Abstract
The hallmark of chlamydial infection is the development of upper genital pathology in the form of hydrosalpinx and oviduct and/or tubal dilatation. Although molecular events leading to genital tissue presentation and cellular architectural remodelling are unclear, early-stage host immune responses are believed to contribute to these long-term sequelae. Recently, we reported the contribution of selected infection-associated microRNAs (miRs) in the generation of host immunity at early-stage infection (day 6 after intravaginal Chlamydia muridarum challenge in C57BL/6 mice). In this report, we describe the contribution of an infection-associated microRNA, i.e. miR-214, to host immunity. Chlamydia muridarum infection in the C57BL/6 mouse genital tract significantly down-regulated miR-214 while up-regulating intracellular adhesion molecule 1 (ICAM1) gene expression. These in vivo observations were confirmed by establishing direct regulation of ICAM-1 by miR-214 in ex vivo genital cell cultures in the presence of miR-214 mimic and inhibitor. Because, ICAM-1 contributes to recruitment of neutrophils following infection, we also demonstrated that alteration of ICAM1 by miR-214 in interleukin-17A-deficient (IL-17A−/−) mice correlated with reduction of neutrophils infiltrating genital tissue at day 6 after challenge. Additionally, these early-stage events resulted in significantly decreased genital pathology in IL-17A−/− mice compared with C57BL/6 mice. This report provides evidence for early-stage regulation of ICAM1 by microRNAs, resulting in reduction of genital pathology associated with chlamydial infection.
Keywords: Chlamydia muridarum, genital pathology, host responses, intracellular adhesion molecule-1, microRNA-214, murine genital tract
Introduction
Genital Chlamydia trachomatis is the leading cause of sexually transmitted infections globally, and is the most commonly reported sexually transmitted infection in the USA.1 The increase in number of C. trachomatis-positive individuals has been attributed to unavailability of a vaccine, lack of organized healthcare programmes, asymptomatic nature of infection (∼75% in women and ∼50% in men), and significant under-reporting of symptomatic cases.2 In infected women, anti-chlamydial immune response results in immunopathology in the uterus and fallopian tube, and subsequently as pelvic inflammatory disease, ectopic pregnancy and infertility.3
Using several genital C. trachomatis animal models (mice, guinea pigs and non-human primates)1,4 it has been established that intravaginal infection of epithelial cells ascends in non-homogeneous fashion to the upper regions of the genital tract, including the uterine horns and oviducts. A single inoculation of Chlamydia muridarum in mice results in vaginal shedding of viable inclusion-forming units (IFUs) up to approximately 24–30 days after challenge with moderate to severe hydrosalpinx formation, and tubal dilatation in uterine horns and/or oviducts.5 Chlamydial infection in genital epithelial cells leads to chemokine–cytokine cascades resulting in targeting of neutrophils, macrophages and T cells to the mucosa.6 It has been speculated that the difference in early-stage anti-chlamydial immunity (i.e. cell activation, cytokine/chemokine profiles) may significantly contribute to genital pathology and reproductive sequelae in infected mice.7,8
Recently, we reported alteration of host microRNA (miR) profiles during the early stage (day 6) of C. muridarum infection.9 These short, non-coding RNA species modulate gene function post-transcriptionally by direct binding to target gene mRNA,10–12 influencing biological processes including immune function13–15 and reproduction,16–19 and constitute potential biomarkers for genital C. trachomatis infection in humans.20 In an attempt to identify potential immune molecule targets for chlamydial pathogenesis-associated miRs,9 in silico bioinformatics yielded several gene targets including possible regulation of intracellular adhesion molecule 1 (ICAM1) gene expression by miR-214. The miR-214 has been reported to be important in cardiac hypertrophy,21 and in cancer progression by inhibiting apoptosis.22 However, the role of miR-214 in infectious diseases has not been established. The putative miR-214 target gene, i.e. ICAM1 is an immunoglobulin-like cell adhesion molecule and involves transmigration of leucocytes into inflamed tissues and initiation of host immunity.23,24 ICAM1 gene expression was significantly elevated in genital tract tissues as early as day 7 after C. muridarum challenge, peaking at day 21, and gradually decreasing while the host continued to clear infection.25 Up-regulation of ICAM-1 on genital endothelial, stromal and epithelial cells facilitated recruitment of circulating memory T cells to the infection site.26 Increased ICAM1 mRNA levels also correlated with massive infiltration of neutrophils to the lung following intranasal inoculation with C. muridarum.27 Furthermore, ICAM-1-mediated interaction of chlamydial antigen-specific CD4+ and CD8+ T cells in Chlamydia-infected epithelial and fibroblast cell lines enhanced bacterial killing through activation of the inducible nitric oxide synthase pathway28 and cytolysis,29 respectively. In this study, we extend our previous findings on C. muridarum-associated murine miR-214,9 and demonstrate regulation of ICAM1 expression in the infected genital tract. To the best of our knowledge, this is the first report on regulation of ICAM1 through miR-214 in the C. muridarum-infected genital tract, suggesting a role for miRs in early immunological events following infection.
Materials and methods
Chlamydia muridarum stocks
Seed stocks of C. muridarum were propagated in HeLa 229 carcinoma cells. At 24 hr after C. muridarum challenge, HeLa cells were mechanically dislodged using glass beads. Cells were collected in a 50-ml Falcon tube containing five or six glass beads, vortexed for 10 min while on ice, and the cellular lysate was centrifuged for 10 min at 900 g at 4°. The resulting supernatant was decanted and subjected to centrifugation at 27 000 g for 1 hr at 4°, and the bacterial pellet material was further purified on a Renografin gradient as previously described.30
Mice
All procedures were carried out in compliance with the University of Texas at San Antonio Institutional Animal Care and Use Committee guidelines. Female, 4- to 6-week-old wild-type C57BL/6 (WT) mice were purchased from Jackson Laboratory (Bar Harbor, ME). Interleukin-17A-deficient (IL-17A−/−) mice31 (C57BL/6 genetic background) were kindly provided by Dr Yoichiro Iwakura of the University of Tokyo, and bred at the University of Texas at San Antonio.
