Abstract
The modification of protein and nanoparticle therapeutics with polyethylene glycol (PEG), a flexible, uncharged and highly hydrophilic polymer, is a widely adopted approach to reduce RES clearance, extend circulation time, and improve drug efficacy. Nevertheless, an emerging body of literature, generated by numerous research groups, demonstrates that the immune system can produce antibodies that specifically bind PEG, which can lead to the “accelerated blood clearance” of PEGylated therapeutics. In animals, anti-PEG immunity is typically robust but short-lived and consists of a predominantly anti-PEG IgM response. Rodent studies suggest that the induction of anti-PEG antibodies (α-PEG Abs) primarily occurs through a type 2 T-cell independent mechanism. Although anti-PEG immunity is less well-studied in humans, the presence of α-PEG Abs has been correlated with reduced efficacy of PEGylated therapeutics in clinical trials. The prevalence of anti-PEG IgG and reports of memory immune responses, as well as the existence of α-PEG Abs in healthy untreated individuals, suggests that the mechanism(s) and features of human anti-PEG immune responses may differ from those of animal models. Many questions, including the incidence rate of pre-existing α-PEG Abs and immunological mechanism(s) of α-PEG Ab formation in humans, must be answered in order to fully address the potential complications of anti-PEG immunity.
Introduction
Extended circulation of proteins and nanoparticle therapeutics is often necessary to achieve adequate drug concentrations in target tissues.1–3 Unfortunately, many peptide and protein drugs are rapidly degraded and/or cleared from the systemic circulation due to their small size,4 and nanoparticulate drug carriers are readily eliminated by the cells of the mononuclear phagocyte system (MPS).3, 5 To overcome these challenges, proteins and nanoparticles are frequently conjugated to various hydrophilic polymers, which can significantly reduce degradation and opsonization, consequently extending the circulation half-lives of the modified therapeutics.1, 6 These polymers are frequently referred to as “stealth” polymers, reflective of their ability to render proteins and particles inert to the biological environment.
Polyethylene glycol (PEG) has been, and continues to be, the most widely used stealth polymer in drug delivery, with over a dozen PEGylated pharmaceuticals currently on the market and many more in clinical testing.2, 3 PEG has a long history of safe use in humans, and the polymer is classified under the Generally Recognized As Safe (GRAS) category by the FDA. Despite the frequent use of PEG to extend circulation kinetics, a number of investigators have observed the rapid clearance of some PEGylated systems upon repeated administration.7, 8 This “accelerated blood clearance” phenomenon was ultimately attributed to the formation of PEG-specific antibodies.9 Indeed, animals that receive repeated doses of PEGylated systems often generate a potent IgM antibody response to PEG, which causes the complete elimination of subsequent doses of PEGylated agents from the circulation within minutes to a few hours.8 The induction of anti-PEG antibodies (α-PEG Abs) in humans was also observed in recent clinical trials of PEGylated proteins and has been correlated with poor drug efficacy. Interestingly, there is emerging evidence that α-PEG Abs can be found in the general population in individuals who likely have never received PEGylated therapeutics injected systemically.10, 11 As many more PEGylated protein and nanoparticle therapeutics are expected to enter the market over the next several years, an improved understanding of the prevalence, induction, and effects of anti-PEG immunity is undoubtedly critical for the continued clinical use of PEGylated systems.
ADVANTAGES AND PHYSICOCHEMICAL PROPERTIES OF EFFECTIVE STEALTH PEGYLATION
The stealth properties of PEG are rooted in several distinctive molecular and physical characteristics. First, PEG is exceedingly hydrophilic, with each ethylene glycol subunit (-CH3-CH3-O-) surrounded by a minimum of 2–3 water molecules.12, 13 Thus, PEG coatings generate a hydration shell with a large excluded volume that sterically prevents biomacromolecules from penetrating into the polymer layer and binding to the underlying core via hydrophobic or electrostatic interactions.14–16 Second, PEG is highly flexible and exhibits high chain mobility, which results in an exceedingly large number of polymer chain conformations. As a result, any substantial reduction in the conformational freedom of PEG, including the displacement of PEG chains by intruding biomacromolecules, is thermodynamically unfavorable.17–19 Together, these features greatly suppress interactions between PEGylated systems and the biological environment.
For proteins, PEG conjugation decreases enzymatic degradation, opsonization, and immunogenicity of the protein core;4 PEGylation can also improve stability and solubility.2 Additionally, the resulting increase in the hydrodynamic diameter can reduce renal elimination and improve the biodistribution and pharmacokinetics of PEGylated proteins.4 For nanocarriers, PEGylation reduces opsonization and MPS cell clearance, resulting in significantly prolonged circulation kinetics.5, 6 For oncological applications, this effect often leads to greater tumor distribution via the enhanced permeability and retention (EPR) effect, while decreasing accumulation in non-targeted organs.3 PEGylation can also improve nanocarrier stability and minimize the premature release of cargo therapeutics. Finally, PEG coatings have been shown to decrease nanocarrier association with structural components of mucus and extracellular matrix, thereby improving distribution and delivery to regions such as mucosal surfaces and brain tissues.20, 21
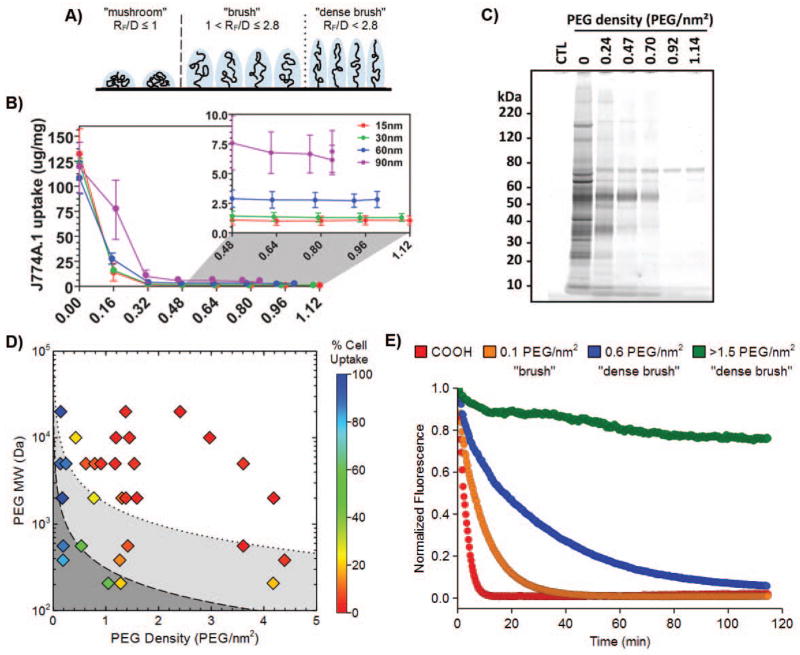
Naturally, the effectiveness of PEG as a nanoparticle coating polymer is critically dependent on the density and resulting conformations assumed by conjugated PEG chains. The thickness of the PEG coating is dictated by its Flory radius (RF; a function of the molecular weight) and the distance between two neighboring PEG chains (D; a function of the PEG coating density).22 When neighboring PEG chains are sparsely packed and do not overlap, PEG occupies a diffuse volume generally termed a “mushroom” conformation (RF/D ≤ 1). As more PEG polymers are introduced, the excluded volume and repulsion by neighboring PEG chains cause the polymer to transition from a diffuse conformation to a more extended “brush” conformation (RF/D > 1),3, 23 eventually reaching a “dense brush” regime, where the height of the PEG layer exceeds the RF by at least two-fold (at RF/D > 2.8) (Fig. 1A).18, 24, 25 The mushroom/brush transition has long been considered to be the critical threshold at which PEG begins to exhibit stealth polymer functions. However, we and others have recently found that both rigid polymeric and metallic nanoparticles require PEG grafting densities far exceeding the minimum for brush conformation to demonstrate effective stealth nanoparticle behavior.25, 26 Indeed, maximal reduction of uptake by mouse and human phagocytes in vitro required at least a dense brush PEG coating (Fig. 1B and D), and PEG grafting densities extending well into the dense brush conformation were necessary for the evasion of serum protein adsorption (Fig. 1C), as well as to achieve sustained circulation in vivo (Fig. 1E).
Figure 1.
A) The conformation adopted by PEG chains at various grafting densities. At low grafting densities (RF/D ≤ 1), the PEG chains adopt a diffuse “mushroom” conformation. At higher densities, the PEG chains are increasingly able to repel opsonization and cell uptake as they transition into a more extended “brush” conformation (RF/D > 1) and eventually reach a “dense brush” regime (RF/D > 2.8). B) Uptake of PEG5k-grafted gold nanoparticles by mouse J774A.1 macrophage-like cells. A PEG5k density of 0.16 PEG/nm2 corresponds to brush conformation; all other PEG densities correspond to dense brush conformation. C) Qualitative analysis of the serum proteins adsorbed onto 30 nm gold nanoparticles modified with varying amounts of PEG5k. A PEG5k density of 0.24 PEG/nm2 corresponds to brush conformation; all other PEG densities correspond to dense brush conformation. D) Phase diagram mapping polymeric nanoparticle uptake by human THP-1 macrophage-like cells as a function of PEG length (MW) and coating density (PEG groups/nm2). The mushroom, brush, and dense brush conformations are indicated in dark gray, gray, and white, respectively, and the transitions between the mushroom-brush and brush-dense brush conformations are indicated by the dashed and dotted lines, respectively. E) Blood circulation profiles, as observed by intravital microscopy, of 100 nm unmodified (COOH) and PEG5k-grafted polystyrene nanoparticles. Panels B and C were reprinted with permission from Ref 26 (copyright 2012 ACS), and panels A, D, and E were adapted with permission from Ref 25 (copyright 2014 ACS).
PEG-SPECIFIC IMMUNITY IN ANIMAL MODELS
The first report of α-PEG Abs in vivo
Because proteins are generally excluded from densely PEG-coated surfaces, it is convenient and intuitive to assume that PEG should be immunologically inert and escape binding by antibodies. However, in 1983, less than a decade after the introduction of protein PEGylation, Richter and Akerblom reported the generation of PEG-specific antibodies following intramuscular (i.m.) or subcutaneous (s.c.) injections of various PEG-modified proteins in Complete Freund’s Adjuvant.27 In contrast, they found that free PEG (MW 10-5.9 x 103 kDa) administered under similar conditions exhibited little to no immunogenicity. This landmark study demonstrated for the first time that antibodies can be formed against PEG polymers. Later studies confirmed that not only can α-PEG Abs be elicited by immunization with PEGylated proteins,28, 29 but also that the induction of PEG-specific immunity can occur in the absence of adjuvants.30, 31
Accelerated blood clearance of PEGylated systems is attributed to α-PEG Abs
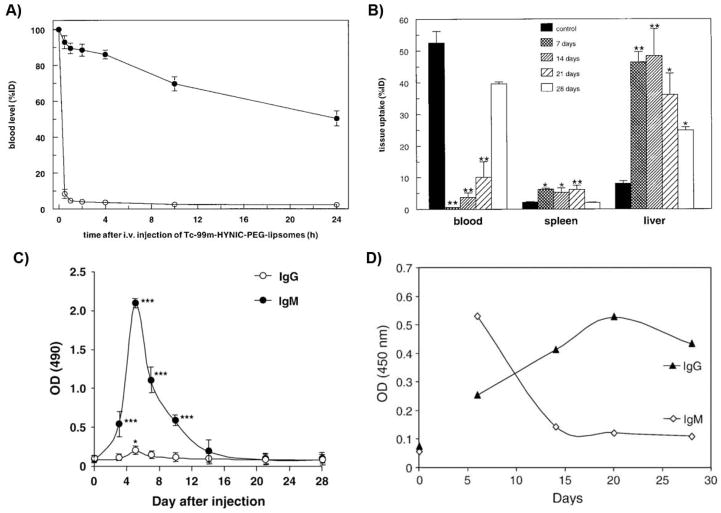
While single doses of PEGylated therapeutics often demonstrate extended system circulation times in vivo, some PEGylated systems exhibit rapid elimination upon repeated administration. For example, Moghimi and Gray reported in 1997 that when long-circulating polystyrene particles coated with poloxamine 908 (a PEG-containing surfactant) were administered 3–4 days after an initial dose, the particles were swiftly cleared from systemic circulation by MPS cells in rats.32 Similarly, Dams et al. and several other groups observed that repeated weekly dosing of empty PEG liposomes also significantly reduced the circulating half-lives of the subsequent doses (Fig. 2A), with a corresponding increase in liver accumulation and hepatic clearance (Fig. 2B), as well as moderate increases in splenic accumulation (Table 1).7, 33–36 This unexpected effect was termed the “accelerated blood clearance” (ABC) phenomenon, and the biological factors underlying the phenomenon remained unclear for a number of years after its discovery. Because the infusion of “naïve” mice with plasma from animals pre-dosed with poloxamine-coated polystyrene beads failed to generate an ABC effect, Moghimi and Gray suggested that the observed phenomenon was not due to plasma factors but rather potentially resulted from an change in phagocyte receptor expression and/or activity elicited by the initial particle dose.32 In contrast, Dams et al. reported that the transfusion of blood or serum from rats pre-treated with PEGylated liposomes generated an ABC effect and observed that this effect was dependent on the presence of a heat-labile, 150-kDa serum factor. Because ABC was observed for serum depleted of IgG or IgM, they proposed that the observed effect was likely due to complement protein(s).7 However, because the extent of IgM depletion appeared incomplete, the involvement of residual IgM could not be discounted. Although Laverman et al. did not identify the specific immune factors responsible, they observed that the ABC phenomenon occurs in two phases: the induction phase, when the immune system is primed by the initial injection, and the effectuation phase, when the pharmacokinetics and biodistribution of the PEGylated therapeutics are affected by the resulting immune response.36
Figure 2.
