Abstract
Introduction
Energy drinks are popular mixers with alcohol. While energy drinks contain many ingredients, caffeine is an important pharmacologically active component and is generally present in larger amounts than in other caffeinated beverages. In these studies, we investigated the hypothesis that caffeine would influence the effects of alcohol (ethanol) on conditioned taste aversion, ataxia and locomotor activity after repeated exposure.
Methods
Four groups of mice were exposed by oral gavage twice daily to vehicle, ethanol (4 g/kg), caffeine (15 mg/kg), or the ethanol/caffeine combination. Conditioned taste aversion to saccharin and ataxia in the parallel rod task were evaluated after 8 or 16 gavages, respectively, using ethanol (1–3 g/kg) or ethanol/caffeine (3mg/kg + 2 g/kg) challenges. In addition, locomotor activity was evaluated initially and after repeated exposure to oral gavage of these drugs and doses.
Results
Repeated oral gavage of ethanol produced significant locomotor sensitization, with those mice increasing total distance traveled by 2-fold. The locomotor response to caffeine, while significantly greater than vehicle gavage, did not change with repeated exposure. On the other hand, repeated gavage of caffeine/ethanol combination produced a substantial increase in total distance traveled after repeated exposure (~4-fold increase). After repeated ethanol exposure, there was significant tolerance to ethanol in the conditioned taste aversion and parallel rod tests. However, neither a history of caffeine exposure nor including caffeine influenced ethanol-induced conditioned taste aversion. Interestingly, a history of caffeine exposure increased the ataxic response to the caffeine/ethanol combination and appeared to reduce the ataxic response to high doses of ethanol.
Conclusion
The data support the general hypothesis that repeated exposure to caffeine influences the response to ethanol. Together with previously published work, these data indicate that caffeine influences some ethanol-related behaviors, notably locomotion and ataxia, but appears not to influence the expression of conditioned behaviors.
Keywords: ethanol, psychostimulant, place preference, ataxia, drug interaction
INTRODUCTION
A growing trend in recent years has been to combine caffeinated energy drinks with alcohol, due in part to the tremendous growth in energy drink sales (Reissig et al., 2009; Seifert et al., 2011). In the United States, epidemiological studies (Arria et al., 2010; Arria et al., 2011), supported by field testing data (Pennay et al., 2014; Thombs et al., 2009), indicate that consumption of alcohol mixed with energy drinks is common. Unfortunately, consuming these mixtures has been associated with increases in emergency room visits (SAMHSA, 2011; SAMHSA, 2013), suggesting that consumption of these drinks can be dangerous. Mixing caffeinated beverages and alcohol is not a new practice nor is it limited to energy drinks since other caffeinated drinks like colas are also popular mixers with alcohol (Rossheim and Thombs, 2011; Thombs et al., 2011). However, many energy drinks typically have more caffeine per serving than other caffeinated beverages, ranging widely from 50 to 500 mg total caffeine per package (Heckman et al., 2010; Reissig et al., 2009). While the higher amount of caffeine can be comparable to brewed coffee, it far exceeds that of a typical cola (~30 mg/serving; see Heckman et al. 2010). Additionally, unlike coffee, some manufacturers of energy drinks promote rapid consumption of their product (Reissig et al., 2009). Thus, energy drinks provide quick access to relatively large amounts of caffeine, and in combination with alcohol, increase the risk of clinically significant interactions between caffeine and alcohol.
Pharmacological interactions of caffeine and alcohol (ethanol) have been studied in humans and animal models. Studies in humans have focused on caffeine’s ability to antagonize deficits caused by ethanol and indicate that caffeine antagonizes some of the cognitive and psychomotor deficits caused by ethanol (Attwood et al., 2012; Hasenfratz et al., 1993; Liguori and Robinson, 2001; Mackay et al., 2002; Marczinski and Fillmore, 2003). Other research using human subjects has focused on energy drinks as the source of caffeine in laboratory tests and report that these combinations can mildly influence subjective intoxication (Ferreira et al., 2006; Peacock et al., 2013), perceived stimulation (Peacock et al., 2012; Peacock et al., 2013, 2014) and can enhance the desire to drink (Marczinski et al., 2012; McKetin and Coen, 2014). With regards to animal models, a recent review nicely summarizes the growing research with caffeine/ethanol combinations (Lopez-Cruz et al., 2013). Previous work has shown that caffeine can increase ethanol drinking in rats (Kunin et al., 2000; Rezvani et al., 2013) and can promote significant ethanol-induced taste aversions (Kunin et al., 2001). Additionally, it has been reported that combinations of caffeine and ethanol, compared to either drug alone, increase locomotor activity after acute injection (Hilbert et al., 2013) or while consuming the drug mixtures in a drinking paradigm (Fritz et al., 2014). Some research has also incorporated energy drinks into animal models and shown that they can antagonize locomotor deficits with higher doses of ethanol (Ferreira et al., 2004) similar to administering only caffeine (Hilbert et al., 2013). Further, when given acutely energy drinks combined ethanol can significantly enhance activity when there is a history of ethanol exposure (Ferreira et al., 2013).
Energy drinks sold by different manufacturers contain a variety of ingredients, but caffeine is common to most of them and many studies have defined caffeine’s pharmacological effects in humans and animal models. Therefore, the present studies focus on the interactive effects of caffeine and ethanol. Since there is relatively little information regarding chronic exposure to the combination of caffeine and ethanol, the primary goal of these studies was to examine how repeated oral exposure to these drugs influences the development of tolerance or sensitization using two established behavioral procedures that are known to be influenced by chronic ethanol exposure. To do this, we used a conditioned taste aversion procedure (Lopez et al., 2012) and another procedure to measure ataxia (Griffin et al., 2013). Prior work by our laboratory and others, indicated that chronic ethanol exposure would produce tolerance to an ethanol challenge (i.e. less aversion and less ataxia) and we hypothesized that caffeine combined with ethanol might augment the development of tolerance in these procedures. Additionally, to extend our earlier work looking at the effects of acute administration of these drugs on locomotion (Hilbert et al., 2013), we measured locomotor activity in an open field after the first oral gavage and then again after repeated oral gavage. Following from our earlier work (Griffin et al., 2013; Griffin et al., 2010; Hilbert et al., 2013; McGovern et al., 2014), our general hypothesis was that caffeine would increase the stimulatory effects of ethanol when administered acutely and that these effects would increase with repeated exposure.
MATERIALS AND METHODS
General study design
These studies examined conditioned taste aversion (CTA), ataxia and locomotor activity in C57BL/6J mice with histories of repeated exposure to ethanol (ethanol; 4 g/kg), caffeine (15 mg/kg), or the combination of these two drugs and doses. The choice of the ethanol dose for the repeated exposure procedure was influenced by earlier work showing that the oral gavage route of administration can achieve high blood ethanol levels (Griffin et al., 2009), but also that it would not completely impair locomotion. We chose to use 15 mg/kg caffeine based on previous work showing that administration of this dose would significantly increase locomotor activity (Hilbert et al., 2013; Kaplan et al., 1989). For the exposure procedure, drugs were administered by oral gavage twice per day (at 0730h and 1530h). After 4 days (i.e. 8 gavages), the exposure procedure was suspended for 3 days while mice were evaluated for CTA induced by ethanol or a caffeine-ethanol combination. After completing the CTA procedure, mice resumed twice daily gavage for 4 additional days and the following day, were evaluated for the ataxic effects of ethanol, caffeine or a caffeine-ethanol combination. These studies were conducted over 3 experiments with the third conducted in 2 replicates (details in Table 1). Additionally, the third experiment included measurement of locomotor activity after the 1st and 15th gavages while conducting the repeated exposure procedure, with CTA and ataxia still evaluated at the same time points as indicated for experiments 1 and 2.
