Abstract
Recent advances in the treatment of hepatitis C virus (HCV) infection have led to the availability of both highly efficacious interferon-containing and interferon-sparing regimens. However, the use of such therapies faces restrictions due to high costs. For patients who are medically eligible to receive interferon, the choice between the two will likely be impacted by preferences surrounding interferon, severity of disease, coverage policies and out-of-pocket costs. We developed a decision model to quantify the trade-offs between immediate, interferon-containing therapy and delayed, interferon-free therapy for patients with chronic, genotype 1 HCV infection. We projected the quality-adjusted life expectancy stratified by the presence or absence of cirrhosis for four strategies: (i) no treatment; (ii) immediate, one-time treatment with an interferon-containing regimen; (iii) immediate treatment as above with the opportunity for retreatment in patients who fail to achieve sustained virologic response with interferon-free therapy in 1 year; and (iv) delayed therapy with interferon-free therapy in 1 year. When compared to one-time immediate treatment with the interferon-containing regimen, delayed treatment with the interferon-free regimen in 1 year resulted in longer life expectancy, with a 0.2 quality-adjusted life year (QALY) increase in noncirrhotic patients, and a 1.1 QALY increase in patients with cirrhosis. This superiority in health benefits was lost when wait time for interferon-free therapy was greater than 3–3.2 years. In this modelling analysis, interferon-free therapy resulted in superior health benefits compared to immediate therapy with interferon until wait time exceeded 3–3.2 years. Such data can inform decision-making regarding treatment initiation for HCV as healthcare financing evolves.
Keywords: decision analysis, HCV, interferon sparing, treatment timing
Hepatitis C virus (HCV) has a high burden of disease globally, where more than 185 million persons are estimated to be living with chronic infection [1]. Over time HCV causes cirrhosis in approximately 40% of individuals due to progressive liver fibrosis, a clinical condition that confers increased risk of hepatocellular cancer, variceal bleeding, hepatic failure and other complications of advanced liver disease [2]. Liver-related morbidity and mortality is projected to increase substantially in the coming years, with an estimated 1.76 million persons with HCV developing cirrhosis and more than 1 million persons dying of liver disease by 2060 in the United States alone [3,4].
While the development of new, oral direct-acting antiviral medications (DAAs) has revolutionized the landscape of hepatitis C therapy, the high price tag has led to increasingly restrictive policies regarding coverage eligibility reported among various US state Medicaid programmes, including limiting access to those with advanced liver fibrosis [5,6]. Internationally, the WHO has endorsed the use of DAAs, although price negotiations have varied between countries [1,7]. As a result, many HCV-infected patients both now, and in the future, will face a decision of whether they would rather be treated immediately, with a less costly, interferon-containing regimen, or wait until such a time that they be considered eligible for an interferon-free treatment.
Deferring therapy until interferon-free options are accessible, however, is not without risk. Progressive liver disease as well as the development of hepatocellular cancer may increase morbidity and mortality in untreated individuals. Additionally, symptoms of HCV, such as malaise, fatigue and abdominal pain, might lower quality of life throughout the time that a patient is forced to wait for interferon-free therapy. The appropriate choice for any individual – treat now or wait? – is a complex function of expected mortality, treatment efficacy and quality of life.
In the light of rapid therapeutic advancement for HCV, data are needed to inform the decision-making between providers and patients regarding the health benefits and risk associated with waiting for interferon-free therapy. We used decision-analytic modelling to quantify the trade-offs between immediate, interferon-containing therapy and delayed, interferon-free therapy for patients with chronic, genotype 1 hepatitis C virus infection.
Methods
Analytic overview
We adapted the Hepatitis C Cost-Effectiveness (HEP-CE) model, a Monte Carlo model to evaluate the clinical impact of immediate, interferon-containing therapy versus delayed, interferon-free therapy for individuals with genotype 1 hepatitis C virus infection. The HEP-CE model simulates the natural history, screening, and treatment of hepatitis C disease and incorporates HCV epidemiology, disease progression, therapy and non-HCV mortality. [8]–[11]. We developed and analysed the model using TreeAge Pro 2012 software (TreeAge Software, Inc., Williamstown, MA, USA). It is described in greater detail below and shown in Fig.1.
Figure 1.
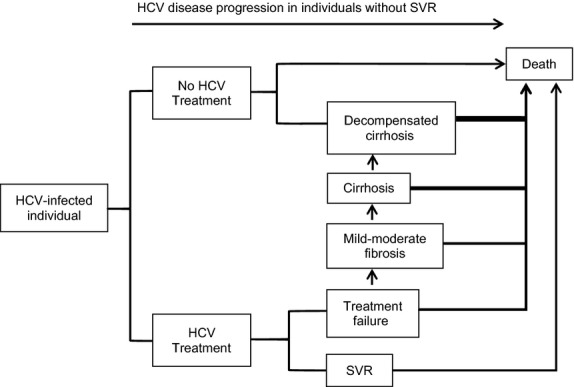
Simplified Model Schema. Treatment versus no treatment of HCV-infected individuals is simulated. Individuals without SVR progress through the natural history of HCV infection. Decompensated cirrhosis represents a composite health state that includes end-stage liver disease, hepatocellular cancer and liver transplant. Death may occur at any state and may be HCV related or HCV unrelated. HCV: hepatitis C virus, SVR: sustained virologic response.
