Abstract
We explored the potential impact of HPV testing on women’s intentions to be screened for cervical cancer in a cohort of Canadian women. Participants aged 25-65 from an ongoing trial were sent a questionnaire to assess women’s intentions to be screened for cervical cancer with HPV testing instead of Pap smears and to be screened every 4 years or after 25 years of age. We created scales for attitudes about HPV testing, perceived behavioural control and direct and indirect subjective norms. Demographic data and scales that were significantly different (p<0.1) between women who intended to be screened with HPV and those who did not intend were included in a stepwise logistic regression model. Of the 2016 invitations emailed, 1538 were received, and 981 completed surveys for a response rate of 63% (981/1538). Eighty-four percent of women (826/981) responded that they intended to attend for HPV-based cervical cancer screening, which decreased to 54.2% when the screening interval was extended, and decreased further to 51.4% when screening start was delayed to age 25. Predictors of intentions to undergo screening were attitudes (OR 1.22; 95%CI 1.15, 1.30), indirect subjective norms (OR 1.02; 95%CI 1.01, 1.03) and perceived behavioural control (OR 1.16; 95% CI 1.10; 1.22). Intentions to be screened for cervical cancer with HPV testing decreased substantially when the screening interval was extended and screening started at age 25. Use of primary HPV testing may optimize the screening paradigm, but programs should ensure robust planning and education to mitigate any negative impact on screening attendance rates.
Keywords: HPV, cervical cancer screening, intention, Theory of Planned Behavior
Introduction
Cervical cancer screening using cytology (the Pap smear) has been an extremely successful public health intervention, achieving reductions in incidence of up to 80% where practiced effectively 1. However, the Pap smear was introduced over 50 years ago, and despite its substantial contributions to cervical cancer reduction, it has limitations as a screening tool. Meta-analyses have found that an individual cytology has sensitivity less than 60%2. There is now ample evidence that infection with high-risk types of the human papillomavirus (hr-HPV) is a requisite step for development of cervical cancer and its precursors 3,4. As a primary screening tool, cross-sectional studies have shown that hr-HPV testing has higher sensitivity and negative predictive value (NPV) for detecting cervical intraepithelial neoplasia 2 or worse (CIN2+) than either the conventional or liquid based cytology (LBC), but with lower specificity and positive predictive value (PPV)5-10. Therefore, one approach for screening would be to use hr-HPV testing as a primary screening test, with cytology reserved only for triage of women with positive test results. Several large, international randomized controlled trials (RCT) are being conducted in Europe and Canada to evaluate the efficacy and effectiveness of HPV testing as the primary screening tool for cervical cancer 7,11-20. Data from these trials show that use of hr-HPV as a primary screen improves detection of CIN2+, and also prevents more cancers than does cytology 21-23.
Although the United States has used co-testing with HPV and cytology for screening for several years24, cervical cancer screening programs across jurisdictions in Canada and Europe are now poised to make a substantial paradigm shift, using hr-HPV testing as the primary screen for cervical cancer 25-27. However, use of HPV testing as a primary screen could lead to changes in the age when cervical cancer screening starts, an extension of the interval between screening and has different implications with a positive screening result. Because a negative hr-HPV test offers greater assurance to clinicians and screening participants that they are not at risk for developing cervical cancer in the near future 8 it has been proposed that screening intervals for hr-HPV negative women could be extended safely to five years instead of every 2 years28. Given the high HPV prevalence in young women, HPV testing in women under 25 will identify many lesions that will ultimately regress; thus, cervical cancer screening using hr-HPV testing likely will be delayed until after age 25 and possibly even later, to age 30, which is more aligned with current European practices. HPV testing represents a shift from an oncological to a communicable disease paradigm. While cytology identifies cellular changes associated with precancerous cervical lesions, HPV testing identifies the infection that precipitates these cellular changes. Use of hr-HPV testing in screening may require practitioners to inform some women, many of whom have been in monogamous relationships, that they are infected with a highly prevalent sexually acquired virus. Even though the virus may have been acquired many years prior and HPV is qualitatively different from other sexually acquired infections due to its high prevalence and long latency, relaying of positive results will create significant challenges both for practitioners and for patients. Practitioners will need both to ensure that they set the appropriate context for HPV infections, as well as manage emotional responses to positive results 29.
Program changes that would occur with the use of HPV testing could influence the acceptability and uptake of cervical cancer screening and thus, ultimately, the success of screening programs. Prior to moving to primary HPV-based cervical cancer screening, clinicians, researchers and policy makers should examine potential impacts of a change from cytology to hr-HPV testing on women’s intentions to participate in cervical cancer screening. In this study, using the Theory of Planned Behavior 30, we examined Canadian women’s intentions to attend cervical cancer screening in the era of primary HPV testing.
Materials and Methods
The primary objective of the study was to determine women’s intentions to be screened with HPV testing instead of Pap smears (cytology) for cervical cancer and women’s intentions to be screened with HPV at a four year screening interval, with screening starting after 25 years of age, and to identify variables that predict intentions to undergo HPV testing instead of having Pap smears for cervical cancer screening.
Participants and recruitment
Study participants were recruited through the HPV FOCAL trial in British Columbia, Canada. HPV-FOCAL is a randomized, controlled, three-armed study conducted in British Columbia 19,20 that completed recruitment of over 25,000 women aged 25-65 through the province’s population-based cervical cancer screening program at the British Columbia Cancer Agency in March 2012. At study exit, all women with email addresses were sent invitations to complete an online web-based survey tool. Women were sent two additional reminders to complete study questionnaires. Data entered by participants were stored at fluid surveys (fluidsurveys.com) and then were automatically populated into an Excel spreadsheet for analysis.
Survey instrument
The survey was based in the Theory of Planned Behavior (TPB) 31, which has been used extensively to assess screening health behaviours 30. TPB is particularly valuable to describe behaviours that are under an individual’s volitional control and identifies intention as the most proximate predictor of behaviour. Survey items were developed from literature review and feedback from content experts. Surveys were drafted and reviewed by a TPB expert and then pilot tested on ten women in the target demographic. A final revised version of the survey was then re-piloted and implemented.
