Abstract
Purpose
To examine the relationship between glaucomatous structural damage and ability to divide attention during simulated driving.
Design
Cross-sectional observational study.
Methods
Setting
Hamilton Glaucoma Center, University of California San Diego.
Patient Population
158 subjects from the Diagnostic Innovations in Glaucoma Study, including 82 with glaucoma and 76 similarly aged controls.
Observation Procedure
Ability to divide attention was investigated by measuring reaction times to peripheral stimuli (at low, medium or high contrast) while concomitantly performing a central driving task (car following or curve negotiation). All subjects had standard automated perimetry (SAP) and optical coherence tomography was used to measured retinal nerve fiber (RNFL) thickness. Cognitive ability was assessed using the Montreal Cognitive Assessment and subjects completed a driving history questionnaire.
Main outcome measures
Reaction times to the driving simulator divided attention task.
Results
The mean reaction times to the low contrast stimulus were 1.05 s and 0.64 s in glaucoma and controls respectively during curve negotiation (P <0.001), and 1.19 s and 0.77 s (P = 0.025) respectively during car following. There was a non-linear relationship between reaction times and RNFL thickness in the better eye. RNFL thickness remained significantly associated with reaction times even after adjusting for age, SAP mean deviation in the better eye, cognitive ability and central driving task performance.
Conclusions
Although worse SAP sensitivity was associated with worse ability to divide attention, RNFL thickness measurements provided additional information. Information from structural tests may improve our ability to determine which patients are likely to have problems performing daily activities, such as driving.
INTRODUCTION
Glaucoma is a progressive optic neuropathy that may result in significant vision-related morbidity.1 As glaucomatous neural loss is irreversible, the central aim of disease management is to slow progression and reduce the risk of patients developing visual impairment and reduction in vision-related quality of life. Visual function in glaucoma is traditionally evaluated using standard automated perimetry (SAP). Although SAP provides a means to quantify glaucomatous damage, the true clinical significance of SAP depends on how well it is able to predict the impact of disease on ability to perform activities of daily living, and an understanding of this relationship remains elusive.2,3 In fact, as SAP attempts to minimize visual distractions during testing, it may be limited in its ability to measure visual impairment related to real-world tasks.4 Visual distractions are present during most daily activities, including during driving and navigation while walking; tasks that require the ability to divide attention or “multi-task”.3,5–8
Divided attention specifically requires processing and/or responding to information from one task while simultaneously conducting another.8 In the case of driving, divided attention involves continuously monitoring information from the roadway to control the vehicle, while simultaneously maintaining awareness of potential hazards surrounding the vehicle. This requires attention to be distributed across the driving scene.9–10 As the cognitive system has a limited amount of attentional resources, the quality and efficiency of performance of a particular task may be compromised if performed under a divided attention situation.11 The ability to divide attention is therefore intrinsically related to the ability to perform tasks such as driving, with failures of divided attention a leading cause of motor vehicle collisions.5,7,10,12–13
The purpose of the present study was to evaluate the ability to divide attention during a simulated driving task and to determine the relationship between ability to divide attention and an objective measure of glaucomatous neural loss, namely, retinal nerve fiber layer (RNFL) thickness measured using spectral domain optical coherence tomography (SDOCT). The contrast characteristics of the visual stimuli presented during driving simulation were varied in order to impose different demands on the visual system, and also to assess damage to the magnocellular pathway, which has been hypothesized as preferentially damaged in glaucoma.14
METHODS
This was a cross-sectional observational study involving participants from the Diagnostic Innovations in Glaucoma Study (DIGS), a prospective longitudinal study designed to evaluate optic nerve structure and visual function in glaucoma. The study was conducted at the Hamilton Glaucoma Center at the Department of Ophthalmology, University of California San Diego (UCSD). Methodological details have been described previously.15 Written informed consent was obtained from all participants, and the institutional review board and human subjects committee at University of California San Diego prospectively approved all methods. All study methods adhered to the tenets of the Declaration of Helsinki for research involving human subjects and the study was conducted in accordance with the regulations of the Health Insurance Portability and Accountability Act. The study was registered at ClinicalTrials.gov with registration number NCT00221897.
Glaucoma was defined by the presence of two or more consecutive abnormal SAP tests or evidence of progressive glaucomatous optic disc changes based on masked assessment of stereophotographs. Suspect glaucoma was defined by the presence of a suspicious appearance of the optic disc (neuroretinal rim thinning, excavation or suspicious RNFL defects) or elevated intraocular pressure (IOP) (>21mmHg). Healthy subjects were recruited from the general population and had IOP of 21 mmHg or less with no history of raised IOP, and normal SAP testing. Categorization was based on the diagnosis in the worse eye.
At each visit, subjects underwent comprehensive ophthalmologic examination including review of medical history, visual acuity, slit-lamp biomicroscopy, IOP measurement, gonioscopy, dilated fundoscopic examination, stereoscopic optic disc photography, SDOCT RNFL imaging (Spectralis, Heidelberg Engineering, Dossenheim, Germany), and SAP using the Swedish interactive threshold algorithm (SITA Standard 24-2, Carl Zeiss Meditec, Inc., Dublin, CA, USA). Only subjects with open angles on gonioscopy were included. Subjects were excluded if they presented with a best-corrected visual acuity of less than 20/40, spherical refraction outside ±5.0 diopters or cylinder correction outside 3.0 diopters, or any other ocular or systemic disease that could affect the optic nerve or the visual field.
Imaging and Standard Automated Perimetry
Spectralis SDOCT (software version 5.4.7.0) was used to obtain average circumpapillary RNFL thickness measurements. Details of its operation have been described elsewhere.16 RNFL thickness measurements were acquired from a 3.45-mm circle centered on the optic disc consisting of 1536 A-scan points. All images were reviewed by the UCSD Imaging Data Evaluation and Analysis Center to ensure the scan was centered, that the signal strength was >15dB and that there were no artifacts. Scans that were inverted, clipped or those that had coexistent retinal pathological abnormalities were excluded. The RNFL segmentation algorithm was also checked for errors, and corrected according to standard protocols.