We considered four treatment strategies (Fig.2): (i) no treatment (for comparison); (ii) immediate, one-time treatment with an interferon (IFN)-containing regimen; (iii) immediate treatment as above with the opportunity for retreatment in patients who fail to achieve sustained virologic response (SVR) with interferon-free therapy in 1 year (IFN + IFN-free); and (iv) delayed therapy with interferon-free therapy in 1 year (IFN-free). For each strategy, we used the model to project the unadjusted life expectancy and quality-adjusted life expectancy, measured in quality-adjusted life years (QALYs) for 1 million individually simulated patients with chronic, genotype 1 hepatitis C virus infection. We discounted QALYs by 3% [12]. We stratified outcomes by the presence or absence of cirrhosis at the start of the simulation. In the base case, we based assumptions for mean age, treatment efficacy, rates of adverse events and quality of life on treatment on published literature. We performed sensitivity analyses around expected time to interferon-free therapy, age at the start of the model, disease progression and quality of life with HCV infection and while on treatment.
Figure 2.
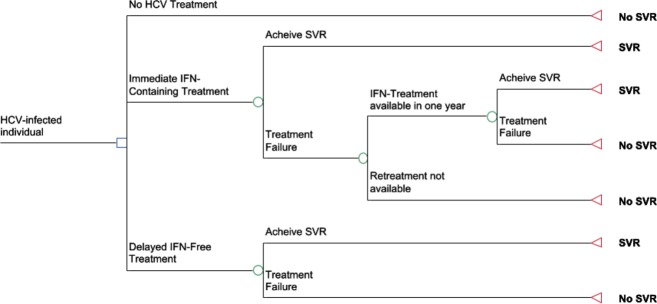
Treatment Strategies. HCV-infected individuals (i) no treatment; (ii) immediate, one-time treatment with an interferon-containing regimen; (iii) immediate treatment as above with the opportunity for retreatment in patients who fail to achieve sustained virologic response with interferon-free therapy in 1 year; and (iv) delayed therapy with interferon-free therapy in 1 year. HCV: hepatitis C virus, IFN: interferon, SVR: sustained virologic response.
HCV disease progression
The model simulates the natural history of treatment-naïve patients with chronic, genotype 1 HCV infection (Fig.1). Patients are individually simulated through the model and progress through three stages of liver disease: mild–moderate fibrosis, cirrhosis and decompensated cirrhosis. The model uses estimates of the time from HCV infection to cirrhosis and of time from cirrhosis to first decompensation event to stochastically determine the fibrosis stage at simulation start, as well as the rate of fibrosis progression throughout the simulation [13]–[15]. As a result, the model generates variable rates of fibrosis progression, such that some individuals progress quickly to cirrhosis, while others die from nonliver-related mortality without ever becoming cirrhotic [2]. Each progressive stage of liver disease is associated with decreased quality of life. Only when individuals reach cirrhosis, however, are they at risk of liver-related mortality, including mortality from hepatocellular cancer. The risk of liver-related death is higher still when individuals reach decompensated cirrhosis.
HCV treatment
In the IFN strategy, patients receive immediate therapy with an IFN-containing regimen. We based the probability of SVR, adverse events and treatment discontinuation due to adverse events for this regimen on a 12-week course of sofosbuvir 400 mg once daily, peginterferon alfa-2a 180 μg subcutaneously once weekly and weight-based ribavirin (1000 mg daily in patients <75 kg and 1200 mg daily in patients ≥75 kg) [16]. In the IFN + IFN-free strategy, patients received immediate therapy with the IFN-containing regimen as above. Those who did not attain SVR with the first regimen waited 12 months and were then retreated with the IFN-free regimen of 12 weeks of sofosbuvir 400 mg once daily and ledipasvir 90 mg once daily (Table1) [17]. Patients with decompensated cirrhosis are ineligible for treatment.
Table 1.
