Abstract
The chemical composition of propolis varies according to factors that could have an influence on its biological properties. Polyphenols from propolis have demonstrated an inhibitory effect on Streptococcus mutans growth. However, it is not known if different years of propolis collection may affect its activity. We aimed to elucidate if the year of collection of propolis influences its activity on Streptococcus mutans. Polyphenol-rich extracts were prepared from propolis collected in three different years, characterized by LC-MS and quantified the content of total polyphenols and flavonoids groups. Finally, was evaluated the antibacterial effect on Streptococcus mutans and the biofilm formation. Qualitative differences were observed in total polyphenols, flavones, and flavonols and the chemical composition between the extracts, affecting the strength of inhibition of biofilm formation but not the antimicrobial assays. In conclusion, chemical composition of propolis depends on the year of collection and influences the strength of the inhibition of biofilm formation.
1. Introduction
The propolis is a resinous substance collected by honeybees (Apis mellifera) used to protect the beehive against the invasion of various pathogenic microorganisms. The main bioactive components of propolis are flavonoids, terpenes, and phenolics compounds. However, it is also composed by sugars, hydrocarbons, and mineral elements [1, 2]. Chemical studies have determined a correlation between the composition of propolis with the season and geographic region of collection, plant sources used for collection, and the bee species involved in the process. Thus, its variable composition may have an influence on the biological properties demonstrated by different extracts [3–6]. The pharmacological properties of propolis are well documented and include previous reports of our group describing antidiabetogenic, antiatherogenic, antimicrobial, and antifungal activities of Chilean propolis extracts [7–10] using well characterized extracts in which pinocembrin appears among its main constituents [11, 12].
Streptococcus mutans (S. mutans) is considered a key player involved in the development of dental caries. Its main virulence is derived from the ability to synthesize water-insoluble and soluble glucans from sucrose, leading to the accumulation of these glucans in a dental biofilm [13], process mediated by the expression of extracellular glucosyltransferases enzymes (GtfS), that in combination with glucan-binding proteins (GBPs) are important for the sucrose-dependent adhesion to the tooth surfaces [14]. The inhibitory capacity of Chilean propolis on the growth of S. mutans has been demonstrated but with high variability depending on the characteristics of the extract evaluated [11]. However, the effect of a propolis sample collected in the same geographical place but in different years has not been evaluated. Thus, the aim of the present study was to evaluate the chemical composition and the effect on Streptococcus mutans growth and biofilm formation of polyphenol-rich extracts from Chilean propolis collected at the same apiary and same season along three different years.
2. Materials and Methods
2.1. Preparation of Crude Extracts from Chilean Propolis (CEP)
To evaluate the effect of propolis-collecting year on the chemical composition and antimicrobial activity of Chilean propolis, three propolis samples were obtained from the Andean region of La Araucanía, Chile, in the spring of the years 2008, 2010, and 2011, to prepare three polyphenol-rich extracts (CEP1, CEP2, and CEP3, resp.). Propolis crude samples were kept frozen (−20°C) and protected from light until when propolis polyphenols were simultaneously extracted and analyzed. Frozen propolis samples were crushed to obtain a powder propolis. Then, 30 g was dissolved in 70% ethanol and macerated for 7 days at room temperature. Finally, the solutions were filtered using a whatman paper number 2 and centrifuged at 327 g for 20 minutes to eliminate the resins from the extract.
2.2. Determination of Phenolic Compounds Groups
2.2.1. Determination of Total Polyphenols
The content of total polyphenols was quantified using the Folin-Ciocalteu method [15]. CEP (100 μL) was mixed with distilled water (100 μL), Folin-Ciocalteu reagent (2 mL), and sodium carbonate 20% w/v (3 mL). The resultant solution was incubated for 2 hours at room temperature and the absorbance measured in a spectrophotometer (Infinite 200 PRO NanoQuant) at 760 nm. Concentrations were obtained from a calibration curve and expressed as mg mL−1 equivalent to the gallic acid.
