Abstract
Background
The non-selective opioid receptor antagonist, naltrexone (NAL), reduces alcohol (ethanol) consumption in animals and humans and is an approved medication for treating alcohol abuse disorders. Proopiomelanocortin (POMC)-derived melanocortin (MC) and opioid peptides are produced in the same neurons in the brain, and recent pre-clinical evidence shows that MC receptor (MCR) agonists reduce excessive ethanol drinking in animal models. Interestingly, there is a growing body of literature revealing interactions between the MC and opioid systems in the modulation of pain, drug tolerance, and food intake.
Method
In the present report, a mouse model of binge ethanol drinking was employed to determine if the MCR agonist, melanotan-II (MTII), would improve the effectiveness of NAL in reducing excessive binge-like ethanol drinking when these drugs were co-administered prior to ethanol access.
Results
Both NAL and MTII blunt binge-like ethanol drinking and associated blood ethanol levels, and when administered together, a low dose of MTII (0.26 mg/kg) produces a 7.6-fold increase in the effectiveness of NAL in reducing binge-like ethanol drinking. Using isobolographic analysis, it is demonstrated that MTII increases the effectiveness of NAL in a synergistic manner.
Conclusions
The current observations suggest that activators of MC signaling may represent a new approach to treating alcohol abuse disorders, and a way to potentially improve existing NAL-based therapies.
Keywords: Melanocortin, Opioid, Binge-Like Drinking, Naltrexone, MTII, Synergistic
INTRODUCTION
Alcohol abuse disorders and alcoholism are major public health problems in the United States and world-wide. The Center for Disease Control places alcohol as the number three cause of preventable deaths following nicotine use and obesity (Mokdad et al., 2004). The economic costs of alcohol misuse in the United States are estimated at about $225 billion per year (Bouchery et al., 2011). Further, alcohol dependence effects on the order of 5–6% of men and 2–3% of women in the United States in a given 12 month period (Grant et al., 2004). Despite these alarming statistics, at present there are only four US Food and Drug Administration (FDA)-approved medications for treating alcohol abuse disorder, and these medications are not effective in all individuals (Garbutt, 2009). Thus, identifying additional pharmacotherapies, or ways to improve existing FDA-approved medications, is of paramount importance.
Neuropeptide systems stemming from the polypeptide precursor proopiomelanocortin (POMC) may be useful targets for treating alcohol (herein referred to as ethanol) abuse disorders. POMC gives rise to β-endorphin, an endogenous opioid peptide, and the melanocortin (MC) peptides including α-melanocyte stimulating hormone (α-MSH), β-MSH, and γ-MSH. These peptides are synthesized primarily in the arcuate nucleus of the hypothalamus (Dores et al., 1986, Hadley and Haskell-Luevano, 1999). β-endorphin neurons provide projections to brain regions implicated in modulating ethanol consumption and the reinforcing properties of ethanol (Khachaturian et al., 1985), and there is a large database suggesting that β-endorphin modulates neurobiological responses to ethanol (Froehlich and Li, 1993, Gianoulakis, 2001, Rasmussen et al., 2002). Furthermore, non-selective opioid receptor antagonists as well as those selective for the μ or δ opioid receptors reduce ethanol consumption (Gianoulakis, 2001) and ethanol intake is reduced in μ opioid receptor knockout mice (Roberts et al., 2000, Hall et al., 2001). Consistent with an important role for opioid peptides, the non-selective opioid receptor antagonist naltrexone (NAL) is the active agent in two of the four currently approved medications for alcoholism in the United States. NAL prevents relapse to heavy drinking and can enhance abstinence in human alcoholics (see (Garbutt, 2010)).
More recent evidence reveals that POMC-derived MC neuropeptides also modulate neurobiological responses to ethanol. Ingestion of an ethanol-containing diet by rats significantly attenuates α-MSH levels in brain regions implicated in the reinforcing properties of ethanol (Navarro et al., 2008), and intraperitoneal injection of ethanol in mice increases brain levels of agouti-related protein (AgRP), an endogenous MC receptor (MCR) antagonist (Cubero et al., 2010). Further, central and peripheral administration of the MCR agonist melanotan-II (MTII) significantly blunts ethanol drinking, while central administration of the MCR antagonist AgRP significantly increases, and genetic deletion of AgRP significantly reduces, ethanol drinking in mice (Navarro et al., 2005, Navarro et al., 2003, Navarro et al., 2009). Both the MC-4 receptor (MC4R) (Navarro et al., 2011) and the MC3R (Olney et al., 2014) modulate the effects of MTII on ethanol intake.
Interactions between the endogenous opioid and MC systems are now well documented and may be a consequence of these systems sharing a similar anatomical distribution in the central nervous system with potentially opposing downstream actions. Administration of MC antagonists prevents and reverses the development of opioid tolerance (Contreras and Takemori, 1984, Starowicz et al., 2005, Starowicz et al., 2003). Further, stimulation of MCRs block, whereas MC4R antagonists enhance, the antinociceptive effects of opioids (Ercil et al., 2005) and MC1R knockout mice display an enhanced sensitivity to opioid antinociception (Mogil et al., 2005). Interestingly, chronic activation of the opioid system decreases MC4R mRNA (Alvaro et al., 1996). More recently, it has been shown that combined administration of NAL and a putative stimulator of MC signaling is a more effective treatment strategy against excessive eating than monotherapy with NAL alone (Greenway et al., 2009, Greenway et al., 2010). MCRs and opioid receptors are Gs and Gi protein-coupled, respectively, thus MCR agonists may exert effects on opioids by opposing the actions of opioids on intracellular signaling cascades in neurons that express both MC and opioid receptors (Alvaro et al., 1997, Contreras and Takemori, 1984).
Given the growing body of evidence suggesting interactions between POMC-derived MC and opioid neuropeptide pathways in pain modulation, opioid tolerance, and food intake, and the observations that NAL and MCR agonists reduce excessive ethanol drinking, here we determined if combining the MCR agonist MTII with NAL would synergistically increase the effectiveness of NAL to blunt binge-like ethanol drinking in mice. First, we established that NAL and MTII can significantly blunt binge-like ethanol drinking in mice using “drinking in the dark” (DID) procedures (Rhodes et al., 2005, Thiele and Navarro, 2014). Then, using drug combination and isobolographic analyses, we showed that a low dose of MTII significantly shifted the dose-response curve of NAL to the left, providing evidence that MTII synergistically increases the effectiveness of NAL in protecting against binge-like ethanol drinking. These observations suggest that using MCR agonists may be a new strategy for treating alcohol abuse disorders, and provide evidence that MCR agonists may increase the effectiveness of currently approved therapies involving NAL.
MATERIAL AND METHODS
Animals
As sex differences in MC neuroanatomy and function have already been described (Lippert et al., 2014, Qu et al., 2014, Gelez et al., 2010), only male C57BL/6J mice (Jackson Laboratories, Jackson, MS), 6–8 weeks of age and weighing between 20–25 g at the beginning of the experiments, were used. Mice were individually housed in plastic cages, were allowed to habituate to their environment for at least 1 week before the start of the experiments, and had ad libitum access to standard rodent (Prolab® RMH 3000, Purina LabDiet®, Inc., St. Louis, MO) and water except when is noted. The colony room was maintained at approximately 22°C with a 12h light/12h dark cycle and lights went off at 10:00 hours. All procedures used were in accordance with the National Institute of Health guidelines, and were approved by the University of North Carolina Institutional Animal Care and Use Committee.
