Abstract
OBJECTIVES
Ninety percent of patients with esophageal adenocarcinoma (EAC) ultimately die of their disease highlighting the need for novel therapeutic targets. The goal of this study was to define the functional significance of overexpression of Dickkopf-3 (DKK3) in EAC.
METHODS
DKK3 expression was analyzed by real-time PCR in 95 chemonaive and 21 chemoresistant EACs. The EAC cell line OE33 was stably transfected with DKK3 (OE33/DKK3) and evaluated using WST-1, matrigel, endothelial tube formation, and chemosensitivity assays. Tumorigenesis was evaluated by injecting 1×106 OE33/DKK3 and vector cells in NOD/SCIDγ mice.
RESULTS
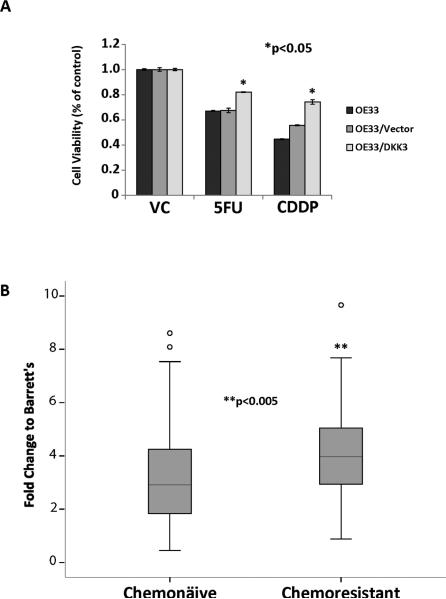
DKK3 was overexpressed (> 2-fold) in 75.8% (72/95) of EACs. DKK3 protein was present at moderate to high levels in 46.8% (29/62) of EACs on tissue microarray. Stable transfection of DKK3 significantly increased proliferation (p<0.05) and matrigel invasion (p<0.001). Levels of SMAD4, a key mediator of the TGFß pathway, increased after activin treatment of OE33/DKK3, and siSMAD4 significantly decreased matrigel invasion suggesting that DKK3 acts through the TGFβ pathway. OE33/DKK3 increased endothelial tube formation, were significantly more resistant to 5-FU and cisplatin, and DKK3 expression was significantly higher in chemoresistant EACs (p<0.005). In NOD/SCIDγ mice, OE33/DKK3 cells resulted in tumors at all sites (8/8) while vector cells grew in only 1/8 sites. Nodal metastases were also significantly increased in patients with EACs highly overexpressing DKK3, 28/32 (88%) versus 42/63 (68%) (p<0.05).
CONCLUSIONS
These findings suggest that DKK3 may be important in mediating invasion in EAC and could be a novel target in the treatment and prevention of metastatic disease.
INTRODUCTION
Dickkopf-3 (DKK3) is a divergent member of the Wnt inhibitor family,1 and the significance of its interaction with the Wnt pathway is unclear. While DKK1, 2, and 4 inhibit the Wnt pathway by binding to LRP5/6, DKK3 does not bind to these proteins.2 DKK3 binds to Kremen1/2;3 however, DKK3 is a secreted protein and the significance of this intracellular interaction is uncertain. DKK3 was found to regulate FGF and Activin/Nodal through SMAD4, a central component of the TGFβ pathway, and stabilization of SMAD4 by DKK3 was found in the induction of mesoderm in Xenopus embryos.4
DKK3 has been proposed as a tumor suppressor, and overexpression of DKK3 suppresses cell growth and invasion of certain cancer cell lines.5 However, DKK3 is overexpressed in other cancers including hepatocellular carcinoma and hepatoblastoma.6 DKK3 is a marker for neoangiogenesis in colon cancer,7 and microvessels expressing DKK3 were increased in glioma, non-Hodgkin's lymphoma, and melanoma.8 DKK3 has been associated with protection from apoptotic stress and with chemoresistance in Saos-2 osteosarcoma cells.9 Using Oncomine (www.oncomine.org), a web-based application that allows evaluation of gene expression using cancer profiling data including 25 esophageal datasets with 751 samples, there was significant overexpression of DKK3 in esophageal adenocarcinomas (EACs) relative to Barrett's metaplasia (BM) and normal esophagus (10.9 fold; p<0.0001).10, 11 Conversely, DKK3 expression was significantly decreased in lung adenocarcinoma. The expression and function of DKK3 appears to be tissue and tumor specific.
The incidence of EAC has increased greatly while the 5-year survival remains only 19%.12 Metastatic disease accounts for the majority of deaths from EAC. While esophagectomy remains the primary treatment, there is an urgent need for novel therapies. In evaluating molecular changes in the progression from BM to EAC, overexpression of DKK3 was identified in a significant subset of tumors. Interestingly, we found that a number of genes mediated by the TGFβ pathway were also overexpressed, suggesting that this pathway is important in EAC. This study was undertaken to delineate the expression and role of DKK3 in EAC. We hypothesized that DKK3 is a mediator of the TGFβ pathway in EAC and plays an important role in the proliferation and invasion of EAC. Inhibition of DKK3 and its downstream mediators could have a significant clinical impact on the treatment and prevention of micrometastatic disease, especially in patients with locally advanced or regional nodal disease.
