Abstract
Background
In recent years much attention has been given to the lack of reproducibility in biomedical research, particularly in pre-clinical animal studies. This is a problem that also plagues the alcohol research field, particularly in consistent consumption in animal models of alcohol use disorders. One often overlooked factor that could affect reproducibility is the maintenance diet used in pre-clinical studies.
Methods
Herein, two well-established models of alcohol consumption, the “drinking in the dark” (DID) procedure and the continuous two-bottle choice paradigm (C2BC), were employed to determine the effects of diet on ethanol consumption. Male C57BL/6J were given one of six standard rodent-chow diets obtained from Purina LabDiet®, Inc. [St. Louis, MO; Prolab® RMH 3000] or Harlan Laboratories Inc. [Indianapolis, IN; Teklad Diets T.2916, T.2918, T.2920X, T.7912, or T.8940]. A separate group of animals were used to test dietary effects on ethanol pharmacokinetics and behavioral measures following intraperitoneal (IP) injections of various doses of ethanol.
Results
Mice eating Harlan diets T.2916 (H2916) and T.2920X (H2920) consumed significantly less ethanol and exhibited lower blood ethanol concentrations (BECs) during DID; however, during C2BC animals maintained on Harlan T.7912 (H7912) consumed more ethanol and had a higher ethanol preference than the other diet groups. Ethanol consumption levels did not stem from changes in alcohol pharmacokinetics, as a separate group of animals administered ethanol IP showed no difference in BECs. However, animals on Harlan diet T.2920X (H2920) were more sensitive to alcohol-induced locomotor activity in an open-field task. No diet dependent differences were seen in alcohol-induced sedation as measured with loss of righting reflex.
Conclusions
Although these data do not identify a specific mechanism, together they clearly show that the maintenance diet impacts ethanol consumption. It is incumbent upon the research community to consider the importance of describing nutritional information in methods, which may help decrease inter-laboratory reproducibility issues.
Keywords: alcohol abuse, standard rodent diet, drinking in the dark, continuous two-bottle choice, scientific reproducibility
Introduction
Animal models of alcohol use disorders (AUDs) allow researchers to explore the neurobiological and behavioral adaptations that influence excessive alcohol consumption. Various paradigms of alcohol abuse have been proposed representing stages in the development of an AUD including models of voluntary consumption, binge-like ethanol drinking, dependence-like drinking, and relapse-like drinking (Vengeliene et al., 2014, Gilpin et al., 2009, Tabakoff and Hoffman, 2000). Two models that are often used to study pre-dependent ethanol drinking include “drinking in the dark” (DID) and continuous two-bottle choice (C2BC) procedures (Thiele and Navarro, 2014, Ozburn et al., 2013). These models are crucial to understanding the neuroplastic changes and maladaptive behavioral events that occur early in the transition to alcohol dependence so attaining reliable results is crucial.
Consistency between laboratories employing models of AUDs is paramount for data reproducibility, and, more importantly, in making progress to unravel the mechanisms that underlie the development of alcoholism. It has previously been shown that even when attempting to equate conditions between laboratory settings, subtle, inconspicuous factors can lead to significant differences in behavioral outcomes including alcohol intake (Crabbe et al., 1999, Crabbe et al., 2012). One variable that is often overlooked but can readily be controlled between laboratories is the specific rodent diet animals are provided in their home-cages. Several companies produce rodent feed, but the constituents of these “standard chows” are not uniform and are therefore an undocumented source of variability in experiments (Barnard et al., 2009). While it is readily accepted that high-fat palatable diets can alter central reward circuity and increase addiction susceptibility (Narayanaswami et al., 2013, Volkow et al., 2013, Thiele et al., 2003), other components of standard rodent diets, like soy, lead to neuroplastic changes that have behavioral repercussions (Lephart et al., 2002, Mead, 2006). For example, the phytoestrogens found within soy products can lead to increased anxiety-like behavior and pain dysregulation, both of which are thought to underlie AUD development (Hartley et al., 2003, Koob and Le Moal, 2001, Tall and Raja, 2004). Moreover, dietary supplements have been shown to influence alcohol-induced changes in peripheral organ systems which could indirectly affect alcohol-related behaviors including ethanol consumption (Tang et al., 2009, Keshavarzian et al., 2001). Maintenance diet can even influence gustatory preferences (Tordoff et al., 2002), which could also influence ethanol consumption (Kampov-Polevoy et al., 1990, Sinclair et al., 1992). Both direct and indirect effects of rodent diet on behavior warrants further exploration of the specific impact of diet on ethanol consumption and ethanol-induced neurobiological responses.
Given the numerous options available for rodent diets produced both between and within vendors, variability of ethanol consumption among laboratories could partially stem from differences in diets offered to animals. To assess this possibility, the present study systematically compares the behavior of male C57BL/6J mice that consumed one of 6 different standard, natural ingredient diets obtained from Harlan Laboratories Inc. or Purina LabDiet, Inc. Binge-like and voluntary ethanol consumption were measured using DID and C2BC, respectively, to determine the influence of diet on non-dependent ethanol consumption. Subsequent experiments determined if diet influenced the consumption of sweet or bitter stimuli. In addition, dietary influences on blood ethanol concentration (BEC), ethanol-induced behaviors in open-field tests (OFT), and ethanol-induced sedation were measured. Results from the present study provide the first systematic evidence that the specific diet made available to mice influences ethanol consumption.
Materials & Methods
Animals
Male C57BL/6J mice (Jackson Laboratories; Bar Harbor, ME) at 6–8 weeks of age were housed individually in a vivarium with a reversed 12:12 hour light:dark cycle maintained at approximately 22°C. Mice were housed in open-top, plastic static microisolator cages (AnCare, Bellmore, NY; 7.5”x11.5”x5”) with wire-lids and irradiated corncob bedding (Andersons Cob Products, Maumee, OH). During experiments, animals had ad libitum access to food and water unless otherwise indicated. Three cohorts of mice were used in these experiments. Cohort one (N = 60) was fed one of six diets (Table 1) to determine the effect of rodent diet on ethanol consumption. Diets were either obtained from Purina LabDiet®, Inc. [St. Louis, MO; Prolab® RMH 3000] or Harlan® Laboratories Inc. [Harlan, Indianapolis, IN; Teklad Diets T.2916, T.2918, T.2920X, T.7912, or T.8940]. In the subsequent text and figures, diets are referred to by the product number and vendor initial (e.g., RMH 3000 as P3000 and T.2916 as H2916). Diets chosen had varying ingredient components and diet texture (Table 1). In a second cohort of animals (N = 30), limited diets (P3000, H2920, and H7912) were selected that had an effect on ethanol consumption within cohort one to determine the influence of diet on ethanol pharmacokinetics and neurobiological responses to ethanol. Finally, a third cohort of mice was used to replicate a subset of results obtained with cohort one by again assessing binge-like ethanol drinking in mice maintained on P3000 and H2920 diet (N = 17). All procedures conducted were approved by the University of North Carolina Institutional Animal Care and Use Committee and were within the Guidelines for the Care and Use of Laboratory Animals (NRC, 2011).
