up-regulation of intercellular adhesion molecule-1 (data not shown). Cultured EPC circulated through the chamber at a constant rate of 0.7 ml/min. (estimated shear stress, 1.0 dynes/cm2). EPC adhesion and transmigration were determined after 5 min. of perfusion by analysis of three to five high-power fields (×10). Transmigrated EPC were determined as being beneath the endothelial monolayer, i.e. in a different plane of focus, distinct from both adherent EPC and the endothelium.
HDL treatment
Male, 12-week-old C57BL/6J mice (wild-type) were used and treated with 40 mg/kg/day recombinant HDL (rHDL; CSL-111, CSL Behring AG, Bern, Switzerland) or placebo intravenously 24 hrs before, at the day and 24 hrs after carotid artery denudation.
Carotid artery denudation
Carotid artery injury was induced as described previously [33]. Briefly, the mice were anaesthetized with 150 mg/kg body weight ketamine hydrochloride (ketanest®, Pharmacia, Erlangen, Germany) and 0.1 mg/kg body weight xylazine hydrochloride (rompun® 2%, Bayer, Leverkusen, Germany). The common carotid artery was exposed and submitted to an electric injury starting at the level of the bifurcation and continuing to the proximal part of the artery (in total 4 mm denudation). The denuded area was determined at day 5 after surgery after intravenous injection of 50 μl Evans blue in an en face preparation of the vessel. Evans blue stained denuded areas and the complete vessel area were measured using AxioVision version 4.5.0 software. The percentage of denudation 5 days after injury is provided.
Flow cytometry
Flow cytometry to enumerate EPC numbers in mice and human beings was performed as recently described by our group [15, 19] and in accordance with the current standards for EPC enumeration using flow cytometry for human cells [34]. Mouse blood was analysed as described previously. The viable lymphocyte population was analysed for Sca-1-FITC (Becton Dickinson) and Flk-1-PE (Becton Dickinson). Isotype-identical antibodies served as controls in every experiment (Becton Dickinson).
Ficoll-concentrated MNCs were used for analysis of human EPC. Blood samples were processed with the fluorescent-conjugated antibodies CD34-FITC (Becton-Dickinson), KDR and CD133-PE (Miltenyi, Bergisch, Gladbach). For identification of KDR positive cells, indirect immunolabelling was performed with a biotinylated goat mononuclear antibody against the extracellular domain of human KDR (R&D Systems, Minneapolis, MN, USA). In pilot experiments, no significant differences in EPC numbers were observed using additional viability markers (PI, 7-AAD). IgG2a-FITC and IgG2a-PE (Pharmingen, San Diego, CA, USA) served as negative controls. Cell fluorescence was measured immediately after staining using a FACS Calibur instrument (Becton Dickinson). Data were analysed using Cellquest software (Becton Dickinson). Units of all measured components are absolute cell counts obtained after measuring of 20,000 events in a pre-specified lymphocyte gate during FACS analysis.
Study subjects
Between March 2003 and January 2004, consecutive patients who underwent a coronary angiography were screened for inclusion into the EPCAD (endothelial progenitor cells in coronary artery disease) study. Informed consent was obtained from all patients and the study protocol was approved by the ethical committee of the University of Saarland. Patient enrolment and patient characteristics have been described in detail previously [19]. In a pre-specified analysis, HDL cholesterol was determined in patients not on lipid-modifying drugs and correlated with the number of circulating EPC.
Statistical analysis
Data are presented as mean ± standard error of mean (S.E.M.). Statistical analysis was performed with unpaired Student’s t-test and the anova test followed by the Neuman–Keuls post-hoc analysis. P < 0.05 indicates statistical significance.
The authors had full access to the data and take responsibility for its integrity. All authors have read and agree to the manuscript as written.
Results
HDL isolation
HDL was isolated from young healthy individuals according to standard procedures. Purity of HDL was checked and the AHDL cholesterol flex method for the Dimension clinical chemistry system was used to quantitatively measure HDL cholesterol. A mean concentration of 65.3 ± 8.1 mg/dl HDL with no detectable LDL contamination was achieved.
HDL enhances differentiation of mononuclear cells into early outgrowth EPC
Differentiation of human MNC into EPC-like DiLDL/lectin positive cells in vitro was determined after incubation with HDL (100–1000 μg/ml) or vehicle for 7 days. The number of DiLDL/lectin positive early outgrowth EPC was significantly enhanced compared to vehicle-treated cells (137.74 ± 4 versus 100 ± 8.14; P < 0.01; Fig. 1). Furthermore, morphological characteristics differed between HDL- and vehicle-treated cells with HDL inducing a characteristic spindle-type phenotype of early outgrowth EPC after 7 days (P < 0.01; Fig. 1). RT-PCR analysis revealed a significant increase in eNOS mRNA abundance after HDL treatment compared to vehicle- treated cells indicating a more mature phenotype of cells after HDL incubation (P < 0.05; Fig. 2A). Western blot experiments confirmed a trend towards an increase in eNOS protein abundance after HDL treatment (P= n.s.; Fig. 2B).
Figure 1.
