Abstract
The introduction of newborn screening and the development of new therapies have led to an expanding population of patients with inherited metabolic disorders, and these patients are now entering adulthood. Dietary therapy is the mainstay of treatment for many of these disorders and thus, trained metabolic dietitians are critical members of the multidisciplinary team required for management of such patients. The main goals of dietary therapy in inborn errors of metabolism are the maintenance of normal growth and development while limiting offending metabolites and providing deficient products. Typically, the offending metabolite is either significantly reduced or removed completely from the diet and then reintroduced in small quantities until blood levels are within the normal range. Such treatment is required in infancy, childhood and adulthood and requires careful monitoring of micronutrient and macronutrient intake throughout the lifespan. The goal of this review is to highlight the basic principles of chronic nutritional management of the inborn errors of protein, carbohydrate and fat metabolism.
Introduction
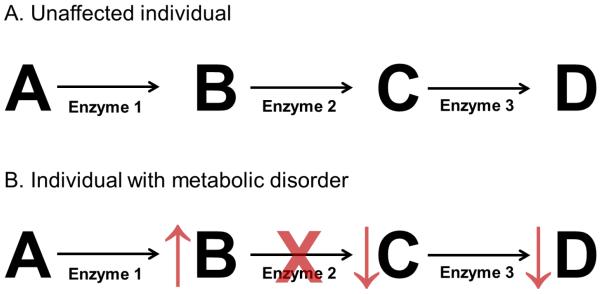
Inborn errors of metabolism result from an enzymatic deficiency in a metabolic pathway. As a result of this enzyme deficiency, substrates may accumulate, and products may become deficient (Figure 1). These substrates or their metabolites may be toxic. Thus, a basic principle of management in these disorders is to reduce the tissue and plasma concentrations of toxic substrates by reducing the consumption of nutrients that produce toxic products or by increasing excretion of such toxic metabolites. Likewise, a second basic principle of management is the provision of deficient products through supplementation or by bypassing the deficient enzyme if a more distal product or products are necessary for normal growth and development. When residual enzyme activity remains, another common strategy is to provide vitamins or cofactors to increase enzyme activity. There is a wide spectrum of metabolic tolerance for patients even with the same disorder. Thus, the prescribed diet is individualized for each patient to account for an individual’s tolerance to the toxic metabolite, their stage of development, and their clinical status.
Figure 1.
Principles of nutritional management of inborn errors of metabolism. A. A model metabolic pathway is depicted in an unaffected individual with metabolites labeled with letters and enzymes numbered. B. In an individual in which enzyme 2 is deficient, product B will accumulate and products C and D will become deficient. To treat this patient, products A and B will be limited in the diet, and products C and D may need to be supplemented in the diet. In addition, excretion of product B may be enhanced, if possible. Cofactors of enzyme 2, if they exist, may be supplemented depending on the disorder to enhance enzyme activity.
The dietary manipulations required for managing patients with inborn errors of metabolism may place patients at risk for an essential amino acid, fatty acid, or micronutrient deficiency. Special medical formulas that include macro- and micronutrients but omit the offending substrate are available to help prevent such deficiencies. In addition, careful monitoring of protein adequacy is necessary in the chronic management of many of these disorders and requires vigilant attention to dietary intake, overall clinical status, measurement of biochemical markers such as prealbumin and albumin, and monitoring of growth over time. Furthermore, routine monitoring and counseling regarding appropriate weight gain and potential long-term health consequences of overweight and obesity should be provided. Several references are available for the metabolic dietitian to use when generating a dietary prescription and calculating macro- and micro-nutrient intake in patients with metabolic disorders1-3.
In this review, we will focus on the chronic dietary management of several of the more common inborn errors of protein metabolism, fatty acid metabolism, and carbohydrate metabolism.
Disorders of Amino Acid and Protein Metabolism
Phenylketonuria (PKU)
PKU results from a deficiency in phenylalanine hydroxylase, the enzyme that converts the amino acid phenylalanine to tyrosine (Figure 2). When this enzyme is deficient, phenylalanine and its metabolites accumulate, and tyrosine, a precursor for neurotransmitters and melanin, becomes deficient. When left untreated, patients with PKU develop irreversible neurocognitive impairment, growth deficiency, eczema, and skin and hair hypopigmentation4-6. These complications are believed to result from a combination of factors including deficiency of tyrosine, the precursor of melanin and numerous neurotransmitters, and disruption of the transport of large neutral amino acids by the accumulation of phenylalanine. Thus, when a diet restricted in phenylalanine and supplemented with tyrosine is introduced early in infancy, permanent clinical sequelae of this disorder can be prevented.
Figure 2.
Phenylalanine hydroxylase enzyme activity is deficient in phenylketonuria (PKU). As a result of this enzyme deficiency, phenylalanine accumulates and tyrosine becomes deficient. Thus, consumption of phenylalanine must be limited and tyrosine supplemented in patients with PKU.
The objective of the phenylalanine-restricted diet is to provide sufficient phenylalanine for growth, while maintaining serum phenylalanine levels within a target range of 2-6 mg/dL throughout the lifespan7, 8. Although previously treatment was restricted to infancy and early childhood, lifelong therapy is now recommended given the emergence of numerous behavioral and psychiatric phenotypes in patients, particularly in those who relaxed their protein restriction in late childhood or early adolescent years9-15. Although some clinicians also monitor the ratio of phenylalanine to tyrosine, evidence for this practice is limited16.
To reduce plasma phenylalanine levels to the target range, individuals with classical forms of PKU typically require total elimination of high protein foods such as meats, eggs, milk and cheese from their diet. Individuals with milder forms of hyperphenylalaninemia may be able to incorporate small amounts of high protein foods into their diet due to increased tolerance to phenylalanine. A thorough knowledge of the phenylalanine content of foods is necessary to plan an appropriate diet with variety and maintenance of essential nutrients. Metabolic formulas, which contain synthetic mixtures of amino acids devoid of phenylalanine, are typically required if the phenylalanine restriction results in protein intake below levels necessary for growth. These special metabolic formulas are supplemented with tyrosine, the deficient product in this disorder. Formula prescriptions must be individualized and a variety of commercial products, such as coolers, gels, and ready-to-drink boxes, are available to meet patient’s preferences for taste and palatability.
Individuals may exhibit significant range in their tolerance for phenylalanine and this tolerance varies during the growth cycle. Consequently, individualized calculations for phenylalanine and tyrosine prescriptions must be made using frequent assessments of serum phenylalanine and tyrosine levels, growth parameters, and “actual” dietary intake through diet records provided by the patient. Due to the potential for nutrient deficiencies with restriction of phenylalanine, comprehensive macronutrient and micronutrient analysis must compliment the physical evaluation and biochemical assessments. Recommended monitoring guidelines have been discussed in more detail elsewhere7, 8. Although families and eventually the patient can master the basic principles of this diet, continued follow-up by health care providers is essential to allow for adjustments to the diet prescription which are necessary at various stages of development, to provide motivation to continue the diet, and to provide continued age-appropriate education. To promote long-term dietary adherence, it is necessary to help families adapt the diet to their personal life-style or cultural preferences.
