Abstract
Rationale
Disrupted social behavior, including occasional aggressive outbursts, is characteristic of withdrawal from long-term alcohol (EtOH) use. Heavy EtOH use and exaggerated responses during withdrawal may be treated using glutamatergic N-methyl-D-aspartate receptor (NMDAR) antagonists.
Objectives
The current experiments explore aggression and medial prefrontal cortex (mPFC) glutamate as consequences of withdrawal from intermittent access to EtOH, and changes in aggression and mPFC glutamate caused by NMDAR antagonists memantine and ketamine.
Methods
Swiss male mice underwent withdrawal following 1-8 weeks of intermittent access to 20% EtOH. Aggressive and non-aggressive behaviors with a conspecific were measured 6-8 h into EtOH withdrawal after memantine or ketamine (0-30 mg/kg, i.p.) administration. In separate mice, extracellular mPFC glutamate after memantine was measured during withdrawal using in vivo microdialysis.
Results
At 6-8 h withdrawal from EtOH, mice exhibited more convulsions and aggression, and decreased social contact compared to age-matched water controls. Memantine, but not ketamine, increased withdrawal aggression at the 5 mg/kg dose in mice with a history of 8 weeks EtOH but not 1 or 4 weeks of EtOH or in water drinkers. Tonic mPFC glutamate was higher during withdrawal after 8 weeks EtOH compared to 1 week EtOH or 8 weeks water. Five mg/kg memantine increased glutamate in 8 week EtOH mice, but also in 1 week EtOH and water drinkers.
Conclusions
These studies reveal aggressive behavior as a novel symptom of EtOH withdrawal in outbred mice and confirm a role of NMDARs during withdrawal aggression and for disrupted social behavior.
Keywords: intermittent access, alcohol withdrawal, aggression, glutamate, microdialysis, mPFC, memantine, ketamine
Aggressive behavior during withdrawal from alcohol (EtOH) is of substantial concern to public health and also criminal justice (Cardoso et al. 2006). Withdrawal-induced violence toward caretakers or in the emergency room (Abeyasinghe and Jayasekera 2003; Citrome and Volavka 1999; Patch et al. 1997) may result from impaired behavioral inhibition (Björk et al. 2004; Wills et al 2009). Features of EtOH withdrawal can include dysfunctional social and aggressive behavior (Broadwater et al. 2011; Overstreet et al. 2002; Varlinskaya and Spear 2004), in addition to common symptoms such as headaches, fever, convulsions, and anxiety (Trevisan et al. 1998). Experimental studies examining either recovering alcoholics or heavy, binge-drinking youths independently link greater increases in frustration and irritability during early abstinence to a higher chance of relapse (Baars et al. 2013; Winward et al. 2014). It would be beneficial to identify a translational model of EtOH withdrawal-induced aggression.
Heightened aggressive and defensive activity are among the most striking behavioral events seen in laboratory mice and rats undergoing opiate withdrawal (Kantak and Miczek 1988; Lal et al. 1971; Vivian and Miczek 1991), but no such aggression-focused model exists for animals undergoing EtOH withdrawal. Early studies document impaired social interactions between animals during EtOH withdrawal (File et al. 1989), which can be augmented by repeated withdrawal cycles (Overstreet et al. 2002). Other behavioral tests, such as the elevated plus maze, suggest anxiety-like behavior during EtOH withdrawal (Kampov-Polevoy et al. 2000). However these tests may not be repeated over the time course of withdrawal nor do they assess aspects of sociability. EtOH dependence may result from a specific pattern of long-term, intermittent heavy drinking, or ‘binge’ episodes, followed by periods of deprivation. In the current study, not only did the mice consume EtOH voluntarily according to an intermittent schedule of access, one which produces excessive drinking that is associated with neurobiological indices of dependence and withdrawal (George et al. 2012; Hwa et al. 2011), but they also were repeatedly tested for social encounters in the home-cage.
One of the cardinal features of EtOH withdrawal is a hyperexcitable state caused by the upregulation of glutamatergic activity in the brain (Chefer et al. 2011; Rossetti et al. 1999). Cycles of heavy EtOH consumption and deprivation are hypothesized to produce a kindling process caused by multiple withdrawals, similar to electrophysiological kindling (Ballenger and Post 1978). In support of this hypothesis, EtOH withdrawal episodes also encompass hypermotility (Poldrugo and Snead, III 1984) and increased seizure scores (Becker and Hale 1993). Microdialysis studies have shown increased glutamate in the striatum (Rossetti and Carboni 1995), nucleus accumbens (Dahchour et al. 1998; Melendez et al. 2005a; Kapasova and Szumlinski 2008; Griffin III et al. 2014) and hippocampus (Dahchour and De Witte 1999; Chefer et al. 2011) during withdrawal in EtOH-exposed rats and mice. Therefore, glutamate and its receptors are of considerable interest for targeting treatments for EtOH dependence.
In particular, the glutamatergic N-methyl-D-aspartate receptors (NMDARs) may mediate the effects of EtOH since acute doses of EtOH inhibit the function of NMDARs (Lovinger et al. 1989). To mimic this action, NMDAR antagonists have been used to decrease EtOH drinking and to alleviate symptoms of EtOH withdrawal, including convulsive events (Erden et al. 1999; Grant et al. 1990). For example, FDA-approved acamprosate can decrease EtOH preference in rats and suppress the elevated glutamatergic tone during repeated withdrawal (Dahchour et al. 1998; Spanagel et al. 1996). The uncompetitive NMDAR antagonist memantine attenuates EtOH withdrawal-induced seizures (Stepanyan et al. 2008) and protects against EtOH withdrawal-induced cell death and NMDAR upregulation in rats (Idrus et al. 2011; Maler et al. 2005). Importantly, memantine also reduces cravings for EtOH in moderate users and in EtOH-dependent subjects (Bisaga and Evans 2004; Krupitsky et al. 2007). The pharmacokinetics of memantine may incorporate a ‘partial trapping’ mechanism where the drug binds to a superficial site on the NMDAR instead of a deep site (Kotermanski et al. 2009). These more rapid on-off kinetics contribute to a favorable clinical profile compared to other NMDAR antagonists like ketamine and phencyclidine (Blanpied et al. 1997; Krystal et al. 2003; Macdonald et al. 1991), prompting its proposed use as a treatment for the symptoms of EtOH withdrawal (Spanagel et al. 1996).
The present study examined aggression in Swiss male outbred mice that underwent withdrawal from intermittently available 20% EtOH. Specifically, we investigated how glutamate and NMDARs play a role in withdrawal aggression through systemic injections of NMDAR antagonists memantine and ketamine at the peak of acute withdrawal symptoms. As EtOH withdrawal is accompanied by increased glutamatergic neurotransmission, we speculate that NMDAR antagonism restores normal glutamate activity, thereby decreasing aggression. An alternative hypothesis predicts that administration of NDMAR antagonists increase aggressive behavior in EtOH-withdrawn mice, possibly as a result of non-specific alterations in medial prefrontal cortex (mPFC) circuitry. The mPFC is a crucial area in the behavioral disinhibition and cognitive decline during the transition to drug and EtOH addiction (George and Koob 2010), as well as in EtOH-heightened aggression (Quadros et al. 2014). PFC pyramidal neurons release excitatory amino acids, like glutamate, locally (Pirot et al. 1995; Thomson and Deuchars 1994) and project to the nucleus accumbens and ventral tegmental area (Sesack and Pickel 1992). A final experiment provides direct measurement of mPFC glutamate and other excitatory amino acids during EtOH withdrawal and its manipulation through memantine and resumption of EtOH intake. Restoration of dysregulated mPFC extracellular glutamate may provide evidence for the neuroprotective effects of memantine during EtOH withdrawal.
METHODS
Animals
Swiss-derived, Carworth Farm Webster (CFW) male mice (Charles River Laboratories International Inc., Wilmington, MA) were eight weeks (wks) old upon arrival and initially housed for three days in groups of six before being housed individually in polycarbonate cages (28 × 17 × 12 cm) with stainless steel wire mesh lids and pine shavings as bedding. Mice were randomly assigned to be on either EtOH-drinking or water-drinking schedules then assessed as resident aggressors or for in vivo microdialysis. Other groups of CFW mice served as intruders that were housed in groups of eight to ten in 46 × 24 × 16 cm polycarbonate cages with corn cob shavings as bedding. All animals were given one wk to habituate to laboratory conditions on an inverse 12 h light/dark cycle (lights off at 7 AM) with constant temperature (21 ± 2º C) and humidity (30%). Mice had unrestricted access to food (LabDiet 5001 Rodent Diet, PMI Nutrition International, Brentwood, MO) and water (H2O). All experimental procedures were approved by the Tufts University Institutional Animal Care and Use Committee and complied with the NIH Guide for Care and Use of Laboratory Animals (2011).
Intermittent access to ethanol
Ethanol drinking procedures
Outbred CFW mice were given intermittent access to a 20% EtOH (w/v) solution according to the procedure used in inbred C57BL/6J mice (Hwa et al. 2011). Three days per wk, with 24-48 h between EtOH access days, EtOH-drinking mice had a two-bottle choice between 20% EtOH and H2O for 24 h. On the remaining days the mice had two bottles of H2O. H2O-drinking controls had continuous access to two bottles of H2O. Drinking tubes were held in the wire mesh cage lid and weighed before and after 24 h access. Mice were weighed prior to every EtOH access day before receiving the two bottles, 3 h into the dark cycle. H2O drinkers were also weighed three days per wk. To control for evaporation or spillage, ‘drip’ measurements (ca. 0.2 mL) were taken from bottles on an empty cage. These values were averaged across one wk, and subtracted from each individual animal’s fluid intake. Daily fluid intake of 20% EtOH and H2O were measured in mL to calculate EtOH consumption in grams of EtOH per kilogram of bodyweight (g/kg) and EtOH preference, defined as volume of EtOH intake divided by volume of total fluid intake (%). In addition to EtOH consumption, blood EtOH concentrations (BECs) were measured after 4 wks of EtOH drinking in a subset of mice (n=24). Blood was collected from the submandibular vein after 1 h access to two-bottle choice. Samples were centrifuged at 3000 rpm for 10 min, and plasma was analyzed using the Analox AM1 Alcohol Analyzer (Analox Instruments Inc., Lunenberg, MA).
