Abstract
Sirtuins are evolutionarily conserved class III histone deacetylases that have been the focus of intense scrutiny and interest since the discovery of Sir2 as a yeast longevity factor. Early reports demonstrated an important role of Sirt1 in aging and metabolism, but its critical regulatory role in the immune system has only been unveiled in recent years. In this review we discuss the latest advances in understanding the regulatory role of Sirt1 in immune responses as well as how Sirt1 translates metabolic cues to immune signals, which would bring new insights into both pathogenesis and potential therapeutic strategies of a variety of immune-related diseases, such as cancer, microbial infection, autoimmune diseases and transplantation.
Keywords: immune diseases, innate immune cells, myeloid-derived suppressor cells, metabolism, regulatory T cells, Sirt1, T-cell activation
Introduction
Sirtuins, initially identified as orthologues of the yeast Sir2 (silent information regulatory 2) protein, belong to the class III histone deacetylase family, using NAD+ as co-factor.1 In Saccharomyces cerevisiae, SIR2 was originally identified as one of the genes encoding a chromatin-silencing complex.2 Then, it was also found involved in transcriptional silencing of telomeres3 and rDNA repeats.4,5 Subsequent studies showed that in budding yeast Sir2 is a limiting factor in promoting yeast longevity as increasing Sir2 activity extended the lifespan, sparking an interest in Sir2 and its orthologues in higher organisms. This aging control phenotype has since been extended to Caenorhabditis elegans, Drosophila melanogaster and mammals.6–9
In mammals, seven Sirtuins, Sirt1–Sirt7, have been identified. They ubiquitously express in brain, heart, liver, testis, ovary, muscle, lung, kidney, blood and spleen, albeit at various levels. All Sirtuins share a c.275-amino-acid core deacetylase domain, but their various N- and C-terminal domains dictate their different subcellular localization and physiological functions.10,11 Sirt1, closest to yeast Sir2 and the most studied sirtuin in mammals, has two nuclear localization signals at the N-terminus and a coiled-coil domain at the C-terminus in addition to the core deacetylase domain.10 Like Sirt1, Sirt6 and Sirt7 generally reside in nucleus and regulate transcription by targeting transcription factors, co-factors or histones.12–16 Sirt2 primarily controls oligodendrocyte differentiation and cell cycle in cytosol.17,18 Sirt3, Sirt4 and Sirt5 regulate the activities of metabolic enzymes and oxidative stress pathways in mitochondria.19,20
Although studies have shown that Sirt1 is extensively involved in physiological as well as pathological conditions associated with aging, including cancer, neurodegenerative diseases and metabolic diseases,21–24 its role as a regulator in the immune system has only been revealed recently. In this review we summarize Sirt1's roles in both innate and adaptive immune regulation through sensing and integrating metabolic cues. We also discuss various immune-related diseases associated with Sirt1 dysregulation as well as potential therapeutics for the diseases.
Sirt1 originally served as a metabolic sensor in aging control
Restricting calorie intake, a reduction of calories by 20–50% known as caloric restriction, has been proved to increase the lifespan of organisms from yeast to mammals. The hypothesis that Sirt1 might mediate the benefits of calorie restriction was based on its enzymatic activity,25,26 which depends on NAD+, elevated in most metabolic tissues under calorie restriction. The strict NAD+-dependence of Sirt1 activity provided the first clue that this deacetylase functions as a global energy status sensor.23,26,27 During the deacetylation reaction, NAD+ is cleaved into nicotinamide and ADP-ribose, an acetyl acceptor to form acetyl-ADP-ribose product. Considering that many enzymes or transcription regulators involved in metabolism need appropriate acetylation of their specific lysine residues to perform normal functions or correct subcellular localization, Sirt1 can serve as a critical sensor/regulator for metabolism and energy homeostasis.23,28 Peroxisome proliferator-activated receptor γ coactivator 1α (PGC1α), a transcriptional co-regulator that controls mitochondrial biogenesis and activity, deacetylation by Sirt1 leads to its activation and induces the expression of gluconeogenic genes for hepatic glucose output during caloric restriction responses.29,30 Liver X receptor deacetylation by Sirt1 results in increased reverse cholesterol transport,31 whereas protein tyrosine phosphatase 1B deacetylation by Sirt1 represses insulin resistance.32 Meanwhile, Sirt1 deacetylates peroxisome proliferator-activated receptor γ to up-regulate insulin expression and secretion in pancreatic β-cells as well as possibly promoting fat mobilization in white adipose tissue.33–35 Sirt1 also deacetylates forkhead box O (FOXO) transcription factors, which are key regulators of lipid and glucose metabolism as well as cell stress responses.36–39
Sirtuins were found to control aging from Saccharomyces cerevisiae to Caenorhabditis elegans and Drosophila melanogaster,6,7and this conserved function has been confirmed recently in a variety of organisms.9,40 In mammals, Sirt1 is also demonstrated as a global metabolic regulator in controlling glucose homeostasis and lipid metabolism, protecting against age-associated diseases, and thereby increasing health span and, in some cases, lifespan.22,23,39,41 The state-of-the-art mechanisms of how Sirt1 regulates metabolism can be found in the latest reviews by Guarente, Cantό and their colleagues.22,23 In this review, we specifically focus on the emerging modulatory role of Sirt1 in the immune system.
Sirt1 recently emerges as a regulator in immune regulation
Sirt1 regulates innate immune responses
Sirt1 determines the fates of innate immune cells
Haematopoietic stem cells can differentiate into all kinds of terminal immune cells,42 and they are tightly regulated by Sirt1 because Sirt1 deficiency compromises embryonic stem cell haematopoietic differentiation, and embryonic and adult haematopoiesis in mice.43,44 Derived from haematopoietic stem cells, macrophages are functionally polarized into classically activated macrophages, termed M1 macrophages, and alternatively activated macrophages, namely M2 macrophages, in response to various microenvironmental signals.45,46 Whereas M1 macrophages, induced by interferon-γ (IFN-γ) in concert with microbial stimuli, are pro-inflammatory and have a central role in host defence against bacterial and viral infections,47,48 M2 macrophages, polarized by T helper type 2 (Th2) cytokines interleukin-4 (IL-4) and IL-13, are associated with responses to anti-inflammatory reactions, helminth infection, tissue remodelling, fibrosis and tumour progression.45 Sirt1-deficient macrophages displayed a significant increase in basal and IFN-γ/lipopolysaccharide-stimulated inducible nitric oxide synthase expression, suggesting that Sirt1 deletion promoted polarization of M1 macrophages, whereas Sirt1-deficient bone marrow derived macrophages exhibited a significant decrease in IL-4-stimulated expression of M2 macrophage marker arginase 1.49 It is well known that increasing obesity triggers a switch in the macrophage phenotype from M2 macrophages towards M1 cells in adipose tissues, leading to tumour necrosis factor-α-induced-insulin resistance.50 Sirt1 deletion in myeloid cells increased infiltration of M1 macrophages and decreased M2 macrophages in adipose tissue in mice on high-fat diets, resulting in insulin resistance.49 Yet, the precise mechanisms of how Sirt1 regulates macrophage polarization are poorly understood, and need detailed future research (Fig.1).
Figure 1.
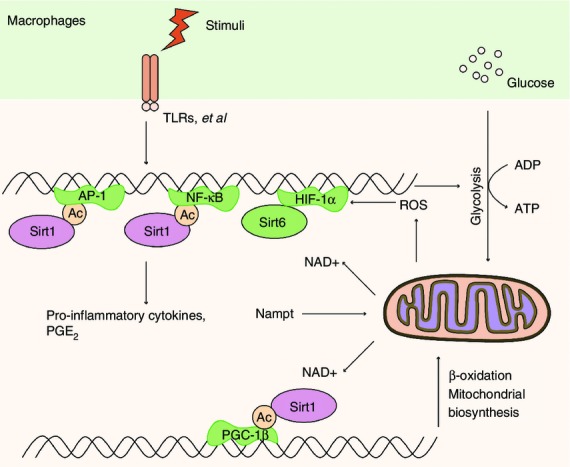
Sirt1 controls the immune responses in macrophages. When Toll-like receptor 4 (TLR4) signal is ignited, reactive oxygen species (ROS) stabilize hypoxia-inducible factor 1α (HIF-1α) protein and activate glycolysis-related genes accompanied by nuclear factor-κB (NF-κB) p65 activation. HIF-1α could further increase glycolysis and inhibit mitochondrial glucose oxidation. When transformed to late stage, Sirt1 and Sirt6 are required for the switch from glycolysis to enhanced fatty acid mitochondrial oxidation, which also requires the enzyme Nampt. Sirt1 deacetylates and undermines the activation of the NF-κB pathway as well as transcription factor activator protein 1 (AP-1). Sirt1 could also support fatty acid oxidation by deacetylating and activating peroxisome proliferator-activated receptor gamma coactivator 1β (PGC-1β), promoting mitochondrial biogenesis and recovering homeostasis, while Sirt6 represses glucose metabolism by epigenetically silencing the HIF-1α pathway, consequently promoting a shift towards fatty acid oxidation. Meanwhile, activation of the TLR signalling pathway could induce Nampt expression during the late stage of macrophage activation, thus causing a negative feedback effect on macrophage activation.
Neutrophilic granulopoiesis involving continuous generation of mature neutrophils from haematopoietic progenitors, is tightly regulated by granulocyte colony-stimulating factor (G-CSF).51 During neutrophilic granulocyte differentiation of CD34+ haematopoietic progenitor cells, NAD+-dependent Sirt1 activity was increased, leading to the activation of the granulocyte-specific transcription factors CCAAT-enhancer-binding proteins α and β and then up-regulating the expression of G-CSF and G-CSF receptor, which led to a positive feedback regulation of G-CSF.52 Consistently, in another study, Sirt1 mRNA levels were much higher in granulocytes of healthy donors compared with haematopoietic CD34+ progenitor cells. Furthermore, up-regulation of Sirt1 mRNA levels was observed upon differentiating therapy with all-trans retinoic acid in patients with acute promyelocytic leukaemia. Interestingly, Sirt1 knockdown by specific short hairpin RNA impaired granulocytic differentiation, pointing to a possible involvement of Sirt1 in the initiation of neutrophil differentiation, providing a possible therapeutic strategy for acute promyelocytic leukaemia.53
Myeloid-derived suppressor cells (MDSCs) are a major component of the immune suppressive network responsible for immune cell tolerance in cancer, autoimmunity, chronic infection and other pathological conditions.54–60 MDSCs exhibit an immature phenotype that can be conditioned into an M1 or M2 in tumours. In our recent study, we found that compared to wild-type (WT) MDSCs, the MDSCs from Sirt1-myeloid-deficient mice bearing tumours displayed an M1 phenotype, producing more NO, tumour necrosis factor-α, IL-12, higher glycolytic activity and lower arginase activity and IL-10, and significantly diminished suppressive activity (Fig.2), indicating the key role of Sirt1 in directing the differentiation of MDSCs during tumour growth.
Figure 2.
