Abstract
Targeting of antigens to the endocytic uptake receptor DEC205 resulted in enhanced antigen presentation by dendritic cells (DCs). In combination with adjuvants for DC maturation, proteins coupled to an antibody against DEC205 induced strong pathogen-specific immune responses, whereas without additional adjuvant tolerance could be induced. As less is known about DNA vaccines encoding DEC205-targeted antigens, we explored the immunogenicity and efficacy of a dendritic cell-targeted DNA vaccine against influenza A virus (IAV) delivered by electroporation. Although coupling of haemagglutinin to a single-chain antibody against DEC205 enhanced antigen presentation on MHC class II and activation of T-cell receptor-transgenic CD4 T cells, the T-cell responses induced by the targeted DNA vaccine in wild-type BALB/c mice were significantly reduced compared with DNA encoding non-targeted antigens. Consistently, these mice were less protected against an IAV infection. Adoptive transfer experiments were performed to assess the fate of the antigen-specific T cells in animals vaccinated with DNA encoding DEC205-targeted antigens. By this, we could exclude the general deletion of antigen-specific T cells as cause for the reduced efficacy, but observed a local expansion of antigen-specific regulatory T cells, which could suppress the activation of effector cells. In conclusion, DNA vaccines encoding DEC205-targeted antigens induce peripheral tolerance rather than immunity in our study. Finally, we evaluated our DNA vaccines as prophylactic or therapeutic treatment in an allergen-induced asthma mouse model.
Keywords: dendritic cells, DNA vaccine, immune response, tolerance
Introduction
DNA immunization is a promising approach to induce stable, prolonged and broad immune responses against pathogens. For instance, administration of antigen-encoding DNA induced protective immune responses against influenza A virus (IAV) infection in different animal models.1–7 Delivery of exogenous DNA containing appropriate expression cassettes into the skeletal muscle allows for prolonged expression of the transgene in terminally differentiated myocytes.8 Secreted antigens can be easily taken up by dendritic cells (DCs), which subsequently present them to T cells in the lymphatic tissues. The immunogenicity and efficacy of DNA vaccines were dramatically improved by using in vivo electroporation.9–11 The short electric pulses have been shown to enhance the DNA uptake and induce inflammation at the injection site, which leads to the recruitment of immune cells.12,13
Dendritic cells take up pathogens from the periphery by phagocytosis or receptor-mediated endocytosis. After processing of the pathogen and loading of restricted epitopes on MHC, the DCs interact with T lymphocytes in lymphatic organs.14,15 Upon stimulation via pathogen recognition receptors they undergo maturation and up-regulate the expression of co-stimulatory molecules like CD80/86. Mature DCs in the lymph nodes activate T cells to differentiate into cytotoxic T cells or T helper cells enabling efficient antibody production by B cells, which finally results in the formation of an immunological memory response. In contrast, peptide presentation by immature DCs under steady-state conditions induces peripheral tolerance to prevent activation of autoreactive T cells. Peripheral tolerance is maintained by several distinct mechanisms, like the induction of suppressive regulatory T (Treg) cells,16 deletion of self-reactive T cells17 or T-cell anergy.18,19 Therefore, the activation of and antigen presentation by DCs are critical steps in the induction of vaccine-specific cellular and humoral immune responses.
Improved peptide presentation by DCs was achieved by targeting the endocytotic receptor DEC205 via an antibody–antigen fusion protein. DEC205 belongs to the C-type lectin receptor family and is expressed at high levels on several subsets of DCs in mice.20 Immunization with these antibody–antigen fusion proteins resulted in enhanced antigen presentation by CD11c DCs, which was reported for both MHC class II21–25 and MHC class I restricted peptides.22–24,26,27 Binding of antibodies to DEC205 alone does not stimulate maturation of DCs28 and therefore additional stimuli for DC maturation like anti-CD40 antibodies and/or poly IC are necessary to induce antigen-specific immunity.23,29–31 This approach had been used to enhance the immunogenicity and efficacy of protein vaccines against infectious diseases or tumours.22,29,31,32 In contrast, targeting the DEC205 receptor without adjuvant led either to a partial activation and proliferation of T cells followed by deletion and/or anergy21,33,34 or to the induction of Treg cells.35,36
The induction of antigen-specific Treg cells via therapeutic immunizations with DEC205-targeted proteins is a promising approach to treat cell-mediated autoimmune diseases, like multiple sclerosis, as recently demonstrated in a mouse model of experimental allergic encephalomyelitis.37
Although the consequences of DEC205-targeted protein immunization seem to be well understood and documented, far less is known for DNA vaccines encoding DEC205-targeted antigens. In contrast to protein vaccines, intramuscular DNA immunizations lead to prolonged antigen production by the transduced myocytes, which might influence the balance between immunity and tolerance. The published studies describing the use of DNA vaccines encoding DEC205-targeted antigens revealed conflicting results. Despite the application of similar protocols including in vivo electroporation, two groups showed an enhanced efficacy of the vaccine38,39 whereas Ettinger et al. reported on the induction of antigen-specific tolerance in mice.40 In our previous experiment, in rhesus macaques, DEC205-targeting of the encoded antigen also had a negative effect on the immunogenicity of simian immunodeficiency virus-specific DNA vaccines.41
Therefore, we further evaluated whether DNA vaccines encoding DEC205-targeted antigens favour induction of immunity or tolerance to the coupled antigen. In this study, we used plasmids encoding DEC205-targeted antigens derived from the haemagglutinin (HA) of the IAV strain Puerto Rico/8/34 (PR8) (H1N1). This allowed us to use the same DNA constructs for tracking antigen-specific T-cell responses in T-cell receptor (TCR) -transgenic models and for the analysis of protective immune responses in a viral infection model. Additionally, the potential of these DNA vaccines to induce peripheral tolerance towards the encoded antigens was analysed in mouse models for allergic asthma.
Materials and methods
Plasmids
Both pVAX-DEC-solHA and pVAX-GL117-solHA were cloned by amplification of the extracellular domain of HA from IAV Puerto Rico/8/34 (PR8) (H1N1) out of pEF-mycHA. pVAX-DEC-HACD8 and pVAX-GL117-HACD8 were constructed by hybridization of the two oligonucleotides AATTCCGGCGGAGGGGGAATCTACTCAACTGTCGCCAGTTCACTGCGAT + CGCAGTGAACTGGCGACAGTTGAGTAGATTCCCCCTCCGCCGG and cloned into the pVAX-DEC/GL117 background. In all constructs the gene of interest is followed by an OLLAS-tag42 for further detection. DNA for immunization was prepared using the NucleoBond® Xtra Maxi EF Kit (Macherey-Nagel, Düren, Germany) and tested for endotoxin levels with the Limulus amoebocyte lysate quantification assay (Cambrex Bio Science, Verviers, Belgium), confirming that the dose used for immunization of mice contained < 0·1 Endotoxin Units.
Transgene expression analysis
HEK293T cells were transiently transfected using 10 μg plasmid DNA and 10 μg polyethylenimine.43 Seventy-two hours post transfection, supernatants were collected and analysed by Western blot. For the detection, a monoclonal αOLLAS-antibody (purified from hybridoma) was used in combination with horseradish peroxidase-conjugated αRat IgG (DakoCytomation, Glostrup, Denmark). The analysis was performed with the software Wasabi! (Hamamatsu Photonics Germany GmbH, Herrsching am Ammersee, Germany).
The binding capacities of the single-chain antibodies to the DEC205 receptor were analysed with DEC205 expressing (CHODEC205) and control (CHOneo) CHO cells as described previously.44 Bound fusion proteins were detected by flow cytometry using an αOLLAS antibody labelled with Alexa647.
Mice and immunizations
Female, 6- to 8-week-old BALB/cJ mice were purchased from Janvier (Le Genest-St-Isle, France). The TCR transgenic mouse strains, CL445 and TCR-HA46 were provided by W. Hansen and J. Buer(Medical Microbiology, Essen, Germany). TCR-HA mice were bred in the animal facility of the medical faculty of the Ruhr-University Bochum. All mice were housed in individually ventilated cages in accordance with the national law and institutional guidelines.
For all immunization experiments, the plasmids were diluted in PBS and 20 μg was used for one intramuscular immunization followed by electroporation (Ichor Medical Inc., San Diego, CA) as described previously.47
Adoptive T-cell transfer and in vivo antigen presentation
CD4 T cells from TCR-HA donor mice or CD8 T cells from CL4 donor mice were purified by negative selection using antibodies against B220, F4/80, NK1.1, MHCII, CD4 or CD8 followed by magnetic affinity cell sorting. CD4 and CD8 T cells were labelled with 5 μm carboxyfluorescein succinimidyl ester (CFSE; Molecular Probes®, Invitrogen, Darmstadt, Germany). Then, 2 × 106 CFSE+ cells were transferred into BALB/cJ recipient mice that had been immunized 4 days before. Three days after transfer, mice were killed and single-cell suspensions were prepared from popliteal lymph nodes and spleens. After surface staining with αCD4-peridinin chlorophyll protein (PerCP) antibodies in PBS/BSA/azide, the samples were analysed on a FACSCanto™ II (BD Bioscience, Heidelberg, Germany). Each proliferation cycle is represented by the bisection of the previous fluorescence intensity.