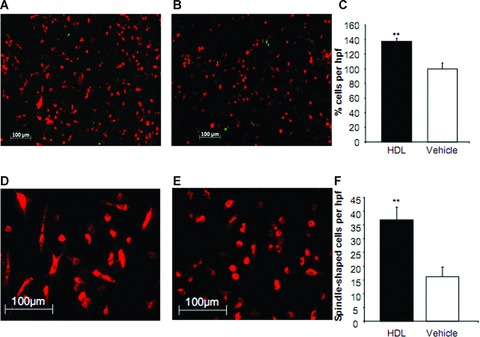
Number of Dil-Ac-LDL and lectin positive early outgrowth endothelial progenitor cells (EPC) after high-density lipoprotein (HDL) incubation. Mononuclear cells were isolated and cultured in supplemented endothelial basal medium in the presence of HDL (A) or vehicle (B). After 7 days, Dil-Ac-LDL and lectin positive early outgrowth EPC were increased after HDL incubation (C). HDL induces a change in the phenotype of cultivated early outgrowth EPC into a more mature, spindle-shaped morphology (D, E, F). n= 3; **P < 0.01. hpf, high-power field.
Figure 2.
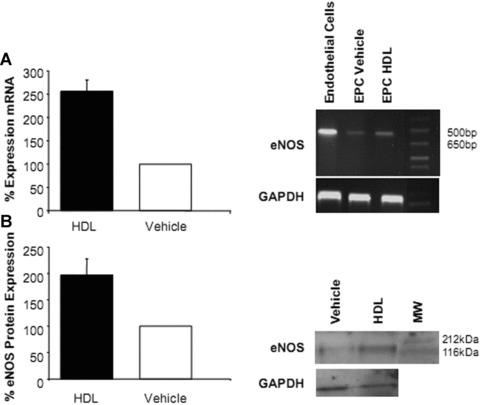
eNOS mRNA and protein abundance in endothelial progenitor cells (EPC) after high-density lipoprotein (HDL) treatment. Early outgrowth EPC were treated with HDL or vehicle. HDL increased eNOS mRNA abundance (*P < 0.05) (A) and protein expression (P= n.s.) (B) compared to vehicle.
HDL inhibits apoptosis of early outgrowth EPC and influences proliferation capacity of EPC after extended cell cultivation
To determine whether HDL protects EPC from apoptosis, the cells were exposed to medium with increasing concentrations of HDL (100–1000 μg/ml for up to 7 days). The minimal concentration that induced reproducible protection assessed using a flow cytometry based TUNEL assay was used for subsequent experiments. A 7-day HDL treatment regimen was associated with a nearly 50% reduction in TUNEL positive cells compared with vehicle alone (P < 0.01, Fig. 3A). HDL induced a decrease in TUNEL expression in all phases of the cell cycle but in particularly G1-Phase (data not shown). Additional apoptosis experiments revealed that the number of annexin V positive cells was similarly reduced in HDL compared to vehicle-treated EPC (P < 0.01, Fig. 3B). At HDL concentrations of 100–1000 μg/ml there was no significant increase or decrease in the total number of proliferating EPC progenitors at 7 days (data not shown). Analysis of the cell cycle, based on measurements of DNA content, indicated that nearly all cells were in G0/1 phase of the cell cycle and only approximately 0.2% of the cells were in S phase. However, when looking at EPC after extended cell cultivation (day 14), HDL was able to increase proliferation of cells by twofold compared to vehicle (P < 0.001; Fig. 4).
Figure 3.
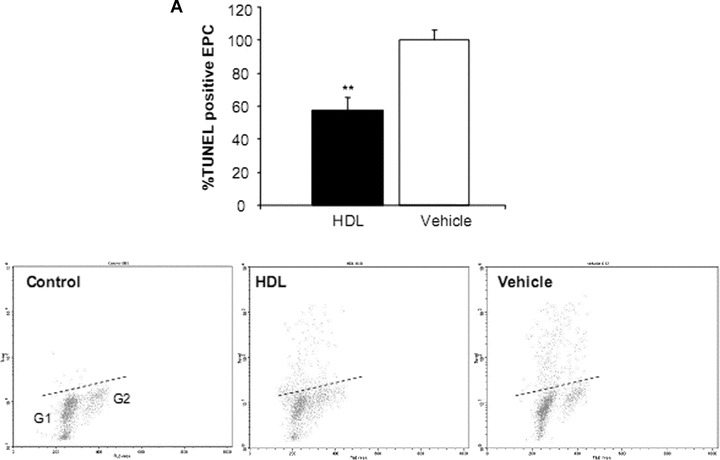
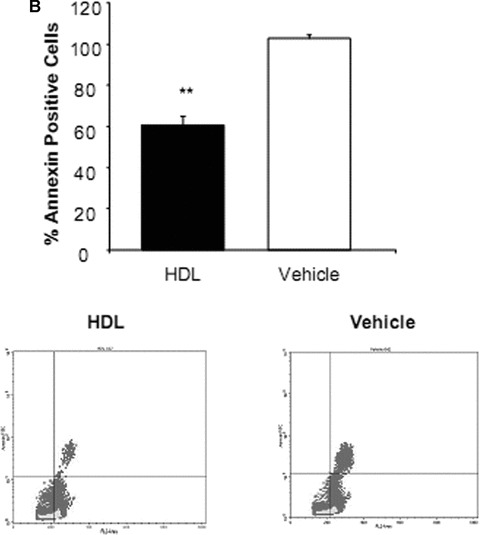
Percentage of apoptotic endothelial progenitor cells (EPC) after incubation with high-density lipoprotein (HDL). EPC were cultured in the presence or absence of HDL. (A) Based on differences in propidium iodide (PI) intensity, cells were subdivided into cell cycle phase G1, S, G2. The presence of DNA strand breaks in TUNEL assay was decreased in all phases of cell cycle but in particularly in G1 cells after HDL treatment. The dashed line represents the upper threshold of TUNEL fluorescence intensity for 99% of the control cells. (B) Analysis of phosphatidylserine exposure of human EPC cultured in the presence of HDL or vehicle. Flow cytometric dot-plot analysis of PI (abscissa) and annexin V (ordinate) double-stained EPC. Annexin V positive cells were enumerated within the right upper quadrant. n= 4; **P < 0.01.