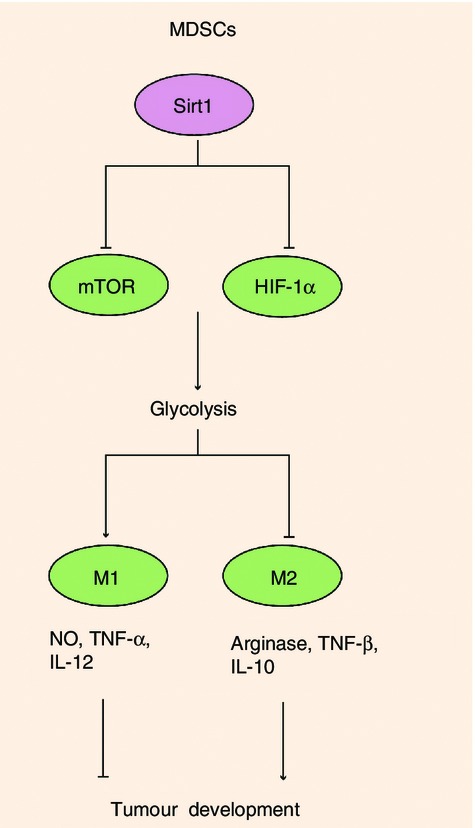
Sirt1 determined the switch of myeloid-derived suppressor cell (MDSC) differentiation into M1 or M2 MDSCs. In MDSCs, Sirt1 deficiency directs a specific switch to the M1 lineage when cells enter the periphery from bone marrow, decreasing suppressive function in favour of a pro-inflammatory M1 phenotype with more NO, tumour necrosis factor-α and IL-12. Glycolytic activation through the mammalian target of rapamycin (mTOR)-HIF1α pathway was required for differentiation to the M1 phenotype, which conferred higher tumoricidal activity.
Sirt1 regulates the functions of innate immune cells
Sirt1 controls the production of pro-inflammatory cytokines in innate immune cells
Sirt1 controls the production of pro-inflammatory cytokines in innate immune cells. Sirt1 has a straightforward regulatory role in macrophages, a main source of pro-inflammatory cytokines secreted in response to infection and environmental stress.61 Sirt1 regulated nuclear factor κ-light-chain-enhancer of activated B cells (NF-κB) signalling by deacetylating RelA/p65 at lysine 310 residue (K310), undermining NF-κB transcriptional activity and so suppressing the pro-inflammatory phenotype of macrophages. Knockdown of Sirt1 by small interfering RNA in the murine macrophage RAW264.7 cell line and in intraperitoneal macrophages led to increased lipopolysaccharide-stimulated inflammation by broadly activating the c-Jun N-terminal kinase and IκB kinase inflammatory pathways (Fig.1).62 Sirt1 levels were reduced in macrophages and lungs of smokers and patients with chronic obstructive pulmonary disease due to its post-translational modifications by cigarette smoke-derived reactive components, leading to increased acetylation of RelA/p65. Whereas in models of chronic obstructive pulmonary disease, Sirt1 activator treatment ameliorated the pro-inflammatory effect of cigarette smoke,63,64 as well as reducing neutrophil flux, neutrophil chemoattractant production and RelA/p65 activation in bronchoalveolar lavage fluid.65 Moreover, Sirt1 activators, i.e. naturally occurring polyphenol resveratrol and synthetic Sirt1-activating compound SRT1720, could attenuate the macrophage pro-inflammatory state in adipose tissue, leading to insulin-sensitizing effects in fatty rats (Fig.1).62 Consistently, myeloid cell-specific Sirt1 knockout (KO) mice were hypersensitive to local and systemic lipopolysaccharide challenges and displayed higher percentages of activated macrophages in liver and adipose tissue when challenged with a high-fat diet, predisposing the animals to the development of systemic insulin resistance and metabolic derangement.66 Besides, the anti-inflammatory effects of 5′ adenosine monophosphate-activated protein kinase (AMPK) activation in lipid-induced inflammation required Sirt1, especially the Sirt1-mediated K310 deacetylation of NF-κB p65, thereby contributing to the protection against obesity, inflammation and insulin resistance.67 Actually, Sirt1 and AMPK develop a reciprocal positive regulating loop in modulating glycolysis and lipid metabolism. Sirt1 activates AMPK by deacetylating liver kinase B1, whereas AMPK reciprocally activates Sirt1 by increasing the NAD/NADH ratio or the expression/activity of nicotinamide phosphoribosyl transferase (Nampt).68 Given all that, these findings suggest that the AMPK-SIRT1 cycle links the cell's energy status and inflammation. It has also been proved that over-expression of Sirt1 and Sirt1 agonist resveratrol addition in central nervous system microglia cells markedly reduced NF-κB signalling stimulated by amyloid-peptides and had strong neuroprotective effects, highlighting the therapeutic potential of Sirt1-activating compounds in Alzheimer disease.69,70
Sirt1 is also involved in dendritic cell (DCs) cytokine programming. Enhanced Sirt1 activity in response to phagocytic stimuli regulated the IL-12p70/IL-23 balance in human DCs by deacetylating histone and reducing the accessibility of c-Rel to the il12a promoter and its transcriptional activation, possibly modulating the Th1/Th17 balance during immune diseases (Fig.3).71 In a murine model of autoimmune inflammation experimental autoimmune encephalomyelitis, genetic deletion of Sirt1 in DCs partially protected mice from myelin oligodendrocyte glycoprotein (MOG)-induced experimental autoimmune encephalomyelitis with statistically reduced clinical disease scores. Sirt1 interacted with and deacetylated interferon regulatory factor 1, a transcription factor that drove IL-27 production,72 which is possibly catalysed by the acetyl-transferase p300,73 to suppress interferon regulatory factor 1 binding to the promoter region of the p28 subunit of IL-27. Combined with the inhibitory effects of IL-27 and IFN-γ on pathogenic Th17 differentiation,74,75 specific deletion of Sirt1 in DCs suppressed Th17 differentiation during inflammation, hence resulting in reduced experimental autoimmune encephalomyelitis in mice,76 indicating that deacetylase Sirt1 could programme DCs to regulate Th17 differentiation during inflammation (Fig.3). Our recent study found that deletion of Sirt1 or Sirt1 inactivation treatment in both murine and human DCs increased the production of IL-12 but decreased transforming growth factor-β levels. Consequently, genetic deletion of Sirt1 in DCs restrained the generation of regulatory T (Treg) cells while promoting Th1 development, resulting in an enhanced T-cell-mediated inflammation against microbial responses (Fig.3).77
Figure 3.
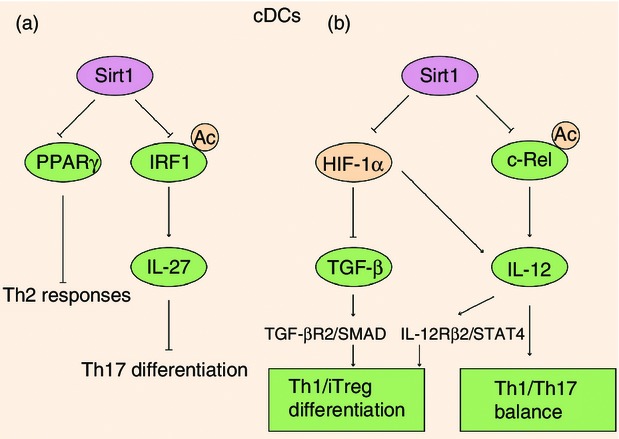
Sirt1 regulated the immune responses in dendritic cells (DCs). (a) Sirt1 inhibition disables conventional DCs to prime T helper type 2 (Th2) responses in the airways by depressing peroxisome proliferator-activated receptor-γ (PPARγ). On the other hand, Sirt1 deacetylates interferon regulatory factor 1 (IRF1) to suppress IRF1 binding to the promoter region of the p28 subunit of interleukin-27 (IL-27), modulating Th17 differentiation during MOG-induced experimental autoimmune encephalomyelitis. (b) Sirt1-HIF-1α signalling axis is required for DCs to guide the Th1 and iTreg cell differentiation through regulating the expression of transforming growth factor-β (TGF-β) and IL-12. Moreover, Sirt1 modulates the IL-12 p70/IL-23 balance in response to phagocytic stimuli by deacetylating histone and reducing the accessibility of c-Rel to the il12a promoter and its transcriptional activation.
The role of Sirt1 in phagocytosis and killing
Another important function of innate immune cells is the clearance of invading pathogenic organisms as well as apoptotic cells and neoplastic cells.78–80 Besides modulating the NF-κB signalling pathway, Sirt1 also played a key role in activator protein 1 (AP-1) signal through deacetylation. Sirt1 could suppress the transcriptional activity of AP-1 by directly interacting with the basic leucine zipper domains of c-Fos and c-Jun, reducing the expression of cyclooxygenase 2, the rate-limiting enzyme for prostaglandin production. Consequently, over-expression of Sirt1 in macrophages reduced the production of prostaglandin E2, an inhibitor of the phagocytosis activity and the bacterial killing function of macrophages,81,82 so leading to higher phagocytic percentage and phagocytic index and tumoricidal activities of peritoneal macrophages, while knockdown Sirt1 by RNAi significantly impaired the anti-tumoral activity of macrophages (Fig.1).83
Subsets of MDSCs also can be divided on the basis of their morphological heterogeneity. Granulocytic MDSCs have a CD11b+ Ly6G+ Ly6Clow phenotype, whereas MDSCs with monocytic morphology are CD11b+Ly6G− Ly6Chigh. Importantly, evidence indicates that these two subpopulations may have different functions in cancer. Generally, granulocytic MDSCs exerted weak or no suppression on CD8+ T cells, whereas monocytic MDSCs displayed strong suppressive potential.84–86 Our laboratory has recently reported that Sirt1 KO MDSCs, preferring an M1 type, had stronger tumour killing capacity, especially CD11b+ Gr1+ Ly6Chi CD115+ (monocytic) MDSCs, so significantly delaying tumour growth (Fig.2).87 However, the specific mechanisms of Sirt1 in controlling the differentiation and functions of granulocytic MDSCs and monocytic MDSCs still need further study. In another study, intraperitoneal macrophages, bone marrow macrophages as well as alveolar macrophages lacking Sirt1 had similar phagocytic and bacterial killing capacity as WT cells. Moreover, mice lacking Sirt1 in myeloid cells displayed no survival advantage when challenged with Gram-negative toxin or Gram-positive bacteria,88,89 suggesting that therapeutic suppression of Sirt1 should be done safely without suppression of myeloid cell-specific immune responses to severe bacterial infection since Sirt1 inhibitors are being investigated as therapeutic agents for the treatment of Huntington's disease and cancer (refs 90–93; clinical trials NCT01521832 and NCT01521585).
Sirt1 plays an important role in antigen processing and presentation
Conventional DCs are specialized in antigen processing and have unrivalled potential, inefficiently stimulating naive Th cells and initiating primary immune responses, and they are also needed for optimal re-stimulation of effector Th cells.94,95 Contrary to the well-reported anti-inflammatory effect of Sirt1, inhibition of Sirt1 by cambinol markedly attenuated ovalbumin (OVA)-induced airway allergy.96,97 Migration and maturation of DCs were attenuated because pharmacological inhibition of Sirt1 substantially reduced the flux of DCs in bronchial lymph node and down-regulated the expression of maturation markers CD40, CD80 and CD86. Consistently, mice conditionally deficient for Sirt1 in DCs displayed significantly reduced parameters of airway inflammation compared with control mice, suggesting that blocking Sirt1 activity in lung DCs might have therapeutic potential in the treatment of airway allergy.97 However, the role of Sirt1 in regulating antigen presentation in DCs shows tissue- and/or disease-dependent characteristics. During the airway allergy, cambinol treatment or Sirt1 deficiency reduced Th2 cytokine production and T-cell proliferation, suggesting the muted function of DCs in pro-Th2 accessory activity when Sirt1 activity was inhibited. And the pre-treatment of OVA-pulsed bone-marrow-derived DCs or sorted lung DCs with either cambinol or sirtinol significantly reduced their capacity to induce T-cell proliferation and Th2 cytokine production by depressing peroxisome proliferator-activated receptor-γ (Fig.3).97 However, in spleen or peripheral lymph nodes, Sirt1-deficient DCs showed similar capacity of antigen presentation since CD4+ OT-IIT donor cells isolated from WT and Sirt1CD11c−/− recipients displayed a comparable proliferation rate in infectious inflammation.77 Overall, exploring the role of Sirt1 in DCs under various diseases remains ripe for increased investigation.
