Abstract
Obsessive Compulsive Disorder (OCD) is a chronic, severe mental illness with up to 2–3% prevalence worldwide, which has been classified as one of the world’s 10 leading causes of illness-related disability according to the World Health Organization, largely because of the chronic nature of disabling symptoms 1. Despite the severity and high prevalence of this chronic and disabling disorder, there is still relatively limited understanding of its pathophysiology. However, this is now rapidly changing due to development of powerful technologies that can be used to dissect the neural circuits underlying pathologic behaviors. In this article, we describe recent technical advances that have allowed neuroscientists to start identifying the circuits underlying complex repetitive behaviors using animal model systems. In addition, we review current surgical and stimulation-based treatments for OCD that target circuit dysfunction. Finally, we discuss how findings from animal models may be applied in the clinical arena to help inform and refine targeted brain stimulation-based treatment approaches.
Keywords: Obsessive Compulsive Disorder (OCD), cortico-striato-thalamo-cortical circuits, prefrontal cortex, orbitofrontal cortex (OFC), ventral striatum, basal ganglia, deep brain stimulation (DBS), transcranial magnetic stimulation (TMS), anxiety, optogenetics
Introduction
Obsessive Compulsive Disorder (OCD) is a chronic, severe mental illness with up to 2–3% prevalence worldwide 2,3. In fact, the World Health Organization has classified OCD as one of the world’s 10 leading causes of illness-related disability, largely because of the chronic nature of disabling symptoms 1. Despite the severity and high prevalence of OCD, there is still relatively limited understanding of its pathophysiology. However, this is rapidly changing due to development of powerful technologies that can be used to dissect the neural circuits underlying pathologic behaviors. In this article, we will describe recent technical advances that have allowed neuroscientists to start identifying circuits underlying complex repetitive behaviors using animal model systems. We also review current surgical and stimulation-based treatments for OCD that target circuit dysfunction. Finally, we discuss how findings from animal models may be applied in the clinical arena to help inform and refine targeted brain stimulation-based treatment approaches.
Clinical Features of OCD
Despite recent changes to the Diagnostic and Statistical Manual (DSM-5), core clinical features of OCD remain the same 4,5. Specifically, OCD is characterized by obsessions, which are recurrent intrusive thoughts, images, or impulses; and compulsions, which are repetitive mental or behavioral rituals. Obsessions and compulsions cause significant distress, are time-consuming, and interfere with patients’ ability to function. Though OCD is no longer classified as an anxiety disorder in DSM-5, obsessions are frequently associated with significant distress, and compulsions are often performed with conscious intent to reduce obsession-associated anxiety 6. For example, an intrusive thought about the house burning down could lead to ritualized checking to make sure the stove is off. While rituals can provide temporary anxiety relief, it is important to note that performing compulsions is actually believed to strengthen dysfunctional neural circuits that underlie OCD, leading to persistence of symptoms and overall long-term increased anxiety.
Though clinical presentation is covered in detail elsewhere, several key features are important for understanding OCD neurobiology. Specifically, both clinical and neurobiological evidence indicates that OCD is a heterogeneous disorder 7, though different metrics for subdividing the illness have been proposed and this is an active area of research. First, there is evidence that tic-related OCD is a biologically distinct entity, with increased prevalence in males, different neurochemical features, distinct striatal pathophysiology, and earlier age of onset 8. Similarly, there have been suggestions that some childhood-onset OCD may correspond to a distinct subtype with different genetic and environmental underpinnings 9. In addition, there is significant variation in level of insight both between different OCD patients and within patients throughout their illness course 10; specifiers are now included in DSM-5 to reflect this spectrum. Finally, there are indications that differences in specific content of obsessions and compulsions may reflect distinct neurobiological substrates 11. This is most clearly demonstrated for hoarding, which is therefore now considered a separate disorder in DSM-5 12,13.
Etiology of OCD
Though our understanding of OCD’s etiology is limited, current evidence implicates both genetic and environmental factors. In the next section, we will briefly describe key genetic factors that have specific links to circuit dissection in animal models, though note that other genes have also been implicated that have not yet been translated into animal models.
GENETIC DISSECTION
Evidence from both twin and family studies supports a role for genetics in OCD. Genetic vulnerability may be even greater in pediatric-onset OCD, since there is more heritability in this group 14. Candidate gene studies have focused on serotonin, glutamate, and dopamine associated-genes, because of the hypothesized roles of these neurotransmitters in OCD. More recently, genome-wide linkage and association studies have provided some candidates 15,16, though in general OCD genome-wide association studies have been underpowered. Definitive genome-wide candidates therefore have yet to be fully elucidated17.
SLC1A1
One of the more consistently replicated genetic findings in OCD is an association with the neuronal glutamate transporter SLC1A1 (protein: EAAT3 or EAAC1) 18–24, although a recent meta-analysis showed only a modest association of 2/9 SNPs with OCD 25, and SLC1A1 has not emerged as a probable locus from recent GWAS studies 15,16. Findings cluster in the 3′ region, with most evidence for association with the rs301430C allele. In cell models and brain tissue, this allele is associated with increased SLC1A1 expression, suggesting that overexpression contributes to OCD susceptibility 19. Coding variants are very rare (3/1400 subjects screened) and do not clearly segregate with OCD 26,27. Thus, noncoding polymorphisms most likely account for the association of SLC1A1 with OCD.
Though SLC1A1 knockout mice do not demonstrate clear OCD-relevant phenotypes, they have not yet been screened in targeted behavioral tests 28. In addition, it is likely that brain-wide deletion is less relevant to OCD pathophysiology than targeted alteration of expression. Ongoing studies are therefore investigating whether tissue-specific manipulations of SLC1A1 may be more relevant to the human clinical phenotype. Examining the outcome of targeted expression changes in specific neural circuits will allow us to directly address the molecular, cellular, and behavioral impact of this OCD candidate gene.
GRIN2B
GRIN2B, which encodes the NR2B subunit of the NMDA glutamate receptor 18,29, has also been implicated in OCD, although the findings are not as strong. Some positive association studies exist18,29, as well as a magnetic resonance spectroscopy study showing an association between GRIN2B polymorphisms and glutamatergic concentrations in anterior cingulate cortex (ACC) of OCD patients 30. NR2B is a conceptually attractive candidate because it is an important mediator of synaptic plasticity, since its incorporation into NMDA receptors renders them more calcium-permeable 31. However, even partial NR2B deficits in the brain lead to significant abnormalities in functioning, since NMDA receptors are essential for basic neurobiological functions necessary for learning and memory 32. In fact, constitutive NR2B knockout mice have an early postnatal lethal phenotype due to impaired suckling 33,34. It is therefore likely that any potential genetic association with OCD is accounted for by NR2B functional abnormalities in specific brain regions; this can now be tested using tissue-specific transgenic mouse models combined with OCD-relevant behavioral tasks.
PHARMACOLOGIC DISSECTION
Serotonin-1B Receptor (5-HT1B)
Several lines of evidence suggest that abnormalities in 5-HT1B receptor function (5-HT1D in the human literature) play a role in OCD 35, including pharmacological challenge studies 36 and some genetic association studies that provide tentative aggregate support 37. In addition, studies in mice demonstrate that administration of a 5-HT1B agonist leads to OCD-relevant perseverative locomotion and prepulse inhibition deficits, both of which are reversed with chronic, but not acute, fluoxetine treatment 38. Further studies have localized the responsible receptors to the orbitofrontal cortex (OFC) 39, demonstrating the utility of pharmacological dissection of neural circuits for understanding OCD pathophysiology.
Neural Circuits Associated With OCD
Despite the need for further studies regarding genetic and environmental causes of OCD, we have a relatively good sense of the involved neural circuits through application of modern neuroimaging technology. Over the past 20 years, functional and structural imaging has led to discovery of aberrant neural circuits in OCD. Despite some discrepancies, particularly regarding directionality of findings (which may be dependent on developmental stage assessed), there is remarkable convergence of neuroanatomy, circuit function, and OCD neurochemistry findings collectively implicating cortico-striato-thalamo-cortical (CSTC) circuits in OCD pathophysiology 40–43. This evidence is described in detail below.