A) Amount of 99mTc-labeled PEGylated liposomes remaining in circulation after i.v. administration in rats quantified by scintigraphic image analysis. (●) indicates the first dose, and (○) indicates the second dose given 7 days later. B) Tissue biodistribution of 99mTc-labeled PEGylated liposomes in rats for the initial injection (control) and for second doses given after 7, 14, 21, or 28 days. *p < 0.05, **p < 0.01. C) PEG-specific antibodies responses after an initial injection of PEGylated liposomes (0.001 μmol/kg) in rats, as determined using ELISA. *p < 0.05, ***p < 0.005. D) PEG-specific antibodies responses after an initial injection of PEGylated liposomes (100 μg/animal) in mice, as determined using ELISA. Panels A and B were reprinted from Ref 7; panel C was reprinted from Ref 39 with permission from Elsevier; and panel D was reprinted from Ref 41 with permission from Elsevier.
Table 1.
Examples of α-PEG Ab and/or accelerated blood clearance responses to PEGylated systems.
| Type of PEGylated system |
Animal model | Dose*,† | Dosing interval* |
Parameters of initial or control dose |
Parameters of subsequent dose(s) |
Fold change in parameters‡ |
α-PEG Ab response# |
Ref |
|---|---|---|---|---|---|---|---|---|
| PEGylated liposome | Balb/c mice | 0.01–1 μmol PL/kg | 5–7 d | N.D. | N.D. | - | IgM + (no to weak) IgG | 48 |
| PEGylated liposome | Std:ddY mice | 25 μmol PL/kg | 10 d | t1/2,β
12.9 h Cl 0.07 mL/h AUC0-24h 486%dose h/mL |
t1/2,β
6.3 h Cl 0.2 mL/h AUC0-24h 221%dose h/mL |
0.5 (t1/2,β) 2.9 (Cl) 0.5 (AUC0-24h) |
N.D. | 33 |
| PEGylated liposome | KM mice | 0.1 μmol PL/kg (initial); 5 μmol PL/kg (subsequent) | 6 d |
44%IDblood,4h 13%IDliver,4h 12%IDspleen,4h |
14%IDblood,4h 28%IDliver,4h 18%IDspleen,4h |
0.3 (%IDblood,4h) 2.2 (%IDliver,4h) 1.5 (%IDspleen,4h) 0.2 (t1/2), 0.2 (AUC)° |
IgM§ | 54 |
| PEGylated liposome | Wistar rats | 0.001 μmol PL/kg | - | N.D | - | - | IgM + (weak) IgG | 39 |
| PEGylated liposome | Wistar rats | 5 μmol PL/kg | 7 d | t1/2,α
2.4 h 52.5%IDblood,4h 8.1%IDliver,4h 2.2%IDspleen,4h |
t1/2,α
0.1 h 0.6%IDblood,4h 46.4%IDliver,4h 6.3%IDspleen,4h |
0.04 (t1/2,α) 0.01 (%IDblood,4h) 5.7 (%IDliver,4h) 2.9 (%IDspleen,4h) |
N.D. | 7 |
| PEGylated liposome | Wistar rats | 0.001 μmol PL/kg (initial); 5 μmol PL/kg (subsequent) | 4–6 d | t1/2
14.8 h Clh <1 mL/min 8%IDliver,24h 8%IDspleen,24h |
t1/2
0.3–1.8 h Clh 25–55 mL/min 67–72%IDliver,24h 8–12%IDspleen,24h |
0.02–0.12 (t1/2) >25–55 (Clh) 8.4–9.0 (%IDliver,24h) 1.0–1.5 (%IDspleen,24h) |
IgM | 47 |
| PEGylated liposome | Wistar rats | 0.001 μmol PL/kg (initial); 5 μmol PL/kg (subsequent) | 5 d |
51%IDblood,24h 6%IDliver,24h |
<2%IDblood,24h 68%IDliver,24h |
<0.02 (%IDblood,24h) 11 (%IDliver,24h) |
IgM + (weak) IgG | 37 |
| PEGylated liposome | Wistar rats | 5 μmol PL/kg | 7 d |
76.4%IDblood,4h 15%IDliver,4h |
0.6%IDblood,4h 68%IDliver,4h |
0.01 (%IDblood,4h) 4.5 (%IDliver,4h) |
N.D. | 36 |
| PEGylated liposome | Sprague-Dawley rats | 7 μmol PL/kg | 7 d | t1/2
16.7 h Cl 1.7 mL/h AUC 856 μg h/mL |
t1/2
0.2 h Cl 74.3 mL/h AUC 17 μg h/mL |
0.01 (t1/2) 43.7 (Cl) 0.02 (AUC) |
IgM§ | 44 |
| PEGylated liposome | Dunkin-Hartley guinea pigs | 0.1 μmol PL/kg (initial); 5 μmol PL/kg (subsequent) | 6 d |
34%IDblood,4h 12%IDliver,4h <2%IDspleen,4h |
12%IDblood,4h 37%IDliver,4h <2%IDspleen,4h |
0.4 (%IDblood,4h) 3.1 (%IDliver,4h) ~1 (%IDspleen,4h) 0.6 (t1/2), 0.6 (AUC)° |
IgM§ | 54 |
| PEGylated liposome | Rabbits | 9 mg PL/animal | 7 d | N.D. | N.D | - | IgG¶,Δ | 49 |
| PEGylated liposome | Japanese white rabbits | 0.1 μmol PL/kg (initial); 5 μmol PL/kg (subsequent) | 6 d |
47%IDblood,4h 15%IDliver 4%IDspleen |
13%IDblood,4h 35%IDliver 6%IDspleen |
0.3 (%IDblood,4h) 2.3 (%IDliver,4h) 1.5 (%IDspleen,4h) 0.5 (t1/2), 0.4 (AUC)° |
IgM§ | 54 |
| PEGylated liposome | Rhesus monkey | 5 μmol PL/kg | 7 d | t1/2
87.5 h 17.6%IDliver,4h |
t1/2
14.2 h 41.2%IDliver,4h |
0.2 (t1/2) 2.3 (%IDliver,4h) |
N.D. | 7 |
| PEGylated pDNA liposome | ICR mice | 100 μg pDNA/animal | 7 d |
80%IDblood,1h 8%IDliver,1h 2%IDspleen,1h |
23%IDblood,1h 41%IDliver,1h 4%IDspleen,1h |
0.3 (%IDblood,1h) 5.1 (%IDliver,1h) 2.0 (%IDspleen,1h) |
IgG + IgMΔ | 41 |
| PEGylated ODN vesicles | ICR mice | 50 mg PL/kg, 10 mg ODN/kg | 7 d | 70%IDblood,1h | 6%IDblood,1h | 0.1 (%IDblood,1h) | IgM§ | 57 |
| PEGylated Gd liposome | C57BL/6 and Balb/c mice | 5 μmol PL/kg | 7 d |
11%IDblood,6h 9%IDliver,6h 10%IDspleen,6h |
<0.5%IDblood,6h 31%IDliver,6h 1%IDspleen,6h |
<0.05(%IDblood,6h) 3.4 (%IDliver,6h) 0.1 (%IDspleen,6h) |
IgMΔ | 45 |
| PEGylated Hb vesicles | ddY mice | 0.1 mg Hb/kg | 7 d | t1/2
2.7 h Cl 3.7 mL/h AUC 27.1%dose h/mL |
t1/2
1.3 h Cl 22.3 mL/h AUC 4.5%dose h/mL |
0.5 (t1/2) 6.0 (Cl) 0.2 (AUC) |
IgM | 98 |
| PEGylated EPI liposome | Wistar rats | 1 μmol PL/kg, 0.08 EPI/kg (initial); 5 μmol PL/kg, 0.4 mg EPI/kg (subsequent) | 7 d |
52%IDblood,4h 16%IDliver,4h 8%IDspleen,4h |
8%IDblood,4h 36%IDliver,4h 15%IDspleen,4h |
0.2 (%IDblood,4h) 2.3 (%IDliver,4h) 1.9 (%IDspleen,4h) |
IgM§ | 53 |
| PEGylated DXR liposome | Beagle dogs | 0.67 μmol PL/kg and 2 mg DXR/m2 | 3 wk | t1/2
24.1 h Cl 1.5 mL/h/kg AUC0-∞ 76.0 μg h/mL |
t1/21.5 h Cl 127.8 mL/h/kg AUC0-∞ 0.6 μg h/mL |
0.06 (t1/2) 85.2 (Cl) 0.01 (AUC0-∞) |
IgM§ | 96 |
| PEGylated TOPO liposome | Beagle dogs | 0.5 mg TOPO/kg | 7 d | Cmax
7.9 mg/L Cl 0.4 mL/min/kg AUC0-t 1.4 mg min/mL |
Cmax
1.7 mg/L Cl 6.7 mL/min/kg AUC0-t 0.1 mg min/mL |
0.2 (C max) 16.8 (Cl) 0.07 (AUC0-t) |
IgM§ | 42 |
| PEGylated solid lipid nanoparticle | Wistar rats | 5 μmol PL/kg (initial s.c., subsequent i.v.) | 7 d | AUC0-4h
27.3 mg h/L 8 μg/g (liver, 4 h) 9 μg/g (spleen, 4 h) |
AUC0-4h
6.6 mg h/L 25 μg/g (liver, 4 h) 26 μg/g (spleen, 4 h) |
0.2 (AUC0-4h) 3.1 (liver, 4 h) 2.9 (spleen, 4 h) |
IgM§ | 64 |
| PEGylated solid lipid nanoparticle | Kunming mice | 10 μmol PL/kg | 7 d |
71.3%IDblood,0.5h 5.4%IDliver,0.5h 4%IDspleen,0.5h |
42.6%IDblood,0.5h 23.3%IDliver,0.5h 9%IDspleen,0.5h |
0.6 (%IDblood,0.5h) 4.3 (%IDliver,0.5h) 2.3 (%IDspleen,0.5h) |
N.D. | 46 |
| PEGylated solid lipid nanoparticle | Beagle dogs | 2 μmol PL/kg | 7 d | t1/2,β
3.4 h Cl 0.2 mL/min/kg AUC0-24h 90.6 mg h/L |
t1/2,β
1.6 h Cl 0.4 mL/min/kg AUC0-24h 34.0 mg h/L |
0.5 (t1/2,β) 2.0 (Cl) 0.4 (AUC0-24h) |
IgM§ | 46 |
| PEGylated emulsion | Wistar rats | 5 μmol PL/kg | 7 d | AUC0-0.5h
30.8 mg h/L 50%IDblood,1h 11 μg/g (liver, 12 h) 14 μg/g (spleen, 12 h) |
AUC0-0.5h
10.8 mg h/L 7%IDblood,1h 22 μg/g (liver, 12 h) 23 μg/g (spleen, 12 h) |
0.4 (AUC0-0.5h) 0.1 (%IDblood,1h) 2.0 (liver, 12 h) 1.6 (spleen, 12 h) |
IgM§ | 68 |
| PEGylated micelle | Sprague-Dawley rats | 7 μmol PL/kg | 7 d | t1/2
8.8 h Cl 3.3 mL/h AUC 442 μg h/mL |
t1/2
9.6 h Cl 3.5 mL/h AUC 408 μg h/mL |
1.1 (t1/2)⋄ 0.9 (Cl)⋄ 1.1 (AUC)⋄ |
IgM§ | 44 |
| PEG-PBLA micelles | C57BL/6 and Balb/c mice | 3 mg/kg | 7 d | 65%IDblood,6h | 50%IDblood,6h | 0.8 (%IDblood,6h)⋄ | IgMΔ | 45 |
| PEG-PLGA ETO nanoparticles | Wistar rats | 0.01–1 mg polymer/kg (initial); 20 mg polymer/kg, 8 mg/kg ETO (subsequent) | 7 d | t1/2
3.5 h Cl 0.6 mL/min AUC 3.7 mg min/mL |
t1/2
1.0–1.2 h Cl 3.2–3.3 mL/min AUC 0.6 mg min/mL |
0.3 (t1/2) 5.3–5.5 (Cl) 0.2 (AUC) |
IgM§ | 43 |
| PEG-PLA PGE1 nanoparticles | Wistar rats | 133 μg PGE1/kg | 7 d | AUC 0-24h
6.4 μg min/mL 50%IDblood,3h |
AUC 1.1 μg min/mL 3% IDblood,3h |
0.2 (AUC0-24h) 0.06 (%IDblood,3h) |
IgM§,Δ | 55 |
| Poloxamine-coated polystyrene nanoparticles | Wistar rats | 3.5 mg polystyrene/kg, 5–7 mg poloxamine/kg | 4 d |
66.3%IDblood,3h 9.4%IDliver,3h 1.1%IDspleen,3h |
11.5%IDblood,3h 50.1%IDliver,3h 6.1%IDspleen,3h |
0.2 (%IDblood,3h) 5.3 (%IDliver,3h) 5.5 (%IDspleen,3h) |
N.D. | 32 |
| PEG-adenovirus | Wistar rats | 1011 particles/animal | - | N.D. | - | - | IgM§,Δ | 30 |
| PEG-BSA | Wistar rats | 1 μg/animal | - | N.D. | - | - | IgM§,Δ | 30 |
| PEG-uricase | Sprague-Dawley rats | 1 mg/kg | 7 d | 20–26 h (t1/2,β) | N.D. | - | IgMΔ | 99 |
| PEG-uricase, PEG-IFNα, PEG-HSA | New Zealand white rabbits | N.D. | 7 d | N.D. | N.D. | - | Igϕ,Δ | 28 |
| PEG-OVA, PEG-SOD, PEG-Rag | New Zealand white rabbits | 100 μg/animal (i.m.) with Freund’s adjuvant | 4 wk | N.D. | N.D. | - | Igϕ,£,Δ | 27 |
Treatment conditions that generated a maximal ABC and/or α-PEG Ab response;
i.v. administration, unless otherwise indicated;
fold change calculated as (subsequent dose)/(initial dose);
α-PEG Ab detection was performed using ELISA, unless otherwise indicated;
data not available for individual injections;
anti-PEG IgG was not evaluated;
anti-PEG IgG detection was performed using Western blotting and IgM was not evaluated;
antibody specificity to PEG confirmed through cross-reactivity and/or competition with other PEGylated agents or free PEG;
no ABC effect was observed;
α-PEG Abs were detected using passive hemagglutination and radial immunodiffusion;
antibody isotype was not evaluated or could not be determined.