Figure 4.
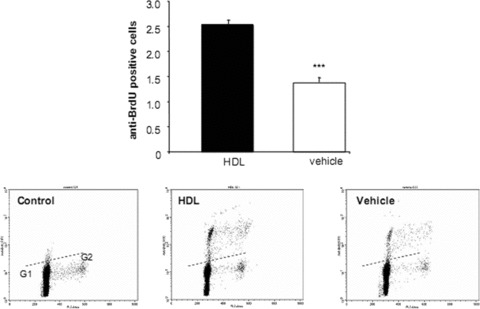
Proliferation of endothelial progenitor cells (EPC) based on measurements of DNA content. EPC were cultured in the presence or absence of high-density lipoprotein (HDL). Based on differences in propidium iodide intensity, cells were subdivided into cell cycle phase G1, S, G2. HDL was able to twofold increase proliferation of EPC after 14 days compared to vehicle. The dashed line represents the upper threshold of BrdU fluorescence intensity for 99% of the control cells. n= 3; ***P < 0.001.
HDL influences adhesion of EPC in vitro
HDL adhesion was assessed using a static adhesion model and an in vitro flow chamber model. EPC incubated with HDL (100–1000 μg/ml) showed a significantly increased adhesion to stimulated HCAEC compared to vehicle-treated EPC as assessed by measuring the optical density of lysed cells after static adhesion (P < 0.01; Fig. 5A). Flow chamber experiments revealed a significantly enhanced adhesion process of HDL pre-incubated EPC on activated HCAEC compared to vehicle-treated EPC after 5 days in culture. In paired experiments at 1.0 dyn/cm2 laminar wall shear stress, 179 ± 19.9 (mean ± S.E.M.) early outgrowth EPC pre-incubated with HDL showed rolling, tethering, and firm adhesion to HCAEC after 2 min. compared to 99 ± 9.8 control (P < 0.001; Fig. 5B). In contrast, HDL pre-treatment of HCAEC significantly prevented adhesion of monocytes (data not shown). In an effort to determine whether HDL influences the expression profile of adhesion molecules, we determined the expression of CD18 (β2-integrin), CD49d (α4-integrin) and CD99 on EPC after HDL or vehicle treatment and their relevance in the adhesion process. Flow cytometry showed a significant increase in CD133+/CD18+ and CD133+/CD49d+ but not CD133+/CD99+ EPC after HDL incubation compared to vehicle (213 ± 14 versus 100 ± 14.8%, P < 0.001; 152 ± 6.44 versus 100 ± 4.55, P < 0.01; 250 ± 69.6 versus 100 ± 24, P= 0.06, respectively). Therefore, we exposed EPC in paired experiments to anti-CD18 antibody in concentrations previously shown to block EPC adhesion [35]. A total of 30 ± 6.4 cells adhered in the presence of anti-CD18 compared to 100 ± 11.0 cells in isotype-blocked EPC (P < 0.01; Fig. 5C). The majority of the positive events after EPC pre-incubation with anti-CD18 were due to rolling. Firm adhesion with subsequent transmigration was not observed. Blocking of the CD49d binding sites lead to a non-significant reduction of EPC adhesion (P= 0.3).
Figure 5.
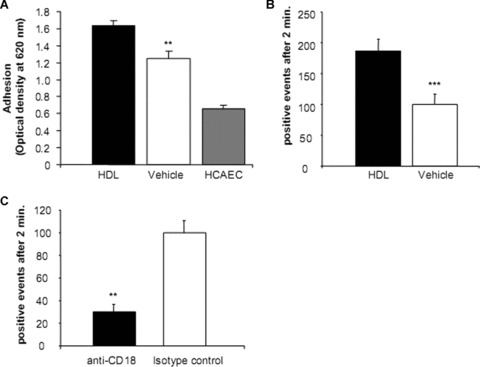
Effect of high-density lipoprotein (HDL) on endothelial progenitor cells (EPC) adhesion to human coronary artery endothelial cells (HCAEC) in a static and flow-mediated adhesion model. EPC were pre-incubated with HDL before adhesion to stimulated HCAEC was determined. (A) Optical density readings of the lysed cells indicated that HDL pre-incubation increased EPC adhesion in a static adhesion model. For control, EPC adhesion to unstimulated HCAEC was determined. (B) EPC adhesion was determined at 1.0 dyn/cm2 laminar wall shear stress after 2 min. HDL pre-incubated EPC showed increased rolling, tethering, and firm adhesion to HCAEC compared to control. (C) EPC were exposed to anti-CD18 antibody or isotype control. Blockade of CD18 was associated with an inhibition of HDL-enhanced EPC adhesion. n= 3–5; **P < 0.01; ***P < 0.001.
HDL improves the migratory capacity of early outgrowth EPC
Enhancing the migratory capacity of EPC is pivotal for an effective EC repair. Early outgrowth EPC incubated with HDL showed a significant better trans-endothelial migration compared to vehicle-treated cells. HDL treatment was associated with an increase in transmigrated events compared to the control group (absolute 108 ± 17.5 versus 52 ± 10.5; P < 0.05, Fig. 6).
Figure 6.