In the survey, women were provided with an introduction to the human papillomavirus, including information on transmission, the role of HPV in cervical cancer and the rationale for use of primary HPV testing and screening intervals and timing. Demographics and items assessing the three specific elements that predict behaviour intentions (attitude towards the behaviour, perceived behavioural control, and subjective norms to the behaviour) were assessed with seven point Likert scales. Attitudes toward the behaviour are an individual’s perspective on the value and utility of behaviour. Subjective norms to behaviour are an individual’s belief about how people they care about will view the behavior in question. Perceived behavioural control describes an individual’s perception of their ability to control the behaviour.
Survey response rate
Survey responses were reviewed for completeness. In the case of duplicate complete surveys, the first complete survey was used, and the second survey was discarded. Response rate was calculated according to the American Association for Public Opinion Research, which is the number of complete surveys divided by the number of returned questionnaires plus eligible non-interview 32. Response rate for women who received the survey was the number of complete surveys divided by number of complete surveys plus refusal, logged on and break off.
Analysis
The primary endpoint was response to the statement ‘I would be willing to have an HPV test to screen for cervical cancer instead of a Pap smear’. Women’s intention to be screened with HPV for cervical cancer every four years was determined based on response to ‘I would be willing to have an HPV test to screen for cervical cancer every four years instead of a Pap smear every year’. Women’s intention to be screened with HPV every four years and after the age of 25 was determined by response to ‘I would be willing to have an HPV test to screen for cervical cancer every four years and after the age of 25 instead of a Pap smear every year after becoming sexually active.’ Women responded to a seven point Likert scale (strongly disagree to strongly agree). Responses for each were dichotomized, with women who responded >4 coded as ‘intending to screen’ and women who responded <= 4 as ‘not intending to screen’.
Demographic characteristics of survey non-respondents were compared using data from the larger clinical trial. Continuous variables were compared with Student’s t-tests, categorical variables were compared with Chi-square and Kruskal-Wallis tests to compare medians, as appropriate. Descriptive analyses of demographic characteristics of the survey respondents were performed, including mean and median age, marital status, education, sexual history, ethnicity and smoking history. Women’s intentions to be screened with HPV for cervical cancer as well as overall rates of intentions to be screened every four years and intentions to be screened every four years after the age of 25 also were calculated with 95% confidence intervals.
Scale items were analyzed according to methods for Theory of Planned Behavior. Briefly, items were re-anchored and re-coded as needed. If items in scales achieved agreement, as measured by Cronbach’s alpha >0.5, a composite variable was created and then included in the univariate, and where appropriate, multivariate analyses. If scales did not achieve a Cronbach’s alpha of >0.5, subscales which did achieve agreement were created and included in analyses 33. Beyond assessing the traditional TPB constructs of attitudes, direct and indirect subjective norms, and perceived behavioural control, an additional item related to the sexual acquisition of HPV was included, as investigators believed this would also be an influential factor in women’s intentions to be screened with HPV for cervical cancer. Previous studies have identified women’s concerns related to stigma around sexually acquired infections, implications of infidelity and the impact of positive HPV results on relationships with partners 34. Two items were designed to assess the impact of informing a partner about HPV status on decision to receive screening. These items were used to determine whether the communicable disease/STI element of HPV would affect women’s intentions to have this test.
Demographic characteristics between women who intend to be screened with HPV for cervical cancer and those who were not were compared overall and between five year age strata, with Chi-square and Student’s t-test as appropriate. Multicollinearity of psychological scales that achieved an item correlation with Cronbach’s α >0.5 was assessed with Pearson correlation coefficient. If two scales were collinear (>0.8), based on investigator judgment, the less influential variable was removed. Following this, overall scale scores and mean scores with standard deviations for scale results between those who intended to be screened and those who did not intend to be screened were calculated. Mean results with standard deviations between scales that had acceptable agreement (Cronbach’s alpha >0.5) and not collinear were compared using Student’s t-tests. All demographic (mean age, marital status, cultural background, educational background, number of male sexual partners, smoking history) and scale variables that achieved p<0.1 in the univariate and bivariate analyses, and variables that were identified by the investigators to be important in predicting women’s decisions to attend screening based on the comprehensive literature review that preceded questionnaire development were entered into the stepwise logistic regression model. We created a logistic regression model to predict factors associated with women’s intentions to be screened for cervical cancer with HPV testing. The dependent variable for the model was ‘intention to be screened for cervical cancer with HPV’ (0=did not intend; 1=intended). We conducted a direct logistic regression analysis and calculated odds ratios for significant variables with 95% confidence intervals to identify variables associated with a women’s intention to be screened for cervical cancer with HPV. Analyses were performed using SAS 9.3.
Results
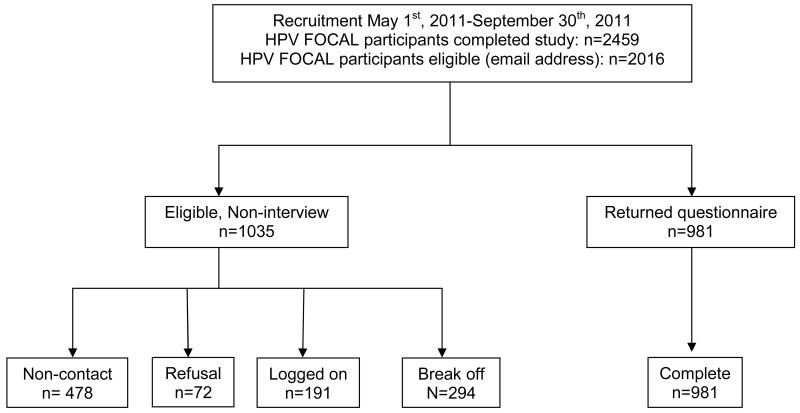
The survey was conducted between May and September, 2011. Among 2459 women who had completed the HPV FOCAL trial, 2016 had email addresses and were sent invitations to participate (Figure 1). This group is the eligible population. Of the 2016 eligible women, 1035 were not surveyed: 478 emails were returned or bounced back (non-contact), 72 replied that they did not want to participate (refusals), 191 individuals logged onto the survey, but did not start it, and 294 started surveys but did not complete them. 981 returned and completed surveys, for an overall response rate of 48.7% (981/2016) for all women and 63% (981/1538) for women who electronically received the survey.
Figure 1.