Intravaginal challenge
To render mice anoestrous and more receptive to the genital infection, animals were injected subcutaneously. with 2·5 mg Depo progesterone (Depo-Provera; Pharmacia & Upjohn Co., New York, NY) 5 days before challenge. Animals were intravaginally infected with purified C. muridarum stock diluted in 15 μl sucrose/phosphate/glutamate buffer at a dose of 5 × 104 IFU. Cervical–vaginal bacterial shedding was monitored every 3 days after challenge.
RNA extraction and real-time PCR
Total RNA was extracted from snap-frozen genital tract samples at days 6 and 15 post challenge. Frozen lower genital tract tissue was crushed using a sterile mortar and pestle on liquid nitrogen. RNA was obtained using an miRNeasy RNA extraction Kit (Qiagen Valencia, CA) according to the manufacturer's instructions. Total RNA was assessed using a Nanodrop Spectrophotometer (ThermoScientific, Asheville, NC). RNA samples exhibiting A260/280 ≅ 2·0 and A260/230 ≅ 1·8 were converted to cDNA using an RT2 First strand cDNA Kit (Qiagen) according to the manufacturer's instructions. RNA (1 μg) was used in all miR PCR amplifications and they were performed using custom-designed miScript Primers (Qiagen) according to manufacturer's instructions. All miR expression analyses were normalized to RNU6-2_1 or SNORD68 expression values.9 For differential gene expression using the T- and B-cell activation RT2 Profiler PCR Array (Qiagen), RNA (160 ng) was extracted from genital tract tissue samples of WT and IL-17−/− mice using an RNeasy RNA extraction kit (Qiagen), and converted to cDNA using the RT2 First strand kit per the manufacturer's instructions and a DNA Engine Opticon 2 continuous fluorescence detection system. For ICAM1, CXCL1, and CXCL2 expression, RNA (1 μg) was converted to cDNA using an iScript™ cDNA Synthesis Kit followed by real-time PCR and the SsoAdvanced™ Universal SYBR® Green Supermix (Bio-Rad, Hercules, CA) with gene-specific primer pair (PrimePCR™ SYBR® Green Assay, BioRad, UniqueAssay ID: ICAM1 qMmuCID0005575; CXCL-1 qMmuCED0047655; CXCL-2 qMmuCED0003897) on a CFX 96 instrument (Bio-Rad). All miR and mRNA expression levels were normalized to the housekeeping gene RNU6-2_19 and heat-shock protein 90, respectively, and expressed relative to the level in the mock sample as indicated in the Results and figure legends using the comparative cycle threshold method.32
Primary culture of mouse genital cells
Thin collagen (collagen I, rat tail; BD, San Jose, CA) coating was prepared in 24-well plates 2–3 hr before genital tract tissue isolation per the manufacturer's instruction. Upper regions of the cervix, and lower regions of the uterine horns were excised from mock-infected mice, and cut into small pieces using fine scissors and forceps. The cut tissue sections were treated with 2 ml Collagenase XI (1 mg/ml in Hanks' balanced salt solution (HBSS; Sigma, St. Louis, MO) for 35 min at 37° while stirring. Collagenase containing HBSS was decanted, and tissues were subsequently immersed in D-10 (Dulbecco's modification Eagle's medium supplemented with 10% heat-inactivated fetal bovine serum), and crushed using a 70-μm nylon cell strainer for single cell isolation. Cells were centrifuged (900 g at 4° for 10 min), and resuspended in enriched D-10 medium (10% L929 supernatant, 1 mm sodium pyruvate, 2 mm l-glutamine, 1% penicillin/streptomycin and 100 μm non-essential amino acids). Viable cells were counted using trypan blue dye, seeded at a density of 5 × 105 per well on collagen-coated wells, and incubated for 15 hr.
In vitro transfection of microRNA mimic and inhibitor
Mouse genital cells were seeded at a density of 5 × 105 per well, and miR-214 agomiR (MiScript miRNA Mimics; Qiagen), and antagomiR (MiScript miRNA Inhibitor) were transfected using Attractene as transfection reagent at a concentration of 20 μm by fast forward transfection per the manufacturer's recommendation (Qiagen). Transfection was carried out for 18 hr followed by C. muridarum challenge (multiplicity of infection of 1). To test the direct effects of IL-17A on miR-214 expression, endotoxin-free murine recombinant IL-17A (10 ng/ml; Peprotech, Rocky Hill, NJ) was added to cells 2 hr before C. muridarum challenge.33 Cells were harvested using Qiazol 24 hr post challenge, and RNA was extracted per the manufacturer's instructions (Qiagen).
Flow cytomtery analysis
WT and IL-17A−/− genital tract tissues, i.e. vagina/cervix (lower genital tract), and uterus/uterine horns (upper genital tract) were removed at designated time-points as previously described.34 Cells were stained with fluorochrome-conjugated CD4, F4/80 and Gr-1-labelled antibodies (Biolegend, San Diego, CA), and flow cytometry analysis was performed using an LSR II instrument (BD Biosciences, San Jose, CA).34
Assessment of gross pathology in the upper genital tract and histopathology
Mice were sacrificed at day 80 post-challenge, genital tract tissues were collected, and the presence of gross hydrosalpinx was noted. Tissues were photographed at a fixed distance (six mega-pixels using a Fuji F10 camera, Fujifilm, Tokyo, Japan). Images were stored at high resolution and printed (A4 size). The cross-sectional diameter of the oviduct was measured in individual mice, and the mean ± standard deviation (SD) for each group was determined as previously described.34
In silico and statistical analyses
Bioinformatic analysis for putative binding sites in ICAM1 observed to be modulated by miR-214 was performed using miR target predictive algorithms: www.microRNA.org (Memorial Sloan-Kettering Cancer Center, New York, NY), and www.targetscan.org (Whitehead Institute for Biomedical Research, Cambridge, MA). The miR analysis was performed using the RT2 Profiler PCR Array Data Analysis (version 3.5; Qiagen). All experimental results were calculated on the mean ± SD of independent experiments. Student's t-test was used for comparison between two groups. Differences were considered statistically significant if P values were < 0·05. These statistical analyses were conducted using the GraphPad Prism 5 software package (La Jolla, CA).
