Abstract
Background
Pediatric, adolescent and young adult (AYA) survivors of bone sarcomas are at risk for poor quality of life (QOL). We conducted a systematic review and meta-analysis to summarize the literature describing QOL in this population and differences in QOL based on local control procedures.
Procedure
Included studies described ≥5 patients <25 years-old who had completed local control treatment for bone sarcoma, defined QOL as a main outcome, and measured it with a validated instrument. Data extraction and quality assessments were conducted with standardized tools. Meta-analyses compared QOL based on surgical procedure (limb-sparing versus amputation) and were stratified by assessment type (objective physical function, clinician-assessed disability, patient-reported disability and patient-reported QOL). Effect sizes were reported as the Standard Mean Difference when multiple instruments were used within a comparison and Weighted Mean Difference otherwise. All were weighted by inverse variance and modeled with random effects.
Results
Twenty-two of 452 unique manuscripts were included in qualitative syntheses, 8 of which were included in meta-analyses. Manuscripts were heterogeneous with respect to included patient populations (age, tumor type, time since treatment) and QOL instruments. Prospective studies suggested that QOL improves over time, and that female sex and older age at diagnosis are associated with poor QOL. Meta-analyses showed no differences in outcomes between patients who underwent limb-sparing versus amputation for local control.
Conclusion
QOL studies among children and AYAs with bone sarcoma are remarkably diverse, making it difficult to detect trends in patient outcomes. Future research should focus on standardized QOL instruments and interpretations.
Keywords: Quality of Life, pediatric cancer, late effects, sarcoma, bone cancer, osteosarcoma, Ewing Sarcoma, survivorship, patient-reported outcomes
Introduction
Bone sarcomas represent fewer than 10% of pediatric cancers, but a disproportionately higher proportion of childhood cancer survivors with long-term disabilities.[1,2] Osteosarcoma and Ewing sarcoma are the most common bone cancers and are commonly diagnosed among adolescents and young adults (AYAs). Patients with these diagnoses are at high risk of poor quality of life (QOL) compared to other childhood cancer survivors and the general population, in part because their intensive chemotherapies may be associated with frequent admissions for febrile neutropenia and risks of cardiac dysfunction, infertility, and recurrent malignancy.[3–7]
Treatment for bone sarcoma also includes local control modalities such as surgery and/or radiation therapy, both of which are associated with short- and long-term risks.[8] Although there is little evidence that one specific surgical local control modality is superior with respect to overall survival, different approaches may be associated with different rates and types of long-term morbidity.[9] The equipoise in efficacy of treatment options creates an opportunity to select those that will minimize late effects and improve patient QOL. Such selection has become an imperative for cancer treatment.[10]
The construct of QOL integrates physical, functional, emotional, social, spiritual, and socio-economic well-being. Comparatively little QOL research has been conducted specifically among survivors of sarcoma who were diagnosed as children or AYAs. Furthermore, existing reports have been inconsistent, likely due to heterogeneity of patient populations and instrument selection.[11–14] The objectives of this systematic review and meta-analysis were to: (1) Summarize the literature describing QOL among pediatric and AYA survivors of bone sarcoma; and, (2) Describe differences in QOL based on surgical procedure (e.g., limb-sparing versus amputation). These findings could inform clinical decision-making, optimize patient survivorship, and guide future research endeavors aimed at improving QOL among patients with bone sarcoma.
Methods
Data Sources and Searches
We followed Cochrane and PRISMA guidelines for the conduct of systematic reviews.[15] A medical librarian developed and executed electronic search strategies. Ovid Medline, Cochrane Database of Systematic Reviews, PsycInfo, Embase, and CINAHL electronic databases were searched for publications between January 1, 2004 and February 27, 2014. This timeline was selected a priori, in order to capture studies reflecting contemporary treatment regimens. Searches were limited to pediatric age range (0–18, with young adults up to age 24 years included where possible) and English language. Retrieval was limited to full text publications; abstracts, meeting presentations, and dissertations were excluded. The search strategy included the following Medical Subject Heading (MeSH) terms: sarcomas, bone neoplasms, soft tissue neoplasms, and quality of life. We also used concepts commonly related to quality of life, including well-being, pain, physical function, activity, and mental health (Supplemental Appendix 1).
Study Selection
We defined inclusion and exclusion criteria a priori. Studies were included if they: (1) described a sample of at least 5 pediatric or young adult patients with osteosarcoma or Ewing Sarcoma; (2) described patients who had completed local control (e.g., surgery and/or radiation therapy) of their tumor; and, (3) defined “quality of life” as a main outcome and measured it with a validated instrument. There was no restriction by study design, inclusion of other tumor types, or inclusion of older adult patients as long as characteristics of pediatric/AYA patients with bone tumors were available. Two authors independently evaluated all titles and abstracts identified by the search strategy; any publication thought to be potentially relevant by either reviewer was retrieved and reviewed in full. Final inclusion of studies was determined by agreement of both reviewers.
Data Extraction and Quality Assessment
Data were extracted from included manuscripts using a modified version of the Cochrane Review template form which included fields for study design, objectives, patient characteristics, QOL and other validated instruments used, main findings, and key limitations (Supplemental Appendix 2).[15] We extracted only reported data; no authors were contacted to obtain additional unreported information.
We assessed study bias and quality with a tool adapted from the “Quality in Prognosis Studies” (QUIPS) and “Strengthening the Reporting of Observational Studies in Epidemiology” (STROBE) reports (Supplemental Appendix 2).[16,17] We defined a possible range of scores (0–44) and corresponding overall quality assessments a priori: <30 points (60%ile) was defined as “poor,” 30–34 points (70%ile) as “moderate,” 35–38 points (80%ile) as “good,” and >38 points as “high” quality. Strength of agreement between reviewers was evaluated with the Kappa statistic.
Data Synthesis Analyses
All identified studies were included in qualitative analysis; only those that included direct comparisons of QOL based on local control modality were included in meta-analyses. In cases where the same population was described in multiple studies, we selected the most recent; no patient population was included more than once in the same meta-analysis. Surgery types were defined as “Limb-Sparing” or “Limb-Salvage” (LS, including endoprosthesis, allograft surgery, and “other conservative surgery”), “Amputation” or “Ablation” [Amp, including Above the Knee Amputation (AKA) and Below the Knee Amputation (BKA)], and Rotationplasty (RP). Synthesis focused on comparing outcomes among patients treated with LS vs. Amp for local control. Data for other patient-groups were extracted when reported in the manuscripts; however, meta-analyses were only conducted when at least 3 papers explicitly described a given population. For example, only 2 papers described patients who were treated with RP separately from other surgical procedures; hence, no meta-analyses comparing RP versus other surgery types were conducted.[18,19] When multiple patient-groups were described in a single manuscript, we included the sub-groups with the largest n for pooled analyses to avoid double-counting. For example, one study compared multiple subgroups of patients (AKA vs. BKA vs. LS-femur vs. LS-tibia vs. RP).[19] In this case, the LS-femur and BKA groups were included in meta-analyses because they were the largest LS and Amp populations in the study, respectively.