Drugs
Ethanol (20% v/v) solutions were prepared using tap water and 95% ethyl alcohol. The opioid antagonist naltrexone (naltrexone hydrochloride; Sigma-Aldrich, Saint Louis, MO) and the melanocortin agonist melanotan-II (MTII; Bachem, Torrance, CA) were dissolved in 0.9% saline. MTII was chosen as this drug is peripherally bioavailable (Navarro et al., 2003).
Blood-Ethanol Concentration (BEC)
Approximately 10μl of blood was collected from the tail vein of each mouse immediately following ethanol access on day 4 (test day) of the drinking in the dark (DID) procedure to analyze BEC. Samples were centrifuged, and 5μl of plasma from each sample was analyzed (Analox Instruments, Lunenburg, MA).
“Drinking in the Dark” (DID) Procedure
For all the experiments, we used a 4-day DID procedure to generate binge-like ethanol drinking (Thiele et al., 2014). On days 1–3, beginning 3 hours into the dark cycle, water bottles were removed from all cages and replaced with a pre-weighted bottle containing 20% (v/v) ethanol solution. Mice had 2 hours of access to ethanol, after which the ethanol bottles were removed from cages and weighed again to calculate ethanol consumption, and water bottles were replaced. On day 4, the test day, the same procedure was followed except that tail blood samples were collected immediately after ethanol intake in Experiments 1 and 2 for analysis of BEC.
Experiments 1 & 2: Naltrexone and MTII Dose-Response Studies
To assess the effect of NAL on binge-like ethanol drinking and to establish effective doses (ED), we performed a dose-response experiment with NAL using the DID procedure. Mice were assigned to one of five groups (n = 9–14/group) so that average body weights were similar between groups: 0, 0.3, 1.0, 3.0, or 10 mg/kg NAL. On days 1–3 animals were weighed and injected intraperitoneally (i.p.) with the appropriate volume (5 ml/kg) of the vehicle to habituate them to the injections. On the test day, i.p. injections of NAL were given approximately 30 minutes before ethanol access. In a separate study using the same procedures, mice were assigned to one of five groups (n = 10–12/group) so that average body weights were similar between groups (0, 0.3, 1.0, 3.0, or 10 mg/kg groups) to assess the effect of MTII on binge-like ethanol drinking and to establish EDs.
Experiments 3 & 4: NAL-MTII Interaction Studies
The drug interaction and isobolographic analyses used in Experiments 3 and 4 required the calculations of EDs from dose-response functions from NAL and MTII alone, as well as these drugs in combination. To allow ED analyses and to facilitate comparisons across groups that had slightly different baseline levels of ethanol consumption, the data from these experiments were converted to % decrease from baseline ethanol consumption for each subject, where baseline consumption was calculated as the average ethanol intake over days 1–3 of the DID procedure.
Experiments 3 and 4 were designed to determine the way MTII and NAL interact (i.e., additively or synergistically) in the modulation of binge-like ethanol drinking. As Experiments 1 and 2 overlapped with the initiation of Experiments 3 and 4, data from a subset of mice from Experiments 1 and 2 were used to calculate ED20, ED30, and ED50 for each drug (n = 45 for NAL, n = 48 for MTII), and these values were used for analyses in Experiments 3 and 4. In Experiment 3, the influence of different doses of NAL (0.3, 1.0, and 3.0 mg/kg) alone (obtained in Experiment 1) or in combination with the ED20 (0.26 mg/kg) or ED30 (0.52 mg/kg) doses of MTII were assessed on binge-like ethanol drinking (for a total 6 groups, n = 9–14/group). Similarly, in Experiment 4 the influence of different doses of MTII (0.3, 1.0, and 3.0 mg/kg) alone (obtained in Experiment 2) or in combination with the ED20 (0.82 mg/kg) or ED30 (1.64 mg/kg) doses of NAL were assessed on binge-like ethanol drinking (for a total 6 groups, n = 8–12/group). On days 1–3 animals were weighed and injected twice with the appropriate volume (5 ml/kg) of the vehicle to habituate them to the injections. On the test day each animal received an i.p. injection of one of the drugs (NAL or MTII) followed immediately by an i.p. injection of the other drug. It should be noted that mice in the NAL and MTII alone groups were tested at a different time and received one, rather than two, i.p. injection on habituation and test days (in Experiment 1). Despite these differences from mice run in Experiments 3 and 4, % baseline ethanol consumption levels were similar between the 0.3 and 1.0 mg/kg doses of NAL alone and groups that included MTII (see Figure 2), and between all doses of MTII alone and groups the included NAL (see Figure 3).
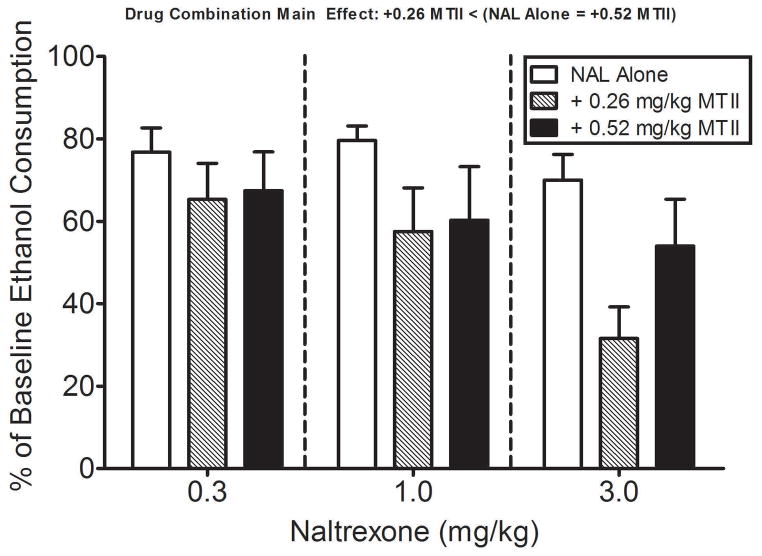
Fig. 2.
The effect of NAL alone, and in combination with the ED20 (0.26 mg/kg) and ED30 (0.52 mg/kg) doses of MTII, on binge-like ethanol intake. When administered in combination with selected doses of NAL (0.3 to 3.0 mg/kg), the ED20 dose of MTII produced leftward shifts in the NAL dose-effect curve. Based on the ED50 values, the 0.26 mg/kg dose of MTII shifted the NAL dose-effect curve (ED50 of 1.07 mg/kg, 0.50 – 2.27 C.L.) to the left by a factor of 7.6; that is, naltrexone was 7.6-fold more potent when administered in combination with MTII relative to when it was administered alone. On the other hand, the ED30 dose of MTII slightly decreased the effects of all doses of NAL to a similar extent. Ethanol consumption data are expressed as percent ethanol consumption (± SEM) on test day (day 4) relative to baseline ethanol consumption averaged over days 1–3 of the DID procedure. Data were analyzed with a two-way, 3 × 3 (NAL dose x MTII dose) ANOVA and post hoc Tukey’s test. Post hoc comparisons of the significant drug combination main effect revealed that combining the 0.26 mg/kg (ED20) dose of MTII with NAL increased the ability of NAL to blunt binge-like ethanol drinking relative to the NAL alone condition.
