Model organisms are essential for biological and biomedical research. They enable scientists to study human diseases faster, cheaper and at a larger scale than in humans, or in human tissue or cell lines. Furthermore, animal models, such as fruit flies, roundworms, mice and rats, allow the study of normal and pathological processes in a controlled and systematic way to investigate specific hypothesis or test the effects of drugs and genes that, with the advent of genetic engineering, can be turned on and off with temporal and spatial precision. Research in model organisms has generated a number of breakthroughs and a huge amount of knowledge about the molecular factors that cause or contribute to many human diseases. Yet, model organisms have a number of caveats that put limits on how this knowledge can be applied to study the human condition. Studying the vast biodiversity among Earth's fauna, in particular “exotic” animals with peculiar characteristics, could therefore help to overcome these limits and yield additional insights into human health, disease and life expectancy.
Animal models clearly are a major paradigm in biomedical research, but they also have considerable limitations as findings do not always translate to humans
In the context of life expectancy and ageing, research has shown that mutations in single genes can make a huge difference by increasing lifespan up to tenfold in the roundworm and up to 50% in mice 1. According to the GenAge database of ageing related genes (http://genomics.senescence.info/genes/), we know hundreds of genes that significantly regulate ageing processes in animal models. Hundreds of genes associated with the risk of multiple diseases have also been identified in mice and a plethora of mouse models was created to study these. Animal models clearly are a major paradigm in biomedical research, but they also have considerable limitations as findings do not always translate to humans. Cholesterol-lowering statins famously did not work as expected in rats. Claims of cancer cures discovered in rodents are common, yet very few make it into the clinic.
In addition, some research fields use a fairly limited number and selection of model systems. Research on ageing and cancer, for example, mostly relies on short-lived rodents—rats and mice in particular—as mammalian models. This is a major problem because biological differences between these short-lived species and humans may confound studies. Moreover, the lack of genetic variation in model organisms—most laboratory mouse strains consist of clones to improve the robustness of the results—means that findings are often relevant for only a fraction of individuals. It is not surprising then that the effect of most genes that were identified as extending longevity in mice have not been observed in human studies, or that the life-extending and health-promoting effects of some diets in rodents are much more modest in monkeys 2. There is another issue, namely that we study species that are less successful in dealing with ageing and diseases.
Animals have always inspired scientists. Early flight pioneers, such as Otto Lilienthal and the Wright brothers, conducted detailed observations of birds to design the first airplanes. Extracts and body parts from animals have also been used as treatments, and some still have applications in modern medicine. For example, hundreds of thousands of horseshoe crabs are harvested each year for their blue blood that is used to test medical products for bacterial contaminations. ACE (angiotensin-converting enzyme) inhibitors to treat hypertension and some types of heart failure were inspired by a peptide found in the venom of a South American pit viper known as “jararaca”. A synthetic version of the protein exendin-4, a toxin produced by the Gila monster lizard, is used to manage type 2 diabetes.