TABLE 1.
| Experiment | Figure | CTA Doses | Ataxia Doses | Activity? |
|---|---|---|---|---|
| 1 | 1A, 2A | 2 g/kg | 2 g/kg | No |
| 2 | 1B, 2B | 1 g/kg | 3 g/kg | No |
| 3 & 4 | 1C, 2C, 3A, 3B | 2 g/kg + 3 mg/kg | 2 g/kg + 3 mg/kg | Yes |
Ethanol doses in g/kg; caffeine doses in mg/kg
Subjects
Adult male C57BL/6J mice were used in these experiments (Jackson Laboratories, Bar Harbor, ME). Mice arrived at 8–9 weeks of age and acclimated at least 1 week before beginning studies. All mice were singly housed under standard conditions (12h light cycle; lights off 1400hr) in an AAALAC accredited facility with free access to food and water. All procedures were approved by the Medical University of South Carolina Institutional Animal Care and Use Committee.
Conditioned Taste Aversion (CTA) procedures
This procedure was conducted similarly to that described by Lopez and colleagues (Lopez et al., 2012). For this procedure, saccharin (1%) was presented on two consecutive days for 30 minutes at the beginning of the dark phase in the circadian cycle. Immediately at the conclusion of the second session, mice were intraperitoneally injected with vehicle (0.9% NaCl), ethanol (1 or 2 g/kg) or a caffeine-ethanol combination (3 mg/kg caffeine + 2 g/kg ethanol). The next day, mice were presented with the saccharin solution for 30 minutes (Test day). Each day saccharin intake was recorded to the nearest 0.1 mL and the amount consumed was corrected for spillage by using bottles placed on empty cages. An important methodological difference for the experiment reported in Figure 1C compared to other two experiments was that only one conditioning session was used, rather than two as for the other experiments This resulted in some mice across the 4 exposure groups consuming <0.2 ml saccharin during the 30 minute conditioning period and being excluded from the CTA analysis. Because of this, an additional replicate of experiment 3 was conducted to increase the number of mice available for CTA analysis and mice in this replicate were treated exactly as described experiment 3.
Figure 1. Caffeine exposure history did not influence conditioning to the aversive effects of ethanol or ethanol plus caffeine.
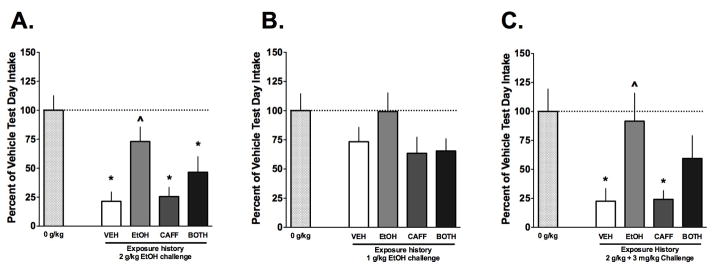
A) Compared with 0 g/kg, a strong aversion was produced using 2 g/kg ethanol as indicated by the significant reduction in saccharin intake in the mice with VEH exposure history. Further, the repeated exposure to ethanol (EtOH group) produced tolerance because no significant reduction occurred and the intake was greater than the VEH group. However, caffeine pre-exposure did not influence the development of aversion or tolerance to ethanol (n=7–10/grp). B) A lower dose of ethanol (1 g/kg) did not produce aversion and a history of caffeine exposure did not influence this (n=9–12/grp). C) The combination of ethanol and caffeine produced aversion in the VEH and CAFF groups and there was tolerance to this effect in the EtOH and BOTH (i.e. EtOH + CAFF) groups, though the EtOH and BOTH groups did not differ in the magnitude of the response (n=8–12/grp). Data are means ± S.E.M. (*=p<0.05 vs. 0 g/kg challenge, ^=p<0.05 vs. vehicle history).
Ataxia apparatus and procedures
The apparatus and procedures have been described previously (Griffin et al., 2013) and were adapted from previous work (Kamens and Crabbe, 2007). In brief, footslips were counted as mice ambulated within a small arena on a floor constructed of parallel rods. Footslips were defined as any hindfoot or forefoot slip between the parallel rods. Additionally, vertical lines on the rear of the arena spaced 5 cm apart allowed the footslip counts to be normalized to the number of lines crossed to control for drug effects on general activity (using the base of the tail as the reference point). For the test, mice were intraperitoneally injected with vehicle (0.9% NaCl), ethanol (2 or 3 g/kg) or a caffeine-ethanol combination (3 mg/kg caffeine + 2 g/kg ethanol) and returned to their home cage for 5 minutes before being placed into the arena. The session was 2 minutes and video recorded for later visual quantification.
Locomotor activity (LA) apparatus and procedures
Modified Med Associates (St. Albans, VT, USA) activity chambers (ENV-510) were used for these experiments. As previously described, the open field arenas were restricted in size to 13.5 cm wide × 27.5 cm long × 20.5 cm deep (Hilbert et al., 2013). In this experiment, the mice were placed immediately into the locomotor activity apparatus after the 1st and 15th gavages and distance traveled (cm) was measured for 45 minutes. Both of these activity measurement sessions occurred during light phase of the circadian cycle.
Drugs
Drugs were administered by either oral gavage using water as the vehicle for the repeated exposure or given intraperitoneally (IP) with normal saline as the vehicle (0.9% NaCl). The volume was 0.02ml/g of body weight for all dosing. When the drug combination is indicated, caffeine and ethanol were administered simultaneously in the same solution. Caffeine used was the anhydrous base (Fluka, a subsidiary of Sigma-Aldrich, St Louis, MO). Ethanol (ethanol; 95%) was obtained from AAPER (Shelbyville, KY) and diluted as needed.
Statistical analyses
The primary dependent variables for these studies were saccharin intake (mL), footslip counts per line cross, and distance traveled (cm). For saccharin intake, the Test day data are presented as a percent of the saccharin intake occurring during the conditioning session 24 hours earlier. Note that for the data shown in Figures 1 and 2, the data in the light shaded bar (0 g/kg) represents approximately equal numbers of mice (n=3–5) from each exposure history that were combined into a single group to represent a baseline comparison for the challenge doses of ethanol and ethanol plus caffeine used for the CTA or the Footslip procedures. Additionally, for the CTA experiments (Figure 1), the data were normalized to the saccharin intake of vehicle conditioning group (0 g/kg) on test day.
Figure 2. Caffeine exposure history mildly influences ethanol-induced or ethanol plus caffeine-induced ataxia.