N.D., not determined or not stated; PL, phospholipid; pDNA, plasmid DNA; ODN, oligonucleotide; Hb, hemoglobin; EPI, epirubicin; DXR, doxorubicin; TOPO, topotecan; ETO, etoposide, PGE1, prostaglandin E1; BSA, bovine serum albumin; IFN, interferon; HSA, human serum albumin; OVA, ovalbumin; SOD, superoxide dismutase; Rag, ragweed pollen extract.
Since then, mounting and irrefutable evidence has established that α-PEG Abs can be elicited by PEGylated systems and is likely responsible for the observed ABC that can greatly alter the pharmacokinetics and efficacy of PEGylated therapeutics in vivo. Ishida et al. first observed that the serum of pre-treated rats, as compared to naïve animals, demonstrated greater antibody adsorption onto PEGylated liposomes, suggesting that antibodies were the dominant serum factor responsible for the ABC effect.37, 38 Soon afterwards, the same group reported that intravenous injection of PEGylated liposomes strongly induced the production of PEG-specific antibodies (Fig. 2C),39 and the presence of these antibodies was correlated with hepatic clearance.9 Cheng et al. demonstrated that the injection of monoclonal α-PEG IgM into naïve mice resulted in the rapid clearance of PEGylated therapeutic proteins (e.g., 38-fold reduction in systemic concentration compared to uninjected control).31, 40 Numerous other groups have subsequently corroborated the relationship between α-PEG Abs and the ABC phenomenon.41–46 The observed α-PEG Ab response is predominantly IgM,9, 45, 47, 48 although the development of anti-PEG IgG has also been reported (Fig. 2C and D, Table 1).29, 41, 49 α-PEG Ab-mediated complement activation may also be involved in the MPS clearance of repeatedly dosed PEGylated therapeutics. Antibodies, particularly IgM, can efficiently activate the complement system, and opsonization by complement proteins such as C3b facilitates particle phagocytosis and clearance. Serum from rats generating an ABC response demonstrated complement activation upon incubation with PEGylated liposomes,50 and heat-treatment (complement inactivation) of this serum abrogated the first-pass hepatic clearance of PEGylated liposomes.51 A proteomics analysis indicated that, after the induction of α-PEG Abs, PEGylated liposomes are predominantly bound by plasma IgM and complement proteins (i.e., C1, C3) in mice.52 Additionally, complement proteins can disrupt liposomal membranes; indeed, the leakage of cargo epirubicin from PEG-liposomes was associated with complement activation.53 Altogether, these results suggest that complement can play an important role in the ABC of PEGylated liposomes, although the role of complement in the ABC of various non-liposomal PEGylated systems (e.g., polymeric nanoparticles, proteins) remains to be further investigated.
The development of α-PEG Abs and its resulting effects on clearance has been reported not only in a variety of animal models, ranging from rodents, rabbits, and canines to non-human primates,7, 28, 46, 54 but also for different classes of PEGylated systems, including polymeric nanoparticles, micelles, adenovirus, and proteins (Table 1).30, 44, 55 Across fifteen studies, the presence of α-PEG Abs reduced the circulation half-lives of PEGylated agents by 2- to 10-fold on average and increased the hepatic and splenic accumulation by roughly 2- to 5-fold and 1- to 2-fold, respectively. These results clearly underscore the potency and impact of anti-PEG immunity, which represents a particularly important concern in light of increasing number of PEGylated therapeutic proteins and nanomedicines that are FDA-approved or currently in clinical development. Indeed, recent FDA guidelines recommend screening for α-PEG Abs when evaluating the potential immunogenicity of therapeutic proteins.56
Immunological mechanism(s) of α-PEG Ab induction
Given PEG’s well-documented anti-fouling properties, the induction of PEG-specific antibodies no doubt appears paradoxical, and the precise mechanism(s) underlying the formation of α-PEG Abs has received much attention. To date, research efforts have primarily focused on elucidating the cellular processes involved in the generation of PEG-specific immunity in rodent models after repeated intravenous (i.v.) dosing of PEGylated liposomes.
In both rats and mice, splenectomy prior to or immediately following the injection of an initial dose of PEGylated liposomes dramatically reduced the extent of anti-PEG IgM responses, whereas splenectomy performed 4 or more days after the initial injection did not eliminate the ABC of PEGylated systems, suggesting that splenic cells serve as the primary site of α-PEG Ab induction.30, 37, 48, 57 In addition, the ABC phenomenon appears to involve B cells functioning through T-cell independent (TI) mechanisms, as T cell-deficient nude mice, but not SCID mice (B and T cell-deficient), generated an ABC response to both empty and nucleic acid-containing PEGylated liposomes.48, 57, 58 In general, marginal zone B cells are involved in immune responses to TI antigens.59 Consistent with a TI response to PEG, splenic marginal zone B cell depletion in rats eliminated the formation of PEG-specific IgM antibodies, and PEGylated liposomes are initially localized in the marginal zone upon repeat injection.60
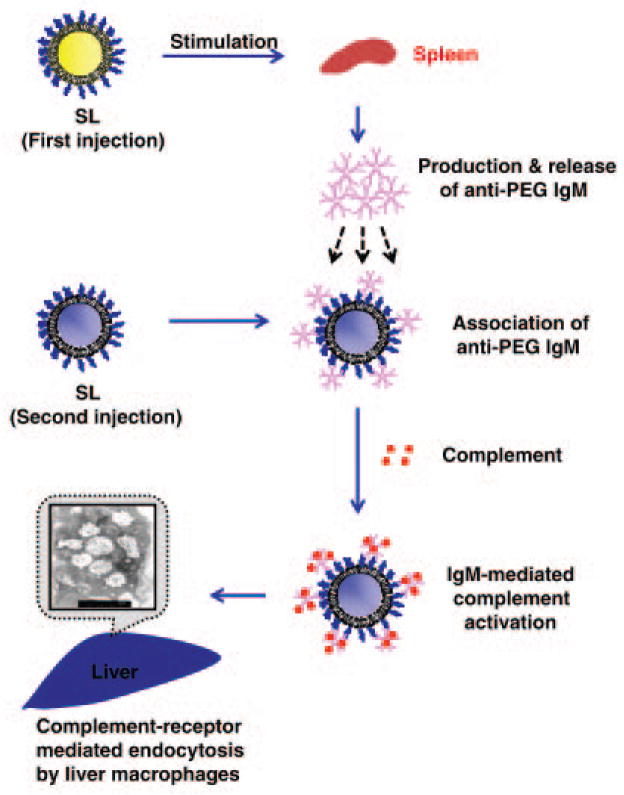
Due to PEG’s structural similarity to other highly repetitive polymeric antigens such as microbial polysaccharides, the research groups of Kiwada and Ishida have explored the possibility of a type 2 T-cell independent (TI-2) mechanism (Fig. 3).8, 59 In this proposed mechanism, the initial dose of a PEGylated therapeutic first enters the spleen, where it comes in contact with marginal zone B cells and crosslinks surface antibodies present on these cells, triggering the production of PEG-specific IgM antibodies. Then, the induced anti-PEG IgM binds to subsequent doses of PEGylated agents in the circulation and activates complement binding, ultimately resulting in hepatic clearance through Kupffer cell uptake.8
Figure 3.
Proposed type-2 T-cell independent (TI-2) response mechanism for the formation of α-PEG Abs and the ABC effect. Splenic B cells are stimulated by an initial dose of PEGylated therapeutic and produce α-PEG IgM. These antibodies then associate with subsequent doses of PEGylated systems and activate complement proteins, which then opsonize PEGylated system and lead to its eventual clearance through hepatic MPS cells. Reprinted from Ref 8 by permission from Macmillan Publishers Ltd.
While the majority of published findings on the induction of α-PEG are consistent with this TI-2 mechanism, there are a small number of studies that present contrasting results. TI responses typically do not induce significant memory or antibody class switching unless there is strong co-stimulation by non-cognate immune cells and/or secreted factors such as cytokines (e.g., IL-1, IL-6, TNFα).61–63 While IgM is indeed the dominant α-PEG Ab isotype observed, a few studies have reported anti-PEG IgG responses.29, 41, 49 For example, Judge et al. observed a strong initial IgM response that was replaced by an elevated IgG response (peaks at day 7 and 20, respectively) after a single dose of PEGylated liposomes (Fig. 2D).41 Whether PEG-specific IgG was formed due to exceptional B cell stimulation that generated class switching or to the induction of α-PEG Abs through non-TI-2 mechanisms remains unclear. The ABC phenomenon was also elicited after the s.c. injection of PEGylated solid nanoparticles, leading Zhao et al. to suggest that regional lymph nodes can also directly produce anti-PEG immune responses.64 However, because a minor amount of the s.c. administered nanoparticles were distributed to the spleen, the involvement of splenic lymphocytes cannot be excluded. Additionally, macrophage depletion prior to an initial dose of PEGylated liposomes completely abrogated the ABC of subsequent doses of PEGylated liposomes in rats, suggesting the potential dependence of α-PEG Ab induction on non-B cell populations as well.36
Reflective of the immunological pathway(s) responsible for the formation of anti-PEG immunity, there are also substantial variations reported for the formation of long-term memory responses. In many studies, the ABC effect is generated 3–7 days after the initial dose (Fig. 2A and B) and diminishes over the period of a couple weeks.43, 48 Nevertheless, Semple et al. did report an anti-PEG IgM response that persisted for at least 50 days in dogs (1, 2, 3, or 7 d dosing intervals), highlighting the potential for long-term ABC responses in vivo and the need to further evaluate not only acute but also long-term α-PEG Ab responses.57
Properties of the α-PEG Ab epitope
How α-PEG Abs specifically bind to PEG polymers remains a mystery, as is the antigenic determinant that leads to α-PEG Ab responses. As noted above, TI-2 antigens are typically composed of identical, repeating epitopes that crosslink B cell receptors to generate significant and prolonged activation of B cells without co-stimulation by T cells.8, 59 The typical PEG chain lengths for modified nanoparticles and proteins are approximately 1–5 kDa and 5–40 kDa, respectively, which covers a range from several tens to hundreds of ethylene glycol subunits and could readily and extensively crosslink any receptors capable of binding PEG. Richter and Akerblom reported hapten inhibition of α-PEG Ab precipitation with PEG of 300 MW, suggesting that the antigenic epitope of PEG may consist of a 6–7 subunit region.27 This value has been commonly cited as the size of the α-PEG Ab binding epitope. Nevertheless, a recent study observed that tri(ethylene glycol) (MW 150–160) was bound by α-PEG Abs in direct and competitive ELISAs (see Sidebar 1 for methods of α-PEG Ab detection).65 We have likewise found that both anti-PEG IgM and IgG can bind to polymers composed of repeating methacrylate PEG300 subunits (unpublished observations, Yang and Lai). Together, these findings suggest that the α-PEG Ab binding epitope could be smaller than the proposed 6–7 subunit length.
