Abstract
Rationale
Numerous substituted cathinone drugs have appeared in recreational use. This variety is often a response to legal actions; the scheduling of 3,4-methylenedioxypyrovalerone (MDPV; “bath salts”) in the U.S.A. was followed by the appearance of the closely related drug α-pyrrolidinopentiophenone (alpha-PVP; “flakka”).
Objectives
To directly compare the efficacy and potency of alpha-PVP with that of MDPV.
Methods
Groups of male Wistar rats were trained in the intravenous self-administration (IVSA) alpha-PVP or MDPV under a fixed-ratio 1 schedule of reinforcement. An additional group was examined for locomotor and body temperature responses to non-contingent administration of MDVP or alpha-PVP (1.0, 5.6, 10.0 mg/kg, i.p.).
Results
Acquisition of alpha-PVP (0.1 mg/kg/infusion) IVSA resulted in low, yet consistent drug intake and excellent discrimination for the drug-paired lever. Dose-substitution (0.05-0.25 mg/kg/infusion) under a fixed-ratio 1 schedule confirmed potency is similar to MDPV in prior studies. In direct comparison to MDPV (0.05 mg/kg/infusion), rats trained on alpha-PVP (0.05 mg/kg/infusion) responded for more infusions but demonstrated similar drug-lever discrimination by the end of acquisition. However, the dose-response (0.018-0.56 mg/kg/inf) functions of these drugs under a progressive-ratio schedule of reinforcement reflected identical efficacy and potency. Peak locomotor responses to MDPV or alpha-PVP were observed after the 1.0 mg/kg, i.p. dose and lasted ~2 hours. Modest body temperature decreases were of similar magnitude (~0.75°C) for each compound.
Conclusions
The potency and efficacy of MDPV and alpha-PVP were very similar across multiple assays, predicting that the abuse liability of alpha-PVP will be significant and similar to that of MDPV.
Keywords: stimulants, substance abuse, bath salts, self-administration, cathinone, reward
INTRODUCTION
A diversity of substituted cathinone stimulants have appeared on the recreational use markets worldwide over the past half-decade. Some of this variety is apparently being driven by legal control actions for one specific compound resulting in the emergence of a closely related replacement. The US Drug Enforcement Administration (DEA) made a temporary scheduling action for 3,4-methylenedioxypyrovalerone (MDPV; aka “bathsalts”) in 2011 (DEA 2011) and Congress permanently scheduled it in 2012. This was followed by the appearance of the closely related α-pyrrolidinopentiophenone (alpha-PVP; aka “gravel” or “flakka”) in the recreational market. Alpha-PVP has already been associated with human fatalities (Nagai et al. 2014; Wurita et al. 2014) and, while the US DEA placed alpha-PVP on the Schedule I in 2014 (Drug Enforcement Administration 2014) in a temporary scheduling action, there are few data attesting to the abuse liability of alpha-PVP. The direct determination of abuse liability using validated animal models is critical for understanding likely risks of specific substituted cathinones because inferences based on similar chemical structure alone can be misleading. For example, the neuropharmacological, locomotor stimulant and thermoregulatory effects of 4-methylmethcathinone/mephedrone (Baumann et al. 2012; Huang et al. 2012; Miller et al. 2013a; Wright et al. 2012) are similar to those of 3,4-methylenedioxymethamphetamine (MDMA). Nevertheless, this compound is considerably more effective than MDMA (De La Garza et al. 2007; Feduccia et al. 2010; Schenk et al. 2007) in rat models of intravenous self-administration (Aarde et al. 2013a; Hadlock et al. 2011; Motbey et al. 2013). The 3,4-methylenedioxy motif that distinguishes MDMA from methamphetamine – a readily self-administered amphetamine-class stimulant - also distinguishes MDPV from alpha-PVP (Figure 1). MDMA is only poorly and inconsistently self-administered by rats as compared with methamphetamine (De La Garza et al. 2007; Feduccia et al. 2010; Schenk et al. 2007). It remains to be determined whether or not this particular molecular change to the readily self-administered MDPV (Aarde et al. 2013f) to generate alpha-PVP yields the same distinction in the reinforcer potency and efficacy with these restricted transporter blockers as it does for the amphetamines which have monamine transporter substrate and monoamine releaser properties.
Figure 1.
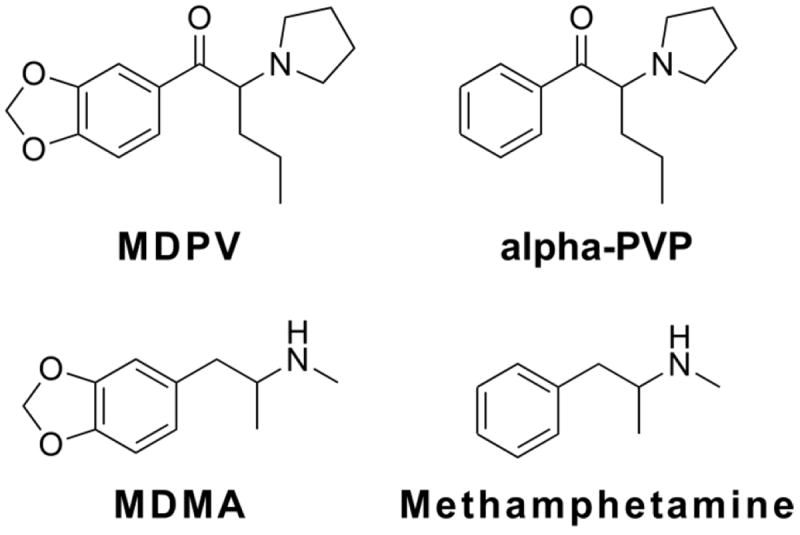
Chemical structures of cathinone stimulants α-pyrrolidinopentiophenone (alpha-PVP) and 3,4-methylenedioxypyrovalerone (MDPV) are compared with the amphetamines methamphetamine and 3,4-methylenedioxymethamphetamine (MDMA).
Alpha-PVP has been recently shown to increase locomotor behavior in mice (Kaizaki et al. 2014; Marusich et al. 2014) with a potency that is less than that of MDPV. It was also slightly less potent at inhibiting the dopamine transporter compared with MDPV (Marusich et al., 2014) which was consistent with the mouse locomotor data, and less effective in reducing intracranial self-stimulation reward thresholds (Watterson et al. 2014a; Watterson et al. 2014b). This predicts that alpha-PVP would be less potent as a reinforcer in supporting intravenous self-administration behavior in rat models (Wee et al. 2006). Nevertheless, higher selectivity of alpha-PVP for the DAT over the serotonin transporter (SERT) compared with MDPV suggests that alpha-PVP might be even more potent, since DAT/SERT ratio is more predictive of reinforcer potency than DAT affinity alone (Roberts et al. 1999; Wee et al. 2005).
This study was undertaken to directly compare the potency and efficacy of alpha-PVP with that of MDPV in established rat models of stimulant abuse liability, thermoregulation and activity. These approaches therefore permit additional inferences to be drawn to related investigations of other psychomotor stimulants. Studies in this study were conducted to compare drug intake across the acquisition of intravenous self-administration and then during subsequent dose-substitution under fixed-ratio and progressive-ratio schedules of reinforcement. The stimulants were also compared for their potential to alter body temperature and locomotor activity using a minimally invasive radiotelemetry system which has the benefit of minimizing potential handling artifacts. Locomotor behavior is a frequently used behavioral assessment of psychomotor stimulants and the potential to disrupt body temperature is relevant both to human medical emergency – including MDPV case reports (Borek and Holstege 2012; Froberg et al. 2014; Kesha et al. 2013) - and lasting neurotoxicity in high dose rodent models. Thus the study extends the comparison of these drugs across additional major endpoints of interest.
MATERIALS AND METHODS
Subjects
Male Wistar rats (Charles River, New York) were used for these investigations. Subjects were housed in groups of 2-3 in humidity and temperature-controlled (23±1 °C) vivaria on 12:12 hour light:dark cycles. Rats entered the laboratory at 10-13 weeks of age and weighed 350-400 grams at the start of the study. All rats had ad libitum access to food and water in their home cages. All procedures were conducted 4-5 days per week in the dark cycle starting 0-3 hours after lights-out. Studies were conducted under protocols approved by the Institutional Care and Use Committees of The Scripps Research Institute
Drugs
The racemic 3,4-methylenedioxypyrovalerone HCl used for this study was obtained from Fox Chase Chemical Diversity Center (Doylestown, PA) from synthetic routes designed by author TJD. Racemic α-pyrrolidinopentiophenone HCl was obtained from Cayman Chemical Company (Ann Arbor, Michigan, USA). All MDPV and alpha-PVP doses are expressed as the salt and drugs were dissolved in physiological saline for intravenous infusions or intraperitoneal injections. Cefazolin (Hikma Farmaceutica, Portugal), Flunixin (Bimeda USA, Oakbrook Terrace, IL) and Brevital (Methohexital Sodium; JHP Pharmaceuticals, Rochester, MI) were used for surgical and catheter care as described below.
