Abstract
Background and Aims
To investigate the associations between selected adipokines and the N-terminal prohormone of B-type natriuretic peptide (NT-proBNP).
Methods and Results
1489 individuals enrolled in the Multi-Ethnic Study of Atherosclerosis were evaluated at 4 clinic visits about every 2 years. The evaluation included fasting venous blood, which was analyzed for NT-proBNP (at visits 1 and 3) and the adipokines adiponectin and leptin (at visits 2 and 3). The mean age was 64.8 ± 9.6 years and 48% were female. After multivariable adjustment, a 1-SD increment in adiponectin was associated with a 14 pg/ml higher NT-proBNP level (p < 0.01), while, compared to the 1st quartile of adiponectin, the 2nd, 3rd and 4th quartiles had 28, 45 and 67% higher NT-proBNP levels (p < 0.01 for all). For changes in NT-proBNP over the follow-up period, and after multivariable adjustment including baseline NT-proBNP, a 1-SD increment in adiponectin was associated with a 25 pg/ml absolute increase in NT-proBNP (p < 0.01), while those in the 2nd, 3rd and 4th quartiles of adiponectin were associated with increases of 5, 28 and 65 pg/ml (p = 0.74, 0.09 and < 0.01, respectively). There was a significant interaction between adiponectin and sex for visit 3 NT-proBNP (p-interaction < 0.01), with significantly stronger associations in men. Leptin was not associated with NT-proBNP.
Conclusion
Higher adiponectin, but not leptin, is significantly associated with higher levels of NT-proBNP, as well as with greater longitudinal increases in NT-proBNP. The associations were stronger in men.
INTRODUCTION
Obese individuals often have higher levels of sympathetic nervous system activity[1], which results in activation of the renin-angiotensinogen-aldosterone system (RAAS) and renal tubular resorption of sodium.[2] Despite elevations in blood pressure due to these effects, and in the presence of excess adiposity, sodium resorption continues and extracellular volume expands.[3] The increased wall stress on the ventricle results in the release of the prohormone pro B-type natriuretic peptide (proBNP) from the myocardium, which is ultimately converted to the active hormone [BNP], and the inactive N-terminal fragment (NT-proBNP).[4] As volume overload is a typical finding in patients with heart failure, BNP and/or NT-proBNP have become standard screening tests for this condition.[6, 7, 8]
Adipokines are cytokines released from adipose tissue that have diverse physiologic effects. For example, leptin is a satiety hormone that increases with obesity, while adiponectin levels correlate inversely with fat mass and has been associated with lower CVD risk. Both are secreted by adipocytes.[9] We have previously shown that higher levels of leptin are associated with smaller left ventricular mass and volume, as well as a significantly lower odds for left ventricular hypertrophy[10], but higher levels of blood pressure and odds for prevalent hypertension. In this same study, higher levels of total adiponectin were associated with higher stroke volume and cardiac output. Given the linkages between excess adiposity and volume overload, and volume status to BNP, we conducted a study to test the hypothesis of significant associations between NT-proBNP and both adiponectin and leptin.
METHODS
Participants
The Multi-Ethnic Study of Atherosclerosis (MESA) is a longitudinal cohort study of African-, Chinese- and Hispanic-Americans, as well as non-Hispanic Whites. Details about the study design have been published.[11] In brief, between July 2000 and August 2002, 6,814 men and women who were 45 to 84 years old and free of clinically apparent cardiovascular disease (CVD) were recruited from 6 United States communities. Individuals with a history of physician-diagnosed heart attack, angina, heart failure, stroke or transient ischemic attack, or having undergone an invasive procedure for CVD (coronary artery bypass graft, angioplasty, valve replacement or pacemaker placement) were excluded from participation. Enrolled participants returned for follow-up clinic examinations on 3 subsequent visits at approximately 18-month intervals. All participants provided written informed consent and the institutional review boards (IRB) at the participating Universities approved the study.
At clinic exams 2 and 3, a random subsample of 1,970 participants (approximately . at each visit) enrolled in an ancillary study on body composition, inflammation and cardiovascular disease. [12] This included measurements for adiponectin and leptin from stored blood collected at those visits.
Data Collection
At all clinic examinations, standardized questionnaires were used to obtain sociodemographic and health history information. Cigarette smoking was defined as current, former, or never. Height and weight were measured with participants wearing light clothing and no shoes. Body mass index (BMI) was calculated as weight in kilograms divided by height in meters squared. Waist and hip circumferences were measured using a standard flexible tape measure. Resting blood pressure was measured 3 times in seated participants with a Dinamap model Pro 100 automated oscillometric sphygmomanometer (Critikon, Tampa, FL), with the second and third readings being averaged and used in the analysis. Hypertension (HTN) was defined as systolic blood pressure ≥ 140 mm Hg, diastolic blood pressure ≥ 90 mm Hg, or current use of antihypertensive medication.
At the baseline clinic visit, MRI exams of the heart were performed using scanners with 1.5-Tesla magnets to determine specific measures of cardiac structure and function. [13] Imaging consisted of fast gradient echo cine LV images using a phased-array surface coil with time resolution of <50 milliseconds and the following scan protocol: 6 mm slice thickness, 400 mm field of view, 256×128 matrix, 20° flip angle, echo time (TE) = 3 – 5 msec and repetition time (TR) = 8 – 10 msec. Imaging data were read using MASS software version 4.2 (Medis, Leiden, the Netherlands) by technologists trained in the MESA protocol but without knowledge of risk factor information.