Cortico-striato-thalamo-cortical circuit function
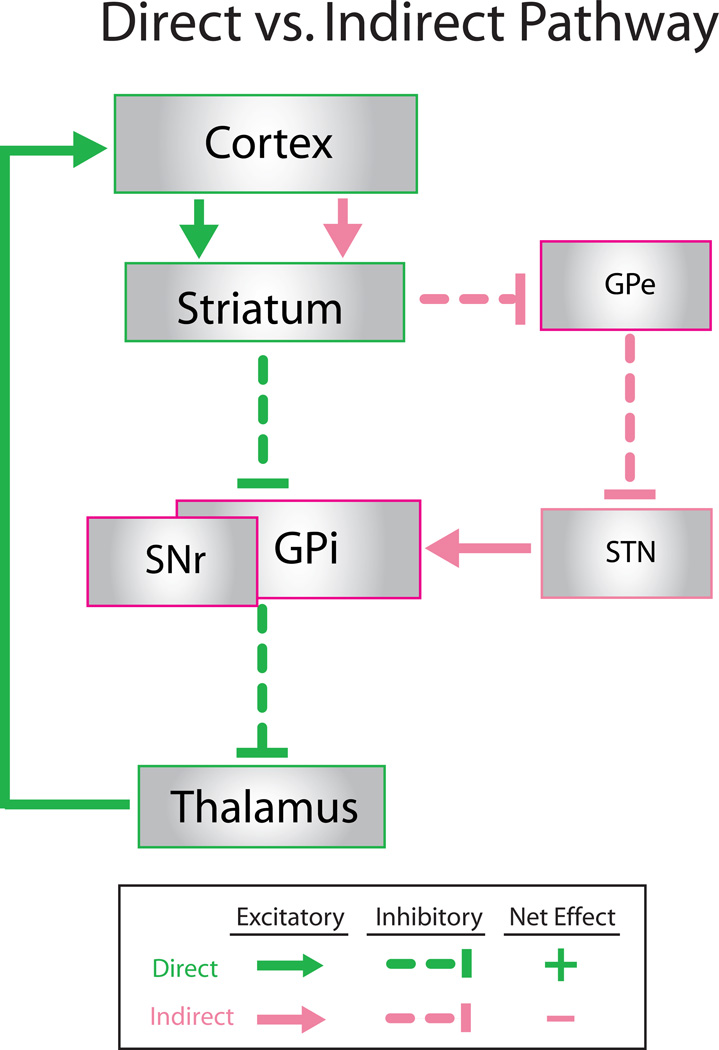
CSTC circuits have been implicated in many higher order cognitive functions, including inhibition of impulsive behavior, action selection/ modulation of motor activity, and attentional allocation. Anatomical studies in humans and nonhuman primates demonstrate that CSTC circuits are composed of multiple parallel and interconnected loops that connect frontocortical and subcortical brain areas 44. These loops are comprised of (1) glutamatergic corticostriatal projections synapsing onto striatal spiny projection neurons and/or interneurons, (2) GABAergic spiny projection neurons targeting basal ganglia output structures (globus pallidus pars internalis [GPi] and substantia nigra pars reticulata [SNr]), (3) GABAergic output neurons from GPi/SNr projecting to thalamic regions, and (4) glutamatergic neurons from thalamus projecting back to cortex 45. Within striatum, spiny projection neurons can connect to GPi/SNr through either the direct (striatonigral) or indirect (striatopallidal) pathways. In a simplified framework, these anatomically distinct pathways have been thought to oppose each other, resulting in net inhibition of thalamus and decrease of movement via activation of the indirect pathway, or net disinhibition (i.e. overall excitation) and increase of movement by activation of the direct pathway (Fig.1) 46,47. However, recent data have suggested this picture is more complex, indicating that a) direct and indirect basal ganglia pathways may both be simultaneously active during sequence initiation 48, and b) bridging collaterals between direct and indirect pathways may permit modulation of information transmission through CSTC circuits 49.
Figure 1. Schematic diagram of direct vs. indirect pathway.
A simplified diagram of the direct and indirect pathways through the cortex and basal ganglia are shown; thalamo-striatal projections and reciprocal connections between striatum and cortex are not shown for simplicity. Direct pathway is represented by green; indirect pathway is represented by pink. Direct pathway exerts a net excitatory effect on thalamic output to the cortex, while indirect pathway exerts a net inhibitory effect. GPi: globus pallidus interna; GPe: globus pallidus externa; STN: subthalamic nucleus; SNr: substantia nigra pars reticulata
In general, it is thought that different CSTC loops may be responsible for dictating particular motor and cognitive functions. Evidence from functional imaging studies suggests that selectivity is determined by the specific frontocortical area included in the loop 45. Multiple models have been proposed suggesting that interplay between frontocortical areas and the basal ganglia determines which actions are selected, and which are screened out as maladaptive. A popular model suggests that changing the balance of activity between direct and indirect pathways can either promote or inhibit the selection of appropriate behavior sequences 42,50. According to this theory, both excessive selection of actions or dysfunction in screening out maladaptive behavior sequences could potentially lead to OCD symptoms.
Structural neuroimaging
Although exact findings have varied across studies, structural abnormalities in CSTC circuits involving orbitofrontal cortex (OFC), anterior cingulate cortex (ACC), and striatum have been repeatedly demonstrated in OCD 51–53. The largest structural MRI study to date reported reduced OFC gray matter and increased gray matter in the highly-connected ventral striatum. In addition, a recent meta-analysis reported reduced volumes of left ACC and bilateral OFC, and increased thalamic volumes bilaterally, but no differences in basal ganglia volumes relative to control samples 54. However, another meta-analysis demonstrated changes in basal ganglia (i.e. increased bilateral caudate gray matter volume) as well as decreased bilateral ACC volume, in OCD patients 55, while a recent mega-analysis demonstrated a reduction in ACC, dorsomedial PFC, and inferior frontal gyrus volumes, with group-by-age interactions in putamen, OFC, and insula 53. Finally, a study combining structural MRI and behavioral testing demonstrated that impairment on a response inhibition task (Stop-Signal Reaction Time task) in both OCD patients and unaffected first-degree relatives was correlated with decreased grey matter in OFC and right inferior frontal cortex, and increased grey matter in cingulate cortex, parietal cortex, and striatum 56. Thus, structural imaging studies in OCD have collectively demonstrated changes in ACC, OFC, and striatal volume despite some inconsistencies across studies.
Functional neuroimaging
Similar to findings from structural studies, OFC, ACC, and caudate (specifically the head) have likewise been implicated in OCD using functional PET and fMRI; functional studies also highlight anterior thalamus 11,40,57. These brain regions are linked by well-described neuroanatomical connections 51. Notably, OCD subjects demonstrate hyperactivity in these areas both at rest and with symptom provocation, though OFC shows the most robust activation 40. In further support of the role of this regional hyperactivity in symptom generation, most studies have found that successful SRI or cognitive behavioral therapy treatment was associated with reduced activity in OFC or caudate, with decreased ACC activity being less prominent 58,59. Finally, recent fMRI studies of resting state connectivity have also generally supported a role for cortical-basal ganglia circuit dysfunction in OCD, demonstrating abnormal connectivity of orbitofrontal cortex 60–62, anterior cingulate 61,63, ventral striatum 61–63, dorsal striatum 62,63, putamen 60,63, and anterior thalamus 63. However, other regions including subthalamic nucleus 60, cerebellum 63,64, and temporal cortex 64 have also been implicated, and directionality of findings varies across studies, potentially depending on medication status, symptom subtype, or specific subregion examined 61.
Cognitive activation studies
Based on the theory that circuit dysfunction in particular mental illnesses may only be unmasked during performance of neurocognitive tasks, there has been a recent shift towards performing OCD imaging studies during cognitive activation paradigms. Many tasks have been used, though executive functions have been particularly emphasized 43). First, several studies have shown hyperactivity of dorsal ACC (dACC) in OCD patients during performance of tasks involving error monitoring and/ or conflict resolution, suggesting that dACC and connected regions might function differently in OCD 65; these findings correlate well with baseline functional studies. In addition, other studies have used Go/NoGo tasks to assess inhibitory control in OCD, and though the directionality of results is in conflict, both studies report altered activation of OFC [66 = increased; 67 = decreased]. Similarly, greater frontostriatal activation has been demonstrated in unmedicated OCD patients during engagement of control and conflict resolution on the Simon task 68. Finally, decreased activation of the lateral OFC, as well as the lateral PFC and parietal cortex, has been demonstrated in both OCD patients and their unaffected first-degree relatives in a reversal learning task 69. Overall, these findings support the idea that cortical-basal ganglia circuits are dysfunctional in OCD, and may contribute to symptom generation.
Working Model of OCD Pathophysiology
By synthesizing the studies reviewed above, several models of OCD pathophysiology have been proposed 42,50. Though models differ in details, they consistently share the idea that obsessions and compulsions somehow result from malfunctioning neural circuits that include OFC, ACC, caudate, and anterior thalamus (Fig.2). The specific regions involved may depend on the particular OCD subtype. For example, based on functional imaging studies, it has been proposed that different OCD symptom dimensions (e.g., symmetry/ordering vs. washing/cleaning) may have different underlying neural substrates within CSTC circuits 7. Thus, different OCD subtypes could have distinct core neurobiologic deficits leading to differences in both neuroimaging findings and neurocognitive task performance.
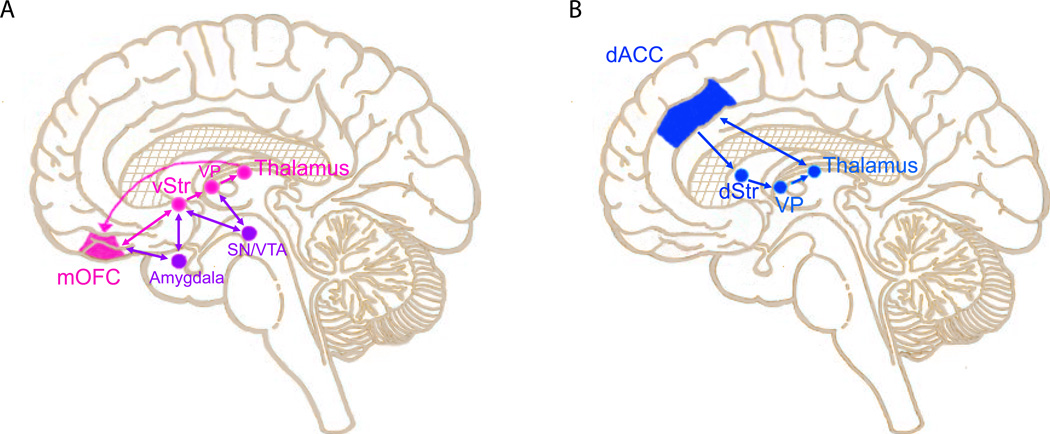
Figure 2. Cortical-basal ganglia circuits implicated in OCD pathophysiology.
Both structural and functional imaging studies provide evidence that orbitofrontal cortex (OFC), anterior cingulate cortex (ACC), caudate, and anterior thalamus are involved in OCD pathophysiology. A) A circuit linking medial OFC (mOFC), ventral striatum (vStr), ventral pallidum (VP), and thalamus is thought to be involved in OCD pathology. This circuit has classically been associated with attribution of value to the outcome of particular actions to facilitate reward learning. Evidence from both stereotactic ablation and deep brain stimulation (DBS) studies indicates that interrupting this dysfunctional circuit can decrease symptoms in OCD patients. Links between this circuit and amygdala provide opportunities for regulation of activity by affect. Dopaminergic projections from substantia nigra/ ventral tegmental area (SN/VTA) provide critical modulatory input. B) Though the role of ACC in OCD symptomatology is unclear, a circuit linking dorsal ACC (dACC), dorsal striatum (dStr), VP (ventral pallidum), and thalamus is critical for action selection. Abnormalities in this loop could therefore contribute to perseverative behaviors in OCD.