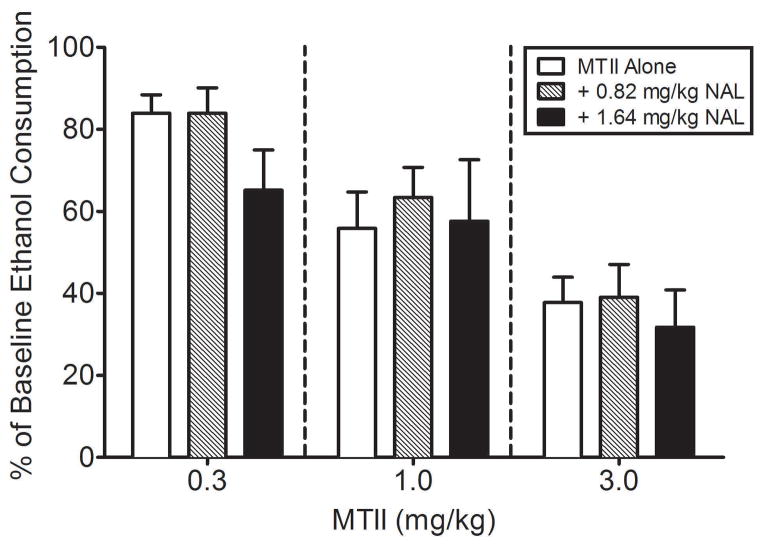
Fig. 3.
The effects of MTII alone, and in combination with the approximate ED20 (0.82 mg/kg) and ED30 (1.64 mg/kg) doses of NAL, on binge-like ethanol drinking. Neither ED20 nor ED30 doses of MTII had substantial influence on the NAL dose-effect curve. Ethanol consumption data are expressed as percent ethanol consumption (± SEM) on test day (day 4) relative to baseline ethanol consumption (averaged intake over days 1–3 of the DID procedure). Data were analyzed with a two-way, 3 × 3 (MTII dose x NAL dose) ANOVA and post hoc Tukey’s test. Post hoc comparisons of the significant MTII dose-response factor revealed that each of the MTII doses differed from each other, reflecting the dose-dependent blunting of binge-like ethanol drinking by MTII.
Statistical Analysis
To obtain a measure that corrected for individual differences in body weight, grams of ethanol consumed per kilogram of body weight were calculated. For all experiments, differences between groups in consumption or BECs were analyzed using analysis of variance (ANOVA). With significant interaction effects, or main effects in the absence of significant interactions, post hoc comparisons were performed using Tukey’s tests to parse out group differences. In all cases, p < 0.05 (two-tailed) was used to indicate statistical significance.
Isobolographic Analysis of Drug Interactions
The dose-addition model, which represents a widely accepted but non-mechanistic model of drug interactions (Tallarida, 2001, Tallarida, 2006, Loewe, 1953), was used to evaluate the interaction effects between MTII and NAL on binge-like ethanol drinking (Experiments 3 and 4). The dose-additional model can be used to assess how drugs interact (additive or synergistic) by plotting data in an isobologram (see Fig. 4). First, dose-effect curves were generated from the data in Figure 1, 2 and 3 by expressing the percentage decrease in ethanol consumption as a function of the dose of each drug or drug combination examined. For NAL and MTII alone, the dose that produced a 20, 30 and 50% (i.e., ED20, ED30, ED50) decrease in ethanol consumption was derived by log-linear interpolation on the linear portion of the group dose-effect curve. For drug combinations, only the ED50 dose was determined.
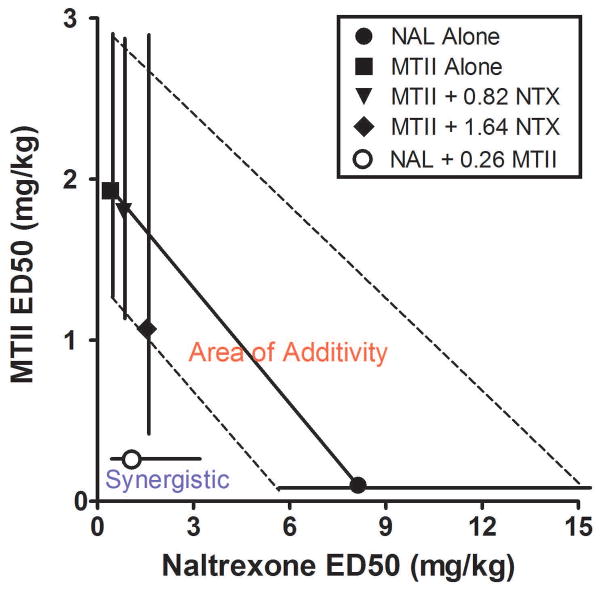
Fig. 4.
Isobolographic analysis of the effects of NAL and MTII administered in selected combinations. For this analysis, the ED50 and 95% confidence levels (C.L.) for the effects of NAL (solid circle) and MTII (solid square) alone on binge-like ethanol drinking were plotted along the abscissa and ordinate, respectively. Then the ED50 and 95% C.L. for drugs when tested in combination with the ED20 or ED30 of the other drug were plotted. The area between dashed lines that connect the 95% C.L. of the ED50 for each drug alone represents the “area of additivity”, the area in which drug interactions were considered to be additive. The region to the left of the dashed line defines synergistic drug interactions. The data show that the low ED20 dose of MTII (0.26 mg/kg) synergistically augmented the ability of NAL to blunt binge-like ethanol drinking, shifting the dose-response ED50 value of NAL to the left beyond the area of additivity and into the region representing synergistic drug interactions. Neither the low ED20 (0.82 mg/kg) nor high ED30 (1.64 mg/kg) doses of NAL synergistically altered the ED50 values for MTII-induced blunting of binge-like ethanol drinking, as the ED50 for these drug dose combinations fell within the area of additivity.
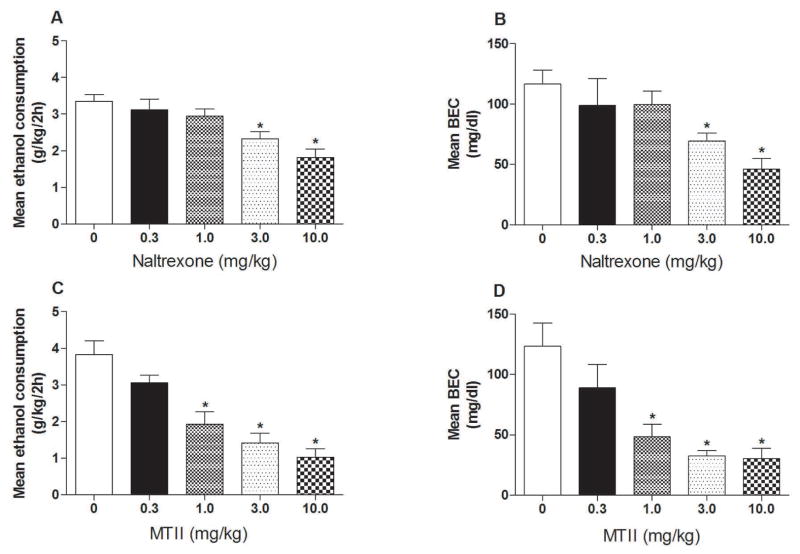
Fig. 1.