Table 1.
| P3000 | H2916 | H2918 | H2920 | H7912 | H8940 | |
|---|---|---|---|---|---|---|
| Product Name | RMH 3000 | T.2916 | T.2918 | T.2920X | T.7912 | T.8940 |
| Main Ingredients (listed in descending order) | Ground Wheat Dehulled Soybean Meal Wheat Middlings Ground Corn Fish Meal Porcine Animal Fat Dehydrated Alfalfa Meal | Ground Wheat Ground Corn Wheat Middlings Corn Gluten meal Mineral Sources Soybean Oil | Ground Wheat Ground Corn Dehulled Soybean Meal Corn Gluten Meal Soybean Oil | Ground Wheat Ground Corn Corn Gluten Meal Wheat Middlings Soybean Oil | Ground Corn Dehulled Soybean Meal Ground Oats Wheat Middlings Dehydrated Alfalfa Meal Soybean Oil Corn Gluten Meal | Dehulled Soybean Meal1 Ground Corn Wheat Middlings Flaked Corn Fish Meal Cane Molasses Soybean Oil Ground Wheat |
| Pellet Form | Compressed | Compressed | Compressed | Extruded | Compressed | Compressed |
| Estimated Isoflavone Concentration2, ppm | 350 – 650 | < 20 | 200 – 250 | < 20 | ~500 (350 – 650) | ~525 (400 – 700) |
| Energy Density3 (kcal/g) | 3.18 | 3 | 3.1 | 3.1 | 3.1 | 3 |
| Crude Protein, % | 22.5 | 16.4 | 18.6 | 19.1 | 19.1 | 22 |
| Fat4, % | 5.5 | 4 | 6.2 | 6.5 | 5.8 | 5.5 |
| Neutral Detergent Fiber, % | 15.1 | 15.2 | 14.7 | 12.3 | 13.7 | 12.8 |
Due to the 2 forms of corn (ground and flake) the first ingredient listed is soybean meal but corn is more abundant than soybean meal when both forms are accounted.
Estimated isoflavone concentrations (daidzein and genistein aglycone equivalents) were based on diet vendor quarterly testing and previously published levels (Thigpen et al., 2004, Jensen and Ritskes-Hoitinga, 2007)
Energy densities were calculated based on estimated metabolizable energy.
Fat levels were calculated based on ether extraction values for compressed pelleted diets and acid hydrolysis for the extruded diet T.2920X.
Drinking-in-the-Dark Procedure
After two-weeks of acclimation to the designated diet and the vivarium, cohort one was subjected to the DID paradigm, a well-established model of binge-like ethanol consumption (Thiele and Navarro, 2014, Thiele et al., 2014). Briefly, three hours into the dark cycle, home-cage water bottles were replaced with modified sipper-tubes that were created as described in Thiele et al. (2014) that were filled with 20% (v/v) ethanol made from a solution of 95% ethanol (Decon Labs, King of Prussia, PA) and tap water. During the first three days, animals were allotted two-hours of access to ethanol; however, on the fourth (test) day, ethanol consumption (g/kg) was measured after four-hours of access. Tail blood samples were collected immediately following the four-hour access period on test days. This 4-day procedure was repeated a second time with a three day rest period in between the two, 4-day DID tests. Following all ethanol testing, cohort one mice were tested using the same DID access procedure but with access to tap water, 1% sucrose (Fisher Scientific, Inc. Pittsburg, PA), 3% sucrose, 0.15% saccharin (Fisher Scientific Inc. Pittsburg, PA), and 0.004%(w/v) quinine (Sigma-Aldrich, St. Louis, MO) in tap water, sequentially. This was done to determine if consumption differences between diet conditions were unique to ethanol or generalized to other non-alcohol tastants.
Two-Bottle Choice Paradigm
The week following ethanol DID procedures, animals were subjected to C2BC procedures to determine the influence of rodent diet on voluntary ethanol consumption as previously described (Cox et al., 2013). Briefly, the mice were given continuous access to two drinking bottles made from Nalgene polycarbonate 50 ml conical bottom centrifuge tubes (Fisher Scientific, Inc. Pittsburg, PA) fitted with #5.5 rubber stoppers with 2.5” straight and non-ball bearing sipper tubes (AnCare, Bellmore, NY) in their home-cage: one containing water and a second bottle containing an ethanol solution. The ethanol solution concentration was changed every 8 days as follows: 10, 15, 20, 15, and 10% (v/v) (Cox et al., 2013). Animals' body weights were recorded every four days while fluid consumptions were measured daily to calculate daily consumption (g/kg). Preference was determined by dividing the volume of ethanol solution consumed by the total fluid intake. The week following C2BC, animals were given access to tap water only and the volume (mL/kg) of water consumed was measured every 24h for four days.
Ethanol Pharmacokinetics
Cohort two was divided into three groups to study the effects of select diets (P3000, H2920, and H7912) on alcohol pharmacokinetics and behavioral measures. Animals in cohort two were given a 0.5g/kg (5mL/kg) intraperitoneal (IP) dose of ethanol (13%(v/v) ethanol-isotonic saline solution). Tail blood samples were taken at 10, 30, 60 and 120 minutes following injection. These procedures were repeated two additional times with the mice receiving IP injections of 1.5g/kg (15mL/kg) and then 3g/kg (30mL/kg) doses of ethanol. Injections were separated by a week to reduce stress and limit the development of metabolic tolerance associated with multiple ethanol injections.
Blood Ethanol Concentration Determination
Tail blood samples were collected from a tail nick (using a sterile razor blade) into capillary tubes (~20μl) and centrifuged in a Haematospin 1400 for 6 minutes at 3100g (Analox, London, UK) to obtain serum. The serum was then stored at −20°C until BEC's were determined using an AM1 Alcohol Analyzer (Analox, London, UK). Samples were averaged from duplicate runs and expressed as mg/dL.
Ethanol-Induced Locomotor-Activity
Following the ethanol pharmacokinetic experiment, mice from cohort two received an IP injection of a 1.5g/kg dose of ethanol (13% (v/v) ethanol-isotonic saline solution) or isotonic saline (15mL/kg). Saline treated animals were selected randomly from each diet group and pooled into a control group for comparison with ethanol treated groups. Immediately after injection, the mouse was placed into an open-field testing (OFT) arena consisting of a Plexiglas chamber lined with bedding consistent with the home-cage environment, identical to previous reports (Fee et al., 2004, Cox et al., 2013). Movement was tracked using the VersaMax® software program (AccuScan Instruments, Inc., Columbus, OH) and the output of five-minute bins were measured over thirty-minutes using VersaDat® 4.00 software (AccuScan Instruments, Inc.). Total distance traveled was measured to assess the interaction of diet and ethanol on locomotor activity; whereas center distance and time were recorded to determine the influence of diet on alcohol-induced anxiolytic effects (Cox et al., 2013, Fee et al., 2004, Prut and Belzung, 2003).
