Abstract
Background
In sub-Saharan Africa (SSA), HIV-infected patients may under-report alcohol consumption. We compared self-reports of drinking to phosphatidylethanol (PEth), an alcohol biomarker. In particular, we assessed beverage-type adjusted fractional graduated frequency (FGF) and quantity frequency (QF) measures of grams of alcohol, novel non-volume measures, and the Alcohol Use Disorders Identification Test – Consumption (AUDIT-C).
Methods
We analyzed cohort-entry data from the Biomarker Research of Ethanol in Those with HIV cohort study (2011-2013). Participants were HIV-infected past year drinkers, newly enrolled into care. Self-report measures included FGF and QF grams of alcohol, the AUDIT-C, number of drinking days, and novel adaptations of FGF and QF methods to expenditures on alcohol, time spent drinking, and symptoms of intoxication. PEth levels were measured from dried blood spots. We calculated Spearman’s rank correlation coefficients of self-reports with PEth and bias-corrected bootstrap 95% confidence intervals (CI) for pairwise differences between coefficients.
Results
A total of 209 subjects (57% male) were included. Median age was 30; inter-quartile range (IQR) 25-38. FGF grams of alcohol over the past 90 days (median 592, IQR 43 to 2137) were higher than QF grams (375, IQR 33 to 1776), p<0.001. However, both measures were moderately correlated with PEth; rho = 0.58, 95% CI 0.47 to 0.66 for FGF grams and 0.54, 95% CI 0.43 to 0.63 for QF grams (95% CI for difference −0.017 to 0.099, not statistically significant). AUDIT-C, time drinking, and a scale of symptoms of intoxication were similarly correlated with PEth (rho = 0.35 to 0.57).
Conclusion
HIV-infected drinkers in SSA likely underreport both any alcohol consumption and amounts consumed, suggesting the need to use more objective measures like biomarkers when measuring drinking in this population. Although the FGF method may more accurately estimate drinking than QF methods, the AUDIT-C and other non-volume measures may provide simpler alternatives.
Keywords: Self-report measures of alcohol consumption, sub-Saharan Africa, Fractional Graduated Frequency, Quantity frequency, Phosphatidylethanol
Introduction
In sub-Saharan Africa (SSA), home to more than 75% of the global HIV-infected population (UNAIDS, 2013), alcohol consumption is common and is among the drivers of the HIV epidemic (Hahn et al., 2011, Woolf-King et al., 2013). Unhealthy drinking, a spectrum of alcohol use behaviors ranging from risky drinking to alcohol dependence that are associated with varying degrees of risk to health (Saitz, 2005), is also common (WHO, 2011). As unhealthy drinking increases risk of non-adherence to HIV treatment (Hendershot et al., 2009), its reduction in those with HIV may lead to better HIV treatment outcomes, as well as reduced risk of HIV transmission and acquisition (Braithwaite et al., 2014).
However, significant limitations remain in the measurement of alcohol consumption and the evaluation of interventions for unhealthy drinking (Greenfield and Kerr, 2008). Quantifying alcohol consumption is particularly difficult in SSA as drinks are not always in standard sizes (Hahn et al., 2010). Also, many drinkers consume locally-made alcohols with variable ethanol contents (Mwesigye and Okurut, 1994). Whereas self-report is the most common way to assess alcohol intake, increasing evidence suggests under-reporting (Hahn et al., 2012b).
Novel biomarkers may measure alcohol consumption more objectively (Greenfield et al., 2014). In our previous study among HIV-infected adults in Uganda, blood concentrations of the alcohol biomarker phosphatidylethanol (PEth) ≥10 ng/ml had sensitivity of 88% and specificity of 89% for detecting any alcohol use in the past 21 days, and sensitivity of 76% and specificity of 100% for detecting any alcohol use in the past 90 days (Hahn et al., 2012a). However, biomarker tests remain expensive and are particularly inaccessible in SSA due to weak laboratory infrastructure. The performance of biomarkers relative to commonly used measures of self-report such as the World Health Organization (WHO)’s Alcohol Use Disorders Identification Test-Consumption (AUDIT-C) (Rubinsky et al., 2013) also remains unclear.
Several self-report measures of drinking obtain information on two dimensions: usual quantity of alcohol consumed and typical frequency of drinking. This method, known as quantity frequency (QF), assumes that drinkers always take the same alcohol in the same way or are capable of averaging consumption on these two dimensions over multiple drinking occasions (Greenfield, 2000, Dawson and Room, 2000). As they do not account for infrequent episodes of heavy drinking, a predominant pattern in SSA (Rehm et al., 2003), QF methods are likely to underestimate alcohol intake in this setting.
The graduated frequency (GF) method, which captures frequency of drinking at varying quantity levels, can capture total volumes consumed more accurately (Wilsnack et al., 2009). However, GF measures are difficult to implement in settings where a standard drink is not the norm (Greenfield and Kerr, 2008, Gmel et al., 2006). To overcome this challenge, the fractional graduated frequency (FGF) method uses the maximum quantity that a drinker consumed on a single occasion to calculate the frequency of consuming fixed fractions (100%, 75%, 50% and 25%) of that maximum quantity (Greenfield et al., 2010). This method has yielded comparable total volumes as 28-day diaries, a rigorous self-report measure of drinking (Greenfield et al., 2009, Greenfield et al., 2010), but has yet to be compared to biomarkers.
Finally, data are lacking on alternative measures of drinking and/or alternative measurement dimensions. As drinkers in SSA commonly consume non-standard drinks and express their drinking in non-volume terms (Papas et al., 2010b), domains such as expenditure, time spent drinking, and symptoms of intoxication, may provide novel measurement options.
Among HIV-infected drinkers in SSA, we compared multiple traditional and novel self-report measures of alcohol consumption to blood PEth levels. Our analysis had two primary aims. Firstly, we sought to compare PEth and the AUDIT-C; we hypothesized that PEth concentration and overall proportions of drinkers that are PEth positive would increase across AUDIT-C categories. Secondly, we sought to determine whether the FGF measure of grams of alcohol more accurately measures alcohol consumption than the QF measure; we hypothesized that FGF estimates would be more highly correlated with blood PEth levels than QF estimates. To address the need for alternative self-report measures, we also performed a secondary analysis assessing the correlations of simpler non-volume measures and screening tools (number of drinking days, expenditures on alcohol, time spent drinking, and symptoms of intoxication) with PEth.
Methods
Study design, setting and population
The Biomarker Research of Ethanol Among Those with HIV (BREATH) Study was a mixed methods prospective cohort study of HIV-infected adults at the Immune Suppression Syndrome (ISS) Clinic in Mbarara, Uganda. The study aimed to quantify changes in alcohol intake and to determine correlates of such changes during the first year of HIV care among HIV-infected drinkers. Eligibility criteria were: HIV-infected adult (≥18 years), newly enrolled into HIV care, not yet initiated on antiretroviral therapy (ART), fluent in the local language (Runyankole) or English, and reported any alcohol consumption in the past year at their initial clinic visit. Patients who had received prior HIV care or lived more than 60 kilometers from the clinic were excluded. Clinic counselors screened all new patients for eligibility and invited those eligible to participate. Research assistants then consecutively enrolled those who provided written informed consent.