Laboratory
At all clinic examinations, total and high density lipoprotein (HDL) cholesterol, triglycerides, and glucose levels were measured from blood samples obtained after a 12-hour fast. Fasting blood was also assayed for measures of systemic inflammation (C-reactive protein [CRP], fibrinogen, interleukin-6) and insulin concentration. Dyslipidemia was defined as a total-cholesterol/HDL-cholesterol ratio > 5.0 or if the participant used medication to reduce cholesterol. Diabetes was defined as fasting glucose ≥ 126 mg/dL or use of hypoglycemic medication.
The core lab at the University of California San Diego analyzed stored blood samples obtained at clinic visits 1 and 3 for NT-proBNP using a highly sensitive and specific Elecsys electrochemiluminescence immunoassay (Roche Diagnostics Corporation, Indianapolis, IN). Samples from visits 2 or 3 were analyzed for leptin and total adiponectin using Bio-Rad Luminex flow cytometry (Millipore, Billerica, MA) at the Laboratory for Clinical Biochemistry Research (University of Vermont, Burlington, VT).
Statistical Analysis
The primary exposure variables were leptin and total adiponectin obtained at visits 2 or 3, while the primary outcome variable was NT-proBNP measured at visit 3. We also examined the associations with the change in NT-proBNP from visit 1 to visit 3. All covariate data (e.g. age, BMI, blood pressure, etc.) was used from values obtained at visit 3.
Among the 1,970 potential participants who had measurements of leptin and total adiponectin, and after accounting for missing covariate data and exclusion of those with incident heart failure and acute myocardial infarction after visit 1, there were 1,489 individuals who had NT-proBNP measured at visit 3 and 1,452 who had NT-proBNP measured at visits 1 and 3 that could be used for analyses using the change in this biomarker. To reduce skewness, it was necessary to log transform visit 3 NT-proBNP. The change in NT-proBNP from visit 1 to 3 was normally distributed.
Differences in the cohort characteristics by quartile of NT-proBNP were tested for statistical significance using ANCOVA (Pr > F for ANCOVA) adjusting for age, sex and race. To determine the linear association between the adipokines and NT-proBNP, we utilized multivariable linear regression and the continuous and categorical (in quartiles) forms of leptin and total adiponectin. Then, to determine if there were significant associations with a relevant clinical cut-point for NT-proBNP (i.e. > 70 pg/mL[14, 15]), we conducted multivariable logistic regression analyses using both the continuous and categorical forms of leptin and total adiponectin. For all regression analyses, the initial model 1 was adjusted for age, sex and race. Models were subsequently adjusted for additional covariates to include visit 1 NT-proBNP (in the analyses utilizing the change in this variable from visit 1 to visit 3). Of note, we compared the associations with adjustment for height and the waist to hip ratio versus adjustment for BMI. In the latter case, the changes in the effect sizes after the adjustment were smaller (i.e. likely accounted for less confounding). As such, we elected to use height and the waist to hip ratio (WHR) in the final models.
Multiplicative interactions between each adipokine and sex, race, BMI group and chronic kidney disease status (separately) for both visit 3 NT-proBNP and the change in this variable were assessed. A two-tailed p-value < 0.05 was considered statistically significant and all statistical analyses were conducted using STATA (Version 13; StataCorp, College Station, TX).
RESULTS
The mean age of the cohort was 64.8 ± 9.6 years and 48% were female (Table 1). Forty-two percent were non-Hispanic White, 26% Hispanic/Latino, 18% African American and 14% Chinese American. The mean BMI and waist circumference values were 28.1 ± 5.2 kg/m2 and 98 ± 14 cm, respectively, and 30% had a BMI greater than 30 kg/m2. Forty-seven percent of the participants were hypertensive, 40% were dyslipidemic, 14% had diabetes mellitus, 14% were classified with chronic kidney disease (i.e. eGFR < 60) and 6% had a positive family history of premature myocardial infarction. Forty-seven percent were never smokers, 43% were former smokers and 10% were current smokers. Mean/median (standard deviation) values for total adiponectin, leptin and NT-proBNP were 20.6/17.3 (13.1) µg/ml, 20.3/13.1 (21.8) ng/ml and 123/67 (215) pg/mL, respectively.
TABLE 1.
