Abstract
Introduction:
Chronic obstructive pulmonary disease (COPD) is characterized by airflow limitation that is not completely reversible by administration of inhaled bronchodilators. Many studies propose that telomere length shortening might have occurred in COPD patients. We aimed to determine the telomere length in COPD patients and compare the results of non-smoking and smoking control subjects.
Materials and Methods:
In our case-control study, 84 clinically stable COPD patients were recruited on admission to Masih Daneshvari Hospital. Eighty-five healthy controls were also selected including 45 non-smokers and 40 smokers admitted for diseases other than COPD. Spirometry was done for all subjects. Telomere length was measured by quantitative real time PCR as described by Cawthon. The telomere repeat copy number (T) to single-gene copy number (S) ratio was calculated using the comparative Ct method.
Results:
The mean ±SD of age was 64.33±10.04 years in patients and 65.06 ±10.02 years in controls (P=0.693). The mean ±SD of FEV1 was 1.62±0.75 L in patients, 2.84±0.54 L in smoker controls and 2.83±0.56 L in non-smoker controls; significant differences were detected in this regard between cases and controls (P<0.001). T/S ratio was significantly lower in COPD patients (0.61±0.08) than in the control subjects (0.69±0.09) (P<0.001). However, telomere length was shorter in the patients than in controls in each age group (P<0.001). Additionally, there were no statistically significant differences in telomere length between the smoker and non-smoker control subjects. Regarding the correlation between BMI and telomere length, there were no significant differences among the patients and control groups.
Conclusion:
In conclusion, we found that telomere length in COPD patients was shorter than that in smoker and non-smoker controls, irrespective of age, sex, spirometric variables, BMI and history of cigarette smoking.
Keywords: Chronic obstructive pulmonary disease, Aging, Telomere, Telomere length
INTRODUCTION
Chronic obstructive pulmonary disease (COPD) is the most important cause of morbidity and mortality in the world imposing a growing burden on developing countries (1, 2). Tobacco use is a known major risk factor of diseases and can alter the structure of tissues and organs especially the respiratory tract (3). According to the World Health Organization (WHO) health topic on the global burden of disease, COPD is estimated to be the third cause of death in 2020 (1).
COPD is characterized by airflow limitation that is not completely reversible by administration of inhaled bronchodilators (4).
Decreased forced expiratory volume in 1 second (FEV1) and reduced FEV1/FVC ratio less than 70% are the main spirometric findings of COPD (5–7).
COPD can significantly affect the lungs but it can have other systemic manifestations as well such as inflammatory response, cancer and cardiovascular diseases that may occur as the disease progresses (8). Cardiovascular events and cancer are the two most common causes of mortality particularly in end-stage patients (9, 10).
Atherosclerosis, anxiety, depression, osteoporosis and low BMI as age-related conditions are the most common systemic manifestations of COPD (9, 11). Such similarities suggest that COPD can relate to advanced age and its prevalence increases with age (5, 12). COPD is rare before the age of 40 years but its prevalence increases to 30% after the age 70 (12). Evidence suggests a correlation between COPD and advanced age. Reactive oxygen species and other causes of oxidative stress such as cigarette smoking and noxious gases can promote the process of premature aging (12–14) when the length of telomere reaches to a condition which is called Hay-flick limit (15, 16).
Telomeres are DNA sequences with high amounts of G-rich nucleotides located at the end of chromosomes, which shorten with age in all somatic cells after birth (17, 18). The end replication problem can lead to telomere shortening with each cellular division in cell cycle (14). Since telomere length determines the number of cell divisions and proliferation rate of somatic cells, it has been considered as a replicometer or biomarker of aging. They protect the end of chromosomes from end to end fusion and genomic instability (19–21).
Impaired balance between the protease and antiprotease and the impaired balance between oxidative stress and antioxidant defense can accelerate the process of telomere shortening.
Furthermore, telomere attrition in circulating blood leukocytes has been suggested as a biomarker of systemic inflammation and oxidative stress and also as a predictor of biological aging (5, 10).
Many studies propose that telomere length shortening might have occurred in COPD patients (8, 18, 12, 22).
Although smoking as a trigger of oxidative stress may affect telomere shortening, there are many investigations showing that telomere length decreases in COPD patients irrespective of smoking (10).
