Abstract
Objective
Declines in cervical cancer incidence and mortality in Canada and in the United States have been widely attributed to the introduction of the Papanicolaou (Pap) test. This article reviews changes in screening and introduction of HPV vaccination.
Method
Sentinel events in cervical cancer screening and primary prevention through HPV vaccination in the US and Canada are described.
Results
Despite commonalities, cervical cancer screening and prevention differ between the two countries. Canada has a combination of opportunistic and organized programs at the provincial and territorial level, while the US has opportunistic screening and vaccination systems. In the US, the HPV test along with the Pap test (co-testing) is part of national recommendations for routine cervical cancer screening for women age 30 and older. Co-testing is not being considered anywhere in Canada, but primary HPV testing is currently recommended (but not implemented) in one province in Canada.
Conclusion
Many prevention strategies are available for cervical cancer. Continued public health efforts should focus on increasing vaccine coverage in the target age groups and cervical cancer screening for women at appropriate intervals. Ongoing evaluation will be needed to ensure appropriate use of health resources, as vaccinated women become eligible for screening.
Keywords: HPV, Screening, Vaccination, North America, US and Canada
Background
In the United States and Canada, cervical cancer screening is a public health success (Centers for Disease Control and Prevention, 2011b; Public Health Agency of Canada, 2012). Declines in cervical cancer incidence and mortality in both countries have been attributed to the introduction of the Papanicolaou (Pap) cytology test, but declines have recently leveled off and disparities continue (Freeman and Wingrove, 2005). The United States (U.S. Cancer Statistics Working Group, 2010) and Canada (Canadian cancer society's steering committee on cancer statistics, 2012) have, respectively, approximately 12,400 and 1350 cases of cervical cancers diagnosed and 4000 and 390 deaths annually.
While screening with a Pap test remains an important prevention tool, several key developments in cervical cancer prevention have slowly shifted focus from cytology-based screening alone to incorporate human papillomavirus (HPV)-based screening with Pap testing and HPV vaccination. A sentinel event in 1975, was Dr. zur Hausen's hypothesis that HPV was the primary cause of cervical cancer (Fig. 1) (zur Hausen et al., 1975). This laid the groundwork for development of HPV-based diagnostics and HPV vaccines in following decades. Better understanding of the natural history of cervical cancer led to more refined screening parameters and options for prevention. While considerable progress has been made in the discovery of new technologies related to cervical cancer screening and HPV vaccination, challenges remain in making public health prevention of cervical cancer more efficient.
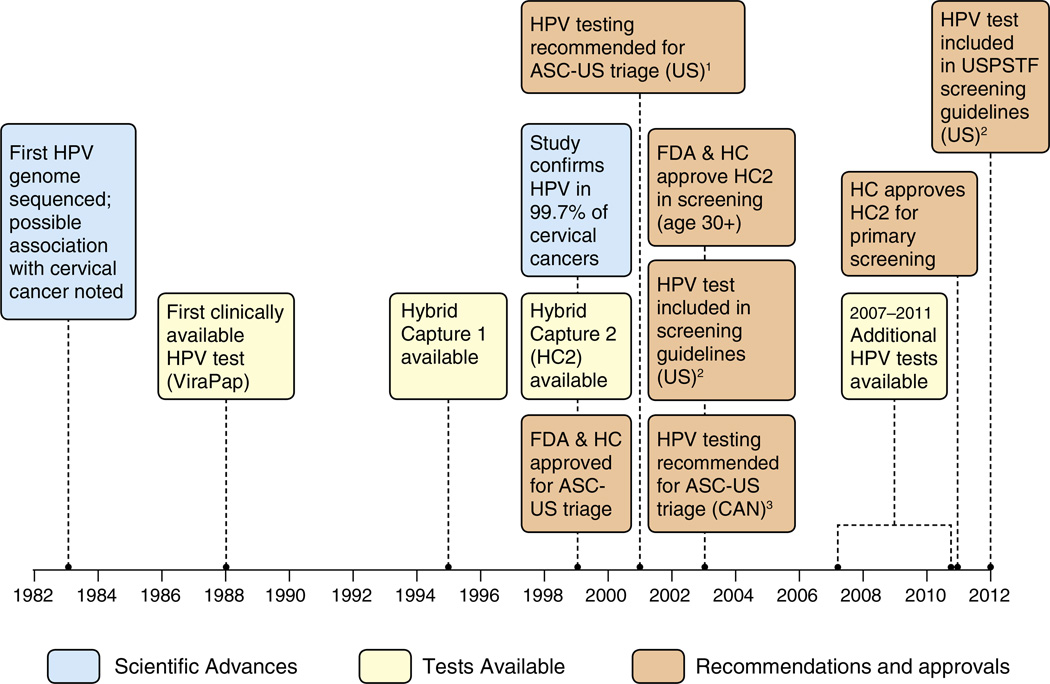
Fig. 1.
Evolution of HPV testing in Canada and the United States. Footnotes: US=United States. CAN=Canada. FDA=US Food and Drug administration. HC=Health Canada. USPSTF=US Preventive Services Task Force. ASC-US=Atypical squamous cells of undetermined significance. HPV=Human Papillomavirus. HPV testing was first recommended for triage of ASC-US lesions by the American Society for Colposcopy and Cervical Pathology in 2001. The American Cancer Society and American College of Obstetricians and Gynecologists recommended HPV tests as an option for screening women 30 years and older starting in 2003. The US Preventive service Task Force made a similar recommendation in 2012. The Pan-Canadian Forum on Cervical Cancer Prevention and Control first recommended HPV testing for triage of ASCUS lesions in Canada in 2003. The Society of Obstetricians and Gynecologists of Canada made a similar recommendation in 2007. SOURCES: Cox, JT. History of the use of HPV testing in cervical screening and in the management of abnormal cervical screening results. Journal of Clinical Virology 2009;45:S3–S12. Centers for Disease Control and Prevention. Cervical Cancer Screening Guidelines for Average-Risk Women. Available at http://www.cdc.gov/cancer/cervical/pdf/guidelines.pdf. Accessed November 6, 2012. FDA. U.S. Food and Drug Administration website. Available at www.fda.gov. Accessed November 6, 2012.
Natural history
HPV infection is common, but cervical cancer is comparatively rare and usually slow to develop. Almost all sexually active persons will be infected with HPV at least once in their lifetime (Weinstock et al., 2004). Most HPV infections clear within a few years (Rodriguez et al., 2008). Screening detects many lesions, but most regress, especially low grade squamous intraepithelial lesions (LSIL) and atypical cells of undetermined significance (ASC-US), confirming that not all lesions need to be treated (Ostor, 1993). Integration of HPV and persistence over time, not merely infection, leads to development of high-grade precancers and sometimes invasive cervical cancer (Schiffman et al., 2011). Of the 14 oncogenic HPV types, HPV 16 and to a lesser degree, HPV 18 are considered the HPV types that progress most rapidly and most often from infection to significant lesions (Schiffman et al., 2011).