SAP was performed using the Humphrey Field Analyzer II (Carl Zeiss Meditec, Dublin, CA, USA). All visual fields were evaluated by the UCSD Visual Field Assessment Center.17 Visual fields with more than 33% fixation losses or false-negative errors, or more than 15% false-positive errors, were excluded. The only exception was the inclusion of visual fields with false-negative errors of more than 33% when the field showed advanced disease. An abnormal SAP test was defined as a visual field with a pattern standard deviation with P <0.05 and/or a Glaucoma Hemifield Test outside normal limits.
Driving Simulator
For the purposes of this study, ability to divide attention was assessed by measuring reaction times to stimuli presented during a divided attention protocol during simulated driving. The driving simulator consisted of a typical driving seat, a steering wheel, brake and accelerator pedals, and a 40-inch screen (Supplemental Material Figure 1 at AJO.com). The position of the seat, wheel and pedals could be adjusted for comfort but the distance between the subject’s head and the center of the screen was set at 43-inches. The screen width was 35-inches resulting in a driving scene with a 45-degree horizontal field of view. Software for the driving simulator was developed at the Hamilton Glaucoma Center, UCSD.
The driving simulator tested the ability to attend simultaneously to one of two central visual tasks of driving (adjusting speed while following another car that varies its speed and staying in a lane on a winding road) and to a peripheral visual task of perceiving a projected stimulus and responding by pushing a button on the steering wheel. The peripheral stimuli were presented at about 20-degrees of visual angle in the upper right and upper left of the driving simulator screen and at three different contrasts (low, medium and high). The contrast of the stimulus was altered using alpha blending techniques to achieve symbol transparencies of 0.1, 0.4 and 0.9. Therefore in the case of 0.1 symbol transparency, the symbol intensity and color that the driver perceived was 10% of the symbol intensity and color and 90% of the background intensity and color. The equivalent Michelson contrasts were 0.04, 0.14 and 0.27 for low, medium and high contrast stimuli, respectively. At maximum screen intensity the divided attention stimulus symbols were pure white, while the background was constant and consisted of a cloudy sky. There were an average of 5 stimuli presented at each contrast for each central driving task (a total of about 15 per 3 minutes or about one every 12 seconds) and stimuli stayed on the screen for a maximum of 3 and 6 seconds (uniform distribution) or until the driver responded. The next stimuli appeared between 3 and 6 seconds (again uniform distribution) after the driver responded or when the maximum display time had elapsed.
The main outcome measure of “reaction time” was defined as the time interval between appearance of the peripheral stimulus and the subject pressing the button, with a longer reaction time indicating worse performance. The mean reaction time for each central task (curve negotiation and car following) and contrast (low, medium, high) was calculated, giving a total of 6 sets of reaction times for each subject, and the false positive percentage, which was defined as the number of button presses occurring when no stimulus had been presented divided by the total number of stimuli presented, was calculated to assess speed-accuracy tradeoffs (see discussion).
Reaction time was chosen as the outcome variable as difficulties with divided attention tasks seem to be related, at least in part, to a slowing of visual processing speed. Visual processing speed, which is defined as the time needed to make a correct judgment about a visual stimulus, is commonly studied in behavioral research by measuring reaction times.3,18–21 The use of reaction times has some limitations as the registration of a reaction requires a motor response (the act of pressing a button), in addition to lower and higher-order sensory functions. However, a large component of reaction time is the speed at which sensory data are carried to the brain, which depends on structural aspects of neural wiring and conduction.22
Reactions times are prolonged under more demanding conditions, such as with low contrast stimuli. However, if a stimulus is perceived, the motor response for a particular subject is likely to be constant regardless of contrast. Therefore, to minimize the possible confounding effect of motor response in reaction times, the difference in reaction times to the low and high contrast stimuli was calculated, with the aim of isolating the visual processing component.
Driving Tasks
1. Curve Negotiation
During the curve negotiation task, the driver was presented with a winding, three-lane road and was instructed to drive in the center lane. The velocity of the vehicle was constant such that the driver only had to operate the steering wheel. The vehicle speed was set at 15 m/s (54 km/h) for the first half of the test, increasing to 25 m/s (90 km/h) for the second half of the test.
As a subject might achieve fast reaction times by adopting a strategy in which the driving task is neglected, it was important to assess central driving task performance.23 This was measured using “curve coherence”, which was defined as the normalized cross-correlation function between the road curvature and the vehicle path curvature as a function of spatial shift. Curve coherence was calculated using the following equation, where n is the number of samples of the two signals and SD is the standard deviation of the signals, with a coherence of 1 indicating the two signals to be an exact match.
2. Car Following
The second task was a car following task, during which the driver was instructed to drive down a straight road following a leading police car. The subject was instructed to follow the lead vehicle at a short distance, controlling the gas pedal and brake. The speed of the lead vehicle fluctuated according to a multi-sine function with frequencies chosen to achieve normal traffic speed fluctuations (0.028Hz, 0.039Hz, 0.061Hz, 0.094Hz and 0.128Hz).24 This yielded a standard deviation in the acceleration profile of 1.4 m/s2 with 3 events with decelerations exceeding 3 m/s2 and 3 events with acceleration exceeding 3.0 m/s2. To facilitate a symmetric acceleration profile, the vehicle was boosted in its acceleration capabilities.
Central driving task performance was assessed using “speed coherence”, which is similar to the curve coherence measure calculated for the curve negotiation task. Speed coherence is a measure of the accuracy with which the driver can reproduce the lead vehicle speed fluctuations and was calculated using the speed cross correlation function, obtained according to the following equation: 23
Where CCF is the cross correlation function, n is the number of samples of the two signals and SD is the standard deviation of the signals. Speed coherence was defined as the maximum correlation observed in the CCF; generally observed as some delay. The larger the coherence the better the driver was able to follow the lead car fluctuations, with a coherence of 1 indicating that the two speed signals match exactly.
To minimize the effect of unreliable tests and learning effect, all subjects underwent driving simulator training prior to test commencement. Training consisted of 2 minutes practice acceleration and deceleration, followed by 1 minute of each of the car following and curve negotiation tasks.
Montreal Cognitive Assessment
All subjects also completed the Montreal Cognitive Assessment. The Montreal Cognitive Assessment is a 30-point, ten-minute cognitive screening tool developed to detect mild cognitive impairment.25 It is similar to the Mini-Mental State Examination but has additional subtests focusing on aspects of attention relative to driving. All subjects also completed a driving habits questionnaire to assess the average number of miles driven over the past 3 years.
