Abstract
Background
This current study was undertaken to carefully assess the accuracy of routinely used laboratory tests in detecting excessive/recent alcohol use. We also determined the kinetics of these markers in subjects who underwent intensive alcohol rehabilitation program.
Methods
The study cohort consisted of 210 non-excessive drinkers, 272 excessive drinkers, and 76 with alcoholic cirrhosis. To determine the kinetics of these markers during alcohol abstinence, we followed 45 subjects with history of excessive alcohol use for 12-week during the intensive alcohol treatment program.
Results
%CDT provided the highest diagnostic performance (AUC 0.77) followed by GGT (AUC 0.68) to detect excessive drinkers. The percentage of excessive drinkers with AST:ALT > 2 was only 2%; whereas, 51% of subjects with alcoholic cirrhosis had AST:ALT > 2. In the multivariate analysis, the levels of GGT and %CDT were associated with the level of alcohol consumed during the past 30 days. The levels of GGT, MCV, and %CDT were significantly lower compared to those at baseline before alcohol rehabilitation whereas the AST, ALT, and AST:ALT ratio were unchanged. The percent reduction was ~ 2.7% (for MCV), 19% (for GGT), and 43% (for %CDT) at the end of 12-week follow up compared to the baseline.
Conclusions
%CDT, are useful markers to screen for excessive alcohol use and for follow up of abstinence. Most subjects with excessive alcohol use do not high AST:ALT ratio. Rather, the AST:ALT > 2 is suggestive of alcoholic cirrhosis. The performance of the %CDT to screen for heavy alcohol use is still not ideal. Further research to identify the non-invasive marker(s) (i.e. using proteomic or metabolomics approach) should be considered.
Keywords: Liver enzymes, MCV, %CDT, Excessive alcohol use
INTRODUCTION
Screening for ongoing and excessive alcohol use is of importance especially in patients with underlying chronic liver diseases. Ongoing alcohol drinking can exacerbate hepatitis C infection and the underlying liver injury, thereby accelerating disease progression (Hajarizadeh, Grebely, and Dore 2013). It also has the adverse effect on the response rates to anti-viral treatment (Younossi et al. 2013). Assessment of ongoing alcohol use is essential in patients with alcoholic liver disease, in which no specific treatment exists and abstinence is the mainstay of therapy (Chayanupatkul and Liangpunsakul 2014). Excessive alcohol use may impose the detrimental effect in those with underlying non-alcoholic fatty liver disease (NAFLD); as it might increase levels of pro-inflammatory cytokines, such as tumor necrotic factor-alpha and monocyte chemotactic protein-1, which can worsen liver injury by causing inflammatory responses in the hepatocytes (Liangpunsakul and Chalasani 2012).
In general clinical practice, identification of patients with excessive alcohol use can lead to early behavioral interventions which will reduce the risk for medical, social, and psychological problems (Lee and Pham 2014). Taken together, the detection of excessive drinking and prompt intervention with counseling or referral to addiction specialist is crucial and should be part of the comprehensive care for patients in general practice.
Excessive drinking is defined as men who drink more than 4 standard drinks in a day (or more than 14 per week) and women who drink more than 3 standard drinks in a day (or more than 7 per week). Screening for excessive drinking can be obtained through the construct interview formats using questionnaires, such as AUDIT, CAGE, or reports from collateral family with direct interaction with patients(Knight et al. 2003). However, limitations of this approach are most apparent in patients with underlying liver disease where individuals have the tendency to minimize the magnitude of drinking behavior to mitigate personal ramifications (such as to prevent the denial for hepatitis C therapy or the eligibility for liver transplant evaluation(Freeman and Vrana 2010).
Besides questionnaires, several lab tests such as gamma-glutaryltransferase (GGT), aspartate aminotransferase (AST), alanine aminotransferase (ALT), mean corpuscular volume (MCV), and carbohydrate deficient transferrin (CDT) have been routinely used clinically to screen for excessive alcohol use (Allen and Anton 2003; Freeman and Vrana 2010; Bearer, Bailey, and Hoek 2010; Liangpunsakul et al. 2010). An elevated serum AST to serum ALT has been proposed as an indicator that alcohol has induced organ damage. When AST/ALT ratio is > 2, it is highly considered as highly suggestive of alcoholic liver cirrhosis (Nyblom et al. 2004). However, the role of AST:ALT > 2 to screen for excessive alcohol use, notably in those without advanced alcoholic liver disease is inconclusive. In fact, one study showed the poor diagnostic performance of this ratio to detect heavy alcohol use (Nyblom et al. 2004). Serum liver enzymes, surprisingly, have been used widely despite the fact that they can be abnormal secondary to underlying liver diseases such as hepatitis C or NAFLD. In this context, other non-hepatic enzyme markers such as CDT might be more accurate and useful (Bearer, Bailey, and Hoek 2010).
Previous studies have determined the sensitivity and specificity of these lab tests to stratify those with and without excessive alcohol use (Das, Dhanya, and Vasudevan 2008). However, none had reported the correlation of these markers with the amount of alcohol consumed. This current study was undertaken to carefully assess the accuracy of routinely used laboratory tests in detecting excessive and recent alcohol use in a large cohort of subjects with history of alcohol use disorder. Further, we also determined the kinetics of these markers once the patient’s alcohol consumption has stopped in the cohort of subjects who underwent intensive alcohol rehabilitation program. The results of this study is of importance as it will provide us the information on the usefulness of these commonly used markers to screen for recent excessive alcohol use and monitor for abstinence.