HPV-based screening
HPV testing identifies individuals at increased risk of developing high-grade cervical precancer or cancer, and has been evaluated as a screening test with cytology (co-testing), as a stand-alone screening test (primary HPV screening), and as part of management and surveillance strategies. Although invasive cervical cancer is rare in screened populations, false-negative screening cytology results may be responsible for up to 30% of invasive cervical cancers (Spence et al., 2007). HPV testing has a higher sensitivity but lower specificity (i.e. more false-positive test results) than cytology in the detection of high-grade lesions (Moyer and USPSTF, 2012).
Available HPV tests
The most commonly used HPV test in the United States and Canada has been the Digene (i.e. Qiagen Inc., Valencia CA) Hybrid Capture 2 (HC2) test (Hogarth et al., 2012). In the last 5 years, additional tests have been approved by the US Food and Drug Administration (FDA) and Health Canada (HC) for detecting clinically significant levels of 13–14 high-risk HPV types (see Table 2).
Table 2.
Currently available HPV tests in the United States and Canada.*
| Test | Manufacturer | Method | HPV types detected | Information obtained from test |
|---|---|---|---|---|
| Hybrid capture 2 | Qiagen (Valencia, California) | Signal amplification | 13 high risk HPV DNA types | Indication of a high risk HPV type present |
| Cervista™ HPV HR test | Hologic (Bedford, MA) | Probe amplification | 14 High Risk HPV DNA types | Indication of a high risk HPV type present |
| Cervista™ 16/18 HPV test | Hologic (Bedford, MA) | Probe amplification | HPV 16/18 (DNA) | Indication of presence of HPV 16/18 |
| Aptima HPV | GenProbe (San Diego, CA) | Target amplification | 14 high risk HPV types (RNA/E6/E7) | Indication of a high risk HPV type present |
| Cobas 4800 HPV test | Roche molecular systems (Pleasanton, CA) | Target amplification | 14 high risk HPV (DNA/L1 region) | Indicates which high risk is present |
Approved by FDA in United States or by Health Canada.
HPV test use in the United States
Although the first HPV test was FDA-approved in 1988, the HC2 test was only FDA-approved in 1999 for follow-up of ASC-US cytology to identify women who may benefit from immediate colposcopy (Fig. 1). Shortly thereafter, the ASC-US, LSIL (ALTS) trial confirmed the efficacy of HPV testing as a triage method for women with ASC-US Pap test results, increasing the use of HPV testing (Solomon et al., 2001). Guidelines from the American Society for Colposcopy and Cervical Pathology and the American College of Obstetricians and Gynecologists (ACOG) recommended using the HPV test for ASC-US management and in other less common scenarios (2003;Wright et al., 2002). In 2003, the HC2 High-Risk test was FDA-approved for screening women ≥ 30 years as a co-test. ACOG and American Cancer Society (ACS) recommended either co-testing or cytology alone, both at a 3-year interval. In 2009, ACOG recommended starting screening at age 21, since lesions among younger women are likelier to regress and treatment may cause adverse pregnancy outcomes. In 2012, because of the high negative predictive value of an HPV test, ACS and ACOG recommended co-testing as the preferred option for screening at a 5-year interval (American College of Obstetricians and Gynecologists, 2009; Saslow et al., 2012) The USPSTF released new recommendations in 2012 and for the first time, also included the option of co-testing as a screening strategy with similar intervals (i.e. 5-year interval for co-testing or 3-year interval with cytology alone) (Moyer and USPSTF, 2012). Despite several randomized clinical trials evaluating the efficacy of primary HPV testing, current U.S. guidelines do not support primary HPV testing alone, because of limited evidence and concerns about the high number of referrals for colposcopy. Both USPSTF and ACS guidelines mention a potential role for primary HPV testing, most likely in the context of an organized screening system. Guidelines also agree that adequately screened women over age 65 can discontinue screening. Thus, current guidelines from all organizations are consistent with regards to starting and stopping screening, intervals, and HPV co-testing.
HPV test use in Canada
In 1995, the first recommendation about HPV testing in Canada counseled against its use (Johnson and Canadian Task Force on the Periodic Health Examination, 1995). The 2003 Pan-Canadian Forum on Cervical Cancer Prevention and Control recommended HPV testing for triage of abnormal results and primary screening, but the recommendation was not implemented in public health programs (Stuart et al., 2004). The 2007 Canadian consensus guidelines of the Society of Obstetricians and Gynecologists of Canada recommended type-specific HPV testing within an appropriate algorithm for eligible women (Provencher DM, 2007). Triage of HPV DNA testing is recommended for women ≥ 30 years with ASC-US and only as an adjunct to cervical cytology, to reduce the false-positive rate of conventional cytology and increase the negative predictive value of testing (HPV Consensus Guidelines Committee, 2007). Since 2005, Ontario has recommended HPV testing for triage of ASC-US Pap tests and in 2012, was the first province to also recommend primary HPV testing for screening (Murphy et al., 2012a,b). However, in Ontario, HPV testing is only funded for ASC-US triage for women ≥ 30, not for primary screening, thus most likely only available to women willing to pay out of pocket or through private insurance. Recently, the Canadian Task Force on Preventive Health Care recommended against routine screening at age<25 years, routine screening at ages 25–69 years every 3 years, and and that routine screening may stop for ≥70 years who have undergone adequate screening (Canadian Task Force on Preventive Health Care, 2013). The recommendations do not address screening with HPV testing due to insufficient evidence.
Adherence to screening guidelines
In response to screening guidelines, providers have rapidly adopted new tests, but have been slow to adopt increased screening intervals. This resistance to interval change could be the result of pre-graduate training, disincentives due to loss of reimbursements with longer intervals, and lack of well-organized information systems to track screening history and ensure patient recall when screening is due. Findings from U.S. national surveys of providers and observational data demonstrate that contrary to guidelines, most providers use HPV testing with the Pap test annually, and that woman <30 are often tested for HPV (Lee et al., 2011; Phelan et al., 2011; Roland et al., 2011; Saraiya et al., 2010; Tatsas et al., 2012).
In the United States, cervical cancer screening prevalence is determined largely from national surveys using self-reported data. Approximately 83% of women with an intact cervix aged 21–65 years reported being screened in the past 3 years (Centers for Disease Control and Prevention, 2012a).Women less likely to receive recommended screening were Asian, Hispanic, foreign-born, less-educated, uninsured and those without a usual source of care. In Canada, the percentage of eligible women in the target population who had at least one Pap test in a three-year period ranged from 72.4% to 79.6%(Screening Performance Indicators Working Group and Control, 2010). The percentage of eligible women who were re-screened within three years after a negative Pap test, called the retention rate, ranged from 74.6% to 87.1% (Screening Performance Indicators Working Group and Control, 2010).
HPV vaccination
Two vaccines are licensed for use in the United States and Canada, quadrivalent HPV vaccine (Gardasil, Merck & Co, Inc.), and bivalent HPV vaccine (Cervarix, GlaxoSmithKline). Quadrivalent vaccine is directed against two oncogenic types, HPV 16 and 18, and two nononcogenic types, HPV 6 and 11. The bivalent vaccine is directed against HPV 16 and 18.