In vitro proliferation assay
To determine the suppressive capacity of the vaccine-induced Treg cells, popliteal and inguinal lymph nodes as well as the spleens of TCR-HA mice were removed 7 days post immunization. Single-cell suspension from all three lymphatic organs were labelled separately with 5 μm CFSE and plated into 96-well round-bottom plates (Nunc, Roskilde, Denmark) at a density of 2 × 106 cell/well. Samples were re-stimulated for 72 hr with the MHC-II restricted HA peptide (SFERFEIFPKE, 5 μg/ml) and the co-stimulatory αCD28 antibody (1 μg/ml) in RPMI-1640 supplemented with 10% fetal calf serum, 2 mm l-glutamine, 10 mm HEPES, 50 μm β-mercaptoethanol and 1% antibiotic/antimycotic (all Gibco (Karlsruhe, Germany)). Finally, cells were stained with αCD4-PerCP in PBS/BSA/azide and the proliferation indicated by loss of CFSE intensity was analysed on a FACSCanto™ II (BD Bioscience).
Alternatively, the suppressive capacity of enriched CD25+ CD4 T cells was analysed. One week after the immunization, CD25+ CD4 cells were isolated from spleen and popliteal lymph nodes (pooled from four immunized mice) via magnetic separation (CD4+ CD25+ Regulatory T-Cell Isolation Kit; Miltenyi Biotec, Bergisch Gladbach, Germany). CD25− CD4 cells were used as controls. 2 × 105 cells, either CD25+ or CD25–, were incubated with 2 × 105 CFSE-labelled CD4 cells isolated from naive TCR-HA mice. The proliferation of the effector cells was assessed as described above. The percentage of dividing TCR-HA cells in the absence of any cells from the immunized mice was set as 100% and the per cent inhibition by the respective population was calculated.
Foxp3 staining and intracellular cytokine staining
For Foxp3 staining, blood samples were collected by retro-orbital puncture and directly mixed with 40 μl of an EDTA solution (10 μm). After lysis of red blood cells, samples were plated into 96-well round-bottom plates (Nunc). Surface staining was performed with αCD25-allophycocyanin (APC; eBioscience, San Diego, CA), αCD4-PerCP (BD Bioscience) and αTCR-HA-FITC (provided by W. Hansen) in PBS/BSA/azide. Intracellular Foxp3 staining was performed by anti-mouse/rat Foxp3 staining set phycoerythrin (PE; eBioscience) according to the manufacturer's instructions.
Intracellular cytokine staining was performed on re-stimulated splenocytes as previously described.48 Briefly, splenocytes were plated in 96-well round-bottom plates (Nunc). CD4 cells were re-stimulated by the MHCII-restricted HA peptide in combination with an αCD28 antibody, whereas CD8 T cells were re-stimulated in the presence of the MHCI-restricted HA peptide (IYSTVASSL) and αCD107a-FITC (eBioscience). After stimulation, surface staining was carried out with αCD8-PerCP or αCD4-PerCP (BD Bioscience) and Fixable Viability Dye-Fluor780 (eBioscience). Cells were fixed, followed by permeabilization and cytokine staining with αTNFα-PE, αIFNγ-PECy7 and αIL2-APC (BD Bioscience). Samples were analysed on a FACSCanto™ II.
Serological assays
Blood was taken retro-orbitally and serum was collected after centrifugation for 5 min at 2700 g in a table-top centrifuge. To quantify HA-specific IgG1 and IgG2a antibody levels, 96-well ELISA plates were coated with 200 ng HA protein (Immune Technology Corp., New York, NY) per well in phosphate buffer at 4° overnight. After blocking with 5% skimmed milk powder in washing buffer PBS-T0.05 (0·05% Tween-20), serum samples were added at 1 : 10 dilutions and incubated for 1 hr, followed by intensive washing. Horseradish peroxidase-coupled antibodies against mouse IgG1 or IgG2a antibodies (BD Bioscience) were used for detection. After addition of enhanced chemiluminescence solution, samples were analysed in a microplate Luminometer (Orion L; Titertek Berthold, Pforzheim, Germany).
To determine influenza-specific neutralizing antibody titres, a microneutralization assay was performed as previously described.48
Influenza A virus challenge
Five weeks after the second immunization, mice were anaesthetized and challenged intranasally with 100 plaque-forming units of IAV Puerto Rico/8/34 (PR8) H1N1 (virus strain collection, Inst. of. Mol. Virology, Muenster, Germany). The body weight was monitored daily after the infection and 6 days after infection the mice were killed. Bronchoalveolar lavage fluids (BALF) were collected by flushing the lung twice with 1 ml Hanks’ balanced salt solution (Gibco) before their removal. Lungs were homogenized in 2 ml PBS using the GentleMACS Dissociator (Miltenyi Biotec) according to the manufacturer's protocol. Viral mRNA copy numbers in the BALF and the lung homogenates were quantified by quantitative RT-PCR as described previously.48 The detection limit was 5 copies/quantitative RT-PCR, which corresponds to 355 copies/ml in lung homogenate or BALF.
Additionally, cells from BALF were analysed by surface staining with αCD4-APC, αCD8-PacificBlue, αCD11c-PE, αCD45-PerCPCy5.5 (all from BD Bioscience), αLy6G-FITC, Fixable Viability Dye-eFluor780 (from eBioscience) in PBS/BSA/azide in vitro and measured by flow cytometry (FACSCanto™ II, BD Bioscience).
Ovalbumin-induced asthma model
The basic protocol to establish the ovalbumin (OVA) -induced asthma phenotype is described elsewhere.49 In the prophylactic set up, BALB/c mice were immunized with 20 μg of the DNA vaccines 2 weeks before the sensitization phase, which consists of two intraperitoneal injections of alum-adjuvanted OVA (20 μg OVA/2·2 mg alum) on days 14 and 28. The sensitization is followed by challenges with 1% OVA aerosol at days 42 and 52. To analyse the eosinophilic infiltrates, BALF were collected 3 days after the last aerosol challenge and the cell composition was analysed by surface staining and FACS. Additionally, single cell suspensions from the lungs were re-stimulated for 48 hr with 50 μg/ml OVA per sample. Cytokine secretion [interleukin-4 (IL-4), IL-5, IL-10, IL-13] into the supernatants was determined by ELISA (eBioscience) to confirm the T helper type 2 (Th2) polarization of the OVA-specific T cells.
In the therapeutic set up, the DNA immunization was performed 14 days after the second sensitization step. Afterwards the protocol continued as described above with the aerosol challenges at days 14 and 24 post immunization.
Statistical analysis
Results are expressed as the means ± standard errors of the means (SEM). Statistical comparisons were performed by one-way analysis of variance test, followed by a Tukey post-test using the Prism 5.0 GraphPad Software (San Diego, CA). A P-value < 0·05 was considered statistically significant.
Results
Construction and characterization of DNA vaccines encoding DEC205-targeted antigens
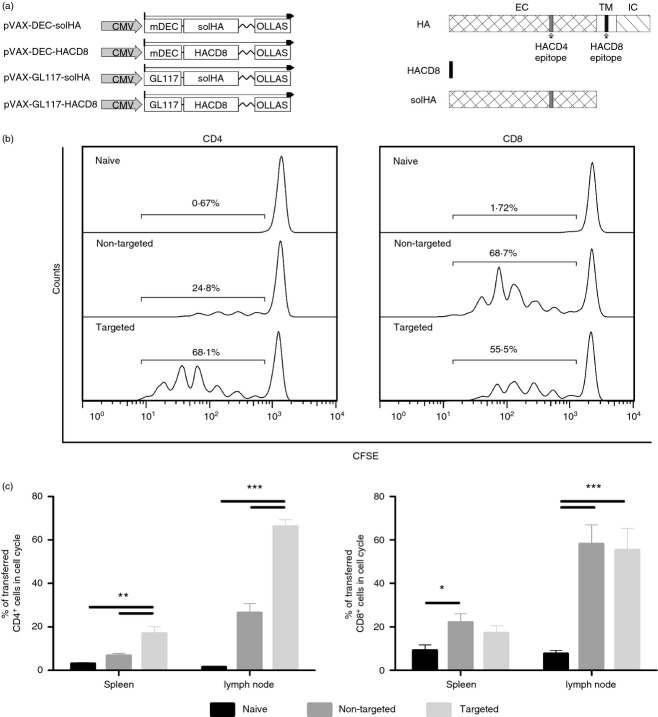
For our studies, we generated DNA vaccines encoding DEC205-targeted HA from the IAV strain Puerto Rico/8/34 (PR8) (H1N1). Secretion of the DEC205-targeted antigen is a prerequisite for our studies and HA contains a transmembrane domain (Fig.1a), so it was not feasible to fuse the complete open reading frame to the DEC205-specific single-chain antibody fragment (scFv), which was described in our earlier studies.44 Therefore the ectodomain, containing the immunodominant CD4 epitope, was fused either to the DEC205-specific or a control scFv resulting in the plasmids pVax-DEC-solHA and pVax-GL117-solHA (Fig.1a). Because the immunodominant CD8 epitope of HA is located in the transmembrane domain, we constructed two additional plasmids and fused the sequence (IYSTVASSL) directly to the respective scFv, pVax-DEC-HACD8 and pVax-GL117-HACD8, to elucidate the effect of DEC205-targeting on CD8 and CD4 T-cell responses in parallel (Fig.1a). To verify expression and secretion of the designed fusion proteins, 293T cells were transfected with the different expression plasmids and supernatants were analysed by Western blot. The DEC205 targeted proteins and the respective controls were detected in equal amounts in the supernatants and appeared at the expected sizes (see Supporting information, Fig. S1A). To ensure the scFv-binding specificity, the transfection supernatants were incubated with DEC205-expressing CHO cells (CHODEC205) or control CHO cells (CHOneo). Bound single-chain molecules were visualized with an Alexa647-labelled anti-OLLAS antibody and subsequent flow cytometric analysis (see Supporting information, Fig. S1B). Incubation of the DEC-specific scFv construct with CHODEC205 resulted in an increase of the mean fluorescence intensity, whereas no changes were observed with the control constructs or the control cells.