Results
Regulation of murine miR-214 and ICAM1 expression in C. muridarum-infected genital tract
Previously, we demonstrated that miR-214 is significantly down-regulated in C. muridarum-infected C57BL/6 (WT) genital tract tissue at day 6 post challenge.9 In this report, we further identify potential miR-214 targets by in silico screening of perspective host gene binding sites previously reported to be critical in the immune response to initial genital chlamydial infection.6 One of the putative gene targets for miR-214 is ICAM1, as shown in Fig.1(a). Typically, C57BL/6 mice resolve genital C. muridarum infection by day 30 post challenge.35 Real-time PCR analyses during the course of infection revealed significant down-regulation (> 50% reduction) of miR-214 in C. muridarum-infected WT genital tract tissue excised at 6 and 15 days post challenge (Fig.1b, upper panel). In contrast, ICAM1 expression was observed significantly up-regulated (more than twofold and fourfold at days 6 and 15, respectively) compared with mock-infected mice (Fig.1b, lower panel) suggesting an inverse correlation between miR-214 and ICAM1 expression (down-regulation of miR-214 and up-regulation of ICAM1 in C. muridarum-infected genital tract tissue). Genital tract tissue excised from mice at 30 (Fig.1b), 55 and 80 (not shown) days post challenge (representative time-points for chlamydial clearance and pathological stage presentation in mice5,36,37) revealed a similar but not statistically significant trend.
Figure 1.
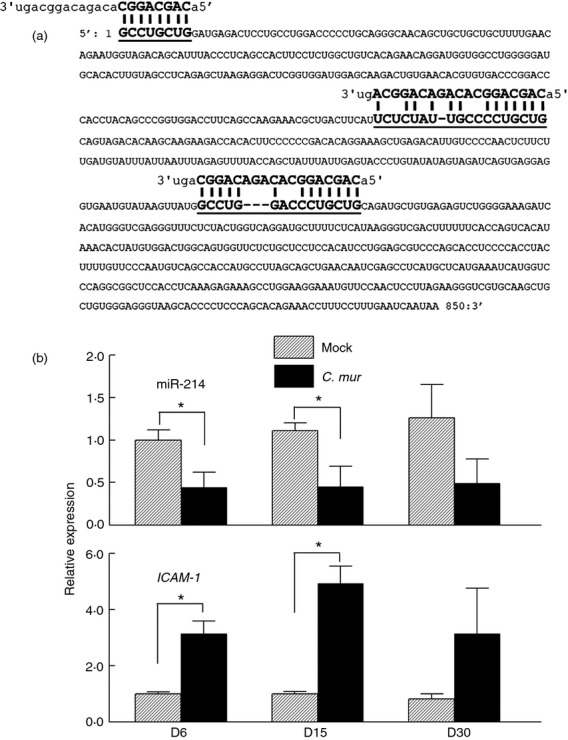
Increased intercellular adhesion molecule 1 (ICAM1) gene expression is correlated with down-regulation of microRNA-214 (miR-214) in Chlamydia muridarum-infected mice. (a) Bioinformatic analysis using algorithms by www.microRNA.org and www.targetscan.org revealed miR-214 putative binding sites in the ICAM1 gene. (b) C57BL/6 (wild-type; WT) mice were challenged intravaginally with 5 × 104 inclusion-forming units (IFU) or treated with sucrose/phosphate/glutamate buffer (mock). Genital tract tissue was excised at days 6, 15 and 30 post challenge. RNA was extracted, converted to cDNA, and real-time PCR was performed to determine relative miR-214 and ICAM1 mRNA levels to D6 mock control. *P < 0·05 (Student's t-test) for C. muridarum-infected group compared with mock group. Data are representative of two individual experiments.
To demonstrate a ‘cause and effect’ relationship, ICAM1 gene expression was determined in ex vivo cultured genital cells transfected with miR-214 mimics and inhibitors (Fig.2). ICAM1 expression was significantly up-regulated (approximately 40% increase) in untransfected genital cells 24 hr after C. muridarum inoculation, similar to in vivo infection (Fig.1b). Transfection with miR-214 inhibitors that compete with endogenous miR-214 in genital cells resulted in higher ICAM1 expression (Fig.2). In contrast, genital cells transfected with miR-214 mimics exhibited a small (15%), but statistically significant (P < 0·05) reduction of ICAM1 mRNA. Genital cells transfected with controls, i.e. scrambled-miRs, exhibited similar ICAM-1 mRNA levels compared with untransfected and non-infected cells. Collectively, these in vivo and ex vivo results demonstrate that genital tract ICAM1 expression is regulated by miR-214 following genital C. muridarum infection.
Figure 2.
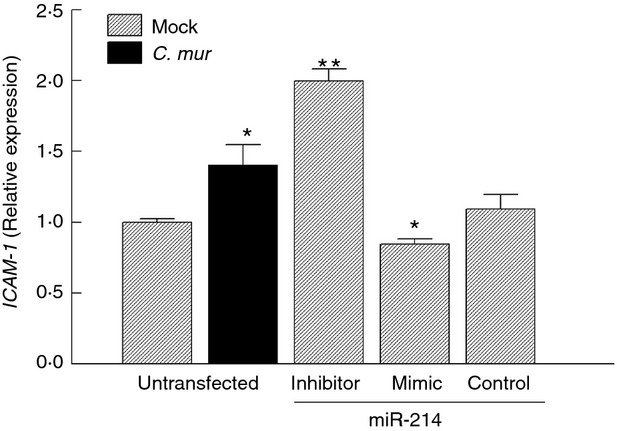
Intercellular adhesion molecule 1 (ICAM1) expression is regulated by microRNA 214 (miR-214) in Chlamydia muridarum-infected murine genital cells. Genital tract tissue from naive C57BL/6 (wild-type; WT) mice was excised and cultured ex vivo. Cells were seeded at a density of 2·5 × 105 and transfected with miR-214 inhibitor, mimic, or scrambled miRs (control). Untransfected genital cells were infected with multiplicity of infection 1 of C. muridarum elementary bodies (resuspended in medium) or incubated with medium alone (mock). ICAM1 expression was assessed by real-time PCR at 24 hr post challenge and expressed as relative value to untransfected mock group. *P < 0·05, **P < 0·01 (Student's t-test) compared with untransfected mock group. Data are representative of three individual experiments.
