Abstract
Background
Combined treatment with a selective serotonin reuptake inhibitor (SSRI) plus mirtazapine has shown superior efficacy in some studies of depression but has not been studied in posttraumatic stress disorder (PTSD). This study aimed to assess acceptability of combined sertraline plus mirtazapine treatment for PTSD and to estimate its effect size relative to sertraline plus placebo.
Methods
Thirty-six adults with PTSD were randomized to 24 weeks of double-blind treatment with sertraline plus mirtazapine or sertraline plus placebo. Outcomes were analyzed with mixed effects models.
Results
The combined treatment group showed a significantly greater remission rate (P = 0.042) and improvement in depressive symptoms (P = 0.023) than the sertraline plus placebo group. There were no significant group differences in the two primary outcomes of treatment retention and PTSD severity, or in other secondary outcomes (sleep impairment, sexual functioning, quality of life, and physical and mental functioning), but the combined treatment group showed numerical advantages on all of these outcomes, and effect sizes relative to sertraline plus placebo ranged from small to moderate (d = 0.26 - 0.63). Both treatments were well-tolerated, with significantly increased appetite but not weight gain in the combined treatment group.
Discussion
Findings suggest that combined treatment of PTSD with sertraline plus mirtazapine may have clinically meaningful advantages in symptomatic improvement, relative to SSRI treatment alone, and acceptable tolerability.
Conclusion
Combined treatment with an SSRI plus mirtazapine in PTSD deserves additional study as initial treatment or as an augmentation strategy for nonresponders to an SSRI.
Clinicaltrials.gov Identifier
Keywords: Posttraumatic stress disorder, Anxiety, Psychopharmacology, Randomized clinical trial, antidepressants, serotonin, norepinephrine, combined treatment
Introduction
Posttraumatic stress disorder (PTSD) is a serious, chronic, and debilitating illness, with a lifetime prevalence of 5-7%.1 PTSD develops in over 15% of persons who have been exposed to a traumatic event,2 and risk factors may include Hispanic ethnicity or other minority status.3,4 Sufferers experience high comorbidity with mood, anxiety, and substance use disorders,1,5 and an increased risk of suicide,6,7 as well as interpersonal difficulties, work impairment, and lower quality of life.1,7 The best-established treatments for PTSD are cognitive behavioral therapies, but these are sometimes unavailable or not fully effective.8
Selective serotonin reuptake inhibitors (SSRIs) are the best-established medication treatment for PTSD, based on more than a dozen randomized controlled trials (RCTs), but they are ineffective for many patients. For example, in large multisite trials of paroxetine and sertraline, only medications with an FDA indication for PTSD, response rates ranged from 53-62%, and remission rates 23-30%.8,9 Drawbacks to SSRI treatment include delayed response, poor benefit for insomnia, and sexual adverse effects.10-12 These limitations may contribute to substantial dropout rates of over 30% in PTSD sertraline trials12-13 and PTSD pharmacotherapy trials in general,14 and reduced likelihood of receiving the longer-term SSRI treatment that has been associated with higher rates of response.15,16 More efficacious treatments for PTSD are sorely needed.
Mirtazapine, a tetracyclic antidepressant, enhances serotonergic neurotransmission indirectly via antagonism at presynaptic α-2-adrenoreceptors, and blocks postsynaptic serotonin 5-HT2 and 5-HT3, and histamine H1 receptors.17 Preclinical data suggest that mirtazapine and SSRIs may have additive or synergistic effects.18 Several RCTs of mirtazapine plus SSRI treatment in major depressive disorder (MDD) and obsessive-compulsive disorder have found the combined treatment to be more efficacious than SSRI alone19-21 or to have more rapid response and less sexual dysfunction,22 although a large single-blind study did not find combined mirtazapine plus the SNRI venlafaxine to be superior to escitalopram monotherapy for depression.23 In PTSD, a single retrospective open label study has suggested efficacy of mirtazapine augmentation for sleep impairment.24 Together, these findings suggest that combined mirtazapine plus SSRI treatment might accelerate response in PTSD, improve insomnia, and minimize sexual side effects, relative to SSRI monotherapy. Such benefits, if present, might reduce the high dropout rates that have plagued SSRI treatments of PTSD and could contribute to greater efficacy.
The goal of this randomized clinical trial was to assess the acceptability and safety of combined mirtazapine-SSRI treatment and to estimate effect sizes for symptomatic improvement relative to SSRI monotherapy, in a culturally diverse sample of civilians with PTSD. We hypothesized that combined treatment from the outset would improve treatment retention and reduction of PTSD symptoms compared to treatment with SSRI plus placebo. Secondary outcomes were response and remission rates, and measures of sleep quality, sexual functioning, depression, physical and mental functioning, and quality of life. We selected sertraline as the SSRI for this study because it has demonstrated efficacy for PTSD,12,13 and as a weak inhibitor of CYP2D6,25 it is unlikely to have significant pharmacokinetic interactions with mirtazapine. A time frame of 24 weeks was selected because prior pharmacotherapy studies in PTSD have found that symptomatic improvement commonly continues to increase after the typical 8-12 week course of an acute trial.15,16
Materials and Methods
Design
This double-blind randomized controlled trial was conducted from January 2011 to February 2014. To acquire a diverse sample, outpatients were recruited at an academic medical center and at a private mental health clinic with primarily Spanish-speaking patients. A single team of investigators conducted the trial at both settings. Individuals with chronic PTSD were randomly assigned to 24 weeks of double-blind treatment with sertraline plus mirtazapine or sertraline plus placebo. This study was conducted in compliance with the Code of Ethics of the World Medical Association (Declaration of Helsinki) and the standards established by an Institutional Review Board and by the National Institutes of Health. Informed consent was obtained from participants after the nature of the procedures was explained.