2.2.2. Determination of Flavones and Flavonols
The content of flavones and flavonols was measured as previously described [16]. CEP samples were diluted 1 : 10 in ethanol 70% (v/v) and 250 μL of this extract was added to 250 μL of aluminum trichloride 5% (v/v) in methanol. The absorbance of the solution was measured at 425 nm in a spectrophotometer (Infinite 200 PRO NanoQuant). Flavonoid concentrations were calculated from a calibration curve and expressed in mg mL−1 equivalent to quercetin.
2.2.3. Determination of Flavanones and Dihydroflavonols
Polyphenol-rich extracts were diluted 1 : 10 in ethanol 70% (v/v). Afterward, 0.5 mL of diluted extract was added to 2 mL of 2.4-dinitrophenylhidrazine (DNP), incubated at 50°C for 50 min, and then decanted [17]. Absorbance was measured at 495 nm and the concentration of flavanones and dihydroflavonols was obtained from a calibration curve. Results were expressed in mg mL−1 equivalent to pinocembrin used as calibration solution.
2.3. Chemical Characterization
To identify the compounds present in the polyphenol-rich extracts we used Liquid Chromatography-tandem Mass Spectrophotometry (LC-MS). For the chromatographic separation RP-C18 Inersil ODS-3 column (2.1 × 150 mm, 3 mm) was used, with 10 μL of injection volume and a flow of 0.2 mL min−1 at 35°C. Standards and samples separation were performed using a gradient elution. The eluents A and B were formic acid (0.1%) and methanol, respectively. Flavonoids were studied in negative and positive polarity using the Multiple Reaction Monitoring (MRM) mode and data was acquired through the software Analyst 1.5.1 (Applied Biosystems, USA). In positive polarity, the flavonoids were optimized using standards of apigenin, daidzein, genistein, kaempferol, myricetin, pinocembrin, quercetin, and rutine (Sigma-Aldrich, St. Louis, MO) using the method of direct injection. In the negative polarity, the flavonoids and phenolic acids were optimized using the MRM mode with the p-coumaric acid, ferulic acid, chlorogenic acid, caffeic acid phenethyl ester (CAPE), caffeic acid, and gallic acid as standards (Sigma-Aldrich, St. Louis, MO).
2.4. Antimicrobial Activity Testing
Clinical isolates of Streptococcus mutans were obtained from the bacterial strain collection of our research center and confirmed by PCR as previously described [18]. Bacteria were grown on Columbia agar plates supplied with sucrose (1%) and incubated in anaerobic atmosphere (Anaerobic Generator GasPak EZ, Becton, Dickinson and Co., NY, USA) at 37°C and for 24 hours. The minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) were determined by the microdilution methodology as described in the Clinical and Laboratory Institute guidelines [19]. Serial dilution tests from the three extract were performed, sterilized in a filters of 0.2 μm, with different total polyphenols concentrations (0.1–100 μg mL−1) using an inoculum of 5 × 105 UFC mL−1 in sterile trypticase soy broth (TSB) supplied with 1% sucrose, and incubated at 37°C and 5% CO2 atmosphere. Sensibility tests were made by triplicate for each extract. Negative controls without treatments and vehicle were also tested.
2.5. Biofilm Formation by Streptococcus mutans under CEP Treatment
Biofilm growth was quantified by crystal violet staining assay [20]. The Streptococcus mutans inoculum (5 × 105 UFC mL−1) was incubated at 37°C and 5% CO2 atmosphere for 24 hours in 96-well microplates. Attachment cells were grown in microplates with TSB and sucrose (1%) with different total polyphenols concentrations ranging from 0.1 to 100 μg mL−1. First, the broth was removed and the plates were washed three times using PBS to eliminate no adherent bacteria and dried at 60°C for 45 minutes. Then, cells were stained using a crystal violet 1% (w/v) solution, incubated for 15 minutes and finally washed with sterile PBS to eliminate the excess of stain. Biofilm formation was determined by adding ethanol 95% to solubilize the crystal violet retained by the cells and optical density (O.D) was measured at 590 nm.