Study flowchart and participant disposition
Survey responders and non-responders did not differ significantly in their socio-demographic characteristics (Table 1). Survey respondents had a mean age of 45.1 (SD 10.1); the age range of respondents was 25 to 65 years of age. Over 85% of women had more than high school education, and 56.1% reported five or fewer sexual partners in their lives. The majority of women were Caucasian, black or South Asian background; 2.4% of women were aboriginal. Six percent of women were current smokers, and 36.1% had smoked at some time in their lives. 84.2% (95% CI 81.9; 86.5) intended to be screened for cervical cancer with HPV. However, women’s intentions to be screened with HPV instead of Pap smears for cervical cancer screening decreased from 84.2% to 54.2% (95%CI 51.1; 57.3) when screening occurred every four years, and decreased further to 51.4% (95%CI 48.3; 54.5) when screening started at age 25.
Table 1.
Comparison of demographic characteristics of survey respondents and survey non-respondents*
| Characteristic | Group | Study Invitees N (%) |
Respondent N (%) |
Non- Respondent N (%) |
P-Value |
|---|---|---|---|---|---|
|
| |||||
| Overall | 2016 | 981 | 1035 | ||
| Age, Recruitment |
Mean (SD) | 45.1(10.1) | 45.0(10.0) | 45.3(10.2) | 0.5248 |
| Median (IQR) † | 45.0 (38.0, 53.0) |
45.0 (38.0,53.) |
46.0 (37.0, 53.0) |
||
| Education | Missing | 130 | 130 | 0.2330 | |
| <High School | 31(1.6%) | 11(1.1%) | 20(2.2%) | ||
| High School (Complete) |
248(13.1%) | 122(12.4%) | 126(13.9%) | ||
| Trade/College/ University (Incomplete) |
692(36.7%) | 356(36.3%) | 336(37.1%) | ||
| University graduate |
584(31.0%) | 311(31.7%) | 273(30.2%) | ||
| University Advanced Degree |
331(17.6%) | 181(18.5%) | 150(16.6%) | ||
| Sexual Partners - Ever |
Missing | 151 | 151 | 0.8514 | |
| 0 | 4(0.2%) | 1(0.1%) | 3(0.3%) | ||
| 1 | 362(19.4%) | 185(18.9%) | 177(20.0%) | ||
| 2 to 5 | 693(37.2%) | 364(37.1%) | 329(37.2%) | ||
| 6 to 10 | 408(21.9%) | 221(22.5%) | 187(21.2%) | ||
| 11 to 50 | 376(20.2%) | 198(20.2%) | 178(20.1%) | ||
| >50 | 22(1.2%) | 12(1.2%) | 10(1.1%) | ||
| Cultural background |
Missing | 128 | 128 | 0.2879 | |
| Chinese | 175(9.3%) | 81(8.3%) | 94(10.4%) | ||
| Aboriginal | 46(2.4%) | 24(2.4%) | 22(2.4%) | ||
| Caucasian and other |
1667(88.3%) | 876(89.3%) | 791(87.2%) | ||
| Smoke, Now | Missing | 188 | 188 | 0.1908 | |
| No | 1707(93.4%) | 923(94.1%) | 784(92.6%) | ||
| Yes | 121(6.6%) | 58(5.9%) | 63(7.4%) | ||
| Smoke, Ever | Missing | 184 | 184 | 0.4382 | |
| No | 1156(63.1%) | 627(63.9%) | 529(62.2%) | ||
| Yes | 676(36.9%) | 354(36.1%) | 322(37.8%) | ||
Pearson’s Chi Square; Student’s t-test;
Kruskal-Wallis
Scale consistency was assessed for each construct; most achieved a Cronbach’s alpha of >0.6 (Table 2). As there was less robust agreement between the items of the direct subjective norms, a scale with two items was included in the analysis, as it had Cronbach’s alpha of >0.5.
Table 2.
Characteristics of scale items: Correlation by Cronbach’s alpha
| Screening Concepts |
Scale items | Scales | Cronbach’s alpha |
|---|---|---|---|
|
Attitudes to HPV testing |
A1: Having an HPV test to screen for cervical cancer instead of a Pap smear would be:
|
A1 | 0.917 |
|
Attitudes to HPV testing every four years |
A20. Having an HPV test to screen for cervical cancer every four years instead of a Pap smear every year would be:
|
A20 | 0.964 |
|
Attitudes to HPV testing every four years and after age of 25 |
A22. Having an HPV test to screen for cervical cancer every four years and after age of 25 instead of a Pap smear every year would be:
|
A22 | 0.968 |
|
Subjective
Norms: Direct |
SND 2: Most people who are important to me would think that I should have an HPV test to screen for cervical cancer instead of a Pap smear SND3: People who are important to me would expect me to have an HPV test to screen for cervical cancer instead of a Pap smear SND4. I would feel under social pressure to have an HPV test to screen for cervical cancer instead of a Pap smear SND4: I would feel under social pressure to have an HPV test to screen for cervical cancer instead of a Pap smear |
SND2-SND4 SND2-3 SND2&SND4 SND3-SND4 |
0.345 0.480 −0.045 0.103 |
|
Subjective
Norms: Indirect |
SNI5. My family physician would think that I should have an HPV test to screen for cervical cancer instead of a Pap smear SNI6. What my family physician thinks is important to me SNI7. My friends would think that I should have an HPV test to screen for cervical cancer instead of a Pap smear SNI8. What my friends think is important to me SNI9. My spouse/partner would think that I should have an HPV test to screen for cervical cancer instead of a Pap smear SNI10. What my spouse/partner thinks is important to me SNI11. The BC Cancer Agency would recommend that I should have an HPV test to screen for cervical cancer instead of a Pap smear SNI12. What the BC Cancer Agency recommends is important to me |
SNI5-SNI12 | 0.823 |
|
Contacting
Partners |
CP13. If I had a cervical cancer screening result that showed I had an HPV infection, I would feel comfortable sharing the results with my partner(s) CP14. My spouse would be understanding if I had an HPV infection |
CP13-CP14 | 0.633 |
|
Perceived
Behavioural Control |
PBC15. I am confident that I could have an HPV test to screen for cervical cancer instead of a Pap smear PBC16. For me to have an HPV test to screen for cervical cancer instead of a Pap smear would be PBC17. Whether or not I would have an HPV test to screen for cervical cancer instead of a Pap smear would be entirely up to me PBC18. How much control would you have over whether you had an HPV test to screen for cervical cancer instead of a Pap smear? |
PBC15- PBC18 |
0.626 |
Of 981 women who completed surveys, 826 reported that they intended to be screened for cervical cancer with HPV tests instead of Pap smears (84.2%) (Table 3). There were no significant differences between the mean age, age strata, marital status, education level, sexual history, cultural background or smoking history of women who intended to be screened with HPV tests instead of Pap smears for cervical cancer (p>0.05) compared to those who did not intend to be screened with HPV. No scale items had p>0.8 on correlation testing, indicating that the variables are measuring non-collinear constructs.