MATERIALS AND METHODS
Patients and Tissues
This study was approved by the IRB, and after obtaining informed consent, tissues were obtained from patients undergoing esophagectomy at the University of Michigan. Specimens were transported in DMEM (Invitrogen) on ice and stored at −80°C. Samples with minimum 70% cellularity were identified using frozen sections including 95 chemonaive and 21 chemoresistant EACs.
Cell Lines
Flo, OE19, and OE33 (Sigma-Aldrich) were derived from EAC. Flo was grown in DMEM (Invitrogen) and OE33 and OE19 were grown in RPMI 1640 with 10% fetal bovine serum (FBS; Atlanta Biologicals) and 1% Antibiotic-Antimycotic (Invitrogen) at 37°C in 5% CO2/95% air. All cell lines and stable subclones underwent genotyping by the University of Michigan Sequencing Core to ensure cell line authenticity. To evaluate for chemosensitivity, cell lines were treated with cisplatin (5 ug/ml) and 5-FU (10 ug/ml) for 48 hours. Viability was assessed by WST-1 (Roche) and repeated in triplicate.
Quantitative Reverse Transcription-Polymerase Chain Reaction
Real-time PCR was performed using 20 ng of total RNA and 0.2 μM of the forward and reverse primers. Cycling parameters included a 50°C hold for 2 minutes; 95°C hold for 10 minutes; 40 cycles at 95°C for 10 seconds; annealing for 15 seconds; and 72°C for 20 seconds. Significant differences in relative quantification were determined using the 2(-ΔΔCt) method. Expression was normalized to GAPDH or β-actin. DKK3 primers were forward 5′-TGAGGAACTGATGGAGGACA-3′ and reverse 5′-TTGCCAGGTTCACTTCTGAT-3′.
Western Blot
Western was performed using a 1:1000 dilution of DKK3 antibody (Santa Cruz) and a 1:5000 dilution of goat anti-rabbit secondary antibody (Vector). While the calculated molecular weight of DKK3 is 38 kDa, the size on Western has been reported as 50-55 kDa in reducing conditions due to glycosylation.13 For SMAD4, a 1:2000 dilution of SMAD4 antibody (Abcam) was used with a 1:8000 dilution of anti-rabbit secondary antibody (Vector). ß-actin was used as a loading control with a 1:10000 dilution of ß-actin antibody (Abcam) and a 1:10000 dilution of anti-mouse secondary antibody (Vector).
Tissue Microarray
Tissue microarrays were constructed with formalin-fixed, paraffin-embedded tissues from 73 patients including 64 EAC, 8 dysplastic Barrett's mucosa, 11 BM, and 2 normal esophageal samples.14 Immunohistochemical staining was done on the DAKO Autostainer using DAKO LSAB+ and 3,3′-diaminobenzidine as the chromogen. Dewaxed and rehydrated sections were labeled with DKK3 antibody (1:200 dilution; Santa Cruz). Microwave citric acid epitope retrieval was performed for 20 minutes. Slides were counterstained with hematoxylin. Samples were scored using a scale of 0 (no staining), 1+ (<10% staining), 2+ (10-50%) staining, or 3+ (≥50%).15 The scoring was repeated to ensure reproducibility.
Construction of DKK3 Stable Cell Line
A DKK3 mammalian expression construct (Plasmid 15496; Addgene) was PCR amplified using primers containing EcoRI and XbaI restriction sites for directional cloning into the pcDNA3(+) Vector (V79020; Invitrogen). The insert was sequenced to confirm that it contained DKK3. DKK3 or empty vector constructs were transfected into OE33 or Flo using FuGENE 6 (Promega). Selected clones were maintained in medium containing 200 μg/mL geneticin.
Matrigel Invasion Assay
1×105 cells in serum-free RPMI 1640 medium were seeded in the upper chamber of the 24 well invasion chamber system (BD Biosciences), and 20% FBS was added to the lower chamber as a chemoattractant. After 48 hours, non-invading cells and Matrigel were removed with a cotton swab. Invasive cells on the lower side of the chamber were stained with crystal violet. Four 100x fields were counted, and assays were performed in duplicate.
SNP Array Analysis
Using the Genome-Wide Human Sty I 250K SNP Array (Affymetrix), 73 EAC DNAs were genotyped.16 DKK3 copy number analysis was performed using a log2 copy number ratio exceeding 0.848 for amplifications and −0.737 for deletions. Genomic positions were mapped in the hg18 genome build. SNP data was visualized using Integrative Genomics Viewer 1.3.1 (www.broadinstitute.org/igv).
Endothelial Tube Formation
HMVEC (Human Microvascular Endothelial Cells; Cascade Biologics) were stably transduced with lentiviral-expressed red fluorescent protein (RFP). OE33/DKK3 and OE33/Vector cells were stably transduced with lentiviral-expressed green fluorescent protein (GFP). RFP and GFP-positive cells were sorted by flow cytometry. Selected HMVECs were cultured as monolayers on Attachment Factor coated plates with Medium 131 mixed with Microvascular Growth Supplement (Cascade Biologics) and 1% FBS.17 Cells were applied to collagen gel polymerized 6 well plates (2×105 cells/well) and incubated for 16 hours. A second collagen gel layer was overlaid with 2×105 OE33/DKK3 or vector cells/ml. VEGF (1 μg/ml; R&D Systems) was added to either OE33/Vector with endothelial cells or endothelial cells alone as positive controls. Cells were incubated at 37°C for 7 days, and media was changed every 3 days. Experiments were performed in triplicate.