2.6. Statistical Analysis
Statistical analysis was performed using the program GraphPad Prism, version 5.0 (US). ANOVA was used for comparison of continuous variables (MBC and MIC). Tukey's Multiple Comparisons posttest was applied when we observed significant differences in ANOVA test, and Dunett's multiple comparisons to compare with the control. The significance level was α = 0.05.
3. Results
3.1. Determination of Different Groups of Phenolic Compounds
Differences were observed in the content of phenolic compounds between the extracts collected along the 3 years. Total polyphenols contained in the CEP2 were superior to CEP1 and CEP3 (p < 0.0001) and the content of flavones and flavonols differed between the three extracts analyzed. No differences were observed regarding the concentration of flavanones and dihydroflavonols (p = 0.228). Quantifications of phenolic compounds in polyphenol-rich extracts from Chilean propolis are listed in Table 1.
Table 1.
Influence of propolis-collecting year on the content of phenolic compounds groups in polyphenol-rich extracts.
| Group of compounds | CEP1 | CEP2 | CEP3 | ∗ p-value |
|---|---|---|---|---|
| Total polyphenols, mg mL−1 | 24.6 ± 0.4a | 29.0 ± 0.8b | 24.7 ± 0.2a | <0.0001 |
| Flavones and flavonols, mg mL−1 | 10.2 ± 0.03a | 11.9 ± 0.05b | 9.8 ± 0.1c | <0.0001 |
| Flavanones and dihydroflavonols, mg mL−1 | 8.3 ± 0.3 | 9.4 ± 0.6 | 8.2 ± 1.3 | 0.228 |
CEP1, CEP2, and CEP3: polyphenol-rich extracts from Chilean propolis collected in 2008, 2010, and 2011, respectively. Results expressed as mean ± standard deviation. Total polyphenols, flavones, and flavonols and flavanones and dihydroflavonols are expressed as gallic acid, quercetin, and pinocembrin equivalent, respectively. ∗ p value from ANOVA test. Different letters indicate significant differences after Tukey's Multiple Comparisons posttest.
3.2. Chemical Characterization
The chemical characterization of three CEP obtained by LC/MS using both retention times and spectra transitions in positive and negative polarity distinguished flavonoids and phenolic acids. The majority of compounds are present in the three analyzed extracts; however, there are some qualitative variations. Among the common flavonoids compounds are apigenin, genistein, kaempferol, myricetin, pinocembrin, and quercetin. Daidzein and rutine were detected depending on the year of collection (Table 2). Regarding phenolic acids, CAPE, caffeic, p-coumaric, and ferulic acids were detected in all extracts analyzed. Chlorogenic and gallic acids were dependent on the year (Table 3).
Table 2.
Flavonoids identified in three polyphenol-rich extracts from Chilean propolis by LC-MS.
| Compound | CEP1 | CEP2 | CEP3 | Retention time | MW | Main fragments |
|---|---|---|---|---|---|---|
| Apigenin | + | + | + | 42.6 | 270 | 269, 254, 226, 167 |
| Daidzein | + | N.D. | + | 40.5 | 254 | 153, 129 |
| Genistein | + | + | + | 44.4 | 270 | 253, 215 |
| Kaempferol | + | + | + | 34.6 | 286 | 269, 241, 229, 183 |
| Myricetin | + | + | + | 30.0 | 318 | 301, 273, 169, 153 |
| Pinocembrin | + | + | + | 42.0 | 256 | 239, 215, 173, 153 |
| Quercetin | + | + | + | 32.5 | 302 | 285, 257 |
| Rutine | + | N.D. | N.D. | 35.9 | 309 | 300, 271 |
CEP1, CEP2, and CEP3: polyphenol-rich extracts from Chilean propolis collected in 2008, 2010, and 2011, respectively. + indicates presence; N.D.: not detected.
Table 3.
