Abstract
Angiogenesis relies on specialized endothelial tip cells to extend toward guidance cues in order to direct growing blood vessels. Although many of the signaling pathways that control this directional endothelial sprouting are well known, the specific cellular mechanisms that mediate this process remain to be fully elucidated. Here, we show that Polo-like kinase 2 (PLK2) regulates Rap1 activity to guide endothelial tip cell lamellipodia formation and subsequent angiogenic sprouting. Using a combination of high-resolution in vivo imaging of zebrafish vascular development and a human umbilical vein endothelial cell (HUVEC) in vitro cell culture system, we observed that loss of PLK2 function resulted in a reduction in endothelial cell sprouting and migration, whereas overexpression of PLK2 promoted angiogenesis. Furthermore, we discovered that PLK2 may control angiogenic sprouting by binding to PDZ-GEF to regulate RAP1 activity during endothelial cell lamellipodia formation and extracellular matrix attachment. Consistent with these findings, constitutively active RAP1 could rescue the endothelial cell sprouting defects observed in zebrafish and HUVEC PLK2 knockdowns. Overall, these findings reveal a conserved PLK2-RAP1 pathway that is crucial to regulate endothelial tip cell behavior in order to ensure proper vascular development and patterning in vertebrates.
Keywords: Polo-like kinase 2, angiogenesis, vascular development, zebrafish, Human, Umbilical Vein Endothelial Cells
Introduction
Angiogenesis is a highly integrative and reiterative cellular process involving the migration and proliferation of endothelial cells (EC) to form new blood vessels from pre-existing ones. It is dependent on the specification of specialized EC subtypes (Adams and Alitalo, 2007; De Smet et al., 2009; Lamalice et al., 2007) that play distinct roles in angiogenic sprouting. To this end, endothelial tip cells can extend filopodia- and lamellipodia-like processes to sense growth factors, extracellular matrix (ECM) components and attractive/repulsive cues that direct EC sprouting and migration (Adams and Alitalo, 2007; De Smet et al., 2009; Lamalice et al., 2007). In contrast, endothelial stalk cells, which reside behind the leading endothelial tip cells, proliferate to elongate the growing blood vessel. Recent studies have shown that Vascular endothelial growth factor (VEGF) and Notch signaling tightly control the differentiation of these EC subtypes (Gerhardt et al., 2003; Hellstrom et al., 2007; Roca and Adams, 2007; Siekmann and Lawson, 2007; Suchting et al., 2007). In particular, VEGF can induce tip cell specification and the expression of the Notch ligand Delta-like 4, which in turn activates Notch signaling in neighboring stalk cells to suppress VEGF receptor 2 expression and tip cell behavior. Furthermore, VEGF and other developmental signaling cues (Bedell et al., 2005; Herbert et al., 2009; Larrivee et al., 2009; Lee et al., 2002; Lu et al., 2004; Park et al., 2003; Torres-Vazquez et al., 2004; Weitzman et al., 2008) can also guide endothelial tip cell membrane extensions to control the proper development and patterning of the vascular network (Carmeliet and Tessier-Lavigne, 2005; Mukouyama et al., 2002; Zacchigna et al., 2008).
In order for ECs to directionally sprout and migrate to form the vasculature, a series of organized cellular events are required for endothelial tip cells to extend their membranes and migrate toward guidance cues. They initially extend multiple filopodia at their distal tips to sense guidance cues in their environment and subsequently form lamellipodia to create an EC leading edge that protrudes toward the chemotactic signal. These EC protrusions then attach to the ECM at focal adhesions to stabilize the sprouting EC membrane and prepare the endothelial tip cell for subsequent migration. A central cellular component in regulating many of these specific cellular events is the activation of small GTPases (Kiosses et al., 2001; Spindler et al., 2010; Wojciak-Stothard et al., 1998). In response to VEGF, Cdc42 promotes the growth of filopodia and mediates cell polarization through microtubule organization (Petrovic et al., 2007), whereas Rac1, together with PAK, controls lamellipodia generation (Kiosses et al., 1999; Kiosses et al., 2001; Somanath and Byzova, 2009). Moreover, RhoA/ROCK and Ras-associated protein 1 (Rap1) have also been discovered to regulate angiogenesis through the phosphorylation of focal adhesion kinase (FAK) as well as the activation of β1 and αVβ3 integrins (Carmona et al., 2009; Lakshmikanthan et al., 2011; van Nieuw Amerongen et al., 2003; Zeng et al., 2002). Consistent with these findings, both loss of function of Rap1 and PDZ-GEF, a guanine nucleotide exchange factor (GEF) for Rap1, results in vascular developmental defects in both mouse and zebrafish (Carmona et al., 2009; Chrzanowska-Wodnicka et al., 2008; Lakshmikanthan et al., 2011; Wei et al., 2007). Thus, specific small GTPases control distinct cellular events during EC sprouting and discovering the factors that regulate these small GTPases may provide insight as to how the activity of these small GTPases are coordinated to promote directional EC angiogenic sprouting and migration.
The polo-like kinase (PLK) family proteins have been previously shown to play pivotal roles in regulating the cell cycle (Simmons et al., 1992), including entry into mitosis, centrosome maturation, and exit from mitosis with the initiation of cytokinesis (Liu and Erikson, 2003). They contain two conserved domains - the canonical serine/threonine kinase domain and the non-catalytic polo box domain (PBD), which binds to substrates and targets the kinase to specific subcellular zones (Lowery et al., 2005; Strebhardt, 2010). Upon ablation of the kinase domain, the PBD domain alone becomes a dominant-negative form of PLKs (Seeburg et al., 2008). Despite their roles in cell cycle regulation, recent reports have suggested PLKs may possess additional cellular functions (Strebhardt, 2010). For example, Plk3 has been shown to suppress tumor angiogenesis, and Plk3−/− mice developed tumors in various organs at advanced age, with enhanced angiogenesis (Xu et al., 2012; Xu et al., 2010a; Xu et al., 2010b; Yang et al., 2008). Additionally, PLK2 has been observed to control dendritic spine sprouting and the density of synapses in neurons by governing RAP1 activity via the regulation of PDZ-GEF (Lee et al., 2011a; Lee et al., 2011b). Interestingly, previous studies have also revealed that human PLK2 transcripts can be observed in human fetal lung, kidney, spleen and heart (Simmons et al., 1992), and additional expression analysis showed that PLK2 may also be found more specifically expressed in the vascular system in early developmental stages (Duncan et al., 2001; Zhong et al., 2010), suggesting that PLK2 may also regulate vascular development. Thus, given its role in controlling RAP1 activity, we speculate that during vascular development, PLK2 may also serve to mediate lamellipodia formation and attachment to the ECM in ECs through regulating EC RAP1 function.
Here, we report that PLK2 is expressed in the vascular system and can control angiogenesis during vascular development by specifically regulating the EC lamellipodia but not filopodia formation. Using a combination of high-resolution in vivo imaging of zebrafish vascular development and a human umbilical vein endothelial cell (HUVEC) in vitro cell culture system, we observed that loss of PLK2 function resulted in a reduction in EC sprouting and migration, whereas overexpression of PLK2 promoted angiogenesis. Moreover, PLK2 appears to impart its function through the regulation of RAP1 to mediate focal adhesion and lamellipodia formation in migrating ECs. Overall, our data reveal a conserved PLK2-RAP1 pathway that is crucial to regulate endothelial tip cell behavior in order to ensure proper vascular development and patterning in vertebrates.
Material and Methods
Zebrafish strains
Embryos and adult fish were maintained under standard laboratory conditions as described previously (Zhang et al., 2013). The following lines were used: Tg(kdrl:mcherry-ras)s896 (Chi et al., 2008), Tg(kdrl:GFP)s843 (Jin et al., 2005), Tg(fli1a:EGFP)y1 (Lawson and Weinstein, 2002), and Tg(hsp70:dn-MAML-GFP)fb10 (Zhao et al., 2014).
PLK2 sequence alignment
PLK2 sequence alignment was performed by ClustalW multi-sequence alignment as described (Hegarty et al., 2013).
Morpholino (MO) knockdown and rescue experiments
To knock down plk2b function, we used an ATG-MO (MO1) against the 5’UTR adjacent to the translation start site of plk2b and a splicing MO (MO2) against the 3’ splice site of exon 2. The MO sequences are: MO1 (ATG-plk2b-MO): 5’- GCTGTGTGTTACTGTGCTTTCTGTC -3’ and MO2 (splicing-plk2b-MO): 5’- TATGCAGTGTTTATCCTACCTTCTC -3’. Five base pairs (bp) of the ATG MO was altered to create a control 5 bp mismatched MO, which did not cause any discernible phenotypes: (ATG-plk2b-control-MO): 5’- GCTcTcTGTTAgTGTcCTTTCTcTC -3’. One-cell stage Tg(kdrl:mcherry-ras), Tg(fli1a:eGFP) embryos were injected with 10 ng of MO1, MO2 or control MO. To confirm that MO1 blocked the translation of plk2b, the following primers were used to PCR amplify a construct that fuses the plk2b 5’UTR region to GFP: plk2b-GFP-F: 5’- actgtgacagaaagcacagtaacacacagccATGGTGAGCAAGGGCGAGGA-3’ and GFP-R: 5’- TTACTTGTACAGCTCGTCCA -3’. This 5’UTR plk2b-GFP construct was subcloned into the pCS2 vector, confirmed by sequencing and then transcribed into capped mRNA using a mMessage mMachine SP6 Transcription kit (Cat. No. AM1340, Life Technologies). 50 pg of the 5’UTR plk2b-GFP RNA with or without 10 ng of plk2b MO1 was injected into zebrafish embryos at the one-cell stage. To evaluate the MO2 function, the following primers were used to detect plk2b exon 1–4 mRNA from 50 24 hours post-fertilization (hpf) control or MO2 injected embryos: plk2b-sMO-rtF: 5’- GGAAATGTTACTGCCGGGGA -3’, plk2b-sMO-rtR: 5’- CGTTTTGCGTCTGTTGCTGA -3’. For the dominant negative plk2b (dn-plk2b) experiments, the plk2b PBD domain fragment was cloned into the pCS2 vector and then in vitro transcribed using the mMessage mMachine SP6 Transcription kit (Cat. No. AM1340, Life Technologies). The following primers were used for PCR of the PBD domain: plk2b-PBDF 5’-ACTCAGGGCTTCATGCCAGAAACGC -3’, plk2b-PBDR: 5’-GTTGCATCGCTGCAGCAGCATGTTGA -3’. One-cell stage Tg(kdrl:mcherry-ras) or Tg(fli1a:eGFP) embryos were injected with 180 pg of dn-plk2b mRNA. For mRNA rescue experiments, the plk2b coding sequence (CDS), which does not include the plk2b ATG MO1 binding site, and the human PLK2 CDS were cloned into the pCS2 vector and then in vitro transcribed using the mMessage mMachine SP6 Transcription kit (Cat. No. AM1340, Life Technologies). The following primers were used for PCR: plk2b-cdsF 5’- ATGGAGACACTAAGGAATAC -3’, plk2b-cdsR: 5’- TCAGTTGCATCGCTGCAGCAGCATG -3’, hPLK2-cdsF 5’- ATGGAGCTTTTGCGGACTAT -3’, hPLK2-cdsR: 5’- TCAGTTACATCTTTGTAAGA -3’. one cell stage Tg(kdrl:mcherry-ras) embryos were co-injected with 10 ng of plk2b MO1 or MO2 and 80 pg of zebrafish plk2b RNA, 80 pg of human PLK2 RNA, or 100 pg, 160 pg of the constitutively active human RAP1 (ca-RAP1) RNA (G12V). For mRNA overexpression experiments, one cell stage Tg(kdrl:mcherry-ras) or Tg(fli1a:eGFP) embryos were injected with 160 pg of plk2b RNA or 80 pg of the ca-RAP1 RNA. For all MO and RNA experiments, the average length of each ISV and the average number of branches per ISV were measured as described in zebrafish microscopy and imaging section.