Selected model inputs
| Variable | Base case value | Range evaluated in sensitivity analyses | Source(s) |
|---|---|---|---|
| Cohort characteristics | |||
| Average age, years (SD) | 52 (14.3) | 40–80 | [17] |
| Proportion male | 0.59 | 0–1 | [17] |
| Average age at HCV infection (years) | 26 | Triangular (16,36) | [31] |
| HCV disease progression | |||
| Median years to cirrhosis from age of infection (10–90%) | 25 | 10–40 | [14] |
| Median years to first liver event after developing cirrhosis (10–90%) | 11 | Triangular (6,19) | [13,15] |
| Liver-related mortality with cirrhosis (deaths/100 PYs) | 1.39 | 1.0–1.8 | [32] |
| Liver-related mortality with decompensated cirrhosis (deaths/100 PYs) | 12 | 8–16 | [32] |
| Reduction in liver mortality after SVR, % | 94% | 81–98 | [18] |
| HCV therapy efficacy | |||
| SVR of IFN-containing therapy | |||
| No to moderate fibrosis (Metavir F0–F3) | 0.92 | Beta (252,21) | [16] |
| Cirrhosis (Metavir F4) | 0.80 | Beta (43,11) | [16] |
| SVR of IFN-free therapy | |||
| No to moderate fibrosis (Metavir F0–F3) | 0.99 | Beta (179,1) | [17] |
| Cirrhosis (Metavir F4) | 0.94 | Beta (32,2) | [17] |
| HCV therapy adverse events | |||
| IFN-containing regimen | |||
| Treatment DC due to AE | |||
| No to moderate fibrosis (Metavir F0–F3) | 0.02 | Beta (6,267) | [16] |
| Cirrhosis (Metavir F4) | 0.02 | Beta (1,53) | [16] |
| IFN-free regimen | |||
| Treatment DC due to AE | |||
| No to moderate fibrosis (Metavir F0–F3) | 0 | Beta (2,481) | [17] |
| Cirrhosis (Metavir F4) | 0 | Beta (0.2,55.8) | [17] |
| Quality of life | |||
| Without HCV infection or after achieving SVR | 0.74–0.92 | 0.60–1.0 | [33]–[35] |
| With HCV infection | |||
| No to moderate fibrosis (Metavir F0–F3) | 0.89 | 0.75–1.00 | [19,36,37] |
| Cirrhosis (Metavir F4) | 0.62 | 0–0.72 | [19,36,37] |
| Decompensated Cirrhosis | 0.48 | 0.40–0.60 | [19,36,37] |
| While receiving IFN-containing regimen | |||
| No to moderate fibrosis (Metavir F0–F3) | 0.80 | 0–0.89 | [38] |
| Cirrhosis (Metavir F4) | 0.59 | 0–0.62 | [38] |
| While receiving IFN-free regimen | |||
| No to moderate fibrosis (Metavir F0–F3) | 0.86 | 0–0.89 | [38] |
| Cirrhosis (Metavir F4) | 0.61 | 0–0.62 | [38] |
| Major toxicity decrement | 0.16 | 0.1–0.25 | [22] |
SD, standard deviation; HCV, hepatitis C virus; PY, person-years; SVR, sustained virologic response; IFN, interferon; DC, discontinued; AE, adverse event.
Triangular sampling distribution (min, max).
Beta sampling distribution (α, β).
Personal communication, Pang PS, Gilead Sciences on June 4, 2014.
Benefits of SVR
In noncirrhotic individuals, attainment of SVR results in cessation of disease progression and returns in quality of life and mortality risk to that of noninfected HCV individuals of the same age and sex. For those individuals with cirrhosis or decompensated cirrhosis at the time of SVR, the risk of liver-related mortality decreases by 94% [18].
Quality-adjusted life years
Quality-adjusted life years (QALYs) is an outcome measure that combines both the quantity and quality of remaining life. The quantity of life is based on the unadjusted life expectancy as calculated by the HEP-CE model and is a function of the natural history of HCV as well as the treatment strategies examined. The quality of life is a reflection of an individual's preference, or utility value, for a given health state. Utility values are obtained from HCV-specific instruments and range from zero to one, in which zero represents death and one represent life with perfect health. Quality of life is a combination of 3 utility functions:
Utility without HCV infection as related to non-HCV comorbidities and determined by age and sex,
Utility with HCV infection as determined by stage of liver disease and
To estimate the combined utility for these health states, their effects on total utility were assumed to be proportional and independent such that these three functions could be multiplied by each other to yield the utility of their combined health state [21]. A utility decrement was also subtracted to individuals who suffered a major toxicity event due to treatment [22].
Sensitivity analysis
To assess for robustness of results, we performed one- and two-way sensitivity analyses around time to interferon-free therapy, age at start of the model, quality of life with HCV infection and while on treatment, calculations of quality of life for joint health states based on additive and minimum health state utility methods and rate of liver-related mortality. Probabilistic sensitivity analyses were performed using second-order Monte Carlo simulation to determine the probability of expected value outcomes. To account for outcome uncertainty due to sampling error surrounding treatment efficacy and adverse events, we assigned beta distributions to these variables based on clinical trial data and sampled randomly from these distributions.
This study was approved by the Institutional Review Board of the University of Chicago.