Pregnancy provides unique challenges for the management of PKU because of the teratogenic effects of elevated maternal phenylalanine levels. Elevated maternal phenylalanine levels are associated with increased risk for intellectual disability, microcephaly, cardiac defects, and poor fetal growth, in infants born to mothers with PKU who have poor metabolic control17. This constellation of phenotypes has been termed maternal PKU17. Thus, to prevent the development of maternal PKU, maintenance of maternal serum phenylalanine levels within the target range are necessary prior to conception and throughout pregnancy18. Thus, close and careful monitoring of serum phenylalanine levels before, during and after pregnancy are necessary for women with PKU7, 8.
A newer strategy for PKU management involves the use of sapropterin dihydrochloride, a synthetic stereoisomer of tetrahydrobiopterin which is a cofactor for the phenylalanine hydroxylase18-22. Sapropterin dihydrochloride is used in a subset of patients deemed tetrahydrobiopterin-responsive18-22. A trial of responsiveness is performed prior to initiating long-term therapy with this cofactor and should be considered in all PKU patients23. Sapropterin dihydrochloride is typically used in the setting of a phenylalanine-restricted diet but, in many cases, may allow for increased phenylalanine intake in the form of increased natural protein in the diet21. Recommendations for initiating and managing patients with PKU using sapropterin dihydrochloride have recently been published23. Clinical data regarding the safety and efficacy of the use of sapropterin dihydrochloride in pregnancy is limited. However, sapropterin dihydrochloride has been used during pregnancy anecdotally, and to our knowledge, no serious adverse events in pregnancy have been definitively linked to use during pregnancy24.
Two other new strategies for management of PKU include supplementation with large neutral amino acids (LNAAs) and the use of glycomacropeptide (GMP), a naturally occurring, low-phenylalanine protein source. LNAAs are proposed to interfere with the transport of phenylalanine across the intestine and blood-brain barrier since phenylalanine and other large neutral amino acids share the same transport systems. Only small studies of LNAAs in older patients have been performed and larger, long-term studies are necessary to determine safety and efficacy of this treatment strategy25, 26. The small studies performed to date have been limited to older patients and thus, LNAAs are not considered in infants, young children, or pregnant adults with PKU since LNAAs may not be safe for use in these settings. GMP is a naturally occurring phenylalanine-free protein source derived from whey27. When isolated, GMP is contaminated with other proteins and thus, contains small amounts of phenylalanine27. However, given the low phenylalanine content of GMP, GMP has been used in the manufacturing of low phenylalanine foods for patients with PKU27, 28. A small study of GMP use in patients with PKU identified no adverse events and demonstrated short-term efficacy in maintaining serum phenylalanine levels as compared to the use of traditional phenylalanine-free medical formulas28. Further, long-term studies of the use of GMP in patients with PKU are necessary.
Maple Syrup Urine Disease
Maple Syrup Urine Disease (MSUD) is a disorder of branched-chain amino acid (BCAA) metabolism caused by a deficiency in activity of the branched-chain α-ketoacid dehydrogenase complex (BCKDC). This enzyme deficiency results in the accumulation of the BCAAs (leucine, isoleucine, and valine) and their corresponding branched-chain α-ketoacids. If left untreated, this disorder can result in poor appetite, vomiting, acidosis, lethargy, coma, and death in the neonatal period 29. Even with treatment, severe, life-threatening metabolic decompensation can be triggered by common illness, such as upper respiratory tract infections and gastroenteritis or other forms of catabolic stress, and excess leucine consumption.
Of the BCAAs and ketoacids, leucine is considered the most neurotoxic, and strict, long-term control of plasma leucine levels has been associated with improved neurocognitive and neuropsychiatric outcomes in patients with MSUD30-33. Thus, the goal of management in patients with MSUD is to maintain plasma leucine and other BCAAs within the normal range while supplying adequate nutrients necessary for growth and development. This goal is achieved with dietary restriction of BCAAs through protein restriction and the use of commercial elemental formulas which consist of synthetic amino acid mixtures devoid of the BCAAs but which contain other essential macro and micro-nutrients. In addition, a trial of thiamine, the cofactor for the BCKDC, may be initiated since a subset of patients have been found to be thiamine-responsive34. The levels of plasma leucine and other BCAAs are carefully monitored in all patients to monitor the efficacy of dietary therapy. Rises in plasma leucine level may occur with dietary non-compliance, over-prescription, inadequate intake of protein or energy and other catabolic stress such as illness or infection. Leucine concentrations may also rise with deficiencies of valine or isoleucine due to decreased protein synthesis or muscle catabolism. Thus, supplementation with isoleucine and/or valine is sometimes necessary in daily management of MSUD. Comprehensive, nutrition management guidelines for MSUD were recently published and may be used as a guide for therapy35.
Urea Cycle Disorders
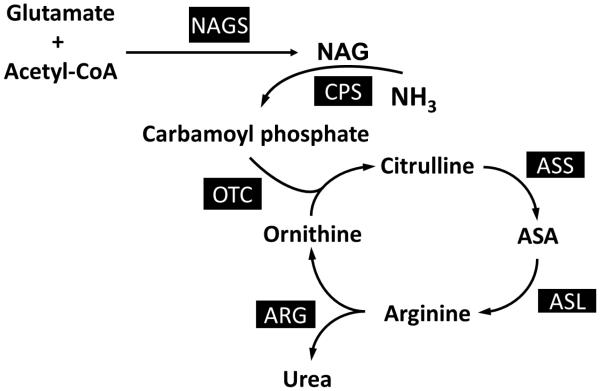
The urea cycle converts ammonia, a toxic metabolite produced from nitrogen in amino acids, to urea, a safely excreted product. Urea cycle disorders (UCDs) result when one of the enzymes of this pathway is deficient (Figure 3). As a result of the enzyme deficiency, ammonia can accumulate and lead to lethargy, coma or even death. Thus, a primary goal of medical nutrition therapy in patients with UCDs is to limit dietary nitrogen intake through protein restriction to prevent hyperammonemia. Patients should be maintained in a positive nitrogen balance by ensuring that protein synthesis is greater than protein catabolism as over-restriction can lead to protein and/or amino acid deficiency and resulting growth deficiency. If nitrogen falls below positive balance and becomes negative, growth failure occurs, and patients may be at increased risk for hyperammonemia. Dietary nitrogen tolerance in patients with defective ureagenesis is substantially greater during infancy because of the requirement of urea synthesis in the rapidly growing infant. As growth slows, nitrogen tolerance decreases, and the dietary protein prescription must be reduced. The FAO/WHO/UNU (Food and Agricultural Organization of the United Nations/World Health Organization/United Nations University) 2007 have set safe levels of protein intake calculated as an age-adjusted mean + 2-standard deviations and can be used as a guide3.
