Abstract
Long-term chronic alcoholism is associated with disparate and widespread residual consequences for brain functioning and behavior, and alcoholics suffer a variety of cognitive deficiencies and emotional abnormalities. Alcoholism has heterogeneous origins and outcomes, depending upon factors such as family history, age, gender, and mental or physical health. Consequently, the neuropsychological profiles associated with alcoholism are not uniform among individuals. Moreover, within and across research studies, variability among participants is substantial and contributes to characteristics associated with differential treatment outcomes after detoxification. In order to refine our understanding of alcoholism-related impaired, spared, and recovered abilities, we focus on five specific functional domains: (1) memory, (2) executive functions, (3) emotion and psychosocial skills, (4) visuospatial cognition, and (5) psychomotor abilities. The brain systems that are most vulnerable to alcoholism are the frontocerebellar and mesocorticolimbic circuitries. Over time, with abstinence from alcohol, the brain appears to become reorganized to provide compensation for structural and behavioral deficits. By relying on a combination of clinical and scientific approaches, future research will help to refine the compensatory roles of healthy brain systems, the degree to which abstinence and treatment facilitate the reversal of brain atrophy and dysfunction, and the importance of individual differences to outcome.
Keywords: Alcoholism, brain, impairments, recovery, MRI, neuropsychology, frontocerebellar system, mesocorticolimbic circuitry
Introduction
In this chapter, we first survey research related to general cognitive competencies and deficiencies in alcoholism, and the broad areas of brain involvement. This is followed by a review of five principal profiles of alcoholism-related neuropsychological functions: memory, executive functions, emotion and psychosocial skills, visuospatial cognition, and psychomotor abilities. Our review of the evidence indicates that while certain features of these functions are impaired, other aspects may be spared or recovered. The research has employed diverse neuropsychological and behavioral assessment procedures, as well as measures of brain structure and function. We begin our review by addressing some concerns regarding the selection of research participants and the tests used to study them. Attention to methodology is important, because variations and inconsistencies among studies can lead to discordant findings and conclusions. We also consider problems related to assessing impaired, spared, and recovered functions. Ultimately, our objective is to reveal areas for future research and to inform clinical strategies in the treatment of alcoholism.
The Participants and the Tests
Our review focuses on studies of uncomplicated alcoholism as distinguished from special cases of alcohol-related disorders such as Korsakoff’s syndrome (Oscar-Berman, 2012), alcohol dementia (Brust, 2010; Vetreno et al., 2011), and Marchiafava-Bignami disease (Kim et al., 2007). Our review excludes studies of adolescent drinkers, as well as research investigating binge drinking, the episodic heavy consumption of alcohol. Additionally, although studies using acute alcohol administration have direct applicability to driving situations, work-related hazards, and other societally relevant concerns, here we consider residual neuropsychological functions associated with long-term chronic alcoholism.
Characteristics of the participants
In understanding the consequences of alcoholism, drinking patterns are important considerations, i.e., the type, amount, and frequency of alcohol consumed; the age of onset of drinking; the severity and duration of the abuse or dependency; and the duration of alcohol abstinence. Repeated episodes of withdrawal also may be a risk factor for cognitive impairment and for decreased cognitive recovery due to neurotoxic lesions and excitotoxicity (Loeber et al., 2010). Studies of residual effects typically include participants who have abused alcohol for a long period of time (e.g., five years or more), as this permits the measurement of cumulative effects. Additionally, investigators interested in studying alcohol’s residual influences generally enroll participants who have been abstinent for a minimum of three to four weeks. This duration of abstinence is important for obtaining stable levels of performance after ethanol and its metabolites have been eliminated from the body (Oscar-Berman and Marinkovic, 2007). In this review, we include studies meeting that criterion unless specified otherwise.
When investigating brain-behavior relationships in individuals with a history of alcoholism, it is imperative to keep in mind that, in addition to drinking patterns, many factors influence the expression and course of the disease, and can interact to aggravate alcoholism’s effects (Petrakis et al., 2002). Consequently, methodological differences with respect to exclusion and inclusion criteria contribute to disparate findings across studies. These differences include the following: family history of alcoholism (Cardenas et al., 2005); treatment history (Di Sclafani et al., 2008); age (Pfefferbaum et al., 2006; Schottenbauer et al., 2007; Westlye et al., 2010); gender (Fattore et al., 2008; Devaud and Prendergast, 2009; Nixon, 2013; Ruiz et al., 2013); the use or abuse of medicines, nicotine, or other drugs (Durazzo et al., 2006; Cosgrove et al., 2011); body mass index (Pfefferbaum et al., 2009; Gazdzinski et al., 2010a); and comorbid medical, neurological, and psychiatric conditions (Di Sclafani et al., 2007; Fama et al., 2009; Oscar-Berman et al., 2009; Charness, 2010; Sameti et al., 2011; Martin, 2013). Common comorbid medical complications are malnutrition, diseases of the liver and the cardiovascular system, HIV/AIDS, neurological conditions such as head injury and inflammation of the brain (encephalopathy), and fetal alcohol spectrum disorders. Common comorbid psychiatric conditions are depression, anxiety, posttraumatic stress disorder, antisocial personality disorder, and schizophrenia.