QOL among adult sarcoma patients has typically been operationalized and measured in four distinct ways: (1) Objective physical function, measured by physiologic or activity-based tests conducted in the clinic; (2) Clinician-assessed disability, often measured by the Musculoskeletal Tumor Society (MSTS) questionnaire; (3) Patient-reported disability, commonly measured by the Toronto Extremity Salvage Score (TESS) among patients treated with lower extremity orthopedic surgery; and, (4) Patient-reported generic or cancer-specific health-related QOL, frequently measured by the generic Medical Outcomes Study Short-Form 36 (SF-36), the Health Utilities Index (HUI),the Functional Assessment of Cancer Therapy (FACT) scale, and/or the European Organization for Research and Treatment of Cancer (EORTC) quality of life scale among adults, and the Pediatric Quality of Life Inventory (Peds-QL) among children.[20–26]
The primary outcome of interest was QOL, as defined by a previously validated instrument. We stratified quantitative analyses based on each study’s operationalization of QOL and its corresponding instrument type, as described above. In order to minimize heterogeneity, we selected the SF-36 measure when it and other QOL instruments were described within a single population. Meta-analyses used instrument scores as continuous variables. The mean and standard deviation (SD) were used as measures of central tendency; where relevant, we assumed the mean could be approximated by the reported median, the range contains 6 SDs, and the 95% Confidence Interval (CI) contains 4 Standard Errors (SE). Where different instruments were used within a comparison, we used standard mean differences (SMD) to describe instrument scores among patients who received radiotherapy vs. surgery and LS vs. Amp surgery. The SMD standardizes study results to a uniform scale before they are combined and expresses differences in mean outcomes between groups relative to study variability. Where the same instrument was used, the weighted mean difference (WMD) was used. Effect sizes were weighted by inverse variance. Because we anticipated heterogeneity between studies, we used a random-effects model for all analyses.[27] Meta-analyses were performed using Review Manager, version 5.3 (Cochrane Collaboration, Oxford, United Kingdom). Kappa statistics were conducted using the STATA statistical software program (StataCorp, College Station, Texas).
Results
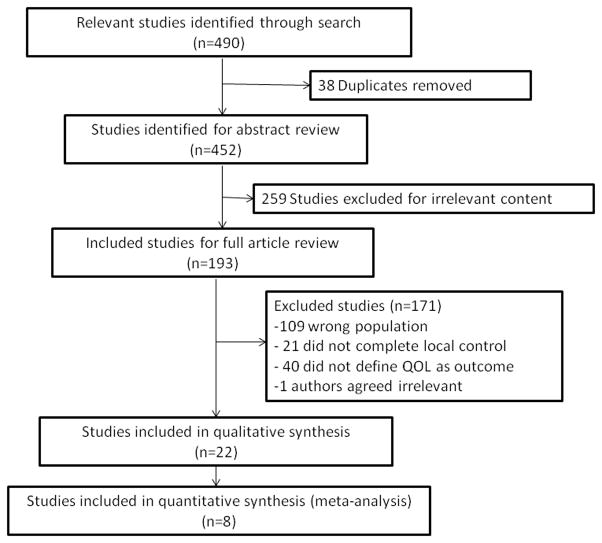
Our search methods identified 452 unique publications (Figure 1). Of these, 193 full-texts were retrieved and 22 included in qualitative synthesis. Agreement between the two reviewers regarding inclusion of articles was excellent (Kappa=0.95, 95% CI 0.94–0.97). Agreement regarding the quality of papers was moderate (Kappa=0.51; 95% CI 0.43–0.63).
Figure 1.
Study Flow Diagram
Objective 1
Of the 22 included studies, 10 described trajectories of QOL during survivorship, comparisons of sarcoma patients to population norms or other cancer survivors, or both (Table I).[11,28–36] Study populations, QOL assessments, and findings were heterogeneous. Some studies were limited to discrete tumor histologies (e.g., only osteosarcoma) whereas others included a variety of sarcomas including osteosarcoma, Ewing sarcoma, and tumors amenable to surgical treatment without concurrent chemotherapy (e.g., giant cell tumors).[11,28–32,35,36] Indeed, treatment histories ranged from surgery alone to multi-modal regimens to high-dose chemotherapy with autologous stem cell rescue. Studies described patients with a variety of tumor locations, ranging from localized lower extremity tumors to widespread metastatic or recurrent disease. Patient age and time since treatment varied as well, with mean ages at diagnosis ranging from 11 to 22 years, and mean time between treatment and study-assessments ranging from <1 to >20 years.[13,18,37,38]
Table I.
Studies describing outcomes over time, compared to population norms, or compared to other cancer-types (in order of publication year)
| Author/ Year |
Study Design |
Population | Assessments | Main Findings | Notes and Limitations | |||
|---|---|---|---|---|---|---|---|---|
| Objective Function |
Clinician- Assessed Disability |
PRO Disability |
PRO QOL | |||||
| Marchese/200425 | Cross-sectional | OS survivors (n=18), median age at diagnosis 12 y (range 5–19); median age at assessment 18 y (range 10–27), median interval since surgery 5 y (range 2–10) | TUG, TUDS, 9-minute run-walk, rate of perceived exertion, PCI | MSTS | . | SF-36 | MSTS did not correlate with quantitative functional outcomes or SF-36. Subjects with higher vitality on SF-36 walked more efficiently and farther, and performed the TUDS and TUG with more speed. | No raw instrument scores reported, only correlative data between instruments. Heterogeneous sample (e.g., multiple surgery types, treatments). Population also described in Marchese/06 and Ginsberg/07. |
| Marchese/200626 | Cross-sectional | LE sarcoma (OS/ES/Synovial Sarcoma) s/p LS surgery, mean age at diagnosis 18.7 y (SD=3.9); mean time since surgery 4.6 y (SD=2.7) | TUG, TUDS, 9-minute run-walk, rate of perceived exertion, PCI | MSTS | . | SF-36 | Range of motion correlates with functional mobility and QOL scores. | Population also described in Marchese/04 and Ginsberg/07 |
| Gerber/200627 | Cross-sectional | ES, Rhabdomyosaroma, other sarcoma patients (n=32); mean age at diagnosis 16.2 (SD=5.2); mean age at study 35.4 y (SD=10.6) | 6-min walk, manual muscle test, grip strength, ROM, limb volume, AMPS | . | HAP, SIP, LSM, vocation development | . | 67% of patients reported moderate-severe loss of range of motion; half at least 1 SD below normal grip strength, half with significantly reduced activity. Motor/Process skills lower, but leisure satisfaction higher than population norms. Loss of abilities associated with negative impact on vocational activities. | Heterogeneous patient group with various diagnosis, treatment experiences (not all treated surgically). Scores reported without measure of variance. |
| Aksnes/200728 | Cross-sectional | Mean age at diagnosis/time from diagnosis, respectively: UE/LE bone tumor survivors (n=57): males −20 y (SD=8.2)/14 y (SD=4.5), females −16 y (SD=4.5/12 y (SD=5.8); Hodgkin Lymphoma survivors (n=89): males −23 y (S=6.3)/12 y (SD=5.8), females 21 y (SD=5.0)/9 y (SD=4.1); Testicular cancer (n=62): 25 y (SD=7.0)/10 y (SD=3.4) | . | . | . | SF-36, HADS, fatigue | Bone tumor survivors reported more fatigue and less depression than population norms, not different from other cancer survivors. Bone tumor survivors reported lower PCS (SF-36) than all other groups. Older age at survey, female, bone tumor survivor, and unemployment were all associated with inferior PCS (SF-36). | Heterogeneous patient groups (e.g., bone tumor group included various locations, diagnoses and surgery types); differences noted between populations based on age and sex. |
