Abstract
BK virus (BKV) is associated with kidney and bladder disease after hematopoietic cell transplantation (HCT) but less is known about the seroprevalence of pre-transplant antibodies to BKV in children. We measured BKV IgG antibody titers in 36 children before HCT. BKV IgG antibodies were detected in all 36 patients, with 28/36 (77.8%) developing BK viremia in the first 100 days. Pre-HCT BKV IgG antibody titers >1:40,960 were protective against later BK viremia ≥10,000 copies/mL. The seroprevalence of antibodies to BKV is high in children undergoing HCT and post-transplant BK viremia, which is associated with bladder and kidney injury, is common.
Keywords: BK virus, hematopoietic cell transplant, humoral immunity, pediatrics
INTRODUCTION
The polyomavirus BK (BKV) infects kidney and bladder cells and can reactivate in immunosuppressed patients [1]. BKV is associated with nephropathy in kidney transplant recipients and with hemorrhagic cystitis and nephropathy after hematopoietic cell transplantation (HCT) [2, 3].
In healthy children, the seroprevalence of BKV infection approaches 90% by 10 years of age [4, 5]. While studies have examined BKV antibodies in children prior to kidney transplant [6–8], in HCT recipients, less is known about whether BKV antibodies confer protection against later disease [9–11].
We describe BKV antibodies in children prior to allogeneic HCT and the association between pre-HCT titers and post-HCT BK viremia. We focused on the development of BK viremia ≥10,000 copies/mL because this degree of viremia is more specific than viruria for bladder and kidney injury after transplant [2, 3].
METHODS
Study population
We analyzed 100 consecutive children and adolescents receiving a HCT at Cincinnati Children’s Hospital Medical Center (CCHMC) from September 2010 to December 2011 who were followed until 100 days after transplant [12] and were originally enrolled to study thrombotic microangiopathy [13]. We included 36 patients after excluding 10 patients undergoing autologous HCT, 2 not consenting to have their samples used for any purpose, 2 without baseline serum available, and 50 who had received pre-HCT intravenous immune globulin (IVIG), which contains antibodies to BKV [14]. The CCHMC Institutional Review Board approved the study.
BKV antibody testing
Serum obtained a median of 6 days (interquartile range (IQR) 5–8.5 days) prior to stem cell infusion was frozen (−80C) and later tested for BKV IgG antibodies. Antibodies to BKV were measured at Viracor-IBT Laboratories (Lee’s Summit, Missouri) [6]. The assay reports titers ranging from 1:640 to >1:163,840 against the VP1 capsid.
BKV PCR testing
Polymerase chain reaction (PCR) testing for BK viremia was performed clinically for unexplained hematuria, cystitis, and/or an elevation in serum creatinine. This was supplemented with an analysis of stored plasma samples from those without clinical testing results [12]. Plasma was stored (−80C) weekly while inpatient and at day 100. These stored samples were tested so that each patient with available plasma had at least three BKV PCR results: at least one measured between days 0–14, 15–85, and 100±14 days post-HCT. The 36 patients had a median of 12 (IQR 3.5–20 tests) plasma BKV PCR tests. All BKV PCR testing was performed at CCHMC using genomic sequence targets common to all BKV genotypes.
Outcome definition
The exact BK viremia PCR cutoff associated with clinical disease is not known [15]. Nevertheless, a blood PCR ≥10,000 copies/mL is sensitive and specific for biopsy proven BKV nephropathy after kidney transplant [2]. We previously reported that higher grade BK viremia (≥10,000 copies/mL) was also associated with kidney injury and hemorrhagic cystitis after HCT [3, 12]. We therefore categorized post-HCT BK viremia using each subject’s peak plasma PCR as 0–9,999, 10,000–100,000, or >100,000 copies/mL [12]. BK viremia has a higher positive predictive value for clinically relevant disease than viruria [2, 3, 7, 12, 16], but we also reported information on viruria, when available.