COHORT CHARACTERISTICS
| Characteristic | Mean (SD)/Median or Frequency (%) |
|---|---|
| Visit 1 NT-pro BNP (pg/ml) | 90.8 (128.5)/54.6 |
| Visit 3 NT-pro BNP (pg/ml) | 122.6 (214.9)/66.7 |
| NT-pro BNP Difference (Visit 3 – Visit 1) | 32.7 (11.6)/11.6 |
| Visit 3 NT-pro BNP > 70 pg/ml | 714 (48.0%) |
| Age (years) | 64.8 (9.6)/65 |
| Gender (male) | 768 (51.6%) |
| Race | |
| -Non-Hispanic White | 627 (42.1%) |
| -Chinese | 210 (14.1%) |
| -African American | 266 (17.9%) |
| -Hispanic | 385 (25.9%) |
| Waist circumference (cm) | 98.3 (14.1)/97 |
| Hip circumference (cm) | 104.3 (10.9)/102.6 |
| BMI (kg/m2) | 28.1 (5.2)/27.3 |
| Waist to Hip Ratio | 0.94 (0.07)/0.95 |
| Visceral Fat Area (cm2) | 148 (69.2)/137 |
| Subcutaneous Fat Area (cm2) | 254 (117.7)/236 |
| Dyslipidemia (Y/N) | 587 (40.2%) |
| Triglycerides (mg/dl) | 135 (98)/116 |
| Glucose (mg/dl) | 98 (26)/91 |
| Diabetes Mellitus | 211 (14.2%) |
| Smoking | |
| -Current | 151 (10.2%) |
| -Former | 636 (43.1%) |
| -Never | 689 (46.7%) |
| Systolic Blood Pressure (mmHg) | 124 (20)/121 |
| Diastolic Blood Pressure (mmHg) | 70 (10)/70 |
| Hypertension | 697 (47.2%) |
| eGFR by CKD Epi (ml/min/1.73 m2) | 79 (17)/79 |
| Chronic Kidney Disease (eGFR < 60) | 209 (14.1%) |
| Family History of Premature Myocardial Infarction | 89 (6%) |
| Adiponectin (µg/ml) | 21 (13)/17 |
| Leptin (ng/ml) | 20 (22)/13 |
| Aldosterone (pg/ml) | 151 (88)/131 |
| Renin (ng/ml) | 1.45 (3.3)/0.57 |
| End-Diastolic LV Mass (grams) | 147 (39)/141 |
| LV Ejection Fraction (%) | 69 (7)/70 |
N = 1,489 for visit 3 variables; N = 1,452 for change in NT-proBNP from visit 1 to visit 3
Higher quartiles of NT-proBNP were significantly associated with increasing age and a higher proportion of women (p < 0.01 for both) (Table 2). After adjustment for age, sex and race, significant differences by quartile of NT-proBNP were found for total and LDL-cholesterol, systolic blood pressure, pulse pressure, diagnosis of hypertension, urine albumin, eGFR, left ventricular mass and total adiponectin (p < 0.05 for all). The differences for left ventricular ejection fraction were not statistically significantly different (p = 0.06).
TABLE 2.
MEANS OF THE COHORT CHARACTERISTICS BY QUARTILE OF NTproBNP
| Characteristic | VISIT 3 NT-proBNP | p-value | |||
|---|---|---|---|---|---|
| Q1 | Q2 | Q3 | Q4 | ||
| Age (years) | 59.4 | 62.3 | 66.3 | 71.1 | < 0.01 |
| Sex (male) | 69.6 | 51.2 | 46.0 | 40.0 | < 0.01 |
| Waist circumference (cm)* | 97.7 | 97.0 | 96.6 | 95.9 | 0.45 |
| Hip circumference (cm)* | 103.5 | 102.7 | 102.6 | 102.5 | 0.60 |
| BMI (kg/m2)* | 28.0 | 27.5 | 27.4 | 27.2 | 0.19 |
| Waist to Hip Ratio* | 0.9 | 0.9 | 0.9 | 0.9 | 0.40 |
| Visceral Fat Area (cm2)* | 147 | 144 | 149 | 140 | 0.25 |
| Subcutaneous Fat Area (cm2)* | 250 | 238 | 243 | 234 | 0.34 |
| Dyslipidemia (Y/N)* | 38.9 | 36.3 | 37.7 | 36.7 | 0.90 |
| Triglycerides (mg/dl)* | 136.3 | 140.6 | 133.2 | 133.6 | 0.75 |
| Glucose (mg/dl)* | 97.0 | 100.5 | 97.4 | 97.2 | 0.22 |
| Diabetes (Y/N)* | 12.7 | 13.2 | 12.9 | 18.0 | 0.18 |
| Ever Smoker* | 50.1 | 46.8 | 52.5 | 50.7 | 0.46 |
| Systolic Blood Pressure (mmHg)* | 120.4 | 123.3 | 126.0 | 129.6 | < 0.01 |
| Diastolic Blood Pressure (mmHg)* | 68.9 | 68.9 | 69.5 | 69.7 | 0.71 |
| Hypertension (Y/N)* | 41.0 | 45.8 | 49.9 | 63.3 | < 0.01 |
| eGFR by CKD Epi (ml/min/1.73 m2)* | 79.3 | 79.5 | 78.9 | 76.2 | 0.03 |
| CKD (eGFR < 60)* | 15.3 | 16.4 | 17.7 | 21.6 | 0.12 |
| Adiponectin (μg/ml)* | 16.5 | 18.3 | 20.2 | 24.0 | < 0.01 |
| Leptin (ng/ml)* | 20.8 | 20.0 | 20.3 | 19.5 | 0.88 |
| Aldosterone (pg/ml)* | 152.6 | 142.2 | 153.6 | 150.4 | 0.32 |
| Renin (ng/ml)* | 1.6 | 1.4 | 1.0 | 1.1 | 0.11 |
| End-Diastolic LV Mass (g)* | 139.3 | 139.8 | 142.8 | 152.9 | < 0.01 |
| LV Ejection Fraction (%)* | 70.1 | 70.2 | 69.9 | 68.6 | 0.06 |
Q1 = Quartile 1 (< 34.4 pg/ml), Q2 = Quartile 2 (34.4 – 66.7 pg/ml), Q3 = Quartile 3 (66.7 – 130.1 pg/ml), Q4 = Quartile 4 (> 130.1 pg/ml)
Adjusted for age, sex and race.
