Abstract
Background:
High sensitive CRP (hs-CRP) is used as a marker of systemic inflammation in chronic obstructive pulmonary disease (COPD). However, we hypothesize that the raised hs-CRP is not closely related to the multiple consequences of COPD.
This study was undertaken to investigate the association of COPD assessment test (CAT) score with SpO2, FEV1, body mass index (BMI), obstruction, dyspnea and exercise capacity (BODE) index and COPD exacerbation rate and compare it with the association to serum hs-CRP level.
Materials and Methods:
Sixty patients with stable COPD referred to the pulmonology clinic of Ardabil Imam Khomeini Hospital were included in this study. SpO2, 6-minute walk distance (6MWD), body mass index, BODE index, and pulmonary function test as well as exacerbation rate were determined in COPD patients. Then, the CAT questionnaire was completed by patients. Serum level of hs-CRP was measured in all patients and 15 controls. We statistically compared the relationships and correlations among the variables.
Results:
Hs-CRP level was significantly raised in patients (P=0.005). In these patients, the correlation of hs-CRP level with BODE index was significant (P=0.008). However, the correlation of hs-CRP with SpO2 and FEV1 was not significant (P=0.47 and P=0.17, respectively). Also, the correlation of CAT score with SpO2, FEV1, BODE index, and exacerbation rate in the previous year was significant (P<0.001, P<0.001, P<0.001 and P=0.017, respectively).
Conclusion:
SpO2, FEV1, BODE index and exacerbation rate are more correlated with CAT scores than with the serum level of hs-CRP in stable COPD patients. The findings of this study should be considered in management of stable COPD patients.
Keywords: C-Reactive Protein, BODE index, COPD, hs-CRP, COPD assessment test
INTRODUCTION
COPD is the fourth cause of mortality afflicting over 200 million people around the world (1,2). It is characterized by irreversible airflow limitation and is associated with a systemic inflammatory response. It is estimated to rank the third most common cause of death in the world by 2020 (3). COPD has a major negative impact on health and quality of life (4). There is a list of major risk factors for COPD in which, smoking holds the first rank (5). Other relatively less common risk factors for COPD include respiratory infections, occupational exposure, air pollution, and genetics (6).
It is well known that in patients with COPD, systemic inflammation in addition to local airway inflammation depending on the severity of COPD, contribute to pulmonary and extra-pulmonary complications of the disease such as pulmonary function impairment, exercise intolerance (even regardless of lung function impairment level), disease exacerbation, hypoxemia, muscle atrophy, activity confinement, cachexia, and osteoporosis (7–10).
The most common causes of mortality in patients with COPD include respiratory insufficiency and cardiovascular complications; both of which can be ascribed to the systemic inflammation and its severity (11).
Systemic inflammation can be determined with markers of inflammation such as CRP, interleukins (IL), and TNFα. Among these markers, hs-CRP is as an important one and is a widely accepted biomarker related to the airflow obstruction (7).
However, the potential role of hs-CRP as a marker to reflect all aspects of pulmonary and extra-pulmonary complications in COPD and its prognostic value in COPD need further investigation.
COPD assessment test (CAT) is a new questionnaire for COPD patients. It is a simple method for assessing the impact of COPD on health and has similar discriminative properties to the much more complex St. George`s Respiratory Questionnaire (SGRQ) to measure the impact of COPD on individual patient’s health (12–15).
This study was undertaken to investigate the association of hs-CRP levels and CAT scores with COPD impairment variables such as hypoxia severity (SpO2), FEV1, BODE index and the frequency of exacerbations.
MATERIALS AND METHODS
This cross sectional study was conducted on 60 patients with COPD diagnosed on the basis of the criteria suggested by the American Thoracic Society (ATS) for diagnosis and treatment of COPD (16). All the patients with symptoms suggestive of COPD underwent spirometry before and after inhalation of bronchodilator.
