Abstract
Objectives
There is a dearth of prospective evidence regarding cancer of the major salivary glands. Outcomes and management of major salivary gland are based largely on retrospective series spanning many decades and changes in surgical, radiation, imaging and systemic therapy strategies and technique. We sought to report contemporary patterns of relapse and prognostic factors for major salivary gland cancer.
Materials and Methods
112 patients with major salivary gland cancers underwent resection with or without adjuvant therapy between January 1997 and September 2010. Outcomes were documented with follow-up until December 2014. Survival was calculated by the Kaplan-Meier method. Log-rank test and Cox proportional hazards regression were performed with locoregional control (LRC), distant control (DC) and overall survival (OS) as the primary outcome variables.
Results
Median follow-up was 55.1 months. Rates of LRC for stage I/II and III/IV at five years were 95.7% and 61.9% respectively. Rates of DC at five years for stage I/II and III/IV were 93% and 56.9% respectively. Multivariate analysis identified larger tumor size, clinical nerve involvement and in parotid cancers, advanced T stage, no adjuvant radiation, and older age at diagnosis to be associated with increased risk of locoregional recurrence (all p<0.05). Distant metastasis was associated with sublingual site, degree of clinical nerve involvement, high grade, tumor size and in parotid tumors additionally deep lobe involvement on multivariate analysis (all p<0.05).
Conclusion
Several prognostic factors were identified that may help guide decisions regarding adjuvant therapy. DM remains a significant concern in the management of this disease.
Keywords: Head and neck cancer, salivary gland cancer, radiation therapy
Introduction
Malignancies of the major salivary glands - parotid, submandibular and sublingual - represent a diverse subset of head and neck cancers. In all, they represent only 3-6.5% of head and neck cancers[1, 2]. The overall annual incidence of this disease is 1.195/100,000[3]. The relative paucity and diverse biology of salivary gland cancer have made progress in their management challenging. Surgery remains the cornerstone for management of this disease site. Although it has never been evaluated in the setting of a randomized clinical trial, postoperative radiation therapy has been increasingly used for patients with recognized high-risk features, including high grade histologies, size greater than 4 cm, extraparenchymal extension, close or positive margins, lymph node involvement, bone involvement, and perineural invasion among others[4, 5]. Local therapies alone are not sufficient in high-risk patients[6-8], and appropriate patient selection for systemic therapy represents another clinical challenge. Unlike many other head and neck cancers, outcomes in salivary cancer have not improved appreciably over time. Unfortunately patient heterogeneity and paucity have precluded prospective, randomized trials that could guide us in the integration of systemic therapy into the therapeutic armamentarium, and we must rely on retrospective data.
Over the past two decades the management of head and neck cancer has evolved significantly. Preoperative imaging, surgical, and radiation techniques have all become more refined. Intensity modulated radiation therapy, aggressive facial nerve preservation and microsurgical free tissue reconstructions have become de facto standards of care[9]. In the present study, we report an updated experience relevant to current standards of practice. With changes in management in head and neck cancer we will particularly focus on prognostic factors for disease recurrence and overall patterns of recurrence within a modern cohort of patients.
Materials and Methods
After obtaining Emory Institutional Review Board approval, we reviewed the records of 112 consecutive patients over the age of 18 with malignancies of the major salivary glands. Patients with suspected squamous cell carcinoma skin metastases, metastatic disease at presentation and no documented follow-up visits were excluded. All patients underwent surgical resection at Emory University between January 1997 and September 2010. Outcomes were documented with follow-up until December 1st 2014. Diagnosis was made by an attending head and neck pathologist. Initial staging for all patients included a detailed physical exam and computed tomography; many additionally underwent positron emission tomography (PET) and/or magnetic resonance imaging (MRI). Deaths were verified either by medical records or the Social Security Death Index.
All patients had primary surgery with curative intent as their initial treatment. Adjuvant chemotherapy and radiation usage was dictated at the discretion of the treating physicians, and the decision to give adjuvant therapy was generally driven by high risk factors, e.g. close or positive margins, T3-T4 tumor, perineural invasion, high grade and/or positive lymph nodes. Radiation was performed both at Emory University and outside facilities. All tumors were prospectively or retrospectively pathologically staged according to the 7th edition of the American Joint Committee on Cancer's staging system[10].
Statistical Analysis
Descriptive statistics were reported for patient and disease characteristics. Kaplan-Meier method was used to produce survival estimates of locoregional recurrence (LRR), distant metastatic (DM) recurrence, and overall survival (OS) along with 5-year event free rate and its 95% confidence interval. Time to events was measured from the date of initial surgical resection. Patients were censored for LRR at time of DM, death or last clinical follow-up. Patients were censored for DM at time of death or last clinical follow-up. Univariate and multivariate survival analysis were carried out with a Cox proportional hazards model. The univariate association with histology and clinical nerve involvement was carried by ANOVA for numerical covariates; and Chi-Square test or Fisher's exact test for categorical covariates, where appropriate. For the multivariate analysis, the initial list of variables contained those with p < 0.2 in the univariate analysis as well as clinical relevant variables, such as adjuvant radiation usage in the LRR analysis, with the final model determined by backward elimination using a removal criterion of p > 0.2. All analyses were done using SAS 9.3 (SAS Institute, Inc., Cary, North Carolina) and SAS macros developed by Biostatistics and Bioinformatics Shared Resource at Winship Cancer Institute with a significance level of 0.05 [11].