Figure 1.
In vivo antigen presentation and T-cell activation. (a) Schematic representation of the DNA vaccines and the corresponding protein domains of the haemagglutinin of influenza A virus (IAV) strain PR8. The single-chain antibody fragment variables were separated from the antigens by a [Gly4Ser]3-linker and all expression cassettes contain a C-terminal OLLAS-tag for detection purposes. (b) To assess the antigen presentation and activation of T-cell receptor (TCR)-specific T cells in vivo, BALB/c mice were immunized with 20 μg of DNA encoding either the DEC205-targeted antigens (DECsolHA/DEC-HACD8) or the control antigens (GL117-solHA/GL117-HACD8). Four days after immunization, 2 × 106 CFSE-labelled TCR-transgenic CD8 T cells (CL4) or CD4 T cells (TCR-HA) were adoptively transferred and their proliferation was determined after 3 days by CFSE dilution. Lymphocytes from spleen and pooled lymph nodes (popliteal, inguinal) were analysed. Representative histograms are shown and percentages of proliferating cells are indicated for the marked region. (c) Mean values and standard errors of the means (SEM) for six animals per group out of two independent experiments are shown for CD4 T cells (left) and CD8 T cells (right), respectively. One-way analysis of variance, Tukey post test, *P < 0·05, **P < 0·01, ***P < 0·001.
Next, we analysed indirectly the presentation of MHC-restricted peptides by the activation of TCR-transgenic T cells after DNA immunization in vivo. Therefore, 2 × 106 HA-specific CFSE-labelled CD4 or CD8 T cells were adoptively transferred into BALB/c mice that had been immunized 3 days before with DNA encoding either the targeted or the non-targeted antigens. One week post immunization, CFSE-labelled cells were isolated from the spleen and lymph nodes and analysed for proliferation indicated by decreasing fluorescence signals (Fig.1b,c). Surprisingly, the activation of TCR-transgenic CD8 T cells was comparable in both immunization groups, indicating a minor influence of DC-targeting on MHC class I presentation in our study, which is in contrast to other reports.22–24,26,27 However, immunization with the targeted antigens resulted in significantly higher percentages of proliferating TCR-transgenic CD4 T cells compared with the group that received the control scFv-antigen encoding plasmids. Although this was also the case in the spleens (17% versus 7%), the more profound proliferation was observed in the lymph nodes (66% versus 26%). These results indicate that the DEC205-targeting enhances the antigen uptake by DCs and increases the antigen presentation on MHC class II, which in turn leads to stronger activation of CD4 T cells. To confirm DCs as presenting cells, we isolated CD11c+ cells from the lymph nodes and spleens of immunized mice and analysed their capacity to activate, naive TCR transgenic CD4 T cells in a proliferation assay (see Supporting information, Fig. S2). Dendritic cells isolated from the lymph nodes of mice receiving the targeted vaccine stimulated the proliferation more efficiently than DCs from the animals receiving the non-targeted DNA. In accordance with the in vivo proliferation assay, the stimulation capacity of spleen-derived CD11c+ cells was less pronounced.
Antigen-targeting to DCs reduced the cellular immune response after DNA immunizations
We demonstrate specific binding to the DEC205 receptor and enhanced activation of TCR-transgenic T cells, so we were interested whether this translates into improved immunogenicity of the targeted DNA vaccines. Therefore mice were injected once intramuscularly with 20 μg of each DNA followed by electroporation and 2 weeks later the cellular immune responses were analysed by intracellular cytokine staining (Fig.2a,b).
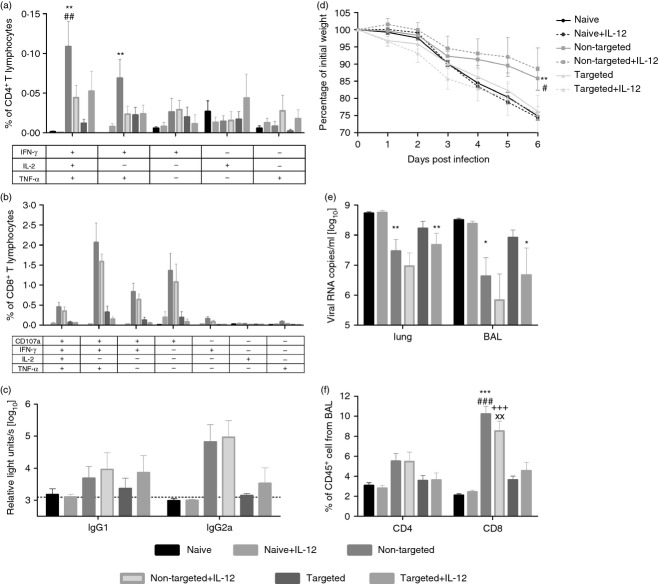
Figure 2.
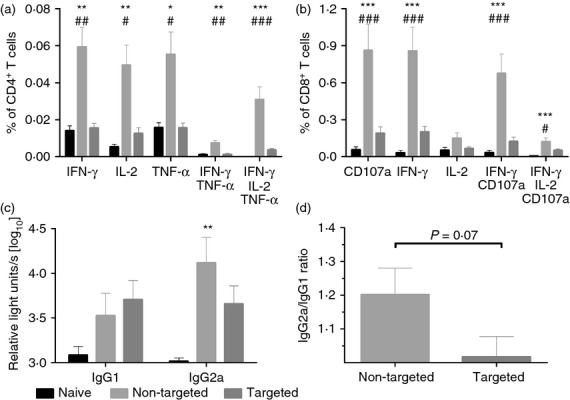
Immunogenicity of DNA vaccines encoding DEC-targeted antigen. (a, b) BALB/c mice were immunized with DNA encoding either the DEC205-targeted antigens (DEC-solHA/DEC-HACD8, 20 μg each) or the control antigens (GL117-solHA/GL117-HACD8, 20 μg each). Antigen-specific CD4 (a) and CD8 (b) T-cell responses in the spleens were analysed 14 days after immunization by intracellular cytokine staining. The percentages of the different subpopulations among the total CD4 (a) resp. CD8 (b) T cells are shown. Mean values and SEM represent seven mice per group out of two independent experiments. (c) Sera were collected at day 35 post immunization and haemagglutinin (HA)- specific antibody titres of the subclasses IgG1 and IgG2a were measured by ELISA. Each bar represents the mean and SEM of 16 animals per group. Log10-transformed relative light units (RLU) are shown. (d) The IgG2a/IgG1 ratio of the log10-transformed RLUs are shown as means and SEM. One-way analysis of variance, Tukey post test, * = significantly different from naive, # = significantly different from targeted, *P < 0·05, **P < 0·01, ***P < 0·001.
DNA immunization with plasmids encoding the antigens fused to the control scFv (non-targeted) induced robust HA-specific CD4 and CD8 T-cell responses, which were significantly higher than the ones observed in the targeted or naive group. Although we demonstrated strong proliferation of TCR-transgenic CD4 T cells after adoptive transfer indicating proper MHC II presentation, the DEC-targeted antigens failed to induce cytokine-producing CD4 T cells. In contrast, the non-targeted antigens induced substantial amounts of HA-specific CD4 T cells producing interferon-γ (IFN-γ), IL-2 or tumour necrosis factor-α with nearly half of them expressing all three cytokines simultaneously (Fig.2a).
A similar observation was made for the HA-specific CD8 T-cell response. Although both the non-targeted and the targeted antigens were able to stimulate the proliferation of TCR-transgenic cells to a comparable extent, the HA-specific CD8 T-cell responses in the wild-type animals were significantly higher after DNA immunization with the non-targeted antigens. In the non-targeted group, around 1% of the CD8 T cells responded to the HA peptide stimulation with the expression of IFN-γ and nearly two-thirds of those were also positive for the degranulation marker CD107a (∼0·6%). As a consequence of the DEC-targeting, the percentages of antigen-specific CD8 T cells were significantly reduced with only 0·2% IFN-γ-producing cells found in animals of the targeted group.
As the whole extracellular domain of HA was fused to the scFv, we were also interested if the antibody responses were influenced by the DEC205-targeting. Therefore, serum samples were collected 5 weeks after a single DNA immunization and IgG1 as well as IgG2a antibody levels were determined by ELISA (Fig.2c,d). Independent of the presence of DEC205-targeting, the amount of HA-specific antibodies was generally low. Only the IgG2a antibody level in the non-targeted group was significantly higher compared with that in the naive group (Fig.2c). Nevertheless, a single DNA immunization with the non-targeted construct revealed the preferential induction of the IgG2a subtype, whereas the targeted construct induced a balanced IgG2a/IgG1 response (Fig.2d).