Xenograft Models
NOD/SCIDγ (NSG) mice were received from the Unit for Laboratory Animal Medicine at 6-8 weeks of age. All procedures were approved by the University Committee on Use and Care of Animals, and all animals received humane care in compliance with the Guide for the Care and Use of Laboratory Animals (www.nap.edu/catalog.php?record_id=12910). To evaluate whether stable transfection of DKK3 increases the tumorigenicity of OE33, the number of cells injected was titrated to the lowest number resulting in a palpable tumor after flank injection of OE33/DKK3 but not OE33/Vector. Cells were passed through a 40-μm filter. A single cell suspension was prepared with 100 μl of saline and 50% Matrigel (BD Biosciences). 2×106, 1×106, 1×105 and 5×104 cells were injected into the flank of NSG mice.18 At 1×106 cells, OE33/DKK3 resulted in tumors at five weeks while OE33/Vector cells did not. Each experimental group consisted of 4 mice injected with 1×106 cells in each flank for a total of 8 sites per cell line. Mice were sacrificed after 5 weeks.
Oligonucleotide Microarray
Total RNA was isolated from stably transfected OE33/DKK3 and OE33/Vector cells using QIAzol (Qiagen) and purified with miRNeasy spin columns (Qiagen). RNA quality was confirmed by 1% agarose gel electrophoresis and A260:280 by NanoDrop 2000 spectrophotometer ratios. RNA quality was reassessed with the Agilent Bioanalyzer (Agilent Technologies) after double-stranded cDNA and cRNA synthesis. Hybridization and normalization of the Human Gene 2.1 ST Gene Chip data (Affymetrix) were performed by the University of Michigan Cancer Center Microarray Core. A summary statistic was calculated for the eleven probe pairs for each gene with the robust multichip average (RMA) method (14) using the Affymetrix library of Bioconductor (www.bioconductor.org). Expression values for OE33/DKK3 were compared with OE33/Vector. Pathway analysis was performed using DAVID Bioinformatics Resources 6.7 (http://david.abcc.ncifcrf.gov). Results were confirmed using real-time PCR.
Statistical Analysis
Statistical analysis was performed using SPSS version 22. Categorical variables were analyzed using the Fisher's exact or Chi-squared tests while continuous variables were analyzed using the Student's t-test. Survival was determined using the Kaplan-Meier method. For the analysis of the clinical characteristics, high DKK3 expression was defined as expression relative to Barrett's mucosa with a threshold greater than 2 standard deviations above the mean.
RESULTS
DKK3 is overexpressed in esophageal adenocarcinoma
DKK3 was overexpressed greater than 2-fold in 75.8% (72/95) of EACs relative to BM using real-time PCR (Supplemental Figure 1A). qRT-PCR analysis of an additional 40 esophageal samples (6 BM, 7 BM/low-grade dysplasia, 6 low-grade dysplasia, 7 high-grade dysplasia, and 14 EACs) showed significant overexpression in EAC in the progression from BM (p<0.05) (Supplemental Figure 1B). The purpose was to identify molecular changes in the progression from BM to adenocarcinoma. Barrett's metaplasia was used as the control to avoid identifying genes with similar expression in BM and EAC but differential expression in normal esophagus. BM has been used as a reference control by other investigators.19 Significant overexpression of DKK3 in 95 primary EACs was also confirmed relative to normal esophagus using real-time PCR.
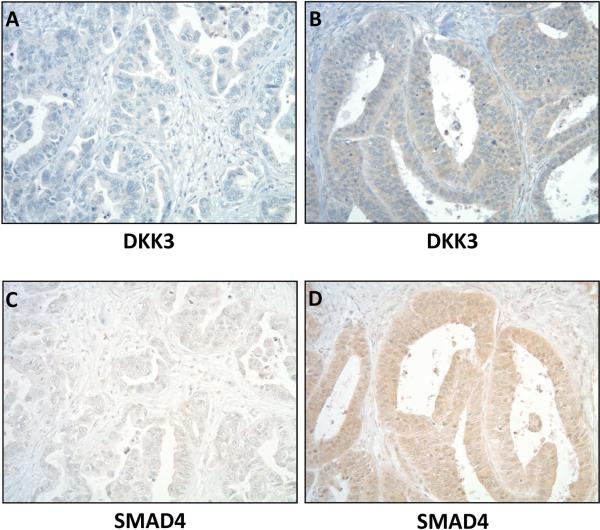
Staining of DKK3 on tissue microarray (Supplemental Table 1) showed moderate to high DKK3 expression (2-3+) in 46.8% (29/62) of EAC samples (Figure 1). 16.7% (2/12) of dysplastic samples and 20% (2/10) of BM samples had 2+ staining, but unlike EAC, none had 3+ staining. Normal esophageal samples showed mild 1+ DKK3 staining (2/2).
Figure 1.
Representative sections from a tissue microarray showing low (A) and high (B) cytoplasmic DKK3 staining in two esophageal adenocarcinomas. Moderate to high expression (2-3+) was found in 46.8% (29/62) of esophageal adenocarcinomas. Low (C) and high (D) SMAD4 staining correlated with DKK3 in the same tumors. Original magnifications are all 100x.