Results
One hundred twelve eligible patients were identified: parotid (n=97 [86.6%]), submandibular (n=11 [9.8%]), and sublingual tumors (n=4 [3.6%]). The median follow-up was 55.1 months, and the median age at diagnosis was 56 years (range: 18-91) Adenocarcinoma was the most common histology encountered (n=39 [34.8%]). Full patient, disease, and treatment characteristics are listed in Table 1.
Table 1.
Patient and Treatment Characteristic
| Characteristic | n=112 (%) | |
|---|---|---|
| Primary Site | Submandibular | 11 (9.8) |
| Parotid | 97 (86.6) | |
| Sublingual | 4 (3.6) | |
| Parotid Lobe | Superficial | 59 (64.8) |
| Deep | 32 (35.2) | |
| Missing | 6 | |
| Gender | Male | 47 (42.0) |
| Female | 65 (58.0) | |
| Age | <55 | 65 (58.0) |
| ≥55 | 46 (42.0) | |
| Tumor Size | <2 cm | 31 (28.4) |
| 2-3 cm | 33 (30.3) | |
| ≥3 cm | 45 (41.3) | |
| Missing | 3 | |
| Lymph Node Dissection | No | 66 (59.5) |
| Yes | 45 (40.5) | |
| Degree of Clinical Nerve Involvement | None | 86 (76.9) |
| Partial | 15 (13.4) | |
| Complete | 11 (9.8) | |
| T Stage | 1 or 2 | 71 (63.4) |
| 3 or 4 | 41 (36.6) | |
| Nodal Involvement | Yes | 34 (30.4) |
| No | 78 (69.6) | |
| Stage | I/II | 63 (56.3) |
| III/IV | 49 (43.8) | |
| Grade | Low or Intermediate | 65 (58.0) |
| High | 47 (42.0) | |
| Positive Margin | Yes | 41 (36.6) |
| No | 71 (63.4) | |
| Bone Invasion | Yes | 4 (3.6) |
| No | 108 (96.4) | |
| Adjuvant Radiation | Yes | 61 (54.5) |
| No | 51 (45.5) | |
| Adjuvant Chemotherapy | No | 105 (93.8) |
| Yes | 7 (6.3) | |
| Histology | Adenocarcinoma | 39 (34.8) |
| Acinic Cell Carcinoma | 22 (19.6) | |
| Adenoid Cystic Carcinoma | 10 (8.9) | |
| Mucoepidermoid Carcinoma | 28 (25.0) | |
| CEP | 9 (8.0) | |
| Salivary Duct Carcinoma | 2 (1.8) | |
| Basal Cell Adenocarcinoma | 1 (0.9) | |
| Basaloid Carcinoma | 1 (0.9) | |
| Radiation Dose (median, Gy) | 63 (53-72) | |
| Radiation field including neck | No | 7 (31.8) |
| Ipsilateral | 13 (59.1) | |
| Bilateral | 2 (9.1) | |
| Missing | 29 | |
Adenocarcinoma (56.4%), acinic cell (22.7%) and mucoepidermoid were the most likely histologies to present with pathologic nodal involvement (p<0.001). Adenocarcinoma (61.6%) and adenoid cystic (30%) were most likely to present with advanced T stage (p=0.002). Lymphovascular space invasion (LVSI) was most common in adenocarcinoma (46%) and adenoid cystic carcinomas (40%) (p<0.001). Clinical nerve involvement was most common in adenocarcinomas (41%) (p=0.018). Patients with clinical nerve involvement were more likely to have high-grade disease (p<0.001). There was no association between histology and the presence of close or positive margins following resection.
At the time of primary surgical resection, 45 (40.5%) patients underwent elective or therapeutic lymph node dissection as indicated by clinical or radiographic findings at time of diagnosis. 61 (54.5%) patients received adjuvant radiation, and 7 (6.3%) patients received concurrent chemotherapy (carboplatin and paclitaxel or cisplatin) (Figure 1). Those receiving radiation by stage were - Stage I 10/36 (28%), stage II 12/27 (44%), stage III 11/15 (73%) and stage IV 28/34 (82%). All patients with available records were treated using intensity modulated radiation therapy. All patients who received adjuvant chemotherapy were stage IV, which represented 7/34 (14%) of stage IV patients.
