Abstract
Rationale
Tobacco use is a serious health problem in the United States and this problem is potentiated in patients with schizophrenia. The reward system is implicated in schizophrenia and may contribute to the high comorbidity between nicotine use and schizophrenia but very little research has been done on the topic. The reward-enhancement effect of nicotine has been shown to be important in nicotine use, but there have been no studies on this effect in animal models of schizophrenia.
Objectives
This study was designed to determine the effects of phencyclidine, used to model negative symptoms of schizophrenia, on self-administration of nicotine with or without a co-occurring sensory reinforcer [i.e., visual stimulus (VS)] in rats.
Methods
Phencyclidine (2.0 mg/kg) was administered before each of 7 nicotine self-administration sessions (0.01 mg/kg/inf) after which rats (n=8–9 per group) were given 7 days of extinction without phencyclidine pretreatment. Reinstatement using phencyclidine (2.0 mg/kg), nicotine (0.2 mg/kg), and yohimbine (1.25 mg/kg, a pharmacological stressor) were tested after extinction to determine if previous exposure to phencyclidine would alter reinstatement of active lever pressing.
Results
Phencyclidine initially decreased nicotine self-administration, but only in the groups with a concurrent VS. This decrease in self-administration dissipated after 5 days. During reinstatement, rats that had previously received phencyclidine during self-administration with a VS were more sensitive to stress-induced reinstatement than any other group.
Conclusions
These results show a transitory effect of phencyclidine on nicotine self-administration. Phencyclidine may induce a potential sensitivity to pharmacological stressors contributing to reinstatement of nicotine.
Keywords: schizophrenia, phencyclidine, nicotine, self-administration, reinstatement
Introduction
Tobacco use is a serious problem in the United States; more than 480,000 people die each year from nicotine-related health concerns (Center for Disease Control 2014). Schizophrenics use tobacco at a much higher rate than the general population, with 50–90% of schizophrenics using nicotine (the primary psychoactive component in tobacco) in some form compared to around 22% of non-mentally ill people (Kumari & Postma 2005). The high rate of smoking and nicotine use in schizophrenia patients leads to an increased incidence of cardiovascular and respiratory problems that contributes to a mortality rate attributed to smoking that is 2 to 3 times higher than the general population (Hennekens et al. 2005; Parks et al. 2006; Saha et al. 2007; Mortenson & Juel 1990; Buda et al 1988). Nicotine addiction is exceedingly detrimental to patients with schizophrenia and potential mechanisms behind this comorbidity need to be explored.
While the comorbidity between nicotine use and schizophrenia is well documented, there is no consensus as to the mechanisms contributing to this comorbidity. The brain reward system has been implicated in nicotine addiction (Merlo-Pich et al. 1997; Koob & LeMoal 2006) and in schizophrenia (AhnAllen et al. 2012; Der-Avakian & Markou 2012; Wolf 2006; Kring & Moran 2008; Ziauddeen & Murray 2010). Given that this system is involved in both pathologies, the lack of research on the role of the dopaminergic reward system in this comorbidity is surprising. The few studies done on reward dysregulation in both schizophrenia and nicotine show a potential connection between the two. In humans, high levels of nicotine dependence are correlated with the severity of anhedonia, one of the primary negative symptoms of schizophrenia (AhnAllen et al. 2012). In another clinical report, patients with schizophrenia rated smoking as more rewarding than other reinforcers (Smith et al. 2002). Finally, the subcortical reward system and prefrontal cortex are hypofunctional in schizophrenics (Watkins et al., 2000; Chambers et al., 2001), suggesting involvement of the reward circuitry in the high rates of smoking seen in patients with schizophrenia.
One factor indicated in nicotine addiction is the reward or reinforcer-enhancement effect of nicotine. The reward-enhancement effect is defined as a potentiation by nicotine of the reinforcing effects of other non-nicotine stimuli. This enhancement effect has been seen as an increase in operant responding maintained by reinforcers such as auditory and visual cues, food, water, and social interaction (Liu et al. 2007; Donny et al. 2003; Chaudhri et al. 2007; Palmatier et al. 2006; Palmatier et al. 2007; Caggiula et al. 2009; Raiff & Dallery 2006; Raiff & Dallery 2008; Raiff & Dallery 2009; Thiel et al. 2009; Barrett & Odum 2011). Notably, some investigators suggest that the relatively robust reward-enhancement effects of nicotine may be more potent and hence more important for nicotine dependence than the relatively weaker primary reinforcing effects (Donny et al. 2003; Caggiula et al. 2009). In fact, self-administration of nicotine has been consistently difficult to find in rodents, being influenced by a number of factors such as feeding regimen (Lang et al., 1977; Cox et al., 1984). When self-administration has been identified, responding maintained by nicotine is relatively low in comparison to other drugs of abuse (Risner & Goldberg 1983). Alternatively, when a cue such as a visual stimulus is coupled to the infusion of nicotine, rats have significantly heightened levels of nicotine self-administration (Caggiula et al. 2002). This evidence suggests that this heightened self-administration reflects the reward-enhancement effect of nicotine increasing responding maintained by the weak sensory reinforcing effects of the visual stimulus (Caggiula et al. 2009; Caggiula et al. 2002).
To date, only one study to our knowledge has investigated nicotine use in a preclinical model of specific aspects of schizophrenia (Berg et al. 2013), which is surprising given that nicotine is used by patients with schizophrenia more than any other drug of abuse (Dolan et al. 2004). Berg and colleagues using the neonatal ventral hippocampal model found increased nicotine self-administration, leading to greater intake of nicotine, and later drug-seeking behaviors (see Discussion). The present study will extend Berg’s work in three important ways. First, this study will utilize phencyclidine to model the negative symptoms of schizophrenia, examining the generalizability of findings between this model and the neonatal ventral hippocampal model used by Berg and colleagues (2013). Given the inherent behavioral differences seen between varying animal models of schizophrenia symptoms (see Jones et al. 2011 for review), exploring the potential similarities and differences between these two paradigms will inform research on preclinical modeling of aspects of schizophrenia. Second, this study will explicitly compare the reward-enhancement effect of nicotine and the primary reinforcing effect of nicotine by utilizing controls based on the seminal study by Caggiula and colleagues (2002). Finally, this study will examine potentially altered reinstatement to a cue (i.e. phencyclidine), a nicotine prime, and a pharmacological stressor (i.e. yohimbine) thereby providing an opportunity to identify other processes involved in the comorbidity between tobacco addiction and negative symptoms of schizophrenia.
Methods
Animals
Thirty-eight adult male Sprague Dawley rats (275–299 g upon arrival) from Harlan, (Indianapolis, IN) completed the study and were included in the final analyses. They were housed individually in clear rectangular polycarbonate tubs (48.3 × 26.7 × 20.3 cm) under 12-hr light/dark conditions (light on at 6:00 am). Experiments were conducted during the light cycle. Room temperature was maintained at 22±1°C with a relative humidity of 45–60%. Water was continuously available and food was available ad libitum until the beginning of the experiment in which rats were food-restricted to 90% of their free-feeding weight except for the day prior to surgery and 5 days following surgery in which food was available ad libitum. After four weeks, the target weight was increased by 2 g. Rats were allowed 5 days of habituation to the animal facility before the start of the experiment. During acclimation they were handled for 5 min per rat by each experimenter. All procedures were approved by the Institutional Animal Care and Use Committee at the University of Nebraska-Lincoln.
