Abstract
Dermal papilla cells (DPCs) in the mammalian hair follicle have been shown to develop hair follicles through epithelial–mesenchymal interactions. A cell therapy to regenerate human hair is theoretically possible by expanding autologous human DPCs (hDPCs) and transplanting them into bald skin, though much remains to be overcome before clinical success. In this study, we compared gene signatures of hDPCs at different passages and human dermal fibroblasts, and found transforming growth factor (TGF)-β2 to be highly expressed in cultured hDPCs. Keratinocyte conditioned medium, which is known to help preserve the hair-inducing capacity of hDPCs, up-regulated TGF-β2 expression of hDPCs and also enhanced their alkaline phosphatase (ALP) activity, a known index for hair-inductive capacity. Through screening of components secreted from keratinocytes, the vitamin D3 analogue was found to promote TGF-β2 expression and ALP activity of hDPCs. In animal hair folliculogenesis models using rat epidermis and expanded hDPCs, inhibition of TGF-β2 signalling at the ligand or receptor level significantly impaired hair folliculogenesis and maturation. These results suggest an important role for TGF-β2 in hair follicle morphogenesis and provide insights into the establishment of future cell therapies for hair regrowth by transplanting expanded DPCs.
Keywords: TGF-β2, dermal papilla cell, hair regrowth, hair regeneration, tissue engineering, cell therapy, vitamin D3
Introduction
The mammalian hair follicle is a complex mini organ that consists of different lineages of cells, including epithelial, mesenchymal and pigmented cells. The dermal papilla, considered as the most important mesenchymal component, plays versatile roles in hair follicle morphogenesis and hair cycling via epithelial–mesenchymal interactions [1–4]. Because cultured dermal papilla cells (DPCs) as well as organ dermal papilla were found to have hair-inductive capacity [5–7], many attempts have been made to regenerate hair follicles by transplanting expanded DPCs, sometimes together with epithelial stem cells. However, challenges in developing regeneration strategies have arisen, as the hair-inductive ability of DPCs is lost upon culture and the molecules and mechanisms responsible for the hair-inducing capacity are not yet fully elucidated [8].
There are six major morphogenetic molecular family systems in hair follicle development and cycling: fibroblast growth factor (FGF), transforming growth factor (TGF)-β, sonic hedgehog, Wingless or Wnt pathway, neurotrophins and homeobox gene families [4, 9, 10]. In each morphogenetic stage, all function as responsible molecules for the reciprocal signalling between hair follicle epithelial and dermal components. In the context of hair follicle ‘neogenesis’, however, it is unclear which signalling molecule(s) among these pathways function in hair induction in transplanted DPCs. Thus far, specific signalling molecules, such as bone morphogenetic protein (BMP)-6 [11], have been shown to enhance mouse hair folliculogenesis. Wnt3a signalling from epithelial component is also required to maintain the inductive capacity of DPCs and to generate hair follicles [12]. These factors have been determined as candidates for hair-inducing activity by employing sophisticated transgenic approaches such as specific knockout or overexpression in vivo. In human beings, however, the difficulty in applying transgenic approaches has hampered such studies for specific in vivo gene function [13]. Therefore, although various biomarkers specifically expressed in human DPCs (hDPCs) have been reported [14, 15], their functions remain to be clarified.
Conditioned media obtained from epidermal keratinocyte culture (keratinocyte conditioned media, or KCM) are known to maintain DPC capacity to proliferate and induce hair follicles for a longer period than control media [16], suggesting that cultured keratinocytes release key factors for DPCs to maintain hair-inducing capacity. Keratinocytes produce a vast variety of soluble factors including growth factors, hormones and chemokines [17, 18]. Screening of biologically active components in KCM may identify the substances that stimulate DPCs to maintain their hair-inducing capability and provide an efficient method for in vitro expansion of hair-inductive DPCs.
We suggested that specific genes relating to hair-inducing capacity are up-regulated in hDPCs and that expression is promoted by particular components contained in human KCM. In this study, the global gene signatures of hDPCs at early and later passages and human dermal fibroblasts (hDFs) with no hair-inducing capacity were compared by microarray analysis. Our results showed that the TGF-β2 gene was specifically expressed in hDPCs and its expression was up-regulated by KCM. We further investigated potential roles of TGF-β2 in hair induction by hDPCs and sought to identify keratinocyte-derived components that can affect the hair-inducing capacity of hDPCs.
Materials and methods
Human DPC and DF culture
Scalp and facial skin with hair were obtained from facelift operations performed at two institutions; informed consent was obtained using protocols approved by institutional review boards from each individual institution. Dermal papillae were isolated from the hair follicles under a microscope, and placed onto a culture dish containing Dulbecco's modified Eagle's medium (DMEM; Gibco, Carlsbad, CA, USA) supplemented with 10% foetal bovine serum (10% DMEM). After 2 weeks of explant culture, expanded hDPCs were subcultured with the same medium. Human DFs were obtained from the explant culture of facial skin dermis of the same individuals and cultured in 10% DMEM.
Human epidermal keratinocyte culture and preparation of the conditioned culture media
Human facial skin was cut into 3 × 3 mm pieces and incubated in 10% DMEM supplemented with 1000 U/ml Dispase™ II (Sankyo, Tokyo, Japan) at 4°C for 15–18 hrs. The epidermis was carefully peeled off from the dermis and incubated in phosphate buffered saline (PBS) supplemented with 0.25% trypsin and ethylenediaminetetraacetic acid (EDTA) mixture at 37°C for 20 min. to obtain fresh keratinocyte cell suspension. Keratinoctyes were cultured in serum free media, DKSFM™ (Gibco), for 7–10 days up to 60–80% confluence; afterwards, the culture medium was switched to 10% DMEM. The culture supernatant was collected after 1 week, centrifuged at 3000 ×g for 30 min., and filtrated through a 0.22 μm membrane filter (Micropore, Madison, NJ, USA). The supernatant was mixed with fresh 10% DMEM at a 1:1 ratio to make KCM for hDPC culture.
Reagents
Reagents supplemented to hDPC culture were as follows: acidic FGF (Peprotech, Rocky Hill, NJ, USA); basic FGF (Peprotech); BMP-2 (Wako, Osaka, Japan); interleukin (IL)-1β (Endogen, Rockford, IL, USA); IL-6 (Peprotech); IL-8 (Wako); vascular endothelial growth factor (VEGF) (Wako); platelet-derived growth factor (PDGF)-BB (Wako); nerve growth factor (Sigma-Aldrich, Louis, MO, USA); heparin-binding EGF-like growth factor (Peprotech); macrophage inflammatory protein (MIP)-3α (R&D systems, Minneapolis, MN, USA); monocyte chemotactic protein (MCP)-1 (R&D systems); insulin-like growth factor (IGF)-1 (Sigma-Aldrich); epithelial cell-derived neutrophil-activating peptide (ENA)-78 (Wako); growth-related oncogene (GRO)-α (Wako); 1,25-dihydroxyvitamin D3[1,25(OH)2D3] (LKT laboratories, St. Paul, MN, USA); cholesterol sulphate (Sigma-Aldrich); all-trans retinoic acid (Biomol, Hamburg, Germany); 17β-estradiol (Cayman Chemical, Ann Arbor, MI, USA) and dihydrotestosterone (Biomol). All reagents were diluted in PBS or ethanol to 1000-fold of the final working concentrations indicated in manufacturer’s instructions and stored in aliquots at –20°C.
Real-time RT-PCR
RNA was isolated from cultured hDPCs or hDFs using an RNeasy™ Mini Kit (Qiagen, Valencia, CA, USA), followed by reverse transcription. PCR amplification of cDNA was performed in a 50 μl reaction consisting of 1× TaqMan™ Universal Master Mix (Applied Biosystems, Foster City, CA, USA) with the ABI 7700 sequence detection system. Gene expression of various hair follicle-related genes was quantified based on measurement of the cycle threshold using the following TaqMan™ pre-designed primers and probes (Applied Biosystems): TGF-β2 (Hs00236092_m1); TGF-β1 (Hs00998129_m1); BMP-2 (Hs00154192_m1); syndecan1 (Hs00896423_m1); integrin-β1 (Hs00559595_m1); keratinocyte growth factor (KGF) (Hs00940253_m1); VEGF (Hs00900054_m1); IGF-1 (Hs01547656_m1); hepatocyte growth factor (HGF; Hs00300159_m1); PDGF (Hs00234042_m1); steroid 5α-reductase II (Hs01399057_m1); versican (Hs00171642_m1); ephrin-A3 (Hs00191913_m1) and androgen receptor (AR; Hs00171172_m1). We used GAPDH (Hs99999905_m1) as an endogenous reference gene.
Microarray generation and analysis
To identify genes differentially expressed in hDPCs responding to the substances secreted from keratinocytes, gene expressions of hDPCs and hDFs of the same individual were compared. tRNA of hDPCs cultured in KCM (passages 2 and 8) and hDFs (passage 2) hDFs in 10% DMEM were isolated using an RNeasy™ Mini Kit. The quality of each sample was assessed by rRNA 28S/18S ratio and RNA integrity number using Agilent 2100 Bioanalyzer™ (Agilent Technology, Palo Alto, CA, USA). cDNA was obtained from 5 μg of tRNA by one-cycle of reverse transcription. The biotin-labelled cRNAs were purified, fragmented, and hybridized to the GeneChip™ Human Genome U133 Plus 2.0 Array (Affymetrix, Santa Clara, CA), which was then scanned by the GeneChip™ 3000 Scanner (Affymetrix) following the manufacturer’s protocol. The numerical data of the signal intensity was analysed using GeneChip™ Command Console™ Software (Affymetrix) and Microsoft Excel™.