Intravenous catheterization
Rats (MDPV, N = 18; alpha-PVP, N = 27) were anesthetized with an isoflurane/oxygen vapor mixture (isoflurane 5% induction, 1-3% maintenance) and prepared with chronic intravenous catheters as described previously (Aarde et al. 2013a; Aarde et al. 2013f). Briefly, the catheters consisted of a 14-cm length of polyurethane based tubing (Micro-Renathane®, Braintree Scientific, Inc, Braintree MA, USA) fitted to a guide cannula (Plastics One, Roanoke, VA) curved at an angle and encased in dental cement anchored to an ~3 cm circle of durable mesh. Catheter tubing was passed subcutaneously from the rat’s back to the right jugular vein. Catheter tubing was inserted into the vein and tied gently with suture thread. A liquid tissue adhesive was used to close the incisions (3M™ Vetbond™ Tissue Adhesive; 1469SB).
A minimum of 4 days was allowed for surgical recovery prior to starting an experiment. For the first three days of the recovery period, the antibiotic Cefazolin (Hikma Farmaceutica, Portugal; 0.4 mg/kg, i.m. in sterile water Day 1, s.c. Day 2-3) and the analgesic flunixin (FlunixiJect, Bimeda USA, Oakbrook Terrace, IL; 2.5 mg/kg, s.c. in saline) were administered daily. During recovery, as well as during testing and training, intravenous catheters were flushed with heparinized saline (before sessions) and heparinized saline containing cefazolan (100 mg/mL; after sessions).
Catheter patency was assessed after the last session of the week via administration through the catheter of ~0.2 ml (10 mg/ml) of the ultra-short-acting barbiturate anesthetic Brevital sodium (1% methohexital sodium in saline; JHP Pharmaceuticals, Rochester, MI). Rats with patent catheters exhibit prominent signs of anesthesia (pronounced loss of muscle tone) within 3 s after infusion. Rats that failed to display these signs were considered to have faulty catheters and were discontinued from the study. Rats that failed to complete a given phase of the study with patent catheters were excluded from analysis.
Radiotelemetry implantation
Sterile radiotelemetry transmitters (Data Sciences International; TA-F40) were implanted in the abdominal cavities of a group of rats (N=8) thru an incision along the abdominal midline posterior to the xyphoid space as previously reported (Miller et al. 2013a; Wright et al. 2012). Absorbable sutures were used to close the abdominal muscle incision and the skin incision was closed with the aforementioned tissue adhesive. Post-operative care and recovery time was the same as that for i.v. catheterization.
Self-administration procedure
Drug self-administration was conducted in one hour (3 h for progressive-ratio, see below) sessions using operant conditioning chambers (Med Associates; interior dimensions (30.5 cm L × 24.1 cm W × 21.0 cm H) located inside sound-attenuating chambers located in an experimental room (ambient temperature 23 ± 1 °C; illuminated by red light) outside of the housing vivarium. To begin a session, the catheter fitting on a rat’s back was connected to polyethylene tubing contained inside a protective spring (the “tether”) suspended into the operant chamber from a liquid swivel attached to a balance arm. The tether was filled with the drug solution prior to connection to the catheter and then the catheter tubing volume was filled after connection. Each operant session started with the extension of two retractable levers into the chamber. Following each completion of the response requirement (response ratio) on the drug-associated lever (right for all rats), a white stimulus light (located above the drug-associated lever) signaled delivery of the reinforcer and remained on during a 20 s post-infusion timeout, during which responses were recorded but had no other scheduled consequences. Responses on the alternate lever were recorded but led to no scheduled consequences. Rats were primed with a single infusion per session if none were observed within 30 min of the start of the session. Drug infusions (0.1 ml/infusion, 4 seconds / infusion) were delivered via syringe pump. Separate groups of rats were trained to self-administer MDPV (N=18; 0.05 mg/kg/inf) or alpha-PVP (N=9; 0.1 mg/kg/inf, N=18; 0.05 mg/kg/inf). The response requirement was fixed-ratio 1 and session duration was 1 h during the acquisition phase which was schedule a priori for 20 sessions.
Fixed-Ratio 1 (FR) Dose-Response Testing
The rats trained on alpha-PVP (0.1 mg/kg/inf) were subjected to randomized dose-substitution conditions in which the per-infusion dose differed (0.0, 0.025, 0.05, 0.1, 0.25 mg/kg/inf) on alternate (daily) sessions (1 h duration) starting after the acquisition interval. The dose orders were counterbalanced by Latin Square design and each rat received two sequential blocks of dose series (an individual’s data were averaged across block for analysis).
Progressive-Ratio (PR), Dose-Response Testing
Following the FR series, the rats were subjected to randomized dose-substitution under a PR schedule of reinforcement. In this self-administration paradigm, the required response ratio increases after each reinforcer delivery (Hodos 1961; Segal and Mandell 1974) as determined by the following equation (rounded to the nearest integer): Response Ratio = 5eˆ(injection number * j) – 5 (Richardson and Roberts 1996). The value of “j” for this study was 0.4 and was based upon a prior study with MDPV (Aarde et al. 2013f) so as to observe a “breakpoint” in ~3 hrs at 0.10 mg/kg/inf. The dose orders were counterbalanced by a Latin Square design and each rat received two sequential blocks of dose series. Thus an individual experienced each dose condition once within each block and the data were averaged across the two determinations of each dose for analysis. Sessions ended after 3 hours or the observation of an inter-infusion interval of 1 hour.
Telemetry procedure
Rats were assessed for 30 min prior to injection to ensure stable temperature and activity baseline, subsequent to any effects of handling or of moving to the procedure room. Rats were evaluated in a normal housing cage, with bedding material, placed on top of the telemetry receiver plate. Seven treatment conditions (Veh; 1, 5.6, 10 mg/kg of alpha-PVP and MDPV) were counterbalanced by a Latin Square design. Drugs were injected i.p. (1.0 ml/kg volume) with a 3-4 day interval between sessions. The procedure room was at 21°C (±1°C) for telemetry studies and all rats were assessed in single-housing conditions. The data (activity rate, core body temperature in degrees Celsius) were recorded at 5 min intervals and were represented as 15 min averages for analysis and presentation.
Data Analysis
Data were analyzed with repeated-measures Analysis of Variance (rmANOVA) with dose, time from injection, treatment condition (for non-contingent drug administration) and session number as within-subjects factors and drug identity as a between-subjects factor as appropriate. Any significant rmANOVA main effects were followed with post-hoc analysis using Tukey (within-subjects effects) or Sidak (between groups effects) correction for all possible comparisons. In the self-administration studies the primary dependent variables were the number of infusions obtained and the drug-associated lever discrimination ratio (drug-associated lever presses / all lever presses). Priming infusions were very uncommon (For MDPV rats priming included: 1 individual on 10 acquisition sessions, 2 individuals on 2 sessions and 3 individuals on 1 session and 4 individuals on 1 session- of these a single individual was primed on 12 sessions. For alpha-PVP rats priming included: 1 individual on 5 sessions, 2 individuals on 3 sessions- of these a single individual was primed on 5 sessions) thus these data were not analyzed. The total number of drug-associated lever responses were analyzed in the progressive-ratio procedure since breakpoints and infusions can violate the assumptions of ANOVA. A Kaplan-Meier survival analysis was used to compare acquisition of alpha-PVP and MDPV under the 0.05 mg/kg/inf dose (non-acquiring rats right censored at 20 sessions; group differences determined by Mantel-Cox Logrank test). The posthoc tests of the temperature and activity data included all possible comparisons but the reporting of results focuses on logical comparisons of interpretive interest as in prior studies of the effects of THC, MDMA, methamphetamine, MDPV and mephedrone (Aarde et al. 2013f; Miller et al. 2013a; Miller et al. 2013f; Taffe et al. 2015; Wright et al. 2012) in rats as well as studies of MDMA, methamphetamine and THC in monkeys (Crean et al. 2007; Crean et al. 2006; Taffe 2011; 2012; Taffe et al. 2006; Von Huben et al. 2007). The first set of comparisons evaluated change from the pre-injection baseline within treatment conditions and the second set compared drug effects with the vehicle condition at corresponding time-points post-injection.