Cell Culture and immunofluorescence
HUVECs were purchased from Lonza (Cat. No. CC-2157). Cells were cultured in Endothelial Basal Medium (Cat. No. CC-3121, Lonza) supplemented with 10% Fetal Calf Serum, bovine brain supplement, human recombinant epidermal growth factor, penicillin (50 U/ml) and streptomycin (50 µg/ml) (Cat. No. CC-4133, Lonza). Cells grown for 24 hours on coverslips were fixed with 4% PFA for 10 min at 37°C and processed for immunohistochemistry, which was performed using the following antibodies: anti-PLK2 (Cat. No. K2392, Sigma, 1:500), antipFAK (Cat. No. 611722, BD Biosciences, 1:500), anti-Integrin αVβ3 clone LM609 (Cat. No. MAB1976, Millipore, 1:500). Primary antibody incubations were followed by incubation with anti-rabbit (Cat. No. A11034, Life Technologies) or anti-mouse IgG Alexa 488 (Cat. No. A11029, Life Technologies). Alexa 568-conjugated phalloidin was used to detect F-actin (Cat. No. A12380, Life Technologies, 1:500) and DAPI (Cat. No. D9564, Sigma) was used to label nuclei. To quantitate the number of filopodia, lamellipodia, pFAK plaques, and integrin αVβ3 plaques in cell cultures, HUVECs were fixed with 4% PFA and stained with phalloidin, anti-pFAK and anti-integrin αVβ3, respectively. The number of filopodia (actin-rich finger-like protrusions crossing the cell edge), lamellipodia (regions of actin-rich fringe adjacent to the leading edge), pFAK plaques, and integrin plaques were counted for each cell (n = 10). Quantitation of the leading edge recruitment of PLK2 protein was performed using ImageJ software (Bivona et al., 2006). Regions of identical size were drawn around an area at the leading edge and at the cytosol faraway from the leading edge. The fluorescence intensity was determined for these areas of interest at each time point. Nine regions were used to calculate each condition from nine cells.
Zebrafish microscopy and imaging
Confocal, fluorescence and bright-field microscopy, as well as live imaging of zebrafish were performed using a Nikon C2 confocal microscope, a Leica DM IL LED and a Leica M205 FA stereomicroscope as previously described (Hegarty et al., 2013). Zebrafish embryos were dechorionated, anesthetized with tricaine, and mounted laterally in 1% agarose/egg water on a glass-bottom Petri dish (MatTek). Because of its strong GFP brightness, the Tg(fli1a:EGFP) transgenic line was used to perform time-lapse imaging as well as to track lamellipodia and filopodia extensions (n = 10 control MO, 10 plk2b MO1, 10 dn-plk2b mRNA, and 10 plk2b mRNA). As previously described (Lamalice et al., 2007; Phng et al., 2013), filopodia are defined as finger-like protrusions that cross the cell membrane’s edge and have widths <1 µm, and lamellipodia are defined as protrusions adjacent to the leading edge that have stronger and broader GFP signal and widths between 1 µm to 5 µm. To evaluate the relative ISV length as well as the number of filopodia, lamellipodia, and branching ECs in the zebrafish vasculature, three ISVs were measured from nine different embryos (total measured ISVs = 27) for each condition.
Flow cytometry
Tg(kdrl:mcherry-ras) embryos were treated with SU5416 or DMSO from 20 to 32 hpf and collected at 32 hpf. Tg(kdrl:mcherry-ras) and Tg(kdrl:mcherry-ras; Tg(hsp70:dn-MAML-GFP) embryos were heat-shocked from 20 to 32 hpf and collected at 32 hpf. 100 embryos for each condition were collected to generate one biologic replicate for cell sorting. Disaggregation into single-cell suspension was achieved as previously described (Bertrand et al., 2010). Endothelial cell sorting was performed on a FACS Aria 2 (Becton Dickinson, San Jose, CA) and quantitiative PCR was performed using cDNA from 10,000 sorted endothelial cells per biological replicate to test plk2b expression.
Expression analysis, quantitative PCR and RT-PCR
Whole-mount in situ hybridization was performed on 16, 24, 36 and 48 hpf zebrafish embryos as described previously (Hegarty et al., 2013) using a plk2a and a plk2b RNA probe. The plk2a and plk2b RNA probes were generated by PCR, using the following primers: plk2aF 5’-TTCTTCGCCATGACTTTTTCTGCCA -3’, plk2aR 5’-cttgatttaggtgacactatagaaAAGCATCCTCGATTTCCCA -3’, plk2bF 5’-ACTCAGGGCTTCATGCCAGAAACGC -3’, plk2bR 5’-cttgatttaggtgacactatagaaGTTGCATCGCTGCAGCAGCATGTTGA -3’, plk2bsenseF 5’-cttgatttaggtgacactatagaaACTCAGGGCTTCATGCCAGAAACGC -3’, plk2bsenseR 5’-GTTGCATCGCTGCAGCAGCATGTTGA -3’. Using Power SYBR Green PCR Master Mix (Cat. No. 4367659, Life Technologies), plk2b quantitative PCR experiments were performed from cDNA obtained from three biologic replicate pooled samples containing flow cytometry sorted fish endothelial cells for each represented condition. Primer sequences used are as follows: plk2aRT-F 5’-GCAGACACCGTGGCCAGAGTACTA -3’ and plk2aRT-R 5’-GCAGGCTCATGTGAGTGCCATTGT -3’; plk2bRT-F 5’-ACAACACGGTGGGCGTCCTTT - 3’ and plk2bRT-R 5’-TCAGCTGGAAGGTAGCGGACG -3’. hPLK2 reverse transcriptase (RT)-PCR experiments were performed from cDNA obtained from HUVECs. Primer sequences used for human RT-PCR are as follows: hPLK2F 5’- GAATGCCTTGAAGACAGTACCA -3’ and hPLK2R 5’- TCAGTTACATCTTTGTAAGA -3’. DNA gel band intensity was quantified using ImageJ software.
Small molecule treatment with zebrafish embryos
The VEGF pathway inhibitor SU5416 (Cat. No. S8442, Sigma) and the Polo-like kinase inhibitor, BI 2536 (Cat. No. 45706, Sigma) (Steegmaier et al., 2007), were dissolved in DMSO (Cat. No. 472301, Sigma) and diluted to 400 µmol/L and 2 µmol/L with egg water, respectively, to make the stock solution. For plk2b zebrafish expression assay, 20 hpf WT embryos were placed in a solution with 1 ml of 50% DMSO or SU5416 stock solution and 19 ml of egg water (final concentration: 2 µmol/L SU5416 with 2.5% DMSO). Embryos were fixed 10 hours post treatment and whole-mount in situ hybridization was performed as described previously (Hegarty et al., 2013). For Polo-like kinase inhibition assay, 20 hpf Tg(kdrl:GFP) embryos were placed in a solution with 1 ml of DMSO or BI 2536 stock solution and 19 ml of egg water (final concentration: 100 nmol/L) and imaging was performed 28 hours post treatment.
Heat shock experiments with zebrafish embryos
Tg(hsp70:dn-MAML-GFP) embryos were initially heat-shocked at 20 hpf in a 42 degree water bath for 5 mins, which was subsequently repeated every 4 hours until 32 hpf. GFP positive and negative labeled embryos were then fixed seperately at 32 hpf, and whole-mount in situ hybridization was performed as described previously (Hegarty et al., 2013).
siRNA transfections
The following human PLK2 siRNA (5’- GGACATGGCTGTGAATCAG -3’), which was previously confirmed to target human PLK2 (Lim et al., 2015; Mbefo et al., 2010; Warnke et al., 2004), as well as a non-targeting control siRNA (scrambled sequence) were purchased from Dharmacon. HUVECs were transfected using Lipofectamine RNAiMAX (Cat. No. 13778030, Life Technologies) according to the manufacturer's instructions.
Lentivirus production and endothelial cell transductions
Lenti-X 293T cells (Cat. No. 632180, Clontech Laboratories) were plated in 100 mmol/L dishes and incubated overnight. The cells were then transfected with second generation viral packaging vectors (Cat. No. TLP5912, Thermo scientific) as well as pWPI empty vectors (control) (Addgene plasmid 12254) or pWPI vector containing a ca-RAP1 expression cassette or dn-PLK2 (which only contains PLK2 PBD domain) expression cassette before the IRES-eGFP cassette. Virus-containing supernatant was collected after 48 hours, then filtered through a 0.45 µmol/L pore-size filter and supplemented with 5 mg/ml polybrene. The filtered virus-containing supernatants were then added to HUVECs that were seeded in 6-well plates one day before transduction, and then incubated overnight. Two days after transduction, the transduction efficiency was checked by GFP signal. This procedure typically resulted in infection of more than 90% of cells. Successfully transduced GFP positive HUVECs were then used for subsequent assays.
Two-dimensional tube formation assay
Basement membrane matrix (Cat. No. 356234, BD Biosciences) was thawed at 4°C overnight and diluted with an equal volume of serum-free Endothelial Basal Medium (Cat. No. CC-3121, Lonza). Each well of a 96-well plate was coated with 100 µl of mixed matrix, then incubated 30 minutes at 37°C. HUVECs transfected with control siRNA, PLK2 siRNA or ca-RAP1 virus were plated on this matrix-coated well, and then allowed to adhere and migrate for 4 hours at 37°C. Tube formation was quantified by counting the length of formed tubes using 100× magnification pictures that were captured and processed as described above. Each sample was assayed in two to three random fields and independently repeated three times.
Wound healing assay
HUVECs transfected with control siRNA, PLK2 siRNA or ca-RAP1 virus were seeded on 8-well chamber slides. To create an injury to the HUVEC monolayer, this cell monolayer was scraped with sterile pipette tips 48 hours after transfection, and then washed twice with PBS to remove floating cells. Photographs were taken 2 hours and 14 hours after wound formation, and the cell-free area percentage of each image was measured between these two time-points to calculate the wound closure ratio. Each sample was assayed in two to three random fields and independently repeated three times.