METHODS
STUDY COHORT
To determine the performance of commonly used lab tests and levels of alcohol consumption, we recruited 272 subjects with history of alcohol use disorder (AUD) who were admitted for alcohol rehabilitation at Fairbanks Drug and Alcohol Treatment Center (Indianapolis, IN). All subjects met the criteria for AUD (defined by the DSM IV criteria) and ‘excessive drinking’ as defined by NIH/NIAAA. They reported the last use of alcohol within 0–72 hrs before the enrollment. Two hundred and ten non-excessive drinkers were recruited from Richard L. Roudebush Veterans Administration Medical Center (RLR VAMC, Indianapolis, IN). These were the healthy subjects who attended the outpatient visit for routine health examination. We used multiple avenues to ensure that each subject did not have excessive alcohol consumption including medical record review, intensive face to face interview with research coordinator, screening using AUDIT-C and time line follow-back questionnaires. All were at least 21 years of age or older and be able to provide informed consent. Subjects were excluded if they had active and serious medical diseases (such as congestive heart failure, chronic obstructive pulmonary disease, cancer, uncontrolled diabetes, and chronic renal failure); past history of jaundice or signs of end stage liver disease (such as ascites, hepatic encephalopathy, or variceal bleeding) had history of chronic hepatitis B/C infection, had history of any systemic infection within 4 weeks prior to the study; or had history of recent major surgeries within the past 3 months. To further explore whether AST:ALT ratio is an indicative of excessive alcohol use or advanced alcoholic liver disease, we also included another cohort of subjects with known diagnosis of alcoholic liver disease (ALD, n=76) who attended the liver clinic or pre liver transplantation clinic at the Indiana University (Whitfield et al. 2015). All subjects with ALD had alcohol consumption averaging at least 80 g per day (for men) or 50 g per day (for women), for at least 10 years. These cases had evidence of cirrhosis as per clinical signs, radiographic imaging results (sonography, computed tomography, magnetic resonance imaging) compatible with cirrhosis and/or history of ascites, grade 2 or higher spontaneous hepatic encephalopathy and/or the presence of esophageal varices on upper gastrointestinal endoscopy, or biopsy-proven cirrhosis, with exclusion of hepatitis B or C, autoimmune liver disease, hemochromatosis, and Wilson’s disease (Whitfield et al. 2015).
To determine the kinetics of the routinely used markers after alcohol abstinence, we prospectively recruited another 45 subjects with history of excessive alcohol use who enrolled in a 12-week intensive alcohol treatment program at Fairbanks. All subjects were closely followed by the psychiatrist/addiction specialist to confirm the abstinence while enrolling in the program. Blood tests were drawn at baseline (before starting the program) and at week 1, 2, 3, 4, 6, 8, 10 and 12 (the end of treatment). All subjects reported the last use of alcohol within 0–72 hrs before the initiation of the program. All complied with the treatment and none relapsed to excessive alcohol use. The study design and protocol were approved by the Institutional Review Board at the Indiana University Purdue University Indianapolis (IUPUI), RLR VAMC Research and Development Program, and at Fairbanks Alcohol Rehabilitation Center. Written informed consent was obtained from each participant at the screening interview.
Data Collection and clinical evaluation
Control subjects and those with excessive alcohol use completed a self-administered questionnaire. Demographic data, smoking history, past medical history, and the Alcohol Use Disorders Identification Test (AUDIT-C) were collected. In this study, the Time Line Follow-Back (TLFB) questionnaire was used to determine the amount of alcohol consumption over the 30-day period before the study date. It was administered in person by trained study coordinators who reviewed the instructions with the subjects prior to administering the questionnaire. The TLFB offers a retrospective report of daily alcohol consumption over the past 30 days; drinks per drinking occasion, and pattern of drinking can be computed (Sobell et al. 1988; Sobell et al. 2003; Vakili et al. 2008). The levels of serum AST, ALT, GGT and MCV were analyzed at our Clinical Pathology laboratory. %CDT (with the reference normal range <2.5%) was performed at Quest Diagnostics.
Statistical analysis
Basic descriptive statistics, including mean, standard deviations (S.D), and frequencies (percentages) were used to characterize the study subjects for each of the cross-sectional study and the follow up cohort. Appropriate comparison tests including chi-square test, student t-test, and ANOVA were used for comparison between/among groups for categorical and continuous variables For the cross-sectional data set (n=210 for non-excessive drinkers and n=272 for excessive drinkers), the receiver operating characteristic (ROC) analysis was utilized to estimate the utility of commonly used serum markers in detecting excessive alcohol use. The area under the curve (AUC) derived from the ROC analysis for each marker was compared. The ROC-based optimized threshold was computed by finding the cutoff that maximized the sum of specificity and sensitivity. Sensitivity, specificity, positive predictive values (PPVs), and negative predictive values (NPVs) for the individual markers were calculated for marker-specific cutoff values. In addition, multivariate linear regression with each marker as the dependent variable and alcohol consumption as the predictor was used to determine the relationship between the concentrations of each marker and the amount of alcohol consumption in the past 30 days before the enrollment. The regression models were assessed for linearity and normality using the residual plots and no violation of model assumption was detected. For the analyses of AST:ALT, we divided the AST:ALT into 3 groups; AST:ALT >2, 1≤AST:ALT≤2, and AST:ALT<1. The percentages of subjects in controls, excessive drinkers, and those with ALD for each AST:ALT group were calculated and compared for the differences using chi-square test.