Figure 3.
Urea Cycle Disorders. Enzymes associated with particular urea cycle disorders include NAGS (n-acetylglutamate synthase), CPS (carbamoyl phosphate synthetase I), OTC (ornithine transcarbamylase), ASS (argininosuccinate synthetase), ASL (argininosuccinate lyase), and ARG (arginase I).
Chronic protein-restriction often requires a combination of limited protein intake from food together with medical formula consisting of essential amino acids with or without other non-protein energy sources. Essential amino acid mixtures provide higher quality nitrogen intake and less nitrogen as compared to whole protein36. A second important strategy for managing patients with UCDs is the provision of deficient products. For example, enzyme deficiencies in the urea cycle (with the exception of arginase deficiency) prevent the production of arginine. Thus, arginine becomes an essential amino acid in these patients. Arginine deficiency results in a catabolic state that stimulates further mobilization of nitrogen from protein breakdown and may place patients at increased risk for hyperammonemia. In addition, arginine deficiency can be associated with growth deficiency, hair abnormalities and skin problems37, 38. In proximal UCDs such as ornithine transcarbamylase (OTC) deficiency, carbamylphosphate synthetase I (CPS I) deficiency, and N-acetylglutamate synthase (NAGS) deficiency, citrulline, rather than arginine can be provided because of the theoretical advantage of incorporating nitrogen from aspartate into the cycle for clearance as urea. In more distal defects, such as argininosuccinate lyase (ASL) and argininosuccinate synthetase (ASS) deficiencies, arginine supplementation is prescribed. With arginine supplementation, water soluble compounds, such as citrulline and argininosuccinic acid, can be formed and excreted, resulting in additional removal of ammonia. However, caution must be used when supplementing with arginine because large doses (500 mg/kg/day) of arginine have been associated with worsening elevation in liver enzymes in a subset of patients with ASL deficiency 39 and because of the possibility the development of spasticity with hyperargininemia from excessive arginine supplementation40. Supplementation of BCAAs may be needed, as medical therapy (eg. nitrogen-scavenging agents) for UCDs can cause decreased BCAAs41, 42. In addition, attention to adequate hydration status is necessary to maintain excretion of nitrogenous compounds. Thus, fluids should be provided to meet 1-1.5x the typical maintenance fluid volume40 . More detailed guidelines for the dietary management of UCDs have been summarized in more details elsewhere 40.
Long-term dietary therapy for UCDs should be monitored by assessing the levels of plasma amino acids, markers of protein status (eg. prealbumin and albumin), and growth parameters. Periodic ammonia measurements as part of routine laboratory monitoring may also be helpful, and such measurements should always be performed in the setting of altered mental status, lethargy, seizures, headaches, vomiting, or any other symptom suggestive of hyperammonemia 43. The timing of blood collections with regards to meals/formula and medication/supplement administration should be considered when results are evaluated.
Methylmalonic aciduria (MMA) and Propionic Aciduria (PA)
The final steps in the catabolism of isoleucine, valine, methionine, threonine and odd chain fatty acids involve the conversion of propionyl-CoA to succinyl-CoA, a citric acid cycle intermediate. The two enzymes that perform this conversion are propionyl-CoA carboxylase, a biotin-dependent enzyme, and methylmalonyl-CoA mutase, an adenosylcobalamin-dependent enzyme. When either of these enzymes is deficient, an inborn error of metabolism (propionic acidemia (PA) or methylmalonic acidemia (MMA), respectively) develops. Although there is a wide variability in clinical presentation of these disorders, patients are at risk for developmental delay and/or intellectual disability, seizures, metabolic strokes, and a complications in a variety of other organ systems (eg. cardiac, renal, ophthalmologic and gastrointestinal)29. As with many other metabolic disorders, such as UCDs and MSUD, patients are at risk for metabolic decompensation which may include severe acidosis and hyperammonemia in the setting of catabolic stress such as intercurrent illness. Although there are clinical differences between these two disorders, the basic management strategies are similar, and thus, these disorders will be discussed together.
The patient with MMA or PA is prescribed a diet which is restricted in isoleucine, methionine, threonine, valine, and odd-chain fatty acids through dietary protein restriction. The amount of natural protein prescribed is determined by age, growth, metabolic stability and severity of condition44. Two different strategies for protein restriction have been proposed. In one strategy, total daily protein is provided as a mixture of natural protein from food or standard infant formula and synthetic amino acid mixtures devoid of the offending amino acids 45, 46. Careful attention to total protein with dietary percentages of natural to synthetic protein (typically no lower than a 50/50 ratio) is necessary. A second strategy uses natural protein alone, often given at near or less than the recommended dietary allowance (RDA)47. Studies have not conclusively determined which strategy is most appropriate, and thus, the therapeutic approach must be individualized. Exogenous administration of hydroxocobalamin is given to patients with MMA who have demonstrated cobalamin responsiveness48.
Regardless of the strategy used for management, careful attention to calories, essential nutrients including essential amino acid levels, and adequate hydration is required to support normal physical and mental development. Some groups also use urea excretion in the urine as a monitoring parameter using pooled random samples collected in a 24 hour period47. Decreased resting energy expenditure has been observed in patients with MMA. Thus, predictive equations that are typically used to determine energy requirements may not be accurate for use in patients with these disorders49.
Disorders of Carbohydrate Metabolism
Classic Galactosemia
Classic galactosemia is an inborn error of galactose metabolism that results from deficiency of the galactose-1-phosphate (Gal-1-P) uridyltransferase, which converts uridine diphosphoglucose (UDP-glucose) and Gal-1-P to uridine diphosphogalactose (UDP-galactose) and glucose-1-phosphate. Glucose-1-phosphate is converted to glucose-6-phosphate which is eventually converted to glucose, lactate and/or pyruvate. As a result of this enzyme deficiency, galactose, Gal-1-P, galactitol and galactonate accumulate. The accumulation of these toxic products results in a variety of clinical sequelae. For example, the undiagnosed neonate with classic galactosemia can quickly develop feeding problems, failure to thrive, hepatocellular damage, bleeding, and E. coli sepsis50. Long-term complications include intellectual disability and learning disabilities and cataracts. Early initiation of a lactose-restricted diet may prevent the life-threatening complications of this disorder. Infants and children require a protein source other than cow’s milk and should be fed powdered infant formulas made with soy protein or elemental formulas. Galactose in fruits and vegetables and some aged cheeses are of unknown consequence and recently published management guidelines suggest that restriction is not necessary51. With the introduction of foods, parents must learn to read nutrition labels and ingredient lists since lactose and galactose are components of many non-dairy foods. In addition, many medications also contain small amounts of lactose and/or galactose as fillers. Recommended calcium and vitamin intakes, especially vitamin D, help prevent decreased bone mineralization, and thus, 25-hydroxy-vitamin D levels should be monitored51. Dietary compliance is assessed by monitoring of the levels of erythrocyte galactose-1-phosphate (random sample), or urine galactitol (fasting or first morning sample is preferred)52. The recommendations for frequency of monitoring is age dependent 53. However, because of endogenous galactose production54-57, the complete normalization of levels of these metabolites is often not possible, and even with optimal management, children with classic galactosemia are at increased risk for developmental delays, childhood apraxia of speech and dysarthria, cataracts, and premature ovarian insufficiency 48, 49, 54-57.