Drugs
(−)Nicotine tartrate salt (Sigma, St. Louis, MO) was dissolved in 0.9% saline and adjusted to a pH of 7.0 ± 0.2 with a dilute NaOH solution. Phencyclidine hydrochloride (PCP, a gift from NIDA Chemical Synthesis and Drug Supply Program [RTI, Research Triangle Park, NC]) and yohimbine (Sigma) were dissolved in 0.9% saline. Saline and PCP (2.0 mg/kg) were administered subcutaneously (SC) 10 min prior to placement in the chamber. The dose and injection-to-placement interval of PCP was based on unpublished data suggesting that this dose would not produce a significant change in motor activity. This dose has also been used to model negative symptoms of schizophrenia in other studies. The overall paradigm used here is unique and aspects of the dosage and regimens were adopted from multiple studies (Sams-Dodd, 1997; Sams-Dodd, 1998; Corbett et al., 1995; Pouzet et al., 2002; Abdul-Monim et al. 2007; Abdul-Monim et al. 2003; Idris et al. 2005; Sams-Dodd, 1991). Yohimbine (1.25 mg/kg) was administered intraperitoneally (IP) 30 min prior to placement. The dose of yohimbine was chosen based on previous findings showing that reinstatement of drug-seeking was reliably seen after administration of 1.25 mg/kg (Shepard et al. 2004). The nicotine dose (0.01 mg/kg/infusion, reported as base) was selected based on previous research in our lab (Charntikov et al. 2013). Nicotine was infused intravenously (IV) at 35.74 μl per infusion across 1 sec. On the nicotine reinstatement day, nicotine (0.2 mg/kg) was injected SC 5 min prior to placement in the chamber.
Self-Administration Chambers
Sessions were conducted in eight chambers (ENV-008CT; Med Associates, Inc., St. Albans, VT; measuring 30.5 × 24.1 × 21.0 cm, l x w x h) enclosed in light and sound-attenuating cubicles fitted with a fan. The sidewalls of the chambers were aluminum, while the ceiling and front and back walls were clear polycarbonate. One sidewall featured a dipper receptacle centered in the chamber, occupying a 5.2 × 5.2 × 3.8 cm (l × w × h) recessed space, into which a dipper arm when raised provided 0.1 ml of 26% sucrose solution (w/v) into the receptacle. Retractable metal levers (147 nN required for micro-switch closure) were featured on either side of the dipper receptacle, approximately 5 cm above the chamber floor. An infrared emitter/detector unit positioned 4 cm above the rod floor bisected the chamber 14.5 cm from the sidewall featuring the dipper receptacle. This unit monitored general locomotor activity during experimental sessions. White 28V DC lamps (2.54 cm diameter, 100 mA) were located 3 cm above each lever; termed the right and left cue lights. Two external 28V DC lamps (100 mA) were also located outside and above the chamber but within the sound attenuating cubicle; termed the houselight. The infusion pump (PMH-100VS; Med Associates, Inc.; St. Albans, VT) for each chamber was located outside the cubicle. A 5-ml syringe mounted on the infusion pump was connected with Tygon® tubing (AAQ04103; VWR; West Chester, PA, USA). The tubing was attached to a swivel coupled with a spring leash (C313C; Plastics One; Roanoke, VA, USA) which were suspended over the ceiling of the chamber on a balanced metal arm. A computer running Med Associates interface and software (MedPC for Windows, IV) started the experimental sessions and collected number of chamber beam breaks, infusions, and lever presses.
Surgical Procedures
Rats were anesthetized with 1 ml/kg ketamine (100 mg/ml)/xylazine (20 mg/ml) mixture at a 2:1 ratio (IM; Sigma, St. Louis, MO). A polyurethane catheter (RJVR-23; Strategic Applications Inc.; Lake Villa, IL, USA) with rounded tip and double suture beads was implanted into the right external jugular vein. The other end of the catheter was placed subcutaneously around the shoulder and exited below the scapula via a polycarbonate back-mount access port (313-000BM; Plastics One Inc.; Roanoke, VA, USA). Catheters were immediately flushed with 0.2 ml of streptokinase (2 mg/ml; Sigma) after surgery diluted in sterile heparinized saline (30 U/ml; Midwest Veterinary Supply; Des Moines, IA, USA). Atipamezole hydrochloride (0.5 mg/kg; IM; Sigma) diluted in saline was used to counteract the anesthesia after the surgery was completed (Wee et al. 2006). Buprenorphine hydrochloride (0.1 mg/kg; SC; Sigma) was administered immediately after the surgery and once a day for two days post-surgery for pain management. Catheters were flushed before each self-administration session with heparinized saline (30 U/ml) and after each self-administration session with the heparinized saline mixed with cefazolin (50 mg/ml, Midwest Veterinary Supply). Catheter patency was assessed after the seven days of self-administration with an infusion of 0.05 ml xylazine (20 mg/ml; IV). This xylazine concentration produces motor ataxia within 5 sec (cf. Bevins 2005; Reichel et al. 2008). If rats failed to exhibit motor ataxia within 5 sec, they were excluded from the analyses and were not included in the subjects. Four animals were excluded from the study for this reason.
Procedure
Autoshaping
All rats were first trained in four consecutive autoshaping sessions. During these sessions, the house light remained on and a randomly selected lever was inserted into the chamber. A lever-press or a lapse of 15 sec resulted in sucrose delivery (4 sec), retraction of the lever, and a 20-sec timeout. Following the timeout, a randomly selected lever was inserted into the chamber with the condition that the same lever was not presented more than twice in a row. The session ended after 60 sucrose deliveries (total time of the session ranging from 65–80 min). All rats reached the criterion of at least 80% of sucrose deliveries received by lever-pressing by the final day of training.
Surgery and Post-surgery Autoshaping
Autoshaping was followed by the surgeries (see surgical procedures) and five days of rest, in which catheters were flushed daily but no testing was performed. After this recovery period, rats underwent three more days of autoshaping as described earlier. Prior to the first day of self-administration, rats had daily nicotine pre-injections (0.4 mg/kg, subcutaneous, 1 h post-autoshaping sessions) to decrease the initial locomotor suppression effects of nicotine (Bevins et al. 2001). All rats were above the lever-pressing criterion by the final day of autoshaping. Rats were then pseudo-randomly assigned to one of four groups with the condition that the last 2 days of lever-pressing performance did not differ statistically. The groups were based on the drug received prior to each self-administration session (saline or PCP) and whether the visual stimulus (VS) was presented with the nicotine infusion resulting in the following four groups: SAL-NOVS, SAL-VS, PCP-NOVS, PCP-VS.
Self-Administration
Following 24 h after the last autoshaping session, there were 7 consecutive days of nicotine self-administration. Rats were injected with either saline or PCP (2 mg/kg) 10 min prior to chamber placement and then permitted to self-administer nicotine with or without the visual stimulus. Before the start of each session, catheters were flushed with 0.2 ml of heparinized saline. The start of each 60-min session was signaled by turning the house light on, priming the catheter with nicotine (ca. 31 μl or 90% of total internal catheter volume), and insertion of both levers. The active lever was randomly assigned for each rat and was reinforced on a fixed ratio (FR) 1 schedule of reinforcement. If a rat pressed on the active lever once, there was a 1-sec infusion of nicotine (0.01 mg/kg/infusion) and termination of the house light for 20 sec as a timeout period for all groups; no additional nicotine infusions were available during this timeout. In addition, there was illumination of the cue lights for the first 5 sec in the PCP-VS and SAL-VS groups. Pressing on the inactive lever had no programmed consequence.
Extinction/Reinstatement
After self-administration, all animals underwent seven days of extinction. During extinction, the session was similar but rats were not injected with any drug before the session and lever-pressing on the active lever did not result in a nicotine infusion. However, cue lights were illuminated in the groups that previously received cue lights in the self-administration phase. Extinction lasted for 7 days. Finally, all rats underwent three tests to determine if reinstatement varied with treatment during the self-administration phase. On the first reinstatement test, all rats were injected with PCP (2 mg/kg, SC, 10 min prior to testing). The second reinstatement test examined nicotine (0.2 mg/kg, SC, 5 min); the final reinstatement test assessed yohimbine (1.25 mg/kg, IP, 30 min). The phencyclidine reinstatement test was performed first in order to most closely mimic the seven-day-on, seven-day-off phencyclidine regimen employed by a number of studies to mimic negative and cognitive symptoms of schizophrenia (Idris et al., 2005; McLean et al., 2009; Sams-Dodd, 1991; Grayson et al., 2007; Cochran et al., 2003; Abdul-Monim et al. 2007; Abdul-Monim et al. 2003; Bruins-Slot et al., 2005). Given the complete lack of knowledge on how yohimbine would interact with previous phencyclidine exposure, the yohimbine reinstatement test was performed last to prevent any carryover effects.