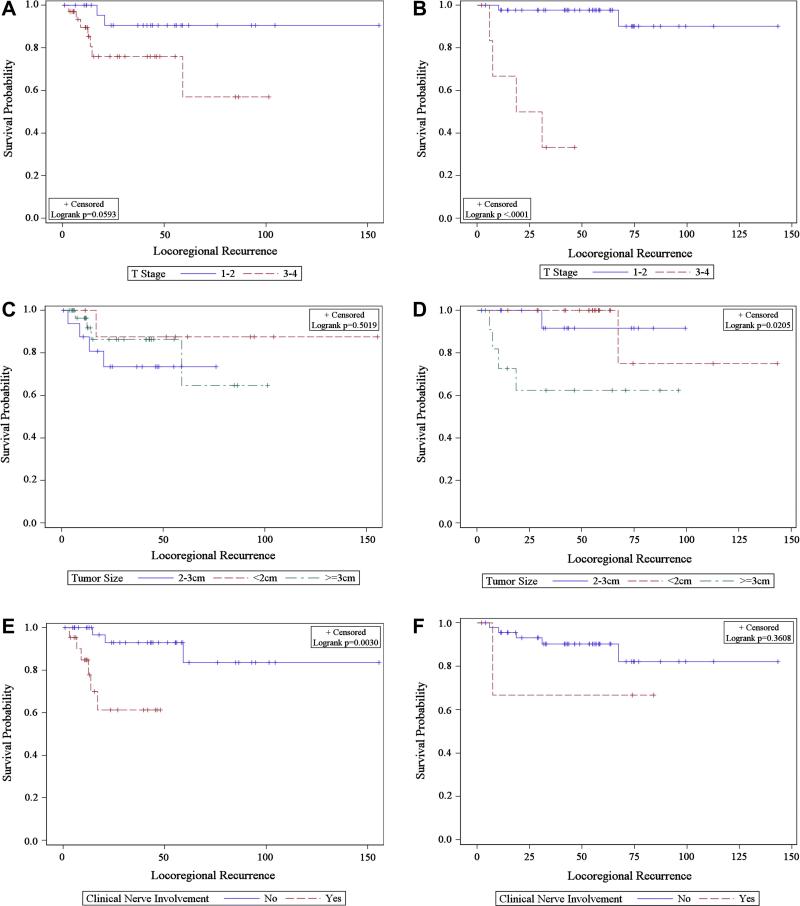
Figure 1.
Kaplan-Meier estimate of locoregional recurrence in T1/2 vs T3/4 treated A) with radiation B) without radiation, in <2, 2-3 and ≥3 cm tumors treated C) with radiation D) without radiation and in patients with and without clinical nerve involvement treated E) with radiation F) without radiation.
Locoregional Control
Actuarial rates of locoregional control at 5 years were 94.6% and 60.4% for stages I/II and III/IV, respectively. Of the 15 patients who had LRR, 7/15 patients had a local failure component, and 11/15 had regional components to their recurrence.
Univariate analysis identified deep parotid location, male gender, lymph node dissection, facial nerve involvement, advanced T stage, nodal involvement, stage III/IV high tumor grade, extracapsular extension, adjuvant chemotherapy, positive margin LVSI, older age and larger tumor size to be associated with inferior locoregional control (Table 2). Notably, close margin (<5 mm), perineural invasion, and bone invasion were not associated with LRR.
Table 2.
Univariate Analysis by Prognostic Factors (All Patients)
| Locoregional Recurrence | Distant Metastasis | |
|---|---|---|
| Prognostic Factor | p | p |
| Primary Site | 0.501 | 0.014 |
| Deep Parotid Lobe | 0.044 | <0.001 |
| Male Gender | 0.017 | 0.059 |
| Smoking History | 0.803 | 0.156 |
| Lymph Node Dissection | 0.032 | <0.001 |
| Clinical Nerve Involvement | 0.003 | <0.001 |
| T Stage | <0.001 | <0.001 |
| N Stage | 0.001 | <0.001 |
| Stage | 0.004 | <0.001 |
| Grade | 0.038 | <0.001 |
| Extracapsular extension | 0.010 | <0.001 |
| Close Margin (<0.5 cm) | 0.440 | 0.801 |
| Positive Margin | 0.011 | 0.004 |
| Bone Invasion | 0.142 | 0.249 |
| Histology | 0.426 | <0.001 |
| LVSI | 0.012 | <0.001 |
| PNI | 0.120 | <0.001 |
| Adjuvant Radiation | 0.328 | <0.001 |
| Adjuvant Chemotherapy | <0.001 | 0.082 |
| Surgery year | 0.806 | 0.966 |
| Age at Diagnosis (continuous) | 0.007 | 0.022 |
| Tumor Size (continuous) | <0.001 | <0.001 |
CM- centimeter, T-tumor, N-node, LVSI-lymphovascular space invasion, PNI-perineural invasion. Bolded values denote statistical significance.
On multivariate analysis, clinical nerve involvement, age at diagnosis and tumor size remained significant in all patients as predictors of LRR. In parotid tumors additionally, advanced T stage, adjuvant radiation (RT) and age at diagnosis as a continuous variable were significant (Table 3,4). RT did appear to abrogate the risk conferred by some tumor related features, as there was no significant difference in LRR with T3/4 vs T1/2, tumor size (<2 v 2-3 v >3 cm), positive vs negative margins in those patients receiving adjuvant RT. However RT did not lower risk of LRR in those with clinical nerve involvement (Figure 1).
Table 3.