HPV vaccination in the United States
Quadrivalent HPV vaccine was licensed by the FDA in June 2006 for use in females aged 9–26 years (Markowitz et al., 2007). In October 2009, the bivalent vaccine was licensed for use in females aged 10–25 years (Centers for Disease Control Prevention, 2010). Following licensure by FDA, national recommendations for vaccine use are made by the Advisory Committee on Immunization Practices (ACIP) (Fig. 2) (Smith et al., 2009). ACIP considers many factors in making recommendations, including efficacy and safety, epidemiology and burden of disease, acceptability and cost effectiveness.
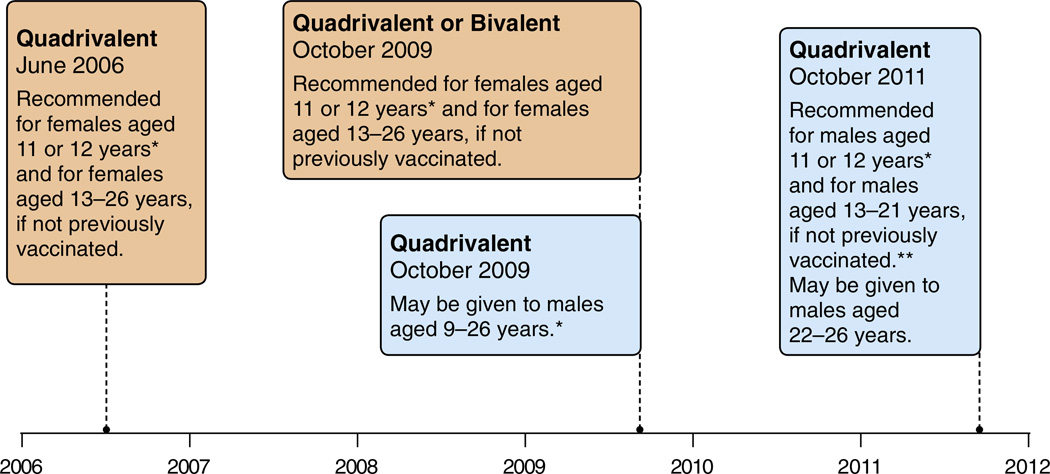
Fig. 2.
United States: evolution of recommendations for HPV vaccination from the Advisory Committee on Immunization Practice (ACIP). Footnotes: Quadrivalent (HPV 6,11,16,18) vaccine; Bivalent (HPV 16,18) vaccine. *Can be given starting at 9 years of age;. **For MSM and immunocompromised males, quadrivalent HPV vaccine through 26 years of age. SOURCES: Centers for Disease Control and Prevention. Quadrivalent Human Papillomavirus Vaccine: Recommendations of the Advisory Committee on Immunization Practices (ACIP), 2007. MMWR Recomm. Rep. 56, 1–24. Centers for Disease Control Prevention, 2010. FDA licensure of bivalent human papillomavirus vaccine (HPV2, Cervarix) for use in females and updated HPV vaccination recommendations from the Advisory Committee on Immunization Practices (ACIP). MMWR Morb. Mortal. Wkly Rep. 59, 626–629. Centers for Disease Control and Prevention, 2011. Recommendations on the use of quadrivalent human papillomavirus vaccine in males—Advisory Committee on Immunization Practices (ACIP), 2011. MMWR Morb. Mortal. Wkly Rep. 60, 1705–1708.
In June 2006, ACIP recommended routine vaccination of females aged 11–12 years with quadrivalent HPV vaccine (Markowitz et al., 2007). This age was selected to reach girls prior to sexual initiation, and to allow incorporation of HPV vaccine into the adolescent vaccination schedule. HPV vaccine was also recommended for previously unvaccinated females aged 13–26 years. When bivalent HPV vaccine was licensed by FDA in 2009, ACIP updated recommendations stating that either HPV vaccine is recommended for females.
In October 2009, quadrivalent HPV vaccine was licensed by FDA for use in males aged 9–26 years for prevention of genital warts. When data from a sub-study in men who have sex with men (MSM) demonstrated efficacy for prevention of vaccine type-related anal precancer lesions (Palefsky), quadrivalent HPV vaccine received an indication for prevention of anal cancer in both males and females (December 2010). After licensure of the vaccine for males, ACIP provided guidance that the vaccine may be used in males ages 9–26 years, but did not include vaccine for males in the routine immunization schedule (Centers for Disease Control and Prevention, 2010). Two years later, after review of additional data including vaccine efficacy for protection against anal precancers in males, the burden of HPV-associated disease in males, the status of the female vaccination program, safety and cost effectiveness, ACIP recommended routine vaccination of males at age 11–12 years, and vaccination through age 21 years for those not previously vaccinated (Centers for Disease Control and Prevention, 2011a; Chesson et al., 2011). For MSM and immunocompromised persons, vaccination is recommended through age 26 years for those not previously vaccinated.
Vaccines recommended by ACIP are usually included in the Vaccines for Children Program (VFC), which supplies providers with federally purchased vaccines as recommended for use among eligible children ages 0–18 years (Centers for Disease Control and Prevention). Both the quadrivalent and bivalent HPV vaccines were included in the VFC program for females at the time recommendations were made for each vaccine. Quadrivalent HPV vaccine for males was included in the VFC program in October 2009. Heath insurance usually covers vaccines routinely recommended by ACIP.
The progress of the immunization program in the United States is measured by the National Immunization Survey (NIS), which uses provider-verified records to determine vaccine coverage. Since 2006, national and state-specific vaccine coverage has been measured by NIS among 13–17 year olds. HPV vaccine initiation (at least one dose) among females increased from 25% in 2007 to 53% in 2011. In 2011, coverage with 3 doses of HPV vaccine was 35% among females and ranged by state (16%–57%) (Centers for Disease Control and Prevention, 2012b).