| Frances/200729 | Prospective | UE/LE/pelvic bone tumor patients (n=43, OS/ES/chondrosarcoma), mean age at surgery 12 y (range 6–16). Assessment at time of surgery, then annually | . | . | PODCI | . | Sports/physical functioning relatively lower in first year compared to other domains of instrument. Larger size tumor and LE location associated with poorer function. Females reported lower scores in sports/physical function, pain, and global function compared to males. | PODCI selected for specificity for musculoskeletal health and pediatric focus; includes some parent report. Although not described as objective, tables include comparisons to population norms and patients with non-oncologic orthopedic diagnoses; bone tumor survivors report worse sports function than other groups. Scores reported without measure of variance. |
| Hinds/200930 | Prospective | Bone tumor patients (n=66, specific diagnoses unclear); mean age at diagnosis 13.3 y (SD=3.9); assessment at diagnosis, weeks 12 and 23, and end-of-therapy | . | . | . | Peds-QL | Patients reported improved physical, emotional, and school domains, worsening nausea from diagnosis through week 23. Symptom distress decreased from diagnosis to weeks 12 and 23 in majority of patients. No sex- or age-related differences except age>12 associated with less procedure anxiety. Parent-patient concordance moderate-good, except in report of treatment anxiety where patients reported less anxiety than parents. | Tumor types not explicitly described. |
| Koopman/200931 | Prospective cohort | Bone tumor survivors (n=20), mean age at diagnosis 14.4 (SD=2.7); assessment at 3- and 8-years following end of therapy; Age/Sex matched population controls (n=1122) | . | . | . | TAC-/TAQ-QOL, Utrecht coping list for Adolescents and Young Adults | Compared to controls, patients and their parents reported inferior motor functioning and autonomy; parents reported children had lower cognitive function and fewer positive emotions. At 8 year mark, young adults reported comparable motor function and autonomy, and higher cognitive, social scores, fewer negative emotions. | Group scores reported without measure of variance. |
| Nagarajan/200915 | Cross-sectional | CCSS participants with LE OS/ES (n=528); mean age at diagnosis 13.5 y (SD=3.8); mean time since diagnosis 20.8 y (SD=4.3) | . | . | TESS, RNL | QOL-CS | Bone tumor survivors reported mild impairment compared to population norms in RNL global function, daily function, and self-perception scales. Female sex and increasing age associated with lower RNL scores. Global function only moderately correlated with physical performance and QOL. | Population also described in Nagarajan/04. |
| Sun/201232 | Prospective cohort | LE OS/ES (n=344), mean age at diagnosis 18.7 y (SD=4.9), assessment 1 year following diagnosis; compared to age/sex-matched controls admitted to hospital at same time for other reasons (n=361), mean age 17.6 (SD=5.8) | . | . | TESS | SF-36 | For bone tumor patients, all QOL scales improve over year but remain significantly lower than controls. No differences in TESS scores at 1 year compared to controls. Older age, female sex and amputation surgery associated with poorer MCS scores. TESS scores correlated with PCS and MCS. | Controls only had one assessment at time of hospitalization; no comparison of trajectories between cancer patients and controls. TESS not conducted at baseline - only at follow-up assessment. |
| Bekkering/201233 | Prospective | LE OS/ES (n=44), mean age at surgery 14.9 y (SD=4.8); assessment at 3, 6, 9, 12, 18, and 24 months post-operatively | 6-minute walk, Actilog, Uptime, PCI | TESS, Baeke physical activity | SF-36, TAC-/TAQ-QOL, Bt-DUX | Over 1st year, QOL, functional ability, physical activity all improved; neither MCS nor Actilog improved. Physical function continued to improve in year 2; however, to varying degrees depending on measure. All other measures unchanged. | RP grouped with amputation patients. Only 24 patients completed full study assessments. Population also described in Bekkering/10. | |
Amp: Amputation; Bt-Dux: Bone Tumor version of DUX-25 quality of life scale; ES: Ewing Sarcoma; HADS: Hospital Anxiety and Depression Scale; HAP: Human Activity Profile; LE: Lower Extremity; LS: Limb Sparing; LSM: Leisure Satisfaction Measure; MCS: Mental Component Summary score (part of SF-36); MSTS: Musculoskeletal Tumor Society score; OS: Osteosarcoma; PCI: Physiologic Cost Index; PCS: Physical Component Summary score (part of SF-36); Peds-QL: Pediatric Quality of Life Inventory; PODCI: Pediatric Outcomes Data Collection Instrument; RNL: Reintegration into Normal Living index; ROM: Range of Motion; SF-36: Short Form-36 of Medical outcomes study; SIP: Sickness Impact Profile; TESS: Toronto Extremity Salvage Score; TUDS: Timed Up and Down Stairs; TUG: Timed Up and Go; RP: Rotationplasty; QOL: Quality of Life; UE: Upper Extremity; QOL-CS: Quality of Life Cancer Survivor
QOL was operationalized with the 4 categories above; most studies included multiple categories and often multiple instruments within each category. Furthermore, several studies included overlapping populations of patients with descriptions of different outcomes.[11,19,28,29,31,36,38–40]
Despite this variability, several trends emerged. First, compared to population norms, survivors of sarcoma tended to have inferior QOL scores across all categories (Table I).[11,13,30,31,34,35,40] Second, in prospective studies, QOL scores tended to improve over time. This improvement was thought to relate to symptom control during treatment, as well as adaptation and coping over time. For example, Hinds et al. measured QOL at initiation of treatment and multiple points over two years. Symptomatology (and corresponding QOL scores) improved as treatment progressed.[33] Bekkering et al. described similar findings, but also noted that healthier patients were more likely to continue participation and may therefore have been overrepresented in the data.[40] Koopman et al. described QOL scores that were inferior to population norms at 3 years after therapy, but no different at year 8. They postulated that patients gradually adapted to their disabilities.[34]
Third, female sex and older age at diagnosis were frequently associated with poorer QOL. Definitions and cut-off criteria for “older” age varied, however.[11,13,31,32,35,38,41] Aksnes et al. found inferior QOL among patients >13 years-old compared to <9 years at treatment;[31] Sun et al. described inferior QOL 1 year after treatment among patients ages 10–20 and >10 years, compared to <10 years.[35] Alternatively, Nagarajan et al. and Barrera et al. surveyed patients later in survivorship and found that older current age (>40 and >26 years, respectively) was associated with inferior QOL.[11,13,38]
There were no other consistent trends across studies, and many reported conflicting findings. For example, while Gerber et al. found that objective functional assessments correlated with patient-reported disability, Marchese et al. and Nagarajan et al. reported inconsistent relationships between objective functional assessments and patient-reported quality of life (Table I).[11,28–30]
Objective 2
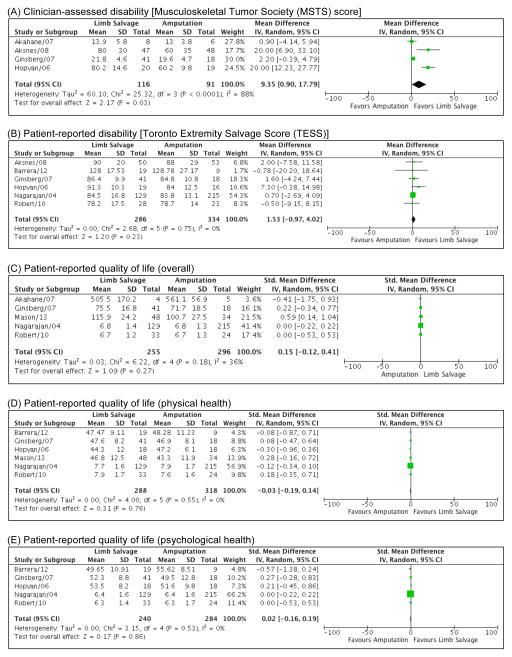
Twelve manuscripts compared sub-groups of patients with sarcoma by surgical procedure, Table II).[12,13,18,19,37–44] Of these, only 8 studies were evaluable for synthesis because 4 did not include a measure of central tendency and variance. We found no significant differences between patients who had received LS vs. Amp in any of the described QOL categories (Figure 2).