For the follow up cohort (n=45), the linear mixed-effect models with time as the main predictor, a random intercept for subject effect, were used to determine whether there were significant time trend in the serum concentration of the markers of interest during the 12-week follow up period and to determine the rate of changes for each marker, if any, over time. All analyses were conducted with SAS software version 9.3 (Cary, NC).
Results
Clinical characteristics of the study cohort
During the study period, 482 subjects were recruited (210 non-excessive drinkers and 272 excessive drinkers). Subject characteristics are summarized in Table 1. Excessive drinkers were older, had higher percentage of divorce/separation, and had lower BMI, when compared to those of non-excessive drinkers. Excessive drinkers had higher AUDIT scores (26.9 vs. 4.7, p<0.001), greater total standard drinks in the past 30 days (257 vs. 13 drinks, p<0.001), higher average drinks per drinking day (12.9 vs 2.5 drinks, p<0.001), and a higher number of drinking days in the past month (20 vs. 4.5 days, p<0.001), when compared to controls. They had significantly higher levels of serum AST, GGT, MCV and %CDT. We observed the higher concentrations of ALT in non-excessive drinkers, likely due to the higher BMI of subjects in this group, compared to excessive drinkers (50.7 vs. 36.5, p < 0.001). When we used the cut off reference for %CDT provided by the laboratory (>2.5% considered being abnormal), we found that 5.2% of controls and 44.2% of excessive drinkers had abnormal %CDT.
Table 1.
Baseline demographic and alcohol drinking characteristics of the study cohort
| Demographic and clinical characterstics | Non excessive drinkers/Controls (n = 210) | Excessive drinkers (n =272) | p-value |
|---|---|---|---|
|
| |||
| Age (years) | 32.5±8.6 | 39.7±11.8 | <.0001 |
|
| |||
| Male sex, n (%) | 180 (85.7%) | 182 (66.9%) | <.0001 |
|
| |||
| Race, White, n (%) | 168 (80%) | 220 (81%) | 0.22 |
|
| |||
| Marital status (%) | 0.001 | ||
| : Married | 54.3 | 35.3 | |
| : Divorced/separated | 18.1 | 28.3 | |
| : Never married | 19.1 | 27.9 | |
| : Others | 8.5 | 8.5 | |
|
| |||
| BMI (kg/m2) | 29.9±14.31 | 27.9±5.51 | 0.05 |
|
| |||
| AUDIT-C | 4.7±5.67 | 26.9±7.75 | <.0001 |
|
| |||
| Alcohol drinking patterns during the last 30 days from TLFB | |||
| : Total drinks | 13.3±15.3 | 257.7±176.5 | <.0001 |
| : Number of days drinking last 30 days | 4.5±5.3 | 20.1±6.2 | <.0001 |
| : Average drinks per drinking day | 2.5±2.9 | 12.9±7.5 | <.0001 |
| : Average drinks per day | 0.5±0.5 | 8.6±5.8 | <.0001 |
| : Greatest number of drinks in one day | 3.9±3.2 | 18.6±8.92 | <.0001 |
|
| |||
| Laboratory measures | |||
| : Serum AST (U/L) | 26.0±22.0 | 38.9±75.5 | 0.008 |
| : Serum ALT (U/L) | 50.7±1.8 | 36.5±1.9 | <.0001 |
| : Serum GGT (U/L) | 35.6±31.1 | 85.5±126.10 | <.0001 |
| : %Carbohydrate deficient transferrin (%CDT) | 1.9±3.7 | 2.6±1.2 | 0.023 |
| : Mean Corpusular volume (fL) | 89.5±4.3 | 93.4±20.1 | 0.002 |
The utility of the routinely used serum markers to detect excessive alcohol use
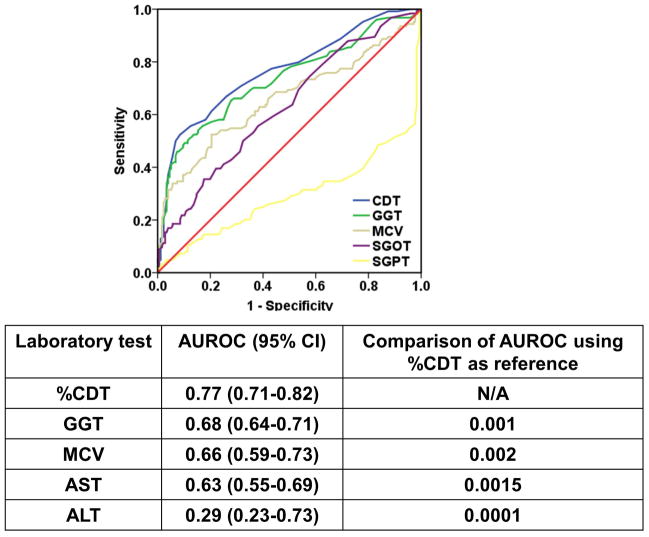
The ROC curves and AUC values for all measured biomarkers in stratifying the excessive drinking status are shown in Figure 1. %CDT provided the highest diagnostic performance to detect excessive alcohol use with an AUC 0.77, followed by GGT with an AUC of 0.68, MCV with an AUC of 0.66, AST with the AUC of 0.61, and ALT with the AUC of 0.29. The AUC for %CDT was significantly higher than that of GGT (p=0.001), MCV (p = 0.002), AST (p=0.0015), and ALT (p=0.0001). The results of application of these markers using the optimal cutoff in detection of excessive alcohol use are shown in Table 2.