Analyses were conducted using Prism 6 for Windows (v. 6.02; GraphPad Software, Inc, San Diego CA) or StatView (SAS Institute, Inc, Cary, NC). Graphs were generated with Excel (Microsoft, Redmond WA) and figures created in Canvas (v.12; ACD Systems of America, Inc, Seattle, WA)
RESULTS
Self-Administration
Experiment 1: Establishing a training dose of alpha-PVP
Acquisition
A group of rats (N=9) was trained to self-administer alpha-PVP with 0.1 mg/kg delivered for each infusion; seven rats’ catheters remained patent through the 20 session interval. As shown in Figure 2, mean infusions obtained were essentially stable for 20 sessions, however, the drug lever discrimination improved substantially across sessions. The one way ANOVA did not confirm any significant effect of training session for infusions obtained but there was a main effect of training session on lever discrimination [F(6,19) = 6.007; P < 0.0001]. Post-hoc comparisons confirmed that discrimination was higher in sessions 7 and 9-20 than 1 and 3 as well as higher in sessions 14-19 than 20. One rat responded for an unusually elevated number of infusions on four sessions (see the large deviations in mean and variance on sessions 7, 11, 12 and 17) which may represent isolated voluntary binge behavior; however, it cannot be excluded that this may have been essentially involuntary repetitive responding (stereotypy).
Figure 2. Acquisition of alpha-PVP (0.1 mg/kg/inf) self-administration.
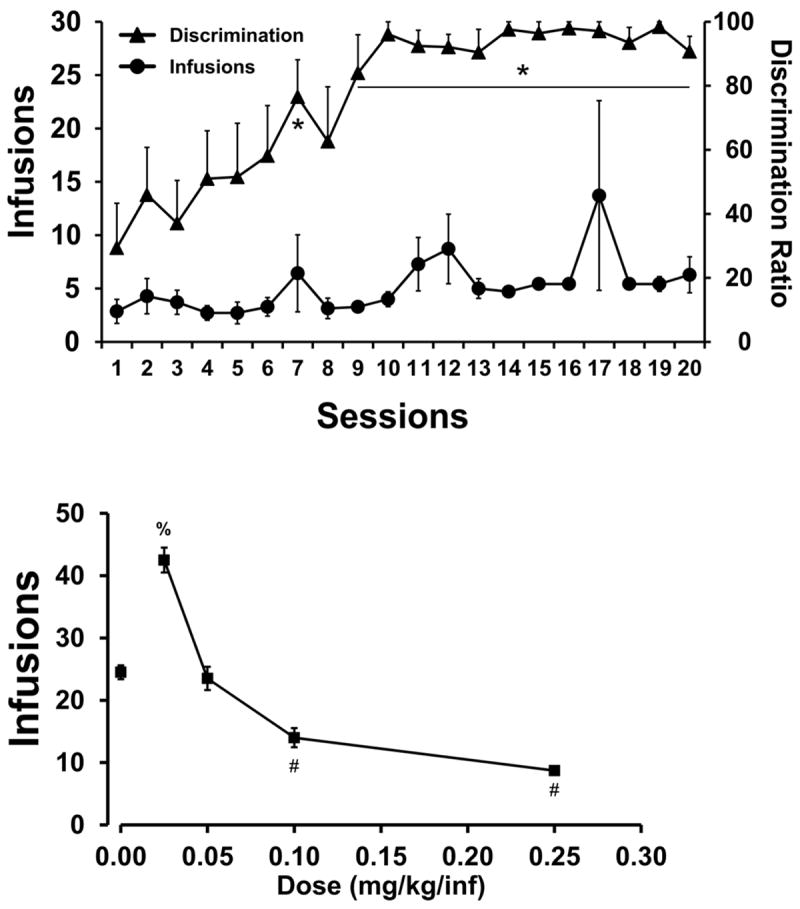
Mean (±SEM) infusions and drug-lever discrimination ratios during acquisition (upper panel; N=9) and infusions obtained during dose-substitution (lower panel; Vehicle, 0.025, 0.05, 0.1, 0.25 mg/kg/inf; N=5) under a fixed-ratio 1 response requirement. Significant differences in lever discrimination from the first and third acquisition session are indicated by * in the upper panel. In the lower panel, significant differences from vehicle, 0.025 and 0.05 are indicated by # and a significant difference from vehicle and 0.05 mg/kg/inf is indicated by %.
Fixed-ratio (FR) dose response
Five rats remained patent through the FR series. Analysis of the dose substitution study (Figure 2, lower panel) confirmed that there was a significant effect of dose on mean infusions obtained [F(1.854, 7.415) = 141.2; P < 0.0001] as well as lever discrimination [F(1.383, 5.532) = 16.50; P < 0.01]. The post-hoc test confirmed that infusions were significantly different between all dose pairs except vehicle vs 0.05 and 0.1 vs 0.25. A post-hoc test also confirmed that discrimination ratio was significantly lower in the vehicle condition (mean 66.1%; SEM 7.5) compared with each active dose (means 95.6-98.8), except 0.05 mg/kg/inf (mean 97.3; SEM 1.9).
Experiment 2: Comparison of alpha-PVP with MDPV
Comparison of our prior results for MDPV IVSA (Aarde et al. 2013f) with the alpha-PVP acquisition (fewer asymptotic infusions) and fixed-ratio dose-substitution data (nearly identical curves) in the first experiment recommended a 0.05 mg/kg/inf training dose of alpha-PVP for direct comparison with MDPV at the same training dose. Likewise, the finding that all rats in this preliminary study reached >= 3 infusions of alpha-PVP (0.1 mg/kg/inf) matches well with a recent MDPV IVSA study (Aarde et al. 2014) in which acquisition criteria of sustained intake >= 6 infusions at 0.05 mg/kg/inf were met.
Acquisition of Intravenous Self-Administration
The mean number of infusions of MDPV and alpha-PVP obtained, as well as the discrimination ratios (drug-paired lever responses /all lever responses), are depicted in Figure 3. Subject loss from the original groups included 2 due to loss of catheter patency (1 from each group) and 8 due to illness (6 MDPV, 2 alpha-PVP), thus affecting completed group sizes for MDPV (N=11) and alpha-PVP (N=15). The MDPV group was further reduced by 2 due to a failure to make a priori acquisition criteria, see below. Infusions of alpha-PVP and MDPV systematically increased over the 20 session acquisition period as reflected in a significant main effect of session [F(19, 418) = 9.710; P < 0.0001], and interaction [F(19, 418) = 3.250; P < 0.0001] but no effect of drug identity, in the ANOVA. Post-hoc analysis confirmed that significantly more infusions of MDPV were taken in session 12 compared with sessions 2, in sessions 9, 11-12 and 19 compared with session 3, in sessions 12 and 19 compared with session 4 and in session 19 compared with session 5. For alpha-PVP rats, significantly more infusions were obtained in sessions 11-20 relative to session 4, in sessions12-20 relative to session 1, 3, 5 and 6 and in sessions 13-20 relative to sessions 2 and 8. Intermittent differences from sessions 7, 9 and 10 were also confirmed within the block of sessions from 13-20 for the alpha-PVP group. No significant differences between groups were confirmed for any session.
Figure 3. Comparing the acquisition of alpha-PVP (0.05 mg/kg/inf) and MDPV (0.05 mg/kg/inf) self-administration.
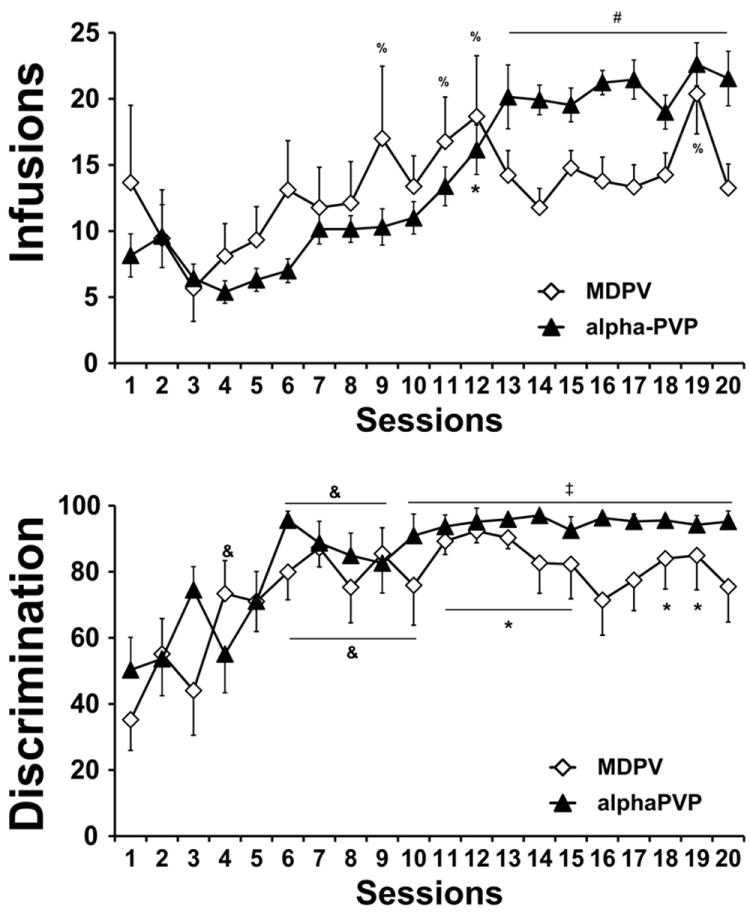
Mean (±SEM) infusions obtained per session (upper panel) and drug-paired lever discrimination ratios (lower panel) across sessions of acquisition of alpha-PVP (N=15) and MDPV (N=9) self-administration for those individuals who met acquisition criteria. Significant differences from the first 6 sessions is indicated by #; from the 1st, 2nd and 4th sessions by ‡; versus the 1st and 3rd sessions by *; compared with the 1st session by & and from the 3rd session by %. See Results for additional significant differences.