Table 3.
Univariate and bivariate comparisons between demographic characteristics of women who intend and do not intent to receive cervical cancer screening with HPV
| Variable | Group | Intend to screen with HPV; PI19 >4 N (%) |
Do not intend to screen with HPV; PI19<=4 N (%) |
P-Value |
|---|---|---|---|---|
|
| ||||
| Intention to be screened with HPV |
826(84.2%) | 155(15.8%) | 0 | |
| Age | Mean (Standard deviation) | 44.9 (10.1) | 45.1 (9.2) | 0.8874 |
| Age | 25-29 | 67 (83.8) | 13 (16.3) | 0.542 |
| 30-34 | 65 (86.7) | 10 (86.7) | ||
| 35-39 | 124 (86.1) | 20 (13.9) | ||
| 40-44 | 151 (82.5) | 32 (17.5) | ||
| 45-49 | 142 (84.0) | 27 (16.0) | ||
| 50-54 | 111 (81.6) | 25 (18.4) | ||
| 55-59 | 94 (81.0) | 22 (19.0) | ||
| 60-64 | 64 (91.4) | 6 (8.6) | ||
| 65+ | 8 (100) | 0 (0) | ||
| Marital Status | Divorced | 88(10.7%) | 20(12.9%) | 0.7427 |
| Common Law/Married | 581(70.3%) | 108(69.7%) | ||
| Never Married | 95(11.5%) | 17(11.0%) | ||
| Widowed | 7(0.8%) | |||
| Did not Answer/Missing | 55(6.7%) | 10(6.5%) | ||
| Education | <High School | 9(1.1%) | 2(1.3%) | 0.6839 |
| High School (Complete) | 105(12.7%) | 17(11.0%) | ||
| Trade/College/University(Incomplete) | 292(35.4%) | 64(41.3%) | ||
| University graduate | 264(32.0%) | 47(30.3%) | ||
| University Advanced Degree | 156(18.9%) | 25(16.1%) | ||
| Education: Combined |
High School or Less | 114(13.8%) | 19(12.3%) | 0.6065 |
| More than High school | 712(86.2%) | 136(87.7%) | ||
| Sexual Partners - Ever |
0 | 1(0.1%) | 0.6869 | |
| 1 | 155(18.8%) | 30(19.4%) | ||
| 2 to 5 | 315(38.1%) | 49(31.6%) | ||
| 6 to 10 | 180(21.8%) | 41(26.5%) | ||
| 11 to 50 | 165(20.0%) | 33(21.3%) | ||
| >50 | 10(1.2%) | 2(1.3%) | ||
| Cultural background | Chinese | 71(8.6%) | 10(6.5%) | 0.3236 |
| Aboriginal | 18(2.2%) | 6(3.9%) | ||
| Caucasian and other | 737(89.2%) | 139(89.7%) | ||
| Smoke, Ever | No | 527(63.8%) | 100(64.5%) | 0.865 |
| Yes | 299(36.2%) | 55(35.5%) | ||
Women who intended to be screened with HPV tests had significantly higher attitudinal scores, indicating their belief that HPV testing was more accurate, safe, protective and acceptable than Pap smears (p<0.01) (Table 4). They were also significantly more likely to report the influence of direct subjective norms on their decisions, with the belief that most who are important to them would think they should have an HPV test, and would expect them to have an HPV test (P<0.01). Women who intended to be screened with HPV were significantly more likely to report the influence of indirect subjective norms as well, including the opinions of family physicians, friends, spouse or partner and the British Columbia Cancer Agency as important in their decision making to screen (p<0.01). Women who were more likely to intend to be screened with HPV testing also reported significantly higher rates of perceived behavioural control (p<0.01). Women who intended to be screened reported greater comfort sharing results with their partners and were more likely to say that partners would be understanding of their HPV results (p=0.05).
Table 4.
Comparison of scale results between women intending to undergo HPV testing instead of Pap smear for cervical cancer screening
| Psychological scales | Mean score Overall (SD) |
Intend to screen (PI19 >4) Mean (SD) |
Do not intend to screen (PI19<=4) Mean (SD) |
P Value* |
|---|---|---|---|---|
|
| ||||
| Attitudes to HPV testing (A1) | 25.7 (3.7) | 26.5 (2.4) | 21.2 (5.7) | <.0001 |
| Subjective norms, Direct (SND2-3) | 11.0 (2.6) | 11.4 (2.3) | 8.8 (2.6) | <.0001 |
| Subjective norms, Indirect (SNI5-12) | 34.8 (31.9) | 40.7 (28.9) | 3.3 (28.8) | <.0001 |
| Perceived Behavioural Control (PBC 15-18) |
23.4 (4.1) | 24.1 (3.7) | 19.6 (4.1) | <.0001 |
| Contacting Partners (CP13-14) |
12.6 (2.2) | 12.7 (2.2) | 12.2 (2.6) | 0.0555 |
Student’s t-test
Based on the univariate and bivariate analysis, the following independent variables were put into the model: psychological scales for attitude; direct subjective norms; indirect subjective norm: perceived behavioural control; and contacting partners. No demographic variables achieved significance, and thus were not entered into the model. Odds ratios and 95% confidence intervals confirmed that positive attitudes regarding the value of HPV testing (OR 1.2; 95%CI 1.1, 1.3) positive indirect subjective norms (OR 1.02; 95% CI 1.01, 1.03) and positive behavioural control (OR 1.16; 95%CI 1.10, 1.23) all significantly predict women’s intentions to be screened with HPV testing (Table 5).
Table 5.