FIGURE 1.
The ROC and Area under the curve with 95% confidence interval for all measured biomarkers in stratifying the excessive drinking status
Table 2.
Diagnostic performance of individual markers for identification of excessive Drinkers
| Biomarkers | Optimal cutoff | Sensitivity (%) | Specificity (%) | Positive predictive value (%) | Negative predictive value (%) |
|---|---|---|---|---|---|
| %CDT | 2.2 | 52.7 | 87.8 | 75.0 | 72.8 |
| GGT | 45.5 | 49.6 | 83.9 | 78.9 | 56.8 |
| MCV | 92.8 | 48.3 | 80.8 | 77.3 | 53.5 |
| AST | 26.8 | 43 | 68 | 64.0 | 47.2 |
| ALT | 26.9 | 4.4 | 50.7 | 6.33 | 41.3 |
Abbreviation: AST (aspartate aminotransferase), ALT (alanine aminotransferase), GGT (Gamma Glutaryltransferase), MCV (mean corpuscular volume), and %CDT (%carbohydrate deficient transferrin), AUROC: Area under the receiver operating characteristic curve for individual diagnostic serum markers
Association between the levels of serum markers and the amount of alcohol consumption in the last 30 days
We next determined the relationship between the levels of serum markers and the amount of alcohol consumption in the past 30 days (Figure 2). The results of the multivariate regression models and Person’s correlation are shown in Table 3. In the multivariate analysis, we found that GGT (r = 0.31, p <0.001) and %CDT (r = 0.15, = 0.04) were associated with the level of alcohol consumed during the past 30 days prior to the enrollment.
FIGURE 2.
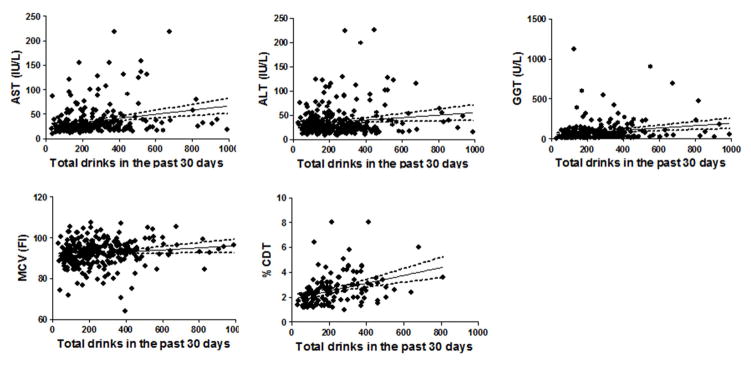
The linear regression analyses between the levels of serum markers and the amount of alcohol consumption in the past 30 days. The dark linear line representing the best-fit line from the linear regression analysis with its 95% confidence interval (dotted line)
Table 3.
Multivariate regression models and Pearson’s correlation coefficient of the association between the levels of serum markers and the amount of alcohol consumed in the past 30 days
| Biomarkers | Regression coefficient (95% CI) | Pearson’s correlation coefficient | P-value* |
|---|---|---|---|
| AST | 0.02 (−0.02−0.06) | 0.12 | 0.06 |
| ALT | −0.01 (−0.03−0.003) | −0.08 | 0.11 |
| GGT | 0.15 (0.10–0.21) | 0.31 | <.0001 |
| MCV | 0.008 (0.00007–0.02) | 0.10 | 0.05 |
| CDT | 0.003 (0.003–0.005) | 0.15 | 0.04 |
p-value for coefficients in the regression models. The model was adjusted for age, gender, race, and BMI
AST:ALT ratio in controls, excessive drinkers and subjects with alcoholic cirrhosis
The baseline characteristics of 76 subjects with alcoholic cirrhosis were shown in Table 4. These subjects had significant higher levels of AST and GGT, but lower levels of ALT, when compared to controls and excessive drinkers without liver diseases (p<0.001). The percentages of subjects in controls, excessive drinkers, and those with alcoholic cirrhosis stratified by the AST:ALT ratio were shown in Figure 3. Among those with excessive alcohol use, 46.3% had AST:ALT < 1 and 51.5% had the ratio between 1 and 2. Interestingly, the percentage of subjects with AST:ALT > 2 was only 2%. Most controls had the AST:ALT ratio < 1 (95%). However, 51% of subjects with alcoholic cirrhosis had AST:ALT > 2; which was statistically higher than that in controls and excessive drinkers (P<0.0001).