Cytokine array analysis
The supernatant of human keratinocyte culture was collected at 1 or 2 weeks after the medium switch from DKSFM to 10% DMEM by the methods described above. Expression levels of multiple cytokines were assayed in each sample using the Human Cytokine Array VI (Ray Biotech, Norcross, GA, USA) following the manufacturer’s instruction. The image of each array was captured by a digital camera (Nikon, Tokyo, Japan) and converted to the binary image format. The signal intensity was calculated using the image processing software, Scion™ Image (Scion Corp., Frederick, MD, USA).
ELISA for TGF-β2
The influence of KCM and other keratinocyte-derived factors on TGF-β2 protein production in hDPCs was assessed by sandwich ELISA, Quantikine™ human ELISA for TGF-β2 (R&D systems). Reagents supplemented to the hDPC culture were as described above. The supernatant of hDPC culture at passage 2 was collected after 96 hrs of incubation, and processed with ELISA following the manufacturer’s instructions.
Alkaline phosphatase (ALP) activity assay
The influence of KCM and other keratinocyte-derived factors on hDPC ALP activity was assessed by fluorescent based ELISA, Sensolyte™ FDP Alkaline Phosphatase Assay Kit (AnaSpec, San Jose, CA, USA). Reagents supplemented to the hDPCs culture were as described above. hDPCs were seeded on a 24-well plate and cell lysates were collected after 48 hrs of incubation. The fluorescence intensity was measured using a microplate fluorescence reader, BTX-880 (Beckman-Coulter, Brea, CA, USA). The fluorescence reading was normalized with the cell number at time of harvest.
MTT proliferation assay
The influence of KCM and other keratinocyte-derived factors on hDPC proliferation was assessed by a MTT cell proliferation assay kit (Roche, Basel, Switzerland). hDPCs were seeded on a 96-well plate and cell lysates were collected after 96 hrs of incubation. The fluorescence intensity was measured using a microplate fluorescence reader, BTX-880 (Beckman-Coulter) and cell number was calculated following the manufacturer’s instruction.
Animal assays for hair folliculogenesis
We generated rat-human chimeric hair follicles in nude mice using a previously described sandwich method [16, 19, 20] with some modifications. Briefly, hDPCs were cultured as described above, and the hDPC sheet was scraped off, cut into 1 × 1 mm pieces and used as transplanted constructs. The follicular foot pad skin of 8-week-old F344 rats was cut into 3-mm2 pieces and incubated in 10% DMEM and 1000 U/ml Dispase™ II at 37°C for 20 min. to separate the epidermis and dermis. The DPC construct was placed between the epidermis and dermis of the foot pad and transplanted to the subcutis of a 6-week-old Balb/c nude mouse (Fig. S1). The transplants were harvested 4 weeks later and processed for histological evaluation of the number and maturation stage of generated hair follicles. The maturation stage was categorized into eight stages (S1 to S8) according to a previously described method [21] (Fig. S2).
A chamber grafting method was also employed according to previously reported methods [11, 20, 22, 23] with some modifications (Fig. S1). A combination of cultured hDPCs (P3) and cultured neonatal B6 mouse keratinocytes was employed to reconstitute human-mouse chimeric hair follicles. In another experiment, a combination of freshly isolated foetal dermal cells and foetal keratinocytes isolated from BL6 mouse embryos was also utilized. For preparation of cultured mKC, the dorsal skin of newborn mice was incubated in 1000 U/ml Dispase™ II at 4°C for 15–18 hrs, and the epidermis was consequently separated from the dermis. The epidermis was digested with a 0.05% Trypsin and 0.2 mM EDTA mixture at 37°C for 20 min. to obtain single cells. The resultant cell suspension was filtrated through a 40-μm cell strainer and cultured in DKSFM™ for 4 days. For preparation of mouse foetal dermal cells and keratinocytes, the dorsal skin of E17.5 BL6 embryos was digested as described above. A dome-shaped polypropylene chamber made from a PCR tube lid (Greiner Bio-One, Frickenhausen, Germany) was transplanted onto the back of a nude mouse 5 days before cell transfer. The number of mesenchymal cells (cultured hDPCs or mouse fresh foetal dermal cells) and epithelial cells (cultured mouse neonatal keratinocytes or fresh foetal keratinocytes) transplanted into each chamber was 106 (2 × 106 in total). Skin samples of recipient nude mice were harvested 4 weeks after cell transplantation. Each group consisted of four chambers on four mice.
TGF-β signal inhibition in vivo
TGF-β signal inhibitors were administered to the recipient nude mice. A selective kinase inhibitor for the TGF-β type I receptor, SB431542 (10 μM; Sigma-Aldrich) or an equivalent amount of vehicle was administrated continuously (0.25 μl/hr) by Alzet™ osmotic pumps (Durect, Cupertino, CA, USA), and transplanted subcutaneously beside the foot pad construct in the sandwich model or the chamber in the chamber model. A specific antibody for human TGF-β2 (BioVision, Mountain View, France) or TGF-β1/2/3 (R&D systems) was used to neutralize TGF-β2 or all three isoforms (TGF-β1/2/3) of TGF-β ligand. One microgram of neutralizing or negative control IgG (R&D systems) was administered every second day by a local injection to the subcutis. Signal inhibition in both models was performed for 4 weeks.
Immunohistochemical staining
After harvest, the foot pad transplant was embedded in the OCT compound (Sakura Finetek, Tokyo, Japan), frozen in liquid nitrogen and stored at –80°C until sectioning. Frozen sections (10 μm) were placed on slides, air dried at room temperature for 1 hr, fixed in paraformaldehyde (4% in PBS) for 1 min. and washed in PBS for 5 min. Every other slide was stained with haematoxylin and eosin by the standard protocol, and the number and maturation of generated hair follicles were evaluated. The other slides were processed by immunohistochemical staining. Briefly, the sections were incubated with 5% goat serum at room temperature for 30 min., followed by incubation with mouse anti-human TGF-β2 (1:100, Neo Markers, Fremont, CA, USA), mouse anti-human TGF-β1 (1:100, Lab Vision, Fremont, CA, USA), rabbit anti-rat phospho-SMAD-2 (1:100, Millipore, Billerica, MA, USA), rabbit anti-rat SMAD-7 (1;100, Santa Cruz Biotechnology, Santa Cruz, CA, USA) or rabbit anti-rat PAI (plasminogen activator inhibitor)-1 (1:100, Innovative Research, Novi, MI, USA) antibodies at room temperature for 60 min. Alexa™546-conjugated goat antimouse IgG (1:100, Molecular Probes, Eugene, CA, USA) or Alexa™ 488 conjugated goat anti-rabbit IgG (1:100, Molecular Probes) was used as a secondary antibody to detect the primary antibodies. Counter staining was performed with Hoechst33342 (Dojindo, Kumamoto, Japan).
Statistical analysis
Data were presented as mean ± S.E. To test the significance of quantitative data, the unpaired Student’s t-test was applied.
Results
TGF-β2 gene is specifically detected in hDPCs by microarray analysis
We performed comparative microarray analysis of the molecular gene signatures of cultured hDPCs and hDFs. We suggested that gene(s) related to hair-inducing pathways are contained in DPs, but not in DFs, and that expression of the gene(s) decreases upon passage. We did not use the same culture conditions for hDPCs and hDFs in this experiment; we used KCM for culturing hDPCs to maximize their hair-inducing capacity but used DMEM (basal medium of KCM) for hDFs not to provide this support. In each comparison, out of 54,613 genes, we first identified genes with signal intensity of at least 100. When we compared cultured hDPCs with hDFs of the same individual at the earlier passage (P2), we found 567 up-regulated and 498 down-regulated genes (‘early-DPC genes’) in hDPCs, of which the fold difference from hDFs was at least 1 or at most –1 in log ratio. At passage 8, the number of up-regulated and down-regulated genes with the same features decreased to 143 and 174 genes, respectively (‘late-DPC genes’). We found 34 overlapping up-regulated genes (Table S1) and 48 overlapping down-regulated genes (Table S2) in both early-DPC and late-DPC genes. When limited to genes whose expression changed significantly from passage 2 to passage 8, only 11 up-regulated and 5 down-regulated genes were listed (Table 1). TGF-β2 was included among the 11 up-regulated genes, suggesting its putative function in hair-inducing capacity.
Table 1.
Selected genes up- or down-regulated in human dermal papilla cells (hDPCs). Signal intensities of gene expression in hDPCs were compared with those of human dermal fibroblasts of the same individual, and the fold changes were expressed as log ratio values in the right two columns (P2 and P8). Among the up-regulated 34 genes in Table S1, 11 genes showed a fold change of at least 2 (log ratio), with a decrease of the fold change from P2 to P8 (‘Up-regulated’, upper panel). Among the down-regulated 44 genes in Table S2, 5 genes showed a difference of at most -2 (log ratio) (‘Down-regulated’, lower panel)
| Symbol | Gene name | P2 | P8 | |
|---|---|---|---|---|
| Up-regulated | CCL2 | Chemokine (C-C motif) ligand 2 | 4.93 | 1.98 |
| MGC5618 | Hypothetical protein MGC5618 | 4.31 | 1.20 | |
| G0S2 | G0/G1 switch 2 | 3.93 | 2.05 | |
| TFPI2 | Tissue factor pathway inhibitor 2 | 2.85 | 1.40 | |
| HNT | Neurotrimin | 2.84 | 1.01 | |
| TGFB2 | Transforming growth factor, beta 2 | 2.47 | 1.49 | |
| PRG1 | Proteoglycan 1, secretory granule | 2.31 | 2.19 | |
| HLA-C | Major histocompatibility complex, class I, C | 2.23 | 1.29 | |
| FGF7 | Fibroblast growth factor 7 (keratinocyte growth factor) | 2.10 | 1.50 | |
| TNFRSF10B | Tumor necrosis factor receptor superfamily, member 10b | 2.10 | 1.08 | |
| PTX3 | Pentraxin-related gene, rapidly induced by IL-1 beta | 2.00 | 1.12 | |
| Down-regulated | EGR1 | Early growth response 1 | –3.46 | –1.62 |
| TK1 | Thymidine kinase 1, soluble | –2.46 | –2.57 | |
| SGK | Serum/glucocorticoid regulated kinase | –2.27 | –1.23 | |
| DOK5 | Docking protein 5 | –2.09 | –1.14 | |
| CDC20 | CDC20 cell division cycle 20 homolog (S. cerevisiae) | –2.02 | –2.86 |
TGF-β2 gene is preferentially expressed in cultured hDPCs
Because cultured DPCs have been shown to contain hair-inducing capacity but lose it upon culture [6, 22], the gene expression profile of cells at early passages of culture was determined. We selected a set of genes previously reported to be related to DPC function [14, 24–36], and examined which genes were up-regulated in hDPC cultured in 10% DMEM at passage 2 in comparison with expression in hDFs cultured in 10% DMEM; the set of genes included TGF-β1, TGF-β2, BMP-2, syndecan-1, integrin-β1, KGF, VEGF, HGF, PDGF, 5α-reductase II (5αRII), versican, ephrin-A3 and AR. Quantitative real-time PCR revealed that TGF-β2 was significantly up-regulated in cultured hDPCs compared to hDFs (Fig. 1A). We also examined hDPCs at later passages. At passage 8, hDPCs were viable enough to keep proliferating and showed no sign of apoptosis or growth arrest. TGF-β2 gene expression was still up-regulated in hDPCs at passage 8 compared to hDFs, and was slightly lower compared to hDPCs at passage 2 (Fig. 1B).