Handling-induced convulsions
The above groups of mice given intermittent access to EtOH for 1, 4, or 8 wks were assessed for handling-induced convulsions (HIC) as a physical measure of EtOH dependence (Goldstein and Pal 1971). HIC scores were assigned according to a 0-4 ordinal scale (Goldstein 1972). Scores of 2 or greater indicated the presence of tonic-clonic convulsions upon handling. Assessments were made every 2 hr for 0-10 hr after the EtOH bottle was removed.
Aggression during withdrawal
Our laboratory has been using CFW mice for almost four decades to study the pharmacology of aggressive behavior (Miczek and O’Donnell 1978; Quadros et al. 2014). These mice are the most commonly studied among outbred stocks (Festing 2014). Before the first presentation of EtOH, resident mice were screened for home-cage aggression against a male conspecific in a resident-intruder protocol (Miczek and O’Donnell 1978). During this initial screening phase, residents encountered an intruder inside a perforated protective cage for 5 min of instigation before the confrontation (Fish et al. 1999). Aggressive encounters lasted for 5 min after the first attack bite or 10 min with no attack bites. Each resident mouse was screened for 5-10 sessions to establish consistent aggression as part of its behavioral repertoire. Animals that did not show any aggression during this phase or showed no aggression during drug testing were not included in the remainder of the study (n=30). After selecting for baseline aggression, mice were divided into EtOH- (n=55) or H2O-drinking (n=25) groups. The emergence of aggression during EtOH withdrawal was tested after 1, 4, or 8 wks of intermittent access to EtOH. HIC scores revealed that 6-8 h after the removal of EtOH was the peak of acute withdrawal in CFW mice, so withdrawal aggression tests also occurred on different days but during this time period.
Withdrawal aggression after NMDAR antagonism
To probe the glutamatergic system during withdrawal from intermittent EtOH, we administered an NMDAR antagonist to mice before aggression tests. Mice were habituated to i.p. injections of dH2O before testing. In a repeated measures design, mice received all doses of memantine or ketamine across 2 wks of intermittent EtOH and withdrawal test periods. H2O-drinking controls were tested for aggression during these same time periods. Specifically, resident-intruder confrontations occurred in the home-cage without prior social instigation, and mice (EtOH 1 wk: n=10, 4 wks: n=24, 8 wks: n=21; H2O 1 wk: n=8, 4 wks: n=10, 8 wks: n=7) received doses of memantine (3-30 mg/kg, i.p. or dH2O vehicle) 20 min prior to the confrontation. A separate group of 8 wk EtOH mice (n=17) received doses of another NMDAR antagonist, ketamine (3, 5, 10 mg/kg, i.p. or d H2O vehicle) 10 min before the confrontation. Memantine and ketamine doses were counterbalanced and delivered in 1ml/100g.
Tests commenced with the introduction of the intruder and lasted for 5 min after the initial attack bite by the resident. Sessions were digitally recorded for later video analysis of aggressive (i.e. attack bites, sideways threats, tail rattles and pursuits), non-aggressive non-social (i.e. walking, rearing and autogrooming) and non-aggressive pro-social (contact with intruder) behaviors (Miczek and O’Donnell 1978). Frequencies and durations (s) of behaviors were scored using the Observer XT 9.0 software (Noldus, Wageningen, The Netherlands).
Glutamate in vivo microdialysis
Surgery
CFW mice underwent stereotaxic surgery for mPFC glutamate microdialysis after EtOH or H2O for 1 wk (EtOH: n=5, H2O: n=7) or 8 wks (EtOH: n=5, H2O: n=10). Mice were given an i.p. ketamine (100 mg/kg) / xylazine (10 mg/kg) combination for anesthesia and s.c. carprofen (5 mg/kg) for analgesia before intracerebral implantation. CMA7 26-gauge guide cannulae (Harvard Apparatus, Holliston, MA) were aimed 1 mm above the mPFC at coordinates AP +1.5 mm and ML +0.3 mm from bregma, DV −1.0 mm from dura and affixed to the skull using carboxylate cement (Durelon®, 3M). Mice recovered for three days then had 2-3 days of intermittent access to EtOH before they were tested for mPFC glutamate.
In vivo microdialysis
Microdialysis sampling of mPFC glutamate after memantine injection was conducted in order to reveal why memantine may increase withdrawal aggression through changes in glutamate signaling. Glutamate samples during peak withdrawal matched the timing of when mice received memantine before a confrontation with an intruder during 6-8 hr withdrawal. On test days, mice were anesthetized with isoflurane (Webster Veterinary, Devens, MA), and the dummy probes were replaced with CMA7 microdialysis probes with a 1 mm active membrane (Harvard Apparatus, Holliston, MA). We believed waiting six hr after probe insertion would be sufficient time to allow tissue to recover before baseline samples were collected. It has been demonstrated that extracellular glutamate levels stabilize following probe insertion after 3-4 hr (Yan et al. 1998; Reznikov et al. 2007), yet some research have measured basal levels immediately after probe insertion (Segovia et al. 1997; Dahchour et al. 1994). Further, mice had 24 hr access to EtOH the evening before, so it was crucial to not interrupt the heavy drinking. Alcohol was removed on the test day when the probe was inserted, beginning the withdrawal process and glutamate stabilization.
After probe insertion, artificial cerebral spinal fluid was perfused at a flow rate of 0.5 µl/min (CMA400 microinfusion pump, Harvard Apparatus, Holliston, MA). Five h later, the flow rate was increased to 1.5 µl/min 1 h before microdialysate collection. The samples were collected every 20 min, during the same period as peak HIC scores and withdrawal aggression, 6-8 h into acute withdrawal. After collecting four baseline samples, mice were injected with dH2O (i.p.) to control for potential injection stress, and then 5 mg/kg (i.p.) memantine. This dose was chosen because it showed pro-aggressive effects during withdrawal in long-term EtOH-drinking animals. Changes in glutamate were calculated in percent change from baseline in µM. There were two samples collected following dH2O injection and three following memantine injection. Since aggressive encounters lasted for five min after drug injection, the first sample after the drug injections during microdialysis was used for analysis. In separate mice, 2 g/kg EtOH was administered via gavage (i.g.) during peak withdrawal to compare between two different pharmacological means of NMDAR antagonism in mice with a history of 8 wk EtOH (n=7) and H2O (n=6). Mice were also habituated to either i.p. or i.g. injections before testing. After testing, animals were given an overdose of ketamine/xylazine, then perfused with saline followed by 4% paraformaldehyde before sectioning. Coronal brain slices were cut using a cryostat, and correct mPFC placements were verified using Nissl staining. Mice with placements outside the mPFC or glutamate samples below the limit of detection were excluded from analysis (n=10).
HPLC
Glutamate was measured using an HPLC-electrochemical detection (ECD) system and a pre-column ortho-phthalaldehyde (OPA) derivatization method. Mobile phase consisted of filtered 0.1M phosphate buffer: methanol: acetonitrile (85:4:11) including 5 mg/l EDTA•2Na, pH 6.0 and ran at a flow rate of 0.5 ml/min through an Eicompak FA-3ODS analytical column (3.0mm, i.d. × 50 mm, Eicom, USA) with a temperature of 30°C and VT-05 detection cell (Antec, The Netherlands). For derivatization, 20 mM OPA was prepared from 0.5 M potassium carbonate including 10% 2N hydrochloric acid with added 0.2% 2-mercaptoethanol, which was then diluted to 5 mM OPA-mercaptoethanol. An autosampler (AS100, Antec, The Netherlands) transferred 5 µl 5 mM OPA-mercaptoethanol reagent to 20 µl microdialysate and left for 2.5 min before 15 µl was injected onto the column for HPLC analysis. Using this method, the following amino acids were separated and measured: glutamate, glutamine, glycine, alanine, taurine, and GABA. All chemicals were obtained from Sigma, unless otherwise specified.
Recovery and probe dynamics were similar between the groups of mice. Although day-to-day variations may have occurred, EtOH and H2O drinking groups of mice were tested concurrently, with 1 wk and 8 wk groups mixed, as well. Amino acid concentrations were determined by using standard curves with known amounts in a range of 1 µM to 1 mM. The average baselines of each experimental group were determined from the standards and could be compared to the other groups.
Statistical Analyses
EtOH drinking (g/kg/24h) and EtOH preference over time were analyzed using one-way repeated measures (RM) analyses of variance (ANOVA). Because of the ordinal nature of HIC scores, convulsions during withdrawal were compared using the Kruskall-Wallis ANOVA on ranks. Also, the proportion of mice that showed aggression during withdrawal was analyzed using a Χ2 test with the Dunn’s method for post-hoc testing. Other behavioral measures during withdrawal from intermittent EtOH over time were analyzed with two-way RM ANOVA (drinking time in wks vs. drinking condition of EtOH or H2O) as well as during drug testing (drinking condition vs. NMDAR antagonist dose). Extracellular glutamate samples in the mPFC were compared over time with two-way RM ANOVA, as well (drinking condition vs. time). Holm-Sidak post-hoc tests were performed when applicable. α = 0.05.
RESULTS
Intermittent EtOH drinking and withdrawal
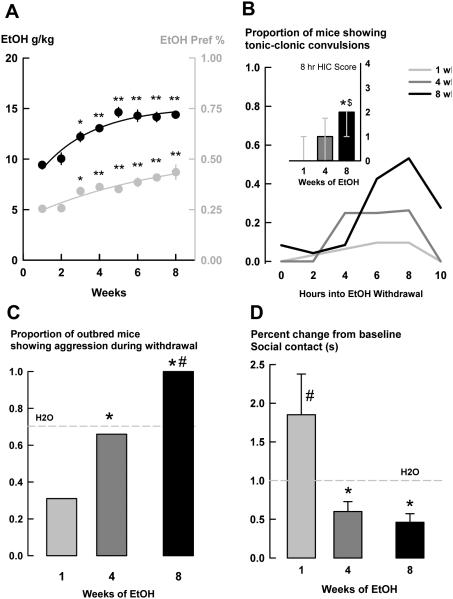
Over time, mice increased their daily EtOH intake to 14.39 ± 0.35 g/kg and their EtOH preference to 0.43 ± 0.04 [g/kg: F(7,630) = 13.65, p<0.001; preference: F(7,630) = 15.27, p<0.001; Fig. 1a]. Holm-Sidak post-hoc tests revealed an increase in EtOH consumption (g/kg) and a larger EtOH preference after wk 3 compared to wk 1 [g/kg and preference: wk 3: p<0.05, wks 4-8: p<0.001]. BECs taken after 4 wks of intermittent access to EtOH, 1 h after bottle presentation, suggest that most CFW mice drank to mild intoxication during the initial h of access [53.48 ± 13.46 mg/dl], but there was a wide range of BECs [11.10 – 284.3 mg/dl].