Statistics
All data are expressed as mean ± SEM. Data from the 7 self-administration days were analyzed using planned comparisons examining specific group differences to address the specific hypotheses of the study in a targeted fashion. Data from the reinstatement sessions were analyzed using a two-way ANOVA. A conventional alpha value of 0.05 was used to determine significance. Additionally, means and standard errors (SEM) are given while discussing specific differences. The discrimination index was calculated by dividing the total active lever presses by the total number of overall lever presses (both active and inactive). All data were analyzed using SPSS version 21.
Results
Self-Administration
Infusions
Total infusions of nicotine are shown in Figure 1. To examine the importance of the sensory reinforcer in general, a two-way mixed measures ANOVA with Group (SAL-VS; SAL-NOVS) as a between-subjects factor and Session as a within subjects factor was utilized. There was no main effect of Session (p=0.089), but there was a main effect of Group [F(1,16)=32.447, p<0.001], with the SAL-VS condition having more infusions that the SAL-NOVS condition (38.8±0.7 vs. 19.5±3.0). There was also a significant Session by Group interaction [F(6,96)=3.323, p=0.005]. This interaction was driven by the decreasing number of infusions in the SAL-NOVS conditions (24.5±4.7 to 8.9±2.2), but no difference in the SAL-VS condition. These findings indicate that nicotine self-administration was significantly increased by the presence of a VS coupled with the infusion.
Figure 1.
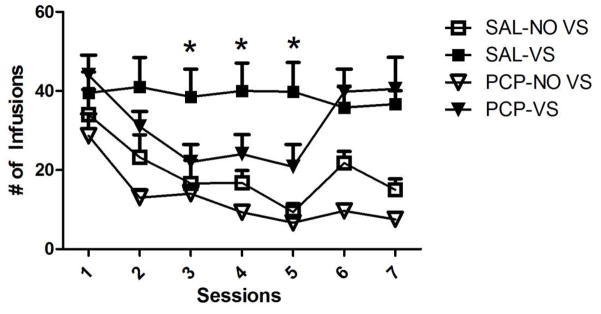
Number of nicotine infusions earned over the 60-min session for the seven days of self-administration. Phencyclidine initially decreased self-administration of nicotine in the concurrent VSs condition but this rebounded to saline levels by day 6. * indicates p<0.05
To examine the hypothesis that PCP alters reward-enhancement by nicotine, a separate two-way mixed measures ANOVA was conducted comparing the SAL-VS and PCP-VS groups with an additional factor of session. There was no main effect of Session and no interaction (ps≥0.12), but the main effect of Group approached criterion for significance [F(1,18)=3.848, p=0.065]. Further examination of the data pattern using planned one-way ANOVAs on the seven individual self-administration revealed a main effect of Group on the third [F(1,18)=7.259, p=0.015], fourth [F(1,18)=5.981, p=0.025] and fifth day [F(1,18)=0.013, p=0.013], but no effect on days 1, 2, 6, and 7 (ps≥0.176). These findings show that PCP altered the reward-enhancement effect of nicotine but this effect was somewhat transient.
Finally, a third two-way mixed measures ANOVA was conducted to determine if PCP changed nicotine self-administration that does not involve enhancement of a sensory reinforcer. The SAL-NOVS and PCP-NOVS groups were compared over daily sessions. There was a main effect of Session [F(6,96)=20.324, p<0.001], with infusions decreasing over days (29.42±3.6 to 20.6±3.9). There was no main effect of Group or interaction (ps≥0.23). This pattern indicates that self-administration decreased across session and that this decrease was not affected significantly by PCP.
Discrimination Index
Figure 2 shows the measure of the discrimination between the active and inactive lever for each group. A value of 0.5 indicates similar responding on the active and inactive lever; a value greater than 0.5 denotes more responding on the active (nicotine) lever. When comparing the SAL-NOVS and SAL-VS groups, there was a marginal main effect of Session [F(6,96)=2.197, p=0.050], a main effect of Group [F(1,16)=16.085, p=0.001] but no significant Session by Group interaction (p=0.55). These findings indicate that the discrimination index is higher in animals with a VS coupled to the infusion and this index increased over days (0.48±0.03 to 0.54±0.04), irrespective of groups. When comparing the SAL-VS groups and PCP-VS groups, there was a main effect of Session [F(6,96)=5.466, p<0.001] but no main effect of Group and no interaction (ps≥0.36), suggesting that PCP did not affect the discrimination index when a VS was coupled to the infusion but the discrimination index increased over days (0.57±0.03 to 0.78±0.04). Finally, when comparing the SAL-NOVS and PCP-NOVS groups, there were no main effects and no interactions (ps≥0.21). Together, this data indicates that the presence of a VS coupled with an infusion increased the discrimination index which also increased over days of self-administration but this was not affected by administration of PCP.
Figure 2.
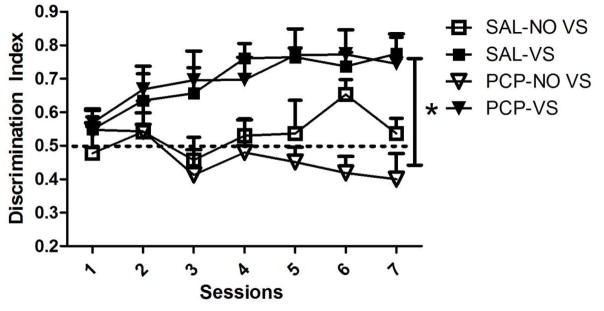
Discrimination index for the seven days of self-administration. Rats in the NOVS condition had a significantly lower discrimination index (indicated by *) than rats in the VS condition. PCP had no effect on discrimination index. The line at 0.5 indicates when active lever-pressing equals inactive lever-pressing.
Activity
Chamber activity for each group is shown in Figure 3. When comparing the SAL-NOVS and SAL-VS groups, there was a main effect of Session [F(6,96)=3.336, p=0.005], a main effect of Group [F(1,16)=4.952, p=0.041] and a significant Session by Group interaction [F(6,96)=2.379, p=0.035]. These findings show that activity was higher in animals with a VS paired with the infusion and this increased over days (436.6±57 to 503.7±58.5). The increase was primarily due to the activity increase in the SAL-VS group (517.5±98.9 to 668.4±74.3). When comparing the SAL-VS groups and PCP-VS groups, there was a main effect of Session [F(6,96)=2.196, p=0.049] with activity increasing over days (583.0±59.0 to 663.6±77.0). There was also a main effect of Group [F(1, 16)=5.676, p=0.28], with activity being higher in the PCP-VS group and an interaction [F(6,96)=3.504, p=0.003], with the session effect being driven by the SAL-VS group, which showed an increase in activity over days (517.5±98.9 to 668.4±74.3). Finally, when comparing the SAL-NOVS and PCP-NOVS groups, there was a main effect of Group [F(1,160=10.695, p=0.004], but no effect of Session and no interaction (ps≥0.22), suggesting that PCP increased activity even in the NOVS condition, but this did not change over session in comparison to SAL-NOVS. Overall, PCP increased activity over days in the VS groups and increased activity overall in the NOVS groups and the presence of a VS increased activity even with no PCP exposure.
Figure 3.
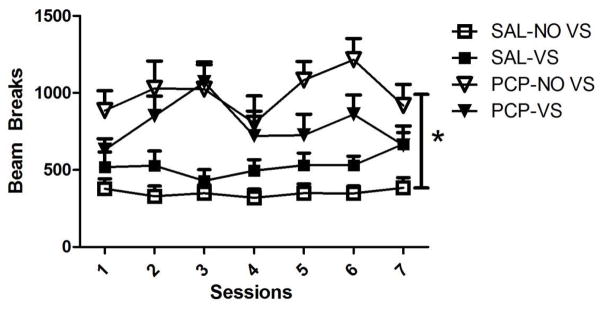
Number of beam breaks over the seven days of self-administration. Activity was higher overall in the PCP condition in comparison to the SAL condition.* indicates a significant difference.