The potential role of Sirt1 in autophagy and endoplasmic reticulum stress
Recent reports showed that autophagy in macrophages might produce protective effects against advanced atherosclerosis,98 and the impairment of autophagy in macrophages led to inflammation, resulting in the progression of atherosclerosis.99 Sirt1 could influence autophagy directly through its deacetylation of key components of the autophagy induction network, such as the products of autophagy genes (Atg) 5, 7 and 8. Nucleus-localized Sirt1 is also known to induce the expression of autophagy pathway components through the activation of FoxO transcription factor family members.100 In human THP-1 cells, treatment with Sirtinol, a chemical inhibitor of Sirt1, induced inflammation through NF-κB activation and dysregulated autophagy through nutrient-sensing pathways such as mammalian target of rapamycin (mTOR) and AMPK pathways.101 Recently, Sirt1-mediated autophagy was proved to be one of the mechanisms for metformin treatment of hepatic steatosis.102 Moreover, genetic polymorphisms at SIRT1 and FOXO1 have been proved to be associated with carotid atherosclerosis, highlighting the need for functional investigation of Sirt1 in atherosclerosis.103 Further studies using animal models are needed to elucidate a detailed mechanism by which Sirt1 dysfunction-induced inflammation through dysregulation of autophagy in monocytes/macrophages causes insulin resistance and atherosclerosis.
Another intracellular signal that has been connected to inflammatory responses is endoplasmic reticulum stress.104 Recent studies suggested that the inositol-requiring enzyme 1 α–X-box binding protein 1 pathway was required for optimal and sustained Toll-like receptor-induced inflammatory cytokine production in macrophages.105 Besides alternatively splicing, X-box binding protein-1 is regulated by post-translational acetylation and deacetylation by the acetyltransferase p300 and deacetylase Sirt1, respectively.106 In dextran sodium sulphate-induced colitis murine models, methyl-deficient diet, which is frequent in patients with inflammatory bowel disease, aggravates experimental colitis through increasing endoplasmic reticulum stress. Meanwhile, Sirt1 is down-regulated by the methyl-deficient diet treatment, leading to impaired chaperone expression through hyperacetylation of heat-shock factor protein 1. In rats, pharmacological activation of Sirt1 by SRT1720 prevented colitis under a methyl-deficient diet by reducing heat-shock factor protein 1 acetylation and increasing expression of binding immunoglobulin protein, heat-shock proteins 27 and 90. Moreover, the increased NF-κB signal and enhanced inflammasome activation was reversed by the SIRT1 agonist.107 However, more studies are needed to explore the role of Sirt1 in endoplasmic reticulum stress-induced inflammation.
Sirt1 modulates adaptive immune responses
Sirt1 controls the activation and proliferation of T cells
The crucial role of Sirt1 in the adaptive immune system was originally concentrated on T-cell activation.108,109 As the result of comparable percentages of CD4+ and CD8+ mature T cells and similar ratios of B220+ B cells to CD3+ T cells in both Sirt1 KO mice and control littermates, disruption of Sirt1 expression in mice might not affect T-cell and B-cell development. In CD4+ T cells of patients with active systemic lupus erythematosus, deacetylase Sirt1 levels were significantly increased compared with controls, whereas mRNA levels of acetylase p300 were significantly down-regulated.110 Consistently, activated T cells as well as anergic T cells displayed higher Sirt1 protein levels than naive T cells in mice.111 Activation of T cells requires the cooperative interactions of several transcription factors, including AP-1, NF-κB, and nuclear factor of activated T-cell transcription factor.111 In T cells, Sirt1 could interact with c-Jun by its C-terminus and suppress c-Jun acetylation, inhibiting AP-1 transcriptional activity. As a result, Sirt1-deficient T cells were hyper-responsive and could be activated by T-cell receptor (TCR) stimulation alone without CD28 co-activation. In OVA-immunized mice, proliferation as well as IL-2 production of Sirt1−/− T cells were dramatically increased compared with control T cells, suggesting that Sirt1 could function as a negative regulator of T-cell activation (Fig.4).111
Figure 4.
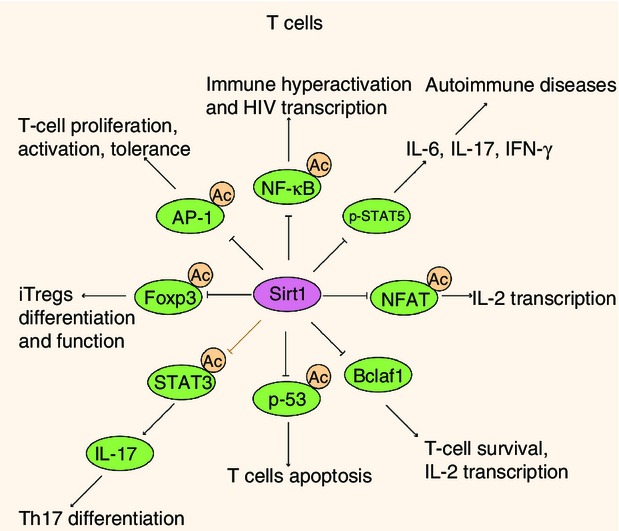
Sirt1 played a role in T-cell activation, differentiation and tolerance. In T cells, Sirt1 serves as a negative regulator of T-cell activation by deacetylating activator protein 1 (AP-1), but over-expression of Sirt1 drives T-cell tolerance. Sirt1 could also limit T-cell proliferation in response to interleukin-2 (IL-2) by both down-regulating signal transducer and activator of transcription 5 (STAT5) expression and suppressing pSTAT5 signalling. During HIV infection, Sirt1 inhibition leads to immune hyperactivation induced by Tat and T-cell apoptosis respectively by targeting p65 and p53. Sirt1 could also regulate T-cell activation and IL-2 transcription by controlling Bclaf1 and nuclear factor of activated T-cell transcription factor (NFAT). Besides, modulating Sirt1 activity in T cells regulates Foxp3 protein levels as well as the number and suppressive capacity of induced regulatory T cells. Although the Sirt1–STAT3 axis has not been proved in T cells directly, it is likely that Sirt1 regulates T-cell differentiation through deacetylating STAT3.
Sirt1 activators could also limit the capacity of T cells to proliferate in response to IL-2 by both down-regulating signal transducer and activator of transcription 5 (STAT5) expression and suppressing p-STAT5 signal (Fig.4). In an experimental autoimmune uveoretinitis model, oral Sirt1 activator treatment suppressed disease by attenuating antigen-specific T-cell responses and markedly inhibiting innate and adaptive pro-inflammatory cytokine production in the eyes. Moreover, oral treatment with Sirt1 activators during the efferent phase of experimental autoimmune uveoretinitis could even suppress disease onset.112 T-cell activation on the other hand created the optimal environment for HIV replication, enhancing viral transcription through the production of cytokines and transcription factors, such as NF-κB.113 The HIV infection itself manipulated the activation status of infected T cells through the expression of viral proteins, including trans-activator of transcription (Tat).114 During HIV infection, HIV Tat protein hyperactivated T cells by impairing the deacetylase activity of Sirt1 by binding to the Sirt1 catalytic site, manifested by abnormally sustained action of p65 and p53 as well as superactivated IL-2 gene expression, which led respectively to increased NF-κB pro-inflammatory signalling and T-cell apoptosis (Fig.4).115,116 On the other hand, Sirt1 acts as a transcriptional co-activator of HIV-1, as it recycles Tat to its unacetylated form, which is the one required for subsequent rounds of HIV transcription. Consequently, the disrupted balance between p300 and Sirt1 drove immune hyperactivation and sustained HIV transcription.117 Moreover, Sirt1 was reported to activate hepatitis B virus core promotor, cooperating with the other two metabolic sensors farnesoid X receptor α and PGC-1α.118
In spite of directly modulating the transcriptional activity of transcription factors NF-κB and AP-1, Sirt1 can also modify some other necessary genes for T-cell activation and proliferation. B-cell lymphoma 2 associated factor 1 (Bclaf1) primarily identified as an inducer of apoptosis,119,120 has been proved to be a critical regulator in T-cell activation.121 Bclaf1 expression in Sirt1-deficient CD4+ T cells upon TCR/CD28 stimuli when compared with WT T cells was greatly increased, suggesting Sirt1 as a negative regulator of Bclaf1. Sirt1 inhibited Bclaf1 expression not only by suppressing NF-κB transcriptional activity but also by localizing to the Bclaf1 locus, and deacetylating histone lysine residues at the promoter region of Bclaf1. Moreover, Bclaf1 knockdown restored Sirt1-null CD4+ T-cell hyper-activation (Fig.4).122 Consequently, Sirt1 does not fight a lone battle but orchestrates a vast and intricate regulatory network in the regulation of T-cell activation.
Sirt1 in T-cell tolerance
Most autoreactive T cells are removed by negative selection during development in the thymus (central tolerance), but escape of self-reactive T cells into the periphery also occurs. One effective mechanism to deal with self-reactive T cells in the periphery is clonal anergy (peripheral tolerance), which is induced by partial or suboptimal stimulation. The failure of peripheral tolerance will contribute to autoimmunity.123–125 Indeed, Sirt1-deficient mice have been found to develop spontaneous autoimmunity.111,126 Importantly, a leucine-to-proline mutation at residue 107 of Sirt1 has been found in a family with type 1 diabetes, leading to a decrease in deacetylase activity, indicating that defects of Sirt1 deacetylase activity could give rise to the development of autoimmunity.127 As described above, anergic T cells displayed higher levels of Sirt1, and Sirt1-deficient CD4+ T cells were hyperproliferative upon TCR/CD28 stimuli and could be activated by TCR stimulation alone without CD28 co-activation, suggesting Sirt1 as an anergic factor in peripheral CD4+ T-cell tolerance. In fact, OT-II TCR Sirt1−/− mice could not be tolerated by OVA323–339 peptide, and Sirt1−/− OT-II T cells exhibited higher proliferation upon OVA stimuli. Moreover, Sirt1 interacted with and deacetylated c-Jun, yielding an inactive AP-1 factor. As a result, breakdown of CD4+ T-cell tolerance due to Sirt1 deficiency promoted the development of autoimmune syndrome (Fig.4). Finally, the finding that Sirt1 inhibited T-cell activation and was required for T-cell tolerance suggests that Sirt1 activator might help in the treatment of autoimmune diseases.111
Interleukin-2 provides an indispensable signal for maintaining the viability and promoting proliferation of activated T cells, and reversing T-cell anergy.108 The forkhead transcription factor, FoxO3a, interacts with early responsive genes 2/3 on the Sirt1 promoter to synergistically regulate Sirt1 expression. The addition of recombinant mouse IL-2 suppresses Sirt1 transcription by sequestering FoxO3a to the cytoplasm because of the phosphorylation of FoxO3a by the activated phosphatidylinositol-4, 5-bisphosphate 3-kinase-AKT (also called as protein kinase B, is serine/threonine-specific protein kinase) pathway. Furthermore, expression of the constitutively active form of FoxO3a blocks IL-2-mediated reversal of T-cell tolerance by retaining Sirt1 expression.128 However, there are still several questions concerning the mechanism of how Sirt1 regulates T-cell tolerance.
Sirt1 regulates the differentiation of T cells
During TCR activation in a particular cytokine milieu, naive CD4+ T cells may differentiate into one of several lineages of T helper cells, including Th1, Th2, Th17 and induced Treg (iTreg) cells, as defined by their pattern of cytokine production and function,129 whereas CD8+ T cells programme into cytotoxic T lymphocytes to kill host cells infected with pathogens.130 Sirt1 is also implicated in the differentiation of activated T cells, although the precise mechanisms are not well studied.