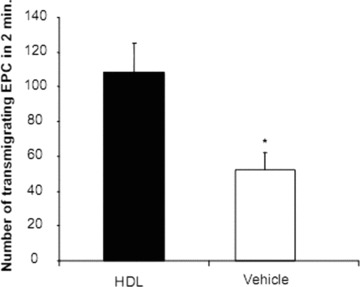
Transmigration of EPC after HDL pre-incubation. Early outgrowth EPC incubated with HDL showed a significant better trans-endothelial migration under flow conditions compared to vehicle-treated cells. n= 5; *P < 0.05.
HDL improves EPC-mediated pseudo-tube formation
Cultured EPC were subjected to a pseudo-tube formation assay in the presence or absence of 500 μg/ml HDL. After 2 days the cell cultures showed networks of branching and anastomosing cord of cells. Analyses evaluating pseudo-tube formation length demonstrated that HDL treatment was associated with increased pseudo-tube length (13.0 ± 0.7 mm versus 8.36 ± 0.06 mm, P < 0.05, Fig. 7).
Figure 7.
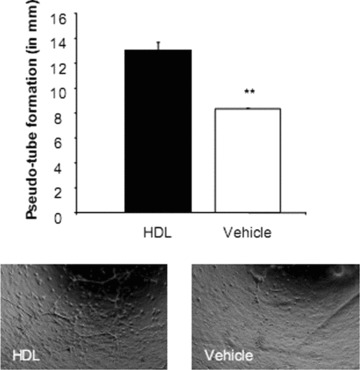
Pseudo-tube formation after high-density lipoprotein (HDL) treatment. Endothelial progenitor cells were plated on a thin layer of matrigel and allowed to settle for 48 hrs under stimulation with HDL or vehicle. After 2 days, analyses evaluating pseudo-tube formation length demonstrated that HDL treatment was associated with increased pseudo-tube length (13.0 ± 0.7 mm versus 8.36 ± 0.06 mm. n= 3; *P < 0.05).
Recombinant HDL increases circulating EPC and enhances re-endothelialization
Treatment of wild-type mice with 40 mg/kg/day rHDL resulted in a significant increase in HDL levels (36.6 ± 1.5 mg/dl to 39.3 ± 2.2 mg/dl, P < 0.05) while LDL levels were unaffected (8.2 ± 0.7 and 8.3 ± 1.5, respectively). A total of 40 mg/kg/day rHDL or placebo was intravenously injected before and on two consecutive days after EC denudation. HDL treatment increased the number of circulating Sca1/flk1 positive EPC as determined by flow cytometry compared to vehicle-treated mice (P= 0.001; n= 6 for each group; Fig. 8). The effects of rHDL on the growth of spleen-derived EPC-CFU are shown in Fig. 8. rHDL caused an increase (P < 0.05) in the number of colonies per power field, when colonies >20 cells were considered. Morphometric analysis of carotid arteries revealed an enhanced re-endothelialization process in rHDL-treated mice compared to placebo-treated mice (P < 0.05; Fig. 9).
Figure 8.
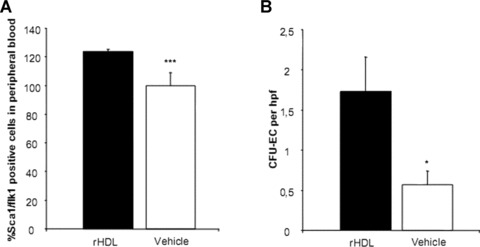
Recombinant HDL (rHDL) influences circulating endothelial progenitor cells and colony forming units-endothelial cells. Treatment of wild-type mice with 40 mg/kg/day rHDL or placebo i.v. resulted in a significantly increased number of circulating Sca1/flk1 positive EPC compared to vehicle-treated mice (A, flow cytometry). rHDL caused an increase in the number of colonies per high-power field (hpf) (B). n= 6 for each group; *P < 0.05; ***P= 0.001.
Figure 9.
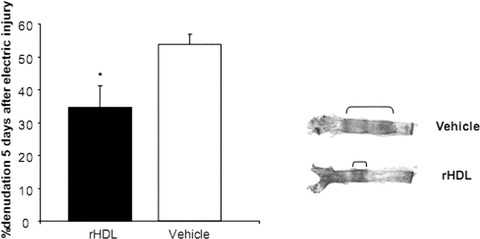
Morphometric analysis of endothelial cell denudation 5 days after perielectric injury of the common carotid artery. Treatment of wild-type mice with 40 mg/kg/day recombinant HDL (rHDL) or placebo i.v. resulted in an enhanced re-endothelialization process in rHDL-treated mice compared to placebo-treated mice. n= 6 for each group; *P < 0.05.
HDL correlates with the number of circulating EPC in human beings with coronary artery disease
In 116 patients without lipid-lowering or lipid-modifying drugs and established coronary artery disease, HDL concentrations were determined. HDL cholesterol significantly correlated with the number of circulating CD34/KDR positive EPC (P < 0.001, r= 0.390, Fig. 10). CD133 positive circulating EPC, CD117 positive haematopoietic progenitors, and CFU-EC/Hill did not correlate with HDL concentrations.
Figure 10.
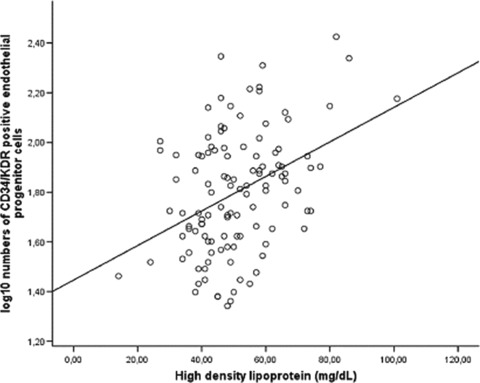
High-density lipoprotein (HDL) concentrations and circulating endothelial progenitor cells in human beings with coronary artery disease. HDL cholesterol correlates with the number of circulating CD34/KDR positive EPC (P < 0.001, r= 0.390).