Glycogen Storage Disease Type 1a
Although multiple types of glycogen storage disease exist, glycogen storage disease type 1a (GSD 1a) is one of the most severe glycogen storage disorders. GSD 1a results from a deficiency of the glucose-6-phosphatase enzyme. As a result of this enzyme deficiency, phosphate cannot be cleaved from glucose-6-phosphate. Thus, glucose becomes trapped in the hepatocyte as glucose-6-phosphate, and patients develop life-threatening hypoglycemia even after relatively short periods of fasting when the body relies on glycogenolysis and gluconeogenesis for glucose production. Patients typically present in the neonatal period or during infancy with recurrent hypoglycemia, hepatomegaly and lactic acidosis 58. In addition, hyperuricemia and hypertriglyceridemia are typically observed 58.
The primary goal of nutritional intervention in GSD 1a is maintaining normoglycemia (70 to 100 milligrams per deciliter (mg/dL)) while minimizing hyperlipidemia, hyperuricemia and other clinical manifestations such as growth impairment. A practice guideline for management of GSD 1a has recently been published59. In the infantile period, normoglycemia is maintained through frequent feeds and in some cases, the addition of glucose polymer to the feeds. Since fructose and galactose are metabolized into glucose-6-phosphate, these sugars are also restricted. Given these dietary restrictions, a soy infant formula is typically recommended. When an infant is able to sleep for longer periods, they must still be wakened every 3-4 hours for feeds to prevent hypoglycemia. Continuous feeds via nasogastric or gastrostomy tube are an alternative treatment strategy. In infancy, continuous feeds should provide a glucose infusion rate of ~8-10 mg/kg/min with lower rates in older children (4-8 mg/kg/min)59. Accidental disconnection of continuous feeds during sleep can lead to life-threatening hypoglycemia. Thus, families must be educated regarding this risk if continuous feeds are used. There are potential risks and benefits to both frequent feeds and continuous night feedings, and although studies have attempted to address which is the best approach, no study has definitively answered this question60, 61.
As children age, the quantity of soy formula consumed will be reduced and replaced with foods restricted in appropriate sugars. In addition, in later infancy, uncooked cornstarch (UCCS), a source of slow-digesting carbohydrate, is introduced as a method for maintaining normoglycemia62. UCCS (1.6 – 2.5 g/kg/dose) is typically provided approximately every 4 hours59. A trial of UCCS is attempted between 6 months and 1 year of age since the digestion of UCCS requires pancreatic amylase, an enzyme that may not be fully active until 1-2 years of age. The introduction of a modified form of cornstarch with an even longer duration of action has allowed for longer periods of normoglycemia and has even precluded the need for frequent nighttime feedings or a continuous infusion of carbohydrate via a gastrostomy tube in some patients63, 64.
The diet in patients with GSD 1a should have a low fat content in order to prevent excessively elevated lipid values. Frequent monitoring of diet via dietary records is crucial to establishing adequate intake of essential fatty acids. The oxidation of long chain fatty acids may also be compromised in GSD 1a65. New literature suggests that this block can be bypassed using medium chain triglycerides (MCT) in the diet and that utilizing MCT may help to prevent hypoglycemia during catabolism65, 66.
Patients with GSD 1a require close follow-up and nutritional monitoring throughout the lifespan. Education of caregivers at diagnosis should include formula preparation, feeding schedules, and glucometer use. In infancy and early childhood, regular glucose monitoring (preprandial) is necessary as it allows for the establishment of glucose trends and indicates when an increase in carbohydrate dose may be needed to prevent hypoglycemia. Glucose monitoring should also occur in the setting of intercurrent illness and when the infant show signs and symptoms of hypoglycemia (eg. lethargy, sweating, jittery, seizures). A concentrated form of glucose (eg. glucose gel) should be available at all times for use in the setting of hypoglycemia until medical care is available as patients with GSD 1a do not respond to glucagon injections. In addition, heavy galactose restriction in children may lead to substantial micronutrient deficits and thus, appropriate vitamin/mineral monitoring (eg. vitamin D) and/or supplementation may be necessary67. Although dietary adherence is typically high in infancy given the ease of administration of frequent feeds, adherence may be more challenging as patients grow older especially because of the unpalatable nature of UCCS. Education should be provided to caregivers and to patients, when appropriate, regarding appropriate food selection and the importance of adherence to cornstarch regimens as deviation from the diet and cornstarch regimens can be life-threatening.
Disorders of Fatty Acid Oxidation
Fatty acid oxidation disorders result from deficiencies in one of the enzymes involved in the β-oxidation of fatty acids. Nutrition management goals of these disorders are to minimize fatty acid oxidation by avoidance of fasting and to provide adequate nonfat energy during stress.
Medium-chain acyl-CoA dehydrogenase (MCAD) deficiency
Medium-chain acyl-CoA dehydrogenase (MCAD) is one of the enzymes involved in mitochondrial fatty acid ß-oxidation, which fuels hepatic ketogenesis, a major source of energy once hepatic glycogen stores are depleted in the setting of a prolonged fast or high energy demands. Prior to the introduction of newborn screening for MCAD deficiency, patients presented with hypoketotic hypoglycemia, vomiting, and lethargy triggered by catabolic stress, such as a cold, flu, gastroenteritis, or decreased caloric intake68, 69. Such an episode may quickly progress to coma and death if unrecognized68, 69. However, with early diagnosis and prevention of situations in which the cells must rely solely on stored fats for energy (i.e., avoid fasting), hypoglycemia and its sequelae can be prevented.
Standard infant formula or breast milk can be continued in infants with MCAD deficiency. If breastfeeding, pumped breast milk will give a more accurate indication of actual intake. Formulas and breast milk enhancers containing MCT oil are typically avoided in this patient population. After infancy, no special dietary restrictions are typically recommended in MCAD deficiency. Although some centers recommend a low fat diet, to our knowledge, no data exists to support this strategy36. Instead, a well-balanced-diet with appropriate calories for age and size is recommended. Counseling regarding the dangers of fasting should be emphasized. A concentrated form of glucose should be available at all times for use in treating hypoglycemia until emergency providers are available.
