Abstract
Background
Individual randomized controlled trials (RCTs) have demonstrated the efficacy of cognitive behavioral therapy (CBT) and serotonin reuptake inhibitors (SRIs) for the treatment of youth with obsessive-compulsive disorder (OCD). While meta-analyses have confirmed these results, there has been minimal examination of treatment moderators or an examination of treatment response and symptom/diagnostic remission for these two treatment types. The present report examined the treatment efficacy, treatment response, and symptom/diagnostic remission for youth with OCD receiving either CBT or SRIs relative to comparison conditions, and examined treatment moderators.
Method
A comprehensive literature search identified 20 RCTs that met inclusion criteria, and produced a sample size of 507 CBT participants and 789 SRI participants.
Results
Random effects meta-analyses of CBT trials found large treatment effects for treatment efficacy (g=1.21), treatment response [relative risk (RR)=3.93], and symptom/diagnostic remission (RR=5.40). Greater co-occurring anxiety disorders, therapeutic contact, and lower treatment attrition were associated with greater CBT effects. The number needed to treat (NNT) was three for treatment response and symptom/diagnostic remission. Random effects meta-analyses of SRI trials found a moderate treatment effect for treatment efficacy (g=0.50), treatment response (RR=1.80), and symptom/diagnostic remission (RR=2.06). Greater methodological quality was associated with a lower treatment response for SRI trials. The NNT was five for treatment response and symptom/diagnostic remission.
Conclusions
Findings demonstrate the treatment effects for CBT and SRIs across three important outcome metrics, and provide evidence for moderators of CBT across trials.
Keywords: obsessive compulsive disorder, cognitive behavior therapy, clomipramine, selective serotonin reuptake inhibitors, treatment outcome, treatment response, diagnostic remission
Introduction
Obsessive compulsive disorder (OCD) is characterized by the presence of obsessions and/or compulsions, and affects approximately 1–2% of youth.[1; 2] Although OCD may develop in adulthood,[3] a majority of cases report symptom onset during childhood.[4] Youth with OCD frequently experience co-occurring psychiatric conditions including anxiety disorders, attention deficit hyperactivity disorders (ADHD), major depressive disorders (MDD), chronic tic disorders (CTDs) and oppositional defiant disorder (ODD).[5; 6] Youth with OCD experience functional impairment,[7] disrupted family functioning,[8] and a poor quality of life.[9] As OCD symptoms rarely remit without treatment,[10] effective and efficient treatments are essential.
The two first-line empirically-supported treatment recommended for youth with OCD include cognitive behavioral therapy (CBT) and serotonin reuptake inhibitors (SRIs).[11] Cognitive behavioral therapy is a psychological treatment that includes psychoeducation, cognitive training, symptom hierarchy development, and exposure with response prevention (ERP).[12; 13] Several randomized controlled trials (RCTs) have evaluated the efficacy of CBT for youth with OCD using manualized treatment protocols that emphasize either cognitive or behavioral components, with most treatments involving both aspects. Irrespective of these distinctions, CBT significantly reduces obsessive-compulsive symptom severity relative to waitlist,[14–18] placebo,[19] treatment-as-usual,[20] and relaxation training (RT) comparison conditions,[21–23] with maintenance up to 7 years after acute treatment.[24] Indeed, CBT is a highly preferred treatment option for parents of children with OCD,[25] and is recommended as a first-line monotherapy for youth with mild-to-moderate OCD symptom severity and together with SRIs for more severe cases.[11]
Medication management with SRIs presents another empirically-supported treatment option for youth with OCD. The SRIs that have been evaluated in RCTs for youth with OCD include clomipramine (CMI),[26–28] sertraline (SERT),[19; 29] fluoxetine (FLUX),[30–32] paroxetine (PAX),[33] and fluvoxamine (FLUV).[34] These medications significantly reduce obsessive-compulsive symptom severity relative to placebo and waitlist control conditions, with some evidence suggesting that CMI is superior to selective SRIs (SERT, FLUX, PAX, FLUV).[35] Long-term benefit from acute SRI treatment has been observed for up to 12 months with maintenance medication,[36] however symptom reemergence can occur with discontinued use.[37; 38] While appropriate safety and tolerability monitoring are needed due to side-effect concerns,[39; 40] SRI medications are recommended as first-line interventions for youth with moderately severe OCD.[11]
When making treatment recommendations, it is important to synthesize empirical evidence to guide clinical decisions.[41] Meta-analyses provide a quantitative synthesis of treatment trials, and provide for a more powerful examination of outcome moderators than individual treatment trials.[38; 42–46] To date, there have been seven published meta-analyses examining the efficacy of CBT,[47–49] SRIs,[35] or both interventions[50–52] for the treatment of pediatric OCD. Findings from these meta-analyses have demonstrated large treatment effects for CBT (1.45–1.98) and moderate effects for SRIs (0.46–0.48) for reducing symptom severity. While these meta-analyses are noteworthy contributions to the literature, they have several limitations, including: small sample size;[48] inclusion of open-label trials that may have inflated treatment effects;[47; 50] combined treatment effects across multiple OCD measures some which have poor treatment sensitivity in youth;[35; 52; 53] limited examination of treatment moderators;[35; 47–51] combined treatment effects across individual and group therapy formats that may have influenced moderator analyses;[51; 52] and inferred values from other placebo-controlled trials for comparison conditions.[52] Additionally, prior meta-analyses did not examine treatment response (i.e., when a patient exhibits a clinically meaningful reduction in obsessive-compulsive severity) and symptoms/diagnostic remission (i.e., when a patient no longer meets syndromal criteria and/or has no more than minimal symptoms),[54; 55] which are two clinically meaningful metrics that are informative to treatment providers.[35; 47–52]
In an effort to address these limitations, this report examined RCTs of individually-delivered empirically supported treatments (SRI or CBT) to determine their efficacy in reducing obsessive-compulsive symptom severity and to identify the risk ratios (RR) of experiencing a clinically meaningful response to treatment and symptom/diagnostic remission. Analyses across these three outcomes can provide clinicians with important probabilistic treatment response and symptom/diagnostic remission rates to aid parents and clinicians in the selection of empirically-supported monotherapies. Based on factors purported to impact treatment efficacy, treatment response, and symptom/diagnostic remission among CBT and SRI trials,[38; 42–45] this report examined the following putative treatment moderators: participant age; comorbidity (CTD, ADHD, depressive disorders, and anxiety disorders); baseline OCD symptom severity; medication status (for CBT trials); therapeutic contact (for CBT trials); attrition; treatment subtype; and methodological quality. Based on findings from individual RCTs, we had several hypotheses. First, we hypothesized that CBT would outperform comparison conditions. Second we hypothesized that SRIs would outperform comparison conditions. Finally, given the variable findings across moderators in individual RCTs, the association between purported moderators and treatment effects were explored across interventions. Although the efficacy of combined treatment (e.g., CBT+SRI) for pediatric OCD has some empirical support,[19; 56; 57] this treatment modality was considered too preliminary for inclusion in the current meta-analyses due to the limited number of published RCTs. Direct comparison trials of CBT and SRIs were also not examined due to their recent meta-analytic evaluation.[58] In this meta-analysis, Romanelli and colleagues[58] found that CBT outperformed SRIs across RCTs of youth and adults (effect size=0.37), but found no significant difference between CBT and CBT+SRI conditions.
Method
Search Strategy
PubMED, PsycInfo, and ProQuest Dissertations and Theses Online were searched using key search terms (i.e., “obsessive compulsive disorder” AND “children” with either “cognitive behavioral therapy” or “exposure response prevention” for CBT trials and either “selective serotonin reuptake inhibitors” OR “clomipramine” for medication trials). Identified abstracts were reviewed independently by two raters for appropriateness. The references of eligible treatment trials, and review articles were also searched. Identified abstracts/citations were evaluated for the following inclusion criteria: (1) a RCT that included an individually delivered treatment that could include family members, but was not a group-based intervention; (2) evaluated the efficacy of CBT or a SRI in treating OCD symptom severity relative to a non-evidence based comparison condition (e.g., placebo, desipramine, waitlist, relaxation training, treatment-as-usual) ; (3) included only participants under the age of 18 with a diagnosis of OCD; (4) available in English; (5) included an adequate dose/duration of CBT (5 session minimum) or SRI medication (5 week minimum); and (6) provided sufficient data to allow calculation of either treatment efficacy, treatment response, or symptom/diagnostic remission.