Phenolic acids identified in three polyphenol-rich extracts from Chilean propolis by LC-MS.
| Compound | CEP1 | CEP2 | CEP3 | Retention time | MW | Main fragments |
|---|---|---|---|---|---|---|
| Caffeic acid | + | + | + | 15.3 | 180 | 135, 105 |
| CAPE | + | + | + | 41.8 | 284 | 139, 135 |
| Chlorogenic acid | N.D. | + | + | 30.9 | 354 | 191, 161 |
| P-coumaric acid | + | + | + | 16.7 | 164 | 119, 104 |
| Ferulic acid | + | + | + | 34.5 | 194 | 178, 134 |
| Gallic acid | + | N.D | N.D | 8.5 | 170 | 125, 107 |
CEP1, CEP2, and CEP3: polyphenol-rich extracts from Chilean propolis collected in 2008, 2010, and 2011, respectively. CAPE: caffeic acid phenethyl ester; + indicates presence; N.D.: not detected.
3.3. Antimicrobial Testing
Antibacterial activity of the analyzed extracts was tested determining MIC and MBC in Streptococcus mutans cultures under treatment with polyphenols. Both parameters showed no variations between the extracts collected in different years (Table 4; MIC, p = 0.177; MBC, p = 0.645).
Table 4.
Antimicrobial activity of polyphenol-rich extracts from Chilean propolis on Streptococcus mutans.
| Antimicrobial activity | CEP1 | CEP2 | CEP3 | ∗ p value |
|---|---|---|---|---|
| MIC μg mL−1 | 0.91 ± 0.59 | 0.22 ± 0.15 | 0.39 ± 0.35 | 0.177 |
| MBC μg mL−1 | 1.30 ± 0.44 | 1.05 ± 0.44 | 0.91 ± 0.59 | 0.645 |
CEP1, CEP2, and CEP3: polyphenol-rich extracts from Chilean propolis collected in 2008, 2010, and 2011, respectively. MIC: minimum inhibitory concentration; MBC: minimum bactericide concentration. Results expressed as mean ± standard deviation. ∗ p value from ANOVA test.
3.4. Biofilm Formation by Streptococcus mutans
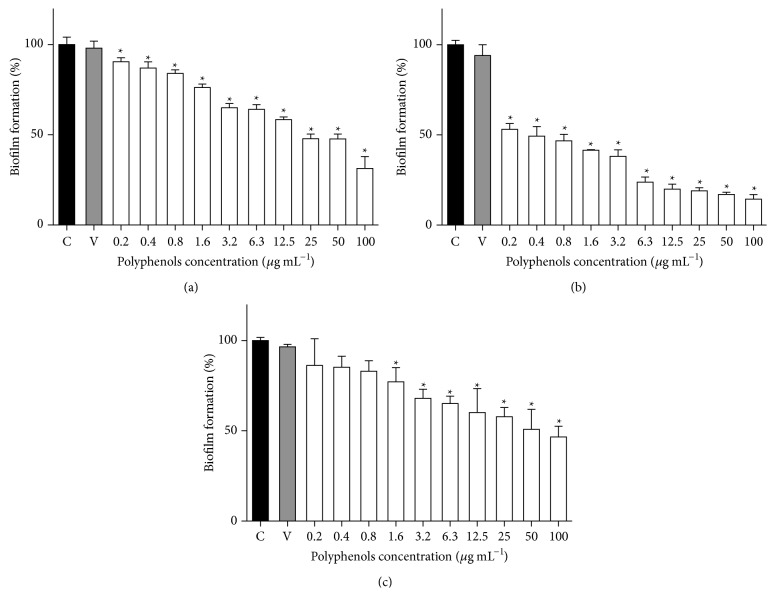
The effect of CEP on biofilm formation by Streptococcus mutans was determined by the Crystal Violet staining assay. The growth of bacterial plaque diminished with dose-dependent effects, starting from 0.2 μg mL−1 for the CEP1 and CEP2. The CEP3 showed a less potent effect with an inhibitory effect starting from 1.6 μg mL−1 (Figure 1).
Figure 1.