Regulation of miR-214/ICAM-1 contributes to neutrophil infiltration and upper genital pathology in C. muridarum-infected mice
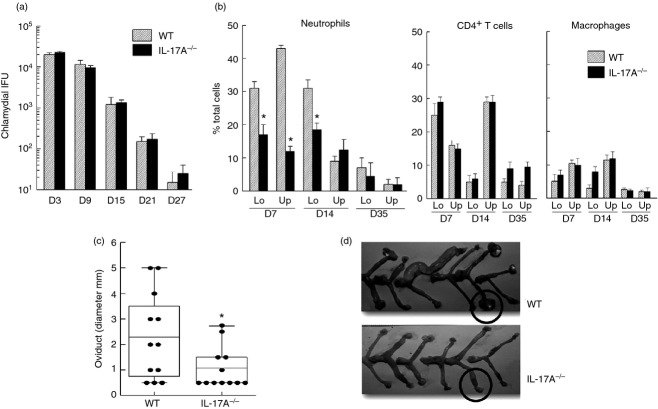
To further characterize the plausible effect of miR-214-mediated ICAM1 expression on chlamydial pathogenesis, we used IL-17A-deficient mice, a cytokine known to regulate ICAM-1 production in primary lung epithelial38 and heart endothelial39 cells. Additionally, IL-17A has been reported to play a role in development of upper genital pathology following primary C. muridarum infection.40 We analysed miR-214 and ICAM1 gene expression in genital tract tissues of IL-17A−/−, and WT mice at day 6 post challenge. As shown in Fig.3(a), under non-infection conditions (mock), miR-214 transcription was significantly higher, whereas ICAM1 transcription in IL-17A−/− animals was lower than that observed in WT animals, indicating an intrinsic difference in gene expression between these two mouse strains. However, the inverse correlation of miR-214 and ICAM-1 was still evident. Infection-induced miR-214 down-regulation, and concomitant ICAM1 up-regulation were observed in WT mice 6 days post C. muridarum challenge. However, this inverse relationship was noticeably absent in IL-17A-deficient animals suggesting that miR-214-regulated ICAM1 expression may be mediated by IL-17A during chlamydial infection. In contrast, increased transcription of CXCL-1 and CXCL-2 (two important chemokines for neutrophil and monocyte/macrophage recruitment) reported in murine oviduct epithelial cells following C. muridarum infection by Johnson41 and observed by our laboratory as well was independent of IL-17A (Fig.3b). To further characterize genital Chlamydia pathogenesis germane to reciprocal miR-214/ICAM1 levels, i.e. WT versus IL-17A−/− mice, we analysed populations of immune cells known to be recruited/retained by ICAM-1 at the infection sites. Chlamydial shedding kinetics in IL-17A−/− animals was similar to WT (Fig.4a). However, flow cytometry analysis of lower and upper regions of C. muridarum-infected IL-17A−/− and WT genital tract tissue revealed a lower (P < 0·05) population of Gr-1+ cells (neutrophils) in IL-17A−/− genital tract tissue compared with WT at days 7 and 14 but not 35 post challenge (Fig.4b). In contrast, composition of F4/80+ (macrophages) and CD4+ (T cells) populations was comparable between WT and IL-17A−/− mice at all time-points examined (days 7, 14 and 35 post challenge; (Fig.4b). Additionally, we observed reduced upper genital pathology in IL-17A−/− mice with less oviduct dilatation (Fig.4c), and hydrosalpinx formation (Fig.4d) at day 80 post challenge. Taken together, these data suggest genital chlamydial infection down-regulation of miR-214 results in higher ICAM1 expression leading to enhanced neutrophil infiltration and pathology. This associated miR-214-mediated response was further supported by the IL-17A−/− chlamydial infection model demonstrating when infection failed to down-regulate miR-214, fewer viable ICAM1 transcripts were available to WT mice (Fig.3a), possibly due to miR-214-mediated degradation resulting in decreased neutrophil infiltration and reduced pathology.
Figure 3.
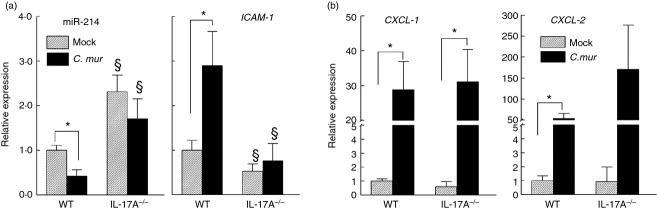
Interleukin-17A (IL-17A) contributes to microRNA (miR) -214 and intercellular adhesion molecule 1 (ICAM1) gene modulation in Chlamydia muridarum-infected genital tract tissue. C57BL/6 wild-type (WT) and IL-17A-deficient (IL17A−/−) mice (n = 3) were infected intravaginally with 5 × 104 inclusion-forming units (IFU) or were treated with sucrose/phosphate/glutamate buffer (mock). Respective reproductive tracts were excised at day 6 post challenge and sectioned. RNA from lower genital tract tissue was extracted, converted to cDNA, and real-time PCR was performed to determine (a) relative miR-214 and ICAM1, and (b) CXCL1 and CXCL2 mRNA levels compared with WT mock control. Student's t-test was used for statistical analyses between two tested groups. *P < 0·05, comparison of mock and infected groups. §P < 0·05, comparison between WT and IL-17A−/− with respective treated groups (i.e. mock versus mock, C. mur versus C. mur). Data are representative of two individual experiments.
Figure 4.
Chlamydia muridarum-associated upper genital pathology is reduced in interleukin-17A-deficient (IL17A−/−) mice. Groups (n = 6) of C57BL/6 (wild-type; WT) and IL17A−/− mice were challenged with 5 × 104 inclusion-forming units (IFU) of C. muridarum on day 0. (a) Bacterial shedding was assessed at designated time-points. (b) Neutrophils CD4+ T cell, and macrophage frequency in lower (Lo) and upper (Up) genital tract tissue of WT and IL17A−/− mice was analysed by flow cytometry at designated time-points. Infection-induced pathology was assessed at day 80 post challenge by (c) measurement of oviduct diameter (dilatation) and (d) documentation of gross pathology (hydrosalpinx formation circled in WT). *P < 0·05 (Student's t-test) between C. muridarum infected WT and IL-17A−/− mice.