Extinction
Findings from the extinction and reinstatement phases are shown in Figure 4. When comparing the SAL-NOVS and SAL-VS groups, there was a main effect of Group [F(1,16)=8.280, p=0.10] but no main effect of Session and no interaction (ps≥0.19), showing that there were higher levels of lever-pressing in extinction for the VS groups, but this did not change over time. There were no main effects and no interaction when comparing the SAL-NOVS and PCP-NOVS groups (ps≥0.33). When comparing the SAL-VS and PCP-VS groups, there was no main effect of Group (p=0.14), but there was an effect of Session [F(6,96)=2.983, p=0.010] and a marginally significant interaction [F(6,96)=2.184, p=0.050]. These results suggest that lever-pressing increased over extinction days in the VS groups (20.9±3.4 to 34.6±5.0) and this increase was specifically in the PCP-VS groups (16.7±3.9 to 39.3±7.7).
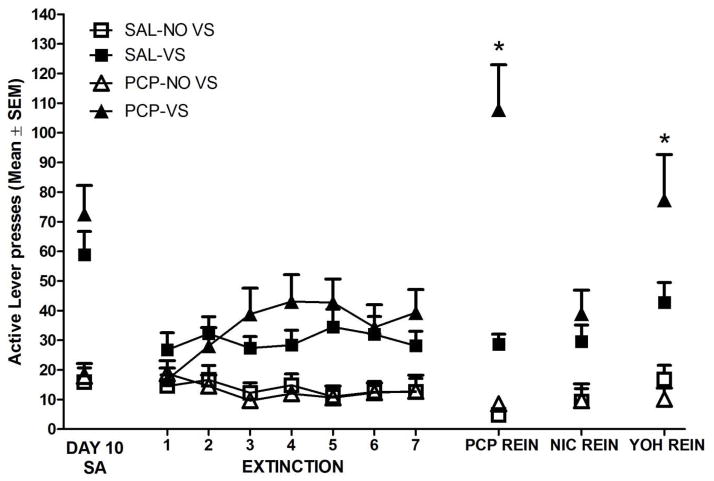
Figure 4.
Final day of self-administration followed by extinction and reinstatement data (all shown in number of active lever presses). Active lever presses includes lever-pressing during the 20-second timeout. Rats in the VS conditions had higher active lever-pressing on all reinstatement days in comparison to the NOVS conditions (which showed no reinstatement behavior). * indicated that rats in the combined PCP/VS condition had potentiated active lever-pressing on the PCP and yohimbine reinstatement days in comparison to all other groups.
Examining Figure 4, there was a clear drop in lever-pressing after nicotine was removed in the VS groups, providing strong support for the reward-enhancement effect of nicotine. The SAL-NOVS and PCP-NOVS groups had low levels of lever-pressing during self-administration and thus had a low percentage drop after removal of nicotine (a 9% and a 5% decrease respectively). The SAL-VS and PCP-VS groups had a 57% and a 74% decrease in lever-pressing from self-administration to the first day of extinction, respectively. However, lever-pressing was higher in groups with the concurrent VS during extinction than in the NO-VS groups suggesting that the VS alone maintained a low level of lever-pressing. This finding supports the idea that the VS is a weak sensory reinforcer.
Reinstatement
PCP Reinstatement
There was a main effect of VS on the phencyclidine reinstatement day [F(1,34)=48.07, p<0.001], with rats in the VS group having higher active lever-pressing than the NO-VS groups. There was a main effect of PCP [F(1,34)=21.827, p<0.001], with rats having higher active lever-pressing if they were previously exposed to PCP. In addition, there was an interaction of PCP and VS [F(1,34)=18.07, p<0.001]. Using LSD tests, rats in the PCP/VS groups had higher active lever-pressing than all other groups (LSDmmd=22.18). Active lever-pressing differed between the final extinction day and the PCP reinstatement test [F(1,34)=15.04, p<0.001] and this effect was specific to the PCP-VS group (p<0.001) as no other groups showed increased responding on the PCP reinstatement day (ps>0.05).
Examining the activity data, there was no main effect of PCP or VS and no interaction of both on the PCP reinstatement day (ps≥0.17). There was also no main effect of PCP or VS and no interaction on the PCP reinstatement day for inactive lever presses (ps≥0.21). Overall, rats that had either received PCP or a VS coupled to the nicotine infusion had higher active lever responding on the PCP reinstatement day than the saline and NO-VS groups. Data from the interaction shows that rats that were exposed to PCP and had a concurrent VS presentation reinstated more to the PCP trigger.
Nicotine Reinstatement
There was a main effect of VS on the nicotine reinstatement day [F(1,34)=15.987, p<0.001], with rats in the VS groups having higher active lever-pressing than rats in the NO-VS groups. There was no main effect of PCP, although the effect was trending towards significance (p≥0.057). There was no interaction of PCP and VS on the nicotine reinstatement day (p≥0.16). Active lever-pressing did not differ between the final extinction day and the nicotine reinstatement test (p=0.59). Examining the activity data, there was a main effect of previous PCP exposure on nicotine reinstatement [F(1,34)=3.809, p=0.039], with higher levels of activity seen in the rats that had nicotine previously paired with PCP (509.7±41.3) than the saline groups (355.95±50.2), but there was no main effect of VS and no interaction of PCP and the VS on the nicotine reinstatement day. For inactive lever-pressing data, there was no main effect of VS and PCP and no interaction (ps≥0.28).
Yohimbine Reinstatement
There was a main effect of VS on yohimbine reinstatement day [F(1,34)=22.856, p<0.001], with rats in the VS groups having higher active lever-pressing than the NO-VS groups. There was no main effect of PCP (p=0.079), yet there was an interaction of PCP and VS [F(1,34)=4.494, p=0.041]. Rats in the PCP-VS groups had higher lever pressing than the SAL-VS and both NOVS groups (LSDmmd=28.36). Active lever-pressing differed between the final extinction day and the yohimbine reinstatement test [F(1,34)=9.590, p=0.0038] and this effect was specific to the PCP-VS group (p<0.001) as no other groups showed increased responding on the PCP reinstatement day (ps>0.05). There were no main effects and no interaction on activity measures (ps≥0.44) or inactive lever-pressing (ps≥0.41). This interaction indicates that rats that were exposed to phencyclidine and had a concurrent VS presentation reinstated to a pharmacological stressor; an effect not seen in any other group.
Discussion
We found that pretreatment with phencyclidine initially attenuated nicotine self-administration but only in the groups with a paired visual stimulus. This attenuation dissipated after 5 days, shown by equivalent lever-pressing between the control and phencyclidine groups. Active lever responding in the VS groups dropped to NOVS levels upon removal of nicotine at the onset of the extinction phase indicating the import of the co-occurrence of nicotine with the VS for maintaining responding. After extinction, reinstatement tests revealed that rats in the PCP-VS group were more sensitive to phencyclidine- and yohimbine-induced reinstatement. These results point to two factors that may be involved in the comorbidity between nicotine abuse and aspects of schizophrenia: differential responsivity to the reward-enhancement effect of nicotine and an increased vulnerability to stress-induced reinstatement.
We found that phencyclidine, used to model the negative symptoms of schizophrenia, decreased nicotine self-administration. These results are dissimilar to those found by Berg and colleagues (2013), in which the neonatal ventral hippocampal lesion preclinical model increased nicotine self-administration. The two models each encapsulate unique aspects of certain components of schizophrenia. The neonatal ventral hippocampal lesion paradigm is better at modeling the developmental aspects of this disorder, whereas PCP more consistently produces symptoms resembling the positive and negative symptomology of schizophrenia, such as anhedonia and memory deficits (Tseng et al. 2008; Javitt & Zukin 1991). The differential nature of the constructs that each model captures could be a potential factor in the disparate results.