Predictors of women’s intentions to receive cervical cancer screening with primary human papillomavirus testing using multivariate logistic regression
| Variable Name | Odds Ratio | 95% Confidence Interval |
|---|---|---|
| Attitudes to HPV (A1) | 1.224 | 1.153; 1.301 |
| Indirect subjective norms (SNI5-12) | 1.022 | 1.014; 1.031 |
| Perceived behavioural control (PBC 15-18) | 1.158 | 1.095; 1.225 |
Discussion
There is increasing evidence demonstrating that cervical cancer screening with primary HPV testing coupled with cytology triage is more effective at detecting relevant precancerous lesions and decreases cervical cancer incidence20,21. Several jurisdictions, including the Netherlands and the province of Ontario, have recommended the use of primary HPV testing for cervical cancer 26,27,35. Although neither the Canadian Task Force on Preventive Health Care 36 nor the US Preventive Service Task Force37 have yet to recommend primary HPV testing for cervical cancer screening, both groups identified that the weight of evidence would likely ultimately lead programs towards this recommendation. With this substantive change on the horizon in one of the most established screening programs, it is important that, prior to introduction of this new technology for screening, broader considerations should be included in planning for its implementation. Women’s experiences and their willingness to participate in screening if the new technology is adopted should be carefully examined. In particular, because of the improvements in the sensitivity and negative predictive values of HPV testing compared to cytology, screening programs using primary HPV testing would have increased screening intervals and later starting dates. These changes, along with the use of a test for a sexually acquired infection, could have unintended impacts on the acceptability of cervical cancer screening with primary HPV testing.
In our study, we found that over 80% of women intended to be screened for cervical cancer with HPV primary screening. However, women’s intentions to be screened with HPV decreased significantly once they were advised of the extended screening interval with HPV testing, from 84.2% to 54.2%. Because of improved sensitivity, high negative predictive value of HPV compared to Pap screening as well as risk for false positive HPV tests, cervical cancer screening using HPV should occur every 4-5 years, not annually as has been the case with Pap smears38. When advised that cervical cancer screening with HPV would not start until age 25, compared to current starting ages of 18 or soon after sexual debut in Canada, women’s intentions to be screened for cervical cancer with HPV remained low at 51.2%. Programs moving to primary HPV testing must effectively communicate the natural history of HPV (emphasizing its prevalence and high rate of regression), added diagnostic capabilities and negative predictive value of HPV testing, in order to reassure women about the safety of the extended screening interval, and to ensure high acceptability of this improved method of cervical cancer screening. In addition, health systems are often poor at communicating the risks of over-screening. For cervical cancer, too frequent use of HPV testing could lead to unwarranted colposcopies and biopsies, unnecessary anxiety, and perhaps create iatrogenic illnesses39, which would be an equally valuable message to convey broadly.
In our logistic regression analyses, the predictors of intention to be screened with HPV were overall positive attitudes to HPV, the endorsement and recommendations for HPV testing from highly regarded health agencies, health practitioners and individuals in women’s lives and women’s ability to gain access to HPV testing. Positive attitudes to HPV testing include positive assessments regarding HPV accuracy, safety, ability to protect health and acceptability of HPV testing. This indicates that substantial efforts should be made to ensure women are aware of the diagnostic attributes of HPV testing, as this is a key element for women to understand the safety, accuracy and acceptability of HPV testing. Our findings also indicate that opinion leaders for women’s decision-making include groups that have established roles in cancer prevention as well as their own practitioners and their peers and families. Agencies with cancer prevention, screening and treatment mandates should adopt strong messages that endorse and support HPV testing for cervical cancer screening. Additionally, education efforts should not be solely targeted at women, but should also include their peers and family members, as these individuals are influential agents in women’s decisions about screening. Finally, women should understand how they can access primary HPV screening, in order for them to plan to receive the testing.
Previous work has found that women report anxiety, distress, and shame when they receive positive HPV results 40-42. Women also report concern about communicating test results to sexual partners, and about stigma and shame associated with having a sexually transmitted infection 38,43. Although these results are illustrative, few studies have examined the impact of these emotions of receiving a positive HPV result on women’s intentions to be screened for cervical cancer. In our study, we explicitly examined the association of the potential need to discuss HPV status with a partner as an influence on intentions to be screened. Although significant in the univariate analysis, in the multivariate analysis, contacting and communicating HPV status to partners did not emerge as a significant predictor of intentions to be screened. This suggests that while important in women’s deliberations for HPV testing, ultimately it was not a predictor of women’s intentions to be screened with HPV.
Further research is needed to understand why women are reluctant to have extended screening intervals. There are no data about why women are unwilling to have extended intervals for primary HPV testing, but in research on Pap smears, women were also reluctant to have extended screening intervals; 69% of women reported that they would continue to receive annual screening, even if advised it was not required44. In Sirovich’s survey, women believed that cost was driving increased intervals between screening, and for HPV testing, women may interpret less frequent screening as a poorer quality screening program. Women may believe that extended intervals and less frequent screening may miss precancerous lesions. Thus, there is a need for comprehensive education for women to improve their understanding about the rationales underpinning the move from cytology to HPV testing and the differences between the two different technologies, which include a higher sensitivity and negative predictive value of HPV compared to cytology, thus decreasing the need for frequent screening. It will be critical to determine the optimal communication strategy through careful evaluation to ensure women are confident that an extended screening offers safe and effective cervical cancer screening. Similarly, changes in age of initiation for screening are based on an improved understanding of the natural history of cervical cancer, as well as increased awareness of the potential long-term consequences of treatment for precancerous lesions, including preterm labour and low birth weight infants, not on a desire to reduce access to screening 39. Messaging that clearly outlines the scientific as opposed to economic underpinning of this decision is needed.
Limitations
This study examined women who were part of the provincial screening program in British Columbia and had completed participation in a randomized controlled trial 20. Participants were emailed invitations, but not all participants had functional email addresses, and not all invitees completed responses, leading to a response rate of 63%. Comparison of survey respondents and non-respondents showed that they were not significantly different (Table 1). Survey participants also received information on HPV as part of their participation in the study, and thus may be more comfortable with the role of HPV and HPV screening than other women. Thus, these study findings are likely to be generalizable to the population of women who were part of the provincial screening program and participated in a large clinical trial. This study does not capture perspectives of women who did not attend for cervical cancer screening, nor the attitudes of women under the age of 25. As this population remains a key consideration for cervical cancer prevention, further explorations of this group are needed to understand both opportunities to improve uptake with HPV and also to ensure there is improved engagement for women who have not attended cervical cancer screening.
Overall, 84.2% of women intended to have cervical cancer screening with HPV instead of Pap screening. No demographic characteristics were significantly different among women who intended to be screened with HPV. In particular, age, marital status, sexual history, smoking history, education and cultural background were not significantly different between women who intended to be screened for cervical cancer with HPV and those who did not. There was also no difference between age strata for women who intended to be screened with HPV and those who did not. This is in contrast to several previous studies, which identified differing anxiety and concerns about HPV and willingness to have HPV-based tests, depending on age 29,45,46, and cultural background34. In particular, previous studies reported certain cultural groups identified concerns about the sexual nature of the infection, implications for fidelity and relationships and the need for disclosure34,38. Regardless, this has relevance for programming. One might expect women who have different educational or cultural backgrounds to be more or less reluctant to move to a different type of screening; particularly one with a communicable disease overtone, and that this ultimately could affect on willingness to be screened.