Results
Immediate treatment with interferon-containing therapy, with the option of retreatment with interferon-free therapy for those do not initially attain SVR led to the greatest gains in quality-adjusted life expectancy (3.1 QALYs and 8.6 QALYs in noncirrhotic and cirrhotic patients, respectively, compared to no treatment). When retreatment was not an option, delayed treatment with interferon-free therapy was superior to immediate treatment with interferon-containing therapy by 0.2 QALY in noncirrhotic patients and 1.1 QALY in patients with cirrhosis (Table2).
Table 2.
Base case and sensitivity analysis results
| Strategy | QALYs | Unadjusted LY |
|---|---|---|
| Base Case Results | ||
| No cirrhosis | ||
| No treatment | 11.5 | 25.4 |
| IFN immediately | 14.4 | 28.0 |
| IFN-free in 1 year | 14.6 | 28.2 |
| IFN+IFN-free in 1 year for treatment failures | 14.6 | 28.2 |
| Cirrhosis | ||
| No treatment | 4.9 | 11.9 |
| IFN immediately | 12.2 | 23.9 |
| IFN-free in 1 year | 13.3 | 26.2 |
| IFN+IFN-free in 1 year for treatment failures | 13.5 | 26.2 |
| Sensitivity Analysis Results | ||
| When QoL with cirrhosis = death | ||
| No cirrhosis | ||
| No treatment | 9.7 | 25.4 |
| Immediate treatment | 14.2 | 28.0 |
| Delayed treatment | 14.5 | 28.2 |
| Immediate treatment with retreatment | 14.6 | 28.3 |
| Cirrhosis | ||
| No treatment | 0.1 | 11.9 |
| Immediate treatment | 10.9 | 23.9 |
| Delayed treatment | 12.4 | 26.1 |
| Immediate treatment with retreatment | 13.0 | 26.4 |
| When QoL on IFN = death | ||
| No cirrhosis | ||
| No treatment | 11.5 | 25.4 |
| Immediate treatment | 14.3 | 28.0 |
| Delayed treatment | 14.6 | 28.2 |
| Immediate treatment with retreatment | 14.5 | 28.3 |
| Cirrhosis | ||
| No treatment | 4.9 | 11.9 |
| Immediate treatment | 12.0 | 23.9 |
| Delayed treatment | 13.3 | 26.1 |
| Immediate treatment with retreatment | 13.5 | 26.4 |
| When QoL on IFN = IFN-Free Tx | ||
| No cirrhosis | ||
| No treatment | 11.5 | 25.4 |
| Immediate treatment | 14.4 | 28.0 |
| Delayed treatment | 14.6 | 28.2 |
| Immediate treatment with retreatment | 14.7 | 28.3 |
| Cirrhosis | ||
| No treatment | 4.9 | 11.9 |
| Immediate treatment | 12.2 | 23.9 |
| Delayed treatment | 13.3 | 26.1 |
| Immediate treatment with retreatment | 13.6 | 26.4 |
| When QoL on IFN-free = death | ||
| No cirrhosis | ||
| No treatment | 11.5 | 25.4 |
| Immediate treatment | 14.42 | 28.0 |
| Delayed treatment | 14.43 | 28.2 |
| Immediate treatment with retreatment | 14.7 | 28.3 |
| Cirrhosis | ||
| No treatment | 4.9 | 11.9 |
| Immediate treatment | 12.1 | 23.9 |
| Delayed treatment | 13.1 | 26.1 |
| Immediate treatment with retreatment | 13.6 | 26.4 |
QALYs, quality-adjusted life years; LY, life years; QoL, quality of life; IFN, interferon.
The superiority of delayed interferon-free therapy compared to one-time, immediate interferon-containing therapy was sensitive to the time that patients were forced to wait before receiving interferon-free treatment. In patients with and without cirrhosis, the health benefit of waiting for interferon-free therapy was lost when wait time for this regimen was greater than 3 years for patients with cirrhosis and 3.2 years for noncirrhotic patients (Fig.3). Increasing delay in interferon-free therapy resulted in decreasing health benefits compared to no therapy, an effect that was greater in patients with cirrhosis compared to noncirrhotic patients.
Figure 3.
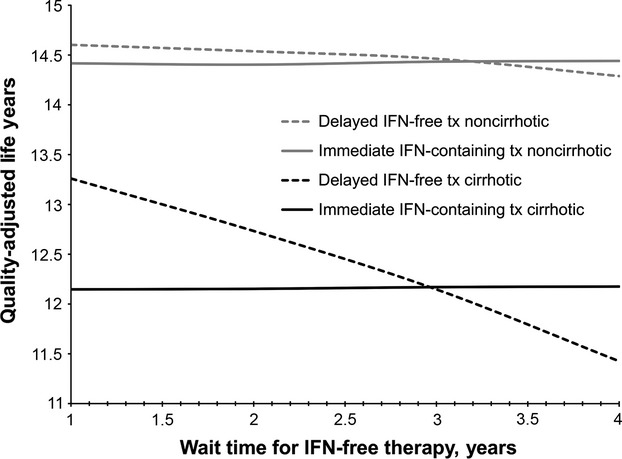
Impact of increased wait time for delayed therapy. Immediate, interferon-containing therapy (solid lines) was compared to delayed, interferon-free therapy (dashed lines) for noncirrhotic (grey lines) and cirrhotic (black lines) patients as wait time for interferon-free therapy was increased. IFN, interferon; tx, treatment.