Multivariate Analysis by Prognostic Factors (All Patients)
| Locoregional Recurrence | Distant Metastasis | ||||
|---|---|---|---|---|---|
| HR 95% CI | p | HR 95% CI | p | ||
| Adjuvant Radiation | No vs Yes | 3.08 (0.72-13.27) | 0.131 | 0.21 (1.31-4.22) | 0.172 |
| Tumor Size (cm) | 1.57 (1.18-2.09) | 0.002 | 2.35 (1.31-4.22) | 0.004 | |
| Age at Diagnosis | 1.05 (1.01-1.09) | 0.027 | |||
| Gender | Male vs Female | 3.04 (0.80-11.60) | 0.104 | 3.12 (0.91-10.65) | 0.069 |
| CNI | No vs Yes | 0.19 (0.05-0.78) | 0.021 | ||
| Positive Margin | No vs Yes | 0.42 (0.12-1.43) | 0.165 | ||
| Bone Invasion | No vs Yes | 0.10 (0.01-1.04) | 0.054 | ||
| Primary Site | Submandibular | 0.08 (0.01-0.94) | 0.045 | ||
| Parotid | 0.07 (0.01-0.40) | 0.003 | |||
| Sublingual | Reference | - | |||
| Grade | Low/Intermediate vs High | 0.19(0.04-0.94) | 0.041 | ||
| Degree of CNI | None | 0.09(0.02-0.32) | <0.001 | ||
| Partial | 0.08(0.01-0.46) | 0.005 | |||
| Complete | Reference | - | |||
| T stage | T1/2 vs T3/4 | 6.81 (1.08-42.83) | 0.041 | ||
| Histology | Adenocarcinoma | 7.14 (0.73-70.01) | 0.092 | ||
| Acinic Cell | 1.89 (0.10-36.37) | 0.673 | |||
| Adenoid Cystic | 9.09 (0.44-188.54) | 0.154 | |||
| Mucoepidermoid | 0.41 (0.01-23.90) | 0.670 | |||
| Other | Reference | - | |||
CM- centimeter, CNI-clinical nerve involvement. Bolded values denote statistical significance.
Table 4.
Multivariate Analysis by Prognostic Factors (Parotid Alone)
| Locoregional Recurrence | Distant Metastasis | ||||
|---|---|---|---|---|---|
| HR 95% CI | p | HR 95% CI | p | ||
| T Stage | T1/2 vs T3/4 | 0.05 (0.01-0.38) | 0.004 | 4.23 (0.58-30.89) | 0.156 |
| Positive Margin | No vs Yes | 0.32 (0.08-1.23) | 0.098 | ||
| Bone Invasion | No vs Yes | 0.06 (0.00-1.04) | 0.053 | ||
| Adjuvant Radiation | No vs Yes | 13.80 (2.11-90.47) | 0.006 | ||
| Degree of CNI | None vs Complete | 0.06 (0.01-0.66) | 0.021 | ||
| Partial vs Complete | 0.18 (0.01-2.23) | 0.182 | |||
| Parotid Gland Location | Superficial vs Deep | 0.08 (0.02-0.38) | 0.001 | ||
| Grade | Low/Intermediate vs High | 0.15 (0.03-0.72) | 0.018 | ||
| PNI | No vs Yes | 6.53 (0.74-57.58) | 0.091 | 0.04 (0.01-0.29) | 0.001 |
| Age at Diagnosis | 1.08 (1.02-1.13) | 0.007 | 1.04 (0.99-1.08) | 0.149 | |
PNI-perineural invasion, T-tumor, CNI-clinical nerve involvement. Bolded values denote statistical significance.
Univariate analysis was performed in the subgroup of patients (n=51) who did not receive radiation to identify factors associated with risk of LRR. Superficial parotid location (p=0.018 HR 0.11 95%CI 0.02-0.68), T stage 1/2 (p=0.001 HR 0.03 95%CI 0.00-0.24), N stage 0 (p=0.042 HR 0.17 95%CI 0.03-0.94), negative margin (p=0.034 HR 0.10 95%CI 0.01-0.84) were all significantly associated with a decreased risk of LRR. Older age at diagnosis (years) (p=0.023 HR 1.09 95%CI 1.01-1.17) and larger tumor size (cm) (p=0.048 HR 1.56 95%CI 1.00-2.41) were associated with an increased risk of LRR.
Distant Metastasis
Actuarial rates of distant control at 5 years were 93.0% and 56.9% for stages I/II and III/IV respectively. No patient developed a DM more than 46 months after surgery.
Sublingual gland primary, deep parotid lobe involvement, tumor size, lymph node dissection, degree of facial nerve involvement, advanced T stage, nodal involvement, stage III/IV, high grade, extracapsular extension, surgical margin involvement, adenocarcinoma histology, the use of adjuvant RT, LVSI, perineural invasion (PNI) and age and tumor size as continuous covariates were all associated with increased risk of DM in univariate analysis (Table 2).
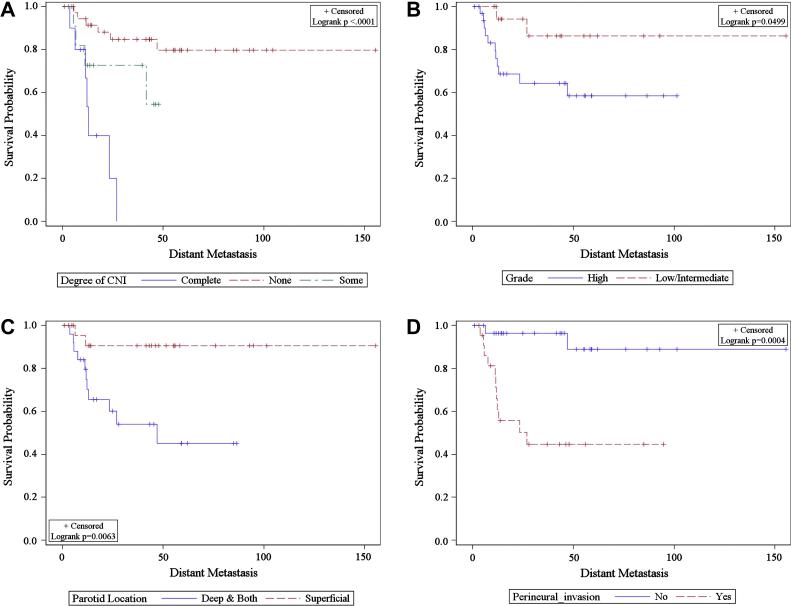
On multivariate analysis of all patients sublingual primary tumors, complete facial nerve paralysis, high grade, advanced T stage and tumor size as a continuous variable all remained associated with risk of DM. Three of four patients with sublingual primaries developed DM. Examining parotid patients alone deep parotid lobe involvement, high grade and PNI remained significant independent predictors of DM. Notably, nodal involvement was not independently associated with DM. (Tables 3,4)
There was no association between the use of RT and risk of DM in multivariate analysis. Radiation did not appear to mitigate the risk conferred by clinical nerve involvement, tumor grade, perineural invasion, parotid location and increased tumor size on the development of DM (Figure 2). Of the seven patients who received adjuvant concurrent chemoradiation, two developed DM and four developed LRR.