Assessing neuropsychological functions and the brain
In an attempt to clarify the deficits and intact abilities of individuals with obvious or suspected central nervous system (CNS) pathology, neuropsychologists have relied on a variety of assessment procedures. Test instruments are selected to engage both general and specific abilities that depend upon the integrity of the brain regions involved in those functions. In general, the primary neuropsychological domains assessed in alcoholics have been memory, executive functions (e.g., attention, abstraction, problem solving, organizing and planning, and inhibition), emotion and psychosocial skills, visuospatial cognition, and psychomotor abilities. Examples of specific assessment instruments are referred to in the sections below, and descriptions of the tests can be found in secondary sources (Strauss et al., 2006; Lezak et al., 2012). Importantly, because these tests tend to measure broad categories of abilities, there is no universally accepted consensus on specific attributes that define the functions they assess.
Neuroimaging techniques have become essential adjunctive tools used to investigate functional, anatomical, and biochemical characteristics of the brain. In the study of alcoholism, commonly used methods include magnetic resonance imaging (MRI), functional MRI (fMRI), diffusion tensor imaging (DTI), positron emission tomography (PET), magnetoencephalography (MEG), MR perfusion, and MR spectroscopy (Schulte et al., 2012c). These measurement tools permit researchers to evaluate attributes of the brain associated with particular neuropsychological processes, and to assess the size and integrity of the underlying neural tissues. Most research endeavors typically employ a single neuroimaging method with individual participants. However, Schulte et al. (2012c) have suggested that the combined use of several techniques would be highly advantageous in defining brain abnormalities, because each method has unique strengths and weaknesses.
The Impaired, the Spared, and the Recovered
The various domains of neuropsychological functioning and their associated brain networks have different patterns of impairment, preservation, and recovery, hence our tripartite categorization. Before reviewing the alcoholism-related functional domains of interest, we first consider separate issues relevant to each of the three categories.
Impairments
Chronic alcoholism has been associated with abnormalities in cognitive, behavioral, and emotional functioning. Although these abnormalities may not be evident through superficial observation, neuropsychological tests have been able to illuminate the impairments (Leber and Parsons, 1982; Tivis et al., 1995; Clark et al., 2007; Rourke and Grant, 2009). Similarly, neuroimaging techniques have revealed reliable differences between alcoholic and nonalcoholic control groups in brain activation patterns and structural integrity, with the most vulnerable networks being the frontocerebellar system and mesocorticolimbic circuitry (Agartz et al., 1999; Makris et al., 2008; Fama et al., 2012; Chanraud et al., 2013). However, relatively few studies have demonstrated definitive and direct links between specific component neuropsychological impairments and measures of highly localized brain abnormalities.
Spared functions and compensation
Individuals with a history of long-term uncomplicated alcoholism appear free from impairments in certain basic neuropsychological functions. Some researchers have suggested that the lack of detectable deficits in these seemingly spared domains could be due to compensatory recruitment of alternative brain regions to facilitate cognitive processes (Sullivan and Pfefferbaum, 2005; Oscar-Berman and Marinkovic, 2007; Chanraud et al., 2013). In support of this notion, fMRI studies have demonstrated altered patterns of neural activation among alcoholics, with reduced activity in some regions (Chanraud et al., 2010; Chanraud et al., 2011), but simultaneous increases in activity in others (Pfefferbaum et al., 2001a; Desmond et al., 2003; Chanraud-Guillermo et al., 2009; Chanraud et al., 2013). As such, it can be difficult to distinguish between functions that have been truly spared and those that appear unimpaired due to compensatory mechanisms or other recovery of function.
Descriptions of spared CNS structures, while not prone to concerns about compensation in the same way as neuropsychological performance, are subject to issues of tissue regrowth with sustained sobriety, which could mask effects that might have been observable earlier in abstinence (Ruiz et al., 2013). The picture is further complicated in cross-sectional studies when trying to identify structures that have been truly spared and those that have recovered after cessation of drinking. In other words, brain regions that appear to be spared from damage might contain tissues that have been repaired or regenerated.