All the participants chosen for this study had stable COPD and had been referred to the pulmonology clinic of Ardabil Imam Khomeini Hospital. All subjects with a history of asthma, bronchiectasis, tuberculosis and debilitating inflammatory diseases such as arthritis, connective tissue disorders, inflammatory bowel disease or malignancy and patients in the exacerbation phase or with a hospitalization record during the past two months were excluded from this study.
All participants signed a consent form for participation in the study. Subsequently, their demographic data (including sex, weight, height, age and the amount and duration of smoking) were recorded.
Each patient was assigned a BODE score. The BMI was calculated and the degree of airflow obstruction was determined by means of FEV1; dyspnea was determined using the MMRC dyspnea scale and exercise capacity was determined with the distance walked in 6 minutes (6MWT). For each value of FEV1, MMRC dyspnea scale and 6MWT, each patient received points that varied from 0 to 3. For the BMI, each patient received 0 or 1 point. The points for each component of the BODE index were added, and the final score varied between 0 and 10 points for each patient. In addition, the BODE score was classified in quartiles: quartile I included patients with a score of 0–2; quartile II, 3–4 points; quartile III, 5–6 points; and quartile IV, 7–10 points (17).
Oxygen saturation was measured in all patients by pulse-oximetry. Exacerbations were defined using the criteria developed by Global Initiative for Obstructive Lung Disease (GLOD) (18).
CAT is a recently introduced, patient-completed instrument to assess and quantify health-related quality of life and symptom burden in patients with COPD (13, 19). It comprises 8 questions, each presented as a semantic 6-point (0–5) differential scale, providing a total score out of 40 (20). All patients completed the CAT questionnaire. The total CAT score was calculated for each individual by summing the points for each variable. The CAT score was classified into four groups of 1) low, 2) medium, 3) high, and 4) very high based on the impact level of disease on health status (13).
For measurement of the serum level of hs-CRP, blood samples were collected from the patients and a control group of 15 healthy non-smokers who were over 40 years old and did not have any record of specific illness. Hs-CRP test was done by means of multidetector BT-3000, using immunoturbidimetric method, 10 minutes after incubation over 5μL serum, and reading after 290 seconds.
Variables were presented as percentage and mean± SD depending on their distributions.
Differences between means were analyzed with Student’s t-test and reported with 95% confidence interval. ANOVA was applied with 95% confidence interval for the estimation of differences. In order to evaluate the correlation between parametric variables, the Pearson’s correlation coefficient was utilized. SPSS was used for analysis of data. P value less than 0.05 in all analyses was considered statistically significant.
RESULTS
A total of 60 patients were recruited out of which, 54 (90%) were males and 6 (10%) were females. Also, 15 healthy subjects comprised the control group. The mean± SD hs-CRP level was 4.86±6.42 mg/L in patients with COPD and 1.96±2.16 mg/L in healthy controls. The difference between the two groups was statistically significant (P=0.005). Physiologic and clinical characteristics of patients are presented in Table 1.
Table 1.
The physiologic and clinical characteristics of patients
| Subjects (Number/percent) | Mean± SD | |
|---|---|---|
| Age (years) | 60.53±13.06 | |
| Sex (M/F) | 54/6 | |
| Smoking history | ||
| Current smoker | 44(74%) | |
| Ex-smoker | 14(23%) | |
| Non smoker | 2(3%) | |
| BMI(Kg/m2) | ||
| BMI≤21 | 4(6.7%) | 25.18±3.37 |
| BMI>21 | 56(93.3%) | |
| FEV1/FVC% | 63.02±7.57 | |
| FEV1% predict | 56.71±18.22 | |
| MMRC (dyspnea scale) | 1.62±0.88 | |
| 6MWD (meter) | 346±182 | |
| SpO2 | 93.33±3.87 | |
| CAT score | ||
| Mild | 2(3.3%) | 7.00±2.82 |
| Moderate | 37(61.7%) | 15.14±3.94 |
| Severe | 18(30%) | 25.50±2.45 |
| Very severe | 3(5%) | 32.00±1.00 |
| BODE score | ||
| Quartile I (0–2) | 30(50%) | 0.90±0.76 |
| Quartile II (3–4) | 17(28.3%) | 3.29±0.47 |
| Quartile III (5–6) | 7(11.7%) | 5.57±0.53 |
| Quartile IV (7–10) | 6(10%) | 7.50±0.55 |
The patients’ CAT scores ranged from 5 to 33 with a mean±SD of 18.82±6.86. The number of patients in CAT groups of 1, 2, 3 and 4 was 2, 37, 18 and 3, respectively. The majority of patients (34 subjects out of 60) were in the first group of 6MWD test (over 350 meters).