Procedures
A hierarchy of preferred rating scales for the primary outcome measure was established a priori to limit possible investigator reporting bias. In order of preference, these OCD ratings scales for treatment efficacy included the CY-BOCS,[59] and the National Institute of Mental Health Global OCD Scale (NIMH-GOCS).[60] For classification of treatment response, preference was placed on the Clinical Global Impression of Improvement (CGI-Improvement),[61] with treatment response considered a rating of “much improved” or “very much improved” consistent with extant RCTs. When the CGI-Improvement was unavailable, a reduction of 25% or greater on the CY-BOCS was used as it corresponds with a treatment response on the CGI-Improvement.[55] For classification of symptom/diagnostic remission, preference was placed on CY-BOCS 14 as it corresponds with a Clinical Global Impression-Severity (CGI-Severity) rating of “no illness” or “mild illness”.[55] If CY-BOCS remission cut-off scores were not reported and/or unavailable, diagnostic remission on the ADIS-P was preferred.[62] Finally, a CY-BOCS reduction of 40–50% was considered to be permissible in the absence of the other two measures, as it corresponds well to CGI-Severity ratings of “no illness” or “mild illness”.[55]
Study Coding
Trials were coded for the following characteristics: (1) participant mean age; (2) percentage of comorbid TS/CTD, ADHD, depressive disorders (major depressive disorder, dysthymia), and anxiety disorders (social phobia, generalized anxiety disorder, separation anxiety disorder, panic disorder); (3) baseline OCD severity; (4) number of 1-hour therapy sessions (for CBT trials); (5) percentage of SRI medication at baseline (for CBT trials); (6) active treatment attrition; (7) measure of treatment efficacy, response, and remission; (8) effect size (Hedges’ g), treatment response, and symptom/diagnostic remission; (9) treatment subtype (ERP or CT for CBT trials; CMI or SSRI for SRI trials); (10) comparison condition; and (11) study methodological quality. Comorbid disorders were selected due to their potential impact on treatment outcome.[42–45] Comparison conditions were classified into two categories: non-active interventions (e.g., waitlist, placebo, treatment-as-usual); and active interventions (e.g., desipramine, relaxation training). Study methodology was assessed using a 23-item scale that has been used in other meta-analyses.[63–65] Possible scores range from 0 to 46, with higher values corresponding with greater methodological rigor. Study investigators were contacted to request the above information if it was not available in published form. Trials were coded by two raters to ascertain reliability. Rater disagreement was resolved through discussion and consensus.
Effect size (ES) calculation
Hedges’ g was chosen as the treatment ES statistic for treatment efficacy since it controls for different sample sizes across studies, and was calculated in Comprehensive Meta-Analysis (CMA)Version 2.[66] Effect sizes were calculated using change scores because this increases the precision of ES estimators by controlling for pretreatment group differences of obsessive-compulsive symptom severity. Pre-and-post treatment means and standard deviations were entered into CMA, and were divided by the pooled post-treatment standard deviation. Effect sizes were standardized so that a positive result indicated that the active treatment (CBT or SRI) performed better than comparison conditions. For treatment response and symptom/diagnostic remission, the RR was selected to serve as the ES. The RR is the ratio of patients exhibiting response or remission in the active treatment condition divided by the probability of patients exhibiting response or remission in the comparison condition.[67] A RR of 1 suggests that response or remission outcomes did not differ between the two treatment conditions, whereas a RR of 4 indicates that the active treatment condition had a fourfold greater probability than the comparison condition of exhibiting response or remission. The number of treatment responders/non-responders and participants experiencing symptom remittance/non-remittance were entered into CMA, which calculated the RR for treatment response and symptom/diagnostic remittance.
Statistical Analyses
Inter-rater agreement of study characteristics and quality ratings was assessed using descriptive statistics and intra-class correlation coefficient (ICC). A random effects model using inverse variance weights examined the ES of CBT and SRIs in CMA.[66] A random effects model was chosen because the true ES were expected to vary across trials due to different study characteristics.[68] Heterogeneity of ES was assessed using the forest plot, Q statistic, and I2 statistic. Publication bias was assessed by visual inspection of the funnel plot and Egger’s test for bias. When publication bias was present, Duval and Tweedie’s trim-and-fill method was used to account for publication bias by producing an adjusted summary effect that takes into account potential within the field.[68] An analog to the analysis of variance (ANOVA) examined the heterogeneity of ES across comparison conditions (non-active versus active comparison conditions). Separate random effect models examined the RR of CBT and SRI in CMA for treatment response and symptom/diagnostic remission. The same procedures noted above assessed for publication bias and sensitivity analyses. The number needed to treat (NNT) was calculated for treatment response and symptom/diagnostic remission for each treatment. The NNT is the number of youth with OCD that would need to be treated with the active intervention for one patient to respond who would not have responded to the comparison intervention. Finally, hypothesized moderator variables were analyzed using either method-of-moments meta-regression or an analog to ANOVA.
Results
Included Studies
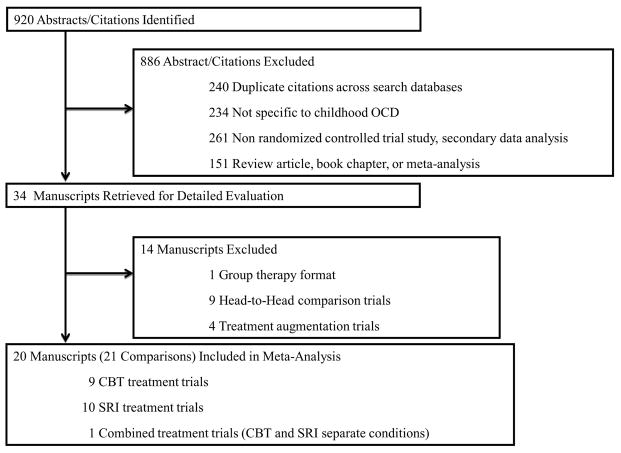
Initial search strategies produced 920 potential abstracts/citations, with 34 abstracts citations being retrieved for detailed review (see Figure 1). Table 1 displays the 20 RCTs that met all inclusion criteria, which produced a total sample size of 507 CBT participants and 789 SRI participants. Table 2 presents the ES and outcome measure of treatment efficacy, treatment response and symptom/diagnostic remission across trials.
Figure 1.
Study Selection and Rationale For Exclusion.
Table 1.
Characteristics of Randomized Controlled Trials of Cognitive Behavioral Therapy (CBT) and Serotonin Reuptake Inhibitores (SRIs) Included in Meta-analysis
| N | Treatment Subtype |
Control Tx | Mean Age |
% Comorbid TS/CTD |
% Comorbid ADHD |
% Comorbid Depressive Disorder |
% Comorbid Anxiety Disorder |
Baseline OCD Severity on CY-BOCS |
% on SRI Med at Baseline |
# of 1hr Therapy Sessions |
% Active Tx Dropout |
Method Quality |
|
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CBT Trials | |||||||||||||
| Barrett et al. 2004 | 48 | ERP | WL | 11.3 | 23 | 0 | 2 | 77 | 23.3 | 17 | 14 | 0 | 29 |
| POTS, 2004a | 56 | ERP | PLBO | 11.9 | 14 | 16 | 7 | 29 | 25.6 | 0 | 14 | 11 | 38 |
| Bolton & Perrin, 2007 | 20 | ERP | WL | 13.2 | 5 | 5 | 10 | 50 | 23.0 | 0 | 9 | 20 | 30 |
| Freeman et al. 2008 | 42 | ERP | RT | 7.1 | 10 | 19 | NR | NR | 22.4 | 14 | 12 | 27 | 32 |
| Williams et al. 2010 | 21 | CT | WL | 13.6 | NR | 10 | 5 | NR | 22.1 | 33 | 10 | 9 | 31 |
| Bolton et al. 2011 | 60 | CT | WL | 14.6 | 3 | 8 | 15 | 38 | 23.1 | 15 | 12 | 6 | 31 |
| Piacentini et al. 2011 | 71 | ERP | RT | 12.2 | 11 | 14 | 4 | 47 | 24.9 | 9 | 12 | 16 | 38 |
| Storch et al. 2011 | 31 | ERP | WL | 11.1 | 3 | 7 | 10 | 45 | 23.4 | 55 | 14 | 13 | 31 |
| Lewin et al. 2014 | 31 | ERP | TAU | 5.8 | 0 | 42 | 0 | 71 | 24.5 | 0 | 12 | 0 | 32 |
| Freeman et al. 2014 | 127 | ERP | RT | 7.2 | 18 | 14 | 2 | 47 | 25.6 | 2 | 12 | 13 | 41 |
|
| |||||||||||||
| SRI Trials | |||||||||||||
| Flament et al. 1985b | 19 | CMI | WL | 14.5 | NR | NR | NR | NR | NR | 0 | NA | 0 | 33 |
| Leonard et al. 1989b | 49 | CMI | DES | 13.9 | NR | NR | NR | NR | NR | 0 | NA | 2 | 28 |
| March et al. 1990c | 16 | CMI | PLBO | 15.0 | 0 | NR | NR | NR | 26.0 | 0 | NA | 25 | 26 |
| DeVeaugh-Geiss et al. 1992c | 60 | CMI | PLBO | 14.3 | 0 | 0 | NR | NR | 27.7 | 0 | NA | 13 | 31 |
| Riddle et al. 1992 | 13 | FLUX | PLBO | 11.7 | 15 | 8 | 15 | 54 | 22.4 | 0 | NA | 14 | 36 |
| March et al. 1998 | 107 | SERT | PLBO | 12.6 | 4 | 5 | 2 | 4 | 22.8 | 0 | NA | 20 | 34 |
| Geller et al. 2001c | 103 | FLUX | PLBO | 11.4 | 0 | 0 | NR | NR | 25.0 | 0 | NA | 31 | 37 |
| Riddle et al. 2001 | 120 | FLUV | PLBO | 13.0 | NR | 2 | 1 | 2 | 24.2 | 0 | NA | 33 | 33 |
| Liebowietz et al. 2002c | 43 | FLUX | PLBO | 12.7 | 0 | 7 | 21 | 47 | 23.2 | 0 | NA | 5 | 39 |
| Geller et al. 2004 | 203 | PARX | PLBO | 11.3 | NR | 9 | NR | 7 | 24.8 | 0 | NA | 34 | 39 |
| POTS, 2004a | 56 | SERT | PLBO | 12.0 | 18 | 16 | 7 | 34 | 23.4 | 0 | NA | 7 | 29 |
placebo group used in both comparisons,
used a cross-over trial design,
Tourette Syndrome in exclusion criteria
NOTE: NR = Not Reported, NA = Not Available/Applicable
Table 2.