Table II.
Studies reporting comparisons of outcomes between patients treated with Limb-Salvage versus Amputation in order of publication year)
| Author/Year | Study Design | Population | Assessments | Main Findings | Notes and Limitations | |||
|---|---|---|---|---|---|---|---|---|
| Objective Function | Clinician-Assessed Disability | PRO Disability | PRO QOL | |||||
| Nagarajan/200435 ** | Cross-sectional | LE OS/ES (n=528), mean age at dx 13.5 y (range 1–20); mean time from surgery 20.8 y(range 13–31). | . | . | TESS | QOL-CS | Results stratified by age (=<12 vs >12) and surgery type. No differences in instrument scores between groups; self-rated “disabled” survivors also reported lower TESS and QOL scores, regardless of age/surgery. =<12/Amp significantly less likely to be in lowest quartile TESS. Females more likely to score <25 %ile than males for TESS. Age/surgery not predictive of QOL in multivariate models, but poor health status, current age >40, not graduating high school all associated with perceived disability. | Patients in =<12 Amp/LS groups included in meta-analyses. “No RP noted” in any patient based on ICD coding; unclear if accurate in this relatively large cohort of Childhood Cancer Survivorship Study participants. Population also described in Nagarajan/09. |
| Tabone/200538 | Cross-sectional | Appendicular OS/ES (n=27); median age at diagnosis 15 y (range 10–18); median time from surgery 4 y (range 1.5–12) | . | . | . | CHQ | LS associated with lower physical function compared to other groups. Female sex, receipt of high dose therapy associated with poorer mental health scores, history of recurrence associated with increased body pain. | LS defined as endoprosthesis; “other conservative surgery’ types were undefined in manuscript but pooled with Amp in comparisons (no clear Amp vs. LS comparison). Heterogeneous subject population (multiple treatment types; not all received surgery). No variance reported with mean instrument scores (hence, not included in meta-analyses). |
| Hopyan/200639 ** | Cross-sectional | LE OS/ES (n=123), mean age at diagnosis 11.9 y (SD=4.2); mean time from surgery 13.9 y (SD=5.7) | Uptime | MSTS | TESS | SF-36 | LS higher TESS/MSTS in some subsets; no differences in SF-36/uptime between groups. RP not included in analyses. | Total SF-36 scores not reported (hence, not included in meta-analyses). |
| Akahane/200722 ** | Cross-sectional | LE localized, metastatic, or secondary OS (n=20), mean age at diagnosis 21.9 y (range 7–79); median time from surgery 59.3 mo (range 7–79). | . | MSTS | TESS | SF-36 | No differences between LS and Amp groups; RP analyzed separately and associated with higher MTST than others. TESS results not reported; only total SF-36 scores reported (no PCS or MCS subscale scores). | LS patients older than others (mean age 28 versus 14 years); only male patients received RP. Investigators selected “best score” after 1yr follow-up; unclear how many assessments available per subject. Heterogeneous patient population (e.g., stage of disease, age, prior treatment). |
| Ginsberg/200723 ** | Cross-sectional | LE OS/ES (n=91), mean age at surgery 14.5y (SD=4); mean time since surgery 4 y (SD=3) for LS, 10 y (SD=6) for Amp | FMA | MSTS | TESS | SF-36 | Amp higher FMA scores than LS, no other differences between groups. Sub-analyses by surgical location showed higher MSTS for RP than LS-femur, higher TESS for below vs. above-knee Amp. | Multiple comparisons and high variance in patient subsets (e.g., time from surgery). Analyses stratified by specific surgery type/location. Population also described in Marchese/04, Marchese/06. |
| Aksnes/200836 ** | Cross-sectional | UE and LE OS/ES (n=118); median age at diagnosis 18 y (range 2–44); median time from diagnosis to surgery 13 y (range 7–79) | . | MSTS | TESS | SF-36 | LS higher MSTS and SF-36 PCS. No differences in TESS between groups. Amp more commonly associated with MSTS and SF-36 PCS <50%ile. Tumor location above knee associated with lower MSTS and TESS scores than below knee. | RP patients grouped with LS. Mean SF-36 scores with variance not reported (hence, not included in meta-analyses). Population also described in Asknes/2007. |
| Bekkering/201037 | Cross-sectional | LE OS/ES (n=81), mean age at surgery 14 y (SD=24.1); mean time since surgery 2.8 y (SD=1.6) | . | . | . | SF-36, TAC-/TAQ-QOL | Amp higher positive emotions than LS among younger patients, no differences among patients 16y and older. Compared to norms: sample had lower QOL scores (all instruments). | RP patients grouped with Amp. No variance reported with mean scores by surgery type (hence, not included in meta-analyses). Population also described in Bekkering/12. |
| Robert/201040 ** | Cross-sectional | UE and LE OS (n=57), mean age at diagnosis 13.8 y (range 3.3–28.2); mean time from diagnosis to study 18.6 y (range 3.8–35.6) | . | . | TESS, ABIS | QOL-CSS | Analyses restricted to patients with LE tumors. No differences in outcomes by surgery type except body image scores lower for those who underwent late Amp or Amp following failed LS. | RP patients grouped with Amp. Multiple procedure types (e.g., shoulder disarticulation, hemi-pelvectomy) contributing to heterogeneous population. Analyses limited to LE tumor groups only. |
| Barrera/201217 ** | Cross-sectional | LE OS/ES (n=28), mean age at diagnosis 11.2 y (SD=3.3); mean age at follow-up 25 y (SD=4.5) | . | . | TESS | SF-36, HUI, EORTC | Findings varied depending on QOL instrument: LS associated with lower emotion (HUI), more fatigue (EORTC), but no differences or similar trends noted in other scales or across instruments (SF-36, HUI, EORTC, TESS.) Female sex associated with lower SF-36 PCS, HUI, EORTC and TESS total scores. Older age (26+ y) associated with lower PCS, HUI and TESS total scores. Compared to norms, sample had lower SF-36 PCS, HUI3, and EORTC total scores. | RP patients grouped with Amp. SF-36 scores (rather than other instruments) used in meta-analyses to limit heterogeneity of pooled samples. SF-36 total score not reported (hence only PCS and MCS used in meta-analyses). |
| Han/201234 | Prospective | LE OS/ES (n=120), mean age at surgery 14.1 y (SD=4.6); assessment conducted 6-and 12-months post-operatively | . | . | . | SF-36 | Odds ratio of SF-36 PCS and MCS <50%il lower for Amp compared to LS. Across all groups: QOL subscales improved over first 6 months (no comparison reported between enrollment and 12 month time-point). Women and older age associated with SF-36 PCS <50%ile. | Mean scores not reported by specific surgery type (hence, not included in meta-analyses). No clear description of RP nor if RP patients included. Comment in text that patients “chose” surgery type; however, unclear description of factors/options involved in choice. |