To evaluate the mechanism of overexpression, SNP array analysis of 73 EACs was performed, and DKK3 was not amplified. While treatment of OE33 with 5-azacytidine did not increase DKK3 expression, a combination of 5-azacytidine and trichostatin A increased DKK3 expression on Western suggesting that histone acetylation is involved in the overexpression of DKK3.
OE33 transfection with DKK3 increases cell proliferation and matrigel invasion
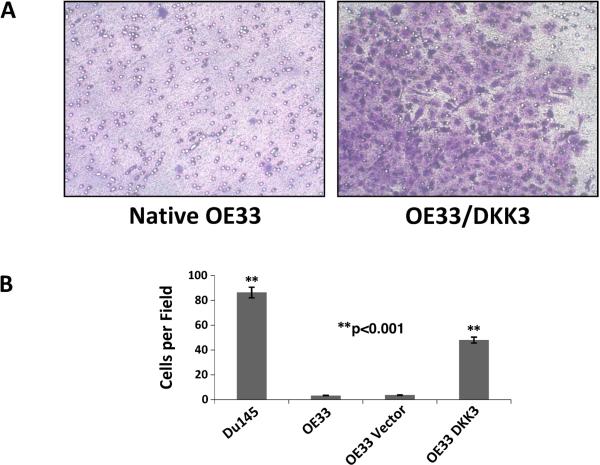
DKK3 overexpression was confirmed on qRT-PCR compared to native and vector controls and on Western, which also confirmed expression of a Flag tag. OE33, which does not natively express DKK3, had significantly increased proliferation on WST-1 assay after DKK3 transfection compared to vector controls at 96 and 120 hours (p<0.005). OE33/DKK3 cells also had significantly greater matrigel invasion (p<0.001) (Figure 2). To ensure that these results were not cell line-specific, the EAC cell line Flo was transfected with DKK3 and confirmed increased proliferation and matrigel invasion compared to vector controls.
Figure 2.
A. OE33/DKK3 had significantly increased matrigel invasion compared to controls (p<0.001). Du145 prostate cancer cells served as a positive control. B. All assays were performed in duplicate.
Transfection of DKK3 leads to stabilization of SMAD4 and activation of the TGFβ pathway
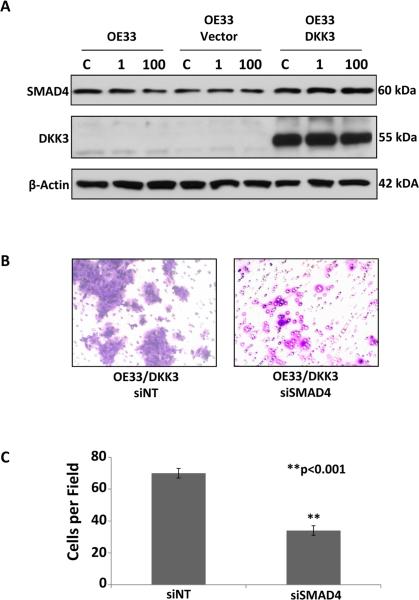
To determine the mechanism of action of DKK3 in EAC, the TOP-flash TCF-reporter assay was performed and showed no significant decrease in canonical Wnt pathway activation after transfection of DKK3, a divergent member of the Wnt inhibitor family, into OE33 compared to native and vector controls. However, treatment of OE33/DKK3 cells with the TGFβ ligand activin increased SMAD4 protein on Western (Figure 3A), suggesting its mechanism of action is through the TGFβ pathway. Activin treatment also significantly increased proliferation in OE33/DKK3 compared with vector controls. Inhibition with siSMAD4 significantly decreased matrigel invasion of OE33/DKK3 (p<0.001) (Figure 3B and C). In addition, low and high SMAD4 staining correlated with DKK3 expression in the same tumors on immunohistochemistry (Figure 1). DAVID pathway analysis of Human Gene ST 2.1 array expression induced by the transfection of DKK3 in OE33 showed that the TGFβ pathway was significantly increased compared with OE33 vector control (p<0.05).
Figure 3.
A. SMAD4 was significantly increased on Western in OE33/DKK3 after treatment with 1 or 100 ng/ml of activin compared to native and vector controls (C, carrier control). B and C. Treatment of OE33/DKK3 cells with siSMAD4 resulted in significantly less matrigel invasion compared to a non-targeting control (**p<0.001).
DKK3 overexpression leads to increased neoangiogenesis
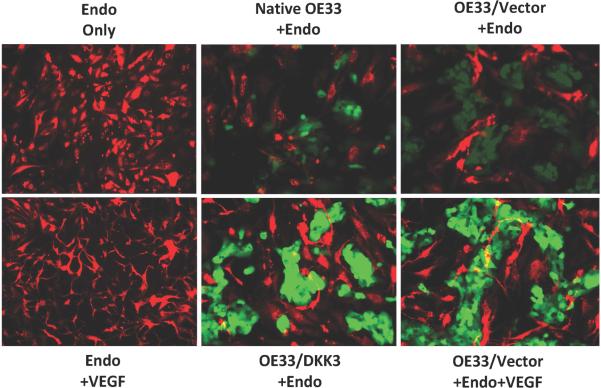
Endothelial tube formation assay demonstrated increased angiogenesis in the presence of OE33/DKK3 compared to OE33 native, vector, and endothelial cell controls (Figure 4). Endothelial tube formation was similar to that seen after adding VEGF to the OE33/Vector and endothelial only positive controls.