Effect sizes and Outcome Measures for Treatment Efficacy, Treatment Response and Symptom/Diagnostic Remission Across Included Trials
| Treatment Efficacy Measure | Effect size (Hedges’ g) | Treatment Response Measure | Relative Risk of Treatment Resposne | Symptom /Diagnostic Remission Measure | Relative Risk of Symptom/Diagnostic Remission | |
|---|---|---|---|---|---|---|
| CBT Trials | ||||||
| Barrett et al. 2004 | CYBOCS | 2.82 | 25% CYBOCS Reduction | 19.00 | ADIS-P | 43.00 |
| POTS, 2004a | CYBOCS | 1.06 | 25% CYBOCS Reduction | 2.50 | CYBOCS ≤10 | 15.00 |
| Bolton & Perrin, 2007 | CYBOCS | 1.02 | 25% CYBOCS Reduction | 13.00 | CYBOCS ≤14 | 2.50 |
| Freeman et al. 2008 | CYBOCS | 0.49 | CGI-I | 1.25 | CYBOCS ≤12 | 2.50 |
| Williams et al. 2010 | CYBOCS | 1.31 | 25% CYBOCS Reduction | 3.18 | CYBOCS ≤14 | 2.73 |
| Bolton et al. 2011 | CYBOCS | 1.45 | 25% CYBOCS Reduction | 3.87 | ADIS-C/P | 7.33 |
| Piacentini et al. 2011 | CYBOCS | 0.38 | CGI-I | 2.10 | CYBOCS ≤10 | 2.54 |
| Storch et al. 2011 | CYBOCS | 1.15 | CGI-I | 6.09 | CYBOCS ≤10 | 4.22 |
| Lewin et al. 2014 | CYBOCS | 1.62 | CGI-I | 9.06 | CYBOCS ≤12 | 17.50 |
| Freeman et al. 2014 | CYBOCS | 1.18 | CGI-I | 1.76 | CYBOCS ≤14 | 1.94 |
|
| ||||||
| SRI Trials | ||||||
| Flament et al. 1985b | NIMH-GOCS | 0.78 | NA | --- | NA | --- |
| Leonard et al. 1989b | NIMH-GOCS | 0.78 | NA | --- | NA | --- |
| March et al. 1990c | CYBOCS | 0.51 | NA | --- | NA | --- |
| DeVeaugh-Geiss et al. 1992 | CYBOCS | 0.73 | CGI-I | 3.56 | NA | --- |
| Riddle et al. 1992 | CYBOCS | 0.78 | NA | --- | NA | --- |
| March et al. 1998 | CYBOCS | 0.62 | CGI-I | 1.61 | NA | --- |
| Geller et al. 2001 | CYBOCS | 0.44 | CGI-I | 2.93 | 40% CYBOCS Reduction | 1.97 |
| Riddle et al. 2001 | CYBOCS | 0.31 | CGI-I | 1.88 | NA | --- |
| Liebowietz et al. 2002 | CYBOCS | 0.24 | CGI-I | 1.80 | CYBOCS ≤14 | 1.44 |
| Geller et al. 2004 | CYBOCS | 0.40 | CGI-I | 1.38 | NA | --- |
| POTS, 2004a | CYBOCS | 0.44 | 25% CYBOCS Reduction | 2.13 | CYBOCS ≤10 | 11.00 |
placebo group used in both,
used a cross-over trial design
NOTE: CY-BOCS = Children’s Yale-Brown Obsessive-Compulsive Scale, NIMH-GOCS = National Institute of Mental Health Global Obsessive Compulsive Disorder Scale, NA = Not Available/Applicable, CGI-I = Clinical Global Impression of Improvement, ADIS-C/P = Anxiety Disorder Interview Schedule-Child and Parent report
Reliability of Coding Study Characteristics
There was excellent inter-rater agreement between the two raters on categorical and continuous study characteristics (100% agreement), as well as overall study methodological quality (ICC=0.90, 95% CI=0.76, 0.96).
Efficacy of CBT, Publication Bias, and Sensitivity Analyses
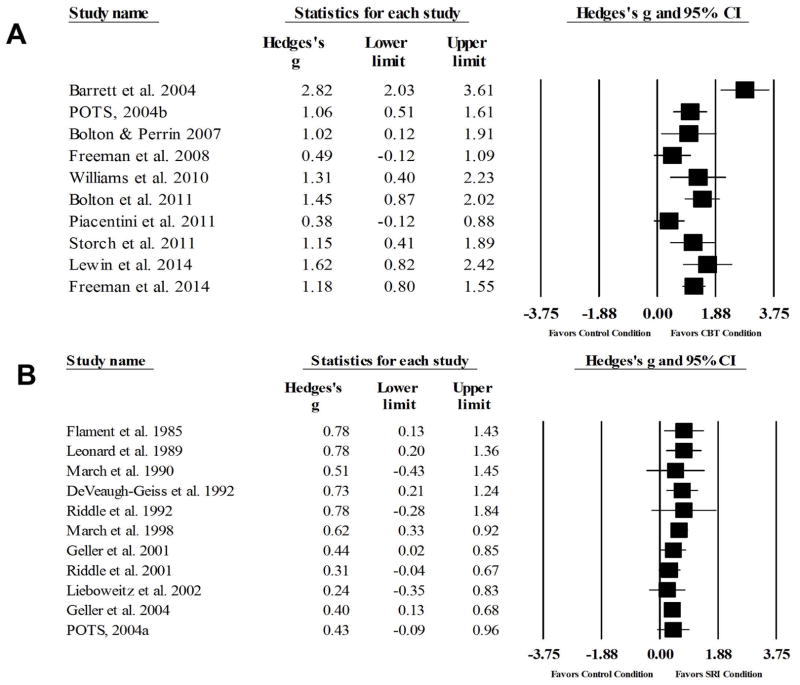
The random effects meta-analysis identified a large effect for CBT relative to comparison conditions (g=1.21, 95% CI: 0.83, 1.59, z=6.17, p<0.001) (Figure 2). Visual inspection of the forest plot, Q statistic, and I2 statistic identified the presence of significant heterogeneity [Q(9)=33.37 p<0.001, I2=73.03%]. Although visual inspection of the funnel plot suggested that publication bias may exist, Egger’s test for bias indicated that publication bias was not significant (t=1.03, p=0.33). The analog-to-ANOVA revealed that a significant difference between active comparison trials and non-active comparison trials [Q(1)=4.56, p=0.03]. Given the significant difference between control comparison conditions, the summary effect was recalculated with active comparison trials excluded. Results identified a larger treatment effect (g=1.48, 95% CI: 1.04, 1.92, z=6.58, p<0.001) with less heterogeneity [Q(6)=15.19, p=0.02, I2=60.50%] among non-active comparison trials. For active comparison trials, a moderate-to-large effect was observed (g=0.71, 95% CI: 0.16, 1.26, z=2.54, p=0.01), with significant heterogeneity [Q(2)=7.60 p=0.02, I2=73.69%] (Figure 2). Visual inspection of the funnel plot and Egger’s test (t=1.89, p=0.31) for bias indicated that publication bias was not significant.
Figure 2.