Biofilm formation in Streptococcus mutans cultures treated with polyphenol-rich extracts from Chilean propolis. (a), (b), and (c) figures show the effect on biofilm formation of CEP1, CEP2, and CEP3, respectively. C: control; V: vehicle. ANOVA: p < 0.0001, ∗Dunett's multiple comparisons versus control: p < 0.05.
4. Discussion
The medicinal properties of propolis have been widely described and include Streptococcus mutans antibacterial capabilities, suggesting the use of propolis as a cariostatic agent [21]. Biological activities of polyphenol-rich extracts from propolis show variations depending on its chemical composition, which in turn depends on its geographical origin, botanical sources, and the season of collection [22]. Thus, propolis samples from the same regions might have similar types of flavonoids and other phenolic compounds [23]. We prepared polyphenolic-rich extracts from Chilean propolis samples collected during spring at the same apiary along three different years. These samples were obtained from a nontranshumant apiary, so it is expected that vegetal species contributing to the production of propolis in the hive should not vary significantly between each year of production. Previously reported data characterizing the botanical origin of Chilean propolis from La Araucanía showed that Lotus uliginosus Schk. was the predominant vegetal source followed by Caldcluvia or Eucryphia, whose distinctive elements are not able to differentiate because of their high similarity [11]. Likewise, the plant debris identified in CEP1, CEP2, and CEP3 samples showed a predominance of structures from Lotus uliginosus Schk. (58–61%) and Caldcluvia or Eucryphia (19–23%).
Colorimetric assays were performed to quantify total polyphenols and flavonoids using two assays to determine flavone and flavonols or total flavanones and dihydroflavonols content. The three extracts analyzed in this study showed differences in total content of polyphenols depending on the year of collection, with the highest content in the propolis from the spring of 2010 (CEP2). The analyzed extracts had a slightly higher content than previously reported for Chilean propolis that have exhibited a high variability according to its geographical origin (from 3.4 to 21.4 mg mL−1), with the highest content observed in propolis from La Araucania [11]. The content of flavonoids, flavones, and flavonols also showed differences according to the year of collection and concordantly with the content of total polyphenols, a higher amount was observed in CEP2. However, the qualitative composition of flavonoids obtained by LC-MS showed the CEP2 as the extract with fewer compounds among the studied flavonoids. These findings suggest a quantitative compensation of absent flavonoids by the other compounds identified in the extract, so the quantification of individual compounds is an interesting issue to consider in further analysis.
The composition of the analyzed extracts was similar to that previously described for propolis from La Araucanía in which pinocembrin is the predominant compound [11]. The composition of Chilean propolis includes several classes of flavonoids and phenolic acids. Fragmentation patterns and retention times showed the presence of specifics members of different chemical families as flavones, represented by apigenin; flavonols such as quercetin, kaempferol; flavanones represented mainly by pinocembrin; and isoflavones as daidzein and genistein, whose characteristics ions in positive polarity were corresponding with previous LC-MS spectrum for propolis from other geographical areas [24, 25]. In comparison with propolis from other geographical origin, the Chilean propolis has a chemical composition with a higher diversity of compounds. Poplar types' propolis from Europe, Asia, and North America is characterized by the presence of pinocembrin, pinobanksin, galangin and benzyl, phenethyl, and prenyl caffeates; Northern Russia samples have significant levels of acacetin, apigenin, ermanin, and kaempferide. Another propolis collected from tropical areas, such as the Brazilian green propolis, contains prenylated p-coumaric acids, prenylated acetophenones, and diterpenic acids [6, 22, 26]. Cinnamic acid derivatives were the most abundant phenolics acids, including caffeic, p-coumaric, and ferulic acid and were present in the samples, but gallic acid was detected only in the CEP1, similarly to tropical and subtropical sources rich in p-coumaric acids and diterpenic and triterpenic acids [15]. Moreover, chemical composition of propolis has been linked to its botanical origin, which corresponds to the botanical sources from which bees produce propolis. Data previously reported for Chilean propolis have shown variations depending on the month of collection [27]. Similar results were described for the content of total polyphenols and flavonoids for propolis from Argentina [28]. The samples analyzed in the present study were collected during the same month along three different years, which could mean that the predominant botanical resources are maintained between samples and thus its chemical composition is only slightly affected. In brief, our results show some quantitative and qualitative variations in the composition of the polyphenol-rich extracts from Chilean propolis collected on the years 2008, 2010, and 2011, which could influence its biological activities.