Enrolled participants were randomized to the main cohort study arm or to a comparison arm in which enrollees were minimally assessed during follow-up to see if the degree of assessment affects study results (Clifford et al., 2007). Main cohort participants completed interviewer-administered questionnaires and provided blood samples at cohort entry and at quarterly visits for one year. As drinking by HIV-infected patients is stigmatized in this setting, study staff interviewing patients were different from usual clinic staff providing treatment. Data collected from patients for study purposes were not shared with clinic staff. The study team explained this procedure to all staff at the clinic and to study participants. The single exception was for participants with high AUDIT scores (≥ 20), for whom, with their permission, we provided referrals to a mental health counselor for treatment of possible alcohol dependence. The BREATH Study protocol was approved by the Institutional Review Committees of Mbarara University of Science and Technology (MUST) and the University of California San Francisco and the Uganda National Council for Science and Technology.
Previous reports from the BREATH Study have explored changes in alcohol consumption during HIV care (Sundararajan et al., 2014) and developed a novel scale on alcohol expectancies, that is, how patients in this setting expect to benefit from alcohol consumption (Woolf-King et al., 2015). In this report, we performed a cross-sectional analysis of participants’ cohort entry data specifically to address challenges to the measurement of alcohol consumption in this setting.
Measurements
We collected socio-demographic information such as age, gender, literacy, and income, and all self-reported alcohol intake data using an interviewer-administered structured questionnaire. The questionnaires were developed in English, translated to the local language (Runyakole), and then back translated to English by a language expert to ensure consistency of the translations (WHO, 2015). Interviews were conducted in Runyankole or English, depending on the participant’s preference. During each interview, data were directly entered into a laptop using a computer program called CASIC (Computer Assisted Survey Information Collection), which allowed entries in either English or Runyankole and facilitated use of complex skip patterns as appropriate.
Self-reported alcohol consumption
We measured self-reported alcohol consumption in the past 3 months in multiple ways (Table 1), including the AUDIT-C, number of drinking days, and beverage-specific FGF and QF grams of alcohol. In addition, we adapted FGF and QF methods to create novel non-volume measures of drinking using expenditures on alcohol, time spent drinking, and symptoms of intoxication.
Table 1.
Traditional and novel self-reported measures of drinking used in the study and how they were implemented. The table shows the methods used to measure drinking in this study and summarizes how each method was implemented.
| Name of measure | Summary of how the measure was obtained | Beverage-type adjustment |
|---|---|---|
| AUDIT-C | Explanation of definition of a standard drink (using commercial quantities in this setting); followed by the three standard AUDIT-C questions (frequency of drinking; number of drinks on a typical drinking day; and frequency of ≥6 drinks on one occasion). |
No |
|
| ||
| Number of drinking days | Those reporting any drinking chose a frequency from: daily/nearly daily, 3 to 4 times a week, once or twice a week, 2 to 3 times a month, about once a month, or once or twice in the entire 3 months; number of drinking days calculated using midpoint of chosen frequency; for example, 2 to 3 times per month = 2.5 times 3 months = 7.5 days. |
No |
|
| ||
| FGF grams of alcohol | For each drink type; maximum-day* grams of alcohol consumed times a graduated beaker fraction (representing 100% of a maximum day, 75%, 50%, 25%) times the frequency of consuming that fraction in past 3 months; resulting beverage-specific quantities summed into a total. |
Yes |
|
| ||
| QF grams of alcohol | For each drink type; typical-day† grams of alcohol consumed times number of drinking days in past 3 months; resulting beverage-specific quantities summed into a total. |
Yes |
|
| ||
| FGF time spent drinking | Time spent drinking on a maximum day times a graduated beaker fraction times the frequency of drinking at that fraction in past 3 months; resulting quantities per fraction summed into a total. |
No |
|
| ||
| QF time spent drinking | Time spent drinking on a typical day times number of drinking days in past 3 months. |
No |
|
| ||
|
FGF symptoms of
intoxication |
Symptoms of intoxication‡ (score) on a maximum day times a graduated beaker fraction times the frequency of drinking at that fraction; the resulting quantities per fraction were summed into a total. |
No |
|
| ||
|
QF symptoms of
intoxication |
Symptoms of intoxication (score) on a typical day times number of drinking days in past 3 months. |
No |
|
| ||
|
FGF expenditure on
alcohol |
Expenditure on alcohol on a maximum day times a graduated beaker fraction times the frequency of drinking at that fraction in past 3 months; resulting quantities per fraction were summed into a total. |
No |
|
| ||
| QF expenditure on alcohol | Expenditure on alcohol on a typical day times number of drinking days No in past 3 months. |
No |
AUDIT-C: Alcohol Use Disorders Identification Test Consumption, QF: Quantity Frequency, FGF: Fractional Graduated Frequency,
Maximum day grams of alcohol = a beverage specific estimate for heaviest drinking day in prior 3 months (calculated, for each drink type reported on a maximum drinking day, according to setting-specific containers/volumes and estimated ethanol content for that drink type).
Typical-day grams = a beverage specific estimate for a typical drinking day in prior 3 months (calculated, for each drink type reported for a typical drinking day, according to setting-specific containers/volumes and estimated ethanol content for that drink type).
Symptoms of intoxication = a description of how a participant felt after drinking alcohol choosing from: becoming unconscious or stuporous; having difficulty speaking or seeing clearly or walking; having difficulty thinking clearly; feeling uninhibited or feeling a false sense of security and confidence; feeling only mild pleasurable effects of alcohol; or feeling no effects at all from the alcohol. The response is scored from 6 (becoming unconscious) down to 1 (no effects).
AUDIT-C
We adapted the AUDIT-C questionnaire (categorizing frequency of any alcohol use, quantity of typical use, and frequency of taking 6 or more drinks on one occasion) (Bradley et al., 2007) to a reference period of the past 3 months. Validation studies of the AUDIT-C have not been conducted in resource-limited settings, although it is commonly used (Peltzer et al., 2007). In primary care populations in the US, the AUDIT-C identified unhealthy drinkers with a sensitivity of 0.73 and specificity of 0.91 using a cut-off of ≥3 for women, and a sensitivity of 0.86 and specificity of 0.89 using a cut-off of ≥4 for men (Bradley et al., 2007). Among HIV-infected adults also in the US, and using the full 10-item AUDIT as the gold standard, the AUDIT-C had a sensitivity of 0.81-0.89 and specificity of 0.91 to 1.0 using a cut-off of 4, and sensitivity of 0.94 to 0.98 and specificity of 0.82 to 0.91 at a cut-off of 3 for detecting unhealthy drinking (Strauss and Rindskopf, 2009). We defined a drink as a 140ml glass of 12%-alcohol wine, 40ml of hard liquor, or a 360ml bottle or can of beer. To aid participants, we used illustrations of containers in which commercial alcoholic drinks are commonly sold in this setting that approximated these volumes. We categorized subjects based on their AUDIT-C scores into lower-risk drinkers (score = 1 to 3 for males or 1 to 2 for females) and unhealthy drinkers (score ≥4 for men or ≥3 for women).
Number of drinking days
Participants reporting any alcohol consumption in the past 3 months were asked on how many days they drank any alcohol, choosing one of six possible responses (every day or nearly every day, 3 to 4 times a week, once or twice a week, 2 to 3 times a month, about once a month, or once or twice in the entire 3 months). We converted these responses to a numeric estimate of drinking days by taking their midpoints.