Discussion
Overall, this study provides an association between a specific miR (miR-214), and its target gene (ICAM1) in early stage C. muridarum infection. This study not only provides evidence of miR-214-dependent regulation of ICAM1, which probably contributes to infiltration of neutrophils and pathology, but also augments the body of evidence supporting the regulatory role of miRs in Chlamydia pathogenesis.9,20,42–44 Possible ICAM1 regulation by miR-214 was first suggested in C. muridarum-infected genital tract tissues (Fig.1b), but later confirmed in miR-214-modulated primary genital cells (Fig.2). Cultured primary genital cells (Fig.2) were used to investigate a ‘regulatory’ relationship between miR-214 and ICAM1. Primary genital cell cultures are comprised mainly of epithelial cells, in addition to macrophages, neutrophils and T cells. Epithelial cells are the primary target cells for C. muridarum infection in vivo. The ex vivo model described here mimicked the natural infection site of genital C. muridarum and would provide information on the contribution of miR-214 to chlamydial pathogenesis in vivo. Although several putative miR-214 binding sites, i.e. in the 3′ untranslated region of ICAM1 mRNA, were identified by in silico prediction, specific binding assays such as those described by Wu et al.45 are needed to verify that ICAM1 mRNA degradation arises from miR-214 binding. Regardless of the underlying mechanisms, i.e. by direct miR-214 ICAM1 binding or indirect inhibition, data reported here clearly demonstrate that increase of miR-214 leads to reduced ICAM1 gene expression (Fig.2).
The IL-17−/− mice were used instead of miR-214−/− to decipher the effect of miR-214-mediated ICAM1 expression on chlamydial pathogenesis. Not using the existing miR-214-deficient mouse46 was premised on the fact that C. muridarum infection would be miR-214 down-regulated (not up-regulated). Hence miR-214 knockout mouse is of less value to this specific study because the phenotype of the knockout and WT would probably be comparable, if not identical. following challenge. Although, the IL-17A−/− mouse is not the only infection model applicable to this study, we consider it to be the most suitable alternative to miR-214−/− based upon two important factors: IL-17A regulates ICAM-1,38,39 and has been shown to play a role in Chlamydia pathogenesis.40 Importantly, decreased ICAM1 transcripts in infected IL-17A−/− genital tract tissues coincided with higher miR-214 copies. Hence, IL-17A deficiency alters miR-214 expression following C. muridarum infection, which provides important insight into the effect of miR-214-mediated ICAM1 expression by identifying differences in the immune response(s) and disease between the knockout and WT mice. Regulation of miR-214 in human autoimmune diseases such as multiple sclerosis and systemic lupus erythematosus and associated mouse models has been reported.47–49 Given that IL-17A and its family members contribute to inflammation and pathogenesis in these disease models,50 and that molecules like CD47 and nuclear factor-κB are regulated by miR-214,48,49,51 further investigation into the role of these molecules to chlamydial pathogenesis is warranted. Importantly, the regulatory role of IL-17A in CD47-mediated neutrophil migration has been established.52
The influence of IL-17 in inflammation-associated neutrophil recruitment has been well documented.53 The significant decrease of neutrophil population in C. muridarum-infected IL-17A−/− (C57BL/6 background) genital tissues is consistent with similar findings in IL-17 receptor A-deficient mice54 and IL-17A−/− in BALB/c background.40 Along with the report of increased neutrophil infiltration associated with ICAM-1 expression following C. muridarum respiratory infection,27 we now propose a novel mechanism that IL-17 down-regulation of miR-214 leads to ICAM1 up-regulation, subsequently enhancing neutrophil infiltration to the infection site. To this end, we found significant down-regulation of miR-214 and up-regulation of ICAM1 in WT genital cells supplemented with murine recombinant IL-17A and subsequently infected with C. muridarum compared with C. muridarum infection alone (see Supplementary material, Fig. S1), corroborating regulation of miR-214/ICAM1 in WT and IL-17A−/− mice (Fig.3a). Despite these findings, further investigation using luciferase reporter assays in genital cell cultures, or HeLa or HEK cell lines is required to determine the direct role(s) of IL-17A, miR-214 in regulating ICAM1. Although not the focus in this study, to further establish the direct link between IL-17 and miR-214, e.g. to compare and contrast the miR-214 expression in recombinant IL-17A-activated IL-17RA sufficient and deficient T cells. Overall, data reported here strongly suggest that miR-214/ICAM1 mediates neutrophil infiltration in the early stages of genital chlamydial infection.
Reduction in upper genital pathology has been attributed to reduced infiltration of neutrophils.40,55 Release of matrix metalloproteinases during movement of neutrophils results in epithelial layer disruption, architectural remodelling, and upper genital pathology in WT55–57 and IL-17A−/− mice.40 We observed significantly reduced neutrophil infiltration (Fig.4b), and upper genital pathology (Fig.4c,d) in IL-17A−/− mice compared with WT mice at day 6 post challenge similar to findings reported by Andrew et al.40 In contrast, no differences in bacterial shedding (Fig.4a) or CD4+ T-cell and macrophage populations (Fig.4b) were observed using C57BL/6 mice background knockou and WT mice (in this study) compared with BALB/c mice in the published report.40 We speculate that these differences could to be the result of differential susceptibility and host responses of murine strains infected with C. muridarum.7,58 Collectively, miR-214/ICAM1-mediated early neutrophil infiltration may be a critically needed event for subsequent development of genital pathology in Chlamydia infection.
Pathological sequelae in Chlamydia-infected mice arise from orchestrated interaction between several early-stage molecules. Using a predesigned panel comprising 84 genes involved in cellular migration, B-cell and T-cell activation, we observed significant up-regulation of Thy1.1, and down-regulation of IL1b, IL12b, SOCS1, TLR4 and ICAM1 in IL-17A−/− mice when compared with WT mice following C. muridarum infection. The contribution of IL-1β, IL-12β and Toll-like receptor-4 in causation of genital pathology has been previously reported.59–62 Current investigation on miRs regulating these genes in IL-17A−/− mice (data not shown) will provide needed additional insight into miR–gene interaction(s) and their contribution to chlamydial pathogenesis.