Table 4.
Baseline demographics and clinical characteristics of subjects with alcoholic cirrhosis (N=76)*
| Demographic and clinical characterstics | N=76 |
|---|---|
|
| |
| Age (yrs) | 49.4±9.2 |
|
| |
| Male sex, n (%) | 75 |
|
| |
| Race, White, n (%) | 91 |
|
| |
| MELD scores | 13.1±7.6 |
|
| |
| Child-Pugh Classification (%) | |
| A | 63 |
| B | 30 |
| C | 7 |
|
| |
| Laboratory measures | |
| : platelet counts (cells/mm3) | 140.2±72 |
| : albumin (g/dl) | 3.0±0.6 |
| : AST (U/L) | 48.9±33 |
| : ALT (U/L) | 27±17 |
| : GGT (U/L) | 156±84 |
FIGURE 3.
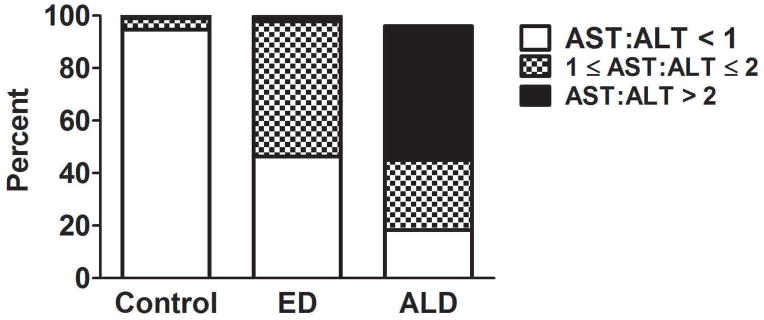
Percentage of subjects in controls, excessive drinkers, and alcoholic cirrhosis with the AST:ALT ratio > 2, 1≤AST:ALT≤2, and AST:ALT < 1
Trends/kinetics of the routinely used serum markers after alcohol abstinence
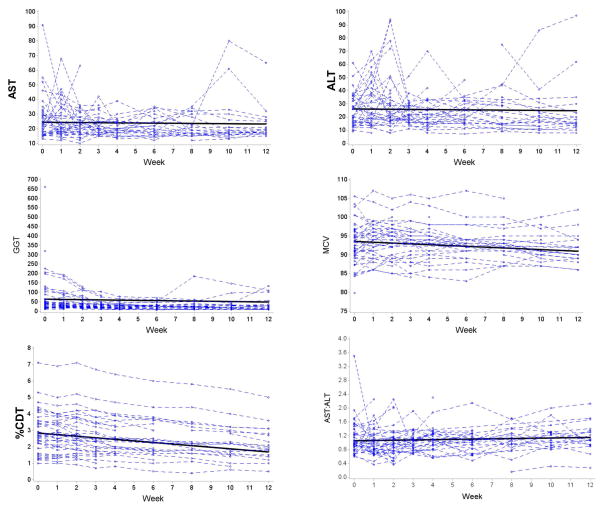
To study the utility of these markers as for follow up of abstinence in clinical practice, we prospectively followed 45 subjects with history of excessive alcohol use who enrolled in a 12-week intensive alcohol treatment program at Fairbanks. The demographic and clinical characteristics of these subjects are shown in Table 5. These subjects had the mean AUDIT scores of 28.7, an average of 234 drinks in the past month, and had ~ 12 drinks per drinking day. The mean serum concentrations for AST, ALT, AST:ALT ratio, and GGT were 26, 24.8, 1.13, and 74.3 U/L, respectively (these levels were not statistically different compared to those of 272 subjects shown in Table 1). In this prospective cohort, we again found that none of the 45 subjects had the AST:ALT ratio > 2, indicating the poor diagnostic performance of using the ratio to screen for excessive alcohol use without advanced liver disease. During the follow up period, only the levels of GGT, MCV, and %CDT were significantly lower compared to those at baseline before alcohol rehabilitation. The rate of decline was 1.2 unit/week for GGT, 0.21 unit/week for MCV, and 0.09 unit/week for %CDT (Figure 4, Table 6). During the course of the follow up, the average levels of AST, ALT, and AST:ALT ratio were unchanged.
Table 5.
Baseline demographic and alcohol drinking characteristics of 45 subjects who underwent intensive alcohol rehabilitation program
| Demographic and clinical characterstics | N=45 |
|---|---|
|
| |
| Age (yrs) | 37.9±11.73 |
|
| |
| Male sex, n (%) | 25 (55.6%) |
|
| |
| Race, White, n (%) | 37 (83%) |
|
| |
| Marital status | |
| : Married | 17 (37.8%) |
| : Divorced/separated | 8 (17.8%) |
| : Never married | 17 (37.8%) |
| : Others | 3 (6.6%) |
|
| |
| BMI (kg/m2) | 26.9±4.40 |
|
| |
| AUDIT-C | 28.7±6.55 |
|
| |
| Alcohol drinking patterns during the last 30 days from TLFB | |
| : Total drinks | 234.0±150.40 |
| : Number of days drinking last 30 days | 18.7±6.89 |
| : Average drinks per drinking day | 12.3±5.51 |
| : Average drinks per day | 7.8±5.01 |
| : Number of heavy drinking days | 15.9±7.78 |
| : Greatest number of drinks in one day | 18.7±8.71 |
|
| |
| Laboratory measures | |
| : Serum AST (U/L) | 26.0±13.87 |
| : Serum ALT (U/L) | 24.8±11.52 |
| : Serum GGT (U/L) | 74.3±111.80 |
| : Mean Corpusular volume (fL) | 92.9±5.32 |
| : Serum %Carbohydrate deficient transferrin (%CDT, %) | 2.83±1.2 |
| : AST:ALT ratio | 1.13±0.4 |
FIGURE 4.