Fig. 1.
a) Mean ± SEM daily EtOH intake (g/kg) and EtOH preference (%) of male outbred mice (n=90) when given intermittent access to 20% EtOH (w/v) and water over time. Black dots and line are the three-day intake averages on the left axis. Grey dots and line are the three-day preference averages on the right axis. b) Proportion of mice showing tonic-clonic convulsions when assessed for handling-induced convulsions (HIC) during initial hours of acute EtOH withdrawal after 1 (grey line), 4 (dark grey line), or 8 (black line) wks of intermittent access to EtOH. Inset graph shows median ± interquartile range HIC scores during 8 h withdrawal. c) Proportion of mice showing aggression during withdrawal from intermittent EtOH after 1, 4, or 8 wks of exposure. Grey striped line represents the average of H2O drinkers across age-matched groups. d) Mean ± SEM percent change from H2O baseline social contact during the resident-intruder confrontation in EtOH withdrawal. Grey striped line is the average of H2O. *p<0.05 vs. wk 1. **p<0.001 vs. wk 1. #p<0.05 vs. H2O. $p<0.05 vs. wk 4.
Several behavioral indicators of EtOH withdrawal were assessed in different groups of mice drinking for 1, 4, or 8 wks. First, mice showed group differences in HIC scores from 0-10 h into acute withdrawal [0 h H(2) = 15.53, p<0.001; 4 h H(2) = 11.22, p<0.05; 6 h H(2) = 13.59, p = 0.001; 8 h H(2) = 28.62, p<0.001; 10 h H(2) = 22.22, p<0.001]. The post-hoc Dunn’s method revealed the 1 wk EtOH group differed from the 8 wk EtOH group at the 0, 4, 6, 8, and 10 h time points [p<0.05], and the 4 wk EtOH group differed from the 8 wk EtOH group at 8 and 10 h [p<0.05]. The proportion of mice showing tonic-clonic convulsions, meaning a score of 2 or higher on the Goldstein (1972) scale, peaked at 8 h into withdrawal, with over half of the mice showing serious convulsions [Fig. 1b].
Because the 6-8 h time period showed peak HIC scores, we assessed aggressive behavior against a male conspecific in the home-cage during 6-8 h withdrawal. Compared to time-matched H2O drinkers, the proportion of mice showing withdrawal aggression increased over time of intermittent access to EtOH [Χ2(2) = 24.69, p<0.001; Fig. 1c]. Specifically, lower proportions of 1 wk EtOH drinkers showing withdrawal aggression compared to both 4 wk [p<0.05] and 8 wk [p<0.001] EtOH drinkers, and higher proportions of 8 wk EtOH drinkers showed aggression compared to 4 wk drinkers [p<0.05]. Compared to age-matched H2O drinkers, only the 8 wk EtOH group showed different levels of aggression than the 8 wk H2O group [p<0.05]. We also measured frequencies of attack bites and sideways threats over time. A two-way RM ANOVA showed that mice decreased the frequency of attack bites across time, regardless of EtOH- or H2O-drinking history [F(2,74) = 5.17, p<0.05; not shown], but there was no interaction between drinking history and time. Holm-Sidak post-hoc tests confirmed 8 wk mice were different from 1 wk mice [p<0.05]. Sideways threats during withdrawal were not different among drinking groups over time [not shown].
Another behavioral measure of withdrawal was an interaction of decreased social contact (s) over time [F(2,72) = 4.63, p<0.05; Table 1] during confrontation with a conspecific. One wk EtOH mice showed increased contact with the intruder compared to 1 wk H2O drinkers [p<0.05], and 8 wk EtOH mice showed suppressed contact with the intruder compared to 8 wk H2O drinkers [p<0.05]. Also, 4 wk and 8 wk EtOH drinkers showed suppressed contact compared to 1 wk EtOH drinkers [both p<0.05]. Since there were no statistical differences in contact duration (s) among H2O drinking groups across time, we transformed the contact data to percent change from the H2O drinking baseline contact for another depiction. There was also a significant interaction between time and drinking history for percent change from baseline social contact [F(2,73) = 3.72, p<0.05; Fig. 1d]. Both the 4 wk and 8 wk EtOH groups had decreased contact compared to the 1 wk EtOH group [both p<0.001], and 1 wk EtOH was different from 1 wk H2O [p<0.05].
Table 1.
Non-aggressive behaviors during ethanol withdrawal after memantine
| Behavior | Memantine (mg/kg) |
1 week | 4 weeks | 8 weeks | |||
|---|---|---|---|---|---|---|---|
| H2O | EtOH | H2O | EtOH | H2O | EtOH | ||
| Contact | dH2O 3 5 10 30 |
40.35 ± 11.08 45.10 ± 16.18 33.51 ± 14.40 56.74 ± 29.03 |
74.70 ±21.17 65.16 ± 19.03 58.99 ± 18.98 41.51 ± 19.74 |
24.42 ± 8.22 9.78 ± 4.90* 7.64 ± 254* 7.25 ± 229* |
14.67 ± 3.12 9.82 ± 215 22.59 ± 3.97# 12.83 ± 3.01 |
67.04 ± 13.32 75.24 ± 24.26 57.77 ± 17.24 52.76 ± 22.63 61.50 ± 33.70 |
32.51 ± 7.61 29.24 ± 8.02 32.09 ± 11.53 39.08 ± 9.98 65.80 ± 15.11 |
| Autogroom | dH2O 3 5 10 30 |
260 ± 1.65 8.06 ± 3.32 4.50 ± 1.51 0.41 ± 0.21 |
6.29 ± 3.18 7.64 ± 3.41 8.54 ± 3.79 6.86 ± 3.74 |
3.42 ± 0.80 5.92 ± 1.62 7.36 ± 4.96 3.98 ± 1.59 |
5.04 ± 0.83 3.89 ± 1.23 4.27 ± 1.09 3.59 ± 0.99 |
2.02 ± 0.84 4.21 ± 2.62 5.45 ± 2.99 8.59 ± 5.79 7.10 ± 4.79 |
7.60 ± 2.03 4.49 ± 1.11 4.52 ± 1.21 4.98 ± 1.58 1.49 ± 0.66*# |
| Rearing | dH2O 3 5 10 30 |
16.67 ± 5.06 24.20 ± 5.57 37.47 ± 9.41* 40.81 ± 966* |
12.65 ±3.67 20.50 ± 5.48 10.52 ± 3.98# 12.20 ± 263# |
15.23 ± 6.13 14.61 ± 3.69 12.94 ± 3.94 16.23 ± 4.24 |
22.47 ± 3.83 14.55 ± 2.20 18.85 ± 3.03 776 ± 1.91 |
16.35 ± 5.82 11.71 ± 3.97 13.98 ± 3.95 12.00 ± 5.88 11.58 ±7.03 |
33.12 ± 5.92 38.35 ± 5.98 30.02 ± 5.08 30.72 ± 6.05 22.39 ± 4.59 |
| Walking | dH2O 3 5 10 30 |
140.11 ± 12.66 10.412 ± 12.90 13.239 ± 15.96 99.14 ± 17.17 |
112.97 ±16.44 10.273 ± 12.19 103.36 ± 17.60 125.99 ± 17.77 |
86.68 ± 5.57 92.26 ± 7.97 76.88 ± 11.39 92.34 ± 10.16 |
96.52 ± 5.90 93.76 ± 7.51 98.78 ± 5.62 89.45 ± 6.85 |
116.14 ± 17.33 135.78 ± 22.99 120.17 ± 17.04 131.56 ± 15.54 79.94 ± 17.65 |
159.61 ± 8.75# 177.31 ± 7.12 184.87 ± 11.54# 160.81 ± 10.51 141.76 ± 13.54# |
Other behavioral measures like baseline autogrooming duration (s) during withdrawal differed in mice with distinctive drinking histories [F(1,74) = 4.81, p<0.05; Table 1], but there was no significant interaction. Mice with an EtOH-drinking history displayed more autogrooming behavior compared to H2O drinkers, irrespective of time [p<0.05]. Walking duration (s) was different in animals across time [F(2,74) = 10.65, p<0.001] and there was an interaction between time and drinking history [F(2,74) = 4.44, p<0.05; Table 1]. 8 wk EtOH mice walked more than 8 wk H2O mice [p<0.05] and 1 wk EtOH mice [p<0.05]. Walking in the 4 wk H2O group was different from the 1 wk H2O group [p<0.05]. Rearing was not different among groups across time [Table 1].
Withdrawal aggression and NMDAR antagonists
In 1 wk EtOH and H2O drinkers, doses of memantine did not significantly alter aggressive or non-aggressive behaviors. However, a two-way RM ANOVA showed that there was an interaction in rearing duration (s) in the 1 wk mice [F(3,46) = 3.03, p<0.05; Table 1]. Within H2O mice, rearing after 5 and 10 mg/kg memantine were different from rearing after the dH2O vehicle [both p<0.05]. There were also group differences in rearing behavior between H2O mice and EtOH mice at the 5 and 10 mg/kg memantine doses [both p<0.05].
In animals that were drinking EtOH and H2O for 4 wks, memantine administration significantly affected aggressive behavior, specifically frequency of attack bites. There was an effect of memantine [F(3,96) = 3.96, p<0.05; not shown], but there was no significant interaction. A moderate dose of memantine (10 mg/kg) decreased attack bites compared to dH2O vehicle in the 4 wk EtOH mice [p<0.05]. Social contact (s) with the intruder during withdrawal was also affected by memantine after 4 wks of drinking [F(3,96) = 3.53, p<0.05; Table 1]. All doses of memantine decreased contact in the H2O drinking group [all p<0.05], and the effect of 5 mg/kg memantine was different between the H2O and EtOH drinkers. No other aggressive or non-aggressive behaviors were affected by memantine at this time point [Table 1].