Drink types
Alcohol in Uganda is available as commercially-made or locally-made alcohol. Unlike commercial alcohols, which are standardized, local alcohols have variable ethanol contents; local spirits can be highly potent (estimated ethanol content = 18 to 53%), while local beers are usually less potent (estimated ethanol content = 6 to 11%) (Hahn et al., 2012a, Mwesigye and Okurut, 1994). We grouped alcohols into six classes: wine (both local and commercial types are fruit-based); locally brewed beer; commercially brewed beer; locally distilled hard liquor or spirits; commercially distilled hard liquor or spirits; and communally consumed beverages such as “malwa”. To evaluate if drinking certain alcohol types was associated with differences in PEth concentrations, we created two summary variables, the first defining those typically drinking only local alcohols versus commercial alcohols versus mixtures of the two production methods, and the second defining those who reported drinking any spirits versus those who reported not taking any spirits (given that spirits in this setting have high ethanol contents we hypothesized that those drinking any spirits are likely to be heavier drinkers than those not drinking any spirits).
Maximum-day and typical-day volumes
We defined the “maximum drinking day” as the day when a participant drank their “maximum amount of alcohol on a single day”; a “typical day” was one where they drank “the most common amount of alcohol consumed on days other than the maximum day”. Participants were asked what drink type(s) they drank and its estimated volume on both days. To aid participants, we used a list of typical volumes/containers specific to each alcohol type. For example, when interviewing a participant, we began by asking: “on your maximum drinking day, which drink type(s) did you consume?” This was then followed by a series of questions regarding the quantity of common beverage-specific containers that were consumed. For example, if someone reported drinking wine on their maximum day, they were asked how many bottles (750 ml) and glasses (140-200 ml) of wine they consumed on that day. The same questions would then be asked for a typical drinking day. As such, volumes taken for the maximum drinking day and the typical drinking day were obtained for each particular beverage type by multiplying the quantity of drinks consumed by the size of each beverage in milliliters.
Grams of alcohol on a typical or maximum drinking day
We used previously reported estimates of the average ethanol content for each drink type (5% for beers, 12.5% for wines, and 40% for spirits, all multiplied by a factor of 0.7893 in volume-to-weight conversions) (Hahn et al., 2012a) to convert volumes to grams of alcohol. Typical-day grams were the sum of the products of typical-day volume for each drink type consumed and the average ethanol content for each drink. Maximum-day grams of alcohol were obtained similarly as the maximum-day volume of each drink type times the ethanol content of that drink type (Greenfield et al., 2010).
Maximum and typical day quantities on the other self-reported domains
For both typical and maximum days, participants were asked: how much money they or someone else spent on all types of alcohol; how much time they spent drinking; and how they felt after drinking using a scale of symptoms of intoxication. This scale includes, in descending order: becoming unconscious or stuporous; having difficulty speaking or seeing clearly or walking; having difficulty thinking clearly; feeling uninhibited or feeling a false sense of security and confidence; feeling only mild pleasurable effects of alcohol; or feeling no effects at all from the alcohol. Participants were asked to choose the highest level of symptoms that best described how they felt after consuming alcohol; their answers were scored correspondingly from 6 (becoming unconscious) down to 1 (no effects). We have previously used variants of this scale to measure alcohol consumption. In young injection drug users in the US, the measure correlated favorably with PEth (rho = 0.69) (Jain et al., 2014). However, a simplification of the measure had low correlation with PEth (rho = 0.24) in ART-treated HIV-infected patients in Uganda (Bajunirwe et al., 2014).
The graduated beaker for fractional graduated frequency estimations
To collect data on the fractional frequencies required for FGF estimates, we showed participants illustrations of four graduated beakers full to different levels (100%, 75%, 50%, and 25%) and asked them how often in the past 3 months they had drank each level (that is, the full amount, about three-quarters, a half, and a quarter) in relation to their maximum consumption.
Quantity frequency and fractional graduated frequency grams of alcohol
We calculated QF grams of alcohol over the past 3 months as the total typical-day grams (the sum of grams for each drink type) times the total number of drinking days; this is a beverage-specific estimate, hence an adaptation of the standard QF method (Rehm, 1998, Heeb and Gmel, 2005). To calculate FGF grams of alcohol, the total grams of alcohol consumed on a maximum drinking day were used; these estimates were also beverage-specific. The total grams consumed on a maximum day were multiplied by each fraction (1.0, 0.75, 0.5, 0.25) and the number of days that the fraction was consumed; these products were summed into a total over the past 3 months (Greenfield et al., 2010).
Adaptation of quantity frequency and fractional graduated frequency methods to non-volume domains
We adapted QF and FGF methods to expenditure on alcohol, time spent drinking, and symptoms of intoxication as follows. For QF estimates, the typical-day measure for each domain (for example, expenditure, time spent drinking) was multiplied by the total number of drinking days in the past 3 months (Table 1). For FGF estimates, the maximum day measure for each domain was multiplied by the frequencies of drinking at maximum and step down fractions and by the respective fractions (1.0, 0.75, 0.5, 0.25). The resulting estimates were then summed for each domain into a total for the past 3 months.
Laboratory measurements
PEth
PEth is a phospholipid derivative of ethanol metabolism, formed only in the presence of ethanol, which may be present in whole blood for at least 3 weeks after alcohol intake (Aradottir et al., 2006, Hansson et al., 1997). It has a biological specificity close to 100% for recent alcohol use and detects excessive drinking in outpatients with sensitivity of up to 98% (Varga et al., 2000, Isaksson et al., 2011). To measure PEth, venous blood samples were collected from patients by clinic staff and transferred to dried blood spots (DBS) on the same day by laboratory staff. The DBS cards were then stored at −80°C with a small amount of desiccant until shipping. The samples were shipped at room temperature to a commercial laboratory (US Drug Testing Laboratories, Des Plaines, Illinois) and tested using liquid chromatography with tandem mass spectrometry (LC-MS) (Jones et al., 2011). Samples were defined as positive if PEth concentration was above the current limit of quantification (≥8ng/ml). Following the laboratory’s standard operating procedures, positive samples were re-run for two batches of DBS cards (97 samples collected between July 2011 and October 2012 and between July 2013 and September 2013), with the final result being reported as an average of the two results. Positive PEth assays run between October 2012 and July 2013 were not re-run per the laboratory’s standard operating procedures during this time. However, among the 97 retested samples, correlations between first and second runs were high (Spearman’s rank correlation coefficient = 0.94).
Other laboratory tests
Additional tests included CD4+ T-cell counts (Coulter Epics XL.MCL Cytometer, Beckman Coulter, Brea, California) and plasma HIV RNA level (Bayer System 340 bDNA analyzer, Bayer Healthcare Corporation, Whippany, New Jersey). These were performed at the MUST Clinical Research Laboratory, which participates in external quality assurance by the National Health Laboratory Service (Johannesburg, South Africa).
Analysis
We summarized participant characteristics as appropriate. PEth results are presented as medians with inter-quartile range (IQR) and as the proportion above the limit of quantification (≥8ng/ml) by AUDIT-C categories, overall, and among only the self-reported drinkers. We compared PEth concentrations across AUDIT-C categories. We also assessed if PEth concentrations varied by gender and drink type. We tested whether any differences in PEth concentrations in these variables were statistically significant using non-parametric tests of equality of medians (the Wilcoxon rank-sum test for 2-category comparisons, and the Kruskal-Wallis equality of populations test for 3-category comparisons); we used chi-squared tests to compare proportions that were PEth positive across these same groups. To evaluate associations between measures of self-reported alcohol consumption and PEth, we calculated Spearman’s rank correlation coefficients and 95% confidence intervals (CI) between each self-report measure and PEth; we determined whether observed associations differed by measure using 95% bias-corrected bootstrap CIs (based on 1000 bootstrap samples) for pairwise differences in correlation coefficients (95% CIs excluding zero were judged as providing evidence of a significant difference). We analyzed only those subjects with complete information; missing values were few (the highest number in any variable was 17). All analyses were performed in STATA 13 (College Station, Texas).