Ethanol-Induced Sedative Effects
One week after the OFT, mice from cohort two were given an IP injection of a 3.5g/kg dose of ethanol (20% (v/v) ethanol-isotonic saline solution; 20mL/kg) to assess the effect of diet on ethanol-induced sedation. Upon the onset of sedation, mice were turned over so that their backs were placed in a U-shaped plastic trough. The loss of righting reflex (LORR) was defined as the inability of a mouse to turn itself onto its abdomen on all four paws three times within a 30s period, as previously described (Fee et al., 2004). Both the latency to the onset and duration of LORR were measured and recorded in seconds.
Statistics
Data were analyzed and graphed using Prism version 5.0 (GraphPad Software, Inc. La Jolla, CA). All data reported are expressed as the mean ± SEM and considered significant if p < 0.05, two-tailed. For all experiments, an analysis of variance (ANOVA) was used to determine differences between groups. Post-hoc tests were conducted if a significant interaction or main effect of diet was observed.
Results
Dietary influence on ethanol consumption with DID procedures
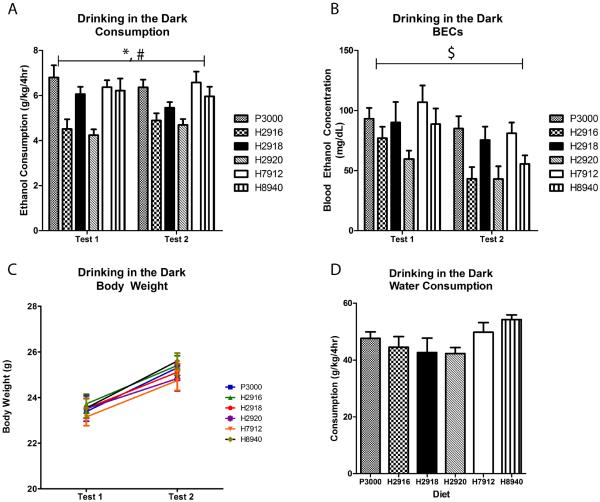
The ethanol consumption on the test days were analyzed using a two-way repeated measures (RM) ANOVA. The two-way RM ANOVA indicated a significant main effect of diet [F(5,54)=10.03, p<0.0001] but no interaction [F(5,54)=0.73, p=0.60] or main effect of time [F(1,54)=0.04, p=0.83] (Figure 1A). After collapsing the groups across Test 1 and Test 2, posthoc Bonferroni analyses indicated that animals maintained on H2916 and H2920 consumed significantly less ethanol compared with mice given P3000, H7912, and H8940 (p<0.05). Moreover, animals on H2920 also consumed less than those on H2918. BECs concurred with consumption data as a two-way RM ANOVA indicated a main effect of diet [F(5,54)=5.28, p=0.0005] and time [F(1,54)=10.91, p=0.0017] but no interaction [F(5,54)=0.42, p=0.84]. Collapsing across Test 1 and Test 2, posthoc Bonferroni analyses showed that animals on H2920 had significantly lower BECs than the group receiving either P3000 or H7912 (p<0.05; Figure1B). Importantly, two-way RM ANOVA indicated no interaction [F(45,486)=1.33, p=0.08] or main effect of diet [F(5,486)=2.27, p=0.06] on body weights, but an expected main effect of time [F(9,486)=130.05, p<0.0001] was observed (Figure 1C). No effect of diet was observed using a one-way ANOVA on water consumption during a 4-hour DID session [F(5,54)=2.03, p=0.09]. (Figure 1D). In a replication experiment, t-tests showed that a separate group of mice given access to the P3000 diet exhibited higher levels of binge-like ethanol drinking [t(15)=2.47, p=0.0262] but not BECs [t(15) =2.05, p=0.0583] than mice on H2920 (Supplementary Figure 1).
Figure 1.
Diet had an effect on ethanol consumption during the DID test days (panel A) as well as the corresponding BECs (panel B) No significant differences were reflected in the body weights (panel C) or in water consumption (panel D) between the six groups. * indicates significantly less than P3000, H7912, and H8940; # indicates significantly less than H2918; $ indicates significantly less than P3000 and H7912. Significance was set at p<0.05 (two-tailed), and all data are expressed as mean ± SEM.
Dietary influence on ethanol consumption with C2BC procedures
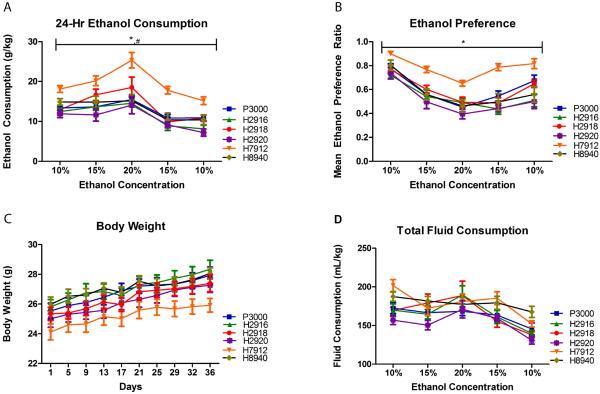
The individual daily ethanol consumed at each concentration was averaged over the 8-day period. A two-way RM ANOVA performed on the average ethanol consumption (Figure 2A) indicated significant main effects of diet [F(5,216)=12.37, p<0.0001] and concentration [F(4,216)=48.86, p<0.0001] but the interaction effect was not significant [F(20,216)=1.47, p=0.09]. Collapsing across concentration, Bonferroni tests indicated that animals on the H7912 diet exhibited significantly higher consumption of ethanol than groups on all other diets and that animals on H2918 consumed more ethanol than those on H2920 (Figure 2A; p<0.05). A twoway RM ANOVA performed on ethanol preference data (Figure 2B) indicated significant main effects of diet [F(5,216)=8.95, p<0.0001] and concentration [F(4,216)=75.94, p<0.0001] but the interaction effect was not significant [F(20,216)=1.03, p=0.57]. Bonferroni tests performed after collapsing across concentration indicated that animals on H7912 showed higher ethanol preference for ethanol than animals on other diets (Figure 2B; p<0.0001). A two-way RM ANOVA performed on body weights collected during C2BC (Figure 2C) revealed no significant main effect of diet [F(5,486)=2.27, p=0.06] or interaction of diet and time [F(45,486)=1.33, p=0.08] but a main effect of time [F(9,486)=130.05, p<0.0001] was significant. A two-way RM ANOVA performed on total fluid consumption (Figure 2D) indicated a significant main effect of ethanol concentration [F(4,216)=25.03, p<0.0001] but not diet [F(5,216)=2.13, p=0.08] or an interaction between concentration and diet [F(20,2165)=0.79, p=0.28]. In agreement with equitable fluid consumption, a one-way ANOVA test revealed no significant effect of diet [F(5,54)=2.22, p=0.07] on daily water consumption averaged over 4 days in the absence of ethanol following C2BC (Supplementary Figure 2).