This study is one of the first to identify the potential impact of a transition to HPV primary screening from cytology on uptake of cervical cancer screening. In a highly motivated population, although over 80% of women were willing to be screened with HPV, women’s intentions to be screened for cervical cancer decreased to 54% when they were made aware of extended screening intervals. Jurisdictions considering the use of primary HPV screening need to ensure comprehensive education program for women, to assure them of the safety and rationale for primary HPV screening and to mitigate any potential negative impact on screening attendance rates.
Impact statement: Despite evidence for primary HPV testing, there is no published data on whether women will accept HPV testing for cervical cancer screening. In this large study, we find women are willing to be screened with HPV, but acceptability decreases significantly when women are advised about the extended screening interval and the later start of screening with HPV-based screening. Study findings will have substantial influence globally as countries consider changing to HPV based cervical cancer screening.
Acknowledgements
The authors are grateful to the Canadian Foundation for Women’s Health, which provided funding for this study.
Appendix 1. Information sheet provided to survey recipients
The human papillomavirus (HPV) is a common virus that can infect the cervix (part of a woman’s womb). It is now known to be the cause of cervical cancer. Women develop HPV infections in the cervix after having sexual activity with a partner who is infected with HPV. However, HPV is so common that over 75% of sexually-active women will have an HPV infection of their cervix sometime during their life. Most women who find out they have an HPV infection in the cervix after the age of 30, were infected with HPV years before. Over 90% of women who are infected with HPV in the cervix get rid of the infection naturally. It is only women who have longstanding infections with certain types of HPV who may be at risk for developing cervical cancer. Women may not have known it in the past, but it is these same HPV infections that are the most common reason for abnormal Pap smears.
Right now in BC, women start cervical cancer screening once they become sexually active. We now know that testing for HPV infections in the cervix is more accurate than the Pap smear for predicting whether or not a woman will develop cervical cancer.
Footnotes
Publisher's Disclaimer: This article has been accepted for publication and undergone full peer review but has not been through the copyediting, typesetting, pagination and proofreading process which may lead to differences between this version and the Version of Record. Please cite this article as an ‘Accepted Article’, doi: 10.1002/ijc.28324
These findings do not represent the official views of the Centers for Disease Control and Prevention.
Conflict of Interest statements
Eduardo Franco has served as an occasional consultant to companies involved with HPV vaccines (Merck Canada Inc., GlaxoSmithKline Inc.), cervical cancer screening (Cytyc Corporation, Iknoisys Inc.) and HPV diagnostics (Qiagen, Roche Diagnostics, BD and Gen-Probe Inc.). Mel Krajden received research contracts relating to HPV testing from Roche Diagnostics, Gen-Probe Inc. and Merck Canada Inc. Andrew Coldman is an investigator on a study sponsored by an equipment manufacturer unrelated to this study.
References
- 1.Franco EL, Duarte-Franco E, Ferenczy A. Cervical cancer: epidemiology, prevention and the role of human papillomavirus infection. CMAJ. 2001;164:1017–1025. [PMC free article] [PubMed] [Google Scholar]
- 2.Nanda K, McCrory DC, Myers ER, Bastian LA, Hasselbad V, Hickey JD. Accuracy of the Papanicoloau test in screening for and follow-up of cervical cytologic abnormalities: a systematic review. Annals of Internal Medicine. 2000;132:810–819. doi: 10.7326/0003-4819-132-10-200005160-00009. [DOI] [PubMed] [Google Scholar]
- 3.Walboomers JM, Jacobs MV, Manos MM, Bosch FX, Kummer JA, Shah KV, Snijders PJ, Peto J, Meijer CJ, Munoz N. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J Pathol. 1999;189:12–19. doi: 10.1002/(SICI)1096-9896(199909)189:1<12::AID-PATH431>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 4.Munoz N, Bosch FX, de Sanjose S, Herrero R, Castellsague X, Shah KV, Snijders PJ, Meijer CJ, International Agency for Research on Cancer Multicenter Cervical Cancer Study Group Epidemiologic classification of human papillomavirus types associated with cervical cancer. N Engl J Med. 2003;348:518–527. doi: 10.1056/NEJMoa021641. [DOI] [PubMed] [Google Scholar]
- 5.Schiffman M. Integration of human papillomavirus vaccination, cytology, and human papillomavirus testing. Cancer. 2007;111:145–153. doi: 10.1002/cncr.22751. [DOI] [PubMed] [Google Scholar]
- 6.Ratnam S, Franco EL, Ferenczy A. Human papillomavirus testing for primary screening of cervical cancer precursors. Cancer Epidemiol Biomarkers Prev. 2000;9:945–951. [PubMed] [Google Scholar]
- 7.Cuzick J, Szarewski A, Cubie H, Hulman G, Kitchener H, Luesley D, McGoogan E, Menon U, Terry G, Edwards R, Brooks C, Desai M, Gie C, Ho L, Jacobs I, Pickles C, Sasieni P. Management of women who test positive for high-risk types of human papillomavirus: the HART study. Lancet. 2003;362:1871–1876. doi: 10.1016/S0140-6736(03)14955-0. [DOI] [PubMed] [Google Scholar]