In the present study, the attenuation of nicotine self-administration seems to be somewhat transient and is seen primarily on days 3 through 5 of nicotine self-administration before returning to saline levels. This finding is similar to those seen after repeated PCP injections in an intracranial self-stimulation (ICSS) and a conditioned place preference task (Amitai et al. 2009; Iwamoto, 1986; Kitaichi et al. 1996). In these tasks, phencyclidine initially produced a decrease in reward-related behavior, shown by increased reward thresholds in the ICSS task and an aversion to the chamber previously paired with phencyclidine in the place conditioning task. After repeated injections, this initial reward deficit was reversed, eventually attenuating reward thresholds and, in a separate study, leading to a conditioned place preference. Combined, these studies suggest that one potential mechanism behind the transient decrease in nicotine self-administration in the PCP-VS is tolerance to the initial decrease in reward seen after PCP exposure.
Tolerance to the reward-attenuating effects of phencyclidine might not be the only mechanism at play. NMDA receptor antagonists such as dizocilpine and D-CCPene decreased nicotine sensitization of the mesolimbic dopaminergic system (Shoaib et al. 1994). Dextrorphan, which substitutes for phencyclidine in drug discrimination studies and antagonizes NMDA receptors, decreases selective nicotinic acetylcholine receptors (nAChRs), such as the α2β3 variety (Nicholson et al. 1999; Franklin & Murray 1992; Damaj et al. 2005; Wright et al. 2006). A competitive NMDA receptor antagonist (LY235959) acutely reduced nicotine self-administration and this finding was related to its actions in the central nucleus of the amygdala and ventral tegmental area (Kenny et al. 2009). In addition, phencyclidine is a noncompetitive inhibitor of nAChRs (Connolly et al. 1992; Fryer & Lukas 1999). A potential hypothesis for the initial decrease of nicotine self-administration is blockade by PCP of nAChRs. A progressive upregulation of nAChRs in response to the inhibition by PCP could lead to the dissipation of this attenuation seen on day 6.
Another potential explanation of the self-administration findings is that the effects of phencyclidine might be specific to the reinforcement-enhancement effect of nicotine. Our findings first support the seminal study done by Caggiula and colleagues (2002) which suggests that the reinforcement of the visual stimulus concurrent with a nicotine infusion is enhanced by nicotine. We found that nicotine self-administration was enhanced by co-administration of a visual stimulus. PCP administration reduced self-administration levels only in the VS-paired group but did not potentiate or attenuate lever-pressing in animals in the NOVS group. The attenuation in the VS-paired group was not due to a suppression of locomotion, as chamber activity was actually increased by PCP administration. The transient reduction could be due to the mechanisms discussed above, such as receptor blockade and potentially progressive upregulation of nAChRs. An alternative hypothesis is that nicotine self-administration in groups without a concurrent visual stimulus is low enough that further reductions might not be possible (i.e. a floor effect). While this is a valid theory in explaining the lack of the effect in the NOVS conditions, a floor effect explanation cannot explain the transient attenuation in the VS group.
The trend of phencyclidine interacting with only the visual stimulus-paired group extended into the reinstatement period as well. When rats were tested in a reinstatement procedure with PCP, only rats in group PCP-VS showed reinstatement. The low levels of nicotine self-administration in the non-visual stimulus groups explain the lack of reinstatement behavior on all three reinstatement days while the reinstatement in the PCP-VS group could be seen as cue-induced. In a typical cue-induced reinstatement paradigm, rats are given a cue (typically a visual stimulus) previously coupled to the infusion and levels of drug-seeking behavior are examined. In this experiment, PCP was only given during the self-administration days and neither PCP nor saline was given during extinction. Thus, the interoceptive stimulus effects of PCP could act as a cue or context for availability of nicotine. Not surprisingly, the PCP-NOVS group did not show enhanced active lever pressing during the PCP reinstatement test. Again, this notes the import of the conjoint presence of the visual stimulus and nicotine to the later effect of PCP.
Finally, the yohimbine reinstatement test showed that only rats previously exposed to phencyclidine with a concurrent visual stimulus increased active lever pressing. Yohimbine, an α2 adrenergic receptor antagonist, has been widely used as a pharmacological stressor as it induces an increase in stress-like behaviors in both human and non-human animals (Li et al. 2012; Stine et al. 2002; Davis et al. 1979; Charney et al. 1983). Yohimbine has been shown to increase drug-seeking behaviors in a reinstatement model for drugs such as methamphetamine, cocaine, and nicotine (Shepard et al. 2004; Lee et al. 2004; Feltenstein et al. 2012). Perhaps the interoceptive stimulus effects of yohimbine might be similar to the effects of phencyclidine and the reinstatement reflects this generalization between yohimbine and PCP. We could find no study to date that has examined whether phencyclidine and yohimbine substitute for one another in a drug discrimination task. Generalizability between the two substances, however, seems unlikely given the dissimilarity between mechanisms of actions. Phencyclidine works as an NMDA antagonist and yohimbine as an α2 adrenergic antagonist. Yohimbine also counteracts phencyclidine-induced behaviors such as head bobbing and hyperlocomotion (Byron et al. 1985; Freed et al., 1980).
There are two potential limitations of this study. First, reinstatement tests were not counterbalanced. This procedural decision reflects our primary interest in the PCP model of negative and cognitive symptoms seen in schizophrenia. By testing phencyclidine first, exactly one week after the last day of PCP exposure, our test of PCP reinstatement was unaffected by other reinstatement ligands. Of course, this decision means that interpretation of the nicotine and yohimbine outcomes should be interpreted with the lack of counterbalancing in mind. The second limitation is the lack of a saline control in reinstatement to assess the possibility the PCP sensitized rats in an manner that allowed an injection to simply reinstate active lever presses. Our data does not support this possibility. First, recall that PCP-NOVS also has similar treatment with PCP in the self-administration phase. Despite having similar PCP exposure as PCP-VS, they do not show any sign of increased responding across all reinstatement tests. Second, this sensitization account would not predict the pattern of reinstatement in the PCP-VS group. That is, reinstatement should be seen on all 3 tests, or only on the first test if the effect was transient. Instead, reinstatement was only seen on the first and third tests, suggesting that reinstatement effects were related to the pharmacological effects of the reinstatement ligand.
The present study revealed important interactions between nicotine, PCP and a pharmacological stressor. Future studies could be conducted to investigate other factors involved in this complex relationship. First, the length of the self-administration period was chosen based on its ability to consistently model many aspects of schizophrenia but it may not have been long enough to fully capture the effects of phencyclidine on nicotine self-administration. Future work extending the length of self-administration and administering phencyclidine during both the maintenance and acquisition of self-administration is integral. Research utilizing an escalating fixed ratio or progressive ratio schedule could explicate behavioral mechanisms involved in this transient disruption of nicotine self-administration, especially using a procedure involving prior food training.
Stress has long been known to be an integral factor in the etiology of schizophrenia (Walker & Diforio 1997; Tennant 1985) and is also widely implicated in the development, continuation, and reemergence of drug addiction in both clinical and preclinical models (Tomkins 1966; Koob & Le Moal, 1997; see Sinha 2008 for review). Reinstatement by yohimbine of nicotine-seeking behavior suggests that a rat may be more sensitive to pharmacological stressors after being exposed to phencyclidine, used here to model the negative symptoms of schizophrenia. Future research to characterize this effect and determine whether this extends to other stressors is necessary. The specificity of these findings (e.g. that reinstatement and disruption of self-administration is only seen when nicotine is paired with a visual cue) implies that the nicotine reinstatement-enhancement effect might be particularly altered in a preclinical model of the negative symptoms of schizophrenia. Together, the self-administration and reinstatement evidence propose a role of the reinforcement-enhancement effect of nicotine in the comorbidity between tobacco use and schizophrenia.