Statistical Analysis
Normality assumption was assessed by inspection of histograms and using Shapiro-Wilk tests. Student t-tests were used for group comparison for normally distributed variables and Wilcoxon rank-sum test for continuous non-normal variables. Reaction times were positively skewed and therefore base-10 logarithms were calculated for further analysis. The relationship between the logarithmic reaction times and RNFL thickness in each subject’s better and worse eyes were examined using scatter plots and locally weighted scatterplot smoothing (LOWESS) curves.
The relationship between reaction times and RNFL thickness was adjusted for performance on the central driving task. Other variables examined as potentially confounding factors included age, Montreal Cognitive Assessment score, SAP MD, distance driven per week and lens status. All statistical analyses were performed with commercially available software (Stata, version 12; StataCorp LP). The α level (type I error) was set at 0.05.
RESULTS
The study included both eyes of 82 subjects with glaucoma and 76 similarly aged controls, including 30 healthy subjects and 46 with suspect glaucoma. The demographic and clinical characteristics of all subjects are summarized in Table 1. There were no significant differences in age, gender, ancestry, cognitive ability or average distance driven per week between controls and those with glaucoma. Subjects with glaucoma had significantly worse SAP MD and RNFL thickness in their better and worse eyes than controls (Table 1).
TABLE 1.
Demographic and clinical characteristics (mean (median, interquartile range)) of patients with glaucoma compared to controls.
| Controls (76 subjects) | Glaucoma (82 subjects) | P-value | |
|---|---|---|---|
| Age (years) | 61.2 ± 9.2 | 64.2 ± 12.2 | 0.093a |
| Sex, female (%) | 34 (44.7%) | 35 (42.7%) | 0.873c |
| Ethnicity | |||
| Caucasian | 53 (69.7%) | 51 (62.2%) | 0.783c |
| African-American | 17 (22.4%) | 19 (23.2%) | |
| MD worse eye (dB) | −0.2 (0.1, −0.8 to 0.7) | −6.4 (−3.9, −8.7 to −1.6) | <0.001b |
| MD better eye (dB) | 0.5 (0.7, −0.2 to 1.3) | −2.3 (−1.2, −3.4 to 0.1) | <0.001b |
| RNFL thickness worse eye (μm) | 90 (91, 83 to 98) | 71 (70, 60 to 80) | <0.001b |
| RNFL thickness better eye (μm) | 94 (94, 87 to 100) | 79 (78, 67 to 90) | <0.001b |
| Pseudophakic in at least one eye (number of patients (%)) | 10 (13.2%) | 17 (20.7%) | 0.290c |
| Montreal Cognitive Assessment score | 28 (29, 27 to 30) | 28 (28, 26 to 30) | 0.350b |
| Average distance driven per week (miles) | 143 ± 18 | 125 ± 18 | 0.065a |
| Curve Negotiation Metrics | |||
| Curve coherence | 0.93 (0.95, 0.90 to 0.97) | 0.91 (0.95, 0.89 to 0.98) | 0.978b |
| False positives (%) | 9.2 (3.1, 2.0 to 6.7) | 9.2 (2.4, 0 to 9.1) | 0.599b |
| Divided Attention Reaction Time – Low contrast (s) | 0.64 (0.59, 0.52 to 0.70) | 1.05 (0.70, 0.61 to 1.02) | <0.001b |
| Divided Attention Reaction Time – Medium contrast (s) | 0.55 (0.52, 0.48 to 0.63) | 0.59 (0.57, 0.49 to 0.64) | 0.142b |
| Divided Attention Reaction Time – High contrast (s) | 0.55 (0.53, 0.46 to 0.60) | 0.58 (0.55, 0.49 to 0.66) | 0.167b |
| Low contrast minus high contrast reaction time (s) | 0.09 (0.07, 0.03 to 0.10) | 0.47 (0.13, 0.07 to 0.40) | <0.001b |
| Car Following Metrics | |||
| Speed coherence | 0.93 (0.95, 0.90 to 0.97) | 0.91 (0.95, 0.89 to 0.98) | 0.978b |
| False positives (%) | 6.5 (2.7, 0 to 5.5) | 6.3 (2.7, 0 to 5.6) | 1.000b |
| Divided Attention Reaction Time – Low contrast (s) | 0.77 (0.62, 0.54 to 0.85) | 1.19 (0.69, 0.58 to 1.22) | 0.025b |
| Divided Attention Reaction Time – Medium contrast (s) | 0.62 (0.54, 0.50 to 0.64) | 0.62 (0.56, 0.50 to 0.66) | 0.956b |
| Divided Attention Reaction Time – High contrast (s) | 0.57 (0.54, 0.50 to 0.61) | 0.65 (0.55, 0.50 to 0.64) | 0.412b |
| Low contrast minus high contrast reaction time (s) | 0.20 (0.08, 0.03 to 0.30) | 0.48 (0.11, 0.03 to 0.60) | 0.199b |
t-test,
Wilcoxon rank sum test.
Fishers exact test.
Abbreviations: MD = Mean deviation, dB = decibels, RNFL = Retinal nerve fiber layer, s = seconds.
Divided Attention Reaction Times
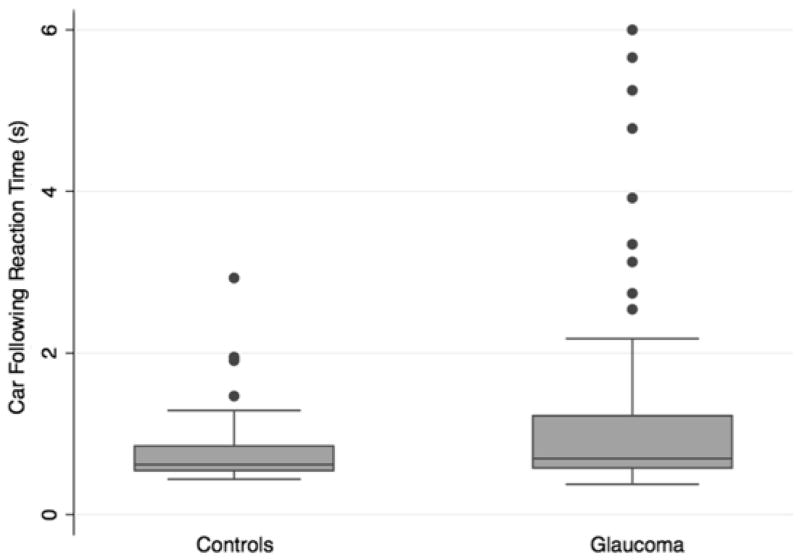
Patients with glaucoma had significantly worse reaction times to low contrast divided attention stimuli compared to controls (Table 1 and Figure 1). Median reaction times to the curve negotiation and car following tasks were 1.05 and 1.19 seconds respectively in those with glaucoma compared to only 0.64 and 0.77 seconds in controls (P < 0.001 for both comparisons). However, there was no significant difference between groups when tested using higher contrast stimuli (Table 1). There was also no significant difference in the false positive percentage between those with glaucoma and controls.