Author contributions
TA carried out all experiments including bacterial inoculum preparation, cell line experiments and real-time microRNA PCR amplification. WL performed IL-17A flow cytometry, shedding and pathology experiments. SW assisted in carrying out the experiments. RG, JJY and BPA conceptualized the project and analysed the data and was assisted by TA. RG, JJY, NG, JPC, LKC and BPA drafted and edited the manuscript. All authors have read and approved the final manuscript.
Acknowledgments
This work was supported by National Institutes of Health Grant (1RO1AI074860) and the Army Research Office, Department of Defense contract No. W911NF-11-1-0136.
Disclosures
The authors declare that they have no competing interests.
Supporting Information
Figure S1. Interleukin-17A contributes to microRNA (miR) -214 and intracellular adhesion molecule l 1 gene (ICAM1) modulation in Chlamydia muridarum-infected murine genital cells.
References
- Brunham RC, Rey-Ladino J. Immunology of Chlamydia infection: implications for a Chlamydia trachomatis vaccine. Nat Rev Immunol. 2005;5:149–61. doi: 10.1038/nri1551. [DOI] [PubMed] [Google Scholar]
- Gottlieb SL, Martin DH, Xu F, Byrne GI, Brunham RC. Summary: the natural history and immunobiology of Chlamydia trachomatis genital infection and implications for Chlamydia control. J Infect Dis. 2010;201(Suppl. 2):S190–204. doi: 10.1086/652401. [DOI] [PubMed] [Google Scholar]
- Gottlieb SL, Berman SM, Low N. Screening and treatment to prevent sequelae in women with Chlamydia trachomatis genital infection: how much do we know? J Infect Dis. 2010;201(Suppl. 2):S156–67. doi: 10.1086/652396. [DOI] [PubMed] [Google Scholar]
- De Clercq E, Kalmar I, Vanrompay D. Animal models for studying female genital tract infection with Chlamydia trachomatis. Infect Immun. 2013;81:3060–7. doi: 10.1128/IAI.00357-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farris CM, Morrison RP. Vaccination against Chlamydia genital infection utilizing the murine C. muridarum model. Infect Immun. 2011;79:986–96. doi: 10.1128/IAI.00881-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darville T, Hiltke TJ. Pathogenesis of genital tract disease due to Chlamydia trachomatis. J Infect Dis. 2010;201(Suppl. 2):S114–25. doi: 10.1086/652397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darville T, Andrews CW, Jr, Sikes JD, Fraley PL, Rank RG. Early local cytokine profiles in strains of mice with different outcomes from chlamydial genital tract infection. Infect Immun. 2001;69:3556–61. doi: 10.1128/IAI.69.6.3556-3561.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stagg AJ, Tuffrey M, Woods C, Wunderink E, Knight SC. Protection against ascending infection of the genital tract by Chlamydia trachomatis is associated with recruitment of major histocompatibility complex class II antigen-presenting cells into uterine tissue. Infect Immun. 1998;66:3535–44. doi: 10.1128/iai.66.8.3535-3544.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta R, Arkatkar T, Yu JJ, et al. Chlamydia muridarum infection associated host MicroRNAs in the murine genital tract and contribution to generation of host immune response. Am J Reprod Immunol. 2015;73:126–40. doi: 10.1111/aji.12281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carletti MZ, Fiedler SD, Christenson LK. MicroRNA 21 blocks apoptosis in mouse periovulatory granulosa cells. Biol Reprod. 2010;83:286–95. doi: 10.1095/biolreprod.109.081448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald JB, Chennathukuzhi V, Koohestani F, Nowak RA, Christenson LK. Role of microRNA-21 and programmed cell death 4 in the pathogenesis of human uterine leiomyomas. Fertil Steril. 2012;98:726–34. doi: 10.1016/j.fertnstert.2012.05.040. e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu LF, Liston A. MicroRNA in the immune system, microRNA as an immune system. Immunology. 2009;127:291–8. doi: 10.1111/j.1365-2567.2009.03092.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumjohann D, Kageyama R, Clingan JM, et al. The microRNA cluster miR-17 approximately 92 promotes TFH cell differentiation and represses subset-inappropriate gene expression. Nat Immunol. 2013;14:840–8. doi: 10.1038/ni.2642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroesen BJ, Teteloshvili N, Smigielska-Czepiel K, Brouwer E, Boots AM, van den Berg A, Kluiver J. Immuno-miRs: critical regulators of T-cell development, function and ageing. Immunology. 2015;144:1–10. doi: 10.1111/imm.12367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seddiki N, Brezar V, Ruffin N, Levy Y, Swaminathan S. Role of miR-155 in the regulation of lymphocyte immune function and disease. Immunology. 2014;142:32–8. doi: 10.1111/imm.12227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christenson LK. MicroRNA control of ovarian function. Anim Reprod. 2010;7:129–33. [PMC free article] [PubMed] [Google Scholar]
- Luense LJ, Carletti MZ, Christenson LK. Role of Dicer in female fertility. Trends Endocrinol Metab. 2009;20:265–72. doi: 10.1016/j.tem.2009.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nothnick WB. The role of micro-RNAs in the female reproductive tract. Reproduction. 2012;143:559–76. doi: 10.1530/REP-11-0240. [DOI] [PubMed] [Google Scholar]
- Pang RT, Liu WM, Leung CO, Ye TM, Kwan PC, Lee KF, Yeung WS. miR-135A regulates preimplantation embryo development through down-regulation of E3 Ubiquitin Ligase Seven In Absentia Homolog 1A (SIAH1A) expression. PLoS ONE. 2011;6:e27878. doi: 10.1371/journal.pone.0027878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeruva L, Myers GS, Spencer N, et al. Early microRNA expression profile as a prognostic biomarker for the development of pelvic inflammatory disease in a mouse model of chlamydial genital infection. MBio. 2014;5:e01241–14. doi: 10.1128/mBio.01241-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang T, Gu H, Chen X, Fu S, Wang C, Xu H, Feng Q, Ni Y. Cardiac hypertrophy and dysfunction induced by overexpression of miR-214 in vivo. J Surg Res. 2014;192:317–25. doi: 10.1016/j.jss.2014.06.044. [DOI] [PubMed] [Google Scholar]