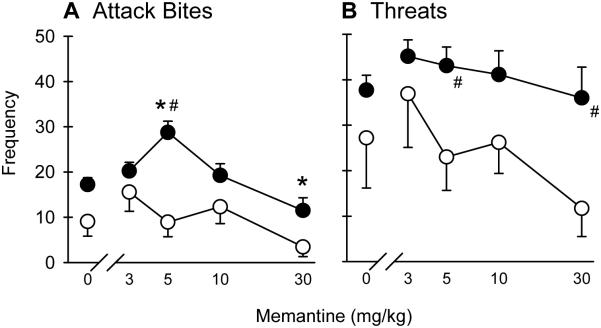
After 8 wks EtOH drinking, aggressive and non-aggressive behaviors were altered by memantine during withdrawal aggression. A two-way RM ANOVA revealed a significant interaction for attack bites during withdrawal [F(4,103) = 3.96, p<0.05; Fig 2a]. There were both main effects of drinking condition [F(1,103) = 7.29, p<0.05] and memantine dose [F(4,103) = 9.18, p<0.001]. In the EtOH drinkers only, treatment with the 5 mg/kg dose of memantine increased attack bites [p<0.001] and 30 mg/kg memantine decreased attack bites compared to dH2O vehicle [p<0.05]. Bites during withdrawal from 8 wk EtOH after 5 mg/kg memantine were also higher than the H2O drinkers [p<0.001]. There were also main effects of drinking history [F(1,103) = 5.19, p<0.05; Fig. 2b] and memantine dose [F(4,103) = 2.56, p<0.05], but no interaction. Overall, 8 wk EtOH mice displayed more threats than the H2O mice [p<0.05], specifically at the 5 and 30 mg/kg memantine doses [both p<0.05]. In addition to the aggressive behaviors, there were group differences in walking duration (s) [F(1,103) = 8.88, p<0.05; Table 1]. Eight wk EtOH mice engaged in more walking behavior than H2O mice [p<0.05], specifically after vehicle, 5, and 30 mg/kg memantine [all p<0.05]. Lastly, there was a significant interaction in autogrooming duration (s) after 8 wks [F(4,102) = 2.70, p<0.05; Table 1]. Administration of the highest dose of memantine (30 mg/kg) decreased autogrooming in the EtOH group compared to vehicle [p<0.05] and compared to the H2O group [p<0.05]. Other behaviors were not affected by memantine, shown in Table 1.
Fig. 2.
a) Attack bites and b) sideways threats during 6-8 h withdrawal from 8 wks intermittent EtOH after NMDAR antagonist memantine (0-30 mg/kg, i.p.). Black dots represent mean ± SEM frequency of bites/threats of EtOH drinkers and white dots represent bites/threats of H2O drinkers. *p<0.05 vs. vehicle. #p<0.05 vs. H2O
After 8 wks EtOH, a separate group of mice (n=17) was given ketamine, another NMDAR antagonist, before confrontations during withdrawal. Unlike memantine, doses of ketamine (3-10 mg/kg) did not significantly alter aggressive or non-aggressive behavior 6-8 h into withdrawal from intermittent EtOH.
mPFC glutamate during ethanol withdrawal
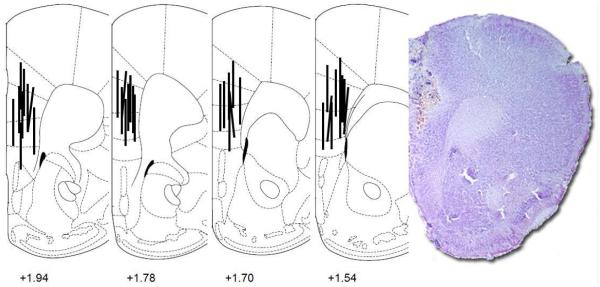
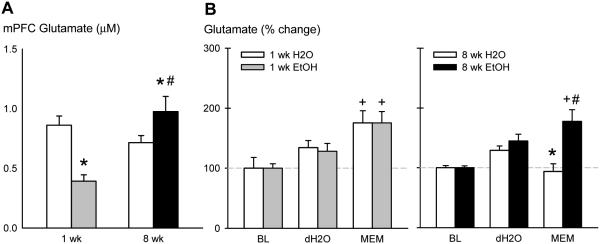
Mice were implanted with microdialysis probes into the mPFC [Fig. 3] to measure glutamate during ethanol withdrawal and changes in glutamate after memantine administration. There was a significant interaction for baseline glutamate concentrations (µM) in the mPFC [F(1,145) = 12.96, p<0.001; Fig. 4a]. Glutamate was higher in 8 wk EtOH mice than 1 wk EtOH mice [p<0.001]. Both EtOH groups differed from their time-matched H2O groups [both p<0.05]. Baseline levels of other amino acids during withdrawal from EtOH are shown in Table 2. There was also an interaction for glutamine during withdrawal [F(1,144) = 9.80, p<0.05]. Glutamine was lower in 1 wk EtOH drinkers compared to 1 wk H2O drinkers [p<0.05] but higher in the 8 wk EtOH drinkers compared to 8 wk H2O drinkers [p<0.05]. H2O mice also differed across time [p<0.001]. There was an EtOH effect for baseline mPFC alanine concentrations [F(1,146) = 7.61, p<0.05] where EtOH-experienced mice had lower alanine levels than H2O drinkers. Similar to glutamate and glutamine, there was an interaction for baseline taurine during withdrawal [F(1,146) = 61.01, p<0.001]. Taurine was lower in 1 wk EtOH mice compared to 1 wk H2O mice [p<0.001], but higher in 8 wk EtOH mice versus 8 wk H2O mice [p<0.001]. Both 8 wk groups differed from the 1 wk groups [H2O: p<0.001, EtOH: p<0.05]. There were no significant differences in mPFC glycine or GABA among treatment groups during withdrawal.
Fig. 3.
Placements of microdialysis probes in the medial prefrontal cortex (mPFC) in coronal brain sections and a representative photomicrograph of the placement. Numbers are mm from bregma
Fig. 4.
a) Baseline glutamate concentrations during 6-8 h EtOH withdrawal in the mPFC (µM) as measured by microdialysis in CFW mice after 1 or 8 wks of intermittent EtOH. The grey bar represents mean ± SEM glutamate of 1 wk EtOH drinkers and the black bar represents glutamate of 8 wk EtOH drinkers. *p<0.05 vs. H2O. #p<0.05 vs. 1 wk. +p<0.05 vs. baseline. b) Glutamate percent change after dH2O or 5 mg/kg memantine i.p. injection in mice that consumed H2O (white bars) or EtOH (grey bars) for 1 wk. c) Glutamate percent change after dH2O or 5 mg/kg memantine i.p. injection in mice that consumed H2O (white bars) or EtOH (black bars) for 8 wks
Table 2.
Extracellular mPFC amino acids during withdrawal from intermittent ethanol
| 1 week | 8 weeks | |||
|---|---|---|---|---|
| H2O | EtOH | H2O | EtOH | |
|
| ||||
| Glutamine | 10.88 ± 0.85 | 6.99 ± 0.40* | 5.59 ± 1.07# | 8.92 ± 1.00* |
| Glycine | 2.28 ± 0.24 | 2.17 ± 0.26 | 2.61 ± 0.37 | 2.64 ± 0.32 |
| Alanine | 1.85 ± 0.14 | 0.96 ± 0.14 | 2.36 ± 0.44 | 1.15 ± 0.09 |
| Taurine | 1.97 ± 0.12 | 0.88 ± 0.05* | 0.75 ± 0.09# | 1.38 ± 0.10*# |
| GABA^ | 0.32 ± 0.10 | 0.32 ± 0.07 | 0.27 ± 0.05 | 0.07 ± 0.00 |
After comparing baseline glutamate concentrations between groups, we challenged the mice with the 5 mg/kg aggression-heightening dose of memantine. There was an interaction in percent change glutamate after dH2O and memantine administration [F(15,112) = 3.46, p<0.001; Fig. 4b]. Memantine administration increased glutamate percent change in 1 wk H2O and EtOH drinkers in a similar manner compared to baseline [both p<0.001]. However, only 8 wk EtOH drinkers showed increased glutamate after memantine compared to baseline [p<0.001], which also differed from the H2O drinkers [p<0.001]. The 1 wk and 8 wk H2O groups differed after memantine injection [p<0.001].
Separate mice with histories of 8 wks of EtOH (n=7) or H2O (n=6) were given 2 g/kg EOH gavage injections to measure changes in glutamate compared to memantine administration. There was an effect of EtOH gavage on both drinking groups [F(4,43) = 5.74, p<0.001; not shown] and no interaction. Glutamate increased 164 ± 18% in EtOH mice and 174 ± 19% in H2O mice after 2 g/kg EtOH.
DISCUSSION
Male outbred mice given intermittent access to EtOH escalated their drinking with evidence for dependence. After 4 and especially 8 wks of EtOH, half of the mice reacted to handling with tonic-clonic convulsions upon 6-8 h withdrawal from EtOH. These physical signs of withdrawal were accompanied by reduced social interaction and higher levels of aggression with a conspecific male during the withdrawal period. The NMDAR antagonist memantine, but not ketamine, exacerbated withdrawal aggression at the 5 mg/kg dose after 8 wk EtOH consumption correlating with increased extracellular mPFC glutamate during the same time period. The key findings of convulsions, impaired social behavior, and increased glutamate during withdrawal emerging after 8 wks of intermittent EtOH indicate elements of dependence.
The current studies highlight intermittent access to EtOH as a protocol that generates excessive intake without the need for sweeteners, EtOH fading, H2O deprivation, or forced exposure. Previously, this escalation was reported in outbred rats given intermittent access (Simms et al. 2008; Wayner et al. 1972; Wise 1973) and then in inbred C57BL/6J mice (Hwa et al. 2011; Melendez et al. 2011). Here, we present the first findings using outbred, Swiss-derived mice that increase EtOH intake to about 15 g/kg/day and approximating 50% ethanol preference. Some individuals show binge-like intake, as confirmed by high BECs during the initial hours of access. Alternating between periods of high EtOH intake and abstinence may produce aspects of EtOH withdrawal syndrome, characterized in rodents by hyperexcitability, rigidity of the tail and body, and increased seizure activity (Adinoff et al. 1988; Little et al. 1986). As mice continued to drink according to this phasic schedule, higher proportions of tonic-clonic convulsions were elicited. During resident-intruder tests, EtOH-withdrawn mice also showed more walking behavior compared to H2O controls, an indicator of hyperexcitability. A similar pattern was observed for social contact during withdrawal from an EtOH liquid diet (File et al. 1989; Overstreet et al. 2002) – mice that drank EtOH for 8 wks showed reduced levels of social contact compared to H2O drinkers. Similarly, administration of NMDAR antagonists may enhance social inhibition (Morales and Spear 2014). Deficits in social interaction have been interpreted to result from anxiety-like states (File and Seth 2003).