Figure 2.
Mice on diet H7912 had increased ethanol consumption and preference compared with the animals receiving other diets at various ethanol concentrations (v/v) (panels A & B). No significant differences were seen in the body weights (panel C) or in total consumption (panel D). * indicates H7912 greater than other groups; # indicates H2918 greater than H2920. Significance was set at p<0.05 (two-tailed), and all data are expressed as mean ± SEM.
Dietary influence on sucrose, saccharin, and quinine solution consumption
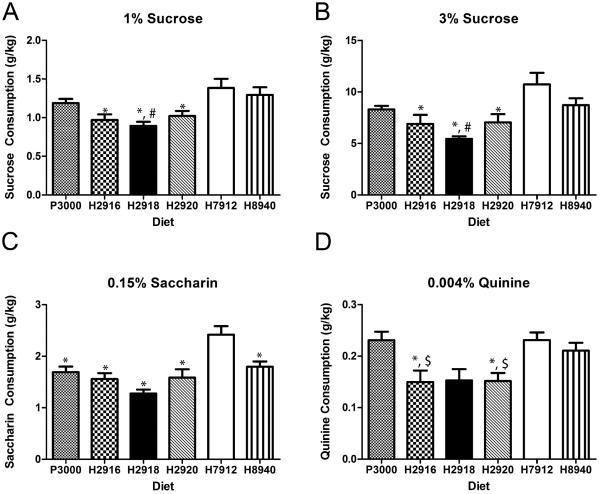
To determine if the influence of diet was specific to ethanol consumption, sucrose, saccharin and quinine solution intake were tested. The consumptions during the day-4, four hour tests were analyzed. One-way ANOVAs showed that diet condition had an effect on consumption of 1% sucrose [F(5,54)=5.81, p=0.0002], 3% sucrose [F(5,54)=6.07, p<0.0001], and 0.15% saccharin [F(5,54)=9.32, p<0.0001], and quinine (F(5,54)=4.71, p=0.0012) solutions. In general, posthoc Bonferroni tests indicated the group with access to the H7912 diet consumed more of the sweet solutions relative to the other diet groups (see Figure 3 for details on group and solution differences). However, the bitter quinine solution was consumed in greater volumes by animals on P3000 and H7912 compared with those on H2916.
Figure 3.
Animals on diet H7912 consumed more sucrose compared with mice on diets H2920, H2916, and H2918 (panels A & B). Mice on diet H8940 also consumed more sucrose than those on diet H2918. In regards to saccharin, only mice on diet H7912 were significantly different, consuming more of the sweetener than all other groups (panel C). Both the P3000 and H7912 diet groups consumed more quinine solution than those on diets H2920 or H2916 (panel D) * indicates less than H7912; # less than H8940; $ less than P3000. Significance was set at p<0.05 (two-tailed), and all data are expressed as mean ± SEM.
Diet condition had no effect on ethanol pharmacokinetics
BECs after various time-points following IP injection of 0.5, 1.5, or 3.0g/kg doses of ethanol are presented in Figure 4. Two-way RM ANOVAs revealed significant main effects of diet and time, but the interaction effects were not significant, following IP injections of ethanol at the 0.5g/kg ([F(2,103) =3.44, p=0.036]; [F(3,103)=99.97, p<0.0001]; [F(6,103)=1.14, p=0.34]) and 1.5g/kg ([F(2,98)=3.78, p=0.026]; [F(3,98)=3.78, p<0.0001]; [F(6,98)=0.81, p=0.57]) doses, but only a significant main effect of time following the 3g/kg dose [F(3,100)=36.35, p<0.0001] and not diet [F(2,100)=2.90, p=0.06] or interaction [F(6,100)=0.55, p=0.77]. However, after collapsing across time, Bonferroni tests comparing BECs between the diet conditions failed to show significant differences at either 0.5 or 1.5g/kg ethanol dose.
Figure 4.
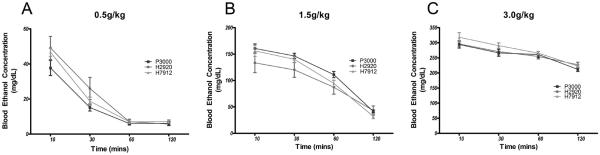
Examination of ethanol pharmacokinetics after IP injection of 0.5 g/kg (panel A), 1.0 g/kg (panel B) or 3.0 g/kg (panel C) ethanol revealed significant no effect of diet at any dose given. Significance was set at p<0.05 (two-tailed), and all data are expressed as mean ± SEM.
Dietary influence on ethanol-induced locomotor-activity and sedation
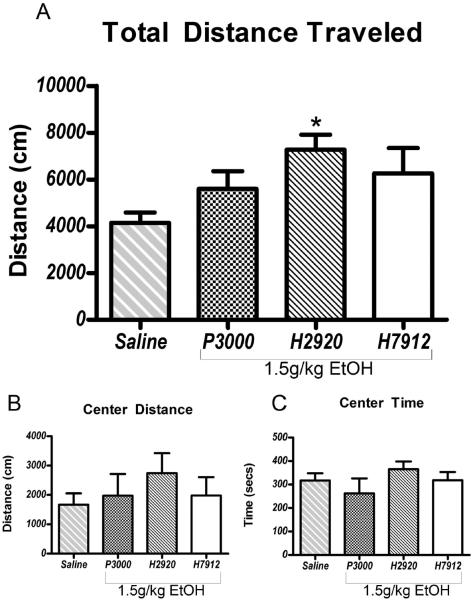
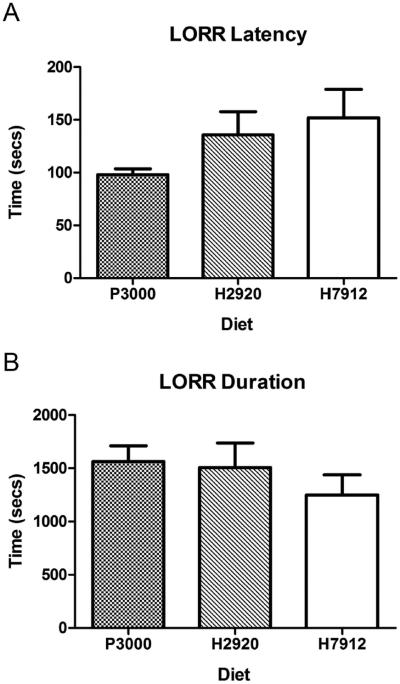
Data from the OFT are presented in Figure 5. A one-way ANOVA showed that diet condition had a significant effect on total distance traveled (Figure 5A; [F(3,24)=4.23, p=0.015]). A post-hoc Bonferroni test showed that only animals on the H2920 diet were significantly different than the saline group. Examining the first fifteen minutes provided similar results (Supplementary Figure 3). However, ANOVAs performed on center distance (Figure 5B; [F(3,24)=0.92, p=0.57]) and center time (Figure 5C; [F(3,24)=0.92, p=0.44]) failed to yield significant differences, suggesting diet condition did not alter anxiety-like behavior. One-way ANOVAs analyzing the latency to the onset of righting reflex [F(2,18)=1.77, p=0.19] and the duration of LORR [F(2,18)=0.84, p=0.45] failed to yield significant differences, indicating that diet condition did not influence sensitivity to the sedative effects of a 3.5g/kg dose of ethanol (Figure 6).