Forest plots of treatment efficacy in CBT trials (A) and SRI trials (B)
Treatment Response with CBT
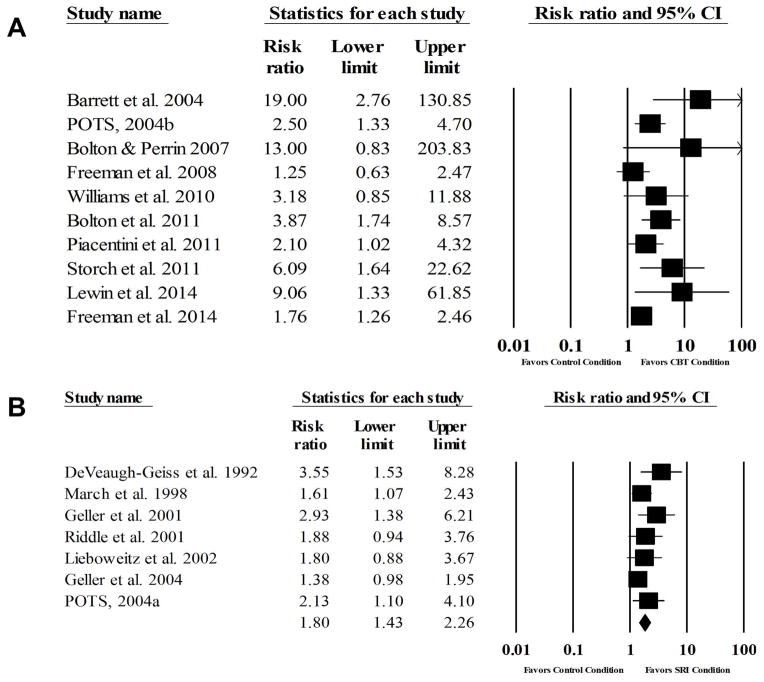
The average response rate across trials (n=10) for CBT, non-active comparison conditions, and active comparison conditions were 68%, 13%, and 36% respectively. A random effects meta-analysis identified a large effect for CBT relative to comparison conditions (RR=2.72, 95% CI: 1.83, 4.04, z=4.94, p<0.001) with significant heterogeneity [Q(9)=17.56, p=0.04, I2=44.79%] (Figure 3). The NNT for CBT treatment response was three. Visual inspection of the funnel plot and Egger’s test for bias indicated that publication bias was present (t=3.99, p=0.004). When Duval and Tweedie’s trim-and-fill method was applied, four studies were trimmed and CBT still exhibited a large significant effect (RR=2.13, 95%CI: 1.39, 3.27). The analog-to-ANOVA revealed a significant difference between active comparison trials (RR=1.71) and non-active comparison trials (RR=3.93) [Q(1)=9.74, p<0.002]. Given the significant difference between comparison conditions, the summary effect was recalculated with active comparison trials removed. Results identified a large effect (RR=3.93, 95% CI: 2.52, 6.14, z=6.02, p<0.001) with minimal heterogeneity [Q(6)=6.50, p=0.37, I2=7.51%] among non-active comparison trials. For active comparison trials, a moderate effect was observed (RR=1.71, 95% CI: 1.29, 2.25, z=3.77, p<0.001), with minimal heterogeneity [Q(2)=1.14 p=0.57, I2=0%]. Visual inspection of the funnel plot and Egger’s test (t=0.24, p=0.85) for bias indicated that publication bias was not present.
Figure 3.
Forest plots of treatment response in CBT trials (A) and SRI trials (B)
Symptom/Diagnostic Remission with CBT
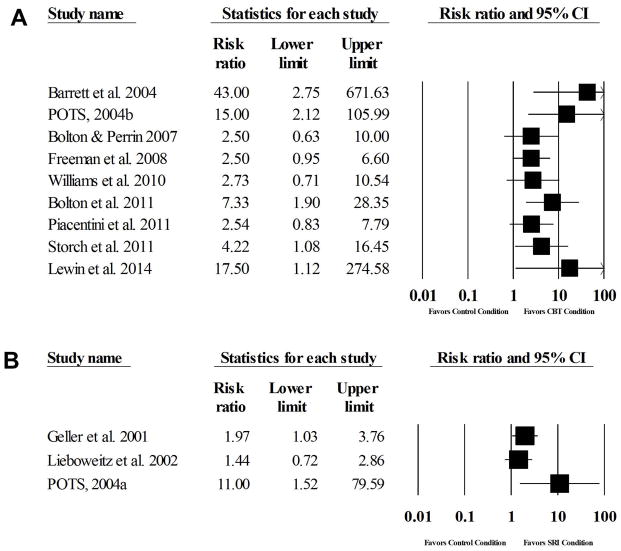
The average remission rate across trials (n=10) for CBT, non-active comparison conditions, and active comparison conditions were 57%, 9%, and 23% respectively. A random effects meta-analysis identified a large effect for CBT relative to comparison conditions (RR=3.42, 95% CI: 2.11, 5.53, z=5.00, p<0.001) with little heterogeneity [Q(9)=13.96, p=0.12, I2=35.52%] (Figure 4). The NNT for CBT remission was three. Visual inspection of the funnel plot and Egger’s test for bias indicated that publication bias was present (t=3.10, p=0.01). When Duval and Tweedie’s trim-and-fill method was applied, six studies were trimmed and CBT still exhibited a large significant effect (RR=2.15, 95%CI: 1.31, 3.54). The analog-to-ANOVA revealed a significant difference between active comparison trials (RR=2.05) and non-active comparison trials (RR=5.40) [Q(1)=6.93, p=0.008]. Given the significant difference between comparison conditions, the summary effect was recalculated with active comparison trials removed. Results identified a large effect (RR=5.40, 95% CI: 2.86, 10.22, z=5.18, p<0.001) with minimal heterogeneity [Q(6)=6.43, p=0.38, I2=6.63%] among non-active comparison trials. For active comparison trials, a large effect was observed (RR=2.05, 95% CI: 1.46, 2.88, z=4.16, p<0.001), with minimal heterogeneity [Q(2)=0.39 p=0.83, I2=0%]. Visual inspection of the funnel plot and Egger’s test for bias indicated that publication bias may be present (t=11.25, p=0.06). When Duval and Tweedie’s trim-and-fill method was applied, two studies were trimmed and CBT still exhibited a moderate significant effect (RR=1.94, 95%CI: 1.43, 2.64).
Figure 4.
Forest plots of symptom/diagnostic remission in CBT trials (A) and SRI trials (B)
Moderators of CBT Across Treatment Efficacy, Treatment Response, and Symptom Remission
Given the significant difference between comparison interventions, moderator analyses were conducted separately on CBT trials using non-active and active comparison conditions. Table 3 presents the results of moderator analyses across all three outcome types. For non-active comparison conditions, there was a positive association between the percentage of youth with co-occurring anxiety disorders and ES for treatment efficacy and response, with trials that had participants with more co-occurring anxiety disorders exhibiting larger ES. Additionally, for treatment efficacy and symptom remission, there was a positive association between the number of therapeutic hours and ES, with trials that had greater therapeutic contact exhibiting larger ES. Furthermore, there was an association between active treatment attrition and ES for treatment efficacy, with trials exhibiting greater attrition yielding lower ES. There was no significant difference between non-active comparison CBT trials that emphasized ERP relative to CT for treatment efficacy (g=1.52 vs. 1.41), treatment response (RR=5.50 vs. 3.67), or symptom diagnostic/remission (RR=6.69 vs. 4.47). There were no other significant moderators of treatment efficacy, treatment response, or symptom/diagnostic remission among non-active comparison trials. Table 3 also presents moderator analyses for active-comparison trials across all three outcome types. For treatment efficacy, a positive association was found between comorbid TS/CTD and ES, with trials that had a greater percentage of TS/CTD exhibited larger ES. Otherwise, there were no significant moderators of treatment efficacy, treatment response, or symptom/diagnostic remission among active-comparison trials.
Table 3.
Regression Analyses and Analog to ANOVA Examining Moderators of Treatment Efficacy, Treatment Response, and Remission for CBT and SRI Trials
| CBT Trials versus Non-active comparisons | Treatment Efficacy (n=7) | Treatment Response (n=7) | Symptom Remission (n=7) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
||||||||||||
| Study Characteristics | B | SE | z | p | B | SE | z | p | B | SE | z | p |
|
|
||||||||||||
| Mean Participant Age | −0.05 | 0.09 | −0.50 | 0.61 | −0.09 | 0.12 | −0.82 | 0.41 | −0.16 | 0.16 | −1.01 | 0.31 |
| Percentage of comorbid TS or CTD | 0.05 | 0.03 | 1.49 | 0.14 | <0.01 | 0.05 | 0.01 | 0.99 | 0.09 | 0.06 | 1.47 | 0.14 |
| Percentage of comorbid ADHD | −0.01 | 0.02 | −0.40 | 0.69 | −0.01 | 0.03 | −0.33 | 0.74 | 0.03 | 0.04 | 0.83 | 0.41 |
| Percentage of comorbid Depressive Disorders | −0.06 | 0.05 | −1.21 | 0.22 | −0.03 | 0.06 | −0.58 | 0.56 | −0.05 | 0.08 | −0.63 | 0.53 |
| Percentage of comorbid Anxiety Disorders | 0.03 | 0.01 | 2.59 | 0.01 | 0.04 | 0.02 | 2.41 | 0.02 | 0.02 | 0.03 | 0.60 | 0.55 |
| Baseline CY-BOCS Symptom Severity | −0.09 | 0.23 | −0.40 | 0.69 | −0.20 | 0.16 | −1. 24 | 0.21 | 0.54 | 0.33 | 1.66 | 0.10 |
| Percentage of participants on a SRI | −0.00 | 0.01 | −0.06 | 0.95 | −0.01 | 0.01 | 0.70 | 0.48 | −0.01 | 0.02 | −0.67 | 0.50 |
| Number of 1-hour Therapy Sessions | 0.13 | 0.05 | 2.75 | 0.005 | 0.08 | 0.10 | 0.84 | 0.40 | 0.23 | 0.11 | 2.01 | 0.04 |