Discussion
Patients with elevated HDL cholesterol plasma levels are less susceptible for the development of endothelial dysfunction and atherosclerosis [21] and suffer significantly less from cardiovascular events [20]. Circulating EPC have been shown to contribute to EC repair, restoration of endothelial function, and are an important predictor for cardiovascular events. Here we give evidence that the functional properties of endothelium-regenerating EPC are influenced by HDL cholesterol resulting in an improvement in functional capacity and enhanced regeneration after endothelial injury.
EC repair by endogenous circulating EPC requires effective mobilization of cells into peripheral blood, firm adhesion at the injury site, and proliferation, migration and differentiation of progenitor cells into mature EC. Various factors including cytokines, chemokines, integrins, and possibly inflammatory cells are involved in this complex, multi-step process. Firm adhesion of EPC on the vascular wall is an integrin-dependent pathway. Involved integrins on leucocytes include α4- and β2-, which mediate cellular binding to VCAM-1/-2 and intercellular adhesion molecules expressed on ECs. Previous studies indicated that these adhesion markers play a pivotal role in the adhesion and homing of leucocytes but also EPC to ischaemic tissue [36–38]. We could demonstrate that cell surface expression of CD18 (integrin β2-, MAC-1, LFA1) on EPC is influenced by HDL incubation. In addition, CD49d (integrin α4) was up-regulated in EPC after HDL incubation. Inhibition of β2- but not α4-integrin using blocking antibodies resulted in the abrogation of HDL-mediated improved EPC adhesion. This observation is of special interest since β2-integrin is involved in the recruitment, homing and engraftment of EPC within ischaemic myocardium [35]. Surprisingly, blockade of integrin α4, which has previously described to play a pivotal role in engraftment and stem cell maintenance within the BM, did not result in an inhibition of EPC adhesion. This observation is in line with the recently published data describing that the functional disruption of α4-integrin results in an effective mobilization of BM-derived haematopoietic progenitor cells as well as EPC without impairing the homing of such cells within ischaemic tissue [39]. BM-derived EPC from α4-integrin deficient mice incorporated into the neovasculature within ischaemic tissue in the same way than control cells. Various explanations may account for this observation: α4-integrin may be dispensable for progenitor cell homing or, more likely, plays a redundant role in EPC homing, which is supported by the findings that α6- and β1-integrins in addition to β2-integrins are involved in EPC adhesion in a comparable manner [40, 41].
The proliferation capacity of cultured, blood-derived EPC is restricted and is the focus of ongoing debate [42]. Here we could demonstrate that HDL induces a moderate increase in cell proliferation in long-term cultured early outgrowth colonies. However, the observed effect was low and the significance of these findings for the in vivo situation is undetermined. In contrast, HDL was able to significantly inhibit EPC apoptosis. The micro-milieu within an atherosclerotic plaque or lesion site is highly pro-apoptotic. Survival of regenerating cells within such a micro-milieu is pivotal for an effective EC repair. EPC have been previously shown to have effective mechanism for coping with oxidative stress and pro-apoptotic triggers [43]. The expression of the intracellular antioxidative enzymes (e.g. catalase, glutathione peroxidase and manganese superoxide dismutase), is significantly higher in EPC compared to EC. The presence of HDL seems to be further protective against EPC apoptosis in addition to its known anti-apoptotic effect on mature EC.
Migratory capacity of EPC has been significantly improved after HDL incubation. In vitro cultured EPC incubated with HDL showed a significantly improved trans-endothelial migration compared to vehicle-treated cells. Recently, similar results were demonstrated for EC [44]. HDL stimulated EC migration in vitro via scavenger receptor B type I (SR-BI)-mediated activation of Rac GTPase and carotid artery re-endothelialization was blunted in apolipoprotein A-I−/− mice and SR-BI−/− mice. The results demonstrate that HDL not only exerts its positive effects regarding migration on mature cells but also on regenerating circulating progenitor cells.
Proliferation and migration of EPC towards the injury site is a pre-requisite for the differentiation of progenitor cells into mature EC. Here we could demonstrate that HDL additionally changes the phenotype of cultivated early outgrowth EPC into a more mature, spindle-shaped morphology. In accordance with recent observations that the level of abundance of eNOS mRNA correlates with the fraction of spindle-shaped cells in culture [45], HDL treatment was associated with up-regulation of eNOS indicating the enhanced differentiation into mature EC. However, the overall eNOS mRNA and protein abundance was less in EPC even after HDL treatment compared to mature EC. Apparently, eNOS expression seems to correlate with maturational stages since Hur et al. recently demonstrated a higher expression of eNOS in late cobblestone-shaped EPC compared to early EPC [46]. These findings may be supported by the fact that HDL concentrations did not correlate with the number of CD133 positive EPC which represent a more immature cell population compared to CD34/KDR positive EPC.