Figure 4.
The presence of stably-transfected OE33 (OE33/DKK3 +Endo) increased endothelial tube formation similar to the tube formation seen after adding VEGF to endothelial cells (Endo +VEGF) and to OE33/Vector (OE33/Vector +Endo +VEGF). Endothelial, native OE33, and vector controls showed minimal tube formation. OE33 and endothelial cells were labeled green and red respectively. Original magnifications were 200x.
DKK3 overexpression increases chemoresistance in EAC cells
OE33/DKK3 cells were significantly more chemoresistant to 5-FU and cisplatin compared to vehicle control (p<0.05) (Figure 5A). DKK3 expression was significantly higher on real-time PCR relative to BM in 21 chemoresistant EACs compared to 96 chemonaive EACs (p<0.005) (Figure 5B). To evaluate whether DKK3 expression is induced by chemotherapy, OE33 and Flo were treated with cisplatin and 5-FU with no significant increase in DKK3 expression.
Figure 5.
A. OE33/DKK3 were significantly more resistant to 5-FU and cisplatin compared to treatment with vehicle control for 48 hours (*p<0.05). B. DKK3 expression was significantly higher on real-time PCR in chemoresistant (n=21) compared to chemonaive esophageal adenocarcinomas (n=95) (**p<0.005).
Injection of OE33/DKK3 cells in NOD/SCIDγ mice significantly increases the incidence of tumor growth
NSG mice were injected in the flank with 1×106 cells, the lowest number of cells to produce tumors with OE33/DKK3 but not OE33/Vector as described in the Methods. Five weeks after injection, only 1/8 OE33/Vector sites produced a palpable tumor (2 mm) while all OE33/DKK3 (8/8) tumor sites resulted in tumors (4-5 mm) (Supplemental Figure 2). Tumors were confirmed pathologically to be consistent with EAC. DKK3 overexpression was also confirmed by RT-PCR and Western with no expression in the OE33/Vector tumor.
Nodal metastases were significantly increased in patients with EACs highly overexpressing DKK3
Clinical characteristics for 94 patients with chemonaive EACs, obtained before the routine use of preoperative chemotherapy, were analyzed for an association with DKK3 overexpression (Supplemental Table 2). Nodal metastases were significantly associated with DKK3 overexpression with 28/32 (88%) with nodal disease compared to 42/62 (68%) with lower DKK3 expression (p=0.047). The pathological stage was also significantly higher with EACs highly overexpressing DKK3 with 26/32 (81%) EACs stage III+IVa versus 36/62 (58%) with lower DKK3 expression (p=0.038). However, DKK3 overexpression was not significantly associated with overall survival.
DISCUSSION
The TGFβ pathway is involved in proliferation, differentiation, and EMT, which has been associated with chemoresistance and tumor invasion. While TGFβ is a tumor suppressor at early stages of carcinogenesis, advanced cancers are resistant to its growth inhibition and TGFβ promotes metastasis and invasion.20-22 TGFβ is overexpressed in EAC and is related to a poor prognosis.23 DKK3 mediates the effects of activin, a TGFβ ligand, in Xenopus.4 Our results suggest that DKK3 acts independently of the canonical Wnt pathway in EAC and that the mechanism of action is mediated through the TGFβ pathway with DKK3 stabilizing SMAD4 protein. Transfection of DKK3 in OE33 resulted in increased activation of the TGFβ pathway. Various tumor biological processes were evaluated focusing on processes mediated by the TGFβ pathway, especially those previously associated with DKK3 overexpression including invasion, angiogenesis, and chemoresistance. While DKK3 has been described as a tumor suppressor in some cancers, DKK3 is overexpressed in hepatocellular carcinoma and hepatoblastoma.6 The expression and function of DKK3, like the TGFβ pathway, depends on the tumor and tissue context. The overexpression and role of DKK3 have not been previously described in EAC.
In the current study, DKK3 was overexpressed in a significant subset of EACs. Esophageal cancers are heterogeneous, and most molecular changes, including DKK3 overexpression, will only be present in a subset of tumors. While DKK3 is overexpressed in 75.8% of EACs on real-time PCR, 2-3+ DKK3 protein expression was found in 46.8% on immunohistochemistry. Discordance in mRNA and protein expression in other genes has been reported in lung adenocarcinoma.24 These differences may be the result of posttranscriptional regulation with changes in mRNA stability,25 transcript localization,26 or translational efficiency as well as differences in sensitivity between mRNA and antibody-based assays.
The mechanism behind DKK3 overexpression in EAC was also evaluated. DKK3 was not amplified on SNP array analysis, and treatment of OE33 cells with 5-azacitidine alone did not increase DKK3 expression. However, a combination of 5-azacytidine and trichostatin A increased expression suggesting histone acetylation is involved in DKK3 overexpression.