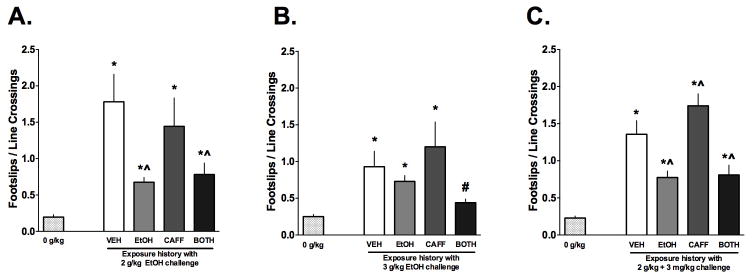
A) Acute injection of 2 g/kg increased footslips in all groups compared to 0 g/kg, however the EtOH and BOTH (i.e. EtOH + CAFF) had smaller increases, indicative of tolerance to the ataxic effects of ethanol (n=7–10/grp. B) A higher dose of ethanol (3 g/kg) increased footslips in the VEH, EtOH and CAFF groups. Footslips recorded in the BOTH group were similar to those of the 0 g/kg group indicating that the history of ethanol/caffeine exposure produced tolerance to high doses of ethanol (n=9–12/grp). C) Acute challenge with ethanol and caffeine also produced ataxia similarly to the results shown in Panel A. The CAFF mice showed an increase in footslips compared to the VEH mice, indicating that the history of caffeine increased the ataxic response to the drug combination (n=12–20/grp). Data are means ± S.E.M. (*=p<0.05 vs. vehicle challenge, ^=p<0.05 vs. vehicle history, #=p>0.05 vs. 0 g/kg).
All data in these experiments were analyzed using Analysis of Variance (ANOVA) with repeated measures (RM) when necessary and significant factor interactions were followed up using Pairwise Multiple comparisons. Additionally, to facilitate comparisons between exposure histories across the different experiments shown in Figure 1 and Figure 2, we calculated Cohen’s d values for each possible combination of groups (Nakagawa and Cuthill, 2007). The Cohen’s d values were compared across relevant pairs using Chi square analyses. Alpha was set to 0.05 for all analyses.
RESULTS
Conditioned Taste Aversion (CTA)
The influence of repeated exposure of ethanol, caffeine or the combination on the conditioned taste aversion was examined using saccharin as a conditioned stimulus and either ethanol or an ethanol-caffeine combination as the unconditioned stimulus (Figure 1).
For the experiment shown Figure 1A, the one way ANOVA was significant (F(4, 42) = 8.513, p < 0.001) indicating that the exposure history of the mice contributed to different intake responses during the CTA test. As can be seen, relative to the 0 g/kg conditioning dose, the 2 g/kg ethanol dose produced a significant aversion (~80% decrease, *p<0.05) in saccharin consumption during the test in mice with a history of vehicle exposure consistent with previous work (Lopez et al., 2012). Also as expected, mice with a history of ethanol exposure (EtOH group) demonstrated tolerance to the aversive effects of 2 g/kg ethanol because there was no significant decrease in saccharin consumption during the CTA test. Further, it was noted that the saccharin consumption was significantly greater in the EtOH group compared to the VEH mice (^p<0.05). Mice with a history of caffeine exposure demonstrated a similar response to 2 g/kg ethanol when compared with the VEH history mice, indicating that a history of CAFF exposure did not alter the conditioned aversive effects of ethanol in this paradigm. Saccharin intake for those mice with a history of exposure to ethanol plus caffeine (BOTH group) was significantly reduced indicating a significant aversion (*p<0.05) although there was not a significant difference compared to the EtOH group response. Thus, the history of combined ethanol and caffeine exposure did not alter the capacity of the mice to develop aversion to an ethanol challenge compared to only a history of ethanol exposure.
In another group of mice, the influence of these same exposure histories on aversive conditioning was examined using a lower dose of ethanol (1 g/kg; Figure 1B). The one way ANOVA did not reveal a significant effect of exposure (F(4, 36) = 2, p > 0.05). The lack of conditioned aversion using 1 g/kg ethanol in both the VEH exposure group and the EtOH exposure group was expected (Lopez et al., 2012). However, this analysis also indicates that a history of caffeine or caffeine in combination with ethanol did not facilitate aversive conditioning with a low dose of ethanol.
The possibility that a combination of ethanol and caffeine would produce CTA to saccharin was also examined (Figure 1C). The one way ANOVA was significant (F(4, 45) = 5.153, p < 0.002), again indicating that the exposure history altered the conditioned response to the drug combination in the CTA paradigm. Post-hoc analysis found that the mice with a history of VEH exposure and CAFF exposure demonstrated significant aversion to the drug combination (both *p<0.05), consistent with a previous report (Kunin et al., 2001).. On the other hand, there was clear evidence of ethanol tolerance since the saccharin intake was not different in the EtOH exposure group relative to the 0 g/kg group and the EtOH group’s intake was also significantly greater than the VEH exposure group (^p<0.05). The mice in the BOTH exposure group demonstrated an intermediate response to conditioning with the drug combination. This group did not have a significant reduction in saccharin intake compared to the 0 g/kg group (p>0.05), indicative that some tolerance developed to the aversive effects of the ethanol plus caffeine combination. However, mice in the BOTH group did not show a significant increase in saccharin intake relative to the VEH exposure group indicating that the tolerance was less robust than that observed in the EtOH exposure group.
As described in the methods, the experiments shown in each panel of Figure 1 were conducted independently. To facilitate comparison across these experiments, we calculated bias corrected Cohen’s D values to serve as a measure of effect size for each possible pair of groups in the experimental design. The Cohen’s D values from relevant pairs of data from the 3 experiments were compared using Chi Square analyses (see Methods). Of interest were comparisons of the response differences between various groups across the 3 experiments mice to determine if caffeine influenced the effects of ethanol-induced CTA. For example, the difference in behavioral responses between the EtOH and BOTH groups resulted in effect sizes (i.e. Cohen’s d) of 0.647, 0.842 and 0.517 for Experiments 1, 2 and 3, respectively. Given the relatively similar effect sizes, the Chi Square analysis did not find a significant effect (X2 = 0.084, p>0.05). Further, the 95% confidence intervals (CI) for the calculated effect sizes overlapped substantially. Other comparisons from these 3 experiments also did not reveal any significant differences in effect sizes between any of the other groups (all X2<1.7, p>0.05; See Supplementary Table 1 for the complete list of comparisons) and there was typically significant overlap in the 95%CI. It is noteworthy that as part of these analyses, Student’s T-tests were conducted for each pair of comparisons and those findings replicate those already described above that used ANOVA and post-hoc tests (Supplementary Table 1).
Ataxia
Next, we examined the influence of repeated exposure of ethanol, caffeine or the combination on ataxia caused by acute ethanol or caffeine-ethanol challenge (Figure 2). The data are reported as footslips normalized according to their activity in the arena (i.e. line crosses) and compared to mice challenged with 0 g/kg.
As shown in Figure 2A, a 2 g/kg ethanol dose increased footslips in all mice relative to the 0 g/kg challenge. A one way ANOVA on these data was significant (F(4, 35) = 7.193, p < 0.01) and confirmed that the history of exposure modified the amount of ataxia demonstrated by the mice. Post-hoc analysis indicated that all mice challenged with 2 g/kg ethanol had increased footslips/line cross compared to the 0 g/kg challenge (*p<0.05). The VEH and CAFF exposure history mice exhibited the most footslips. Compared to the VEH exposure group, EtOH and BOTH exposure histories had reduced ataxia (^p<0.05), consistent of the development of tolerance to the ataxic effects of ethanol as reported by others (Linsenbardt et al., 2011) when ethanol was part of the exposure history. However, in this study, previous CAFF exposure did not influence the ataxic effects of acute ethanol challenge.