Figure 2.
Kaplan-Meier estimate of distant metastasis by A) Clinical nerve involvement B) Grade C) Parotid lobe location D) Perineural invasion
Overall Survival
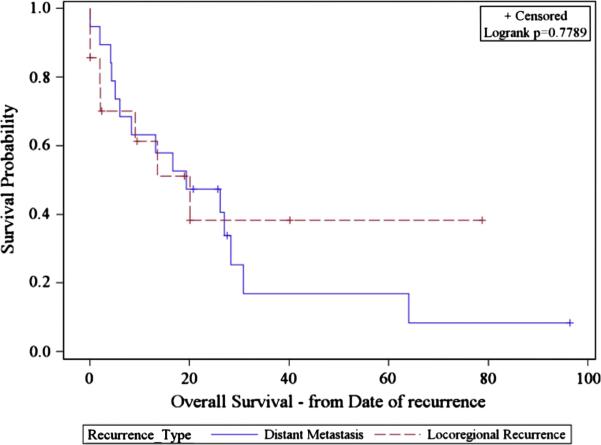
The actuarial rate of OS at 5 years was 76%: 94 and 50.6% for stage I/II and III/IV respectively. In patients who developed DM, subsequent survival was 47.4% at 2 years and 16.9% at five years. In patients who developed LRR, subsequent survival was 38.4% at two years and five years (Figure 3).
Figure 3.
Kaplan-Meier estimate of survival after locoregional and distant metastasis
Multivariate analysis identified male gender (p=0.001 HR 4.29 95%CI 1.75-10.48) alone as associated with worse OS. In parotid patients male gender (p=0.030 HR3.03 95%CI 1.12-8.24), complete CNI (p=0.008 HR 4.8 95%CI 1.5-16.7) and advanced T stage (p=0.032 HR 4.7 95%CI 1.2-20.0) were associated with worse OS.
Discussion
Cancer of the major salivary glands represents a heterogeneous disease with a low overall incidence. These factors have partially contributed to our inability to make substantive improvement in outcomes related to this disease. Notably, there is a lack of randomized or prospective evidence guiding practice. Improvements that have been seen in head and neck cancer outcomes as a whole are not reflected within the salivary cancer population. The findings in our contemporary series, which spans a relatively short treatment period, closely follow the studies that precede it regardless of era[12-14]. Overall survival at five years in our series was 74.7%. Overall rates of distant and locoregional control are similar to previously published series at 79% and 82.6% respectively. Survival following distant recurrence is exceedingly poor.
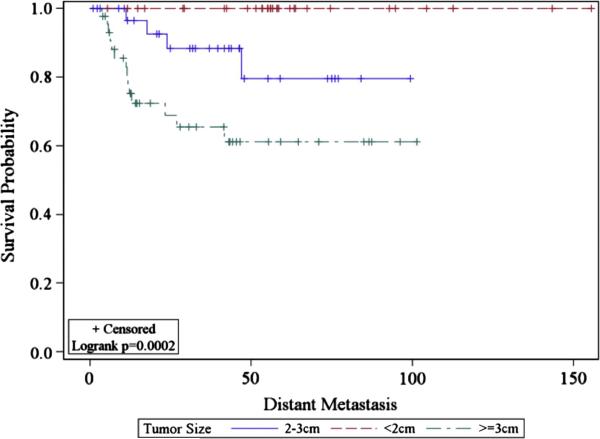
Our experience identifies LRR to be associated with tumor size and age at diagnosis and additionally in parotid primary tumors, advanced T stage and use of radiation. Sublingual primaries, complete facial nerve paralysis, tumor size, high grade, advanced T stage and in parotid patients additionally deep lobe involvement and perineural invasion were all associated with increased risk of DM on multivariable analysis. Patients with tumors ≥3 cm in size appeared to fare particularly poorly (Figure 4). Neither histologic subtype nor nodal involvement remained significant for recurrence on multivariate analysis. This is the first series to identify sublingual primary and deep parotid lobe involvement as a predictor of DM. Prior series have identified submandibular primary as a predictor of DM[15]. Both submandibular and sublingual tumors presumably are at higher risk for DM due to the rich lymphatic network that traverses their space. Similarly deep parotid lobe cancers are more proximal to the deep parotid and deep cervical lymphatics, which speculatively may play a role in increased DM. These findings are valuable as we have little evidence to base the usage of adjuvant therapy. A comparison of our series and prognostic factors identified on multivariate analysis in previously published series is detailed in Table 5.