FIGURE 1.
Boxplots showing the distribution of reaction times to the low contrast stimulus curve negotiation (Top) and car following (Bottom) divided attention driving simulator tasks in subjects with glaucoma compared to controls.
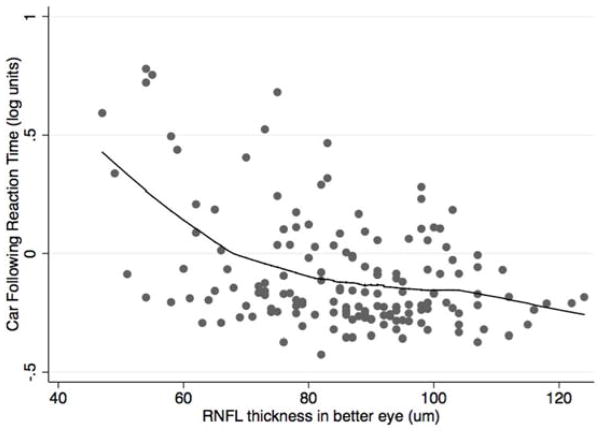
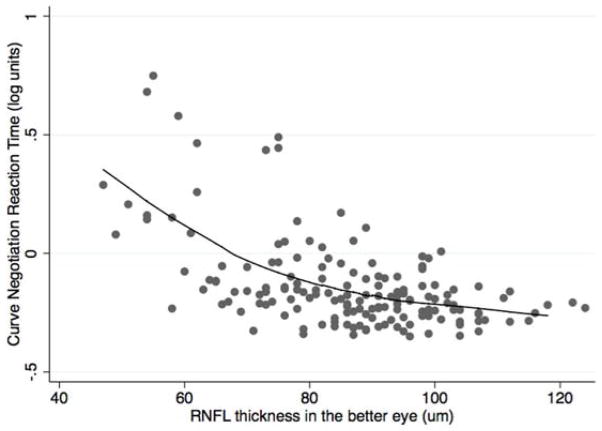
Although on average subjects with glaucoma had longer reaction times to low contrast divided attention stimuli compared with controls, longer reaction times primarily occurred in those with more severe disease. There were significant, albeit nonlinear, relationships between the divided attention reaction times to low contrast stimuli and RNFL thickness in the better and worse eyes for both the curve negotiation and car following tasks (Figure 2). The spearman rank correlation coefficients examining the relationship between RNFL thickness and curve negotiation divided attention reaction time were ρ = −0.480; P <0.001 and ρ = −0.481; P <0.001 for the better and worse eyes respectively, with corresponding values of ρ = −0.280; P <0.001 and ρ = −0.281; P <0.001 for better and worse eyes using the car following task. Similar nonlinear relationships were found between RNFL thickness and reaction times using the medium and high contrast stimuli; however, the strongest relationship was with the more demanding low contrast stimulus. Further analyses were conducted using only the low contrast reaction times.
FIGURE 2.
Scatterplots showing the relationship between retinal nerve fiber layer thickness in the better eye of subjects with glaucoma and controls, and reaction times (in logarithmic units) for the low contrast stimulus curve negotiation (Top) and car following (Bottom) driving simulator divided attention tasks.
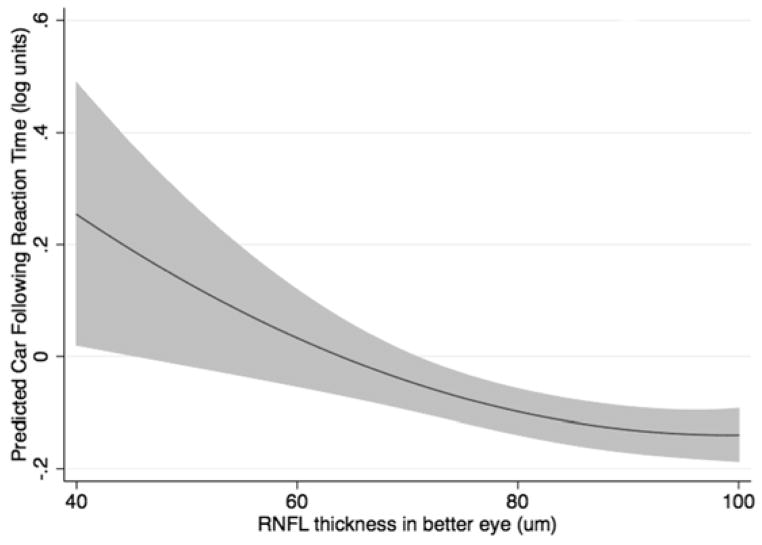
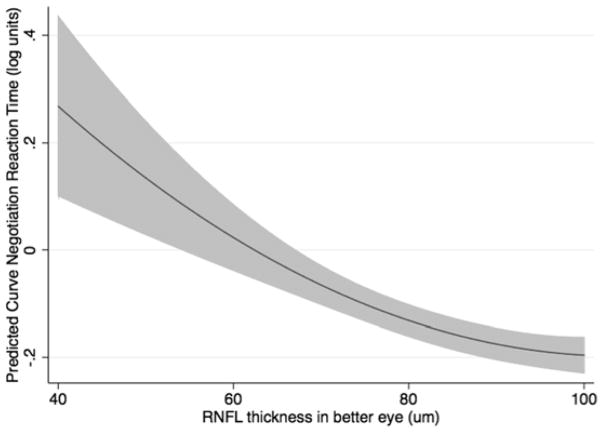
To account for nonlinearity, a quadratic model was fitted to characterize the relationship between reaction times and RNFL thickness. The model adjusted for age, MD in the better eye, performance on the central driving task, and the cognitive assessment score. RNFL thickness in the better eye was still significantly predictive of reaction times in the multivariable model for both the curve negotiation (P = 0.001; joint Wald test) and car following tasks (P = 0.019; joint Wald test), even after adjustment for possible confounding variables (Table 2). SAP MD in the better eye and central task performance (curve coherence or speed coherence) were also significantly predictive of reaction times, as was age for the curve negotiation task. The multivariable models performed well in predicting reaction times, with adjusted R2 of 46.9% and 31.6% for the curve negotiation and car following tasks, respectively. The modeled relationship between RNFL thickness in the better eye and reaction times during both divided attention driving simulation tasks is shown in Figure 3.