Levels of biomarkers of interest during the 12-week alcohol rehabilitation
Table 6.
Adjusted associations between average weekly decline, and percent of reduction compared to baseline in the serum markers of interest and time of follow up in 45 subjects who underwent 12-week intensive alcohol rehabilitation program$
| Biomarker | Coefficient of the time effect | Reduction (%)* | P-value** |
|---|---|---|---|
| MCV | −0.21 | 2.7 | <.0001 |
| AST | −0.09 | N/A | 0.5893 |
| ALT | 0.33 | N/A | 0.2868 |
| GGT | −1.20 | 19 | 0.0040 |
| CDTA | −0.10 | 43 | <.0001 |
| AST:ALT | 0.21 | N/A | 0.47 |
percent reduction was based off decrease in these values to the average levels of these markers at baseline before the alcohol rehabilitation program
p-value is reflective of the coefficient of the time effect. The model was adjusted for age, gender, race, and BMI
DISCUSSION
Excessive alcohol use is not uncommon among patients with underlying liver disease and those who are seen in general medicine clinics (Lee and Pham 2014; Jackson et al. 2010). Numerous studies have shown the adverse effects of ongoing excessive alcohol use (Taylor et al. 2007; Mathurin 2005). Therefore, screening for alcohol use, appropriate counselling, and ensuring abstinence are necessary when providing medical cares to these patients.
The under detection of excessive alcohol use in the routine care of patients has been found when using the self-report or questionnaire administration to estimate alcohol consumption(Bearer, Bailey, and Hoek 2010). In contrast to self-report questionnaires, serum markers can objectively and reliably detect the problem drinking or track the abstinence. Several routinely used markers for excessive alcohol use have been studied such as serum AST, ALT, and GGT(Bearer, Bailey, and Hoek 2010; Liangpunsakul et al. 2010). Unfortunately, the baseline levels of these markers, a panel of liver enzymes, may be perturbed from underlying liver disease such as hepatitis C or NAFLD, but not from excessive alcohol use per se. Other non-hepatic enzyme markers have been used, e.g. MCV and %CDT (Das, Dhanya, and Vasudevan 2008). Heavy alcohol use causes the alteration in the lipid structure of red cell membrane, leading to the increase in the MCV values (Liangpunsakul et al. 2010). Transferrin molecules in the blood usually contain several carbohydrate components. In chronic heavy drinkers, however, the number of carbohydrate components in each transferrin molecule is reduced, resulting in the increase in %CDT(Golka et al. 2004).
We found that the commonly used markers have fairly low sensitivities to detect excessive alcohol use. %CDT had the highest sensitivity, followed by GGT. However, few conditions other than heavy drinking will elevate CDT levels, thus decreasing the probability of false positive results by using CDT as the marker. %CDT might also be a better marker than GGT as other chronic liver diseases also can increase GGT levels, increasing the likelihood of false-positive results. The sensitivity of AST and ALT was ~43% and 4%, respectively, and thus they should not be used as the surrogates to screen for excessive alcohol use.
Traditionally, AST:ALT ratio > 2 is considered as highly suggestive of alcohol-induced liver injury, notably alcoholic cirrhosis (Nyblom et al. 2004). However, the diagnostic performance of using the cut-off ratio to detect excessive drinking without significant liver disease is still debatable. Our study provided the important insight into the use of AST:ALT ratio in the context of excessive alcohol use, and in advanced alcoholic liver disease/cirrhosis. We found that most patients with excessive alcohol consumption but without clinical evidence of advanced liver disease did not have AST:ALT ratio > 2. In fact, our results are in accordance with previous report showed that many patients who consume high amounts of alcohol and in deed are alcohol dependent with elevated serum aminotransferase levels do not show the high AST:ALT ratio (Nyblom et al. 2004). High AST:ALT ratio (>2) suggests the presence of advanced alcoholic liver disease/cirrhosis. The explanation for AST:ALT>2 in alcoholic liver cirrhosis is the decreasing in ALT activity or pyridoxal 5′-phosphate depletion (Diehl et al. 1984; Matloff, Selinger, and Kaplan 1980). However, the reason on why in excessive drinkers without alcoholic liver disease did not have high AST:ALT ratio is not clear, though it is possible that ongoing and excessive alcohol drinking might have direct toxic effect on both AST and ALT (Nyblom et al. 2004).