Drug-lever discrimination also significantly increased across the 20 day acquisition interval, as reflected in a significant main effect of session [F (19, 418) = 9.642; P < 0.0001], but no effect of drug identity or of the interaction, in the ANOVA. Post-hoc analysis confirmed that MDPV lever discrimination was significantly higher in sessions 4, 6-15 and 17-20 compared with the first session and in sessions 7, 9, 11-15 and 18-19 compared with the third session. Lever discrimination in the alpha-PVP group was also significantly improved compared with the first (sessions 6-20), second (sessions 6-20), fourth (sessions 6-7, 10-20) and fifth (session 18) sessions.
Two of the 11 MDPV rats that completed the acquisition interval failed to make a priori acquisition criteria of intake >=6 infusions (Aarde et al. 2014) for three sessions. To determine if there was any difference in the group rate of acquisition rats were defined as having acquired self-administration on the first of three sequential days in which they responded for 6 or more infusions; these data were subjected to a survival analysis with rats that failed to make criteria censored at 20 sessions (Figure 4). There was no significant difference between groups even when using the most liberal Logrank (Mantel-Cox) procedure [χ2 (1, N=24)=0.721; P = 0.396]. Exploration with an even more stringent criterion of sustaining >=6 infusions without any subsequent sessions below 6 infusions only reduced the terminal acquisition percentages to 87% of the alpha-PVP group and 73% of the MDPV group but again, there was no significant difference in group acquisition.
Figure 4. Percent of Subjects Acquiring self-administration.
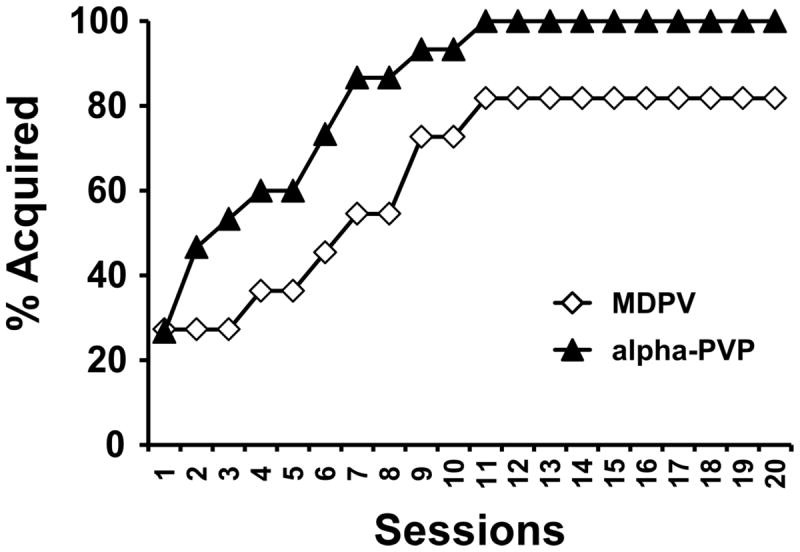
The cumulative percentage of rats that reached acquisition criteria are depicted as a function of session and the drug available (MDPV, N=11; alpha-PVP, N = 15) over the 20-session acquisition period. Rats were defined as having acquired self-administration on the first of three sequential sessions for which >= 6 infusions were acquired.
Progressive Ratio Dose Response
Two sequential rounds of the PR dose-substitution were completed in MDPV-trained (N=9) and alpha-PVP (N=12) trained rats (Figure 5). The analysis of drug-associated lever pressing confirmed a significant main effect of dose [F (6, 114) = 7.20; P < 0.0001] but not of drug identity nor any interaction between factors. For reference, the mean infusions obtained ranged from 3.7 (0.018 mg/kg/inf MDPV) to 7.5 (0.18 mg/kg/inf alphaPVP) in this study.
Figure 5. Progressive Ratio Dose Substitution.
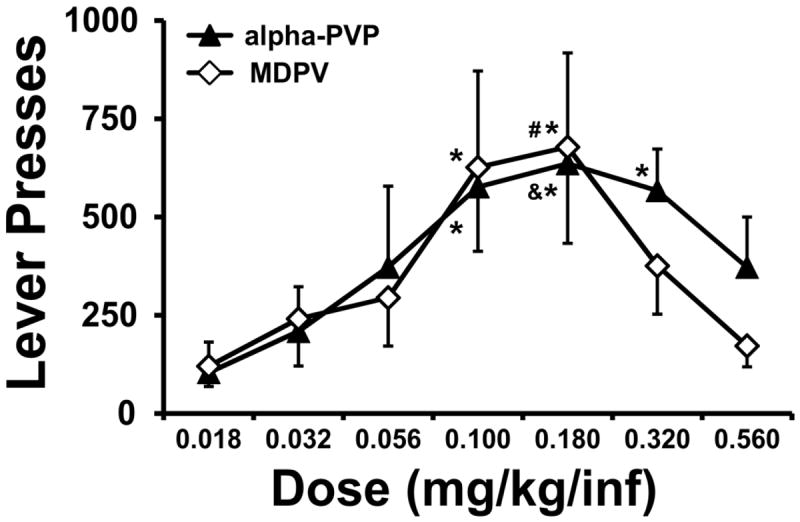
Mean (±SEM) drug-associated lever presses during the self-administration of alpha-PVP (N=12) or MDPV (N=8) under a progressive-ratio schedule of reinforcement. Significant differences from the 0.018 mg/kg/inf dose condition are signified by *, from the 0.032 dose by & and differences from the 0.56 dose by #.
Post-hoc comparisons confirmed that the alpha-PVP rats emitted significantly less lever pressing when the per-infusion dose was 0.018 compared with 0.1, 0.18 and 0.32 mg/kg/inf doses. They also pressed significantly less for 0.032 mg/kg/inf compared with the 0.018 mg/kg/inf sessions. The MDPV rats responded significantly less on the drug-associated lever when the dose was 0.018 mg/kg/inf compared with the 0.1 and 0.18 mg/kg/inf conditions; in addition lever pressing differed between 0.18 and 0.56 mg/kg/inf condition. All rats for both drugs reached a breakpoint before the end of their session for doses at or below 0.1 mg/kg, thus confirming that the selection of the PR schedule worked as intended.
Experiment 3: Physiological Effects
Locomotor Activity
Locomotor activity was altered by intraperitoneal administration of both alpha-PVP and MDPV as depicted in Figure 6. The initial analysis including all treatment conditions (vehicle, 1.0, 5.6, 10.0 mg/kg of each of alpha-PVP and MDPV) confirmed a significant main effect of drug treatment condition [F(6, 42) = 3.154; P = 0.0121], of time post-injection [F(12, 84) = 10.67; P < 0.0001] and of the interaction between factors [F(72, 504) = 1.664; P = 0.0010]. Post-hoc analysis confirmed that the 1.0 mg/kg alpha-PVP activity was significantly different after vehicle (30 min post-injection) compared with the 5.6 mg/kg (30, 75 min post-injection) and 10.0 mg/kg (60, 165 min post-injection) alpha-PVP conditions. Activity after 1.0 mg/kg alpha-PVP differed significantly from the higher MDPV doses at several time points but did not differ from activity following the 1.0 mg/kg MDPV dose at any time post-injection. The post-hoc test also confirmed that activity following the 1.0 mg/kg MDPV dose differed significantly from activity after the 5.6 and 10.0 mg/kg doses of MDPV 30-75 minutes post-injection. Activity after 5.6 mg/kg MDPV differed from both the 1.0 and 10.0 mg/kg conditions 105 minutes after administration.
Figure 6. Locomotor Activity.