Our set of experiments additionally described an understudied symptom of EtOH withdrawal: aggression. For example, a Sri Lankan hospital documented alcohol withdrawal as the most common cause (45.7%) of violence in the monitored year (Abeyasinghe and Jayasekera 2003). In addition to violence, high emotional reactivity during sustained abstinence may be a large contributing factor to relapse, in heavy binge-drinking adolescents (Winward et al. 2014). Relapse was most highly correlated with aggression, more so than with impulsivity, anxiety, and depression in abstinent male alcoholics (Baars et al. 2013). We report that high proportions of mice show aggression during withdrawal from 8 wks EtOH. However, frequency of attack bites tended to decrease over time in both H2O and EtOH groups. This may be attributed to an inhibition of aggression that occurs with age into later adulthood, documented both in some mice and humans (Clement et al. 1987; Gray et al. 1991; Leober and Hay 1997). A more detailed analysis of the social confrontations promises to reveal whether aggression during withdrawal is reactive and defensive or proactive and offensive. As is the case with opiate and benzodiazepine withdrawal-induced aggression, we speculate that EtOH withdrawal-induced aggression is also reactive in nature (Gianutsos and Lal 1978, Kantak and Miczek 1986; Krŝiak et al. 1998).
The current study also found that increased extracellular glutamate concentrations in the mPFC during withdrawal characterized long-term alcohol use. Past research has shown increased glutamate during the first 12 h of withdrawal from forced alcohol exposure in the striatum (Dahchour et al. 1998; Griffin III et al. 2014; Melendez et al. 2005a; Rossetti and Carboni 1995) and hippocampus (Chefer et al. 2011; Dahchour and De Witte 2003). Molecular work also demonstrates an upregulation of NMDAR following chronic EtOH and withdrawal (Clapp et al. 2010; Kalluri et al. 1998; Rani and Ticku 2006). Our results extend these findings by measuring intra-mPFC glutamate in mice withdrawn from two-bottle choice drinking (Ding et al. 2013). Prefrontal cortical pyramidal neurons are rich in glutamate projections that release excitatory amino acids in the nucleus accumbens and ventral tegmental area (Sesack and Pickel 1992; Taber and Fibiger 1995). Extracellular glutamate in the mPFC has been shown to be regulated by the cysteine glutamate exchanger and group I metabotropic glutamate receptors (Melendez et al. 2005b) and derived from vesicular synaptic and non-vesicular glial release (Danbolt 2001; Haydon 2001). Future studies need to examine the source of the glutamate and glutamatergic afferents to the mPFC during EtOH withdrawal. We also found increased glutamine concentrations in the mPFC, so astrocytes may be a candidate for the source of glutamate. In support of this hypothesis, others have found altered glial plasticity in the mPFC (Kim et al. 2014) and decreased glutamate clearance caused by the excitatory amino acid transporter, which normalizes after 2 wks of abstinence (Ding et al. 2013; Kalinine et al. 2014). An unexpected finding was that glutamate activity was different after memantine injection in the 1 wk versus 8 wk H2O mice. It is possible that social isolation for 8 wks could have altered glutamate activity. Past research has found increased mGluR6 and AMPA3 glutamate receptor subunits in the mPFC of socially isolated rats (Levine et al. 2007). However, Melendez et al. (2004) found decreased mGluR1 and mGluR5 protein in the dorsal mPFC and reduced PFC glutamate after mGluR1 agonist or mGluR2 antagonism in social isolates. Isolation through single-housing is a facet of the intermittent EtOH procedure.
Our results confirm that the mPFC plays a large role in the transition to EtOH dependence (George et al. 2013; Kroener et al. 2012; Holmes et al. 2012), withdrawal from drugs of abuse (Williams and Steketee 2004) and EtOH-related aggression (Quadros et al. 2014). The mPFC has long been associated with the inhibitory control of emotional outbursts, including aggression and violence (Nelson and Trainor 2007; Siegel et al. 1974). Aggressive behavior has been linked to PFC hypo-activation, especially in the dorsal anterior cingulate in humans (Sterzer et al. 2005; Meyer-Lindenberg and Weinberger 2006), and alcohol further impairs inhibitory control through diminished PFC activity (Anderson et al. 2011). Recent studies using optogenetics confirm that the silencing of excitatory neurons in the mPFC causes increased aggressive behavior and its activation suppresses aggression (Takahashi et al. 2014). The current experiments showing changes in mPFC glutamate and social behavior are consistent with this body of literature. One limitation of our microdialysis study was that the probes spanned both the infralimbic and prelimbic subregions of the mPFC. It is thought that the infralimbic region is associated with visceromotor activity, whereas the prelimbic region is connected more with cognitive functions (Vertes 2004; Hoover and Vertes 2007). However, complex goal-directed behaviors entail an integration of visceral and cognitive elements, as in the case of negative affect during withdrawal from long-term drug abuse.
One distinct feature of the experimental design is that we used intermittent access to EtOH to induce high levels of voluntary drinking leading to dependence and withdrawal. It is thought that the progressive intensification of EtOH withdrawal symptoms following cycles of intoxication and withdrawal represents a kindling mechanism (Becker and Hale 1993). These long-term changes in neuronal hyperexcitability might be related to the progression of alcohol withdrawal symptoms from tremor to seizures, as well as the alcoholic personality changes between episodes of withdrawal (Ballenger and Post 1978). EtOH vapor exposure can elicit robust HICs for the first 10 hr during acute withdrawal in male C3H mice (Becker and Hale 1993; Becker, Diaz-Granados, and Weathersby 1997). The intensity and severity of withdrawal HIC scores in mice that have been withdrawn multiple times are greater than in mice that were tested following a single episode of withdrawal from the same cumulative vapor exposure. Though we cannot account for the effects of cumulative EtOH exposure in a continuous drinking protocol, we speculate that the multiple withdrawals in the intermittent access protocol would yield similar results as the intermittent EtOH vapor. Our laboratory and others have studied withdrawal seizures during withdrawal from EtOH liquid diet (Freund and Walker 1971), EtOH vapor (Becker and Lopez 2004; Crabbe et al. 1983) and intermittent access to 20% EtOH (Hwa et al. 2011) in C57BL/6J mice. Despite high BECs with the repeated drinking-in-the-dark protocol, some mice still do not show HIC during acute withdrawal, so the emergence of HIC after intermittent access in the CFW strain is a novel observation. With intermittent access to alcohol, there are repeated episodes of EtOH intoxication and withdrawal. While previous research has measured glutamate at a single time point during EtOH withdrawal, the present studies assessed levels of prefrontal cortical amino acids at different time points along the progression to dependence.
Interestingly, we found that NMDAR antagonism via memantine treatment can precipitate withdrawal aggression. Memantine biphasically increased aggression in mice undergoing withdrawal from 8 wks of intermittent EtOH at the 5 mg/kg dose. This effect differed from ketamine, which did not change aggressive behavior in withdrawn mice at any dose. Memantine may differ from ketamine affecting aggressive behavior perhaps caused by differential binding kinetics of the two compounds, like the partial trapping of memantine to a superficial site on NMDA receptors (Kotermanski, Wood and Johnson 2009). Our laboratory has also found that moderate doses of memantine, neramexane, and MTEP interacted with acutely administered EtOH to increase aggression while ketamine did not (Newman et al. 2012). With increasing doses of memantine, other pharmacological targets are recruited , including serotonin and dopamine uptake, serotonin receptors, nicotinic acetylcholine receptors, sigma-1 receptors, and voltage-activated sodium channels (Brau et al. 2001; Danysz et al. 1997). For example, memantine’s action as a 5-HT3 receptor antagonist is greater than that of ketamine (Rammes et al. 2001). Memantine may escalate aggression by reducing serotonin while ketamine does not; however, memantine may block serotonin and dopamine reuptake as well as MAOA and MAOB activity (Onogi et al. 2009). A future direction could measure PFC serotonin following memantine treatment during EtOH withdrawal (Shearman et al. 2006). Altogether, it is the binding characteristics of these compounds, including the ‘partial trapping,’ and the non-specificity of higher doses that may contribute to the aggression-heightening effects. Furthermore, there is a known significant positive correlation between CSF glutamate concentrations and impulsive aggression in human subjects (Coccaro et al. 2013). Repeated episodes of withdrawal characterized by increased glutamate may increase the likelihood of aggression during periods of abstinence.
In sum, our results highlight a novel asocial behavioral phenomenon in mice, increased aggression towards an intruder, during EtOH withdrawal. These studies further show how intermittent access drinking protocols can generate several dependence-like symptoms in outbred animals. In addition to examining the deficits in social behavior from long-term drinking, it was unexpected to observe pro-social behavior during the initial stages of drinking. Mice were screened for aggression before access to EtOH, so the decreased aggression and increased social contact may result from heightened social exploration linked to the start of EtOH consumption. Perhaps there were carry over effects from low-dose EtOH to facilitate social behavior and novelty-seeking which may predict heavy drinking (Kampov-Polevoy et al. 2004) in the form of a novel stimulus animal. Another limitation was that we did not measure glutamate concentrations in the same individuals that underwent social confrontations. This was a correlative study, in which sets of mice had either short-term or long-term access to EtOH or H2O with similar memantine manipulations. But, we cannot ignore the fact that a history of aggressive behavior could change tonic glutamate levels in the mPFC. Nevertheless, we demonstrate a role for memantine in escalating aggression in the absence of long-term EtOH intake, which is related to our previous findings that memantine interacts with acutely self-administered 1 g/kg EtOH to increase aggression (Newman et al. 2012). Intermittency may be a defining principle for the kindling of NMDAR activity during withdrawal within periods of heavy intoxication in EtOH-use disorders.
ACKNOWLEDGEMENTS
This research was supported by NIH grants R01 AA013983 (KAM) and F31 AA021622 (LSH). The authors would like to acknowledge Justin Choi and Alexandra Kiesling for their behavioral research as well as Dr. Lucas Albrechet-Souza for assistance with histology.
This research was supported by NIH funding R01AA013983 (Miczek) and F31AA021622 (Hwa).
Footnotes
The authors declare no conflicts of interest.