HPV vaccination in Canada
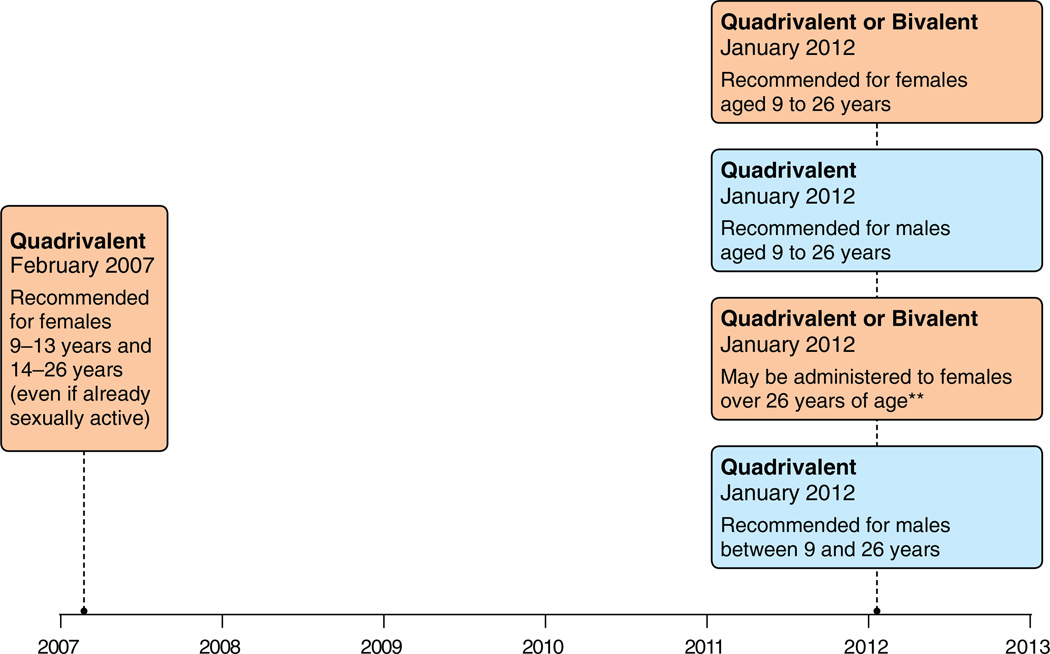
In July 2006, quadrivalent vaccine was approved by HC for use in females 9–26 years of age (Fig. 1). In February 2010, bivalent vaccine was approved for use in females 9–25 years of age (GSK). Also in February 2010, quadrivalent vaccine was approved for males 9–26 years of age for the prevention of HPV 6, 11, 16, and 18 infections as well as genital warts. In April 2011, quadrivalent vaccine was approved for use in females 9–45 years of age, and in May 2011, quadrivalent vaccine was approved for use in persons aged 9–26 years of age for the prevention of anal cancer and anal precancer lesions (Merck Canada Inc., 2012).
Following approval by HC, national recommendations for vaccine use are made by the National Advisory Committee on Immunization (NACI) (Fig. 3). NACI recommends bivalent or quadrivalent vaccines for females 9–26 years of age. Quadrivalent vaccine is recommended for males 9–26 years of age and males ≥9 years of age who have sex with men. Quadrivalent vaccine or bivalent vaccine may be administered to females older than (National Advisory Committee on, 2007, 2012; National Advisory Committee on Immunization (NACI), 2007). Provincial and territorial public health authorities adapt NACI recommendations into local programs, explaining differences in vaccine use across Canada (Table 1). These programs identify specific target age groups for the vaccination program. Providers can vaccinate patients outside the free programs of their province or territory but within HC licensures; patients may be covered by private insurance or pay out-of-pocket.
Fig. 3.
Canada: Evolution of Recommendations for HPV Vaccination from the National Advisory Committee on Immunization (NACI)*. Footnotes: Quadrivalent (HPV 6,11,16,18) vaccine; Bivalent (HPV 16,18) vaccine. *Recommendations are adapted by provincial and territorial programs. *Grade A for quadrivalent and Grade B for bivalent. National Advisory Committee on Immunization (NACI). Statement on human papillomavirus vaccine. An Advisory Committee Statement (ACS). Can Commun Dis Rep 2007;33 ACS-2: 1–31. Advisory Committee Statement (ACS) National Advisory Committee on Immunization (NACI) Update on Human Papillomavirus (HPV) Can Commun Dis Rep 2012;38 ACS-1:1–62. Note: NACI makes recommendations, but it is up to the various provincial and territorial authorities to adapt them to their specific vaccine programs.
Table 1.
HPV immunization programs in Canada.
| Province/territory | Routine schedule (0, 2 and 6 months) |
Date of implementation of routine program |
Catch-up programs (date of implementation) | Coverage |
|---|---|---|---|---|
| British Columbia | Grade 6 | September 2008 | Grade 9 (2008–2011) | 62% |
| Alberta | Grade 5 | September 2008 | Grade 9 (2009–2012) | 50–60% |
| Saskatchewan | Grade 6 | September 2008 | Grade 7 (2008–2009) | 58–66% |
| Manitoba | Grade 6 | September 2008 | 52–61% | |
| Ontario | Grade 8 | September 2007 | 53% | |
| Quebec | Grade 4* | September 2008 | 9–17 years oldwith school based program in Grade 4 and Grade 9 and “catch up” by participating clinics (2008–2013) Immunocompromised women 9–26 years old |
Grade 4: 66–94% (mean 77%) Grade 9: 63–93% (mean 76%) |
| New Brunswick | Grade 7 | September 2008 | Grade 8 (2008–2009) | n/a |
| Nova Scotia | Grade 7 | September 2007 | Grade 10 (2009–2010 only) | 85% |
| Grade 8 (2010–2011 only) | ||||
| Prince Edward Island | Grade 6 | September 2007 | Grade 9 (2009–2010 only) | 85% |
| Newfoundland and Labrador | Grade 6 | September 2007 | Grade 9 (2008–2010) | 85% |
| Northwest Territories | Grade 4 | September 2009 | Grades 11 & 12 (2009–2010) | |
| Grades 10 & 11 (2010–2011) | ||||
| Grades 9 & 10 (2011–2012) | ||||
| Grade 9 (2012–2014) | ||||
| Yukon | Grade 6 | September 2009 | Grades 7 & 8 | |
| Nunavut | Grade 6 | March 2010 |
Source:
Advisory Committee Statement (ACS) National Advisory Committee on Immunization (NACI) Update on Human Papillomavirus (HPV) CCDR. 2012;38 ACS-1:1–62.
British Columbia: Naus M, Ogilvie G. Human papillomavirus vaccine program in British Columbia: A good start with room for improvement. BCMJ, Vol. 52, No. 2, March 2010, p. 95
BC Centre for Disease Control.
Québec: Flash vigie, bulletin québécois de vigie et d'intervention en maladies infectieuses. Vol. 7, no. 7: 3–4.
Coverage rates provided Julie Laroche, Public Health Agency of Canada and appropriate provincial and territorial HPV immunization.
Coverage rates for completed school-based programs range from 50 to 94% depending on the region or the province (Naus and Ogilvie, 2010). For example, rates in Quebec vary regionally: for Grade 4, from 66 to 94% (mean 77%), and for Grade 9: 63–93% (mean 76%). Canadian programs for females of the target age but not reached by the school-based program have lower rates of coverage.
Achieving efficiency in cervical cancer prevention
Newer HPV prevention strategies are costlier initially, but may reduce cancers and precancers, ultimately reducing morbidity and treatment costs (Goldhaber-Fiebert et al., 2007). Current guidelines are developed using evidence-based methods incorporating natural history, epidemiology, burden of disease, cost-effectiveness, sustainability, feasibility and acceptability.