The majority of EAC patients die of metastatic disease.12 DKK3 overexpression may be important in tumor invasion, and matrigel invasion was increased following transfection of DKK3 in Flo and OE33. Knockdown experiments could not be performed since none of the three available EAC cell lines overexpress DKK3. However, downstream inhibition of SMAD4 was able to decrease matrigel invasion of OE33/DKK3. Deckers, et al. found that inhibiting SMAD4 in MDA-MB-231 breast carcinoma cells inhibited bone metastases in nude mice.27 While SMAD4 inhibition may have effects independent of DKK3 overexpression, it is notable for its ability to inhibit matrigel invasion of OE33/DKK3.
Neoangiogenesis is important in the progression from BM to adenocarcinoma and in supporting tumor invasion.28 DKK3 supports capillary formation in gliomas and lymphoma,8 and our results show an increase in endothelial tube formation in the presence of OE33/DKK3. While transfection of DKK3 resulted in a more malignant phenotype with increased invasion and proliferation, there was no evidence that DKK3 is oncogenic in EAC. The three EAC cell lines available to us are cancer cell lines and therefore, were not able to be specifically tested for oncogenic transformation. However, this chromosomal region was not amplified on SNP array.
To determine if DKK3 transfection was able to increase the tumorigenicity of OE33, the lowest number of OE33/DKK3, but not OE33/Vector, cells able to produce tumors was determined to be 1×106 cells. This is several fold less than previously described mice experiments using OE33 (2×106 to 1×107 cells).29-32 In a tumorigenic assay, Zhao, et al. injected 1×105, 1×106, and 1×107 OE33 cells at 15 sites in BALB/c OlaHsd-Foxn1nu mice.18 OE33 cells produced tumors at 1/15 sites at 10 weeks and 2/15 at 29 weeks. In the current study, OE33/Vector cells produced tumors in 1/8 sites at 5 weeks while OE33/DKK3 cells resulted in tumors at all 8 sites supporting that DKK3 expression is important in invasion and growth in EAC.
EACs highly overexpressing DKK3 were significantly associated with nodal metastases in 88% (p=0.047) and higher stage III or IVa in 81% of EACs (p=0.038), consistent with the increased matrigel invasion seen with DKK3 overexpression. While T and M stage were not significantly associated with DKK3 expression, this is likely due to the fact that patients with invasion of surrounding structures or metastatic disease were not included in this series since they were not treated surgically.
Neoadjuvant chemoradiation followed by esophagectomy is standard in most large centers for EACs greater than T2 or with regional nodal disease to “downstage” the tumor and limit micrometastatic disease. However, only 21% of patients have a complete response.34 DKK3 overexpression is associated with chemoresistance in Saos-2 osteosarcoma,9 and our results show that OE33/DKK3 cells were significantly more chemoresistant to cisplatin and 5-FU. Targeting DKK3 and other TGFβ pathway mediators may decrease this chemoresistance. If inhibiting DKK3 alone is not sufficient, combination with other therapies targeting the TGFβ pathway or downstream proteins may be a successful strategy.
DKK3 -/- knockout mice have significantly decreased natural killer cells, increased IgM and hemoglobin, and are hyperactive.35 Dkk3 does not appear to function as a tumor suppressor in these knockout mice as the authors did not report any increase in tumors. In addition, these knockout mice show that DKK3 is a non-lethal target since the DKK3 -/- mice were viable.
There are some limitations to our study. None of the three EAC cell lines overexpress DKK3 so we were unable to evaluate targeted inhibition of DKK3. However, we were able to evaluate the effect of transfection of DKK3 in two EAC cell lines. Inhibition of DKK3 using siRNA in an oral squamous cell carcinoma cell line natively overexpressing DKK3 significantly decreased migration and invasion providing further support that DKK3 plays a role in these biological processes.33
While DKK3 overexpression was not associated with decreased overall survival, the sample size was relatively small and only included operable patients. DKK3 overexpression was increased in chemoresistant tumors, and transfection increased chemoresistance and invasion. DKK3 overexpression may be associated with decreased survival in other populations including patients with metastatic disease or those treated with preoperative chemotherapy. We were not able evaluate these groups directly since our tumor bank consists mostly of chemonaive tumors obtained when patients were not treated routinely with preoperative chemotherapy. Katase, et al. reported DKK3 negative oral squamous cell carcinoma patients had decreased nodal metastases and significantly longer disease-free survival.33 These findings support that DKK3 overexpression is important clinically in cancers overexpressing DKK3.
The overall survival of patients with EAC remains poor with the majority of patients ultimately dying of metastatic disease. The results of the current study suggest that DKK3 may play an important role in tumor growth and invasion in EAC. DKK3 is overexpressed in a significant subset of esophageal adenocarcinomas, and targeting DKK3 and its downstream mediators may be beneficial in the prevention and treatment of micrometastatic disease and potentially decreasing disease recurrence.
Supplementary Material
PERSPECTIVE STATEMENT.
Most esophageal adenocarcinoma patients die of metastases. DKK3 was significantly overexpressed in esophageal adenocarcinoma and was associated with advanced tumors and nodal disease. Stable transfection of DKK3 increases proliferation, invasion, and chemoresistance. DKK3 may be important in mediating invasion and could be a novel target in the treatment and prevention of metastatic disease.
ACKNOWLEDGEMENTS
We would like to thank David Erdody for his help in preparing and formatting the images for this manuscript.