Intraperitoneal (i.p.) injection of naltrexone (NAL) or melanotan-II (MTII) blunt binge-like ethanol drinking (A & C) and associated blood ethanol concentrations (B & D) in C57BL/6J mice when administered over a range of concentrations (0, 0.3, 1.0, 3.0, & 10.0 mg/kg). Ethanol consumption is expressed as mean intake (± SEM) over the 2-h test in g/kg, and BECs are expressed as mean (± SEM) mg/dl of ethanol in blood. Data were analyzed with one-way ANOVAs and post hoc Tukey’s test. * P < 0.05 (two tailed).
Next, the ED50 and 95% confidence levels (C.L). for the effects of NAL (solid circle) and MTII (solid square) alone on binge-like ethanol drinking were plotted along the abscissa and ordinate, respectively. Then the ED50 and 95% C.L. for each drug when tested in combination with the ED20 or ED30 of the other drug were then plotted. Graphically, the solid diagonal line that connects the two ED50 points of MTII and NAL alone represents the dose combinations that would be predicted to decrease binge-like ethanol drinking by 50% if the two drugs interacted in an additive manner. The area between dashed lines that connect the 95% C.L. of the ED50 for each drug alone represents the “area of additivity”, or the area in which drug interactions were considered to be additive. The region to the left of the dashed line defines synergistic drug interactions. When the ED50 and C.L. for a particular drug combination fell within the area of additivity, the drug interaction was considered additive. When the ED50 and C.L.s for a particular drug combination fell to the left of the theoretical area of additivity, the interaction was considered to be supra-additive or synergistic.
RESULTS
Experiment 1 & 2: NAL and MTII Dose-Response Studies
Ethanol consumption and blood ethanol concentrations (BECs) from the NAL dose-response study are presented in the top row of Fig. 1(A–B). One-way ANOVAs performed on ethanol consumption [F(4, 49) = 8.24, p < 0.001] and BEC [F(4, 49) = 5.57, p < 0.05] data both achieved statistical significance. Post hoc analyses revealed that the groups treated with the 3.0 and 10.0 mg/kg doses of NAL drank significantly less ethanol and had lower BECs relative to the vehicle treated control group. Ethanol consumption and BECs from the MTII dose-response study are presented in the bottom row of Fig. 1(C–D). One-way ANOVAs performed on ethanol consumption [F(4, 53) = 16.04, p < 0.001] and BEC [F(4, 53) = 8.69, p < 0.001] data both achieved statistical significance. Post hoc analyses revealed that the groups treated with the 1.0, 3.0, and 10.0 mg/kg doses of MTII drank significantly less ethanol and had lower BECs relative to the vehicle treated control group. Based on effective dose (ED) ED50 values, MTII (1.92 mg/kg, 1.25 – 2.94 95% C.L.) was 4.2-fold more potent than NAL (8.13 mg/kg, 4.31 – 15.34 C.L.) in blunting binge-like ethanol drinking. MTII was also more effective, as the highest dose tested (10.0 mg/kg) produced a 72% decrease in ethanol consumption, whereas with NAL the maximal effect was only 47% (at the 10.0 mg/kg dose).
Experiment 3 & 4: NAL-MTII Interaction Studies
Experiment 3
Average baseline ethanol consumption (average of days 1–3 of DID testing) among the groups were as follows: 0.3 mg/kg NAL (4.02 ± 0.21, 3.15 ± 0.31, & 2.70 ± 0.32 g/kg at each dose level of MTII, respectively), 1.0 mg/kg NAL (3.70 ± 0.15, 3.15 ± 0.22, & 2.75 ± 0.26 g/kg at each dose level of MTII, respectively), and 3.0 mg/kg NAL (3.29 ± 0.25, 3.15 ± 0.23, & 2.50 ± 0.34 g/kg at each dose level of MTII, respectively). Fig. 2 shows the effects of NAL alone, and in combination with the approximate ED20 (0.26 mg/kg) and ED30 (0.52 mg/kg) doses of MTII, on binge-like ethanol intake. When administered in combination with selected doses of NAL (0.3 to 3.0 mg/kg), the ED20 dose of MTII produced leftward shifts in the NAL dose-effect curve. Based on the ED50 values, the 0.26 mg/kg dose of MTII shifted the NAL dose-effect curve (ED50 of 1.07 mg/kg, 0.50 – 2.27 C.L.) to the left by a factor of 7.6; that is, naltrexone was 7.6-fold more potent when administered in combination with MTII relative to when it was administered alone. The largest effect produced by this combination of NAL and MTII (68% reduction relative to control consumption), which was obtained at the 3.0 mg/kg dose of NAL, was considerably larger than that obtained when the 3.0 mg/kg dose of NAL was administered alone (30%). Somewhat different effects were obtained when doses of NAL were combined with the higher ED30 (0.52 mg/kg) dose of MTII, as each dose of NAL decreased the level of ethanol consumption to a similar extent, with the range across doses of 53% (0.3 mg/kg) to 67% (3.0 mg/kg). Consequently, an ED50 value for the NAL dose-response curve, when combined with the ED30 dose of MTII, could not be determined (and is thus absent from the isobolographic analysis shown in Fig. 4 below). Further, when compared to the NAL alone condition, the 0.52 mg/kg dose of MTII did not further increase the ability of NAL to reduce binge-like ethanol drinking. A two-way, 3 × 3 (NAL dose x MTII dose) ANOVA performed on the data in Fig. 2 revealed a significant main effect of NAL dose [F(2, 90) = 3.46, p < 0.05] and MTII dose [F(2, 90) = 5.64, p < 0.01], but the interaction effect did not attain statistical significance [F(4, 90) = 0.75, p > 0.05]. Post hoc comparisons of the MTII dose main effect revealed that combining the 0.26 mg/kg (ED20) dose of MTII with NAL increased the ability of NAL to blunt binge-like ethanol drinking relative to the NAL alone condition. Post hoc comparisons of the NAL dose main effect revealed no significant group differences.