Participants
Participants were adults ages 18-75, referred by clinicians or responding to advertisements. After a preliminary telephone screening, eligibility was determined by clinical interview and confirmed by structured interview with trained raters using the Clinician-Administered PTSD Scale (CAPS)26 and the Structured Clinical Interview for DSM-IV Axis I Disorders -- Patient Edition.27 Participants had a principal DSM-IV diagnosis of chronic PTSD of at least moderate severity (CAPS score ≥50), and English or Spanish fluency. Bilingual clinicians treated and assessed individuals with Spanish language preference. Exclusion criteria were significant suicidal ideation; lifetime psychotic disorder, bipolar disorder, organic mental disorder, or seizure disorder; alcohol or substance use disorder in the past 3 months; unstable medical illness; history of traumatic brain injury of greater than moderate severity; pregnancy or nursing; unwillingness to use contraception (for women of childbearing potential); prior nonresponse to sertraline or combined treatment, or intolerance of sertraline or mirtazapine); and psychotropic medication use during the prior 2 weeks (4 weeks for monoamine oxidase inhibitors or fluoxetine), except that zolpidem for insomnia was allowed up to three times per week during the week prior to randomization; psychotherapy initiated within 3 months before randomization. Concomitant psychotropic medications were not permitted during the study.
Randomization and Blinding
Randomization used randomly permuted blocks stratified by patient language preference (English vs. Spanish), implemented by the data manager who had no patient contact. Mirtazapine 15 mg capsules or matching placebo capsules were packaged by a pharmacist with no patient contact. Patients were reminded at each visit with the independent evaluator (IE) to not discuss medication or adverse events, and allocations were concealed from all research personnel throughout each patient’s participation.
Treatments
A single psychiatrist saw each patient for medication management, with an initial visit of 45 minutes and subsequent 30 minute visits weekly for two weeks, biweekly through week 12, then at 4-week intervals. At each visit the psychiatrist assessed clinical improvement and adverse events. Mirtazapine/placebo was initiated at 30 mg (two capsules) at bedtime for four weeks, after which patients without significant adverse events and with persistent PTSD symptoms had dose increased to a maximum of 45 mg/day. Dose could be decreased for intolerable adverse events, to a minimum of 15mg/day. Sertraline was initiated at 25 mg/day for four days, then increased as tolerated to 50 mg/day for the remainder of Week 1, 100 mg/day for Weeks 2-4, 150 mg/d for Weeks 5-6, and then 200 mg/day. Dosage could be decreased as clinically indicated to a minimum of 50 mg/day. Compliance was assessed with patient diaries and pill counts.
Assessments
Master’s- or doctoral-level clinicians, blinded to dosage and adverse events, conducted independent evaluations at 4-week intervals. The CAPS was administered to assess PTSD severity, the Clinical Global Impression of Change scale28 (CGI-C, ranging from 7, very much worse, to 1, very much improved) was used to define responder status (see Statistical Analyses), and depression severity was assessed with the 17-item Hamilton Rating Scale for Depression (HRSD).29 IEs met regularly to compare taped interview ratings and achieved excellent inter-rater reliability on the CAPS (Shrout-Fleiss interclass reliability coefficient = 0.93) and HRSD (0.89). Treating psychiatrists rated 29 potential adverse effects on a 0- to 3-point scale (none, mild, moderate, or severe) using a checklist (available on request from the authors) at every visit. Sexual dysfunction was also assessed with the Arizona Sexual Experience Scale (ASEX), a valid and reliable five-item self-rated scale.30 Patients also completed the Quality of Life Enjoyment and Satisfaction Questionnaire (QLESQ),31 a measure with eight domains; the Posttraumatic Stress Disorder Checklist (PCL),32 a widely used measure of PTSD severity; the Pittsburgh Sleep Quality Index (PSQI), a well-validated assessment of sleep quality, comprised of 24 items;33 the Pittsburgh Sleep Quality Index Addendum for PTSD (PSQI-A), ten additional items assessing disruptive nocturnal behaviors specific to PTSD;34 and the Medical Outcomes Study Short Form 12 (SF-12),35 a widely-used measure of functional impairment assessing eight dimensions of physical and mental health on a 0-100 scale. Vital signs and weight were measured at each visit. Patients who prematurely discontinued study medication were encouraged to return for all assessments through week 24.
Statistical Analyses
Primary outcomes were treatment retention (days on study treatment) and PTSD severity (CAPS total score). Secondary outcomes included remission (defined by CAPS score < 20) and response (defined by >30% decrease in CAPS score and CGI-C score = 1 or 2, very much or much improved) assessed at each time point, and measures of depression, PTSD severity, sleep, sexual function, quality of life, and overall functioning. An adverse event was designated treatment-emergent if its severity at any point during the study exceeded its baseline severity. Analyses used data from all assessments of the intent-to-treat (ITT) sample of randomized participants (excepting two excluded from analyses for eligibility violations). Rates of treatment-emergent adverse events, however, were based on only those assessments occurring while on study medication. All tests were two-sided and performed at a significance level alpha = 0.05, except where otherwise noted.
Treatment retention was analyzed between treatment groups by t-test, and rates of each treatment-emergent adverse event were compared using Fisher’s Exact Test. Longitudinal outcomes were modeled for continuous measures (e.g., CAPS) using linear mixed effects models (MEM), and for dichotomous measures (e.g., remission) using generalized estimating equations (GEE).36 Time, treatment, and interaction between time and treatment were included in the models. Baseline value was also included for continuous measures. Time was treated as discrete for MEM and as continuous for GEE. Within-subject correlation among repeated measures was modeled using either a random subject effect in MEM or a compound symmetric working correlation matrix in GEE to provide valid inferences in the presence of ignorable nonresponse. MEM and GEE are available-case methods of analysis that provide accurate estimates of treatment effects when dropout and missing data are present. If the interaction between time and treatment was found significant at an alpha level of 0.10, contrasts were tested between treatment groups at each time point (for MEM) or at week 24 (for GEE) and presented as effect sizes (Cohen’s d) or odds ratios. If the interaction between time and treatment was not significant, the interaction term was dropped and a model with only main effect was fit and an overall between-groups treatment effect was tested and quantified by effect size or odds ratio.