Figure 4.
Kaplan-Meier estimate of risk of distant metastasis stratified by tumor size
Table 5.
Significant Prognostic Factors on Multivariate Analysis
| Institution (years) |
Number - sites |
Prognostic factors on Multivariate Analysis |
||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| T Stage |
N stage |
Stage | Grade | RT | Margin | Histology | CNI | Bone invasion |
Age | Gender | Subsite | PNI | Tumor size |
Deep parotid lobe |
RT | |||
| Terhaard[14] | Dutch (1985-94) | 565 – P, S, SL, M | LRR, DM, OS | LRR, DM, OS | LRR | DM | OS | OS | LRR | LRR, DM | ||||||||
| Mendenhall[12] | Florida (1964-2003) | 224 - P, S, SL, M | LRR | OS | LRR, DM, OS | LRR | ||||||||||||
| Garden[13] | MDACC (1961-1990) | 166 - P | OS | LRR | ||||||||||||||
| Chen[28] | UCSF (1960-2004) | 207 – P, S, SL | LRR | LRR | LRR | LRR | ||||||||||||
| Pohar[29] | SUNY/Kansas (1960-2000) | 163 -P | LRR | OS | LRR | LRR, OS | ||||||||||||
| Current | Emory (1997-2010) | 112 – P, S, SL | DM, LRR, OS | DM | LRR | LRR DM, OS | LRR | OS | DM | DM | LRR, DM | DM | LRR | |||||
DM – distant metastasis, LRR- locoregional recurrence, PFS – progression free survival, OS- overall survival, P-parotid, S- submandibular, SL-sublingual, CNI –clinical nerve involvement, PNI-perineural invasion
The absolute indications for postoperative RT in salivary cancer remain controversial, as no prospective evidence exists to guide practice. In our series use of RT was strongly associated with an improved risk of LRR in parotid primary cancer with a HR of 13.80 on multivariate analysis. RT did appear to mitigate LRR risk conferred by some adverse prognostic features identified in our study (Figure 1). Several disease and treatment characteristics were identified that increase risk of LRR in patients not receiving RT. These factors may help guide usage of adjuvant RT. When examining the DM risk of adverse prognostic features stratified by the receipt of radiation, there did not appear to be any treatment interaction. In our cohort 17/18 patients who developed DM received adjuvant radiation therapy. It does not appear that the improvement in local control conferred by radiation has any role in preventing DM.
One of the most significant advancements of the past decades in management of head and neck cancer has been the adoption of concurrent chemotherapy in both the definitive and postoperative settings[16-19]. Improvements in local control, overall survival and possibly distant control can be seen in selected squamous cell head and neck cancer patients with high-risk features. There is little evidence, however, that chemotherapy can improve outcomes in salivary cancer. The high rates of DM and poor outcomes following emphasize the need to develop effective systemic therapies.
A recent small series reported results with the use of concurrent chemoradiation in largely high-risk patients (n=22)[20]. These investigators reported excellent 3-year locoregional and distant control of 92 and 83%, respectively. While the results of this study are promising, the small size of the cohort and limited follow-up (median 2.3 years) preclude definitive conclusions regarding the value of chemotherapy in this setting. A similar retrospective series with short follow-up included 12 high-risk patients receiving chemoradiation. They showed that the use of chemoradiation was associated with significantly better local control with no difference in overall survival compared to patients receiving RT alone[21]. Seven patients in our study received adjuvant chemoradiation. This relatively low number limits our ability draw conclusions on the efficacy of added chemotherapy. The use of chemotherapy was not associated with a lower risk of LRR or DM in multivariate analysis. Notably, 5 of 7 high-risk patients who received combined chemoradiation experienced either a distant or locoregional recurrence.
The role of concurrent chemotherapy in the setting of high-risk salivary gland cancer is currently being examined (RTOG 1008: A Randomized Phase II Study of Adjuvant Concurrent Radiation and Chemotherapy Versus Radiation Alone in Resected High-Risk Malignant Salivary Gland Tumors, clinical trial registry: NCT01272037.) The results of this trial will hopefully elucidate the role of concurrent chemotherapy in high-risk patients. The role of full dose adjuvant chemotherapy in the non-metastatic setting requires further study. Traditional chemotherapies in the metastatic setting have had poor response rates from 15-50% with time to relapse typically 5-6 months[7, 22, 23]. Targeted therapies that exploit actionable mutations identified within the various salivary cancer histologies are needed[23]. While trials to date evaluating targeted therapies have also had poor results[24-26], recent studies have identified translocation generated gene fusions and their signaling pathways, present in multiple salivary subtypes. This will hopefully lead to the identification of further novel therapeutic strategies[27].
Limitations of our study primarily involve the retrospective nature of the series with heterogeneity of pathologists, surgical and radiation treatment. We are unable to ascertain the reason why some patients with involved margins and advanced T stage did not receive adjuvant therapy. As well, given the year of diagnosis of included patients the recently described histologic subtype, mammary analog secretory carcinoma, was not identified.
In conclusion, our series identifies prognostic features associated with local and distant disease recurrence in cancer of the major salivary glands treated with current era management that may help guide decisions regarding adjuvant therapy. Adjuvant radiation therapy reduces risk of locoregional failure, however this does not translate to improvement in distant metastatic control. We establish that distant disease recurrence remains a significant concern in the treatment of major salivary gland cancer, and the identification of active systemic agents is paramount in this disease.