Acknowledgments
Source of funding: This work was supported by F31 DA034407 awarded to Natashia Swalve, MH085635 awarded to Ming Li, and DA034389 to Rick Bevins
This work was supported by DA034407 (NS), MH085635 (ML), and DA034389 (RAB). All Med PC programs are available upon request.
Footnotes
Conflicts of interest: None
References
- Abdul-Monim Z, Reynolds GP, Neill JC. The atypical antipsychotic ziprasidone, but not haloperidol, improves phencyclidine-induced cognitive deficits in a reversal learning task in the rat. Journal of Psychopharmacology. 2003;17:57–65. doi: 10.1177/0269881103017001700. [DOI] [PubMed] [Google Scholar]
- Abdul-Monim Z, Neill JC, Reynolds GP. Sub-chronic psychotomimetic phencyclidine induces deficits in reversal learning and alterations in parvalbumin-immunoreactive expression in the rat. Journal of Psychopharmacology. 2007;21:198–205. doi: 10.1177/0269881107067097. [DOI] [PubMed] [Google Scholar]
- AhnAllen CG, Liverant GI, Gregor KL, Kamholz BW, Levitt JJ, Gulliver SB, Kaplan GB. The relationship between reward-based learning and nicotine dependence in smokers with schizophrenia. Psychiatry research. 2012;196(1):9–14. doi: 10.1016/j.psychres.2011.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amitai N, Semenova S, Markou A. Clozapine attenuates disruptions in response inhibition and task efficiency induced by repeated phencyclidine administration in the intracranial self-stimulation procedure. European Journal of Pharmacology. 2009;602:78–84. doi: 10.1016/j.ejphar.2008.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett ST, Odum AL. The effects of repeated exposure on the reward-enhancing effects of nicotine. Behavioural Pharmacology. 2011;22:283–290. doi: 10.1097/FBP.0b013e3283473c25. [DOI] [PubMed] [Google Scholar]
- Berg SA, Sentir AM, Cooley BS, Engleman EA, Chambers RA. Nicotine is more addictive, not more cognitively therapeutic in a neurodevelopmental model of schizophrenia produced by neonatal ventral hippocampal lesions. Addiction Biology. 2013 doi: 10.1111/adb.12082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bevins RA. The reference-dose place conditioning procedure yields a graded dose-effect function. Int J Comp Psychol. 2005;18(2) [Google Scholar]
- Bevins RA, Besheer J, Pickett KS. Nicotine-conditioned locomotor activity in rats: Dopaminergic and GABAergic influences on conditioned expression. Pharmacology, Biochemistry and Behavior. 2001;68:135–145. doi: 10.1016/s0091-3057(00)00451-2. [DOI] [PubMed] [Google Scholar]
- Bruins-Slot LA, Kleven MS, Newman-Tancredi A. Effects of novel antipsychotics with mixed D2 antagonist/5-HT1A agonist properties on PCP-induced social interaction deficits in the rat. Neuropharmacology. 2005;49(7):996–1006. doi: 10.1016/j.neuropharm.2005.05.013. [DOI] [PubMed] [Google Scholar]
- Buda M, Tsuang MT, Fleming JA. Causes of death in DSM-III schizophrenics and other psychotics (atypical group). A comparison with the general population. Archives of General Psychiatry. 1988;45:283–285. doi: 10.1001/archpsyc.1988.01800270101012. [DOI] [PubMed] [Google Scholar]
- Byron K, Young GA, Khazan N, Hong O. Suppression of PCP-induced behavioral arousal in the rat by yohimbine pretreatment. European Journal of Pharmacology. 1985;117:271–273. doi: 10.1016/0014-2999(85)90613-2. [DOI] [PubMed] [Google Scholar]
- Caggiula AR, Donny EC, Chaudhri N, Perkins KA, Evans-Martin FF, Sved AF. Importance of nonpharmacological factors in nicotine self-administration. Physiology and Behavior. 2002;77:683–687. doi: 10.1016/s0031-9384(02)00918-6. [DOI] [PubMed] [Google Scholar]
- Caggiula AR, Donny EC, Palmatier MI, Liu X, Chaudhri N, Sved AF. The role of nicotine in smoking: a dual-reinforcement model. Nebraska Symposium on Motivation. 2009;55:91–109. doi: 10.1007/978-0-387-78748-0_6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Center for Disease Control. Adult Cigarette Smoking in the United States: Current Estimates. Centers for Disease Control and Prevention; 2014. Feb 14, Retrieved May 1, 2014, from http://www.cdc.gov/tobacco/data_statistics/fact_sheets/adult_data/cig_smoking/index.htm. [Google Scholar]
- Chambers RA, Krystal JH, Self DW. A neurobiological basis for substance abuse comorbidity in schizophrenia. Biological Psychiatry. 2001;50:71–83. doi: 10.1016/s0006-3223(01)01134-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charney DS, Heninger GR, Redmond DE., Jr Yohimbine induced anxiety and increased noradrenergic function in humans: effects of diazepam and clonidine. Life Science. 1983;33:19–29. doi: 10.1016/0024-3205(83)90707-5. [DOI] [PubMed] [Google Scholar]
- Charntikov S, Swalve N, Pittenger S, Fink K, Schepers S, Hadlock GC, Fleckenstein AE, Hu G, Li M, Bevins RA. Iptakalim attenuates self-administration and acquired goal-tracking behavior controlled by nicotine. Neuropharmacology. 2012;75:138–144. doi: 10.1016/j.neuropharm.2013.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhri N, Caggiula AR, Donny EC, Booth S, Gharib M, Craven L, Palmatier MI, Liu X, Sved AF. Self-administered and noncontingent nicotine enhance reinforced operant responding in rats: impact of nicotine dose and reinforcement schedule. Psychopharmacology (Berlin) 2007;190(3):353–362. doi: 10.1007/s00213-006-0454-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cochran SM, Kennedy M, McKerchar CE, Steward LJ, Pratt JA, Morris BJ. Induction of metabolic hypofunction and neurochemical deficits after chronic intermittent exposure to phencyclidine: differential modulation by antipsychotic drugs. Neuropsychopharmacology: official publication of the American College of Neuropsychopharmacology. 2003;28(2):265–275. doi: 10.1038/sj.npp.1300031. [DOI] [PubMed] [Google Scholar]
- Connolly J, Boulter J, Heinemann SF. Alpha 4-2 beta 2 and other nicotinic acetylcholine receptor subtypes as targets of psychoactive and addictive drugs. British Journal of Pharmacology. 1992;105(3):657–666. doi: 10.1111/j.1476-5381.1992.tb09035.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbett R, Camacho F, Woods AT, Kerman LL, Fishkin RJ, Brooks K, Dunn RW. Antipsychotic agents antagonize non-competitive N-methyl-d-aspartate antagonist-induced behaviors. Psychopharmacology. 1995;120(1):67–74. doi: 10.1007/BF02246146. [DOI] [PubMed] [Google Scholar]
- Cox BM, Goldstein A, Nelson WT. Nicotine self-administration in rats. British Journal of Pharmacology. 1984;83(1):49–55. doi: 10.1111/j.1476-5381.1984.tb10118.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damaj MI, Flood P, Ho KK, May EL, Martin BR. Effect of dextrometorphan and dextrorphan on nicotine and neuronal nicotinic receptors: in vitro and in vivo selectivity. Journal of Pharmacology and Experimental Therapeutics. 2005;312(2):780–785. doi: 10.1124/jpet.104.075093. [DOI] [PubMed] [Google Scholar]
- Davis M, Redmond DE, Jr, Baraban JM. Noradrenergic agonists and antagonists: effects on conditioned fear as measured by the potentiated startle paradigm. Psychopharmacology. 1979;65(2):111–118. doi: 10.1007/BF00433036. [DOI] [PubMed] [Google Scholar]