Base case results remained robust despite broad variation in age at start of the simulation (varied from 40 to 80 years old), mortality rate associated with HCV infection, quality of life with HCV infection and while on treatment and method for calculating joint health states. For example, interferon-free therapy at 1 year remained superior to one-time immediate interferon-containing therapy when the quality of life with cirrhosis and decompensated cirrhosis was equivalent to death (Table2). When quality of life while receiving interferon was equivalent to death, waiting for interferon-free therapy remained superior to immediate one-time interferon-containing therapy. The superiority of waiting 1 year for interferon-free therapy persisted until the incidence of liver-related death reached 98 of 100 person-years (approximately 70 times the base case incidence) in noncirrhotic individuals and 12 of 100 person-years (approximately 9 times the base case incidence) in cirrhotic individuals (not shown). Two-way sensitivity analyses of the impact of age at start of simulation and wait time for interferon-free therapy are shown in Fig.4.
Figure 4.
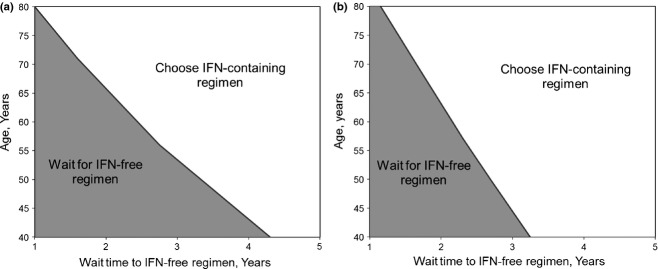
Two-way sensitivity analysis of age and time to delayed therapy, noncirrhotic patients. Health benefits for (a) noncirrhotic patients and (b) patients with cirrhosis of immediate, interferon-containing therapy were compared to delayed, interferon-free therapy as patient age and wait time for interferon-free therapy were varied. Waiting for interferon-free therapy was favoured in the grey shaded area, whereas immediate, interferon-containing therapy was favoured in the un-shaded area. IFN, interferon.
In probabilistic sensitivity analysis, interferon-free therapy with 1-year delay was superior to one-time immediate interferon-containing therapy for patients without cirrhosis in 88.2% of one thousand cohorts of one thousand simulated patients. For patients with cirrhosis, the interferon-free therapy was superior in 95.4% of one thousand cohorts of one thousand simulated patients.
Discussion
The decision to initiate treatment in patients with genotype 1 HCV infection who are eligible to receive pegylated interferon is complex and must balance, among other factors, quality of life, patient preferences, available regimens and risks of disease progression. Using decision-analytic modelling, we found that the benefits of waiting for all oral therapy in patients who wished to avoid pegylated interferon as well as retreatment were time-limited. In an era of interferon-free therapy with restrictions to access, waiting for interferon-free therapy is beneficial so far as this delay did not exceed 3–3.2 years. These findings were more pronounced with increasing age at the time of decision.
For patients who have no absolute contraindication to interferon-containing therapy, but wish to avoid the medication given its poor tolerability, this analysis may inform in several ways the process of shared decision-making with providers regarding the common question, ‘How long can I wait before I need to be treated?’ Firstly, assuming that retreatment is not an option, the decision around the timing of HCV therapy is driven to the greatest extent by treatment efficacy. Although the use of health utilities and QALYs remain somewhat controversial as a measure of health benefit, it is interesting to note that results remained stable even when the quality of life on either treatment was equivalent to zero, or death. This may provide information on how the discussion regarding preferences of treatment acceptability may be framed for those patients who are fearful of the side effects of treatment and believe that their quality of life during this time would be very low. Secondly, we see that patients, even those who are not currently cirrhotic, cannot wait for therapy forever. The health benefits conferred by interferon-free therapy over interferon-containing therapy, including superior efficacy and tolerability, are lost after approximately 3 years as they are outweighed by increasing HCV-related morbidity and mortality.