Results
Sample
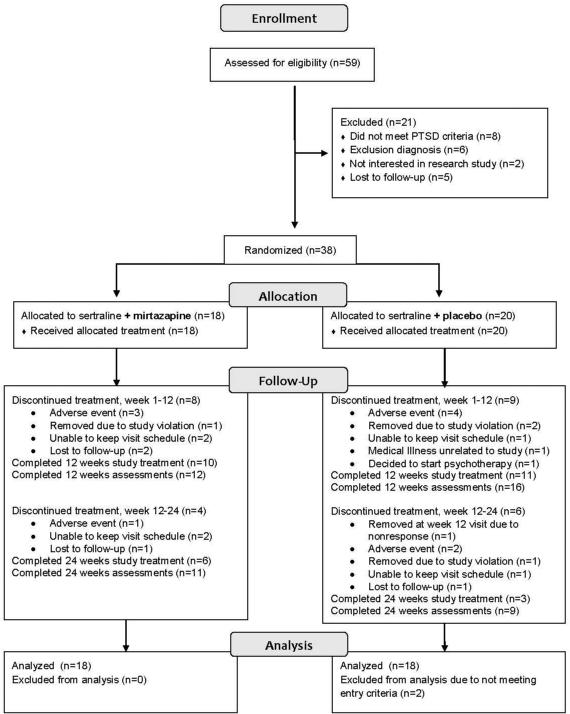
A total of 59 adults completed clinical assessments of eligibility, yielding 38 participants who were randomized (see Figure 1). Two placebo group participants were excluded (blind to treatment assignment) from analyses: One was discovered to have falsified eligibility information; another had been mistakenly randomized with a subthreshold CAPS score at baseline. We separately report rates of discontinuation of study medication, and rates of discontinuation of study assessments (as patients were encouraged to complete all assessments regardless of treatment adherence status), as follows. Premature discontinuation of study medication during the first 12 weeks occurred for eight patients (44%) in the combined treatment group and nine (50%) in the sertraline plus placebo group; and during the second 12 weeks for four patients (22%) in the combined treatment group and six (33%) in the sertraline plus placebo group (see Figure 1). Among patients who discontinued assigned medication prematurely, at least one post-discontinuation assessment was completed by six in the combined treatment group and nine in the sertraline plus placebo. Four of these patients continued study assessments after switching to non-study open label medication: One who discontinued sertraline plus mirtazapine was treated openly with sertraline monotherapy, and of three who discontinued sertraline plus placebo, one received escitalopram, one sertraline plus mirtazapine, and one sertraline followed by citalopram. Premature discontinuation of assessments occurred for seven patients in the combined treatment group and nine in the sertraline plus placebo group. The 16 patients who discontinued participation in study assessments before week 24 did not differ on baseline measures from the 20 who completed 24 weeks of assessments.
Figure 1.
Flow diagram
Baseline demographic and clinical characteristics were similar for the two treatment groups (see Table 1). The sample was about 2/3 female, half of the participants preferred to complete study procedures in Spanish, PTSD was moderate to severe in both groups, and depression symptoms were moderate. The primary trauma for most participants had been physical and interpersonal, and no participants had experienced combat-related PTSD. Both groups had received little prior treatment: Five patients (28%) in the combined treatment group and six (33%) in the sertraline plus placebo group had previously received medication treatment. Prior medication had been discontinued for at least 32 weeks, except for one patient in the sertraline plus placebo group who had discontinued an ineffective SSRI two weeks prior to randomization. Although zolpidem was permitted in the week prior to randomization, no participants used it.
Table 1.
Sample Characteristics
| Characteristic | Sertraline plus Mirtazapine |
Sertraline plus Placebo |
||
|---|---|---|---|---|
| N=18 | N=18 | |||
| Mean | SD | Mean | SD | |
| Age (years) | 37.6 | 11.7 | 42.4 | 13.3 |
| Education (years) | 12.2 | 4.9 | 12.5 | 5.5 |
| Age of onset of PTSD (years) | 28.6 | 15.9 | 29.7 | 16.3 |
| Body Mass Index | 25.6 | 4.4 | 29.7 | 6.5 |
| n | % | n | % | |
| Male | 6 | 33.3 | 7 | 38.9 |
| Race | ||||
| White | 4 | 22.2 | 5 | 27.8 |
| Black | 4 | 22.2 | 3 | 27.8 |
| Other* | 10 | 55.6 | 10 | 44.4 |
| Hispanic | 10 | 55.6 | 12 | 66.7 |
| Treated in Spanish | 8 | 44.4 | 9 | 50.0 |
| Marital Status | ||||
| Single (never married) | 10 | 55.6 | 9 | 50.0 |
| Married or Living with Partner | 5 | 27.8 | 5 | 27.8 |
| Divorced or Separated | 3 | 16.7 | 4 | 22.2 |
| Full-time employment, school or Homemaker |
7 | 38.9 | 5 | 27.8 |
| Any prior trials of medication | 5 | 27.8 | 6 | 33.3 |
| Any prior trials of psychotherapy | 5 | 27.8 | 6 | 33.3 |
| Characteristics of Primary Trauma** | n | % | n | % |
| Interpersonal (e.g. assault) | 13 | 76.5 | 11 | 61.1 |
| Physical (non-sexual) | 12 | 70.6 | 8 | 44.4 |
| Sexual | 5 | 29.4 | 5 | 27.8 |
Most Hispanic participants self-identified race as “other.”