| Malek/201216 | Cross-sectional | LE tumors (n=20, various types including OS, ES, and others), mean age at dx not described, mean age at study-entry 34 y (range 15–76); median time from surgery 56 mo (range 12–108) | PCI | . | TESS, RNL | SF-36 | LS higher RNL and lower PCI (both suggest better function). No differences in TESS or SF-36 scores. | Heterogeneous subject population (age, diagnosis, prior treatment). Unclear if RP included, and if so, in which surgical subset. Patients younger than 15 y excluded from study. No raw scores or variance reported by surgery group (hence, not included in meta-analyses). |
| Mason/201341 ** | Cross-sectional | LE tumors (n=82, tumor types not described), mean age at surgery and follow-up time 19.5 y (SD=9.7) and 8.1 y (SD=6.6) for Amp, 20.7 y (SD=7.7) and 5.6 y (SD=4.0) for LS | . | . | . | MMPI, QLQ | LS associated with superior scores in overall QOL, material well-being, occupational relations, job satisfaction, creative-esthetic behavior, sports activities, trend to suggest LS associated with higher social desirability. MMPI scores similar by groups except higher defensiveness, lower college maladjustment in LS group. | RP explicitly excluded from analyses. Specific cancer types not specified. Multiple comparisons including instrument subscales. No overall mental health score reported (hence, not included in meta-analyses). |
Included in meta-analyses. ABIS: Amputee Body Image Scale; Amp: Amputation; CHQ: Child Health Questionnaire; EORTC: European Organization for Research and Treatment of Cancer Quality of Life scale; ES: Ewing Sarcoma; FMA: Functional Mobility Assessment; HUI: Health Utilities Index; LE: Lower Extremity; LS: Limb Sparing; MCS: Mental Component Summary score (part of SF-36); MSTS: Musculoskeletal Tumor Society score; MMPI: Minnesota Multiphasic Personality Inventory; OS: Osteosarcoma; PCI: Physiologic Cost Index; PCS: Physical Component Summary score (part of SF-36): RNL: Reintegration into Normal Living index; SF-36: Short Form-36 of Medical outcomes study; TAC/TAQ-QOL: Netherlands Organization for Applied Scientific Research Academic Medical Center Quality of Life Questionnaires; TESS: Toronto Extremity Salvage Score; RP: Rotationplasty; QLQ: Quality of life Questionnaire; QOL: Quality of Life; UE: Upper Extremity; QOL-CS: Quality of Life Cancer Survivor.
Figure 2.
Forrest Plots comparing outcomes among patients who underwent Limb Salvage vs. Amputation surgery. (A) Clinician-assessed disability [Musculoskeletal Tumor Society (MSTS) Score]; (B) Patient-reported disability [Toronto Extremity Salvage Score (TESS)]; (C) Patient-reported quality of life (overall scores, multiple instruments); (D) Patient-reported quality of life (physical health scores, multiple instruments); (E) Patient-reported quality of life (psychological health scores, multiple instruments).
Additionally, correlations between QOL instrument types were highly variable. For example, Barrera et al., used multiple patient-reported QOL instruments including the SF-36, Health Utilities Index (HUI), and European Organization for Research and Treatment of Cancer (EORTC) instruments within the same population of sarcoma patients (Table II).[13] Findings varied depending on individual instruments. Comparing LS to Amp, the HUI (but not the SF-36 or EORTC) suggested that patients treated with LS suffered from inferior emotional health, and the EORTC (but not the SF-36 or HUI) suggested patients treated with LS suffered more from fatigue.
Discussion
Unlike many other cancers in young people, bone sarcomas often require significant surgical interventions that can dramatically impact mobility, function, and body image. Patients and families face complex decisions between amputation and limb-sparing procedures; having some assessment of expected quality of life would therefore be helpful for practitioners, patients, and families alike. However, patient-reported quality of life is multifactorial and highly subjective. Our systematic review identified remarkably diverse study populations and methods assessing QOL. Meta-analyses comparing LS vs. Amp as options for surgical approach did not show differences in clinician-assessed disability, patient-reported disability, or patient-reported QOL. Better data on QOL, stratified by local control modality, has the potential to impact the importance of QOL in clinical decision-making in sarcoma treatments.
Our findings add to the weight of evidence that describes the heterogeneity of QOL research within pediatric and AYA cancer.[45–48] In addition, they underscore critical challenges in QOL research: How do objective functional measures relate to patient’s own definitions of “quality” and what do patients value? How sensitive are individual instruments to specific QOL subdomains and how completely do they assess patient-reported QOL?
These questions have been raised in other ways among pediatric and AYA bone tumor patients. For example, Barrera et al. compared sexual health among young adult survivors of bone tumors and found that those who were treated with LS reported fewer sexual thoughts and experiences than those treated with Amp.[49] In another analysis of the same patients, Teall et al. reported that survivors of bone cancer reported fewer depressive symptoms than population norms, that patients treated with LS vs. Amp were no different in reported benefit-finding, and that male survivors reported stronger social support than females.[14] Arguably, sexual health, depression, benefit-finding, and social support are all important aspects of both function and QOL; however, these related patient-reported outcomes may not routinely be integrated into standard QOL assessments.
Similarly, historical studies among sarcoma patients have described objective- and patient-reported-function, and health-related quality of life separately. The rationale for this practice may be that narrowing the outcome of interest avoids bias and allows for clearer identification of associations. Indeed, we chose to stratify our meta-analyses by function versus health-related quality of life in order to limit pooled study heterogeneity. However, focusing on single subdomains may have been incomplete because it failed to address patient-centered priorities.