Transwell migration assay
HUVECs transfected with control siRNA, PLK2 siRNA or ca-RAP1 virus were counted and seeded on membrane inserts (8 µm pore size) (Cat. No. 3421, Costar) in the presence of 200 µl of Endothelial Basal Medium supplemented with 0.1 % BSA. The lower chamber contained 800 µl of Endothelial Basal Medium supplemented with 4% Fetal Calf Serum. Following a four hour incubation, cells that had migrated into the lower compartment were fixed in methanol for 10 minutes and stained with Crystal Violet for 10 minutes. Transmigrated cells were counted under a Leica M205 FA stereomicroscope. Each sample was assayed in two to three random fields and independently repeated three times.
HUVEC adhesion assay
Glass coverslips were coated with type I collagen (Cat. No. 354236, BD Biosciences, 10 µg/ml) or fibronectin (Cat. No. 354008, BD Biosciences, 5 µg/ml), and incubated one hour at 37°C. HUVECs were trypsinized, and 3000 cells were seeded per coverslip. After a 50 minute incubation at 37°C, the adherent HUVECs were fixed with 4% paraformaldehyde, and cell numbers were counted with DAPI staining.
HUVEC proliferation assay
To detect proliferating cells, HUVECs transfected with control or PLK2 siRNA were counted and seeded on 4-well chamber slides and incubated with 10 µM 5-bromo-2'-deoxyuridine (BrdU, Cat. No. B5002, Sigma) for 6 hours. HUVECs were then fixed with 4% PFA and imaged by immunofluorescence microscopy using an antibody against BrdU (Cat. No. OBT0030, abdserotec) which was then detected with a secondary antibody conjugated to Alexa Fluor 568. The percentage of cells undergoing proliferation was determined by dividing the number of BrdU positive cells by the total number of cells labeled with DAPI nuclear stain.
HUVEC cell death assay
HUVECs transfected with control or PLK2 siRNA were counted and seeded on 4-well chamber slides and grown for 48 hours. Transfected HUVECs were then fixed with 4% PFA and apoptotic cells were detected using an in situ cell death detection kit from Roche (# 2156792). The percentage of cells undergoing apoptosis was determined by dividing the number of TUNEL positive cells by the total number of cells labeled with DAPI nuclear stain.
HUVEC RAP1 activity assay
RAP1 activity was measured using the RAP1 activation assay kit (Cat. No. 17–321, Millipore), which is based on the specific binding of a GST fusion protein containing the Rap-binding domain of RalGDS (RBD-GST) to the active GTP-bound form of RAP1. Briefly, the GTP-bound RAP1 was pulled down using RBD-GST, which was immobilized to glutathione agarose beads. Precipitated GTP-bound RAP1 and total RAP1 as input control were identified by SDS-PAGE (12%) gels and western blot using a polyclonal anti-RAP1 antibody. RAP1-GTP and total RAP1 band intensities were quantified using ImageJ software.
Western blot analysis
Western blots were performed according to the manufacturer’s instructions using the following primary antibodies: anti-PLK2 (Cat. No. K2392, Sigma, 1:1000), anti-GAPDH (Cat. No. GT239, GeneTex, 1:500) and anti-PDZ-GEF1 (Cat. No. WH0009693M1, Sigma, 1:1000). Gel band intensity was quantified using ImageJ software.
PLK2 and PDZ-GEF1 co-immunoprecipitation assay
HUVECs were rapidly lysed at 4°C, and the whole-cell lysates were precleared with protein A-agarose beads (Cat. No. 11719408001, Roche) for three hours at 4°C. The supernatants were incubated overnight at 4°C with 1 µg of rabbit anti-PLK2 antibody (Cat. No. K2392, Sigma) or rabbit immunoglobulin G (IgG) as a negative control. On the next day, 50 µL of protein A-agarose beads were added to the immunoprecipitation tube and incubated at 4°C for three hours. After washing, the bead/protein complexes were boiled in sample loading buffer and then identified by SDS-PAGE and western blot using anti-PDZ-GEF1 (Cat. No. WH0009693M1, Sigma).
Statistical analyses
Quantitative statistical data are shown as the mean ± standard error of the mean (SEM) for 3 to 4 separate experiments. P-values of group comparisons were obtained by using unpaired two tailed Student’s t-test analysis. Multi-group statistical significance was evaluated by one-way analysis of variance (ANOVA) with Turkey's multiple comparison test. Statistical significance was concluded when P-values were <0.05.
Results
Endothelial cell expression of PLK2 is conserved in vertebrates
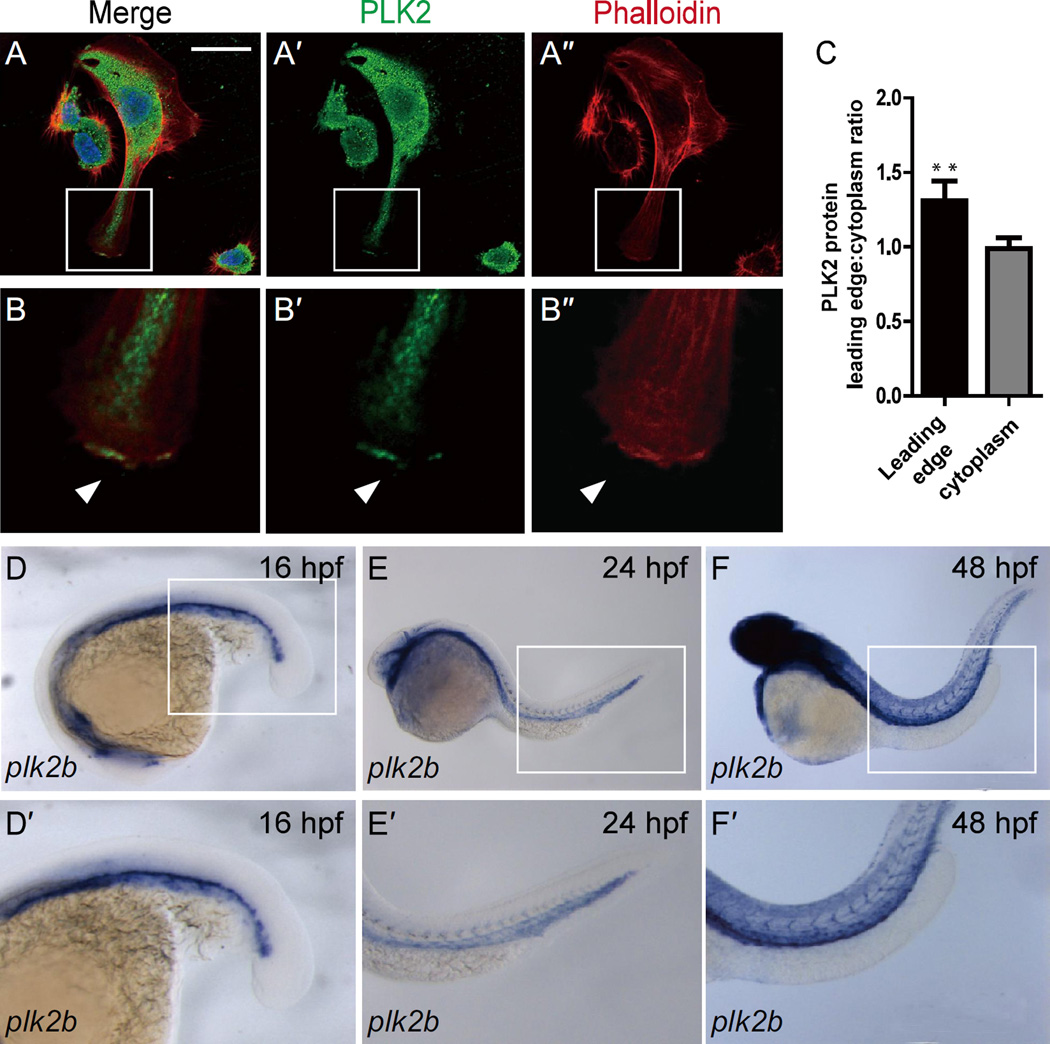
Because PLK2 has been recently reported to regulate RAP1 activity in neurons (Lee et al., 2011a; Lee et al., 2011b) and RAP1 is required for angiogenesis (Carmona et al., 2009; Lakshmikanthan et al., 2011), we postulated that PLK2 may also mediate endothelial cell sprouting. Thus, we initially examined the expression of PLK2 in both HUVECs and the developing zebrafish vasculature (Figure 1; Figure S1, S2). Reverse transcription (RT)-PCR and western blot analysis revealed that PLK2 transcript and protein, respectively, were expressed in HUVECs (Figure S1A–C). Furthermore, immunohistochemistry showed that PLK2 can also aggregate in regions of the lamellipodia of migrating HUVECs as detected by the colocalization of PLK2 with the thick cortical network of actin filaments in the extending EC membrane (Figure 1A–C, arrowheads). Protein sequence comparison using the ClustalW program revealed that PLK2 is highly conserved across vertebrate species (Figure S3A) and genetic analysis showed that the plk2 zebrafish ortholog may be gene duplicated, resulting in a plk2a and a plk2b gene on chromosome 10 and 8, respectively (Figure S3B, C). Additionally, zebrafish Plk2a and Plk2b proteins share 74% and 70% homology with human PLK2, and plk2b appeared to be more closely syntenic than plk2a to mammalian PLK2 (Figure S3B). Whole mount in situ analysis showed that plk2b was expressed predominantly in the developing vasculature further supporting the conserved EC role of PLK2 (Figure 1D–F, Figure S2), whereas plk2a appeared to be mainly expressed in the nervous system consistent with PLK2’s function in neurons (Figure S4A–D, yellow arrowheads). As a result, we focused on the role of zebrafish Plk2b to further understand the role of PLK2 in vascular development.
Figure 1. PLK2 is expressed in endothelial cells.
(A–B”’) Immunostaining of HUVECs reveals that PLK2 is expressed in ECs. (B-B”’) Enlarged image of the boxed area in A-A”’ shows that PLK2 can aggregate at the leading edge of extending ECs (arrowhead). (A, B) - merge; (A’, B’) - anti-PLK2 immunostaining (green); (A”, B”) - phalloidin actin staining (red). DAPI nuclear staining (blue). Scale bar, 40 µm. (C) Quantitative measurements of PLK2 localized at the leading edge and in the cytoplasm of HUVECs (Mean +/− s.e.m. *p = 0.0056 by Student's t-test). (D–F) in situ hybridization shows that plk2b is expressed in the zebrafish vasculature at 16 hpf (n = 31/31), 24 hpf (n = 46/47), and 48 hpf (n = 28/30). (D’–F’) Enlarged image of the boxed area of D–F shows that plk2b is expressed in the cardinal vein, aorta, and intersomitic vessels of the zebrafish body and tail.