In line with this vein of thinking, evidence from recent human studies suggests that OCD patients have dysfunction in core neural processes mapped onto CSTC circuits, such as response inhibition 70,71,72 and sensorimotor gating 73. In addition, a group of studies that examined the balance between goal-directed versus habitual behavior in OCD patients is particularly interesting. Although the ways in which goal-directed and habitual performance cooperate and/ or interfere with each other in healthy subjects is still an area of active investigation (see Balleine & O’Doherty for comprehensive review 74), there is growing evidence that patients with OCD are biased to perform habits, sometimes at the expense of goal-directed actions 75. Interestingly, this bias towards increased habit formation in OCD not only applies to appetitive habits, it also extends to avoidant habits that may be more relevant to the clinical symptoms seen in patients76,77. Though it is challenging in general to make direct links between dysfunctional neural processes and symptoms in patients (highlighted by Gillan et al’s finding that avoidance habits did not correlate with the YBOCS compulsion subscale), the possibility that impaired regulation of the goal-directed behavior/ habit balance contributes to symptom generation is intriguing.
As described briefly above, a leading pathophysiologic model that is not mutually exclusive proposes that different populations of striatal spiny projection neurons differentially regulate the direct and indirect basal ganglia pathways, ultimately leading to stereotypic motor behaviors. Given the known functions of the direct pathway (i.e. striatum, globus pallidus interna, substantia nigra) and indirect pathway (i.e. striatum, globus pallidus externa, subthalamic nucleus) in modulating thalamic input to cortex and in generating motor patterns, this has led to the hypothesis that OCD symptoms result from excess activity in direct versus indirect OFC-subcortical pathways. This imbalance could lead to OCD symptoms in a variety of ways. For example, increased direct pathway activity could lead to decreased inhibition of thalamus, which in turn would decrease filtering of intrusive thoughts and images to cortex, triggering compulsions. Another model suggests that OCD symptoms stem from increased glutamatergic activity in OFC and ACC, which generates intrusive thoughts and images that override other sensorimotor input. In turn, this could trigger ritualistic compulsions driven by striatum through persistent activation of the direct pathway. As described below, studies in rodent models can be used to test these models.
Other candidate regions
Though current models suggest that dysfunctional CSTC circuits are important in generation and/or maintenance of OCD symptoms, evidence for involvement of other structures is beginning to accumulate 65. For example, while CSTC models do not provide a satisfying explanation for increased anxiety observed in OCD, exaggerated responses in amygdala observed after presentation of OCD-specific stimuli could be responsible 78. Furthermore, although dACC has been classically linked to conflict monitoring/obsessions in OCD, there is evidence that it also plays a role in expression of fear responses 79. dACC hyperactivation could therefore explain increased anxiety observed in OCD patients. Finally, recent studies have demonstrated that OCD patients have impaired extinction recall in a fear-conditioning paradigm, with accompanying alterations in cerebellum, posterior cingulate, and putamen activity during extinction recall, and reduced hippocampus and caudate activation during fear extinction 80. Integration of other brain structures may therefore be necessary to generate a satisfying explanatory model of OCD.
Translating Circuit Findings from Humans into Animal Models
Though there is strong evidence from human studies that dysfunction in CSTC circuits is linked to OCD symptoms, it is difficult (and perhaps impossible) to test causality in humans. Researchers have therefore turned to animal models to 1) test the causal role of specific circuits in generation and resolution of OCD-like symptoms; and 2) determine precise localization of neurochemical abnormalities that lead to abnormal repetitive behaviors. In this section, we will review new technologies that allow precise dissection of neural circuits involved in repetitive behaviors, and discuss recent studies in the OCD animal literature that exploit these techniques.
OCD Rodent Models
Since valid animal models are essential for identifying molecular and cellular events that lead to pathology, substantial effort has gone towards establishing rodent models of OCD 50,51,81. Though it is generally accepted that no one animal model will be able to recreate all aspects of any complex neuropsychiatric disorder, including OCD 82, powerful models can nevertheless be generated to recreate particular aspects of a clinical disorder. However, it is important for models to be carefully assessed to ensure relevance to the human disorder in question, typically by examining the extent of predictive and construct validity. In this section, we will provide an overview of established OCD animal models, and discuss associated circuit abnormalities.
SYMPTOM MODELING
OCD animal models have classically emphasized the presence of stereotyped and compulsive behaviors, although reliance on face validity may lead to discrepancies in the field since identical phenotypes can result from different underlying biological processes (for review see Wang et al, 2009) 81. These include barbering (repetitive hair biting and pulling), acral paw-lick (repetitive canine paw-licking), zoo-related stereotypies, and marble burying. In addition to these models of spontaneously-generated behaviors, many groups have studied induced repetitive behaviors including: 1) perseverative lever-pressing in the absence of reward 83; 2) persistent revisiting of unrewarded arms in a T-maze 84; and 3) pharmacologically-induced compulsive checking 85 and perseverative locomotion 38. All of these models can be used to dissect circuits underlying stereotyped behaviors via either targeted lesions or drug injections through stereotactically-placed cannulae. For example, pharmacologic studies in wild-type rats have shown that striatal NMDA-antagonist injections led to increased perseveration on a T-maze delayed alternation task 86. Similarly, in vivo microdialysis in corticostriatal projections in deer mice demonstrated increased glutamate directly preceding stereotypic behaviors 87.
GENETIC MODELING
There has been a recent explosion in the use of transgenic technology to generate animal models of neuropsychiatric disorders, due to increasing sophistication of available techniques. These strategies allow investigators to upregulate or downregulate genes of interest in specific brain regions at particular developmental timepoints, with temporal and spatial precision that has not previously been achievable 88. Thus, circuit-specific function of candidate genes identified in human studies can now be directly tested in mice. However, generation of targeted transgenics relevant to OCD is still in its infancy, largely due to the difficulty of identifying candidate genes because of lack of replication in genetic studies.
Although targeted transgenics based on OCD candidate genes are therefore still in development, serendipitously-generated OCD models have advanced the field in the meantime. In several recent cases, OCD-like behaviors have emerged following disruption of genes not previously implicated in OCD 50. For example, knockout of the developmentally expressed Hoxb8lox gene leads to perseverative grooming, which is surprisingly reversed by bone marrow transplant from wild-type mice 89, while disruption of the serotonin 2C receptor leads to perseverative chewing 90. However, the link between these genes and human OCD remains unclear.
Two other recently-generated knockout mice have stronger evidence for relevance to OCD and related disorders. First, in an elegant study, Welch et al. 91 created a transgenic knockout of SAPAP3, a corticostriatal postsynaptic density protein. Mutant mice demonstrated both anxiety and perseverative grooming that was so severe it led to facial lesions, calling to mind OCD patients with contamination obsessions and corresponding washing rituals. Interestingly, these investigators also discovered a synaptic mechanism that correlated with the OCD-related behaviors–i.e. abnormal glutamate signaling at striatal synapses corresponding with a ‘juvenile’ developmental stage (increased NMDA-dependent and decreased AMPA-dependent fEPSPs). Both behavioral and electrophysiologic changes were rescued after either lentiviral-mediated SAPAP3 expression broadly throughout striatum or acute treatment with low-dose fluoxetine. Further characterization of these mice has demonstrated that electrophysiologic abnormalities are specifically localized to corticostriatal, and not thalamostriatal, synapses 92.
In a more recent study, Shmelkov et al 93 inactivated Slitrk5, a member of a gene family implicated in obsessive-compulsive spectrum disorders and Tourette’s Syndrome, which encodes a postsynaptic density transmembrane protein. Slitrk5 KOs demonstrate increased anxiety and perseverative grooming that are reversed by chronic treatment with fluoxetine, demonstrating relevance to human OCD. Interestingly, Slitrk5 KOs also have OFC overactivation as measured with baseline c-fos staining, paralleling findings from human neuroimaging studies.
Current efforts from the groups who made the SAPAP3 and Slitrk5 KO mice are focused on the challenge of linking these mechanistic observations back to the human disorder. For example, a recent human genetics study found no association of SAPAP3 single nucleotide polymorphisms with OCD, but did find associations with grooming disorders such as pathologic skin picking, trichotillomania, and/or nail biting 94. In addition, though preliminary evidence from Slitrk5 genetic studies is promising, identifying rare Slitrk5 genetic variants in OCD patients, these findings must still be validated (F. Lee, personal communication). Regardless, both models clearly link molecular changes at corticostriatal synapses with abnormal repetitive behaviors, and therefore yield new insight into potential molecular and cellular pathologic mechanisms in OCD.
CIRCUIT MODELING
Recent technologic advances now permit both acute and chronic manipulation of activity in specific neural circuits, allowing direct simulation of human neuroimaging findings in mice. This approach was first elegantly applied to OCD research using a transgenic line that expresses the active subunit of cholera toxin under control of the D1 receptor promoter (D1CT-7 transgenic mice) 95. Expression of this stimulatory subunit yields constitutive hyperactivation of a subset of D1-positive neurons, providing some construct validity by generating strong overactivation of prefrontal-cortex and striatal neurons as observed in OCD imaging studies. At baseline, D1CT-7 mice demonstrate perseverative climbing, leaping, and biting behaviors that are exacerbated by increased NMDA-dependent glutamatergic transmission.
Other new technologies can also be used to mimic circuit abnormalities from human imaging studies 96. For example, recently-developed chemogenetic technology can generate sustained activation and inhibition in specific circuits. This is achieved by expressing mutated G-protein coupled receptors known as DREADDs (Designer Receptors Exclusively Activated by Designer Drugs) in specific cell-types, and then activating the DREADDs by oral, intraperitoneal, or intracranial administration of inert small molecules. Though this technology has not yet been used to directly investigate OCD, it has already been used to probe circuits underlying complex neuropsychiatric disorders such as schizophrenia 97 and major depressive disorder 98. Furthermore, chemogenetic studies have demonstrated the importance of OFC in switching between goal-directed behaviors and habits, a process that may be disrupted in OCD 99.