There are several additional limitations to this systematic review. First, the heterogeneity of tumor types, location, stage, treatment experience, and surgical procedures precluded significant data syntheses. In fact, perhaps such diverse patient experiences should not be pooled; rather, they are too distinct and should be described individually. For example, there may be significant differences among QOL outcomes among patients treated for lower-extremity sarcomas compared to those with upper extremity or pelvic tumors because tumor location impacts physical function and reconstructive options differently. Likewise, pooling the subtypes of LS and amputation groups together might have masked important differences between subsets of patients. For example, QOL among patients with AKA versus BKA may be discrepant, but only 3 papers described amputation site explicitly, limiting our ability to conduct meta-analyses for these subsets. Second, most of the included studies were cross-sectional assessments of relatively few patients, limiting the power and quality of findings. Several did not report complete quantitative data and we did not contact authors to access such information. Due to the small sizes of these studies, it is unlikely that including those data would have changed our meta-analysis results. Third, we deliberately focused on pediatric and AYA patient populations to ensure that we would not bias our analysis with older adults who may have different expectations or perceptions of mobility and pain. In doing so, we may have excluded important papers conducted in the larger sarcoma survivor population. While these are certainly relevant to the pediatric experience, we felt that pediatric versus adult experiences were distinct enough to warrant this approach. Fourth, we found no papers comparing QOL outcomes by radiation versus surgical local control. This comparison may be particularly important for patients with Ewing Sarcoma, because neither modality has been consistently associated with superior survival outcomes and therefore QOL may direct clinical decision-making.[50].
Finally, the variety of instruments limited our ability to conduct qualitative syntheses across studies. Barrera et al. demonstrated that different interpretations could be drawn even from the same population of patients based on which QOL instrument was used. The fact that we found no differences between groups in our meta-analyses, for example, may have been due to instrument variability rather than true differences in patient experiences.
Barriers associated with instrument diversity are inescapable in pediatric survey-based research. Pediatric patient-report is highly contingent on age. Validated instruments often include parent-report up to certain ages and many pediatric QOL studies include both parents and patient respondents when describing “patient-report.” How to interpret these data remains unclear. Furthermore, instrument validation studies often include either adult patients (ages 18 and up) or pediatric patients (under 18). The SF-36, EORTC, and HUI, for example, are all adult-validated scales that would not be appropriate yet in pediatrics.
Recognizing these challenges, the Children’s Oncology Group of North America recently convened a subcommittee dedicated to advancing the rigorous study of patient QOL in pediatric cancer.[10] The group has also described the challenges inherent to this research and underscored the need to develop comprehensive, age-appropriate and cancer-specific instruments.[51] Future efforts will include standard integration of QOL study into pediatric cancer clinical trials, as well as initiatives to create patient-valued measures that will direct meaningful clinical care and research endeavors. Meanwhile, we prefer the PedsQL instruments because they include generic and cancer-specific QOL domains and are well validated across a wide spectrum of patient ages, including pediatrics, adolescents and young adults.[24,52]
Quality of life is a clinically important, yet poorly operationalized, and understudied construct. Pediatric and AYA sarcoma patients are at high risk of poor QOL; however, the landscape of research to date has been relatively sparse and remarkably heterogeneous. The fact that AYA patients have comparatively poorer QOL than younger pediatric patients suggests these patients have potentially greater unmet needs. Many centers are working on building AYA-specific programs which may help support these patients. With survival rates for non-metastatic osteosarcoma and Ewing sarcoma now exceeding 70%, there is a growing cohort of long-term sarcoma survivors; research and treatment must focus not only on survivability, but also on better understanding, operationalizing, measuring, and improving patient-reported quality of life.
Supplementary Material
Systematic Review Search Methodology
Systematic Review Data Extraction Form
Acknowledgments
The authors would like to thank Susan Klawansky, librarian at Seattle Children’s Hospital, for performing an extensive search of the literature, and the Children’s Oncology Group Quality of Life subcommittee for their advice, direction, and contribution to the development of this manuscript. Dr. Rosenberg was supported by the National Center For Advancing Translational Sciences of the National Institutes of Health under award number KL2TR000421 and the National Cancer Institute loan repayment program under award number L40CA170049. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Abbreviations
- Amp
Amputation
- AYA
Adolescent and Young Adult
- LS
Limb-Sparing
- QOL
Quality of Life
Footnotes
Conflict of Interest Statement
None of the authors has a financial or other conflict of interest related to this research.
References
- 1.Ries LAGSM, Gurney JG, Linet M, Tamra T, Young JL, Bunin GR, editors. NIH Pub No 99-4649. Bethesda, MD: 1999. Cancer Incidence and Survival among Children and Adolescents: United States SEER Program 1975–1995, National Cancer Institute, SEER Program. [Google Scholar]
- 2.Hoffman MC, Mulrooney DA, Steinberger J, Lee J, Baker KS, Ness KK. Deficits in physical function among young childhood cancer survivors. J Clin Oncol. 2013;31(22):2799–2805. doi: 10.1200/JCO.2012.47.8081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zebrack BJ, Zevon MA, Turk N, Nagarajan R, Whitton J, Robison LL, Zeltzer LK. Psychological distress in long-term survivors of solid tumors diagnosed in childhood: a report from the childhood cancer survivor study. Pediatr Blood Cancer. 2007;49(1):47–51. doi: 10.1002/pbc.20914. [DOI] [PubMed] [Google Scholar]
- 4.Oeffinger KC, Mertens AC, Sklar CA, Kawashima T, Hudson MM, Meadows AT, Friedman DL, Marina N, Hobbie W, Kadan-Lottick NS, Schwartz CL, Leisenring W, Robison LL. Chronic health conditions in adult survivors of childhood cancer. N Engl J Med. 2006;355(15):1572–1582. doi: 10.1056/NEJMsa060185. [DOI] [PubMed] [Google Scholar]