Results
General characteristics of the study population
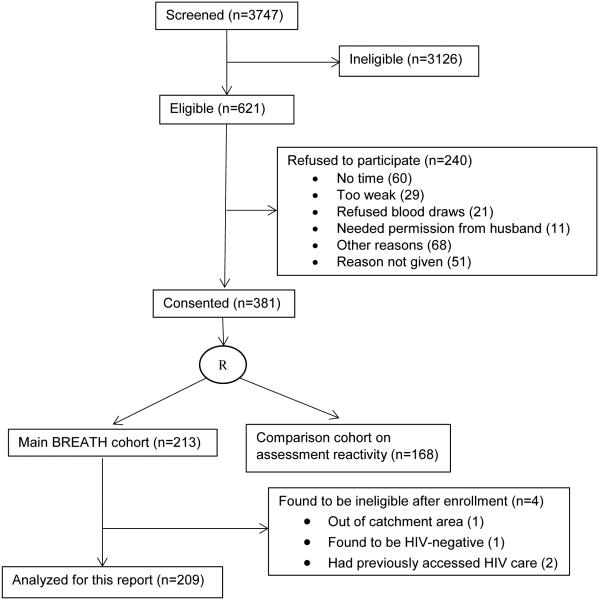
From July 2011 through September 2013, clinic counselors screened 3747 new patients; 621 were eligible for the study (Figure). Sixty-one percent of those eligible (n=381) provided informed consent to participate. Enrolled patients were similar by gender (55% male) to those who were eligible but declined to participate (53% male, p=0.59). Of the 381 consenting, 213 were randomized to the main BREATH cohort (4 were later found to be ineligible, leaving 209 for analysis); 168 were randomized to the comparison cohort examining assessment reactivity. For the 209 participants analyzed for this report, median values were: age 30 (IQR 25 to 38); time since HIV diagnosis 0.3 months (IQR 0.1 to 1.3); CD4+ T-cell count 349 (IQR 221 to 535); and plasma HIV RNA level 1.6 × 104 copies/ml (IQR 0.34 to 8.4) (Table 2).
Figure.
Enrollment flow diagram for the BREATH Cohort study (July 2011 to September 2013) of HIV-infected adults, who were newly enrolled into care (not yet initiated on antiretroviral therapy) at the Immune Suppression Syndrome Clinic in Mbarara, Uganda.
Table 2.
Personal characteristics and drinking patterns of 209 subjects who were interviewed at entry into the BREATH Cohort study (July 2011-September 2013). Participants were HIV-infected adults newly enrolled into care (and not yet on antiretroviral therapy) at the Immune Suppression Syndrome Clinic in Mbarara, Uganda
| Characteristic | |
|---|---|
| Demographic and socioeconomic information | |
| Sex male, n (%) | 120 (57%) |
| Age, median (IQR) | 30 (25 to 38) |
| BMI, median (IQR) | 22 (20-24) |
| Literacy, n (%) | |
| Cannot read at all | 21 (10%) |
| Reads parts of sentence | 24 (12%) |
| Reads whole sentence | 161 (77%) |
| Not assessed | 3 (1.4%) |
| Monthly income in USD, median (IQR) | 40 (20-80) |
| Time in months since HIV diagnosis, median (IQR) | 0.3 (0.1-1.3) |
| Drinking behavior among drinkers, n=169, n(%) | |
| Usual drinking place | |
| Home | 39 (23%) |
| Bar | 130 (77%) |
| Work | 5 (3.0%) |
| Drinking companion | |
| Drinks with friends | 124 (77%) |
| Drinks alone | 20 (12%) |
| Drinks with spouse | 21 (13%) |
| Typical alcohol production type* | |
| Commercial alcohol only | 111 (66%) |
| Local alcohol only | 36 (21%) |
| Both types | 22 (13%) |
| Consumption of spirits† | |
| Drank any spirits | 69 (41%) |
| Did not drink any spirits | 100 (59%) |
| Frequency of alcohol consumption in the last 3 months, n (%) |
|
| No alcohol | 40 (19%) |
| Monthly or less | 58 (28%) |
| 2-4 times/month | 30 (14%) |
| 2-3 times/week | 57 (27%) |
| 4+ times a week | 24 (12%) |
| Number of drinking days in the past 3 months, median (IQR) |
7.5 (1.5-19) |
| AUDIT-C score, median (IQR) | 3 (1-4) |
| AUDIT-C-score risk categories‡, n (%) | |
| Unhealthy drinkers | 93 (45%) |
| Lower-risk drinkers | 76 (36%) |
| Abstainers | 40 (19%) |
|
Self-report alcohol quantities, in past 3 months, median
(IQR) |
|
| Grams of alcohol | |
| Grams on a typical drinking day | 41 (20-79) |
| Grams on maximum drinking days | 59 (20-99) |
| Total grams by the FGF method | 592 (43-2137) |
| Total grams by the QF method | 375 (33-1776) |
| Expenditure on alcohol ($), median (IQR) | |
| Maximum-day expenditure on any alcohol | 2 (0.72-4) |
| Typical-day-expenditure on any alcohol | 1.8 (0.56-3) |
| Total expenditure on alcohol by the FGF method | 19 (2.1-65) |
| Total expenditure by the QF method | 14 (1.8-57) |
| Sum of intoxication symptoms, median (IQR) | |
| FGF method | 19 (3-64) |
| QF method | 15 (1.5-57) |
| Total time spent drinking, hours, median (IQR) | |
| FGF method | 19 (2.0-74) |
| QF method | 9.5 (0.75-38) |
| Laboratory measurements | |
| CD4+ T-cell counts, median (IQR) | 349 (221-535) |
| Plasma HIV RNA PCR level , IU/ml × 104, median (IQR) | 1.6 (0.34-8.4) |
| PEth results, ng/ml, median (IQR) | 57 (0-211) |
AUDIT-C: alcohol use disorders identification test-consumption, FGF: Fractional graduated frequency, IQR: interquartile range, QF: Quantity Frequency, PEth: phosphpatidyl ethanol
Refers to whether or not the alcohol typically drank was commercially produced or locally produced irrespective of whether the drink was considered a spirit, a beer, or a wine.
Refers to whether or not participants reported drinking any spirits.
Unhealthy drinking = AUDIT-C score ≥4 for men or ≥3 for women; low risk drinking = AUDIT-C score <4 for men or <3 for women.
Drinking patterns and quantity estimates
By self-report, nearly half of the participants (45%) were unhealthy drinkers in the past 3 months. Among all self-reported drinkers (n = 169), the majority (77%) drank from bars and typically drank commercial beer (66%). In general, the FGF method yielded higher estimates than the QF method (Table 2). For example, median FGF grams of alcohol over 90 days was 592 (IQR 43 to 2137), which was higher (p<0.001) than 375 (IQR 33 to 1776), the median QF grams of alcohol.