Very long-chain acyl-CoA dehydrogenase (VLCAD) deficiency
The very long chain acyl-CoA dehydrogenase enzyme is required for β-oxidation of long chain fatty acids (14 to 20 carbons in length). When this enzyme is deficient, long chain fatty acids and CoA derivatives accumulate. In addition, significant hypoglycemia can result in the setting of fasting when liver glycogen stores are depleted and fatty acids cannot be adequately oxidized as an energy source. Without early diagnosis and initiation of treatment, the severe infantile presentation may include poor feeding, dehydration, failure to thrive, hypoketotic hypoglycemia, metabolic acidosis, hyperammonemia, cardiomyopathy, cardiac arrhythmias, hepatic dysfunction, vomiting, lethargy, seizures, coma, and even death69. Milder forms of the disorder may present later in childhood or early adulthood with hypoglycemia and/or rhabdomyolysis69.
The primary nutritional strategy for managing VLCAD deficiency is to limit the dependence on long chain fatty acids as an energy substrate. To this end, the consumption of dietary long chain fatty acids is limited and catabolism (eg. fasting) is avoided. Families should be educated to seek medical care in the setting of intercurrent illnesses (eg. poor appetite, vomiting, febrile illness, etc) which may place the patient at risk for metabolic decompensation as hospitalization may be necessary in the setting of such illnesses. In addition, the deficiency can be by-passed by providing medium chain fatty acids in the form of MCT oil since the β-oxidation of medium chain fatty acids is intact in this disorder. Special formulas containing MCT are available for use in patients with long chain fatty acid oxidation disorders, and breast milk or standard infant formulas may be used as the source of long chain fat. In addition, supplementation with specific oils, such as canola, walnut, or flaxseed oil may be used for meeting the recommended requirements for essential fatty acids.
Challenges of Nutritional Management of Inborn Errors of Metabolism
Numerous dietary challenges may be encountered during the course of the long-term management of various inborn errors of metabolism. Palatability of metabolic formulas may be a challenge for young children and adults. Furthermore, as children grow older and gain greater responsibility for dietary choices, compliance may become an issue. An increasing number of metabolic formulas with varied flavors have become available for some disorders and can be trialed to determine the most palatable for any given patient. In addition to liquids and powders, amino acid mixtures may be available in tablet or gel form for some disorders to ease administration. In addition, safe additives can often be used to improve palatability. However, consultation with a metabolic dietitian should be done prior to providing such additives to determine which are safe for a particular disorder. Lastly, special “low protein” medical foods have become commercially available and may be used as substitutes for higher protein food sources in the diet. Because dietary therapy is lifelong in these disorders, education regarding the diet and formula options must begin at early ages and be age-appropriate to meet the needs of young children, adolescents and adults. Many of the obstacles to formula and diet adherence in the inborn errors of metabolism has been reviewed elsewhere70.
Intercurrent illness may also complicate the management of many inborn errors of metabolism, especially UCDs, MSUD, PA, MMA, and the fatty acid oxidation disorders. Special “sick day” formulas with increased caloric content and decreased content of toxic metabolites may be used for management during illness with the supervision of the metabolic team caring for the patient. In addition, gastrostomy tubes may be recommended to improve both compliance and provision of calories during intercurrent illness and during times of wellness. Patients are often provided emergency metabolic letters that describe the diagnosis, initial management, and contact numbers for the metabolic team caring for the patient and such letters should be presented at the initial presentation so that dietary management and medical management can be initiated early in the course of illness.
Conclusion
The management of patients with many inborn errors of metabolism requires a multi-disciplinary approach which combines both nutritional and medical management. In these disorders, nutritional therapy is a critical component of the management to prevent acute complications, such as metabolic decompensation, and/or to prevent long-term complications of the given disorder. Such nutritional therapy is customized for the specific disorder and individualized for each patient to promote compliance. Furthermore, nutritional therapy for the patient with an inborn error of metabolism is lifelong. Thus, registered dietitians and/or nutritionists trained in the area of rare metabolic disorders are an essential part of the multi-disciplinary team including nurses, physicians, social workers, and other health care providers that delivers care for patients with metabolic disorders.
Acknowledgements
We would like to thank William Craigen for critically reading this manuscript.
Financial Disclosures:
L.C.B. is supported by a fellowship from the National Urea Cycle Disorders Foundation and a fellowship from the Urea Cycle Disorders Consortium (UCDC; U54HD061221), which is a part of the National Institutes of Health (NIH) Rare Disease Clinical Research Network (RDCRN), supported through collaboration between the Office of Rare Diseases Research (ORDR), the National Center for Advancing Translational Science (NCATS and the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD).
REFERENCES
- 1.Acosta PB, Yannicelli S. Nutrition support protocols. Ross Products Division, Abbott Laboratories; Columbus, OH: 2001. [Google Scholar]