Studying how some animals manage to survive in extreme environments may also provide insights for how to protect our organs during hypothermia, hypoxia or ischemia
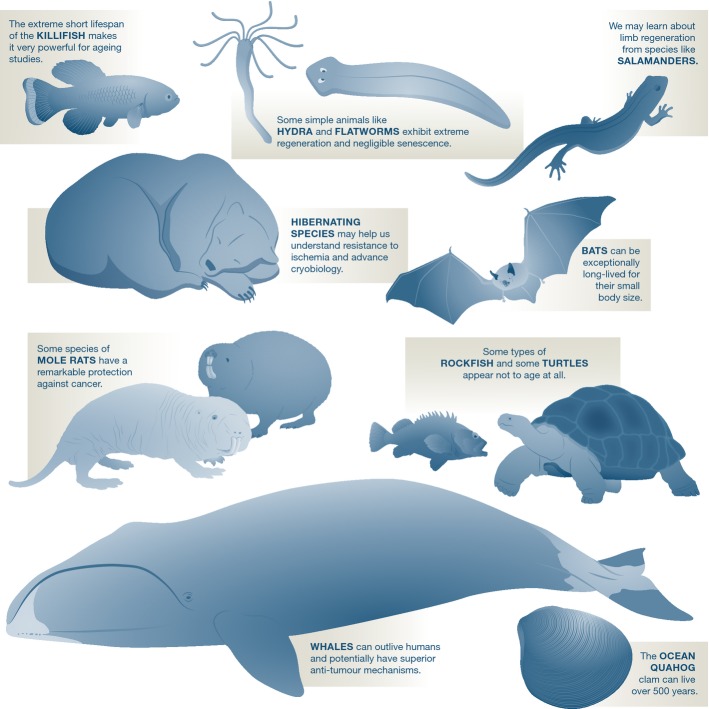
And yet, the number of medical applications inspired by animals is tiny compared to the incredible biodiversity on Earth and the extraordinary characteristics of some animals. The idea that, like some salamanders, we could regrow lost limbs has been a recurring theme in science fiction and has inspired regenerative medicine. Many other species evolved adaptations that may have applications for human health: take, for instance, the different strategies employed by hibernating animals to survive the winter months. Hibernating bears may help us understand natural obesity and its relation to insulin resistance, and studies of the wood frog, which partially freezes solid during winter, may help advance cryobiology. Studying how some animals manage to survive in extreme environments may also provide insights for how to protect our organs during hypothermia, hypoxia or ischaemia.
Whales are certainly not an ideal model organism for laboratory studies, but they have some characteristics that make them uniquely interesting for biomedical research
One problem is that tissue regeneration and many responses to extreme environments involve much more complex processes than inhibiting a single enzyme. Understanding such processes requires at least a modest understanding of the molecular and cellular mechanisms involved. But then, studying non-traditional organisms is not trivial. Traditional animal models come with a variety of resources, such as protocols, reagents, knock-out lines, mutants and so on; developing such a set of resources for a new model organism would require considerable time and money.
What is more, some animals are hard to study. Whales are certainly not an ideal model organism for laboratory studies, but they have some characteristics that make them uniquely interesting for biomedical research. Since the 1970s, scientists have suspected that whales have superior anti-tumour mechanisms that are not present in humans. The rationale goes like this: if cancer is caused by one rogue cell dividing uncontrollably, then large animals like whales, which have over 1,000 times more cells than humans, must have a much higher probability of developing cancer and thus should be relatively short-lived. Only they are not—some species can live up to 200 years—and therefore must have additional or more efficient mechanism(s) to protect them against cancer. Given the impracticality of studying whales, however, this has been impossible to find out until the advent of genomics.
Since the Human Genome Project (HGP), the costs of sequencing have declined dramatically; sequencing a human genome now costs about US$1,000 compared to the HGP's US$3 billion price tag. Even though the cost of sequencing the genome of a species for the first time is still one or two orders of magnitude higher than the cost of sequencing a human genome, we can now sequence its genome at a high quality in a cost-efficient way. Since sequencing DNA samples is also much easier than keeping and studying animals in the laboratory, comparative genomics provides an opportunity to investigate species that otherwise would be difficult to study. This is exemplified by the recent genome sequencing of the bowhead whale. These magnificent animals (Fig1) live in Arctic and sub-Arctic waters, can grow to 20 meters in length and up to 100 tons in weight and can live for more than 200 years. Bowhead whales also have a much reduced disease incidence compared with humans. After sequencing the genome, we identified several promising candidate genes to explain the bowhead's higher life expectancy and disease resistance, including genes associated with DNA repair, cell cycle regulation, cancer and ageing 3. Although much work remains to be done to demonstrate the biological importance of these findings, it showcases the power of high-throughput approaches to study animals that exhibit extreme disease resistance.
Figure 1.
Photograph of a bowhead whale
© Mads Peter Heide-Jorgensen.
The idea that long-lived animals can yield clues about ageing and disease resistance is not new, but the sequencing of genomes opened new opportunities to investigate the molecular mechanisms involved. In 2007, I led a white paper proposal to the NIH/NHGRI to sequence the genomes of three long-lived mammalian species: the naked mole rat, the capuchin monkey and the bowhead whale. The proposal was not deemed cost-effective at the time—we estimated the costs of full genome sequencing at US$15–20 M per organism—but the declining costs of DNA sequencing eventually paved the way for the sequencing of the bowhead and naked mole rat genomes by my laboratory and others. Both genomes are now available online for anyone to download and study in silico: (http://naked-mole-rat.org/ and http://bowhead-whale.org/).