Figure 5.
Mice on diet H2920 showed a greater sensitivity to ethanol-induced locomotor activity, as evidenced by increased total distance traveled compared with the saline group (panel A;). No differences were seen in center distance traveled or time spent in the center of the chamber (panels B & C). Significance was set at p<0.05 (two-tailed), and all data are expressed as mean ± SEM.
Figure 6.
Examination of ethanol induced sedation after IP injection of 3.5g/kg ethanol revealed no effect of diet as there was no change in the latency of onset (panel A) or duration (panel B) of the loss of righting reflex (LORR). Significance was set at p<0.05 (two-tailed), and all data are expressed as mean ± SEM.
Discussion
The implications of different rodent diets fed between laboratories and research institutions are often overlooked in behavioral experiments. The data presented herein provide the first direct evidence that the specific rodent diet fed is a particularly important variable to describe and control within ethanol consumption studies. To the best of our knowledge, this is the first study to show that the specific laboratory diet used had significant effects on ethanol consumption in both DID and C2BC. In the DID study, mice that had access to either H2916 or H2920, drank significantly less ethanol than mice that had access to any of the other 4 diets. Further, in a replication study, mice with access to the H2920 were again found to drink significantly less ethanol than mice on the P3000 diet using DID procedures, emphasizing the reproducibility of the diet-induced variability. In accord with consumption, the H2920 group attained BECs that consistently fell below 80mg/dl, commonly used as a minimal limit for modeling binge-like ethanol drinking within DID procedures (Thiele and Navarro, 2014) and one of the defining criteria of binge drinking set forth by the National Institute on Alcohol Abuse and Alcoholism (NIAAA, 2004). Thus, in addition to showing that the level of ethanol consumption can vary with changes in diet, the present results also show that certain diets could prevent successful binge-like ethanol drinking using DID procedures. Because pelleted diets “powderize” into the bedding and lead to overestimation of consumption relative to animals with extruded diet (personnel communication with Harlan Laboratories, Inc. and also observed during food intake assessment of mice in this report while they were acclimated to the laboratory and diets), food intake data was not collected throughout these studies. However, there were no observed diet-related differences in body weight between groups during DID testing, making it unlikely that changes in ethanol drinking were secondary to altered energy balance. Assessment of food consumption and determining if potential caloric intake differences between various extruded diet groups influences ethanol drinking levels will be an important future direction.
Within the C2BC procedure, less variability in the level of ethanol consumption between the diet conditions was observed. Only the group of animals with access to H7912 showed consistently different behavior relative to the other diet conditions, exhibiting greater preference for ethanol and increased ethanol consumption. While increased consumption may be preferable in modeling AUDs, over time the animals on H7912 diet non-significantly trended towards reduced age-related weight gain relative to the other groups. This could present an issue in studies looking at prolonged ethanol consumption. However, it is important to note that the mice maintained on H7912 had an average mass (μ=27.8g) which falls well within the 95% confidence interval for their age group (Jackson-Laboratory, 2007) and that the energy densities are very similar between the diets tested (Table 1).
While these results provide compelling evidence that diet can impact ethanol consumption, it is not enough to make firm conclusions on the underlying causes of these differences. However, several possible factors were considered. First, differences in ethanol drinking could be due to the consistency of the diets. All diets were in a compressed form with the exception of diet H2920 which was in an “extruded” form. Ethanol consumption in mice on the H2916 (compressed) and H2920 (extruded) diets, which have very common components, were comparable during DID procedures. Moreover, mice on the extruded H2920 diet drank similar amounts of ethanol relative to all other pelleted diet groups (except H7912) during C2BC. Dietary influence on ethanol consumption is also not likely due to diet influence on ethanol pharmacokinetics as no differences were seen in ethanol clearance between groups of mice with access to the P3000, H2920, and H7912 diets. A third possibility is that diet had an influence on general fluid consumption. The observations herein indicate that differences in ethanol consumption are not likely related to altered fluid balance or chow-induced polydipsia as no differences were seen in baseline water consumption between the diet conditions during limited access measures (consistent with DID) or 24h measures (consistent with C2BC) in the absence of ethanol access. Together, these data suggest that the source of ethanol consumption variability is not related to the consistency of the diet, dietary influence on pharmacokinetics, or chow-induced polydipsia.
One potential mechanism that is supported by these data is that diet influences gustatory preference and therefore ethanol consumption. Previous studies indicate the specific chow available influences the consumption of sweet and bitter solutions (Tordoff et al., 2002, Tordoff, 2007). The data herein agrees as animals maintained on diet H7912 consumed greater amounts of sucrose, saccharin, and quinine compared with several other diet groups. Intriguingly, this group also showed increased ethanol consumption and preference in C2BC. High ethanol drinking was associated with increased sucrose and quinine intake in C57BL/6ByJ mice relative to 129/J mice (Bachmanov et al., 1996a), though only with increased sucrose drinking (and not increased quinine intake) in a similar study (Bachmanov et al., 1996b). Since ethanol entails a sweet and bitter component (Kiefer et al., 1990), the increased ethanol consumption during C2BC of animals maintained on H7912 diet could be secondary to gustatory preference changes, such as increased sweet and bitter preference. It should be noted that the exact influence of diet on tastant consumption is confounded by previous exposure to ethanol, but dietary changes in gustatory preferences remains a potential underlying mechanism for altered ethanol consumption.