In the OVA-induced asthma murine model, pharmacological inhibition of Sirt1 dampens adaptive Th2 responses, demonstrated by reduced production of IL-4, IL-5 and IL-13, so attenuating subsequent allergic inflammation. Although Sirt1 promotes Th2 response indirectly through interfering with the lung DC pro-Th2 phenotype, Sirt1 might play an important role in Th2 differentiation because the depressed Th2 response might be the result of impaired Th2 differentiation.97
In mice with collagen-induced arthritis, administration of the Sirt1 activator resveratrol either before or after disease onset attenuated clinical parameters and bone erosion. The arthritis-protective effects of resveratrol were associated with the markedly reduced number of total CD4+ T cells, especially Th17 cells, as well as the production of pro-inflammatory cytokines IL-17 in draining lymph nodes.131 In the lamina propria mononuclear cells of patients with Crohn's disease and those with ulcerative colitis, Sirt1 activation by Cay10591 significantly reduced the fractions of IFN-γ and IL-17A-expressing CD3+ T cells.132 Indeed, small molecule activators of Sirt1 are currently used in clinical trials toward the treatment of autoimmune diseases such as rheumatoid arthritis.109
Sirt1 might also have a potential role in modulating Th differentiation given that Sirt1 could suppress STAT3 activity by deacetylation.133 Activated by both IL-6 and IL-23, STAT3 plays a critical role in Th17 development by regulating RAR-related orphan receptor γt.134 Interestingly, STAT3 phosphorylation and function in the liver were tightly regulated by Sirt1-mediated deacetylation, activating the stimulatory effect of PGC-1α and FoXO1 on gluconeogenesis, so ensuring maximal activation of gluconeogenic gene transcription.133 Despite the fact that the Sirt1–STAT3 pathway has not been demonstrated in T cells directly, it is likely that Sirt1 regulates T-cell differentiation through the same mechanisms (Fig.4).
Sirt1 was also demonstrated to regulate the differentiation of CD4+ T cells to Treg cells. Both natural Treg cells and iTreg cells are characterized by expression of the transcription factor Foxp3, which is essential to their suppressive function.129 Foxp3 protein has a short half-life and acetylation prevents proteasomal degradation, dramatically increasing Foxp3 levels. Sirt1, together with histone acetyltransferase p300, was found to reciprocally regulate the acetylation and activity of Foxp3. Consequently, modulating Sirt1 activity in T cells regulated Foxp3 protein levels as well as the number and suppressive capacity of Treg cells (Fig.4).135,136 Meanwhile, Sirt1 has also been shown to destabilize mothers against decapentaplegic homologue 7 (Smad7), an important mediator of the signalling pathway of transforming growth factor-β,137 further implying that Sirt1 was involved in Treg cell development and function. Targeting Sirt1 by specific deletion in Treg cells or treatment with Sirt1 inhibitors EX-527 promoted the expression of Foxp3, and increased Treg cell suppressive function, so prolonging the allograft survival.138 In another study, adoptive transfer of CD4+ CD25+ Foxp3− T effector cells from Sirt1fl/CD4cre mice but not Sirt1fl-Foxp3cre natrual Treg cells to B6 Rag1−/− mice, resulted in a 2·8-fold increase in iTreg cell formation compared with mice receiving WT effector cells and correlated with attenuated colitis and reduced weight loss. Similarly, in mice with dextran sodium sulphate-induced irritable bowel disease, pharmacological inhibition of Sirt1 also increased iTreg cell formation and alleviated colitis.139 As a result, Sirt1 inhibitor therapy could be used to enhance iTreg cell functions in vivo and have beneficial effects on allograft survival and autoimmune diseases. Given the fact that therapeutic inhibition of other histone deacetylases (HDACs), HDAC6 and HDAC9, could also augment the suppressive function of Treg cells, combined inhibition of HDACs might be a potential therapeutic strategy in the management of autoimmunity and organ transplantation. Of note, in spite of shared mechanisms involved in the efficacy of HDACs targeting Treg cells, the distinct mechanism of each HDAC should be noted.140
Moreover, Sirt1 was the specific target of basic leucine zipper transcription factor, ATF-like (BATF), a member of the AP-1 family, in CD8+ T cells.141,142 BATF, together with c-Jun, transcriptionally inhibited Sirt1 expression, resulting in increased histone acetylation of the T-box transcription factor (T-bet) locus and elevated cellular NAD+ levels, which increased T-bet expression and ATP production, so promoting effector differentiation and cell survival. As a result, BATF deficiency inhibited effector CD8 T-cell differentiation.141
The role of Sirt1 in B cells
Compared to the above immune cells, the potential role of Sirt1 in B cells has not been well discussed. Sirt1 null mice displayed the lupus-like autoimmune syndrome, such as higher titres of autoantibodies in serum, deposition of autoantibodies in kidney,111,126,131 suggesting that Sirt1 might regulate B-cell activation and function. It is possible that Sirt1 modulated the activation and maturation of B cells along with CD38 expression and NAD+ availability.143,144 Despite the above findings, further work is needed to illuminate the role of Sirt1 in regulating B-cell activation, maturation and function.
Sirt1 translates metabolic cues during regulation of the immune responses
Given the fact that Sirt1 is a NAD+-dependent deacetylase, Sirt1 regulates the inflammatory responses in coordination with metabolic changes of the cells.145 The acute stage of sepsis triggered a rapid and transient decline in ATP concentration, Sirt1 and AMPK protein expression levels along with the increase of hypoxia-inducible factor 1α (HIF-1α) expression and autophagy in peripheral blood lymphocytes and liver.146 When sepsis is ignited, the acute inflammatory stage generates reactive oxygen species, which could stabilize HIF-1α protein and activate glycolysis-related genes accompanied by NF-κB p65 activation. HIF-1α could further increase glycolysis and inhibit mitochondrial glucose oxidation.147–149 As the early inflammatory response transforms to late adaptation, NAD+ sensors Sirt1 and Sirt6 are required for the switch from glycolysis to enhanced fatty acid mitochondrial oxidation, which also requires the enzyme Nampt. Sirt1 deacetylates and inactivates the p65 component of the NF-κB pathway as well as c-Jun element of transcription factor AP-1, so limiting the expression of NF-κB- and AP-1-dependent genes (Fig.1).63,69,111,150,151 Besides, Sirt1 could support fatty acid oxidation by deacetylating and activating PGC-1β,152 promoting mitochondrial biogenesis and recovering homeostasis in sepsis survivors, while Sirt6 represses glucose metabolism by epigenetically silencing the HIF-1α pathway, consequently promoting a shift towards fatty-acid oxidation.16,153,154 Meanwhile, activation of the Toll-like receptor signalling pathway could induce Nampt expression during the late stage of macrophage activation, so causing a negative feedback effect on macrophage activation (Fig.1).155 In the liver of WT mice, the metabolic homeostasis is re-established within 24 hr. However, in Sirt1 Liver-KO mice, this recovery does not occur.146 Sirt1 was originally identified as a gene prolonging lifespan through metabolic control,39,156 but the beneficial effects of Sirt1 activation in mammals might owe more to its anti-inflammatory capacity.145 The anti-inflammatory effects of calorie restriction, or pharmacological up-regulation of Sirt1, might occur through reprogramming macrophages to an anti-inflammatory status.
Recently, we reported that compared to WT MDSCs, MDSCs from Sirt1-myeloid-deficient mice bearing tumours displayed an M1 phenotype and delayed the tumour growth.87 Given that immune cell activation is accompanied by metabolic switch,145 we found that myeloid deficiency in Sirt1 led to much higher glycolytic activity in splenic MDSCs in response to lipopolysaccharide and/or IFN-γ. Consistently, Sirt1 KO MDSCs screened from tumour tissues contained much higher glycolytic activity compared with that of WT MDSCs. To prove that glycolytic activity is required for the role of Sirt1 in directing MDSC differentiation during protection against tumour, we blocked glycolysis with 2-deoxy-d-glucose and found reciprocally reduced M1- but promoted M2-type MDSC differentiation in Sirt1 KO mice bearing tumour. We then found that mTOR and HIF1α orchestrated glycolytic metabolism during Sirt1-mediated MDSC programming (Fig.2).87 In another study, we reported that the interplay between Sirt1 and HIF1α but independent of mTOR in DCs instructed Th1 and iTreg cell differentiation under infectious inflammation through modulating the production of DC-derived T-cell polarizing cytokines, including IL-12 and transforming growth factor-β1 (Fig.3).77 It was demonstrated that Sirt1 and HIF1α, two metabolic sensors of redox and oxygen, respectively, helped immune cells adapt to the inflammatory microenvironment and changing metabolic states during immune responses. Similar to the metabolic switch in pro-inflammatory M1 cells and anti-inflammatory M2 cells, DCs activated by Toll-like receptor 4 stimulation and the Th17 lymphocytes that produce the pro-inflammatory cytokine IL-17 undergo aerobic glycolysis while anti-inflammatory T lymphocytes such as Treg cells have a metabolism that is characterized by mitochondrial oxidative metabolism.145 Sirt1 might also translate metabolic cues into immune signals in controlling the fates and functions of T lymphocytes as well as other activated immune cells. During the inflammatory responses, Sirt1 bridges metabolic changes and immune signals to ensure a rapid high energy supply and timely inflammatory response that will clear the pathogen and then switch to a lower energy restorative state that rebalances immunity and inflammation to regain homeostasis.
Concluding remarks
Sirt1 was originally identified as a metabolism and lifespan regulator, but its regulatory role in immune responses has been uncovered in recent years and this area remains ripe for increased investigation. The existing studies demonstrated that Sirt1 was engaged in macrophage and T-cell activation mainly through two of the major pro-inflammatory pathways in the immune response, NF-κB and AP-1 pathways (Figs1 and 4).62,111 Sirt1 could also regulate the differentiation and function of iTreg cells through deacetylating and destabilizing Foxp3,138 whereas the roles of Sirt1 in the activation of B cells and DCs as well as programming effector T-cell differentiation are only beginning to be explored.111,126,131 Since Sirt1 plays a pivotal role in immune responses, pharmacological control of Sirt1 provides potential therapeutic strategies in clinics.109,131,139,140,157,158 As a matter of fact, small molecule activators or inhibitors of Sirt1 are currently used in clinical trials into the treatment of immune diseases, such as rheumatoid arthritis, Huntington's disease and cancer.90–93,109
Recently, metabolism has not been simply viewed as a means to generate a store of energy and macromolecules for cell maintenance and growth, but a closely integrated system participating in metabolic diseases, cancer and inflammation. During the inflammatory responses, immune cells switch from a resting state to a highly active state and undergo metabolic changes, such as a shift towards aerobic glycolysis in M1 inflammatory macrophages, and in Th17 lymphocytes, while M2 macrophages and regulatory T cells have lower glycolytic rates and higher levels of oxidative metabolism.145 On the other hand, adipose tissue expansion in obesity, the hallmark of the metabolic syndrome, is characterized by increasing infiltration of pro-inflammatory immune cells into adipose tissue causing chronic, low-grade inflammation. Phenotypic switching of macrophages is an important mechanism of adipose tissue inflammation, and there is also the involvement of cells from the adaptive immune system in this process.159 But how does the cross-talk between metabolic cues and the immune signals happen or how does metabolism integrate with immune responses to regulate disease progression? Sirt1 might be one of the key answers. Sirt1 was primarily described as having a role in prolonging lifespan, and was involved in the beneficial effects of diet restriction since it is an NAD+ sensor.23,39,108,145,160 Importantly, Sirt1 could not only deacetylate and inactivate the p65 component of the NF-κB pathway as well as c-Jun element of transcription factor AP-1,63,69,111,150,151 Sirt1 could also support fatty acid oxidation by deacetylating and activating the PGC-1β.152 Under the tumour microenvironment, we verified that Sirt1 was engaged in regulating the differentiation and functions of MDSCs through orchestrating mTOR- and HIF-1α-mediated glycolysis.87 Under infectious inflammation, we proved that the Sirt1-HIF-1α signalling axis was required for DCs to guide the Th1 and iTreg cell differentiation, indicating that the DC-directed adaptive immunity requires the combination of inflammatory responses and metabolic signals.77 In a word, Sirt1 fills the gap between metabolic cues and immune signals during immune-related diseases. The prospect of targeting Sirt1 to rectify the metabolic responses in inflammation holds substantial therapeutic promise.