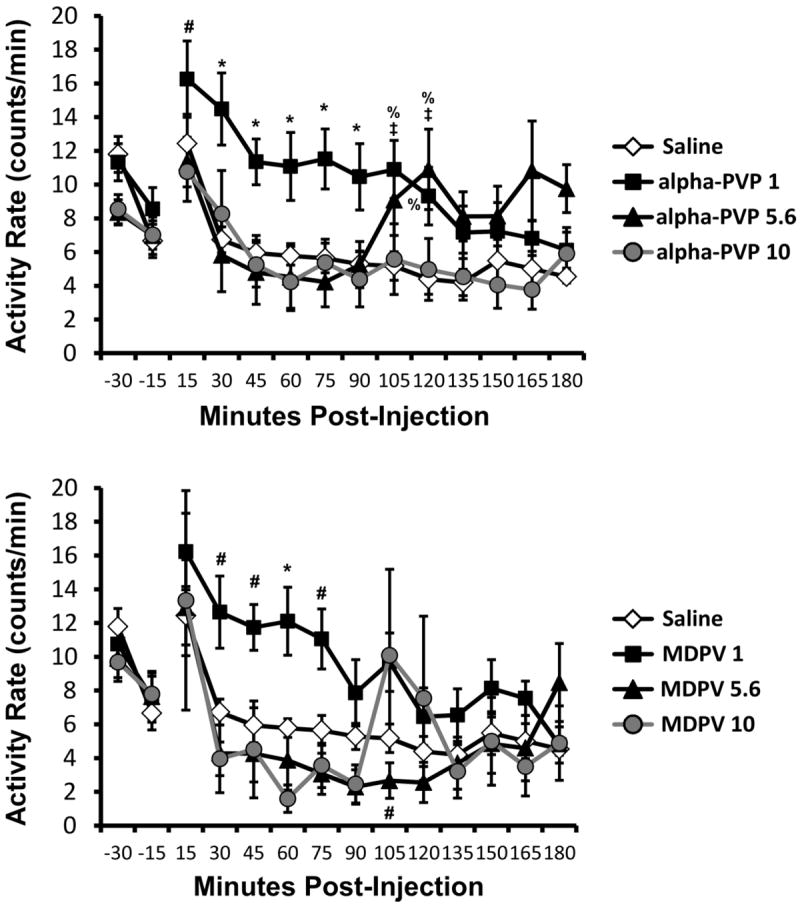
Mean (N=8; ±SEM) activity rate after alpha-PVP (upper panel) or MDPV (lower panel) was administered i.p. The vehicle condition is the same in both panels as all drug doses were randomized with a single vehicle condition. Significant differences from vehicle alone are indicated by %; from vehicle and the other two active doses by *; from the other two active doses by #; and from the 10 mg/kg dose is indicated by ‡
Body Temperature
Body temperature was altered by both MDPV and alpha-PVP as depicted in Figure 7. The analysis including all treatment conditions (vehicle, 1.0, 5.6, 10.0 mg/kg of each of alpha-PVP and MDPV) confirmed a significant main effect of drug treatment condition [F(6, 42) = 4.463; P = 0.0014], of time post-injection [F(12, 84) = 13.16; P < 0.0001] and of the interaction between factors [F(72, 504) = 1.756; P < 0.0005]. The post-hoc test confirmed that body temperature was lower compared with the vehicle condition and the pre-treatment baseline (-15 min) within condition after 5.6 mg/kg (45-60 min post-injection) and 10 mg/kg (45-180 min) alpha-PVP as well as after 5.6 mg/kg (45-120 min) and 10 mg/kg (30-105, 150-180 min) MDPV. In addition, body temperature was significantly reduced relative to the vehicle condition (but not the baseline after 5.6 mg/kg alpha-PVP (30, 75-105 min post-injection) and 5.6 mg/kg MDPV (30 min post-injection). Within a drug compound, temperature was significantly lower than the 1.0 mg/kg dose after 5.6 mg/kg (60, 180 min post-injection) and 10.0 mg/kg (45-180 min) alpha-PVP as well as after 5.6 mg/kg (30-90, 135-150 min) and 10.0 mg/kg (30-90, 150-180 min) MDPV. The only time point in which temperature differed between equal doses of MDPV and alpha-PVP was 120 min after the administration of 10 mg/kg. Finally, body temperature was significantly different from the pre-treatment baseline (-15 min) after 5.6 mg/kg (135-150 min post-injection) or 10.0 mg/kg (120-135 min) MDPV.
Figure 7. Thermoregulation.
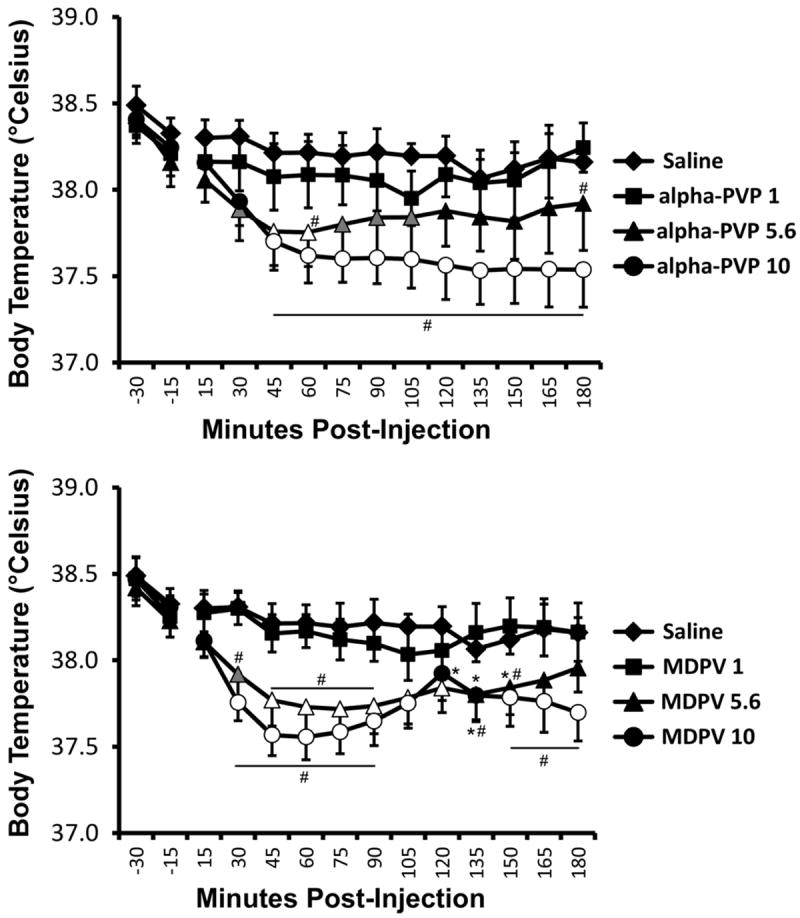
Mean (N=8; ±SEM) body temperature after alpha-PVP (upper panel) or MDPV (lower panel) was administered i.p. The vehicle condition is the same in both panels as all drug doses were randomized with a single vehicle condition. Shaded figures indicate a significant difference from vehicle at the respective timepoint. Open figures indicate a significant difference from vehicle at the respective timepoint and the baseline within treatment condition (15 min prior to injection). Significant differences from baseline (only) are indicated with * and differences from the 1.0 mg/kg dose (within compound) with #.
DISCUSSION
The results of this investigation show that alpha-PVP is similar to MDPV in both potency and efficacy as a reinforcer as assessed with intravenous self-administration in rats. The rates of group acquisition under equal training doses (0.05 mg/kg/inf) did not significantly differ in terms of a Kaplan-Meier analysis of sessions to criteria, although intake was higher and more stable for alpha-PVP than MDPV at the end of the acquisition period as confirmed by a significant interaction of sessions and training drug. Lever discrimination was also more consistently higher in the alpha-PVP trained group which might indicate a more consistently reinforcing effect. Together these outcomes may possibly indicate increased addiction liability for alpha-PVP over MDPV on population-wide basis. It should be noted that two groups trained on MDPV (0.05 mg/kg/inf) in another recent study (Aarde et al. 2014) from this laboratory generated mean intakes in the final sessions that were comparable to the present MDPV group. The similarity of the drugs was particularly apparent in the nearly identical dose-response functions generated in a progressive-ratio procedure following initial acquisition. Indirect comparison of the 0.1 mg/kg/inf trained alpha-PVP group’s responding under FR1 dose substitution with prior results for MDPV (Aarde et al. 2013f) also show this similarity, although a direct comparison would be required for a definitive conclusion. Likewise, a similar potency and efficacy of the two drugs was observed on locomotor stimulation and thermoregulatory disruption (Figures 6,7). Thus, the human abuse liability of these two compounds is predicted to be nearly indistinguishable, despite the presence of the 3,4-methylenedioxy motif which differentiates MDPV. This moiety is associated with significantly reduced potency and efficacy as a reinforcer when added to methamphetamine to produce 3,4-methylenedioxymethamphetamine (MDMA) as has been shown in prior studies (De La Garza et al. 2007; Feduccia et al. 2010; Kitamura et al. 2006; Schenk et al. 2007). The present findings show, therefore, that inferences about the role of this structural motif based solely on comparing reinforcer potency or efficacy of methamphetamine with 3,4-methylenedioxymethamphetamine (MDMA) may be misleading, particularly after regular use has been established. It also raises the possibility that relative DA/5HT effects of compounds that act (only) as transporter inhibitors are less important compared with the compounds which release monoamines. This shows that it is imperative to evaluate the abuse liability of novel cathinone derivatives directly with in vivo models since reliance on structure-activity relationships established with the amphetamines are poorly predictive. The amphetamines act as monoamine transporter substrates and monoamine releasers whereas MDPV and alpha-PVP appear to only function as transporter blockers (Baumann et al. 2013; Simmler et al. 2013), likely due in part to the presence of the pyrrolidine ring (Kolanos et al. 2013). It remains to be further determined if the unimportance of the methylenedioxy motif is associated with cathinones or with the subset of cathinones that act only as transporter inhibitors but not substrates or monoamine releasers.