In addition, the recent development of optogenetics allows precise modulation of neural circuit activity using light-activated microbial ion channels. Though optogenetics initially focused on channelrhodopsin-2 (ChR2), an excitatory sodium channel gated by 480nm blue light; and halorhodopsin, an inhibitory chloride pump gated by 570nm yellow light; many opsin variants have now been synthesized to yield a wider range of activation wavelengths, kinetics, and open-channel current strengths 100,101. Through tissue-specific expression and local stimulation of light-activated proteins, distinct neural circuits can therefore be rapidly activated or inhibited without affecting neighboring cells 102,103.
Optogenetics has recently been applied to the study of OCD pathology and treatment in two back-to-back studies. Using the SAPAP3 KO mice described above to investigate treatment mechanisms, Burguiere et al 104 demonstrated impaired response inhibition in a conditioned grooming task. By selectively stimulating projections from the lateral OFC to the striatum, they were able to restore normal response inhibition, likely by compensating for SAPAP3 KO deficits in fast-spiking interneurons. In contrast, Ahmari et al 105 directly tested whether hyperstimulating OFC-ventral striatal projections, thus simulating hyperactivity seen in OCD patients, would lead to OCD-like behaviors in wild-type mice. While acute stimulation did not generate repetitive behaviors, repeated hyperactivation for multiple days in a row led to a progressive and persistent increase in grooming that correlated with an increased evoked-firing rate at OFC-VMS synapses. Increased grooming and evoked firing were both reversed by chronic fluoxetine treatment. Ongoing studies are attempting to synthesize these two sets of findings.
Though this review is focused on studies explicitly examining pathophysiology of OCD, optogenetic approaches have also been applied to the study of neurocognitive domains that are relevant to OCD, particularly the switch from goal-directed behaviors to habits. Briefly, these studies implicate the infralimbic cortex and the sensorimotor striatum in the development of habitual behavior, and suggest that modulation of these circuits may serve as a targeted treatment for disorders with excessive habit formation 106,107. As cumulative evidence begins to highlight abnormalities of particular neurocognitive functions in OCD, such as sensorimotor gating, response inhibition, goal-directed versus habitual behavior, and fear-extinction, we will be able to apply findings from the rich literature investigating the basic neurobiology of these core neural processes to gain improved understanding of circuit dysfunction in OCD.
Limitations of Animal Models
The above examples clearly demonstrate the utility of animal models for investigation of pathologic processes in OCD. However, critical evaluation of models to determine their relevance to OCD is crucial. Translatable probes of neural circuits that are reliably abnormal in OCD patients can be used for validation, helping ensure that dissection of molecular and cellular abnormalities will ultimately yield information relevant for treatment development 108.
Circuit-based Treatments in OCD Patients
Evidence from both patients and animal models converges on the idea that dysfunction in CSTC circuits leads to OCD symptoms. Significant efforts have therefore recently been made to develop new therapeutic approaches that directly target dysfunctional circuits. We will discuss these developments below; current pharmacologic and psychotherapeutic OCD treatments are reviewed elsewhere 109–111.
Stereotactic Lesions
Paralleling the neuroimaging findings described above, disruption of CSTC loops via multiple different surgical procedures has been found to decrease symptom severity in OCD 52. Though many of these procedures were developed empirically, they are all consistent with the theory that interrupting hyperactive connections and/or severing tracts between key circuit nodes with excess connectivity will lead to decreased abnormal transmission and fewer symptoms. Specific ablation procedures used to treat refractory OCD unresponsive to medications and psychotherapy include anterior cingulotomy, capsulotomy, subcaudate tractotomy, and limbic leucotomy 112. In anterior cingulotomy, bilateral lesions in the cingulum bundle are thought to disrupt hyperactive connections between frontocortical and subcortical areas 113,114; likewise, capsulotomy is thought to sever white matter bundles in the anterior limb of the internal capsule connecting OFC with mediodorsal thalamus 115,116. Similarly, both subcaudate tractotomy, which relies on a lesion made below and immediately anterior to the head of the caudate117, and limbic leucotomy, which is a combination of subcaudate tractotomy and cingulotomy, are thought to employ a similar mechanism of action–i.e. interruption of hyperactive frontothalamic circuits leading to symptom relief 113. Although in aggregate stereotactic lesions are moderately efficacious, with success rates ranging from 27–86% depending on criteria used to measure improvement, gauging success of these procedures is complicated by the challenges associated with performing double-blind studies118. Only one double-blind study has been performed to date 116, which demonstrated symptom improvement in active gamma knife ventral capsulotomy when compared to sham treatment.
Deep Brain Stimulation (DBS)
Following its success as a relatively safe, efficacious, adjustable, and reversible treatment for many movement disorders, including Parkinson’s disease, deep brain stimulation (DBS) has recently emerged as an investigative treatment for several neuropsychiatric disorders, including OCD 119,120. Multiple studies and research groups worldwide have attempted to build on the success of ablative procedures by performing targeted tunable stimulation that avoids the permanence and possible degeneration associated with lesions. Though there are caveats [the sham-control multicenter trial for DBS in OCD is still underway (ClinicalTrials.gov: NCT00640133A); different groups have different criteria for patient inclusion and treatment efficacy), many independent studies have indicated that DBS for OCD is a promising approach for treatment-resistant patients. DBS currently has a Humanitarian Device Exemption from the FDA, so that severe patients can obtain treatment before completion of the multi-center trial. Below, we will briefly describe current DBS targets and theories of mechanism of action; for meta-analysis see 121.
Targets
Optimizing brain targets for DBS in OCD remains an area of active investigation 122. One of the current most promising targets is the subthalamic nucleus (STN), which was initially targeted in OCD patients with co-morbid Parkinson’s Disease 123. Based on encouraging findings in this co-morbid population, the first controlled DBS study in primary OCD patients demonstrated significant response rates following DBS in bilateral limbic STN 124. Additional investigations of DBS broadly targeted the anterior limb of the internal capsule based on the success of stereotactic capsulotomy procedures, with resulting clinical improvement and decreased frontal cortical activity on PET scan. Subsequent studies by Greenberg and colleagues focused in on a smaller region, the ventral aspect of the anterior limb of the ventral capsule/ventral striatum (VC/VS) 112,125. These studies demonstrated clinical efficacy, with responders showing 35% reduction in symptoms on average, and some patients showing evidence for decreased OFC, ACC, and thalamus activity on PET. Other groups have also reported positive outcomes when targeting this region 112,126.
Based in part on the fact that shifting the electrode towards the VS led to improved efficacy and need for lower voltage stimulation, multiple groups have now focused on targeting nucleus accumbens (NAc), a structure classically involved in reward processing that lies within the VS and has extensive connectivity with both prefrontal cortical and thalamic regions. Particularly promising results have been reported by Denys and colleagues, who have performed open-label treatment trials demonstrating efficacy up to 2 years following surgery 127. Though multiple groups are now converging on targets in VC/VS, NAc, and limbic STN based on the results described above, research to find new targets with increased efficacy and decreased side effects is still ongoing.
Mechanism of Action
Although results from clinical studies are promising, the mechanism of action for effective DBS treatment is still unknown 125,128. Even in the more mature field of DBS for movement disorders, questions regarding mechanism remain, including importance of orthodromic vs. antidromic propagation of stimulation. However, recent studies in OCD are beginning to reveal clues. Though initial theories partly based on animal models suggested that DBS has an overall inhibitory effect on CSTC network transmission, effectively interrupting hyperactive circuits in a manner similar to stereotactic ablation, accumulation of clinical data suggests a potentially more complicated picture. For example, studies from Greenberg and colleagues indicate that acute VC/VS DBS leads to activation in OFC, ACC, striatum, globus pallidus, and thalamus 129, while chronic internal capsule activation has been shown to resolve OFC and ACC hyperactivity 130, as has been observed following effective pharmacologic treatment or exposure therapy. Activity normalization in mPFC and OFC has also been observed following effective STN DBS 131.
Other mechanistic studies of NAc DBS from the Denys group have used neuroimaging in humans combined with neurocognitive tasks to identify possible circuit-based mechanisms underlying symptom resolution. A recent study demonstrated decreased D2/3 receptor availability in putamen following both acute and chronic NAc DBS, suggesting that effective DBS induced striatal dopamine release 132. Another elegant study showed normalization of NAc activity accompanied by a decrease in excessive PFC-NAc connectivity following DBS 133. Future studies combining DBS with neuroimaging, high resolution EEG, and neurocognitive tasks will be necessary to further define mechanism of action.
Transcranial Magnetic Stimulation (TMS)
Despite promising initial results from DBS studies, it will be challenging to put this treatment into large-scale use if it continues to prove efficacious, due to barriers and risks associated with any neurosurgical procedure. Attempts have therefore recently been made to determine if TMS, a targeted, less invasive method of circuit stimulation, is effective in decreasing OCD symptoms. The most promising results to date target the supplementary motor area (SMA), which exhibits hyperexcitability in functional imaging studies; this suggests potential impairments in either excitatory or inhibitory activity within SMA 134. Based on these findings, multiple studies have applied low frequency TMS in SMA to reverse its hyperactivity with promising results, including two sham-controlled trials 135,136. In addition, a single-blind sham-control trial of TMS in left OFC showed decreased symptoms after 3 months despite not showing efficacy at 3 weeks, which raises interesting questions about possible plasticity changes induced by neural stimulation. In contrast, sham-controlled trials of TMS in DLPFC demonstrated no benefit 137. Notably, though TMS is currently limited to superficial brain structures, targeting of deep structures is now being attempted in an ongoing clinical trial (NCT01343732). If efficacious, deep TMS could greatly expand potential treatment targets for this non-invasive procedure.