In the current study, we aimed to determine the telomere length in COPD patients and compare the results of non-smoking and smoking control subjects.
MATERIALS AND METHODS
Study population
Our case-control study was performed on 84 clinically stable COPD patients confirmed by a pulmonologist according to ATS/ERS criteria recruited on admission to Masih Daneshvari Hospital, Tehran, Iran.
The inclusion criteria consisted of at least 10/pack/year history of tobacco consumption and the ratio of EFV1/FVC less than 70%. The exclusion criteria were presence of cardiovascular diseases, malignancies or other chronic inflammatory diseases.
Eighty-five healthy controls were selected including 45 non-smokers and 40 smokers who were admitted for diseases other than COPD. Their FEV1 was greater than 80% and FEV1/FVC ratio was more than 70%. We tried to select sex-matched and age-matched control subjects with±2 years of age.
This study was approved by the Ethical Committee of Tehran University of Medical Sciences and the National Research Institute of Tuberculosis and Lung Disease (NRITLD).
All patients and controls underwent spirometry and their FEV1, FVC, FEV1/FVC and BMI were recoded.
Laboratory investigations:
Blood samples were drawn from all patients and controls (5ml) and transferred into EDTA-containing tubes.
All samples were centrifuged at 1000×g for 10 minutes at 4°C and stored at −70°C for DNA extraction.
Genomic DNA was extracted from buffy coats with Qiagen (QIAamp DNA Blood Mini Kit, Germany) and quantified by spectrophotometer (Hitachi 1800, Japan).
Telomere length was measured by quantitative Real Time-PCR as described by Cawthon (23)
All samples were run in triplicate, using the SYBR green method and 35 ng of DNA. The sequences of the telomere primers for tel-1 and tel-2 and 36B4 as single copy gene for normalized technique were as follows:
| Tel-1F | 5′-CGGTTTGTTTGGGTTTGGGTTTGGGTTTGGGTTTGGGTT-3′ |
| Tel-2R | 5′-GGCTTGCCTTACCCTTACCCTTACCCTTACCCTTACCCT-3′ |
| Single-1F | 36B4F, 5′-CAGCA AGTGGGAAGGTGTAATCC-3 |
| Single-2R | 36B4R, 5′-CCCATTCTATCATCAACGGGTACAA-3′ |
The telomere repeat copy number (T) to single-gene copy number (S) ratio was calculated using the comparative Ct method.
Positive controls (extracted from a normal healthy person) and negative controls (DDW+ master mix) were added for every PCR run.
PCR was performed by a BioRad RT-PCR system (BioRad, USA).
Statistical analysis
The data were compared between COPD patients and control subjects including smoker and non-smoker controls. Differences between patients and controls were tested using the Student’s t test.
Quantitative variables were recorded as mean± SD and their correlation was analyzed by Pearson’s correlation or by non-parametric Kendalls’ correlation tests.
P values less than 0.05 were considered significant. Data were analyzed using SPSS for windows (version 22.00, IBM).
RESULTS
The characteristics of understudy subjects are shown in Table 1. Patients and controls did not have statistically significant differences regarding age or sex because of the type of selection as age- and sex-matched.
Table 1.
Age and sex characteristics of understudy subjects
| Variables | Case N=84 | Control N=85 | Total | P-value |
|---|---|---|---|---|
| Age (Yrs.) | 64.33±10.04 | 65.06±10.04 | 64.70±10.02 | 0.693 |
| Sex | ||||
| Male | 68 (81.0%) | 69 (81.2%) | 137 (81.1%) | 0.970 |
| Female | 16 (19.0%) | 16 (18.8%) | 32 (18.9%) | |
The mean ±SD of age was 64.33±10.04 years in patients and 65.06 ±10.02 years in controls (P=0.693) (Table 1).
The mean ±SD of FEV1 was 1.62±0.75 L in patients, 2.84±0.54 L in smoker controls, and 2.83±0.56 L in non- smoker controls, which were significantly different between cases and controls (P<0.001) (Table 2).
Table 2.