When we analyzed the antibacterial activity using both MIC and MBC assays, no differences were observed between the three polyphenol-rich extracts. This finding suggests that antibacterial activity of the polyphenol-rich extracts analyzed results from the action of common compounds present in these three extracts as apigenin; a flavonoid identified in all CEP samples showed antimicrobial activity in previous studies [29], although the mechanisms of antimicrobials properties of propolis have not been completely elucidated. As pointed out previously, it can also be associated with a synergistic effect of its components [21]. Finally, the inhibitory effect of polyphenol-rich extracts on biofilm formation was variable between the three extracts. Concordantly with the observed in the content of polyphenols and flavonoids, the second extract (CEP2) showed a highest inhibition at lower concentrations of polyphenols, starting from 0.2 μg mL−1 with an inhibition of about 50% on biofilm formation. Other authors obtained similar results in microplates assays starting with 100 μg mL−1 of EEP and observed decreased biofilm formation percentages and dose-dependent effects [30]. Glucosyltransferases B and C (GtfB and GtfC) play a key role on biofilm formation in oral cavity. Several reports show multiple inhibitory activities at concentrations as low as 25 μg mL−1 of 6 propolis types and an effective inhibition of GtfB and GtfC, affecting the process of dental caries and plaque formation [31, 32]. Therefore, this may be a possible responsible mechanism of the effect of polyphenol-rich extracts on biofilm formation by Streptococcus mutans.
5. Conclusion
In summary, our results indicate that polyphenol-rich extracts from Chilean propolis present qualitative differences in its composition that are dependent on the year of collection. The year-related content differences distinctively inhibit the biofilm formation of Streptococcus mutans. However, they do not have an influence on bacterial growth.
Acknowledgments
The authors would like to express their gratitude to CONICYT and the financial support of DIUFRO (Grant no. DI10-0031).
Conflict of Interests
The authors declare that there is no conflict of interests regarding the publication of this paper.
References
- 1.Castaldo S., Capasso F. Propolis, an old remedy used in modern medicine. Fitoterapia. 2002;73(1):S1–S6. doi: 10.1016/s0367-326x(02)00185-5. [DOI] [PubMed] [Google Scholar]
- 2.Huang S., Zhang C. P., Wang K., Li G. Q., Hu F. L. Recent advances in the chemical composition of propolis. Molecules. 1961;19(12):19610–19632. doi: 10.3390/molecules191219610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Daugsch A., Moraes C. S., Fort P., Park Y. K. Brazilian red propolis—chemical composition and botanical origin. Evidence-Based Complementary and Alternative Medicine. 2008;5(4):435–441. doi: 10.1093/ecam/nem057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Moreno M. I. N., Isla M. I., Cudmani N. G., Vattuone M. A., Sampietro A. R. Screening of antibacterial activity of Amaicha del Valle (Tucumán, Argentina) propolis. Journal of Ethnopharmacology. 1999;68(1–3):97–102. doi: 10.1016/s0378-8741(99)00051-3. [DOI] [PubMed] [Google Scholar]
- 5.Silici S., Kutluca S. Chemical composition and antibacterial activity of propolis collected by three different races of honeybees in the same region. Journal of Ethnopharmacology. 2005;99(1):69–73. doi: 10.1016/j.jep.2005.01.046. [DOI] [PubMed] [Google Scholar]