The reported potency differences between MDPV and alpha-PVP on dopamine transporter inhibition and mouse locomotor assays in a prior study (Marusich et al. 2014) predicted that MDPV would be slightly more potent as a reinforcer, and yet no such effect was found. Indeed the most striking feature of the PR study was the similarity of the curves. This observation may reflect the (low) affinity for the serotonin transporter (SERT) found for MDPV contrasted with essentially no SERT affinity for alpha-PVP (Baumann et al. 2013; Marusich et al. 2014) given that DAT/SERT inhibition ratio is related to potency in intravenous self-administration (Roberts et al. 1999; Wee et al. 2005). Similarly, it is that case that rat self-administration of MDMA is marked by inter-subject inconsistency (Dalley et al. 2007) with up to half of a sample failing to meet acquisition criteria (Colussi-Mas et al. 2010; Oakly et al. 2014; Schenk et al. 2007); this is one reason discrimination ratio is used in this study as a dependent variable rather than a screening criterion. Therefore it may be that the modest SERT affinity of MDPV counteracts the increased DAT potency relative to alpha-PVP, thereby rendering the two compounds very similar in IVSA potency in vivo. Alternately it may be that there is no functional effect on SERT at self-administered doses and that therefore the small differences in DAT potency as determined in vitro for MDPV do not translate to discernible potency differences for-intravenous self-administration.
The locomotor stimulant effects of alpha-PVP were also very similar to those of MDPV, particularly in the finding that the peak locomotor stimulant dose was 1.0 mg/kg and effects lasted about 2 hours after injection and the lack of statistical differences between drugs at identical doses. The MDPV results were themselves roughly consistent with those previously reported for rats (Aarde et al. 2013f; Huang et al. 2012) in terms of duration of action and dose-relationships. In contrast, peak locomotor stimulant doses in mouse have been reported at 10-30 mg/kg, i.p., with effects lasting up to 6 hours post-administration (Fantegrossi et al. 2013; Gatch et al. 2013; Marusich et al. 2012). It was also notable that the 5.6 mg/kg dose of alpha-PVP engendered increased activity in a sustained manner after about 105 min post-injection. This multiphasic pattern is similar to the effects of higher doses of methamphetamine in inducing initial stereotyped movement (and low locomotion) which is replaced after a few hours with increased locomotion as drug levels subside (Segal and Kuczenski 1997). This pattern is similar to one previously reported for MDPV using a wheel-running assay, however, in that case the suppression/rebound was observed after 1.0 mg/kg MDPV, but not 5.6 mg/kg, within a one hour observation interval (Huang et al. 2012). Qualitatively there was some indication that the higher two doses of MDPV may have suppressed activity below that observed after vehicle injection. Combining this finding with the rebound of activity 2 hours after injection of 5.6 mg/kg alpha-PVP may indicate slightly increased potency of MDPV in terms of inducing stereotyped behavior (which was not directly measured in this study).
As noted above, MDPV has been shown to elevate body temperature in some cases of human medical emergency and/or death (Borek and Holstege 2012; Froberg et al. 2014; Kesha et al. 2013). In this study, a modest, but consistent, alteration in body temperature (~0.75°C) was observed after either MDPV or alpha-PVP; this is similar to the magnitude of change produced by methamphetamine, 4-methylmethcathinone (mephedrone) or MDPV in our prior studies (Aarde et al. 2013f; Miller et al. 2013a; Miller et al. 2013f; Wright et al. 2012) and a finding for MDPV in mice (Fantegrossi et al. 2013). The effects were dose-dependent and lasted up to 3 hours after dosing. The temperature change in the hypothermic direction was what would be predicted for rats evaluated under the 21°C ambient temperature condition of this study based on prior findings for MDMA (Malberg and Seiden 1998), methamphetamine (Gilpin et al. 2011; Miller et al. 2013f; Myles et al. 2008) and mephedrone (Aarde et al. 2013a; Miller et al. 2013a; Wright et al. 2012) as well as the non-substrate, transporter inhibitor cocaine (Cox and Lee 1979) and dopamine direct agonist apomophine (Brown et al. 2007). Differences with prior reports of hyperthermic effects of MDPV in rats are likely due to ambient temperature, strain and especially light cycle differences (King et al. 2014; Kiyatkin et al. 2015).
There has been a great deal of confusion in the popular media and lay public resulting from grouping a diverse array of new and emerging substituted cathinone drugs under the single label “bath salts”. The scientific literature has provided some initial clues as to distinctions between individual entities in a handful of initial reports on the pharmacological (Baumann et al. 2012; Baumann et al. 2013; Simmler et al. 2013; Simmler et al. 2014) and even locomotor / subjective properties of these diverse stimulants. So far only limited self-administration data are available for mephedrone (Aarde et al. 2013a; Hadlock et al. 2011; Motbey et al. 2013), MDPV (Aarde et al. 2013f; Watterson et al. 2014b), methylone (Watterson et al. 2012) and now alpha-PVP. These data have shown that inferences about reinforcer efficacy and abuse liability based on molecular structure alone are not sufficient. Some entities such as MDPV and alpha-PVP are restricted monoamine transporter inhibitors, not monoamine releasers like many amphetamine and cathinones (Marusich et al. 2014; Simmler et al. 2013; Simmler et al. 2014) and have been shown to be highly potent and efficacious reinforcers (Aarde et al. 2013f; Watterson et al. 2014b). Other entities such as mephedrone have been demonstrated to have unexpectedly high abuse liability in rodent self-administration assays (Aarde et al. 2013a; Hadlock et al. 2011), compared with what would be expected based on the MDMA-like neurochemical response in the nucleus accumbens (Baumann et al. 2012; Kehr et al. 2011; Wright et al. 2012); limited data for methylone are mixed (Creehan et al. 2015; Watterson et al. 2012). As we show here in the direct comparison of alpha-PVP with MDPV, the 3,4-methylenedioxy motif does not strongly predict reduced reinforcer efficacy. In conclusion, this study predicts that the human abuse liability of alpha-PVP will be significant, and similar to that of MDPV.
Acknowledgments
This work was funded by support from the United States Public Health Service National Institutes of Health (R01 DA024105) which had no direct input on the design, conduct, analysis or publication of the findings. This is manuscript #28012 from The Scripps Research Institute.
Footnotes
Financial Disclosures
The authors report no financial conflicts that are relevant to the conduct of this study.