Highlights.
Evaluated outcomes of a recent cohort of patients with major salivary gland cancer
Distant metastasis was associated with deep parotid lobe involvement
Radiation abrogated locoregional recurrence risk from associated factors
Radiation did not reduce distant metastasis risk from associated factors
Distant metastasis remains the primary concern in management of this disease
Acknowledgement
Research reported in this publication was supported in part by the Biostatistics and Bioinformatics Shared Resource of Winship Cancer Institute of Emory University and NIH/NCI under award number P30CA138292. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Naresh Jegadeesh had full access to all of the data in the study and takes responsibility for the integrity of the data and accuracy of the data analysis.
Abbreviations
- LRC
locoregional control
- DC
distant control
- LRR
locoregional recurrence
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of interest: None declared
References
- 1.Carvalho AL, Nishimoto IN, Califano JA, Kowalski LP. Trends in incidence and prognosis for head and neck cancer in the United States: a site-specific analysis of the SEER database. International journal of cancer Journal international du cancer. 2005;114:806–16. doi: 10.1002/ijc.20740. [DOI] [PubMed] [Google Scholar]
- 2.Eveson JW, Cawson RA. Salivary gland tumours. A review of 2410 cases with particular reference to histological types, site, age and sex distribution. The Journal of pathology. 1985;146:51–8. doi: 10.1002/path.1711460106. [DOI] [PubMed] [Google Scholar]
- 3.Boukheris H, Curtis RE, Land CE, Dores GM. Incidence of carcinoma of the major salivary glands according to the WHO classification, 1992 to 2006: a population-based study in the United States. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2009;18:2899–906. doi: 10.1158/1055-9965.EPI-09-0638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Armstrong JG, Harrison LB, Spiro RH, Fass DE, Strong EW, Fuks ZY. Malignant tumors of major salivary gland origin. A matched-pair analysis of the role of combined surgery and postoperative radiotherapy. Archives of otolaryngology--head & neck surgery. 1990;116:290–3. doi: 10.1001/archotol.1990.01870030054008. [DOI] [PubMed] [Google Scholar]
- 5.Terhaard CH. Postoperative and primary radiotherapy for salivary gland carcinomas: indications, techniques, and results. International journal of radiation oncology, biology, physics. 2007;69:S52–5. doi: 10.1016/j.ijrobp.2007.04.079. [DOI] [PubMed] [Google Scholar]
- 6.Chandana SR, Conley BA. Salivary gland cancers: current treatments, molecular characteristics and new therapies. Expert review of anticancer therapy. 2008;8:645–52. doi: 10.1586/14737140.8.4.645. [DOI] [PubMed] [Google Scholar]
- 7.Laurie SA, Licitra L. Systemic therapy in the palliative management of advanced salivary gland cancers. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2006;24:2673–8. doi: 10.1200/JCO.2005.05.3025. [DOI] [PubMed] [Google Scholar]
- 8.Rizk S, Robert A, Vandenhooft A, Airoldi M, Kornek G, Machiels JP. Activity of chemotherapy in the palliative treatment of salivary gland tumors: review of the literature. European archives of oto-rhino-laryngology : official journal of the European Federation of Oto-Rhino-Laryngological Societies. 2007;264:587–94. doi: 10.1007/s00405-007-0297-x. [DOI] [PubMed] [Google Scholar]
- 9.Spiro JD, Spiro RH. Cancer of the parotid gland: role of 7th nerve preservation. World journal of surgery. 2003;27:863–7. doi: 10.1007/s00268-003-7112-7. [DOI] [PubMed] [Google Scholar]
- 10.Edge SB. AJCC cancer staging manual. 7th ed. Springer; New York: 2010. American Joint Committee on Cancer. [DOI] [PubMed] [Google Scholar]
- 11.Nickleach D, Liu Y, Shrewsberry A, Ogan K, Kim S, Wang Z. SAS® Macros to Conduct Common Biostatistical Analyses and Generate Reports. SESUG 2013: The Proceeding of the SouthEast SAS User Group. 2013 [Google Scholar]
- 12.Mendenhall WM, Morris CG, Amdur RJ, Werning JW, Villaret DB. Radiotherapy alone or combined with surgery for salivary gland carcinoma. Cancer. 2005;103:2544–50. doi: 10.1002/cncr.21083. [DOI] [PubMed] [Google Scholar]
- 13.Garden AS, el-Naggar AK, Morrison WH, Callender DL, Ang KK, Peters LJ. Postoperative radiotherapy for malignant tumors of the parotid gland. International journal of radiation oncology, biology, physics. 1997;37:79–85. doi: 10.1016/s0360-3016(96)00464-6. [DOI] [PubMed] [Google Scholar]
- 14.Terhaard CH, Lubsen H, Van der Tweel I, Hilgers FJ, Eijkenboom WM, Marres HA, et al. Salivary gland carcinoma: independent prognostic factors for locoregional control, distant metastases, and overall survival: results of the Dutch head and neck oncology cooperative group. Head & neck. 2004;26:681–92. doi: 10.1002/hed.10400. discussion 92-3. [DOI] [PubMed] [Google Scholar]