- 6.Bankova V. Recent trends and important developments in propolis research. Evidence-Based Complementary and Alternative Medicine. 2005;2(1):29–32. doi: 10.1093/ecam/neh059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Saavedra N., Barrientos L., Herrera C. L., Alvear M., Montenegro G., Salazar L. A. Effect of Chilean propolis on cariogenic bacteria Lactobacillus fermentum . Ciencia e Investigación Agraria. 2011;38(1):117–125. [Google Scholar]
- 8.Daleprane J. B., da Silva Freitas V., Pacheco A., et al. Anti-atherogenic and anti-angiogenic activities of polyphenols from propolis. The Journal of Nutritional Biochemistry. 2012;23(6):557–566. doi: 10.1016/j.jnutbio.2011.02.012. [DOI] [PubMed] [Google Scholar]
- 9.Pacheco A., Daleprane J. B., Freitas V. S., et al. Efecto del propóleos chileno sobre el metabolismo de glucosa en ratones diabéticos. International Journal of Morphology. 2011;29(3):754–761. doi: 10.4067/s0717-95022011000300015. [DOI] [Google Scholar]
- 10.Herrera C. L., Alvear M., Barrientos L., Montenegro G., Salazar L. A. The antifungal effect of six commercial extracts of Chilean propolis on Candida spp. Ciencia e Investigación Agraria. 2010;37(1):75–84. [Google Scholar]
- 11.Barrientos L., Herrera C. L., Montenegro G., et al. Chemical and botanical characterization of chilean propolis and biological activity on cariogenic bacteria Streptococcus mutans and Streptococcus sobrinus . Brazilian Journal of Microbiology. 2013;44(2):577–585. doi: 10.1590/s1517-83822013000200038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cuevas A., Saavedra N., Cavalcante M. F., Salazar L. A., Abdalla D. S. P. Identification of microRNAs involved in the modulation of pro-angiogenic factors in atherosclerosis by a polyphenol-rich extract from propolis. Archives of Biochemistry and Biophysics. 2014;557:28–35. doi: 10.1016/j.abb.2014.04.009. [DOI] [PubMed] [Google Scholar]
- 13.Beighton D. The complex oral microflora of high-risk individuals and groups and its role in the caries process. Community Dentistry and Oral Epidemiology. 2005;33(4):248–255. doi: 10.1111/j.1600-0528.2005.00232.x. [DOI] [PubMed] [Google Scholar]
- 14.Krzyściak W., Jurczak A., Kościelniak D., Bystrowska B., Skalniak A. The virulence of Streptococcus mutans and the ability to form biofilms. European Journal of Clinical Microbiology and Infectious Diseases. 2014;33(4):499–515. doi: 10.1007/s10096-013-1993-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Popova M., Bankova V., Butovska D., et al. Validated methods for the quantification of biologically active constituents of poplar-type propolis. Phytochemical Analysis. 2004;15(4):235–340. doi: 10.1002/pca.777. [DOI] [PubMed] [Google Scholar]
- 16.Popova M. P., Bankova V. S., Bogdanov S., et al. Chemical characteristics of poplar type propolis of different geographic origin. Apidologie. 2007;38(3):306–311. doi: 10.1051/apido:2007013. [DOI] [Google Scholar]
- 17.Chang C.-C., Yang M.-H., Wen H.-M., Chern J.-C. Estimation of total flavonoid content in propolis by two complementary colometric methods. Journal of Food and Drug Analysis. 2002;10(3):178–182. [Google Scholar]
- 18.Salazar L. A., Vásquez C., Almuna A., et al. Detección molecular de estreptococos cariogénicos en Saliva. International Journal of Morphology. 2008;26(4):951–958. doi: 10.4067/S0717-95022008000400027. [DOI] [Google Scholar]
- 19.CLSI. Methods for Dilution Antimicrobial Susceptibility Test for Bacteria That Grow Aerobically. Approved Standard. 8th. Wayne, Pa, USA: Clinical and Laboratory Standards Institute; 2009. (CLSI Document M07-A8). [Google Scholar]