DNA immunization with targeted antigens does not protect mice against lethal IAV infection
Previously, it was reported that not only the quantity, but also the quality, of the immune response could be altered by DEC205-targeting, so the efficacy of our DNA vaccines was analysed in a homologous IAV challenge experiment. This time, we immunized twice with a 6-week interval and co-administered DNA encoding for the pro-inflammatory cytokine IL-12 to polarize the CD4 T-cell response towards Th1 for an improved CD8 T-cell response. Eighteen days after the first immunization, HA-specific CD4 and CD8 T lymphocytes from the blood were analysed for their cytokine expression by intracellular cytokine staining. Co-administration of DNA encoding IL-12 increased the frequency of polyfunctional CD4 T cells and CD8 T cells in both the non-targeted and the targeted groups. Nevertheless, the responses were still stronger in the animals receiving the DNA encoding the non-targeted antigens (data not shown).
Two weeks after the second immunization, the influence of the co-expressed IL-12 on the CD4 and CD8 T-cell responses was no longer detectable in the intracellular cytokine staining. The only exception is the frequency of polyfunctional CD4 T cells observed after immunization with the targeted antigens, which seemed to be higher in the group where the additional IL-12-encoding plasmid was applied (Fig.3a). The CD8 responses were still highest in the non-targeted groups independent of the delivery of IL-12 (Fig.3b).
Figure 3.
Protective efficacy against influenza A virus (IAV) challenge. BALB/c mice were immunized twice in a 6-week interval with DNA encoding either the DEC205-targeted (DEC-solHA/DEC-HACD8, 20 μg each) or the control antigens (GL117-solHA/GL117-HACD8, 20 μg each). We included three additional groups which received 10 μg of an interleukin-12 (IL-12) -encoding plasmid as genetic adjuvant. Antigen-specific CD4 (a) and CD8 (b) T-cell responses in the blood were analysed 13 days after the second immunization by intracellular cytokine staining. The percentages of the different subpopulations among the total CD4 (a) or CD8 (b) T cells are shown, respectively. At the same time-point, haemagglutinin (HA) -specific antibody titres of the subclasses IgG1 and IgG2a were measured by ELISA. Each bar represents the mean and SEM of six animals per group. Log10-transformed relative light units (RLU) are shown (c). (d) Three weeks after the second immunization the animals were challenged with 100 plaque-forming units (PFU) of IAV PR8 and their body weights were documented daily and presented as means and SEM of six animals per group. (e) Six days post infection, mice were killed and the viral loads in bronchoalveolar lavage fluid (BALF) as well as lung homogenates were determined by quantitative RT-PCR. Each bar represents the logarithmically transformed amount of viral copies per ml. To analyse if vaccine-induced CD4 or CD8 T cells infiltrated the infected lung, cells from the BALF were analysed by FACS. Mean values and SEM for the percentages of CD4 and CD8 among total CD45+ cells are shown in (f). One-way analysis of variance, Tukey post test, * = significantly different from naive, # = significantly different from targeted, + = significantly different from naive+IL-12, x = significantly different from targeted+IL-12, *P < 0·05, **P < 0·01, ***P < 0·001.
Furthermore, the IL-12 co-expression had only a minor influence on the vaccine-induced antibody levels measured 2 weeks after the boost immunization (Fig.3c). Generally, the second immunization slightly enhanced the overall antibody levels and the dominant induction of IgG2a in the non-targeted groups was even more pronounced compared with the antibody responses after a single immunization (Fig.2c). Nevertheless, two immunizations with DNA encoding the DEC205-targeted HA failed to induce significant levels of IgG2a antibodies (Fig.3c). These results indicate that not only the cellular response but also the humoral immune response is altered by the DEC-targeting. Of note, none of the animals among all groups developed neutralizing antibodies against the homologous IAV strain, which was analysed in a microneutralization assay (data not shown).
Finally, the mice were infected with the homologous IAV strain PR8 (∼10 LD50) to determine the protective capacity of these immune responses. We documented daily the body weight loss as a sign for disease progression (Fig.3d) and the viral load was measured in the BALF and lung at day 6 post infection (Fig.3e). From day 3 post infection, all animals showed a steady decline of body weight until day 6, at this time-point nearly all control mice had reached the end-point of 25% loss of the initial body weight (Fig.3d). In comparison to the control group, significantly less weight loss was observed in the groups that received the non-targeted vaccines, whereas no significant difference was observable in the groups that received the targeted vaccines. This indicates a partial protection against virus-induced disease progression in animals vaccinated with the non-targeted constructs. Accordingly, these two groups had reduced viral loads in the lungs and BALFs (1·5–2·5 log reduction compared with naive mice, Fig.3e). Although not significant in multi-variant analysis, the animals that received the targeted vaccine alone also showed a lower viral burden than the naive mice (0·5–1·5 log reduction). Interestingly, the viral loads were further reduced significantly in the group that additionally received plasmids encoding IL-12. Of note, this reduction was not observed in the control animals that received only the IL-12-encoding plasmid, suggesting that non-specific activation of immune cells by IL-12 is unlikely to contribute to this protection.
Furthermore, the cellular composition of the lungs was analysed 6 days post infection by flow cytometry (Fig.3f). This revealed higher frequencies of CD4 and CD8 T cells in the lungs of the non-targeted groups in comparison to the targeted or control groups, which might indicate control of viral replication by anamnestic T-cell responses. In the case of the targeted vaccines, the tendency of increased cellular infiltrates was lower and confined to CD8 T cells, which is in line with the reduction in viral mRNA copy numbers.
These results clearly demonstrate that the enhanced antigen presentation after immunization with DNA encoding DEC205-targeted antigens did not translate into improved immunogenicity or vaccine efficacy. Even in the presence of the pro-inflammatory cytokine IL-12, we observed poor cellular responses, suggesting that our vaccination protocol more likely suppresses the immune responses, possibly by the induction of Treg cells or anergy.
Induction of antigen-specific Treg cells by DNA vaccines encoding DEC205-targeted antigens
To analyse if the overall number of Treg cells was altered by the DEC-targeted vaccine, we first determined the levels of CD4+ CD25+ Foxp3+ T cells in the spleen after the first and second immunizations. There were no differences with regard to the absolute number or the frequency of Treg cells between the naive mice and the two immunized groups (data not shown).
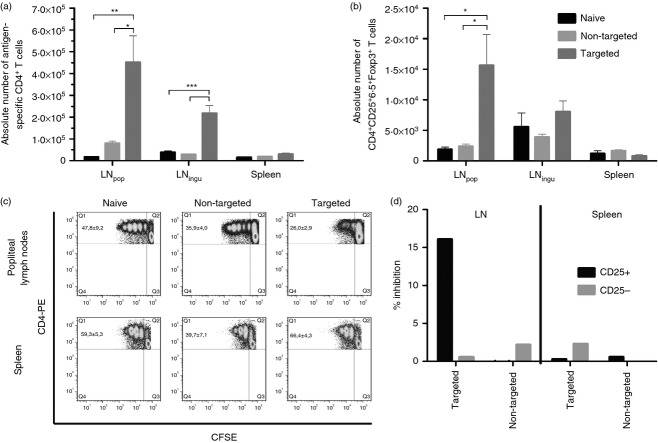
As we could demonstrate that the antigen presentation on MHC II by DCs was enhanced in mice immunized with the targeted vaccine, we were interested in the fate of the antigen-specific CD4 T cells in these mice. Because the frequency of antigen-specific T cells in the bulk CD4 population was rather low, we adoptively transferred 1 × 106 TCR-transgenic CD4 T cells (TCR-HA) into BALB/c mice 1 day before vaccination. One week later, the popliteal and inguinal lymph nodes, as well as the spleens, were analysed for the presence of HA-specific CD4 T cells (Fig.4a,b). Significantly more HA-specific CD4 T cells were found in the lymph nodes of the animals vaccinated with the targeted antigens than in the two other groups (Fig.4a), this argues against an early depletion of the HA-specific T cells after DEC205-mediated antigen presentation by immature DCs. In addition, we demonstrate that the pool of antigen-specific Treg cells was locally expanded in the draining lymph nodes after the immunization with DEC-targeted antigens (Fig.4b).
Figure 4.
Induction of antigen-specific regulatory T (Treg) cells. One day before BALB/c mice were immunized as described in Fig.2, 1 × 106 T-cell receptor (TCR) -transgenic CD4 T cells (TCR-HA) were adoptively transferred. One week after vaccination, lymphocytes were isolated from spleen, popliteal (LNpop) and inguinal (LNingu) lymph nodes and the absolute numbers of antigen-specific CD4 (a) and Treg (b) cells were determined by FACS. Each bar represents the mean and SEM of four animals per group of one experiment. One representative is shown out of two independent experiments. In a second experiment, TCR-HA mice were directly immunized with 20 μg of either pV-DEC-solHA or pV-GL117-solHA or left untreated as controls. One week post immunization, lymphocytes were isolated from the spleen and popliteal LN and the proliferative capacity of the CD4 T cells was analysed by CFSE-dilution assay (c). The cells were re-stimulated in vitro with the CD4-specific haemagglutinin (HA) peptide for 3 days. Representative dot plots for one animal per group are shown and the figures in the upper left quadrants represent the means and SEM of three animals per group. In (d), CD25+ CD4 T cells isolated from LNpop or spleens from immunized mice (pooled from four animals) were analysed for their capacity to suppress HA-driven proliferation of naive, CFSE-labelled TCR-HA cells. 1 × 105 CD25+ or CD25– cells were incubated with 1 × 105 TCR-HA cells in the presence of HA peptide. The percentage of dividing TCR-HA cells in the absence of any cells from the immunized mice was set as 100% and the per cent inhibition by the respective populations was shown. One-way analysis of variance, Tukey post test, *P < 0·05, **P < 0·01, ***P < 0·001.