Analyses
We compared categorical variables with the Fischer exact test and continuous variables with the Wilcoxon rank-sum test. Data were collected using Research Electronic Data Capture [17] and analyzed with STATA (version 12, College Station, Texas).
RESULTS
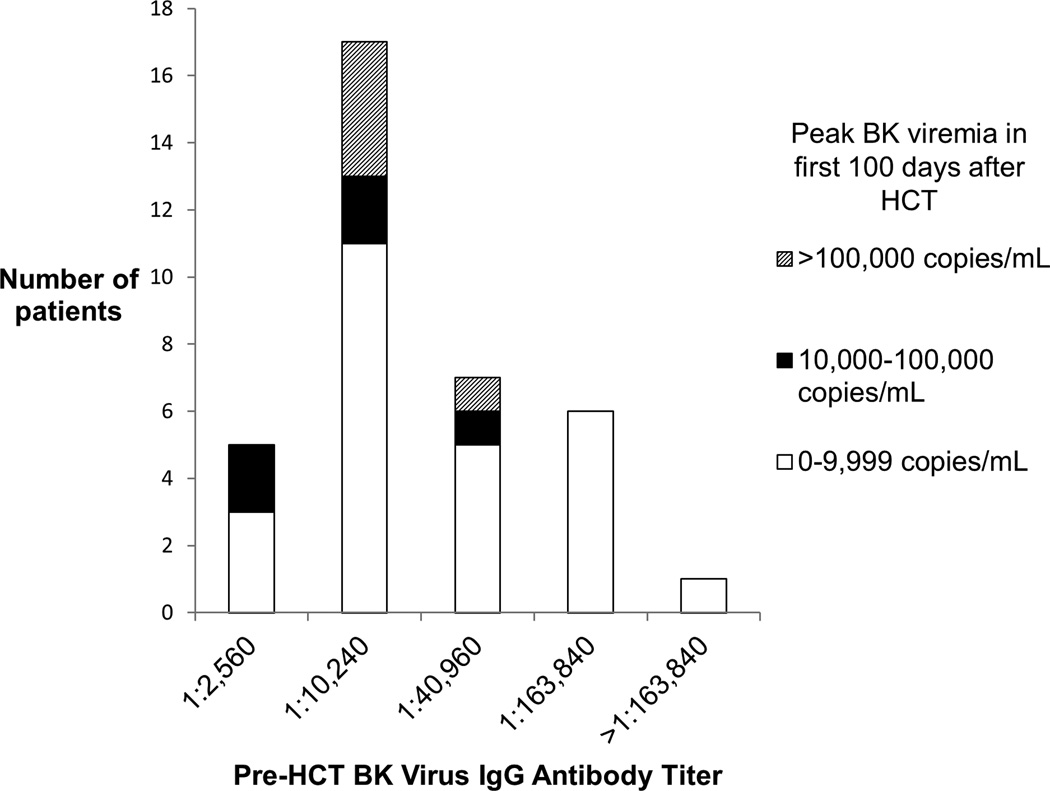
The clinical characteristics of the 36 patients undergoing HCT are shown in Table I, of whom 5 (13.9%) had a pre-HCT BKV IgG titer=1:2,560, 17 (47.2%) had a titer=1:10,240, 7 (19.4%) had a titer=1:40,960, 6 (16.7%) had a titer=1:163,840, and 1 (2.8%) had a titer>1:163,840.
Table I.
Characteristics of the 36 children undergoing allogeneic hematopoietic cell transplant
| Age (years) | 7.9 [5.1–14.6] | |
| Male gender | 20 (55.6%) | |
| Diagnosis group* | ||
| Bone marrow failure | 16 (44.4%) | |
| Malignancy | 14 (38.9%) | |
| Metabolic | 3 (8.3%) | |
| Immunodeficiency | 2 (5.6%) | |
| Sickle cell anemia | 1 (2.8%) | |
| Donor Cell source | ||
| Un-Related | 25 (69.4%) | |
| Related | 11 (30.6%) | |
| Donor Cell product | ||
| Marrow | 22 (61.1%) | |
| Peripheral blood | 8 (22.2%) | |
| Cord blood | 6 (16.7%) | |
| Conditioning therapy | ||
| Myeloablative (versus reduced intensity) | 27 (75.0%) | |
| Conditioning agents received (yes versus no) | ||
| Total body irradiation | 6 (16.7%) | |
| Cyclophosphamide | 27 (75.0%) | |
| Alemtuzumab | 6 (16.7%) | |
Data shown as median [interquartile range] or n (%).