References
- Aarde SM, Angrish D, Barlow DJ, Wright MJ, Jr, Vandewater SA, Creehan KM, Houseknecht KL, Dickerson TJ, Taffe MA. Mephedrone (4-methylmethcathinone) supports intravenous self-administration in Sprague-Dawley and Wistar rats. Addict Biol. 2013a;18:786–99. doi: 10.1111/adb.12038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aarde SM, Huang PK, Creehan KM, Dickerson TJ, Taffe MA. The novel recreational drug 3,4-methylenedioxypyrovalerone (MDPV) is a potent psychomotor stimulant: self-administration and locomotor activity in rats. Neuropharmacology. 2013f;71:130–40. doi: 10.1016/j.neuropharm.2013.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aarde SM, Huang PK, Dickerson TJ, Taffe MA. Binge-like acquisition of 3,4-methylenedioxypyrovalerone (MDPV) self-administration and wheel activity in rats. Psychopharmacology (Berl) 2014 doi: 10.1007/s00213-014-3819-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann MH, Ayestas MA, Jr, Partilla JS, Sink JR, Shulgin AT, Daley PF, Brandt SD, Rothman RB, Ruoho AE, Cozzi NV. The designer methcathinone analogs, mephedrone and methylone, are substrates for monoamine transporters in brain tissue. Neuropsychopharmacology. 2012;37:1192–203. doi: 10.1038/npp.2011.304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann MH, Partilla JS, Lehner KR, Thorndike EB, Hoffman AF, Holy M, Rothman RB, Goldberg SR, Lupica CR, Sitte HH, Brandt SD, Tella SR, Cozzi NV, Schindler CW. Powerful cocaine-like actions of 3,4-Methylenedioxypyrovalerone (MDPV), a principal constituent of psychoactive ‘bath salts’ products. Neuropsychopharmacology. 2013;38:552–62. doi: 10.1038/npp.2012.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borek HA, Holstege CP. Hyperthermia and Multiorgan Failure After Abuse of “Bath Salts” Containing 3,4-Methylenedioxypyrovalerone. Ann Emerg Med. 2012 doi: 10.1016/j.annemergmed.2012.01.005. [DOI] [PubMed] [Google Scholar]
- Brown PL, Bae D, Kiyatkin EA. Relationships between locomotor activation and alterations in brain temperature during selective blockade and stimulation of dopamine transmission. Neuroscience. 2007;145:335–43. doi: 10.1016/j.neuroscience.2006.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colussi-Mas J, Wise RJ, Howard A, Schenk S. Drug seeking in response to a priming injection of MDMA in rats: relationship to initial sensitivity to self-administered MDMA and dorsal striatal dopamine. Int J Neuropsychopharmacol. 2010:1–13. doi: 10.1017/S1461145710000283. [DOI] [PubMed] [Google Scholar]
- Cox B, Lee TF. Evidence for an endogenous dopamine-mediated hypothermia in the rat. Br J Pharmacol. 1979;67:605–10. doi: 10.1111/j.1476-5381.1979.tb08707.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crean RD, Davis SA, Taffe MA. Oral administration of (+/-)3,4-methylenedioxymethamphetamine and (+)methamphetamine alters temperature and activity in rhesus macaques. Pharmacol Biochem Behav. 2007 doi: 10.1016/j.pbb.2007.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crean RD, Davis SA, Von Huben SN, Lay CC, Katner SN, Taffe MA. Effects of (+/-)3,4-methylenedioxymethamphetamine, (+/-)3,4-methylenedioxyamphetamine and methamphetamine on temperature and activity in rhesus macaques. Neuroscience. 2006;142:515–25. doi: 10.1016/j.neuroscience.2006.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creehan KM, Vandewater SA, Taffe MA. Intravenous self-administration of mephedrone, methylone and MDMA in female rats. Neuropharmacology. 2015 doi: 10.1016/j.neuropharm.2015.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalley JW, Laane K, Theobald DE, Pena Y, Bruce CC, Huszar AC, Wojcieszek M, Everitt BJ, Robbins TW. Enduring deficits in sustained visual attention during withdrawal of intravenous methylenedioxymethamphetamine self-administration in rats: results from a comparative study with d-amphetamine and methamphetamine. Neuropsychopharmacology. 2007;32:1195–206. doi: 10.1038/sj.npp.1301220. [DOI] [PubMed] [Google Scholar]
- De La Garza R, 2nd, Fabrizio KR, Gupta A. Relevance of rodent models of intravenous MDMA self-administration to human MDMA consumption patterns. Psychopharmacology (Berl) 2007;189:425–34. doi: 10.1007/s00213-005-0255-5. [DOI] [PubMed] [Google Scholar]
- DEA. Schedules of controlled substances: temporary placement of three synthetic cathinones in Schedule I. Final Order. Fed Regist. 2011;76:65371–5. [PubMed] [Google Scholar]
- Drug Enforcement Administration DoJ. Schedules of controlled substances: temporary placement of 10 synthetic cathinones into Schedule I. Final order. Fed Regist. 2014;79:12938–43. [PubMed] [Google Scholar]
- Fantegrossi WE, Gannon BM, Zimmerman SM, Rice KC. In vivo effects of abused ‘bath salt’ constituent 3,4-methylenedioxypyrovalerone (MDPV) in mice: drug discrimination, thermoregulation, and locomotor activity. Neuropsychopharmacology. 2013;38:563–73. doi: 10.1038/npp.2012.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feduccia AA, Kongovi N, Duvauchelle CL. Heat increases MDMA-enhanced NAcc 5-HT and body temperature, but not MDMA self-administration. Eur Neuropsychopharmacol. 2010 doi: 10.1016/j.euroneuro.2010.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Froberg BA, Levine M, Beuhler MC, Judge BS, Moore PW, Engebretsen KM, McKeown NJ, Rosenbaum CD, Young AC, Rusyniak DE On behalf of the ATIC. Acute Methylenedioxypyrovalerone Toxicity. J Med Toxicol. 2014 doi: 10.1007/s13181-014-0446-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatch MB, Taylor CM, Forster MJ. Locomotor stimulant and discriminative stimulus effects of ‘bath salt’ cathinones. Behav Pharmacol. 2013 doi: 10.1097/FBP.0b013e328364166d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilpin NW, Wright MJ, Jr, Dickinson G, Vandewater SA, Price JU, Taffe MA. Influences of activity wheel access on the body temperature response to MDMA and methamphetamine. Pharmacol Biochem Behav. 2011;99:295–300. doi: 10.1016/j.pbb.2011.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadlock GC, Webb KM, McFadden LM, Chu PW, Ellis JD, Allen SC, Andrenyak DM, Vieira-Brock PL, German CL, Conrad KM, Hoonakker AJ, Gibb JW, Wilkins DG, Hanson GR, Fleckenstein AE. 4-Methylmethcathinone (mephedrone): neuropharmacological effects of a designer stimulant of abuse. The Journal of Pharmacology and Experimental Therapeutics. 2011;339:530–6. doi: 10.1124/jpet.111.184119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodos W. Progressive ratio as a measure of reward strength. Science (New York, N Y) 1961;134:943–944. doi: 10.1126/science.134.3483.943. [DOI] [PubMed] [Google Scholar]
- Huang PK, Aarde SM, Angrish D, Houseknecht KL, Dickerson TJ, Taffe MA. Contrasting effects of d-methamphetamine, 3,4-methylenedioxymethamphetamine, 3,4-methylenedioxypyrovalerone, and 4-methylmethcathinone on wheel activity in rats. Drug Alcohol Depend. 2012;126:168–75. doi: 10.1016/j.drugalcdep.2012.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaizaki A, Tanaka S, Numazawa S. New recreational drug 1-phenyl-2-(1-pyrrolidinyl)-1-pentanone (alpha-PVP) activates central nervous system via dopaminergic neuron. J Toxicol Sci. 2014;39:1–6. doi: 10.2131/jts.39.1. [DOI] [PubMed] [Google Scholar]
- Kehr J, Ichinose F, Yoshitake S, Goiny M, Sievertsson T, Nyberg F, Yoshitake T. Mephedrone, compared with MDMA (ecstasy) and amphetamine, rapidly increases both dopamine and 5-HT levels in nucleus accumbens of awake rats. Br J Pharmacol. 2011;164:1949–58. doi: 10.1111/j.1476-5381.2011.01499.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kesha K, Boggs CL, Ripple MG, Allan CH, Levine B, Jufer-Phipps R, Doyon S, Chi P, Fowler DR. Methylenedioxypyrovalerone (“bath salts”), related death: case report and review of the literature. J Forensic Sci. 2013;58:1654–9. doi: 10.1111/1556-4029.12202. [DOI] [PubMed] [Google Scholar]
- King HE, Wetzell B, Rice KC, Riley AL. 3,4-Methylenedioxypyrovalerone (MDPV)-induced conditioned taste avoidance in the F344/N and LEW rat strains. Pharmacol Biochem Behav. 2014;126:163–9. doi: 10.1016/j.pbb.2014.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitamura O, Wee S, Specio SE, Koob GF, Pulvirenti L. Escalation of methamphetamine self-administration in rats: a dose-effect function. Psychopharmacology (Berl) 2006;186:48–53. doi: 10.1007/s00213-006-0353-z. [DOI] [PubMed] [Google Scholar]