To analyse if the induced Treg cells could suppress the activation of CD4 effector T cells, we immunized directly the TCR-transgenic animals with either the non-targeted or the DEC205-targeted vaccines and kept one naive control group. Seven days later, CD4 T cells were isolated from the popliteal lymph nodes and the spleen, labelled with CFSE, re-stimulated with the respective HA-specific peptide for 3 days in vitro and then the proliferation was analysed by flow cytometry. In case the DEC-targeted antigens increased the numbers of antigen-specific Treg cells, we expected reduced proliferative responses of respective lymphocyte cultures. Indeed, we observed strong proliferation of the lymphocytes isolated from the popliteal lymph nodes of the naive and the non-targeted group, reaching up to six divisions (Fig.4c). In contrast, only a small fraction of lymphocytes isolated from animals immunized with the targeted antigen started to proliferate (26%) and the proliferation did not exceed three division cycles. Interestingly, in cultures from the spleen of the same animals this specific suppression could not be observed and a higher percentage of cells (66%) showed a reduction of CFSE intensity. Generally, there was less proliferation of the spleen-derived CD4 T cells in all groups (up to four cycles), but only the targeted group showed this discrepancy between the two lymphatic organs, which would be in accordance with a local expansion of antigen-specific Treg cells in the draining lymph nodes. In a second experiment, we adoptively transferred again TCR-HA cells before vaccination and isolated via magnetic cell separation CD25+ CD4+ T cells from the lymph nodes and the spleens 1 week later. To address the suppressive capacity of the Treg cells, they were incubated with naive, CFSE-labelled TCR-HA cells in the presence of HA peptide. Since the amount of antigen-specific Treg cells among the whole CD25+ FoxP3+ population was still rather low, we observed only minor effects. Nevertheless, the Treg cells isolated from the lymph node of mice immunized with the targeted antigens were the only ones that could partially inhibit the HA-driven proliferation of the CFSE-labelled TCR-HA cells (16% specific inhibition). There was no inhibition seen by the CD25– cells from the same animals or by Treg cells isolated from the control mice (4D). Furthermore, there was no suppression observed for the spleen-derived Treg cells. This further supported the hypothesis that the reduced T-cell responses might be explained by the induction of antigen-specific Treg cells instead of effector cells.
Induction of antigen specific tolerance by DEC205 targeted DNA vaccines in an allergic asthma model
To explore if DNA vaccines encoding DEC205-targeted antigens could mediate an antigen-specific tolerance, we used OVA as model antigen to induce an allergic asthma phenotype in BALB/c mice. During the sensitization phase, a Th2-dominated immune response is induced by two intraperitoneal injections of alum-adjuvanted OVA protein, which results in strong eosinophilia in the lungs after subsequent OVA-aerosol challenges. In case of a vaccine-induced tolerance, the Th2 response induced by the protein injections should be less pronounced and so the eosinophilia after the aerosol-challenges should be reduced.
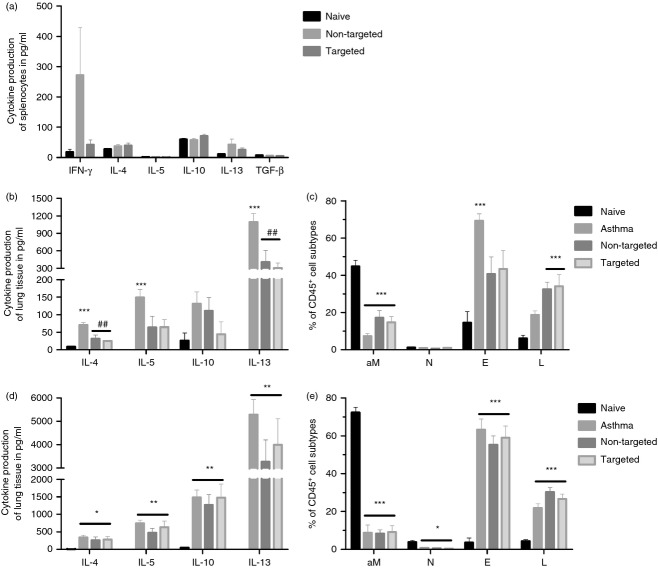
For that purpose, we used DNA vaccines encoding either DEC-OVA or GL117-OVA 38 and immunized animals either 2 weeks before the first sensitization (prophylactic; Fig.5b,c) or after the sensitization and 2 weeks before the aerosol challenge (therapeutic; Fig.5d,e). First, we analysed the OVA-specific cellular response 2 weeks after DNA immunization and confirmed that the non-targeted antigen induced stronger IFN-γ responses compared with the targeted antigen as measured by cytokine-specific ELISA (Fig.5a). Furthermore, none of the DNA vaccines induced substantial amounts of Th2-specific helper cells indicated by the absence of the Th2-associated cytokines IL-4, IL-5 and IL-13 (Fig.5a).
Figure 5.
Potential of DNA vaccines encoding DEC205-targeted antigens as therapy for allergen-induced asthma. (a) BALB/c mice were immunized with 20 μg of either pV-DEC-OVA or pV-GL117-OVA and cellular immune responses were analysed 14 days post immunization. Splenocytes were re-stimulated with 50 μg/ml of ovalbumin (OVA) protein for 48 hr in vitro and the culture supernatants were analysed for secreted cytokines by ELISA. The potential of DNA serving as prophlyactic (b, c) or therapeutic (d, e) vaccine was anaylsed in an OVA-induced asthma model as described in the Materials and methods section. Three days after the final aerosol challenge, the cytokine profiles of the OVA-specific T cells in the lungs were determined by ELISA specific for T helper type 2 cytokines (b, d). The composition of the cellular infiltrates was characterized by surface staining of the cells in the broncholalveolar lavage fluid (c, e) Each bar represents the mean with SEM of six animals per group. aM represents alveolar macrophages, N represents neutrophils, E, represents eosinophil and L represents lymphocytes. One-way analysis of variance, Tukey post test, * = significantly different from naive, # = significantly different from asthma, *P < 0·05, **P < 0·01, ***P < 0·001.
Surprisingly, in the prophylactic set up both DNA vaccines could reduce the development of the OVA-specific Th2 response (Fig.5b) and consequently the infiltration of eosinophils into the lungs after the aerosol challenges (Fig.5c). In consideration of our previous results, the underlying mechanisms seem to differ substantially for the different DNA constructs. The non-targeted antigen induced strong Th1 responses, thereby limiting the Th2 response (Fig.5a), whereas the DEC205-targeted antigens could induce a state of peripheral tolerance.
In contrast to the prophylactic regimen, DNA immunization after the sensitization phase had no effect on the outcome of the asthmatic phenotype. Therefore, the amounts of Th2-resticted cytokines produced by the lung-infiltrating lymphocytes were comparable for all groups independent of the vaccination (Fig.5d). In addition, the influx of eosinophils into the lung lumen was not influenced by the therapeutic DNA immunization (Fig.5e). These results indicate that once the antigen-specific T cells were systemically primed, they could not be silenced or suppressed after therapeutic immunization with a single-dose of plasmid encoding the DEC205-targeted antigen.
Discussion
The potential of DC-targeted vaccines had been demonstrated in a variety of different models including induction of tolerance by targeting immature DCs21,33–36 or protective immune responses against infectious diseases22,32,38,50 or tumours when delivered together with adjuvants for DC maturation.29,31 Most of these studies deal with protein vaccines and only a few studies using gene-based vaccines had been described to date. Therefore, we wanted to address the influence of DEC205-targeted antigens on the immunogenicity and efficacy of a DNA vaccine against IAV.
For this reason, we genetically fused the HA protein of Pr8/34 either to the DEC205-specific or a control scFv (GL117). Since HA is a transmembrane protein, we had to generate two separate scFv fusion proteins to include the whole extracellular domain with the immunodominant CD4 epitope as well as the immunodominant CD8 epitope, which resides in the transmembrane domain. Both, the DEC205-targeted and the control antigens were efficiently secreted by 293T cells after transient transfection, but only the DEC205-targeted antigens bound specifically to CHO cells expressing the DEC205 receptor, demonstrating that the receptor-targeting of our scFv-HA worked properly. Surprisingly, the presentation on MHC I after the DNA immunization seemed to be equally efficient in the targeted and non-targeted group indicated by comparable activation of TCR-transgenic CD8 T cells. This is in sharp contrast to previous studies using plasmids encoding OVA as antigen,38 where the DNA encoding DEC205-OVA resulted in enhanced MHC I presentation by CD11c+ DC. A major difference between these two studies is based on the nature of the fused antigen, which is in our study only the short immunodominant epitope of HA and a full-length protein in the study of Nchinda et al.38 Although this was never analysed in the context of DNA vaccines, it was shown for protein vaccines that synthetic long peptides are more efficiently presented on MHC I by murine DCs than full-length protein and result in stronger CD8 T-cell activation.51 This was shown in the context of HIVgag and OVA and originated from different antigen processing after uptake. Hence, it might be possible that the DEC205-targeting has less impact on MHC I presentation in our set up, because we used the peptide instead of the whole protein. Nevertheless, the antigen delivery to the DC via DEC205 impacted on the priming of CD8 T cells as discussed below. In accordance with previous studies,38,44 immunization with DEC205-targeted antigens resulted in a stronger activation of TCR-specific CD4 T cells than immunization with the control scFv construct indicating enhanced antigen presentation on MHC class II molecules. This was supported by the fact that CD11c+ DCs isolated from draining lymph nodes of mice immunized with the DEC205 targeted DNA were capable of activating naive, TCR-transgenic CD4 T cells ex vivo, which is in accordance with the previous report.38 The consequences of DEC205 targeting in the context of DNA vaccines might be different for MHC I and MHC II presentation, which also makes perfect sense considering the proposed model of antigen presentation after DNA delivery.52 The MHC I presentation and the subsequent CD8 T-cell activation by DCs depends either on direct transfection of DCs or on cross-presentation by DCs after the uptake of apoptotic bodies. Both pathways are more dependent on the nature of DNA delivery than on the secretion of the antigen. In contrast, MHC class II presentation and CD4 T-cell activation are considered to occur most efficiently after the uptake of secreted proteins, which might explain that the DEC-targeting affects these pathways more than the MHC I pathway.