| Percentage of Active Treatment Dropout | −0.07 | 0.03 | −2.57 | 0.01 | −0.05 | 0.06 | −0.97 | 0.33 | −0.09 | 0.05 | −1.79 | 0.07 |
| Methodological Quality | −0.09 | 0.07 | −1.29 | 0.20 | −0.11 | 0.06 | −1.84 | 0.07 | 0.14 | 0.14 | 0.96 | 0.34 |
| Q | (df) | p | Q | (df) | p | Q | (df) | p | ||||
|
|
|
|
||||||||||
| Treatment Subtype (ERP vs. CT) | 0.08 | 1 | 0.78 | 0.55 | 1 | 0.46 | 0.34 | 1 | 0.56 | |||
| Outcome Measure | NA | NA | NA | 1.04 | 1 | 0.31 | 1.39 | 1 | 0.24 | |||
|
| ||||||||||||
| CBT Trials versus Active Comparisons | Treatment Efficacy (n=3) | Treatment Response (n=3) | Symptom Remission (n=3) | |||||||||
|
|
||||||||||||
| Study Characteristics | B | SE | z | p | B | SE | z | p | B | SE | z | p |
|
|
||||||||||||
| Mean Participant Age | −0.10 | 0.12 | −0.79 | 0.43 | 0.05 | 0.08 | 0.62 | 0.53 | 0.05 | 0.12 | 0.39 | 0.70 |
| Percentage of comorbid TS or CTD | 0.10 | 0.04 | 2.67 | 0.01 | 0.02 | 0.04 | 0.46 | 0.64 | −0.03 | 0.05 | −0.61 | 0.54 |
| Percentage of comorbid ADHD | −0.06 | 0.15 | −0.37 | 0.71 | −0.08 | 0.08 | −1.00 | 0.32 | 0.04 | 0.11 | 0.40 | 0.69 |
| Percentage of comorbid Depressive Disorders | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| Percentage of comorbid Anxiety Disorders | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| Baseline CY-BOCS Symptom Severity | −0.16 | 0.22 | 0.71 | 0.48 | 0.10 | 0.12 | 0.87 | 0.39 | −0.08 | 0.17 | −0.51 | 0.61 |
| Percentage of participants on a SRI | −0.06 | 0.04 | −1.67 | 0.10 | −0.02 | 0.03 | −0.64 | 0.52 | 0.02 | 0.04 | 0.58 | 0.56 |
| Number of 1-hour Therapy Sessions | −0.10 | 0.12 | −0.82 | 0.41 | 0.05 | 0.08 | 0.60 | 0.55 | 0.05 | 0.12 | 0.40 | 0.69 |
| Percentage of Active Treatment Dropout | −0.04 | 0.05 | −0.79 | 0.43 | −0.02 | 0.03 | −0.85 | 0.40 | 0.02 | 0.04 | 0.52 | 0.61 |
| Methodological Quality | −0.07 | 0.07 | 0.96 | 0.34 | 0.03 | 0.04 | 0.79 | 0.43 | −0.03 | 0.06 | −0.54 | 0.59 |
| Q | (df) | p | Q | (df) | p | Q | (df) | p | ||||
|
|
|
|
||||||||||
| Treatment Subtype (ERP vs. CT) | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | |||
| Outcome Measure | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | |||
|
| ||||||||||||
| SRI Trials versus Non-Active Comparisons | Treatment Efficacy (n=11) | Treatment Response (n=7) | Symptom Remission (n=3) | |||||||||
|
|
||||||||||||
| Study Characteristics | B | SE | z | p | B | SE | Z | p | B | SE | z | p |
|
|
||||||||||||
| Mean Participant Age | 0.08 | 0.06 | 1.26 | 0.21 | 0.18 | 0.12 | 1.44 | 0.15 | −0.29 | 1.42 | −0.21 | 0.83 |
| Percentage of comorbid TS or CTD | < 0.01 | 0.02 | 0.03 | 0.97 | −0.01 | 0.03 | −0.33 | 0.74 | 0.10 | 0.06 | 1.80 | 0.07 |
| Percentage of comorbid ADHD | < −0.01 | 0.02 | −0.31 | 0.76 | −0.03 | 0.02 | −1.22 | 0.22 | 0.09 | 0.09 | 0.99 | 0.32 |
| Percentage of comorbid Depressive Disorders | −0.01 | 0.02 | −0.47 | 0.63 | <0.01 | 0.02 | 0.19 | 0.85 | −0.03 | 0.08 | −0.34 | 0.73 |
| Percentage of comorbid Anxiety Disorders | < −0.01 | 0.01 | −0.26 | 0.79 | 0.01 | 0.01 | 0.74 | 0.46 | NA | NA | NA | NA |
| Baseline CY-BOCS Symptom Severity | < 0.01 | 0.05 | 0.09 | 0.93 | 0.10 | 0.09 | 1.14 | 0.26 | −0.20 | 0.96 | −0.21 | 0.84 |
| Percentage of Active Treatment Dropout | −0.01 | 0.01 | −1.40 | 0.16 | −0.01 | 0.01 | −0.93 | 0.35 | −0.02 | 0.07 | −0.24 | 0.81 |
| Methodological Quality | −0.02 | 0.02 | −1.44 | 0.15 | −0.06 | 0.03 | −2.01 | 0.04 | 0.34 | 0.18 | 1.91 | 0.06 |
| Q | (df) | p | Q | (df) | p | Q | (df) | p | ||||
|
|
|
|
||||||||||
| Treatment Subtype (SSRI v. CMI) | 2.55 | 1 | 0.11 | 2.85 | 1 | 0.09 | NA | NA | NA | |||
| Outcome Measure | 1.79 | 1 | 0.18 | 0.21 | 1 | 0.64 | 3.66 | 2 | 0.16 | |||
Note: TS = Tourette Syndrome, CTD = Chronic Tic Disorder, CY-BOCS = Children’s Yale-Brown Obsessive-Compulsive Scale, SRI = Serotonin Reuptake Inhibitor, NA = Not Applicable,
Efficacy of SRIs
A random effects meta-analysis identified a moderate effect of SRIs compared to all control conditions (g=0.50, 95% CI: 0.37, 0.63, z=7.33, p<0.001) (Figure 2). Visual inspection of the forest plot, Q statistic, and I2 statistic identified minimal heterogeneity across SRI trials [Q(10)=5.70 p=0.94, I2=0%]. Visual inspection of the funnel plot and Egger’s test for bias indicated that publication bias was not significant (t=0.98, p=0.35). The analog-to-ANOVA revealed no significant difference between active comparison trials (g=0.78) and non-active comparison trials (g=0.48) [Q(1)=0.94, p=0.33].
SRI Treatment Response
The average response rate across trials (n=7) for SRI and non-active comparison conditions were 50% and 25%, respectively. A random effects meta-analysis identified a moderate effect for SRIs relative to comparison conditions (RR=1.80, 95% CI: 1.43, 2.26, z=5.06 p<0.001) with minimal heterogeneity [Q(6)=6.89, p=0.33, I2=12.88%] (Figure 3). The NNT for SRI treatment response was five. Visual inspection of the funnel plot and Egger’s test for bias indicated that publication bias was present (t=4.46, p=0.006). When Duval and Tweedie’s trim-and-fill method was applied, four studies were trimmed and SRIs still exhibited a moderate effect (RR=1.52, 95%CI: 1.18, 1.95). All trials with treatment response data used a placebo control condition.
SRI Symptom/Diagnostic Remission
The average remission rate across trials (n=3) for SRI and non-active comparison conditions were 47% and 22%, respectively. A random effects meta-analysis identified a moderate effect for SRIs relative to comparison conditions (RR=2.06, 95% CI: 1.03, 4.13, z=2.05 p=0.04) with minimal heterogeneity [Q(2)=3.66, p=0.16, I2=45.35%] (Figure 4). The NNT for SRI symptom remission was five. Visual inspection of the funnel plot and Egger’s test for bias indicated that publication bias was not present (t=2.25, p=0.27). All trials with treatment response data used a placebo control condition.
Moderators of SRIs Across Treatment Efficacy, Treatment Response, and Symptom Remission
Given that there was no significant difference between active and non-active comparison conditions, all comparison conditions were included in moderator analyses. Table 3 presents the results of moderator analyses across all three outcome types. Although there was a non-significant trend for larger treatment effects in CMI trials relative to SSRI trials, there were no significant differences in treatment efficacy (g=0.73 vs. 0.45) or treatment response (RR=3.56 vs. 1.68). There was a small negative association between methodological quality and ES for treatment response (p=0.04), with trials that had greater methodological rigor exhibiting smaller ES. There were no other significant moderators of treatment efficacy, treatment response, or symptom/diagnostic remission among SRI trials.