TABLE 2.
Results of multivariable regression analyses examining the relationship between retinal nerve fiber layer thickness in the better eye in subjects with glaucoma and controls, and driving simulator divided attention reaction times at low contrast (in logarithmic units).
| Curve Negotiation Task | |||
|---|---|---|---|
| Log10 Curve Negotiation Divided Attention Reaction Time, adjusted R2 = 0.469. | |||
| Coefficient | 95% CI | P-Value | |
| RNFL thickness in better eye (per 10μm) | −0.24 | −0.40 to −0.10 | 0.001 |
| RNFL thickness in better eye squared (per 100μm2) | 0.01 | 0.00 to 0.02 | 0.004 |
| Age (per 10 years) | 0.03 | 0.02 to 0.05 | 0.035 |
| MD in better eye (dB) | −0.02 | −0.03 to −0.01 | <0.001 |
| Curve coherence | −0.63 | −1.10 to −0.15 | 0.010 |
| Montreal Cognitive Assessment score | −0.01 | −0.02 to 0.01 | 0.337 |
| Constant | 1.59 | 0.74 to 2.44 | <0.001 |
| Car Following Task | |||
|---|---|---|---|
| Log10 Car Following Divided Attention Reaction Time, adjusted R2 = 0.316. | |||
| Coefficient | 95% CI | P-Value | |
| RNFL thickness in better eye (per 10μm) | −0.22 | −0.41 to −0.04 | 0.019 |
| RNFL thickness in better eye squared (per 100μm2) | 0.01 | 0.00 to 0.02 | 0.004 |
| Age (per 10 years) | 0.03 | 0.00 to 0.06 | 0.057 |
| MD in better eye (dB) | −0.02 | −0.03 to −0.01 | 0.001 |
| Speed coherence | −0.34 | −0.65 to −0.04 | 0.029 |
| Montreal Cognitive Assessment score | 0.00 | −0.01 to 0.02 | 0.712 |
| Constant | 0.99 | −0.05 to 2.02 | 0.062 |
Abbreviations: RNFL = Retinal nerve fiber layer, MD = mean deviation.
FIGURE 3.
Results of the multivariable models showing predicted reaction times to the low contrast curve negotiation (Top) and car following (Bottom) divided attention driving simulator tasks for given values of retinal nerve fiber layer thickness in the better eye of subjects with glaucoma and controls. The results are for a subject with the mean sample age of 62.8 years.
The difference in reaction times to the low and high contrast stimuli, calculated with the aim of isolating the visual processing component, was also significantly related to RNFL thickness in the better eye for the car following (spearman rank correlation ρ = −0.458; P <0.001) and curve negotiation tasks (spearman rank correlation ρ= −0.262; P <0.001). RNFL thickness was still significantly associated with the difference in reaction times in the multivariable models (Table 3).
Table 3.
Results of multivariable regression analyses examining the relationship between retinal nerve fiber layer thickness in the better eye in subjects with glaucoma and controls, and the difference in reaction times to the curve negotiation (and car following) driving simulator divided attention task at high and low contrast.
| Curve Negotiation Task | |||
|---|---|---|---|
| Driving simulator divided attention reaction time at low contrast minus reaction time at high contrast, adjusted R2 = 0.327. | |||
| Coefficient | 95% CI | P-Value | |
| RNFL thickness in better eye (per 10μm) | −0.78 | −1.28 to −0.27 | 0.003 |
| RNFL thickness in better eye squared (per 100μm2) | 0.04 | 0.01 to 0.07 | 0.010 |
| Age (per 10 years) | 0.06 | −0.03 to 0.15 | 0.207 |
| MD in best eye (dB) | −0.06 | −0.09 to −0.02 | 0.001 |
| Curve coherence | −1.10 | −2.91 to 0.72 | 0.235 |
| Montreal Cognitive Assessment score | −0.01 | −0.05 to 0.03 | 0.759 |
| Constant | 4.78 | 1.54 to 8.03 | 0.004 |
| Car Following Task | |||
|---|---|---|---|
| Driving simulator divided attention reaction time at low contrast minus reaction time at high contrast, adjusted R2 = 0.256. | |||
| Coefficient | 95% CI | P-Value | |
| RNFL thickness in better eye (per 10μm) | −0.85 | −1.51 to −0.18 | 0.014 |
| RNFL thickness in better eye squared (per 100μm2) | 0.04 | 0.01 to 0.08 | 0.026 |
| Age (per 10 years) | 0.05 | −0.01 to 0.16 | 0.421 |
| MD in best eye (dB) | −0.07 | −0.12 to −0.03 | 0.002 |
| Speed coherence | −0.41 | −1.48 to 0.65 | 0.444 |
| Montreal Cognitive Assessment score | 0.01 | −0.04 to 0.06 | 0.738 |
| Constant | 4.09 | 0.39 to 7.79 | 0.030 |
Abbreviations: RNFL = Retinal nerve fiber layer, MD = mean deviation.
We also investigated the relationship between reaction times during simulated driving and stimulus contrast using a mixed effects linear regression model. A 0.1 decrease in Michelson contrast was associated with a 0.077 (95% CI 0.059 to 0.096) increase in curve negotiation and 0.075 (95% CI 0.053 to 0.098) increase in car following reaction times (in logarithmic units) in those with glaucoma, compared to increases of only 0.026 (95% CI 0.016 to 0.035) and 0.046 (95% CI 0.032 to 0.059) for the curve negotiation and car following tasks, respectively, in controls.