PEth values
The median PEth concentration for the entire sample was 57 ng/ml (IQR 0 to 221). PEth concentrations increased across AUDIT-C categories (for example median concentration was 32 ng/ml, IQR 0-133, in all low-risk drinkers versus 133 ng/ml, IQR 46-412, in all high risk drinkers, P <0.001). Also, proportions that were PEth positive increased across AUDIT-C categories (for example 68% in all low-risk drinkers versus 90% in all high-risk drinkers, P <0.001) (Table 3). PEth concentrations were higher in males (median = 112 ng/ml, IQR 15-326) compared to females (median = 19 ng/ml, IQR 0-84, P<0.001). Among self-reported drinkers, those drinking any spirits had higher PEth concentrations (median = 156 ng/ml, IQR 21-411) than those not drinking any spirits (median = 57 ng/ml, IQR 15-148, P = 0.0029); those drinking locally-made alcohols also had higher PEth concentrations. For example, median PEth concentration was 217 ng/ml, IQR 26-440, in those drinking only locally-made alcohols versus 60 ng/ml, IQR 13-170, P = 0.0146, for those drinking only commercial alcohols.
Table 3.
PEth levels and proportions with detectable PEth at cohort entry, presented overall, and by drinker- and drink-types in 209 enrollees in the BREATH Cohort study (July 2011-September 2013). The table show median PEth levels and interquartile ranges and proportions that were PEth positive for participants grouped according to their characteristics. Participants were HIV-infected adults, newly enrolled into care (and not yet on antiretroviral therapy) at the Immune Suppression Syndrome Clinic in Mbarara, Uganda.
| PEth concentration, ng/ml, median (IQR) |
Proportion PEth- positive |
|
|---|---|---|
| Full sample (n=209) | ||
| All subjects | 57 (0-211) | 70% |
| No drinking in past 3 months | 0 (0-8.5) | 25% |
| Low risk drinkers* | 32 (0-133) | 68% |
| Unhealthy drinkers | 133 (46-412) | 90% |
| Females only(n=89) | ||
| All females | 19 (0-84) | 56% |
| No drinking in past 3 months | 0 (0-0) | 0% |
| Low risk drinkers | 16 (0-68) | 57% |
| Unhealthy drinkers | 82 (32-170) | 89% |
| Males only (n=120) | ||
| All males | 112 (15-326) | 80% |
| No drinking in past 3 months | 15 (0-113) | 59% |
| Low risk drinkers | 63 (7.5-187) | 75% |
| Unhealthy drinkers | 257 (71-554) | 91% |
|
Stratified by beverage production and type (drinkers
only) |
||
| Beverage production type | ||
| Locally-made alcohol only drinkers (n=36) | 217 (26-440) | 83% |
| Commercially made alcohol only drinkers (n=111) | 60 (13-170) | 78% |
| Both types | 82 (57-304) | 86% |
| Consumption of spirits | ||
| Drank any spirits (n=69) | 156 (21-411) | 86% |
| Did not drink any spirits (n=100) | 57 (15-148) | 77% |
Unhealthy drinking = Alcohol Use Disorders Identification Test – Consumption (AUDIT-C) score ≥4 for men or ≥3 for women; low risk drinking = AUDIT-C score <4 for men or <3 for women.
Correlation of self-reported grams of alcohol with PEth
Both FGF grams (rho = 0.58, 95% CI 0.47-0.66) and QF grams (rho = 0.54, 95% CI 0.43-0.63) of alcohol were only moderately correlated with PEth concentration; the difference between the correlation coefficients was not statistically significant (95% CI of estimated difference = −0.017 to 0.099). Restricting the analysis to current (past 3 months) drinkers did not improve correlations with PEth: rho = 0.48, 95% CI 0.35 to 0.59 for FGF grams versus rho = 0.44, 95% CI 0.30 to 0.55 for QF grams; the difference between these coefficients was also not statistically significant (95% CI of the difference = −0.046 to 0.144) (Table 4).
Table 4.
Spearman’s rank correlation coefficients and 95% confidence intervals (CI) between self-report measures of drinking and PEth concentration overall, and among current drinkers only, for 209 HIV-infected adults, who participated in the BREATH Cohort study (July 2011-September 2013) at the Immune Suppression Syndrome Clinic in Mbarara, Uganda. The table shows coefficients and 95% CIs for the correlation of FGF measures, QF measures, and two screeners, that is, AUDIT-C and number of drinking days with PEth.
| Measure | Full Sample (n=209) PEth concentration |
Self-reported drinkers (n=169) PEth concentration |
||
|---|---|---|---|---|
| rho | 95% CI | rho | 95% CI | |
| Grams of alcohol | ||||
| FGF | 0.58 | 0.47-0.66 | 0.48 | 0.35-0.59 |
| QF-BS | 0.54 | 0.43-0.63 | 0.44 | 0.30-0.55 |
| Expenditure on alcohol | ||||
| FGF | 0.52 | 0.40-0.61 | 0.37 | 0.22-0.50 |
| QF | 0.44 | 0.32-0.55 | 0.25 | 0.10-0.39 |
| Intoxication | ||||
| FGF | 0.56 | 0.46-0.65 | 0.45 | 0.32-0.57 |
| QF | 0.49 | 0.38-0.57 | 0.35 | 0.21-0.48 |
| Time drinking | ||||
| FGF | 0.54 | 0.43-0.63 | 0.43 | 0.29-0.55 |
| QF | 0.50 | 0.39-0.59 | 0.37 | 0.23-0.50 |
| Number of drinking days | 0.49 | 0.38-0.59 | 0.36 | 0.22-0.49 |
| AUDIT-C score | 0.57 | 0.47-0.65 | 0.48 | 0.35-0.59 |
FGF: Fractional graduated frequency, QF: Quantity frequency
Correlation of other measures of alcohol consumption with PEth
Among the non-volume measures, only expenditure on alcohol (rho for FGF expenditure = 0.52, 95% CI 0.40 to 0.61) had a lower correlation with PEth than FGF grams of alcohol (95% CI for the difference = 0.009 to 0.12); symptoms of intoxication and time spent drinking had similar correlations with PEth as was grams of alcohol. For all measures, any differences correlations with PEth between FGF and QF measures were not statistically significant. The correlation of AUDIT-C with PEth (rho = 0.57, 95% 0.47 to 0.65 overall) was similar to the correlation of FGF grams of alcohol with PEth (95% of estimated difference = −0.078 to 0.069, not statistically significant) (Table 4).
Discussion
In SSA, self-reported measurement of alcohol consumption is complicated by the lack of standard drinks. In particular, when measured using self-report, drinking may be underestimated in those with HIV infection, in part because drinking in this population is stigmatized and may be under-reported. Among ART-naive HIV-infected adults, we assessed correlations of multiple self-report measures of alcohol consumption with the alcohol biomarker PEth. The correlations were moderate (rho = 0.44 to 0.58) and lower than those observed in our prior study (0.65 to 0.74), which aimed to characterize PEth (Hahn et al., 2012a), and were not improved by restriction to self-reported past 3 months drinkers. We interpret these findings to mean that HIV-infected drinkers in this setting under-report both any alcohol intake and amounts consumed. Our findings highlight the need for increased use of objective measures such as biomarkers (Greenfield et al., 2014) to determine and quantify alcohol intake in this setting.
As both under-reporting and over-reporting are possible, self-reports may under-estimate (Hahn et al., 2012b, Bajunirwe et al., 2014) or over-estimate (Gmel et al., 2006) true alcohol consumption. In our data, FGF estimates were higher than QF ones. However, since both measures were only moderately correlated with PEth, we suspect the moderate correlations to be due to underreporting. In our previous study where we found higher correlations with PEth, self-reports had been corroborated by daily home or drinking establishment visits during which we carried out drinking surveys and breathalyzer tests and interviewed friends/relatives of the study participants to obtain a collateral report of the participant’s drinking (Hahn et al., 2012a). Aware of such additional measures, patients may have reported more truthfully.