Sidebar. Detection of α-PEG Abs by validated ELISA methods.
α-PEG Abs have been detected in animal models and humans using a variety of methods, including passive hemagglutination, immunodiffusion, flow cytometry, Western blotting, and enzyme-linked immunosorbent assays (ELISAs).27, 41, 49, 82 Of these methods, ELISAs can simultaneously provide high sensitivity, rapid screening of multiple samples, and antibody isotype/subclass detection. As a result, most recent studies have used ELISAs almost exclusively to analyze α-PEG Ab responses. However, for many reports of α-PEG Abs, particularly those using animal models, antibody specificity to the PEG moiety was not always thoroughly confirmed. Indeed, most in vivo studies of treated animals only performed direct ELISAs using plates coated with the same PEGylated material (e.g., PEG-lipid) that was injected; thus the possibility that induced antibodies were actually bound to the carrier rather than PEG itself cannot be fully discounted.
As noted by others, there is a critical need for more rigorous, validated α-PEG Ab detection methods.11, 66 In our opinion, both direct and competitive ELISAs should be used in combination to confirm the PEG-specificity of α-PEG Abs with the application of proper controls and conditions (see Fig. 5), as was carried out in some recent human trials.75, 81, 86 Standard curves can be generated using commercially available α-PEG Abs (e.g., mouse, rat, rabbit, chicken, goat, and monkey host Abs) to quantify induced or pre-existing α-PEG Abs. Additionally, the validation of ELISA protocols (e.g., determination of precision, sensitivity, reagent interference) must be performed and reported.81, 86 Importantly, the use of Tween and other PEG-containing detergents must be avoided, as they can significantly reduce the sensitivity of α-PEG Ab detection assays.28 The development of standardized laboratory tests that can quantitatively and accurately measure α-PEG Ab levels is crucial to furthering our understanding of anti-PEG immunity.
Since free PEG is known to be non-immunogenic, the antigenic determinant for α-PEG Abs has been suggested to occur at the linkage between PEG and other materials. Based on the observations that α-PEG Abs induced by hydrophobic PEGylated micelles were able to bind to PEGylated liposomes, and vice versa, whereas hydrophilic PEGylated micelles avoided the induction of and opsonization by α-PEG Abs, Shiraishi et al. proposed that the α-PEG Ab epitope is the interphase between a hydrophobic core and conjugated PEG groups.45 Nevertheless, because free PEG can inhibit α-PEG Ab binding in competitive ELISAs and hemagglutination assays,27, 28 at least some of the observed α-PEG Ab responses must be specific to PEG itself. Due to the disparity in the immune responses to free PEG versus PEGylated therapeutics, as well as the frequent immunogenicity of therapeutic agents that require PEGylation, PEG has been proposed to function as a hapten (i.e., a molecule that elicits immune responses only when conjugated to a carrier agent).10, 66
Factors influencing the formation of anti-PEG immunity and ABC in animals
The ABC phenomenon and immune responses to PEG are affected by a number of factors such as the dosing regimen,43 animal model,54 drug/cargo incorporation,67 nanocarrier/protein identity and composition,27, 35 and PEG structure.30, 68 For example, the dosing interval that generates a maximal ABC response is dependent on the timing for α-PEG Ab formation, which typically peaks at 3–7 days post-injection (Fig. 2A and B). Additional doses administered after less than 48 h or more than 4 weeks typically exhibit extended circulation times comparable to the initial dose.69, 70 High doses of PEGylated therapeutics can also influence α-PEG Ab formation, likely due to the induction of immune tolerance or B cell anergy.9, 38 Encapsulated drug cargo can also play a key role in PEG-specific immune responses to PEGylated nanocarriers. For example, the incorporation of immunostimulatory substances such as CpG DNA enhances α-PEG Ab responses,71 while the loading of cytotoxic chemotherapeutics can directly suppress α-PEG Ab induction,36, 72, 73 likely through the direct killing or impaired proliferation of B cells. Additionally, the presence of endotoxins, which can elicit strong inflammatory responses, may have potentially affected the immunogenicity of the administered PEGylated agents; however, only a small number of studies reported testing for endotoxin contaminants.32, 41, 57 The composition and physicochemical properties (e.g., size,44, 74 lipid membrane rigidity,54 curvature, PEG density and terminal groups28, 30, 42) of PEGylated systems can also affect α-PEG Ab induction and ABC responses. For an excellent review of these various factors, please refer to Ref 8.
ANTI-PEG IMMUNITY IN HUMANS
PEGylation has been critical to the success of numerous therapeutic agents currently on the market, including uricase, interferon-α, and liposomal doxorubicin, as well as many protein and nanomedicines currently drugs in clinical trials.4, 75, 76 However, a growing body of evidence clearly suggests that the induction of α-PEG Abs is possible in humans. In contrast to most animal studies, the α-PEG Ab response in humans is more skewed towards IgG isotype antibodies (Table 2). Interestingly, we and others have found that a significant fraction of the normal population actually possesses pre-existing α-PEG (i.e., the presence of PEG-specific antibodies in the absence of treatment with PEGylated therapeutics), which may become even more prevalent in the years ahead.77 Both pre-existing and induced α-PEG Abs present significant challenges to the clinical efficacy of PEGylated therapeutics.10, 11
Table 2.
Human studies and clinical trials demonstrating α-PEG Ab responses.
| PEGylated therapeutic | Dosing regimen | α-PEG Ab isotype* | Pre-existing α-PEG Ab incidence rate | Induced/post-treatment α-PEG Ab incidence rate | Effects of α-PEG Abs | Method of α-PEG Ab detection | Ref |
|---|---|---|---|---|---|---|---|
| PEGylated bee venom or ragweed extract | 47–4,630 μg (median cumulative dose), 6–40 total weekly or biweekly injections | IgM† | Healthy donors: 0.2% (1/453) high titer (≥1:32), 4.9% (22/453) any titer Untreated allergy patients: 3.3% (3/92) high titer (≥1:32), 20.6% (19/92) any titer |
1 treatment course: 50% (29/58) high titer (≥1:32), 78% (45/58) any titer 2 treatment courses: 29% (8/28) high titer (≥1:32), 86% (24/28) any titer |
N.D. | Agglutination | 78 |
| PEG-IFN-2α (Pegasys®) or PEG-IFN-2β (PegIntron®) | N.D. | N.D. | Healthy controls: 7% (2/29) NASH patients: 7% (2/30) SLE patients: 8% (3/40) HCV patients: 44% (30/68) |
No observed increase in α-PEG | No observed effects on HCV antiviral treatment | ELISA | 79 |
| PEG-asparaginase (Oncaspar®) | 1,000 U/m2 i.v. infusion | IgG + IgM (69%) IgM (31%) |
Unmodified asparaginase group: 38% (6/16) PEG-asparaginase group: 46% (13/28)# |
Strongly correlated with rapid PEG-ASNase clearance and loss of activity | Agglutination, flow cytometryΔ | 82 | |
| PEG-PAL | 0.01–0.10 mg/kg single s.c. dose | IgG + IgM (72%) IgG (18%) |
Treatment-naïve patients: 16% (4/25) | 100% (21/21) | No observed effect on drug efficacy; associated with adverse reactions to subsequent administration of other PEGylated therapeutics | N.D. | 80 |
| PEG-uricase (Krystexxa®/Puricase) | 4–24 mg single s.c. dose | IgG + IgM | Treatment-naïve patients: 0% (0/13) | 38% (5/13) | Associated with rapid PEG-uricase clearance and loss of activity, as well as late injection site reactions | ELISAΔ | 75 |
| PEG-uricase (Krystexxa®/Puricase) | 0.5–12 mg single i.v. infusion | IgG§ | Treatment-naïve patients: 4% (1/24) | 35% (8/23) | Associated with rapid PEG- uricase clearance and loss of activity | ELISAΔ | 84 |
| PEG-uricase (Krystexxa®/Puricase) | 8 mg biweekly or monthly i.v. infusions over 6 months | IgG + IgM¶ | N.D. | 33% (69/212) | Correlated with rapid PEG- uricase clearance and loss of activity, as well as increased risk of infusion reactions¶ | ELISAΔ | 91 |
| PEG-uricase (Krystexxa®/Puricase) | 8 mg i.v. infusion at 3-week intervals, 5 doses | IgG + IgMϕ | Treatment-naïve patients: 19% (5/27) Previously treated patients: 100% (3/3) |
23% (5/22) | Associated with rapid PEG-uricase clearance and loss of efficacy, as well as a two-fold increase in the risk of infusion reactions | ELISAΔ | 81 |
| - | - | IgG (69%) IgM (18%) IgG + IgM (12%) |
Healthy donors: 27–28% (94/350–97/350) | - | - | Agglutination, flow cytometryΔ | 11 |
| - | - | IgG (61%) IgG + IgM (31%) IgM (8%) |
Healthy donors: 42% (13/31) | - | - | ELISAΔ | £ |
Indicated as percentage of total α-PEG-positive individuals;
as determined by mercaptoethanol denaturation;
serum samples were typically collected after the initial dose;
pre-existing and induced α-PEG Abs could not be differentiated because α-PEG Ab levels were determined in post-analysis;
antibody specificity to PEG confirmed through cross-reactivity and/or competition with other PEGylated agents or free PEG;
anti-PEG IgM was not evaluated;
results for anti-PEG-uricase antibodies, no comparable results available for α-PEG;
anti-PEG IgM results available for only 2 patients;
unpublished observations, Yang and Lai.
N.D., not determined or not reported; IFN, interferon; NASH, non-alcoholic steatohepatitis; SLE, systemic lupus erythematous; HCV, hepatitis C virus; PAL, phenylalanine ammonia lyase.
Pre-existing α-PEG Abs in the general population
In 1984, Richter and Akerblom first observed that 0.2% and 3.3% of normal subjects and untreated allergy patients, respectively, exhibited relatively high titers of mostly anti-PEG IgM (Table 2).78 Almost 20 years later, Armstrong et al. reported a much higher incidence rate of 27–28% among normal healthy subjects.11 Interestingly, they observed predominantly PEG-specific IgG, with 19%, 5%, and 3% of the total individuals possessing IgG only, IgM only, and both IgM and IgG antibodies, respectively. The reasons for the discrepancy in the observed α-PEG Ab incidence rates are unclear. Both studies utilized passive hemagglutination of PEG-modified RBCs to detect PEG-specific antibodies, so the differences are unlikely to be caused by the method of detection. In light of the decades-long gap between the reports, these variations could reflect a substantial increase in the prevalence of pre-existing α-PEG Abs in the general population, but this hypothesis has not been carefully assessed.
How pre-existing α-PEG are generated in individuals who have never received any formal treatment with PEGylated therapeutics remains largely unknown. As a GRAS product, PEG is widely used in cosmetics, processed foods, pharmaceuticals, agriculture, and industrial manufacturing. PEG-containing surfactants, as well as PEG itself, are found in the vast majority of household and hygiene products (e.g., soap, shampoo, toothpaste, lotion, detergent). It is natural to assume that frequent exposure to PEG could lead to the inevitable formation of α-PEG Abs, but this constant exposure does not offer insight into the actual mechanism(s) underlying anti-PEG immunity. While we have no direct supportive evidence to date, we wish to offer the following speculation: the human body is frequently subjected to insults (e.g., abrasions, lacerations, skin tears) that may result in local inflammatory responses and recruitment of immune cells. Due to the ubiquitous presence of PEG in products used in daily life, as well as in many disinfecting agents (e.g., soaps and detergents used to clean wounds), PEG is likely present at or introduced to sites of inflammation. The presence of PEG in close proximity to highly active immune cells, particularly in an immunostimulatory environment containing microbes and/or bactericidal chemicals, may be sufficient to drive the induction of α-PEG Abs. Subsequent persistent exposure to PEG-containing products may further induce a robust memory immune response to the polymer.