Because VEGF and Notch signaling can promote and inhibit angiogenesis, respectively, we investigated whether these signaling pathways may alter PLK2 expression. As a result, we observed that both PLK2 transcript and protein levels were increased in VEGF-treated HUVECs (Figure S1A–C), whereas zebrafish embryos treated with the VEGF inhibitor SU5416 at 20 hpf exhibited significantly reduced plk2b EC expression by 32 hpf (Figure S1E, H). In contrast, Notch inhibition in Tg(hsp70:dn-MAML-GFP) zebrafish embryos at 20 hpf resulted in increased plk2b expression by 32 hpf (Figure S1G, I). Finally, we observed that expression of plk2b, but not plk2a, was overall significantly downregulated by in situ and quantitative PCR in cloche mutants which lack ECs (Stainier et al., 1995) (Figure S5).
PLK2 is required for endothelial cell sprouting and migration
Because of its EC expression and localization at the leading edge of sprouting ECs, we next performed PLK2 knockdown and overexpression experiments in vivo and in vitro in zebrafish and HUVECs, respectively, to investigate whether PLK2 could regulate EC sprouting and migration. To this end, we generated a plk2b ATG MO (MO1) and a plk2b splice MO (MO2), which target the translation initiation site and its surrounding sequences and the splice junction boundary sequences between exon 2 and intron 2, respectively (Figure S6A–H). 10 ng of either MO1 or MO2 injected into zebrafish Tg(kdrl:mcherry-ras) embryos, in which endothelial cells are labeled in red, resulted in a significant reduction in intersomitic vessel (ISV) length (Figure 2B, C, I; Figure S6I). In particular, some ISVs failed to migrate dorsally beyond the horizontal myoseptum to form the dorsal longitudinal anastomotic vessel (DLAV) (Figure 2B, C, open arrowheads). Furthermore, injecting dominant negative plk2b RNA (dn-plk2b), which lacks the Plk kinase domain, into Tg(kdrl:mcherry-ras) zebrafish embryos or treating Tg(kdrl:GFP) zebrafish embryos with the polo-like kinase inhibitor, BI 2536, could also reduce ISV length (Figure 2D, I; Figure S6I, K). Finally, we observed that these loss of plk2b function embryos appeared to otherwise develop and swim normally compared to age-matched MO controls (Figure S7), supporting that the phenotypic defects of the loss of plk2b function embryos were vascular-specific.
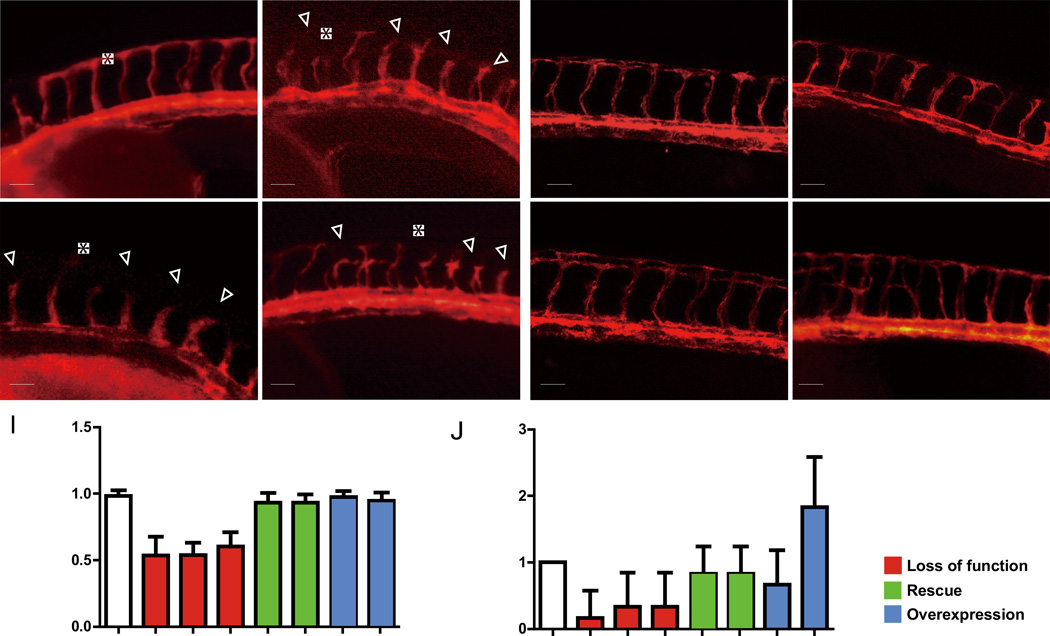
Figure 2. plk2b regulates endothelial cell sprouting.
(A–D) Fluorescence micrographs show that loss of plk2b function in Tg(kdrl:mcherry-ras) zebrafish embryos results in underdeveloped intersomitic vessel sprouting (open arrowheads) at 36 hpf. (A) control MO (n = 0/149); (B) plk2b ATG MO (MO1) (n = 125/171); (C) plk2b splice MO (MO2) (n = 89/137); (D) dn-plk2b RNA – dominant negative plk2b (n = 49/85). Injecting (E) 80 pg of plk2b (n = 31/103) or (F) 80 pg of hPLK2 (n = 24/71) can rescue the plk2b MO1 vascular sprouting defect. (G) Injecting 80 pg of plk2b RNA into wild-type Tg(kdrl:mcherry-ras) embryos did not cause any significant vascular phenotypes (n = 0/105). However, (H) injecting 160 pg of plk2b resulted in increased ISV spouting and branches (arrowheads) (n = 91/145). Top, dorsal longitudinal anastomotic vessel (yellow asterisk); bottom, dorsal aorta/cardinal vein. Scale bar, 80 µm. Quantitative measurements of (I) intersomitic vessel length and (J) the number of ISV branches for each corresponding condition. Mean +/− s.e.m. *p<0.05 by ANOVA.
To validate the effect of the plk2b MOs, we attempted to rescue the plk2b morphant knockdowns by co-injecting zebrafish plk2b or human PLK2 RNA. Co-injecting 80 pg of wild-type zebrafish plk2b mRNA or human PLK2 mRNA with plk2b MOs significantly reduced the ability of plk2b MOs to cause the EC sprouting defect (Figure 2E, F, I; Figure S6I), in effect rescuing the plk2b MO knockdowns. Although injecting 80 pg of wild-type plk2b into zebrafish wild-type embryos had no effect on zebrafish development (Figure 2G), injecting 160 pg of wild-type plk2b interestingly led to increased endothelial ISV branching and connections between neighboring ISVs at the horizontal myoseptum (Figure 2H, J, arrowheads; Figure S6I).
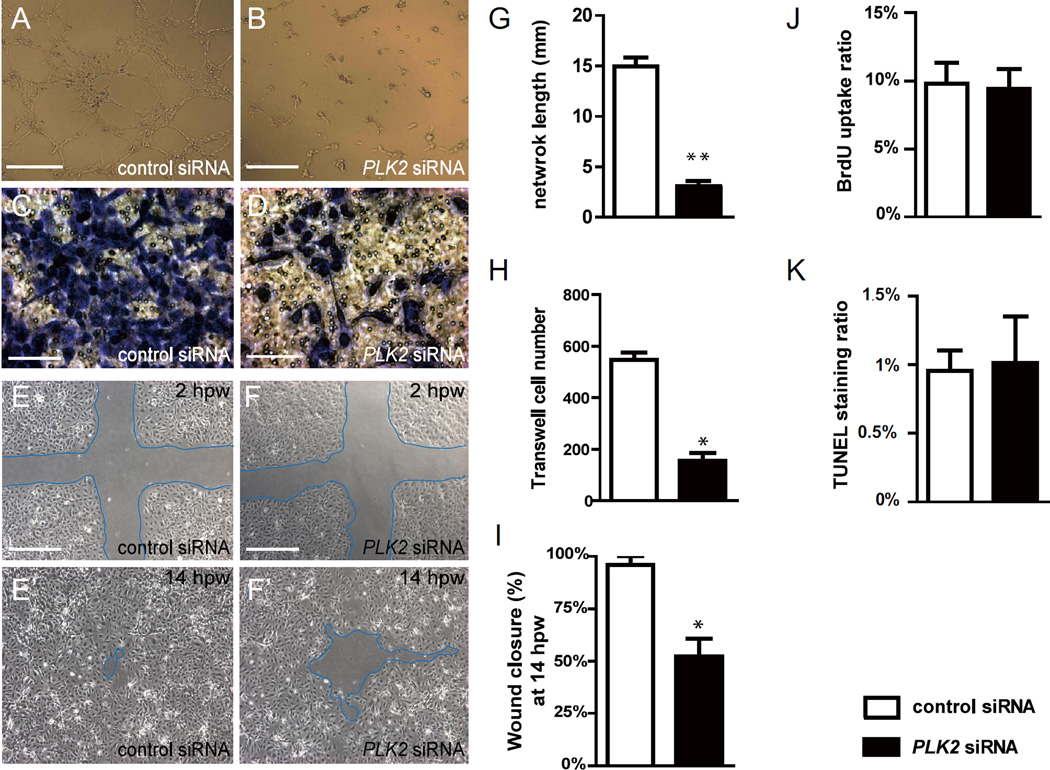
To further analyze the direct effects of PLK2 deficiency on EC migration, we disrupted PLK2 function in HUVECs using a PLK2 siRNA that has been shown to successfully inhibit PLK2 protein expression in human cells (Lim et al., 2015; Mbefo et al., 2010; Warnke et al., 2004). When compared to control siRNA, PLK2 siRNA knockdown inhibited the ability of VEGF and bFGF stimulated HUVECs to form a network of interconnecting branches as well as to migrate as detected in in vitro tube formation assays and transwell migration assays, respectively (Figure 3A–D, G, H). Additionally, using wound healing assays where confluent monolayer cultures of HUVECs were wounded with a pipette tip, we observed that PLK2 siRNA HUVECs exhibited significantly delayed migration and wound healing closure when compared to controls (Figure 3E–F’, I). To confirm that the delay in wound healing was due to a cell migration defect rather than a cell proliferation or cell death defect, we performed BrdU labeling and TUNEL staining experiments and observed no significant difference in cell proliferation or cell apoptosis between PLK2 and control siRNA knockdowns (Figure 3J, K). Finally, we transfected HUVECs with human dominant negative PLK2 RNA (dn-PLK2) to confirm the PLK2 siRNA findings and discovered that dn-PLK2 RNA can also block the endothelial cell migration in in vitro tube formation, transwell migration and wound healing assays (Figure S8). Together, these studies show that PLK2 functions to regulate the migration of endothelial cells in vivo and in vitro.
Figure 3. PLK2 regulates endothelial cell migration.
(A, B) EC tube formation assays, (C, D) transwell EC migration assays, and (E–F’) wound healing EC assays show that when compared to control siRNA knockdown, PLK2 siRNA knockdown in HUVECs results in (B and G) decreased EC network/tube formation (p = 0.0028), (D and H) reduced EC migration formation (p = 0.0185), and (F, F’ and I) slower EC wound healing/closure, respectively (p = 0.0114). (J–K) Quantitative measurements of the percentage of (J) BrdU positive cells and (K) TUNEL staining positive cells in control and PLK2 siRNA transfected HUVECs show that PLK2 knockdown does not affect HUVEC cell proliferation (p = 0.6007) and apoptosis (p = 0.7970). (A, B, E–F’) Scale bar, 1.6 mm. (C, D) Scale bar, 400 µm. hpw – hours post wounding. Mean +/− s.e.m. *p<0.05, **p<0.01 by Student's t-test.