The hs-CRP level had a statistically significant correlation with BODE severity (r=0.34, P=0.008). Groups with higher BODE score had higher hs-CRP (Figure 1).
Figure 1.

The relationship between hs-CRP and BODE groups
Drawing a comparison between patients’ CAT scores and their BODE index scores revealed a significant relationship between CAT scores and disease severity in patients based on the BODE criterion (r=0.65, P<0.001) (Figure 2).
Figure 2.
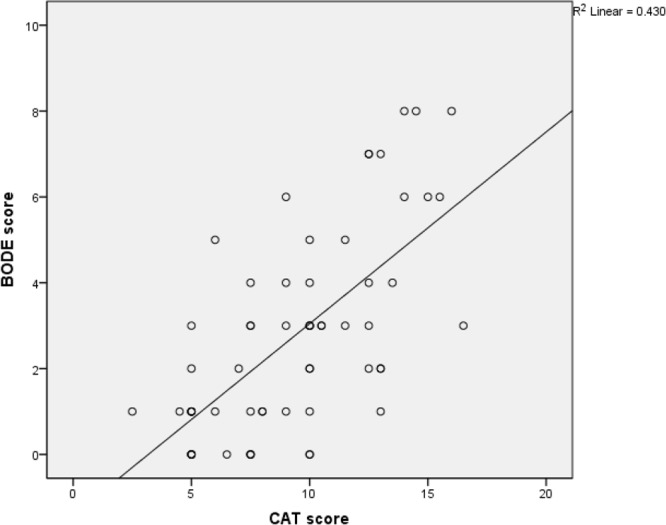
The relationship between BODE score and CAT groups
The relationship between hs-CRP and BMI was examined. ANOVA did not reveal any significant relationship between hs-CRP and low (less than 21) / high (≥ 21) BMI in patients (P=0.16) (Table 3). Similarly, the relationship between CAT score and BMI was not statistically significant (r=−0.01, P=0.89).
Table 3.
The relationship of hs-CRP level and each conducted test
| Variables | Patients (No.) | Mean hs-CRP ±SD | P Value* |
|---|---|---|---|
|
Subjects 6MWD/meters | |||
| First group ≥ 350 | 34 | 3.50±4.55 | 0.008 |
| Second group 250–349 | 9 | 5.96±8.03 | |
| Third group 150–249 | 9 | 2.95±3.73 | |
| Fourth group < 150 | 8 | 11.53±9.71 | |
| BODE score | |||
| 1th Quartile | 30 | 3.50±4.87 | 0.03 |
| 2th Quartile | 17 | 3.87±5.88 | |
| 3th Quartile | 7 | 7.91±7.15 | |
| 4th Quartile | 6 | 10.88±10.53 | |
shows between-group differences
The mean±SD of FEV1/FVC% and FEV1% predicted was 63.21±7.53 and 56.71±18.22, respectively in patients with COPD. The correlation analysis conducted on hs-CRP and FEV1 did not indicate any significant relationship (r=−0.17, P=0.17). However, the correlation coefficient indicated a significant relationship between FEV1 and CAT scores (r=−0.49, P<0.001).
There was also a significant relationship between CAT scores and MMRC amounts (P<0.001). The correlation between hs-CRP and MMRC did not reach significance (P=0.06).