To evaluate the in vivo relevance of our findings, we evaluated the effect of HDL on re-endothelialization after focal EC denudation of the common carotid artery. In order to prevent immunological reactions by using HDL derived from human beings, we used rHDL which has been previously evaluated in human studies. rHDL was able to influence in vitro EPC function in the same way than HDL derived from human beings (data not shown). rHDL increased the number of circulating Sca1/flk1 positive EPC within peripheral blood. This increase in cells was associated with a significantly enhanced re-endothelialization compared to placebo-treated animals. At present, we cannot rule out that species differences (mouse, human) and additional HDL-mediated effects on mature EC account for the observed effects. However, we and others have demonstrated in a number of studies that enhancement of EPC numbers as well as functional properties results in a significantly improved restoration of the damaged endothelium mediated by BM-derived cells [2, 4, 5, 15, 17, 47, 48]. Furthermore, we could demonstrate that in human beings, EPC levels but not haematopoietic progenitor cells correlate with HDL concentrations indicating that like other vasculoprotective agents, HDL is a potent mediator of EPC number and function. Beyond the function of EPC and HDL in the arterial system, Eichinger et al. recently demonstrated that patients with high levels of apolipoprotein A-I and HDL have a decreased risk of recurrent venous thromboembolism [49]. Interestingly, EPC seem to have additional anticoagulant and antifibrinolytic properties as recently demonstrated by Smadja et al., indicating another potentially HDL-mediated function of EPC within the venous system [50].
More insights into the multiple actions of HDL on the vascular wall are urgently needed. Here we provide evidence that HDL in addition to its effects on mature ECs mediate important action via the improvement of function of circulating EPC.
Acknowledgments
Kathrin Paul and Isabel Paez-Maletz provided outstanding technical assistance. rHDL was kindly provided by CSL Behring AG, Switzerland. This work was supported by Deutsche Gesellschaft für Kardiologie, Deutsche Forschungsgemeinschaft, Bonfor, the Ernst und Berta Grimmke-Stiftung, and by the European Vascular Genomics Network, a Network of Excellence granted by the European Commission (Contract N° LSHM-CT-2003-503254).
References
- 1.Shi Q, Rafii S, Wu MH, et al. Hammond WP. Evidence for circulating bone marrow-derived endothelial cells. Blood. 1998;92:362–7. [PubMed] [Google Scholar]
- 2.Walter DH, Rittig K, Bahlmann FH, et al. Statin therapy accelerates reendothelialization: a novel effect involving mobilization and incorporation of bone marrow-derived endothelial progenitor cells. Circulation. 2002;105:3017–24. doi: 10.1161/01.cir.0000018166.84319.55. [DOI] [PubMed] [Google Scholar]
- 3.Wassmann S, Werner N, Czech T, et al. Improvement of endothelial function by systemic transfusion of vascular progenitor cells. Circ Res. 2006;99:e74–83. doi: 10.1161/01.RES.0000246095.90247.d4. [DOI] [PubMed] [Google Scholar]
- 4.Werner N, Priller J, Laufs U, et al. Bone marrow-derived progenitor cells modulate vascular reendothelialization and neointimal formation: effect of 3-hydroxy-3-methylglutaryl coenzyme a reductase inhibition. Arterioscler Thromb Vasc Biol. 2002;22:1567–72. doi: 10.1161/01.atv.0000036417.43987.d8. [DOI] [PubMed] [Google Scholar]
- 5.Werner N, Junk S, Laufs U, et al. Intravenous transfusion of endothelial progenitor cells reduces neointima formation after vascular injury. Circ Res. 2003;93:e17–24. doi: 10.1161/01.RES.0000083812.30141.74. [DOI] [PubMed] [Google Scholar]
- 6.Crosby JR, Kaminski WE, Schatteman G, et al. Endothelial cells of hematopoietic origin make a significant contribution to adult blood vessel formation. Circ Res. 2000;87:728–30. doi: 10.1161/01.res.87.9.728. [DOI] [PubMed] [Google Scholar]
- 7.Dimmeler S, Zeiher AM. Vascular repair by circulating endothelial progenitor cells: the missing link in atherosclerosis. J Mol Med. 2004;82:671–7. doi: 10.1007/s00109-004-0580-x. [DOI] [PubMed] [Google Scholar]
- 8.Werner N, Nickenig G. Clinical and therapeutical implications of EPC biology in atherosclerosis. J Cell Mol Med. 2006;10:318–32. doi: 10.1111/j.1582-4934.2006.tb00402.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Imanishi T, Hano T, Sawamura T, et al. Oxidized low-density lipoprotein induces endothelial progenitor cell senescence, leading to cellular dysfunction. Clin Exp Pharmacol Physiol. 2004;31:407–13. doi: 10.1111/j.1440-1681.2004.04022.x. [DOI] [PubMed] [Google Scholar]
- 10.Rauscher FM, Goldschmidt-Clermont PJ, Davis BH, et al. Aging, progenitor cell exhaustion, and atherosclerosis. Circulation. 2003;108:457–63. doi: 10.1161/01.CIR.0000082924.75945.48. [DOI] [PubMed] [Google Scholar]
- 11.Tepper OM, Galiano RD, Capla JM, et al. Human endothelial progenitor cells from type II diabetics exhibit impaired proliferation, adhesion, and incorporation into vascular structures. Circulation. 2002;106:2781–6. doi: 10.1161/01.cir.0000039526.42991.93. [DOI] [PubMed] [Google Scholar]