PLK2 regulates lamellipodia but not filopodia formation through mediating EC cell adhesion
Angiogenesis is dependent on the initiation and stabilization of EC cell membrane extensions to form sprouting EC branches. In order to further investigate how PLK2 may regulate angiogenesis, we examined both filopodia and lamellipodia formation in vivo using time-lapse imaging of the zebrafish vasculature as well as in vitro using HUVECs. We observed that loss of plk2b function in zebrafish Tg(fli1a:eGFP) embryos and loss of PLK2 function in HUVECs resulted in significantly reduced lamellipodia numbers when compared to controls (Figure 4A, B, G, H; Figure S9A–C; Movie S1–S3). Conversely, in zebrafish overexpressing plk2b, we observed increased lamellipodia numbers and endothelial branches (Figure 2H, J; Figure 4G; Figure S6I; Figure S9D, Movie S4). However, we did not observe a significant difference in filopodia numbers after perturbing PLK2 function in HUVECs and zebrafish embryos (Figure 4G, H).
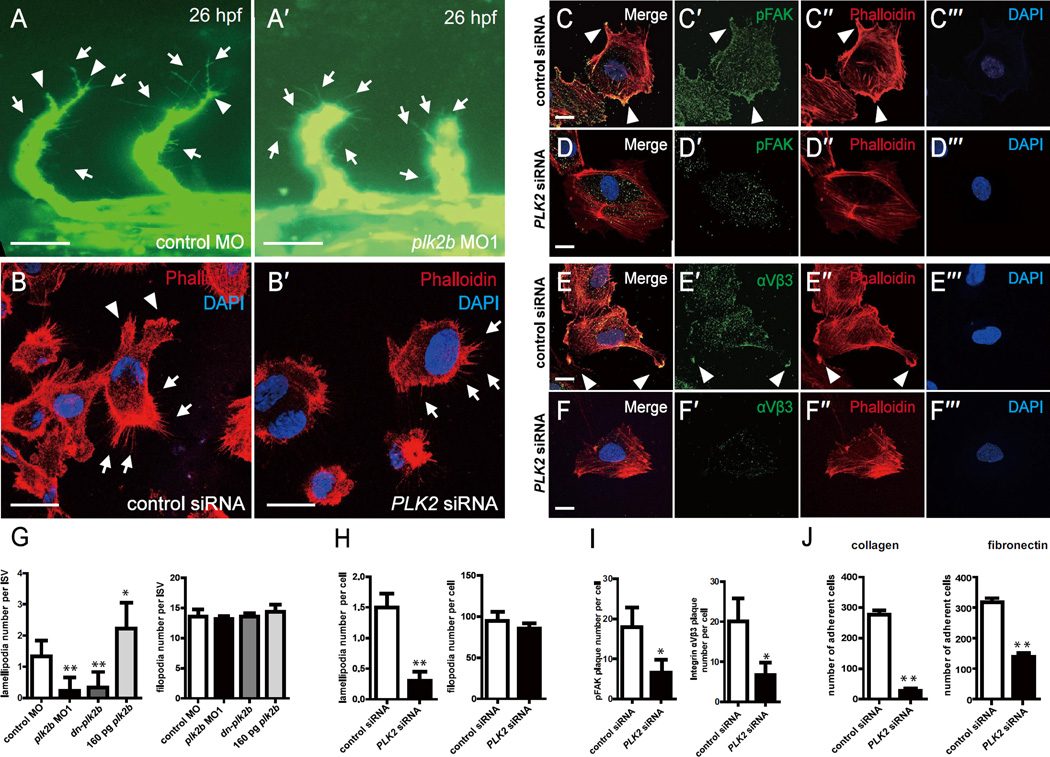
Figure 4. PLK2 regulates endothelial cell lamellipodia and cell adhesion formation.
(A-A’) 26 hpf plk2b MO1 injected Tg(fli1a:eGFP) fish (n = 31/40) exhibit fewer lamellipodia, and extending intersomitic vessels compared to age-matched control MO injected Tg(fli1a:eGFP) fish (n = 0/48). Scale bar, 40 µm. Top, dorsal longitudinal anastomotic vessel; bottom, dorsal aorta/cardinal vein. (B-B’) Phalloidin staining reveals that PLK2 siRNA transfected HUVECs display reduced lamellipodia when compared to control siRNA transfected HUVECs. Scale bar, 20 µm. Immunostaining of (C-C”’, D-D”’) pFAK and (E-E”’, F-F”’) integrin αVβ3 shows that focal adhesions and integrins, respectively, are localized to the lamellipodia of migrating/extending (C, E) control siRNA transfected HUVECs, but they fail to organize and aggregate in (D, F) PLK2 siRNA transfected HUVECs. Scale bar, 10 µm. (G) Quantitative measurements of the number of lamellipodia and filopodia reveal that zebrafish plk2b knockdowns have reduced lamellipodia but relatively the same number of filopodia (finger-like protrusions crossing the cell edge) when compared to controls. Conversely, zebrafish RNA injection of 160 pg plk2b resulted in more lamellipodia only. (H) Quantitative measurements of the number of lamellipodia and filopodia reveal that human PLK2 knockdowns have reduced lamellipodia (p = 0.0023) but relatively the same number of filopodia (p = 0.2615) when compared to controls. (I) Quantitative measurements of the number of pFAK (p = 0.0143) and integrin αVβ3 plaques (p = 0.0224) reveals that knockdown of PLK2 reduced the number of cell adhesions in HUVECs. (J) EC adhesion assays show that PLK2 siRNA HUVECs adhered less to type I collagen (p = 0.0017) and fibronectin-coated coverslips (p = 0.0061) compared to control siRNA HUVECs. Arrowheads and arrows point to lamellipodia and filopodia, respectively. Red – phalloidin/actin staining; Blue – DAPI staining; Green – (A) GFP, (C–D) pFAK, or (E, F) integrin αVβ3. Mean +/− s.e.m. *p<0.05, **p<0.01 by ANOVA for G and Student's t-test for H–J.
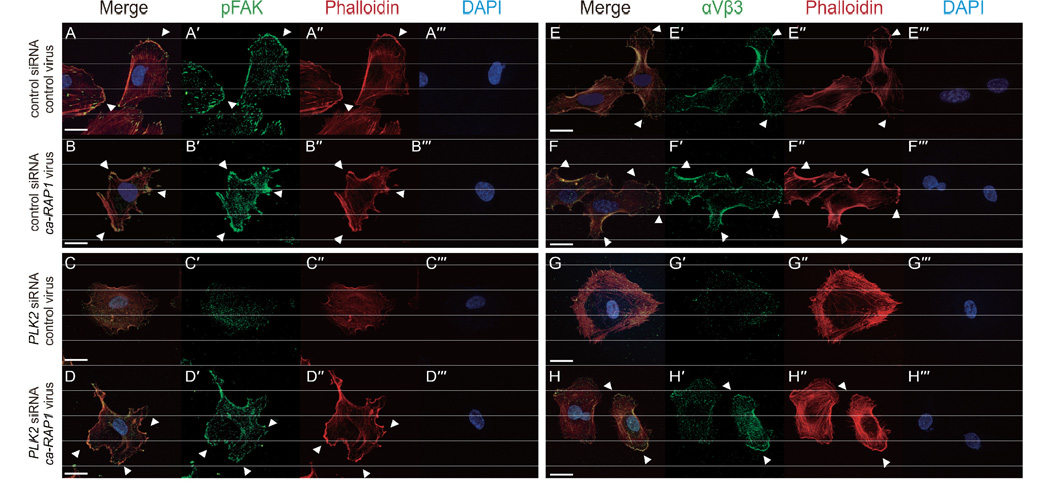
Because integrin-dependent focal adhesions help to stabilize and maintain EC lamellipodia protrusions during angiogenesis, we next examined the localization of phosphorylated focal adhesion kinase (pFAK) and integrin αVβ3 in control and PLK2 siRNA transfected HUVECs. PLK2 siRNA transfected HUVECs failed to form stable cell adhesion plaques as detected by pFAK and integrin αVβ3 immunostaining and to organize actin filaments to develop a leading edge for lamellipodia protrusions (Figure 4C–F, I). As a result, we observed reduced numbers of PLK2 siRNA transfected HUVECs adhering to either collagen or fibronectin coated coverslips (Figure 4J). Overall, these studies reveal that PLK2 may mediate angiogenesis through regulating EC adhesion and lamellipodia stabilization.
RAP1 acts downstream of PLK2 to regulate endothelial cell sprouting and migration
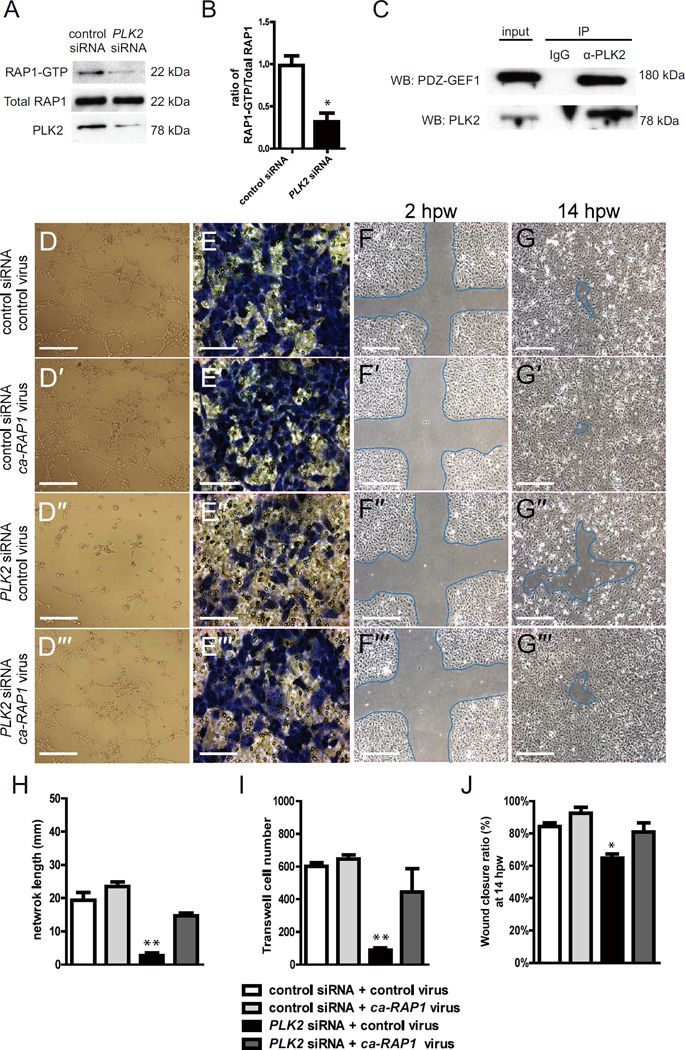
Previous studies have shown that PLK2 can mediate sprouting in neuronal cells through regulating the ability of PDZ-GEF1 to exchange GDP for GTP on RAP1 and thereby activating RAP1 (Lee et al., 2011b). Because RAP1 has also been recently reported to mediate angiogenesis through the phosphorylation of FAK and the subsequent activation of integrins (Carmona et al., 2009), we investigated whether PLK2 may regulate RAP1 during EC focal adhesion formation and EC migration. Using a RAP1-GTP binding assay (de Rooij and Bos, 1997), which monitors RAP1 activity through the analysis of RAP1-GTP binding to a GST fusion protein containing the RAP-binding domain (RBD) of RalGDS (RBD-GST), we examined the levels of activated RAP1-GTP in PLK2 and control siRNA transfected HUVECs. As a result, we observed reduced activated RAP1-GTP in PLK2 siRNA transfected HUVECs when compared to controls, suggesting that PLK2 may be required to activate RAP1 (RAP1-GTP) in ECs to bind to its effector (Figure 5A, B). Finally, to investigate whether PLK2 in ECs may regulate RAP1 activity through PDZ-GEF1 as observed in neurons, we performed PLK2 immunoprecipitations in HUVECs and observed that PDZ-GEF1 could interact in a protein complex with PLK2 (Figure 5C).