Underlying diagnoses (number of patients): Bone marrow failure: Fanconi anemia (7), myelodysplastic syndrome (3), aplastic anemia (3), dyskeratosis congenita (2), congenital macrothrombocytopenia (1); Malignancy: acute myelogenous leukemia (6), acute lymphoblastic leukemia (4), biphenotypic leukemia (1), non-Hodgkin lymphoma (1), juvenile myelomonocytic leukemia (1), myelodysplastic syndrome (1); Metabolic: Hurler syndrome (1), metachromatic leukodystrophy (1), Krabbe disease (1); Immunodeficiency: chronic granulomatous disease (1), Wiskott-Aldrich syndrome (1)
BK viremia >0 copies/mL was detected in 28 (77.8%) recipients. Among the 36 patients, the peak BKV blood PCR was 0–9,999 copies/mL in 26 (72.2%), was 10,000–100,000 copies/mL in 5 (13.9%), and was >100,000 copies/mL in 5 (13.9%) patients (Supplemental Table I).
The association between pre-transplant BKV antibody titers and post-transplant BK viremia is shown in Figure 1, illustrating that none of the 7 HCT recipients with a pre-transplant titer >1:40,960 developed BK viremia ≥10,000 copies/mL (p=0.16).
Figure 1.
Association between pre-transplant BKV IgG antibody titers and post-HCT BK viremia in 36 children and young adults undergoing HCT
There were 8 cases (22.2%) of cystitis, with 7/8 (87.5%) in patients with a titer ≤1:40,960 (p=1.0). Of these 8 cases, 4 (50.0%) had a peak BK blood PCR of <10,000 copies/mL and 4 (50.0%) had a peak BK blood PCR of ≥10,000 copies/mL. In the 29/36 (80.6%) patients with day 100 data, the median (IQR) creatinine-estimated glomerular filtration rate was 93.8 (87.6–97.6 ml/min/1.73m2) in the 9 patients with a peak BK blood PCR of ≥10,000 copies/mL and was 109.4 (87.9–140.7 ml/min/1.73m2) in the 20 patients with a peak BK blood PCR of <10,000 copies/mL (p=0.11).
DISCUSSION
All 36 children undergoing HCT had BKV antibodies, post-transplant BK viremia was common, and higher baseline titers were associated with protection against BK viremia ≥10,000 copies/mL.
Koskenvuo et al [9] reported BKV antibodies in 6 children developing cystitis after HCT and found that increasing titers were associated with less severe disease. Titers increased in 4 children with decreasing BK viremia and resolution of cystitis. In the remaining 2 children, BKV titers did not increase, viremia persisted, and cystitis continued.
The literature regarding BK viruria is conflicting. Drummond et al [11] found that pre-HCT BKV titers were high in those with viruria but decreased in those without viruria. Bogdanovic et al [10] observed that 87% of 45 children undergoing HCT were seropositive for BKV IgG pre-transplant, but antibody titers did not predict later cystitis. Wong et al [18] reported a higher pre-transplant BKV titer was associated with an increased risk of viruria in 76 adult HCT recipients, but there was no association between the titer and cystitis. Finally, Lee et al measured BKV antibodies in 98 adults undergoing HCT and reported that increasing titers were associated with viruria. Using the same assay as our analysis and similar to our findings, all 98 subjects (100%) had a detectable BKV IgG of >1:640 [19].