Experiment 4
Average baseline ethanol consumption (average of days 1–3 of DID testing) among the groups were as follows: 0.3 mg/kg MTII (3.56 ± 0.31, 3.25 ± 0.20, & 2.77 ± 0.33 g/kg at each dose level of NAL, respectively), 1.0 mg/kg MTII (3.39 ± 0.26, 3.08 ± 0.23, & 2.69 ± 0.27 g/kg at each dose level of NAL, respectively), and 3.0 mg/kg MTII (3.52 ± 0.24, 3.71 ± 0.30, & 2.66 ± 0.25 g/kg at each dose level of NAL, respectively). Fig. 3 shows the effects of MTII alone, and in combination with the approximate ED20 (0.82 mg/kg) and ED30 (1.64 mg/kg) doses of NAL, on binge-like ethanol drinking. Based on the ED50 values, the 0.82 mg/kg (ED50 of 1.80 mg/kg, 1.11 – 2.91 C.L.) and 1.64 mg/kg (ED50 of 1.07 mg/kg, 0.39 – 2.93 C.L.) doses of NAL minimally shifted the MTII dose-effect curve (0.3 to 3.0 mg/kg) to the left by a factor of 1.08 and 1.79, respectively. The largest effect on binge-like ethanol drinking produced by 0.82 and 1.64 mg/kg doses of NAL (61% and 68% reduction of binge-like ethanol drinking relative to the control, respectively), which were obtained at the 3.0 mg/kg dose of MTII, were similar to that obtained when this dose of MTII was administered alone (61% reduction relative to the control condition). A two-way, 3 × 3 (MTII dose x NAL dose) ANOVA performed on the data in Fig. 3 revealed a significant main effect of MTII dose [F(2, 81) = 18.58, p < 0.001], but the NAL dose [F(2, 81) = 1.16, p > 0.05], and the interaction effect [F(4, 81) = 0.41, p > 0.05] did not achieve statistical significance. Post hoc comparisons of the MTII dose-response factor revealed that each of the MTII doses differed from each other, reflecting the dose-dependent blunting of binge-like ethanol drinking by MTII.
Isobolographic Analysis of Drug Interactions
Fig. 4 shows the isobolographic analysis of the effects of NAL and MTII administered in selected combinations. The low ED20 dose of MTII (0.26 mg/kg) synergistically augmented the ability of NAL to blunt binge-like ethanol drinking, shifting the ED50 value of NAL to the left beyond the area of additivity and into the region representing synergistic drug interactions. Although administering 0.52 (ED30) mg/kg of MTII in combination with selected doses of NAL decreased ethanol consumption in a dose-dependent manner, as noted above calculations of the ED50 values (and 95% C.L.) could not be obtained as all doses of MTII produced comparable decreases in ethanol consumption. Also evident in Fig. 4 is that neither the low ED20 (0.82 mg/kg) nor moderate ED30 (1.64 mg/kg) doses of NAL synergistically altered the ED50 values for MTII-induced blunting of binge-like ethanol drinking, as the ED50 for these drug dose combinations fell within the area of additivity, though the ED30 dose of NAL moved the ED50 for MTII to the borderline between additive and synergistic interactions. Taken together, these data show that a low dose of MTII synergistically augments the ability of NAL to blunt binge-like ethanol drinking.
Discussion
The outcomes from the experiments described herein are as follows: 1) In mice exhibiting binge-like levels of ethanol intake (i.e., consumption that led to BECs greater than 100 mg/dL under control conditions), both NAL and MTII significantly reduced binge-like ethanol drinking in a dose-dependent manner. As noted above, MTII was 4.2-fold more potent than NAL, and was more effective at reducing binge-like drinking. 2) When administered together, a low dose (0.26 mg/kg) of MTII increased the effectiveness of NAL to blunt binge-like ethanol drinking in mice. Based on the ED50 values, the 0.26 mg/kg dose of MTII shifted the NAL dose-effect curve to the left by a factor of 7.6, indicating that NAL was 7.6-fold more potent when administered in combination with MTII relative to when it was administered alone. Interestingly, a moderate dose of MTII (0.52 mg/kg) failed to increase the effectiveness of NAL in reducing binge-like ethanol drinking. 3) On the other hand, 0.82 and 1.64 mg/kg doses of NAL failed to alter the effectiveness of MTII in reducing binge-like ethanol drinking. However, the ED30 dose of NAL moved the ED50 for MTII to the borderline between additive and synergistic interactions (see Fig. 4), suggesting the possibility that slightly higher doses of NAL (e.g., ED35 or ED40) may also be effective in synergistically augmenting the effectiveness of MTII to reduce binge-like ethanol drinking. 4) Isobolographic analysis of the drug interactions confirmed that the 0.26 mg/kg dose of MTII synergistically augmented the ability of NAL to reduce binge-like ethanol drinking.
These data show that a low (ED20), but not moderate (ED30), dose of MTII increased the effectiveness of NAL in reducing excessive ethanol intake. This somewhat surprising observation is likely related to the binding properties of MTII to the different MCRs, and the different ways in which the MC3R versus the MC4R influence ethanol drinking. We have previously shown that MTII fails to reduce ethanol drinking in MC4R knockout mice (Navarro et al., 2011), and that a MC4R-selective agonist blunts ethanol drinking (Navarro et al., 2005), indicating that stimulation of MC4R signaling is protective against excessive ethanol intake. More recently, we found that MTII was more effective in blunting binge-like ethanol drinking in mutant mice lacking the MC3R (Olney et al., 2014), indicating that stimulation of the MC3R counteracts against the protective effects of MTII. While often considered to be a non-selective agonist, MTII has 5-fold to 7-fold higher affinity for the MC4R relative to the MC3R (Bednarek et al., 1999, Schioth et al., 1997). Thus, when given in lower subthreshold doses (e.g., the 0.26 mg/kg), MTII would be expected to have greater activity at the MC4R relative to the MC3R. At moderate subthreshold doses (e.g., the 0.52 mg/kg dose), MTII would be expected to be more likely to activate both the MC3R and MC4R. We speculate that the low dose of MTII synergistically augmented NAL-induced reductions of binge-like ethanol drinking through primary actions on the MC4R, but that the higher dose of MTII failed to alter the effectiveness of NAL because stimulation of the MC3R counteracted the beneficial effects of MC4R activity. While the mechanism underlying the interaction between MC3R and MC4R signaling is not clear, MC3Rs exert inhibitory control over neurons (Cowley et al., 2001), while MC4Rs can either excite or inhibit neurons depending on brain location (Kawashima et al., 2003). One possibility is that MC3Rs inhibit post-receptor excitation caused by MC4R activation on neurons that these receptors are co-expressed. On the other hand, when given at high enough doses, MTII alone reduces ethanol drinking (see Figure 1), suggesting that doses of MTII that clear threshold levels can overcome, at least in part, the opposing actions of MC3R activation on the MC4R.