- Der-Avakian A, Markou A. The neurobiology of anhedonia and other reward-related deficits. Trends Neuroscience. 2012;35(1):68–77. doi: 10.1016/j.tins.2011.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolan SL, Sacco KA, Termine A, Seyal AA, Dudas MM, Vessicchio JC, Wexler BE, George TP. Neuropsychological deficits are associated with smoking cessation treatment failure in patients with schizophrenia. Schizophrenia Research. 2004;70(2–3):263–275. doi: 10.1016/j.schres.2004.01.006. [DOI] [PubMed] [Google Scholar]
- Donny EC, Chaudhri N, Caggiula AR, Evans-Martin FF, Booth S, Gharib MA, Clements LA, Sved AF. Operant responding for a visual reinforcer in rats is enhanced by noncontingent nicotine: implications for nicotine self-administration and reinforcement. Psychopharmacology (Berlin) 2003;169(1):68–76. doi: 10.1007/s00213-003-1473-3. [DOI] [PubMed] [Google Scholar]
- Feltenstein MW, Ghee SM, See RE. Nicotine self-administration and reinstatement of nicotine-seeking in male and female rats. Drug and Alcohol Dependence. 2012;121(3):240–246. doi: 10.1016/j.drugalcdep.2011.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin PH, Murray TF. High affinity [3H] dextrorphan binding in rat brain is localized to a noncompetitive antagonist site of the activated N-methyl-D-aspartate receptor-cation channel. Molecular pharmacology. 1992;41(1):134–146. [PubMed] [Google Scholar]
- Freed WJ, Weinberger DR, Bing LA, Wyatt RJ. Neuropharmacological studies of phencyclidine (PCP)-induced behavioral stimulation in mice. Psychopharmacology. 1980;71(3):291–297. doi: 10.1007/BF00433064. [DOI] [PubMed] [Google Scholar]
- Fryer JD, Lukas RJ. Noncompetitive functional inhibition at diverse, human nicotinic acetylcholine receptor subtypes by bupropion, phencyclidine, and ibogaine. Journal of Pharmacology and Experimental Therapeutics. 1999;288(1):88–92. [PubMed] [Google Scholar]
- Grayson B, Idris NF, Neill JC. Atypical antipsychotics attenuate a sub-chronic PCP-induced cognitive deficit in the novel object recognition task in the rat. Behavioural brain research. 2007;184(1):31–38. doi: 10.1016/j.bbr.2007.06.012. [DOI] [PubMed] [Google Scholar]
- Hennekens CH, Hennekens AR, Hollar D, Casey DE. Schizophrenia and increased risks of cardiovascular disease. American Heart Journal. 2005;150(6):1115–1121. doi: 10.1016/j.ahj.2005.02.007. [DOI] [PubMed] [Google Scholar]
- Idris NF, Repeto P, Neill JC, Large CH. Investigation of the effects of lamotrigine and clozapine in improving reversal-learning impairments induced by acute phencyclidine and D-amphetamine in the rat. Psychopharmacology (Berlin) 2005;179(2):336–348. doi: 10.1007/s00213-004-2058-5. [DOI] [PubMed] [Google Scholar]
- Iwamoto ET. Place-aversion conditioned by phencyclidine in rats: Development of tolerance and pharmacologic antagonism. Alcohol & Drug Research. 1986 [PubMed] [Google Scholar]
- Javitt DC, Zukin SR. Recent advances in the phencyclidine model of schizophrenia. Am J Psychiatry. 1991;148(10):1301–1308. doi: 10.1176/ajp.148.10.1301. [DOI] [PubMed] [Google Scholar]
- Jentsch JD, Tran A, Le D, Youngren KD, Roth RH. Subchronic phencyclidine administration reduces mesoprefrontal dopamine utilization and impairs prefrontal cortical-dependent cognition in the rat. Neuropsychopharmacology. 1997;17(2):92–99. doi: 10.1016/S0893-133X(97)00034-1. [DOI] [PubMed] [Google Scholar]
- Jones CA, Watson DJG, Fone KCF. Animal models of schizophrenia. British journal of pharmacology. 2011;164(4):1162–1194. doi: 10.1111/j.1476-5381.2011.01386.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenny PJ, Chartoff E, Roberto M, Carlezon WA, Jr, Markou A. NMDA receptors regulate nicotine-enhanced brain reward function and intravenous nicotine self-administration: role of the ventral tegmental area and central nucleus of the amygdala. Neuropsychopharmacology. 2009;34(2):266–281. doi: 10.1038/npp.2008.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitaichi K, Noda Y, Hasegawa T, Furukawa H, Nabeshima T. Acute phencyclidine induces aversion, but repeated phencyclidine induces preference in the place conditioning test in rats. European Journal of Pharmacology. 1996;318(1):7–9. doi: 10.1016/s0014-2999(96)00875-8. [DOI] [PubMed] [Google Scholar]
- Koob GF, Le Moal M. Addiction and the brain antireward system. Annu Rev Psychol. 2008;59:29–53. doi: 10.1146/annurev.psych.59.103006.093548. [DOI] [PubMed] [Google Scholar]
- Koob GF, Le Moal M. Drug abuse: hedonic homeostatic dysregulation. Science. 1997;278(5335):52–58. doi: 10.1126/science.278.5335.52. [DOI] [PubMed] [Google Scholar]
- Kring AM, Moran EK. Emotional response deficits in schizophrenia: insights from affective science. Schizophrenia bulletin. 2008;34(5):819–834. doi: 10.1093/schbul/sbn071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumari V, Postma P. Nicotine use in schizophrenia: the self medication hypotheses. Neuroscience and Biobehavioral Review. 2005;29(6):1021–1034. doi: 10.1016/j.neubiorev.2005.02.006. [DOI] [PubMed] [Google Scholar]
- Lee B, Tiefenbacher S, Platt DM, Spealman RD. Pharmacological blockade of alpha2-adrenoceptors induces reinstatement of cocaine-seeking behavior in squirrel monkeys. Neuropsychopharmacology. 2004;29(4):686–693. doi: 10.1038/sj.npp.1300391. [DOI] [PubMed] [Google Scholar]
- Li S, Zou S, Coen K, Funk D, Shram MJ, Le AD. Sex differences in yohimbine-induced increases in the reinforcing efficacy of nicotine in adolescent rats. Addiction Biology. 2012;19(2):156–164. doi: 10.1111/j.1369-1600.2012.00473.x. [DOI] [PubMed] [Google Scholar]
- Liu X, Palmatier MI, Caggiula AR, Donny EC, Sved AF. Reinforcement enhancing effect of nicotine and its attenuation by nicotinic antagonists in rats. Psychopharmacology (Berlin) 2007;194(4):463–473. doi: 10.1007/s00213-007-0863-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLean SL, Woolley ML, Thomas D, Neill JC. Role of 5-HT receptor mechanisms in sub-chronic PCP-induced reversal learning deficits in the rat. Psychopharmacology. 2009;206(3):403–414. doi: 10.1007/s00213-009-1618-0. [DOI] [PubMed] [Google Scholar]
- Merlo-Pich EM, Pagliusi SR, Tessari M, Talabot-Ayer D, van Huijsduijnen RH, Chiamulera C. Common neural substrates for the addictive properties of nicotine and cocaine. Science. 1997;275(5296):83–86. doi: 10.1126/science.275.5296.83. [DOI] [PubMed] [Google Scholar]
- Mortensen PB, Juel K. Mortality and causes of death in schizophrenic patients in Denmark. Acta Psychiatrica Scandinavica. 1990;81(4):372–377. doi: 10.1111/j.1600-0447.1990.tb05466.x. [DOI] [PubMed] [Google Scholar]
- Nicholson KL, Hayes BA, Balster RL. Evaluation of the reinforcing properties and phencyclidine-like discriminative stimulus effects of dextromethorphan and dextrorphan in rats and rhesus monkeys. Psychopharmacology (Berlin) 1999;146(1):49–59. doi: 10.1007/s002130051087. [DOI] [PubMed] [Google Scholar]