- Kiyatkin EA, Kim AH, Wakabayashi KT, Baumann MH, Shaham Y. Effects of Social Interaction and Warm Ambient Temperature on Brain Hyperthermia Induced by the Designer Drugs Methylone and MDPV. Neuropsychopharmacology. 2015;40:436–45. doi: 10.1038/npp.2014.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolanos R, Solis E, Jr, Sakloth F, De Felice LJ, Glennon RA. “Deconstruction” of the abused synthetic cathinone methylenedioxypyrovalerone (MDPV) and an examination of effects at the human dopamine transporter. ACS Chem Neurosci. 2013;4:1524–9. doi: 10.1021/cn4001236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malberg JE, Seiden LS. Small changes in ambient temperature cause large changes in 3,4-methylenedioxymethamphetamine (MDMA)-induced serotonin neurotoxicity and core body temperature in the rat. J Neurosci. 1998;18:5086–94. doi: 10.1523/JNEUROSCI.18-13-05086.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marusich JA, Antonazzo KR, Wiley JL, Blough BE, Partilla JS, Baumann MH. Pharmacology of novel synthetic stimulants structurally related to the “bath salts” constituent 3,4-methylenedioxypyrovalerone (MDPV) Neuropharmacology. 2014 doi: 10.1016/j.neuropharm.2014.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marusich JA, Grant KR, Blough BE, Wiley JL. Effects of synthetic cathinones contained in “bath salts” on motor behavior and a functional observational battery in mice. Neurotoxicology. 2012;33:1305–1313. doi: 10.1016/j.neuro.2012.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller ML, Creehan KM, Angrish D, Barlow DJ, Houseknecht KL, Dickerson TJ, Taffe MA. Changes in ambient temperature differentially alter the thermoregulatory, cardiac and locomotor stimulant effects of 4-methylmethcathinone (mephedrone) Drug Alcohol Depend. 2013a;127:248–53. doi: 10.1016/j.drugalcdep.2012.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller ML, Moreno AY, Aarde SM, Creehan KM, Vandewater SA, Vaillancourt BD, Wright MJ, Jr, Janda KD, Taffe MA. A methamphetamine vaccine attenuates methamphetamine-induced disruptions in thermoregulation and activity in rats. Biol Psychiatry. 2013f;73:721–8. doi: 10.1016/j.biopsych.2012.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motbey CP, Clemens KJ, Apetz N, Winstock AR, Ramsey J, Li KM, Wyatt N, Callaghan PD, Bowen MT, Cornish JL, McGregor IS. High levels of intravenous mephedrone (4-methylmethcathinone) self-administration in rats: neural consequences and comparison with methamphetamine. J Psychopharmacol. 2013;27:823–36. doi: 10.1177/0269881113490325. [DOI] [PubMed] [Google Scholar]
- Myles BJ, Jarrett LA, Broom SL, Speaker HA, Sabol KE. The effects of methamphetamine on core body temperature in the rat--part 1: chronic treatment and ambient temperature. Psychopharmacology (Berl) 2008;198:301–11. doi: 10.1007/s00213-007-1061-z. [DOI] [PubMed] [Google Scholar]
- Nagai H, Saka K, Nakajima M, Maeda H, Kuroda R, Igarashi A, Tsujimura-Ito T, Nara A, Komori M, Yoshida K. Sudden death after sustained restraint following self-administration of the designer drug alpha-pyrrolidinovalerophenone. Int J Cardiol. 2014;172:263–5. doi: 10.1016/j.ijcard.2013.12.262. [DOI] [PubMed] [Google Scholar]
- Oakly AC, Brox BW, Schenk S, Ellenbroek BA. A genetic deletion of the serotonin transporter greatly enhances the reinforcing properties of MDMA in rats. Mol Psychiatry. 2014;19:534–5. doi: 10.1038/mp.2013.75. [DOI] [PubMed] [Google Scholar]
- Richardson NR, Roberts DC. Progressive ratio schedules in drug self-administration studies in rats: a method to evaluate reinforcing efficacy. Journal of Neuroscience Methods. 1996;66:1–11. doi: 10.1016/0165-0270(95)00153-0. [DOI] [PubMed] [Google Scholar]
- Roberts DC, Phelan R, Hodges LM, Hodges MM, Bennett B, Childers S, Davies H. Self-administration of cocaine analogs by rats. Psychopharmacology (Berl) 1999;144:389–97. doi: 10.1007/s002130051022. [DOI] [PubMed] [Google Scholar]
- Schenk S, Hely L, Lake B, Daniela E, Gittings D, Mash DC. MDMA self-administration in rats: acquisition, progressive ratio responding and serotonin transporter binding. Eur J Neurosci. 2007;26:3229–36. doi: 10.1111/j.1460-9568.2007.05932.x. [DOI] [PubMed] [Google Scholar]
- Segal DS, Kuczenski R. Repeated binge exposures to amphetamine and methamphetamine: behavioral and neurochemical characterization. J Pharmacol Exp Ther. 1997;282:561–73. [PubMed] [Google Scholar]
- Segal DS, Mandell AJ. Long-term administration of d-amphetamine: progressive augmentation of motor activity and stereotypy. Pharmacol Biochem Behav. 1974;2:249–55. doi: 10.1016/0091-3057(74)90060-4. [DOI] [PubMed] [Google Scholar]
- Simmler L, Buser T, Donzelli M, Schramm Y, Dieu LH, Huwyler J, Chaboz S, Hoener M, Liechti M. Pharmacological characterization of designer cathinones in vitro. British Journal of Pharmacology. 2013;168:458–70. doi: 10.1111/j.1476-5381.2012.02145.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmler LD, Rickli A, Hoener MC, Liechti ME. Monoamine transporter and receptor interaction profiles of a new series of designer cathinones. Neuropharmacology. 2014;79:152–60. doi: 10.1016/j.neuropharm.2013.11.008. [DOI] [PubMed] [Google Scholar]
- Taffe MA. A comparison of intraperitoneal and subcutaneous temperature in freely moving rhesus macaques. Physiol Behav. 2011;103:440–4. doi: 10.1016/j.physbeh.2011.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taffe MA. Delta9-Tetrahydrocannabinol attenuates MDMA-induced hyperthermia in rhesus monkeys. Neuroscience. 2012;201:125–33. doi: 10.1016/j.neuroscience.2011.11.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taffe MA, Creehan KM, Vandewater SA. Cannabidiol fails to reverse hypothermia or locomotor suppression induced by Delta -tetrahydrocannabinol in Sprague-Dawley rats. Br J Pharmacol. 2015;172:1783–1791. doi: 10.1111/bph.13024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taffe MA, Lay CC, Von Huben SN, Davis SA, Crean RD, Katner SN. Hyperthermia induced by 3,4-methylenedioxymethamphetamine in unrestrained rhesus monkeys. Drug Alcohol Depend. 2006;82:276–81. doi: 10.1016/j.drugalcdep.2005.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Von Huben SN, Lay CC, Crean RD, Davis SA, Katner SN, Taffe MA. Impact of ambient temperature on hyperthermia induced by (+/-)3,4-methylenedioxymethamphetamine in rhesus macaques. Neuropsychopharmacology. 2007;32:673–81. doi: 10.1038/sj.npp.1301078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watterson LR, Burrows BT, Hernandez RD, Moore KN, Grabenauer M, Marusich JA, Olive MF. Effects of alpha-Pyrrolidinopentiophenone and 4-Methyl-N-Ethylcathinone, Two Synthetic Cathinones Commonly Found in Second-Generation “Bath Salts,” on Intracranial Self-Stimulation Thresholds in Rats. Int J Neuropsychopharmacol. 2014a;18 doi: 10.1093/ijnp/pyu014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watterson LR, Hood L, Sewalia K, Tomek SE, Yahn S, Johnson CT, Wegner S, Blough BE, Marusich JA, Olive MF. The Reinforcing and Rewarding Effects of Methylone, a Synthetic Cathinone Commonly Found in “Bath Salts”. J Addict Res Ther. 2012;(S9):1–8. doi: 10.4172/2155-6105.S9-002. 002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watterson LR, Kufahl PR, Nemirovsky NE, Sewalia K, Grabenauer M, Thomas BF, Marusich JA, Wegner S, Olive MF. Potent rewarding and reinforcing effects of the synthetic cathinone 3,4-methylenedioxypyrovalerone (MDPV) Addict Biol. 2014b;19:165–74. doi: 10.1111/j.1369-1600.2012.00474.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wee S, Anderson KG, Baumann MH, Rothman RB, Blough BE, Woolverton WL. Relationship between the serotonergic activity and reinforcing effects of a series of amphetamine analogs. J Pharmacol Exp Ther. 2005;313:848–54. doi: 10.1124/jpet.104.080101. [DOI] [PubMed] [Google Scholar]
- Wee S, Carroll FI, Woolverton WL. A reduced rate of in vivo dopamine transporter binding is associated with lower relative reinforcing efficacy of stimulants. Neuropsychopharmacology. 2006;31:351–62. doi: 10.1038/sj.npp.1300795. [DOI] [PubMed] [Google Scholar]
- Wright MJ, Jr, Angrish D, Aarde SM, Barlow DJ, Buczynski MW, Creehan KM, Vandewater SA, Parsons LH, Houseknecht KL, Dickerson TJ, Taffe MA. Effect of ambient temperature on the thermoregulatory and locomotor stimulant effects of 4-methylmethcathinone in Wistar and Sprague-Dawley rats. PLoS One. 2012;7:e44652. doi: 10.1371/journal.pone.0044652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wurita A, Hasegawa K, Minakata K, Gonmori K, Nozawa H, Yamagishi I, Suzuki O, Watanabe K. Postmortem distribution of alpha-pyrrolidinobutiophenone in body fluids and solid tissues of a human cadaver. Leg Med (Tokyo) 2014 doi: 10.1016/j.legalmed.2014.05.001. [DOI] [PubMed] [Google Scholar]