REFERENCES
- Abeyasinghe R, Jayasekera R. Violence in a general hospital psychiatry unit for men. Ceylon Med J. 2003;48:45–47. doi: 10.4038/cmj.v48i2.3369. [DOI] [PubMed] [Google Scholar]
- Adinoff B, Bone GH, Linnoila M. Acute ethanol poisoning and the ethanol withdrawal syndrome. Med Toxicol Adverse Drug Exp. 1988;3:172–196. doi: 10.1007/BF03259881. [DOI] [PubMed] [Google Scholar]
- Anderson BM, Stevens MC, Meda SA, Jordan K, Calhoun VD, Pearlson GD. Functional imaging of cognitive control during acute alcohol intoxication. Alcohol Clin Exp Res. 2011;35:156–165. doi: 10.1111/j.1530-0277.2010.01332.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baars MY, Muller MJ, Gallhofer B, Netter P. Relapse (number of detoxifications) in abstinent male alcohol-dependent patients as related to personality traits and types of tolerance to frustration. Neuropsychobiology. 2013;67:241–248. doi: 10.1159/000350483. [DOI] [PubMed] [Google Scholar]
- Ballenger JC, Post RM. Kindling as a model for alcohol withdrawal syndromes. Br J Psychiatry. 1978;133:1–14. doi: 10.1192/bjp.133.1.1. [DOI] [PubMed] [Google Scholar]
- Becker HC, Diaz-Granados JL, Weathersby RT. Repeated ethanol withdrawal experience increases the severity and duration of subsequent withdrawal seizures in mice. Alcohol. 1997;14:319–326. doi: 10.1016/s0741-8329(97)87949-9. [DOI] [PubMed] [Google Scholar]
- Becker HC, Hale RL. Repeated episodes of ethanol withdrawal potentiate the severity of subsequent withdrawal seizures: an animal model of alcohol withdrawal "kindling". Alcohol Clin Exp Res. 1993;17:94–98. doi: 10.1111/j.1530-0277.1993.tb00731.x. [DOI] [PubMed] [Google Scholar]
- Becker HC, Lopez MF. Increased ethanol drinking after repeated chronic ethanol exposure and withdrawal experience in C57BL/6J mice. Alcohol Clin Exp Res. 2004;28:1829–1838. doi: 10.1097/01.alc.0000149977.95306.3a. [DOI] [PubMed] [Google Scholar]
- Bisaga A, Evans SM. Acute effects of memantine in combination with alcohol in moderate drinkers. Psychopharmacology (Berl) 2004;172:16–24. doi: 10.1007/s00213-003-1617-5. [DOI] [PubMed] [Google Scholar]
- Bjork JM, Hommer DW, Grant SJ, Danube C. Impulsivity in abstinent alcohol-dependent patients: relation to control subjects and type 1-/type 2-like traits. Alcohol. 2004;34:133–150. doi: 10.1016/j.alcohol.2004.06.012. [DOI] [PubMed] [Google Scholar]
- Blanpied TA, Boeckman FA, Aizenman E, Johnson JW. Trapping channel block of NMDA-activated responses by amantadine and memantine. J Neurophysiol. 1997;77:309–323. doi: 10.1152/jn.1997.77.1.309. [DOI] [PubMed] [Google Scholar]
- Brau ME, Dreimann M, Olschewski A, Vogel W, Hempelmann G. Effect of drugs used for neuropathic pain management on tetrodotoxin-resistant Na(+) currents in rat sensory neurons. Anesthesiology. 2001;94:137–144. doi: 10.1097/00000542-200101000-00024. [DOI] [PubMed] [Google Scholar]
- Broadwater M, Varlinskaya EI, Spear LP. Chronic intermittent ethanol exposure in early adolescent and adult male rats: effects on tolerance, social behavior, and ethanol intake. Alcohol Clin Exp Res. 2011;35:1392–1403. doi: 10.1111/j.1530-0277.2011.01474.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardoso JM, Barbosa A, Ismail F, Pombo S. NETER alcoholic typology (NAT) Alcohol Alcohol. 2006;41:133–139. doi: 10.1093/alcalc/agh247. [DOI] [PubMed] [Google Scholar]
- Chefer V, Meis J, Wang G, Kuzmin A, Bakalkin G, Shippenberg T. Repeated exposure to moderate doses of ethanol augments hippocampal glutamate neurotransmission by increasing release. Addict Biol. 2011;16:229–237. doi: 10.1111/j.1369-1600.2010.00272.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Citrome L, Volavka J. Violent patients in the emergency setting. Psychiatric Clinics of North America. 1999;22:789–801. doi: 10.1016/s0193-953x(05)70126-x. [DOI] [PubMed] [Google Scholar]
- Clapp P, Gipson ES, Dell’Acqua ML, Hoffman PL. Phosphorylation regulates removal of synaptic N-methyl-D-aspartate receptors after withdrawal from chronic ethanol exposure. J Pharm Exp Ther. 2010;332:720–729. doi: 10.1124/jpet.109.158741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clement J, Simler S, Ciesielski L, Mandel P, Cabib S, Puglisi-Allegra S. Age-dependent changes of brain GABA levels, turnover rates and shock-induced aggressive behavior in inbred strains of mice. Pharmacology, Biochemistry and Behavior. 1987;26:83–88. doi: 10.1016/0091-3057(87)90538-7. [DOI] [PubMed] [Google Scholar]
- Coccaro EF, Lee R, Vezina P. Cerebrospinal fluid glutamate concentration correlates with impulsive aggression in human subjects. J Psychiatr Res. 2013;47:1247–1253. doi: 10.1016/j.jpsychires.2013.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crabbe JC, Kosobud A, Young ER. Genetic selection for ethanol withdrawal severity: differences in replicate mouse lines. Life Sci. 1983;33:955–962. doi: 10.1016/0024-3205(83)90751-8. [DOI] [PubMed] [Google Scholar]
- Dahchour A, De WP. Effect of repeated ethanol withdrawal on glutamate microdialysate in the hippocampus. Alcohol Clin Exp Res. 1999;23:1698–1703. doi: 10.1111/j.1530-0277.1999.tb04063.x. [DOI] [PubMed] [Google Scholar]
- Dahchour A, De WP. Excitatory and inhibitory amino acid changes during repeated episodes of ethanol withdrawal: an in vivo microdialysis study. Eur J Pharmacol. 2003;459:171–178. doi: 10.1016/s0014-2999(02)02851-0. [DOI] [PubMed] [Google Scholar]
- Dahchour A, De WP, Bolo N, Nedelec JF, Muzet M, Durbin P, Macher JP. Central effects of acamprosate: part 1. Acamprosate blocks the glutamate increase in the nucleus accumbens microdialysate in ethanol withdrawn rats. Psychiatry Res. 1998;82:107–114. doi: 10.1016/s0925-4927(98)00016-x. [DOI] [PubMed] [Google Scholar]
- Dahchour A, Quertemont E, De Witte P. Acute ethanol increases taurine but neither glutamate nor GABA in the nucleus accumbens of male rats: a microdialysis study. Alcohol Alcohol. 1994;29:485–487. [PubMed] [Google Scholar]
- Danbolt NC. Glutamate uptake. Prog Neurobiol. 2001;65:1–105. doi: 10.1016/s0301-0082(00)00067-8. [DOI] [PubMed] [Google Scholar]
- Danysz W, Parsons CG, Kornhuber J, Schmidt WJ, Quack G. Aminoadamantanes as NMDA receptor antagonists and antiparkinsonian agents--preclinical studies. Neurosci Biobehav Rev. 1997;21:455–468. doi: 10.1016/s0149-7634(96)00037-1. [DOI] [PubMed] [Google Scholar]
- Ding ZM, Rodd ZA, Engleman EA, Bailey JA, Lahiri DK, McBride WJ. Alcohol drinking and deprivation alter basal extracellular glutamate concentrations and clearance in the mesolimbic system of alcohol-preferring (P) rats. Addict Biol. 2013;18:297–306. doi: 10.1111/adb.12018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erden BF, Ozdemirci S, Yildiran G, Utkan T, Gacar N, Ulak G. Dextromethorphan attenuates ethanol withdrawal syndrome in rats. Pharmacol Biochem Behav. 1999;62:537–541. doi: 10.1016/s0091-3057(98)00175-0. [DOI] [PubMed] [Google Scholar]
- Festing MF. Evidence should trump intuition by preferring inbred strains to outbred stocks in preclinical research. ILAR Journal. 2014;55:399–404. doi: 10.1093/ilar/ilu036. [DOI] [PubMed] [Google Scholar]
- File SE, Baldwin HA, Hitchcott PK. Flumazenil but not nitrendipine reverses the increased anxiety during ethanol withdrawal in the rat. Psychopharmacology. 1989;98:262–264. doi: 10.1007/BF00444702. [DOI] [PubMed] [Google Scholar]
- File SE, Seth P. A review of 25 years of the social interaction test. Eur J Pharmacol. 2003;463:35–53. doi: 10.1016/s0014-2999(03)01273-1. [DOI] [PubMed] [Google Scholar]
- Fish EW, Faccidomo S, Miczek KA. Aggression heightened by alcohol or social instigation in mice: reduction by the 5-HT1B receptor agonist CP-94,253. Psychopharmacology. 1999;146:391–399. doi: 10.1007/pl00005484. [DOI] [PubMed] [Google Scholar]
- Freund G, Walker DW. Sound-induced seizures during ethanol withdrawal in mice. Psychopharmacologia. 1971;22:45–49. doi: 10.1007/BF00401465. [DOI] [PubMed] [Google Scholar]
- George O, Koob GF. Individual differences in prefrontal cortex function and the transition from drug use to drug dependence. Neurosci Biobehav Rev. 2010;35:232–247. doi: 10.1016/j.neubiorev.2010.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George O, Sanders C, Freiling J, Grigoryan E, Vu S, Allen CD, Crawford E, Mandyam CD, Koob GF. Recruitment of medial prefrontal cortex neurons during alcohol withdrawal predicts cognitive impairment and excessive alcohol drinking. Proc Natl Acad Sci U S A. 2012;109:18156–18161. doi: 10.1073/pnas.1116523109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gianutsos G, Lal H. Narcotic analgesics and aggression. In: Valzelli L, editor. Psychopharmacology of Aggression. Vol. 13. S.Karger; New York: 1978. pp. 114–138. Modern Problems of Pharmacopsychiatry. [DOI] [PubMed] [Google Scholar]
- Goldstein DB. Relationship of alcohol dose to intensity of withdrawal signs in mice. J Pharmacol Exp Ther. 1972;180:203–215. [PubMed] [Google Scholar]