Supported by the Thoracic Surgery Foundation for Research and Education Research Grant [J.L.], the Department of Surgery, University of Michigan Research Advisory Committee Grant [J.L.], and U54 CA163059 from the National Institutes of Health [D.G.B].
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest: The authors have no conflicts of interest or financial disclosures.
CENTRAL MESSAGE
DKK3 may mediate invasion in esophageal adenocarcinoma and could be a novel target in the treatment and prevention of metastases.
CENTRAL PICTURE
Figure 2A has been selected as the Central Picture.
CENTRAL PICTURE LEGEND
OE33/DKK3 had significantly increased matrigel invasion compared to controls (p<0.001).
REFERENCES
- 1.Krupnik VE, Sharp JD, Jiang C, et al. Functional and structural diversity of the human Dickkopf gene family. Gene. 1999;238:301–313. doi: 10.1016/s0378-1119(99)00365-0. [DOI] [PubMed] [Google Scholar]
- 2.Mao B, Wu W, Li Y, et al. LDL-receptor-related protein 6 is a receptor for Dickkopf proteins. Nature. 2001;411:321–325. doi: 10.1038/35077108. [DOI] [PubMed] [Google Scholar]
- 3.Nakamura RE, Hackam AS. Analysis of Dickkopf3 interactions with Wnt signaling receptors. Growth factors. 2010;28:232–242. doi: 10.3109/08977191003738832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pinho S, Niehrs C. Dkk3 is required for TGF-beta signaling during Xenopus mesoderm induction. Differentiation; research in biological diversity. 2007;75:957–967. doi: 10.1111/j.1432-0436.2007.00185.x. [DOI] [PubMed] [Google Scholar]
- 5.Tsuji T, Miyazaki M, Sakaguchi M, et al. A REIC gene shows down-regulation in human immortalized cells and human tumor-derived cell lines. Biochemical and biophysical research communications. 2000;268:20–24. doi: 10.1006/bbrc.1999.2067. [DOI] [PubMed] [Google Scholar]
- 6.Pei Y, Kano J, Iijima T, et al. Overexpression of Dickkopf 3 in hepatoblastomas and hepatocellular carcinomas. Virchows Archiv : an international journal of pathology. 2009;454:639–646. doi: 10.1007/s00428-009-0772-4. [DOI] [PubMed] [Google Scholar]
- 7.Zitt M, Untergasser G, Amberger A, et al. Dickkopf-3 as a new potential marker for neoangiogenesis in colorectal cancer: expression in cancer tissue and adjacent non-cancerous tissue. Disease markers. 2008;24:101–109. doi: 10.1155/2008/160907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Untergasser G, Steurer M, Zimmermann M, et al. The Dickkopf-homolog 3 is expressed in tumor endothelial cells and supports capillary formation. International journal of cancer. Journal international du cancer. 2008;122:1539–1547. doi: 10.1002/ijc.23255. [DOI] [PubMed] [Google Scholar]
- 9.Hoang BH, Kubo T, Healey JH, et al. Dickkopf 3 inhibits invasion and motility of Saos-2 osteosarcoma cells by modulating the Wnt-beta-catenin pathway. Cancer research. 2004;64:2734–2739. doi: 10.1158/0008-5472.can-03-1952. [DOI] [PubMed] [Google Scholar]
- 10.Hao Y, Triadafilopoulos G, Sahbaie P, et al. Gene expression profiling reveals stromal genes expressed in common between Barrett's esophagus and adenocarcinoma. Gastroenterology. 2006;131:925–933. doi: 10.1053/j.gastro.2006.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rhodes DR, Kalyana-Sundaram S, Mahavisno V, et al. Oncomine 3.0: genes, pathways, and networks in a collection of 18,000 cancer gene expression profiles. Neoplasia. 2007;9:166–180. doi: 10.1593/neo.07112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA: a cancer journal for clinicians. 2012;62:10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 13.Hsieh SY, Hsieh PS, Chiu CT, et al. Dickkopf-3/REIC functions as a suppressor gene of tumor growth. Oncogene. 2004;23:9183–9189. doi: 10.1038/sj.onc.1208138. [DOI] [PubMed] [Google Scholar]
- 14.Kononen J, Bubendorf L, Kallioniemi A, et al. Tissue microarrays for high-throughput molecular profiling of tumor specimens. Nature medicine. 1998;4:844–847. doi: 10.1038/nm0798-844. [DOI] [PubMed] [Google Scholar]
- 15.Raso MG, Behrens C, Herynk MH, et al. Immunohistochemical expression of estrogen and progesterone receptors identifies a subset of NSCLCs and correlates with EGFR mutation. Clinical cancer research : an official journal of the American Association for Cancer Research. 2009;15:5359–5368. doi: 10.1158/1078-0432.CCR-09-0033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bass AJ, Watanabe H, Mermel CH, et al. SOX2 is an amplified lineage-survival oncogene in lung and esophageal squamous cell carcinomas. Nature genetics. 2009;41:1238–1242. doi: 10.1038/ng.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang B, Xiao Y, Ding BB, et al. Induction of tumor angiogenesis by Slit-Robo signaling and inhibition of cancer growth by blocking Robo activity. Cancer cell. 2003;4:19–29. doi: 10.1016/s1535-6108(03)00164-8. [DOI] [PubMed] [Google Scholar]