NT-proBNP levels were significantly higher among women (81 vs. 52 pg/mL; p < 0.01), while Non-Hispanic Whites had the highest levels of NT-proBNP (78 pg/mL), followed by Hispanic/Latino (62 pg/mL), Chinese (53 pg/mL) and African Americans (52 pg/mL). Although there was a trend toward lower NT-proBNP levels with higher BMI, the difference was not statistically significant (p = 0.17): BMI 18.5 – 25 [n = 431]: 69 pg/mL, 25 – 30 [n = 588]: 65 pg/mL, 30 – 40 [n = 393]: 59 pg/mL, > 40 [n = 40]: 67 pg/mL) while adiponectin was different by BMI group (p < 0.01): 18.5 – 25: 25 µg/mL, 25 – 30: 20 µg/mL, 30 – 40: 18 µg/mL, > 40: 15 µg/mL). NT-pro BNP did increase significantly across quartiles of total adiponectin (p < 0.01): Q1: 50 pg/mL, Q2: 59 pg/mL, Q3: 69 pg/mL, Q4: 87 pg/mL; p < 0.01), but decreased modestly across quartiles of leptin (p = 0.40): Q1: 70 pg/mL, Q2: 65 pg/mL, Q3: 64 pg/mL, Q4: 60 pg/mL; p = 0.40).
Total Adiponectin
In multivariable linear regression models adjusted for age, sex and race/ethnicity, a 1-standard deviation (SD) increment in total adiponectin was associated with a 21 pg/ml higher visit 3 NT-proBNP concentration (p < 0.01), which decreased minimally to 21 pg/ml (p < 0.01) with additional adjustment for height, visceral and subcutaneous fat areas, dyslipidemia, diabetes, smoking, hypertension, eGFR, leptin, aldosterone and plasma renin activity (Table 3). The association was further attenuated, but remained statistically significant, with adjustment for left ventricular mass and ejection fraction (14 pg/ml, p <0.01). When total adiponectin was categorized into quartiles (Q1: < 11.8, Q2: 11.8 – 17.5, Q3: 17.5 – 26.3, Q4: > 26.3), and after full adjustment with all of the variables listed above, quartiles 2, 3 and 4 were associated with 15, 42 and 58% higher NT-proBNP concentrations respectively, compared to the first quartile.
TABLE 3.
MULTIVARIABLE LINEAR ASSOCIATIONS BETWEEN TOTAL ADIPONECTIN AND NT-proBNP
| OUTCOME: Visit 3 NT-Pro BNP (pg/ml) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Adipokine | Model 1 | Model 2 | Model 3* | |||||
| Estimate | p-value | Estimate | p-value | Estimate | p-value | |||
| Adiponectin (1-SD) | 21.16 | < 0.01 | 21.0 | < 0.01 | 14.0 | < 0.01 | ||
| Adiponectin Quartiles | ||||||||
| -Q2* | 1.16 | 0.03 | 1.16 | 0.06 | 1.15 | 0.09 | ||
| -Q3* | 1.40 | <0.01 | 1.41 | <0.01 | 1.42 | <0.01 | ||
| -Q4* | 1.74 | <0.01 | 1.65 | <0.01 | 1.58 | <0.01 | ||
| OUTCOME: NT-Pro BNP Change from Visit 1 to Visit 3 (pg/ml) | ||||||||
| Adipokine | Model 1 | Model 2 | Model 3 | Model 4** | ||||
| Estimate | p-value | Estimate | p-value | Estimate | p-value | Estimate | p-value | |
| Adiponectin (1-SD) | 16.9 | < 0.01 | 16.3 | < 0.01 | 19.8 | < 0.01 | 23.9 | < 0.01 |
| Adiponectin Quartiles | ||||||||
| -Q2 | −7.9 | 0.55 | −15.0 | 0.35 | −7.7 | 0.65 | −5.0 | 0.77 |
| -Q3 | 13.1 | 0.34 | 14.8 | 0.37 | 13.9 | 0.43 | 18.9 | 0.27 |
| -Q4 | 34.4 | 0.02 | 27.6 | 0.13 | 31.9 | 0.11 | 43.0 | 0.03 |
1-SD = 1-standard deviation increment of total adiponectin (13.1 μg/ml), Parameter estimate is a percent (i.e. 1.16 = 16% higher than reference)
R2 (1-SD) = 0.36, R2 (Quartiles) = 0.37;
R2 = 0.09, R2 = 0.09
Model 1: age, sex and race
Model 2: Model 1 + height, visceral and subcutaneous fat areas, dyslipidemia, diabetes, smoking, hypertension, eGFR, leptin, aldosterone & renin
Model 3: Model 2 + left ventricular mass and left ventricular ejection fraction
Model 4: Model 3 + NT-proBNP (visit 1 value)
After adjustment for age, sex and race/ethnicity, a 1- SD increment in total adiponectin was associated with an increase of 17 pg/ml in NT-proBNP from visit 1 to visit 3 (p < 0.01) (Table 3). This association was accentuated (20 pg/ml, p < 0.01) with additional adjustment for height, visceral and subcutaneous fat areas, dyslipidemia, diabetes, smoking, hypertension, eGFR, leptin, aldosterone, renin, left ventricular mass and left ventricular ejection fraction. The association was accentuated even more (24 pg/ml, p < 0.01) when the baseline (visit 1) NT-proBNP level was added to the model. Moreover, compared to the 1st quartile, and in models that included all of the covariates listed above, the 3rd and 4th quartiles of total adiponectin were associated with 19 (p = 0.27) and 43 pg/ml (p = 0.03) higher increases in NT-proBNP from visit 1 to visit 3, while the increase among those in the 2nd quartile was not significantly different (−5 pg/ml, p = 0.74).