Acknowledgments
The authors are grateful for researchers who have contributed to this field and whose work was not cited owing to space limitations. We would like to thank Dr Ge Ying for helpful suggestion and critical reading of the manuscript. The work is supported by grants from the National Natural Science Foundation for General Programmes of China (31171407 and 81273201, G.L.), National Natural Science Foundation for Young Programmes of China (81401740, Y.H), Key Basic Research Project of the Science and Technology Commission of Shanghai Municipality (12JC1400900, G.L.), Innovation Programme of Shanghai Municipal Education Commission (14Z Z009, G.L.), Shanghai City Health Committee of planning of key projects (C704688, G.L.) and Excellent Youth Foundation of Chinese Academy of Sciences (KSCX2-EW-Q-7, G.L.).
Glossary
- ADP
adenosine diphosphate
- AMPK
5′ adenosine monophosphate-activated protein kinase
- AP-1
activator protein 1
- Atg
autophagy gene
- ATP
adenosine 5′-triphosphate
- BALF
bronchoalveolar lavage fluid
- BATF
basic leucine zipper transcription factor, ATF-like
- Bclaf1
B-cell lymphoma 2 associated factor 1
- DCs
dendritic cells
- Foxp3
forkhead box P3
- G-CSF
granulocyte-colony stimulating factor
- HDACs
histone deacetylases
- HIF-1α
hypoxia-inducible factor 1α
- HIV
human immunodeficiency virus
- IFN-γ
interferon γ
- IL-2
interleukin 2
- iTreg cells
induced T regulatory cells
- MDSCs
myeloid-derived suppressor cells
- MOG
myelin oligodendrocyte glycoprotein
- mTOR
mammalian target of rapamycin
- NAD+
nicotinamide adenine dinucleotide
- NAMPT
nicotinamide phosphoribosyl transferase
- NF-κB
nuclear factor kappa-light-chain-enhancer of activated B cells
- NO
nitric oxide
- OVA
ovalbumin
- PGC1α
peroxisome proliferator-activated receptor gamma coactivator 1alpha
- Sirt1
silent mating type information regulation 2 homolog 1
- Smad7
mothers against decapentaplegic homolog 7
- STAT5
signal transducer and activator of transcription 5
- Tat
trans-activator of transcription
- T-bet
T-box transcription factor
- TCR
T-cell receptor
- Th1
T helper cells 1
Disclosures
The authors declare no competing financial interests.
References
- Imai S, Armstrong CM, Kaeberlein M, Guarente L. Transcriptional silencing and longevity protein Sir2 is an NAD-dependent histone deacetylase. Nature. 2000;403:795–800. doi: 10.1038/35001622. [DOI] [PubMed] [Google Scholar]
- Klar AJ, Fogel S, Macleod K. MAR1-a Regulator of the HMa and HMalpha Loci in Saccharomyces cerevisiae. Genetics. 1979;93:37–50. doi: 10.1093/genetics/93.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JS, Boeke JD. An unusual form of transcriptional silencing in yeast ribosomal DNA. Genes Dev. 1997;11:241–54. doi: 10.1101/gad.11.2.241. [DOI] [PubMed] [Google Scholar]
- Gottlieb S, Esposito RE. A new role for a yeast transcriptional silencer gene, SIR2, in regulation of recombination in ribosomal DNA. Cell. 1989;56:771–6. doi: 10.1016/0092-8674(89)90681-8. [DOI] [PubMed] [Google Scholar]
- Bryk M, Banerjee M, Murphy M, Knudsen KE, Garfinkel DJ, Curcio MJ. Transcriptional silencing of Ty1 elements in the RDN1 locus of yeast. Genes Dev. 1997;11:255–69. doi: 10.1101/gad.11.2.255. [DOI] [PubMed] [Google Scholar]
- Tissenbaum HA, Guarente L. Increased dosage of a sir-2 gene extends lifespan in Caenorhabditis elegans. Nature. 2001;410:227–30. doi: 10.1038/35065638. [DOI] [PubMed] [Google Scholar]
- Rogina B, Helfand SL, Frankel S. Longevity regulation by Drosophila Rpd3 deacetylase and caloric restriction. Science. 2002;298:1745. doi: 10.1126/science.1078986. [DOI] [PubMed] [Google Scholar]
- Rogina B, Helfand SL. Sir2 mediates longevity in the fly through a pathway related to calorie restriction. Proc Natl Acad Sci USA. 2004;101:15998–6003. doi: 10.1073/pnas.0404184101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satoh A, Brace CS, Rensing N, Cliften P, Wozniak DF, Herzog ED, Yamada KA, Imai S. Sirt1 extends life span and delays aging in mice through the regulation of Nk2 homeobox 1 in the DMH and LH. Cell Metab. 2013;18:416–30. doi: 10.1016/j.cmet.2013.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frye RA. Characterization of five human cDNAs with homology to the yeast SIR2 gene: Sir2-like proteins (sirtuins) metabolize NAD and may have protein ADP-ribosyltransferase activity. Biochem Biophys Res Commun. 1999;260:273–79. doi: 10.1006/bbrc.1999.0897. [DOI] [PubMed] [Google Scholar]
- Michishita E, Park JY, Burneskis JM, Barrett JC, Horikawa I. Evolutionarily conserved and nonconserved cellular localizations and functions of human SIRT proteins. Mol Biol Cell. 2005;16:4623–35. doi: 10.1091/mbc.E05-01-0033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liszt G, Ford E, Kurtev M, Guarente L. Mouse Sir2 homolog SIRT6 is a nuclear ADP-ribosyltransferase. J Biol Chem. 2005;280:21313–20. doi: 10.1074/jbc.M413296200. [DOI] [PubMed] [Google Scholar]
- Mostoslavsky R, Chua KF, Lombard DB, et al. Genomic instability and aging-like phenotype in the absence of mammalian SIRT6. Cell. 2006;124:315–29. doi: 10.1016/j.cell.2005.11.044. [DOI] [PubMed] [Google Scholar]
- Ford E, Voit R, Liszt G, Magin C, Grummt I, Guarente L. Mammalian Sir2 homolog SIRT7 is an activator of RNA polymerase I transcription. Genes Dev. 2006;20:1075–80. doi: 10.1101/gad.1399706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michishita E, McCord RA, Berber E, et al. SIRT6 is a histone H3 lysine 9 deacetylase that modulates telomeric chromatin. Nature. 2008;452:492–6. doi: 10.1038/nature06736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong L, D'Urso A, Toiber D, et al. The histone deacetylase Sirt6 regulates glucose homeostasis via Hif1alpha. Cell. 2010;140:280–93. doi: 10.1016/j.cell.2009.12.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dryden SC, Nahhas FA, Nowak JE, Goustin AS, Tainsky MA. Role for human SIRT2 NAD-dependent deacetylase activity in control of mitotic exit in the cell cycle. Mol Cell Biol. 2003;23:3173–85. doi: 10.1128/MCB.23.9.3173-3185.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Zhang B, Tang J, et al. Sirtuin 2, a mammalian homolog of yeast silent information regulator-2 longevity regulator, is an oligodendroglial protein that decelerates cell differentiation through deacetylating alpha-tubulin. J Neurosci. 2007;27:2606–16. doi: 10.1523/JNEUROSCI.4181-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura Y, Ogura M, Tanaka D, Inagaki N. Localization of mouse mitochondrial SIRT proteins: shift of SIRT3 to nucleus by co-expression with SIRT5. Biochem Biophys Res Commun. 2008;366:174–9. doi: 10.1016/j.bbrc.2007.11.122. [DOI] [PubMed] [Google Scholar]
- Ahuja N, Schwer B, Carobbio S, Waltregny D, North BJ, Castronovo V, Maechler P, Verdin E. Regulation of insulin secretion by SIRT4, a mitochondrial ADP-ribosyltransferase. J Biol Chem. 2007;282:33583–92. doi: 10.1074/jbc.M705488200. [DOI] [PubMed] [Google Scholar]
- Hubbard BP, Sinclair DA. Small molecule SIRT1 activators for the treatment of aging and age-related diseases. Trends Pharmacol Sci. 2014;35:146–54. doi: 10.1016/j.tips.2013.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boutant M, Canto C. SIRT1 metabolic actions: integrating recent advances from mouse models. Mol Metab. 2014;3:5–18. doi: 10.1016/j.molmet.2013.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang HC, Guarente L. SIRT1 and other sirtuins in metabolism. Trends Endocrinol Metab. 2014;25:138–45. doi: 10.1016/j.tem.2013.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herskovits AZ, Guarente L. SIRT1 in neurodevelopment and brain senescence. Neuron. 2014;81:471–83. doi: 10.1016/j.neuron.2014.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park S, Mori R, Shimokawa I. Do sirtuins promote mammalian longevity? A critical review on its relevance to the longevity effect induced by calorie restriction. Mol Cells. 2013;35:474–80. doi: 10.1007/s10059-013-0130-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guarente L. Calorie restriction and sirtuins revisited. Genes Dev. 2013;27:2072–85. doi: 10.1101/gad.227439.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalkiadaki A, Guarente L. Sirtuins mediate mammalian metabolic responses to nutrient availability. Nat Rev Endocrinol. 2012;8:287–96. doi: 10.1038/nrendo.2011.225. [DOI] [PubMed] [Google Scholar]
- Verdin E. The many faces of sirtuins: Coupling of NAD metabolism, sirtuins and lifespan. Nat Med. 2014;20:25–27. doi: 10.1038/nm.3447. [DOI] [PubMed] [Google Scholar]
- Rodgers JT, Lerin C, Haas W, Gygi SP, Spiegelman BM, Puigserver P. Nutrient control of glucose homeostasis through a complex of PGC-1alpha and SIRT1. Nature. 2005;434:113–8. doi: 10.1038/nature03354. [DOI] [PubMed] [Google Scholar]
- Lerin C, Rodgers JT, Kalume DE, Kim SH, Pandey A, Puigserver P. GCN5 acetyltransferase complex controls glucose metabolism through transcriptional repression of PGC-1alpha. Cell Metab. 2006;3:429–38. doi: 10.1016/j.cmet.2006.04.013. [DOI] [PubMed] [Google Scholar]
- Li X, Zhang S, Blander G, Tse JG, Krieger M, Guarente L. SIRT1 deacetylates and positively regulates the nuclear receptor LXR. Mol Cell. 2007;28:91–106. doi: 10.1016/j.molcel.2007.07.032. [DOI] [PubMed] [Google Scholar]
- Sun C, Zhang F, Ge X, Yan T, Chen X, Shi X, Zhai Q. SIRT1 improves insulin sensitivity under insulin-resistant conditions by repressing PTP1B. Cell Metab. 2007;6:307–19. doi: 10.1016/j.cmet.2007.08.014. [DOI] [PubMed] [Google Scholar]
- Picard F, Kurtev M, Chung N, et al. Sirt1 promotes fat mobilization in white adipocytes by repressing PPAR-gamma. Nature. 2004;429:771–6. doi: 10.1038/nature02583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moynihan KA, Grimm AA, Plueger MM, Bernal-Mizrachi E, Ford E, Cras-Meneur C, Permutt MA, Imai S. Increased dosage of mammalian Sir2 in pancreatic beta cells enhances glucose-stimulated insulin secretion in mice. Cell Metab. 2005;2:105–17. doi: 10.1016/j.cmet.2005.07.001. [DOI] [PubMed] [Google Scholar]
- Banks AS, Kon N, Knight C, Matsumoto M, Gutierrez-Juarez R, Rossetti L, Gu W, Accili D. SirT1 gain of function increases energy efficiency and prevents diabetes in mice. Cell Metab. 2008;8:333–41. doi: 10.1016/j.cmet.2008.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunet A, Sweeney LB, Sturgill JF, et al. Stress-dependent regulation of FOXO transcription factors by the SIRT1 deacetylase. Science. 2004;303:2011–5. doi: 10.1126/science.1094637. [DOI] [PubMed] [Google Scholar]