We found the statistical significance between the serum GGT and %CDT and the alcohol consumed in the past 30 days before testing. However, the results may not be clinically meaningful. We found that the regression coefficient, representing the mean change in the response variable (the serum levels of each marker) for one unit of change in the predictor variable (the level of alcohol consumption), was low. This indicated that for every additional drink, we can expect the changes in the markers of interest by an average of 0.003 (for CDT) to 0.15 (for GGT). So while both might be useful in detection of excessive alcohol use, it might not be helpful for its use to correlate to the amount of alcohol being consumed by patient in the past month.
The rate of decline of the marker of interest after abstinence is a crucial information in clinical practice. In patients in whom the regression in the level of these markers is not what we expected, it might be an indicative of concealed, ongoing alcohol drinking. Among these markers, we found that only MCV, GGT, and %CDT had the significant decline over time, at the rate of 0.21, 1.20, and 0.10 unit/week, respectively. At the end of 12 weeks follow up, the total decline for MCV, GGT, and %CDT was ~2.52 fL, 14.4 U/L, and 1.2%, respectively. When compared the decrease in these values to the average levels of these markers at baseline (MCV 93 fL, GGT 74 U/L, and % CDT 2.8%), the reduction was ~ 2.7% (for MCV), 19% (for GGT), and 43% (for %CDT) as shown in Table 6. There were no changes in the AST, ALT and AST:ALT ratio during the follow up period. Our data indicated that, among these markers, %CDT is the best marker to track the abstinence with the significant reduction ~43% (compared to baseline when patient stopped drinking) at 12 weeks follow up.
Several limitations deserve discussion. It is difficult to determine the ‘true’ cases for specific diseases; in which no ‘objective’ tests exist – in our scenario – subjects with excessive alcohol drinking. To minimize this drawback, we thus recruited the subjects from an alcohol rehabilitation hospital, in whom they have been screened and diagnosed by the addiction specialist with alcohol use disorder. On the average, these subjects have history of excessive alcohol consumption for at least 5 years, consumed alcohol almost daily, and the last alcohol consumption was within 72 hours prior to enrollment. For the recent alcohol consumption history (during the past 30 days), we relied on self-report questionnaire, TLFB, as our reference. This instrument rates high in validity and reliability for determining daily alcohol intake, and is the standard instrument used by alcohol researchers for quantifying daily drinking(Carey et al. 2001).
In summary, our data showed that %CDT, are useful markers to screen for excessive alcohol use and for follow up of abstinence. Most subjects with excessive alcohol use do not high AST:ALT ratio. Rather, the AST:ALT > 2 is suggestive of alcoholic cirrhosis. The performance of the %CDT to screen for heavy alcohol use is still not ideal. Further research to identify the non-invasive marker(s) (i.e. using proteomic or metabolomics approach) should be considered.
Acknowledgments
Sources of funding: This study is supported by K08 AA016570 from the NIH/NIAAA, 1I01CX000361-01 from the Veterans Affairs Research and Administration, Indiana University Research Support Fund Grant, and W81XWH-12-1-0497 from United States Department of Defense (All to S.L), 5P60AA007611 and U01AA021840 (to DWC).
LIST OF ABBREVIATIONS
- AST
Aspatate alanine transferase
- ALT
alanine aminotransferase
- %CDT
Percentage of carbohydrate deficient transferin
- GGT
Gamma-glutaryltransferase
- MCV
Mean Corpuscular volume
- MELD
Model for end stage liver disease
- TLFB
Timeline follow back
Footnotes
Conflicts of interest: None
References
- Allen JP, Anton R. Biomarkers as aids to identification of relapse in alcoholic patients. Recent Dev Alcohol. 2003;16:25–38. doi: 10.1007/0-306-47939-7_4. [DOI] [PubMed] [Google Scholar]
- Bearer CF, Bailey SM, Hoek JB. Advancing alcohol biomarkers research. Alcohol Clin Exp Res. 2010;34:941–945. doi: 10.1111/j.1530-0277.2010.01168.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carey MP, Carey KB, Maisto SA, Gordon CM, Weinhardt LS. Assessing sexual risk behaviour with the Timeline Followback (TLFB) approach: Continued development and psychometric evaluation with psychiatric outpatients. International Journal of STD & AIDS. 2001;2:365–375. doi: 10.1258/0956462011923309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chayanupatkul M, Liangpunsakul S. Alcoholic hepatitis: a comprehensive review of pathogenesis and treatment. World J Gastroenterol. 2014;20:6279–6286. doi: 10.3748/wjg.v20.i20.6279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das SK, Dhanya L, Vasudevan DM. Biomarkers of alcoholism: an updated review. Scand J Clin Lab Invest. 2008;68:81–92. doi: 10.1080/00365510701532662. [DOI] [PubMed] [Google Scholar]