Beyond the initial reports by Richter and Akerblom and by Armstrong et al., the prevalence of pre-existing α-PEG Abs has been further reported in both healthy donors and untreated controls of clinical trials (Table 2). Tillmann et al. observed an incidence rate of 7%–8% in healthy individuals and in hepatitis and lupus patients, whereas 44% of hepatitis C patients were found to be positive for α-PEG Abs prior to treatment with PEGylated interferon.79 Treatment-naïve gout patients and patients with phenylketonuria demonstrated pre-existing α-PEG Ab incidence rates of 19% and 16%, respectively.80, 81 In addition, 38% of pediatric leukemia patients receiving unmodified asparaginase were also found to possess α-PEG Abs.82 Importantly, the relatively high incidence rate in this study was observed for patients with a mean age of 8.8 years, suggesting that α-PEG Abs can be developed relatively early in life. In a pilot study, we observed that α-PEG Abs were present in 42% (13/31) of healthy adult individuals, with 26%, 3%, and 13% of the total individuals exhibiting IgG only, IgM only, and both IgM and IgG antibodies, respectively (unpublished observations, Yang and Lai).
Induction and effects of α-PEG Abs in individuals treated with PEGylated therapeutics
Studies of PEGylated therapeutics in humans began nearly three decades ago, but early results indicated that α-PEG Ab responses were non-existent or clinically insignificant in humans. In a clinical trial of PEG-modified allergens, 50% of allergy patients had high α-PEG Ab titers after one year of hyposensitization treatment, compared to 3.3% of untreated patients.78 However, the occurrence of α-PEG Abs did not appear to prime further immune responses, as the α-PEG Ab incidence rate decreased to 28.5% in patients receiving two years of treatment. The potential effect of α-PEG Abs on the efficacy of hyposensitization treatment or adverse effects was not examined. Ten of seventeen patients (59%) treated with PEG-modified bovine adenosine deaminase (PEG-ADA, Adagen) generated IgG anti-PEG-ADA antibodies, but competitive ELISAs using ADA and different PEGylated proteins indicated that these antibodies were formed against ADA rather than the PEG moiety.83 In a study of hepatitis C (HCV) patients, the presence of pre-existing α-PEG Abs in 44% of the patients did not appear to affect the efficacy of antiviral PEG-interferon therapy.79 The potential reasons for the apparent lack of α-PEG Ab effects, including immune impairment and hepatic damage caused by HCV, were not explored.
Unlike most studies that report α-PEG Ab responses in only a subset of patients, 100% of phenylketonuria patients developed PEG-specific Abs within 6 weeks of a s.c. injection of PEGylated phenylalanine ammonia lyase (PEG-PAL).80 Although the authors found that neither pre-existing nor induced α-PEG Abs appeared to influence the efficacy of a single dose of PEG-PAL, peak therapeutic efficacy was observed on day 6, whereas testing for α-PEG Abs was performed on days 0, 14, 28, and 42. Thus, the potential effects of the observed α-PEG responses on the activity of multiply-dosed PEG-PAL is unclear. Importantly, two patients in the study later experienced severe adverse reactions to i.m. injections of medroxyprogesterone acetate, which contains both free PEG and polysorbate as excipients. While there is insufficient data to prove causation or statistical significance, this observation indicates that future studies should also investigate whether α-PEG responses may impact not only the repeated administration of PEGylated therapeutics but also the use of pharmaceutical formulations comprising free PEG or PEG-containing chemicals excipients.
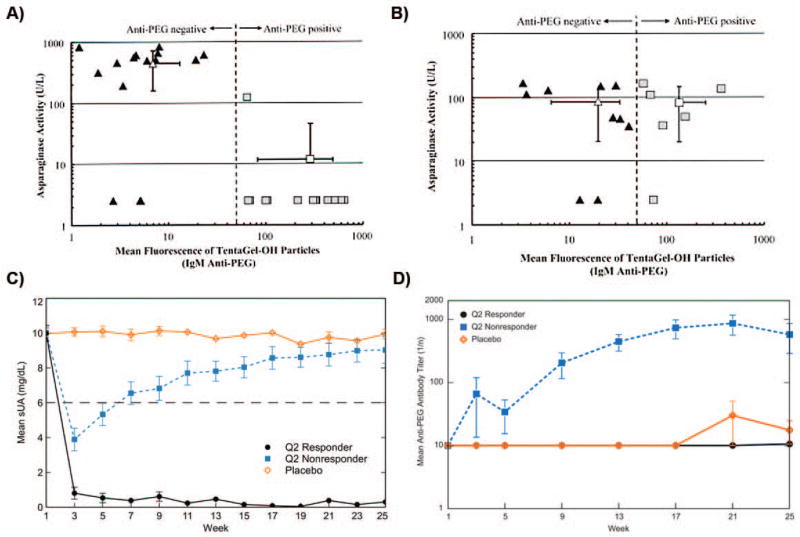
The correlation between the presence of α-PEG Abs and reduced therapeutic efficacy of PEGylated drugs has been observed for only two PEG-modified proteins to date: PEG-asparaginase (PEG-ASNase) and PEG-urate oxidase (PEG-uricase, pegloticase) (Table 2). In pediatric acute lymphoblastic leukemia patients treated with PEG-ASNase, anti-PEG IgM antibodies were observed in 46% of the patients, and the presence of α-PEG Abs was strongly correlated with the rapid clearance of PEG-ASNase and loss of protein activity (Fig. 4A).82 In contrast, α-PEG Abs present in patients treated with unmodified ASNase exhibited no effect on therapeutic protein clearance or activity (Fig. 4B). Because serum samples were only collected after treatment, it is not clear whether the observed α-PEG Abs were induced or pre-existing. However, given that 38% of patients treated with control ASNase also exhibited α-PEG Abs, the authors suggested that the antibodies observed in the PEG-ASNase group were likely pre-existing.
Figure 4.
Anti-PEG IgM vs. asparaginase (ASNase) activity for patients treated with A) PEG-ASNase and B) ASNase. Flow cytometry was used to detect α-PEG Abs bound to PEG hydrogel (TentaGel-OH) particles. C) Mean serum uric acid (sUA) levels in patients receiving biweekly i.v. infusions of PEG-uricase. Normal sUA levels are typically defined as ≤6 mg/dL (indicated by gray dashed line). D) Mean α-PEG Ab titers in patients receiving biweekly i.v. infusions of PEG-uricase. Panels A and B were reprinted from Ref 82, and panels C and D were modified from Ref 86.
In the earliest clinical trial of PEG-uricase, 38% of refractory gout patients developed α-PEG Abs, which was correlated with poor efficacy, after a single s.c. injection of the PEGylated drug.75 This PEG-specific antibody response demonstrated apparent class switching, with IgM and IgG predominating at days 3–7 and 7–14, respectively, after injection. One patient was later re-challenged with PEG-uricase and demonstrated an anamnestic antibody response to the PEGylated protein.75 In a separate study, α-PEG Ab responses were generated in 35% of gout patients after a single i.v. infusion of PEG-uricase, and α-PEG Ab formation also associated with rapid protein clearance.84 Additionally, one patient with a pre-existing α-PEG Ab response exhibited a correspondingly reduced half-life for PEG-uricase.
Repeated dosing of PEG-uricase generated two distinct patient populations: responders (sustained low plasma uric acid levels) and non-responders (early decrease in plasma uric acid levels followed by a rebound to baseline levels) (Fig. 4C).85, 86 The production of high titer anti-PEG-uricase antibodies, most of which appeared to be specific to PEG (Fig. 4D), was correlated with the loss of PEG-uricase activity.86 Similar results were obtained in a study by Hershfield et al., with 37% of treatment-naïve patients establishing an α-PEG Ab response and non-responsive to PEG-uricase treatment by the end of the clinical trial; half of these α-PEG Ab responses were pre-existing.81 Three patients that received PEG-uricase during previous studies (1–3 years prior) were also non-responsive to the new round of treatment, exhibiting loss of PEG-uricase efficacy earlier than the affected treatment-naïve patients (2–7 days vs. ~2 weeks). Interestingly, of the demographic characteristics (e.g., age, gender, BMI, renal function) examined during the repeated dosing studies, only age (>60–70 years) and organ recipient status, both of which involve some level of immunoinsufficiency, were found to be associated with reduced α-PEG Ab formation.81, 86 In addition to rapid PEG-uricase clearance, α-PEG Ab-positive individuals also demonstrated an increased rate of infusion reactions,75, 81, 85 but the precise involvement of α-PEG Abs in adverse reactions to PEG-uricase and other PEGylated therapeutics remains unclear.66
Clinical implications of and strategies to overcome α-PEG Abs
The reduced efficacy of PEG-ASNase and PEG-uricase in the presence of α-PEG Abs highlights the potential impact of PEG-specific immune responses on the growing clinical use of PEGylated therapeutics and underscores the need to incorporate testing for α-PEG Abs in clinical trials of PEG-containing drugs. Standard laboratory tests (see Sidebar 1) that can quantitatively and accurately measure α-PEG Ab levels and determine a patient’s α-PEG Ab status are crucial to this effort, as suggested by others.11, 66, 87 Importantly, clinical trial designs must screen for pre-existing anti-PEG immunity, as well as monitor treatment history, since previous exposure to PEGylated therapeutics could prime future responses to subsequent therapy with PEGylated drugs. Furthermore, the true extent of α-PEG Abs in the human population and the factors that lead to α-PEG Ab immunity must be further investigated.
In addition to an improved understanding of the prevalence and development of anti-PEG immunity in humans, strategies to avert or overcome α-PEG Ab responses must be developed. Unfortunately, the effect of important dosing regimen factors identified in animal studies remains to be fully evaluated in human subjects. In the clinical trials of PEG-uricase, neither the dose (0.5–24 mg/patient), dosing interval (2–4 weeks), nor route of administration (s.c. or i.v.) appeared to affect α-PEG Ab induction or its effects,75, 81, 86 but these results must be corroborated for other PEGylated drugs. The use of cleavable or sheddable PEG has been demonstrated to decrease or eliminate α-PEG Ab and ABC responses in vivo,41, 57, 88 but the rapid loss of the stealth PEG coating also significantly reduces the circulation half-life and may render the modified therapeutics ineffective. As was observed for a small number of organ transplant recipients, co-treatment with immunosuppressive agents may be able to effectively reduce α-PEG Ab induction,81 but these drugs also can generate undesirable side effects and health risks that may contraindicate their use in patients with existing illnesses.89 Once the precise mechanisms of human α-PEG Ab induction are better understood, the use of drugs that specifically target immunological pathways related anti-PEG immunity may allow the specific suppression of α-PEG Ab generation while avoiding unwanted side effects.
The use of alternative stealth polymers such as chitosan, poly(carboxybetaine), poly(2-oxazaline), XTEN peptide, and poly(glycerol) has also received growing attention.90–93 These polymers are less ubiquitous in everyday household items and thus may not encounter the problem of pre-existing antibodies. Nevertheless, antibodies against various natural and synthetic repeating polymers have been reported,94, 95 suggesting that stealth polymers other than PEG may also prove immunogenic upon repeated administration in humans. In individuals with induced or pre-existing α-PEG Abs, the elimination of circulating α-PEG Abs could be achieved through selective plasmapheresis, although the use of such a complicated procedure clearly poses additional cost burdens and may not be warranted if alternative strategies to remove α-PEG Abs are available. Additionally, it may be possible to overwhelm PEG-specific immune responses by simply administering a much greater dose of the PEGylated therapeutic.36, 57, 96 Nevertheless, dosage increases will obviously be limited by the maximum tolerated dose and potential toxicity to various clearance organs. A conceptually similar but more desirable approach would be to first saturate pre-existing α-PEG Abs with free, low molecular weight PEG. Indeed, Moghimi reported that the administration of free PEG and PEG-containing molecules 1–3 h prior to a second dose of poloxamine-modified polystyrene beads reduced the ABC of these particles in rats.97 Further animal and human studies are needed to confirm the safety and efficacy of such a strategy.