Guidelines for cervical cancer screening among fully-vaccinated girls have not yet changed in Canada or in the United States, because coverage is not optimal and few actual changes in HPV prevalence or HPV-based outcomes have been documented (e.g., decreases in high-grade precancers like CIN2/3) (Saslow et al., 2012). With decreased prevalence of HPV 16/18 infection, decreases in precancers may be observed sooner in younger women since HPV16 attributable precancers and cancers develop more rapidly and are more frequent than those due to other HPV types. Furthermore, the positive predictive value of cytology-based testing will be impacted, possibly resulting in HPV-genotype-specific screening strategies (Castle et al., 2008; Saslow et al., 2012; Wheeler et al., 2009).
Increase adherence to screening guidelines
Current evidence-based cervical cancer screening guidelines are targeted towards average-risk women who could benefit the most from screening, (Table 3) by: 1) beginning screening at age 21, minimizing discovery of frequently self-limiting lesions which need colposcopic assessment and which might result in adverse birth outcomes; 2) stopping screening at recommended ages (65 years in US and 65–70 years in Canada) among women with a history of adequate screening or after a total hysterectomy for benign reasons; and, 3) adopting HPV testing starting at a later age, to increase sensitivity while also increasing time between screening, lowering the number of lifetime screenings. More provinces in Canada are considering primary HPV testing. As screening becomes less frequent, the available time in a visit may be used to provide other recommended evidence-based, age-appropriate screening or counseling (Institute of Medicine, 2011; Stormo et al., 2011, 2012).
Table 3.
Current cervical cancer screening guidelines in Canada and United States.
| Canada: province | Organized program | Primary screening test | Initiation (age) | Interval after negative test (years) |
Cessation (age) |
| Canadian Task Force (2013) | Cytology | 25 | 3 | 69 | |
| Alberta | Yes | Cytology | 21 | 3 (after 3 normal) | 69 |
| British Columbia | Partially | Cytology | 21 | 2 (after 3 normal) | 69 |
| Manitoba | Partially | Cytology | Within 3 years of sexual activity | 2 | 69 |
| Ontario | Partially | Cytology | 21 | 3 | 70 |
| HPV test | 30 | 5 | 65 | ||
| Newfoundland2 | Partially | Cytology | 20 | 3 (after 3 normal) | 70 |
| New Brunswick3 | No | Cytology | 21 | 2–3 (after 3 normal) | 69 |
| Northwest Territories | No | Cytology | 21 | 2 (after 3 normal) | 69 |
| Nova Scotia | Partially | Cytology | 21 | 2 (after 3 normal) | 75 |
| Nunavut | No | Cytology | 16 | 2 (after 3 normal) | 70 |
| Prince Edward Island | No | Cytology | 20 | 2 | 69 |
| Quebec4 | No | Cytology | 21 | 2 to 3 | 65 |
| Saskatchewan | Yes | Cytology | 21 | 3 (after 3 normal) | 69 |
| Yukon | No | Cytology | 18 | 2 (after 3 normal) | – |
| United States: organization providing guidelines* | Primary screening test | Initiation (age) | Interval after negative test (years) |
Cessation (age) |
|
| American Cancer Society | Cytology | 21 | 3 | 65 | |
| HPV co-test† (preferred method) | 30 | 5 | |||
| U.S. Preventive Services Task Force | Cytology | 21 | 3 | 65 | |
| HPV co-test (for women who want to extend screening interval) | 30 | 5 | |||
| American College of Obstetricians and Gynecologists | Cytology | 21 | 3 | 65 | |
| HPV co-test | 30 | 5 | |||
Canadian guidelines provided by Lori Elliott, senior policy analyst, Cancer Care Ontario.
US information from http://www.cdc.gov/cancer/cervical/pdf/guidelines.pdf.
Note, the US does not have an organized screening program. Programs for specific populations (e.g. National Breast and Cervical Cancer Early Detection Program, Indian Health Service, Family Planning (Title X) etc.) do exist, but guidelines are generally based on those of the above organizations.
HPV co-test consists of cytology+HPV test administered together.
Some European countries have achieved efficiency and favorable outcomes through organized screening programs. Many countries with organized screening programs, including Canada, have not adopted co-testing, citing this strategy as cost-ineffective (Ronco et al., 2012). Given the lower specificity of the HPV test, overtreatment is a concern, thus recommendations generally use primary HPV testing among women 30 years of age and older, with a triage cytology test before referral to colposcopy. The Netherlands is contemplating adoption of primary HPV testing with cytology triage (Meijer, 2011). On the other hand, in Finland there is considerable debate on whether a more efficient screening program could be achieved by limiting opportunistic screening, limiting cytology-based screening to women 25–35 years of age, and then using HPV testing as a primary screening test for older women (Niemenen, 2012). A community-based randomized clinical trial found that HPV testing may lead to over-diagnosis of self-limiting low-grade disease leading to unnecessary procedures, raising concerns about switching from cytology-based screening to HPV-based screening, especially in an organized infrastructure where invasive cancer is rare and cytology sensitivity is high (Malila et al., 2013).
Ensuring screening coverage of women at highest risk of getting cervical cancer
In the United States and Canada, the population at greatest risk of getting and dying from are women who have irregular or no access to screening. This includes immigrant women, poor women, women belonging to certain racial/ethnic groups (e.g. Hispanic, Asian/Pacific Islander, Indigenous populations), and women from rural or isolated areas (e.g. Appalachia, Border, Deep South) (Freeman and Wingrove, 2005; Horner et al., 2011; Vasilevska et al., 2012).
Both U.S. and Canadian systematic reviews have found that client-based interventions (e.g., client reminders, small media, one-on-one education) improve screening rates for cervical cancer (Community Preventive Services Task Force, 2012; Sabatino et al., 2012; Brouwers et al., 2011a,b). Effective provider-based interventions include prompts to inform health care providers that a client should present for screening (called a “reminder”) or that the client is overdue for screening (called a “recall”). Provider assessment and feedback interventions evaluate provider performance in delivering or offering screening to clients (assessment) and present providers with information about their performance in providing screening services (feedback). Feedback may describe the performance of a group of providers or an individual provider, and may be compared with a goal or standard (Community Preventive Services Task Force, 2012).
Organized screening programs with centralized data collection and follow-up strategies have shown greater reductions of cervical cancer than opportunistic screening, which often excludes women with no connection to a primary care organization (Albrow et al., 2012). The United States has largely relied on the model of more frequent screening to allow for lapses in follow up, feedback, and communication inherent in opportunistic screening (Habbema et al., 2012). As more organized systems are established for routine clinical preventive services, adherence to guideline-based screening can be achieved. In Canada, there is a mix of organized and opportunistic screening; provinces with centralized systems are opting for more efficient screening algorithms.
In spite of provider reminder and recall systems and organized screening programs, certain women will remain at higher risk of not getting screened in a clinical setting, either due to loss of privacy, having a male provider, or other barriers. Self-sampling techniques have been shown to be similar to physician-collected tests and can increase access for women with low access to care (Cerigo et al., 2012; Scarinci et al., 2010; Stewart et al., 2007). However, organized screening systems must be in place to ensure proper follow-up in terms of interval for screening as well as follow up of abnormal results of these women.