Antibodies against BKV may neutralize infection or signal cell-mediated viral control [9, 20]. After cell attachment, endocytosis of BKV occurs relatively slowly, potentially allowing time for neutralizing antibodies to prevent viral entry [21]. Alternatively, antibodies may clear BK viremia but be less effective at the site of tissue injury [22].
Reduction of immunosuppression is the most effective treatment for BKV [1, 23, 24], but may not be feasible in HCT recipients at risk for graft versus host disease. Novel strategies may include using IVIG, a BKV vaccine, or infusion of virus-specific T-cells [14, 25–27].
BKV infection after HCT is associated with significant kidney and bladder disease [3, 12]. While our observations are limited by a small sample and a post hoc analysis of a completed cohort, we observed that higher pre-transplant BKV titers were associated with a lower risk of high grade BK viremia. In contrast to other viruses (CMV), guidelines do not support routine monitoring for BKV antibodies or screening for infection after HCT because currently available treatments are often ineffective [28]. However, as novel therapies become available, such as the infusion of T-cells [27] or a vaccine [26], it will be important to target patients at highest risk for disease. Studies are needed to test if BKV-related disease could be predicted by measuring pre-transplant antibodies and following blood PCR levels after HCT.
Supplementary Material
Acknowledgements
This work was supported by a Career Development Award in Comparative Effectiveness Research from the National Institutes of Health [grant number KM1CA156715-01] and an American Society for Blood and Marrow Transplantation/Genentech New Investigator Award [to BL]. The REDCap database is supported by a Cincinnati Children’s Hospital Center for Clinical and Translational Science and Training grant [grant number UL1-RR026314-01 NCRR/NIH]. This work was also funded in part by the National Center for Advancing Translational Sciences [grant number UL1TR000003] and by the National Institutes of Health [grant number UL1RR024134].
Abbreviations
- BKV
BK virus
- HCT
Hematopoietic cell transplant
- PCR
Polymerase chain reaction
Footnotes
Conflict of Interest Statement
Dr. Hester is an employee of Viracor-IBT Laboratories, which performed the BKV antibody testing free of charge.
REFERENCES
- 1.Ramos E, Drachenberg CB, Wali R, Hirsch HH. The decade of polyomavirus BK-associated nephropathy: state of affairs. Transplantation. 2009;87:621–630. doi: 10.1097/TP.0b013e318197c17d. [DOI] [PubMed] [Google Scholar]
- 2.Hirsch HH, Knowles W, Dickenmann M, Passweg J, Klimkait T, Mihatsch MJ, Steiger J. Prospective study of polyomavirus type BK replication and nephropathy in renal-transplant recipients. N Engl J Med. 2002;347:488–496. doi: 10.1056/NEJMoa020439. [DOI] [PubMed] [Google Scholar]
- 3.Haines HL, Laskin BL, Goebel J, Davies SM, Yin HJ, Lawrence J, Mehta PA, Bleesing JJ, Filipovich AH, Marsh RA, Jodele S. Blood, and not urine, BK viral load predicts renal outcome in children with hemorrhagic cystitis following hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2011;17:1512–1519. doi: 10.1016/j.bbmt.2011.02.012. [DOI] [PubMed] [Google Scholar]
- 4.Egli A, Infanti L, Dumoulin A, Buser A, Samaridis J, Stebler C, Gosert R, Hirsch HH. Prevalence of polyomavirus BK and JC infection and replication in 400 healthy blood donors. J Infect Dis. 2009;199:837–846. doi: 10.1086/597126. [DOI] [PubMed] [Google Scholar]