Comparison between case and control subjects regarding spirometric variables
| Variables | Case N=84 | Control Smoker N=40 | Control Non-smoker N=45 | P-value |
|---|---|---|---|---|
| FEV1 (L) | 1.62±0.75 | 2.84±0.54 | 2.83±0.56 | <0.001 |
| FEV1/FVC (%) | 62.11±7.63 | 79.2±4.78 | 79.93±4.48 | <0.001 |
In comparison between smoker and non-smoker control subjects, no significant difference was shown in FEV1 (P=0.968).
The mean ±SD of FEV1/FVC was 62.11±7.63% in patients, 79.93±4.48% in smoker controls and 79.20±4.78 percent in non-smoker controls; these values were significantly different between cases and controls (P<0.001) (Table 2).
In comparison between smoker and non-smoker control subjects, no significant difference was noted in FEV1/FVC (P=0.499).
T/S ratio was significantly lower in COPD patients (0.61±0.08) than in control subjects (0.69±0.09) (P<0.001) (Figures 1 and 2).
Figure 1.
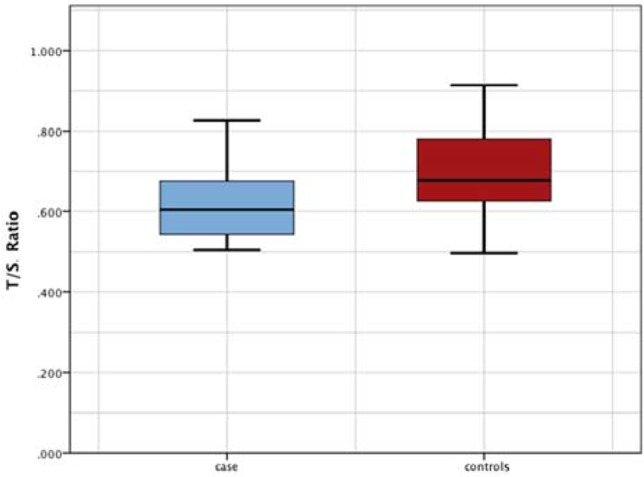
Comparision between COPD patients and control subjects regarding telomere length
Figure 2.
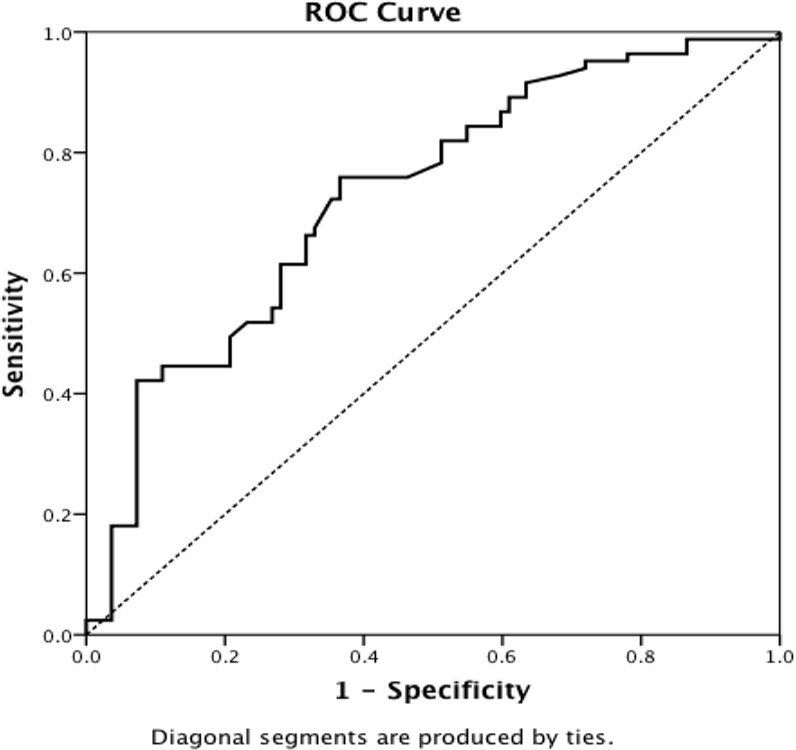
ROC curve distinguishing control subjects from patients by telomere length.
However, telomere length was shorter in the patients than in the controls in each age group (P<0.001).
Additionally, there were no statistically significant differences in telomere length between the smoker and non-smoker control subjects.