- Zhang ZC, Li YY, Wang HY, Fu S, Wang XP, Zeng MS, Zeng YX, Shao JY. Knockdown of miR-214 promotes apoptosis and inhibits cell proliferation in nasopharyngeal carcinoma. PLoS ONE. 2014;9:e86149. doi: 10.1371/journal.pone.0086149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denucci CC, Mitchell JS, Shimizu Y. Integrin function in T-cell homing to lymphoid and nonlymphoid sites: getting there and staying there. Crit Rev Immunol. 2009;29:87–109. doi: 10.1615/critrevimmunol.v29.i2.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu XW, Gong JP. Expression and role of ICAM-1 in the occurrence and development of hepatocellular carcinoma. Asian Pac J Cancer Prev. 2013;14:1579–83. doi: 10.7314/apjcp.2013.14.3.1579. [DOI] [PubMed] [Google Scholar]
- Belay T, Eko FO, Ananaba GA, Bowers S, Moore T, Lyn D, Igietseme JU. Chemokine and chemokine receptor dynamics during genital chlamydial infection. Infect Immun. 2002;70:844–50. doi: 10.1128/IAI.70.2.844-850.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry LL, Feilzer K, Portis JL, Caldwell HD. Distinct homing pathways direct T lymphocytes to the genital and intestinal mucosae in Chlamydia-infected mice. J Immunol. 1998;160:2905–14. [PubMed] [Google Scholar]
- Tang X, Bu X, Zhang N, Li X, Huang H, Bai H, Yang X. Inefficiency of C3H/HeN mice to control chlamydial lung infection correlates with downregulation of neutrophil activation during the late stage of infection. Cell Mol Immunol. 2009;6:253–60. doi: 10.1038/cmi.2009.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Igietseme JU, Uriri IM, Hawkins R, Rank RG. Integrin-mediated epithelial-T cell interaction enhances nitric oxide production and increased intracellular inhibition of Chlamydia. J Leukoc Biol. 1996;59:656–62. doi: 10.1002/jlb.59.5.656. [DOI] [PubMed] [Google Scholar]
- Beatty PR, Stephens RS. CD8+ T lymphocyte-mediated lysis of Chlamydia-infected L cells using an endogenous antigen pathway. J Immunol. 1994;153:4588–95. [PubMed] [Google Scholar]
- Kamalakaran S, Chaganty BK, Gupta R, Guentzel MN, Chambers JP, Murthy AK, Arulanandam BP. Vaginal chlamydial clearance following primary or secondary infection in mice occurs independently of TNF-α. Front Cell Infect Microbiol. 2013;3:11. doi: 10.3389/fcimb.2013.00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakae S, Komiyama Y, Nambu A, et al. Antigen-specific T cell sensitization is impaired in IL-17-deficient mice, causing suppression of allergic cellular and humoral responses. Immunity. 2002;17:375–87. doi: 10.1016/s1074-7613(02)00391-6. [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2–ΔΔCT method. Methods. 2001;25:402–8. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Wang H, Ren J, et al. IL-17A synergizes with IFN-gamma to upregulate iNOS and NO production and inhibit chlamydial growth. PLoS ONE. 2012;7:e39214. doi: 10.1371/journal.pone.0039214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Murthy AK, Guentzel MN, Seshu J, Forsthuber TG, Zhong G, Arulanandam BP. Antigen-specific CD4+ T cells produce sufficient IFN-γ to mediate robust protective immunity against genital Chlamydia muridarum infection. J Immunol. 2008;180:3375–82. doi: 10.4049/jimmunol.180.5.3375. [DOI] [PubMed] [Google Scholar]
- Murthy AK, Cong Y, Murphey C, Guentzel MN, Forsthuber TG, Zhong G, Arulanandam BP. Chlamydial protease-like activity factor induces protective immunity against genital chlamydial infection in transgenic mice that express the human HLA-DR4 allele. Infect Immun. 2006;74:6722–9. doi: 10.1128/IAI.01119-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cong Y, Jupelli M, Guentzel MN, Zhong G, Murthy AK, Arulanandam BP. Intranasal immunization with chlamydial protease-like activity factor and CpG deoxynucleotides enhances protective immunity against genital Chlamydia muridarum infection. Vaccine. 2007;25:3773–80. doi: 10.1016/j.vaccine.2007.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farris CM, Morrison SG, Morrison RP. CD4+ T cells and antibody are required for optimal major outer membrane protein vaccine-induced immunity to Chlamydia muridarum genital infection. Infect Immun. 2010;78:4374–83. doi: 10.1128/IAI.00622-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawaguchi M, Kokubu F, Kuga H, et al. Modulation of bronchial epithelial cells by IL-17. J Allergy Clin Immunol. 2001;108:804–9. doi: 10.1067/mai.2001.119027. [DOI] [PubMed] [Google Scholar]
- Griffin GK, Newton G, Tarrio ML, et al. IL-17 and TNF-α sustain neutrophil recruitment during inflammation through synergistic effects on endothelial activation. J Immunol. 2012;188:6287–99. doi: 10.4049/jimmunol.1200385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrew DW, Cochrane M, Schripsema JH, Ramsey KH, Dando SJ, O'Meara CP, Timms P, Beagley KW. The duration of Chlamydia muridarum genital tract infection and associated chronic pathological changes are reduced in IL-17 knockout mice but protection is not increased further by immunization. PLoS ONE. 2013;8:e76664. doi: 10.1371/journal.pone.0076664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson RM. Murine oviduct epithelial cell cytokine responses to Chlamydia muridarum infection include interleukin-12-p70 secretion. Infect Immun. 2004;72:3951–60. doi: 10.1128/IAI.72.7.3951-3960.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuse Y, Finethy R, Saka HA, et al. Search for microRNAs expressed by intracellular bacterial pathogens in infected mammalian cells. PLoS ONE. 2014;9:e106434. doi: 10.1371/journal.pone.0106434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W, Stassen FR, Surcel HM, et al. Analyses of polymorphisms in the inflammasome-associated NLRP3 and miRNA-146A genes in the susceptibility to and tubal pathology of Chlamydia trachomatis infection. Drugs Today (Barc) 2009;45(Suppl. B):95–103. [PubMed] [Google Scholar]