- Goldstein DB, Pal N. Alcohol dependence produced in mice by inhalation of ethanol: grading the withdrawal reaction. Science. 1971;172:288–290. doi: 10.1126/science.172.3980.288. [DOI] [PubMed] [Google Scholar]
- Grant KA, Valverius P, Hudspith M, Tabakoff B. Ethanol withdrawal seizures and the NMDA receptor complex. Eur J Pharmacol. 1990;176:289–296. doi: 10.1016/0014-2999(90)90022-x. [DOI] [PubMed] [Google Scholar]
- Gray A, Jackson DN, McKinlay JB. The relation between dominance, anger, and hormones in normally aging men: results from the Massachusetts Male Aging Study. Psychosom Med. 1991;53:375–385. doi: 10.1097/00006842-199107000-00003. [DOI] [PubMed] [Google Scholar]
- Griffin, Haun HL, Hazelbaker CL, Ramachandra VS, Becker HC. Increased extracellular glutamate in the nucleus accumbens promotes excessive ethanol drinking in ethanol dependent mice. Neuropsychopharmacology. 2014;39:707–717. doi: 10.1038/npp.2013.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haydon PG. GLIA: listening and talking to the synapse. Nat Rev Neurosci. 2001;2:185–193. doi: 10.1038/35058528. [DOI] [PubMed] [Google Scholar]
- Holmes A, Fitzgerald PJ, MacPherson KP, DeBrouse L, Colacicco G, Flynn SM, Mesneuf S, et al. Chronic alcohol remodels prefrontal neurons and disrupts NMDAR-mediated fear extinction encoding. Nat Neurosci. 2012;15:1359–1361. doi: 10.1038/nn.3204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoover WB, Vertex RP. Anatomical analysis of afferent projections to the medial prefrontal cortex in the rat. Brain Structure Function. 2007;212:149–179. doi: 10.1007/s00429-007-0150-4. [DOI] [PubMed] [Google Scholar]
- Hwa LS, Chu A, Levinson SA, Kayyali TM, DeBold JF, Miczek KA. Persistent escalation of alcohol drinking in C57BL/6J mice with intermittent access to 20% ethanol. Alcohol Clin Exp Res. 2011;35:1938–1947. doi: 10.1111/j.1530-0277.2011.01545.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Idrus NM, McGough NN, Riley EP, Thomas JD. Administration of memantine during ethanol withdrawal in neonatal rats: effects on long-term ethanol-induced motor incoordination and cerebellar Purkinje cell loss. Alcohol Clin Exp Res. 2011;35:355–364. doi: 10.1111/j.1530-0277.2010.01351.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalinine E, Zimmer ER, Zenki KC, Kalinine I, Kazlauckas V, Haas CB, Hansel G, Zimmer AR, Souza DO, Muller AP, Portela LV. Nandrolone-induced aggressive behavior is associated with alterations in extracellular glutamate homeostasis in mice. Horm Behav. 2014;66:383–392. doi: 10.1016/j.yhbeh.2014.06.005. [DOI] [PubMed] [Google Scholar]
- Kalluri HSG, Mehta AK, Ticku MK. Up-regulation of NMDA receptor subunits in rat brain following chronic ethanol treatment. Mol Br Res. 1998 doi: 10.1016/s0169-328x(98)00112-0. [DOI] [PubMed] [Google Scholar]
- Kampov-Polevoy AB, Eick C, Boland G, Khalitov E, Crews FT. Sweet liking, novelty seeking, and gender predict alcoholic status. Alcohol Clin Exp Res. 2004;28:1291–1298. doi: 10.1097/01.alc.0000137808.69482.75. [DOI] [PubMed] [Google Scholar]
- Kampov-Polevoy AB, Matthews DB, Gause L, Morrow AL, Overstreet DS. P rats develop physical dependence on alcohol via voluntary drinking: changes in seizure thresholds, anxiety, and patterns of alcohol drinking. Alcoholism: Clinical and Experimental Research. 2000;24:278–284. [PubMed] [Google Scholar]
- Kantak KM, Miczek KA. Pharmacological separation of aggression from other symptoms of morphine withdrawal. Neuroscience Abstracts. 1982;8:592. [Google Scholar]
- Kantak KM, Miczek KA. Aggression during morphine withdrawal: Effects of method of withdrawal, fighting experience and social role. Psychopharmacology. 1986;90:451–456. doi: 10.1007/BF00174059. [DOI] [PubMed] [Google Scholar]
- Kapasova Z, Szumlinski KK. Starin differences in alcohol-induced neurochemical plasticity: a role for accumbens glutamate in alcohol intake. Alcohol Clin Exp Res. 2008;32:617–631. doi: 10.1111/j.1530-0277.2008.00620.x. [DOI] [PubMed] [Google Scholar]
- Kim A, Zamora-Martinez ER, Edwards S, Mandyam CD. Structural reorganization or pyramidal neurons in the medial prefrontal cortex of alcohol dependent rats is associated with altered glial plasticity. Brain Struct Funct. 2014:1–16. doi: 10.1007/s00429-014-0755-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotermanski SE, Wood JT, Johnson JW. Memantine binding to a superficial site on NMDA receptors contributes to partial trapping. J Physiol. 2009;587:4589–4604. doi: 10.1113/jphysiol.2009.176297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroener S, Mulholland PJ, New NN, Gass JT, Becker HC, Chandler LJ. Chronic alcohol exposure alters behavioral and synaptic plasticity of the rodent prefrontal cortex. PlOS One. 2012;7:e37541. doi: 10.1371/journal.pone.0037541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krŝiak M, Podhorna J, Miczek KA. Aggressive and social behavior after alprazolam withdrawal: experimental therapy with Ro 19-8022. Neurosci Biobehav Rev. 1998;23:155–161. doi: 10.1016/s0149-7634(98)00017-7. [DOI] [PubMed] [Google Scholar]
- Krupitsky EM, Neznanova O, Masalov D, Burakov AM, Didenko T, Romanova T, Tsoy M, Bespalov A, Slavina TY, Grinenko AA, Petrakis IL, Pittman B, Gueorguieva R, Zvartau EE, Krystal JH. Effect of memantine on cue-induced alcohol craving in recovering alcohol-dependent patients. Am J Psychiatry. 2007;164:519–523. doi: 10.1176/ajp.2007.164.3.519. [DOI] [PubMed] [Google Scholar]
- Krystal JH, Petrakis IL, Limoncelli D, Webb E, Gueorgueva R, D'Souza DC, Boutros NN, Trevisan L, Charney DS. Altered NMDA glutamate receptor antagonist response in recovering ethanol-dependent patients. Neuropsychopharmacology. 2003;28:2020–2028. doi: 10.1038/sj.npp.1300252. [DOI] [PubMed] [Google Scholar]
- Lal H, O'Brien J, Puri SK. Morphine-withdrawal aggression: Sensitization by amphetamines. Psychopharmacologia. 1971;22:217–223. doi: 10.1007/BF00401783. [DOI] [PubMed] [Google Scholar]
- Levine JB, Youngs RM, MacDonale ML, Chu M, Leeder AD, Berthiaume F, Konradi C. Isolation rearing and hyperlocomotion are associated with reduced immediate early gene expresion levels in the medial prefrontal cortex. Neurosci. 2007;145:42–55. doi: 10.1016/j.neuroscience.2006.11.063. [DOI] [PubMed] [Google Scholar]
- Little HJ, Dolin SJ, Halsey MJ. Calcium channel antagonists decrease the ethanol withdrawal syndrome. Life Sci. 1986;39:2059–2065. doi: 10.1016/0024-3205(86)90356-5. [DOI] [PubMed] [Google Scholar]
- Loeber R, Hay D. Key issues in the development of aggression and violence from childhood to early adulthood. Annu Rev Psychol. 1997;48:371–410. doi: 10.1146/annurev.psych.48.1.371. [DOI] [PubMed] [Google Scholar]
- Macdonald JF, Bartlett MC, Mody I, Pahapill P, Reynolds JN, Salter MW, Schneiderman JH, Pennefather PS. Actions of ketamine, phencyclidine and MK-801 on NMDA receptor currents in cultured mouse hippocampal neurones. J Physiol. 1991;432:483–508. doi: 10.1113/jphysiol.1991.sp018396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maler JM, Esselmann H, Wiltfang J, Kunz N, Lewczuk P, Reulbach U, Bleich S, Ruther E, Kornhuber J. Memantine inhibits ethanol-induced NMDA receptor up-regulation in rat hippocampal neurons. Brain Res. 2005;1052:156–162. doi: 10.1016/j.brainres.2005.06.017. [DOI] [PubMed] [Google Scholar]
- Melendez RI. Intermittent (Every-Other-Day) Drinking Induces Rapid Escalation of Ethanol Intake and Preference in Adolescent and Adult C57BL/6J Mice. Alcohol Clin Exp Res. 2011;35:652–658. doi: 10.1111/j.1530-0277.2010.01383.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melendez RI, Gregory ML, Bardo MT, Kalivas PW. Improverished rearing environment alters metabotropic glutamate receptor expression and function in the prefrontal cortex. Neuropyschopharmacology. 2004;29:1980–1987. doi: 10.1038/sj.npp.1300507. [DOI] [PubMed] [Google Scholar]
- Melendez RI, Hicks MP, Cagle SS, Kalivas PW. Ethanol exposure decreases glutamate uptake in the nucleus accumbens. Alcohol Clin Exp Res. 2005a;29:326–333. doi: 10.1097/01.alc.0000156086.65665.4d. [DOI] [PubMed] [Google Scholar]
- Melendez RI, Vuthiganon J, Kalivas PW. Regulation of extracellular glutamate in the prefrontal cortex: focus on the cystine glutamate exchanger and group I metabotropic glutamate receptors. J Pharmacol Exp Ther. 2005b;314:139–147. doi: 10.1124/jpet.104.081521. [DOI] [PubMed] [Google Scholar]
- Meyer-Lindenberg A, Weinberger DR. Intermediate phenotypes and genetic mechanisms of psychiatric disorders. Nat Rev Neurosci. 2006;7:818–827. doi: 10.1038/nrn1993. [DOI] [PubMed] [Google Scholar]
- Miczek KA, O'Donnell JM. Intruder-evoked aggression in isolated and nonisolated mice: Effects of psychomotor stimulants and l-dopa. Psychopharmacology. 1978;57:47–55. doi: 10.1007/BF00426957. [DOI] [PubMed] [Google Scholar]