We have previously shown the administration of MTII to mice does not alter blood ethanol levels (Navarro et al., 2003), suggesting that activation of MCRs does not alter the absorption of ethanol into the bloodstream or change the rate of ethanol metabolism and elimination. It has also been shown that NAL over a dose range of 1.5 to 4.5 mg/kg produced a small but significant reduction of ethanol absorption into the bloodstream of rats, an effect that was not dose-related. The authors concluded that the very modest and non-dose-dependent effect of NAL on BECs were not a sufficient condition to induce changes in ethanol intake (Linseman and Le, 1997). Further, reduced BECs would be expected to increase ethanol intake, yet NAL blunts ethanol drinking. Taken together, the effects of NAL and MTII on binge-like ethanol drinking, and the synergistic interaction between these drugs, are unlikely related to pharmacokinetics factors. We have also shown that MTII, in addition to reducing ethanol drinking, attenuates food intake, and sucrose (caloric) and saccharin (non-caloric) drinking, without influencing water intake (Navarro et al., 2011). Similarly, in addition to blunting binge-like ethanol drinking (Kamdar et al., 2007), NAL has been shown to reduce food (Tannenbaum and Pivorun, 1984) and saccharin (Yirmiya et al., 1988) intake in mice. Together, these observations suggest that MCR agonists and opioid antagonists reduce the consumption of salient reinforcers regardless of caloric content, and though not tested these observations would suggest that combined administration of MTII and NAL would also blunt consumption of a range of salient reinforcers. While the neurobiological mechanisms underlying the synergistic interaction between NAL and MTII are at present unknown, one interesting possibility involves interactions between the opioid and MC systems within the hypothalamus, as opioid receptors negatively regulate MC-producing neurons in this brain region (Ibrahim et al., 2003, Loose and Kelly, 1990, Kelly et al., 1990). Additionally, the opposing effects of NAL and MTII on G protein-coupled receptor signaling, as discussed in the introduction (Alvaro et al., 1997, Contreras and Takemori, 1984), represents another potential mechanism of synergistic interaction between these systems.
A large body of pre-clinical and clinical evidence demonstrate that NAL reduces ethanol drinking in humans, yet NAL based therapies are not successful with all individuals (Garbutt, 2009), and recent data suggest that NAL produces only modest reductions of ethanol drinking relative to placebo controls (Del Re et al., 2013). Thus, approaches that increase the effectiveness of NAL in managing excessive ethanol drinking would have high clinical value. We have shown that agonists of MCRs reduce excessive binge-like drinking in animal models, and we report here that the MCR agonist MTII synergistically augments the ability of NAL to blunt excessive ethanol drinking. These pre-clinical observations suggest that a) pharmaceutical compounds that stimulate MC signaling may be effective in reducing frequent binge drinking and heavy alcohol use in humans and b), that combining drugs that stimulate MC signaling with NAL may increase the overall effectiveness of NAL-based therapies for treating alcohol abuse disorders. However, it should be emphasized that the present study is only the first work suggesting a synergistic interaction between opioids and MC system in modulating of ethanol consumption. Additional proof-of-concept pre-clinical studies are clearly necessary before clinical applications are justifiable. Once sufficient pre-clinical data are gathered, and as safe MCR agonists become available, assessment of the effectiveness of these compounds, alone and in combination with NAL, in curbing alcohol abuse disorders should be considered. In fact, MK-0493, an orally bioavailable MC4R agonist, was recently tested in human phase I/II clinical trial studies for obesity treatment (Krishna et al., 2009) and may be effective in treating alcohol abuse disorders. Interestingly, as our studies employed an animal model of non-dependent binge drinking (Rhodes et al., 2005, Thiele and Navarro, 2014), our results specifically speak to the possibility that these targets could be useful for curbing excessive binge drinking, an approach that may be useful for preventing the transition to dependence. Such an approach has been argued as having the potential of being a more effective therapeutic strategy than treating ethanol dependence that has already emerged (Thiele, 2012, Thiele and Navarro, 2014). Future studies employing appropriate models will be necessary to determine if MC agonists, alone and in combination with NAL, represents an effective approach for curing dependence-induced excessive ethanol drinking.
In conclusion, here we provide evidence that a MCR agonist synergistically augments that ability of NAL to blunt excessive ethanol intake in a mouse model of binge ethanol drinking. While NAL-based therapies are effective in reducing abusive ethanol drinking, this approach is not effective in all individuals (Garbutt, 2009). The current observations suggest that activators of MC signaling may represent a new approach to treating alcohol abuse disorders, and a way to potentially improve existing NAL-based therapies. Interestingly, the drug Contrave, which combines NAL and bupropion (a drug which has been reported to increase MC signaling (Hasegawa et al., 2005, Billes and Cowley, 2007)) into one medication, has recently been approved by US Food and Drug Administration (FDA) for treating eating disorders (Gohil, 2014); repurposing this drug to treat alcohol abuse disorders represents an exciting possibility.
Acknowledgments
This work was supported by National Institute of Health grants AA022048, AA013573, and AA015148 and Department of Defense grant W81XWH-09-1-0293 to T.E.T., and AA022044 to M.N.
Footnotes
None of the authors have conflicts of interest related to the research described in this report.
References
- Alvaro JD, Tatro JB, Duman RS. Melanocortins and opiate addiction. Life Sci. 1997;61:1–9. doi: 10.1016/s0024-3205(97)00029-5. [DOI] [PubMed] [Google Scholar]
- Alvaro JD, Tatro JB, Quillan JM, Fogliano M, Eisenhard M, Lerner MR, Nestler EJ, Duman RS. Morphine down-regulates melanocortin-4 receptor expression in brain regions that mediate opiate addiction. Mol Pharmacol. 1996;50:583–591. [PubMed] [Google Scholar]
- Bednarek MA, Macneil T, Kalyani RN, Tang R, Van der Ploeg LH, Weinberg DH. Analogs of MTII, lactam derivatives of alpha-melanotropin, modified at the N-terminus, and their selectivity at human melanocortin receptors 3, 4, and 5. Biochem Biophys Res Com. 1999;261:209–213. doi: 10.1006/bbrc.1999.0981. [DOI] [PubMed] [Google Scholar]