Under-reporting is common in populations where drinking is prohibited, among HIV-infected patients (Bilal et al., 1990, Hormes et al., 2012), and in SSA (Michalak and Trocki, 2009). New HIV patients are especially likely to under-report drinking in fear of being denied ART (Sorsdahl et al., 2012, Papas et al., 2012). In our study, 25% of those self-reporting as current abstainers were PEth-positive; they were all male, consistent with prior findings of underreport by males starting ART in Uganda (Bajunirwe et al., 2014). Using shorter reference periods such as 21 or 30 days can aid recall (Ekholm et al., 2011) and possibly improve correlations (Bajunirwe et al., 2014). However, shorter reference periods can also reduce sensitivity of self-reports when drinking patterns are irregular (Rehm et al., 1999).
While these findings suggest that more objective measures such as biomarkers should be used to measure drinking in these patients, alcohol biomarkers like PEth remain inaccessible in resource-limited settings in terms of both cost and technology. Development of less expensive and/or simpler assays is required. Also, attempts should be made to improve self-reports. For example, approaches such as the Audio-guided Computer-Assisted Self-Interview (Simoes et al., 2006) which use technology to obtain self-reports of drinking should be considered. Low computer/technology literacy in this setting may affect the utility of such methods. However, these methods could reduce the pressure on patients to give socially desirable drinking reports.
FGF estimates were consistently higher than QF estimates. This is consistent with the hypothesis that FGF methods more accurately estimate consumption when drinking patterns are irregular (Greenfield, 2000, Greenfield et al., 2009, Greenfield et al., 2010, Rehm et al., 2003). Also, QF approaches may be less accurate when heavy drinking is stigmatized; patients may try to “normalize” high levels of consumption via underreporting in response to questions about “typical intake” (Greenfield and Kerr, 2008).
We found non-volume measures of drinking such as time spent drinking and a scale of symptoms of intoxication, as well as the AUDIT-C, to have similar correlations with PEth as FGF and QF measures of grams of alcohol. As they are substantially easier to calculate than the beverage-specific grams of alcohol, these measures may provide a simpler alternative to measuring drinking in this setting. Non-volume measures also may aid recall; heavy drinkers can forget volumes (Northcote and Livingston, 2011), but may, in theory, be more likely to remember their expenditure (Papas et al., 2010a) or degree of intoxication. In particular, the AUDIT-C, FGF grams of alcohol, and QF grams of alcohol had similar correlations with PEth. Given that FGF and QF grams of alcohol were more rigorous and were beverage-type adjusted to account for the lack of standard drinks in this setting, this finding suggests the robustness of the AUDIT-C measure.
Asking about specific drink types when measuring drinking can increase the accuracy of volume estimations (Feunekes et al., 1999, Greenfield et al., 2010). In our data, a drink-type-adjusted QF measure of grams of alcohol correlated similarly with PEth as the FGF measure. This finding suggests that drink type information may improve measure performance in this setting. We also observed that patients who drank locally-made alcohols (versus commercially-made alcohols) and those who drank any spirits (versus not drinking any spirits) had higher PEth concentrations, suggesting that drink type may independently predict unhealthy drinking and/or alcohol-associated clinical outcomes (Razvodovsky, 2015).
Our findings have some limitations. PEth is not a perfect gold-standard. For example, 10% of self-reported unhealthy drinkers were PEth-negative, consistent with previously reported estimates of PEth sensitivity for measuring unhealthy drinking (61-91%) (Hahn et al., 2012a, Stewart et al., 2014, Stewart et al., 2010). Also, 25% of abstainers were PEth-positive; these however are likely to have under-reported. In theory, PEth only forms in presence of ethanol and has near-perfect specificity (Aradottir et al., 2006). A remote possibility is that positive tests could result from over-the-counter medications containing ethanol such as cough syrup. This possibility, a common source of controversy in failed drug tests (Skipper et al., 2013), has not been investigated in relation to PEth, and PEth is usually detectable only with high amounts of alcohol that are unlikely to be in these over-the-counter products. Our estimates of grams of alcohol also are based on average ethanol contents; yet substantial variations may exist, especially in the locally-made alcohols. Finally, as drinks are not always standard in this setting, our AUDIT-C estimates may be less accurate than those among patients in resource-rich settings. However, despite this limitation, the correlation of AUDIT-C estimates with PEth was similar to the correlation of FGF and QF grams of alcohol with PEth suggesting robustness of the AUDIT-C even in settings where drinks are not easily standardized.
Our findings apply mainly to HIV-infected persons since they are more likely to underreport alcohol consumption in fear of being denied services such as ART. However, we expect our findings to be applicable to other groups such as adolescents where drinking may also be stigmatized and/or prohibited. The strength of our study is that we focus on the HIV-infected, especially those drinking at less than dependent levels. Compared to their HIV-negative counterparts, HIV-infected, less-than-dependent-drinkers are more accessible via structured HIV treatment programs. Interventions in this group may be integrated into routine HIV care and may reduce overall risk of HIV transmission. As heavy drinking is an important comorbidity in HIV-infected patients, interventions to reduce drinking may also improve HIV treatment outcomes. It is therefore important that alcohol consumption be measured more accurately in this population.
In conclusion, among HIV-infected past year drinkers in Uganda, multiple self-report measures of alcohol intake were only moderately correlated with the alcohol biomarker PEth. Our findings suggest the need for increased use of objective measures like biomarkers to measure alcohol consumption in this setting and, as biomarker tests are expensive and inaccessible, the development of less expensive and simpler assays. Future studies may also attempt to improve existing self-report measures. For example, using FGF methods may lead to higher total estimates of alcohol consumption as these methods better capture variability in drinking patterns. Alternative methods of reporting like self-administered surveys may also reduce socially desirable reporting. Using simpler and/or non-volume measures, which, in our study, showed similar performance as the more complex measures, could facilitate the implementation of such surveys. Finally, future studies should assess how different measures of drinking predict clinical outcomes in this setting; ultimately, measures that predict clinical outcomes would be preferred.
Acknowledgements
Funding was provided by the National Institutes of Health, R01 AA018631, U01AA020776, K24 AA022586, P50 AA005595 (ARG, PHI), and P30 DK026743 (UCSF Liver Center).
Funding: National Institutes of Health, R01 AA018631, U01 AA020776, P50 AA005595, and K24 AA022586
Footnotes
Conflicts of interest
The authors have no conflicts of interest to declare.