DISCUSSION
The present study has shown that patients with glaucoma may have significantly impaired ability to divide attention during simulated driving compared to controls, particularly under demanding low contrast conditions. This finding has important implications, as previous studies have shown that impaired ability to divide attention has significant implications for driving,3,5,7,9,10,18,20,26 with attentional problems implicated in 22% to 50% of motor vehicle collisions.3,13 Failures of divided attention are also associated with difficulties performing other activities of daily living such as walking.6
We found reaction times to the divided attention simulated driving task were significantly associated with measures of glaucoma severity, with longer reaction times observed in patients with more severe disease. As one might expect, there was a significant relationship between a measure of visual function, worse SAP MD, and longer reaction times during simulated driving. However, even after accounting for SAP MD, RNFL thickness measured by OCT provided additional information, with RNFL thinning significantly associated with longer reaction times. To the best of our knowledge these results are the first reported demonstration of a relationship between a structural test in glaucoma and a performance-based functional measure. This is an important finding for clinical practice as although RNFL thickness measurements have been widely used for diagnosis and monitoring of glaucoma progression,27,28 the real clinical relevance of these measurements has not been well established. For example, while it is known that glaucomatous eyes have thinner RNFL than healthy eyes, it has not been established at what point RNFL thinning becomes associated with decrease in the ability to perform daily activities. The present study goes someway towards answering this question.
Although clinicians generally rely on assessment by SAP to evaluate the impact of functional losses in glaucoma, the relationship between SAP and metrics of quality of life and ability to perform daily activities has been generally weak.2,4,5,29,30 It is widely acknowledged that agreement between structural and functional tests is imperfect and therefore we decided to examine whether inclusion of information from OCT could improve the ability to determine which patients with glaucoma may have problems with divided attention tasks. The results of the study show a significant exponential relationship between RNFL thinning and longer reaction times to the divided attention tasks (Figure 2). A consequence of the non-linear relationship between RNFL thinning and longer reaction times is that although patients with mild glaucoma are likely to have preserved ability to divide attention during simulated driving, worsening neural loss with disease progression could lead to large decreases in ability to divide attention. There was significant correlation between SAP MD and RNFL thickness (ρ = 0.346; P <0.001 for better eye), however as one might expect given the imperfect relationship between structure and function, information from OCT was of additional value to SAP in predicting reaction times to the divided attention simulated driving tasks. This is demonstrable from the multivariable models shown in Tables 2 and 3.
An interesting finding of the present study was that testing with a more demanding, low contrast stimulus was required to detect a significant difference in reaction times between those with glaucoma and controls. This finding is consistent with previous studies that have shown patients with glaucoma to be more affected by low contrast conditions than healthy subjects.31,32 We found that reaction times during simulated driving increased as contrast decreased for both patients with glaucoma and controls, however, those with glaucoma were affected more. During the curve negotiation task, patients with glaucoma had a 3-fold greater increase in reaction times (in logarithmic units) compared to controls. Problems with low contrast are of particular importance for driving and have previously been shown to adversely affect driving performance.32
Murray and Plainis suggested that reaction times are regulated by characteristics of neurons at the early stages of visual processing and demonstrated that at low contrasts only a relatively small number of neurons with high contrast gain and fast responses are activated, corresponding to magnocellular (M) neurons.33,34 With higher contrast stimuli, M dominated pathways become saturated and additional parvocellular (P) neurons are recruited, which reduces the synaptic delay, probably due to a probability summation mechanism.33,34 Previous studies have suggested preferential damage to the M pathway in glaucoma14 and this might explain the greater sensitivity of a low-contrast stimulus to detect differences in reaction time in our study. Although the theory of selective cell damage in glaucoma has been challenged,35,36 it is likely that the use of stimuli affecting a smaller population of ganglion cells may help detect differences not seen with the use of non-selective stimuli.
Differences in reaction time to low and high contrast stimuli were calculated to minimize the effect of the motor response component. From Table 3 it is apparent that thinner RNFL in the better eye was significantly associated with larger differences in reaction time to low and high contrast stimuli for both curve negotiation and car following tasks. This suggests that the relationship between RNFL thickness and reaction time during simulated driving was not due to differences in motor response. The descriptive statistics in Table 1 show that the difference in reaction time between low and high contrast stimuli did not reach statistical significance for the car following driving simulator task, however, this is likely due to the relatively large number of patients with early and moderate glaucoma. The important finding is the observation of a significant association between continuous measures of disease severity such as MD and RNFL thickness and the “motor-response corrected reaction time”.
It is possible that subjects with greater driving experience may achieve better results on driving simulation than novice drivers. We therefore examined the effect of previous driving experience in the multivariable model, by including a variable of average distance driven per week. However, this measure of driving experience was not significantly associated with reaction times to the divided attention tasks (P = 0.074 for curve negotiation and P = 0.114 for car following).
The typical instruction when measuring reaction times is for the patient to respond as rapidly as possible, however, this may result in high rates of false-positive errors. On the other hand, subjects who attempt to make fewer errors may end up having longer reaction times by being more careful. This is known as the speed-accuracy tradeoff. We found no evidence of difference in speed-accuracy tradeoffs between glaucomatous and healthy individuals as indicated by similar rates of false-positive responses.
This study has some limitations. Divided attention was assessed using a driving simulator rather than during on-road driving. Although driving simulators have been widely used to assess divided attention and driving skills3, it is possible that participants may show differences in behavior in real world driving, when the risks to safety are also real. However, on-road driving assessments are expensive, potentially hazardous, and it is difficult to create uniform repeatable driving scenarios.3 Furthermore, results from driving simulation have been shown to correlated well with on-road driving assessments and data regarding history of motor vehicle collisions.37 A second limitation is that as driving is a complex task, influenced by many variables, it is possible that important variables may have been omitted from the multivariable models. Nevertheless, the models had good ability to predict reaction times to the divide attention tasks with adjusted R2 values of 32% to 47%. It is however important to emphasize that thinning of the RNFL and worsening SAP MD account for only some of the variability in ability to divide attention during driving simulation. This is to be expected, as many people without glaucoma have failures of divided attention when driving.