Figure 1.
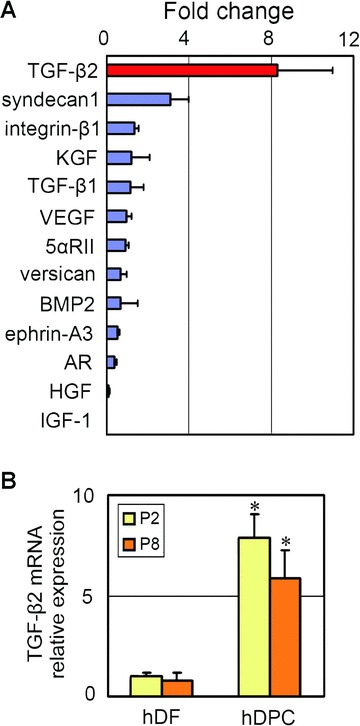
TGF-β2 mRNA is preferentially expressed in cultured human dermal papilla cells (hDPCs). (A) Relative mRNA expression of hair follicle-related genes in cultured hDPCs. mRNA was isolated from cultured hDPCs and hDFs of the same individual (passage 2: P2) and expression levels were quantified by real-time PCR. The expression levels were normalized to GAPDH mRNA expression and shown as fold changes (hDPC/hDF). n= 3. (B) TGF-β2 mRNA relative expression in cultured hDPCs and hDFs of the same individual at passage 2 (P2) or 8 (P8). mRNA expression levels were normalized to GAPDH mRNA expression and fold changes to hDF (P2) were shown. n= 3. *Significant difference from hDFs (P < 0.01).
TGF-β2 gene expression in cultured hDPCs is enhanced by epidermal KCM
It is well documented that rodent DPCs obtained from the vibrissa hair follicles maintain their proliferative and hair-inducing capacities when they are cultured in KCM [16]. To assess whether hDPCs would exhibit similar properties, we first tested the proliferative effect of human KCM on cultured hDPCs. KCM showed a marked effect on promoting hDPC proliferation to the extent of an approximate 1000-fold increase in cell number within eight passages (60–70 days) (Fig. 2A). Although KCM did not cause apparent alterations in the morphology of hDPCs, TGF-β2 mRNA expression was significantly up-regulated in hDPCs cultured in KCM compared to control media or other kind of commercially available growth media (Amniomax™ II) (Fig. 2B).
Figure 2.
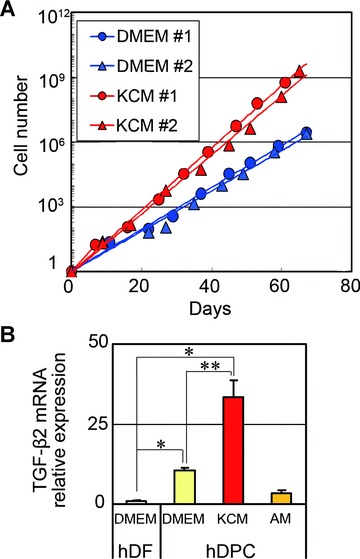
Effects of keratinocyte conditioned medium (KCM) on proliferation and TGF-β2 gene expression in human dermal papilla cells (hDPCs). (A) Integral cell numbers of hDPCs cultured in 10% DMEM or KCM. hDPCs and DFs from two different individuals (#1 and #2) were cultured in 10% DMEM or KCM up to passage 8. Each plot indicates the integral cell number (shown as fold increase from initial seeding) at the time of each subculture. (B) TGF-β2 mRNA relative expression in hDPCs and hDFs (passage 2) cultured in 10% DMEM, KCM or Amniomax™ II (AM). mRNA expression levels were normalized to GAPDH mRNA expression and fold changes to hDF were shown. n= 4. *Significant difference from hDFs (P < 0.01). **Significant difference from hDPCs cultured in 10% DMEM (P < 0.05).
hDPCs respond to soluble factors from epidermal keratinocytes
We next sought to identify possible KCM components that enhance TGF-β2 expression in hDPCs. We first examined the cytokine expression profiles of human KCM using a cytokine antibody array. Among 79 cytokines examined, IL-8, IL-6, IL-1β, MCP-1, Gro, MIP-3α, tissue inhibitor of metalloproteinase (TIMP)-1, TIMP-2, ENA-78, macrophage-derived chemokine, PDGF-BB, VEGF, insulin-like growth factor binding protein (IFGBP)-2 and regulated upon activation, normal T-cell expressed and secreted (RANTES) were highly expressed in KCM compared with control media (Fig. 3A). In addition to these cytokines, soluble factors considered to have biological activity on the hair follicles [17, 18, 37–46] were tested for induction of TGF-β2 expression in hDPCs cultured in 10% DMEM. Among 21 factors tested, a biologically active metabolite of vitamin D3, 1,25(OH)2D3, and IL-1β, as well as KCM, showed a marked effect on promoting TGF-β2 mRNA expression in hDPCs (Fig. 3B). The promotion of TGF-β2 mRNA expression by 1,25(OH)2D3 or IL-1β was independent of serum supplementation (Fig. S3). ELISA for TGF-β2 protein revealed that TGF-β2 secretion from hDPCs was highly elevated upon supplementation with 1,25(OH)2D3 or KCM, while no effect was observed with the other factors (Fig. 3C). Furthermore, ALP activity, a well-established marker for DPCs and hair-inducing property in hDPCs [11, 47], was significantly higher in the presence of 1,25(OH)2D3 as well as KCM (Fig. 3D). In contrast, MTT assay revealed that hDPC proliferation was impaired by 1,25(OH)2D3, while enhanced by KCM (Fig. 3E).
Figure 3.
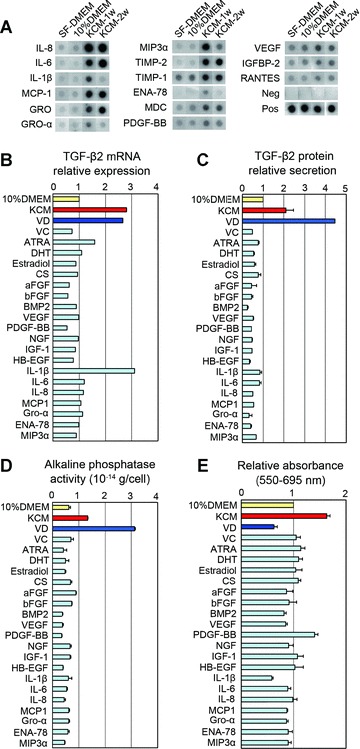
Influences of keratinocyte conditioned media (KCM) components and other factors on human dermal papilla cells (hDPCs) in vitro. (A) Cytokine expression in human KCM. Each panel shows expression of each indicated cytokine released by hDPCs cultured in serum-free DMEM (SF-DMEM), DMEM with 10% foetal bovine serum (10% DMEM), and KCM harvested 1 or 2 weeks after the medium switch (KCM-1w or KCM-2w). (B) TGF-β2 mRNA expression of hDPCs 48 hrs after supplementation of each factor. The data are shown as fold change compared to control media (10% DMEM). (C) TGF-β2 protein in hDPCs 48 hrs after supplementation of each factor. The data are shown as fold change compared to control media (10% DMEM). n= 3. (D) Alkaline phosphatase activity of cultured hDPCs 48 hrs after supplementation of each factor. The data are shown in 10−14 g/cell equivalent. n= 3. (E) Relative cell number of hDPCs measured by the MTT assay. The data are shown as fold change compared to control media (10% DMEM). n= 3. Supplements and concentrations used in (B), (C), (D) and (E) were as follows: acidic FGF (aFGF, 100 ng/ml), basic FGF (bFGF, 100 ng/ml). BMP-2 (100 ng/ml), IL-1β (100 ng/ml), IL-6 (100 ng/ml), IL-8 (100 ng/ml), vascular endothelial growth factor (100 ng/ml), PDGF-BB (100 ng/ml), nerve growth factor (100 ng/ml), heparin-binding EGF-like growth factor (100 ng/ml), MIP-3α (100 ng/ml), MCP-1 (100 ng/ml), IGF-1 (100 ng/ml), ENA-78 (100 ng/ml), GRO-α (100 ng/ml), 1,25(OH)2D3 (VD, 100 nM), ascorbic acid (VC, 100 μM), cholesterol sulphate (CS, 100 μM), all-trans retinoic acid (ATRA, 10 nM), 17β-estradiol (10 nM) and dihydrotestosterone (DHT, 10 nM).