- 2.Mitchell ML, Hsu HW, Sahai I. The increased incidence of congenital hypothyroidism: fact or fancy? Clin Endocrinol (Oxf) 2011;75(6):806–810. doi: 10.1111/j.1365-2265.2011.04128.x. [DOI] [PubMed] [Google Scholar]
- 3.Organization WH. Protein and Amino Acid Requirements in Human Nutrition. Geneva, Switzerland: 2007. [PubMed] [Google Scholar]
- 4.Folling A. Excretion of phenylpyruvic acid in urine as a metabolic anomaly in connection with imbecility. Nord Med Tidskr. 1934;8:1054–1059. [Google Scholar]
- 5.Centerwall SA, Centerwall WR. The discovery of phenylketonuria: the story of a young couple, two retarded children, and a scientist. Pediatrics. 2000;105(1 Pt 1):89–103. doi: 10.1542/peds.105.1.89. [DOI] [PubMed] [Google Scholar]
- 6.Centerwall WR. Phenylketonuria. J Am Med Assoc. 1957;165(4):392. doi: 10.1001/jama.1957.02980220076022. [DOI] [PubMed] [Google Scholar]
- 7.Vockley J, Andersson HC, Antshel KM, Braverman NE, Burton BK, Frazier DM, et al. Phenylalanine hydroxylase deficiency: diagnosis and management guideline. Genet Med. 2014;16(2):188–200. doi: 10.1038/gim.2013.157. [DOI] [PubMed] [Google Scholar]
- 8.Singh RH, Rohr F, Frazier D, Cunningham A, Mofidi S, Ogata B, et al. Recommendations for the nutrition management of phenylalanine hydroxylase deficiency. Genet Med. 2014;16(2):121–131. doi: 10.1038/gim.2013.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brumm VL, Azen C, Moats RA, Stern AM, Broomand C, Nelson MD, et al. Neuropsychological outcome of subjects participating in the PKU adult collaborative study: a preliminary review. J Inherit Metab Dis. 2004;27(5):549–566. doi: 10.1023/b:boli.0000042985.02049.ff. [DOI] [PubMed] [Google Scholar]
- 10.Brumm VL, Bilder D, Waisbren SE. Psychiatric symptoms and disorders in phenylketonuria. Mol Genet Metab. 2010;99(Suppl 1):S59–63. doi: 10.1016/j.ymgme.2009.10.182. [DOI] [PubMed] [Google Scholar]
- 11.Smith I, Beasley MG, Wolff OH, Ades AE. Behavior disturbance in 8-year-old children with early treated phenylketonuria. Report from the MRC/DHSS Phenylketonuria Register. J Pediatr. 1988;112(3):403–408. doi: 10.1016/s0022-3476(88)80320-2. [DOI] [PubMed] [Google Scholar]
- 12.Smith I, Knowles J. Behaviour in early treated phenylketonuria: a systematic review. Eur J Pediatr. 2000;159(Suppl 2):S89–93. doi: 10.1007/pl00014392. [DOI] [PubMed] [Google Scholar]
- 13.Burton BK, Leviton L, Vespa H, Coon H, Longo N, Lundy BD, et al. A diversified approach for PKU treatment: routine screening yields high incidence of psychiatric distress in phenylketonuria clinics. Mol Genet Metab. 2013;108(1):8–12. doi: 10.1016/j.ymgme.2012.11.003. [DOI] [PubMed] [Google Scholar]
- 14.Weglage J, Fromm J, van Teeffelen-Heithoff A, Moller HE, Koletzko B, Marquardt T, et al. Neurocognitive functioning in adults with phenylketonuria: results of a long term study. Mol Genet Metab. 2013;110(Suppl):S44–48. doi: 10.1016/j.ymgme.2013.08.013. [DOI] [PubMed] [Google Scholar]
- 15.Trefz F, Maillot F, Motzfeldt K, Schwarz M. Adult phenylketonuria outcome and management. Mol Genet Metab. 2011;104(Suppl):S26–30. doi: 10.1016/j.ymgme.2011.08.025. [DOI] [PubMed] [Google Scholar]
- 16.Sharman R, Sullivan KA, Young RM, McGill JJ. Tyrosine monitoring in children with early and continuously treated phenylketonuria: results of an international practice survey. J Inherit Metab Dis. 2010;33(Suppl 3):S417–420. doi: 10.1007/s10545-010-9211-6. [DOI] [PubMed] [Google Scholar]
- 17.Rohr FJ, Doherty LB, Waisbren SE, Bailey IV, Ampola MG, Benacerraf B, et al. New England Maternal PKU Project: prospective study of untreated and treated pregnancies and their outcomes. J Pediatr. 1987;110(3):391–398. doi: 10.1016/s0022-3476(87)80500-0. [DOI] [PubMed] [Google Scholar]
- 18.Lee P, Treacy EP, Crombez E, Wasserstein M, Waber L, Wolff J, et al. Safety and efficacy of 22 weeks of treatment with sapropterin dihydrochloride in patients with phenylketonuria. Am J Med Genet A. 2008;146A(22):2851–2859. doi: 10.1002/ajmg.a.32562. [DOI] [PubMed] [Google Scholar]
- 19.Burton BK, Grange DK, Milanowski A, Vockley G, Feillet F, Crombez EA, et al. The response of patients with phenylketonuria and elevated serum phenylalanine to treatment with oral sapropterin dihydrochloride (6R-tetrahydrobiopterin): a phase II, multicentre, open-label, screening study. J Inherit Metab Dis. 2007;30(5):700–707. doi: 10.1007/s10545-007-0605-z. [DOI] [PubMed] [Google Scholar]
- 20.Levy HL, Milanowski A, Chakrapani A, Cleary M, Lee P, Trefz FK, et al. Efficacy of sapropterin dihydrochloride (tetrahydrobiopterin, 6R-BH4) for reduction of phenylalanine concentration in patients with phenylketonuria: a phase III randomised placebo-controlled study. Lancet. 2007;370(9586):504–510. doi: 10.1016/S0140-6736(07)61234-3. [DOI] [PubMed] [Google Scholar]
- 21.Trefz FK, Burton BK, Longo N, Casanova MM, Gruskin DJ, Dorenbaum A, et al. Efficacy of sapropterin dihydrochloride in increasing phenylalanine tolerance in children with phenylketonuria: a phase III, randomized, double-blind, placebo-controlled study. J Pediatr. 2009;154(5):700–707. doi: 10.1016/j.jpeds.2008.11.040. [DOI] [PubMed] [Google Scholar]
- 22.Burton BK, Nowacka M, Hennermann JB, Lipson M, Grange DK, Chakrapani A, et al. Safety of extended treatment with sapropterin dihydrochloride in patients with phenylketonuria: results of a phase 3b study. Mol Genet Metab. 2011;103(4):315–322. doi: 10.1016/j.ymgme.2011.03.020. [DOI] [PubMed] [Google Scholar]
- 23.Cunningham A, Bausell H, Brown M, Chapman M, DeFouw K, Ernst S, et al. Recommendations for the use of sapropterin in phenylketonuria. Mol Genet Metab. 2012;106(3):269–276. doi: 10.1016/j.ymgme.2012.04.004. [DOI] [PubMed] [Google Scholar]
- 24.Grange DK, Hillman RE, Burton BK, Yano S, Vockley J, Fong CT, et al. Sapropterin dihydrochloride use in pregnant women with phenylketonuria: an interim report of the PKU MOMS sub-registry. Mol Genet Metab. 2014;112(1):9–16. doi: 10.1016/j.ymgme.2014.02.016. [DOI] [PubMed] [Google Scholar]