REFERENCES
- ARADOTTIR S, ASANOVSKA G, GJERSS S, HANSSON P, ALLING C. PHosphatidylethanol (PEth) concentrations in blood are correlated to reported alcohol intake in alcohol-dependent patients. Alcohol Alcohol. 2006;41:431–7. doi: 10.1093/alcalc/agl027. [DOI] [PubMed] [Google Scholar]
- BAJUNIRWE F, HABERER JE, BOUM Y, 2ND, HUNT P, MOCELLO R, MARTIN JN, BANGSBERG DR, HAHN JA. Comparison of Self-Reported Alcohol Consumption to Phosphatidylethanol Measurement among HIV-Infected Patients Initiating Antiretroviral Treatment in Southwestern Uganda. PLoS One. 2014;9:e113152. doi: 10.1371/journal.pone.0113152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BILAL AM, MAKHAWI B, AL-FAYEZ G, SHALTOUT AF. Attitudes of a sector of the Arab-Muslim population in Kuwait towards alcohol and drug misuse: an objective appraisal. Drug Alcohol Depend. 1990;26:55–62. doi: 10.1016/0376-8716(90)90083-q. [DOI] [PubMed] [Google Scholar]
- BRADLEY KA, DEBENEDETTI AF, VOLK RJ, WILLIAMS EC, FRANK D, KIVLAHAN DR. AUDIT-C as a brief screen for alcohol misuse in primary care. Alcohol Clin Exp Res. 2007;31:1208–17. doi: 10.1111/j.1530-0277.2007.00403.x. [DOI] [PubMed] [Google Scholar]
- BRAITHWAITE RS, NUCIFORA KA, KESSLER J, TOOHEY C, MENTOR SM, UHLER LM, ROBERTS MS, BRYANT K. Impact of interventions targeting unhealthy alcohol use in Kenya on HIV transmission and AIDS-related deaths. Alcohol Clin Exp Res. 2014;38:1059–67. doi: 10.1111/acer.12332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CLIFFORD PR, MAISTO SA, DAVIS CM. Alcohol treatment research assessment exposure subject reactivity effects: part I. Alcohol use and related consequences. J Stud Alcohol Drugs. 2007;68:519–28. doi: 10.15288/jsad.2007.68.519. [DOI] [PubMed] [Google Scholar]
- DAWSON DA, ROOM R. Towards agreement on ways to measure and report drinking patterns and alcohol-related problems in adult general population surveys: the Skarpo conference overview. J Subst Abuse. 2000;12:1–21. doi: 10.1016/s0899-3289(00)00037-7. [DOI] [PubMed] [Google Scholar]
- EKHOLM O, STRANDBERG-LARSEN K, GRONBAEK M. Influence of the recall period on a beverage-specific weekly drinking measure for alcohol intake. Eur J Clin Nutr. 2011;65:520–5. doi: 10.1038/ejcn.2011.1. [DOI] [PubMed] [Google Scholar]
- FEUNEKES GI, VAN 'T VEER P, VAN STAVEREN WA, KOK FJ. Alcohol intake assessment: the sober facts. Am J Epidemiol. 1999;150:105–12. doi: 10.1093/oxfordjournals.aje.a009909. [DOI] [PubMed] [Google Scholar]
- GMEL G, GRAHAM K, KUENDIG H, KUNTSCHE S. Measuring alcohol consumption--should the 'graduated frequency' approach become the norm in survey research? Addiction. 2006;101:16–30. doi: 10.1111/j.1360-0443.2005.01224.x. [DOI] [PubMed] [Google Scholar]
- GREENFIELD T, BOND J, KERR W. Biomonitoring for improving alcohol consumption surveys: the new gold standard? Alcohol Research: Current Reviews. 2014 doi: 10.35946/arcr.v36.1.05. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GREENFIELD TK. Ways of measuring drinking patterns and the difference they make: experience with graduated frequencies. J Subst Abuse. 2000;12:33–49. doi: 10.1016/s0899-3289(00)00039-0. [DOI] [PubMed] [Google Scholar]
- GREENFIELD TK, KERR WC. Alcohol measurement methodology in epidemiology: recent advances and opportunities. Addiction. 2008;103:1082–99. doi: 10.1111/j.1360-0443.2008.02197.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GREENFIELD TK, KERR WC, BOND J, YE Y, STOCKWELL T. Graduated Frequencies alcohol measures for monitoring consumption patterns: Results from an Australian national survey and a US diary validity study. Contemp Drug Probl. 2009;36 doi: 10.1177/009145090903600320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GREENFIELD TK, NAYAK MB, BOND J, PATEL V, TROCKI K, PILLAI A. Validating alcohol use measures among male drinkers in Goa: implications for research on alcohol, sexual risk, and HIV in India. AIDS Behav. 2010;14(Suppl 1):S84–93. doi: 10.1007/s10461-010-9734-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAHN JA, BWANA MB, JAVORS MA, MARTIN JN, EMENYONU NI, BANGSBERG DR. Biomarker testing to estimate under-reported heavy alcohol consumption by persons with HIV initiating ART in Uganda. AIDS Behav. 2010;14:1265–8. doi: 10.1007/s10461-010-9768-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAHN JA, DOBKIN LM, MAYANJA B, EMENYONU NI, KIGOZI IM, SHIBOSKI S, BANGSBERG DR, GNANN H, WEINMANN W, WURST FM. Phosphatidylethanol (PEth) as a biomarker of alcohol consumption in HIV-positive patients in sub-Saharan Africa. Alcohol Clin Exp Res. 2012a;36:854–62. doi: 10.1111/j.1530-0277.2011.01669.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAHN JA, FATCH R, KABAMI J, MAYANJA B, EMENYONU NI, MARTIN J, BANGSBERG DR. Self-Report of Alcohol Use Increases When Specimens for Alcohol Biomarkers Are Collected in Persons With HIV in Uganda. J Acquir Immune Defic Syndr. 2012b;61:e63–4. doi: 10.1097/QAI.0b013e318267c0f1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAHN JA, WOOLF-KING SE, MUYINDIKE W. Adding fuel to the fire: alcohol's effect on the HIV epidemic in Sub-Saharan Africa. Curr HIV/AIDS Rep. 2011;8:172–80. doi: 10.1007/s11904-011-0088-2. [DOI] [PubMed] [Google Scholar]
- HANSSON P, CARON M, JOHNSON G, GUSTAVSSON L, ALLING C. Blood phosphatidylethanol as a marker of alcohol abuse: levels in alcoholic males during withdrawal. Alcohol Clin Exp Res. 1997;21:108–10. [PubMed] [Google Scholar]
- HEEB JL, GMEL G. Measuring alcohol consumption: a comparison of graduated frequency, quantity frequency, and weekly recall diary methods in a general population survey. Addict Behav. 2005;30:403–13. doi: 10.1016/j.addbeh.2004.04.022. [DOI] [PubMed] [Google Scholar]
- HENDERSHOT CS, STONER SA, PANTALONE DW, SIMONI JM. Alcohol use and antiretroviral adherence: review and meta-analysis. J Acquir Immune Defic Syndr. 2009;52:180–202. doi: 10.1097/QAI.0b013e3181b18b6e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HORMES JM, GERHARDSTEIN KR, GRIFFIN PT. Under-reporting of alcohol and substance use versus other psychiatric symptoms in individuals living with HIV. AIDS Care. 2012;24:420–3. doi: 10.1080/09540121.2011.608795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ISAKSSON A, WALTHER L, HANSSON T, ANDERSSON A, ALLING C. Phosphatidylethanol in blood (B-PEth): a marker for alcohol use and abuse. Drug Test Anal. 2011;3:195–200. doi: 10.1002/dta.278. [DOI] [PubMed] [Google Scholar]
- JAIN J, EVANS JL, BRICENO A, PAGE K, HAHN JA. Comparison of phosphatidylethanol results to self-reported alcohol consumption among young injection drug users. Alcohol Alcohol. 2014;49:520–4. doi: 10.1093/alcalc/agu037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JONES J, JONES M, PLATE C, LEWIS D. The detection of 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphoethanol in human dried blood spots. Analytical Methods. 2011:1101–1106. [Google Scholar]