The correlation between hs-CRP and 6MWD was statistically significant (r=−0.29, P=0.02), and also there was a significant relationship between CAT scores and 6MWD (r=−0.47, P<0.001).
The examination of the correlation of hs-CRP level with SpO2 did not display any significant relationship (r=−0.09, P=0.47). Whereas, CAT scores and SpO2 values were significantly correlated (r= −0.45, P<0.001).
The frequency of exacerbations (number of hospitalizations per year) did not indicate any statistically significant relationship with hs-CRP levels (r=0.10 and P=0.42) but had a statistically significant correlation with CAT scores of the subjects (r= 0.30, P<0.017). Tables 2 and 3 display hs-CRP and CAT score relationships with disease severity variables. The analysis also showed a weak correlation between serum hs-CRP level and CAT score (r=0.25, P=0.05).
Table 2.
The relationship of serum hs-CRP and CAT score with variables
| Variable | CAT | Hs-CRP | ||
|---|---|---|---|---|
| P-value | Correlation | P-value | Correlation | |
| BODE | 0.33 | 0.008 | 0.65 | <0.001 |
| FEV1 | −0.17 | 0.17 | −0.49 | <0.001 |
| 6MWD | −0.29 | 0.02 | −0.47 | <0.001 |
| SpO2 | −0.09 | 0.47 | −0.45 | <0.001 |
| Exacerbation frequency in the past year | 0.10 | 0.42 | 0.30 | 0.017 |
DISCUSSION
The results of our study demonstrated a significant relationship between the CAT scores and exacerbation frequency in the past year. CAT as an instrument, which evaluates severity of impact of disease on quality of life, can be used as a tool for evaluating multiple effects of disease on different aspects of life such as sleep, activity, energy and dyspnea. Also, CAT can be helpful for assessing the effectiveness of treatment during the course of disease and progression and severity of disease in COPD patients.
Furthermore, in this study a significant relationship was found between the CAT scores and FEV1. This is in line with the results of a study by Ghobadi et al (21). Another study by Jones, however, found weak correlations between CAT scores and FEV1 values (12).
There was a significant difference in the hs-CRP levels between the two groups. It means that the level of hs-CRP in patients with COPD was higher than that in the control group. Gan and co-workers found elevated levels of hs-CRP in COPD patients (5).
However, our study did not show any significant relationship between hs-CRP level and the number of exacerbations. This is in line with the results of studies by Halvani et al. and Pinto-Plata et al. that found no relationship between the exacerbation rate and hs-CRP level (22, 23). A study by Takemura et al. in Japan found a significant relationship between hs-CRP level and number of exacerbations (24). Likewise, Alavi et al, (2011) in Guilan reported hs-CRP as a predictor of exacerbation frequency (25).
Lack of a relationship between hs-CRP level and frequency of exacerbations, as well as the inability of hs-CRP for prediction of disease exacerbation according to CAT confirm our proposed hypothesis that serum level of hs-CRP, as an inflammatory marker, alone cannot reflect the effect of disease on various organs in patients with COPD. There are also other factors that play a role in development of symptoms. Existence of a relationship between exacerbation periods and CAT supported our hypothesis.
Hs-CRP is an inflammatory factor, which is released by hepatocytes and its elevation can be a sign of severity of systemic inflammation. Some researchers showed a correlation between hs-CRP level and FEV1 (25–27). However in the present study there was no significant correlation between hs-CRP and FEV1. In our opinion, the serum hs-CRP does not adequately reflect all the pulmonary and extra-pulmonary manifestations of disease. For example, in this study the serum level of hs-CRP did not show a significant correlation with exacerbation rate.
The patients’ BODE index was also calculated and the gained score was placed in the fourth quartile. The results showed that CAT score and hs-CRP levels in patients had a highly significant relationship with their BODE scores (P<0.001 and P=0.008, respectively).