Figure 5. PLK2 regulates HUVEC migration in vitro via RAP1 activity.
(A) RAP1 immunoblot of RAP1-GTP binding assays shows that there is less RAP1-GTP in PLK2 siRNA HUVECs than in control siRNA HUVECs (22 kDa). PLK2 immunoblot shows the efficiency of PLK2 siRNA knockdown in HUVECs. (B) Quantitative measurements shows that the ratio of RAP1-GTP/Total RAP1 is less in PLK2 siRNA (Mean +/− s.e.m. *p = 0.015 by Student's t-test). (C) Immunoprecipitation (IP) of HUVEC lysates with anti-PLK2 antibody reveals that PLK2 interacts in a complex with PDZ-GEF1 (180 kDa). Western analyses of immunoprecipitations were performed with anti-PDZ-GEF (WB: PDZ-GEF) and anti-PLK2 antibodies (WB: PLK2). IP IgG – control antibody; α-PLK2 – anti-PLK2 antibody. (D) EC tube formation assays, (E) Transwell EC migration assays, and (F, G) wound healing EC assays show that ca-RAP1 virus can rescue the PLK2 siRNA knockdown HUVEC (compare D” to D”’) EC network/tube formation, (compare E” to E”’) cell migration, and (compare F”, G” to F”’, G”’) wound healing defects. (D-D”’, F-F”’, G-G”’) Scale bar, 1.6 mm. (E-E”’) Scale bar, 400 µm. (H–J) Quantitative measurements were performed on (H) EC tube formation assays, (I) transwell EC migration assays, and (J) wound healing EC assays. hpw - hours post wounding. Mean +/− s.e.m. *p<0.05, **p<0.01 by ANOVA for H–J.
Because these data suggest that PLK2 may interact with PDZ-GEF to activate Rap1 to mediate focal adhesion assembly, we investigated whether a constitutively active RAP1 (ca-RAP1) (Brinkmann et al., 2002) protein might be able to rescue the PLK2 knockdown angiogenesis phenotype. To this end, we infected the ca-RAP1 virus into PLK2 siRNA HUVECs and discovered that the ca-RAP1 virus could partially rescue the EC migration and sprouting defects observed in PLK2 knockdown HUVECs (compare Figure 5D”–G” to D”’–G”’, H–J). Additionally, we attempted to rescue the zebrafish plk2b MO knockdowns with ca-RAP1 RNA. Co-injecting 100 pg of ca-RAP1 RNA with plk2b MO1 significantly reduced the ability of plk2b MO1 to cause the EC sprouting defect (Figure S10A; Figure S6I), in effect rescuing the plk2b MO1 knockdowns. Moreover, co-injecting higher amounts of ca-RAP1 RNA (160 pg) led to not only rescue of the plk2b MO1 EC defect, but also some ectopic EC sprouting into the somites (Figure 10B, yellow arrowheads, D; Figure S6I). Consistent with these findings, injecting 80 pg of ca-RAP1 RNA into wild-type embryos also resulted in ectopic EC sprouting as well (Figure S10C, yellow arrowheads, D; Figure S6I). To investigate how ca-RAP1 might rescue the PLK2 knockdown HUVEC migration defects, we examined the ability of ca-RAP1 infected PLK2 siRNA HUVECs to develop focal adhesions. We discovered that the ca-RAP1 virus could rescue the reduced numbers of focal adhesions as well as the type I collagen and fibronectin cell adhesion defects in PLK2 siRNA HUVECs when compared to control virus infected PLK2 siRNA HUVECs (compare Figure 6C, G to D, H; Figure S11 A–D). Although ca-RAP1 could rescue the PLK2 knockdown angiogenesis phenotype through restoring lost focal adhesions in HUVECs, interestingly, we did not observe a significant increase in EC sprouting, migration and focal adhesion formation between control and ca-RAP1 infected HUVECs (compare Figure 5D–G to D’–G’, H–J; Figure 6A, E to B, F; Figure S11A, B), suggesting that RAP1 may be required but not sufficient for EC sprouting in vitro. Overall, these studies reveal that PLK2 may mediate angiogenesis through regulating RAP1 activity and localization.
Figure 6. PLK2 regulates focal adhesion formation and integrin localization through RAP1 activity.
(A–H) Immunofluorescence studies show that ca-RAP1 virus can rescue the reduced EC sprouting and lamellipodia defects observed in PLK2 siRNA HUVECs through restoring focal adhesion formation (compare C to D) and integrin αVβ3 organization (compare G to H). Arrowheads point to lamellipodia extension as detected by the organization of F-actin (phalloidin) at the leading edge of extending HUVEC membranes. Green – (A’–−D’) anti-pFAK; or (E’–H’) anti-integrin αVβ3; Red – phalloidin; Blue – DAPI. Scale bar, 20 µm.
Discussion
Angiogenesis is dependent on endothelial tip cells to lead new blood vessels during vascular development. In response to guidance cues, these endothelial tip cells directionally extend their membranes through a series of organized cellular processes including the development of filopodia and lamellipodia. Here, we show that PLK2 specifically regulates EC lamellipodia but not filopodia formation to direct EC sprouting and angiogenesis. Consistent with its recently reported role in neurons (Lee et al., 2011a; Lee et al., 2011b), we observed that PLK2 can control RAP1 activity in ECs. Although it remains unclear as to precisely how PLK2 may regulate neuronal sprouting, we discovered that in ECs, PLK2 interacts with PDZ-GEF to activate RAP1 in order to regulate the formation of EC focal adhesions and the subsequent growth of lamellipodia. Similar to the loss of RAP1 function EC defects (Carmona et al., 2009; Lakshmikanthan et al., 2011), loss of PLK2 function results in reduced EC attachment, lamellipodia structures, and overall angiogenesis.
Previous studies have reported that PLK2 is expressed in Xenopus and mouse embryonic brains and blood vessels (Duncan et al., 2001; Visel et al., 2004). Furthermore, it has been shown to be more than 2.5-fold greater in mouse tip ECs than in stalk ECs (del Toro et al., 2010). In line with these findings, we discovered that PLK2 is also expressed in both HUVECs and the zebrafish vasculature and is further regulated by the VEGF and Notch signaling pathways, which are known to control angiogenesis. Thus, these findings suggest that PLK2’s function may be conserved across vertebrates to regulate blood vessel formation.
In support of its role in angiogenesis, PLK2 knockdowns resulted in EC sprouting and migration defects in both HUVECs and zebrafish. Because PLK2 was previously shown to regulate RAP1 through interactions with PDZ-GEF in neurons (Lee et al., 2011b) and RAP1 is crucial for EC sprouting (Carmona et al., 2009; Chrzanowska-Wodnicka et al., 2008; Lakshmikanthan et al., 2011; Wei et al., 2007), we attempted to rescue the PLK2 knockdown EC defect using a constitutively activated RAP1 (ca-RAP1) protein. We discovered that ca-RAP1 could partially rescue this phenotype suggesting that PLK2 may regulate more than RAP1 activity during EC sprouting and migration. Given that other small GTPases, including RhoA, Rac1, and Cdc42, have been reported to regulate endothelial tip cell filopodia, lamellipodia, cell adhesion, and stress fiber formation (Kiosses et al., 2001; Spindler et al., 2010; Wojciak-Stothard et al., 1998), it is possible that PLK2 may regulate these other small GTPases through interactions with their respective GEFs. In particular, we postulate that a likely additional candidate that PLK2 may regulate would be Rac1 because of its role in lamellipodia formation. Finally, it is noteworthy that ca-RAP1 in control ECs did not result in a significant increase in EC sprouting, suggesting that RAP1 is required but not sufficient for EC sprouting. Thus, it will be particularly interesting in the future to examine whether PLK2 may regulate other small GTPases during angiogenesis.
Actin-rich filopodia and lamellipodia protrusions have been proposed to drive EC sprouting and vascular patterning. Filopodia are long spike-like plasma membrane extensions comprised of tight parallel bundles of filamentous actin (F-actin). On the other hand, lamellipodia are short veil-like structures that are in close proximity to the plasma membrane and exhibit a highly branched actin network (Huber et al., 2003; Mattila and Lappalainen, 2008; Pollard and Borisy, 2003). In many cell types, both cellular processes are able to explore the environment for attractive and repulsive guidance cues (Huber et al., 2003), and form focal adhesions between the cytoskeleton and the ECM to control directional migration (Defilippi et al., 1999). Interestingly, in our knockdown studies, we discovered that PLK2-deficient ECs, which exhibit arrested EC sprouting and migration, were able to form filopodia but not lamellipodia, thus suggesting that filopodia may not be sufficient to promote EC sprouting. These results are consistent with those recently reported where ECs treated with low levels of Latrunculin B, which inhibits F-actin polymerization and filopodia formation, were still able to form lamellipodia and sprout, albeit inefficiently (Phng et al., 2013). However, these EC findings are in contrast to studies that report that filopodia may be sufficient to guide neuronal and retinal growth cones (Bentley and Toroian-Raymond, 1986; Chien et al., 1993; Zheng et al., 1996). Although filopodia may not be enough to promote EC sprouting and migration, they may be still required for proper vascular development including efficient EC migration and anastomosis as previously suggested (Phng et al., 2013). Lastly, our data also suggests that lamellipodia may not be required to form filopodia as we did not observe any demonstrable filopodia defects in the PLK2 knockdown ECs, which exhibited significantly decreased lamellipodia. Thus, future detailed EC cellular studies will be necessary to further examine the specific roles of filopodia and lamellipodia in EC sprouting and migration.