- Zhao GJ, Mo ZC, Tang SL, et al. Chlamydia pneumoniae negatively regulates ABCA1 expression via TLR2-Nuclear factor-κB and miR-33 pathways in THP-1 macrophage-derived foam cells. Atherosclerosis. 2014;235:519–25. doi: 10.1016/j.atherosclerosis.2014.05.943. [DOI] [PubMed] [Google Scholar]
- Wu Z, Lu H, Sheng J, Li L. Inductive microRNA-21 impairs anti-mycobacterial responses by targeting IL-12 and Bcl-2. FEBS Lett. 2012;586:2459–67. doi: 10.1016/j.febslet.2012.06.004. [DOI] [PubMed] [Google Scholar]
- Aurora AB, Mahmoud AI, Luo X, et al. MicroRNA-214 protects the mouse heart from ischemic injury by controlling Ca2+ overload and cell death. J Clin Invest. 2012;122:1222–32. doi: 10.1172/JCI59327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jr Ode F, Moore CS, Kennedy TE, Antel JP, Bar-Or A, Dhaunchak AS. MicroRNA dysregulation in multiple sclerosis. Front Genet. 2012;3:311. doi: 10.3389/fgene.2012.00311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Junker A, Krumbholz M, Eisele S, et al. MicroRNA profiling of multiple sclerosis lesions identifies modulators of the regulatory protein CD47. Brain. 2009;12:3342–52. doi: 10.1093/brain/awp300. [DOI] [PubMed] [Google Scholar]
- Zhu S, Pan W, Song X, et al. The microRNA miR-23b suppresses IL-17-associated autoimmune inflammation by targeting TAB2, TAB3 and IKK-α. Nat Med. 2012;18:1077–86. doi: 10.1038/nm.2815. [DOI] [PubMed] [Google Scholar]
- Waite JC, Skokos D. Th17 response and inflammatory autoimmune diseases. Int J Inflamm. 2012;2012:819467. doi: 10.1155/2012/819467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan Q, Wang X, Gong W, et al. ER stress negatively modulates the expression of the miR-199a/214 cluster to regulates tumor survival and progression in human hepatocellular cancer. PLoS ONE. 2012;7:e31518. doi: 10.1371/journal.pone.0031518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bian Z, Guo Y, Luo Y, Tremblay A, Zhang X, Dharma S, Mishra A, Liu Y. CD47 deficiency does not impede polymorphonuclear neutrophil transmigration but attenuates granulopoiesis at the postacute stage of colitis. J Immunol. 2013;190:411–7. doi: 10.4049/jimmunol.1201963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouyang W, Kolls JK, Zheng Y. The biological functions of T helper 17 cell effector cytokines in inflammation. Immunity. 2008;28:454–67. doi: 10.1016/j.immuni.2008.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scurlock AM, Frazer LC, Andrews CW, Jr, et al. Interleukin-17 contributes to generation of Th1 immunity and neutrophil recruitment during Chlamydia muridarum genital tract infection but is not required for macrophage influx or normal resolution of infection. Infect Immun. 2011;79:1349–62. doi: 10.1128/IAI.00984-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HY, Schripsema JH, Sigar IM, Murray CM, Lacy SR, Ramsey KH. A link between neutrophils and chronic disease manifestations of Chlamydia muridarum urogenital infection of mice. FEMS Immunol Med Microbiol. 2010;59:108–16. doi: 10.1111/j.1574-695X.2010.00668.x. [DOI] [PubMed] [Google Scholar]
- Imtiaz MT, Distelhorst JT, Schripsema JH, Sigar IM, Kasimos JN, Lacy SR, Ramsey KH. A role for matrix metalloproteinase-9 in pathogenesis of urogenital Chlamydia muridarum infection in mice. Microbes Infect. 2007;9:1561–6. doi: 10.1016/j.micinf.2007.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imtiaz MT, Schripsema JH, Sigar IM, Kasimos JN, Ramsey KH. Inhibition of matrix metalloproteinases protects mice from ascending infection and chronic disease manifestations resulting from urogenital Chlamydia muridarum infection. Infect Immun. 2006;74:5513–21. doi: 10.1128/IAI.00730-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Maza LM, Pal S, Khamesipour A, Peterson EM. Intravaginal inoculation of mice with the Chlamydia trachomatis mouse pneumonitis biovar results in infertility. Infect Immun. 1994;62:2094–7. doi: 10.1128/iai.62.5.2094-2097.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, Lei L, Zhou Z, et al. Contribution of interleukin-12 p35 (IL-12p35) and IL-12p40 to protective immunity and pathology in mice infected with Chlamydia muridarum. Infect Immun. 2013;81:2962–71. doi: 10.1128/IAI.00161-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darville T, O'Neill JM, Andrews CW, Jr, Nagarajan UM, Stahl L, Ojcius DM. Toll-like receptor-2, but not Toll-like receptor-4, is essential for development of oviduct pathology in chlamydial genital tract infection. J Immunol. 2003;171:6187–97. doi: 10.4049/jimmunol.171.11.6187. [DOI] [PubMed] [Google Scholar]
- den Hartog JE, Lyons JM, Ouburg S, et al. TLR4 in Chlamydia trachomatis infections: knockout mice, STD patients and women with tubal factor subfertility. Drugs Today (Barc) 2009;45(Suppl. B):75–82. [PubMed] [Google Scholar]
- Prantner D, Darville T, Sikes JD, Andrews CW, Jr, Brade H, Rank RG, Nagarajan UM. Critical role for interleukin-1β (IL-1β) during Chlamydia muridarum genital infection and bacterial replication-independent secretion of IL-1β in mouse macrophages. Infect Immun. 2009;77:5334–46. doi: 10.1128/IAI.00883-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Interleukin-17A contributes to microRNA (miR) -214 and intracellular adhesion molecule l 1 gene (ICAM1) modulation in Chlamydia muridarum-infected murine genital cells.