An additional group of mice with the same exposure histories was challenged with a higher dose of ethanol (3 g/kg; Figure 2B). The one way ANOVA on these data was significant (F(4, 43) = 4.914, p < 0.05), indicating that the exposure history influenced the ataxic response to ethanol. Post-hoc analysis found that only mice in the VEH, EtOH and CAFF groups exhibited footslips greater than 0 g/kg (*p<0.05) while mice in the BOTH group did not (#p>0.05). The reduced number of footslips in the BOTH group suggests that the exposure to caffeine and ethanol influenced the development of tolerance to the ataxic effects of the high dose of ethanol (3 g/kg).
Lastly, we examined the acute effects of ethanol plus caffeine on ataxia in mice with a history of exposure to VEH, EtOH, CAFF or BOTH (Figure 2C). The one way ANOVA on these data was significant (F(4, 67) = 26.42, p < 0.05), indicating that exposure history influenced ataxia in response to a challenge with 2 g/kg ethanol plus 3 mg/kg caffeine. However, the analysis revealed that the response to the drug combination in this experiment were similar to that for 2 g/kg ethanol alone (Figure 2A) with the exception that the history of CAFF exposure increased the footslips recorded compared to the VEH group. This small, but significant (^p<0.05), increase in footslips following a history of CAFF exposure suggests that sensitization developed to the ataxic effects of caffeine.
Similar to the CTA studies above, Cohen’s d values for the footslip data were also calculated to facilitate comparison across the three experiments. Interestingly, these analyses found a significant difference in effect sizes for the response differences between the EtOH and BOTH groups across the three experiments (X2 = 10.687, p<0.01). The Cohen’s d values were 0.19, 1.364 and 0.079 for Experiments 1, 2 and 3, respectively. In this case, the significant X2 statistic was driven by the relatively large effect size for the difference between footslips for the EtOH and BOTH mice from Experiment 2 using the high dose of ethanol (3 g/kg), compared to the relatively small differences between those same groups for Experiments 1 and 2. The 95% confidence intervals for the Cohen’s d values diverged to a larger extent than for other comparisons. This analysis corroborates the findings in Figure 2B described above that combined exposure to ethanol and caffeine appears to increase tolerance to heavy doses of ethanol. The same analyses for the other footslip data sets did not reveal any other significant effects (all X2 <2.5, p>0.05; See Supplementary Table 2).
Locomotor Activity
We measured locomotor activity immediately after the 1st and 15th oral gavages (see Table 1). As described in the methods, these gavages were with VEH, EtOH (4 g/kg), CAFF (15mg/kg) and BOTH (EtOH 4g/kg + CAFF 15 mg/kg).
These data were initially analyzed using a 4(History) × 2(Gavage) × 9(Bin) ANOVA with Gavage and Bin as repeated measures. The 3-way interaction was significant (F (24, 536) =12.069, p<0.0001) as were all of the 2-way interactions (all F’s >28, p<0.0001) and factors (all F’s >11, p<0.0001). This analysis confirmed what can be seen in Figure 3, that locomotor activity was increased with repeated gavage, particularly in the ethanol plus caffeine group.
Figure 3. Repeated gavage of ethanol or ethanol plus caffeine produces locomotor sensitization.
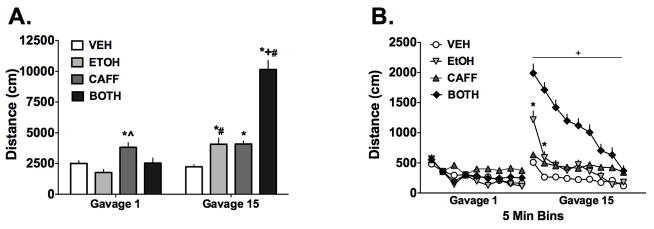
A) Total distances traveled following the 1st gavage were only increased in the CAFF group compared to vehicle but by the 15th gavage, the EtOH, CAFF and BOTH (i.e. EtOH + CAFF) groups showed increased activity compared to VEH. Further, the EtOH and BOTH groups demonstrated significantly increased activity by the last gavage compared to the first with the increase in activity for the BOTH group being particularly robust (n=12–20/grp). B) The same data shown as a within-session time course and demonstrate that for the EtOH and BOTH groups, the increased activity after the 15th gavage was greatest at the beginning of the session and declined with time. Data are means ± S.E.M. (*=p<0.05 compared to vehicle group, ^=p<0.05 compared to ethanol group, #=p<0.05 compared to same exposure group gavage 1)
To determine how exposure history interacted with repeated gavage challenge, the 4(History) × 2(Gavage) repeated measures ANOVA, which is part of the overall 3-way ANOVA, was examined further and the analysis is summarized in Figure 3A. The factor interaction was significant (F (3, 67) = 32, p<0.0001) as were effects of History (F (3,67) = 64, p<0.0001) and Gavage (F (1,67) = 40, p<0.0001). Post-hoc analysis of the data after the 1st gavage found that only the CAFF challenge increased distance traveled more than VEH (*p<0.05) and EtOH (^p<0.05). By the 15th gavage, the distances traveled increased for the EtOH, CAFF and BOTH groups compared to VEH during that gavage (*p<0.05). In particular, there was a dramatic increase in distance traveled for the BOTH group that was significant compared to all other groups (+p<0.05). Additionally, when compared with activity following Gavage 1, the activity of the EtOH and BOTH groups increased significantly (#p<0.05) but this was not found for the VEH and CAFF groups. These data indicate the development of sensitization following repeated gavage to ethanol and the combination of ethanol plus caffeine. Repeated exposure to ethanol resulted in a ~2-fold increase in activity from Gavage 1 to Gavage 15 while the same comparison for the BOTH group showed a ~4-fold increase in activity.
Given the large increase in distance traveled during the 15th gavage in the drug combination group, we examined how locomotor activity changed within session for all 4 groups. These data are summarized in Figure 3B. The analysis used separate 4(History) × 9(Bin) ANOVAs with Bin as a repeated measure for both Gavage 1 and Gavage 15. For Gavage 1, the factor interaction was significant (F (24, 544)= 2.991, p<0.0001) as were History (F (3,68)= 6.6, p=0.001) and Bin (F (8, 544)= 32, p<0.0001). Due to the number of comparisons being made, Bonferroni’s correction was used in the post-hoc analysis. The post-hoc analysis for Gavage 1 did not reveal very many specific differences between the four groups during any given Bin and therefore are not shown. However, the analysis did support the general observation that activity declined over time, though this was not evident for the CAFF group (for example, Bin 1 vs Bin 9, p>0.05).
For Gavage 15, the factor interaction for the 2 way ANOVA was significant (F (24, 536)=14, p<0.0001) as were the History (F (3,67)=50, p<0.0001) and Bin (F (8, 544) = 74, p<0.0001) factors. Again, Bonferroni’s correction was applied to the post-hoc comparisons for this analysis. The post-hoc analysis revealed that the BOTH group traveled significantly more distance during Bins 1 through 9 compared to VEH and EtOH groups as well as Bins 1 through 6 for the CAFF group (all these comparisons denoted as +p<0.05). Additionally, the EtOH group showed increased activity during Bins 1 and 2 (*p<0.05) compared to the VEH group and Bin 1 versus the CAFF group (*p<0.05). These findings support the analysis described for Figure 3A and further indicate that the large increases in distance traveled for the BOTH and EtOH groups occurred at the beginning of the session and declined with time.