- MICHALAK L, TROCKI K. Comments on surveying alcohol in Africa. Addiction. 2009;104:1155–6. doi: 10.1111/j.1360-0443.2009.02628.x. [DOI] [PubMed] [Google Scholar]
- MWESIGYE P, OKURUT T. A Survey of the Production and Consumption of Traditional Alcoholic Beverages in Uganda. Process Biochemistry. 1994;30:497–501. [Google Scholar]
- NORTHCOTE J, LIVINGSTON M. Accuracy of self-reported drinking: observational verification of 'last occasion' drink estimates of young adults. Alcohol Alcohol. 2011;46:709–13. doi: 10.1093/alcalc/agr138. [DOI] [PubMed] [Google Scholar]
- PAPAS RK, GAKINYA BN, BALIDDAWA JB, MARTINO S, BRYANT KJ, MESLIN EM, SIDLE JE. Ethical issues in a stage 1 cognitive-behavioral therapy feasibility study and trial to reduce alcohol use among HIV-infected outpatients in western Kenya. J Empir Res Hum Res Ethics. 2012;7:29–37. doi: 10.1525/jer.2012.7.3.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PAPAS RK, SIDLE JE, MARTINO S, BALIDDAWA JB, SONGOLE R, OMOLO OE, GAKINYA BN, MWANIKI MM, ADINA JO, NAFULA T, OWINO-ONG'OR WD, BRYANT KJ, CARROLL KM, GOULET JL, JUSTICE AC, MAISTO SA. Systematic cultural adaptation of cognitive-behavioral therapy to reduce alcohol use among HIV-infected outpatients in western Kenya. AIDS Behav. 2010a;14:669–78. doi: 10.1007/s10461-009-9647-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PAPAS RK, SIDLE JE, WAMALWA ES, OKUMU TO, BRYANT KL, GOULET JL, MAISTO SA, BRAITHWAITE RS, JUSTICE AC. Estimating alcohol content of traditional brew in Western Kenya using culturally relevant methods: the case for cost over volume. AIDS Behav. 2010b;14:836–44. doi: 10.1007/s10461-008-9492-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PELTZER K, SIMBAYI L, KALICHMAN S, JOOSTE S, CLOETE A, MBELLE Alcohol Use in Three Different Inner Cities in South Africa: AUDIT-C and CAGE. Journal of Psychology in Africa. 2007:1–2. [Google Scholar]
- RAZVODOVSKY YE. The effect of beverage type on alcoholic psychoses rate in Russia. Alcohol Alcohol. 2015;50:200–5. doi: 10.1093/alcalc/agu104. [DOI] [PubMed] [Google Scholar]
- REHM J. Measuring quantity, frequency, and volume of drinking. Alcohol Clin Exp Res. 1998;22:4S–14S. doi: 10.1097/00000374-199802001-00002. [DOI] [PubMed] [Google Scholar]
- REHM J, GREENFIELD TK, WALSH G, XIE X, ROBSON L, SINGLE E. Assessment methods for alcohol consumption, prevalence of high risk drinking and harm: a sensitivity analysis. Int J Epidemiol. 1999;28:219–24. doi: 10.1093/ije/28.2.219. [DOI] [PubMed] [Google Scholar]
- REHM J, REHN N, ROOM R, MONTEIRO M, GMEL G, JERNIGAN D, FRICK U. The global distribution of average volume of alcohol consumption and patterns of drinking. Eur Addict Res. 2003;9:147–56. doi: 10.1159/000072221. [DOI] [PubMed] [Google Scholar]
- RUBINSKY AD, DAWSON DA, WILLIAMS EC, KIVLAHAN DR, BRADLEY KA. AUDIT-C scores as a scaled marker of mean daily drinking, alcohol use disorder severity, and probability of alcohol dependence in a U.S. general population sample of drinkers. Alcohol Clin Exp Res. 2013;37:1380–90. doi: 10.1111/acer.12092. [DOI] [PubMed] [Google Scholar]
- SAITZ R. Clinical practice. Unhealthy alcohol use. N Engl J Med. 2005;352:596–607. doi: 10.1056/NEJMcp042262. [DOI] [PubMed] [Google Scholar]
- SIMOES AA, BASTOS FI, MOREIRA RI, LYNCH KG, METZGER DS. A randomized trial of audio computer and in-person interview to assess HIV risk among drug and alcohol users in Rio De Janeiro, Brazil. J Subst Abuse Treat. 2006;30:237–43. doi: 10.1016/j.jsat.2005.12.002. [DOI] [PubMed] [Google Scholar]
- SKIPPER GE, THON N, DUPONT RL, BAXTER L, WURST FM. Phosphatidylethanol: the potential role in further evaluating low positive urinary ethyl glucuronide and ethyl sulfate results. Alcohol Clin Exp Res. 2013;37:1582–6. doi: 10.1111/acer.12121. [DOI] [PubMed] [Google Scholar]
- SORSDAHL K, STEIN DJ, MYERS B. Negative attributions towards people with substance use disorders in South Africa: variation across substances and by gender. BMC Psychiatry. 2012;12:101. doi: 10.1186/1471-244X-12-101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STEWART SH, KOCH DG, WILLNER IR, ANTON RF, REUBEN A. Validation of blood phosphatidylethanol as an alcohol consumption biomarker in patients with chronic liver disease. Alcohol Clin Exp Res. 2014;38:1706–11. doi: 10.1111/acer.12442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STEWART SH, LAW TL, RANDALL PK, NEWMAN R. Phosphatidylethanol and alcohol consumption in reproductive age women. Alcohol Clin Exp Res. 2010;34:488–92. doi: 10.1111/j.1530-0277.2009.01113.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STRAUSS SM, RINDSKOPF DM. Screening patients in busy hospital-based HIV care centers for hazardous and harmful drinking patterns: the identification of an optimal screening tool. J Int Assoc Physicians AIDS Care (Chic) 2009;8:347–53. doi: 10.1177/1545109709350509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SUNDARARAJAN R, WYATT MA, WOOLF-KING S, PISARSKI EE, EMENYONU N, MUYINDIKE WR, HAHN JA, WARE NC. Qualitative Study of Changes in Alcohol Use Among HIV-Infected Adults Entering Care and Treatment for HIV/AIDS in Rural Southwest Uganda. AIDS Behav. 2014 doi: 10.1007/s10461-014-0918-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- UNAIDS UNAIDS report on the global AIDS epidemic 2013. 2013.
- VARGA A, HANSSON P, JOHNSON G, ALLING C. Normalization rate and cellular localization of phosphatidylethanol in whole blood from chronic alcoholics. Clin Chim Acta. 2000;299:141–50. doi: 10.1016/s0009-8981(00)00291-6. [DOI] [PubMed] [Google Scholar]
- WHO . Global Status Report on Alcohol and Health 2011. The World Health Organization; Geneva, Switzerland: 2011. [Google Scholar]
- WHO Process of translation and adaptation of instruments. 2015 Available: http://www.who.int/substance_abuse/research_tools/translation/en/ [Accessed 02/01/2011]
- WILSNACK RW, WILSNACK SC, KRISTJANSON AF, VOGELTANZ-HOLM ND, GMEL G. Gender and alcohol consumption: patterns from the multinational GENACIS project. Addiction. 2009;104:1487–500. doi: 10.1111/j.1360-0443.2009.02696.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WOOLF-KING SE, FATCH R, EMENYONU N, MUYINDIKE W, CARRICO AW, MAISTO SA, HAHN JA. Development and Validation of the East Africa Alcohol Expectancy Scale (AFEXS) J Stud Alcohol Drugs. 2015;76:336–43. doi: 10.15288/jsad.2015.76.336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WOOLF-KING SE, STEINMAUS CM, REINGOLD AL, HAHN JA. An update on alcohol use and risk of HIV infection in sub-Saharan Africa: Meta-analysis and future research directions. International Journal of Alcohol and Drug Research. 2013;2:99–110. [Google Scholar]