- 5.Knowles WA, Pipkin P, Andrews N, Vyse A, Minor P, Brown DW, Miller E. Population-based study of antibody to the human polyomaviruses BKV and JCV and the simian polyomavirus SV40. J Med Virol. 2003;71:115–123. doi: 10.1002/jmv.10450. [DOI] [PubMed] [Google Scholar]
- 6.Ali AM, Gibson IW, Birk P, Blydt-Hansen TD. Pretransplant serologic testing to identify the risk of polyoma BK viremia in pediatric kidney transplant recipients. Pediatr Transplant. 2011;15:827–834. doi: 10.1111/j.1399-3046.2011.01583.x. [DOI] [PubMed] [Google Scholar]
- 7.Ginevri F, Azzi A, Hirsch HH, Basso S, Fontana I, Cioni M, Bodaghi S, Salotti V, Rinieri A, Botti G, Perfumo F, Locatelli F, Comoli P. Prospective monitoring of polyomavirus BK replication and impact of pre-emptive intervention in pediatric kidney recipients. Am J Transplant. 2007;7:2727–2735. doi: 10.1111/j.1600-6143.2007.01984.x. [DOI] [PubMed] [Google Scholar]
- 8.Smith JM, McDonald RA, Finn LS, Healey PJ, Davis CL, Limaye AP. Polyomavirus nephropathy in pediatric kidney transplant recipients. Am J Transplant. 2004;4:2109–2117. doi: 10.1111/j.1600-6143.2004.00629.x. [DOI] [PubMed] [Google Scholar]
- 9.Koskenvuo M, Dumoulin A, Lautenschlager I, Auvinen E, Mannonen L, Anttila VJ, Jahnukainen K, Saarinen-Pihkala UM, Hirsch HH. BK polyomavirus-associated hemorrhagic cystitis among pediatric allogeneic bone marrow transplant recipients: Treatment response and evidence for nosocomial transmission. J Clin Virol. 2013;56:77–81. doi: 10.1016/j.jcv.2012.09.003. [DOI] [PubMed] [Google Scholar]
- 10.Bogdanovic G, Priftakis P, Taemmeraes B, Gustafsson A, Flaegstad T, Winiarski J, Dalianis T. Primary BK virus (BKV) infection due to possible BKV transmission during bone marrow transplantation is not the major cause of hemorrhagic cystitis in transplanted children. Pediatr Transplant. 1998;2:288–293. [PubMed] [Google Scholar]
- 11.Drummond JE, Shah KV, Saral R, Santos GW, Donnenberg AD. BK virus specific humoral and cell mediated immunity in allogeneic bone marrow transplant (BMT) recipients. J Med Virol. 1987;23:331–344. doi: 10.1002/jmv.1890230405. [DOI] [PubMed] [Google Scholar]
- 12.Laskin BL, Denburg M, Furth S, Diorio D, Goebel J, Davies SM, Jodele S. BK viremia precedes hemorrhagic cystitis in children undergoing allogeneic hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2013;19:1175–1182. doi: 10.1016/j.bbmt.2013.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jodele S, Davies SM, Lane A, Khoury J, Dandoy C, Goebel J, Myers K, Grimley M, Bleesing J, El-Bietar J, Wallace G, Chima RS, Paff Z, Laskin BL. Diagnostic and risk criteria for HSCT-associated thrombotic microangiopathy: a study in children and young adults. Blood. 2014;124:645–653. doi: 10.1182/blood-2014-03-564997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Randhawa PS, Schonder K, Shapiro R, Farasati N, Huang Y. Polyomavirus BK neutralizing activity in human immunoglobulin preparations. Transplantation. 2010;89:1462–1465. doi: 10.1097/tp.0b013e3181daaaf1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Trofe-Clark J, Sparkes T, Gentile C, Van Deerlin V, Sawinski D, Bloom RD. BK Virus Genotype Variance and Discordant BK Viremia PCR Assay Results. Am J Transplant. 2013;13:1112–1113. doi: 10.1111/ajt.12169. [DOI] [PubMed] [Google Scholar]