The mean±SD values of telomere length in patients and controls based on sex are listed in Table 3. There were no statistically significant differences in telomere length and sex between patients and control subjects.
Table 3.
Comparison of patients and control subjects in terms of sex and telomere length
| Group | Sex | N | Mean | Std. deviation | P-value | |
|---|---|---|---|---|---|---|
| Case | T/S ratio | Male | 68 | .61448 | .080547 | 0.841 |
| Female | 16 | .60975 | .098851 | |||
| Control smoker | T/S ratio | Male | 35 | .66082 | .087778 | 0.645 |
| Female | 5 | .66560 | .123488 | |||
| Control non-smoker | T/S ratio | Male | 34 | .65991 | .088084 | 0.944 |
| Female | 11 | .66209 | .094236 | |||
After stratification of patients into three age groups of <50 years, 50–60 years and > 60 years, the differences between patients and controls in telomere length remained (Figure 3).
Figure 3.
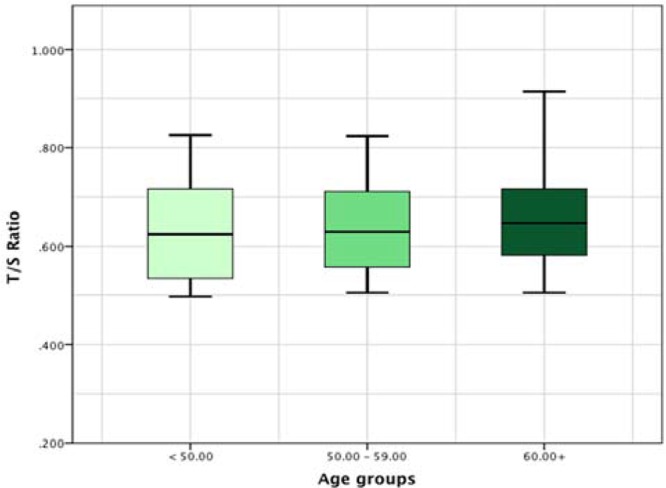
Comparision among the three age groups regarding telomere length
Regarding the correlation between BMI and telomere length, there were no significant differences among the three understudy groups (Table 4).
Table 4.
Correlation between BMI and telomere length among patients and control subjects
| Group | BMI | |||
|---|---|---|---|---|
| Case | Kendall’s tau_b | T/S Ratio | Correlation coefficient | −0.038 |
| Sig. (2-tailed) | 0.614 | |||
| N | 84 | |||
| Control smoker | Kendall’s tau_b | T/S Ratio | Correlation coefficient | −0.109 |
| Sig. (2-tailed) | 0.339 | |||
| N | 40 | |||
| Control non smoker | Pearson’s correlation | T/S Ratio | Correlation coefficient | −0.042 |
| Sig. (2-tailed) | 0.783 | |||
| N | 45 |
DISCUSSION
The main finding of the present study was that the telomere length in COPD patients was shorter than that in smoker and non-smoker controls, irrespective of age, sex, spirometric data, BMI and smoking. Our results were in accord with those of Savale et al (10).
Telomere length decreased in both male and female COPD patients compared with the control subjects.
Savale et al, (10) in their study showed that telomere length was shorter in males than females only in control subjects. We found no sex-related statistically significant difference in telomere length between patients and controls.
We found no relationship between telomere length and smoking in patients or controls.
Morla et al. (24), Valdes et al. (25), and Chan et al. (26) showed the effect of cigarette smoking on telomere shortening.
Morla and colleagues (24) in their study, done by fluorescence in situ hybridization (FISH) reported a dose-dependent relationship between telomere length and exposure to smoking. They could not show the influence of smoking on telomere length based on presence of COPD.
In contrast with their results, we showed that telomere length was affected by COPD irrespective of cigarette smoking. Our data are in accordance with those of other previous studies (10, 12, 14).
Although cigarette smoking, among the oxidative stress agents, can play an important role in cellular senescence and possibly telomere shortening, our findings similar to those of other studies (10, 12) confirm that the role of COPD in telomere shortening is more prominent than that of smoking.
Tsuji et al. found that the telomere length in alveolar type II cells of patients with emphysema was shorter than in control subjects (27). Muller et al. reported that telomere length did not differ in cultured parenchymal fibroblasts of patients with emphysema (28).