- 18.Zhao R, Quaroni L, Casson AG. Identification and characterization of stemlike cells in human esophageal adenocarcinoma and normal epithelial cell lines. The Journal of thoracic and cardiovascular surgery. 2012;144:1192–1199. doi: 10.1016/j.jtcvs.2012.08.008. [DOI] [PubMed] [Google Scholar]
- 19.Helm J, Enkemann SA, Coppola D, et al. Dedifferentiation precedes invasion in the progression from Barrett's metaplasia to esophageal adenocarcinoma. Clinical cancer research : an official journal of the American Association for Cancer Research. 2005;11:2478–2485. doi: 10.1158/1078-0432.CCR-04-1280. [DOI] [PubMed] [Google Scholar]
- 20.Derynck R, Zhang YE. Smad-dependent and Smad-independent pathways in TGF-beta family signalling. Nature. 2003;425:577–584. doi: 10.1038/nature02006. [DOI] [PubMed] [Google Scholar]
- 21.Derynck R, Akhurst RJ, Balmain A. TGF-beta signaling in tumor suppression and cancer progression. Nat Genet. 2001;29:117–129. doi: 10.1038/ng1001-117. [DOI] [PubMed] [Google Scholar]
- 22.Kang YB. Pro-metastasis function of TGF beta mediated by the Smad pathway. J Cell Biochem. 2006;98:1380–1390. doi: 10.1002/jcb.20928. [DOI] [PubMed] [Google Scholar]
- 23.Seder CW, Hartojo W, Lin L, et al. Upregulated INHBA Expression May Promote Cell Proliferation and Is Associated with Poor Survival in Lung Adenocarcinoma. Neoplasia. 2009;11:388–396. doi: 10.1593/neo.81582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen G, Gharib TG, Huang CC, et al. Discordant protein and mRNA expression in lung adenocarcinomas. Molecular & cellular proteomics : MCP. 2002;1:304–313. doi: 10.1074/mcp.m200008-mcp200. [DOI] [PubMed] [Google Scholar]
- 25.Zhang Z, Sheng H, Shao J, et al. Posttranscriptional regulation of cyclooxygenase-2 in rat intestinal epithelial cells. Neoplasia. 2000;2:523–530. doi: 10.1038/sj.neo.7900117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lipshitz HD, Smibert CA. Mechanisms of RNA localization and translational regulation. Current opinion in genetics & development. 2000;10:476–488. doi: 10.1016/s0959-437x(00)00116-7. [DOI] [PubMed] [Google Scholar]
- 27.Deckers M, van Dinther M, Buijs J, et al. The tumor suppressor Smad4 is required for transforming growth factor beta-induced epithelial to mesenchymal transition and bone metastasis of breast cancer cells. Cancer research. 2006;66:2202–2209. doi: 10.1158/0008-5472.CAN-05-3560. [DOI] [PubMed] [Google Scholar]
- 28.Sihvo EI, Ruohtula T, Auvinen MI, et al. Simultaneous progression of oxidative stress and angiogenesis in malignant transformation of Barrett esophagus. The Journal of thoracic and cardiovascular surgery. 2003;126:1952–1957. doi: 10.1016/j.jtcvs.2003.08.014. [DOI] [PubMed] [Google Scholar]
- 29.Zhang YF, Zhang AR, Zhang BC, et al. MiR-26a regulates cell cycle and anoikis of human esophageal adenocarcinoma cells through Rb1-E2F1 signaling pathway. Molecular biology reports. 2013;40:1711–1720. doi: 10.1007/s11033-012-2222-7. [DOI] [PubMed] [Google Scholar]
- 30.Sehdev V, Katsha A, Ecsedy J, et al. The combination of alisertib, an investigational Aurora kinase A inhibitor, and docetaxel promotes cell death and reduces tumor growth in preclinical cell models of upper gastrointestinal adenocarcinomas. Cancer. 2013;119:904–914. doi: 10.1002/cncr.27801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ford SJ, Obeidy P, Lovejoy DB, et al. Deferasirox (ICL670A) effectively inhibits oesophageal cancer growth in vitro and in vivo. British journal of pharmacology. 2013;168:1316–1328. doi: 10.1111/bph.12045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sehdev V, Peng D, Soutto M, et al. The aurora kinase A inhibitor MLN8237 enhances cisplatin-induced cell death in esophageal adenocarcinoma cells. Molecular cancer therapeutics. 2012;11:763–774. doi: 10.1158/1535-7163.MCT-11-0623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Katase N, Lefeuvre M, Gunduz M, et al. Absence of Dickkopf (Dkk)-3 protein expression is correlated with longer disease-free survival and lower incidence of metastasis in head and neck squamous cell carcinoma. Oncology letters. 2012;3:273–280. doi: 10.3892/ol.2011.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Orringer MB, Marshall B, Chang AC, et al. Two thousand transhiatal esophagectomies: changing trends, lessons learned. Annals of surgery. 2007;246:363–372. doi: 10.1097/SLA.0b013e31814697f2. discussion 372-364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Barrantes Idel B, Montero-Pedrazuela A, Guadano-Ferraz A, et al. Generation and characterization of dickkopf3 mutant mice. Molecular and cellular biology. 2006;26:2317–2326. doi: 10.1128/MCB.26.6.2317-2326.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.