DISCUSSION
Our results demonstrate that caffeine alters the behavioral effects of ethanol, in particular, when measured immediately after drug administration but there is evidence that a history of caffeine exposure influences the response to ethanol as well. The most substantial finding was that the combination of caffeine and ethanol produced a robust locomotor sensitization. After repeated exposure, the effect of the drug combination on activity was approximately 2.5 times greater than either that produced by ethanol or caffeine alone, suggesting that caffeine/ethanol co-intoxication has a synergistic effect on the development of locomotor sensitization. Although ethanol tolerance was established after repeated exposure in both the conditioned aversion and ataxia procedures, only the ataxic effects of acutely administered ethanol or the ethanol/caffeine combination were influenced by the presence of caffeine in the exposure history.
When given by oral gavage for the first time, the combination of caffeine and ethanol tended to antagonize the slight (non-significant) locomotor depression caused by ethanol given alone and by the 15th gavage there was a striking locomotor sensitization effect with the drug combination group compared to the other three groups after either the 1st or 15th gavage. Although this large effect declined with time during the session, the large increase in distance traveled was sustained for most of the 45 minute session compared to the other three groups. These results indicate that repeated co-exposure to ethanol and caffeine produces a much larger effect than repeated exposure to either drug alone. A recent report by Fritz and colleagues using a voluntary consumption model in the home cage hinted at the possibility that repeated exposure to a combination of ethanol and caffeine could produce a sensitized locomotor response (Fritz et al., 2014), though the effect was not reported as significant. Another report indicated that an ethanol sensitization effect could be enhanced in mice when acutely challenged with a mixture of an energy drink and ethanol, particularly in those mice in which the ethanol sensitization effect is small (Ferreira et al., 2013). Taken together, these data indicate that a history of exposure to the drug combination influences the locomotor response when subjects are acutely challenged.
The experimental design of the locomotor activity measurements also afforded the opportunity to examine the effects of repeated ethanol and caffeine exposure, separately, on locomotor activity in the mice. The initial activity response to ethanol oral gavage was reduced compared to vehicle gavage (albeit non-significantly) but then increased significantly more than the vehicle response, indicative of sensitization. Locomotor sensitization following repeated ethanol exposure has been reported in mice in a variety of studies (Carrara-Nascimento et al., 2011; Fish et al., 2002; Lessov et al., 2001; Lessov and Phillips, 1998; Phillips et al., 1997). With regards to caffeine, although it acutely increased activity consistent with previous reports (Buckholtz and Middaugh, 1987; Hilbert et al., 2013; Kaplan et al., 1989), repeated exposure to caffeine did not produce locomotor sensitization in our model. While several other reports indicate that caffeine can induce a small, albeit significant, locomotor sensitization effect (Ball and Poplawsky, 2011; Hsu et al., 2009; Hsu et al., 2010; Zancheta et al., 2012), the reason for the lack of caffeine locomotor sensitization in our model is unclear but may be related to methodological differences such as dose, route of administration and frequency of administration.
When given acutely to the mice with a history of vehicle exposure (VEH group), ethanol and the ethanol/caffeine combination both produced significant conditioned taste aversion and increased footslips (i.e. ataxia). For the conditioned taste aversion experiments, the effect of ethanol was dose-dependent, with the 2 g/kg dose of ethanol producing significant aversion while the 1 g/kg dose did not, as expected (Lopez et al. 2012). The addition of caffeine to ethanol during conditioning did not further enhance aversion. Perhaps future testing with the smaller ethanol dose (1g/kg) in combination with caffeine would yield significant aversion as previously reported (Kunin et al., 2001). Alternatively, examining conditioned taste aversion after 16 gavage exposures (as done for the parallel rod test) could also reveal an influence of caffeine on this behavior. Similarly, in the ataxia measurements, acutely administered ethanol significantly increased footslips as expected but the addition of caffeine did not further augment the increase in VEH exposed mice. Interestingly, the higher dose of ethanol (3 g/kg) produced slightly less footslips than the 2 g/kg dose (Figure 2), which reflects similar numbers of footslips but less overall activity due to sedation from the high dose. Earlier, we reported that adding methylphenidate to ethanol intoxication further increased footslips (Griffin et al., 2013) and another laboratory indicated this possibility using caffeine in rats (Dar et al., 1987), though at higher doses. However the present data do not indicate that this occurred with the combination of ethanol and caffeine given acutely in this experiment.
Repeated exposure to ethanol leads to tolerance in several behavioral paradigms, resulting in diminished effects to ethanol during a subsequent challenge, including measures of ataxia (Linsenbardt et al., 2011) and conditioned taste aversion (Lopez et al., 2012). Indeed, as expected, significant ethanol tolerance was found in both the ataxia and the aversive conditioning procedures after repeated oral gavage of ethanol. Of interest in the present studies was whether caffeine increased or decreased the development or expression of ethanol tolerance. In our conditioned taste aversion procedure, we found that a history of caffeine or caffeine/ethanol did not significantly increase or decrease either the development or expression of ethanol tolerance. However, in contrast, a history of caffeine exposure did significantly affect ethanol-induced ataxia in the parallel rod test. First, for mice having a history of ethanol/caffeine exposure and then challenged with a high dose of ethanol (3 g/kg) there was not a significant increase in footslips compared to the 0 g/kg challenge as with the other groups (Figure 2B). This effect was not found using a lower ethanol dose, consistent with other work (Linsenbardt et al., 2011), and suggests the development of tolerance to the ataxic effects of a high ethanol dose when caffeine is included as part of the ethanol exposure history, though more experimental work is needed for confirmation. Secondly, mice with a history of only caffeine exposure showed increased footslips when challenged with the ethanol/caffeine combination (Figure 2C). There was a trend for this same behavioral effect when mice were challenged only with ethanol (Figure 2B), although it did not reach statistical significance. This finding indicates that a history of caffeine exposure negatively influences fine motor coordination after a ethanol challenge, consistent with an earlier study using 10 days of caffeine exposure and a 1.5 g/kg ethanol challenge dose (Dar and Wooles, 1986).
As previously reviewed (Ferre and O’Brien, 2011), the adenosinergic system is likely to be an important mediator in the behavioral effects reported here since both caffeine and ethanol interact with this neurotransmitter system. It is well established that caffeine antagonizes central A1 and A2a adenosine receptors (Fredholm et al., 2011) and it was reported that these pharmacological actions underlie the locomotor activating effects of caffeine (Kuzmin et al., 2006). Additionally, ethanol increases adenosine concentrations by inhibiting transporters and increases in adenosine may underlie the sedative and ataxic effects of ethanol (Dunwiddie and Masino, 2001; Nam et al., 2012; Ruby et al., 2014). Further, chronic exposure to either ethanol or caffeine results in adaptations of the adenosine receptors (Butler and Prendergast, 2012; Fredholm et al., 2011). In fact, evidence suggests that chronic antagonism of the A2a receptor contributes to caffeine sensitization (Hsu et al., 2009; Hsu et al., 2010). While it is clear that caffeine and ethanol can interact at the level of adenosine transmission, the downstream effects of A1 and A2a receptor blockade likely involve alterations in glutamate and dopamine neurotransmission (Quarta et al., 2004) to affect behavior. Thus, adaptations in the adenosinergic system and its subsequent role in modulating glutamate and dopamine transmission may underlie the behavioral changes that reported here.