Adler et al, in their experimental investigation found that a short telomere can act as a predisposing factor, which increases the susceptibility to cigarette smoking-induced emphysema in mice (29).
In our study, similar to a previous study (10), no correlation was found between telomere shortening and change in spirometric variables such as FEV1, FVC and FEV1/FVC.
We found no relationship between BMI and telomere length in our current investigation similar to Savale’s study. In contrast, Valdes et al, (25) and Cui et al. (30) found a correlation between telomere shortening and obesity in women.
Finally, our findings regarding decreased telomere length in COPD patients can support the involvement of accelerated aging in pathogenesis of this disease, and measurement of telomere length can be considered as a diagnostic biomarker for COPD as well as premature aging.
Similarities between COPD and aging suggest that COPD can relate to advanced age, and its prevalence increases with age (5, 12, 22, 31).
In conclusion, we found that telomere length in COPD patients was shorter than in smoker and non-smoker controls, irrespective of age, sex, spirometric variables, BMI and history of cigarette smoking.
Acknowledgement
This research project (No. 10936-30-02-89) was supported by Tehran University of Medical Sciences (TUMS). The authors are grateful to Dr. Habib Emami and Mrs. Golnar Radmand for helpful tips in statistical analysis.
REFERENCES
- 1. Buist AS, McBurnie MA, Volmer WM. International variation in the prevalence of COPD : a population-based prevalence study. Lancet 2007; 370: 741– 50. [DOI] [PubMed] [Google Scholar]
- 2. Burney P, Jithoo A, Kato B, Janson C, Mannino D, Nizankowska-Mogilnicka E, et al. Chronic obstructive pulmonary disease mortality and prevalence: the associations with smoking and poverty--a BOLD analysis. Thorax 2014; 69 ( 5): 465– 73. 10.1136/thoraxjnl-2013-204460. Epub 2013 Dec 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Karrasch S, Holz O, Jörres RA. Aging and induced senescence as factors in the pathogenesis of lung emphysema. Respir Med 2008; 102 ( 9): 1215– 30. [DOI] [PubMed] [Google Scholar]
- 4. Celli BR, NacNee W. Standards for the diagnosis and treatment of patients with COPD: a summary of the ATS/ERS position paper. Eur Respir J 2004; 23: 932– 46. [DOI] [PubMed] [Google Scholar]
- 5. Ito K, Barnes PJ. COPD as a disease of accelerated lung aging. Chest 2009; 135 ( 1): 173– 80. [DOI] [PubMed] [Google Scholar]
- 6. Ito K, Mercado N. STOP accelerating lung aging for the treatment of COPD. Exp Gerontol 2014. [DOI] [PubMed] [Google Scholar]
- 7. MacNee W. Accelerated lung aging: a novel pathogenic mechanism of chronic obstructive pulmonary disease (COPD). Biochem Soc Trans 2009; 37 ( Pt 4): 819– 23. [DOI] [PubMed] [Google Scholar]
- 8. Tuder RM, Kern JA, Miller YE. Senescence in chronic obstructive pulmonary disease. Proc Am Thorac Soc 2012; 9 ( 2): 62– 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sabit R, Bolton CE, Edwards PH, Pettit RJ, Evans WD, McEniery CM, Wilkinson IB, Cockroft JR, Shale DJ. Arterial stiffness and osteoprosis in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2007; 175: 1259– 65. [DOI] [PubMed] [Google Scholar]
- 10. Savale L, Chaouat A, Bastuji-Garin S, Marcos E, Boyer L, Maitre B, et al. Shortened telomeres in circulating leukocytes of patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2009; 179: 566– 71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bolton CE, Ionescu AA, Shiels KM, Pettit RJ, Edwards PH, Stone MD, Nixon LS, Evans WD, Griffiths TL, Shale DJ. Associated loss of fat-free mass and bone mineral density in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2004; 170: 1286– 93. [DOI] [PubMed] [Google Scholar]