Finally, although Polo-like kinase proteins have been shown to regulate the cell cycle, they may also participate in regulating angiogenesis and vascular development. Here, we showed that PLK2 is expressed in the vascular system and functions to control vascular development through regulating small GTPases involved in lamellipodia formation of ECs during angiogenesis. Furthermore, PLK3 has been shown to suppress tumor angiogenesis through phosphorylating HIF-1α and PTEN, which destabilizes HIF-1α and stabilizes PTEN, a known regulator of HIF-1α and tumor angiogenesis (Xu et al., 2012; Xu et al., 2010a; Xu et al., 2010b; Yang et al., 2008). Given the biological relationship between angiogenesis, tissue development, and tumor growth, these findings raise the possibility that other PLK family members may also regulate angiogenesis and support further studies to explore their role in vascular development.
Supplementary Material
Highlights.
The Polo-like Kinase, PLK2, is expressed in zebrafish and human endothelial cells.
PLK2 is required for endothelial cell sprouting.
PLK2 regulates endothelial cell lamellipodia and cell adhesion formation.
PLK2 regulates endothelial cell migration and sprouting via Rap1 activity.
PLK2 regulates focal adhesion formation and integrin localization through Rap1.
Acknowledgements
We thank Neil Tedeschi for expert help with the fish and Mark Ginsberg’s lab for ca-RAP1 plasmids. This work was supported in part by grants from the American Heart Association to H.Y. (12POST12050080); and the NIH to N.C.C (HD069305), N.C.C. and J.G.G. (HD070494), T.K.H. (HL111437), and Y.I.M. (HL093767).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference
- Adams RH, Alitalo K. Molecular regulation of angiogenesis and lymphangiogenesis. Nature reviews. Molecular cell biology. 2007;8:464–478. doi: 10.1038/nrm2183. [DOI] [PubMed] [Google Scholar]
- Bedell VM, Yeo SY, Park KW, Chung J, Seth P, Shivalingappa V, Zhao J, Obara T, Sukhatme VP, Drummond IA, Li DY, Ramchandran R. roundabout4 is essential for angiogenesis in vivo. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:6373–6378. doi: 10.1073/pnas.0408318102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentley D, Toroian-Raymond A. Disoriented pathfinding by pioneer neurone growth cones deprived of filopodia by cytochalasin treatment. Nature. 1986;323:712–715. doi: 10.1038/323712a0. [DOI] [PubMed] [Google Scholar]
- Bertrand JY, Chi NC, Santoso B, Teng S, Stainier DY, Traver D. Haematopoietic stem cells derive directly from aortic endothelium during development. Nature. 2010;464:108–111. doi: 10.1038/nature08738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bivona TG, Quatela S, Philips MR. Analysis of Ras activation in living cells with GFP-RBD. Methods in enzymology. 2006;407:128–143. doi: 10.1016/S0076-6879(05)07012-6. [DOI] [PubMed] [Google Scholar]
- Brinkmann T, Daumke O, Herbrand U, Kuhlmann D, Stege P, Ahmadian MR, Wittinghofer A. Rap-specific GTPase activating protein follows an alternative mechanism. The Journal of biological chemistry. 2002;277:12525–12531. doi: 10.1074/jbc.M109176200. [DOI] [PubMed] [Google Scholar]
- Carmeliet P, Tessier-Lavigne M. Common mechanisms of nerve and blood vessel wiring. Nature. 2005;436:193–200. doi: 10.1038/nature03875. [DOI] [PubMed] [Google Scholar]
- Carmona G, Gottig S, Orlandi A, Scheele J, Bauerle T, Jugold M, Kiessling F, Henschler R, Zeiher AM, Dimmeler S, Chavakis E. Role of the small GTPase Rap1 for integrin activity regulation in endothelial cells and angiogenesis. Blood. 2009;113:488–497. doi: 10.1182/blood-2008-02-138438. [DOI] [PubMed] [Google Scholar]
- Chi NC, Shaw RM, De Val S, Kang G, Jan LY, Black BL, Stainier DY. Foxn4 directly regulates tbx2b expression and atrioventricular canal formation. Genes & development. 2008;22:734–739. doi: 10.1101/gad.1629408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chien CB, Rosenthal DE, Harris WA, Holt CE. Navigational errors made by growth cones without filopodia in the embryonic Xenopus brain. Neuron. 1993;11:237–251. doi: 10.1016/0896-6273(93)90181-p. [DOI] [PubMed] [Google Scholar]
- Chrzanowska-Wodnicka M, Kraus AE, Gale D, White GC, 2nd, Vansluys J. Defective angiogenesis, endothelial migration, proliferation, and MAPK signaling in Rap1b-deficient mice. Blood. 2008;111:2647–2656. doi: 10.1182/blood-2007-08-109710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Rooij J, Bos JL. Minimal Ras-binding domain of Raf1 can be used as an activationspecific probe for Ras. Oncogene. 1997;14:623–625. doi: 10.1038/sj.onc.1201005. [DOI] [PubMed] [Google Scholar]
- De Smet F, Segura I, De Bock K, Hohensinner PJ, Carmeliet P. Mechanisms of vessel branching: filopodia on endothelial tip cells lead the way. Arteriosclerosis, thrombosis, and vascular biology. 2009;29:639–649. doi: 10.1161/ATVBAHA.109.185165. [DOI] [PubMed] [Google Scholar]
- Defilippi P, Olivo C, Venturino M, Dolce L, Silengo L, Tarone G. Actin cytoskeleton organization in response to integrin-mediated adhesion. Microscopy research and technique. 1999;47:67–78. doi: 10.1002/(SICI)1097-0029(19991001)47:1<67::AID-JEMT7>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- del Toro R, Prahst C, Mathivet T, Siegfried G, Kaminker JS, Larrivee B, Breant C, Duarte A, Takakura N, Fukamizu A, Penninger J, Eichmann A. Identification and functional analysis of endothelial tip cell-enriched genes. Blood. 2010;116:4025–4033. doi: 10.1182/blood-2010-02-270819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan PI, Pollet N, Niehrs C, Nigg EA. Cloning and characterization of Plx2 and Plx3, two additional Polo-like kinases from Xenopus laevis. Experimental cell research. 2001;270:78–87. doi: 10.1006/excr.2001.5333. [DOI] [PubMed] [Google Scholar]
- Gerhardt H, Golding M, Fruttiger M, Ruhrberg C, Lundkvist A, Abramsson A, Jeltsch M, Mitchell C, Alitalo K, Shima D, Betsholtz C. VEGF guides angiogenic sprouting utilizing endothelial tip cell filopodia. The Journal of cell biology. 2003;161:1163–1177. doi: 10.1083/jcb.200302047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hegarty JM, Yang H, Chi NC. UBIAD1-mediated vitamin K2 synthesis is required for vascular endothelial cell survival and development. Development. 2013;140:1713–1719. doi: 10.1242/dev.093112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellstrom M, Phng LK, Hofmann JJ, Wallgard E, Coultas L, Lindblom P, Alva J, Nilsson AK, Karlsson L, Gaiano N, Yoon K, Rossant J, Iruela-Arispe ML, Kalen M, Gerhardt H, Betsholtz C. Dll4 signalling through Notch1 regulates formation of tip cells during angiogenesis. Nature. 2007;445:776–780. doi: 10.1038/nature05571. [DOI] [PubMed] [Google Scholar]
- Herbert SP, Huisken J, Kim TN, Feldman ME, Houseman BT, Wang RA, Shokat KM, Stainier DY. Arterial-venous segregation by selective cell sprouting: an alternative mode of blood vessel formation. Science. 2009;326:294–298. doi: 10.1126/science.1178577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber AB, Kolodkin AL, Ginty DD, Cloutier JF. Signaling at the growth cone: ligand-receptor complexes and the control of axon growth and guidance. Annual review of neuroscience. 2003;26:509–563. doi: 10.1146/annurev.neuro.26.010302.081139. [DOI] [PubMed] [Google Scholar]
- Jin SW, Beis D, Mitchell T, Chen JN, Stainier DY. Cellular and molecular analyses of vascular tube and lumen formation in zebrafish. Development. 2005;132:5199–5209. doi: 10.1242/dev.02087. [DOI] [PubMed] [Google Scholar]
- Kiosses WB, Daniels RH, Otey C, Bokoch GM, Schwartz MA. A role for p21-activated kinase in endothelial cell migration. The Journal of cell biology. 1999;147:831–844. doi: 10.1083/jcb.147.4.831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiosses WB, Shattil SJ, Pampori N, Schwartz MA. Rac recruits high-affinity integrin alphavbeta3 to lamellipodia in endothelial cell migration. Nature cell biology. 2001;3:316–320. doi: 10.1038/35060120. [DOI] [PubMed] [Google Scholar]
- Lakshmikanthan S, Sobczak M, Chun C, Henschel A, Dargatz J, Ramchandran R, Chrzanowska-Wodnicka M. Rap1 promotes VEGFR2 activation and angiogenesis by a mechanism involving integrin alphavbeta(3) Blood. 2011;118:2015–2026. doi: 10.1182/blood-2011-04-349282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamalice L, Le Boeuf F, Huot J. Endothelial cell migration during angiogenesis. Circulation research. 2007;100:782–794. doi: 10.1161/01.RES.0000259593.07661.1e. [DOI] [PubMed] [Google Scholar]
- Larrivee B, Freitas C, Suchting S, Brunet I, Eichmann A. Guidance of vascular development: lessons from the nervous system. Circulation research. 2009;104:428–441. doi: 10.1161/CIRCRESAHA.108.188144. [DOI] [PubMed] [Google Scholar]
- Lawson ND, Weinstein BM. In vivo imaging of embryonic vascular development using transgenic zebrafish. Developmental biology. 2002;248:307–318. doi: 10.1006/dbio.2002.0711. [DOI] [PubMed] [Google Scholar]
- Lee KJ, Hoe HS, Pak DT. Plk2 Raps up Ras to subdue synapses. Small GTPases. 2011a;2:162–166. doi: 10.4161/sgtp.2.3.16454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KJ, Lee Y, Rozeboom A, Lee JY, Udagawa N, Hoe HS, Pak DT. Requirement for Plk2 in orchestrated ras and rap signaling, homeostatic structural plasticity, and memory. Neuron. 2011b;69:957–973. doi: 10.1016/j.neuron.2011.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee P, Goishi K, Davidson AJ, Mannix R, Zon L, Klagsbrun M. Neuropilin-1 is required for vascular development and is a mediator of VEGF-dependent angiogenesis in zebrafish. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:10470–10475. doi: 10.1073/pnas.162366299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim J, Choi HS, Choi HJ. Estrogen receptor-related receptor gamma regulates dopaminergic neuronal phenotype by activating GSK3beta/NFAT signaling in SH-SY5Y cells. Journal of neurochemistry. 2015 doi: 10.1111/jnc.13085. [DOI] [PubMed] [Google Scholar]