Each subject’s primary trauma may have had more than one characteristic
Primary Outcomes
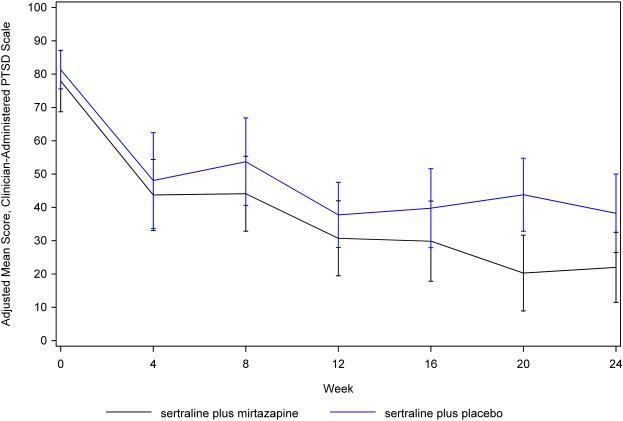
Treatment groups did not differ significantly in days on study treatment (95.2+/−73.1 days for sertraline plus mirtazapine vs. 90.0+/−14.8 days for sertraline plus placebo, df = 34, t = 0.23, P = 0.82). In the CAPS models, time by treatment interaction terms were not significant and were removed from the final models. All longitudinal results (primary and secondary outcomes) are displayed from the main effects models, unless otherwise noted. Treatment groups did not differ significantly in CAPS total score (P = 0.17), although the combined treatment group had numerically greater improvement at all post-baseline assessments, and the between-group effect size was moderate (d = 0.51, 95% CI 1.23, −0.22) (see Figure 2). In secondary analyses, the combined treatment group had nonsignificant numerical advantages on the three CAPS subscales: re-experiencing/intrusion (P = 0.11, d = 0.52), avoidance/numbing (P = 0.44, d = 0.27), and hyperarousal (P = 0.39, d = 0.28).
Figure 2.
Adjusted mean Clinician-Administered PTSD Scale scores by week
Secondary Outcomes
Remission rates increased significantly over time in the sertraline plus mirtazapine group compared to the sertraline plus placebo group. The week x treatment interaction met the P < .10 threshold for significance (num df = 1, den df = 115, F = 3.14, P = 0.08). By week 24 there was a significant difference in remission rates between groups (estimate = 1.56, SE = 0.73, df = 30, t = 2.12, P = 0.042, and OR = 4.7, 95% CI 1.1, 19.9). At week 24, in the intention-to-treat sample, 7 of 18 (39%) on mirtazapine were remitted, versus 2 of 18 (11%) on placebo. The number needed to treat to achieve an additional remission was 3.5. Post hoc analyses that excluded data from the visits in which four patients received non-study medication found a slightly diminished group difference in remission rates at week 24 (estimate = 1.50, SE = 0.79, df = 29, t = 1.91, P = 0.066, and OR = 4.5, 95% CI 0.90, 22.4).
Response rates were not significantly different between groups, with OR = 1.8, P = 0.31 for main effect of treatment, although they were numerically greater in the combined treatment group at each time point. For example, in the intention-to-treat sample at week 24 response rates were 10 of 18 (56%) vs. 4 of 18 (22%) for the placebo group. Among completers at week 24, response rates were 10/11 (91%) for combined treatment vs. 4/9 (44%) sertraline plus placebo, and remission rates were 7/11 (64%) vs. 2/9 (22%).
Depression severity also improved significantly more over the 24 weeks of assessment in the sertraline plus mirtazapine group (P = 0.023, d = −0.63, CI −1.17, −0.09) (See Table 2). Post hoc analyses that excluded data from the visits in which four patients received non-study medication found a slightly greater group difference in depression severity (P = 0.020, d = −0.68, CI −1.24,−0.10). Patient self-ratings of PTSD symptoms, sleep impairment, sleep impairment related to PTSD, physical and mental functioning, and quality of life all had no statistically significant differences, but numerical advantages for the combined treatment group were present for all these outcome measures, (d = 0.26 – 0.63) (see Table 2). No significant moderators of treatment response were identified.
Table 2.
Secondary Measures of Outcome over 24 Weeks of Treatment
| Outcome | Sertraline plus Mirtazapine (N=18) |
Sertraline plus Placebo (N=18) |
Treatment Group Effect | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | Mean | SD | n | Mean | SD | Esti- mate |
Standard error |
df | t | P |
d
(95% CI) |
|
| Hamilton Rating Scale for Depression |
−3.77 | 1.64 | 112 | −2.30 | 0.023 | −0.63 (−1.17, −0.09) |
||||||
| Baseline | 18 | 20.8 | 6.2 | 18 | 20.4 | 5.9 | ||||||
| Week 12 | 12 | 8.7 | 6.7 | 16 | 10.4 | 5.7 | ||||||
| Week 24 | 11 | 6.4 | 6.1 | 9 | 11.6 | 6.4 | ||||||
| PCL | −2.82 | 4.91 | 100 | −0.57 | 0.57 | −0.27 (−1.17, 0.64) |
||||||
| Baseline | 18 | 58.9 | 11.0 | 18 | 60.0 | 10.5 | ||||||
| Week 12 | 11 | 35.2 | 15.3 | 15 | 41.8 | 16.7 | ||||||
| Week 24 | 9 | 33.2 | 18.4 | 10 | 39.2 | 18.2 | ||||||
| QLESQ* | 3.52 | 5.06 | 87 | 0.70 | 0.49 | 0.26 (−0.48, 1.01) |
||||||
| Baseline | 17 | 39.1 | 13.6 | 17 | 41.4 | 13.5 | ||||||
| Week 12 | 10 | 61.9 | 11.7 | 13 | 62.2 | 22.1 | ||||||
| Week 24 | 8 | 60.8 | 21.7 | 10 | 59.4 | 18.4 | ||||||
| SF12 Physical* |
3.78 | 2.72 | 91 | 1.39 | 0.17 | 0.37 (−0.15, 0.90) |
||||||
| Baseline | 18 | 43.9 | 9.8 | 17 | 41.4 | 10.6 | ||||||
| Week 12 | 10 | 46.2 | 6.7 | 14 | 46.3 | 9.0 | ||||||
| Week 24 | 7 | 52.6 | 7.6 | 10 | 44.0 | 9.3 | ||||||
| SF12 Mental* |
5.36 | 3.49 | 91 | 1.53 | 0.13 | 0.46 (−0.13, 1.04) |
||||||
| Baseline | 18 | 30.3 | 23.5 | 17 | 30.4 | 10.0 | ||||||
| Week 12 | 10 | 44.9 | 11.4 | 14 | 41.6 | 11.6 | ||||||
| Week 24 | 7 | 53.5 | 7.2 | 10 | 40.1 | 10.1 | ||||||
| PSQI Global |
−1.90 | 1.50 | 92 | −1.27 | 0.21 | −0.46 (−1.17, 0.25) |
||||||
| Baseline | 18 | 11.5 | 4.9 | 17 | 12.8 | 3.1 | ||||||
| Week 12 | 11 | 7.9 | 3.7 | 11 | 7.8 | 3.9 | ||||||
| Week 24 | 8 | 4.4 | 4.2 | 10 | 9.1 | 4.9 | ||||||
| PSQI Addendum |
−1.59 | 1.27 | 100 | −1.24 | 0.22 | −0.30 (−0.78, 0.17) |
||||||
| Baseline | 18 | 10.0 | 4.9 | 18 | 11.2 | 5.6 | ||||||
| Week 12 | 10 | 6.0 | 5.8 | 15 | 7.3 | 5.2 | ||||||
| Week 24 | 9 | 5.1 | 5.4 | 10 | 7.3 | 6.2 | ||||||
For these items, positive values of estimates and d represent greater improvement in the combined treatment group, whereas for other items negative values of estimates represent greater improvement in the combined treatment group.