- 5.Audino AN, Yeager ND, Asti L, Miao Y, O’Brien SH. Length of stay and treatment-related complications are similar in pediatric and AYA patients with bone sarcoma in United States children’s hospitals. Pediatr Blood Cancer. 2013;60(3):415–419. doi: 10.1002/pbc.24231. [DOI] [PubMed] [Google Scholar]
- 6.Ginsberg JP, Goodman P, Leisenring W, Ness KK, Meyers PA, Wolden SL, Smith SM, Stovall M, Hammond S, Robison LL, Oeffinger KC. Long-term survivors of childhood Ewing sarcoma: report from the childhood cancer survivor study. J Natl Cancer Inst. 2010;102(16):1272–1283. doi: 10.1093/jnci/djq278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mansky P, Arai A, Stratton P, Bernstein D, Long L, Reynolds J, Chen D, Steinberg SM, Lavende N, Hoffman K, Nathan PC, Parks R, Augustine E, Chaudhry U, Derdak J, Wiener L, Gerber L, Mackall C. Treatment late effects in long-term survivors of pediatric sarcoma. Pediatr Blood Cancer. 2007;48(2):192–199. doi: 10.1002/pbc.20871. [DOI] [PubMed] [Google Scholar]
- 8.Eiser C, Darlington AS, Stride CB, Grimer R. Quality of life implications as a consequence of surgery: limb salvage, primary and secondary amputation. Sarcoma. 2001;5(4):189–195. doi: 10.1080/13577140120099173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Marina N, Hudson MM, Jones KE, Mulrooney DA, Avedian R, Donaldson SS, Popat R, West DW, Fisher P, Leisenring W, Stovall M, Robison LL, Ness KK. Changes in health status among aging survivors of pediatric upper and lower extremity sarcoma: a report from the childhood cancer survivor study. Arch Phys Med Rehabil. 2013;94(6):1062–1073. doi: 10.1016/j.apmr.2013.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sung L, Zaoutis T, Ullrich NJ, Johnston D, Dupuis L, Ladas E. Children’s Oncology Group’s 2013 blueprint for research: cancer control and supportive care. Pediatr Blood Cancer. 2013;60(6):1027–1030. doi: 10.1002/pbc.24426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nagarajan R, Mogil R, Neglia JP, Robison LL, Ness KK. Self-reported global function among adult survivors of childhood lower-extremity bone tumors: a report from the Childhood Cancer Survivor Study (CCSS) J Cancer Surviv. 2009;3(1):59–65. doi: 10.1007/s11764-008-0073-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Malek F, Somerson JS, Mitchel S, Williams RP. Does limb-salvage surgery offer patients better quality of life and functional capacity than amputation? Clin Orthop Relat Res. 2012;470(7):2000–2006. doi: 10.1007/s11999-012-2271-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Barrera M, Teall T, Barr R, Silva M, Greenberg M. Health related quality of life in adolescent and young adult survivors of lower extremity bone tumors. Pediatr Blood Cancer. 2012;58(2):265–273. doi: 10.1002/pbc.23017. [DOI] [PubMed] [Google Scholar]
- 14.Teall T, Barrera M, Barr R, Silva M, Greenberg M. Psychological resilience in adolescent and young adult survivors of lower extremity bone tumors. Pediatr Blood Cancer. 2013;60(7):1223–1230. doi: 10.1002/pbc.24441. [DOI] [PubMed] [Google Scholar]
- 15.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151(4):264–269. W264. doi: 10.7326/0003-4819-151-4-200908180-00135. [DOI] [PubMed] [Google Scholar]
- 16.Hayden JA, van der Windt DA, Cartwright JL, Cote P, Bombardier C. Assessing bias in studies of prognostic factors. Ann Intern Med. 2013;158(4):280–286. doi: 10.7326/0003-4819-158-4-201302190-00009. [DOI] [PubMed] [Google Scholar]
- 17.von Elm E, Altman DG, Egger M, Pocock SJ, Gotzsche PC, Vandenbroucke JP. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Ann Intern Med. 2007;147(8):573–577. doi: 10.7326/0003-4819-147-8-200710160-00010. [DOI] [PubMed] [Google Scholar]
- 18.Akahane T, Shimizu T, Isobe K, Yoshimura Y, Fujioka F, Kato H. Evaluation of postoperative general quality of life for patients with osteosarcoma around the knee joint. J Pediatr Orthop B. 2007;16(4):269–272. doi: 10.1097/BPB.0b013e3280925670. [DOI] [PubMed] [Google Scholar]
- 19.Ginsberg JP, Rai SN, Carlson CA, Meadows AT, Hinds PS, Spearing EM, Zhang L, Callaway L, Neel MD, Rao BN, Marchese VG. A comparative analysis of functional outcomes in adolescents and young adults with lower-extremity bone sarcoma. Pediatr Blood Cancer. 2007;49(7):964–969. doi: 10.1002/pbc.21018. [DOI] [PubMed] [Google Scholar]
- 20.Ware JE, Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992;30(6):473–483. [PubMed] [Google Scholar]
- 21.Furlong WJ, Feeny DH, Torrance GW, Barr RD. The Health Utilities Index (HUI) system for assessing health-related quality of life in clinical studies. Ann Med. 2001;33(5):375–384. doi: 10.3109/07853890109002092. [DOI] [PubMed] [Google Scholar]
- 22.Cella DF, Tulsky DS, Gray G, Sarafian B, Linn E, Bonomi A, Silberman M, Yellen SB, Winicour P, Brannon J, et al. The Functional Assessment of Cancer Therapy scale: development and validation of the general measure. J Clin Oncol. 1993;11(3):570–579. doi: 10.1200/JCO.1993.11.3.570. [DOI] [PubMed] [Google Scholar]
- 23.Aaronson NK, Ahmedzai S, Bergman B, Bullinger M, Cull A, Duez NJ, Filiberti A, Flechtner H, Fleishman SB, de Haes JC, et al. The European Organization for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst. 1993;85(5):365–376. doi: 10.1093/jnci/85.5.365. [DOI] [PubMed] [Google Scholar]
- 24.Varni JW, Burwinkle TM, Katz ER, Meeske K, Dickinson P. The PedsQL in pediatric cancer: reliability and validity of the Pediatric Quality of Life Inventory Generic Core Scales, Multidimensional Fatigue Scale, and Cancer Module. Cancer. 2002;94(7):2090–2106. doi: 10.1002/cncr.10428. [DOI] [PubMed] [Google Scholar]
- 25.Enneking WF, Dunham W, Gebhardt MC, Malawar M, Pritchard DJ. A system for the functional evaluation of reconstructive procedures after surgical treatment of tumors of the musculoskeletal system. Clin Orthop Relat Res. 1993;(286):241–246. [PubMed] [Google Scholar]
- 26.Davis AM, Wright JG, Williams JI, Bombardier C, Griffin A, Bell RS. Development of a measure of physical function for patients with bone and soft tissue sarcoma. Qual Life Res. 1996;5(5):508–516. doi: 10.1007/BF00540024. [DOI] [PubMed] [Google Scholar]
- 27.Lipsey MW, Wilson DB. Practical Meta-Analysis. Thousand Oaks, CA: Sage Publications, Inc; 2000. [Google Scholar]
- 28.Marchese VG, Ogle S, Womer RB, Dormans J, Ginsberg JP. An examination of outcome measures to assess functional mobility in childhood survivors of osteosarcoma. Pediatr Blood Cancer. 2004;42(1):41–45. doi: 10.1002/pbc.10462. [DOI] [PubMed] [Google Scholar]
- 29.Marchese VG, Spearing E, Callaway L, Rai SN, Zhang L, Hinds PS, Carlson CA, Neel MD, Rao BN, Ginsberg J. Relationships among range of motion, functional mobility, and quality of life in children and adolescents after limb-sparing surgery for lower-extremity sarcoma. Pediatr Phys Ther. 2006;18(4):238–244. doi: 10.1097/01.pep.0000232620.42407.9f. [DOI] [PubMed] [Google Scholar]