- Billes SK, Cowley MA. Inhibition of dopamine and norepinephrine reuptake produces additive effects on energy balance in lean and obese mice. Neuropsychopharmacology. 2007;32:822–834. doi: 10.1038/sj.npp.1301155. [DOI] [PubMed] [Google Scholar]
- Bouchery EE, Harwood HJ, Sacks JJ, Simon CJ, Brewer RD. Economic costs of excessive alcohol consumption in the U.S., 2006. Am J Prev Med. 2011;41:516–524. doi: 10.1016/j.amepre.2011.06.045. [DOI] [PubMed] [Google Scholar]
- Contreras PC, Takemori AE. Antagonism of morphine-induced analgesia, tolerance and dependence by alpha-melanocyte-stimulating hormone. JPET. 1984;229:21–26. [PubMed] [Google Scholar]
- Cowley MA, Smart JL, Rubinstein M, Cerdan MG, Diano S, Horvath TL, Cone RD, Low MJ. Leptin activates anorexigenic POMC neurons through a neural network in the arcuate nucleus. Nature. 2001;411:480–484. doi: 10.1038/35078085. [DOI] [PubMed] [Google Scholar]
- Cubero I, Navarro M, Carvajal F, Lerma-Cabrera JM, Thiele TE. Ethanol-induced increase of agouti-related protein (AgRP) immunoreactivity in the arcuate nucleus of the hypothalamus of C57BL/6J, but not 129/SvJ, inbred mice. Alcohol Clin Exp Res. 2010;34:693–701. doi: 10.1111/j.1530-0277.2009.01138.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Re AC, Maisel N, Blodgett J, Finney J. The declining efficacy of naltrexone pharmacotherapy for alcohol use disorders over time: a multivariate meta-analysis. Alcohol Clin Exp Res. 2013;37:1064–1068. doi: 10.1111/acer.12067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dores RM, Jain M, Akil H. Characterization of the forms of beta-endorphin and alpha-MSH in the caudal medulla of the rat and guinea pig. Brain Res. 1986;377:251–260. doi: 10.1016/0006-8993(86)90866-8. [DOI] [PubMed] [Google Scholar]
- Ercil NE, Galici R, Kesterson RA. HS014, a selective melanocortin-4 (MC4) receptor antagonist, modulates the behavioral effects of morphine in mice. Psychopharmacology (Berl) 2005;180:279–285. doi: 10.1007/s00213-005-2166-x. [DOI] [PubMed] [Google Scholar]
- Froehlich JC, Li TK. Recent developments in alcoholism:opioid peptides. Recent Dev Alcohol. 1993;11:187–205. [PubMed] [Google Scholar]
- Garbutt JC. The state of pharmacotherapy for the treatment of alcohol dependence. J Subst Abuse Treat. 2009;36:S15–23. quiz S24–15. [PubMed] [Google Scholar]
- Garbutt JC. Efficacy and tolerability of naltrexone in the management of alcohol dependence. Curr Pharm Des. 2010;16:2091–2097. doi: 10.2174/138161210791516459. [DOI] [PubMed] [Google Scholar]
- Gelez H, Poirier S, Facchinetti P, Allers KA, Wayman C, Bernabe J, Alexandre L, Giuliano F. Neuroanatomical distribution of the melanocortin-4 receptors in male and female rodent brain. J Chem Neuroanat. 2010;40:310–324. doi: 10.1016/j.jchemneu.2010.09.002. [DOI] [PubMed] [Google Scholar]
- Gianoulakis C. Influence of the endogenous opioid sytem on high alcohol consumption and genetic predisposition to alcoholism. J Psychiatry Neurosci. 2001;26:304–318. [PMC free article] [PubMed] [Google Scholar]
- Gohil K. Pharmaceutical approval update. P & T: a peer-reviewed journal for formulary management. 2014;39:746–772. [PMC free article] [PubMed] [Google Scholar]
- Grant BF, Stinson FS, Dawson DA, Chou SP, Dufour MC, Compton W, Pickering RP, Kaplan K. Prevalence and co-occurrence of substance use disorders and independent mood and anxiety disorders: results from the National Epidemiologic Survey on Alcohol and Related Conditions. Arch Gen Psychiatry. 2004;61:807–816. doi: 10.1001/archpsyc.61.8.807. [DOI] [PubMed] [Google Scholar]
- Greenway FL, Dunayevich E, Tollefson G, Erickson J, Guttadauria M, Fujioka K, Cowley MA. Comparison of combined bupropion and naltrexone therapy for obesity with monotherapy and placebo. J Clin Endocrinol Metab. 2009;94:4898–4906. doi: 10.1210/jc.2009-1350. [DOI] [PubMed] [Google Scholar]
- Greenway FL, Fujioka K, Plodkowski RA, Mudaliar S, Guttadauria M, Erickson J, Kim DD, Dunayevich E. Effect of naltrexone plus bupropion on weight loss in overweight and obese adults (COR-I): a multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2010;376:595–605. doi: 10.1016/S0140-6736(10)60888-4. [DOI] [PubMed] [Google Scholar]
- Hadley ME, Haskell-Luevano C. The proopiomelanocortin system. Ann NY Acad Sci. 1999;885:1–21. doi: 10.1111/j.1749-6632.1999.tb08662.x. [DOI] [PubMed] [Google Scholar]
- Hall FS, Sora I, Uhl GR. Ethanol consumption and reward are decreased in mu-opiate receptor knockout mice. Psychopharmacology. 2001;154:43–49. doi: 10.1007/s002130000622. [DOI] [PubMed] [Google Scholar]
- Hasegawa H, Meeusen R, Sarre S, Diltoer M, Piacentini MF, Michotte Y. Acute dopamine/norepinephrine reuptake inhibition increases brain and core temperature in rats. J Appl Physiol. 2005;99:1397–1401. doi: 10.1152/japplphysiol.00435.2005. [DOI] [PubMed] [Google Scholar]
- Ibrahim N, Bosch MA, Smart JL, Qiu J, Rubinstein M, Ronnekleiv OK, Low MJ, Kelly MJ. Hypothalamic proopiomelanocortin neurons are glucose responsive and express K(ATP) channels. Endocrinology. 2003;144:1331–1340. doi: 10.1210/en.2002-221033. [DOI] [PubMed] [Google Scholar]
- Kamdar NK, Miller SA, Syed YM, Bhayana R, Gupta T, Rhodes JS. Acute effects of naltrexone and GBR 12909 on ethanol drinking-in-the-dark in C57BL/6J mice. Psychopharmacology (Berl) 2007;192:207–217. doi: 10.1007/s00213-007-0711-5. [DOI] [PubMed] [Google Scholar]
- Kawashima N, Chaki S, Okuyama S. Electrophysiological effects of melanocortin receptor ligands on neuronal activities of monoaminergic neurons in rats. Neurosci Lett. 2003;353:119–122. doi: 10.1016/j.neulet.2003.09.024. [DOI] [PubMed] [Google Scholar]
- Kelly MJ, Loose MD, Ronnekleiv OK. Opioids hyperpolarize beta-endorphin neurons via mu-receptor activation of a potassium conductance. Neuroendocrinology. 1990;52:268–275. doi: 10.1159/000125597. [DOI] [PubMed] [Google Scholar]
- Khachaturian H, Alessi NE, Lewis ME, Munfakh N, Fitzsimmons MD, Watson SJ. Development of hypothalamic opioid neurons: a combined immunocytochemical and [3H]thymidine autoradiographic study. Neuropeptides. 1985;5:477–480. doi: 10.1016/0143-4179(85)90058-7. [DOI] [PubMed] [Google Scholar]
- Krishna R, Gumbiner B, Stevens C, Musser B, Mallick M, Suryawanshi S, Maganti L, Zhu H, Han TH, Scherer L, Simpson B, Cosgrove D, Gottesdiener K, Amatruda J, Rolls BJ, Blundell J, Bray GA, Fujioka K, Heymsfield SB, Wagner JA, Herman GA. Potent and selective agonism of the melanocortin receptor 4 with MK-0493 does not induce weight loss in obese human subjects: energy intake predicts lack of weight loss efficacy. Clin Pharmacol Ther. 2009;86:659–666. doi: 10.1038/clpt.2009.167. [DOI] [PubMed] [Google Scholar]