The naked mole rat has a maximum lifespan of more than 30 years. This is exceptional for a small rodent since, in general, larger animals tend to live longer 4. Living in an underground environment and in colonies with a single queen is among the reasons why naked mole rats may have evolved such an extreme lifespan compared to other rodents. They also exhibit an exceptional cancer resistance: To date, no one has observed cancer in more than 1,000 animals examined 5. As in the case of the bowhead, the analysis of the naked mole rat genome provided similar candidate genes 6,7. Contrary to the bowhead, however, the naked mole rat can be studied in the laboratory: a handful of research institutions keep colonies. Such studies have, for example, shown that hyaluronan, a sugar that is secreted by the rat's connective tissue cells presumably to make their skins elastic, can protect from cancer 6. No doubt other mechanisms must be involved—resistance to some forms of stress, including some types of DNA damage, has also been observed. My view is that we have only scratched the surface when it comes to finding potential anti-cancer mechanisms in these bizarre animals (Fig2).
Figure 2.
Photograph of a naked mole rat
© Lorna Faulkes.
With so much promise and breakthroughs in stem cell-based therapies, I could envision engineering human cells based on knowledge from extreme species
We do not even need to keep whales or colonies of naked mole rats in the laboratory in order to investigate candidate genes and health-promoting molecular mechanisms. We can study genes from extreme animals in traditional model systems, either in cell lines or by genetically engineering mice or rats. Various transgenic mice have already been created to study genes from other species in the context of development, DNA repair mechanisms and so on. Furthermore, proof-of-concept that expressing transgenes from a longer-lived species in short-lived model organisms extends lifespan has been demonstrated by extending the lifespan of worms using zebrafish genes 8. Induced pluripotent stem cells, assuming the technology can be applied to non-traditional species, also opens new perspectives in studying the exceptional traits and responses of extreme animals in vitro. One caveat is that resistance to complex diseases such as cancer and ageing is likely to involve various genes and processes, and engineering multiple genes in cell lines or in mice is still technically challenging. Nonetheless, advances in genome editing techniques, notably CRISPR/Cas, may permit such studies in the foreseeable future. But how can we apply this knowledge to clinical research?
Traditional medical applications derived from extreme animals relied on obtaining some product, like the blood from the abovementioned horseshoe crab. I find it unlikely, however, that we will start hunting whales or naked mole rats for some therapeutic compound against ageing or cancer in humans. Instead, it will be necessary to mimic the molecular processes that provide these animals with increased disease resistance. The most obvious method is drugs. For example, if we find that a given gene is activated in hibernating ground squirrels to protect their organs from ischaemia, we could identify drugs that activate the equivalent human gene as a therapy to prevent ischaemic damage in patients. It is interesting to note that some current pharmaceutical targets were actually identified in “extreme” human individuals, such as the ongoing development of cholesterol-lowering drugs based on the finding that people with certain rare mutations have astoundingly low levels of cholesterol.
I am convinced that many more such mutations are waiting to be found in extreme animals, and, given recent advances in regenerative medicine and genome editing, we could develop much more powerful clinical approaches in the future. With so much promise and breakthroughs in stem cell-based therapies, I could envision engineering human cells based on knowledge from extreme species. For instance, we could engineer genes into therapeutic human stem cells based on insights gained from the bowhead whale to prevent that these cells develop into cancer. Further into the future, gene therapy might become sufficiently advanced to express transgenes in human organs that improve function or resilience. As mentioned above, the complexity and the number of genes involved in some of these traits will be a limitation; for instance, it seems that limb regeneration could involve hundreds of genes, which may hinder its clinical application for the foreseeable future. Many other traits, however, notably cancer resistance, are likely influenced by single or a few genes and therefore insights gained from extreme animals may generate clinical applications in the nearer future (Fig3).
Figure 3.