Active form of vitamin D promotes TGF-β2 gene expression of hDPCs
Real-time RT-PCR revealed that supplementation of 10–1000 nM of 1,25(OH)2D3 significantly up-regulated TGF-β2 mRNA expression in hDPCs after 24 or 48 hrs of incubation (Fig. 4A), and this up-regulation of TGF-β2 expression was seen as early as 8 hrs (Fig. 4B). ELISA analysis of secreted TGF-β2 protein showed that 1,25(OH)2D3 significantly promoted TGF-β2 secretion from hDPCs in a dose-dependent manner (Fig. 4C).
Figure 4.
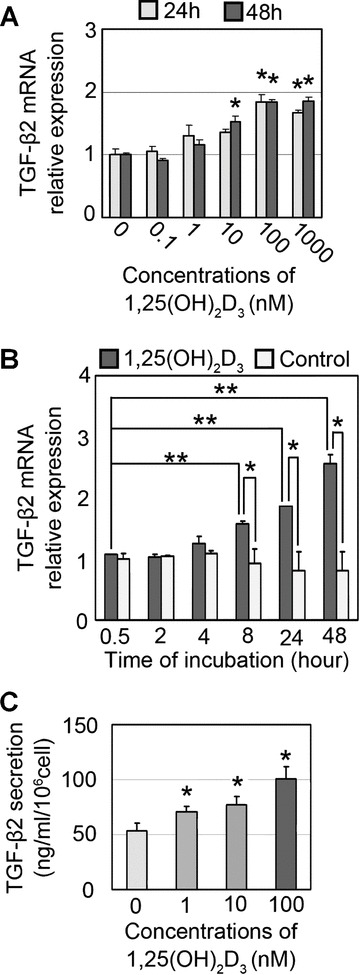
1,25-dihydroxyvitamin D3 (1,25(OH)2D3) up-regulates TGF-β2 expression in human dermal papilla cells (hDPCs). (A) Quantitative real-time PCR detection of TGF-β2 mRNA expression in hDPCs (passage 2) cultured for 24 or 48 hrs in the presence of various concentrations (0, 0.1, 1, 10, 100, 1000 nM) of 1,25(OH)2D3. The data are shown as fold changes to the baseline expression (at 0 nM). n= 5. *Significant difference from baseline expression (P < 0.05). (B) Quantitative real-time PCR detection of TGF-β2 mRNA expression in hDPCs (passage 2) at various times of incubation (0.5, 2, 4, 8, 24, 48) in the presence of 100 nM of 1,25(OH)2D3. The data are shown as the fold changes to the baseline expression (at 0 hr). n= 3. *Significant difference between each pair (P < 0.05). **Significant difference from baseline (P < 0.05). (C) Quantitative detection by ELISA of TGF-β2 protein secretion from cultured hDPCs (passage 2, normalized to 106 cells equivalent, 3 days incubation) in the presence of various concentrations of 1,25(OH)2D3 (0, 1, 10, 100 nM). *Significant difference from 0 nM (P < 0.05).
Inhibition of TGF-β2 signalling at either the receptor or ligand level suppresses hair folliculogenesis in vivo
The functional capacity of TGF-β2 in contributing to hair induction was assessed in an animal model for hair folliculogenesis. Rat-human chimeric hair follicles were generated in nude mice using the sandwich method described above. In control mice, the chimeric hair follicles were generated in 3–4 weeks after grafting of cultured hDPCs and showed histological features at a variety of developmental stages (Fig. S2), mimicking those of foetal hair follicle morphogenesis as described previously [20, 48]. Immunohistochemistry confirmed that TGF-β2, but not TGF-β1, was expressed in the dermal sheath of newly developed hair follicles (Fig. 5A).
Figure 5.
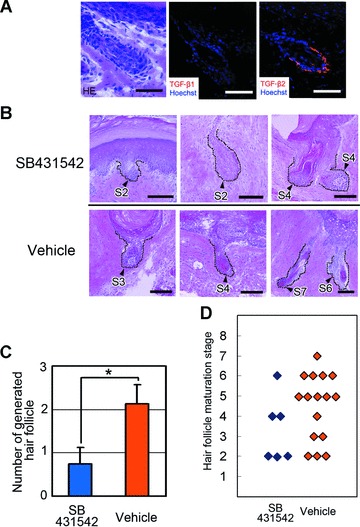
Histological analysis of generated chimeric hair follicles in sandwich models with or without TGF-β signal inhibition. Cell sheets of human dermal papilla cells (hDPCs) were inserted between the dermis and epidermis of rat foot pad skin. The sandwiched construct was transplanted into the subcutis of nude mice (see Fig. 1), and harvested 4 weeks after transplantation. (A) The bulb region of a generated hair follicle. Left: Haematoxylin and eosin staining, middle and right: immunohistochemical stainings for TGF-β1 and TGF-β2. Nuclear staining was performed with Hoechst33342. Bars = 20 μm. (B) Histology of generated hair follicles with or without SB431542-mediated inhibition of TGF-β signal. Generated hair follicles and boundaries are indicated with black arrowheads and dashed lines. The maturation stage is indicated beside each follicle, e.g.‘S2’, ‘S4’ or ‘S7’, and was evaluated according to the classification proposed by Paus et al.[21] after modification. (See Table S3 and Fig. S2 for details). Haematoxylin and eosin staining. Bars = 50 μm. (C) Number of generated hair follicles per transplant. Hair follicle morphogenesis was significantly smaller in number in the SB431542-administered mice compared to control mice. Four recipient mice were used for each group; three hDPC sheet fragments were sandwiched for each mouse. *Significant difference from the control mice (P < 0.05). (D) Maturation stage data of generated hair follicles with or without SB431542-mediated inhibition of TGF-β signal. Note the decreased distribution of well-maturated hair follicles (S5 to S7) in the SB431542-administered mice.
To evaluate the dependency of TGF-β signalling in hair folliculogenesis in the above animal models, signal transduction was inhibited by a continuous infiltration of SB431542, a highly selective kinase inhibitor for TGF-β type I receptor [49]via osmotic pump. Histological evaluation revealed that SB431542 suppressed both the frequency and maturation of hair follicle development. In control mice, well-maturated hair follicles were observed that could be categorized into high maturation stages, with structures such as the hair shaft and the sebaceous gland (Fig. 5B). On the other hand, in the SB431542-administered mice, signs of impaired maturation, such as pseudo-keratosis of the inner root sheath, were seen (Fig. 5B). The total number of generated hair follicles was significantly decreased in the SB431542-administered mice (Fig. 5C). Furthermore, a morphometric categorization of maturation stages of generated hair follicles demonstrated the suppressive effects of SB431542 on folliculogenesis (Fig. 5D); follicles of control mice were observed to be in well-maturated stages (stage 5 to 7), while follicles of SB431542-administered mice were classified as poorly maturated stages (stages 2 to 4).
Two neutralizing antibodies were employed for the TGF-β ligand-neutralizing approach and administered via local injections: a neutralizing antibody specific for TGF-β2 with no effect on TGF-β1 or TGF-β3 and a pan TGF-β neutralizing antibody that inhibits TGF-β1, TGF-β2 and TGF-β3 activity. Although histological analysis did not reveal any significant differences in maturation stages of generated hair follicles among the groups, the number of inducted hair follicles was significantly decreased in the antibody-administered mice compared with the nonspecific IgG-administered mice (Fig. 6A–C).
Figure 6.
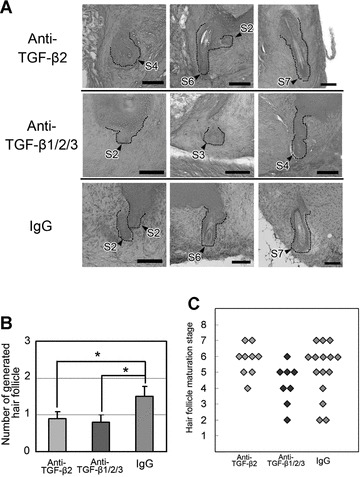
Neutralizing antibodies against TGF-βs impair hair folliculogenesis in sandwich models. (A) Histology of generated hair follicles with or without inhibition of TGF-β signal by neutralizing antibody against TGF-β2 or TGF-β1/2/3. Generated hair follicles and their boundaries are indicated with black arrowheads and dashed lines. The maturation stage is indicated beside each follicle, e.g.‘S2’, ‘S4’ or ‘S7’, and was evaluated according to the classification proposed by Paus et al.[21] after modification. (See Table S3 and Fig. S2 for details). Haematoxylin and eosin staining. Bars = 50 μm. (B) Number of generated hair follicles per transplant. Hair follicle morphogenesis was significantly smaller in number in mice treated with anti-TGF-β2 antibody or anti-TGF-β1/2/3 antibody than in those treated with control IgG. Five recipient mice were used for each group; three hDPC sheet fragments were sandwiched for each mouse. *Significant difference from the control mice (P < 0.05). (C) Maturation stage data of hair follicles generated in mice with administration of anti-TGF-β2 or anti-TGF-β1/2/3 neutralizing antibody or nonspecific control IgG.
In addition, a hair reconstitution assay with or without signal inhibition by anti-TGF-β2 antibody was also performed with the chamber method. The number of generated hair follicles was significantly smaller in mice treated with anti-TGF-β2 antibody compared to control mice (Fig. 7A and B). In chamber models using mouse DPCs (foetal dermal cells), SB431542 and anti-TGF-β2 antibody substantially decreased the average number of generated hair follicles, though the differences did not reach statistical significance (Fig. S4).
Figure 7.