In contrast to protein vaccines, DNA vaccines have been proven to induce robust cellular and humoral immune responses without additional adjuvants, especially when the delivery was followed by electroporation.53,54 It was a unexpected that both HA-specific CD4 and CD8 T-cell responses were significantly reduced in wild-type animals receiving the DNA encoding the DEC205-targeted antigens. Even in the presence of an IL-12 encoding plasmid as genetic adjuvant known to polarize the T cells toward IFN-γ producing Th1 cells and cytotoxic T cells,55,56 the targeted vaccine hardly induced HA-specific T-cell responses, whereas the non-targeted DNA performed well. This is in contrast to the studies of Nchinda et al.38 and Cao et al.,39 in which the cellular responses were enhanced by the DEC205-targeted constructs in comparison to the control-scFv or non-fused proteins. Interestingly, DNA electroporation was applied in all three studies, so that a different influence in the DC maturation induced by the electroporation mediated inflammation at the site of injection12,13 is unlikely to be the reason. A difference between these studies is found in the chosen antigen, which might influence the overall immunogenicity for a so far unknown reason. Since we demonstrated efficient antigen presentation after immunization with the targeted DNA, the reduced immunogenicity could not be explained by a general deficit of our vectors, but must be a consequence of targeting the DEC205 receptor. Possibly, DCs directly transfected during the immunization have different stimulatory properties with regard to T-cell activation than DEC205+ DCs, which took up the secreted scFv-antigens later. If these different DC populations were counteracting each other (e.g. mature DCs versus immature DCs), this might explain the different T-cell responses in the two vaccine groups. Therefore, it would be interesting to address which subpopulations of DCs take up and present the non-targeted and the DEC-targeted antigen after the DNA delivery.
In accordance with the reduced immunogenicity of the DEC-targeted antigens, animals of those groups were less protected from an experimental IAV infection than animals from the non-targeted groups, which showed significantly less weight loss after IAV challenge. This translated into lower viral loads in the lungs, most probably due to anamnestic T-cell responses as indicated by higher percentages of CD4 and CD8 T cells detected in the BALF. In contrast, animals of the targeted group had comparable weight loss to naive mice and only marginally reduced viral loads. Further, there was no sign of enhanced migration of CD4 T cells into the lungs when the cell counts in the BALF were compared with those of naive mice. The reduced efficacy of the targeted vaccine is in accordance with our previous findings in the context of scDEC-OVA encoded by adenoviral vectors.44 These results clearly demonstrate that targeting the antigen to immature DCs could negatively affect the immunogenicity and efficacy of DNA vaccines, suggesting that our vaccination protocol more probably induce peripheral tolerance towards the antigen, possibly by the induction of Treg cells or anergy. Although Ettinger et al. did not include a control scFV in their study, their results support our hypothesis, because gene gun immunizations with DNA encoding DEC-hNC16A could induce peripheral tolerance and improved the graft-acceptance after transplantation.40 Hence, the effect of DC-targeting has to be carefully evaluated for each antigen and in the appropriate disease model.
We observed strong activation of TCR-transgenic CD4 T cells indicating efficient antigen presentation on MHC class II molecules but very low CD4 responses in wild-type mice, as a result we were interested in the fate of the antigen-specific CD4 T cells after the DNA vaccination. In accordance with studies using DEC-targeted protein vaccines, we detected a strong expansion of TCR-HA cells after adoptive transfer in lymph nodes and there was no evidence of T-cell depletion as it was described for antigen-specific CD8 T cells after targeting immature DCs by anti-DEC-OVA.34 As described by Mahnke et al.36and Kretschmer et al.,35 there was an expansion of antigen-specific Treg cells by the targeted vaccine, which seemed to be restricted to the draining lymph nodes close to the injection site in our study. Isolated CD25+ FoxP3+ T cells from these lymph nodes were also capable of suppressing the HA-driven proliferation of naive TCR-HA cells ex vivo. This inhibition was not seen with CD25+ FoxP3+ cells from mice immunized with the non-targeted DNA. This supports our hypothesis that the DEC-targeted DNA vaccine induced immunosuppressive mechanisms counteracting the efficient priming of effector cells. This became even more obvious when TCR-HA mice were immunized and the proliferative capacity of the CD4 T cells was analysed in vitro. The expansion of Treg cells resulted in suppressed proliferation of CD4 cells in lymph node cultures, but not in splenocyte cultures of the same animals. The suppression of proliferative responses by Treg cells is in line with the studies using protein vaccines,35,36 but such a local restriction was not reported before and might be a consequence of the different application routes.
Therefore it would be interesting to analyse the potential of the DEC-targeted DNA vaccines in a model for an autoimmune disease, in which the disease-bearing animal could be directly immunized, such as experimental allergic encephalomyelitis.37 As an alternative, we analysed the suppressive effect of DNA vaccines encoding DEC205-targeted antigens in a model of allergy, in which a Th2-dominated immune response induces an asthmatic phenotype after aerosol challenges with the respective antigen (OVA).
Animals immunized with DNA encoding scDEC-OVA before starting the sensitization and aerosol challenge protocol did not develop the asthma-typical Th2 response and showed reduced eosinophilia. Together with the fact that these animals did not develop any OVA-specific T-cell responses after the vaccination, it supports the hypothesis that OVA-specific tolerance is induced through antigen presentation by immature DCs either by antigen-specific Treg cells or CD4 T-cell anergy. The latter was already reported for protein vaccines, but so far not for gene-based vaccines. In contrast, as expected vaccination with the non-targeted antigens induced robust Th1-biased T-cell responses indicated by IFN-γ production and thereby also inhibited the Th2 phenotype after OVA aerosol challenges. This is in line with previous reports on DNA, liposomes or adjuvanted protein vaccines, which induced strong Th1 responses.57,58 However, it is not desirable to induce strong Th1 responses against harmless antigens that the recipient is likely to be repeatedly exposed to. For this reason, protection against asthma development by inducing a state of unresponsiveness or active suppression, as is most probably the case after specific immunotherapy (reviewed in refs 59 and 60), would be the better choice. Furthermore, a Th1-driven autoimmune disease, like experimental allergic encephalomyelitis, would be more suitable to analyse the underlying mechanisms, as the Th1 response induced by the non-targeted DNA would enhance disease severity whereas peripheral tolerance induced by the targeted DNA should suppress disease progression. This is an objective for future studies.
Unfortunately, therapeutic immunization with DEC205-targeted DNA after the sensitization neither revert or suppress the Th2 response, nor prevent the eosinophilia after the aerosol challenge. Once polarized, T cells could not be suppressed by the vaccine-induced immune response, which supports the idea of induction of anergy in naive, but not in already primed antigen-specific T cells by immature DCs presenting the antigen on MHC II. Ring et al. demonstrated partial regression of experimental allergic encephalomyelitis after therapeutic immunization with DEC-targeted protein indicating active suppression of effector cells by vaccine-induced Treg cells.37 In the above-quoted study, the vaccine was applied systemically via intravenous injection, which might have an impact on immune suppression. Our transfer experiments revealed a more localized expansion of Treg cells after DNA immunization, so it might also be possible that the immune-suppressive environment is locally confined and thereby not able to control T-cell responses in the airways. It would be interesting to see if mucosal vaccinations with DNA encoding DEC-targeted antigens might also improve the therapeutic efficacy in this model.
In summary, we demonstrate that DNA vaccines encoding DEC205-targeted antigens improved the antigen presentation on MHC class II molecules, but appear to be biased towards induction of tolerance rather than immunity towards the coupled antigen. Further investigations are necessary to point out the underlying mechanism of this tolerance in detail, but our studies suggest that the local expansion of antigen-specific Treg cells as well as the induction of anergy in CD4 T cells play a role. The role of different DC subpopulations would also be an important factor to address in future studies. This will be of significant importance for the further use of DC-targeted antigens in the context of gene-based vaccines. The induction of local antigen-specific tolerance by gene-based DC-targeted vaccines might be an interesting approach for prophylactic or therapeutic approaches to autoimmune diseases or allergies without inducing systemic immune suppression.
Acknowledgments
This work was supported by grants from the German Research Foundation (GK1045/3), the Heinrich and Alma Vogelsang-Stiftung and the Mercator Research Centre Ruhr (St-2010-0004).