ONGOING QUESTIONS REGARDING ANTI-PEG ANTIBODIES
There are many questions related the phenomenon of α-PEG Abs, particularly as it applies to human patients, that are of great interest to the scientific and clinical communities. While a full discussion of these questions is beyond the scope of this review, we wish to highlight a few of them below:
How are α-PEG Abs able to specifically bind PEG polymers? Due to PEG’s flexible, neutral, and hydrophilic character, the precise antibody-polymer interactions that allow α-PEG Abs to specifically bind to such an amorphous target in the absence of hydrophobic and electrostatic interactions are of interest.
What is the immunological pathway of α-PEG Ab formation in humans? The features of human α-PEG Ab responses (i.e., pre-existing α-PEG Abs, high prevalence of IgG, and memory responses to PEGylated products) suggests that the mechanisms underlying PEG-specific immunity may differ greatly between humans and animal models currently used to study anti-PEG immunity. Due to the difficulty of performing mechanistic studies in humans, the use of animal models that more accurately recapitulate human α-PEG Ab responses are necessary to improve our understanding of anti-PEG immunity, including that elicited by long-term exposure to PEG and PEG-containing products.
What factors predispose individuals towards α-PEG Ab formation and are certain portions of the human population more, or less, inclined towards anti-PEG immunity? The majority (≥50%) of patients treated with PEGylated therapeutics do not appear to develop α-PEG Abs, and the reasons underlying the incongruity of anti-PEG immune responses remain largely unknown. Increased age and immunosuppressive treatments were found to be associated with reduced α-PEG Ab induction in response to PEG-uricase.81, 86 Further analysis of patients receiving PEGylated therapeutics may identify other factors that affect PEG-specific immunity, as well as reveal additional strategies to manage α-PEG Ab responses.
What is the current and likely future prevalence of pre-existing α-PEG Abs? The reported prevalence of α-PEG Abs varies significantly, with values ranging from as low as 5% to over 40%. Thus, a precise estimate of the level of α-PEG Abs in both the general and special populations is sorely needed. Additionally, given the disparity in the incidence rate between early and more recent studies, the potential for further increases in the prevalence of pre-existing α-PEG Abs must be explored.
How can anti-PEG immunity be efficiently and effectively managed in a clinical setting? Strategies to overcome pre-existing and/or induced α-PEG Abs, including the administration of an excess dose of PEGylated therapeutic or prior injection of free PEG polymer, should be further investigated.
Conclusion
Because of its ability to significantly prolong the circulation of nanoparticles and proteins, as well as its presumed lack of immunogenicity, PEG has been widely used to modify various therapeutic agents. However, a growing body of evidence indicates that potent and specific antibody responses can be generated against the PEG polymer, and α-PEG Ab induction can lead to substantial reductions in the circulation half-life and therapeutic efficacy of PEGylated drugs in both animal models and humans. In light of the relatively high prevalence of pre-existing and induced α-PEG Ab responses observed in clinical studies, PEG-specific immunity will likely pose a major challenge to the increasing number of PEGylated therapeutics used in the clinic. An improved understanding of the precise means by which α-PEG Abs develop and are able to bind to the polymer are needed, as are strategies to overcome the challenge of PEG-specific immunity.
Figure 5.
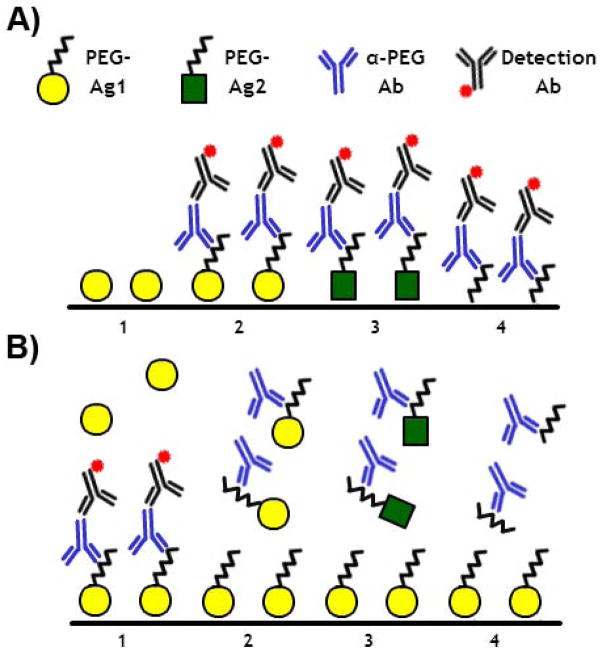
A) Direct and B) competitive enzyme-linked immunosorbent assays (ELISAs) should be used in combination to determine the PEG-specificity of Ab responses induced after treatment with a PEGylated agent (PEG-Ag1), as well as pre-existing α-PEG Abs. In direct ELISAs, PEG specificity can be confirmed by the cross-reactivity of α-PEG Abs to plates coated with pure PEG polymers (see A4) or other PEGylated materials (see A3). In competitive ELISAs, PEG specificity can be confirmed via the inhibition of α-PEG Ab binding by increasing concentrations of free PEG (see B4) or other PEGylated materials (see B3). Additionally, α-PEG Abs should not directly bind to non-PEGylated treatment agents (see A1) nor be competitively inhibited in their presence (see B1).
Acknowledgments
Financial support was provided by the PhRMA Foundation Predoctoral Fellowship (Q.Y.), National Science Foundation CAREER Award (DMR-1151477, S.K.L.), The David and Lucile Packard Foundation (2013-39274), National Institutes of Health (R21EB017938, S.K.L.), and startup funds from the Eshelman School of Pharmacy and Lineberger Comprehensive Cancer Center (S.K.L.).
Footnotes
The authors declare no conflicts of interest.
Contributor Information
Qi Yang, Division of Molecular Pharmaceutics, University of North Carolina at Chapel Hill.
Samuel K. Lai, Division of Molecular Pharmaceutics and UNC/NCSU Joint Department of Biomedical Engineering, University of North Carolina at Chapel Hill.
References
- 1.Amoozgar Z, Yeo Y. Recent advances in stealth coating of nanoparticle drug delivery systems. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2012;4:219–233. doi: 10.1002/wnan.1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Veronese FM, Pasut G. PEGylation, successful approach to drug delivery. Drug Discov Today. 2005;10:1451–1458. doi: 10.1016/S1359-6446(05)03575-0. [DOI] [PubMed] [Google Scholar]
- 3.Jokerst JV, Lobovkina T, Zare RN, Gambhir SS. Nanoparticle PEGylation for imaging and therapy. Nanomedicine (Lond) 2011;6:715–728. doi: 10.2217/nnm.11.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Harris JM, Chess RB. Effect of pegylation on pharmaceuticals. Nat Rev Drug Discov. 2003;2:214–221. doi: 10.1038/nrd1033. [DOI] [PubMed] [Google Scholar]
- 5.Zahr AS, Davis CA, Pishko MV. Macrophage uptake of core-shell nanoparticles surface modified with poly(ethylene glycol) Langmuir. 2006;22:8178–8185. doi: 10.1021/la060951b. [DOI] [PubMed] [Google Scholar]
- 6.Klibanov AL, Maruyama K, Torchilin VP, Huang L. Amphipathic polyethyleneglycols effectively prolong the circulation time of liposomes. FEBS Lett. 1990;268:235–237. doi: 10.1016/0014-5793(90)81016-h. [DOI] [PubMed] [Google Scholar]
- 7.Dams ET, Laverman P, Oyen WJ, Storm G, Scherphof GL, van Der Meer JW, Corstens FH, Boerman OC. Accelerated blood clearance and altered biodistribution of repeated injections of sterically stabilized liposomes. J Pharmacol Exp Ther. 2000;292:1071–1079. [PubMed] [Google Scholar]
- 8.Abu Lila AS, Kiwada H, Ishida T. The accelerated blood clearance (ABC) phenomenon: clinical challenge and approaches to manage. J Control Release. 2013;172:38–47. doi: 10.1016/j.jconrel.2013.07.026. [DOI] [PubMed] [Google Scholar]
- 9.Wang X, Ishida T, Kiwada H. Anti-PEG IgM elicited by injection of liposomes is involved in the enhanced blood clearance of a subsequent dose of PEGylated liposomes. J Control Release. 2007;119:236–244. doi: 10.1016/j.jconrel.2007.02.010. [DOI] [PubMed] [Google Scholar]
- 10.Garay RP, El-Gewely R, Armstrong JK, Garratty G, Richette P. Antibodies against polyethylene glycol in healthy subjects and in patients treated with PEG-conjugated agents. Expert Opin Drug Deliv. 2012;9:1319–1323. doi: 10.1517/17425247.2012.720969. [DOI] [PubMed] [Google Scholar]
- 11.Armstrong JK. The occurence, induction, specificity and potential effect of antibodies against poly(ethylene glycol) In: Veronese FM, editor. PEGylated Protein Drugs: Basic Science and Clinical Applications. 2009. pp. 147–168. [Google Scholar]
- 12.Tirosh O, Barenholz Y, Katzhendler J, Priev A. Hydration of polyethylene glycol-grafted liposomes. Biophys J. 1998;74:1371–1379. doi: 10.1016/S0006-3495(98)77849-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Branca C, Magazù S, Maisano G, Migliardo F, Migliardo P, Romeo G. Hydration Study of PEG/Water Mixtures by Quasi Elastic Light Scattering, Acoustic and Rheological Measurements. The Journal of Physical Chemistry B. 2002;106:10272–10276. [Google Scholar]
- 14.Jeon SI, Lee LH, Andrade JD, De Gennes PG. Protein-surface interactions in the presence of polyethylene oxide. I. Simplified theory. J Colloid Interf Scie. 1991;142:149–158. [Google Scholar]
- 15.Lasic DD, Martin FJ, Gabizon A, Huang SK, Papahadjopoulos D. Sterically stabilized liposomes: a hypothesis on the molecular origin of the extended circulation times. Biochim Biophys Acta. 1991;1070:187–192. doi: 10.1016/0005-2736(91)90162-2. [DOI] [PubMed] [Google Scholar]
- 16.Needham D, McIntosh TJ, Lasic DD. Repulsive interactions and mechanical stability of polymer-grafted lipid membranes. Biochim Biophys Acta. 1992;1108:40–48. doi: 10.1016/0005-2736(92)90112-y. [DOI] [PubMed] [Google Scholar]
- 17.Sharma S, Johnson RW, Desai TA. XPS and AFM analysis of antifouling PEG interfaces for microfabricated silicon biosensors. Biosens Bioelectron. 2004;20:227–239. doi: 10.1016/j.bios.2004.01.034. [DOI] [PubMed] [Google Scholar]
- 18.Damodaran VB, Fee CJ, Ruckh T, Popat KC. Conformational studies of covalently grafted poly(ethylene glycol) on modified solid matrices using X-ray photoelectron spectroscopy. Langmuir. 2010;26:7299–7306. doi: 10.1021/la9041502. [DOI] [PubMed] [Google Scholar]
- 19.Vonarbourg A, Passirani C, Saulnier P, Benoit JP. Parameters influencing the stealthiness of colloidal drug delivery systems. Biomaterials. 2006;27:4356–4373. doi: 10.1016/j.biomaterials.2006.03.039. [DOI] [PubMed] [Google Scholar]