While this analysis explores the trade-off between waiting for interferon-free therapy from a pure health benefit perspective, the high costs and variable cost-effectiveness of new DAAs, including sofosbuvir, have been previously discussed [23]–[27]. Deuffic-Burban and colleagues have reported that in genotype 1 HCV-infected patients, interferon-free therapy was cost-effective compared to telaprevir- or boceprevir-based triple therapy in patients with greater than stage F2 fibrosis [27]. Based on current costs of sofosbuvir and simeprevir, an analysis by Hagan et al. [28] found that interferon-free therapy could be considered cost-effective based on a range of frequently reported willingness to pay thresholds of $80 000/QALY to $100 000/QALY. We explored the additional strategy of offering retreatment with interferon-free therapy for individuals who have failed immediate therapy with an interferon-containing regimen. While this strategy results in the greatest QALY gain for patients, it is unlikely to meet societal cost-effectiveness thresholds given the high cost of repeated therapy. In the United States, the heterogeneity of coverage for DAAs by state Medicaid programmes and commercial insurers, including increased cost sharing to patient, limiting treatment courses and/or limiting coverage of treatment to only patients with cirrhosis, may increase out-of-pocket costs and impact some patients’ decisions regarding treatment initiation [25]. In developing countries, price negotiations and prioritization of regimens that allow for simplified models of care may define therapeutic options [7]. This analysis provides projections for the clinical consequences of preferences regarding treatment options, retreatment and timing for a population facing highly variable healthcare financing.
This analysis has several limitations. The phase 3 trials on which treatment efficacy and probability of adverse effects were based had limited enrolment of patients with cirrhosis [16,17]. We examined the impact on this uncertainty through probabilistic sensitivity analysis and found our results to be stable. Similarly, data on quality of life while on treatment were obtained from a single study on patient-reported outcomes. Deterministic sensitivity analysis broadly varying these values did not alter results. Risks of loss to follow-up during the wait for interferon-free therapy may increase with longer wait times; however, this was not modelled. It is important to note that results are based on population-based estimates of disease progression. This was evaluated through probability distributions to account for uncertainty in the primary data. In our analysis, we used a multiplicative method to combine the utilities for outcomes that comprised of multiple health states, a technique that is widely used but criticized for lack of empirical support [21,29,30]. Our results remained robust with sensitivity analyses using additive and minimum health state models for calculating joint utilities. Finally, a treatment strategy in which noncirrhotic patients are made to wait until they are cirrhotic prior to receiving treatment, while reflective of some insurer practices, was beyond the scope of this analysis.
In conclusion, the selection of optimal treatment for chronic hepatitis C and timing thereof is complex, and patient choices may be impacted by preferences surrounding interferon, desire for immediate treatment and access to interferon-free therapy. We used decision-analytic modelling to evaluate the clinical consequences of immediate, interferon-containing therapy versus delayed, interferon-free therapy for chronic hepatitis infection in patients with and without cirrhosis. We found that while waiting 1 year for interferon-free therapy resulted in superior health benefits compared to one-time immediate therapy with interferon over a broad range of sensitivity analyses, these benefits were time-limited. Such data can improve shared decision-making by informing the communication of the risks and benefits of various treatment options.
Acknowledgments
This analysis was funded in part by the Agency for Healthcare Research and Quality (K99HS022433 to M.T.P.) and the National Institute of Drug Abuse (R01DA031059 to B.P.L.). The funders did not have any role in the study design, the collection, analysis and interpretation of data, drafting of the manuscript or decision to submit for publication.
Glossary
- DAAs
direct-acting antiviral medications
- HCV
hepatitis C virus
- HEP-CE
hepatitis C cost-effectiveness
- IFN
interferon
- QALY
quality-adjusted life year
- SVR
sustained virologic response
References
- 1.Global Burden Of Hepatitis CWG. Global burden of disease (GBD) for hepatitis C. J Clin Pharmacol. 2004;44(1):20–29. doi: 10.1177/0091270003258669. [DOI] [PubMed] [Google Scholar]
- 2.Thein HH, Yi Q, Dore GJ, Krahn MD. Estimation of stage-specific fibrosis progression rates in chronic hepatitis C virus infection: a meta-analysis and meta-regression. Hepatology. 2008;48(2):418–431. doi: 10.1002/hep.22375. [DOI] [PubMed] [Google Scholar]
- 3.Rein DB, Wittenborn JS, Weinbaum CM, Sabin M, Smith BD, Lesesne SB. Forecasting the morbidity and mortality associated with prevalent cases of pre-cirrhotic chronic hepatitis C in the United States. Dig Liver Dis. 2011;43(1):66–72. doi: 10.1016/j.dld.2010.05.006. [DOI] [PubMed] [Google Scholar]
- 4.Smith BD, Morgan RL, Beckett GA, et al. Recommendations for the identification of chronic hepatitis C virus infection among persons born during 1945-1965. MMWR Recomm Rep. 2012;61(RR-4):1–32. [PubMed] [Google Scholar]
- 5.Armstrong D. Hepatitis C drug price limiting state medicaid approvals 2014. Accessed at http://www.bloomberg.com/news/2014-03-05/hepatitis-c-drug-price-limiting-state-medicaid-approvals.html on April 17, 2014. [updated 4 MARCH 2014]. Available from: http://www.bloomberg.com/news/2014-03-05/hepatitis-c-drug-price-limiting-state-medicaid-approvals.html.