- Morales M, Spear LP. The effects of an acute challenge with the NMDA receptor antagonists, MK-801, PEAQX, and ifenprodil, on social inhibition in adolescent and adult male rats. Psychopharmacology. 2014;231:1797–1807. doi: 10.1007/s00213-013-3278-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson RJ, Trainor BC. Neural mechanisms of aggression. Nat Rev Neurosci. 2007;8:536–546. doi: 10.1038/nrn2174. [DOI] [PubMed] [Google Scholar]
- Newman EL, Chu A, Bahamon B, Takahashi A, DeBold JF, Miczek KA. NMDA receptor antagonism: escalation of aggressive behavior in alcohol-drinking mice. Psychopharmacology. 2012;224:167–177. doi: 10.1007/s00213-012-2734-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onogi H, Ishigaki S, Nakagawasai O, Arai-Kato Y, Arai Y, Watanabe H, Miyamoto A, Tan-No K, Tadano T. Influence of memantine on brain monoaminergic neurotransmission parameters in mice: neurochemical and behavioral study. Biol Pharm Bull. 2009;32:850–855. doi: 10.1248/bpb.32.850. [DOI] [PubMed] [Google Scholar]
- Overstreet DH, Knapp DJ, Breese GR. Accentuated decrease in social interaction in rats subjected to repeated ethanol withdrawals. Alcohol Clin Exp Res. 2002;26:1259–1268. doi: 10.1097/01.ALC.0000023983.10615.D7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patch PB, Phelps GL, Cowan G. Alcohol withdrawal in a medical-surgical setting: the 'too little, too late' phenomenon. Medsurg Nurs. 1997;6:79–94. 9. [PubMed] [Google Scholar]
- Pirot S, Glowinski J, Thierry AM. Excitatory responses evoked in prefrontal cortex by mediodorsal thalamic nucleus stimulation: influence of anaesthesia. Eur J Pharmacol. 1995;285:45–54. doi: 10.1016/0014-2999(95)00377-w. [DOI] [PubMed] [Google Scholar]
- Poldrugo F, Snead OC., III Electroencephalographic and behavioral correlates in rats during repeated ethanol withdrawal syndromes. Psychopharmacology (Berl) 1984;83:140–146. doi: 10.1007/BF00429722. [DOI] [PubMed] [Google Scholar]
- Quadros IM, Hwa LS, Shimamoto A, Carlson J, DeBold JF, Miczek KA. Prevention of Alcohol-Heightened Aggression by CRF-R1 Antagonists in Mice: Critical Role for DRN-PFC Serotonin Pathway. Neuropsychopharmacology. 2014;39:2874–2883. doi: 10.1038/npp.2014.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rammes G, Rupprechet R, Ferrari U, Zieglegansberger W, Parsons CG. The N-methyl-D-aspartate receptor channel blockers memantine, MRZ 2/579 and other amino-alkl-cyclohexanes antagonize 5-HT3 receptor currents in cultrured HEC-293 and N1E-115 cell systems in a non-competitive manner. Neurosci Lett. 2001;306:81–84. doi: 10.1016/s0304-3940(01)01872-9. [DOI] [PubMed] [Google Scholar]
- Rani SCS, Ticku MK. Comparison of chronic ethanol and chonic intermittent ethanol treatments on the expression of GABAA and NMDA receptor subunits. Alcohol. 2006;38:89–97. doi: 10.1016/j.alcohol.2006.05.002. [DOI] [PubMed] [Google Scholar]
- Reznikov LR, Crillo CA, Piroli GG, Pasumarthi RK, Reagan LP, Fadel J. Acute stress-mediated increases in extracellular glutamate levels in the rat amygdala: differential effects of antidepressant treatment. Eur J Neurosci. 2007;25:3109–3114. doi: 10.1111/j.1460-9568.2007.05560.x. [DOI] [PubMed] [Google Scholar]
- Rossetti ZL, Carboni S. Ethanol withdrawal is associated with increased extracellular glutamate in the rat striatum. Eur J Pharmacol. 1995;283:177–183. doi: 10.1016/0014-2999(95)00344-k. [DOI] [PubMed] [Google Scholar]
- Rossetti ZL, Carboni S, Fadda F. Glutamate-induced increase of extracellular glutamate through N-methyl-D-aspartate receptors in ethanol withdrawal. Neuroscience. 1999;93:1135–1140. doi: 10.1016/s0306-4522(99)00250-x. [DOI] [PubMed] [Google Scholar]
- Segovia G, Porras A, Mora F. Effects of 4-Aminopyridine on extracellular concentrations of glutamate in striatum of the freely moving rat. Neurochem Res. 1997;22:1491–1497. doi: 10.1023/a:1021958613125. [DOI] [PubMed] [Google Scholar]
- Sesack SR, Pickel VM. Prefrontal cortical efferents in the rat synapse on unlabeled neuronal targets of catecholamine terminals in the nucleus accumbens septi and on dopamine neurons in the ventral tegmental area. J Comp Neurol. 1992;320:145–160. doi: 10.1002/cne.903200202. [DOI] [PubMed] [Google Scholar]
- Shearman E, Rossi S, Szasz B, Juranyi Z, Fallon S, Pomara N, Sershen H, Lajtha A. Changes in cerebral neurotransmitters and metabolites induced by acute donepezil and memantine administrations: a microdialysis study. Br Res Bull. 2006;69:204–213. doi: 10.1016/j.brainresbull.2005.12.001. [DOI] [PubMed] [Google Scholar]
- Siegel A, Edinger H, Lowenthal H. Effects of electrical stimulation of the medial aspect of the prefrontal cortex upon attack behavior in cats. Br Res. 1974;66:467–479. doi: 10.1016/0006-8993(75)90185-7. [DOI] [PubMed] [Google Scholar]
- Simms JA, Steensland P, Medina B, Abernathy KE, Chandler LJ, Wise R, Bartlett SE. Intermittent access to 20% ethanol induces high ethanol consumption in Long-Evans and Wistar rats. Alcohol Clin Exp Res. 2008;32:1816–1823. doi: 10.1111/j.1530-0277.2008.00753.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spanagel R, Holter SM, Allingham K, Landgraf R, Zieglgansberger W. Acamprosate and alcohol: I. Effects on alcohol intake following alcohol deprivation in the rat. Eur J Pharmacol. 1996;305:39–44. doi: 10.1016/0014-2999(96)00174-4. [DOI] [PubMed] [Google Scholar]
- Stepanyan TD, Farook JM, Kowalski A, Kaplan E, Barron S, Littleton JM. Alcohol withdrawal-induced hippocampal neurotoxicity in vitro and seizures in vivo are both reduced by memantine. Alcohol Clin Exp Res. 2008;32:2128–2135. doi: 10.1111/j.1530-0277.2008.00801.x. [DOI] [PubMed] [Google Scholar]
- Sterzer P, Stadler C, Krebs A, Kleinschmidt A, Poustka F. Abnormal neural responses to emotional visual stimuli in adolescents with conduct disorder. Biol Psych. 2005;57:7–15. doi: 10.1016/j.biopsych.2004.10.008. [DOI] [PubMed] [Google Scholar]
- Taber MT, Fibiger HC. Electrical stimulation of the prefrontal cortex increases dopamine release in the nucleus accumbens of the rat: modulation by metabotropic glutamate receptors. J Neurosci. 1995;15:3896–3904. doi: 10.1523/JNEUROSCI.15-05-03896.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi A, Nagayasu K, Nishitani N, Kaneko S, Koide T. Control of intermale aggression by medial prefrontal cortex activation in the mouse. PloS One. 2014;9:e94657. doi: 10.1371/journal.pone.0094657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson AM, Deuchars J. Temporal and spatial properties of local circuits in neocortex. Trends Neurosci. 1994;17:119–126. doi: 10.1016/0166-2236(94)90121-x. [DOI] [PubMed] [Google Scholar]
- Trevisan LA, Boutros N, Petrakis IL, Krystal JH. Complications of alcohol withdrawal: pathophysiological insights. Alcohol Health Res World. 1998;22:61–66. [PMC free article] [PubMed] [Google Scholar]
- Varlinskaya EI, Spear LP. Acute ethanol withdrawal (hangover) and social behavior in adolescent and adult male and female Sprague-Dawley rats. Alcohol Clin Exp Res. 2004;28:40–50. doi: 10.1097/01.ALC.0000108655.51087.DF. [DOI] [PubMed] [Google Scholar]
- Vertes RP. Differential projections of the infralimbic and prelimbic cortex in the rat. Synapse. 2004;51:32–58. doi: 10.1002/syn.10279. [DOI] [PubMed] [Google Scholar]
- Vivian JA, Miczek KA. Ultrasounds during morphine withdrawal in rats. Psychopharmacology. 1991;104:187–193. doi: 10.1007/BF02244177. [DOI] [PubMed] [Google Scholar]
- Wayner MJ, Greenberg I, Tartaglione R, Nolley D, Fraley S, Cott A. A new factor affecting the consumption of ethyl alcohol and other sapid fluids. Physiol Behav. 1972;8:345–362. doi: 10.1016/0031-9384(72)90383-6. [DOI] [PubMed] [Google Scholar]
- Williams JM, Steketee JD. Cocaine increases medial prefrontal cortical glutamate overflow in cocaine-sensitized rats: a time course study. Eur J Neurosci. 2004;20:1639–1646. doi: 10.1111/j.1460-9568.2004.03618.x. [DOI] [PubMed] [Google Scholar]
- Wills TA, Knapp DJ, Overstreet DH, Breese GR. Sensitization, duration, and pharmacological blockade of anxiety-like behavior following repeated ethanol withdrawal in adolescent and adult rats. Alcohol Clin Exp Res. 2009;33:455–463. doi: 10.1111/j.1530-0277.2008.00856.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winward JL, Bekman NM, Hanson KL, Lejuez CW, Brown SA. Changes in emotional reactivity and distress tolerance among heavy drinking adolescents during sustained abstinence. Alcohol Clin Exp Res. 2014;38:1761–1769. doi: 10.1111/acer.12415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise RA. Voluntary ethanol intake in rats following exposure to ethanol on various schedules. Psychopharmacologia. 1973;29:203–210. doi: 10.1007/BF00414034. [DOI] [PubMed] [Google Scholar]
- Yan Q, Reith MEA, Yan SG, Jobe PC. Effects of systemic ethanol on basal and stimulated glutamate releases in the nucleus accumbens of freely moving Sprage-Dawley rats: a microdialysis study. Neurosci Lett. 1998;258:29–32. doi: 10.1016/s0304-3940(98)00840-4. [DOI] [PubMed] [Google Scholar]