Achieving high vaccine coverage
A variety of factors impact vaccine coverage, including delivery systems, financing and acceptability. In the United States most vaccinations are delivered by primary care providers during preventive healthcare visits. As for all vaccinations, a strong provider recommendation has been found to be important for HPV vaccination (Dorell et al., 2011); lack of strong provider recommendations results in many missed opportunities. Use of reminder systems could also help increase coverage (Suh et al., 2012). In Canada, the HPV vaccination program is primarily school-based for the target age group, resulting in higher coverage. School-based programs in Canada (even for adolescents) have a long history, and HPV vaccines have generally been added to other vaccines already given at such ages.
Conclusion
Cervical cancer screening and follow-up alone are estimated to cost $6.6 billion annually in the United States (Chesson et al., 2012). Screening in the US is costly compared to other countries for several reasons: rapid adoption of expensive new technologies, opportunistic screening, private insurance reimbursement not tied to evidence based guidelines, and provider fear of litigation. Compared to the Netherlands which has observed a similar decrease in cervical cancer mortality, a woman in the United States has approximately 3 to 4 times as many Pap tests over her lifetime (Habbema et al., 2012).
With the advent of HPV vaccines, the United States and Canada have both primary as well as secondary prevention strategies for cervical cancer. In Canada, the HPV vaccination program is primarily school-based for the target age group, resulting in generally high coverage. Continued public health efforts should be directed at increasing vaccine coverage in the target age groups and at cervical cancer screening for women at highest risk. Vaccinated women should be aware of the need for continued cervical cancer screening at recommended intervals. As vaccine coverage increases and vaccinated women move into ages targeted for screening, further evaluation of screening programs will be needed to ensure appropriate use of health resources.
Acknowledgments
We would like to acknowledge Dr. Tom Cox for sharing his slides/ graphs with regards to timeline. We would like to thank Dr. Lori Elliott, Dr. Joan Murphy, Dr. Meg McLachlin, Dr. Eduardo L Franco, Dr. François Coutlée, Dr. Gina Ogilvie and Dr. Monika Naus for their contributions on tables and figures.
Financial disclosure
Marc Steben personally or through his affiliations has received research, travel grants, lecture honorarium and advisory honorarium from Abbott Molecular, Beckton-Dickinson, Copan, Digene-Qiagen, Genomica, Gen-Probe, Greiner Bio-One, GSK/GSK biologicals, Graceway pharma/Medicis,Hologic, IncellDx, Innogenetics NV, Laboratoire Biron, Marubeni, Merck/Merck Sharp Dohme, MTM laboratories, NeoDiagnostix, Roche molecular systems and Warnex.
Footnotes
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Source of funding: This work was conducted as part of national public health employees' regular duties.
Conflict of interest
The authors declare that there are no conflicts of interests.
References
- ACOG. ACOG practice bulletin: clinical management guidelines for obstetrician-gynecologists. Number 45, August 2003. Cervical cytology screening. Obstet. Gynecol. 2003;102:417–427. doi: 10.1016/s0029-7844(03)00745-2. [DOI] [PubMed] [Google Scholar]
- Albrow R, Kitchener H, Gupta N, Desai M. Cervical screening in England: the past, present, and future. Cancer Cytopathol. 2012;120:87–96. doi: 10.1002/cncy.20203. [DOI] [PubMed] [Google Scholar]
- American College of Obstetricians and Gynecologists. ACOG Practice Bulletin: Cervical Cytology Screening. 2009;(109):1409–1420. [Google Scholar]
- Brouwers MC, De Vito C, Bahirathan L, et al. Effective interventions to facilitate the uptake of breast, cervical and colorectal cancer screening: an implementation guideline. Implement. Sci. 2011a;6:112. doi: 10.1186/1748-5908-6-112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brouwers MC, De Vito C, Bahirathan L, et al. What implementation interventions increase cancer screening rates? A systematic review. Implement. Sci. 2011b;6:111. doi: 10.1186/1748-5908-6-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canadian Cancer Society's Steering Committee on Cancer Statistics. Canadian cancer statistics 2012. Canada: Ontario; 2012. [Google Scholar]
- Canadian Task Force on Preventive Health Care. Recommendations on screening for cervical cancer. CMAJ. 2013;185:35–45. doi: 10.1503/cmaj.121505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castle PE, Solomon D, Saslow D, Schiffman M. Predicting the effect of successful human papillomavirus vaccination on existing cervical cancer prevention programs in the United States. Cancer. 2008;113:3031–3035. doi: 10.1002/cncr.23762. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. FDA licensure of quadrivalent human papillomavirus vaccine (HPV4, Gardasil) for use in males and guidance from the Advisory Committee on Immunization Practices (ACIP) MMWR Morb. Mortal. Wkly Rep. 2010;59:630–632. [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. Recommendations on the use of quadrivalent human papillomavirus vaccine in males—Advisory Committee on Immunization Practices (ACIP), 2011. MMWR Morb. Mortal. Wkly Rep. 2011a;60:1705–1708. [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. Ten great public health achievements—United States, 2001–2010. MMWR Morb. Mortal. Wkly Rep. 2011b;60:619–623. [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. Cancer screening — United States, 2010. MMWR Morb. Mortal. Wkly Rep. 2012a;61:41–45. [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. National and state vaccination coverage among adolescents aged 13–17 years — United States, 2011. MMWR Morb. Mortal. Wkly Rep. 2012b;61:671–677. [PubMed] [Google Scholar]
- Centers for Disease Control Prevention. FDA licensure of bivalent human papillomavirus vaccine (HPV2, Cervarix) for use in females and updated HPV vaccination recommendations from the Advisory Committee on Immunization Practices (ACIP) MMWR Morb. Mortal. Wkly Rep. 2010;59:626–629. [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. Vaccine For Children. http://www.cdc.gov/vaccines/programs/vfc/about/index.html.