- Palmatier MI, Evans-Martin FF, Hoffman A, Caggiula AR, Chaudhri N, Donny EC, et al. Dissociating the primary reinforcing and reinforcement-enhancing effects of nicotine using a rat self-administration paradigm with concurrently available drug and environmental reinforcers. Psychopharmacology (Berlin) 2006;184(3–4):391–400. doi: 10.1007/s00213-005-0183-4. [DOI] [PubMed] [Google Scholar]
- Palmatier MI, Matteson GL, Black JJ, Liu X, Caggiula AR, Craven L, Donny EC, Sved AF. The reinforcement enhancing effects of nicotine depend on the incentive value of non-drug reinforcers and increase with repeated drug injections. Drug and Alcohol Dependence. 2007;89(1):52–59. doi: 10.1016/j.drugalcdep.2006.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parks KA, Pardi AM, Bradizza CM. Collecting data on alcohol use and alcohol-related victimization: a comparison of telephone and Web-based survey methods. Journal of Studies on Alcohol. 2006;67(2):318–323. doi: 10.15288/jsa.2006.67.318. [DOI] [PubMed] [Google Scholar]
- Pouzet B, Didriksen M, Arnt J. Effects of the 5-HT 6 receptor antagonist, SB-271046, in animal models for schizophrenia. Pharmacology Biochemistry and Behavior. 2002;71(4):635–643. doi: 10.1016/s0091-3057(01)00743-2. [DOI] [PubMed] [Google Scholar]
- Raiff BR, Dallery J. Effects of acute and chronic nicotine on responses maintained by primary and conditioned reinforcers in rats. Experimental and Clinical Psychopharmacology. 2006;14(3):296–305. doi: 10.1037/1064-1297.14.3.296. [DOI] [PubMed] [Google Scholar]
- Raiff BR, Dallery J. The generality of nicotine as a reinforcer enhancer in rats: effects on responding maintained by primary and conditioned reinforcers and resistance to extinction. Psychopharmacology (Berlin) 2008;201(2):305–314. doi: 10.1007/s00213-008-1282-9. [DOI] [PubMed] [Google Scholar]
- Raiff BR, Dallery J. Responding maintained by primary reinforcing visual stimuli is increased by nicotine administration in rats. Behavioural Processes. 2009;82(1):95–99. doi: 10.1016/j.beproc.2009.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichel CM, Linkugel JD, Bevins RA. Bupropion differentially impacts acquisition of methamphetamine self-administration and sucrose-maintained behavior. Pharmacol Biochem Behav. 2008;89(3):463–472. doi: 10.1016/j.pbb.2008.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risner ME, Goldberg SR. A comparison of nicotine and cocaine self-administration in the dog: fixed-ratio and progressive-ratio schedules of intravenous drug infusion. Journal of Pharmacology and Experimental Therapeutics. 1983;224(2):319–326. [PubMed] [Google Scholar]
- Saha S, Chant D, McGrath J. A systematic review of mortality in schizophrenia: is the differential mortality gap worsening over time? Archives of General Psychiatry. 2007;64(10):1123–1131. doi: 10.1001/archpsyc.64.10.1123. [DOI] [PubMed] [Google Scholar]
- Sams-Dodd F. Effect of novel antipsychotic drugs on phencyclidine-induced stereotyped behaviour and social isolation in the rat social interaction test. Behavioural pharmacology. 1997;8(2–3):196–215. [PubMed] [Google Scholar]
- Sams-Dodd F. Effects of dopamine agonists and antagonists on PCP-induced stereotyped behaviour and social isolation in the rat social interaction test. Psychopharmacology. 1998;135(2):182–193. doi: 10.1007/s002130050500. [DOI] [PubMed] [Google Scholar]
- Shepard JD, Bossert JM, Liu SY, Shaham Y. The anxiogenic drug yohimbine reinstates methamphetamine seeking in a rat model of drug relapse. Biological Psychiatry. 2004;55(11):1082–1089. doi: 10.1016/j.biopsych.2004.02.032. [DOI] [PubMed] [Google Scholar]
- Shoaib M, Benwell ME, Akbar MT, Stolerman IP, Balfour DJ. Behavioural and neurochemical adaptations to nicotine in rats: influence of NMDA antagonists. British Journal of Pharmacology. 1994;111(4):1073–1080. doi: 10.1111/j.1476-5381.1994.tb14854.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha R. Chronic stress, drug use, and vulnerability to addiction. Annual New York Academy of Science. 2008;1141:105–130. doi: 10.1196/annals.1441.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith RC, Singh A, Infante M, Khandat A, Kloos A. Effects of cigarette smoking and nicotine nasal spray on psychiatric symptoms and cognition in schizophrenia. Neuropsychopharmacology. 2002;27(3):479–497. doi: 10.1016/S0893-133X(02)00324-X. [DOI] [PubMed] [Google Scholar]
- Stine SM, Southwick SM, Petrakis IL, Kosten TR, Charney DS, Krystal JH. Yohimbine-induced withdrawal and anxiety symptoms in opioid-dependent patients. Biological Psychiatry. 2002;51(8):642–651. doi: 10.1016/s0006-3223(01)01292-6. [DOI] [PubMed] [Google Scholar]
- Tennant CC. Stress and schizophrenia: A review. Integrative Psychiatry 1985 [Google Scholar]
- Thiel KJ, Sanabria F, Neisewander JL. Synergistic interaction between nicotine and social rewards in adolescent male rats. Psychopharmacology (Berlin) 2009;204(3):391–402. doi: 10.1007/s00213-009-1470-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomkins SS. Psychological model for smoking behavior. American Journal of Public Health. 1966;56:17. doi: 10.2105/ajph.56.12_suppl.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng KY, Lewis BL, Hashimoto T, Sesack SR, Kloc M, Lewis DA, O’Donnell P. A neonatal ventral hippocampal lesion causes functional deficits in adult prefrontal cortical interneurons. The Journal of Neuroscience. 2008;28(48):12691–12699. doi: 10.1523/JNEUROSCI.4166-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker EF, Diforio D. Schizophrenia: a neural diathesis-stress model. Psychology Review. 1997;104(4):667–685. doi: 10.1037/0033-295x.104.4.667. [DOI] [PubMed] [Google Scholar]
- Watkins SS, Koob GF, Markou A. Neural mechanisms underlying nicotine addiction: acute positive reinforcement and withdrawal. Nicotine and Tobacco Research. 2000;2(1):19–37. doi: 10.1080/14622200050011277. [DOI] [PubMed] [Google Scholar]
- Wee S, Wang Z, He R, Zhou J, Kozikowski AP, Woolverton WL. Role of the increased noradrenergic neurotransmission in drug self-administration. Drug Alcohol Depend. 2006;82 (2):151–157. doi: 10.1016/j.drugalcdep.2005.09.002. [DOI] [PubMed] [Google Scholar]
- Wolf DH. Anhedonia in schizophrenia. Current psychiatry reports. 2006;8(4):322–328. doi: 10.1007/s11920-006-0069-0. [DOI] [PubMed] [Google Scholar]
- Wright MJ, Jr, Vann RE, Gamage TF, Damaj MI, Wiley JL. Comparative effects of dextromethorphan and dextrorphan on nicotine discrimination in rats. Pharmacology Biochemistry and Behavior. 2006;85(3):507–513. doi: 10.1016/j.pbb.2006.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang XY, Tan YL, Zhou DF, Haile CN, Wu GY, Cao LY, Kosten TA, Kosten TR. Nicotine dependence, symptoms and oxidative stress in male patients with schizophrenia. Neuropsychopharmacology. 2007;32(9):2020–2024. doi: 10.1038/sj.npp.1301317. [DOI] [PubMed] [Google Scholar]
- Ziauddeen H, Murray GK. The relevance of reward pathways for schizophrenia. Current opinion in psychiatry. 2010;23(2):91–96. doi: 10.1097/YCO.0b013e328336661b. [DOI] [PubMed] [Google Scholar]