- 12.Vasa M, Fichtlscherer S, Aicher A, et al. Number and migratory activity of circulating endothelial progenitor cells inversely correlate with risk factors for coronary artery disease. Circ Res. 2001;89:E1–7. doi: 10.1161/hh1301.093953. [DOI] [PubMed] [Google Scholar]
- 13.Heeschen C, Lehmann R, Honold J, et al. Profoundly reduced neovascularization capacity of bone marrow mononuclear cells derived from patients with chronic ischaemic heart disease. Circulation. 2004;109:1615–22. doi: 10.1161/01.CIR.0000124476.32871.E3. [DOI] [PubMed] [Google Scholar]
- 14.Dimmeler S, Aicher A, Vasa M, et al. HMG-CoA reductase inhibitors (statins) increase endothelial progenitor cells via the PI 3-kinase/Akt pathway. J Clin Invest. 2001;108:391–7. doi: 10.1172/JCI13152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Laufs U, Werner N, Link A, et al. Physical training increases endothelial progenitor cells, inhibits neointima formation, and enhances angiogenesis. Circulation. 2004;109:220–6. doi: 10.1161/01.CIR.0000109141.48980.37. [DOI] [PubMed] [Google Scholar]
- 16.Laufs U, Wassmann S, Czech T, et al. Physical inactivity increases oxidative stress, endothelial dysfunction, and atherosclerosis. Arterioscler Thromb Vasc Biol. 2005;25:809–14. doi: 10.1161/01.ATV.0000158311.24443.af. [DOI] [PubMed] [Google Scholar]
- 17.Strehlow K, Werner N, Berweiler J, et al. Estrogen increases bone marrow-derived endothelial progenitor cell production and diminishes neointima formation. Circulation. 2003;107:3059–65. doi: 10.1161/01.CIR.0000077911.81151.30. [DOI] [PubMed] [Google Scholar]
- 18.Schmidt-Lucke C, Rossig L, Fichtlscherer S, et al. Reduced number of circulating endothelial progenitor cells predicts future cardiovascular events: proof of concept for the clinical importance of endogenous vascular repair. Circulation. 2005;111:2981–7. doi: 10.1161/CIRCULATIONAHA.104.504340. [DOI] [PubMed] [Google Scholar]
- 19.Werner N, Kosiol S, Schiegl T, et al. Circulating endothelial progenitor cells and cardiovascular outcomes. N Engl J Med. 2005;353:999–1007. doi: 10.1056/NEJMoa043814. [DOI] [PubMed] [Google Scholar]
- 20.Barter P, Gotto AM, LaRosa JC, et al. HDL cholesterol, very low levels of LDL cholesterol, and cardiovascular events. N Engl J Med. 2007;357:1301–10. doi: 10.1056/NEJMoa064278. [DOI] [PubMed] [Google Scholar]
- 21.Spieker LE, Sudano I, Hurlimann D, et al. High-density lipoprotein restores endothelial function in hypercholesterolemic men. Circulation. 2002;105:1399–1402. doi: 10.1161/01.cir.0000013424.28206.8f. [DOI] [PubMed] [Google Scholar]
- 22.Buring JE, O’Connor GT, Goldhaber SZ, et al. Decreased HDL2 and HDL3 cholesterol, Apo A-I and Apo A-II, and increased risk of myocardial infarction. Circulation. 1992;85:22–9. doi: 10.1161/01.cir.85.1.22. [DOI] [PubMed] [Google Scholar]
- 23.Thompson MM, Reed SC, Cockerill GW. Therapeutic approaches to raising plasma HDL-cholesterol levels. Nat Clin Pract Cardiovasc Med. 2004;1:84–9. doi: 10.1038/ncpcardio0044. [DOI] [PubMed] [Google Scholar]
- 24.Tso C, Martinic G, Fan WH, et al. High-density lipoproteins enhance progenitor-mediated endothelium repair in mice. Arterioscler Thromb Vasc Biol. 2006;26:1144–9. doi: 10.1161/01.ATV.0000216600.37436.cf. [DOI] [PubMed] [Google Scholar]
- 25.Noor R, Shuaib U, Wang CX, et al. High-density lipoprotein cholesterol regulates endothelial progenitor cells by increasing eNOS and preventing apoptosis. Atherosclerosis. 2007;192:92–9. doi: 10.1016/j.atherosclerosis.2006.06.023. [DOI] [PubMed] [Google Scholar]
- 26.Sumi M, Sata M, Miura S, et al. Reconstituted high-density lipoprotein stimulates differentiation of endothelial progenitor cells and enhances ischemia-induced angiogenesis. Arterioscler Thromb Vasc Biol. 2007;27:813–8. doi: 10.1161/01.ATV.0000259299.38843.64. [DOI] [PubMed] [Google Scholar]
- 27.Van Oostrom O, Nieuwdorp M, Westerweel PE, et al. Reconstituted HDL increases circulating endothelial progenitor cells in patients with type 2 diabetes. Arterioscler Thromb Vasc Biol. 2007;27:1864–5. doi: 10.1161/ATVBAHA.107.143875. [DOI] [PubMed] [Google Scholar]
- 28.Tardif JC, Gregoire J, L’Allier PL, et al. Effects of reconstituted high-density lipoprotein infusions on coronary atherosclerosis: a randomized controlled trial. JAMA. 2007;297:1675–82. doi: 10.1001/jama.297.15.jpc70004. [DOI] [PubMed] [Google Scholar]
- 29.Kastelein JJ, Van Leuven SI, Burgess L, et al. Effect of torcetrapib on carotid atherosclerosis in familial hypercholesterolemia. N Engl J Med. 2007;356:1620–30. doi: 10.1056/NEJMoa071359. [DOI] [PubMed] [Google Scholar]
- 30.Nissen SE, Tardif JC, Nicholls SJ, et al. Effect of torcetrapib on the progression of coronary atherosclerosis. N Engl J Med. 2007;356:1304–16. doi: 10.1056/NEJMoa070635. [DOI] [PubMed] [Google Scholar]
- 31.Redgrave TG, Roberts DC, West CE. Separation of plasma lipoproteins by density-gradient ultracentrifugation. Anal Biochem. 1975;65:42–9. doi: 10.1016/0003-2697(75)90488-1. [DOI] [PubMed] [Google Scholar]