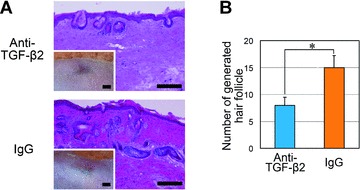
Histological analysis of hair folliculogenesis in chamber models with or without TGF-β2 signal inhibition. Cultured human dermal papilla cells (hDPCs) and cultured keratinocytes derived from newborn BL6 mice (mKC) were mixed as a cell suspension and implanted into the chamber on the back of nude mice. Skin samples were harvested 4 weeks after cell transfer; hair folliculogenesis was evaluated with histological sections. Four chambers were prepared on four mice per group. (A) Histology (haematoxylin and eosin staining) and macroscopic views (inset). Recipient nude mice were treated with a neutralizing antibody against TGF-β2 (top) or a negative control IgG (bottom). Bars = 200 μm (haematoxylin and eosin staining) or 2 μm (inset). (B) Average number of generated hair follicles per section (n= 4). Hair follicle morphogenesis was significantly smaller in number in mice treated with anti-TGF-β2 antibody than those treated with control IgG. *Significant difference from control mice (P < 0.05).
To further assess effects of augmentation of TGF-β2 signal on hair follicle induction, TGF-β2-releasing gelatin hydrogel microsphere beads (GMBs) were incorporated into the sandwich models; no positive effect of TGF-β2 signal augmentation was observed with this approach (Fig. S5).
Immunohistological analysis of TGF-β signal transduction in animal models
To analyse TGF-β2 signal transduction in hair folliculogenesis in the sandwich models, phosphorylation of SMAD-2 and expression of two SMAD-2 target genes (SMAD-7 and PAI-1) [50] were evaluated by immunohistology (Fig. 8). In control mice, phosphorylated SMAD-2 (pSMAD-2) translocation to nuclei, and positive signals of SMAD-7 and PAI-1 were observed in the epithelium of generated hair follicles. In regions in which no hair follicle development was observed despite the presence of DiI-labelled hDPCs beneath the basal lamina, signs of TGF-β2 signalling activation were not observed in either SB431542- or anti-TGF-β2 antibody-treated mice. In SB431542- and anti-TGF-β2 antibody-treated mice, generated hair follicles expressed pSMAD-2, SMAD-7 or PAI-1 in the epithelium, especially when the follicles were well matured, though expression levels were not as strong compared to the control.
Figure 8.
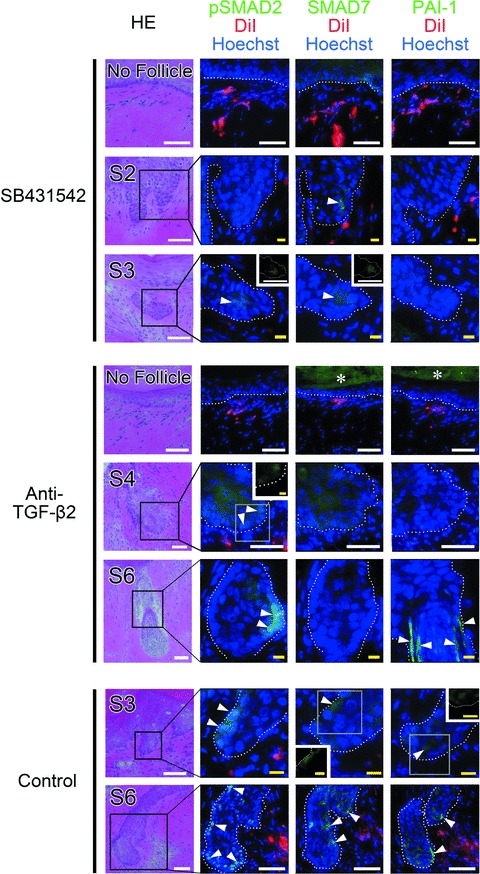
Immunohistochemical staining for TGF-β signal transduction related factors in sandwich models. Cell sheet fragments of human dermal papilla cells (hDPCs) were placed between the dermis and epidermis of rat foot pad skin; the sandwiched transplant was then inserted into the subcutis of a nude mouse and harvested 4 weeks after transplantation. SB431542, anti-TGF-β2 neutralizing antibody, or vehicle was administered to the nude mice during the 4 weeks. Each sample was serially sectioned and stained for haematoxylin and eosin or immunostained against phosphorylated SMAD2 (pSMAD-2) and two SMAD-2 target genes (SMAD-7 and PAI-1). Hair follicle maturation stages were indicated in the haematoxylin and eosin images as ‘S2’ or ‘S6’. Representative images are also shown in which hDPCs were present but folliculogenesis was absent (shown as ‘No follicle’). Immunostaining for pSMAD-2, SMAD-7 and PAI-1 were visualized in green fluorescence, red colour indicates DiI-labelled hDPCs, and Hoechst33342 was used for nuclear staining. In vehicle-administered control mice, positive pSMAD-2 signal was detected predominantly in the nuclei and positive SMAD-7 signals were located in the nuclei and/or cytoplasm of epithelial cells (arrowheads). PAI-1 signal was detected in the cytoplasm of epithelial cells and/or interstitial spaces in generated hair follicles (arrowheads). Similar findings were also observed in well-matured generated follicles, but no signals for pSMAD-2, SMAD-7 and PAI-1 were found in the ‘No follicle’ area. White dotted lines indicate the boundary of hair follicles and asterisks (*) indicate non-specific fluorescence in the stratum corneum. Insets are single-immunostained images for pSMAD-2, SMAD-7 or PAI-1. White bar = 50 μm, yellow bar = 10 μm.
Discussion
Attempts over the last several decades to regenerate hair follicles by transplanting expanded hDPCs have been hampered by the lack of knowledge of the signal and mechanism in hDPCs to induce hair folliculogenesis. How the hair-inducing capacity of hDPCs can be maintained upon expansion culture also remains unclear. Here we sought to identify a gene(s) in cultured hDPCs responsible for or contributing to hair-inductive capability. In addition, we tried to optimize the cultured method to help preserving hair-inducing capacity of hDPCs. By comparison of the gene expression profiles of hDPCs and hDFs, along with additional gene analysis of DPC biomarkers, TGF-β2 was identified as a factor specifically expressed by cultured hDPCs. Our results showed that TGF-β2 expression slightly decreased over culture time, as was the hair-inductive property of hDPCs reported in the literature [8, 12, 16].
For developing an expansion method of hDPCs while maintaining the hair-inductive activity, one piece of evidence may provide some insight: a previous observation that KCM showed a positive effect in maintaining the proliferative and hair-inductive ability of rodent DPCs [16]. Our results demonstrated that TGF-β2 gene expression was up-regulated in KCM-treated hDPCs compared to non-treated hDPCs. ELISA further revealed that KCM promoted TGF-β2 protein secretion from hDPCs. In addition, a concomitant elevation of ALP activity in KCM-treated hDPCs suggested KCM-mediated effects on the hair-inductive ability of hDPCs.
These effects of KCM indicated the possibility that KCM contains a key component to maintain hair-inductive property of hDPCs, and the key component may also stimulate cultured hDPCs to express TGF-β2. Because hair follicle epithelium and dermal papilla are in contact with each other and send reciprocal signals to induce hair folliculogenesis and maintain hair cycles [1–3], it is not surprising that KCM contains a key component in this process. Cytokine array analysis of KCM detected inflammatory cytokines such as IL-1β, IL-6, IL-8, MCP-1, RANTES, ENA-78 and Gro, which are known to be secreted from keratinocytes as an acute or late-phase response to inflammation [17, 18]. Our results also revealed that KCM contained known mitotic growth factors for DPCs, such as PDGF-BB [40] or VEGF [39, 43], suggesting that the proliferative effect of KCM is attributed to such growth factors.
Screening analysis of components in KCM detected an unexpected function of vitamin D3: promoting effects on TGF-β2 expression and ALP activity of hDPCs. Cultured hDPCs express vitamin D receptor (VDR) (data not shown), and thus 1,25(OH)2D3-induced TGF-β2 mRNA up-regulation may be mediated via VDR. However, TGF-β2 mRNA expression increases over time up to 48 hrs and remains high for 5 to 7 days (data not shown), which may suggest the involvement of other signal pathways. A similar observation was made in hDFs: 1,25(OH)2D3 specifically induces TGF-β2 mRNA expression in hDFs in the early-phase of signal transduction, followed by induction of all TGF-β isoforms (TGF-β1, β2 and β3 mRNA) in an autocrine manner [51]. Thus, TGF-β2 mRNA induction by 1,25(OH)2D3 shows a monophasic increase with time [51]. Other studies showed that TGF-β signal positively regulates the vitamin D signalling pathway by formation of the Smad3-VDR complex [52], and vitamin D3 induces strong activation of Smad2/Smad3 within 24 hrs in HL-60 cells [53]. These data indicate a direct interplay between TGF-β and vitamin D signalling pathways mediated by VDR and Smad proteins. Taken together, there appears to be a prolonged activation of both TGF-β and vitamin D signalling pathways in cultured hDPCs as the result of a close interplay and positive feedback loop, although further investigation should be performed.
Ablation of VDR in mice [54, 55] and mutations of VDR in human beings result in the development of alopecia [56, 57]. VDR is expressed in the two major cell components that make up hair follicles: the mesenchymal component, dermal papilla, and the epithelial component, outer root sheath keratinocytes [58]. Recently, VDR expression in follicular keratinocytes was shown to be essential in maintaining hair follicle homeostasis [59, 60]. However, less is known of the effects of vitamin D3 signal on dermal papilla thus far. The biologically active metabolite of vitamin D3, 1,25(OH)2D3, is mainly produced in kidneys [61], but is also produced and secreted by keratinocytes in the presence of endogenous 1α-hydroxylase [37, 38, 62]. Thus, it is suggested that 1,25(OH)2D3 secreted by keratinocytes likely works as a signalling molecule to stimulate DPCs to secret TGF-β2 and initiate the vitamin D3 and TGF-β signalling loop.