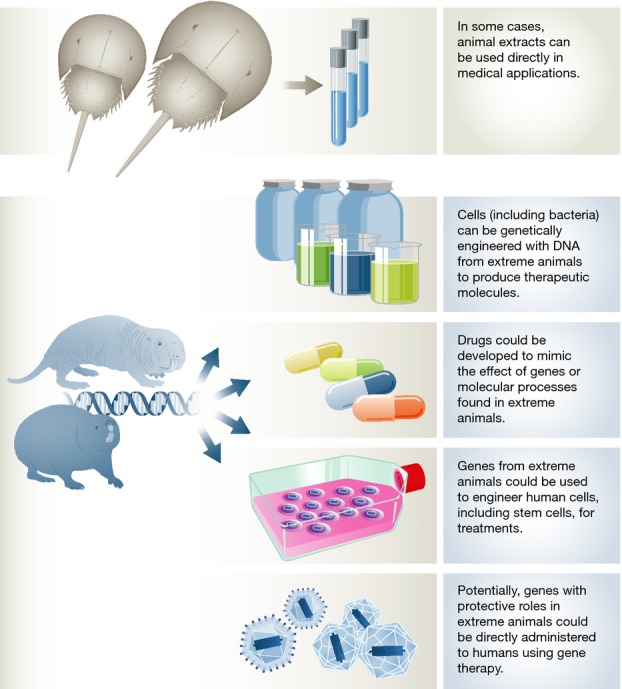
Potential human clinical applications of findings from extreme animals
Another important consideration is that different species may have evolved different mechanisms to overcome the same problems. For example, different species of mole rat seem to have different mechanisms that confer cancer resistance. In the blind mole rat, cancer resistance is not related to hyaluronan but seems to involve one or more secreted factors that trigger death of cancerous cells 6. My view is that different species will often have evolved different “tricks”, adaptations that result in a longer lifespan, and therefore, a variety of long-lived species could lead to the discovery of new ways to combat age-related diseases. Some of these adaptations will be easier to translate into humans than others, and we would do well to identify as many “tricks” as possible in as many species as possible.
Other long-lived organisms include the capuchin monkey, which can live up to 54 years and is, after humans and apes, the best example of a long-lived primate; bats (some small bats can live over 40 years in the wild); and species exhibiting negligible senescence 4,9. The latter are arguably most fascinating in terms of the comparative biology of ageing. All primates and, as far as we know, all mammals eventually age. Even old bowhead whales show signs of ageing: The oldest known specimen, a male caught as part of the annual hunts by Alaskan natives and estimated to be 211 years old, had tough meat and blubber. Old naked mole rats show muscle loss. We know that natural selection can considerably shorten and lengthen lifespans, even in closely related species 1. This is encouraging, as it demonstrates that studying species differences can provide clues about ageing and age-related diseases.
Given the aforementioned concerns about traditional model organisms, expanding our bestiary in biomedical research is essential
Some animals appear not to age at all. These include simple ones like a tiny immortal jellyfish that can reverse its life cycle, the equally minuscule hydra and planarian flatworms, which have extreme regenerative abilities, or the ocean quahog clam, which has been estimated to live more than 500 years, but also complex vertebrates like some turtles, certain species of rockfish and the cave salamander (http://genomics.senescence.info/species/nonaging.php). Various studies, in some cases spanning decades, showed that these animals failed to exhibit functional or physiological decline, or an increase in mortality with age. Even if they do eventually become senescent, they age much slower than humans. Because these are not mammals, genome and physiological comparisons are trickier, since specific adaptations related to their longevity will be harder to pinpoint; though, again with the declining costs of sequencing, I expect attention to turn to these animals as well.
Given the aforementioned concerns about traditional model organisms, expanding our bestiary in biomedical research is essential (Fig4). In the context of longevity, such efforts are already underway. One exciting non-traditional model organism is the African killifish. Like the bowhead and the naked mole rat, it is an extreme animal, only that it sits at the other end of the spectrum and is one of the shortest-lived vertebrates known with a lifespan of less than six months. Efforts to sequence its genome and develop tools to create genetically modified animals could facilitate its use by scientists 10. No doubt, additional models of ageing and age-related diseases would be much welcomed to improve our understanding of the discoveries made thus far in short-lived organisms and their relevance to humans.