The per capita consumption of caffeine appears to be increasing and many investigators have expressed concerns regarding caffeine consumption in general (Attwood, 2012; Higgins et al., 2010; Reissig et al., 2009; Wolk et al., 2012). The half-life of caffeine in humans varies with the dose with higher doses having a longer half-life (Kaplan et al., 1997). A single 500 mg dose of caffeine yields peak blood concentrations of ~18 μg/ml that, after 4 hours, declines to 7–8 μg/ml, a concentration range still associated with significant behavioral effects when lower doses are administered (Kaplan et al., 1997). Interestingly, a 15 mg/kg dose in mice yields nearly similar blood levels of caffeine (~15 μg/ml) as the 500 mg dose in humans (Kaplan et al., 1989), providing some relevance to our choice of dose in this set of experiments. Moreover, ethanol appears to increase caffeine’s half-life in humans (George et al., 1986) though this may not be true in mice (Fritz et al., 2014). Therefore, consideration of higher caffeine doses is important in the context of energy drink consumption, not only because of the higher concentration of caffeine in some of these drinks, but also that many people could already have significant amounts of caffeine in their body from other sources. With caffeine already on board at the beginning of a drinking episode that includes energy drinks, caffeine levels increase even further. However, examining higher doses of caffeine (and ethanol) in human laboratory experiments is obviously problematic due to the development of pleasant side effects and safety concerns once doses rise above 200mg caffeine (Wolk et al., 2012), underscoring the crucial role of animal models for examining the interactive effects of caffeine and ethanol over a wide range of doses (Griffin, 2013).
In conclusion, our results support the general hypothesis that caffeine influences the effects of ethanol, particularly locomotion and ataxia. We found that pre-exposure to the caffeine/ethanol combination tempered the acute ataxic effects of a high ethanol dose and, similar to an earlier study (Dar and Wooles, 1986), that the drug combination itself slightly increased ataxia when administered to mice with a history of caffeine exposure. A history of caffeine exposure (with or without ethanol) did not influence conditioned aversion to ethanol or the caffeine/ethanol combination. This finding recalls results from our recent place-preference experiment (Hilbert et al., 2013), suggesting that caffeine does not influence conditioned effects of ethanol, though more work is necessary to draw a firm conclusion. When given by oral gavage, caffeine also tended to acutely antagonize the locomotor depressant effects of a high ethanol dose, consistent with our earlier work (Hilbert et al., 2013) and other reports (Ferreira et al., 2004; Fritz et al., 2014). Moreover, we found that with repeated exposure, caffeine co-exposure with ethanol leads to large increases in activity compared to either drug given alone. Though speculative, the implication of these findings is that the pro-stimulant effects of this drug combination give people more time to drink and to engage in risky behaviors before ethanol-induced sedation curtails activity. Indeed, emerging data appears to support the idea that drinkers who mix energy drinks and alcohol have longer drinking episodes (Pennay et al., 2014). Therefore, caffeine’s influence on ethanol-related behaviors may have important implications both acutely as well as over the long term.
Supplementary Material
Acknowledgments
Support from NIAAA P50 AA10761.
Bibliography
- Arria AM, Caldeira KM, Kasperski SJ, O'Grady KE, Vincent KB, Griffiths RR, Wish ED. Increased alcohol consumption, nonmedical prescription drug use, and illicit drug use are associated with energy drink consumption among college students. J Add Med. 2010;4:74–80. doi: 10.1097/ADM.0b013e3181aa8dd4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arria AM, Caldeira KM, Kasperski SJ, Vincent KB, Griffiths RR, O'Grady KE. Energy drink consumption and increased risk for alcohol dependence. Alcohol Clin Exp Res. 2011;35:365–375. doi: 10.1111/j.1530-0277.2010.01352.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attwood AS. Caffeinated alcohol beverages: a public health concern. Alcohol Alcohol. 2012;47:370–371. doi: 10.1093/alcalc/ags062. [DOI] [PubMed] [Google Scholar]
- Attwood AS, Rogers PJ, Ataya AF, Adams S, Munafo MR. Effects of caffeine on alcohol-related changes in behavioural control and perceived intoxication in light caffeine consumers. Psychopharmacol. 2012;221:551–560. doi: 10.1007/s00213-011-2601-0. [DOI] [PubMed] [Google Scholar]
- Ball KT, Poplawsky A. Low-dose oral caffeine induces a specific form of behavioral sensitization in rats. Pharmacol Rep. 2011;63:1560–1563. doi: 10.1016/s1734-1140(11)70721-6. [DOI] [PubMed] [Google Scholar]
- Buckholtz NS, Middaugh LD. Effects of caffeine and L-phenylisopropyladenosine on locomotor activity of mice. Pharmacol Biochem Behav. 1987;28:179–185. doi: 10.1016/0091-3057(87)90211-5. [DOI] [PubMed] [Google Scholar]
- Butler TR, Prendergast MA. Neuroadaptations in adenosine receptor signaling following long-term ethanol exposure and withdrawal. Alcohol Clin Exp Res. 2012;36:4–13. doi: 10.1111/j.1530-0277.2011.01586.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrara-Nascimento PF, Griffin WC, 3rd, Pastrello DM, Olive MF, Camarini R. Changes in extracellular levels of glutamate in the nucleus accumbens after ethanol-induced behavioral sensitization in adolescent and adult mice. Alcohol. 2011;45:451–460. doi: 10.1016/j.alcohol.2011.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dar MS, Jones M, Close G, Mustafa SJ, Wooles WR. Behavioral interactions of ethanol and methylxanthines. Psychopharmacol. 1987;91:1–4. doi: 10.1007/BF00690916. [DOI] [PubMed] [Google Scholar]
- Dar MS, Wooles WR. Effect of chronically administered methylxanthines on ethanol-induced motor incoordination in mice. Life Sci. 1986;39:1429–1437. doi: 10.1016/0024-3205(86)90547-3. [DOI] [PubMed] [Google Scholar]
- Dunwiddie TV, Masino SA. The role and regulation of adenosine in the central nervous system. Annual review of neuroscience. 2001;24:31–55. doi: 10.1146/annurev.neuro.24.1.31. [DOI] [PubMed] [Google Scholar]
- Ferre S, O’Brien MC. Alcohol and Caffeine: The Perfect Storm. J Caffeine Res. 2011;1:153–162. doi: 10.1089/jcr.2011.0017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira SE, Abrahao KP, Souza-Formigoni ML. Expression of behavioral sensitization to ethanol is increased by energy drink administration. Pharmacol Biochem Behav. 2013;110:245–248. doi: 10.1016/j.pbb.2013.07.014. [DOI] [PubMed] [Google Scholar]