In most types of cells, the fundamental functions of TGF-β isoforms are growth inhibition and deposition of extracellular matrix [63]. Especially during foetal development, TGF-βs are found in a broad range of organs, such as epithelium, myocardium, cartilage and bone of extremities, and in the nervous system, suggesting its critical functions in organogenesis. In hair follicle physiology, TGF-βs have been shown to exert unique multidirectional effects [4], i.e. both positive and suppressive effects on hair growth. TGF-β1 blocks anagen and induces catagen [64, 65] and inhibits hair growth [66]. TGF-β1 and TGF-β2 stimulate proliferation of outer root sheath keratinocytes [67, 68]. TGF-β2 induces premature hair follicle regression in adult hair cycling [35, 69], while TGF-β2 was also shown to be required for hair folliculogensis [31, 70].
In the context of adult hair cycle, TGF-β2 is synthesized in the dermal papilla by the stimulation of dihydrotestosterone at the initiation of catagen, triggering the intrinsic caspase network and subsequent apoptotic cell death of hair follicle epithelial cells [35, 69]. In contrast, during hair development, TGF-β2 receptors are focally expressed initially in the placode and subsequently in the outer root sheath [71], and TGF-β2 exerts its morphogenetic function [31] through transient induction of the transcriptional factor Snail in the hair bud [70]. These highly elaborate spatio-temporal manners of expression suggest critical roles of the TGF-β ligand-receptor system in hair folliculogenesis. In our study, phosphorylation of SMAD-2 and expression of SMAD-2 targeted gene products (SMAD-7 and PAI-1) were seen in the epithelium of generated follicles but not in epithelium in which folliculogenesis was not induced despite the adjacent localization of transplanted hDPCs; together this suggests that the SMAD-2-mediated signal may be required for generated hair follicle maturation.
The hair folliculogenesis in our animal models depends on the epithelial–mesenchymal interaction, mimicking foetal hair follicle morphogenesis, not transition to anagen in the adult hair cycle. Suppression of hair folliculogenesis in this study by inhibition of TGF-β2 signal transduction both at the receptor and ligand level may reflects TGF-β signalling function observed in foetal hair follicle morphogenesis [31]. Although careful considerations should be given to differences between our animal models and normal physiological conditions [72], our findings may draw attention to the underestimated TGF-β2 function in hair folliculogenesis and provide insights into clinical hair regeneration with expanded hDPCs.
In conclusion, TGF-β2 was specifically expressed in hDPCs at higher levels compared to hDFs, and inhibition of TGF-β2 signal at either the ligand or receptor level impaired hair folliculogenesis in an hDPC transplantation animal model. The vitamin D3 analogue promoted TGF-β2 expression and ALP activity in hDPCs and may be a critical functional factor in KCM in the enhancement and preservation of the hair-inducing capacity of cultured hDPCs, suggesting its potential use for treatment of alopecia with expanded hDPC transplantation. The results of this study suggest a critical role for TGF-β2 and vitamin D3 signalling pathways in hair folliculogenesis.
Acknowledgments
We gratefully acknowledge Prof. Kohei Miyazono (Department of Molecular Pathology, University of Tokyo Graduate School of Medicine) for valuable suggestions on the analysis of TGF-β signal transduction in vivo. Contract grant sponsor: Grants-in-Aid by the Japanese Ministry of Education, Culture, Sports, Science, and Technology (MEXT); contract grant numbers: B2–1730474 and B2–18591964.
Conflict of interests
The authors declare that they have no competing financial interests.
Supporting Information
Materials and methods
TGF-β2 releasing gelatin hydrogel microspherebeads
Gelatin hydrogel microsphere beads (GMBs) containing 1 μgTGF-β2 in 40 μl of PBS or PBS alone wereprepared according to the previously reported method[73]. hDPC sheet fragments were inserted betweenthe epidermis and dermis of rat foot pad with 2 μl of GMBsolution on top of a hDPC sheet. The sandwich transplants weretransferred to the subcutis of nude mice, harvested 4 weeks aftertransplantation and processed for histological examination of hairfolliculogenesis.
Fig. S1 Animal assays for hair folliculogenesis.(A) Sandwich model. Human dermal papilla cells (hDPCs)cultured at full confluence were scraped off and cut into pieces.The follicular foot pad skin of a F344 rat was cut and digestedwith a medium supplemented with Dispase™ II to separate theepidermis from the dermis. After placement of the hDPC sheetbetween the epidermis and dermis, the sandwiched transplant wasinserted into the subcutis of a Balb/c nude mouse. (B)Chamber model. The chamber was transplanted on the back of a Balb/cnude mouse prior to cell transfer. hDPCs and keratinocytes derivedfrom newborn BL6 mice (mKC) were cultured, combined in a cellsuspension and transplanted into the chamber.
Fig. S2 Representative examples of generated hairfollicles at various stages. Maturation of generated hair follicleswas classified according to the scale (eight stages from S1 to S8)proposed by Paus et al. [21] aftermodification, as listed in Table S3. Haematoxylin and eosinstaining. Bars = 50 μm. DP: dermal papilla; HS: hairshaft; IRS: inner root sheath; SG: sebaceous gland.
Fig. S3 Influence of serum supplementation onTGF-β2 mRNA expression in cultured human dermalpapilla cells (hDPCs) mRNA was isolated from hDPCs cultured for 48hrs in the presence of 1,25-dihydroxyvitamin D3(1,25(OH)2D3, 100 nM) or IL-1β (100ng/ml) with or without 10% serum (foetal bovine serum).TGF-β2 mRNA expression was assessed by real-timePCR and relative expression levels normalized by GAPDH expressionare shown. Serum supplementation did not influenceTGF-β2 mRNA up-regulation by1,25(OH)2D3 or IL-1β. n= 4. *P < 0.05, **P < 0.01.
Fig. S4 Histological analysis of generated murine hairfollicles in chamber models with or without TGF-β signalinhibition. Fresh dermal cells and fresh keratinocytes isolatedfrom foetal BL6 mice were mixed as a cell suspension and implantedinto the chamber on the back of a nude mouse. Skin samples wereharvested 4 weeks after cell transfer, and hair folliculogenesiswas evaluated with histological sections. Four chambers wereprepared on four mice per group. (A) Histology (haematoxylinand eosin staining) and macroscopic views (inset) of samplestreated with SB431542 (top) or vehicle (bottom). Bars= 200 μm (haematoxylin and eosin staining) or 2 mm(inset). (B) Average number of generated hairfollicles per section. n = 4. SB431542substantially decreased the average number of regeneratedfollicles, though the difference between mice treated with SB431542and the control mice was not significant (P =0.075). (C) Histology (haematoxylin and eosin staining) andmacroscopic views (inset) of samples treated with aneutralizing antibody against TGF-β2 (top)or a negative control IgG (bottom). (D) Averagenumber of generated hair follicles per section. n= 4. TGF-β2 decreased the average numberof regenerated follicles, though the difference between micetreated with TGF-β2 and the control mice was notsignificant (P = 0.117).Bars = 200 μm (haematoxylin and eosinstaining) or 2 mm (inset).
Fig. S5 Effects of augmented TGF-β2signal on hair folliculogenesis. TGF-β2 signal wasaugmented by implanting TGF-β2 releasing gelatinhydrogel microsphere beads (GMBs) together with sheets ofDiI-labelled cultured human dermal papilla cells (hDPCs) in oursandwich models; after placement of the hDPC sheet between theepidermis and dermis of rat foot pad skin, the sandwichedtransplant was inserted into the subcutis of a Balb/c nude mouseand harvested 4 weeks after transplantation. GMBs with PBS alonewere used as a negative control. (A) Histology of sandwichmodel samples with or without TGF-β signal augmentation.Serial sections were stained for haematoxylin and eosin orHoechst33342. GMBs and DiI-labelled hDPCs were observed in yellow(due to non-specific fluorescence) and red under a fluorescentmicroscope, respectively. Asterisks (*) indicate GMBs. Bar= 50 μm. (B, C) Maturation stage(B) and number per sample (C) of generated hairfollicles (TGF-β2-GMBs: n = 9,PBS-GMBs, n = 10). No significant difference inmaturation or number of generated hair follicles between the twogroups (P = 0.283).
Table S1 Genes up-regulated in human dermal papilla cells (hDPCs) both at passage 2 (P2) and passage 8 (P8) in comparison with human dermal fibroblasts (hDFs). Signal intensities of gene expression in hDPCs were compared with those in hDFs of the same individual, and the fold changes were expressed as log ratio values in the right two columns of P2 and P8. There were 567 up-regulated genes in hDPCs at P2 with a difference of at least 1 (log ratio), and 143 genes in hDPCs at P8. Thirty-four genes in P2 and P8 overlapped and are shown in order of the fold change at P2.
Table S2 Genes down-regulated in human dermal papillacells (hDPCs) both at passage 2 (P2) and passage 8 (P8) incomparison with human dermal fibroblasts (hDFs). Signal intensitiesof gene expression in hDPCs were compared with those in hDFs of thesame individual, and the fold changes were expressed as log ratiovalues in the right two columns of P2 and P8. There were 498down-regulated genes in hDPCs at P2 with a difference of at most–1 (log ratio), and 174 genes in hDPCs at P8. Forty-fourgenes in P2 and P8 overlapped and are shown in order of the foldchange at P2.
Table S3 Classification scale of generated hairfollicles. The classification was originally proposed by Paus etal. [21]. Maturation stage is classified intoeight categories (S1–S8) according to histological features.See Fig. S1 for representative histological views.