- 20.Kouidhi B., Zmantar T., Bakhrouf A. Anti-cariogenic and anti-biofilms activity of Tunisian propolis extract and its potential protective effect against cancer cells proliferation. Anaerobe. 2010;16(6):566–571. doi: 10.1016/j.anaerobe.2010.09.005. [DOI] [PubMed] [Google Scholar]
- 21.Libério S. A., Pereira A. L. A., Araújo M. J. A. M., et al. The potential use of propolis as a cariostatic agent and its actions on mutans group streptococci. Journal of Ethnopharmacology. 2009;125(1):1–9. doi: 10.1016/j.jep.2009.04.047. [DOI] [PubMed] [Google Scholar]
- 22.Bankova V. S., de Castro S. L., Marcucci M. C. Propolis: Recent advances in chemistry and plant origin. Apidologie. 2000;31(1):3–15. doi: 10.1051/apido:2000102. [DOI] [Google Scholar]
- 23.Gülçin I., Bursal E., Şehitoğlu M. H., Bilsel M., Gören A. C. Polyphenol contents and antioxidant activity of lyophilized aqueous extract of propolis from Erzurum, Turkey. Food and Chemical Toxicology. 2010;48(8-9):2227–2238. doi: 10.1016/j.fct.2010.05.053. [DOI] [PubMed] [Google Scholar]
- 24.Cavaliere C., Cucci F., Foglia P., Guarino C., Samperi R., Laganà A. Flavonoid profile in soybeans by high-performance liquid chromatography/tandem mass spectrometry. Rapid Communications in Mass Spectrometry. 2007;21(14):2177–2187. doi: 10.1002/rcm.3049. [DOI] [PubMed] [Google Scholar]
- 25.Gardana C., Scaglianti M., Pietta P., Simonetti P. Analysis of the polyphenolic fraction of propolis from different sources by liquid chromatography-tandem mass spectrometry. Journal of Pharmaceutical and Biomedical Analysis. 2007;45(3):390–399. doi: 10.1016/j.jpba.2007.06.022. [DOI] [PubMed] [Google Scholar]
- 26.Fabris S., Bertelle M., Astafyeva O., et al. Antioxidant properties and chemical composition relationship of Europeans and Brazilians propolis. Pharmacology & Pharmacy. 2013;4(1):46–51. doi: 10.4236/pp.2013.41006. [DOI] [Google Scholar]
- 27.Montenegro G., Peña R. C., Avila G., Timmermann B. N. Botanical origin and seasonal production of propolis in hives of central Chile. Boletim de Botânica da Universidade de São Paulo. 2001;19:1–6. [Google Scholar]
- 28.Isla M. I., Zampini I. C., Ordóñez R. M., et al. Effect of seasonal variations and collection form on antioxidant activity of propolis from San Juan, Argentina. Journal of Medicinal Food. 2009;12(6):1334–1342. doi: 10.1089/jmf.2008.0286. [DOI] [PubMed] [Google Scholar]
- 29.Koo H., Rosalen P. L., Cury J. A., Park Y. K., Bowen W. H. Effects of compounds found in propolis on Streptococcus mutans growth and on glucosyltransferase activity. Antimicrobial Agents and Chemotherapy. 2002;46(5):1302–1309. doi: 10.1128/aac.46.5.1302-1309.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Islam B., Khan S. N., Haque I., Alam M., Mushfiq M., Khan A. U. Novel anti-adherence activity of mulberry leaves: inhibition of Streptococcus mutans biofilm by 1-deoxynojirimycin isolated from Morus alba . The Journal of Antimicrobial Chemotherapy. 2008;62(4):751–757. doi: 10.1093/jac/dkn253. [DOI] [PubMed] [Google Scholar]
- 31.Mattos-Graner R. O., Napimoga M. H., Fukushima K., Duncan M. J., Smith D. J. Comparative analysis of Gtf isozyme production and diversity in isolates of Streptococcus mutans with different biofilm growth phenotypes. Journal of Clinical Microbiology. 2004;42(10):4586–4592. doi: 10.1128/jcm.42.10.4586-4592.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Duarte S., Koo H., Bowen W. H., et al. Effect of a novel type of propolis and its chemical fractions on glucosyltransferases and on growth and adherence of mutans streptococci. Biological & Pharmaceutical Bulletin. 2003;26(4):527–531. doi: 10.1248/bpb.26.527. [DOI] [PubMed] [Google Scholar]