Both treatments were well tolerated, with no serious adverse events. Increased appetite was the only adverse effect to emerge significantly more often in the combined treatment group. The sertraline plus placebo group had significantly more treatment-emergent nausea, with a trend for more fatigue (See Table 3). Body mass index (BMI) did not differ significantly between groups over the 24 weeks of treatment (estimate = −0.66, SE = 0.42, df = 143, t = 1.60, P = 0.11), and ≥5% weight gain occurred with a frequency of 2 (11.1%) of 18 sertraline plus mirtazapine group patients vs. 5 (27.8%) of 18 sertraline plus placebo patients. There was no significant group difference in sexual functioning on the Arizona Sexual Experiences Scale over the course of treatment (estimate = 0.63, SE = 1.36, df = 100, t = 0.46, P = 0.65, d= 0.10). Treatment was discontinued prematurely due to adverse events by four patients in the combined treatment group (three in week 1, due to anxiety & insomnia, somnolence & insomnia, and syncope (a pre-existing condition), respectively; one at week 12 due to dry skin), and by six patients in the sertraline plus placebo group (three in week 1, due to headaches, palpitations, and nausea, dizziness & somnolence, respectively; one at week 4 due to apathy; and two at week 12 due to insomnia and HA, respectively.
Table 3.
Treatment-Emergent Adverse Events1
| Sertraline plus Mirtazapine n (%) |
Sertraline plus Placebo n (%) |
P
(Fishers’ Exact Test) |
|
|---|---|---|---|
| Headache | 4 (30.8) | 3 (21.4) | 0.68 |
| Heartburn | 5 (38.5) | 3 (37.5) | 0.42 |
| Nausea | 2 (15.4) | 8 (57.1) | 0.046* |
| Vomiting | 2 (15.4) | 3 (21.4) | 1.00 |
| Decreased appetite | 3 (23.1) | 5 (35.8) | 0.68 |
| Increased appetite | 9 (69.2) | 2 (18.2) | 0.006* |
| Dry mouth | 3 (23.1) | 5 (35.7) | 0.68 |
| Constipation | 3 (23.1) | 5 (35.7) | 0.68 |
| Diarrhea/Gas | 6 (46.2) | 5 (35.7) | 0.70 |
| Excessive Sweating | 4 (30.8) | 5 (35.7) | 1.00 |
| Skin problems | 4 (30.8) | 1 (7.1) | 0.16 |
| Bruising Easily | 0 (0.0) | 1 (7.1) | 1.00 |
| Restlessness | 2 (15.4) | 1 (7.1) | 0.60 |
| Tremor | 5 (38.5) | 5 (35.7) | 1.00 |
| Nervousness | 1 (7.7) | 1 (7.1) | 1.00 |
| Impaired Coordination | 3 (23.1) | 2 (14.3) | 0.65 |
| Insomnia | 2 (15.4) | 2 (14.3) | 1.00 |
| Fatigue | 1 (7.7) | 6 (42.9) | 0.08 |
| Somnolence | 6 (46.2) | 5 (35.7) | 0.70 |
| Decreased Libido (men)2 | 0 (0.0) | 2 (33.3) | 0.47 |
| Decreased Libido (women)2 | 5 (55.6) | 3 (37.5) | 0.64 |
| Sexual Dysfunction (men)2 | 0 (0.0) | 3 (50.0) | 0.20 |
| Sexual Dysfunction (women)2 | 1 (11.1) | 2 (25.0) | 0.58 |
| Urinary Dysfunction | 5 (38.5) | 5 (35.7) | 1.00 |
| Blurry Vision | 4 (30.8) | 2 (14.3) | 0.38 |
| Lightheadedness | 9 (69.2) | 4 (28.6) | 0.06 |
| Forgetfulness | 4 (30.8) | 4 (28.6) | 1.00 |
| Impaired Concentration | 2 (15.4) | 4 (28.6) | 0.65 |
| Apathy | 2 (15.4) | 3 (21.4) | 1.00 |
P < .05
Treatment emergent adverse event data was available for 27 patients (13 mirtazapine, 14 placebo) who returned for assessments on treatment.
Treatment emergent adverse event data was available for 10 men (4 mirtazapine, 6 placebo) and 17 women (9 mirtazapine, 8 placebo)
Each group received a similar maximum dose of sertraline (118.1 +/−59.2 mg/d in the sertraline plus mirtazapine group vs. 122.2 +/−72.2 mg/d in the sertraline plus placebo group, df = 34, t = 0.19, P = 0.85). Groups also did not differ in maximum dose of mirtazapine vs. placebo-equivalent (32.5 +/− 11.8 mg/d for mirtazapine vs. 36.7 +/− 7.7 mg/d for placebo, df = 34, t = −1.26, P = 0.22).