Discussion
Prior meta-analyses of childhood OCD treatments have focused primarily on treatment efficacy, with minimal to no attention dedicated to treatment response or symptom/diagnostic remission. This is the first meta-analysis to collectively examine the treatment efficacy, treatment response, and symptom/diagnostic remission of evidence-based treatments for youth with OCD. Findings suggest that CBT has a large treatment effect for treatment efficacy (g=1.21), and an excellent RR for both treatment response (RR=2.72) and symptom/diagnostic remission (RR=3.42). Interestingly, there was little difference between treatment response and remission rates for CBT, which may suggest that youth who respond to CBT likely experience marked reductions in symptom severity that typically reach symptom/diagnostic remission. Several CBT treatment moderators in non-active comparison trials were identified that included the percentage of co-occurring anxiety disorders (treatment efficacy and treatment response), the number of therapeutic contact hours (treatment efficacy and symptom/diagnostic remission), and active treatment attrition (treatment efficacy). Additionally, TS/CTD was found to moderate treatment outcome in active comparison trials. The finding that CBT trials that had a greater incidence of co-occurring anxiety disorders exhibited larger treatment effects may suggest that OCD comorbid with other anxiety disorders could be driven by a more fear-based psychopathology which then leads to a more robust CBT response. The relationship between a greater number of CBT contact hours and larger treatment effects is consistent with psychosocial interventions for related disorders.[64; 65] Similarly, CBT trials that had higher treatment dropout rates were associated with lower therapeutic benefit. Taken together, these findings suggest that youth who persist in treatment and receive a full-course of CBT may likely experience optimal therapeutic benefit. While trials that emphasized ERP exhibited larger effects relative to CT, the difference was not statistically significant. Notably, the power to detect this statistical difference was largely constrained by the small number of trials that emphasized CT, as well as the possible overlap between “behavioral experiments” used in CT trials and “exposures” in ERP trials. Finally, the association between greater ES and a greater incidence of TS/CTD in active comparison trials is consistent with recent findings highlighting the benefit of CBT for tic-related OCD.[69]
Findings across SRI trials suggest that SRIs have a moderate-to-large treatment effect for treatment efficacy (g=0.50), and a moderate effect for treatment response (RR=1.80) and remission (RR=2.06). When examining treatment moderators of SRI trials, only methodological quality moderated treatment response. Thus, SRI trials with greater methodological rigor exhibited lower treatment response rates, which is likely attributed to reduced error variance in well-controlled medication trials. Interestingly, the difference between SSRI and CMI trended toward significance for both treatment efficacy (p=0.11) and treatment response (p=0.09) favoring CMI. Contrary to findings from individual SRI trials,[38; 44] TS/CTD and ADHD were not significant moderators of treatment efficacy, treatment response, or symptom/diagnostic remission. Although further examination is still warranted, this suggests that youth with TS/CTD or ADHD may still benefit from SRIs to treat their obsessive-compulsive symptoms.
Several limitations should be considered. First, there was inconsistent reporting of variables needed to calculate treatment efficacy, treatment response, and symptom/diagnostic remission across RCTs. Although study investigators were contacted to obtain these data, this resulted in a limited number of RCTs included in treatment response and symptom/diagnostic remission analyses and may have influenced findings. Second, most of these RCTs focused on acute outcomes and were not designed with the goal of symptom/diagnostic remission. Thus, it may be that longer treatment durations and/or higher doses may yield improved symptoms/diagnostic remission rates for CBT and SRIs. Third, some moderator analyses had appropriate power to detect effects, but others had less power. Borenstein and colleagues [68] recommend 10 studies for moderator analyses. Thus, non-significant moderator findings should not be interpreted as a conclusive lack of association. Moreover, an examination of patient-level data may yield different moderator results, however such data are unavailable at this time.[46] Finally, there were limited characteristics available for extraction across RCTs. Although theoretically driven moderators were selected, there may be unexamined factors (e.g., homework compliance, medication adherence) omitted from these reports that influence treatment effects.
In summary, findings suggest that CBT produces large treatment effects for treatment efficacy, treatment response, and symptom/diagnostic remission. The presence of greater comorbid anxiety disorders and TS/CTD, greater therapeutic contact, and decreased treatment attrition were found to be associated with greater CBT treatment effects. Meanwhile, SRIs produce moderate treatment effects for treatment efficacy, treatment response, and symptom/diagnostic remission. Although methodological quality was associated with smaller treatment effects, no other characteristics influenced treatment effects across trials. Although we encourage the future examination of patient-level moderator analyses from these combined RCTs, in their absence, these findings provide some guidance to practicing clinicians. From a clinical perspective, these findings provide three practical implications. First, these findings provide clinicians with probabilistic treatment response and symptom/diagnostic remission rates in response to empirically-supported monotherapies, which can be useful to inform families in the treatment selection process and aid patient/parent expectations. Second, these findings suggest that youth with either comorbid anxiety disorders or TS/CTD are good candidates for CBT. Third, these findings indicate that improved CBT therapeutic outcomes were associated with greater therapeutic contact and lower attrition rates. Thus, practicing clinicians should encourage families who wish to discontinue treatment early to stay the course for a full CBT trial.
Acknowledgments
The authors would like to express their appreciation to the following study investigators (in alphabetical order) for their responsiveness and/or assistance in obtaining data not published in their original article: Paula Barrett PhD, Derek Bolton PhD, Scott Compton PhD, Joseph DeVeaugh-Geiss MD, Lara Farrell PhD, Jennifer Freeman PhD, Daniel Geller MD, John March MD, Mark Riddle MD, Jeffrey Sapyta PhD, H. Blair Simpson MD PHD, and Timothy Williams PhD.
Footnotes
Financial Disclosures
Mr. McGuire and Ms. Brennan report no potential conflicts of interest.
Dr. Piacentini reports supported from National Institute of Mental Health (NIMH), Tourette Syndrome Association (TSA), Pfizer and the Pettit Family Foundation; book royalties from Oxford University Press and Guilford Publications, and speaking honoraria from the Tourette Syndrome Association and the International OCD Foundation (IOCDF).
Dr. Lewin has received research support from the IOCDF, Joseph Drown Foundation, and National Alliance for Research on Schizophrenia and Depression (NARSAD), has an agreement for a publishing honorarium from Springer Publishing, speakers honorarium from the TSA, reviewer honorarium from Children’s Tumor Foundation, travel support from Rogers Memorial Hospital, NIMH, the Society for Clinical Child and Adolescent Psychology (SCCAP) and American Academy of Child and Adolescent Psychiatry (AACAP), and consulting fees from Prophase LLC.
Dr. Murphy receives or has received research funding from the Florida Agency for Healthcare Administration, AstraZeneca Research &Development, Brain and Behavior Research Foundation, the Center for Disease Control and Prevention, F. Hoffmann-La Roche Ltd., Indevus Pharmaceuticals, IOCDF, National Institutes of Health/National Institute of Mental Health (NIH/NIMH), Otsuka Pharmaceuticals, Pfizer, Inc., Psyadon Pharmaceuticals, Inc, and Shire Pharmaceuticals. She has received travel support from the TSA and honoraria from grand rounds/CME lectures.
Dr. Storch has received support from the NIH, CDCP, Agency for Healthcare Research and Quality, IOCDF, TSA, and Janssen Scientific Affairs. He receives textbook honorarium from Springer publishers, American Psychological Association, Lawrence Erlbaum and Wiley-Blackwell. Dr. Storch has been an educational consultant for Rogers Memorial Hospital. He is a consultant for Prophase, Inc. and CroNos, Inc., and is on the Speaker’s Bureau and Scientific Advisory Board for the IOCDF. He receives research support from the All Children’s Hospital Guild Endowed Chair.