- Motta MC, Divecha N, Lemieux M, et al. Mammalian SIRT1 represses forkhead transcription factors. Cell. 2004;116:551–63. doi: 10.1016/s0092-8674(04)00126-6. [DOI] [PubMed] [Google Scholar]
- van der Horst A, Tertoolen LG, de Vries-Smits LM, Frye RA, Medema RH, Burgering BM. FOXO4 is acetylated upon peroxide stress and deacetylated by the longevity protein hSir2(SIRT1) J Biol Chem. 2004;279:28873–9. doi: 10.1074/jbc.M401138200. [DOI] [PubMed] [Google Scholar]
- Houtkooper RH, Pirinen E, Auwerx J. Sirtuins as regulators of metabolism and healthspan. Nat Rev Mol Cell Biol. 2012;13:225–38. doi: 10.1038/nrm3293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee KK, Ayyub C, Ali SZ, Mandot V, Prasad NG, Kolthur-Seetharam U. dSir2 in the adult fat body, but not in muscles, regulates life span in a diet-dependent manner. Cell Rep. 2012;2:1485–91. doi: 10.1016/j.celrep.2012.11.013. [DOI] [PubMed] [Google Scholar]
- Sinclair D, Verdin E. The longevity of sirtuins. Cell Rep. 2012;2:1473–4. doi: 10.1016/j.celrep.2012.12.004. [DOI] [PubMed] [Google Scholar]
- Liu G, Yang H, Chen X, Wang X, Chu Y. Modulation of neutrophil development and homeostasis. Curr Mol Med. 2013;13:1270–83. doi: 10.2174/15665240113139990062. [DOI] [PubMed] [Google Scholar]
- Ou X, Chae HD, Wang RH, et al. SIRT1 deficiency compromises mouse embryonic stem cell hematopoietic differentiation, and embryonic and adult hematopoiesis in the mouse. Blood. 2011;117:440–50. doi: 10.1182/blood-2010-03-273011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leko V, Varnum-Finney B, Li H, Gu Y, Flowers D, Nourigat C, Bernstein ID, Bedalov A. SIRT1 is dispensable for function of hematopoietic stem cells in adult mice. Blood. 2012;119:1856–60. doi: 10.1182/blood-2011-09-377077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sica A, Mantovani A. Macrophage plasticity and polarization: in vivo veritas. J Clin Invest. 2012;122:787–95. doi: 10.1172/JCI59643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantovani A, Sica A, Sozzani S, Allavena P, Vecchi A, Locati M. The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol. 2004;25:677–86. doi: 10.1016/j.it.2004.09.015. [DOI] [PubMed] [Google Scholar]
- Mege JL, Mehraj V, Capo C. Macrophage polarization and bacterial infections. Curr Opin Infect Dis. 2011;24:230–4. doi: 10.1097/QCO.0b013e328344b73e. [DOI] [PubMed] [Google Scholar]
- Mosser DM, Edwards JP. Exploring the full spectrum of macrophage activation. Nat Rev Immunol. 2008;8:958–69. doi: 10.1038/nri2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z, Wang X, He Y, Qi L, Yu L, Xue B, Shi H. The full capacity of AICAR to reduce obesity-induced inflammation and insulin resistance requires myeloid SIRT1. PLoS ONE. 2012;7:e49935. doi: 10.1371/journal.pone.0049935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lumeng CN, Bodzin JL, Saltiel AR. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J Clin Invest. 2007;117:175–84. doi: 10.1172/JCI29881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welte K, Gabrilove J, Bronchud MH, Platzer E, Morstyn G. Filgrastim (r-metHuG-CSF): the first 10 years. Blood. 1996;88:1907–29. [PubMed] [Google Scholar]
- Skokowa J, Lan D, Thakur BK, et al. NAMPT is essential for the G-CSF-induced myeloid differentiation via a NAD(+)-sirtuin-1-dependent pathway. Nat Med. 2009;15:151–8. doi: 10.1038/nm.1913. [DOI] [PubMed] [Google Scholar]
- Wampfler J, Tschan MP, Shan D, et al. SIRT1 is downregulated during neutrophil differentiation of acute promyelocytic leukaemia cells. Br J Haematol. 2009;146:337–41. doi: 10.1111/j.1365-2141.2009.07749.x. [DOI] [PubMed] [Google Scholar]
- Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol. 2009;9:162–74. doi: 10.1038/nri2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medzhitov R, Shevach EM, Trinchieri G, et al. Highlights of 10 years of immunology in Nature Reviews Immunology. Nat Rev Immunol. 2011;11:693–702. doi: 10.1038/nri3063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murdoch C, Muthana M, Coffelt SB, Lewis CE. The role of myeloid cells in the promotion of tumour angiogenesis. Nat Rev Cancer. 2008;8:618–31. doi: 10.1038/nrc2444. [DOI] [PubMed] [Google Scholar]
- Mauti LA, Le Bitoux MA, Baumer K, Stehle JC, Golshayan D, Provero P, Stamenkovic I. Myeloid-derived suppressor cells are implicated in regulating permissiveness for tumor metastasis during mouse gestation. J Clin Invest. 2011;121:2794–807. doi: 10.1172/JCI41936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crook KR, Jin M, Weeks MF, et al. Myeloid-derived suppressor cells regulate T cell and B cell responses during autoimmune disease. J Leukoc Biol. 2015;97:573–82. doi: 10.1189/jlb.4A0314-139R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- du Plessis N, Loebenberg L, Kriel M, et al. Increased frequency of myeloid-derived suppressor cells during active tuberculosis and after recent mycobacterium tuberculosis infection suppresses T-cell function. Am J Respir Crit Care Med. 2013;188:724–32. doi: 10.1164/rccm.201302-0249OC. [DOI] [PubMed] [Google Scholar]
- Pereira WF, Ribeiro-Gomes FL, Guillermo LV, Vellozo NS, Montalvao F, Dosreis GA, Lopes MF. Myeloid-derived suppressor cells help protective immunity to Leishmania major infection despite suppressed T cell responses. J Leukoc Biol. 2011;90:1191–7. doi: 10.1189/jlb.1110608. [DOI] [PubMed] [Google Scholar]
- Geissmann F, Manz MG, Jung S, Sieweke MH, Merad M, Ley K. Development of monocytes, macrophages, and dendritic cells. Science. 2010;327:656–61. doi: 10.1126/science.1178331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshizaki T, Schenk S, Imamura T, et al. SIRT1 inhibits inflammatory pathways in macrophages and modulates insulin sensitivity. Am J Physiol Endocrinol Metab. 2010;298:E419–28. doi: 10.1152/ajpendo.00417.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang SR, Wright J, Bauter M, Seweryniak K, Kode A, Rahman I. Sirtuin regulates cigarette smoke-induced proinflammatory mediator release via RelA/p65 NF-kappaB in macrophages in vitro and in rat lungs in vivo: implications for chronic inflammation and aging. Am J Physiol Lung Cell Mol Physiol. 2007;292:L567–76. doi: 10.1152/ajplung.00308.2006. [DOI] [PubMed] [Google Scholar]
- Rajendrasozhan S, Yang SR, Kinnula VL, Rahman I. SIRT1, an antiinflammatory and antiaging protein, is decreased in lungs of patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2008;177:861–70. doi: 10.1164/rccm.200708-1269OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hongwei Y, Sangwoon C, Jaewoong H, Isaac KS, Michael WM, Leonard G, Wei G, Irfan R. D27 NF-kB: Regulation and Exploitation A5769–A5769. New York: American Thoracic Society; 2011. [Google Scholar]
- Schug TT, Xu Q, Gao H, Peres-da-Silva A, Draper DW, Fessler MB, Purushotham A, Li X. Myeloid deletion of SIRT1 induces inflammatory signaling in response to environmental stress. Mol Cell Biol. 2010;30:4712–21. doi: 10.1128/MCB.00657-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z, Kahn BB, Shi H, Xue BZ. Macrophage alpha1 AMP-activated protein kinase (alpha1AMPK) antagonizes fatty acid-induced inflammation through SIRT1. J Biol Chem. 2010;285:19051–9. doi: 10.1074/jbc.M110.123620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruderman NB, Carling D, Prentki M, Cacicedo JM. AMPK, insulin resistance, and the metabolic syndrome. J Clin Investig. 2013;123:2764–72. doi: 10.1172/JCI67227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Zhou Y, Mueller-Steiner S, Chen LF, Kwon H, Yi S, Mucke L, Gan L. SIRT1 protects against microglia-dependent amyloid-beta toxicity through inhibiting NF-kappaB signaling. J Biol Chem. 2005;280:40364–74. doi: 10.1074/jbc.M509329200. [DOI] [PubMed] [Google Scholar]
- Nimmagadda VK, Bever CT, Vattikunta NR, et al. Overexpression of SIRT1 protein in neurons protects against experimental autoimmune encephalomyelitis through activation of multiple SIRT1 targets. J Immunol. 2013;190:4595–607. doi: 10.4049/jimmunol.1202584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez Y, Rodriguez M, Municio C, Hugo E, Alonso S, Ibarrola N, Fernandez N, Crespo MS. Sirtuin 1 is a key regulator of the interleukin-12 p70/interleukin-23 balance in human dendritic cells. J Biol Chem. 2012;287:35689–701. doi: 10.1074/jbc.M112.391839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remoli ME, Gafa V, Giacomini E, Severa M, Lande R, Coccia EM. IFN-beta modulates the response to TLR stimulation in human DC: involvement of IFN regulatory factor-1 (IRF-1) in IL-27 gene expression. Eur J Immunol. 2007;37:3499–508. doi: 10.1002/eji.200737566. [DOI] [PubMed] [Google Scholar]
- Masumi A, Wang IM, Lefebvre B, Yang XJ, Nakatani Y, Ozato K. The histone acetylase PCAF is a phorbol-ester-inducible coactivator of the IRF family that confers enhanced interferon responsiveness. Mol Cell Biol. 1999;19:1810–20. doi: 10.1128/mcb.19.3.1810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox JH, Kljavin NM, Ramamoorthi N, Diehl L, Batten M, Ghilardi N. IL-27 promotes T cell-dependent colitis through multiple mechanisms. J Exp Med. 2011;208:115–23. doi: 10.1084/jem.20100410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apetoh L, Quintana FJ, Pot C, et al. The aryl hydrocarbon receptor interacts with c-Maf to promote the differentiation of type 1 regulatory T cells induced by IL-27. Nat Immunol. 2010;11:854–61. doi: 10.1038/ni.1912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H, Lee SM, Gao B, Zhang J, Fang D. Histone deacetylase sirtuin 1 deacetylates IRF1 protein and programs dendritic cells to control Th17 protein differentiation during autoimmune inflammation. J Biol Chem. 2013;288:37256–66. doi: 10.1074/jbc.M113.527531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu G, Bi Y, Xue L, et al. Dendritic cell SIRT1-HIF1alpha axis programs the differentiation of CD4+ T cells through IL-12 and TGF-beta1. Proc Natl Acad Sci USA. 2015;112:E957–65. doi: 10.1073/pnas.1420419112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wynn TA, Chawla A, Pollard JW. Macrophage biology in development, homeostasis and disease. Nature. 2013;496:445–55. doi: 10.1038/nature12034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amulic B, Cazalet C, Hayes GL, Metzler KD, Zychlinsky A. Neutrophil function: from mechanisms to disease. Annu Rev Immunol. 2012;30:459–89. doi: 10.1146/annurev-immunol-020711-074942. [DOI] [PubMed] [Google Scholar]