- 25.Matalon R, Michals-Matalon K, Bhatia G, Grechanina E, Novikov P, McDonald JD, et al. Large neutral amino acids in the treatment of phenylketonuria (PKU) J Inherit Metab Dis. 2006;29(6):732–738. doi: 10.1007/s10545-006-0395-8. [DOI] [PubMed] [Google Scholar]
- 26.Matalon R, Michals-Matalon K, Bhatia G, Burlina AB, Burlina AP, Braga C, et al. Double blind placebo control trial of large neutral amino acids in treatment of PKU: effect on blood phenylalanine. J Inherit Metab Dis. 2007;30(2):153–158. doi: 10.1007/s10545-007-0556-4. [DOI] [PubMed] [Google Scholar]
- 27.Ney DM, Gleason ST, van Calcar SC, MacLeod EL, Nelson KL, Etzel MR, et al. Nutritional management of PKU with glycomacropeptide from cheese whey. J Inherit Metab Dis. 2009;32(1):32–39. doi: 10.1007/s10545-008-0952-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.van Calcar SC, MacLeod EL, Gleason ST, Etzel MR, Clayton MK, Wolff JA, et al. Improved nutritional management of phenylketonuria by using a diet containing glycomacropeptide compared with amino acids. Am J Clin Nutr. 2009;89(4):1068–1077. doi: 10.3945/ajcn.2008.27280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ogier de Baulny H, Dionisi-Vici C, Wendel U. Branched-Chain Organic Acidurias/Acidaemias. In: Saudubray JM, van den Berghe G, Walter JH, editors. Inborn Metabolic Diseases: Diagnosis and Treatment. Springer-Verlag; Berlin: 2012. pp. 277–296. [Google Scholar]
- 30.Hilliges C, Awiszus D, Wendel U. Intellectual performance of children with maple syrup urine disease. Eur J Pediatr. 1993;152(2):144–147. doi: 10.1007/BF02072492. [DOI] [PubMed] [Google Scholar]
- 31.Kaplan P, Mazur A, Field M, Berlin JA, Berry GT, Heidenreich R, et al. Intellectual outcome in children with maple syrup urine disease. J Pediatr. 1991;119(1 Pt 1):46–50. doi: 10.1016/s0022-3476(05)81037-6. [DOI] [PubMed] [Google Scholar]
- 32.Muelly ER, Moore GJ, Bunce SC, Mack J, Bigler DC, Morton DH, et al. Biochemical correlates of neuropsychiatric illness in maple syrup urine disease. J Clin Invest. 2013;123(4):1809–1820. doi: 10.1172/JCI67217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hoffmann B, Helbling C, Schadewaldt P, Wendel U. Impact of longitudinal plasma leucine levels on the intellectual outcome in patients with classic MSUD. Pediatr Res. 2006;59(1):17–20. doi: 10.1203/01.pdr.0000190571.60385.34. [DOI] [PubMed] [Google Scholar]
- 34.Scriver CR, Mackenzie S, Clow CL, Delvin E. Thiamine-responsive maple-syrup-urine disease. Lancet. 1971;1(7694):310–312. doi: 10.1016/s0140-6736(71)91041-5. [DOI] [PubMed] [Google Scholar]
- 35.Frazier DM, Allgeier C, Homer C, Marriage BJ, Ogata B, Rohr F, et al. Nutrition management guideline for maple syrup urine disease: an evidence- and consensus-based approach. Mol Genet Metab. 2014;112(3):210–217. doi: 10.1016/j.ymgme.2014.05.006. [DOI] [PubMed] [Google Scholar]
- 36.Brusilow SW, Horwich AL. In: Urea Cycle Enzymes. Valle D, Beaudet AL, Vogelstein B, Kinzler KW, Antonarakis SE, Ballabio A, et al., editors. 2014. [Google Scholar]
- 37.Kline JJ, Hug G, Schubert WK, Berry H. Arginine deficiency syndrome. Its occurrence in carbamyl phosphate synthetase deficiency. Am J Dis Child. 1981;135(5):437–442. [PubMed] [Google Scholar]
- 38.Wijburg F, Nassogne M. Disorders of the Urea Cycle and Related Enzymes. In: Saudubray JM, van den Berghe G, Walter JH, editors. Inborn Metabolic Diseases:Diagnosis and Treatment. Springer-Verlag; Berlin: 2012. pp. 297–310. [Google Scholar]
- 39.Nagamani SC, Shchelochkov OA, Mullins MA, Carter S, Lanpher BC, Sun Q, et al. A randomized controlled trial to evaluate the effects of high-dose versus low-dose of arginine therapy on hepatic function tests in argininosuccinic aciduria. Mol Genet Metab. 2012;107(3):315–321. doi: 10.1016/j.ymgme.2012.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Singh RH, Rhead WJ, Smith W, Lee B, Sniderman King L, Summar M. Nutritional management of urea cycle disorders. Crit Care Clin. 2005;21(4 Suppl):S27–35. doi: 10.1016/j.ccc.2005.08.003. [DOI] [PubMed] [Google Scholar]
- 41.Burrage LC, Jain M, Gandolfo L, Lee BH, Nagamani SC. Sodium phenylbutyrate decreases plasma branched-chain amino acids in patients with urea cycle disorders. Mol Genet Metab. 2014;113(1-2):131–135. doi: 10.1016/j.ymgme.2014.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Scaglia F, Carter S, O’Brien WE, Lee B. Effect of alternative pathway therapy on branched chain amino acid metabolism in urea cycle disorder patients. Mol Genet Metab. 2004;81(Suppl 1):S79–85. doi: 10.1016/j.ymgme.2003.11.017. [DOI] [PubMed] [Google Scholar]
- 43.Berry GT, Steiner RD. Long-term management of patients with urea cycle disorders. J Pediatr. 2001;138(1 Suppl):S56–60. doi: 10.1067/mpd.2001.111837. discussion S60-51. [DOI] [PubMed] [Google Scholar]
- 44.Baumgartner MR, Horster F, Dionisi-Vici C, Haliloglu G, Karall D, Chapman KA, et al. Proposed guidelines for the diagnosis and management of methylmalonic and propionic acidemia. Orphanet J Rare Dis. 2014;9:130. doi: 10.1186/s13023-014-0130-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yannicelli S, Acosta PB, Velazquez A, Bock HG, Marriage B, Kurczynski TW, et al. Improved growth and nutrition status in children with methylmalonic or propionic acidemia fed an elemental medical food. Mol Genet Metab. 2003;80(1-2):181–188. doi: 10.1016/j.ymgme.2003.08.012. [DOI] [PubMed] [Google Scholar]
- 46.Sutton VR, Chapman KA, Gropman AL, MacLeod E, Stagni K, Summar ML, et al. Chronic management and health supervision of individuals with propionic acidemia. Mol Genet Metab. 2012;105(1):26–33. doi: 10.1016/j.ymgme.2011.08.034. [DOI] [PubMed] [Google Scholar]
- 47.Touati G, Valayannopoulos V, Mention K, de Lonlay P, Jouvet P, Depondt E, et al. Methylmalonic and propionic acidurias: management without or with a few supplements of specific amino acid mixture. J Inherit Metab Dis. 2006;29(2-3):288–298. doi: 10.1007/s10545-006-0351-7. [DOI] [PubMed] [Google Scholar]