It should also be acknowledged that the present study included only patients with good visual acuity and no ocular comorbidities. Although both phakic and pseudophakic subjects were included, there was no significant difference in lens status between glaucomatous and control subjects and lens status was not significant in the multivariable models for predicting reaction times to the divided attention driving tasks (P = 0.381 for curve negotiation and P = 0.532 for car following). However, as only patients with good visual acuity were included, it would be interesting to conduct further studies on the effect of cataract on ability to divide attention.
In conclusion, we found that patients with glaucoma had significantly slower reaction times to demanding divided attention tasks during simulated driving. RNFL thinning measured by OCT provided additional information besides that provided by visual field assessment in explaining the observed reaction times of glaucoma patients. This suggests that structural measures such as RNFL thickness may have the potential to improve our ability to determine which patients might have problems performing daily activities, such as driving.
Supplementary Material
Acknowledgments
Funding/Support: Supported in part by National Institutes of Health/National Eye Institute grants EY021818 (FAM), EY11008 (LMZ), EY14267 (LMZ), EY019869 (LMZ) and core grant P30EY022589; Unrestricted grant from Research to Prevent Blindness; Brazilian National Research Council-CNPq grant 200178/2012-1 (DMF); grants for participants’ glaucoma medications from Alcon, Allergan, Pfizer, Merck and Santen.
Other Acknowledgements: None.
Biography
 Andrew Tatham Biosketch
Andrew Tatham Biosketch
Dr. Andrew J. Tatham is Consultant Ophthalmic Surgeon at Princess Alexandra Eye Pavilion and University of Edinburgh, UK. Prior to this he was Glaucoma Fellow at the Hamilton Glaucoma Center, University of California, San Diego. He also completed clinical glaucoma fellowship training at Moorfields Eye Hospital, London. He has achieved several prizes including the Pfizer Ophthalmic Fellowship from the Royal College of Ophthalmologists and highest score at the European Board of Ophthalmology examinations. His research interests include glaucoma imaging and structure-function relationships in glaucoma.
Footnotes
Supplemental Material available at AJO.com
Contributions of the Authors: Design and concept of the study: (AJT, ERB, PNR, MDP, RNW, LMZ, FAM). Collection, management, analysis and interpretation of the data: (AJT, ERB, PNR, MDP, DMF, RNW, LMZ, FAM). Preparation, review or approval of the manuscript: (AJT, ERB, PNR, MDP, DMF, RNW, LMZ, FAM).
Financial Disclosures: Drs Weinreb, Zangwill, and Medeiros receive research support from Carl-Zeiss Meditec; Drs Tatham, Weinreb, Zangwill, and Medeiros receive research support from Heidelberg Engineering; Dr Weinreb receives resource support from Nidek and Topcon; and Drs Medeiros and Weinreb are consultants to Carl-Zeiss Meditec, Inc. and Topcon. Drs Boer, Rosen and Meira-Freitas and Mr Della Penna have no financial disclosures.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Weinreb RN, Aung T, Medeiros FA. The pathophysiology and treatment of glaucoma: a review. JAMA. 2014;311(18):1901–11. doi: 10.1001/jama.2014.3192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McKean-Cowdin R, Varma R, Wu J, et al. Severity of visual field loss and health related quality of life. Am J Ophthalmol. 2007;143(6):1013–1023. doi: 10.1016/j.ajo.2007.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Medeiros FA, Weinreb RN, Boer RE, et al. Driving simulation as a performance-based test of visual impairment in glaucoma. J Glaucoma. 2012;21(4):221–227. doi: 10.1097/IJG.0b013e3182071832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Richman J, Lorenzana LL, Lankaranian D, et al. Relationships in glaucoma patients between standard vision tests, quality of life, and ability to perform daily activities. Ophthalmic Epidemiol. 2010;17(3):144–151. doi: 10.3109/09286581003734878. [DOI] [PubMed] [Google Scholar]
- 5.Owsley C, McGwin G., Jr Vision and driving. Vision Res. 2010;50(23):2348–2361. doi: 10.1016/j.visres.2010.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Broman AT, West SK, Muñoz B, et al. Divided visual attention as a predictor of bumping while walking: the Salisbury Eye Evaluation. Invest Ophthalmol Vis Sci. 2004;45(9):2955–2960. doi: 10.1167/iovs.04-0219. [DOI] [PubMed] [Google Scholar]
- 7.Owsley C, Ball K, McGwin G, et al. Visual processing impairment and risk of motor vehicle crash among older adults. JAMA. 1998;279(14):1083–1088. doi: 10.1001/jama.279.14.1083. [DOI] [PubMed] [Google Scholar]
- 8.Ball K, Owsley C. The useful field of view test: a new technique for evaluating age-related declines in visual function. J Am Optom Assoc. 1993;64(1):71–79. [PubMed] [Google Scholar]
- 9.Brouwer WH, Waterink W, Van Wolffelaar PC, et al. Divided attention in experienced young and older drivers: lane tracking and visual analysis in a dynamic driving simulator. Hum Factors. 1991;33(5):573–582. doi: 10.1177/001872089103300508. [DOI] [PubMed] [Google Scholar]
- 10.Klauer SG, Dingus TA, Neale VL, et al. The impact of driver inattention on near-crash/crash risk: an analysis using the 100-car naturalistic driving study data. Washington DC: US Department of Transportation, National Highway Traffic Safety Administration; 2006. DOT HS 810 594. [Google Scholar]
- 11.McDowd JM, Shaw RJ. Attention and aging: A functional perspective. In: Craik FIM, Salthouse TA, editors. Handbook of aging and cognition. 2. Mahwah, NJ: Erlbaum; 2007. pp. 221–292. [Google Scholar]
- 12.Rubin GS, Ng ES, Bandeen-Roche K, et al. A prospective, population-based study of the role of visual impairment in motor vehicle crashes among older drivers: the SEE study. Invest Ophthalmol Vis Sci. 2007;48(4):1483–1491. doi: 10.1167/iovs.06-0474. [DOI] [PubMed] [Google Scholar]