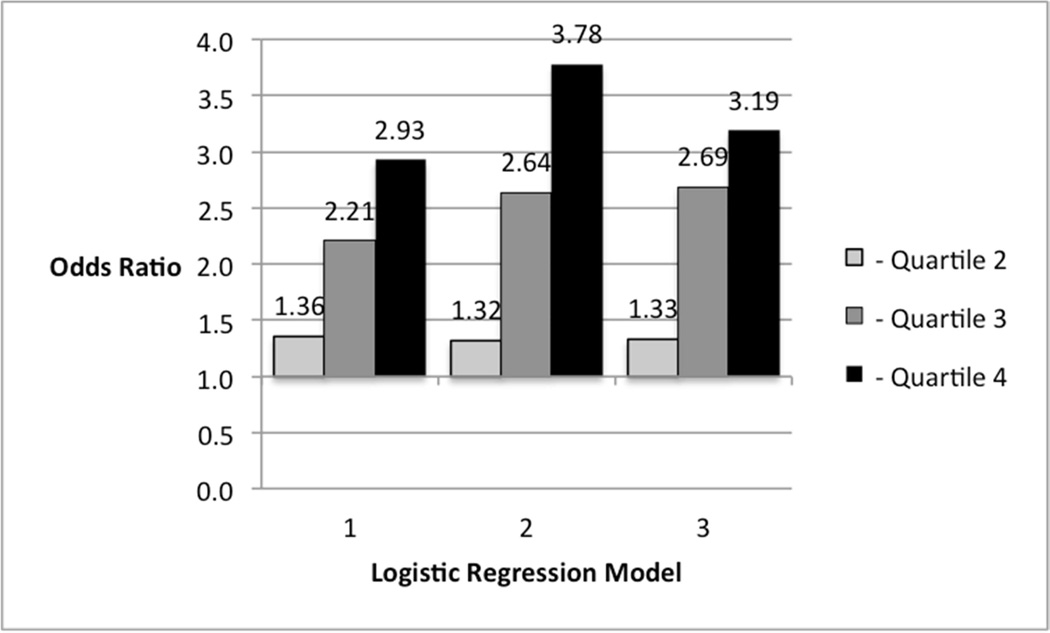
We then conducted multivariable logistic regression analyses to test the association between total adiponectin and the odds for having an NT-proBNP level greater than 70 pg/ml at visit 3. After adjustment for age, sex and race, a 1-SD increment in total adiponectin was associated with 56% higher odds of an NT-proBNP greater than this cut-point. The magnitude of the association was not materially changed with adjustment for all of the covariates used in the previous analyses. When total adiponectin was categorized into quartiles, and after adjustment for age, sex and race, the 3rd quartile was associated with over twice the odds (Odds Ratio: 2.21, p < 0.01) for an elevated NT-proBNP, while those in the 4th quartile had nearly 3-times the odds (2.93, p < 0.01), compared to those in the 1st quartile (Figure 1). Those in the 2nd quartile did not have significantly different odds for an elevated NT-proBNP (1.36, p = 0.08). Further adjustments accentuated the magnitudes of these associations.
FIGURE 1. ASSOCIATIONS BETWEEN QUARTILES OF TOTAL ADIPONECTIN AND A VISIT 3 NT-PROBNP VALUE > 70 pg/ml.
Referent Category = Quartile 1
Model 1: age, sex, race
Model 2: Model 1 + height, waist to hip ratio, dyslipidemia, diabetes, smoking, hypertension, eGFR, leptin, aldosterone and renin
Model 3: Model 2 + LV mass and LV ejection fraction
The test for effect modification between total adiponectin and sex for NT-proBNP at visit 3 was significant (p < 0.01). As such, we conducted analyses stratified by sex and found that the associations were significantly stronger among men such that, in the fully adjusted model, a 1-SD increment of total adiponectin was associated with a 25 pg/ml higher NT-proBNP level in men (p < 0.01) and a 10 pg/ml higher in women (p < 0.01). No other significant interactions were found.
Leptin
Leptin (as a continuous or categorical [quartiles] variable) was not associated with NT-proBNP (data not shown).
DISCUSSION
In this study of a relatively large, multi-ethnic population-based cohort from multiple sites across the United States, higher adiponectin concentrations were significantly associated with higher NT-proBNP levels independent of relevant covariates. The associations were significant for not only cross-sectional associations, but also for absolute changes in NT-proBNP over approximately 5 years. Moreover, a total adiponectin level above the median was associated with at least twice the odds for having an NT-proBNP value greater than 70 pg/ml. At the same time, there were no significant associations between leptin and NT-proBNP in analyses using cross-sectional or longitudinal data. These findings indicate a complex relationship between selected cytokines released from adipose tissue and NT-proBNP.