- 6.Wang AL. 2014. Illinois’ Medicaid restricts who can get ‘game-changing’ hepatitis drug: Crain's Chicago Business. Available at: http://www.chicagobusiness.com/article/20140729/NEWS03/140729819/illinois-medicaid-restricts-who-can-get-game-changing-hepatitis-drug# (accessed 29 August 2013)
- 7.Ford N, Singh K, Cooke GS, et al. Expanding access to treatment for hepatitis C in resource-limited settings: lessons from HIV/AIDS. Clin Infect Dis. 2012;54(10):1465–1472. doi: 10.1093/cid/cis227. [DOI] [PubMed] [Google Scholar]
- 8.Linas BP, Barter DM, Leff JA, et al. The cost-effectiveness of improved hepatitis C virus therapies in HIV/hepatitis C virus coinfected patients. AIDS. 2014;28(3):365–376. doi: 10.1097/QAD.0000000000000093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Linas BP, Barter DM, Leff JA, et al. The hepatitis C cascade of care: identifying priorities to improve clinical outcomes. PLoS ONE. 2014;9(5):e97317. doi: 10.1371/journal.pone.0097317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schackman BR, Leff JA, Barter DM, et al. Cost-effectiveness of rapid hepatitis C virus (HCV) testing and simultaneous rapid HCV and HIV testing in substance abuse treatment programs. Addiction. 2015;110(1):129–143. doi: 10.1111/add.12754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Linas BP, Wong AY, Schackman BR, Kim AY, Freedberg KA. Cost-effective screening for acute hepatitis C virus infection in HIV-infected men who have sex with men. Clin Infect Dis. 2012;55(2):279–290. doi: 10.1093/cid/cis382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gold MR. Cost-effectiveness in Health and Medicine. New York: Oxford University Press; 1996. p. xxiii. 425 p. [Google Scholar]
- 13.Pineda JA, Aguilar-Guisado M, Rivero A, et al. Natural history of compensated hepatitis C virus-related cirrhosis in HIV-infected patients. Clin Infect Dis. 2009;49(8):1274–1282. doi: 10.1086/605676. [DOI] [PubMed] [Google Scholar]
- 14.Poynard T, Bedossa P, Opolon P. Natural history of liver fibrosis progression in patients with chronic hepatitis C. The OBSVIRC, METAVIR, CLINIVIR, and DOSVIRC groups. Lancet. 1997;349(9055):825–832. doi: 10.1016/s0140-6736(96)07642-8. [DOI] [PubMed] [Google Scholar]
- 15.Sangiovanni A, Prati GM, Fasani P, et al. The natural history of compensated cirrhosis due to hepatitis C virus: A 17-year cohort study of 214 patients. Hepatology. 2006;43(6):1303–1310. doi: 10.1002/hep.21176. [DOI] [PubMed] [Google Scholar]
- 16.Lawitz E, Mangia A, Wyles D, et al. Sofosbuvir for previously untreated chronic hepatitis C infection. N Engl J Med. 2013;368(20):1878–1887. doi: 10.1056/NEJMoa1214853. [DOI] [PubMed] [Google Scholar]
- 17.Afdhal N, Zeuzem S, Kwo P, et al. Ledipasvir and sofosbuvir for untreated HCV genotype 1 infection. N Engl J Med. 2014;370(20):1889–1898. doi: 10.1056/NEJMoa1402454. [DOI] [PubMed] [Google Scholar]
- 18.van der Meer AJ, Veldt BJ, Feld JJ, et al. Association between sustained virological response and all-cause mortality among patients with chronic hepatitis C and advanced hepatic fibrosis. JAMA. 2012;308(24):2584–2593. doi: 10.1001/jama.2012.144878. [DOI] [PubMed] [Google Scholar]
- 19.Chong CA, Gulamhussein A, Heathcote EJ, et al. Health-state utilities and quality of life in hepatitis C patients. Am J Gastroenterol. 2003;98(3):630–638. doi: 10.1111/j.1572-0241.2003.07332.x. [DOI] [PubMed] [Google Scholar]
- 20.Younossi ZM, Stepanova M, Zeuzem S, et al. Patient-reported outcomes assessment in chronic hepatitis C treated with sofosbuvir and ribavirin: the VALENCE study. J Hepatol. 2014;61(2):228–234. doi: 10.1016/j.jhep.2014.04.003. [DOI] [PubMed] [Google Scholar]
- 21.Naglie G, Krahn MD, Naimark D, Redelmeier DA, Detsky AS. Primer on medical decision analysis: part 3–Estimating probabilities and utilities. Med Decis Making. 1997;17(2):136–141. doi: 10.1177/0272989X9701700203. [DOI] [PubMed] [Google Scholar]
- 22.Schackman BR, Teixeira PA, Weitzman G, Mushlin AI, Jacobson IM. Quality-of-life tradeoffs for hepatitis C treatment: do patients and providers agree? Med Decis Making. 2008;28(2):233–242. doi: 10.1177/0272989X07311753. [DOI] [PubMed] [Google Scholar]
- 23.Hoofnagle JH, Sherker AH. Therapy for hepatitis C–the costs of success. N Engl J Med. 2014;370(16):1552–1553. doi: 10.1056/NEJMe1401508. [DOI] [PubMed] [Google Scholar]
- 24.Petta S, Cabibbo G, Enea M, et al. Cost-effectiveness of sofosbuvir-based triple therapy for untreated patients with genotype 1 chronic hepatitis C. Hepatology. 2014;59(5):1692–1705. doi: 10.1002/hep.27010. [DOI] [PubMed] [Google Scholar]
- 25.Pho MT, Linas BP. Valuing cure: bridging cost-effectiveness and coverage decisions for hepatitis C therapy. Hepatology. 2014;60(1):12–14. doi: 10.1002/hep.27220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tice JA, Ollendorf DA, Pearson SD. 2014. The comparative clinical effectiveness and value of simeprevir and sofosbuvir in the treatment of chronic hepatitis C infection 12 FEBRUARY 2014. Report No.