- Liu X, Erikson RL. Polo-like kinase 1 in the life and death of cancer cells. Cell Cycle. 2003;2:424–425. [PubMed] [Google Scholar]
- Lowery DM, Lim D, Yaffe MB. Structure and function of Polo-like kinases. Oncogene. 2005;24:248–259. doi: 10.1038/sj.onc.1208280. [DOI] [PubMed] [Google Scholar]
- Lu X, Le Noble F, Yuan L, Jiang Q, De Lafarge B, Sugiyama D, Breant C, Claes F, De Smet F, Thomas JL, Autiero M, Carmeliet P, Tessier-Lavigne M, Eichmann A. The netrin receptor UNC5B mediates guidance events controlling morphogenesis of the vascular system. Nature. 2004;432:179–186. doi: 10.1038/nature03080. [DOI] [PubMed] [Google Scholar]
- Mattila PK, Lappalainen P. Filopodia: molecular architecture and cellular functions. Nature reviews. Molecular cell biology. 2008;9:446–454. doi: 10.1038/nrm2406. [DOI] [PubMed] [Google Scholar]
- Mbefo MK, Paleologou KE, Boucharaba A, Oueslati A, Schell H, Fournier M, Olschewski D, Yin G, Zweckstetter M, Masliah E, Kahle PJ, Hirling H, Lashuel HA. Phosphorylation of synucleins by members of the Polo-like kinase family. The Journal of biological chemistry. 2010;285:2807–2822. doi: 10.1074/jbc.M109.081950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukouyama YS, Shin D, Britsch S, Taniguchi M, Anderson DJ. Sensory nerves determine the pattern of arterial differentiation and blood vessel branching in the skin. Cell. 2002;109:693–705. doi: 10.1016/s0092-8674(02)00757-2. [DOI] [PubMed] [Google Scholar]
- Park KW, Morrison CM, Sorensen LK, Jones CA, Rao Y, Chien CB, Wu JY, Urness LD, Li DY. Robo4 is a vascular-specific receptor that inhibits endothelial migration. Developmental biology. 2003;261:251–267. doi: 10.1016/s0012-1606(03)00258-6. [DOI] [PubMed] [Google Scholar]
- Petrovic N, Schacke W, Gahagan JR, O'Conor CA, Winnicka B, Conway RE, Mina-Osorio P, Shapiro LH. CD13/APN regulates endothelial invasion and filopodia formation. Blood. 2007;110:142–150. doi: 10.1182/blood-2006-02-002931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phng LK, Stanchi F, Gerhardt H. Filopodia are dispensable for endothelial tip cell guidance. Development. 2013;140:4031–4040. doi: 10.1242/dev.097352. [DOI] [PubMed] [Google Scholar]
- Pollard TD, Borisy GG. Cellular motility driven by assembly and disassembly of actin filaments. Cell. 2003;112:453–465. doi: 10.1016/s0092-8674(03)00120-x. [DOI] [PubMed] [Google Scholar]
- Roca C, Adams RH. Regulation of vascular morphogenesis by Notch signaling. Genes & development. 2007;21:2511–2524. doi: 10.1101/gad.1589207. [DOI] [PubMed] [Google Scholar]
- Seeburg DP, Feliu-Mojer M, Gaiottino J, Pak DT, Sheng M. Critical role of CDK5 and Polo-like kinase 2 in homeostatic synaptic plasticity during elevated activity. Neuron. 2008;58:571–583. doi: 10.1016/j.neuron.2008.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siekmann AF, Lawson ND. Notch signalling limits angiogenic cell behaviour in developing zebrafish arteries. Nature. 2007;445:781–784. doi: 10.1038/nature05577. [DOI] [PubMed] [Google Scholar]
- Simmons DL, Neel BG, Stevens R, Evett G, Erikson RL. Identification of an early-growth-response gene encoding a novel putative protein kinase. Molecular and cellular biology. 1992;12:4164–4169. doi: 10.1128/mcb.12.9.4164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somanath PR, Byzova TV. 14-3-3beta-Rac1-p21 activated kinase signaling regulates Akt1-mediated cytoskeletal organization, lamellipodia formation and fibronectin matrix assembly. Journal of cellular physiology. 2009;218:394–404. doi: 10.1002/jcp.21612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spindler V, Schlegel N, Waschke J. Role of GTPases in control of microvascular permeability. Cardiovascular research. 2010;87:243–253. doi: 10.1093/cvr/cvq086. [DOI] [PubMed] [Google Scholar]
- Stainier DY, Weinstein BM, Detrich HW, 3rd, Zon LI, Fishman MC. Cloche, an early acting zebrafish gene, is required by both the endothelial and hematopoietic lineages. Development. 1995;121:3141–3150. doi: 10.1242/dev.121.10.3141. [DOI] [PubMed] [Google Scholar]
- Steegmaier M, Hoffmann M, Baum A, Lenart P, Petronczki M, Krssak M, Gurtler U, Garin-Chesa P, Lieb S, Quant J, Grauert M, Adolf GR, Kraut N, Peters JM, Rettig WJ. BI 2536, a potent and selective inhibitor of polo-like kinase 1, inhibits tumor growth in vivo. Current biology : CB. 2007;17:316–322. doi: 10.1016/j.cub.2006.12.037. [DOI] [PubMed] [Google Scholar]
- Strebhardt K. Multifaceted polo-like kinases: drug targets and antitargets for cancer therapy. Nature reviews. Drug discovery. 2010;9:643–660. doi: 10.1038/nrd3184. [DOI] [PubMed] [Google Scholar]
- Suchting S, Freitas C, le Noble F, Benedito R, Breant C, Duarte A, Eichmann A. The Notch ligand Delta-like 4 negatively regulates endothelial tip cell formation and vessel branching. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:3225–3230. doi: 10.1073/pnas.0611177104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres-Vazquez J, Gitler AD, Fraser SD, Berk JD, Van NP, Fishman MC, Childs S, Epstein JA, Weinstein BM. Semaphorin-plexin signaling guides patterning of the developing vasculature. Developmental cell. 2004;7:117–123. doi: 10.1016/j.devcel.2004.06.008. [DOI] [PubMed] [Google Scholar]
- van Nieuw Amerongen GP, Koolwijk P, Versteilen A, van Hinsbergh VW. Involvement of RhoA/Rho kinase signaling in VEGF-induced endothelial cell migration and angiogenesis in vitro. Arteriosclerosis, thrombosis, and vascular biology. 2003;23:211–217. doi: 10.1161/01.atv.0000054198.68894.88. [DOI] [PubMed] [Google Scholar]
- Visel A, Thaller C, Eichele G. GenePaint.org: an atlas of gene expression patterns in the mouse embryo. Nucleic acids research. 2004;32:D552–D556. doi: 10.1093/nar/gkh029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warnke S, Kemmler S, Hames RS, Tsai HL, Hoffmann-Rohrer U, Fry AM, Hoffmann I. Polo-like kinase-2 is required for centriole duplication in mammalian cells. Current biology : CB. 2004;14:1200–1207. doi: 10.1016/j.cub.2004.06.059. [DOI] [PubMed] [Google Scholar]
- Wei P, Satoh T, Edamatsu H, Aiba A, Setsu T, Terashima T, Kitazawa S, Nakao K, Yoshikawa Y, Tamada M, Kataoka T. Defective vascular morphogenesis and mid-gestation embryonic death in mice lacking RA-GEF-1. Biochemical and biophysical research communications. 2007;363:106–112. doi: 10.1016/j.bbrc.2007.08.149. [DOI] [PubMed] [Google Scholar]
- Weitzman M, Bayley EB, Naik UP. Robo4: a guidance receptor that regulates angiogenesis. Cell adhesion & migration. 2008;2:220–222. doi: 10.4161/cam.2.4.7061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wojciak-Stothard B, Entwistle A, Garg R, Ridley AJ. Regulation of TNF-alpha-induced reorganization of the actin cytoskeleton and cell-cell junctions by, Rho, Rac, and Cdc42 in human endothelial cells. Journal of cellular physiology. 1998;176:150–165. doi: 10.1002/(SICI)1097-4652(199807)176:1<150::AID-JCP17>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- Xu D, Wang Q, Jiang Y, Zhang Y, Vega-Saenzdemiera E, Osman I, Dai W. Roles of Polo-like kinase 3 in suppressing tumor angiogenesis. Experimental hematology & oncology. 2012;1:5. doi: 10.1186/2162-3619-1-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu D, Yao Y, Jiang X, Lu L, Dai W. Regulation of PTEN stability and activity by Plk3. The Journal of biological chemistry. 2010a;285:39935–39942. doi: 10.1074/jbc.M110.166462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu D, Yao Y, Lu L, Costa M, Dai W. Plk3 functions as an essential component of the hypoxia regulatory pathway by direct phosphorylation of HIF-1alpha. The Journal of biological chemistry. 2010b;285:38944–38950. doi: 10.1074/jbc.M110.160325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Bai J, Shen R, Brown SA, Komissarova E, Huang Y, Jiang N, Alberts GF, Costa M, Lu L, Winkles JA, Dai W. Polo-like kinase 3 functions as a tumor suppressor and is a negative regulator of hypoxia-inducible factor-1 alpha under hypoxic conditions. Cancer research. 2008;68:4077–4085. doi: 10.1158/0008-5472.CAN-07-6182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zacchigna S, Ruiz de Almodovar C, Carmeliet P. Similarities between angiogenesis and neural development: what small animal models can tell us. Current topics in developmental biology. 2008;80:1–55. doi: 10.1016/S0070-2153(07)80001-9. [DOI] [PubMed] [Google Scholar]
- Zeng H, Zhao D, Mukhopadhyay D. KDR stimulates endothelial cell migration through heterotrimeric G protein Gq/11-mediated activation of a small GTPase RhoA. The Journal of biological chemistry. 2002;277:46791–46798. doi: 10.1074/jbc.M206133200. [DOI] [PubMed] [Google Scholar]
- Zhang R, Han P, Yang H, Ouyang K, Lee D, Lin YF, Ocorr K, Kang G, Chen J, Stainier DY, Yelon D, Chi NC. In vivo cardiac reprogramming contributes to zebrafish heart regeneration. Nature. 2013;498:497–501. doi: 10.1038/nature12322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao L, Borikova AL, Ben-Yair R, Guner-Ataman B, MacRae CA, Lee RT, Burns CG, Burns CE. Notch signaling regulates cardiomyocyte proliferation during zebrafish heart regeneration. Proceedings of the National Academy of Sciences of the United States of America. 2014;111:1403–1408. doi: 10.1073/pnas.1311705111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng JQ, Wan JJ, Poo MM. Essential role of filopodia in chemotropic turning of nerve growth cone induced by a glutamate gradient. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1996;16:1140–1149. doi: 10.1523/JNEUROSCI.16-03-01140.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong H, Xin S, Zhao Y, Lu J, Li S, Gong J, Yang Z, Lin S. Genetic approach to evaluate specificity of small molecule drug candidates inhibiting PLK1 using zebrafish. Molecular bioSystems. 2010;6:1463–1468. doi: 10.1039/b919743e. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.