- 16.O'Donnell PH, Swanson K, Josephson MA, Artz AS, Parsad SD, Ramaprasad C, Pursell K, Rich E, Stock W, van Besien K. BK virus infection is associated with hematuria and renal impairment in recipients of allogeneic hematopoetic stem cell transplants. Biol Blood Marrow Transplant. 2009;15:1038–1048. e1031. doi: 10.1016/j.bbmt.2009.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wong AS, Chan KH, Cheng VC, Yuen KY, Kwong YL, Leung AY. Relationship of pretransplantation polyoma BK virus serologic findings and BK viral reactivation after hematopoietic stem cell transplantation. Clin Infect Dis. 2007;44:830–837. doi: 10.1086/511863. [DOI] [PubMed] [Google Scholar]
- 19.Lee YJ, Zheng J, Kolitsopoulos Y, Chung D, Amigues I, Son T, Choo K, Hester J, Giralt SA, Glezerman IG, Jakubowski AA, Papanicolaou GA. Relationship of BK Polyoma Virus (BKV) in the Urine with Hemorrhagic Cystitis and Renal Function in Recipients of T Cell-Depleted Peripheral Blood and Cord Blood Stem Cell Transplantations. Biol Blood Marrow Transplant. 2014;20:1204–1210. doi: 10.1016/j.bbmt.2014.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Comoli P, Binggeli S, Ginevri F, Hirsch HH. Polyomavirus-associated nephropathy: update on BK virus-specific immunity. Transpl Infect Dis. 2006;8:86–94. doi: 10.1111/j.1399-3062.2006.00167.x. [DOI] [PubMed] [Google Scholar]
- 21.Dugan AS, Eash S, Atwood WJ. Update on BK virus entry and intracellular trafficking. Transpl Infect Dis. 2006;8:62–67. doi: 10.1111/j.1399-3062.2006.00153.x. [DOI] [PubMed] [Google Scholar]
- 22.Chen Y, Trofe J, Gordon J, Du Pasquier RA, Roy-Chaudhury P, Kuroda MJ, Woodle ES, Khalili K, Koralnik IJ. Interplay of cellular and humoral immune responses against BK virus in kidney transplant recipients with polyomavirus nephropathy. J Virol. 2006;80:3495–3505. doi: 10.1128/JVI.80.7.3495-3505.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zaia J, Baden L, Boeckh MJ, Chakrabarti S, Einsele H, Ljungman P, McDonald GB, Hirsch H. Viral disease prevention after hematopoietic cell transplantation. Bone Marrow Transplant. 2009;44:471–482. doi: 10.1038/bmt.2009.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dropulic LK, Jones RJ. Polyomavirus BK infection in blood and marrow transplant recipients. Bone Marrow Transplant. 2008;41:11–18. doi: 10.1038/sj.bmt.1705886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schachtner T, Muller K, Stein M, Diezemann C, Sefrin A, Babel N, Reinke P. BK virus-specific immunity kinetics: a predictor of recovery from polyomavirus BK-associated nephropathy. Am J Transplant. 2011;11:2443–2452. doi: 10.1111/j.1600-6143.2011.03693.x. [DOI] [PubMed] [Google Scholar]
- 26.Pastrana DV, Brennan DC, Cuburu N, Storch GA, Viscidi RP, Randhawa PS, Buck CB. Neutralization serotyping of BK polyomavirus infection in kidney transplant recipients. PLoS Pathog. 2012;8:e1002650. doi: 10.1371/journal.ppat.1002650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gerdemann U, Christin AS, Vera JF, Ramos CA, Fujita Y, Liu H, Dilloo D, Heslop HE, Brenner MK, Rooney CM, Leen AM. Nucleofection of DCs to generate Multivirus-specific T cells for prevention or treatment of viral infections in the immunocompromised host. Mol Ther. 2009;17:1616–1625. doi: 10.1038/mt.2009.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tomblyn M, Chiller T, Einsele H, Gress R, Sepkowitz K, Storek J, Wingard JR, Young JA, Boeckh MJ. Guidelines for preventing infectious complications among hematopoietic cell transplantation recipients: a global perspective. Biol Blood Marrow Transplant. 2009;15:1143–1238. doi: 10.1016/j.bbmt.2009.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.