Please note: Wiley-Blackwell are not responsible for the content or functionality of any supporting materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
Supporting info item
References
- 1.Millar SE. Molecular mechanisms regulating hair follicle development. J Invest Dermatol. 2002;118:216–25. doi: 10.1046/j.0022-202x.2001.01670.x. [DOI] [PubMed] [Google Scholar]
- 2.Cotsarelis G. Epithelial stem cells: a folliculocentric view. J Invest Dermatol. 2006;126:1459–68. doi: 10.1038/sj.jid.5700376. [DOI] [PubMed] [Google Scholar]
- 3.Fuchs E. Scratching the surface of skin development. Nature. 2007;445:834–42. doi: 10.1038/nature05659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stenn KS, Paus R. Controls of hair follicle cycling. Physiol Rev. 2001;81:449–94. doi: 10.1152/physrev.2001.81.1.449. [DOI] [PubMed] [Google Scholar]
- 5.Cohen J. The transplantation of individual rat and guinea pig whisker papillae. J Embryol Exp Morphol. 1961;9:117–27. [PubMed] [Google Scholar]
- 6.Jahoda CAB, Horne KA, Oliver RF. Induction of hair growth by implantation of cultured dermal papilla cells. Nature. 1984;311:560–2. doi: 10.1038/311560a0. [DOI] [PubMed] [Google Scholar]
- 7.Jahoda CAB. Induction of follicle formation and hair growth by vibrissa dermal papillae implanted into rat ear wounds: vibrissa-type fibres are specified. Development. 1992;115:1103–9. doi: 10.1242/dev.115.4.1103. [DOI] [PubMed] [Google Scholar]
- 8.Stenn KS, Cotsarelis G. Bioengineering the hair follicle: fringe benefits of stem cell technology. Curr Opin Biotechnol. 2005;16:493–7. doi: 10.1016/j.copbio.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 9.Philpott M, Paus R. Principles of hair follicle morphogenesis. In: Chuong CM, editor. Molecular basis of epithelial appendage morphogenesis. Landes; 1998. pp. 75–110. In:, editor.. Austin:. [Google Scholar]
- 10.Oro AE, Scott MP. Splitting hairs: dissecting roles of signaling systems in epidermal development. Cell. 1998;95:575–8. doi: 10.1016/s0092-8674(00)81624-4. [DOI] [PubMed] [Google Scholar]
- 11.Rendl M, Polak L, Fuchs E. BMP signaling in dermal papilla cells is required for their hair follicle-inductive properties. Genes Dev. 2008;22:543–57. doi: 10.1101/gad.1614408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kishimoto J, Burgeson RE, Morgan BA. Wnt signaling maintains the hair-inducing activity of the dermal papilla. Genes Dev. 2000;14:1181–5. [PMC free article] [PubMed] [Google Scholar]
- 13.Randall VA, Sundberg JP, Philpott MP. Animal and in vitro models for the study of hair follicles. J Invest Dermatol Sym Proc. 2003;8:39–45. doi: 10.1046/j.1523-1747.2003.12170.x. [DOI] [PubMed] [Google Scholar]
- 14.Midorikawa T, Chikazawa T, Yoshino T, et al. Different gene expression profile observed in dermal papilla cells related to androgenic alopecia by DNA macroarray analysis. J Dermatol Sci. 2004;36:25–32. doi: 10.1016/j.jdermsci.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 15.Rutberg SE, Kolpak ML, Gourley JA, et al. Differences in expression of specific biomarkers distinguish human beard from scalp dermal papilla cells. J Invest Dermatol. 2006;126:2583–95. doi: 10.1038/sj.jid.5700454. [DOI] [PubMed] [Google Scholar]
- 16.Inamatsu M, Matsuzaki T, Iwanari H, et al. Establishment of rat dermal papilla cell lines that sustain potency to induce hair follicles from afollicular skin. J Invest Dermatol. 1998;111:767–75. doi: 10.1046/j.1523-1747.1998.00382.x. [DOI] [PubMed] [Google Scholar]
- 17.Uchi H, Terao H, Koga T, et al. Cytokines and chemokines in the epidermis. J Dermatol Sci. 2000;24:S29–38. doi: 10.1016/s0923-1811(00)00138-9. [DOI] [PubMed] [Google Scholar]
- 18.Sebastiani S, Albanesi C, De Pita O, et al. The role of chemokines in allergic contact dermatitis. Arch Dermatol Res. 2002;293:552–9. doi: 10.1007/s00403-001-0276-9. [DOI] [PubMed] [Google Scholar]
- 19.Reynolds AJ, Jahoda CAB. Cultured dermal papilla cells induce follicle formation and hair growth by transdifferentiation of an adult epidermis. Development. 1992;115:587–93. doi: 10.1242/dev.115.2.587. [DOI] [PubMed] [Google Scholar]
- 20.Inoue K, Kato H, Sato T, et al. Evaluation animal models for the hair inducing capacity of cultured human dermal papilla cells. Cells Tissues Organs. 2009;190:102–10. doi: 10.1159/000178021. [DOI] [PubMed] [Google Scholar]
- 21.Paus R, Muller-Rover S, Van Der Veen C, et al. A comprehensive guide for the recognition and classification of distinct stages of hair follicle morphogenesis. J Invest Dermatol. 1999;113:523–32. doi: 10.1046/j.1523-1747.1999.00740.x. [DOI] [PubMed] [Google Scholar]
- 22.Weinberg WC, Goodman LV, George C, et al. Reconstitution of hair follicle development in vivo: determination of follicle formation, hair growth, hair quality by dermal cells. J Invest Dermatol. 1993;100:229–36. doi: 10.1111/1523-1747.ep12468971. [DOI] [PubMed] [Google Scholar]
- 23.Kishimoto J, Ehama R, Wu L, et al. Selective activation of the versican promoter by epithelial-mesenchymal interactions during hair follicle development. Proc Natl Acad Sci USA. 1999;96:7336–41. doi: 10.1073/pnas.96.13.7336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Itami S, Kurata S, Sonoda T, et al. Characterization of 5α-reductase in cultured human dermal papilla cells from beard and occipital scalp hair. J Invest Dermatol. 1991;96:57–60. doi: 10.1111/1523-1747.ep12514729. [DOI] [PubMed] [Google Scholar]
- 25.Trautman MS, Kimelman J, Bernfield M. Developmental expression of syndecan, an integral membrane proteoglycan, correlates with cell differentiation. Development. 1991;111:213–20. doi: 10.1242/dev.111.1.213. [DOI] [PubMed] [Google Scholar]
- 26.Couchman JR. Hair follicle proteoglycans. J Invest Dermatol. 1993;101:S60–4. doi: 10.1111/1523-1747.ep12362642. [DOI] [PubMed] [Google Scholar]
- 27.Shimaoka S, Tsuboi R, Jindo T, et al. Hepatocyte growth factor/scatter factor expressed in follicular papilla cells stimulates human hair growth in vitro. J Cell Physiol. 1995;165:333–8. doi: 10.1002/jcp.1041650214. [DOI] [PubMed] [Google Scholar]
- 28.Rosenquist TA, Martin GR. Fibroblast growth factor signaling in the hair growth cycle: expression of the fibroblast growth factor receptor and ligand genes in the murine hair follicle. Dev Dyn. 1996;205:379–86. doi: 10.1002/(SICI)1097-0177(199604)205:4<379::AID-AJA2>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 29.Almond-Roesler B, Schön M, Schön MP, et al. Cultured dermal papilla cells of the rat vibrissa follicle. Proliferative activity, adhesion properties and reorganization of the extracellular matrix in vitro. Arch Dermatol Res. 1997;289:698–704. doi: 10.1007/s004030050264. [DOI] [PubMed] [Google Scholar]
- 30.Kozlowska U, Blume-Peytavi U, Kodelja V, et al. Expression of vascular endothelial growth factor (VEGF) in various compartments of the human hair follicle. Arch Dermatol Res. 1998;290:661–8. doi: 10.1007/s004030050370. [DOI] [PubMed] [Google Scholar]
- 31.Foitzik K, Paus R, Doetschman T, et al. The TGF-β2 isoform is both a required and sufficient inducer of murine hair follicle morphogenesis. Dev Biol. 1999;212:278–89. doi: 10.1006/dbio.1999.9325. [DOI] [PubMed] [Google Scholar]
- 32.Inui S, Fukuzato Y, Nakajima T, et al. Androgen-inducible TGF-β1 from balding dermal papilla cells inhibits epithelial cell growth: a clue to understand paradoxical effects of androgen on human hair growth. FASEB J. 2002;16:1967–9. doi: 10.1096/fj.02-0043fje. [DOI] [PubMed] [Google Scholar]
- 33.Bayer-Garner IB, Sanderson RD, Smoller BR. Syndecan-1 is strongly expressed in the anagen hair follicle outer root sheath and in the dermal papilla but expression diminishes with involution of the hair follicle. Am J Dermatopathol. 2002;24:484–9. doi: 10.1097/00000372-200212000-00005. [DOI] [PubMed] [Google Scholar]
- 34.Kamp H, Geilen CC, Sommer C, et al. Regulation of PDGF and PDGF receptor in cultured dermal papilla cells and follicular keratinocytes of the human hair follicle. Exp Dermatol. 2003;12:662–72. doi: 10.1034/j.1600-0625.2003.00089.x. [DOI] [PubMed] [Google Scholar]
- 35.Foitzik K, Spexard T, Nakamura M, et al. Towards dissecting the pathogenesis of retinoid-induced hair loss: all-trans retinoic acid induces premature hair follicle regression (catagen) by upregulation of transforming growth factor-β2 in the dermal papilla. J Invest Dermatol. 2005;124:1119–26. doi: 10.1111/j.0022-202X.2005.23686.x. [DOI] [PubMed] [Google Scholar]