Based on previous findings linking BNP to left ventricular structure and function[16], and the results we have published that showed significant associations between leptin and left ventricular mass, volume and hypertrophy[10], we hypothesized that leptin would be inversely associated with NT-proBNP levels. The results of the current study do not support this supposition. Rather, adiponectin was strongly and robustly associated with NT-proBNP levels, which has been demonstrated previously in patients after acute coronary syndromes.[17] The logical next question is, why the difference in the associations for adiponectin and leptin with NT-proBNP?
Both of these hormones appear to be important in glucose regulation by increasing insulin sensitivity. In this regard, tumor necrosis factor - alpha (TNF-α) has been shown to decrease insulin sensitivity and leptin secretion[18], while adiponectin decreases both the production and activity of TNF-α.[19] Despite these differences, both adiponectin and leptin increase fatty acid oxidation and thermogenesis.[20] From this, it is not readily apparent why adiponectin is significantly associated with NT-proBNP and leptin is not.
We further explored this question by examining several published relationships of adiponectin and NT-proBNP with common anatomic or physiologic characteristics. First, adiponectin is inversely associated with measures of diastolic dysfunction, but not ejection fraction (i.e. systolic dysfunction).[21] Since diastolic dysfunction is related to higher ventricular wall stress, and NT-proBNP would rise correspondingly to the latter, diastolic dysfunction would predict lower levels of adiponectin but higher levels of NT-proBNP. Notably, previous studies have shown associations between adiponectin and incident CVD are no longer significant after adjustment for NT-proBNP suggesting a complex relationship between these variables.[22] Second, adiponectin is inversely associated with insulin resistance, waist circumference and triglycerides.[20, 23–25] However, using existing data in the MESA, none of the correlations between NT-proBNP and waist circumference and the waist to hip ratio, as well as fasting triglycerides, insulin and glucose were significant (r = −0.03, −0.02, −0.01, −0.05 and 0.01, respectively). Similarly, NT-proBNP is lower among those who are obese[26], which, in some circumstances, may be due to treatment with antihypertensive agents.[27] In the analysis for this report, we tested for effect modification of NT-proBNP by BMI category, which was not significant. Also, our findings were persistant after adjustment for blood pressure medications. Third, higher adiponectin is inversely associated with aldosterone[28] and, as mentioned above, NT-proBNP influences the RAAS system resulting in sodium natriuresis to reduce fluid volume. As an increase in aldosterone would increase fluid volume, and thereby NT-proBNP, and adiponectin is inversely associated with aldosterone, it does not appear that this RAAS hormone could explain the positive association between adiponectin and NT-proBNP. Moreover, from our data, NT-proBNP was not correlated with aldosterone (r = 0.01).
Given that adiponectin is secreted predominately by visceral fat and leptin by subcutaneous fat, physiologic differences between these fat depots may provide insight into the association between adiponectin and NT-proBNP.[9] In this regard, TNF-α is released from visceral adipose tissue and results in decreased expression of adiponectin.[29] As higher levels of obesity are associated with lower NT-proBNP levels, it seems plausible that visceral adipose tissue may be the common link. Indeed, there is some evidence for a strong correlation between visceral fat and higher NTproBNP. [30] This hypothesis requires further investigation.
As can be surmised from the discussion provided above, the physiology between adiponectin and NT-proBNP is quite complex. In some ways, the available data suggests that the association could be inverse. On the other hand, nonconcomitant studies suggest that visceral adipose tissue may be the common factor that links these two biomarkers physiologically. Given the burgeoning nature of both obesity and heart failure, we believe further study of this complex relationship is indicated.
Strengths of this study include a relatively large, well-characterized, multi-ethnic cohort from across the United States that is free of clinical CVD and both valid and reproducible measures of two different adipokines. Limitations include few subjects at the highest levels of obesity and a pseudo longitudinal study design whereby the adipokine measures were collected during the follow-up period, which may limit inferences on causality. Additionally, it may be possible that there is residual confounding.
In conclusion, total adiponectin, and not leptin, is significantly and independently associated with NT-proBNP among a multi-ethnic cohort that had no clinical evidence of cardiovascular disease at baseline. Moreover, the associations appear to be stronger in men, but not by race/ethnic group. These findings suggest a potentially important, albeit potentially paradoxical, relationship between adiponectin and NT-proBNP.
HIGHLIGHTS.
We examined the cross-sectional and longitudinal associations between two adipokines (adiponectin and leptin) and NT-pro BNP.
Higher adiponectin was found to be associated with higher NT-pro BNP, larger increases in NT-pro BNP over 5 years and a higher odds of having an NT-pro BNP greater than 70 pg/mL.
The association between adiponectin and NT-pro BNP was significantly larger in men.
Leptin was not associated with NT-pro BNP.
ACKOWLEDGMENTS
The authors thank the other investigators, the staff, and the participants of the MESA study for their valuable contributions. A full list of participating MESA investigators and institutions can be found at http://www.mesa-nhlbi.org.