Discussion
This randomized controlled trial found 24 weeks of combined treatment with sertraline plus mirtazapine to be superior to sertraline plus placebo in achieving remission and improving depressive symptoms among patients with PTSD. The combined treatment had non-significant numerical advantages for every symptomatic and functional outcome measure, but most of these differences were not statistically significant in this small study that was not powered to provide a definitive test of efficacy. Effect sizes were small to medium, which is clinically meaningful considering that they represent advantages over an SSRI treatment recognized as the first-line pharmacotherapy for PTSD, yet acknowledged to provide only partial benefit for most patients.8
Contrary to our expectations, the combined treatment did not result in greater duration of retention in treatment, nor did it improve sleep or sexual functioning significantly more than sertraline plus placebo. Instead the mirtazapine plus sertraline group showed greatest advantages in maximizing achievement of remission, and in improving depressive symptoms and the re-experiencing/intrusion domain of PTSD symptoms. Re-experiencing is considered a core feature of PTSD,37 and in a meta-analysis of clinical trials of medication (mostly SSRIs) it showed the least improvement of the three CAPS domains,14 suggesting that combined treatment might have a broader effect across PTSD domains than SSRI treatment alone. Depression is extremely common in PTSD, due to both the likely influence of common etiologic factors, and PTSD being a risk factor for secondary depression.38 This overlap of PTSD and depression is reflected in the recent addition of a criterion for negative cognitions and mood for the diagnosis of PTSD in DSM-5.39
The findings here of advantages in efficacy for mirtazapine plus sertraline, and specifically for depressive symptoms, are consistent with two small RCTs of treatment with mirtazapine combined with an SSRI or SNRI for MDD,19,20 but they differ from the larger COMED trial in MDD, which reported thoroughly null results for combined mirtazapine + venlafaxine versus the SSRI escitalopram.23 This PTSD study had a design similar to the prior small RCTs, and it differed from the COMED study in several ways, including double-blind vs. single blind design, and greater mirtazapine dosage: The maximal mean dose of mirtazapine here was 32.5 mg/day, versus a final mean dose of 18 mg/day in the COMED trial.
Patients in this study tolerated the combined treatment similarly to sertraline plus placebo. Those in the combined treatment group had a greater rate of increased appetite, as expected, yet weight gain did not differ between groups during this 24-week treatment. The absence of expected group differences in weight gain, insomnia, and sexual dysfunction increases the likelihood that treatment allocation was not unmasked by side effect differences in this study.
Dropout rates in this study were substantial, but they did not differ significantly between groups. High dropout rates can reduce power to detect group differences in treatment outcome, but the problem was mitigated here by the lack of group differences in dropout rate and by statistical analyses using MEM and GEE models that can provide accurate estimates of treatment effects when missing data are present. Attrition was similar to that reported in prior PTSD pharmacotherapy trials. For example, the 39% treatment discontinuation rate in the sertraline plus placebo group over the first 12 weeks of treatment here was similar to rates of 30-31% reported for sertraline treatment in 12-week RCTs in PTSD. Studies of patient preferences have reported that individuals who have experienced trauma or who have PTSD generally favor psychotherapy approaches over medication, in part due to concerns about adverse effects of medication.40,41 So while the attrition in this study did not prevent detection of significant group differences in treatment outcome on some measures, the effectiveness of pharmacotherapies for PTSD continues to be limited by relatively low rates of retention in treatment.
The planned outcome analyses for this study utilized all data collected during the 24-week period post-randomization. Patients were encouraged to attend all scheduled assessments regardless of whether they had continued to adhere to their assigned study treatment, to facilitate the standard intention-to-treat analysis. Intention-to-treat analysis recognizes noncompliance and protocol deviations to be part of actual clinical practice, and avoids overoptimistic estimates of the efficacy of an intervention that can result from the removal of non-compliant patients. 42,43 A potential drawback to this data analysis strategy, however, is that to the extent that patients are assessed while receiving active nonstudy treatments, outcomes may become less specifically attributable to the study treatments. In this study, four participants were assessed at visits that occurred while they received nonstudy treatments after they had discontinued study medication. Post hoc analyses that excluded these visits showed only small differences that were of inconsistent direction from the original analyses of PTSD and depression severity, suggesting that nonstudy treatments did not significantly impact the findings.
Overall, the findings suggest that the combination of mirtazapine plus an SSRI warrants further study as a PTSD treatment. Given that the advantage of combined treatment did not seem to stem from early improvement in sleep, superior tolerability, or increased retention in treatment, the rationale for combined treatment from the outset was not supported. It may therefore be a more efficient strategy to utilize mirtazapine as an augmentation for SSRI non-remitters, who might be more accepting of a dual treatment strategy than treatment-naïve patients being offered both medicines from the outset. Another alternative, mirtazapine monotherapy, has been little studied for PTSD, and its efficacy relative to combined treatment is unknown.
The main limitation of this study is that small sample size limited power to detect significant group differences and the effects of moderators. For the same reason, the significant findings for greater remission rate and improvement of depression on the combined treatment have effect sizes with wide confidence intervals, rendering the magnitude of their clinical significance uncertain. This sample included no combat-related PTSD sample, so generalizability to combat-related PTSD patients is not known. The sample also had received relatively little prior treatment, so the generalizability of these findings to SSRI-nonresponders or to more highly treatment-refractory PTSD patients will require further study.
In conclusion, this is the first PTSD study to show that combining mirtazapine with SSRI treatment may have advantages in efficacy over SSRI treatment alone, similar to some findings for depression. Combined treatment with mirtazapine plus sertraline was well-tolerated and resulted in greater remission rates and greater improvement in depressive symptoms over 24 weeks of treatment. Although this study offered combined treatment from the outset in the hopes that this would enhance acute benefits and result in superior treatment retention, this mechanism was not confirmed. Future work should help identify specific subgroups, such as SSRI nonresponders, who might benefit most from combined mirtazapine plus SSRI treatment.