References
- 1.Zohar AH. The epidemiology of obsessive-compulsive disorder in children and adolescents. Child and Adolescent Psychiatric Clinics of North America. 1999;8(3):445–460. [PubMed] [Google Scholar]
- 2.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. Arlington, VA: American Psychiatric Association; 2013. [Google Scholar]
- 3.Ruscio AM, Stein DJ, Chiu WT, Kessler RC. The epidemiology of obsessive-compulsive disorder in the National Comorbidity Survey Replication. Molecular Psychiatry. 2010;15(1):53–63. doi: 10.1038/mp.2008.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nestadt G, Samuels J, Riddle M, et al. A family study of obsessive-compulsive disorder. Archives of General Psychiatry. 2000;57(4):358–363. doi: 10.1001/archpsyc.57.4.358. [DOI] [PubMed] [Google Scholar]
- 5.Geller DA, Biederman J, Faraone S, et al. Developmental aspects of obsessive compulsive disorder: Findings in children, adolescents, and adults. Journal of Nervous and Mental Disease. 2001;189(7):471–477. doi: 10.1097/00005053-200107000-00009. [DOI] [PubMed] [Google Scholar]
- 6.Farrell L, Barrett P, Piacentini J. Obsessive-Compulsive Disorder Across the Developmental Trajectory: Clinical Correlates in Children, Adolescents and Adults. Behaviour Change. 2006;23(2):103–120. [Google Scholar]
- 7.Piacentini J, Peris TS, Bergman RL, et al. Functional impairment in childhood OCD : Development and psychometrics properties of the Child Obsessive-Compulsive Impact Scale--Revised (COIS--R) Journal of Clinical Child and Adolescent Psychology. 2007;36(4):645–653. doi: 10.1080/15374410701662790. [DOI] [PubMed] [Google Scholar]
- 8.Lebowitz ER, Panza KE, Su J, Bloch MH. Family accommodation in obsessive-compulsive disorder. Expert Review Neurotherapeutics. 2012;12(2):229–38. doi: 10.1586/ern.11.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lack CW, Storch EA, Keeley ML, et al. Quality of life in children and adolescents with obsessive-compulsive disorder: Base rates, parent–child agreement, and clinical correlates. Social Psychiatry and Psychiatric Epidemiology. 2009;44(11):935–942. doi: 10.1007/s00127-009-0013-9. [DOI] [PubMed] [Google Scholar]
- 10.Stewart SE, Geller DA, Jenike M, et al. Long-term outcome of pediatric obsessive-compulsive disorder: A meta-analysis and qualitative review of the literature. Acta Psychiatrica Scandinavica. 2004;110(1):4–13. doi: 10.1111/j.1600-0447.2004.00302.x. [DOI] [PubMed] [Google Scholar]
- 11.Geller DA, March J. Practice Parameter for the Assessment and Treatment of Children and Adolescents With Obsessive-Compulsive Disorder. Journal of the American Academy of Child and Adolescent Psychiatry. 2012;51(1):98–113. doi: 10.1016/j.jaac.2011.09.019. [DOI] [PubMed] [Google Scholar]
- 12.Lewin AB, Wu MS, McGuire JF, Storch EA. Cognitive Behavioral Therapy for Obsessive-Compulsive and Related Disorders. Psychiatric Clinics of North America. 2014 doi: 10.1016/j.psc.2014.05.002. [DOI] [PubMed] [Google Scholar]
- 13.Freeman J, Garcia A, Frank H, et al. Evidence base update for psychosocial treatments for pediatric obsessive-compulsive disorder. J Clin Child Adolesc Psychol. 2014;43(1):7–26. doi: 10.1080/15374416.2013.804386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Williams TI, Salkovskis PM, Forrester L, et al. A randomised controlled trial of cognitive behavioural treatment for obsessive compulsive disorder in children and adolescents. European Child & Adolescent Psychiatry. 2010;19(5):449–456. doi: 10.1007/s00787-009-0077-9. [DOI] [PubMed] [Google Scholar]
- 15.Barrett P, Healy-Farrell L, March JS. Cognitive-behavioral family treatment of childhood obsessive-compulsive disorder: a controlled trial. J Am Acad Child Adolesc Psychiatry. 2004;43(1):46–62. doi: 10.1097/00004583-200401000-00014. [DOI] [PubMed] [Google Scholar]
- 16.Bolton D, Perrin S. Evaluation of exposure with response-prevention for obsessive compulsive disorder in childhood and adolescence. Journal of Behavior Therapy and Experimental Psychiatry. 2008;39(1):11–22. doi: 10.1016/j.jbtep.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 17.Bolton D, Williams T, Perrin S, et al. Randomized controlled trial of full and brief cognitive-behaviour therapy and wait-list for paediatric obsessive-compulsive disorder. Journal of Child Psychology and Psychiatry. 2011;52(12):1269–1278. doi: 10.1111/j.1469-7610.2011.02419.x. [DOI] [PubMed] [Google Scholar]
- 18.Storch EA, Caporino NE, Morgan JR, et al. Preliminary investigation of web-camera delivered cognitive-behavioral therapy for youth with obsessive-compulsive disorder. Psychiatry Res. 2011;189(3):407–12. doi: 10.1016/j.psychres.2011.05.047. [DOI] [PubMed] [Google Scholar]
- 19.POTS. Cognitive-behavior therapy, sertraline, and their combination for children and adolescents with obsessive-compulsive disorder: the Pediatric OCD Treatment Study (POTS) randomized controlled trial. JAMA. 2004;292(16):1969–1976. doi: 10.1001/jama.292.16.1969. [DOI] [PubMed] [Google Scholar]
- 20.Lewin AB, Park JM, Jones AM, et al. Family-based exposure and response prevention therapy for preschool-aged children with obsessive-compulsive disorder: a pilot randomized controlled trial. Behaviour Research and Therapy. 2014 doi: 10.1016/j.brat.2014.02.001. [DOI] [PubMed] [Google Scholar]
- 21.Freeman JB, Garcia AM, Coyne L, et al. Early childhood OCD: preliminary findings from a family-based cognitive-behavioral approach. J Am Acad Child Adolesc Psychiatry. 2008;47(5):593–602. doi: 10.1097/CHI.0b013e31816765f9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Piacentini J, Bergman RL, Chang S, et al. Controlled comparison of family cognitive behavioral therapy and psychoeducation/relaxation training for child obsessive-compulsive disorder. J Am Acad Child Adolesc Psychiatry. 2011;50(11):1149–61. doi: 10.1016/j.jaac.2011.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Freeman J, Sapyta J, Garcia A, et al. Family-Based Treatment of Early Childhood Obsessive-Compulsive Disorder: The Pediatric Obsessive-Compulsive Disorder Treatment Study for Young Children (POTS Jr)-A Randomized Clinical Trial. JAMA Psychiatry. 2014 doi: 10.1001/jamapsychiatry.2014.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.O’Leary EMM, Barrett P, Fjermestad KW. Cognitive-behavioral family treatment for childhood obsessive-compulsive disorder: A 7-year follow-up study. Journal of Anxiety Disorders. 2009;23(7):973–978. doi: 10.1016/j.janxdis.2009.06.009. [DOI] [PubMed] [Google Scholar]
- 25.Lewin AB, McGuire JF, Murphy TK, Storch EA. Editorial Perspective: The importance of considering parent’s preferences when planning treatment for their children - the case of childhood obsessive-compulsive disorder. J Child Psychol Psychiatry. 2014;55(12):1314–6. doi: 10.1111/jcpp.12344. [DOI] [PubMed] [Google Scholar]
- 26.de Haan E, Hoogduin KAL, Buitelaar JK, Keijsers GPJ. Behavior therapy versus clomipramine for the treatment of obsessive-compulsive disorder. Journal of the American Academy of Child & Adolescent Psychiatry. 1998;37(10):1022–1029. doi: 10.1097/00004583-199810000-00011. [DOI] [PubMed] [Google Scholar]
- 27.DeVeaugh-Geiss J, Moroz G, Biederman J, Cantwell DP. Clomipramine hydrochloride in childhood and adolescent obsessive-compulsive disorder: A multicenter trial. Journal of the American Academy of Child & Adolescent Psychiatry. 1992;31(1):45–49. doi: 10.1097/00004583-199201000-00008. [DOI] [PubMed] [Google Scholar]
- 28.Flament MF, Rapoport JL, Berg CJ, et al. Clomipramine treatment of childhood obsessive-compulsive disorder: A double-blind controlled study. Archives of General Psychiatry. 1985;42(10):977–983. doi: 10.1001/archpsyc.1985.01790330057007. [DOI] [PubMed] [Google Scholar]
- 29.March JS, Biederman J, Wolkow R, et al. Sertraline in children and adolescents with obsessive–compulsive disorder: A multicenter randomized controlled trial. JAMA. 1998;280(20):1752–1756. doi: 10.1001/jama.280.20.1752. [DOI] [PubMed] [Google Scholar]
- 30.Liebowitz MR, Turner SM, Piacentini J, et al. Fluoxetine in children and adolescents with OCD: A placebo-controlled trial. Journal of the American Academy of Child & Adolescent Psychiatry. 2002;41(12):1431–1438. doi: 10.1097/00004583-200212000-00014. [DOI] [PubMed] [Google Scholar]
- 31.Geller DA, Hoog SL, Heiligenstein JH, et al. Fluoxetine treatment for obsessive-compulsive disorder in children and adolescents: A placebo-controlled clinical trial. Journal of the American Academy of Child & Adolescent Psychiatry. 2001;40(7):773–779. doi: 10.1097/00004583-200107000-00011. [DOI] [PubMed] [Google Scholar]
- 32.Riddle MA, Scahill L, King RA, Hardin MT. Double-blind, crossover trial of fluoxetine and placebo in children and adolescents with obsessive-compulsive disorder. Journal of the American Academy of Child & Adolescent Psychiatry. 1992;31(6):1062–1069. doi: 10.1097/00004583-199211000-00011. [DOI] [PubMed] [Google Scholar]
- 33.Geller DA, Wagner KD, Emslie G, et al. Paroxetine treatment in children and adolescents with obsessive-compulsive disorder: A randomized, multicenter, double-blind, placebo-controlled trial. Journal of the American Academy of Child & Adolescent Psychiatry. 2004;43(11):1387–1396. doi: 10.1097/01.chi.0000138356.29099.f1. [DOI] [PubMed] [Google Scholar]