- 48.Morrow G, 3rd, Burkel GM. Long-term management of a patient with vitamin B12-responsive methylmalonic acidemia. J Pediatr. 1980;96(3 Pt 1):425–426. doi: 10.1016/s0022-3476(80)80687-1. [DOI] [PubMed] [Google Scholar]
- 49.Hauser NS, Manoli I, Graf JC, Sloan J, Venditti CP. Variable dietary management of methylmalonic acidemia: metabolic and energetic correlations. Am J Clin Nutr. 2011;93(1):47–56. doi: 10.3945/ajcn.110.004341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Berry GT, Walter JH. Disorders of Galactose Metabolism. In: Saudubray JM, van den Berghe G, Walter JH, editors. Inborn Metabolic Diseases: Diagnosis and Treatment. Springer-Verlag; Berlin: 2012. pp. 141–150. [Google Scholar]
- 51.Van Calcar SC, Bernstein LE, Rohr FJ, Scaman CH, Yannicelli S, Berry GT. A re-evaluation of life-long severe galactose restriction for the nutrition management of classic galactosemia. Mol Genet Metab. 2014;112(3):191–197. doi: 10.1016/j.ymgme.2014.04.004. [DOI] [PubMed] [Google Scholar]
- 52.Schadewaldt P, Killius S, Kamalanathan L, Hammen HW, Strassburger K, Wendel U. Renal excretion of galactose and galactitol in patients with classical galactosaemia, obligate heterozygous parents and healthy subjects. J Inherit Metab Dis. 2003;26(5):459–479. doi: 10.1023/a:1025173311030. [DOI] [PubMed] [Google Scholar]
- 53.Walter JH, Collins JE, Leonard JV. Recommendations for the management of galactosaemia. UK Galactosaemia Steering Group. Arch Dis Child. 1999;80(1):93–96. doi: 10.1136/adc.80.1.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Berry GT, Moate PJ, Reynolds RA, Yager CT, Ning C, Boston RC, et al. The rate of de novo galactose synthesis in patients with galactose-1-phosphate uridyltransferase deficiency. Mol Genet Metab. 2004;81(1):22–30. doi: 10.1016/j.ymgme.2003.08.026. [DOI] [PubMed] [Google Scholar]
- 55.Schadewaldt P, Kamalanathan L, Hammen HW, Kotzka J, Wendel U. Endogenous galactose formation in galactose-1-phosphate uridyltransferase deficiency. Arch Physiol Biochem. 2014;120(5):228–239. doi: 10.3109/13813455.2014.962547. [DOI] [PubMed] [Google Scholar]
- 56.Berry GT, Nissim I, Lin Z, Mazur AT, Gibson JB, Segal S. Endogenous synthesis of galactose in normal men and patients with hereditary galactosaemia. Lancet. 1995;346(8982):1073–1074. doi: 10.1016/s0140-6736(95)91745-4. [DOI] [PubMed] [Google Scholar]
- 57.Schadewaldt P, Kamalanathan L, Hammen HW, Wendel U. Age dependence of endogenous galactose formation in Q188R homozygous galactosemic patients. Mol Genet Metab. 2004;81(1):31–44. doi: 10.1016/j.ymgme.2003.10.007. [DOI] [PubMed] [Google Scholar]
- 58.Laforet P, Weinstein DA, Smit PA. The Glycogen Storage Diseases and Related Disorders. In: Saudubray JM, van den Berghe G, Walter JH, editors. Inborn Metabolic Diseases: Diagnosis and Treatment. Springer-Verlag; Berlin: 2012. pp. 115–139. [Google Scholar]
- 59.Kishnani PS, Austin SL, Abdenur JE, Arn P, Bali DS, Boney A, et al. Diagnosis and management of glycogen storage disease type I: a practice guideline of the American College of Medical Genetics and Genomics. Genet Med. 2014;16(11):e1. doi: 10.1038/gim.2014.128. [DOI] [PubMed] [Google Scholar]
- 60.Derks TG, Martens DH, Sentner CP, van Rijn M, de Boer F, Smit GP, et al. Dietary treatment of glycogen storage disease type Ia: uncooked cornstarch and/or continuous nocturnal gastric drip-feeding? Mol Genet Metab. 2013;109(1):1–2. doi: 10.1016/j.ymgme.2013.02.005. [DOI] [PubMed] [Google Scholar]
- 61.Shah KK, O’Dell SD. Effect of dietary interventions in the maintenance of normoglycaemia in glycogen storage disease type 1a: a systematic review and meta-analysis. J Hum Nutr Diet. 2013;26(4):329–339. doi: 10.1111/jhn.12030. [DOI] [PubMed] [Google Scholar]
- 62.Chen YT, Cornblath M, Sidbury JB. Cornstarch therapy in type I glycogen-storage disease. N Engl J Med. 1984;310(3):171–175. doi: 10.1056/NEJM198401193100306. [DOI] [PubMed] [Google Scholar]
- 63.Correia CE, Bhattacharya K, Lee PJ, Shuster JJ, Theriaque DW, Shankar MN, et al. Use of modified cornstarch therapy to extend fasting in glycogen storage disease types Ia and Ib. Am J Clin Nutr. 2008;88(5):1272–1276. doi: 10.3945/ajcn.2008.26352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bhattacharya K, Orton RC, Qi X, Mundy H, Morley DW, Champion MP, et al. A novel starch for the treatment of glycogen storage diseases. J Inherit Metab Dis. 2007;30(3):350–357. doi: 10.1007/s10545-007-0479-0. [DOI] [PubMed] [Google Scholar]
- 65.Das AM, Lucke T, Meyer U, Hartmann H, Illsinger S. Glycogen storage disease type 1: impact of medium-chain triglycerides on metabolic control and growth. Ann Nutr Metab. 2010;56(3):225–232. doi: 10.1159/000283242. [DOI] [PubMed] [Google Scholar]
- 66.Nagasaka H, Hirano K, Ohtake A, Miida T, Takatani T, Murayama K, et al. Improvements of hypertriglyceridemia and hyperlacticemia in Japanese children with glycogen storage disease type Ia by medium-chain triglyceride milk. Eur J Pediatr. 2007;166(10):1009–1016. doi: 10.1007/s00431-006-0372-0. [DOI] [PubMed] [Google Scholar]
- 67.Bhattacharya K. Dietary dilemmas in the management of glycogen storage disease type I. J Inherit Metab Dis. 2011;34(3):621–629. doi: 10.1007/s10545-011-9322-8. [DOI] [PubMed] [Google Scholar]
- 68.Derks TG, Reijngoud DJ, Waterham HR, Gerver WJ, van den Berg MP, Sauer PJ, et al. The natural history of medium-chain acyl CoA dehydrogenase deficiency in the Netherlands: clinical presentation and outcome. J Pediatr. 2006;148(5):665–670. doi: 10.1016/j.jpeds.2005.12.028. [DOI] [PubMed] [Google Scholar]
- 69.Morris AAM, Spiekerkoetter U. Disorders of Mitochondrial Fatty Acid Oxidation and Related Metabolic Pathways. In: Saudubray JM, van den Berghe G, Walter JH, editors. Inborn Metabolic Disorders: Diagnosis and Traetment. Springer-Verlag; Berlin: 2012. pp. 201–216. [Google Scholar]
- 70.MaCdonald A, van Rijn M, Feillet F, Lund AM, Bernstein L, Bosch AM, et al. Adherence issues in inherited metabolic disorders treated by low natural protein diets. Ann Nutr Metab. 2012;61(4):289–295. doi: 10.1159/000342256. [DOI] [PubMed] [Google Scholar]