Another potential mechanism that was explored in these studies was the effect of diet on ethanol pharmacodynamics through assessment of ethanol-induced locomotor activity and sedation. The data showed that animals maintained on the H2920 diet were more sensitive to ethanol-induced locomotor activity in the OFT relative to the saline injected control group. Increased sensitivity to the locomotor stimulant effects of ethanol has been associated with decreased ethanol intake (Naassila et al., 2004, Hodge et al., 1999). Although this inverse relationship is not always observed (Brabant et al., 2014), reduced ethanol consumption by mice maintained on H2920 may be somehow related to the increased sensitivity to ethanol-induced locomotor stimulation. The lack of alcohol sensitivity in the other diet groups is similar to previous studies that indicate that C57/BL6J mice are less susceptible to alcohol-induced locomotor effects (Homanics et al., 1999). Diet condition did not interact with ethanol-induced activity in center distance traveled or time relative to the saline condition, indicating that ethanol did not promote anxiolysis in any of the diet conditions tested. Thus, changes in anxiety are an unlikely explanation for differences in ethanol consumption between the diet conditions. Finally, while reduced sensitivity to the sedative effects of ethanol correlates with increased ethanol intake (Thiele et al., 2000, Fee et al., 2004), there were no differences between diet groups in the onset or duration of ethanol-induced sedation. Together these data suggest that diet-induced effect on ethanol sensitivity to sedation did not contribute to diet-related differences in ethanol drinking.
While the diets tested shared many components, there were a few ingredients that differed between groups which may have influenced ethanol consumption. Animals fed the H2920 and H2916 diets consumed less ethanol in the DID paradigm compared with the other diet groups. The one characteristic that was uniquely shared by these diets compared with the other diets tested was the very low level of added soy, resulting in very low levels of estimated isoflavones (< 20 ppm). Diets low in isoflavones are often desirable as this ingredient has been shown to affect reproductive and endocrine health (Jefferson et al., 2007, Ryokkynen et al., 2006, Jensen and Ritskes-Hoitinga, 2007, Thigpen et al., 2004). Soy entails several isoflavones, including the phytoestrogen diadzein. Interestingly, diadzein, and the related isoflavone diadzin, have both been shown to have efficacy as potential antidipsotropic agents that reduce alcohol consumption in pre-clinical and clinical studies (Lukas et al., 2013, Penetar et al., 2012). Paradoxically, while soy-related isoflavones appear to be protective against excessive ethanol intake, here elimination of isoflavones from diet H2916 and H2920 was associated with reduced binge-like ethanol drinking in the DID study. Importantly, while soy/isoflavone was associated with the level of ethanol drinking, the present data only provides a correlational relationship between these factors. Further, soy/isoflavone levels did not predict ethanol intake in C2BC. One ingredient unique to the H7912 diet compared with the other diets tested is ground oats. There is evidence that the inclusion of oats in diets ameliorates alcohol-induced liver dysfunction (Keshavarzian et al., 2001), which theoretically could impact long-term ethanol drinking. In the end, while the present work provides compelling evidence that dietary ingredients impact ethanol drinking in a paradigm specific manner, additional research is necessary to pin-point the components of these diets that modulate the level of ethanol drinking.
One caveat of this present study that requires consideration is that mice went through multiple testing paradigms. Three cohorts of animals were used, one that experienced DID, C2BC, and non-alcoholic tastant consumption testing, a second that was used to replicate the DID study, and a third that experienced BEC assessment, ethanol-induced locomotor activity testing, and then ethanol-induced sedation testing. Thus, with the exception of the replication study, the potential influence of carry-over effects between experimental procedures cannot be ignored with the exception of the very first study in each cohort of mice. It may therefore be important to replicate subsequent experiments (i.e., diet influence on C2BC ethanol drinking) in naïve animals before drawing finite conclusions (e.g., that chow influences C2BC drinking). Assessment of the effects of diet on ethanol consumption in other laboratories to determine the resilience of these findings, as well as to assess the generalizability of the observations made herein across different strains of mice, species, and sex are needed as each variable alters ethanol consumption and may interact differently with chow.
The present data set is not independently sufficient to designate a diet or dietary constituents for voluntary alcohol consumption studies, but it does imply that the alcohol research field, specifically scientists measuring ethanol consumption in C57/BL6J mice, should carefully consider the rodent diet provided to subjects. For example, mice on diet H7912 consumed high levels of ethanol in C2BC and DID paradigms, and thus diet H7912 might be considered a good option. However, both P3000 and H7912 contain alfalfa, an ingredient that elevates background fluorescence with imaging procedures and would not be a viable option for neuroimaging studies (Inoue et al., 2008) . Alternatively, mice on diets H2918 and H8940 drank considerable amounts of ethanol in both paradigms, and achieved high, physiologically relevant BECs during DID procedures, making these diets potential considerations.
Researchers attempt to control and standardize as many factors as possible when designing experiments to decrease inter and intra-laboratory variability and increase reproducibility of data, a problem that is quite prevalent in biomedical research and has gained recent attention from the National Institutes of Health (Landis et al., 2012, Macleod, 2011, Collins and Tabak, 2014). There are others who believe that over standardization increases test sensitivity leading to increased false-positive results (Richter et al., 2009, Paylor, 2009). Moreover, if measured outcomes are consistent despite inter-laboratory differences, the results may be more valid for translational purposes considering the heterogeneity of the human population (Martic-Kehl et al., 2012, Ritskes-Hoitinga et al., 2014). While the impact of the validity of standardization in preclinical studies is an ongoing debate, it is clear that diet is an easily controllable but often overlooked component of experimental design as manuscripts often simply report “standard rodent chow”. The data presented herein provides direct evidence that what is often dubbed “standard” rodent chow can influence ethanol consumption. At minimum, the results from the present study demand that A) careful attention be given to diets used in pre-clinical biomedical research studies and B) diets used be standardly reported in detail, particularly in relation to ethanol consumption studies.
Supplementary Material
Acknowledgements
The authors thank Timothy Gilliam and Rhiannon Thomas for their technical assistance. This work was supported by National Institutes of Health grants AA022048, AA013573, AA015148, AA021611, & GM000678. Tina Herfel is an employee of Harlan Laboratories, Inc. and has an interest in the commercial use of laboratory animal diets.