- 20.Lai SK, Wang YY, Hanes J. Mucus-penetrating nanoparticles for drug and gene delivery to mucosal tissues. Adv Drug Deliv Rev. 2009;61:158–171. doi: 10.1016/j.addr.2008.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nance EA, Woodworth GF, Sailor KA, Shih TY, Xu Q, Swaminathan G, Xiang D, Eberhart C, Hanes J. A dense poly(ethylene glycol) coating improves penetration of large polymeric nanoparticles within brain tissue. Sci Transl Med. 2012;4:149ra119. doi: 10.1126/scitranslmed.3003594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.de Gennes PG. Conformation of polymers attached to an interface. Macromolecules. 1980;13:1069–1075. [Google Scholar]
- 23.Owens DE, 3rd, Peppas NA. Opsonization, biodistribution, and pharmacokinetics of polymeric nanoparticles. Int J Pharm. 2006;307:93–102. doi: 10.1016/j.ijpharm.2005.10.010. [DOI] [PubMed] [Google Scholar]
- 24.Perry JL, Reuter KG, Kai MP, Herlihy KP, Jones SW, Luft JC, Napier M, Bear JE, DeSimone JM. PEGylated PRINT nanoparticles: the impact of PEG density on protein binding, macrophage association, biodistribution, and pharmacokinetics. Nano Lett. 2012;12:5304–5310. doi: 10.1021/nl302638g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yang Q, Jones SW, Parker CL, Zamboni WC, Bear JE, Lai SK. Evading Immune Cell Uptake and Clearance Requires PEG Grafting at Densities Substantially Exceeding the Minimum for Brush Conformation. Mol Pharm. 2014 doi: 10.1021/mp400703d. [DOI] [PubMed] [Google Scholar]
- 26.Walkey CD, Olsen JB, Guo H, Emili A, Chan WC. Nanoparticle size and surface chemistry determine serum protein adsorption and macrophage uptake. J Am Chem Soc. 2012;134:2139–2147. doi: 10.1021/ja2084338. [DOI] [PubMed] [Google Scholar]
- 27.Richter AW, Akerblom E. Antibodies against polyethylene glycol produced in animals by immunization with monomethoxy polyethylene glycol modified proteins. Int Arch Allergy Appl Immunol. 1983;70:124–131. doi: 10.1159/000233309. [DOI] [PubMed] [Google Scholar]
- 28.Sherman MR, Williams LD, Sobczyk MA, Michaels SJ, Saifer MG. Role of the methoxy group in immune responses to mPEG-protein conjugates. Bioconjug Chem. 2012;23:485–499. doi: 10.1021/bc200551b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wunderlich DA, Macdougall M, Mierz DV, Toth JG, Buckholz TM, Lumb KJ, Vasavada H. Generation and characterization of a monoclonal IgG antibody to polyethylene glycol. Hybridoma (Larchmt) 2007;26:168–172. doi: 10.1089/hyb.2007.006. [DOI] [PubMed] [Google Scholar]
- 30.Shimizu T, Ichihara M, Yoshioka Y, Ishida T, Nakagawa S, Kiwada H. Intravenous administration of polyethylene glycol-coated (PEGylated) proteins and PEGylated adenovirus elicits an anti-PEG immunoglobulin M Response. Biol Pharm Bull. 2012;35:1336–1342. doi: 10.1248/bpb.b12-00276. [DOI] [PubMed] [Google Scholar]
- 31.Cheng TL, Wu PY, Wu MF, Chern JW, Roffler SR. Accelerated clearance of polyethylene glycol-modified proteins by anti-polyethylene glycol IgM. Bioconjug Chem. 1999;10:520–528. doi: 10.1021/bc980143z. [DOI] [PubMed] [Google Scholar]
- 32.Moghimi SM, Gray T. A single dose of intravenously injected poloxamine-coated long-circulating particles triggers macrophage clearance of subsequent doses in rats. Clin Sci (Lond) 1997;93:371–379. doi: 10.1042/cs0930371. [DOI] [PubMed] [Google Scholar]
- 33.Ishida T, Masuda K, Ichikawa T, Ichihara M, Irimura K, Kiwada H. Accelerated clearance of a second injection of PEGylated liposomes in mice. Int J Pharm. 2003;255:167–174. doi: 10.1016/s0378-5173(03)00085-1. [DOI] [PubMed] [Google Scholar]
- 34.Ishida T, Maeda R, Ichihara M, Irimura K, Kiwada H. Accelerated clearance of PEGylated liposomes in rats after repeated injections. J Control Release. 2003;88:35–42. doi: 10.1016/s0168-3659(02)00462-5. [DOI] [PubMed] [Google Scholar]
- 35.Bendas G, Rothe U, Scherphof GL, Kamps JA. The influence of repeated injections on pharmacokinetics and biodistribution of different types of sterically stabilized immunoliposomes. Biochim Biophys Acta. 2003;1609:63–70. doi: 10.1016/s0005-2736(02)00655-7. [DOI] [PubMed] [Google Scholar]
- 36.Laverman P, Carstens MG, Boerman OC, Dams ET, Oyen WJ, van Rooijen N, Corstens FH, Storm G. Factors affecting the accelerated blood clearance of polyethylene glycol-liposomes upon repeated injection. J Pharmacol Exp Ther. 2001;298:607–612. [PubMed] [Google Scholar]
- 37.Ishida T, Ichihara M, Wang X, Kiwada H. Spleen plays an important role in the induction of accelerated blood clearance of PEGylated liposomes. J Control Release. 2006;115:243–250. doi: 10.1016/j.jconrel.2006.08.001. [DOI] [PubMed] [Google Scholar]
- 38.Ishida T, Harada M, Wang XY, Ichihara M, Irimura K, Kiwada H. Accelerated blood clearance of PEGylated liposomes following preceding liposome injection: effects of lipid dose and PEG surface-density and chain length of the first-dose liposomes. J Control Release. 2005;105:305–317. doi: 10.1016/j.jconrel.2005.04.003. [DOI] [PubMed] [Google Scholar]
- 39.Ishida T, Wang X, Shimizu T, Nawata K, Kiwada H. PEGylated liposomes elicit an anti-PEG IgM response in a T cell-independent manner. J Control Release. 2007;122:349–355. doi: 10.1016/j.jconrel.2007.05.015. [DOI] [PubMed] [Google Scholar]
- 40.Cheng TL, Chen BM, Chern JW, Wu MF, Roffler SR. Efficient clearance of poly(ethylene glycol)-modified immunoenzyme with anti-PEG monoclonal antibody for prodrug cancer therapy. Bioconjug Chem. 2000;11:258–266. doi: 10.1021/bc990147j. [DOI] [PubMed] [Google Scholar]
- 41.Judge A, McClintock K, Phelps JR, Maclachlan I. Hypersensitivity and loss of disease site targeting caused by antibody responses to PEGylated liposomes. Mol Ther. 2006;13:328–337. doi: 10.1016/j.ymthe.2005.09.014. [DOI] [PubMed] [Google Scholar]
- 42.Li C, Cao J, Wang Y, Zhao X, Deng C, Wei N, Yang J, Cui J. Accelerated blood clearance of pegylated liposomal topotecan: influence of polyethylene glycol grafting density and animal species. J Pharm Sci. 2012;101:3864–3876. doi: 10.1002/jps.23254. [DOI] [PubMed] [Google Scholar]
- 43.Saadati R, Dadashzadeh S, Abbasian Z, Soleimanjahi H. Accelerated Blood Clearance of PEGylated PLGA Nanoparticles Following Repeated Injections: Effects of Polymer Dose, PEG Coating, and Encapsulated Anticancer Drug. Pharm Res. 2013;30:985–995. doi: 10.1007/s11095-012-0934-y. [DOI] [PubMed] [Google Scholar]
- 44.Kaminskas LM, McLeod VM, Porter CJ, Boyd BJ. Differences in colloidal structure of PEGylated nanomaterials dictate the likelihood of accelerated blood clearance. J Pharm Sci. 2011;100:5069–5077. doi: 10.1002/jps.22682. [DOI] [PubMed] [Google Scholar]
- 45.Shiraishi K, Hamano M, Ma H, Kawano K, Maitani Y, Aoshi T, Ishii KJ, Yokoyama M. Hydrophobic blocks of PEG-conjugates play a significant role in the accelerated blood clearance (ABC) phenomenon. J Control Release. 2013;165:183–190. doi: 10.1016/j.jconrel.2012.11.016. [DOI] [PubMed] [Google Scholar]
- 46.Zhao Y, Wang L, Yan M, Ma Y, Zang G, She Z, Deng Y. Repeated injection of PEGylated solid lipid nanoparticles induces accelerated blood clearance in mice and beagles. Int J Nanomedicine. 2012;7:2891–2900. doi: 10.2147/IJN.S30943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ishida T, Ichihara M, Wang X, Yamamoto K, Kimura J, Majima E, Kiwada H. Injection of PEGylated liposomes in rats elicits PEG-specific IgM, which is responsible for rapid elimination of a second dose of PEGylated liposomes. Journal of Controlled Release. 2006;112:15–25. doi: 10.1016/j.jconrel.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 48.Ichihara M, Shimizu T, Imoto A, Hashiguchi Y, Uehara Y, Ishida T, Kiwada H. Anti-PEG IgM response against PEGylated liposomes in mice and rats. Pharmaceutics. 2011;3:1–11. doi: 10.3390/pharmaceutics3010001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sroda K, Rydlewski J, Langner M, Kozubek A, Grzybek M, Sikorski AF. Repeated injections of PEG-PE liposomes generate anti-PEG antibodies. Cell Mol Biol Lett. 2005;10:37–47. [PubMed] [Google Scholar]
- 50.Ishida T, Atobe K, Wang X, Kiwada H. Accelerated blood clearance of PEGylated liposomes upon repeated injections: effect of doxorubicin-encapsulation and high-dose first injection. J Control Release. 2006;115:251–258. doi: 10.1016/j.jconrel.2006.08.017. [DOI] [PubMed] [Google Scholar]
- 51.Ishida T, Kashima S, Kiwada H. The contribution of phagocytic activity of liver macrophages to the accelerated blood clearance (ABC) phenomenon of PEGylated liposomes in rats. J Control Release. 2008;126:162–165. doi: 10.1016/j.jconrel.2007.11.009. [DOI] [PubMed] [Google Scholar]
- 52.Kawanishi M. Comprehensive analysis of PEGylated liposome-asscociated proteins relating to the accelerated blood clearance phenomenon by combination with shotgun analysis and conventional methods. Biotechnology and applied biochemistry. 2014 doi: 10.1002/bab.1291. [DOI] [PubMed] [Google Scholar]
- 53.Yang Q, Ma Y, Zhao Y, She Z, Wang L, Li J, Wang C, Deng Y. Accelerated drug release and clearance of PEGylated epirubicin liposomes following repeated injections: a new challenge for sequential low-dose chemotherapy. Int J Nanomedicine. 2013;8:1257–1268. doi: 10.2147/IJN.S41701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Xu H, Ye F, Hu M, Yin P, Zhang W, Li Y, Yu X, Deng Y. Influence of phospholipid types and animal models on the accelerated blood clearance phenomenon of PEGylated liposomes upon repeated injection. Drug Delivery. 2014:1–10. doi: 10.3109/10717544.2014.885998. [DOI] [PubMed] [Google Scholar]
- 55.Ishihara T, Takeda M, Sakamoto H, Kimoto A, Kobayashi C, Takasaki N, Yuki K, Tanaka K, Takenaga M, Igarashi R, et al. Accelerated blood clearance phenomenon upon repeated injection of PEG-modified PLA-nanoparticles. Pharm Res. 2009;26:2270–2279. doi: 10.1007/s11095-009-9943-x. [DOI] [PubMed] [Google Scholar]
- 56.Guidance for industry: immunogenicity assessment for therapeutic protein products. Available at: http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM338856.pdf.