Summary
The Future: Applying Findings from Animal Models to OCD Treatment
Though findings from studies involving stereotactic ablation, DBS, and TMS are all highly suggestive that targeted disruption of hyperactive CSTC circuits is therapeutic in OCD, several caveats remain in addition to those described above. First, although the area of intervention is known (e.g. the stereotactic coordinates of the lesion or electrode implant), the specific cell populations affected by the intervention are unknown. For example, although DBS in VC/VS likely stimulates VS projections originating in OFC, it also affects striatal projections from other cortical areas, as well as fibers of passage. Similarly, TMS in the SMA may stimulate both excitatory projections going broadly to all SMA target areas, as well as local inhibitory networks. Thus, the exact circuits and cell-types targeted by these interventions remain unclear. Studies in animal models using the optogenetic and chemogenetic approaches described above may help delineate the specific circuit-based mechanisms underlying therapeutic efficacy of these procedures.
In the short-term, understanding these mechanisms of action could assist in improved targeting of stimulation-based treatments. Though far in the future, mechanistic animal studies may also ultimately provide the foundation for either activation or inhibition of specific neural circuits for the treatment of neuropsychiatric illness. Specifically, variability in treatment response and side effects may be due to the fact that DBS and TMS broadly stimulate cortical projections, striatal cell bodies, and fibers of passage; conversely, specific stimulation of particular cell types could potentially lead to more targeted symptom reduction. Although minimal success has been achieved to date, several groups are currently investing significant resources in using optogenetics to generate behavioral changes in non-human primates 138–143. This is the first step in the process of performing cell-type specific interventions in people, which may have superior efficacy and fewer side effects compared to the more general stimulation afforded by DBS and TMS.
Acknowledgments
Financial Disclosures for the previous 12 months
The following grants are reported over the past 12 months. Dr. Ahmari: NIMH K08MH087718, NIMH R21 MH096200, NARSAD Young Investigator Award, Burroughs Wellcome CAMS Award, MQ Fellows Award, and NIMH R01MH104255. Dr. Dougherty: DARPA W911NF-14-2-0045, NIMH R01 MH091078, NIH/NCCAM R01 AT006364-01A1, NIH P50MH086400, NIH 7R01MH068376-07, Medtronic RECLAIM Grant, NIH U01 MH0766179-05, NIH/NIA 2R01AG013241, Genentech, Inc Grant.
Footnotes
Disclosure/ Conflicts of Interest
No specific funding was received for this work. Dr. Dougherty reports the following potential conflicts of interest: grants and honoraria from Medtronic, Inc; grants from Cyberonics, grants from Eli Lilly, grants and personal fees from Roche, honoraria from Insys, and honoraria from J&J. Dr. Ahmari has no conflicts of interest to disclose.
References
- 1.Koran LM. Quality of life in obsessive-compulsive disorder. Psychiatric Clinics of North America. 2000;23:509–517. doi: 10.1016/s0193-953x(05)70177-5. [DOI] [PubMed] [Google Scholar]
- 2.Kessler RC, et al. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Archives of General Psychiatry. 2005;62:593–602. doi: 10.1001/archpsyc.62.6.593. [DOI] [PubMed] [Google Scholar]
- 3.Weissman MM, et al. The cross national epidemiology of obsessive compulsive disorder. The Cross National Collaborative Group. J Clin Psychiatry. 1994;(55 Suppl):5–10. [PubMed] [Google Scholar]
- 4.Goodman WK, Grice DE, Lapidus KA, Coffey BJ. Obsessive-Compulsive Disorder. Psychiatr Clin North Am. 2014;37:257–267. doi: 10.1016/j.psc.2014.06.004. [DOI] [PubMed] [Google Scholar]
- 5.Leckman JF, et al. Obsessive-compulsive disorder: a review of the diagnostic criteria and possible subtypes and dimensional specifiers for DSM-V. Depress Anxiety. 2010;27:507–527. doi: 10.1002/da.20669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bienvenu OJ, et al. Is obsessive-compulsive disorder an anxiety disorder, and what, if any, are spectrum conditions? A family study perspective. Psychol Med. 2012;42:1–13. doi: 10.1017/S0033291711000742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mataix-Cols D, Rosario-Campos MC, Leckman JF. A multidimensional model of obsessive-compulsive disorder. American Journal of Psychiatry. 2005;162:228–238. doi: 10.1176/appi.ajp.162.2.228. [DOI] [PubMed] [Google Scholar]
- 8.Baxter LR, Jr, et al. Cerebral glucose metabolic rates in nondepressed patients with obsessive-compulsive disorder. Am J Psychiatry. 1988;145:1560–1563. doi: 10.1176/ajp.145.12.1560. [DOI] [PubMed] [Google Scholar]
- 9.Eichstedt JA, Arnold SL. Childhood-onset obsessive-compulsive disorder: a tic-related subtype of OCD? Clin Psychol Rev. 2001;21:137–157. doi: 10.1016/s0272-7358(99)00044-6. [DOI] [PubMed] [Google Scholar]
- 10.Leckman JF, et al. Obsessive-compulsive disorder: a review of the diagnostic criteria and possible subtypes and dimensional specifiers for DSM-V. Depression and Anxiety. 2010;27:507–527. doi: 10.1002/da.20669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mataix-Cols D, et al. Distinct neural correlates of washing, checking, and hoarding symptom dimensions in obsessive-compulsive disorder. Arch Gen Psychiatry. 2004;61:564–576. doi: 10.1001/archpsyc.61.6.564. [DOI] [PubMed] [Google Scholar]
- 12.Mataix-Cols D, et al. Hoarding disorder: a new diagnosis for DSM-V? Depress Anxiety. 2010;27:556–572. doi: 10.1002/da.20693. [DOI] [PubMed] [Google Scholar]
- 13.Saxena S. Is compulsive hoarding a genetically and neurobiologically discrete syndrome? Implications for diagnostic classification. Am J Psychiatry. 2007;164:380–384. doi: 10.1176/ajp.2007.164.3.380. [DOI] [PubMed] [Google Scholar]
- 14.Pauls DL. The genetics of obsessive compulsive disorder: a review of the evidence. American journal of medical genetics. 2008;148C:133–139. doi: 10.1002/ajmg.c.30168. [DOI] [PubMed] [Google Scholar]
- 15.Mattheisen M, et al. Genome-wide association study in obsessive-compulsive disorder: results from the OCGAS. Mol Psychiatry. 2014 doi: 10.1038/mp.2014.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stewart SE, et al. Genome-wide association study of obsessive-compulsive disorder. Mol Psychiatry. 2013;18:788–798. doi: 10.1038/mp.2012.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Taylor S. Molecular genetics of obsessive-compulsive disorder: a comprehensive meta-analysis of genetic association studies. Mol Psychiatry. 2013;18:799–805. doi: 10.1038/mp.2012.76. [DOI] [PubMed] [Google Scholar]
- 18.Wu K, Hanna GL, Rosenberg DR, Arnold PD. The role of glutamate signaling in the pathogenesis and treatment of obsessive-compulsive disorder. Pharmacol Biochem Behav. 2012;100:726–735. doi: 10.1016/j.pbb.2011.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Arnold PD, Sicard T, Burroughs E, Richter MA, Kennedy JL. Glutamate transporter gene SLC1A1 associated with obsessive-compulsive disorder. Arch Gen Psychiatry. 2006;63:769–776. doi: 10.1001/archpsyc.63.7.769. [DOI] [PubMed] [Google Scholar]
- 20.Dickel DE, et al. Association testing of the positional and functional candidate gene SLC1A1/EAAC1 in early-onset obsessive-compulsive disorder. Arch Gen Psychiatry. 2006;63:778–785. doi: 10.1001/archpsyc.63.7.778. [DOI] [PubMed] [Google Scholar]
- 21.Shugart YY, et al. A family-based association study of the glutamate transporter gene SLC1A1 in obsessive-compulsive disorder in 378 families. Am J Med Genet B Neuropsychiatr Genet. 2009;150B:886–892. doi: 10.1002/ajmg.b.30914. [DOI] [PubMed] [Google Scholar]
- 22.Stewart SE, et al. Association of the SLC1A1 glutamate transporter gene and obsessive-compulsive disorder. Am J Med Genet B Neuropsychiatr Genet. 2007;144B:1027–1033. doi: 10.1002/ajmg.b.30533. [DOI] [PubMed] [Google Scholar]
- 23.Wendland JR, et al. A haplotype containing quantitative trait loci for SLC1A1 gene expression and its association with obsessive-compulsive disorder. Arch Gen Psychiatry. 2009;66:408–416. doi: 10.1001/archgenpsychiatry.2009.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Samuels J, et al. Comprehensive family-based association study of the glutamate transporter gene SLC1A1 in obsessive-compulsive disorder. Am J Med Genet B Neuropsychiatr Genet. 2011;156B:472–477. doi: 10.1002/ajmg.b.31184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stewart SE, et al. Meta-analysis of association between obsessive-compulsive disorder and the 3’ region of neuronal glutamate transporter gene SLC1A1. Am J Med Genet B Neuropsychiatr Genet. 2013;162B:367–379. doi: 10.1002/ajmg.b.32137. [DOI] [PubMed] [Google Scholar]
- 26.Veenstra-VanderWeele JXT, Ruggiero AM, Anderson LR, Jones ST, Himle JA, Kennedy JL, Richter MA, Hanna GL, Arnold PD. Functional studies and rare variant screening of SLC1A1/EAAC1/EAAT3 in males with obsessive-compulsive disorder. Psychiatric Genetics. In Press doi: 10.1097/YPG.0b013e328353fb63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Veenstra-VanderWeele J, et al. Genomic organization of the SLC1A1/EAAC1 gene and mutation screening in early-onset obsessive-compulsive disorder. Mol Psychiatry. 2001;6:160–167. doi: 10.1038/sj.mp.4000806. [DOI] [PubMed] [Google Scholar]