Furthermore, there was a significant correlation between CAT scores and 6MWD in COPD patients in this study. A significant relationship between 6MWD and hs-CRP level was also found (P=0.02). All the prior studies have found significant relationships between CRP and 6MWD (7). Lee et al. in 2008 conducted a study in Taiwan and assessed the effect of pravastatin on hs-CRP level. They indicted that after 6 months of pharmacotherapy, and retesting of 6MWD and hs-CRP, a significant change was observed in patients’ 6MWD test results, as their hs-CRP level decreased. They concluded that controlling systemic inflammation had a positive effect on patients’ activity (28). The results of the current study manifested that as hs-CRP level increased the amount of muscular activity and walked distance in 6 minutes decreased.
In the current study, the patients’ dyspnea was examined using MMRC scoring system and the patients were categorized into different stages (0–4). The results revealed no statistically significant correlation between hs-CRP and MMRC scores (P=0.06). While, there was a significant correlation between the patients’ CAT and MMRC scores. Also, the Pinto-Plata’s study in 2006 did not find any significant correlation between hs-CRP and MMRC (23).
The patients’ BMI varied from 19–34 and their hs-CRP and BMI were not correlated significantly (P=0.79). Contrary to our expectation that chronic inflammatory disease would result in cachexia and weight loss, studies by Bridevaux et al. and Pinto-Plata et al. showed contrary results and reported higher levels of hs-CRP in higher weights (23, 29). On the other hand, in a study in Ohio, Sahebjami and Sathianpitayakul discovered a correlation between dyspnea severity and lower weights. It can be argued that, as much as CRP level is correlated with the severity of COPD, in severe COPD (with higher levels of CRP), patients become cachectic and thin (1). Lack of a correlation between hs-CRP and BMI in the current study may be a result of presence of a large number of stage II and III patients and low number of stage IV patients.
The majority of the patients were over 60 years old, which is similar/parallel to the characteristic of participants partaken in other studies and indicates that in Iran, like other countries, COPD mostly occurs in elderly men (2).
The mean hs-CRP level in the current study was not significantly different between males and females. Previous studies on this issue have presented contradictory results. For example, Kony et al, in their study revealed that hs-CRP level in females was lower than that in males (30); while another study conducted by Bridevaux et al. exhibited the more frequent increase of hs-CRP level and decrease of FEV1 in females compared to males (29). Although the age of patients under study ranged from 32 to 85, no significant relationship was detected between level of hs-CRP and age of patients.
This study had some limitations. The first limitation was not assessing the serum level of other inflammatory markers such as IL-6 and TNFα to evaluate the correlation of these markers with CAT score, 6MWD, BODE score and airflow obstruction. Secondly, relatively few women were recruited in this study. It probably resulted in under diagnosis of COPD in women.
CONCLUSION
CAT can assess the severity of pulmonary and extra pulmonary symptoms. Moreover, it can measure an individual’s energy and strength, energy level decease, and sleep disorder resulting from COPD and systemic inflammation.
Hs-CRP level mainly shows the quantitative degree of systemic inflammation; while CAT reflects the effect of inflammatory markers on patients’ health status and their organ functions. Assessment of hs-CRP level cannot determine the severity of airways obstruction (FEV1) in patients with COPD. Considering the relationship of the exacerbation rate and other disease severity parameters determined by CAT, it may serve as a good predictor of patient outcome.
Acknowledgements
This study was financially supported by the Research Council of Ardabil University of Medical Sciences.
Conflicts of Interest
The authors declare that there are no conflicts of interest.