- 13.Hendricks DL, Fell JC, Freeman M. US Department of Transportation Summary Technical Report. Washington DC: National Highway Traffic Safety Administration; 1999. The relative frequency of unsafe driving acts in serious traffic crashes. [Google Scholar]
- 14.Quigley HA, Sanchez RM, Dunkelberger GR, et al. Chronic glaucoma selectively damages large optic nerve fibers. Invest Ophthalmol Vis Sci. 1987;28(6):913–920. [PubMed] [Google Scholar]
- 15.Sample PA, Girkin CA, Zangwill LM, et al. The African Descent and Glaucoma Evaluation Study (ADAGES): design and baseline data. Arch Ophthalmol. 2009;127(9):1136–1145. doi: 10.1001/archophthalmol.2009.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Leite MT, Rao HL, Zangwill LM, et al. Comparison of the diagnostic accuracies of the Spectralis, Cirrus, and RTVue optical coherence tomography devices in glaucoma. Ophthalmology. 2011;118(7):1334–1339. doi: 10.1016/j.ophtha.2010.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Racette L, Liebmann JM, Girkin CA, et al. African Descent and Glaucoma Evaluation Study (ADAGES): III. Ancestry differences in visual function in healthy eyes. Arch Ophthalmol. 2010;128(5):551–559. doi: 10.1001/archophthalmol.2010.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ball K, Owsley C, Sloane ME, et al. Visual attention problems as a predictor of vehicle crashes in older drivers. Invest Ophthalmol Vis Sci. 1993;34(11):3110–3123. [PubMed] [Google Scholar]
- 19.Potter MC, Faulconer BA. Time to understand pictures and words. Nature. 1975;253(5491):437–438. doi: 10.1038/253437a0. [DOI] [PubMed] [Google Scholar]
- 20.Friedman C, McGwin G, Ball KK, et al. Association between higher order visual processing abilities and a history of motor vehicle collision involvement by drivers ages 70 and over. Invest Ophthalmol Vis Sci. 2013;54(1):778–782. doi: 10.1167/iovs.12-11249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.O’Hare F, Rance G, Crowston JG, et al. Auditory and visual temporal processing disruption in open angle glaucoma. Invest Ophthalmol Vis Sci. 2012;53(10):6512–6518. doi: 10.1167/iovs.12-10188. [DOI] [PubMed] [Google Scholar]
- 22.Hultsch DF, MacDonald SW, Dixon RA. Variability in reaction time performance of younger and older adults. Journal of Gerontology Series B: Psychological Sciences and Social Sciences. 2002;57(2):101–115. doi: 10.1093/geronb/57.2.p101. [DOI] [PubMed] [Google Scholar]
- 23.Brookhuis KA, De Waard D, Mulder B. Measuring driving performance by car-following in traffic. Ergonomics. 1994;37(3):427–434. [Google Scholar]
- 24.Marsden G, McDonald M, Brackstone M. Towards an understanding of adaptive cruise control. Transportation Research Part C: Emerging Technologies. 2001;9(1):33–51. [Google Scholar]
- 25.Nasreddine ZS, Phillips NA, Bédirian V, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53(4):695–699. doi: 10.1111/j.1532-5415.2005.53221.x. [DOI] [PubMed] [Google Scholar]
- 26.Ball KK, Roenker DL, Wadley VG, et al. Can high-risk older drivers be identified through performance-based measures in a Department of Motor Vehicles setting? J Am Geriatr Soc. 2006;54(1):77–84. doi: 10.1111/j.1532-5415.2005.00568.x. [DOI] [PubMed] [Google Scholar]
- 27.Leung CK, Chiu V, Weinreb RN, et al. Evaluation of retinal nerve fiber layer progression in glaucoma: a comparison between spectral-domain and time-domain optical coherence tomography. Ophthalmology. 2011;118(8):1558–1562. doi: 10.1016/j.ophtha.2011.01.026. [DOI] [PubMed] [Google Scholar]
- 28.Mansouri K, Leite MT, Medeiros FA, et al. Assessment of rates of structural change in glaucoma using imaging technologies. Eye. 2011;25(3):269–277. doi: 10.1038/eye.2010.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Van Gestel A, Webers CA, Beckers HJ, et al. The relationship between visual field loss in glaucoma and health-related quality-of-life. Eye. 2010;24(12):1759–1769. doi: 10.1038/eye.2010.133. [DOI] [PubMed] [Google Scholar]
- 30.McKean-Cowdin R, Wang Y, Wu J, et al. Impact of visual field loss on health-related quality of life in glaucoma: the Los Angeles Latino Eye Study. Ophthalmology. 2008;115(6):941–948. doi: 10.1016/j.ophtha.2007.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Burton R, Crabb DP, Smith ND, et al. Glaucoma and reading: exploring the effects of contrast lowering of text. Optom Vis Sci. 2012;89(9):1282–1287. doi: 10.1097/OPX.0b013e3182686165. [DOI] [PubMed] [Google Scholar]
- 32.Richman J, Lorenzana LL, Lankaranian D, et al. Importance of visual acuity and contrast sensitivity in patients with glaucoma. Arch Ophthalmol. 2010;128(12):1576–1582. doi: 10.1001/archophthalmol.2010.275. [DOI] [PubMed] [Google Scholar]
- 33.Murray IJ, Plainis S. Contrast coding and magno/parvo segregation revealed in reaction time studies. Vision Res. 2003;43(25):2707–2719. doi: 10.1016/s0042-6989(03)00408-5. [DOI] [PubMed] [Google Scholar]
- 34.Plainis S, Murray IJ. Neurophysiological interpretation of human visual reaction times: effect of contrast, spatial frequency and luminance. Neuropsychologia. 2000;38(12):1555–1564. doi: 10.1016/s0028-3932(00)00100-7. [DOI] [PubMed] [Google Scholar]
- 35.Sample PA, Medeiros FA, Racette L, et al. Identifying glaucomatous vision loss with visual-function-specific perimetry in the diagnostic innovations in glaucoma study. Invest Ophthalmol Vis Sci. 2006;47(8):3381–3389. doi: 10.1167/iovs.05-1546. [DOI] [PubMed] [Google Scholar]
- 36.Pearson P, Swanson WH, Fellman RL. Chromatic and achromatic defects in patients with progressing glaucoma. Vision Res. 2001;41(9):1215–1227. doi: 10.1016/s0042-6989(00)00311-4. [DOI] [PubMed] [Google Scholar]
- 37.Shechtman O, Classen S, Awadzi K, et al. Comparison of driving errors between on-the-road and simulated driving assessment: a validation study. Traffic Inj Prev. 2009;10(4):379–385. doi: 10.1080/15389580902894989. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.