- 57.Semple SC, Harasym TO, Clow KA, Ansell SM, Klimuk SK, Hope MJ. Immunogenicity and rapid blood clearance of liposomes containing polyethylene glycol-lipid conjugates and nucleic Acid. J Pharmacol Exp Ther. 2005;312:1020–1026. doi: 10.1124/jpet.104.078113. [DOI] [PubMed] [Google Scholar]
- 58.Koide H, Asai T, Hatanaka K, Akai S, Ishii T, Kenjo E, Ishida T, Kiwada H, Tsukada H, Oku N. T cell-independent B cell response is responsible for ABC phenomenon induced by repeated injection of PEGylated liposomes. Int J Pharm. 2010;392:218–223. doi: 10.1016/j.ijpharm.2010.03.022. [DOI] [PubMed] [Google Scholar]
- 59.Cerutti A, Cols M, Puga I. Marginal zone B cells: virtues of innate-like antibody-producing lymphocytes. Nat Rev Immunol. 2013;13:118–132. doi: 10.1038/nri3383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Shimizu T, Ishida T, Kiwada H. Transport of PEGylated liposomes from the splenic marginal zone to the follicle in the induction phase of the accelerated blood clearance phenomenon. Immunobiology. 2012;218:725–732. doi: 10.1016/j.imbio.2012.08.274. [DOI] [PubMed] [Google Scholar]
- 61.Sha Z, Compans RW. Induction of CD4(+) T-cell-independent immunoglobulin responses by inactivated influenza virus. J Virol. 2000;74:4999–5005. doi: 10.1128/jvi.74.11.4999-5005.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Colombo MJ, Sun G, Alugupalli KR. T-Cell-Independent Immune Responses Do Not Require Cxc Ligand 13-Mediated B1 Cell Migration. Infection and Immunity. 2010;78:3950–3956. doi: 10.1128/IAI.00371-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Balázs M, Martin F, Zhou T, Kearney JF. Blood Dendritic Cells Interact with Splenic Marginal Zone B Cells to Initiate T-Independent Immune Responses. Immunity. 2002;17:341–352. doi: 10.1016/s1074-7613(02)00389-8. [DOI] [PubMed] [Google Scholar]
- 64.Zhao Y, Wang C, Wang L, Yang Q, Tang W, She Z, Deng Y. A frustrating problem: Accelerated blood clearance of PEGylated solid lipid nanoparticles following subcutaneous injection in rats. European Journal of Pharmaceutics and Biopharmaceutics. 2012;81:506–513. doi: 10.1016/j.ejpb.2012.04.023. [DOI] [PubMed] [Google Scholar]
- 65.Saifer MG, Williams LD, Sobczyk MA, Michaels SJ, Sherman MR. Selectivity of binding of PEGs and PEG-like oligomers to anti-PEG antibodies induced by methoxyPEG-proteins. Mol Immunol. 2014;57:236–246. doi: 10.1016/j.molimm.2013.07.014. [DOI] [PubMed] [Google Scholar]
- 66.Verhoef JJ, Carpenter JF, Anchordoquy TJ, Schellekens H. Potential induction of anti-PEG antibodies and complement activation toward PEGylated therapeutics. Drug Discov Today. 2014 doi: 10.1016/j.drudis.2014.08.015. [DOI] [PubMed] [Google Scholar]
- 67.Tagami T, Nakamura K, Shimizu T, Ishida T, Kiwada H. Effect of siRNA in PEG-coated siRNA-lipoplex on anti-PEG IgM production. Journal of Controlled Release. 2009;137:234–240. doi: 10.1016/j.jconrel.2009.04.006. [DOI] [PubMed] [Google Scholar]
- 68.Wang C, Cheng X, Sui Y, Luo X, Jiang G, Wang Y, Huang Z, She Z, Deng Y. A noticeable phenomenon: Thiol terminal PEG enhances the immunogenicity of PEGylated emulsions injected intravenously or subcutaneously into rats. European Journal of Pharmaceutics and Biopharmaceutics. 2013;85:744–751. doi: 10.1016/j.ejpb.2013.10.002. [DOI] [PubMed] [Google Scholar]
- 69.Oussoren C, Storm G. Effect of repeated intravenous administration on the circulation kinetics of poly(ethyleneglycol)-liposomes in rats. J Liposome Res. 1999;9:349–355. [Google Scholar]
- 70.Goins B, Phillips WT, Klipper R. Repeat injection studies of technetium-99m-labeled PEG-liposomes in the same animal. J Liposome Res. 1998;8:265–281. [Google Scholar]
- 71.Tagami T, Nakamura K, Shimizu T, Yamazaki N, Ishida T, Kiwada H. CpG motifs in pDNA-sequences increase anti-PEG IgM production induced by PEG-coated pDNA-lipoplexes. Journal of Controlled Release. 2010;142:160–166. doi: 10.1016/j.jconrel.2009.10.017. [DOI] [PubMed] [Google Scholar]
- 72.Nagao A, Abu Lila AS, Ishida T, Kiwada H. Abrogation of the accelerated blood clearance phenomenon by SOXL regimen: promise for clinical application. Int J Pharm. 2013;441:395–401. doi: 10.1016/j.ijpharm.2012.11.015. [DOI] [PubMed] [Google Scholar]
- 73.Cui J, Li C, Wang C, Li Y, Zhang L, Zhang L, Yang H. Repeated injection of pegylated liposomal antitumour drugs induces the disappearance of the rapid distribution phase. Journal of Pharmacy and Pharmacology. 2008;60:1651–1657. doi: 10.1211/jpp/60.12.0011. [DOI] [PubMed] [Google Scholar]
- 74.Koide H, Asai T, Kato H, Ando H, Shiraishi K, Yokoyama M, Oku N. Size-dependent induction of accelerated blood clearance phenomenon by repeated injections of polymeric micelles. Int J Pharm. 2012;432:75–79. doi: 10.1016/j.ijpharm.2012.04.049. [DOI] [PubMed] [Google Scholar]
- 75.Ganson NJ, Kelly SJ, Scarlett E, Sundy JS, Hershfield MS. Control of hyperuricemia in subjects with refractory gout, and induction of antibody against poly(ethylene glycol) (PEG), in a phase I trial of subcutaneous PEGylated urate oxidase. Arthritis Res Ther. 2006;8:R12. doi: 10.1186/ar1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Gabizon A, Shmeeda H, Barenholz Y. Pharmacokinetics of PEGylated liposomal doxorubicin. Clin Pharmacokinet. 2003;42:419–436. doi: 10.2165/00003088-200342050-00002. [DOI] [PubMed] [Google Scholar]
- 77.Armstrong JK, Leger R, Wenby RB, Meiselman HJ, Garratty G, Fisher TC. Occurrence of an antibody to poly(ethylene glycol) in normal donors. Blood. 2003;102:556. [Google Scholar]
- 78.Richter AW, Akerblom E. Polyethylene glycol reactive antibodies in man: titer distribution in allergic patients treated with monomethoxy polyethylene glycol modified allergens or placebo, and in healthy blood donors. Int Arch Allergy Appl Immunol. 1984;74:36–39. doi: 10.1159/000233512. [DOI] [PubMed] [Google Scholar]
- 79.Tillmann H, Ganson NJ, Patel K, Thompson AJ, Abdelmalek M, Moody T, McHutchison JG, Hershfield MS. High prevalence of pre-existing antibodies against polyethylene glycol (PEG) in hepatitis C (HCV) patients which is not associated with impaired response to PEG-interferon. Journal of Hepatology. 2010;52:S129. [Google Scholar]
- 80.Longo N, Harding CO, Burton BK, Grange DK, Vockley J, Wasserstein M, Rice GM, Dorenbaum A, Neuenburg JK, Musson DG, et al. Single-dose, subcutaneous recombinant phenylalanine ammonia lyase conjugated with polyethylene glycol in adult patients with phenylketonuria: an open-label, multicentre, phase 1 dose-escalation trial. Lancet. 2014;384:37–44. doi: 10.1016/S0140-6736(13)61841-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hershfield MS, Ganson NJ, Kelly SJ, Scarlett EL, Jaggers DA, Sundy JS. Induced and pre-existing anti-polyethylene glycol antibody in a trial of every 3-week dosing of pegloticase for refractory gout, including in organ transplant recipients. Arthritis Res Ther. 2014;16:R63. doi: 10.1186/ar4500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Armstrong JK, Hempel G, Koling S, Chan LS, Fisher T, Meiselman HJ, Garratty G. Antibody against poly(ethylene glycol) adversely affects PEG-asparaginase therapy in acute lymphoblastic leukemia patients. Cancer. 2007;110:103–111. doi: 10.1002/cncr.22739. [DOI] [PubMed] [Google Scholar]
- 83.Chaffee S, Mary A, Stiehm ER, Girault D, Fischer A, Hershfield MS. IgG antibody response to polyethylene glycol-modified adenosine deaminase in patients with adenosine deaminase deficiency. J Clin Invest. 1992;89:1643–1651. doi: 10.1172/JCI115761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Sundy JS, Ganson NJ, Kelly SJ, Scarlett EL, Rehrig CD, Huang W, Hershfield MS. Pharmacokinetics and pharmacodynamics of intravenous PEGylated recombinant mammalian urate oxidase in patients with refractory gout. Arthritis Rheum. 2007;56:1021–1028. doi: 10.1002/art.22403. [DOI] [PubMed] [Google Scholar]
- 85.Sundy JS, Baraf HSB, Yood RA, Edwards NL, Gutierrez–Urena SR, Treadwell EL, Vazquez-Mellado J, White WB, Lipsky PE, Horowitz Z, et al. Efficacy and tolerability of Pegloticase for the treatment of chronic gout in patients refractory to conventional treatment. JAMA. 2011;306:711–720. doi: 10.1001/jama.2011.1169. [DOI] [PubMed] [Google Scholar]
- 86.Lipsky PE, Calabrese LH, Kavanaugh A, Sundy JS, Wright D, Wolfson M, Becker MA. Pegloticase immunogenicity: the relationship between efficacy and antibody development in patients treated for refractory chronic gout. Arthritis Res Ther. 2014;16:R60. doi: 10.1186/ar4497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Schellekens H, Hennink WE, Brinks V. The immunogenicity of polyethylene glycol: facts and fiction. Pharm Res. 2013;30:1729–1734. doi: 10.1007/s11095-013-1067-7. [DOI] [PubMed] [Google Scholar]
- 88.Chen D, Liu W, Shen Y, Mu H, Zhang Y, Liang R, Wang A, Sun K, Fu F. Effects of a novel pH-sensitive liposome with cleavable esterase-catalyzed and pH-responsive double smart mPEG lipid derivative on ABC phenomenon. Int J Nanomedicine. 2011;6:2053–2061. doi: 10.2147/IJN.S24344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Abeles AM. PEG-ing down (and preventing?) the cause of pegloticase failure. Arthritis Res Ther. 2014;16:112. doi: 10.1186/ar4572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Cavadas M, Gonzalez-Fernandez A, Franco R. Pathogen-mimetic stealth nanocarriers for drug delivery: a future possibility. Nanomedicine. 2011;7:730–743. doi: 10.1016/j.nano.2011.04.006. [DOI] [PubMed] [Google Scholar]
- 91.Knop K, Hoogenboom R, Fischer D, Schubert US. Poly(ethylene glycol) in Drug Delivery: Pros and Cons as Well as Potential Alternatives. Angewandte Chemie International Edition. 2010;49:6288–6308. doi: 10.1002/anie.200902672. [DOI] [PubMed] [Google Scholar]
- 92.Abu Lila AS, Nawata K, Shimizu T, Ishida T, Kiwada H. Use of polyglycerol (PG), instead of polyethylene glycol (PEG), prevents induction of the accelerated blood clearance phenomenon against long-circulating liposomes upon repeated administration. Int J Pharm. 2013;456:235–242. doi: 10.1016/j.ijpharm.2013.07.059. [DOI] [PubMed] [Google Scholar]
- 93.Ishihara T, Maeda T, Sakamoto H, Takasaki N, Shigyo M, Ishida T, Kiwada H, Mizushima Y, Mizushima T. Evasion of the Accelerated Blood Clearance Phenomenon by Coating of Nanoparticles with Various Hydrophilic Polymers. Biomacromolecules. 2010;11:2700–2706. doi: 10.1021/bm100754e. [DOI] [PubMed] [Google Scholar]
- 94.Soshee A, Zürcher S, Spencer ND, Halperin A, Nizak C. General In Vitro Method to Analyze the Interactions of Synthetic Polymers with Human Antibody Repertoires. Biomacromolecules. 2013;15:113–121. doi: 10.1021/bm401360y. [DOI] [PubMed] [Google Scholar]
- 95.Specht C, Schlüter B, Rolfing M, Brüning K, Pauels H-G, Kölsch E. Idiotype-specific CD4+CD25+ T suppressor cells prevent, by limiting antibody diversity, the occurrence of anti-dextran antibodies crossreacting with histone H3. Eur J Immunol. 2003;33:1242–1249. doi: 10.1002/eji.200323273. [DOI] [PubMed] [Google Scholar]
- 96.Suzuki T, Ichihara M, Hyodo K, Yamamoto E, Ishida T, Kiwada H, Ishihara H, Kikuchi H. Accelerated blood clearance of PEGylated liposomes containing doxorubicin upon repeated administration to dogs. Int J Pharm. 2012;436:636–643. doi: 10.1016/j.ijpharm.2012.07.049. [DOI] [PubMed] [Google Scholar]
- 97.Moghimi SM. Re-establishing the long circulatory behaviour of poloxamine-coated particles after repeated intravenous administration: applications in cancer drug delivery and imaging. Biochimica et Biophysica Acta (BBA) - General Subjects. 1999;1472:399–403. doi: 10.1016/s0304-4165(99)00157-9. [DOI] [PubMed] [Google Scholar]
- 98.Taguchi K, Urata Y, Anraku M, Watanabe H, Kadowaki D, Sakai H, Horinouchi H, Kobayashi K, Tsuchida E, Maruyama T, et al. Hemoglobin vesicles, polyethylene glycol (PEG)ylated liposomes developed as a red blood cell substitute, do not induce the accelerated blood clearance phenomenon in mice. Drug Metab Dispos. 2009;37:2197–2203. doi: 10.1124/dmd.109.028852. [DOI] [PubMed] [Google Scholar]
- 99.Zhang C, Fan K, Ma X, Wei D. Impact of large aggregated uricases and PEG diol on accelerated blood clearance of PEGylated canine uricase. PLoS One. 2012;7:e39659. doi: 10.1371/journal.pone.0039659. [DOI] [PMC free article] [PubMed] [Google Scholar]