- 28.Aoyama K, et al. Neuronal glutathione deficiency and age-dependent neurodegeneration in the EAAC1 deficient mouse. Nat Neurosci. 2006;9:119–126. doi: 10.1038/nn1609. [DOI] [PubMed] [Google Scholar]
- 29.Arnold PD, et al. Association of a glutamate (NMDA) subunit receptor gene (GRIN2B) with obsessive-compulsive disorder: a preliminary study. Psychopharmacology (Berl) 2004;174:530–538. doi: 10.1007/s00213-004-1847-1. [DOI] [PubMed] [Google Scholar]
- 30.Arnold PD, et al. Glutamate receptor gene (GRIN2B) associated with reduced anterior cingulate glutamatergic concentration in pediatric obsessive-compulsive disorder. Psychiatry Res. 2009;172:136–139. doi: 10.1016/j.pscychresns.2009.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Malenka RC, Nicoll RA. NMDA-receptor-dependent synaptic plasticity: multiple forms and mechanisms. Trends Neurosci. 1993;16:521–527. doi: 10.1016/0166-2236(93)90197-t. [DOI] [PubMed] [Google Scholar]
- 32.Akashi K, et al. NMDA receptor GluN2B (GluR epsilon 2/NR2B) subunit is crucial for channel function, postsynaptic macromolecular organization, and actin cytoskeleton at hippocampal CA3 synapses. J Neurosci. 2009;29:10869–10882. doi: 10.1523/JNEUROSCI.5531-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kutsuwada T, et al. Impairment of suckling response, trigeminal neuronal pattern formation, and hippocampal LTD in NMDA receptor epsilon 2 subunit mutant mice. Neuron. 1996;16:333–344. doi: 10.1016/s0896-6273(00)80051-3. [DOI] [PubMed] [Google Scholar]
- 34.Forrest D, et al. Targeted disruption of NMDA receptor 1 gene abolishes NMDA response and results in neonatal death. Neuron. 1994;13:325–338. doi: 10.1016/0896-6273(94)90350-6. [DOI] [PubMed] [Google Scholar]
- 35.Hemmings SM, Stein DJ. The current status of association studies in obsessive-compulsive disorder. Psychiatr Clin North Am. 2006;29:411–444. doi: 10.1016/j.psc.2006.02.011. [DOI] [PubMed] [Google Scholar]
- 36.Barr LC, Goodman WK, Price LH, McDougle CJ, Charney DS. The serotonin hypothesis of obsessive compulsive disorder: implications of pharmacologic challenge studies. Journal of Clinical Psychiatry. 1992;(53 Suppl):17–28. [PubMed] [Google Scholar]
- 37.Camarena B, Aguilar A, Loyzaga C, Nicolini H. A family-based association study of the 5-HT-1Dbeta receptor gene in obsessive-compulsive disorder. Int J Neuropsychopharmacol. 2004;7:49–53. doi: 10.1017/S1461145703003869. [DOI] [PubMed] [Google Scholar]
- 38.Shanahan NA, et al. Chronic reductions in serotonin transporter function prevent 5-HT1B–induced behavioral effects in mice. Biol Psychiatry. 2009;65:401–408. doi: 10.1016/j.biopsych.2008.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shanahan NA, Velez LP, Masten VL, Dulawa SC. Essential role for orbitofrontal serotonin 1B receptors in obsessive-compulsive disorder-like behavior and serotonin reuptake inhibitor response in mice. Biol Psychiatry. 2011;70:1039–1048. doi: 10.1016/j.biopsych.2011.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rauch SL, Savage CR, Alpert NM, Fischman AJ, Jenike MA. The functional neuroanatomy of anxiety: a study of three disorders using positron emission tomography and symptom provocation. Biol Psychiatry. 1997;42:446–452. doi: 10.1016/S0006-3223(97)00145-5. [DOI] [PubMed] [Google Scholar]
- 41.Rotge JY, et al. Anatomical alterations and symptom-related functional activity in obsessive-compulsive disorder are correlated in the lateral orbitofrontal cortex. Biol Psychiatry. 2010;67:37–38. doi: 10.1016/j.biopsych.2009.10.007. [DOI] [PubMed] [Google Scholar]
- 42.Saxena S, Bota RG, Brody AL. Brain-behavior relationships in obsessive-compulsive disorder. Semin Clin Neuropsychiatry. 2001;6:82–101. doi: 10.1053/scnp.2001.21833. [DOI] [PubMed] [Google Scholar]
- 43.Maia TV, Cooney RE, Peterson BS. The neural bases of obsessive-compulsive disorder in children and adults. Dev Psychopathol. 2008;20:1251–1283. doi: 10.1017/S0954579408000606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Parent A, Hazrati LN. Functional anatomy of the basal ganglia. I The cortico-basal ganglia-thalamo-cortical loop. Brain Res Brain Res Rev. 1995;20:91–127. doi: 10.1016/0165-0173(94)00007-c. [DOI] [PubMed] [Google Scholar]
- 45.Pennartz CM, et al. Corticostriatal Interactions during Learning, Memory Processing, and Decision Making. J Neurosci. 2009;29:12831–12838. doi: 10.1523/JNEUROSCI.3177-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kravitz AV, et al. Regulation of parkinsonian motor behaviours by optogenetic control of basal ganglia circuitry. Nature. 2010;466:622–626. doi: 10.1038/nature09159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kravitz AV, Kreitzer AC. Striatal mechanisms underlying movement, reinforcement, and punishment. Physiology (Bethesda) 2012;27:167–177. doi: 10.1152/physiol.00004.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jin X, Tecuapetla F, Costa RM. Basal ganglia subcircuits distinctively encode the parsing and concatenation of action sequences. Nat Neurosci. 2014;17:423–430. doi: 10.1038/nn.3632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cazorla M, et al. Dopamine D2 receptors regulate the anatomical and functional balance of basal ganglia circuitry. Neuron. 2014;81:153–164. doi: 10.1016/j.neuron.2013.10.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ting JT, Feng G. Neurobiology of obsessive-compulsive disorder: insights into neural circuitry dysfunction through mouse genetics. Curr Opin Neurobiol. 2011;21:842–848. doi: 10.1016/j.conb.2011.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pittenger C, Bloch MH, Williams K. Glutamate abnormalities in obsessive compulsive disorder: neurobiology, pathophysiology, and treatment. Pharmacol Ther. 2011;132:314–332. doi: 10.1016/j.pharmthera.2011.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rodman AM, et al. Neuroimaging contributions to novel surgical treatments for intractable obsessive-compulsive disorder. Expert review of neurotherapeutics. 2012;12:219–227. doi: 10.1586/ern.11.189. [DOI] [PubMed] [Google Scholar]
- 53.de Wit SJ, et al. Multicenter voxel-based morphometry mega-analysis of structural brain scans in obsessive-compulsive disorder. Am J Psychiatry. 2014;171:340–349. doi: 10.1176/appi.ajp.2013.13040574. [DOI] [PubMed] [Google Scholar]
- 54.Rotge JY, et al. Meta-analysis of brain volume changes in obsessive-compulsive disorder. Biol Psychiatry. 2009;65:75–83. doi: 10.1016/j.biopsych.2008.06.019. [DOI] [PubMed] [Google Scholar]
- 55.Radua J, Mataix-Cols D. Voxel-wise meta-analysis of grey matter changes in obsessive-compulsive disorder. Br J Psychiatry. 2009;195:393–402. doi: 10.1192/bjp.bp.108.055046. [DOI] [PubMed] [Google Scholar]
- 56.Menzies L, et al. Neurocognitive endophenotypes of obsessive-compulsive disorder. Brain. 2007;130:3223–3236. doi: 10.1093/brain/awm205. [DOI] [PubMed] [Google Scholar]
- 57.Rotge JY, et al. Provocation of obsessive-compulsive symptoms: a quantitative voxel-based meta-analysis of functional neuroimaging studies. J Psychiatry Neurosci. 2008;33:405–412. [PMC free article] [PubMed] [Google Scholar]
- 58.Rauch SL, et al. Predictors of fluvoxamine response in contamination-related obsessive compulsive disorder: a PET symptom provocation study. Neuropsychopharmacology. 2002;27:782–791. doi: 10.1016/S0893-133X(02)00351-2. [DOI] [PubMed] [Google Scholar]
- 59.Nakao T, et al. Brain activation of patients with obsessive-compulsive disorder during neuropsychological and symptom provocation tasks before and after symptom improvement: a functional magnetic resonance imaging study. Biol Psychiatry. 2005;57:901–910. doi: 10.1016/j.biopsych.2004.12.039. [DOI] [PubMed] [Google Scholar]
- 60.Beucke JC, et al. Abnormally high degree connectivity of the orbitofrontal cortex in obsessive-compulsive disorder. JAMA Psychiatry. 2013;70:619–629. doi: 10.1001/jamapsychiatry.2013.173. [DOI] [PubMed] [Google Scholar]
- 61.Posner J, et al. Reduced functional connectivity within the limbic cortico-striato-thalamo-cortical loop in unmedicated adults with obsessive-compulsive disorder. Hum Brain Mapp. 2014;35:2852–2860. doi: 10.1002/hbm.22371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Harrison BJ, et al. Altered corticostriatal functional connectivity in obsessive-compulsive disorder. Arch Gen Psychiatry. 2009;66:1189–1200. doi: 10.1001/archgenpsychiatry.2009.152. [DOI] [PubMed] [Google Scholar]
- 63.Anticevic A, et al. Global resting-state functional magnetic resonance imaging analysis identifies frontal cortex, striatal, and cerebellar dysconnectivity in obsessive-compulsive disorder. Biol Psychiatry. 2014;75:595–605. doi: 10.1016/j.biopsych.2013.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hou JM, et al. Resting-state functional connectivity abnormalities in patients with obsessive-compulsive disorder and their healthy first-degree relatives. J Psychiatry Neurosci. 2014;39:304–311. doi: 10.1503/jpn.130220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Milad MR, Rauch SL. Obsessive-compulsive disorder: beyond segregated cortico-striatal pathways. Trends Cogn Sci. 2012;16:43–51. doi: 10.1016/j.tics.2011.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Elliott R, Agnew Z, Deakin JF. Hedonic and informational functions of the human orbitofrontal cortex. Cereb Cortex. 2010;20:198–204. doi: 10.1093/cercor/bhp092. [DOI] [PubMed] [Google Scholar]