This research was supported by contracts N01-HC-95159, N01-HC-95160, N01-HC-95161, N01-HC-95162, N01-HC-95163, N01-HC-95164, N01-HC-95165, N01-HC-95166, N01-HC-95167, N01-HC-95168 and N01-HC-95169 from the National Heart, Lung, and Blood Institute, as well as by grants HL088451 from the NHLBI and UL1-TR- 000040 and UL1-TR-001079 from NCRR.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Rahmouni K, Correia MLG, Haynes WG, Mark AL. Obesity-associated hypertension: new insights into mechanisms. Hypertension. 2005;45:9–14. doi: 10.1161/01.HYP.0000151325.83008.b4. [DOI] [PubMed] [Google Scholar]
- 2.DiBona GF, Kopp UC. Neural control of renal function. Physiol Rev. 1997;77:75–197. doi: 10.1152/physrev.1997.77.1.75. [DOI] [PubMed] [Google Scholar]
- 3.Tuck ML, Sowers J, Dornfeld L, Kledzik G, Maxwell M. The effect of weight reduction on blood pressure, plasma renin activity, and plasma aldosterone levels in obese patients. N Engl J Med. 1981;304:930–933. doi: 10.1056/NEJM198104163041602. [DOI] [PubMed] [Google Scholar]
- 4.Yasue H, Yoshimura M, Sumida H, Kikuta K, Kugiyama K, Jougasaki M, et al. Localization and mechanism of secretion of B-type natriuretic peptide in comparison with those of A-type natriuretic peptide in normal subjects and patients with heart failure. Circulation. 1994;90:195–203. doi: 10.1161/01.cir.90.1.195. [DOI] [PubMed] [Google Scholar]
- 5.Nakao K, Ogawa Y, Suga S, Imura H. Molecular biology and biochemistry of the natriuretic peptide system. I: Natriuretic peptides. J Hypertens. 1992;10:907–912. [PubMed] [Google Scholar]
- 6.Battaglia M, Pewsner D, Juni P, Egger M, Bucher HC, Bachmann LM. Accuracy of B-Type Natriuretic Peptide Tests to Exclude Congestive Heart Failure: Systematic Review of Test Accuracy Studies. Arch Intern Med. 2006;166:1073–1080. doi: 10.1001/archinte.166.10.1073. [DOI] [PubMed] [Google Scholar]
- 7.Bruch C, Rothenburger M, Gotzmann M, Sindermann J, Scheld HH, Breithardt GN, et al. Risk Stratification in Chronic Heart Failure: Independent and Incremental Prognostic Value of Echocardiography and Brain Natriuretic 19 Peptide and its N-terminal Fragment. Journal of the American Society of Echocardiography. 2006;19:522–528. doi: 10.1016/j.echo.2005.12.027. [DOI] [PubMed] [Google Scholar]
- 8.Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE, Drazner MH, et al. 2013 ACCF/AHA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Jac. 2013;62:e147–e239. doi: 10.1016/j.jacc.2013.05.019. [DOI] [PubMed] [Google Scholar]
- 9.Fantuzzi G. Adipose tissue, adipokines, and inflammation. J Allergy Clin Immunol. 2005;115 doi: 10.1016/j.jaci.2005.02.023. 911–9–quiz920. [DOI] [PubMed] [Google Scholar]
- 10.Allison MA, Bluemke DA, McClelland R, Cushman M, Criqui MH, Polak JF, et al. Relation of Leptin to Left Ventricular Hypertrophy (from the Multi-Ethnic Study of Atherosclerosis) Am J Cardiol. 2013;112:726–730. doi: 10.1016/j.amjcard.2013.04.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bild DE, Bluemke DA, Burke GL, Detrano R, Diez Roux AV, Folsom AR, et al. Multi-ethnic study of atherosclerosis: objectives and design. Am J Epidemiol. 2002;156:871–881. doi: 10.1093/aje/kwf113. [DOI] [PubMed] [Google Scholar]
- 12.Criqui MH, Denenberg JO, McClelland R, Allison MA, Ix JH, Guerci A, et al. Abdominal Aortic Calcium, Coronary Artery Calcium, and Cardiovascular Morbidity and Mortality in the Multi-Ethnic Study of Atherosclerosis. Arteriosclerosis, Thrombosis, and Vascular Biology. 2014 doi: 10.1161/ATVBAHA.114.303268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nasir K, Katz R, Mao S, Takasu J, Bomma C, Lima JAC, et al. Comparison of left ventricular size by computed tomography with magnetic resonance imaging 20 measures of left ventricle mass and volumes: the multi-ethnic study of atherosclerosis. Journal of Cardiovascular Computed Tomography. 2008;2:141–148. doi: 10.1016/j.jcct.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 14.Daniels LB, Allison MA, Clopton P, Redwine L, Siecke N, Taylor K, et al. Use of natriuretic peptides in pre-participation screening of college athletes. International Journal of Cardiology. 2008;124:411–414. doi: 10.1016/j.ijcard.2006.12.076. [DOI] [PubMed] [Google Scholar]
- 15.Daniels LB, Maisel AS. Natriuretic peptides. J Am Coll Cardiol. 2007;50:2357–2368. doi: 10.1016/j.jacc.2007.09.021. [DOI] [PubMed] [Google Scholar]