- 36.Iino M, Ehama R, Nakazawa Y, et al. Adenosine stimulates fibroblast growth factor-7 gene expression via adenosine A2b receptor signaling in dermal papilla cells. J Invest Dermatol. 2007;127:1318–25. doi: 10.1038/sj.jid.5700728. [DOI] [PubMed] [Google Scholar]
- 37.Bikle DD, Nemanic MK, Gee E, et al. 1,25-Dihydroxyvitamin D3 production by human keratinocytes kinetics and regulation. J Clin Invest. 1986;78:557–66. doi: 10.1172/JCI112609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pillai S, Bikle DD, Elias PM. 1,25-Dihydroxyvitamin D production and receptor binding in human keratinocytes varies with differentiation. J Biol Chem. 1988;15:5390–5. [PubMed] [Google Scholar]
- 39.Lachgar S, Moukadiri H, Jonca F, et al. Vascular endothelial growth factor is an autocrine growth factor for hair dermal papilla cells. J Invest Dermatol. 1996;106:17–23. doi: 10.1111/1523-1747.ep12326964. [DOI] [PubMed] [Google Scholar]
- 40.Goodman LV, Ledbetter SR. Secretion of stromelysin by cultured dermal papilla cells: differential regulation by growth factors and functional role in mitogen-induced cell proliferation. J Cell Physiol. 1992;151:41–9. doi: 10.1002/jcp.1041510108. [DOI] [PubMed] [Google Scholar]
- 41.Dooley TP. Molecular biology of the human cytosolic sulfotransferase gene superfamily implicated in the bioactivation of minoxidil and cholesterol in skin. Exp Dermatol. 1999;8:328–9. doi: 10.1159/000026112. [DOI] [PubMed] [Google Scholar]
- 42.Randall VA, Hibberts NA, Thornton MJ, et al. The hair follicle: a paradoxical androgen target organ. Horm Res. 2000;54:243–50. doi: 10.1159/000053266. [DOI] [PubMed] [Google Scholar]
- 43.Yano K, Brown LF, Detmar M. Control of hair growth and follicle size by VEGF-mediated angiogenesis. J Clin Invest. 2001;107:409–17. doi: 10.1172/JCI11317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Edmondson SR, Thumiger SP, Werther GA, et al. Epidermal homeostasis: the role of the growth hormone and insulin-like growth factor systems. Endocr Rev. 2003;24:737–64. doi: 10.1210/er.2002-0021. [DOI] [PubMed] [Google Scholar]
- 45.Osada A, Iwabuchi T, Kishimoto J, et al. Long-term culture of mouse vibrissal dermal papilla cells and de novo hair follicle induction. Tissue Eng. 2007;13:975–82. doi: 10.1089/ten.2006.0304. [DOI] [PubMed] [Google Scholar]
- 46.Paus R, Arck P, Tiede S. (Neuro-) endocrinology of epithelial hair follicle stem cells. Mol Cell Endocrinol. 2008;288:38–51. doi: 10.1016/j.mce.2008.02.023. [DOI] [PubMed] [Google Scholar]
- 47.Iida M, Ihara S, Matsuzaki T. Hair cycle-dependent change of alkaline phosphatase activity in the mesenchyme and epithelium in mouse vibrissal follicles. Develop Growth Differ. 2007;49:185–95. doi: 10.1111/j.1440-169X.2007.00907.x. [DOI] [PubMed] [Google Scholar]
- 48.Hardy MH. The secret life follicle of the hair. Trends Genet. 1992;8:55–61. doi: 10.1016/0168-9525(92)90350-d. [DOI] [PubMed] [Google Scholar]
- 49.Laping NJ, Grygielko E, Mathur A, et al. Inhibition of transforming growth factor (TGF)-β1-induced extracellular matrix with a novel inhibitor of the TGF-β type I receptor kinase activity: SB-431542. Mol Pharmacol. 2002;62:58–64. doi: 10.1124/mol.62.1.58. [DOI] [PubMed] [Google Scholar]
- 50.Miyazono K, Ten Dijke P, Hedlin C-H. TGF-β signaling by SMAD proteins. Adv. Immunol. 2000;75:115–57. doi: 10.1016/s0065-2776(00)75003-6. [DOI] [PubMed] [Google Scholar]
- 51.Oyama N, Iwatsuki K, Satoh M, et al. Dermal fibroblasts are one of the therapeutic targets for topical application of 1α,25-dihydroxyvitamin D3: the possible involvement of transforming growth factor-β induction. Br J Dermatol. 2000;143:1140–8. doi: 10.1046/j.1365-2133.2000.03880.x. [DOI] [PubMed] [Google Scholar]
- 52.Yanagi Y, Suzawa M, Kawabata M, et al. Positive and negative modulation of vitamin D receptor function by transforming growth factor-β signaling through Smad proteins. J Biol Chem. 1999;274:12971–4. doi: 10.1074/jbc.274.19.12971. [DOI] [PubMed] [Google Scholar]
- 53.Cao Z, Flanders KC, Bertolette D, et al. Levels of phospho-Smad2/3 are sensors of the interplay between effects of TGF-β and retinoic acid on monocytic and granulocytic differentiation of HL-60 cells. Blood. 2003;101:498–507. doi: 10.1182/blood-2002-05-1549. [DOI] [PubMed] [Google Scholar]
- 54.Li YC, Pirro AE, Amling M, et al. Targeted ablation of the vitamin D receptor: an animal model of vitamin D-dependent rickets type II with alopecia. Proc Natl Acad Sci USA. 1997;94:9831–5. doi: 10.1073/pnas.94.18.9831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yoshizawa T, Handa Y, Uematsu Y, et al. Mice lacking the vitamin D receptor exhibit impaired bone formation, uterine hypoplasia and growth retardation after weaning. Nat Genet. 1997;16:391–6. doi: 10.1038/ng0897-391. [DOI] [PubMed] [Google Scholar]
- 56.Malloy PJ, Hochberg Z, Tiosano D, et al. The molecular basis of hereditary 1,25-dihydroxyvitamin D3 resistant rickets in seven related families. J Clin Invest. 1990;86:2071–9. doi: 10.1172/JCI114944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Arita K, Nanda A, Wessagowit V, et al. A novel mutation in the VDR gene in hereditary vitamin D-resistant rickets. Br J Dermatol. 2008;158:168–71. doi: 10.1111/j.1365-2133.2007.08232.x. [DOI] [PubMed] [Google Scholar]
- 58.Reichrath J, Schilli M, Kerber A, et al. Hair follicle expression of 1,25-dihydroxyvitamin D3 receptors during the murine hair cycle. Br J Dermatol. 1994;131:477–82. doi: 10.1111/j.1365-2133.1994.tb08547.x. [DOI] [PubMed] [Google Scholar]
- 59.Skorija K, Cox M, Sisk JM, et al. Ligand-independent actions of the vitamin D receptor maintain hair follicle homeostasis. Mol Endocrinol. 2005;19:855–62. doi: 10.1210/me.2004-0415. [DOI] [PubMed] [Google Scholar]
- 60.Sakai Y, Kishimoto J, Demay MB. Evaluation of keratinocyte proliferation and differentiation in vitamin D receptor knockout mice. Endocrinology. 2000;141:2043–9. doi: 10.1210/endo.141.6.7515. [DOI] [PubMed] [Google Scholar]
- 61.Sutton ALM, MacDonald PN. Vitamin D: more than a “bone-a-fide” hormone. Mol Endocrinol. 2003;17:777–91. doi: 10.1210/me.2002-0363. [DOI] [PubMed] [Google Scholar]
- 62.Reichrath J. Vitamin D and the skin: an ancient friend, revisited. Exp Dermatol. 2007;16:618–25. doi: 10.1111/j.1600-0625.2007.00570.x. [DOI] [PubMed] [Google Scholar]
- 63.Massagué J. How cells read TGF-beta signals. Nat Rev Mol Cell Biol. 2000;1:169–78. doi: 10.1038/35043051. [DOI] [PubMed] [Google Scholar]
- 64.Schmid P, Cox D, Bilbe G, et al. Differential expression of TGF β1, β2, β3 genes during mouse embryogenesis. Development. 1991;111:117–30. doi: 10.1242/dev.111.1.117. [DOI] [PubMed] [Google Scholar]
- 65.Millan FA, Denhez F, Kondaiah P, et al. Embryonic gene expression patterns of TGFb1, b2 and b3 suggest different developmental functions in vivo. Development. 1991;111:131–43. doi: 10.1242/dev.111.1.131. [DOI] [PubMed] [Google Scholar]
- 66.Foitzik K, Lindner G, Muller-Rover S, et al. Control of murine hair follicle regression (catagen) by TFG-β1 in vivo. FASEB J. 2000;14:752–60. doi: 10.1096/fasebj.14.5.752. [DOI] [PubMed] [Google Scholar]
- 67.Philpott MP, Green MR, Kealey T. Human hair growth in vitro. J Cell Sci. 1990;97:463–71. doi: 10.1242/jcs.97.3.463. [DOI] [PubMed] [Google Scholar]
- 68.Weinberg WC, Brown PD, Stetler-Stevenson WG, et al. Growth factors specifically alter hair follicle cell proliferation and collagenolytic activity alone or in combination. Differentiation. 1990;45:168–78. doi: 10.1111/j.1432-0436.1990.tb00470.x. [DOI] [PubMed] [Google Scholar]
- 69.Hibino T, Nishiyama T. Role of TGF-β2 in the human hair cycle. J Dermatol Sci. 2004;35:9–18. doi: 10.1016/j.jdermsci.2003.12.003. [DOI] [PubMed] [Google Scholar]
- 70.Jamora C, Lee P, Kocieniewski P, et al. A signaling pathway involving TGF-β2 and snail in hair follicle morphogenesis. PLoS Biol. 2004;3:e11. doi: 10.1371/journal.pbio.0030011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Paus R, Foitzik K, Welker P, et al. Transforming growth factor-b, receptor type I and type II expression during murine hair follicle development and cycling. J. Invest. Dermatol. 1997;109:518–26. doi: 10.1111/1523-1747.ep12336635. [DOI] [PubMed] [Google Scholar]
- 72.Inamatsu M, Tochio T, Makabe A, et al. Embryonic dermal condensation and adult dermal papilla induce hair follicles in adult glabrous epidermis through different mechanisms. Develop Growth Differ. 2006;48:73–86. doi: 10.1111/j.1440-169X.2006.00848.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting info item