REFERENCES
- 1. Sahebjami H, Sathianpitayakul E. Influence of body weight on the severity of dyspnea in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2000; 161 ( 3 Pt 1): 886– 90. [DOI] [PubMed] [Google Scholar]
- 2. Zachariades AG, Zachariadou T, Adamide T, Anagnostopoulou U, Georgiou A, Gourgoulianis KI. Prevalence of chronic obstructive pulmonary disease in Cyprus: a population-based study. COPD 2012; 9 ( 3): 259– 67. [DOI] [PubMed] [Google Scholar]
- 3. Murray CJ, Lopez AD. Mortality by cause for eight regions of the world: Global Burden of Disease Study. Lancet 1997; 349 ( 9061): 1269– 76. [DOI] [PubMed] [Google Scholar]
- 4. Janson C, Marks G, Buist S, Gnatiuc L, Gislason T, McBurnie MA, et al. The impact of COPD on health status: findings from the BOLD study. Eur Respir J 2013; 42 ( 6): 1472– 83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gan WQ, Man SF, Sin DD. The interactions between cigarette smoking and reduced lung function on systemic inflammation. Chest 2005; 127 ( 2): 558– 64. [DOI] [PubMed] [Google Scholar]
- 6. Hooper R, Burney P, Vollmer WM, McBurnie MA, Gislason T, Tan WC, et al. Risk factors for COPD spirometrically defined from the lower limit of normal in the BOLD project. Eur Respir J 2012; 39 ( 6): 1343– 53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. de Torres JP, Cordoba-Lanus E, López-Aguilar C, Muros de Fuentes M, Montejo de Garcini A, Aguirre-Jaime A, et al. C-reactive protein levels and clinically important predictive outcomes in stable COPD patients. Eur Respir J 2006; 27 ( 5): 902– 7. [DOI] [PubMed] [Google Scholar]
- 8. Agustí AG, Noguera A, Sauleda J, Sala E, Pons J, Busquets X. Systemic effects of chronic obstructive pulmonary disease. Eur Respir J 2003; 21 ( 2): 347– 60. [DOI] [PubMed] [Google Scholar]
- 9. Maltais F, Simard AA, Simard C, Jobin J, Desgagnés P, LeBlanc P. Oxidative capacity of the skeletal muscle and lactic acid kinetics during exercise in normal subjects and in patients with COPD. Am J Respir Crit Care Med 1996; 153 ( 1): 288– 93. [DOI] [PubMed] [Google Scholar]
- 10. Pinto-Plata V, Toso J, Lee K, Park D, Bilello J, Mullerova H, De Souza MM, Vessey R, Celli B. Profiling serum biomarkers in patients with COPD: associations with clinical parameters. Thorax 2007; 62 ( 7): 595– 601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sin DD, Man SF. Why are patients with chronic obstructive pulmonary disease at increased risk of cardiovascular diseases? The potential role of systemic inflammation in chronic obstructive pulmonary disease. Circulation 2003; 107 ( 11): 1514– 9. [DOI] [PubMed] [Google Scholar]
- 12. Jones PW, Brusselle G, Dal Negro RW, Ferrer M, Kardos P, Levy ML, et al. Properties of the COPD assessment test in a cross-sectional European study. Eur Respir J 2011; 38 ( 1): 29– 35. [DOI] [PubMed] [Google Scholar]
- 13. Jones PW, Harding G, Berry P, Wiklund I, Chen WH, Kline Leidy N. Development and first validation of the COPD Assessment Test. Eur Respir J 2009; 34 ( 3): 648– 54. [DOI] [PubMed] [Google Scholar]
- 14. Lee SD, Huang MS, Kang J, Lin CH, Park MJ, Oh YM, Kwon N, et al. The COPD assessment test (CAT) assists prediction of COPD exacerbations in high-risk patients. Respir Med 2014; 108 ( 4): 600– 8. [DOI] [PubMed] [Google Scholar]
- 15. Jones PW, Adamek L, Nadeau G, Banik N. Comparisons of health status scores with MRC grades in COPD: implications for the GOLD 2011 classification. Eur Respir J 2013; 42 ( 3): 647– 54. [DOI] [PubMed] [Google Scholar]