- 67.Roth RM, et al. Event-related functional magnetic resonance imaging of response inhibition in obsessive-compulsive disorder. Biol Psychiatry. 2007;62:901–909. doi: 10.1016/j.biopsych.2006.12.007. [DOI] [PubMed] [Google Scholar]
- 68.Marsh R, Maia TV, Peterson BS. Functional disturbances within frontostriatal circuits across multiple childhood psychopathologies. Am J Psychiatry. 2009;166:664–674. doi: 10.1176/appi.ajp.2009.08091354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Chamberlain SR, et al. Orbitofrontal dysfunction in patients with obsessive-compulsive disorder and their unaffected relatives. Science. 2008;321:421–422. doi: 10.1126/science.1154433. [DOI] [PubMed] [Google Scholar]
- 70.van Velzen LS, Vriend C, de Wit SJ, van den Heuvel OA. Response inhibition and interference control in obsessive-compulsive spectrum disorders. Frontiers in Human Neuroscience. 2014;8:419. doi: 10.3389/fnhum.2014.00419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.van Velzen LS, Vriend C, de Wit SJ, van den Heuvel OA. Response inhibition and interference control in obsessive-compulsive spectrum disorders. Front Hum Neurosci. 2014;8:419. doi: 10.3389/fnhum.2014.00419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Chamberlain SR, et al. Impaired cognitive flexibility and motor inhibition in unaffected first-degree relatives of patients with obsessive-compulsive disorder. Am J Psychiatry. 2007;164:335–338. doi: 10.1176/appi.ajp.164.2.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ahmari SE, Risbrough VB, Geyer MA, Simpson HB. Impaired sensorimotor gating in unmedicated adults with obsessive-compulsive disorder. Neuropsychopharmacology. 2012;37:1216–1223. doi: 10.1038/npp.2011.308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Balleine BW, O’Doherty JP. Human and rodent homologies in action control: corticostriatal determinants of goal-directed and habitual action. Neuropsychopharmacology. 2010;35:48–69. doi: 10.1038/npp.2009.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gillan CM, et al. Disruption in the balance between goal-directed behavior and habit learning in obsessive-compulsive disorder. Am J Psychiatry. 2011;168:718–726. doi: 10.1176/appi.ajp.2011.10071062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Gillan CM, et al. Enhanced avoidance habits in obsessive-compulsive disorder. Biol Psychiatry. 2014;75:631–638. doi: 10.1016/j.biopsych.2013.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gillan CM, et al. Functional Neuroimaging of Avoidance Habits in Obsessive-Compulsive Disorder. Am J Psychiatry. 2014 doi: 10.1176/appi.ajp.2014.14040525. appiajp201414040525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Simon D, Adler N, Kaufmann C, Kathmann N. Amygdala hyperactivation during symptom provocation in obsessive-compulsive disorder and its modulation by distraction. Neuroimage Clin. 2014;4:549–557. doi: 10.1016/j.nicl.2014.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Milad MR, et al. A role for the human dorsal anterior cingulate cortex in fear expression. Biol Psychiatry. 2007;62:1191–1194. doi: 10.1016/j.biopsych.2007.04.032. [DOI] [PubMed] [Google Scholar]
- 80.Milad MR, et al. Deficits in conditioned fear extinction in obsessive-compulsive disorder and neurobiological changes in the fear circuit. JAMA Psychiatry. 2013;70:608–618; quiz 554. doi: 10.1001/jamapsychiatry.2013.914. [DOI] [PubMed] [Google Scholar]
- 81.Wang L, Simpson HB, Dulawa SC. Assessing the validity of current mouse genetic models of obsessive-compulsive disorder. Behav Pharmacol. 2009;20:119–133. doi: 10.1097/FBP.0b013e32832a80ad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Arguello PA, Gogos JA. Modeling madness in mice: one piece at a time. Neuron. 2006;52:179–196. doi: 10.1016/j.neuron.2006.09.023. [DOI] [PubMed] [Google Scholar]
- 83.Malkki HA, Donga LA, de Groot SE, Battaglia FP, Pennartz CM. Towards mouse models of perseveration: a heritable component in extinction of operant behavior in fourteen standard and recombinant inbred mouse lines. Neurobiol Learn Mem. 2011;96:280–287. doi: 10.1016/j.nlm.2011.05.005. [DOI] [PubMed] [Google Scholar]
- 84.Kontis D, et al. Dopaminergic and serotonergic modulation of persistent behaviour in the reinforced spatial alternation model of obsessive-compulsive disorder. Psychopharmacology (Berl) 2008;200:597–610. doi: 10.1007/s00213-008-1241-5. [DOI] [PubMed] [Google Scholar]
- 85.Szechtman H, Sulis W, Eilam D. Quinpirole induces compulsive checking behavior in rats: a potential animal model of obsessive-compulsive disorder (OCD) Behav Neurosci. 1998;112:1475–1485. doi: 10.1037//0735-7044.112.6.1475. [DOI] [PubMed] [Google Scholar]
- 86.Hauber W, Schmidt WJ. Effects of intrastriatal blockade of glutamatergic transmission on the acquisition of T-maze and radial maze tasks. J Neural Transm Gen Sect. 1989;78:29–41. doi: 10.1007/BF01247111. [DOI] [PubMed] [Google Scholar]
- 87.Presti MF, Watson CJ, Kennedy RT, Yang M, Lewis MH. Behavior-related alterations of striatal neurochemistry in a mouse model of stereotyped movement disorder. Pharmacol Biochem Behav. 2004;77:501–507. doi: 10.1016/j.pbb.2003.12.004. [DOI] [PubMed] [Google Scholar]
- 88.Donaldson ZR, Nautiyal KM, Ahmari SE, Hen R. Genetic approaches for understanding the role of serotonin receptors in mood and behavior. Curr Opin Neurobiol. 2013;23:399–406. doi: 10.1016/j.conb.2013.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Chen SK, et al. Hematopoietic origin of pathological grooming in Hoxb8 mutant mice. Cell. 2010;141:775–785. doi: 10.1016/j.cell.2010.03.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Chou-Green JM, Holscher TD, Dallman MF, Akana SF. Compulsive behavior in the 5-HT2C receptor knockout mouse. Physiol Behav. 2003;78:641–649. doi: 10.1016/s0031-9384(03)00047-7. [DOI] [PubMed] [Google Scholar]
- 91.Welch JM, et al. Cortico-striatal synaptic defects and OCD-like behaviours in Sapap3-mutant mice. Nature. 2007;448:894–900. doi: 10.1038/nature06104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wan Y, et al. Circuit-selective striatal synaptic dysfunction in the Sapap3 knockout mouse model of obsessive-compulsive disorder. Biol Psychiatry. 2014;75:623–630. doi: 10.1016/j.biopsych.2013.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Shmelkov SV, et al. Slitrk5 deficiency impairs corticostriatal circuitry and leads to obsessive-compulsive-like behaviors in mice. Nat Med. 2010;16:598–602. doi: 10.1038/nm.2125. 591p following 602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Bienvenu OJ, et al. Sapap3 and pathological grooming in humans: Results from the OCD collaborative genetics study. Am J Med Genet B Neuropsychiatr Genet. 2009;150B:710–720. doi: 10.1002/ajmg.b.30897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Campbell KM, et al. OCD-Like behaviors caused by a neuropotentiating transgene targeted to cortical and limbic D1+ neurons. J Neurosci. 1999;19:5044–5053. doi: 10.1523/JNEUROSCI.19-12-05044.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Rogan SC, Roth BL. Remote control of neuronal signaling. Pharmacol Rev. 2011;63:291–315. doi: 10.1124/pr.110.003020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Parnaudeau S, et al. Inhibition of mediodorsal thalamus disrupts thalamofrontal connectivity and cognition. Neuron. 2013;77:1151–1162. doi: 10.1016/j.neuron.2013.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Soumier A, Sibille E. Opposing effects of acute versus chronic blockade of frontal cortex somatostatin-positive inhibitory neurons on behavioral emotionality in mice. Neuropsychopharmacology. 2014;39:2252–2262. doi: 10.1038/npp.2014.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Gremel CM, Costa RM. Orbitofrontal and striatal circuits dynamically encode the shift between goal-directed and habitual actions. Nat Commun. 2013;4:2264. doi: 10.1038/ncomms3264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Gradinaru V, et al. Targeting and readout strategies for fast optical neural control in vitro and in vivo. J Neurosci. 2007;27:14231–14238. doi: 10.1523/JNEUROSCI.3578-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Gradinaru V, et al. Molecular and cellular approaches for diversifying and extending optogenetics. Cell. 2010;141:154–165. doi: 10.1016/j.cell.2010.02.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Zhang F, et al. Optogenetic interrogation of neural circuits: technology for probing mammalian brain structures. Nat Protoc. 2010;5:439–456. doi: 10.1038/nprot.2009.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Sparta DR, et al. Construction of implantable optical fibers for long-term optogenetic manipulation of neural circuits. Nat Protoc. 2012;7:12–23. doi: 10.1038/nprot.2011.