Acknowledgments
This study was funded by NIMH 5R34MH091336 to Dr. Schneier and by the Sycamore Fund.
Dr. Schneier reports grants from the National Institute of Mental Health and from the Sycamore Fund during the conduct of the study; grants from Forest Laboratories, and other from Genentech outside the submitted work; Dr. Lewis-Fernandez reports a grant from Eli Lilly Inc., outside the submitted work.
Footnotes
Conflicts of interest and financial disclosures: The other authors have no conflicts of interest to disclose.
References
- 1.Kessler RC, Sonnega A, Bromet E, et al. Posttraumatic stress disorder in the National Comorbidity Survey. Arch Gen Psychiatry. 1995;52:1048–1060. doi: 10.1001/archpsyc.1995.03950240066012. [DOI] [PubMed] [Google Scholar]
- 2.Breslau N. The epidemiology of posttraumatic stress disorder: What is the extent of the problem? J. Clin Psychiatry. 2001;62:16–22. [PubMed] [Google Scholar]
- 3.Neria Y, Nandi A, Galea S. Post-traumatic stress disorder following disasters: a systematic review. Psychol Med. 2008;38:467–80. doi: 10.1017/S0033291707001353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Roberts AL, Gilman SE, Breslau J, et al. Race/ethnic differences in exposure to traumatic events, development of post-traumatic stress disorder, and treatment-seeking for post-traumatic stress disorder in the United States. Psychol Med. 2011;41:71–83. doi: 10.1017/S0033291710000401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kessler RC. Posttraumatic stress disorder : the burden to the individual and to society. J Clin Psychiatry. 2000;61:4–12. [PubMed] [Google Scholar]
- 6.Gradus JL, Qin P, Lincoln AK, et al. Posttraumatic stress disorder and completed suicide. Am. J Epidemiol. 2010;171:721–727. doi: 10.1093/aje/kwp456. [DOI] [PubMed] [Google Scholar]
- 7.McFarlane AC. The longitudinal course of posttraumatic morbidity. The range of outcomes and their predictors. J Nerv Ment Dis. 1988;176:30–39. doi: 10.1097/00005053-198801000-00004. [DOI] [PubMed] [Google Scholar]
- 8.Institute of Medicine. Committee on Treatment of Posttraumatic Stress Disorder, Board on Population Health and Public Health Practice Treatment of Posttraumatic Stress Disorder: An Assessment of the Evidence. 2008 http://www.nap.edu/catalog/11955.html.
- 9.Davidson JRT. Remission in post-traumatic stress disorder (PTSD): effects of sertraline as assessed by the Davidson Trauma Scale, Clinical Global Impressions and the Clinician-Administered PTSD scale. Psychopharmacol. 2004;19:85–87. doi: 10.1097/00004850-200403000-00005. [DOI] [PubMed] [Google Scholar]
- 10.Tucker P, Potter-Kimball R, Wyatt DB, et al. Can physiologic assessment and side effects tease out differences in PTSD trials? A double-blind comparison of citalopram, sertraline, and placebo. General Psychopharmacology. 2003;37:135–149. [PubMed] [Google Scholar]
- 11.Meltzer-Brody S, Connor KM, Churchill E, Davidson JR. Symptom-specific effects of fluoxetine in post-traumatic stress disorder. Int Clin Psychopharmacol. 2000;15:227–231. doi: 10.1097/00004850-200015040-00006. [DOI] [PubMed] [Google Scholar]
- 12.Brady K, Pearlstein T, Asnis GM, Baker D, Rothbaum B, Sikes CR, Farfel GM. Efficacy and safety of sertraline treatment of posttraumatic stress disorder. JAMA. 2000;283:1837–1844. doi: 10.1001/jama.283.14.1837. [DOI] [PubMed] [Google Scholar]
- 13.Davidson J, Pearlstein T, Londborg P, et al. Efficacy of sertraline in preventing relapse of posttraumatic stress disorder: results of a 28-week double-blind, placebo-controlled study. Am J Psychiatry. 2001;158:1974–1981. doi: 10.1176/appi.ajp.158.12.1974. [DOI] [PubMed] [Google Scholar]
- 14.Stein DJ, Ipser JC, Seedat S. Pharmacotherapy for post traumatic stress disorder (PTSD) Cochrane Database Syst Rev. 2006 Jan 25;(1) doi: 10.1002/14651858.CD002795.pub2. CD002795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Londborg PD, Hegel MT, Goldstein S, et al. Sertraline treatment of posttraumatic stress disorder: results of 24 weeks of open-label continuation treatment. J Clin Psychiatry. 2001;62:325–331. doi: 10.4088/jcp.v62n0503. [DOI] [PubMed] [Google Scholar]
- 16.Marshall RD, Lewis-Fernandez R, Blanco C, et al. A controlled trial of paroxetine for chronic PTSD, dissociation, and interpersonal problems in mostly minority adults. Depress Anxiety. 2007;24:77–84. doi: 10.1002/da.20176. [DOI] [PubMed] [Google Scholar]
- 17.de Boer T. The pharmacological profile of mirtazapine. J Clin Psychiatry. 1995;57(Suppl. 4):19–26. [PubMed] [Google Scholar]
- 18.Besson A, Haddjeri N, Blier P, de Montigny C. Effects of the co-administration of mirtazapine and paroxetine on serotonergic neurotransmission in the rat brain. Eur Neuropsychopharmacol. 2000;10:177–188. doi: 10.1016/s0924-977x(00)00069-9. [DOI] [PubMed] [Google Scholar]
- 19.Blier P, Gobbi G, Turcotte JE, et al. Mirtazapine and paroxetine in major depression: a comparison of monotherapy versus their combination from treatment initiation. Eur Neuropsychopharmacol. 2009;19:457–465. doi: 10.1016/j.euroneuro.2009.01.015. [DOI] [PubMed] [Google Scholar]