Glossary
- BALF
bronchoalveolar lavage fluid
- DCs
dendritic cells
- IAV
influenza A virus
- IFN-γ
interferon-γ
- IL-2
interleukin-2
- OVA
ovalbumin
- PerCP
peridinin chlorophyll protein
- scFv
single-chain antibody fragment variable
- TCR
T-cell receptor
- Th2
T helper type 2
- Treg
regulatory T
Author's contributions
TN, WH, KÜ and MT conceived and designed the experiments and wrote the paper. TN, AM, MSgB, VT and VH performed the experiments. TN, VH, WH and MT analysed the data; and DH, JB, CE and WH contributed reagents, materials and analysis tools.
Disclosures
The authors declare no financial or commercial conflict of interest. D. Hannaman is an employee of the company Ichor Medical Systems. There are no further patents, products in development or marketed products to declare.
Supporting Information
Figure S1. Expression and DEC205-specific binding of the scFv fusion proteins encoded by the DNA vaccines. Characterization of the DNA vaccines.
Figure S2. Enhanced antigen-presentation by CD11c+ and not by CD11c- cells after DNA immunization with pV-DEC-solHA is indicated by increased number of proliferating TCR-HA cells.
References
- Ulmer JB, Fu TM, Deck RR, et al. Protective CD4+ and CD8+ T cells against influenza virus induced by vaccination with nucleoprotein DNA. J Virol. 1998;72:5648–53. doi: 10.1128/jvi.72.7.5648-5653.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ljungberg K, Kolmskog C, Wahren B, van Amerongen G, Baars M, Osterhaus A, Linde A, Rimmelzwaan G. DNA vaccination of ferrets with chimeric influenza A virus hemagglutinin (H3) genes. Vaccine. 2002;20:2045–52. doi: 10.1016/s0264-410x(02)00049-x. [DOI] [PubMed] [Google Scholar]
- Ulmer JB, Donnelly JJ, Parker SE, et al. Heterologous protection against influenza by injection of DNA encoding a viral protein. Science. 1993;259:1745–9. doi: 10.1126/science.8456302. [DOI] [PubMed] [Google Scholar]
- Donnelly JJ, Friedman A, Martinez D, Montgomery DL, Shiver JW, Motzel SL, Ulmer JB, Liu MA. Preclinical efficacy of a prototype DNA vaccine: enhanced protection against antigenic drift in influenza virus. Nat Med. 1995;1:583–7. doi: 10.1038/nm0695-583. [DOI] [PubMed] [Google Scholar]
- Robinson HL, Hunt LA, Webster RG. Protection against a lethal influenza virus challenge by immunization with a haemagglutinin-expressing plasmid DNA. Vaccine. 1993;11:957–60. doi: 10.1016/0264-410x(93)90385-b. [DOI] [PubMed] [Google Scholar]
- Epstein SL, Lo CY, Misplon JA, Bennink JR. Mechanism of protective immunity against influenza virus infection in mice without antibodies. J Immunol. 1998;160:322–7. [PubMed] [Google Scholar]
- Fu TM, Guan L, Friedman A, Schofield TL, Ulmer JB, Liu MA, Donelly JJ. Dose dependence of CTL precursor frequency induced by a DNA vaccine and correlation with protective immunity against influenza virus challenge. J Immunol. 1999;162:4163–70. [PubMed] [Google Scholar]
- Wolff JA, Malone RW, Williams P, Chong W, Acsadi G, Jani A, Felgner PL. Direct gene transfer into mouse muscle in vivo. Science. 1990;247:1465–8. doi: 10.1126/science.1690918. [DOI] [PubMed] [Google Scholar]
- Zucchelli S, Capone S, Fattori E, et al. Enhancing B- and T-cell immune response to a hepatitis C virus E2 DNA vaccine by intramuscular electrical gene transfer. J Virol. 2000;74:11598–607. doi: 10.1128/jvi.74.24.11598-11607.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachy M, Boudet F, Bureau M, Girerd-Chambaz Y, Wils P, Scherman D, Meric C. Electric pulses increase the immunogenicity of an influenza DNA vaccine injected intramuscularly in the mouse. Vaccine. 2001;19:1688–93. doi: 10.1016/s0264-410x(00)00406-0. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Fang F, Chen J, Wang H, Chang H, Yang Z, Chen Z. Electroporation at low voltages enables DNA vaccine to provide protection against a lethal H5N1 avian influenza virus challenge in mice. Intervirology. 2008;51:241–6. doi: 10.1159/000156483. [DOI] [PubMed] [Google Scholar]
- Babiuk S, Baca-Estrada ME, Foldvari M, Storms M, Rabussay D, Widera G, Babiuk L. Electroporation improves the efficacy of DNA vaccines in large animals. Vaccine. 2002;20:3399–408. doi: 10.1016/s0264-410x(02)00269-4. [DOI] [PubMed] [Google Scholar]
- Babiuk S, Baca-Estrada ME, Foldvari M, Middleton DM, Rabussay D, Widera G, Babiuk L. Increased gene expression and inflammatory cell infiltration caused by electroporation are both important for improving the efficacy of DNA vaccines. J Biotechnol. 2004;110:1–10. doi: 10.1016/j.jbiotec.2004.01.015. [DOI] [PubMed] [Google Scholar]
- Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–52. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- Steinman RM, Banchereau J. Taking dendritic cells into medicine. Nature. 2007;449:419–26. doi: 10.1038/nature06175. [DOI] [PubMed] [Google Scholar]
- Asano M, Toda M, Sakaguchi N, Sakaguchi S. Autoimmune disease as a consequence of developmental abnormality of a T cell subpopulation. J Exp Med. 1996;184:387–96. doi: 10.1084/jem.184.2.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Critchfield JM, Racke MK, Zúñiga-Pflücker JC, Cannella B, Raine CS, Goverman J, Lenardo MJ. T cell deletion in high antigen dose therapy of autoimmune encephalomyelitis. Science. 1994;263:1139–43. doi: 10.1126/science.7509084. [DOI] [PubMed] [Google Scholar]
- Perez VL, Van Parijs L, Biuckians A, Zheng XX, Strom TB, Abbas AK. Induction of peripheral T cell tolerance in vivo requires CTLA-4 engagement. Immunity. 1997;6:411–7. doi: 10.1016/s1074-7613(00)80284-8. [DOI] [PubMed] [Google Scholar]
- Latchman Y, Wood CR, Chernova T, et al. PD-L2 is a second ligand for PD-1 and inhibits T cell activation. Nat Immunol. 2001;2:261–8. doi: 10.1038/85330. [DOI] [PubMed] [Google Scholar]
- Jiang W, Swiggard WJ, Heufler C, Peng M, Mirza A, Steinman RM, Nussenzweig MC. The receptor DEC-205 expressed by dendritic cells and thymic epithelial cells is involved in antigen processing. Nature. 1995;375:151–5. doi: 10.1038/375151a0. [DOI] [PubMed] [Google Scholar]
- Hawiger D, Inaba K, Dorsett Y, et al. Dendritic cells induce peripheral T cell unresponsiveness under steady state conditions in vivo. J Exp Med. 2001;194:769–79. doi: 10.1084/jem.194.6.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trumpfheller C, Finke JS, López CB, et al. Intensified and protective CD4+ T cell immunity in mice with anti-dendritic cell HIV gag fusion antibody vaccine. J Exp Med. 2006;203:607–17. doi: 10.1084/jem.20052005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boscardin SB, Hafalla JCR, Masilamani RF, et al. Antigen targeting to dendritic cells elicits long-lived T cell help for antibody responses. J Exp Med. 2006;203:599–606. doi: 10.1084/jem.20051639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonifaz LC, Bonnyay DP, Charalambous A, et al. In vivo targeting of antigens to maturing dendritic cells via the DEC-205 receptor improves T cell vaccination. J Exp Med. 2004;199:815–24. doi: 10.1084/jem.20032220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahnke K, Guo M, Lee S, Sepulveda H, Swain SL, Nussenzweig M, Steinman RM. The dendritic cell receptor for endocytosis, DEC-205, can recycle and enhance antigen presentation via major histocompatibility complex class II – positive lysosomal compartments. J Cell Biol. 2000;151:673–83. doi: 10.1083/jcb.151.3.673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bozzacco L, Trumpfheller C, Siegal FP, et al. DEC-205 receptor on dendritic cells mediates presentation of HIV gag protein to CD8+ T cells in a spectrum of human MHC I haplotypes. Proc Natl Acad Sci U S A. 2007;104:1289–94. doi: 10.1073/pnas.0610383104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudziak D, Kamphorst AO, Heidkamp GF, et al. Differential antigen processing by dendritic cell subsets in vivo. Science. 2007;315:107–11. doi: 10.1126/science.1136080. [DOI] [PubMed] [Google Scholar]
- Birkholz K, Schwenkert M, Kellner C, et al. Targeting of DEC-205 on human dendritic cells results in efficient MHC class II-restricted antigen presentation. Blood. 2010;116:2277–85. doi: 10.1182/blood-2010-02-268425. [DOI] [PubMed] [Google Scholar]
- Charalambous A, Oks M, Nchinda G, Yamazaki S, Steinman RM. Dendritic cell targeting of survivin protein in a xenogeneic form elicits strong CD4+ T cell immunity to mouse survivin. J Immunol. 2006;177:8410–21. doi: 10.4049/jimmunol.177.12.8410. [DOI] [PubMed] [Google Scholar]