- 15.Yu GY, Ma DQ. Carcinoma of the salivary gland: a clinicopathologic study of 405 cases. Seminars in surgical oncology. 1987;3:240–4. doi: 10.1002/ssu.2980030405. [DOI] [PubMed] [Google Scholar]
- 16.Bernier J, Domenge C, Ozsahin M, Matuszewska K, Lefebvre JL, Greiner RH, et al. Postoperative irradiation with or without concomitant chemotherapy for locally advanced head and neck cancer. The New England journal of medicine. 2004;350:1945–52. doi: 10.1056/NEJMoa032641. [DOI] [PubMed] [Google Scholar]
- 17.Cooper JS, Pajak TF, Forastiere AA, Jacobs J, Campbell BH, Saxman SB, et al. Postoperative concurrent radiotherapy and chemotherapy for high-risk squamous-cell carcinoma of the head and neck. The New England journal of medicine. 2004;350:1937–44. doi: 10.1056/NEJMoa032646. [DOI] [PubMed] [Google Scholar]
- 18.Forastiere AA, Goepfert H, Maor M, Pajak TF, Weber R, Morrison W, et al. Concurrent chemotherapy and radiotherapy for organ preservation in advanced laryngeal cancer. The New England journal of medicine. 2003;349:2091–8. doi: 10.1056/NEJMoa031317. [DOI] [PubMed] [Google Scholar]
- 19.Harari PM, Harris J, Kies MS, Myers JN, Jordan RC, Gillison ML, et al. Postoperative chemoradiotherapy and cetuximab for high-risk squamous cell carcinoma of the head and neck: Radiation Therapy Oncology Group RTOG-0234. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2014;32:2486–95. doi: 10.1200/JCO.2013.53.9163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schoenfeld JD, Sher DJ, Norris CM, Jr., Haddad RI, Posner MR, Balboni TA, et al. Salivary gland tumors treated with adjuvant intensity-modulated radiotherapy with or without concurrent chemotherapy. International journal of radiation oncology, biology, physics. 2012;82:308–14. doi: 10.1016/j.ijrobp.2010.09.042. [DOI] [PubMed] [Google Scholar]
- 21.Tanvetyanon T, Qin D, Padhya T, McCaffrey J, Zhu W, Boulware D, et al. Outcomes of postoperative concurrent chemoradiotherapy for locally advanced major salivary gland carcinoma. Archives of otolaryngology--head & neck surgery. 2009;135:687–92. doi: 10.1001/archoto.2009.70. [DOI] [PubMed] [Google Scholar]
- 22.Gilbert J, Li Y, Pinto HA, Jennings T, Kies MS, Silverman P, et al. Phase II trial of taxol in salivary gland malignancies (E1394): a trial of the Eastern Cooperative Oncology Group. Head & neck. 2006;28:197–204. doi: 10.1002/hed.20327. [DOI] [PubMed] [Google Scholar]
- 23.Haddad R, Colevas AD, Krane JF, Cooper D, Glisson B, Amrein PC, et al. Herceptin in patients with advanced or metastatic salivary gland carcinomas. A phase II study. Oral oncology. 2003;39:724–7. doi: 10.1016/s1368-8375(03)00097-6. [DOI] [PubMed] [Google Scholar]
- 24.Laurie SA, Ho AL, Fury MG, Sherman E, Pfister DG. Systemic therapy in the management of metastatic or locally recurrent adenoid cystic carcinoma of the salivary glands: a systematic review. The Lancet Oncology. 2011;12:815–24. doi: 10.1016/S1470-2045(10)70245-X. [DOI] [PubMed] [Google Scholar]
- 25.Agulnik M, Cohen EW, Cohen RB, Chen EX, Vokes EE, Hotte SJ, et al. Phase II study of lapatinib in recurrent or metastatic epidermal growth factor receptor and/or erbB2 expressing adenoid cystic carcinoma and non adenoid cystic carcinoma malignant tumors of the salivary glands. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2007;25:3978–84. doi: 10.1200/JCO.2007.11.8612. [DOI] [PubMed] [Google Scholar]
- 26.Locati LD, Bossi P, Perrone F, Potepan P, Crippa F, Mariani L, et al. Cetuximab in recurrent and/or metastatic salivary gland carcinomas: A phase II study. Oral oncology. 2009;45:574–8. doi: 10.1016/j.oraloncology.2008.07.010. [DOI] [PubMed] [Google Scholar]
- 27.Stenman G, Persson F, Andersson MK. Diagnostic and therapeutic implications of new molecular biomarkers in salivary gland cancers. Oral oncology. 2014;50:683–90. doi: 10.1016/j.oraloncology.2014.04.008. [DOI] [PubMed] [Google Scholar]
- 28.Chen AM, Granchi PJ, Garcia J, Bucci MK, Fu KK, Eisele DW. Local-regional recurrence after surgery without postoperative irradiation for carcinomas of the major salivary glands: implications for adjuvant therapy. International journal of radiation oncology, biology, physics. 2007;67:982–7. doi: 10.1016/j.ijrobp.2006.10.043. [DOI] [PubMed] [Google Scholar]
- 29.Pohar S, Gay H, Rosenbaum P, Klish D, Bogart J, Sagerman R, et al. Malignant parotid tumors: presentation, clinical/pathologic prognostic factors, and treatment outcomes. International journal of radiation oncology, biology, physics. 2005;61:112–8. doi: 10.1016/j.ijrobp.2004.04.052. [DOI] [PubMed] [Google Scholar]