Figure 4.
Selected examples of extreme animals and their potential for biomedical research
The British chemist Leslie Orgel famously stated that “evolution is cleverer than you are”. Given our recent advances in synthetic biology and genomics, I do not fully agree. Yet, I agree with the implication that evolution has overcome biological problems that still elude contemporary research, including the age-related diseases that are the major killers of modern society: cancer, coronary heart disease and neurodegenerative diseases. The use of long-lived animals is uncommon in biomedical research, but I believe it offers unique opportunities to identify genes, mechanisms and processes that protect against rather than cause disease. Advances in our capacity to read and write DNA make it possible to study such extremes animals, even if they are as big as bowhead, as bad (from a longevity perspective) as the killifish or as ugly as the naked mole rat.
Acknowledgments
I am grateful to the UK Biotechnology and Biological Sciences Resource Council and the Wellcome Trust for supporting current work in my laboratory, and to Shaun Calvert for comments on a previous draft of this manuscript. Further thanks to the Life Extension Foundation and the Methuselah Foundation for supporting the sequencing and analysis of the bowhead whale genome and a Marie Curie International Reintegration Grant within EC-FP7 and the Ellison Medical Foundation for supporting our work on the naked mole rat genome and transcriptome. I apologize to colleagues whose work I could not cite owing to lack of space.
Conflict of interest
The author declares that he has no conflict of interest.
References
- 1.Tacutu R, Craig T, Budovsky A, Wuttke D, Lehmann G, Taranukha D, Costa J, Fraifeld VE, de Magalhaes JP. Human ageing genomic resources: integrated databases and tools for the biology and genetics of ageing. Nucleic Acids Res. 2013;41:D1027–D1033. doi: 10.1093/nar/gks1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.de Magalhaes JP. The scientific quest for lasting youth: prospects for curing aging. Rejuvenation Res. 2014;17:458–467. doi: 10.1089/rej.2014.1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Keane M, Semeiks J, Webb AE, Li YI, Quesada V, Craig T, Madsen LB, van Dam S, Brawand D, Marques PI, et al. Insights into the evolution of longevity from the bowhead whale genome. Cell Rep. 2015;10:112–122. doi: 10.1016/j.celrep.2014.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Austad SN. Cats, “rats”, and bats: the comparative biology of aging in the 21st century. Integr Comp Biol. 2010;50:783–792. doi: 10.1093/icb/icq131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Buffenstein R. The naked mole-rat: a new long-living model for human aging research. J Gerontol A Biol Sci Med Sci. 2005;60:1369–1377. doi: 10.1093/gerona/60.11.1369. [DOI] [PubMed] [Google Scholar]
- 6.Gorbunova V, Seluanov A, Zhang Z, Gladyshev VN, Vijg J. Comparative genetics of longevity and cancer: insights from long-lived rodents. Nat Rev Genet. 2014;15:531–540. doi: 10.1038/nrg3728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Keane M, Craig T, Alfoldi J, Berlin AM, Johnson J, Seluanov A, Gorbunova V, Di Palma F, Lindblad-Toh K, Church GM, et al. The naked mole rat genome resource: facilitating analyses of cancer and longevity-related adaptations. Bioinformatics. 2014;30:3558–3560. doi: 10.1093/bioinformatics/btu579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sagi D, Kim SK. An engineering approach to extending lifespan in C. elegans. PLoS Genet. 2012;8:e1002780. doi: 10.1371/journal.pgen.1002780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.de Magalhaes JP. Species selection in comparative studies of aging and antiaging research. In: Conn PM, editor. Handbook of Models for Human Aging. Burlington, MA: Elsevier Academic Press; 2006. pp. 9–20. [Google Scholar]
- 10.Harel I, Benayoun BA, Machado B, Singh PP, Hu CK, Pech MF, Valenzano DR, Zhang E, Sharp SC, Artandi SE, et al. A platform for rapid exploration of aging and diseases in a naturally short-lived vertebrate. Cell. 2015;160:1013–1026. doi: 10.1016/j.cell.2015.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]