- 16. Qaseem A, Wilt TJ, Weinberger SE, Hanania NA, Criner G, van der Molen T, et al. Diagnosis and management of stable chronic obstructive pulmonary disease: a clinical practice guideline update from the American College of Physicians, American College of Chest Physicians, American Thoracic Society, and European Respiratory Society. Ann Intern Med 2011; 155 ( 3): 179– 91. [DOI] [PubMed] [Google Scholar]
- 17. Celli BR, Cote CG, Marin JM, Casanova C, Montes de Oca M, Mendez RA, et al. The body-mass index, airflow obstruction, dyspnea, and exercise capacity index in chronic obstructive pulmonary disease. N Engl J Med 2004; 350 ( 10): 1005– 12. [DOI] [PubMed] [Google Scholar]
- 18. Rabe KF, Hurd S, Anzueto A, Barnes PJ, Buist SA, Calverley P, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med 2007; 176 ( 6): 532– 55. [DOI] [PubMed] [Google Scholar]
- 19. Jones P, Harding G, Wiklund I, Berry P, Leidy N. Improving the process and outcome of care in COPD: development of a standardised assessment tool. Prim Care Respir J 2009; 18 ( 3): 208– 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. CAT development steering group : COPD assessment test-healthcare professional user guide. http://www.catestonline.org
- 21. Ghobadi H, Ahari SS, Kameli A, Lari SM. The Relationship between COPD Assessment Test (CAT) Scores and Severity of Airflow Obstruction in Stable COPD Patients. Tanaffos 2012; 11 ( 2): 22– 6. [PMC free article] [PubMed] [Google Scholar]
- 22. Halvani A, Nadooshan HH, Shoraki FK, Nasiriani K. Serum C-Reactive Protein Level in COPD Patients and Normal Population. Tanaffos 2007; 6: 51– 55. [Google Scholar]
- 23. Pinto-Plata VM, Müllerova H, Toso JF, Feudjo-Tepie M, Soriano JB, Vessey RS, et al. C-reactive protein in patients with COPD, control smokers and non-smokers. Thorax 2006; 61 ( 1): 23– 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Takemura M, Matsumoto H, Niimi A, Ueda T, Matsuoka H, Yamaguchi M, et al. High sensitivity C-reactive protein in asthma. Eur Respir J 2006; 27 ( 5): 908– 12. [DOI] [PubMed] [Google Scholar]
- 25. Alavi SA, Soati F, Forghanparast K, Amani H. HsCRP in Patients with Acute Exacerbation of Chronic Obstructive Pulmonary Disease. Iran Red Crescent Med J 2011; 13 ( 10): 713– 8. [PMC free article] [PubMed] [Google Scholar]
- 26. Tkacova R, Kluchova Z, Joppa P, Petrasova D, Molcanyiova A. Systemic inflammation and systemic oxidative stress in patients with acute exacerbations of COPD. Respir Med 2007; 101 ( 8): 1670– 6. [DOI] [PubMed] [Google Scholar]
- 27. de Torres JP, Pinto-Plata V, Casanova C, Mullerova H, Córdoba-Lanús E, Muros de Fuentes M, et al. C-reactive protein levels and survival in patients with moderate to very severe COPD. Chest 2008; 133 ( 6): 1336– 43. [DOI] [PubMed] [Google Scholar]
- 28. Lee TM, Lin MS, Chang NC. Usefulness of C-reactive protein and interleukin-6 as predictors of outcomes in patients with chronic obstructive pulmonary disease receiving pravastatin. Am J Cardiol 2008; 101 ( 4): 530– 5. [DOI] [PubMed] [Google Scholar]
- 29. Bridevaux PO, Gerbase MW, Schindler C, Dietrich DF, Curjuric I, Dratva J, et al. Sex-specific effect of body weight gain on systemic inflammation in subjects with COPD: results from the SAPALDIA cohort study 2. Eur Respir J 2009; 34 ( 2): 332– 9. [DOI] [PubMed] [Google Scholar]
- 30. Kony S, Zureik M, Driss F, Neukirch C, Leynaert B, Neukirch F. Association of bronchial hyperresponsiveness and lung function with C-reactive protein (CRP): a population based study. Thorax 2004; 59 ( 10): 892– 6. [DOI] [PMC free article] [PubMed] [Google Scholar]