- 20.Blier P, Ward HE, Tremblay P, et al. Combination of antidepressant medications from treatment initiation for major depressive disorder: a double-blind randomized study. Am J Psychiatry. 2010;167:281–288. doi: 10.1176/appi.ajp.2009.09020186. [DOI] [PubMed] [Google Scholar]
- 21.Carpenter LL, Yasmin S, Price LH. A double-blind, placebo-controlled study of antidepressant augmentation with mirtazapine. Biol Psychiatry. 2002;51:183–188. doi: 10.1016/s0006-3223(01)01262-8. [DOI] [PubMed] [Google Scholar]
- 22.Pallanti S, Quercioli L, Bruscoli M. Response acceleration with mirtazapine augmentation of citalopram in obsessive-compulsive disorder patients without comorbid depression: a pilot study. J Clin Psychiatry. 2004;65:1394–1399. doi: 10.4088/jcp.v65n1015. [DOI] [PubMed] [Google Scholar]
- 23.Rush AJ, Trivedi MH, Stewart JW, et al. Combining medications to enhance depression outcomes (CO-MED): acute and long-term outcomes of a single-blind randomized study. Am J Psychiatry. 2011;168:689–701. doi: 10.1176/appi.ajp.2011.10111645. [DOI] [PubMed] [Google Scholar]
- 24.Lewis JD. Mirtazapine for PTSD nightmares (Letter) Am J Psychiatry. 2002;159:1948–1949. doi: 10.1176/appi.ajp.159.11.1948-a. [DOI] [PubMed] [Google Scholar]
- 25.Alfaro CL, Lam YW, Simpson J, Ereshefsky L. CYP2D6 inhibition by fluoxetine, paroxetine, sertraline, and venlafaxine in a crossover study: intraindividual variability and plasma concentration correlations. J Clin Pharmacol. 2000;40:58–66. doi: 10.1177/00912700022008702. [DOI] [PubMed] [Google Scholar]
- 26.Weathers FW, Keane TM, Davidson JRT. Clinician-administered PTSD scale: a review of the first ten years of research. Depression and Anxiety. 2001;13:132–156. doi: 10.1002/da.1029. [DOI] [PubMed] [Google Scholar]
- 27.First MB, Spitzer RL, Gibbon M, et al. User’s Guide for the Structured Clinical Interview for DSM-IV Axis II Personality Disorders. American Psychiatric Press; Washington, D.C.: 1997. [Google Scholar]
- 28.Guy W. ECDEU Assessment Manual for Psychopharmacology - Revised (DHEW Publ No ADM 76-338) Rockville, MD: 1976. [Google Scholar]
- 29.Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McGahuey CA, Gelenberg AJ, Laukes CA, et al. The Arizona Sexual Experience Scale (ASEX): reliability and validity. J Sex Marital Ther. 2000;26:25–40. doi: 10.1080/009262300278623. [DOI] [PubMed] [Google Scholar]
- 31.Endicott J, Nee J, Harrison W, et al. Quality of life, enjoyment, and satisfaction questionnaire: a new measure. Psychopharm Bull. 1993;29:321–326. [PubMed] [Google Scholar]
- 32.Foa E. The Post Traumatic Diagnostic Scale Manual. Minneapolis, NCS: 1995. [Google Scholar]
- 33.Buysse DJ, Reynolds CF, 3rd, Monk TH, et al. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 34.Germain A, Hall M, Krakow B, et al. A brief Sleep Scale for Posttraumatic Stress Disorder: Pittsburgh Sleep Quality Index Addendum for PTSD. J Anxiety Disord. 2005;19:233–244. doi: 10.1016/j.janxdis.2004.02.001. [DOI] [PubMed] [Google Scholar]
- 35.Ware JE, Snow KK, Kosinski M, et al. SF-36 Health Survey: Manual and Interpretation Guide. Health Institute, New England Medical Center; Boston, Mass: 1993. [Google Scholar]
- 36.Fitzmaurice GM, Laird NM, Ware JH. Applied Longitudinal Analysis. Wiley; Hoboken, NJ: 2004. [Google Scholar]
- 37.Ehlers A, Hackmann A, Michael T. Intrusive re-experiencing in post-traumatic stress disorder: phenomenology, theory, and therapy. Memory. 2004;12:403–415. doi: 10.1080/09658210444000025. [DOI] [PubMed] [Google Scholar]
- 38.Stander VA, Thomsen CJ, Highfill-McRoy RM. Etiology of depression comorbidity in combat-related PTSD: a review of the literature. Clin Psychol Rev. 2014;34:87–98. doi: 10.1016/j.cpr.2013.12.002. [DOI] [PubMed] [Google Scholar]
- 39.Gentes EL, Dennis PA, Kimbrel NA, et al. DSM-5 posttraumatic stress disorder: Factor structure and rates of diagnosis. J Psychiatr Res. 2014;59:60–67. doi: 10.1016/j.jpsychires.2014.08.014. [DOI] [PubMed] [Google Scholar]
- 40.Roy-Byrne P, Berliner L, Russo J, et al. Treatment preferences and determinants in victims of sexual and physical assault. J Nerv Ment Dis. 2003;191:161–165. doi: 10.1097/01.NMD.0000055343.62310.73. [DOI] [PubMed] [Google Scholar]
- 41.Zoellner LA, Feeny NC, Cochran B, Pruitt L. Treatment choice for PTSD. Behav Res Ther. 2003;41:879–886. doi: 10.1016/s0005-7967(02)00100-6. [DOI] [PubMed] [Google Scholar]
- 42.Ten Have TR, Normand SL, Marcus SM, et al. Intent-to-Treat vs. Non-Intent-to-Treat Analyses under Treatment Non-Adherence in Mental Health Randomized Trials. Psychiatr Ann. 2008;38:772–783. doi: 10.3928/00485713-20081201-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Heritier SR, Gebski VJ, Keech AC. Inclusion of patients in clinical trial analysis: The intention-to-treat principle. Med J Aust. 2003;179:438–40. doi: 10.5694/j.1326-5377.2003.tb05627.x. [DOI] [PubMed] [Google Scholar]