- 34.Riddle MA, Reeve EA, Yaryura-Tobias JA, et al. Fluvoxamine for children and adolescents with Obsessive-Compulsive Disorder: A randomized, controlled, multicenter trial. Journal of the American Academy of Child & Adolescent Psychiatry. 2001;40(2):222–229. doi: 10.1097/00004583-200102000-00017. [DOI] [PubMed] [Google Scholar]
- 35.Geller DA, Biederman J, Stewart SE, et al. Which SSRI? A Meta-Analysis of Pharmacotherapy Trials in Pediatric Obsessive-Compulsive Disorder. American Journal of Psychiatry. 2003;160(11):1919–1928. doi: 10.1176/appi.ajp.160.11.1919. [DOI] [PubMed] [Google Scholar]
- 36.Wagner KD, Cook EH, Chung H, Messig M. Remission status after long-term sertraline treatment of pediatric obsessive-compulsive disorder. Journal of Child and Adolescent Psychopharmacology. 2003;13(2, Suppl):S53–S60. doi: 10.1089/104454603322126340. [DOI] [PubMed] [Google Scholar]
- 37.Semerci ZB, Unal F. An open trial and discontinuation study of fluoxetine in children and adolescents with obsessive-compulsive disorder. Turk J Pediatr. 2001;43(4):323–8. [PubMed] [Google Scholar]
- 38.Geller DA, Biederman J, Stewart SE, et al. Impact of comorbidity on treatment response to paroxetine in pediatric obsessive-compulsive disorder: is the use of exclusion criteria empirically supported in randomized clinical trials? J Child Adolesc Psychopharmacol. 2003;13 (Suppl 1):S19–29. doi: 10.1089/104454603322126313. [DOI] [PubMed] [Google Scholar]
- 39.Murphy TK, Segarra A, Storch EA, Goodman WK. SSRI adverse events: how to monitor and manage. Int Rev Psychiatry. 2008;20(2):203–8. doi: 10.1080/09540260801889211. [DOI] [PubMed] [Google Scholar]
- 40.Bridge JA, Iyengar S, Salary CB, et al. Clinical response and risk for reported suicidal ideation and suicide attempts in pediatric antidepressant treatment: a meta-analysis of randomized controlled trials. JAMA. 2007;297(15):1683–96. doi: 10.1001/jama.297.15.1683. [DOI] [PubMed] [Google Scholar]
- 41.Murad MH, Montori VM. Synthesizing evidence: shifting the focus from individual studies to the body of evidence. JAMA. 2013;309(21):2217–8. doi: 10.1001/jama.2013.5616. [DOI] [PubMed] [Google Scholar]
- 42.Ginsburg GS, Kingery JN, Drake KL, Grados MA. Predictors of treatment response in pediatric obsessive-compulsive disorder. J Am Acad Child Adolesc Psychiatry. 2008;47(8):868–78. doi: 10.1097/CHI.0b013e3181799ebd. [DOI] [PubMed] [Google Scholar]
- 43.Garcia AM, Sapyta JJ, Moore PS, et al. Predictors and moderators of treatment outcome in the Pediatric Obsessive Compulsive Treatment Study (POTS I) J Am Acad Child Adolesc Psychiatry. 2010;49(10):1024–33. doi: 10.1016/j.jaac.2010.06.013. quiz 1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.March JS, Franklin ME, Leonard H, et al. Tics moderate treatment outcome with sertraline but not cognitive-behavior therapy in pediatric obsessive-compulsive disorder. Biol Psychiatry. 2007;61(3):344–7. doi: 10.1016/j.biopsych.2006.09.035. [DOI] [PubMed] [Google Scholar]
- 45.Storch EA, Merlo LJ, Larson MJ, et al. Impact of comorbidity on cognitive-behavioral therapy response in pediatric obsessive-compulsive disorder. Journal of the American Academy of Child & Adolescent Psychiatry. 2008;47(5):583–592. doi: 10.1097/CHI.0b013e31816774b1. [DOI] [PubMed] [Google Scholar]
- 46.Bloch MH. Meta-analysis and moderator analysis: can the field develop further? J Am Acad Child Adolesc Psychiatry. 2014;53(2):135–7. doi: 10.1016/j.jaac.2013.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Freeman JB, Choate-Summers ML, Moore PS, et al. Cognitive Behavioral Treatment for Young Children with Obsessive-Compulsive Disorder. Biological Psychiatry. 2007;61(3):337–343. doi: 10.1016/j.biopsych.2006.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.O’Kearney R. Benefits of cognitive-behavioural therapy for children and youth with obsessive-compulsive disorder: re-examination of the evidence. Aust N Z J Psychiatry. 2007;41(3):199–212. doi: 10.1080/00048670601172707. [DOI] [PubMed] [Google Scholar]
- 49.O’Kearney RT, Anstey KJ, von Sanden C. Behavioural and cognitive behavioural therapy for obsessive compulsive disorder in children and adolescents. Cochrane Database Syst Rev. 2010;(4):CD004856. doi: 10.1002/14651858.CD004856.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Abramowitz J, Whiteside SP, Deacon B. The effectiveness of treatment for pediatric Obsessive-Compulsive Disorder: A meta-analysis. Behavior Therapy. 2005;36:55–63. [Google Scholar]
- 51.Watson HJ, Rees CS. Meta-analysis of randomized, controlled treatment trials for pediatric obsessive-compulsive disorder. Journal of Child Psychology and Psychiatry. 2008;49(5):489–498. doi: 10.1111/j.1469-7610.2007.01875.x. [DOI] [PubMed] [Google Scholar]
- 52.Sanchez-Meca J, Rosa-Alcazar AI, Iniesta-Sepulveda M, Rosa-Alcazar A. Differential efficacy of cognitive-behavioral therapy and pharmacological treatments for pediatric obsessive-compulsive disorder: A meta-analysis. J Anxiety Disord. 2013;28(1):31–44. doi: 10.1016/j.janxdis.2013.10.007. [DOI] [PubMed] [Google Scholar]
- 53.Storch EA, Park JM, Lewin AB, et al. The Leyton Obsessional Inventory-Child Version survey form does not demonstrate adequate psychometric properties in american youth with pediatric obsessive-compulsive disorder. Journal of Anxiety Disorders. 2011;25(4):574–578. doi: 10.1016/j.janxdis.2011.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Frank E, Prien RF, Jarrett RB, et al. Conceptualization and rationale for consensus definitions of terms in major depressive disorder. Remission, recovery, relapse, and recurrence. Arch Gen Psychiatry. 1991;48(9):851–5. doi: 10.1001/archpsyc.1991.01810330075011. [DOI] [PubMed] [Google Scholar]
- 55.Storch EA, Lewin AB, De Nadai AS, Murphy TK. Defining treatment response and remission in obsessive-compulsive disorder: a signal detection analysis of the Children’s Yale-Brown Obsessive Compulsive Scale. J Am Acad Child Adolesc Psychiatry. 2010;49(7):708–17. doi: 10.1016/j.jaac.2010.04.005. [DOI] [PubMed] [Google Scholar]
- 56.Franklin ME, Sapyta J, Freeman JB, et al. Cognitive behavior therapy augmentation of pharmacotherapy in pediatric obsessive-compulsive disorder: the Pediatric OCD Treatment Study II (POTS II) randomized controlled trial. JAMA. 2011;306(11):1224–32. doi: 10.1001/jama.2011.1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Storch EA, Bussing R, Small BJ, et al. Randomized, placebo-controlled trial of cognitive-behavioral therapy alone or combined with sertraline in the treatment of pediatric obsessive–compulsive disorder. Behaviour research and therapy. 2013;51(12):823–829. doi: 10.1016/j.brat.2013.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Romanelli RJ, Wu FM, Gamba R, et al. Behavioral therapy and serotonin reuptake inhibitor pharmacotherapy in the treatment of obsessive-compulsive disorder: a systematic review and meta-analysis of head-to-head randomized controlled trials. Depress Anxiety. 2014;31(8):641–52. doi: 10.1002/da.22232. [DOI] [PubMed] [Google Scholar]
- 59.Scahill L, Riddle MA, McSwiggin-Hardin M, et al. Children’s Yale-Brown Obsessive Compulsive Scale: reliability and validity. J Am Acad Child Adolesc Psychiatry. 1997;36(6):844–852. doi: 10.1097/00004583-199706000-00023. [DOI] [PubMed] [Google Scholar]
- 60.Insel TR, Murphy DL, Cohen RM, et al. Obsessive-compulsive disorder. A double-blind trial of clomipramine and clorgyline. Arch Gen Psychiatry. 1983;40(6):605–12. doi: 10.1001/archpsyc.1983.04390010015002. [DOI] [PubMed] [Google Scholar]
- 61.Guy W. ECDEU Assessment Manual for Psychopharmacology. Rockville, MD: National Institute for Mental Health; 1976. Clinical Global Impressions; pp. 218–222. [Google Scholar]
- 62.Silverman WK, Albano AM. The Anxiety Disorders Interview Schedule for DSM-IV-Child and Parent Versions. San Antonio, TX: Graywinds Publications; 1996. [Google Scholar]
- 63.Moncrieff J, Churchill R, Drummond DC, McGuire H. Development of a quality assessment instrument for trials of treatments for depression and neurosis. International Journal of Methods in Psychiatric Research. 2001;10(3):126–133. [Google Scholar]
- 64.McGuire JF, Piacentini J, Brennan EA, et al. A meta-analysis of behavior therapy for Tourette Syndrome. J Psychiatr Res. 2014;50:106–12. doi: 10.1016/j.jpsychires.2013.12.009. [DOI] [PubMed] [Google Scholar]
- 65.McGuire JF, Ung D, Selless RR, et al. Treating Trichotillomania: A Meta-Analysis of Treatment Effects and Moderators for Behavior Therapy and Serotonin Reuptake Inhibitors. Journal of Psychiatric Research. 2014;58:76–83. doi: 10.1016/j.jpsychires.2014.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Borenstein M, Hedges L, Higgins J, Rothstein H. Comprehensive Meta-analysis. Englewood, NJ: Biostat; 2005. Version 2. [Google Scholar]
- 67.McGough JJ, Faraone SV. Estimating the size of treatment effects: Moving beyond P values. Psychiatry. 2009;6(10):21–29. [PMC free article] [PubMed] [Google Scholar]
- 68.Borenstein M, Hedges LV, Higgins JPT, Rothstein HR. Introduction to Meta-Analysis. Wiley; 2009. [Google Scholar]
- 69.Conelea CA, Walther MR, Freeman JB, et al. Tic-related obsessive-compulsive disorder (OCD): phenomenology and treatment outcome in the Pediatric OCD Treatment Study II. J Am Acad Child Adolesc Psychiatry. 2014;53(12):1308–16. doi: 10.1016/j.jaac.2014.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]