- Soares H, Waechter H, Glaichenhaus N, et al. A subset of dendritic cells induces CD4+ T cells to produce IFN-γ by an IL-12-independent but CD70-dependent mechanism in vivo. J Exp Med. 2007;204:1095–106. doi: 10.1084/jem.20070176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang B, Kuroiwa JMY, He LZ, Charalambous A, Keler T, Steinman RM. The human cancer antigen mesothelin is more efficiently presented to the mouse immune system when targeted to the DEC-205/CD205 receptor on dendritic cells. Ann N Y Acad Sci. 2009;1174:6–17. doi: 10.1111/j.1749-6632.2009.04933.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurer C, Strowig T, Brilot F, et al. Targeting the nuclear antigen 1 of Epstein–Barr virus to the human endocytic receptor DEC-205 stimulates protective T-cell responses. Blood. 2008;112:1231–9. doi: 10.1182/blood-2008-03-148072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawiger D, Masilamani RF, Bettelli E, Kuchroo VK, Nussenzweig MC. Immunological unresponsiveness characterized by increased expression of CD5 on peripheral T cells induced by dendritic cells in vivo. Immunity. 2004;20:695–705. doi: 10.1016/j.immuni.2004.05.002. [DOI] [PubMed] [Google Scholar]
- Bonifaz L, Bonnyay D, Mahnke K, Rivera M, Nussenzweig MC, Steinman RM. Efficient targeting of protein antigen to the dendritic cell receptor DEC-205 in the steady state leads to antigen presentation on major histocompatibility complex class I products and peripheral CD8+ T cell tolerance. J Exp Med. 2002;196:1627–38. doi: 10.1084/jem.20021598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kretschmer K, Apostolou I, Hawiger D, Khazaie K, Nussenzweig MC, Von Boehmer H. Inducing and expanding regulatory T cell populations by foreign antigen. Nat Immunol. 2005;6:1219–27. doi: 10.1038/ni1265. [DOI] [PubMed] [Google Scholar]
- Mahnke K, Qian Y, Knop J, Enk AH. Induction of CD4+/CD25+ regulatory T cells by targeting of antigens to immature dendritic cells. Blood. 2003;101:4862–9. doi: 10.1182/blood-2002-10-3229. [DOI] [PubMed] [Google Scholar]
- Ring S, Maas M, Nettelbeck DM, Enk AH, Mahnke K. Targeting of autoantigens to DEC205+ dendritic cells in vivo suppresses experimental allergic encephalomyelitis in mice. J Immunol. 2013;191:2938–47. doi: 10.4049/jimmunol.1202592. [DOI] [PubMed] [Google Scholar]
- Nchinda G, Kuroiwa J, Oks M, et al. The efficacy of DNA vaccination is enhanced in mice by targeting the encoded protein to dendritic cells. J Clin Invest. 2008;118:427–36. doi: 10.1172/JCI34224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao J, Jin Y, Li W, et al. DNA vaccines targeting the encoded antigens to dendritic cells induce potent antitumor immunity in mice. BMC Immunol. 2013;14:1. doi: 10.1186/1471-2172-14-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ettinger M, Gratz IK, Gruber C, et al. Targeting of the hNC16A collagen domain to dendritic cells induces tolerance to human type XVII collagen. Exp Dermatol. 2012;21:395–8. doi: 10.1111/j.1600-0625.2012.01474.x. [DOI] [PubMed] [Google Scholar]
- Tenbusch M, Ignatius R, Nchinda G, et al. Immunogenicity of DNA vaccines encoding Simian immunodeficiency virus antigen targeted to dendritic cells in rhesus macaques. PLoS ONE. 2012;7:1–9. doi: 10.1371/journal.pone.0039038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park SH, Cheong C, Idoyaga J, et al. Generation and application of new rat monoclonal antibodies against synthetic FLAG and OLLAS tags for improved immunodetection. J Immunol Methods. 2008;331:27–38. doi: 10.1016/j.jim.2007.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aricescu AR, Lu W, Jones EY. A time- and cost-efficient system for high-level protein production in mammalian cells. Acta Crystallogr D Biol Crystallogr. 2006;62(Pt 10):1243–50. doi: 10.1107/S0907444906029799. [DOI] [PubMed] [Google Scholar]
- Tenbusch M, Nchinda G, Storcksdieck Genannt Bonsmann M, Temchura V, Uberla K. Targeting the antigen encoded by adenoviral vectors to the DEC205 receptor modulates the cellular and humoral immune response. Int Immunol. 2015;25:247–58. doi: 10.1093/intimm/dxs112. [DOI] [PubMed] [Google Scholar]
- Morgan DJ, Liblau R, Scott B, Fleck S, McDevitt HO, Sarvetnick N, Lo D, Sherman L. CD8+ T cell-mediated spontaneous diabetes in neonatal mice. J Immunol. 1996;157:978–83. [PubMed] [Google Scholar]
- Kirberg J, Baron A, Jakob S, Rolink A, Karjalainen K, von Boehmer H. Thymic selection of CD8+ single positive cells with a class II major histocompatibility complex-restricted receptor. J Exp Med. 1994;180:25–34. doi: 10.1084/jem.180.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tenbusch M, Grunwald T, Niezold T, Storcksdieck Genannt Bonsmann M, Hannaman D, Norley S, Überla K. Codon-optimization of the hemagglutinin gene from the novel swine origin H1N1 influenza virus has differential effects on CD4+ T-cell responses and immune effector mechanisms following DNA electroporation in mice. Vaccine. 2010;28:3273–7. doi: 10.1016/j.vaccine.2010.02.090. [DOI] [PubMed] [Google Scholar]
- Stab V, Nitsche S, Niezold T, et al. Protective efficacy and immunogenicity of a combinatory DNA vaccine against influenza A virus and the respiratory syncytial virus. PLoS ONE. 2013;8:e72217. doi: 10.1371/journal.pone.0072217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters M, Kauth M, Schwarze J, et al. Inhalation of stable dust extract prevents allergen induced airway inflammation and hyperresponsiveness. Thorax. 2006;61:134–9. doi: 10.1136/thx.2005.049403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trumpfheller C, Caskey M, Nchinda G, et al. The microbial mimic poly IC induces durable and protective CD4+ T cell immunity together with a dendritic cell targeted vaccine. Proc Natl Acad Sci U S A. 2008;105:2574–9. doi: 10.1073/pnas.0711976105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosalia RA, Quakkelaar ED, Redeker A, et al. Dendritic cells process synthetic long peptides better than whole protein, improving antigen presentation and T-cell activation. Eur J Immunol. 2013;43:2554–65. doi: 10.1002/eji.201343324. [DOI] [PubMed] [Google Scholar]
- Shedlock DJ, Weiner DB. DNA vaccination: antigen presentation and the induction of immunity. J Leukoc Biol. 2000;68:793–806. [PubMed] [Google Scholar]
- Kutzler MA, Weiner DB. DNA vaccines: ready for prime time? Nat Rev Genet. 2008;9:776–88. doi: 10.1038/nrg2432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sardesai NY, Weiner DB. Electroporation delivery of DNA vaccines?: prospects for success. Curr Opin Immunol. 2011;23:421–9. doi: 10.1016/j.coi.2011.03.008. Elsevier Ltd; [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JJ, Maguire HC, Nottingham LK, Morrison LD, Tsai A, Sin JI, Chalian AA, Weiner DB. Coadministration of IL-12 or IL-10 expression cassettes drives immune responses toward a Th1 phenotype. J Interferon Cytokine Res. 1998;18:537–47. doi: 10.1089/jir.1998.18.537. [DOI] [PubMed] [Google Scholar]
- Stobie L, Gurunathan S, Prussin C, Sacks DL, Glaichenhaus N, Wu CY, Seder RA. The role of antigen and IL-12 in sustaining Th1 memory cells in vivo: IL-12 is required to maintain memory/effector Th1 cells sufficient to mediate protection to an infectious parasite challenge. Proc Natl Acad Sci U S A. 2000;97:8427–32. doi: 10.1073/pnas.160197797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda K, Dow SW, Miyahara N, et al. Vaccine-induced CD8+ T cell-dependent suppression of airway hyperresponsiveness and inflammation. J Immunol. 2009;183:181–90. doi: 10.4049/jimmunol.0803967. [DOI] [PubMed] [Google Scholar]
- Trujillo-Vargas CM, Mayer KD, Bickert T, et al. Vaccinations with T-helper type 1 directing adjuvants have different suppressive effects on the development of allergen-induced T-helper type 2 responses. Clin Exp Allergy. 2005;35:1003–13. doi: 10.1111/j.1365-2222.2005.02287.x. [DOI] [PubMed] [Google Scholar]
- Frew AJ. Allergen immunotherapy. J Allergy Clin Immunol. 2010;125(2 Suppl 2):S306–13. doi: 10.1016/j.jaci.2009.10.064. Elsevier Ltd; [DOI] [PubMed] [Google Scholar]
- Chua KY, Huangfu T, Liew LN. DNA vaccines and allergic diseases. Clin Exp Pharmacol Physiol. 2006;33:546–50. doi: 10.1111/j.1440-1681.2006.04405.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Expression and DEC205-specific binding of the scFv fusion proteins encoded by the DNA vaccines. Characterization of the DNA vaccines.
Figure S2. Enhanced antigen-presentation by CD11c+ and not by CD11c- cells after DNA immunization with pV-DEC-solHA is indicated by increased number of proliferating TCR-HA cells.