- 32.Wassmann S, Wassmann K, Jung A, et al. Induction of p53 by GKLF is essential for inhibition of proliferation of vascular smooth muscle cells. J Mol Cell Cardiol. 2007;43:301–7. doi: 10.1016/j.yjmcc.2007.06.001. [DOI] [PubMed] [Google Scholar]
- 33.Fontaine V, Filipe C, Werner N, et al. Essential role of bone marrow fibroblast growth factor-2 in the effect of estradiol on reendothelialization and endothelial progenitor cell mobilization. Am J Pathol. 2006;169:1855–62. doi: 10.2353/ajpath.2006.060260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Duda DG, Cohen KS, Scadden DT, et al. A protocol for phenotypic detection and enumeration of circulating endothelial cells and circulating progenitor cells in human blood. Nat Protoc. 2007;2:805–10. doi: 10.1038/nprot.2007.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wu Y, Ip JE, Huang J, et al. Essential role of ICAM-1/CD18 in mediating EPC recruitment, angiogenesis, and repair to the infarcted myocardium. Circ Res. 2006;99:315–22. doi: 10.1161/01.RES.0000235986.35957.a3. [DOI] [PubMed] [Google Scholar]
- 36.Voermans C, Rood PM, Hordijk PL, et al. Adhesion molecules involved in transendothelial migration of human hematopoietic progenitor cells. Stem Cells. 2000;18:435–43. doi: 10.1634/stemcells.18-6-435. [DOI] [PubMed] [Google Scholar]
- 37.Chavakis E, Aicher A, Heeschen C, et al. Role of beta2-integrins for homing and neovascularization capacity of endothelial progenitor cells. J Exp Med. 2005;201:63–72. doi: 10.1084/jem.20041402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Duan H, Cheng L, Sun X, et al. LFA-1 and VLA-4 involved in human high proliferative potential-endothelial progenitor cells homing to ischemic tissue. Thromb Haemost. 2006;96:807–15. [PubMed] [Google Scholar]
- 39.Qin G, Ii M, Silver M, et al. Functional disruption of alpha4 integrin mobilizes bone marrow-derived endothelial progenitors and augments ischemic neovascularization. J Exp Med. 2006;203:153–63. doi: 10.1084/jem.20050459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ip JE, Wu Y, Huang J, et al. Mesenchymal stem cells use integrin beta1 not CXC chemokine receptor 4 for myocardial migration and engraftment. Mol Biol Cell. 2007;18:2873–82. doi: 10.1091/mbc.E07-02-0166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Smadja DM, Bieche I, Helley D, et al. Increased VEGFR2 expression during human late endothelial progenitor cells expansion enhances in vitro angiogenesis with up-regulation of integrin alpha(6) J Cell Mol Med. 2007;11:1149–61. doi: 10.1111/j.1582-4934.2007.00090.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ingram DA, Caplice NM, Yoder MC. Unresolved questions, changing definitions, and novel paradigms for defining endothelial progenitor cells. Blood. 2005;106:1525–31. doi: 10.1182/blood-2005-04-1509. [DOI] [PubMed] [Google Scholar]
- 43.Dernbach E, Urbich C, Brandes RP, et al. Antioxidative stress-associated genes in circulating progenitor cells: evidence for enhanced resistance against oxidative stress. Blood. 2004;104:3591–7. doi: 10.1182/blood-2003-12-4103. [DOI] [PubMed] [Google Scholar]
- 44.Seetharam D, Mineo C, Gormley AK, et al. High-density lipoprotein promotes endothelial cell migration and reendothelialization via scavenger receptor-B type I. Circ Res. 2006;98:63–72. doi: 10.1161/01.RES.0000199272.59432.5b. [DOI] [PubMed] [Google Scholar]
- 45.Harraz M, Jiao C, Hanlon HD, et al. CD34 negative blood-derived human endothelial cell progenitors. Stem Cells. 2001;19:304–12. doi: 10.1634/stemcells.19-4-304. [DOI] [PubMed] [Google Scholar]
- 46.Hur J, Yoon CH, Kim HS, et al. Characterization of two types of endothelial progenitor cells and their different contributions to neovasculogenesis. Arterioscler Thromb Vasc Biol. 2004;24:288–93. doi: 10.1161/01.ATV.0000114236.77009.06. [DOI] [PubMed] [Google Scholar]
- 47.Iwakura A, Luedemann C, Shastry S, et al. Estrogen-mediated, endothelial nitric oxide synthase-dependent mobilization of bone marrow-derived endothelial progenitor cells contributes to reendothelialization after arterial injury. Circulation. 2003;108:3115–21. doi: 10.1161/01.CIR.0000106906.56972.83. [DOI] [PubMed] [Google Scholar]
- 48.Sandri M, Adams V, Gielen S, et al. Effects of exercise and ischemia on mobilization and functional activation of blood-derived progenitor cells in patients with ischemic syndromes: results of 3 randomized studies. Circulation. 2005;111:3391–9. doi: 10.1161/CIRCULATIONAHA.104.527135. [DOI] [PubMed] [Google Scholar]
- 49.Eichinger S, Pecheniuk NM, Hron G, et al. High-density lipoprotein and the risk of recurrent venous thromboembolism. Circulation. 2007;115:1609–14. doi: 10.1161/CIRCULATIONAHA.106.649954. [DOI] [PubMed] [Google Scholar]
- 50.Smadja DM, Basire A, Amelot A, et al. Thrombin bound to a fibrin clot confers angiogenic and haemostatic properties on endothelial progenitor cells. J Cell Mol Med. 2008;12:975–86. doi: 10.1111/j.1582-4934.2008.00161.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