- 30.Gerber LH, Hoffman K, Chaudhry U, Augustine E, Parks R, Bernad M, Mackall C, Steinberg S, Mansky P. Functional outcomes and life satisfaction in long-term survivors of pediatric sarcomas. Arch Phys Med Rehabil. 2006;87(12):1611–1617. doi: 10.1016/j.apmr.2006.08.341. [DOI] [PubMed] [Google Scholar]
- 31.Aksnes LH, Hall KS, Jebsen N, Fossa SD, Dahl AA. Young survivors of malignant bone tumours in the extremities: a comparative study of quality of life, fatigue and mental distress. Support Care Cancer. 2007;15(9):1087–1096. doi: 10.1007/s00520-007-0227-x. [DOI] [PubMed] [Google Scholar]
- 32.Frances JM, Morris CD, Arkader A, Nikolic ZG, Healey JH. What is quality of life in children with bone sarcoma? Clin Orthop Relat Res. 2007;459:34–39. doi: 10.1097/BLO.0b013e31804f545d. [DOI] [PubMed] [Google Scholar]
- 33.Hinds PS, Gattuso JS, Billups CA, West NK, Wu J, Rivera C, Quintana J, Villarroel M, Daw NC. Aggressive treatment of non-metastatic osteosarcoma improves health-related quality of life in children and adolescents. Eur J Cancer. 2009;45(11):2007–2014. doi: 10.1016/j.ejca.2009.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Koopman HM, Koetsier JA, Taminiau AH, Hijnen KE, Bresters D, Egeler RM. Health-related quality of life and coping strategies of children after treatment of a malignant bone tumor: a 5-year follow-up study. Pediatr Blood Cancer. 2005;45(5):694–699. doi: 10.1002/pbc.20408. [DOI] [PubMed] [Google Scholar]
- 35.Sun YJ, Hu YJ, Jin D, Li JW, Yu B. Health-related quality of life after treatment for malignant bone tumors: a follow-up study in China. Asian Pac J Cancer Prev. 2012;13(7):3099–3102. doi: 10.7314/apjcp.2012.13.7.3099. [DOI] [PubMed] [Google Scholar]
- 36.Bekkering WP, Vliet Vlieland TP, Koopman HM, Schaap GR, Beishuizen A, Anninga JK, Wolterbeek R, Nelissen RG, Taminiau AH. A prospective study on quality of life and functional outcome in children and adolescents after malignant bone tumor surgery. Pediatr Blood Cancer. 2012;58(6):978–985. doi: 10.1002/pbc.23328. [DOI] [PubMed] [Google Scholar]
- 37.Han G, Wang Y, Bi WZ. Study on the health-related quality of life in patients after surgery for malignant bone tumors. Asian Pac J Cancer Prev. 2012;13(1):127–130. doi: 10.7314/apjcp.2012.13.1.127. [DOI] [PubMed] [Google Scholar]
- 38.Nagarajan R, Clohisy DR, Neglia JP, Yasui Y, Mitby PA, Sklar C, Finklestein JZ, Greenberg M, Reaman GH, Zeltzer L, Robison LL. Function and quality-of-life of survivors of pelvic and lower extremity osteosarcoma and Ewing’s sarcoma: the Childhood Cancer Survivor Study. Br J Cancer. 2004;91(11):1858–1865. doi: 10.1038/sj.bjc.6602220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Aksnes LH, Bauer HC, Jebsen NL, Folleras G, Allert C, Haugen GS, Hall KS. Limb-sparing surgery preserves more function than amputation: a Scandinavian sarcoma group study of 118 patients. J Bone Joint Surg Br. 2008;90(6):786–794. doi: 10.1302/0301-620X.90B6.19805. [DOI] [PubMed] [Google Scholar]
- 40.Bekkering WP, Vliet Vlieland TP, Koopman HM, Schaap GR, Schreuder HW, Beishuizen A, Tissing WJ, Hoogerbrugge PM, Anninga JK, Taminiau AH. Quality of life in young patients after bone tumor surgery around the knee joint and comparison with healthy controls. Pediatr Blood Cancer. 2010;54(5):738–745. doi: 10.1002/pbc.22439. [DOI] [PubMed] [Google Scholar]
- 41.Tabone MD, Rodary C, Oberlin O, Gentet JC, Pacquement H, Kalifa C. Quality of life of patients treated during childhood for a bone tumor: assessment by the Child Health Questionnaire. Pediatr Blood Cancer. 2005;45(2):207–211. doi: 10.1002/pbc.20297. [DOI] [PubMed] [Google Scholar]
- 42.Hopyan S, Tan JW, Graham HK, Torode IP. Function and upright time following limb salvage, amputation, and rotationplasty for pediatric sarcoma of bone. J Pediatr Orthop. 2006;26(3):405–408. doi: 10.1097/01.bpo.0000203016.96647.43. [DOI] [PubMed] [Google Scholar]
- 43.Robert RS, Ottaviani G, Huh WW, Palla S, Jaffe N. Psychosocial and functional outcomes in long-term survivors of osteosarcoma: a comparison of limb-salvage surgery and amputation. Pediatr Blood Cancer. 2010;54(7):990–999. doi: 10.1002/pbc.22419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mason GE, Aung L, Gall S, Meyers PA, Butler R, Krug S, Kim M, Healey JH, Gorlick R. Quality of life following amputation or limb preservation in patients with lower extremity bone sarcoma. Front Oncol. 2013;3:210. doi: 10.3389/fonc.2013.00210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Anthony SJ, Selkirk E, Sung L, Klaassen RJ, Dix D, Scheinemann K, Klassen AF. Considering quality of life for children with cancer: a systematic review of patient-reported outcome measures and the development of a conceptual model. Qual Life Res. 2014;23(3):771–789. doi: 10.1007/s11136-013-0482-x. [DOI] [PubMed] [Google Scholar]
- 46.Klassen AF, Anthony SJ, Khan A, Sung L, Klaassen R. Identifying determinants of quality of life of children with cancer and childhood cancer survivors: a systematic review. Support Care Cancer. 2011;19(9):1275–1287. doi: 10.1007/s00520-011-1193-x. [DOI] [PubMed] [Google Scholar]
- 47.Klassen AF, Strohm SJ, Maurice-Stam H, Grootenhuis MA. Quality of life questionnaires for children with cancer and childhood cancer survivors: a review of the development of available measures. Support Care Cancer. 2010;18(9):1207–1217. doi: 10.1007/s00520-009-0751-y. [DOI] [PubMed] [Google Scholar]
- 48.Hinds PS. Progress in quality of life in children and adolescents with cancer. Semin Oncol Nurs. 2010;26(1):18–25. doi: 10.1016/j.soncn.2009.11.004. [DOI] [PubMed] [Google Scholar]
- 49.Barrera M, Teall T, Barr R, Silva M, Greenberg M. Sexual function in adolescent and young adult survivors of lower extremity bone tumors. Pediatr Blood Cancer. 2010;55(7):1370–1376. doi: 10.1002/pbc.22761. [DOI] [PubMed] [Google Scholar]
- 50.DuBois SG, Krailo MD, Gebhardt MC, Donaldson SS, Marcus KJ, Dormans J, Shamberger RC, Sailer S, Nicholas RW, Healey JH, Tarbell NJ, Randall RL, Devidas M, Meyer JS, Granowetter L, Womer RB, Bernstein M, Marina N, Grier HE. Comparative evaluation of local control strategies in localized Ewing sarcoma of bone: A report from the Children’s Oncology Group. Cancer. 2014 doi: 10.1002/cncr.29065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Johnston DL, Nagarajan R, Caparas M, Schulte F, Cullen P, Aplenc R, Sung L. Reasons for non-completion of health related quality of life evaluations in pediatric acute myeloid leukemia: a report from the Children’s Oncology Group. PLoS One. 2013;8(9):e74549. doi: 10.1371/journal.pone.0074549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Varni JW, Seid M, Knight TS, Uzark K, Szer IS. The PedsQL 4. 0 Generic Core Scales: sensitivity, responsiveness, and impact on clinical decision-making. J Behav Med. 2002;25(2):175–193. doi: 10.1023/a:1014836921812. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Systematic Review Search Methodology
Systematic Review Data Extraction Form