- 16.Hsich EM, Grau-Sepulveda MV, Hernandez AF, Eapen ZJ, Xian Y, Schwamm LH, et al. Relationship between sex, ejection fraction, and B-type natriuretic peptide levels in patients hospitalized with heart failure and associations with inhospital outcomes: findings from the Get With The Guideline-Heart Failure Registry. Am Heart J. 2013;166:1063–1063. doi: 10.1016/j.ahj.2013.08.029. [DOI] [PubMed] [Google Scholar]
- 17.Ang DSC, Welsh P, Watt P, Nelson SM, Struthers A, Sattar N. Serial changes in adiponectin and BNP in ACS patients: paradoxical associations with each other and with prognosis. Clin Sci. 2009;117:41–48. doi: 10.1042/CS20080506. [DOI] [PubMed] [Google Scholar]
- 18.Kirchgessner TG, Uysal KT, Wiesbrock SM, Marino MW, Hotamisligil GS. Tumor Necrosis Factor-alpha Contributes to Obesity-related Hyperleptinemia by Regulating Leptin Release from Adipocytes. J Clin Invest. 1997;100:2777–2782. doi: 10.1172/JCI119824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kappes A, Löffler G. Influences of ionomycin, dibutyryl-cycloAMP and tumour necrosis factor-alpha on intracellular amount and secretion of apM1 in differentiating primary human preadipocytes. Horm Metab Res. 2000;32:548–554. doi: 10.1055/s-2007-978684. [DOI] [PubMed] [Google Scholar]
- 20.Dyck DJ, Heigenhauser GJF, Bruce CR. The role of adipokines as regulators of skeletal muscle fatty acid metabolism and insulin sensitivity. Acta Physiol (Oxf) 2006;186:5–16. doi: 10.1111/j.1748-1716.2005.01502.x. [DOI] [PubMed] [Google Scholar]
- 21.Fukuta H, Ohte N, Wakami K, Goto T, Tani T, Kimura G. Relation of plasma levels of adiponectin to left ventricular diastolic dysfunction in patients undergoing cardiac catheterization for coronary artery disease. Am J Cardiol. 2011;108:1081–1085. doi: 10.1016/j.amjcard.2011.06.005. [DOI] [PubMed] [Google Scholar]
- 22.Wannamethee SG, Welsh P, Whincup PH, Sawar N, Thomas MC, Gudnarsson V, et al. High adiponectin and increased risk of cardiovascular disease and mortality in asymptomatic older men: does NT-proBNP help to explain this association? European Journal of Cardiovascular Prevention & Rehabilitation. 2011;18:65–71. doi: 10.1097/HJR.0b013e32833b09d9. [DOI] [PubMed] [Google Scholar]
- 23.Gannage-Yared M-H, Khalife S, Semaan M, Fares F, Jambart S, Halaby G. Serum adiponectin and leptin levels in relation to the metabolic syndrome, androgenic profile and somatotropic axis in healthy non-diabetic elderly men. Eur J Endocrinol. 2006;155:167–176. doi: 10.1530/eje.1.02175. [DOI] [PubMed] [Google Scholar]
- 24.Kwon K, Jung SH, Choi C, Park S-H. Reciprocal association between visceral obesity and adiponectin: in healthy premenopausal women. International Journal of Cardiology. 2005;101:385–390. doi: 10.1016/j.ijcard.2004.03.050. [DOI] [PubMed] [Google Scholar]
- 25.Yatagai T, Nagasaka S, Taniguchi A, Fukushima M, Nakamura T, Kuroe A, et al. Hypoadiponectinemia is associated with visceral fat accumulation and insulin resistance in Japanese men with type 2 diabetes mellitus. Metabolism. 2003;52:1274–1278. doi: 10.1016/s0026-0495(03)00195-1. [DOI] [PubMed] [Google Scholar]
- 26.Taylor JA, Christenson RH, Rao K, Jorge M, Gottlieb SS. B-type natriuretic peptide and N-terminal pro B-type natriuretic peptide are depressed in obesity despite higher left ventricular end diastolic pressures. Am Heart J. 2006;152:1071–1076. doi: 10.1016/j.ahj.2006.07.010. [DOI] [PubMed] [Google Scholar]
- 27.Clerico A, Giannoni A, Vittorini S, Emdin M. The paradox of low BNP levels in obesity. Heart Failure Reviews. 2012;17:81–96. doi: 10.1007/s10741-011-9249-z. [DOI] [PubMed] [Google Scholar]
- 28.Allison MA, Jenny NS, McClelland RL, Cushman M, Rifkin D. The associations of adipokines with selected markers of the renin-angiotensinogen-aldosterone system: the multi-ethnic study of atherosclerosis. J Hum Hypertens. 2015:127–133. doi: 10.1038/jhh.2014.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bruun JM, Verdich C, Toubro S, Astrup A, Richelsen B. Association between measures of insulin sensitivity and circulating levels of interleukin-8, interleukin-6 and tumor necrosis factor-alpha. Effect of weight loss in obese men. Eur J Endocrinol. 2003;148:535–542. doi: 10.1530/eje.0.1480535. [DOI] [PubMed] [Google Scholar]
- 30.Malavazos AE, Morricone L, Marocchi A, Ermetici F, Ambrosi B, Corsi MM. N-Terminal Pro-B-Type Natriuretic Peptide and Echocardiographic Abnormalities in Severely Obese Patients: Correlation with Visceral Fat. Clin Chem. 2006;52:1211–1213. doi: 10.1373/clinchem.2006.067736. [DOI] [PubMed] [Google Scholar]