- Yamada H, Kuroda E, Matsumoto S, Matsumoto T, Yamada T, Yamashita U. Prostaglandin E2 down-regulates viable Bacille Calmette-Guerin-induced macrophage cytotoxicity against murine bladder cancer cell MBT-2 in vitro. Clin Exp Immunol. 2002;128:52–58. doi: 10.1046/j.1365-2249.2002.01686.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aronoff DM, Canetti C, Peters-Golden M. Prostaglandin E2 inhibits alveolar macrophage phagocytosis through an E-prostanoid 2 receptor-mediated increase in intracellular cyclic AMP. J Immunol. 2004;173:559–65. doi: 10.4049/jimmunol.173.1.559. [DOI] [PubMed] [Google Scholar]
- Serezani CH, Chung J, Ballinger MN, Moore BB, Aronoff DM, Peters-Golden M. Prostaglandin E2 suppresses bacterial killing in alveolar macrophages by inhibiting NADPH oxidase. Am J Respir Cell Mol Biol. 2007;37:562–70. doi: 10.1165/rcmb.2007-0153OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang R, Chen HZ, Liu JJ, et al. SIRT1 suppresses activator protein-1 transcriptional activity and cyclooxygenase-2 expression in macrophages. J Biol Chem. 2010;285:7097–110. doi: 10.1074/jbc.M109.038604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Condamine T, Gabrilovich DI. Molecular mechanisms regulating myeloid-derived suppressor cell differentiation and function. Trends Immunol. 2011;32:19–25. doi: 10.1016/j.it.2010.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peranzoni E, Zilio S, Marigo I, Dolcetti L, Zanovello P, Mandruzzato S, Bronte V. Myeloid-derived suppressor cell heterogeneity and subset definition. Curr Opin Immunol. 2010;22:238–44. doi: 10.1016/j.coi.2010.01.021. [DOI] [PubMed] [Google Scholar]
- Umemura N, Saio M, Suwa T, et al. Tumor-infiltrating myeloid-derived suppressor cells are pleiotropic-inflamed monocytes/macrophages that bear M1- and M2-type characteristics. J Leukoc Biol. 2008;83:1136–44. doi: 10.1189/jlb.0907611. [DOI] [PubMed] [Google Scholar]
- Liu G, Bi Y, Shen B, et al. SIRT1 limits the function and fate of myeloid-derived suppressor cells in tumors by orchestrating HIF1 -dependent glycolysis. Cancer Res. 2013;74:727–37. doi: 10.1158/0008-5472.CAN-13-2584. [DOI] [PubMed] [Google Scholar]
- Crotty Alexander LE, Marsh BJ, Timmer AM, Lin AE, Zainabadi K, Czopik A, Guarente L, Nizet V. Myeloid cell sirtuin-1 expression does not alter host immune responses to Gram-negative endotoxemia or Gram-positive bacterial infection. PLoS ONE. 2013;8:e84481. doi: 10.1371/journal.pone.0084481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenda M, Timer A, Leonard G, Victor N, Laura C-A. B42 Molecular aspects of Innate Immunity. A2789–A2789. New York: American Thoracic Society; 2013. [Google Scholar]
- Lara E, Mai A, Calvanese V, et al. Salermide, a Sirtuin inhibitor with a strong cancer-specific proapoptotic effect. Oncogene. 2009;28:781–91. doi: 10.1038/onc.2008.436. [DOI] [PubMed] [Google Scholar]
- Kozako T, Aikawa A, Shoji T, et al. High expression of the longevity gene product SIRT1 and apoptosis induction by sirtinol in adult T-cell leukemia cells. Int J Cancer. 2012;131:2044–55. doi: 10.1002/ijc.27481. [DOI] [PubMed] [Google Scholar]
- Li L, Wang L, Li L, et al. Activation of p53 by SIRT1 inhibition enhances elimination of CML leukemia stem cells in combination with imatinib. Cancer Cell. 2012;21:266–81. doi: 10.1016/j.ccr.2011.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q, Zeng SX, Zhang Y, Zhang Y, Ding D, Ye Q, Meroueh SO, Lu H. A small molecule Inauhzin inhibits SIRT1 activity and suppresses tumour growth through activation of p53. EMBO Mol Med. 2012;4:298–312. doi: 10.1002/emmm.201100211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mildner A, Jung S. Development and function of dendritic cell subsets. Immunity. 2014;40:642–56. doi: 10.1016/j.immuni.2014.04.016. [DOI] [PubMed] [Google Scholar]
- Banchereau J, Briere F, Caux C, Davoust J, Lebecque S, Liu YJ, Pulendran B, Palucka K. Immunobiology of dendritic cells. Annu Rev Immunol. 2000;18:767–811. doi: 10.1146/annurev.immunol.18.1.767. [DOI] [PubMed] [Google Scholar]
- Kim SR, Lee KS, Park SJ, et al. Involvement of sirtuin 1 in airway inflammation and hyperresponsiveness of allergic airway disease. J Allergy Clin Immunol. 2010;125:449–60. doi: 10.1016/j.jaci.2009.08.009. e14. [DOI] [PubMed] [Google Scholar]
- Legutko A, Marichal T, Fievez L, et al. Sirtuin 1 promotes Th2 responses and airway allergy by repressing peroxisome proliferator-activated receptor-gamma activity in dendritic cells. J Immunol. 2011;187:4517–29. doi: 10.4049/jimmunol.1101493. [DOI] [PubMed] [Google Scholar]
- Liao X, Sluimer JC, Wang Y, et al. Macrophage autophagy plays a protective role in advanced atherosclerosis. Cell Metab. 2012;15:545–53. doi: 10.1016/j.cmet.2012.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razani B, Feng C, Coleman T, et al. Autophagy links inflammasomes to atherosclerotic progression. Cell Metab. 2012;15:534–44. doi: 10.1016/j.cmet.2012.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng F, Tang BL. Sirtuins’ modulation of autophagy. J Cell Physiol. 2013;228:2262–70. doi: 10.1002/jcp.24399. [DOI] [PubMed] [Google Scholar]
- Takeda-Watanabe A, Kitada M, Kanasaki K, Koya D. SIRT1 inactivation induces inflammation through the dysregulation of autophagy in human THP-1 cells. Biochem Biophys Res Commun. 2012;427:191–6. doi: 10.1016/j.bbrc.2012.09.042. [DOI] [PubMed] [Google Scholar]
- Song YM, Lee YH, Kim JW, Ham DS, Kang ES, Cha BS, Lee HC, Lee BW. Metformin alleviates hepatosteatosis by restoring SIRT1-mediated autophagy induction via an AMP-activated protein kinase-independent pathway. Autophagy. 2015;11:46–59. doi: 10.4161/15548627.2014.984271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kedenko L, Lamina C, Kedenko I, Kollerits B, Kiesslich T, Iglseder B, Kronenberg F, Paulweber B. Genetic polymorphisms at SIRT1 and FOXO1 are associated with carotid atherosclerosis in the SAPHIR cohort. BMC Med Genet. 2014;15:112–5. doi: 10.1186/s12881-014-0112-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu P, Han Z, Couvillon AD, Kaufman RJ, Exton JH. Autocrine tumor necrosis factor alpha links endoplasmic reticulum stress to the membrane death receptor pathway through IRE1alpha-mediated NF-kappaB activation and down-regulation of TRAF2 expression. Mol Cell Biol. 2006;26:3071–84. doi: 10.1128/MCB.26.8.3071-3084.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinon F, Chen X, Lee AH, Glimcher LH. TLR activation of the transcription factor XBP1 regulates innate immune responses in macrophages. Nat Immunol. 2010;11:411–8. doi: 10.1038/ni.1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang FM, Chen YJ, Ouyang HJ. Regulation of unfolded protein response modulator XBP1s by acetylation and deacetylation. Biochem J. 2011;433:245–52. doi: 10.1042/BJ20101293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melhem H, Hansmannel F, Bressenot A, Battaglia-Hsu SF, Billioud V, Alberto JM, Gueant JL, Peyrin-Biroulet L. Methyl-deficient diet promotes colitis and SIRT1-mediated endoplasmic reticulum stress. Gut. 2015 doi: 10.1136/gutjnl-2014-307030. doi:10·1136/gutjnl-2014-307030. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Kong S, McBurney MW, Fang D. Sirtuin 1 in immune regulation and autoimmunity. Immunol Cell Biol. 2012;90:6–13. doi: 10.1038/icb.2011.102. [DOI] [PubMed] [Google Scholar]
- Kong S, Yeung P, Fang D. The class III histone deacetylase sirtuin 1 in immune suppression and its therapeutic potential in rheumatoid arthritis. J Genet Genomics. 2013;40:347–54. doi: 10.1016/j.jgg.2013.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu N, Qiu X, Luo Y, et al. Abnormal histone modification patterns in lupus CD4+ T cells. J Rheumatol. 2008;35:804–10. [PubMed] [Google Scholar]
- Zhang J, Lee SM, Shannon S, et al. The type III histone deacetylase Sirt1 is essential for maintenance of T cell tolerance in mice. J Clin Invest. 2009;119:3048–58. doi: 10.1172/JCI38902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner PJ, Joshi L, Lee RW, Dick AD, Adamson P, Calder VL. SIRT1 activation protects against autoimmune T cell-driven retinal disease in mice via inhibition of IL-2/Stat5 signaling. J Autoimmun. 2013;42:117–29. doi: 10.1016/j.jaut.2013.01.011. [DOI] [PubMed] [Google Scholar]
- Nabel G, Baltimore D. An inducible transcription factor activates expression of human immunodeficiency virus in T cells. Nature. 1987;326:711–3. doi: 10.1038/326711a0. [DOI] [PubMed] [Google Scholar]
- Peruzzi F. The multiple functions of HIV-1 Tat: proliferation versus apoptosis. Front Biosci. 2006;11:708–17. doi: 10.2741/1829. [DOI] [PubMed] [Google Scholar]
- Pinzone MR, Cacopardo B, Condorelli F, Di Rosa M, Nunnari G. Sirtuin-1 and HIV-1: an overview. Curr Drug Targets. 2013;14:648–52. doi: 10.2174/1389450111314060005. [DOI] [PubMed] [Google Scholar]
- Luo J, Nikolaev AY, Imai S, Chen D, Su F, Shiloh A, Guarente L, Gu W. Negative control of p53 by Sir2alpha promotes cell survival under stress. Cell. 2001;107:137–48. doi: 10.1016/s0092-8674(01)00524-4. [DOI] [PubMed] [Google Scholar]
- Kwon HS, Brent MM, Getachew R, et al. Human immunodeficiency virus type 1 Tat protein inhibits the SIRT1 deacetylase and induces T cell hyperactivation. Cell Host Microbe. 2008;3:158–67. doi: 10.1016/j.chom.2008.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtil C, Enache LS, Radreau P, et al. The metabolic sensors FXRalpha, PGC-1alpha, and SIRT1 cooperatively regulate hepatitis B virus transcription. FASEB J. 2014;28:1454–63. doi: 10.1096/fj.13-236372. [DOI] [PubMed] [Google Scholar]