References
- Bachmanov AA, Reed DR, Tordoff MG, Price RA, Beauchamp GK. Intake of ethanol, sodium chloride, sucrose, citric acid, and quinine hydrochloride solutions by mice: a genetic analysis. Behavior genetics. 1996a;26:563–573. doi: 10.1007/BF02361229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachmanov AA, Tordoff MG, Beauchamp GK. Ethanol consumption and taste preferences in C57BL/6ByJ and 129/J mice. Alcohol Clin Exp Res. 1996b;20:201–206. doi: 10.1111/j.1530-0277.1996.tb01630.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnard DE, Lewis SM, Teter BB, Thigpen JE. Open- and closed-formula laboratory animal diets and their importance to research. Journal of the American Association for Laboratory Animal Science : JAALAS. 2009;48:709–713. [PMC free article] [PubMed] [Google Scholar]
- Brabant C, Guarnieri DJ, Quertemont E. Stimulant and motivational effects of alcohol: lessons from rodent and primate models. Pharmacology, biochemistry, and behavior. 2014;122:37–52. doi: 10.1016/j.pbb.2014.03.006. [DOI] [PubMed] [Google Scholar]
- Collins FS, Tabak LA. Policy: NIH plans to enhance reproducibility. Nature. 2014;505:612–613. doi: 10.1038/505612a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox BR, Olney JJ, Lowery-Gionta EG, Sprow GM, Rinker JA, Navarro M, Kash TL, Thiele TE. Repeated cycles of binge-like ethanol (EtOH)-drinking in male C57BL/6J mice augments subsequent voluntary EtOH intake but not other dependence-like phenotypes. Alcohol Clin Exp Res. 2013;37:1688–1695. doi: 10.1111/acer.12145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crabbe JC, Harkness JH, Spence SE, Huang LC, Metten P. Intermittent availability of ethanol does not always lead to elevated drinking in mice. Alcohol and alcoholism (Oxford, Oxfordshire) 2012;47:509–517. doi: 10.1093/alcalc/ags067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crabbe JC, Wahlsten D, Dudek BC. Genetics of mouse behavior: interactions with laboratory environment. Science. 1999;284:1670–1672. doi: 10.1126/science.284.5420.1670. [DOI] [PubMed] [Google Scholar]
- Fee JR, Sparta DR, Knapp DJ, Breese GR, Picker MJ, Thiele TE. Predictors of high ethanol consumption in RIIbeta knock-out mice: assessment of anxiety and ethanol-induced sedation. Alcohol Clin Exp Res. 2004;28:1459–1468. doi: 10.1097/01.ALC.0000141809.53115.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilpin NW, Smith AD, Cole M, Weiss F, Koob GF, Richardson HN. Operant behavior and alcohol levels in blood and brain of alcohol-dependent rats. Alcohol Clin Exp Res. 2009;33:2113–2123. doi: 10.1111/j.1530-0277.2009.01051.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartley DE, Edwards JE, Spiller CE, Alom N, Tucci S, Seth P, Forsling ML, File SE. The soya isoflavone content of rat diet can increase anxiety and stress hormone release in the male rat. Psychopharmacology (Berl) 2003;167:46–53. doi: 10.1007/s00213-002-1369-7. [DOI] [PubMed] [Google Scholar]
- Hodge CW, Mehmert KK, Kelley SP, McMahon T, Haywood A, Olive MF, Wang D, Sanchez-Perez AM, Messing RO. Supersensitivity to allosteric GABA(A) receptor modulators and alcohol in mice lacking PKCepsilon. Nat Neurosci. 1999;2:997–1002. doi: 10.1038/14795. [DOI] [PubMed] [Google Scholar]
- Homanics GE, Quinlan JJ, Firestone LL. Pharmacologic and behavioral responses of inbred C57BL/6J and strain 129/SvJ mouse lines. Pharmacology, biochemistry, and behavior. 1999;63:21–26. doi: 10.1016/s0091-3057(98)00232-9. [DOI] [PubMed] [Google Scholar]
- Inoue Y, Izawa K, Kiryu S, Tojo A, Ohtomo K. Diet and abdominal autofluorescence detected by in vivo fluorescence imaging of living mice. Molecular imaging. 2008;7:21–27. [PubMed] [Google Scholar]
- Jackson-Laboratory . In: Physiological Data Summary – C57BL/6J (000664), in Series Physiological Data Summary – C57BL/6J (000664) LABORATORY TJ, editor. 2007. [Google Scholar]
- Jefferson WN, Padilla-Banks E, Newbold RR. Disruption of the developing female reproductive system by phytoestrogens: genistein as an example. Molecular nutrition & food research. 2007;51:832–844. doi: 10.1002/mnfr.200600258. [DOI] [PubMed] [Google Scholar]
- Jensen MN, Ritskes-Hoitinga M. How isoflavone levels in common rodent diets can interfere with the value of animal models and with experimental results. Laboratory animals. 2007;41:1–18. doi: 10.1258/002367707779399428. [DOI] [PubMed] [Google Scholar]
- Kampov-Polevoy AB, Kasheffskaya OP, Sinclair JD. Initial acceptance of ethanol: gustatory factors and patterns of alcohol drinking. Alcohol (Fayetteville, N.Y. 1990;7:83–85. doi: 10.1016/0741-8329(90)90065-k. [DOI] [PubMed] [Google Scholar]
- Keshavarzian A, Choudhary S, Holmes EW, Yong S, Banan A, Jakate S, Fields JZ. Preventing gut leakiness by oats supplementation ameliorates alcohol-induced liver damage in rats. J Pharmacol Exp Ther. 2001;299:442–448. [PubMed] [Google Scholar]
- Koob GF, Le Moal M. Drug addiction, dysregulation of reward, and allostasis. Neuropsychopharmacology. 2001;24:97–129. doi: 10.1016/S0893-133X(00)00195-0. [DOI] [PubMed] [Google Scholar]
- Landis SC, Amara SG, Asadullah K, Austin CP, Blumenstein R, Bradley EW, Crystal RG, Darnell RB, Ferrante RJ, Fillit H, Finkelstein R, Fisher M, Gendelman HE, Golub RM, Goudreau JL, Gross RA, Gubitz AK, Hesterlee SE, Howells DW, Huguenard J, Kelner K, Koroshetz W, Krainc D, Lazic SE, Levine MS, Macleod MR, McCall JM, Moxley RT, 3rd, Narasimhan K, Noble LJ, Perrin S, Porter JD, Steward O, Unger E, Utz U, Silberberg SD. A call for transparent reporting to optimize the predictive value of preclinical research. Nature. 2012;490:187–191. doi: 10.1038/nature11556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lephart ED, West TW, Weber KS, Rhees RW, Setchell KD, Adlercreutz H, Lund TD. Neurobehavioral effects of dietary soy phytoestrogens. Neurotoxicol Teratol. 2002;24:5–16. doi: 10.1016/s0892-0362(01)00197-0. [DOI] [PubMed] [Google Scholar]
- Lukas SE, Penetar D, Su Z, Geaghan T, Maywalt M, Tracy M, Rodolico J, Palmer C, Ma Z, Lee DY. A standardized kudzu extract (NPI-031) reduces alcohol consumption in nontreatment-seeking male heavy drinkers. Psychopharmacology (Berl) 2013;226:65–73. doi: 10.1007/s00213-012-2884-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macleod M. Why animal research needs to improve. Nature. 2011;477:511. doi: 10.1038/477511a. [DOI] [PubMed] [Google Scholar]