- 27.Deuffic-Burban S, Schwarzinger M, Obach D, et al. Should we await IFN-free regimens to treat HCV genotype 1 treatment-naive patients? A cost-effectiveness analysis (ANRS 95141) J Hepatol. 2014;61(1):7–14. doi: 10.1016/j.jhep.2014.03.011. [DOI] [PubMed] [Google Scholar]
- 28.Hagan LM, Yang Z, Ehteshami M, Schinazi RF. All-oral, interferon-free treatment for chronic hepatitis C: cost-effectiveness analyses. J Viral Hepat. 2013;20(12):847–857. doi: 10.1111/jvh.12111. [DOI] [PubMed] [Google Scholar]
- 29.Basu A, Dale W, Elstein A, Meltzer D. A linear index for predicting joint health-states utilities from single health-states utilities. Health Econ. 2009;18(4):403–419. doi: 10.1002/hec.1373. [DOI] [PubMed] [Google Scholar]
- 30.Dale W, Basu A, Elstein A, Meltzer D. Predicting utility ratings for joint health States from single health States in prostate cancer: empirical testing of 3 alternative theories. Med Decis Making. 2008;28(1):102–112. doi: 10.1177/0272989X07309639. [DOI] [PubMed] [Google Scholar]
- 31.Freeman AJ, Dore GJ, Law MG, et al. Estimating progression to cirrhosis in chronic hepatitis C virus infection. Hepatology. 2001;34(4 Pt 1):809–816. doi: 10.1053/jhep.2001.27831. [DOI] [PubMed] [Google Scholar]
- 32.Bruno S, Zuin M, Crosignani A, et al. Predicting mortality risk in patients with compensated HCV-induced cirrhosis: a long-term prospective study. Am J Gastroenterol. 2009;104(5):1147–1158. doi: 10.1038/ajg.2009.31. [DOI] [PubMed] [Google Scholar]
- 33.Barnett PG, Zaric GS, Brandeau ML. The cost-effectiveness of buprenorphine maintenance therapy for opiate addiction in the United States. Addiction. 2001;96(9):1267–1278. doi: 10.1046/j.1360-0443.2001.96912676.x. [DOI] [PubMed] [Google Scholar]
- 34.Sherman K, Muir A, Aggarwal J, et al. Health-related quality-of-life among genotype 1 treatment-experienced chronic hepatitis C patients: post-hoc analyses from the realize study. J Hepatol. 2013;58:S372. [Google Scholar]
- 35.Yoshida H, Arakawa Y, Sata M, et al. Interferon therapy prolonged life expectancy among chronic hepatitis C patients. Gastroenterology. 2002;123(2):483–491. doi: 10.1053/gast.2002.34785. [DOI] [PubMed] [Google Scholar]
- 36.Grieve R, Roberts J, Wright M, et al. Cost effectiveness of interferon alpha or peginterferon alpha with ribavirin for histologically mild chronic hepatitis C. Gut. 2006;55(9):1332–1338. doi: 10.1136/gut.2005.064774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stein K, Dalziel K, Walker A, et al. Screening for hepatitis C among injecting drug users and in genitourinary medicine clinics: systematic reviews of effectiveness, modelling study and national survey of current practice. Health Technol Assess. 2002;6(31):1–122. [PubMed] [Google Scholar]
- 38.Younossi ZM, Stepanova M, Nader F, et al. Patient-reported outcomes in chronic hepatitis C patients with cirrhosis treated with sofosbuvir-containing regimens. Hepatology. 2014;59(6):2161–2169. doi: 10.1002/hep.27161. [DOI] [PubMed] [Google Scholar]