- Cerigo H, Coutlee F, Franco EL, Brassard P. Dry self-sampling versus provider-sampling of cervicovaginal specimens for human papillomavirus detection in the Inuit population of Nunavik, Quebec. J. Med. Screen. 2012;19:42–48. doi: 10.1258/jms.2012.012011. [DOI] [PubMed] [Google Scholar]
- Chesson HW, Ekwueme DU, Saraiya M, Dunne EF, Markowitz LE. The cost-effectiveness of male HPV vaccination in the United States. Vaccine. 2011;29:8443–8450. doi: 10.1016/j.vaccine.2011.07.096. [DOI] [PubMed] [Google Scholar]
- Chesson HW, Ekwueme DU, Saraiya M, Watson M, Lowy DR, Markowitz LE. Estimates of the annual direct medical costs of the prevention and treatment of disease associated with human papillomavirus in the United States. Vaccine. 2012;30:6016–6019. doi: 10.1016/j.vaccine.2012.07.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Community Preventive Services Task Force. Updated recommendations for client- and provider-oriented interventions to increase breast, cervical, and colorectal cancer screening. Am. J. Prev. Med. 2012;43:92–96. doi: 10.1016/j.amepre.2012.04.008. [DOI] [PubMed] [Google Scholar]
- Dorell CG, Yankey D, Santibanez TA, Markowitz LE. Human papillomavirus vaccination series initiation and completion, 2008–2009. Pediatrics. 2011;128:830–839. doi: 10.1542/peds.2011-0950. [DOI] [PubMed] [Google Scholar]
- Freeman HP, Wingrove BK. Excess Cervical Cancer Mortality: a Marker for Low Access to Health Care in Poor Communities. Rockville, MD: National Cancer Institue, Center to Reduce Cancer Health Disparities; 2005. [Google Scholar]
- Goldhaber-Fiebert JD, Stout NK, Ortendahl J, Kuntz KM, Goldie SJ, Salomon JA. Modeling human papillomavirus and cervical cancer in the United States for analyses of screening and vaccination. Popul. Health Metrics. 2007;5:11. doi: 10.1186/1478-7954-5-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GSK, a. G.S.K. ttp://www.gsk.ca/english/docs-pdf/product-monographs/Cervarix.pdf (p. GSK Product Monograph for Cervarix). [Google Scholar]
- Habbema D, De Kok IM, Brown ML. Cervical cancer screening in the United States and the Netherlands: a tale of two countries. Milbank Q. 2012;90:5–37. doi: 10.1111/j.1468-0009.2011.00652.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogarth S, Hopkins MM, Rodriguez V. A molecular monopoly? HPV testing, the Pap smear and the molecularisation of cervical cancer screening in the USA. Sociol. Health Illn. 2012;34:234–250. doi: 10.1111/j.1467-9566.2011.01411.x. [DOI] [PubMed] [Google Scholar]
- Horner MJ, Altekruse SF, Zou Z, Wideroff L, Katki HA, Stinchcomb DG. U.S. geographic distribution of prevaccine era cervical cancer screening, incidence, stage, and mortality. Cancer Epidemiol. Biomarkers Prev. 2011;20:591–599. doi: 10.1158/1055-9965.EPI-10-1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HPV Consensus Guidelines Committee. Summary recommendations: Canadian consensus guidelines on human papillomavirus. J. Obstet. Gynaecol. Can. 2007;29:S5. [Google Scholar]
- Institute of Medicine. Clinical Preventive Services for Women: Closing the Gaps. Washington, DC: 2011. [Google Scholar]
- Johnson K Canadian Task Force on the Periodic Health Examination. Periodic Health Examination, 1995 update: 1. Screening for human papillomavirus infection in asymptomatic women. Can. Med. Assoc. J. 1995;152:483–493. [PMC free article] [PubMed] [Google Scholar]
- Lee JW, Berkowitz Z, Saraiya M. Low-risk human papillomavirus testing and other nonrecommended human papillomavirus testing practices among U.S. health care providers. Obstet. Gynecol. 2011;118:4–13. doi: 10.1097/AOG.0b013e3182210034. [DOI] [PubMed] [Google Scholar]
- Malila N, Leinonen M, Kotaniemi-Talonen L, Laurila P, Tarkkanen J, Hakama M. The HPV Test has Similar Sensitivity but more Overdiagnosis than the Pap test — a Randomised Health Services Study on Cervical Cancer Screening in Finland. Int J. Cancer. 2013 May 1;132(9):2141–2147. doi: 10.1002/ijc.27850. [DOI] [PubMed] [Google Scholar]
- Markowitz LE, Dunne EF, Saraiya M, et al. Quadrivalent human papillomavirus vaccine: recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR Recomm. Rep. 2007;56:1–24. [PubMed] [Google Scholar]
- Meijer C. BYPASS EDICIONES. Brussels, Belgium: 2011. Changing the Primary Screening Tool of the Netherlands Program: Why and How? HPV Today; p. 2. [Google Scholar]
- Merck Canada Inc. Gardasil Product Monograph. 2012 [Google Scholar]
- Moyer VA U.S. Preventive Services Task Force. Screening for cervical cancer: U.S. preventive services task force recommendation statement. Ann. Intern. Med. 2012 Jun 19;156(12):880–891. doi: 10.7326/0003-4819-156-12-201206190-00424. [DOI] [PubMed] [Google Scholar]
- Murphy J, Kennedy EB, Dunn S, et al. Cervical screening: a guideline for clinical practice in Ontario. J. Obstet. Gynaecol. Can. 2012a;34:453–458. doi: 10.1016/S1701-2163(16)35242-2. [DOI] [PubMed] [Google Scholar]
- Murphy J, Kennedy EB, Dunn S, et al. HPV testing in primary cervical screening: a systematic review and meta-analysis. J. Obstet. Gynaecol. Can. 2012b;34:443–452. doi: 10.1016/S1701-2163(16)35241-0. [DOI] [PubMed] [Google Scholar]
- National Advisory Committee on Immunization (NACI) Statement on human papillomavirus vaccine. An Advisory Committee Statement (ACS) Accessed 23rd of September 2012 at Can. Commun. Dis. Rep. 2007;33:1–31. [PubMed] [Google Scholar]
- National Advisory Committee on Immunization (NACI) Update on human papillomavirus (HPV) vaccines. An Advisory Committee Statement (ACS) Can. Commun. Dis. Rep. 2012;38:1–31. doi: 10.14745/ccdr.v38i00a01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naus M, Ogilvie G. Human papillomavirus vaccine program in BC: a good start with room for improvement. BCMJ. 2010;52:95. [Google Scholar]
- Niemenen P. BYPASS EDICIONES. Belgium: 2012. Optimizing Cervical Cancer Screening in Finland, HPV Today, Sept 2012 ed; p. 2. [Google Scholar]
- Ostor AG. Natural history of cervical intraepithelial neoplasia: a critical review. Int. J. Gynecol. Pathol. 1993;12:186–192. [PubMed] [Google Scholar]
- Palefsky JM, Giuliano AR, Goldstone S, et al. HPV vaccine against anal HPV infection and anal intraepithelial neoplasia. N. Engl. J. Med. 2011 Oct 27;365(17):1576–1585. doi: 10.1056/NEJMoa1010971. [DOI] [PubMed] [Google Scholar]
- Phelan DF, Boitnott JK, Clark DP, et al. Trends of human papillomavirus testing in cervical cancer screening at a large academic cytology laboratory. Obstet. Gynecol. 2011 Aug;118(2 Pt 1):289–295. doi: 10.1097/AOG.0b013e3182253c33. http://dx.doi.org/10.1097/AOG.0b013e3182253c33 (PubMed PMID:21775844). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Provencher DMMK. The role of HPV testing. Canadian consensus guidelines on human papillomavirus. J. Obstet. Gynaecol. Can. 2007;29:s15–s21. doi: 10.1016/S1701-2163(16)32807-9. [DOI] [PubMed] [Google Scholar]