- Martic-Kehl MI, Ametamey SM, Alf MF, Schubiger PA, Honer M. Impact of inherent variability and experimental parameters on the reliability of small animal PET data. EJNMMI research. 2012;2:26. doi: 10.1186/2191-219X-2-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mead MN. The feed factor: estrogenic variability in lab animal diets. Environ Health Perspect. 2006;114:A640–642. doi: 10.1289/ehp.114-a640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naassila M, Pierrefiche O, Ledent C, Daoust M. Decreased alcohol self-administration and increased alcohol sensitivity and withdrawal in CB1 receptor knockout mice. Neuropharmacology. 2004;46:243–253. doi: 10.1016/j.neuropharm.2003.09.002. [DOI] [PubMed] [Google Scholar]
- Narayanaswami V, Thompson AC, Cassis LA, Bardo MT, Dwoskin LP. Diet-induced obesity: dopamine transporter function, impulsivity and motivation. Int J Obes (Lond) 2013;37:1095–1103. doi: 10.1038/ijo.2012.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NIAAA . NIAAA Council Approves Definition of Binge Drinking, in Series NIAAA Council Approves Definition of Binge Drinking. National Institute on Alcohol Abuse and Alcoholism Newsletter, National Institute of Health; Bethesda, MD: 2004. [Google Scholar]
- NRC . Guide for the care and use of laboratory animals. 8th ed. National Academies Press; Washington, D.C: 2011. [PubMed] [Google Scholar]
- Ozburn AR, Harris RA, Blednov YA. Chronic voluntary alcohol consumption results in tolerance to sedative/hypnotic and hypothermic effects of alcohol in hybrid mice. Pharmacology, biochemistry, and behavior. 2013;104:33–39. doi: 10.1016/j.pbb.2012.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paylor R. Questioning standardization in science. Nature methods. 2009;6:253–254. doi: 10.1038/nmeth0409-253. [DOI] [PubMed] [Google Scholar]
- Penetar DM, Toto LH, Farmer SL, Lee DY, Ma Z, Liu Y, Lukas SE. The isoflavone puerarin reduces alcohol intake in heavy drinkers: a pilot study. Drug Alcohol Depend. 2012;126:251–256. doi: 10.1016/j.drugalcdep.2012.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prut L, Belzung C. The open field as a paradigm to measure the effects of drugs on anxiety-like behaviors: a review. Eur J Pharmacol. 2003;463:3–33. doi: 10.1016/s0014-2999(03)01272-x. [DOI] [PubMed] [Google Scholar]
- Richter SH, Garner JP, Wurbel H. Environmental standardization: cure or cause of poor reproducibility in animal experiments? Nature methods. 2009;6:257–261. doi: 10.1038/nmeth.1312. [DOI] [PubMed] [Google Scholar]
- Ritskes-Hoitinga M, Leenaars M, Avey M, Rovers M, Scholten R. Systematic reviews of preclinical animal studies can make significant contributions to health care and more transparent translational medicine. Cochrane Database Syst Rev. 2014;3:ED000078. doi: 10.1002/14651858.ED000078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryokkynen A, Kukkonen JV, Nieminen P. Effects of dietary genistein on mouse reproduction, postnatal development and weight-regulation. Animal reproduction science. 2006;93:337–348. doi: 10.1016/j.anireprosci.2005.08.007. [DOI] [PubMed] [Google Scholar]
- Sinclair JD, Kampov-Polevoy A, Stewart R, Li TK. Taste preferences in rat lines selected for low and high alcohol consumption. Alcohol (Fayetteville, N.Y. 1992;9:155–160. doi: 10.1016/0741-8329(92)90027-8. [DOI] [PubMed] [Google Scholar]
- Tabakoff B, Hoffman PL. Animal models in alcohol research. Alcohol Res Health. 2000;24:77–84. [PMC free article] [PubMed] [Google Scholar]
- Tall JM, Raja SN. Dietary constituents as novel therapies for pain. The Clinical journal of pain. 2004;20:19–26. doi: 10.1097/00002508-200401000-00005. [DOI] [PubMed] [Google Scholar]
- Tang Y, Forsyth CB, Banan A, Fields JZ, Keshavarzian A. Oats supplementation prevents alcohol-induced gut leakiness in rats by preventing alcohol-induced oxidative tissue damage. J Pharmacol Exp Ther. 2009;329:952–958. doi: 10.1124/jpet.108.148643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiele TE, Crabbe JC, Boehm SL., 2nd “Drinking in the Dark” (DID): a simple mouse model of binge-like alcohol intake. Curr Protoc Neurosci. 2014;68:9, 49, 41–49, 49, 12. doi: 10.1002/0471142301.ns0949s68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiele TE, Navarro M. “Drinking in the dark” (DID) procedures: A model of binge-like ethanol drinking in non-dependent mice. Alcohol (Fayetteville, N.Y. 2014;48:235–241. doi: 10.1016/j.alcohol.2013.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiele TE, Navarro M, Sparta DR, Fee JR, Knapp DJ, Cubero I. Alcoholism and obesity: overlapping neuropeptide pathways? Neuropeptides. 2003;37:321–337. doi: 10.1016/j.npep.2003.10.002. [DOI] [PubMed] [Google Scholar]
- Thiele TE, Willis B, Stadler J, Reynolds JG, Bernstein IL, McKnight GS. High ethanol consumption and low sensitivity to ethanol-induced sedation in protein kinase A-mutant mice. J Neurosci. 2000;20:RC75. doi: 10.1523/JNEUROSCI.20-10-j0003.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thigpen JE, Setchell KD, Saunders HE, Haseman JK, Grant MG, Forsythe DB. Selecting the appropriate rodent diet for endocrine disruptor research and testing studies. ILAR journal / National Research Council, Institute of Laboratory Animal Resources. 2004;45:401–416. doi: 10.1093/ilar.45.4.401. [DOI] [PubMed] [Google Scholar]
- Tordoff MG. Taste solution preferences of C57BL/6J and 129X1/SvJ mice: influence of age, sex, and diet. Chemical senses. 2007;32:655–671. doi: 10.1093/chemse/bjm034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tordoff MG, Pilchak DM, Williams JA, McDaniel AH, Bachmanov AA. The maintenance diets of C57BL/6J and 129X1/SvJ mice influence their taste solution preferences: implications for large-scale phenotyping projects. J Nutr. 2002;132:2288–2297. doi: 10.1093/jn/132.8.2288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vengeliene V, Bilbao A, Spanagel R. The alcohol deprivation effect model for studying relapse behavior: a comparison between rats and mice. Alcohol (Fayetteville, N.Y. 2014;48:313–320. doi: 10.1016/j.alcohol.2014.03.002. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Tomasi D, Baler RD. Obesity and addiction: neurobiological overlaps. Obesity reviews : an official journal of the International Association for the Study of Obesity. 2013;14:2–18. doi: 10.1111/j.1467-789X.2012.01031.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.