- 8.Dillner J, Rebolj M, Birembaut P, Petry KU, Szarewski A, Munk C, de Sanjose S, Naucler P, Lloveras B, Kjaer S, Cuzick J, van Ballegooijen M, Clavel C, Iftner T, Joint European CS. Long term predictive values of cytology and human papillomavirus testing in cervical cancer screening: joint European cohort study. BMJ. 2008;337:a1754. doi: 10.1136/bmj.a1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Franco EL. Chapter 13: Primary screening of cervical cancer with human papillomavirus tests. J Natl Cancer Inst Monogr. 2003;31:89–96. doi: 10.1093/oxfordjournals.jncimonographs.a003488. [DOI] [PubMed] [Google Scholar]
- 10.Franco EL, Ferenczy A. Is HPV testing with cytological triage a more logical approach in cervical cancer screening? Lancet Oncology. 2006;7:527–529. doi: 10.1016/S1470-2045(06)70735-5. [DOI] [PubMed] [Google Scholar]
- 11.Kotaniemi-Talonen L, Nieminen P, Anttila A, Hakama M. Routine cervical screening with primary HPV testing and cytology triage protocol in a randomised setting. British Journal of Cancer. 2005;93:862–867. doi: 10.1038/sj.bjc.6602799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kotaniemi-Talonen L, Anttila A, Malila N, Tarkkanen J, Laurila P, Hakama M, Nieminen P. Screening with a primary human papillomavirus test does not increase detection of cervical cancer and intraepithelial neoplasia 3. European Journal of Cancer. 2008;44:565–571. doi: 10.1016/j.ejca.2007.12.002. [DOI] [PubMed] [Google Scholar]
- 13.Bulkmans NW, Berkhof J, Rozendaal L, van Kemenade FJ, Boeke AJ, Bulk S, Voorhorst FJ, Verheijen RH, van Groningen K, Boon ME, Ruitinga W, van Ballegooijen M, et al. Human papillomavirus DNA testing for the detection of cervical intraepithelial neoplasia grade 3 and cancer: 5-year follow-up of a randomised controlled implementation trial. Lancet. 2007;370:1764–1772. doi: 10.1016/S0140-6736(07)61450-0. [DOI] [PubMed] [Google Scholar]
- 14.Kitchener HC, Almonte M, Wheeler P, Desai M, Gilham C, Bailey A, Sargent A, Peto J, ARTISTIC Trial Study Group HPV testing in routine cervical screening: cross sectional data from the ARTISTIC trial. British Journal of Cancer. 2006;95:56–61. doi: 10.1038/sj.bjc.6603210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Naucler P, Ryd W, Tornberg S, Strand A, Wadell G, Elfgren K, Radberg T, Strander B, Forslund O, Hansson BG, Rylander E, Dillner J. Human papillomavirus and Papanicolaou tests to screen for cervical cancer. N Engl J Med. 2007;357:1589–1597. doi: 10.1056/NEJMoa073204. [DOI] [PubMed] [Google Scholar]
- 16.Mayrand MH, Duarte-Franco E, Rodrigues I, Walter SD, Hanley J, Ferenczy A, Ratnam S, Coutlee F, Franco EL, Canadian Cervical Cancer Screening Trial Study Group Human papillomavirus DNA versus Papanicolaou screening tests for cervical cancer. N Engl J Med. 2007;357:1579–1588. doi: 10.1056/NEJMoa071430. [DOI] [PubMed] [Google Scholar]
- 17.Ronco G, Giorgi-Rossi P, Carozzi F, Confortini M, Dalla PP, Del Mistro A, Gillio-Tos A, Minucci D, Naldoni C, Rizzolo R, Schincaglia P, Volante R, et al. Results at recruitment from a randomized controlled trial comparing human papillomavirus testing alone with conventional cytology as the primary cervical cancer screening test. J Natl Cancer Inst. 2008;100:492–501. doi: 10.1093/jnci/djn065. [DOI] [PubMed] [Google Scholar]
- 18.Giorgi-Rossi P, Segnan N, Zappa M, Naldoni C, Zorzi M, Confortini M, Merito M, Cuzick J, Ronco G. The impact of new technologies in cervical cancer screening: results of the recruitment phase of a large randomised controlled trial from a public health perspective. International Journal of Cancer. 2007;121:2729–2734. doi: 10.1002/ijc.23055. [DOI] [PubMed] [Google Scholar]
- 19.Ogilvie GS, vanNiekerk D, Krajden M, Martin RE, Ehlen TG, Ceballos K, Smith LW, Kan L, Cook DA, Peacock S, Stuart GC, Franco EL, Coldman AJ. A randomized controlled trial of Human Papillomavirus (HPV) testing for cervical cancer screening: trial design and preliminary results (HPV FOCAL Trial) BMC Cancer. 2010:10. doi: 10.1186/1471-2407-10-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ogilvie GS, Krajden M, van Niekerk DJ, Martin RE, Ehlen TG, Ceballos K, Smith LW, Kan L, Cook DA, Peacock S, Stuart GC, Franco EL, Coldman AJ. Primary cervical cancer screening with HPV testing compared with liquid-based cytology: results of round 1 of a randomised controlled trial - the HPV FOCAL Study. Br J Cancer. 2012;107:1917–1924. doi: 10.1038/bjc.2012.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ronco G, Giorgi-Rossi P, Carozzi F, Confortini M, Dalla PP, Del Mistro A, Ghiringhello B, Girlando S, Gillio-Tos A, De Marco L, Naldoni C, Pierotti P, et al. Efficacy of human papillomavirus testing for the detection of invasive cervical cancers and cervical intraepithelial neoplasia: a randomised controlled trial. Lancet Oncol. 2010;11:249–257. doi: 10.1016/S1470-2045(09)70360-2. [DOI] [PubMed] [Google Scholar]
- 22.Leinonen M, Nieminen P, Kotaniemi-Talonen L, Malila N, Tarkkanen J, Laurila P, Anttila A. Age-specific evaluation of primary human papillomavirus screening vs conventional cytology in a randomized setting. J Natl Cancer Inst. 2009;101:1612–1623. doi: 10.1093/jnci/djp367. [DOI] [PubMed] [Google Scholar]
- 23.Anttila A, Kotaniemi-Talonen L, Leinonen M, Hakama M, Laurila P, Tarkkanen J, Malila N, Nieminen P. Rate of cervical cancer, severe intraepithelial neoplasia, and adenocarcinoma in situ in primary HPV DNA screening with cytology triage: randomised study within organised screening programme. BMJ. 2010;340:c1804. doi: 10.1136/bmj.c1804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Saslow D, Castle PE, Cox JT, Davey DD, Einstein MH, Ferris DG, Goldie SJ, Harper DM, Kinney W, Moscicki AB, Noller KL, Wheeler CM, et al. American Cancer Society Guideline for human papillomavirus (HPV) vaccine use to prevent cervical cancer and its precursors. CA: a Cancer Journal for Clinicians. 2007;57:7–28. doi: 10.3322/canjclin.57.1.7. [DOI] [PubMed] [Google Scholar]