- Public Health Agency of Canada. Cervical Cancer Facts and Figures. 2012 [Google Scholar]
- Rodriguez AC, Schiffman M, Herrero R, et al. Rapid clearance of human papillomavirus and implications for clinical focus on persistent infections. J. Natl. Cancer Inst. 2008;100:513–517. doi: 10.1093/jnci/djn044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roland KB, Soman A, Benard VB, Saraiya M. Human papillomavirus and Papanicolaou tests screening interval recommendations in the United States. Am. J. Obstet. Gynecol. 2011;205(447):e441–e448. doi: 10.1016/j.ajog.2011.06.001. [DOI] [PubMed] [Google Scholar]
- Ronco G, Biggeri A, Confortini M, et al. Health technology assessment report: HPV DNA based primary screening for cervical cancer precursors. Epidemiol. Prev. 2012;36:e1–e72. [PubMed] [Google Scholar]
- Sabatino SA, Lawrence B, Elder R, et al. Effectiveness of interventions to increase screening for breast, cervical, and colorectal cancers: nine updated systematic reviews for the guide to community preventive services Updated recommendations for client- and provider-oriented interventions to increase breast, cervical, and colorectal cancer screening. Am. J. Prev. Med. 2012;43:97–118. doi: 10.1016/j.amepre.2012.04.009. [DOI] [PubMed] [Google Scholar]
- Saraiya M, Berkowitz Z, Yabroff KR, Wideroff L, Kobrin S, Benard V. Human papillomavirus co-testing: what screening intervals are physicians recommending. Arch. Intern. Med. 2010 Jun 14;170(11):977–985. doi: 10.1001/archinternmed.2010.134. [DOI] [PubMed] [Google Scholar]
- Saslow D, Solomon D, Lawson HW, et al. American Cancer Society, American Society for Colposcopy and Cervical Pathology, and American Society for Clinical Pathology Screening Guidelines for the Prevention and Early Detection of Cervical Cancer. J. Low. Genit. Tract Dis. 2012;16:175–204. doi: 10.1097/LGT.0b013e31824ca9d5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scarinci IC, Garcia FA, Kobetz E, et al. Cervical cancer prevention: new tools and old barriers. Cancer. 2010;116:2531–2542. doi: 10.1002/cncr.25065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiffman M, Wentzensen N, Wacholder S, Kinney W, Gage JC, Castle PE. Human papillomavirus testing in the prevention of cervical cancer. J. Natl. Cancer Inst. 2011;103:368–383. doi: 10.1093/jnci/djq562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- C.C.P. Screening Performance Indicators Working Group, N. Control. Executive summary—performance monitoring for cervical cancer screening programs in Canada. Chronic Dis. Can. 2010;31:45. [PubMed] [Google Scholar]
- Smith JC, Snider DE, Pickering LK Advisory Committee on Immunization, P. Immunization policy development in the United States: the role of the Advisory Committee on Immunization Practices. Ann. Intern. Med. 2009;150:45–49. doi: 10.7326/0003-4819-150-1-200901060-00009. [DOI] [PubMed] [Google Scholar]
- Solomon D, Schiffman M, Tarone R. Comparison of three management strategies for patients with atypical squamous cells of undetermined significance: baseline results from a randomized trial. J. Natl. Cancer Inst. 2001;93:293–299. doi: 10.1093/jnci/93.4.293. [DOI] [PubMed] [Google Scholar]
- Spence AR, Goggin P, Franco EL. Process of care failures in invasive cervical cancer: systematic review and meta-analysis. Prev. Med. 2007;45:93–106. doi: 10.1016/j.ypmed.2007.06.007. [DOI] [PubMed] [Google Scholar]
- Stewart DE, Gagliardi A, Johnston M, et al. Self-collected samples for testing of oncogenic human papillomavirus: a systematic review. J. Obstet. Gynaecol. Can. 2007;29:817–828. doi: 10.1016/s1701-2163(16)32636-6. [DOI] [PubMed] [Google Scholar]
- Stormo AR, Hawkins NA, Cooper CP, Saraiya M. The pelvic examination as a screening tool: practices of US physicians. Arch. Intern. Med. 2011;171:2053–2054. doi: 10.1001/archinternmed.2011.575. [DOI] [PubMed] [Google Scholar]
- Stormo AR, Cooper CP, Hawkins NA, Saraiya M. Physician characteristics and beliefs associated with use of pelvic examinations in asymptomatic women. Prev. Med. 2012;54:415–421. doi: 10.1016/j.ypmed.2012.03.012. [DOI] [PubMed] [Google Scholar]
- Stuart G, Taylor G, Bancej C, et al. Report of the 2003 Pan-Canadian forum on cervical cancer prevention and control. Obstet. Gynaecol. Can. 2004;26:1004–1014. doi: 10.1016/s1701-2163(16)30423-6. [DOI] [PubMed] [Google Scholar]
- Suh CA, Saville A, Daley MF, et al. Effectiveness and net cost of reminder/recall for adolescent immunizations. Pediatrics. 2012;129:e1437–e1445. doi: 10.1542/peds.2011-1714. [DOI] [PubMed] [Google Scholar]
- Tatsas AD, Phelan DF, Gravitt PE, Boitnott JK, Clark DP. Practice patterns in cervical cancer screening and human papillomavirus testing. Am. J. Clin. Pathol. 2012;138:223–229. doi: 10.1309/AJCPPVX91HQMNYZZ. [DOI] [PubMed] [Google Scholar]
- U.S. Cancer Statistics Working Group. United States Cancer Statistics 1999–2008 Incidence and Mortality Web-based Report. Atlanta: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention and National Cancer Institute; 2010. [Google Scholar]
- Vasilevska M, Ross SA, Gesink D, Fisman DN. Relative risk of cervical cancer in indigenous women in Australia, Canada, New Zealand, and the United States: a systematic review and meta-analysis. J. Public Health Policy. 2012;33:148–164. doi: 10.1057/jphp.2012.8. [DOI] [PubMed] [Google Scholar]
- Weinstock H, Berman S, Cates W., Jr Sexually transmitted diseases among American youth: incidence and prevalence estimates, 2000. Perspect. Sex. Reprod. Health. 2004;36:6–10. doi: 10.1363/psrh.36.6.04. [DOI] [PubMed] [Google Scholar]
- Wheeler CM, Hunt WC, Joste NE, Key CR, Quint WG, Castle PE. Human papillomavirus genotype distributions: implications for vaccination and cancer screening in the United States. J. Natl. Cancer Inst. 2009;101:475–487. doi: 10.1093/jnci/djn510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright TC, Jr, Cox JT, Massad LS, Twiggs LB, Wilkinson EJ. 2001 Consensus guidelines for the management of women with cervical cytological abnormalities. JAMA. 2002;287:2120–2129. doi: 10.1001/jama.287.16.2120. [DOI] [PubMed] [Google Scholar]
- zur Hausen H, Gissmann L, Steiner W, Dippold W, Dreger I. Human papilloma viruses and cancer. Bibl. Haematol. 1975:569–571. doi: 10.1159/000399220. [DOI] [PubMed] [Google Scholar]