- Linseman MA, Le AD. Effects of opioids on the absorption of alcohol. Pharmacol Biochem Behav. 1997;58:79–84. doi: 10.1016/s0091-3057(97)00002-6. [DOI] [PubMed] [Google Scholar]
- Lippert RN, Ellacott KL, Cone RD. Gender-specific roles for the melanocortin-3 receptor in the regulation of the mesolimbic dopamine system in mice. Endocrinology. 2014;155:1718–1727. doi: 10.1210/en.2013-2049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loewe S. The problem of synergism and antagonism of combined drugs. Arzneimittelforsch. 1953;3:285–290. [PubMed] [Google Scholar]
- Loose MD, Kelly MJ. Opioids act at mu-receptors to hyperpolarize arcuate neurons via an inwardly rectifying potassium conductance. Brain Res. 1990;513:15–23. doi: 10.1016/0006-8993(90)91084-t. [DOI] [PubMed] [Google Scholar]
- Mogil JS, Ritchie J, Smith SB, Strasburg K, Kaplan L, Wallace MR, Romberg RR, Bijl H, Sarton EY, Fillingim RB, Dahan A. Melanocortin-1 receptor gene variants affect pain and mu-opioid analgesia in mice and humans. J Med Genet. 2005;42:583–587. doi: 10.1136/jmg.2004.027698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mokdad AH, Marks JS, Stroup DF, Gerberding JL. Actual causes of death in the United States, 2000. JAMA. 2004;291:1238–1245. doi: 10.1001/jama.291.10.1238. [DOI] [PubMed] [Google Scholar]
- Navarro M, Cubero I, Chen AS, Chen HY, Knapp DJ, Breese GR, Marsh DJ, Thiele TE. Effects of melanocortin receptor activation and blockade on ethanol intake: A possible role for the melanocortin-4 receptor. Alcohol Clin Exp Res. 2005;29:949–957. doi: 10.1097/01.ALC.0000167740.19702.8C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarro M, Cubero I, Knapp DJ, Breese GR, Thiele TE. Decreased immunoreactivity of the melanocortin neuropeptide alpha-melanocyte-stimulating hormone (alpha-MSH) after chronic ethanol exposure in Sprague-Dawley rats. Alcohol Clin Exp Res. 2008;32:266–276. doi: 10.1111/j.1530-0277.2007.00578.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarro M, Cubero I, Knapp DJ, Thiele TE. MTII-induced reduction of voluntary ethanol drinking is blocked by pretreatment with AgRP-(83–132) Neuropeptides. 2003;37:338–344. doi: 10.1016/j.npep.2003.10.003. [DOI] [PubMed] [Google Scholar]
- Navarro M, Cubero I, Ko L, Thiele TE. Deletion of agouti-related protein blunts ethanol self-administration and binge-like drinking in mice. Genes Brain Behav. 2009;8:450–458. doi: 10.1111/j.1601-183X.2009.00493.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarro M, Lerma-Cabrera JM, Carvajal F, Lowery EG, Cubero I, Thiele TE. Assessment of voluntary ethanol consumption and the effects of a melanocortin (MC) receptor agonist on ethanol intake in mutant C57BL/6J mice lacking the MC-4 receptor. Alcohol Clin Exp Res. 2011;35:1058–1066. doi: 10.1111/j.1530-0277.2011.01438.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olney JJ, Sprow GM, Navarro M, Thiele TE. The protective effects of the melanocortin receptor (MCR) agonist, melanotan-II (MTII), against binge-like ethanol drinking are facilitated by deletion of the MC3 receptor in mice. Neuropeptides. 2014;48:47–51. doi: 10.1016/j.npep.2013.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu H, Li J, Chen W, Li Y, Jiang Q, Jiang H, Huo J, Zhao Z, Liu B, Zhang Q. Differential expression of the melanocortin-4 receptor in male and female C57BL/6J mice. Mol Biol Rep. 2014;41:3245–3256. doi: 10.1007/s11033-014-3187-5. [DOI] [PubMed] [Google Scholar]
- Rasmussen DD, Boldt BM, Wilkinson CW, Mitton DR. Chronic daily ethanol and withdrawal: 3. Forebrain pro-opiomelanocortin gene expression and implications for dependence, relapse, and deprivation effect. Alcoholism: Clinical & Experimental Research. 2002;26:535–546. [PubMed] [Google Scholar]
- Rhodes JS, Best K, Belknap JK, Finn DA, Crabbe JC. Evaluation of a simple model of ethanol drinking to intoxication in C57BL/6J mice. Physiol Behav. 2005;84:53–63. doi: 10.1016/j.physbeh.2004.10.007. [DOI] [PubMed] [Google Scholar]
- Roberts AJ, McDonald JS, Heyser CJ, Kieffer BL, Matthes HW, Koob GF, Gold LH. mu-Opioid receptor knockout mice do not self-administer alcohol. J Pharm Exp Ther. 2000;293:1002–1008. [PubMed] [Google Scholar]
- Schioth HB, Muceniece R, Mutulis F, Prusis P, Lindeberg G, Sharma SD, Hruby VJ, Wikberg JE. Selectivity of cyclic [D-Nal7] and [D-Phe7] substituted MSH analogues for the melanocortin receptor subtypes. Peptides. 1997;18:1009–1013. doi: 10.1016/s0196-9781(97)00079-x. [DOI] [PubMed] [Google Scholar]
- Starowicz K, Obara I, Przewlocki R, Przewlocka B. Inhibition of morphine tolerance by spinal melanocortin receptor blockade. Pain. 2005;117:401–411. doi: 10.1016/j.pain.2005.07.003. [DOI] [PubMed] [Google Scholar]
- Starowicz K, Sieja A, Bilecki W, Obara I, Przewlocka B. The effect of morphine on MC4 and CRF receptor mRNAs in the rat amygdala and attenuation of tolerance after their blockade. Brain Res. 2003;990:113–119. doi: 10.1016/s0006-8993(03)03444-9. [DOI] [PubMed] [Google Scholar]
- Tallarida RJ. Drug synergism: its detection and applications. J Pharmacol Exp Ther. 2001;298:865–872. [PubMed] [Google Scholar]
- Tallarida RJ. An overview of drug combination analysis with isobolograms. J Pharmacol Exp Ther. 2006;319:1–7. doi: 10.1124/jpet.106.104117. [DOI] [PubMed] [Google Scholar]
- Tannenbaum MG, Pivorun EB. Effect of naltrexone on food intake and hoarding in white-footed mice (Peromyscus) Pharmacol Biochem Behav. 1984;20:35–37. doi: 10.1016/0091-3057(84)90096-0. [DOI] [PubMed] [Google Scholar]
- Thiele TE. Commentary: studies on binge-like ethanol drinking may help to identify the neurobiological mechanisms underlying the transition to dependence. Alcohol Clin Exp Res. 2012;36:193–196. doi: 10.1111/j.1530-0277.2011.01734.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiele TE, Crabbe JC, Boehm SL., 2nd . “Drinking in the Dark” (DID): a simple mouse model of binge-like alcohol intake. In: Crawley Jacqueline N, et al., editors. Current protocols in neuroscience. 9. Vol. 68. 2014. pp. 49 41–49 49 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiele TE, Navarro M. “Drinking in the dark” (DID) procedures: A model of binge-like ethanol drinking in non-dependent mice. Alcohol. 2014;48:235–241. doi: 10.1016/j.alcohol.2013.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yirmiya R, Lieblich I, Liebeskind JC. Reduced saccharin preference in CXBK (opioid receptor-deficient) mice. Brain Res. 1988;438:339–342. doi: 10.1016/0006-8993(88)91360-1. [DOI] [PubMed] [Google Scholar]