- Liu H, Lu ZG, Miki Y, Yoshida K. Protein kinase C delta induces transcription of the TP53 tumor suppressor gene by controlling death-promoting factor Btf in the apoptotic response to DNA damage. Mol Cell Biol. 2007;27:8480–91. doi: 10.1128/MCB.01126-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haraguchi T, Holaska JM, Yamane M, Koujin T, Hashiguchi N, Mori C, Wilson KL, Hiraoka Y. Emerin binding to Btf, a death-promoting transcriptional repressor, is disrupted by a missense mutation that causes Emery-Dreifuss muscular dystrophy. Eur J Biochem. 2004;271:1035–45. doi: 10.1111/j.1432-1033.2004.04007.x. [DOI] [PubMed] [Google Scholar]
- McPherson JP, Sarras H, Lemmers B, et al. Essential role for Bclaf1 in lung development and immune system function. Cell Death Differ. 2009;16:331–9. doi: 10.1038/cdd.2008.167. [DOI] [PubMed] [Google Scholar]
- Kong S, Kim SJ, Sandal B, Lee SM, Gao B, Zhang DD, Fang D. The type III histone deacetylase Sirt1 protein suppresses p300-mediated histone H3 lysine 56 acetylation at Bclaf1 promoter to inhibit T cell activation. J Biol Chem. 2011;286:16967–75. doi: 10.1074/jbc.M111.218206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anasetti C, Tan P, Hansen JA, Martin PJ. Induction of specific nonresponsiveness in unprimed human T cells by anti-CD3 antibody and alloantigen. J Exp Med. 1990;172:1691–700. doi: 10.1084/jem.172.6.1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins MK, Schwartz RH. Antigen presentation by chemically modified splenocytes induces antigen-specific T cell unresponsiveness in vitro and in vivo. J Exp Med. 1987;165:302–19. doi: 10.1084/jem.165.2.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaSalle JM, Tolentino PJ, Freeman GJ, Nadler LM, Hafler DA. Early signaling defects in human T cells anergized by T cell presentation of autoantigen. J Exp Med. 1992;176:177–86. doi: 10.1084/jem.176.1.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sequeira J, Boily G, Bazinet S, et al. sirt1-null mice develop an autoimmune-like condition. Exp Cell Res. 2008;314:3069–74. doi: 10.1016/j.yexcr.2008.07.011. [DOI] [PubMed] [Google Scholar]
- Biason-Lauber A, Boni-Schnetzler M, Hubbard BP, et al. Identification of a SIRT1 mutation in a family with type 1 diabetes. Cell Metab. 2013;17:448–55. doi: 10.1016/j.cmet.2013.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao B, Kong Q, Kemp K, Zhao YS, Fang D. Analysis of sirtuin 1 expression reveals a molecular explanation of IL-2-mediated reversal of T-cell tolerance. Proc Natl Acad Sci USA. 2012;109:899–904. doi: 10.1073/pnas.1118462109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J, Yamane H, Paul WE. Differentiation of effector CD4 T cell populations (*) Annu Rev Immunol. 2010;28:445–89. doi: 10.1146/annurev-immunol-030409-101212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang R, Green DR. Metabolic checkpoints in activated T cells. Nat Immunol. 2012;13:907–15. doi: 10.1038/ni.2386. [DOI] [PubMed] [Google Scholar]
- Xuzhu G, Komai-Koma M, Leung BP, Howe HS, McSharry C, McInnes IB, Xu D. Resveratrol modulates murine collagen-induced arthritis by inhibiting Th17 and B-cell function. Ann Rheum Dis. 2012;71:129–35. doi: 10.1136/ard.2011.149831. [DOI] [PubMed] [Google Scholar]
- Caruso R, Marafini I, Franze E, et al. Defective expression of SIRT1 contributes to sustain inflammatory pathways in the gut. Mucosal Immunol. 2014;7:1467–79. doi: 10.1038/mi.2014.35. [DOI] [PubMed] [Google Scholar]
- Nie Y, Erion DM, Yuan Z, Dietrich M, Shulman GI, Horvath TL, Gao Q. STAT3 inhibition of gluconeogenesis is downregulated by SirT1. Nat Cell Biol. 2009;11:492–500. doi: 10.1038/ncb1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang XO, Panopoulos AD, Nurieva R, Chang SH, Wang D, Watowich SS, Dong C. STAT3 regulates cytokine-mediated generation of inflammatory helper T cells. J Biol Chem. 2007;282:9358–63. doi: 10.1074/jbc.C600321200. [DOI] [PubMed] [Google Scholar]
- van Loosdregt J, Vercoulen Y, Guichelaar T, et al. Regulation of Treg functionality by acetylation-mediated Foxp3 protein stabilization. Blood. 2010;115:965–74. doi: 10.1182/blood-2009-02-207118. [DOI] [PubMed] [Google Scholar]
- van Loosdregt J, Brunen D, Fleskens V, Pals CE, Lam EW, Coffer PJ. Rapid temporal control of Foxp3 protein degradation by sirtuin-1. PLoS ONE. 2011;6:e19047. doi: 10.1371/journal.pone.0019047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kume S, Haneda M, Kanasaki K, et al. SIRT1 inhibits transforming growth factor beta-induced apoptosis in glomerular mesangial cells via Smad7 deacetylation. J Biol Chem. 2007;282:151–8. doi: 10.1074/jbc.M605904200. [DOI] [PubMed] [Google Scholar]
- Beier UH, Wang L, Bhatti TR, Liu Y, Han R, Ge G, Hancock WW. Sirtuin-1 targeting promotes Foxp3+ T-regulatory cell function and prolongs allograft survival. Mol Cell Biol. 2011;31:1022–29. doi: 10.1128/MCB.01206-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akimova T, Xiao H, Liu Y, et al. Targeting sirtuin-1 alleviates experimental autoimmune colitis by induction of Foxp3 T-regulatory cells. Mucosal Immunol. 2014;7:1209–20. doi: 10.1038/mi.2014.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beier UH, Wang L, Han R, Akimova T, Liu Y, Hancock WW. Histone deacetylases 6 and 9 and sirtuin-1 control Foxp3+ regulatory T cell function through shared and isoform-specific mechanisms. Sci Signal. 2012;5:ra45. doi: 10.1126/scisignal.2002873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuroda S, Yamazaki M, Abe M, Sakimura K, Takayanagi H, Iwai Y. Basic leucine zipper transcription factor, ATF-like (BATF) regulates epigenetically and energetically effector CD8 T-cell differentiation via Sirt1 expression. Proc Natl Acad Sci USA. 2011;108:14885–9. doi: 10.1073/pnas.1105133108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorsey MJ, Tae HJ, Sollenberger KG, Mascarenhas NT, Johansen LM, Taparowsky EJ. B-ATF: a novel human bZIP protein that associates with members of the AP-1 transcription factor family. Oncogene. 1995;11:2255–65. [PubMed] [Google Scholar]
- Yasue T, Baba M, Mori S, Mizoguchi C, Uehara S, Takatsu K. IgG1 production by sIgD+ splenic B cells and peritoneal B-1 cells in response to IL-5 and CD38 ligation. Int Immunol. 1999;11:915–23. doi: 10.1093/intimm/11.6.915. [DOI] [PubMed] [Google Scholar]
- Aksoy P, Escande C, White TA, Thompson M, Soares S, Benech JC, Chini EN. Regulation of SIRT 1 mediated NAD dependent deacetylation: a novel role for the multifunctional enzyme CD38. Biochem Biophys Res Commun. 2006;349:353–9. doi: 10.1016/j.bbrc.2006.08.066. [DOI] [PubMed] [Google Scholar]
- O'Neill LA, Hardie DG. Metabolism of inflammation limited by AMPK and pseudo-starvation. Nature. 2013;493:346–55. doi: 10.1038/nature11862. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Lowry SF, Guarente L, Haimovich B. Roles of SIRT1 in the acute and restorative phases following induction of inflammation. J Biol Chem. 2010;285:41391–401. doi: 10.1074/jbc.M110.174482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tannahill GM, Curtis AM, Adamik J, et al. Succinate is an inflammatory signal that induces IL-1beta through HIF-1alpha. Nature. 2013;496:238–42. doi: 10.1038/nature11986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandel NS, McClintock DS, Feliciano CE, Wood TM, Melendez JA, Rodriguez AM, Schumacker PT. Reactive oxygen species generated at mitochondrial complex III stabilize hypoxia-inducible factor-1alpha during hypoxia: a mechanism of O2 sensing. J Biol Chem. 2000;275:25130–8. doi: 10.1074/jbc.M001914200. [DOI] [PubMed] [Google Scholar]
- Sumbayev VV. LPS-induced Toll-like receptor 4 signalling triggers cross-talk of apoptosis signal-regulating kinase 1 (ASK1) and HIF-1alpha protein. FEBS Lett. 2008;582:319–26. doi: 10.1016/j.febslet.2007.12.024. [DOI] [PubMed] [Google Scholar]
- Singh UP, Singh NP, Singh B, Hofseth LJ, Price RL, Nagarkatti M, Nagarkatti PS. Resveratrol (trans-3,5,4′-trihydroxystilbene) induces silent mating type information regulation-1 and down-regulates nuclear transcription factor-kappaB activation to abrogate dextran sulfate sodium-induced colitis. J Pharmacol Exp Ther. 2010;332:829–39. doi: 10.1124/jpet.109.160838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeung F, Hoberg JE, Ramsey CS, Keller MD, Jones DR, Frye RA, Mayo MW. Modulation of NF-kappaB-dependent transcription and cell survival by the SIRT1 deacetylase. EMBO J. 2004;23:2369–80. doi: 10.1038/sj.emboj.7600244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly TJ, Lerin C, Haas W, Gygi SP, Puigserver P. GCN5-mediated transcriptional control of the metabolic coactivator PGC-1beta through lysine acetylation. J Biol Chem. 2009;284:19945–52. doi: 10.1074/jbc.M109.015164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin J, Handschin C, Spiegelman BM. Metabolic control through the PGC-1 family of transcription coactivators. Cell Metab. 2005;1:361–70. doi: 10.1016/j.cmet.2005.05.004. [DOI] [PubMed] [Google Scholar]
- Liu TF, Vachharajani VT, Yoza BK, McCall CE. NAD+-dependent sirtuin 1 and 6 proteins coordinate a switch from glucose to fatty acid oxidation during the acute inflammatory response. J Biol Chem. 2012;287:25758–69. doi: 10.1074/jbc.M112.362343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu TF, Yoza BK, El Gazzar M, Vachharajani VT, McCall CE. NAD+-dependent SIRT1 deacetylase participates in epigenetic reprogramming during endotoxin tolerance. J Biol Chem. 2011;286:9856–64. doi: 10.1074/jbc.M110.196790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longo VD, Kennedy BK. Sirtuins in aging and age-related disease. Cell. 2006;126:257–68. doi: 10.1016/j.cell.2006.07.002. [DOI] [PubMed] [Google Scholar]
- Zou T, Yang Y, Xia F, Huang A, Gao X, Fang D, Xiong S, Zhang J. Resveratrol Inhibits CD4+ T cell activation by enhancing the expression and activity of Sirt1. PLoS ONE. 2013;8:e75139. doi: 10.1371/journal.pone.0075139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milne JC, Lambert PD, Schenk S, et al. Small molecule activators of SIRT1 as therapeutics for the treatment of type 2 diabetes. Nature. 2007;450:712–6. doi: 10.1038/nature06261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sell H, Habich C, Eckel J. Adaptive immunity in obesity and insulin resistance. Nat Rev Endocrinol. 2012;8:709–16. doi: 10.1038/nrendo.2012.114. [DOI] [PubMed] [Google Scholar]
- Christopher L, Brooks WG. How does SIRT1 affect metabolism, senescence and cancer? Nat Rev Cancer. 2009;9:123–8. doi: 10.1038/nrc2562. [DOI] [PMC free article] [PubMed] [Google Scholar]