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Burguiere E, Monteiro P, Feng G, Graybiel AM. Optogenetic stimulation of lateral orbitofronto-striatal pathway suppresses compulsive behaviors. Science. 2013;340:1243–1246. doi: 10.1126/science.1232380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Ahmari SE, et al. Repeated cortico-striatal stimulation generates persistent OCD-like behavior. Science. 2013;340:1234–1239. doi: 10.1126/science.1234733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Smith KS, Graybiel AM. A dual operator view of habitual behavior reflecting cortical and striatal dynamics. Neuron. 2013;79:361–374. doi: 10.1016/j.neuron.2013.05.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Smith KS, Virkud A, Deisseroth K, Graybiel AM. Reversible online control of habitual behavior by optogenetic perturbation of medial prefrontal cortex. Proc Natl Acad Sci U S A. 2012;109:18932–18937. doi: 10.1073/pnas.1216264109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Ahmari SE, Eich T, Cebenoyan D, Smith EE, Blair Simpson H. Assessing neurocognitive function in psychiatric disorders: A roadmap for enhancing consensus. Neurobiol Learn Mem. 2014 doi: 10.1016/j.nlm.2014.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.American Psychiatric Association. Practice guideline for the treatment of patients with obsessive-compulsive disorder. American Journal of Psychiatry. 2007;164:1–56. [PubMed] [Google Scholar]
- 110.Simpson HB. Pharmacological treatment of obsessive-compulsive disorder. Curr Top Behav Neurosci. 2010;2:527–543. doi: 10.1007/7854_2009_12. [DOI] [PubMed] [Google Scholar]
- 111.Stein DJ, et al. A 2012 evidence-based algorithm for the pharmacotherapy for obsessive-compulsive disorder. Curr Psychiatry Rep. 2012;14:211–219. doi: 10.1007/s11920-012-0268-9. [DOI] [PubMed] [Google Scholar]
- 112.Greenberg BD, Rauch SL, Haber SN. Invasive circuitry-based neurotherapeutics: stereotactic ablation and deep brain stimulation for OCD. Neuropsychopharmacology. 2010;35:317–336. doi: 10.1038/npp.2009.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Sheth SA, et al. Limbic system surgery for treatment-refractory obsessive-compulsive disorder: a prospective long-term follow-up of 64 patients. J Neurosurg. 2013;118:491–497. doi: 10.3171/2012.11.JNS12389. [DOI] [PubMed] [Google Scholar]
- 114.Rauch SL, et al. Cerebral metabolic correlates as potential predictors of response to anterior cingulotomy for obsessive compulsive disorder. Biol Psychiatry. 2001;50:659–667. doi: 10.1016/s0006-3223(01)01188-x. [DOI] [PubMed] [Google Scholar]
- 115.Ruck C, Larsson KJ, Mataix-Cols D. Predictors of medium and long-term outcome following capsulotomy for obsessive-compulsive disorder: one site may not fit all. Eur Neuropsychopharmacol. 2012;22:406–414. doi: 10.1016/j.euroneuro.2011.11.003. [DOI] [PubMed] [Google Scholar]
- 116.Lopes AC, et al. Gamma ventral capsulotomy for obsessive-compulsive disorder: a randomized clinical trial. JAMA Psychiatry. 2014;71:1066–1076. doi: 10.1001/jamapsychiatry.2014.1193. [DOI] [PubMed] [Google Scholar]
- 117.Jenike MA. Neurosurgical treatment of obsessive-compulsive disorder. Br J Psychiatry Suppl. 1998:79–90. [PubMed] [Google Scholar]
- 118.Patel SR, Aronson JP, Sheth SA, Eskandar EN. Lesion procedures in psychiatric neurosurgery. World Neurosurg. 2013;80 doi: 10.1016/j.wneu.2012.11.038. S31 e39-16. [DOI] [PubMed] [Google Scholar]
- 119.Bais M, Figee M, Denys D. Neuromodulation in Obsessive-Compulsive Disorder. Psychiatr Clin North Am. 2014;37:393–413. doi: 10.1016/j.psc.2014.06.003. [DOI] [PubMed] [Google Scholar]
- 120.Hamani C, et al. Deep Brain Stimulation for Obsessive-Compulsive Disorder: Systematic Review and Evidence-based Guideline Sponsored by the American Society for Stereotactic and Functional Neurosurgery (ASSFN) and the Congress of Neurological Surgeons (CNS) and Endorsed by the CNS and American Association of Neurological Surgeons (AANS) Neurosurgery. 2014 doi: 10.1227/NEU.0000000000000499. [DOI] [PubMed] [Google Scholar]
- 121.Kisely S, et al. Deep brain stimulation for obsessive-compulsive disorder: a systematic review and meta-analysis. Psychol Med. 2014:1–10. doi: 10.1017/S0033291714000981. [DOI] [PubMed] [Google Scholar]
- 122.de Koning PP, Figee M, van den Munckhof P, Schuurman PR, Denys D. Current status of deep brain stimulation for obsessive-compulsive disorder: a clinical review of different targets. Curr Psychiatry Rep. 2011;13:274–282. doi: 10.1007/s11920-011-0200-8. [DOI] [PubMed] [Google Scholar]
- 123.Mallet L, et al. Compulsions, Parkinson’s disease, and stimulation. Lancet. 2002;360:1302–1304. doi: 10.1016/S0140-6736(02)11339-0. [DOI] [PubMed] [Google Scholar]
- 124.Mallet L, et al. Subthalamic nucleus stimulation in severe obsessive-compulsive disorder. N Engl J Med. 2008;359:2121–2134. doi: 10.1056/NEJMoa0708514. [DOI] [PubMed] [Google Scholar]
- 125.Greenberg BD, et al. Deep brain stimulation of the ventral internal capsule/ventral striatum for obsessive-compulsive disorder: worldwide experience. Mol Psychiatry. 2010;15:64–79. doi: 10.1038/mp.2008.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Aronson JP, Katnani HA, Eskandar EN. Neuromodulation for obsessive-compulsive disorder. Neurosurg Clin N Am. 2014;25:85–101. doi: 10.1016/j.nec.2013.08.003. [DOI] [PubMed] [Google Scholar]
- 127.Ooms P, et al. Deep brain stimulation for obsessive-compulsive disorders: long-term analysis of quality of life. J Neurol Neurosurg Psychiatry. 2014;85:153–158. doi: 10.1136/jnnp-2012-302550. [DOI] [PubMed] [Google Scholar]
- 128.Bourne SK, Eckhardt CA, Sheth SA, Eskandar EN. Mechanisms of deep brain stimulation for obsessive compulsive disorder: effects upon cells and circuits. Front Integr Neurosci. 2012;6:29. doi: 10.3389/fnint.2012.00029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Rauch SL, et al. A functional neuroimaging investigation of deep brain stimulation in patients with obsessive-compulsive disorder. J Neurosurg. 2006;104:558–565. doi: 10.3171/jns.2006.104.4.558. [DOI] [PubMed] [Google Scholar]
- 130.Abelson JL, et al. Deep brain stimulation for refractory obsessive-compulsive disorder. Biol Psychiatry. 2005;57:510–516. doi: 10.1016/j.biopsych.2004.11.042. [DOI] [PubMed] [Google Scholar]
- 131.Le Jeune F, et al. Decrease of prefrontal metabolism after subthalamic stimulation in obsessive-compulsive disorder: a positron emission tomography study. Biol Psychiatry. 2010;68:1016–1022. doi: 10.1016/j.biopsych.2010.06.033. [DOI] [PubMed] [Google Scholar]
- 132.Figee M, et al. Deep brain stimulation induces striatal dopamine release in obsessive-compulsive disorder. Biol Psychiatry. 2014;75:647–652. doi: 10.1016/j.biopsych.2013.06.021. [DOI] [PubMed] [Google Scholar]
- 133.Figee M, et al. Deep brain stimulation restores frontostriatal network activity in obsessive-compulsive disorder. Nat Neurosci. 2013;16:386–387. doi: 10.1038/nn.3344. [DOI] [PubMed] [Google Scholar]
- 134.Richter MA, et al. Evidence for cortical inhibitory and excitatory dysfunction in obsessive compulsive disorder. Neuropsychopharmacology. 2012;37:1144–1151. doi: 10.1038/npp.2011.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Mantovani A, et al. Modulation of motor cortex excitability in obsessive-compulsive disorder: an exploratory study on the relations of neurophysiology measures with clinical outcome. Psychiatry Res. 2013;210:1026–1032. doi: 10.1016/j.psychres.2013.08.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Mantovani A, Simpson HB, Fallon BA, Rossi S, Lisanby SH. Randomized sham-controlled trial of repetitive transcranial magnetic stimulation in treatment-resistant obsessive-compulsive disorder. Int J Neuropsychopharmacol. 2010;13:217–227. doi: 10.1017/S1461145709990435. [DOI] [PubMed] [Google Scholar]
- 137.Lapidus KA, Stern ER, Berlin HA, Goodman WK. Neuromodulation for obsessive-compulsive disorder. Neurotherapeutics. 2014;11:485–495. doi: 10.1007/s13311-014-0287-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Dai J, Brooks DI, Sheinberg DL. Optogenetic and electrical microstimulation systematically bias visuospatial choice in primates. Curr Biol. 2014;24:63–69. doi: 10.1016/j.cub.2013.11.011. [DOI] [PubMed] [Google Scholar]
- 139.Ozden I, et al. A coaxial optrode as multifunction write-read probe for optogenetic studies in non-human primates. J Neurosci Methods. 2013;219:142–154. doi: 10.1016/j.jneumeth.2013.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Ruiz O, et al. Optogenetics through windows on the brain in the nonhuman primate. J Neurophysiol. 2013;110:1455–1467. doi: 10.1152/jn.00153.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Galvan A, Hu X, Smith Y, Wichmann T. In vivo optogenetic control of striatal and thalamic neurons in non-human primates. PLoS One. 2012;7:e50808. doi: 10.1371/journal.pone.0050808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Cavanaugh J, et al. Optogenetic inactivation modifies monkey visuomotor behavior. Neuron. 2012;76:901–907. doi: 10.1016/j.neuron.2012.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Diester I, et al. An optogenetic toolbox designed for primates. Nat Neurosci. 2011;14:387–397. doi: 10.1038/nn.2749. [DOI] [PMC free article] [PubMed] [Google Scholar]