- Ferreira SE, de Mello MT, Pompéia S, de Souza-Formigoni MLO. Effects of energy drink ingestion on alcohol intoxication. Alcohol Clin Exp Res. 2006;30:598–605. doi: 10.1111/j.1530-0277.2006.00070.x. [DOI] [PubMed] [Google Scholar]
- Ferreira SE, Hartmann Quadros IM, Trindade AA, Takahashi S, Koyama RG, Souza-Formigoni MLO. Can energy drinks reduce the depressor effect of ethanol? An experimental study in mice. Physiol Behav. 2004;82:841–847. doi: 10.1016/j.physbeh.2004.06.017. [DOI] [PubMed] [Google Scholar]
- Fish EW, DeBold JF, Miczek KA. Repeated alcohol: behavioral sensitization and alcohol-heightened aggression in mice. Psychopharmacol. 2002;160:39–48. doi: 10.1007/s00213-001-0934-9. [DOI] [PubMed] [Google Scholar]
- Fredholm BB, APIJ, Jacobson KA, Linden J, Muller CE. International Union of Basic and Clinical Pharmacology. LXXXI. Nomenclature and classification of adenosine receptors--an update. Pharmacol Rev. 2011;63:1–34. doi: 10.1124/pr.110.003285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritz BM, Companion M, Boehm SL. “Wired,” yet intoxicated: modeling binge caffeine and alcohol co-consumption in the mouse. Alcohol Clin Exp Res. 2014;38:2269–2278. doi: 10.1111/acer.12472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George J, Murphy T, Roberts R, Cooksley WG, Halliday JW, Powell LW. Influence of alcohol and caffeine consumption on caffeine elimination. Clin Exp Pharmacol Physiol. 1986;13:731–736. doi: 10.1111/j.1440-1681.1986.tb02414.x. [DOI] [PubMed] [Google Scholar]
- Griffin WC., 3rd Commentary on Marczinski and colleagues: mixing an energy drink with an alcoholic beverage increases motivation for more alcohol in college students. Alcohol Clin Exp Res. 2013;37:188–190. doi: 10.1111/acer.12020. [DOI] [PubMed] [Google Scholar]
- Griffin WC, 3rd, Lopez MF, Becker HC. Intensity and duration of chronic ethanol exposure is critical for subsequent escalation of voluntary ethanol drinking in mice. Alcohol Clin Exp Res. 2009;33:1893–1900. doi: 10.1111/j.1530-0277.2009.01027.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin WC, 3rd, McGovern RW, Bell GH, Randall PK, Middaugh LD, Patrick KS. Interactive effects of methylphenidate and alcohol on discrimination, conditioned place preference and motor coordination in C57BL/6J mice. Psychopharmacol. 2013;225:613–625. doi: 10.1007/s00213-012-2849-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin WC, 3rd, Novak AJ, Middaugh LD, Patrick KS. The interactive effects of methylphenidate and ethanol on ethanol consumption and locomotor activity in mice. Pharmacol Biochem Behav. 2010;95:267–272. doi: 10.1016/j.pbb.2010.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasenfratz M, Bunge A, Dal Pra G, Battig K. Antagonistic effects of caffeine and alcohol on mental performance parameters. Pharmacol Biochem Behav. 1993;46:463–465. doi: 10.1016/0091-3057(93)90380-c. [DOI] [PubMed] [Google Scholar]
- Heckman MA, Sherry K, Gonzalez de Mejia E. Energy Drinks: An assessment of their market size, consumer demographics, ingredient profile, functionality, and regulations in the United States. Compr Rev Food Sci Food Saf. 2010;9:303–317. doi: 10.1111/j.1541-4337.2010.00111.x. [DOI] [PubMed] [Google Scholar]
- Higgins JP, Tuttle TD, Higgins CL. Energy beverages: content and safety. Mayo Clin Proc. 2010;85:1033–1041. doi: 10.4065/mcp.2010.0381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilbert ML, May CE, Griffin WC., 3rd Conditioned reinforcement and locomotor activating effects of caffeine and ethanol combinations in mice. Pharmacol Biochem Behav. 2013;110:168–173. doi: 10.1016/j.pbb.2013.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu CW, Chen CY, Wang C-S, Chiu TH. Caffeine and a selective adenosine A2A receptor antagonist induce reward and sensitization behavior associated with increased phospho-Thr75-DARPP-32 in mice. Psychopharmacol. 2009;204:313–325. doi: 10.1007/s00213-009-1461-3. [DOI] [PubMed] [Google Scholar]
- Hsu CW, Wang CS, Chiu TH. Caffeine and a selective adenosine A2A receptor antagonist induce sensitization and cross-sensitization behavior associated with increased striatal dopamine in mice. J Biomed Sci. 2010;17:4. doi: 10.1186/1423-0127-17-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamens HM, Crabbe JC. The parallel rod floor test: a measure of ataxia in mice. Nat Protoc. 2007;2:277–281. doi: 10.1038/nprot.2007.19. [DOI] [PubMed] [Google Scholar]
- Kaplan GB, Greenblatt DJ, Ehrenberg BL, Goddard JE, Cotreau MM, Harmatz JS, Shader RI. Dose-dependent pharmacokinetics and psychomotor effects of caffeine in humans. J Clin Pharmacol. 1997;37:693–703. doi: 10.1002/j.1552-4604.1997.tb04356.x. [DOI] [PubMed] [Google Scholar]
- Kaplan GB, Greenblatt DJ, Leduc BW, Thompson ML, Shader RI. Relationship of plasma and brain concentrations of caffeine and metabolites to benzodiazepine receptor binding and locomotor activity. J Pharmacol Exp Ther. 1989;248:1078–1083. [PubMed] [Google Scholar]
- Kunin D, Bloch RT, Terada Y, Rogan F, Smith BR, Amit Z. Caffeine promotes an ethanol-induced conditioned taste aversion: a dose-dependent interaction. Exp Clin Psychopharmacol. 2001;9:326–333. doi: 10.1037//1064-1297.9.3.326. [DOI] [PubMed] [Google Scholar]
- Kunin D, Gaskin S, Rogan F, Smith BR, Amit Z. Caffeine promotes ethanol drinking in rats. Examination using a limited-access free choice paradigm. Alcohol. 2000;21:271–277. doi: 10.1016/s0741-8329(00)00101-4. [DOI] [PubMed] [Google Scholar]
- Kuzmin A, Johansson B, Gimenez L, Ogren S-O, Fredholm BB. Combination of adenosine A1 and A2A receptor blocking agents induces caffeine-like locomotor stimulation in mice. Eur Neuropsychopharmacol. 2006;16:129–136. doi: 10.1016/j.euroneuro.2005.07.001. [DOI] [PubMed] [Google Scholar]
- Lessov CN, Palmer AA, Quick EA, Phillips TJ. Voluntary ethanol drinking in C57BL/6J and DBA/2J mice before and after sensitization to the locomotor stimulant effects of ethanol. Psychopharmacol. 2001;155:91–99. doi: 10.1007/s002130100699. [DOI] [PubMed] [Google Scholar]
- Lessov CN, Phillips TJ. Duration of sensitization to the locomotor stimulant effects of ethanol in mice. Psychopharmacol. 1998;135:374–382. doi: 10.1007/s002130050525. [DOI] [PubMed] [Google Scholar]
- Liguori A, Robinson JH. Caffeine antagonism of alcohol-induced driving impairment. Drug Alcohol Depend. 2001;63:123–129. doi: 10.1016/s0376-8716(00)00196-4. [DOI] [PubMed] [Google Scholar]