- Diehl AM, Potter J, Boitnott J, Van Duyn MA, Herlong HF, Mezey E. Relationship between pyridoxal 5′-phosphate deficiency and aminotransferase levels in alcoholic hepatitis. Gastroenterology. 1984;86:632–636. [PubMed] [Google Scholar]
- Freeman WM, Vrana KE. Future prospects for biomarkers of alcohol consumption and alcohol-induced disorders. Alcohol Clin Exp Res. 2010;34:946–954. doi: 10.1111/j.1530-0277.2010.01169.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golka K, Sondermann R, Reich SE, Wiese A. Carbohydrate-deficient transferrin (CDT) as a biomarker in persons suspected of alcohol abuse. Toxicol Lett. 2004;151:235–241. doi: 10.1016/j.toxlet.2004.01.023. [DOI] [PubMed] [Google Scholar]
- Hajarizadeh B, Grebely J, Dore GJ. Epidemiology and natural history of HCV infection. Nat Rev Gastroenterol Hepatol. 2013;10:553–562. doi: 10.1038/nrgastro.2013.107. [DOI] [PubMed] [Google Scholar]
- Jackson CB, Varon J, Ho A, Marks KM, Talal AH, Kreek MJ. Identification of substance use and dependence among patients with viral hepatitis. Dig Liver Dis. 2010;42:650–656. doi: 10.1016/j.dld.2010.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight JR, Sherritt L, Harris SK, Gates EC, Chang G. Validity of brief alcohol screening tests among adolescents: a comparison of the AUDIT, POSIT, CAGE, and CRAFFT. Alcohol Clin Exp Res. 2003;27:67–73. doi: 10.1097/01.ALC.0000046598.59317.3A. [DOI] [PubMed] [Google Scholar]
- Lee KC, Pham A. Screening and behavioral counseling interventions in primary care to reduce alcohol misuse. Am Fam Physician. 2014;89:971–972. [PubMed] [Google Scholar]
- Liangpunsakul S, Chalasani N. What should we recommend to our patients with NAFLD regarding alcohol use? Am J Gastroenterol. 2012;107:976–978. doi: 10.1038/ajg.2012.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liangpunsakul S, Chalasani N. What should we recommend to our patients with NAFLD regarding alcohol use? Am J Gastroenterol. 2012;107:976–978. doi: 10.1038/ajg.2012.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liangpunsakul S, Qi R, Crabb DW, Witzmann F. Relationship between alcohol drinking and aspartate aminotransferase:alanine aminotransferase (AST:ALT) ratio, mean corpuscular volume (MCV), gamma-glutamyl transpeptidase (GGT), and apolipoprotein A1 and B in the U.S. population. J Stud Alcohol Drugs. 2010;71:249–252. doi: 10.15288/jsad.2010.71.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathurin P. Is alcoholic hepatitis an indication for transplantation? Current management and outcomes. Liver Transpl. 2005;11:S21–S24. doi: 10.1002/lt.20601. [DOI] [PubMed] [Google Scholar]
- Matloff DS, Selinger MJ, Kaplan MM. Hepatic transaminase activity in alocholic liver disease. Gastroenterology. 1980;78:1389–1392. [PubMed] [Google Scholar]
- Nyblom H, Berggren U, Balldin J, Olsson R. High AST/ALT ratio may indicate advanced alcoholic liver disease rather than heavy drinking. Alcohol Alcohol. 2004;39:336–339. doi: 10.1093/alcalc/agh074. [DOI] [PubMed] [Google Scholar]
- Sobell LC, Agrawal S, Sobell MB, Leo GI, Young LJ, Cunningham JA, Simco ER. Comparison of a quick drinking screen with the timeline followback for individuals with alcohol problems. J Stud Alcohol. 2003;64:858–861. doi: 10.15288/jsa.2003.64.858. [DOI] [PubMed] [Google Scholar]
- Sobell LC, Sobell MB, Riley DM, Schuller R, Pavan DS, Cancilla A, Klajner F, Leo GI. The reliability of alcohol abusers’ self-reports of drinking and life events that occurred in the distant past. J Stud Alcohol. 1988;49:225–232. doi: 10.15288/jsa.1988.49.225. [DOI] [PubMed] [Google Scholar]
- Taylor JE, Haddock K, Poston WSC, Talcott WG. Relationship between patterns of alcohol use and negative alcohol-related outcomes among U.S. Air Force recruits. Mil Med. 2007;172:379–382. doi: 10.7205/milmed.172.4.379. [DOI] [PubMed] [Google Scholar]
- Vakili S, Sobell LC, Sobell MB, Simco ER, Agrawal S. Using the Timeline Followback to determine time windows representative of annual alcohol consumption with problem drinkers. Addict Behav. 2008;33:1123–1130. doi: 10.1016/j.addbeh.2008.03.009. [DOI] [PubMed] [Google Scholar]
- Whitfield JB, Rahman K, Haber PS, Day CP, Masson S, Daly AK, Cordell HJ, Mueller S, Seitz HK, Liangpunsakul S, Westerhold C, Liang T, Lumeng L, Foroud T, Nalpas B, Mathurin P, Stickel F, Soyka M, Botwin GJ, Morgan TR, Seth D. GenomALC Consortium (2015) Brief Report: Genetics of Alcoholic Cirrhosis-GenomALC Multinational Study. Alcohol Clin Exp Res. 39:836–842. doi: 10.1111/acer.12693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Younossi ZM, Stepanova M, Afendy M, Lam BP, Mishra A. Knowledge about infection is the only predictor of treatment in patients with chronic hepatitis C. J Viral Hepat. 2013;20:550–555. doi: 10.1111/jvh.12080. [DOI] [PubMed] [Google Scholar]