- 25.Murphy J, Kennedy E, Dunn S, McLachlin M, Fung Kee Fung M, Gzik D, Shier M, Paszat L. HPV testing in Primary Cervical Screening: A Systematic Review and Meta-Analysis. J Obstetrics and Gynecology of Canada. 2012;34:443–452. doi: 10.1016/S1701-2163(16)35241-0. [DOI] [PubMed] [Google Scholar]
- 26.Murphy J, Kennedy EB, Dunn S, McLachlin CM, Fung Kee FM, Gzik D, Shier M, Paszat L. Cervical screening: a guideline for clinical practice in Ontario. J Obstet Gynaecol Can. 2012;34:453–458. doi: 10.1016/S1701-2163(16)35242-2. [DOI] [PubMed] [Google Scholar]
- 27.Health Council of the Netherlands . Population screening for cervical cancer. Health Council of the Netherlands; The Hague: 2011. Publication no. 2011/07. [Google Scholar]
- 28.Franceschi S, Cuzick J, Herrero R, Dillner J, Wheeler CM. EUROGIN 2008 roadmap on cervical cancer prevention. Int J Cancer. 2009;125:2246–2255. doi: 10.1002/ijc.24634. [DOI] [PubMed] [Google Scholar]
- 29.Huang AJ, Perez-Stable EJ, Kim SE, Wong ST, Kaplan CP, Walsh JM, Iwaoka-Scott AY, Sawaya GF, et al. Preferences for human papillomavirus testing with routine cervical cancer screening in diverse older women. Journal of General Internal Medicine. 2008;23:1324–1329. doi: 10.1007/s11606-008-0633-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ajzen The theory of planned behavior. Organizational Behavior and Human Decision. 1991;50:179–211. [Google Scholar]
- 31.Hankins M, French D, Horne R. Statistical Guidelines for Studies of the Theory of Reasoned Action and the Theory of Planned Behaviour. Psychology and Health. 2000;15:151–161. [Google Scholar]
- 32.The American Association for Public Opinion Research . Final disposition of case codes and outcome rates for surveys. 7th edition AAPOR; 2011. [Google Scholar]
- 33.Francis JJ, Eccles MP, Johnston M, Walker A, Grimshaw J, Foy R, Kaner EFS, Smith L, Bonetti D. Constructing Questionnaires based on the theory of planned behaviour: A manual for health services researchers. Quality of Life and Management of Living Resources. University of Newcastle; 2004. [Google Scholar]
- 34.McCaffery K, Forrest S, Waller J, Desai M, Szarewski A, Wardle J. Attitudes towards HPV testing: a qualitative study of beliefs among Indian, Pakistani, African-Caribbean and white British women in the UK. Br J Cancer. 2003;88:42–46. doi: 10.1038/sj.bjc.6600686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Murphy J, Kennedy EB, Dunn S, McLachlin CM, Fung Kee FM, Gzik D, Shier M, Paszat L. HPV testing in primary cervical screening: a systematic review and meta-analysis. J Obstet Gynaecol Can. 2012;34:443–452. doi: 10.1016/S1701-2163(16)35241-0. [DOI] [PubMed] [Google Scholar]
- 36.Canadian Task Force on Preventive Health Care Recommendations on screening for cervical cancer. Canadian Medical Association Journal. 2013 DOI:10.1503/cmaj.121505. [Google Scholar]
- 37.Whitlock EP, Vesco KK, Eder M, Lin JS, Senger CA, Burda BU. Liquid-based cytology and human papillomavirus testing to screen for cervical cancer: a systematic review for the U.S. Preventive Services Task Force. Ann Intern Med. 2011;155:687–5. doi: 10.7326/0003-4819-155-10-201111150-00376. [DOI] [PubMed] [Google Scholar]
- 38.Fernandez ME, McCurdy SA, Arvey SR, Tyson SK, Morales-Campos D, Flores B, Useche B, Mitchell-Bennett L, Sanderson M. HPV knowledge, attitudes, and cultural beliefs among Hispanic men and women living on the Texas-Mexico border. Ethn Health. 2009;14:607–624. doi: 10.1080/13557850903248621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kyrgiou M, Koliopoulos G, Martin-Hirsch P, Arbyn M, Prendiville W, Paraskevaidis E. Obstetric outcomes after conservative treatment for intraepithelial or early invasive cervical lesions: systematic review and meta-analysis. Lancet. 2006;367:489–498. doi: 10.1016/S0140-6736(06)68181-6. [DOI] [PubMed] [Google Scholar]
- 40.Waller J, McCaffery K, Kitchener H, Nazroo J, Wardle J. Women’s experiences of repeated HPV testing in the context of cervical cancer screening: a qualitative study. Psychooncology. 2007;16:196–204. doi: 10.1002/pon.1053. [DOI] [PubMed] [Google Scholar]
- 41.Waller J, Marlow LA, Wardle J. The association between knowledge of HPV and feelings of stigma, shame and anxiety. Sex Transm Infect. 2007;83:155–159. doi: 10.1136/sti.2006.023333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Waller J, Marlow LA, Wardle J. Anticipated shame and worry following an abnormal Pap test result: the impact of information about HPV. Prev Med. 2009;48:415–419. doi: 10.1016/j.ypmed.2008.11.004. [DOI] [PubMed] [Google Scholar]
- 43.Daley EM, Perrin KM, McDermott RJ, Vamos CA, Rayko HL, Packing-Ebuen JL, Webb C, McFarlane M. The psychosocial burden of HPV: a mixed-method study of knowledge, attitudes and behaviors among HPV+ women. J Health Psychol. 2010;15:279–290. doi: 10.1177/1359105309351249. [DOI] [PubMed] [Google Scholar]
- 44.Sirovich BE, Woloshin S, Schwartz LM. Screening for cervical cancer: will women accept less? Am J Med. 2005;118:151–158. doi: 10.1016/j.amjmed.2004.08.021. [DOI] [PubMed] [Google Scholar]
- 45.Anhang R, Wright TC, Jr., Smock L, Goldie SJ. Women’s desired information about human papillomavirus. Cancer. 2004;100:315–320. doi: 10.1002/cncr.20007. [DOI] [PubMed] [Google Scholar]
- 46.Maissi E, Marteau TM, Hankins M, Moss S, Legood R, Gray A. Psychological impact of human papillomavirus testing in women with borderline or mildly dyskaryotic cervical smear test results: cross sectional questionnaire study. BMJ. 2004;328:1293. doi: 10.1136/bmj.328.7451.1293. [DOI] [PMC free article] [PubMed] [Google Scholar]