- Linsenbardt DN, Moore EM, Griffin KD, Gigante ED, Boehm SL., 2nd Tolerance to ethanol’s ataxic effects and alterations in ethanol-induced locomotion following repeated binge-like ethanol intake using the DID model. Alcohol Clin Exp Res. 2011;35:1246–1255. doi: 10.1111/j.1530-0277.2011.01459.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez MF, Griffin WC, 3rd, Melendez RI, Becker HC. Repeated cycles of chronic intermittent ethanol exposure leads to the development of tolerance to aversive effects of ethanol in C57BL/6J mice. Alcohol Clin Exp Res. 2012;36:1180–1187. doi: 10.1111/j.1530-0277.2011.01717.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Cruz L, Salamone JD, Correa M. The Impact of Caffeine on the Behavioral Effects of Ethanol Related to Abuse and Addiction: A Review of Animal Studies. J Caffeine Res. 2013;3:9–21. doi: 10.1089/jcr.2013.0003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackay M, Tiplady B, Scholey AB. Interactions between alcohol and caffeine in relation to psychomotor speed and accuracy. Human Psychopharmacol. 2002;17:151–156. doi: 10.1002/hup.371. [DOI] [PubMed] [Google Scholar]
- Marczinski CA, Fillmore MT. Dissociative antagonistic effects of caffeine on alcohol-induced impairment of behavioral control. Exp Clin Psychopharmacol. 2003;11:228–236. doi: 10.1037/1064-1297.11.3.228. [DOI] [PubMed] [Google Scholar]
- Marczinski CA, Fillmore MT, Henges AL, Ramsey MA, Young CR. Mixing an Energy Drink with an Alcoholic Beverage Increases Motivation for More Alcohol in College Students. Alcohol Clin Exp Res. 2012 doi: 10.1111/j.1530-0277.2012.01868.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGovern R, Luderman L, Knecht K, Griffin WC., 3rd Influence of sensitization on the discriminative stimulus effects of methylphenidate in mice. Behav Pharmacol. 2014;25:766–774. doi: 10.1097/FBP.0000000000000095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKetin R, Coen A. The effect of energy drinks on the urge to drink alcohol in young adults. Alcohol Clin Exp Res. 2014;38:2279–2285. doi: 10.1111/acer.12498. [DOI] [PubMed] [Google Scholar]
- Nakagawa S, Cuthill IC. Effect size, confidence interval and statistical significance: a practical guide for biologists. Biol Rev Camb Philos Soc. 2007;82:591–605. doi: 10.1111/j.1469-185X.2007.00027.x. [DOI] [PubMed] [Google Scholar]
- Nam HW, McIver SR, Hinton DJ, Thakkar MM, Sari Y, Parkinson FE, Haydon PG, Choi DS. Adenosine and glutamate signaling in neuron-glial interactions: implications in alcoholism and sleep disorders. Alcohol Clin Exp Res. 2012;36:1117–1125. doi: 10.1111/j.1530-0277.2011.01722.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peacock A, Bruno R, Martin FH. The subjective physiological, psychological, and behavioral risk-taking consequences of alcohol and energy drink co-ingestion. Alcohol Clin Exp Res. 2012;36:2008–2015. doi: 10.1111/j.1530-0277.2012.01820.x. [DOI] [PubMed] [Google Scholar]
- Peacock A, Bruno R, Martin FH, Carr A. The impact of alcohol and energy drink consumption on intoxication and risk-taking behavior. Alcohol Clin Exp Res. 2013;37:1234–1242. doi: 10.1111/acer.12086. [DOI] [PubMed] [Google Scholar]
- Peacock A, Bruno R, Martin FH, Carr A. Self-reported physiological and psychological side-effects of an acute alcohol and energy drink dose. Appetite. 2014;76:60–65. doi: 10.1016/j.appet.2014.01.003. [DOI] [PubMed] [Google Scholar]
- Pennay A, Miller P, Busija L, Jenkinson R, Droste N, Quinn B, Jones SC, Lubman DI. ‘Wide-awake drunkenness’? Investigating the association between alcohol intoxication and stimulant use in the night-time economy. Addiction. 2014 doi: 10.1111/add.12742. [DOI] [PubMed] [Google Scholar]
- Phillips TJ, Roberts AJ, Lessov CN. Behavioral sensitization to ethanol: genetics and the effects of stress. Pharmacol Biochem Behav. 1997;57:487–493. doi: 10.1016/s0091-3057(96)00448-0. [DOI] [PubMed] [Google Scholar]
- Quarta D, Ferre S, Solinas M, You ZB, Hockemeyer J, Popoli P, Goldberg SR. Opposite modulatory roles for adenosine A1 and A2A receptors on glutamate and dopamine release in the shell of the nucleus accumbens. Effects of chronic caffeine exposure. J Neurochem. 2004;88:1151–1158. doi: 10.1046/j.1471-4159.2003.02245.x. [DOI] [PubMed] [Google Scholar]
- Reissig CJ, Strain EC, Griffiths RR. Caffeinated energy drinks--a growing problem. Drug Alcohol Depend. 2009;99:1–10. doi: 10.1016/j.drugalcdep.2008.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rezvani AH, Sexton HG, Johnson J, Wells C, Gordon K, Levin ED. Effects of caffeine on alcohol consumption and nicotine self-administration in rats. Alcohol Clin Exp Res. 2013;37:1609–1617. doi: 10.1111/acer.12127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossheim ME, Thombs DL. Artificial sweeteners, caffeine, and alcohol intoxication in bar patrons. Alcohol Clin Exp Res. 2011;35:1891–1896. doi: 10.1111/j.1530-0277.2011.01534.x. [DOI] [PubMed] [Google Scholar]
- Ruby CL, O’Connor KM, Ayers-Ringler J, Choi DS. Adenosine and glutamate in neuroglial interaction: implications for circadian disorders and alcoholism. Adv Neurobiol. 2014;11:103–119. doi: 10.1007/978-3-319-08894-5_6. [DOI] [PubMed] [Google Scholar]
- SAMHSA. The DAWN Report: Emergency Department Visits Involving Energy Drinks. Substance Abuse and Mental Health Services Administration; 2011. pp. 1–6. [PubMed] [Google Scholar]
- SAMHSA. The DAWN Report: Update on Emergency Department Visits Involving Energy Drinks: A Continuing Public Health Concern. Substance Abuse and Mental Health Services Administration; 2013. [PubMed] [Google Scholar]
- Seifert SM, Schaechter JL, Hershorin ER, Lipshultz SE. Health effects of energy drinks on children, adolescents, and young adults. Pediatrics. 2011;127:511–528. doi: 10.1542/peds.2009-3592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thombs D, Rossheim M, Barnett TE, Weiler RM, Moorhouse MD, Coleman BN. Is there a misplaced focus on AmED? Associations between caffeine mixers and bar patron intoxication. Drug Alcohol Depend. 2011;116:31–36. doi: 10.1016/j.drugalcdep.2010.11.014. [DOI] [PubMed] [Google Scholar]
- Thombs DL, O’Mara RJ, Tsukamoto M, Rossheim ME, Weiler RM, Merves ML, Goldberger BA. Event-level analyses of energy drink consumption and alcohol intoxication in bar patrons. Addict Behav. 2009;35:325–330. doi: 10.1016/j.addbeh.2009.11.004. [DOI] [PubMed] [Google Scholar]
- Wolk BJ, Ganetsky M, Babu KM. Toxicity of energy drinks. Curr Opin Pediatr. 2012;24:243–251. doi: 10.1097/MOP.0b013e3283506827. [DOI] [PubMed] [Google Scholar]
- Zancheta R, Possi AP, Planeta CS, Marin MT. Repeated administration of caffeine induces either sensitization or tolerance of locomotor stimulation depending on the environmental context. Pharmacol Rep. 2012;64:70–77. doi: 10.1016/s1734-1140(12)70732-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