- 12. Lee J, Sandford AJ, Connett JE, Yan J, Mui T, Li Y, et al. The relationship between telomere length and mortality in chronic obstructive pulmonary disease (COPD). PLoS One 2012; 7 ( 4): e35567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Repine JE, Bast A, Lankhorst I. Oxidative stress in chronic obsctructive pulmonary disease. Oxidative Stress Study Group. Am J Respir Crit Care Med 1997; 156: 341– 57. [DOI] [PubMed] [Google Scholar]
- 14. Houben JM, Moonen HJ, van Schooten FJ, Hageman GJ. Telomere length assessment: biomarker of chronic oxidative stress? Free Radic Biol Med 2008; 44 ( 3): 235– 46. [DOI] [PubMed] [Google Scholar]
- 15. Hayflick L. The limited in vitro lifetime of human diploid cell strains. Exp Cell Res 1965; 37: 614– 36. [DOI] [PubMed] [Google Scholar]
- 16. Harley CB, Vaziri H, Counter CM, Allsopp RC. The telomere hypothesis of cellular aging. Exp Gerontol 1992; 27: 375– 82 [DOI] [PubMed] [Google Scholar]
- 17. Harley CB, Futcher AB, Greider CW. Telomeres shorten during ageing of human fibroblasts. Nature 1990; 345: 458– 60. [DOI] [PubMed] [Google Scholar]
- 18. Houben JM, Mercken EM, Ketelslegers HB, Bast A, Wouters EF, Hageman GJ, et al. Telomere shortening in chronic obstructive pulmonary disease. Respir Med 2009; 103 ( 2): 230– 6. [DOI] [PubMed] [Google Scholar]
- 19. von Zglinicki T, Martin-Ruiz C, Saretzki G. Telomeres, cell senescence and human ageing. Signal Transduct 2005; 3. [Google Scholar]
- 20. von Zglinicki T. Role of oxidative stress in telomere length regulation and replicative senescence. Ann N Y Acad Sci 2000; 908: 99– 110. [DOI] [PubMed] [Google Scholar]
- 21. Jang JS, Choi YY, Lee WK, Choi JE, Cha SI, Kim YJ, et al. Telomere length and the risk of lung cancer. Cancer Sci 2008; 99 ( 7): 1385– 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gansner JM, Rosas IO. Telomeres in lung disease. Transl Res 2013; 162 ( 6): 343– 52. [DOI] [PubMed] [Google Scholar]
- 23. Cawthon RM. Telomere measurement by quantitative PCR. Nucleic Acids Res 2002; 30: e47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Morla M, Buequets X, Pons J, Sauleda J, MacNee W, Agusti AG. Telomere shortening in smokers with and without COPD. Eur Respir J 2006; 27: 525– 8. [DOI] [PubMed] [Google Scholar]
- 25. Valdes AM, Andrew T, Gardner JP, et al. Obesity, cigarette smoking, and telomere length in women. Lancet 2005; 366: 662– 4. [DOI] [PubMed] [Google Scholar]
- 26. Chan SW, Blackburn EH. New ways not to make ends meet: telomerase, DNA damage proteins and heterochromatin. Oncogene 2002; 21: 553– 63. [DOI] [PubMed] [Google Scholar]
- 27. Tsuji T, Aoshiba K, Nagai A. Alveolar cell senescence in patients with pulmonary emphysema. Am J Respir Crit Care Med 2006; 174 ( 8): 886– 93. [DOI] [PubMed] [Google Scholar]
- 28. Müller KC, Welker L, Paasch K, Feindt B, Erpenbeck VJ, Hohlfeld JM, et al. Lung fibroblasts from patients with emphysema show markers of senescence in vitro. Respir Res 2006; 7: 32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Alder JK, Guo N, Kembou F, Parry EM, Anderson CJ, Gorgy AI, et al. Telomere length is a determinant of emphysema susceptibility. Am J Respir Crit Care Med 2011; 184 ( 8): 904– 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Cui Y, Gao YT, Cai Q, Qu S, Cai H, Li HL, et al. Associations of leukocyte telomere length with body anthropometric indices and weight change in Chinese women. Obesity (Silver Spring) 2013; 21 ( 12): 2582– 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Liu JP. Molecular mechanisms of ageing and related diseases. Clin Exp Pharmacol Physiol 2014; 41 ( 7): 445– 58. [DOI] [PubMed] [Google Scholar]