Another cautionary note is in order with regard to neuropsychological processes or brain structures in which there has been a failure to detect alcoholism-related relationships. That is, although negative results from research studies may be due to genuinely preserved abilities and brain tissue, it is possible that the data sets lack the statistical power needed to detect bona fide differences between populations. Accordingly, studies that have examined certain functions and structures but have not found alcoholism-related abnormalities cannot characterize confidently the functions or regions as spared, because the results may have been obtained from inadequate sample sizes or because the variability within the measures was too large. In many of those studies, the probability that real associations exist but were not detected (Type II error) might be high but not reported. Thus, conclusions regarding spared functions cannot always be drawn with adequate certainty from the current literature. In any case, the identification of preserved functions — whether they are spared, compensatory, or recovered — is of great benefit to the scientific and healthcare communities. Once intact functions are identified, clinicians can work with patients to harness and strengthen these abilities in order to accelerate recovery and improve their quality of life.
Recovery
Although differences in outcomes observed in neuropsychological and brain abnormalities are complicated by the fact that the course of recovery can be dependent on the extent of impairment at the time of drinking cessation, abstinent alcoholics have shown improvements in neuropsychological functioning with continued sobriety (Kish et al., 1980; Oscar-Berman et al., 2004; Rosenbloom et al., 2004; Erickson and White, 2009). Likewise, abstinence is accompanied by brain structural changes, such as increased cortical thickness in the brain’s extended reward and oversight system (Durazzo et al., 2011). Processes proposed to account for recovery of cognitive functioning in alcoholism include neural repair (regeneration) and reorganization, such that new or additional neural networks are recruited to accomplish a task (Crews et al., 2005; Sullivan and Pfefferbaum, 2005; Chanraud et al., 2013).
Neuropsychological findings provided the earliest insights into restored function in abstinent alcoholics (Oscar-Berman and Marinkovic, 2007), and neuroimaging has permitted the quantification of neural tissue repair and recovery with abstinence (Buhler and Mann, 2011). Early neuroimaging evidence for structural brain damage and subsequent improvement among abstinent alcoholics was reported by Carlen et al. (1978), who demonstrated reduced atrophy in recently abstinent alcoholics; improvement was not seen in those who relapsed. Modern MRI studies have confirmed and extended those findings to include demonstrations of recovery in brain volume, microstructure, and neurochemistry (Pfefferbaum et al., 1995; Demirakca et al., 2011; Durazzo et al., 2011; Alhassoon et al., 2012). This body of knowledge has grown steadily to include manifestations of recovery in a wide spectrum of tissue properties across several imaging modalities (Oscar-Berman and Marinkovic, 2007; Schulte et al., 2012c). In particular, improvements in brain tissue volumes following periods of abstinence from alcohol have been observed in gray matter (Durazzo et al., 2011) and in white matter (Ruiz et al., 2013), often with corresponding decreases in the volume of cerebrospinal fluid (CSF). Potential mechanisms underlying recovery of neural tissue during sobriety include remyelination, neurogenesis, and rehydration (Nixon, 2006; Rosenbloom and Pfefferbaum, 2008). Accordingly, recovery of neuropsychological functioning has been directly associated with improvements in the structure of neural tissue (Muller-Oehring et al., 2009; Stavro et al., 2012).
Profiles of Damage and Repair
In the following sections, we review neuropsychological functioning in alcoholism. We begin with an overview of general abilities and intelligence in conjunction with findings related to widespread structural brain abnormalities and evidence of recovery. We then review profiles of five functional domains and associated brain regions.
General neuropsychological abilities
Methods for evaluating general abilities and overall intelligence have been in use for more than a century, and continue to be employed regularly today (Strauss et al., 2006; Lezak et al., 2012). Here we describe examples from research that has looked at general abilities in the study of long-term chronic alcoholism, and we conclude that their utility in characterizing deficits in alcoholism has been limited, in part because of the variety of functions they assess.
Many studies have reported poorer performance by alcoholics as compared to nonalcoholic controls in general cognitive abilities or intelligence (Sullivan et al., 2000b; Sullivan et al., 2002b; Davies et al., 2005; Rosenbloom et al., 2007), but the deficits have been mild, or they tended to improve within a year of abstinence (Bates et al., 2002). Other studies have reported no alcoholism-related impairments in general intelligence (Oscar-Berman et al., 2004; Fein et al., 2006; Foisy et al., 2007; Marinkovic et al., 2009; Sullivan et al., 2010; Pitel et al., 2012b). When general abilities are assessed according to discrete component factors and functional domains, a different picture emerges. That is, investigators who have examined performance on specific IQ subtests or have employed extensive batteries of specialized neuropsychological tests have observed alcoholism-related impairments in some domains but not in others (Tivis et al., 1995; Beatty et al., 2000; Davies et al., 2005; Fein et al., 2006; Oscar-Berman et al., 2009). For example, Davies et al. (2005) reported no differences between alcoholics and controls on prorated Full Scale IQ scores, but alcoholics performed worse on Vocabulary and Digit Symbol subtests. Stavro et al. (2012) found only a minimal impairment in general intelligence in a meta-analysis of 62 studies examining cognitive abilities in alcoholism. However, they did find moderate impairments across 11 separate cognitive functions: verbal fluency/language, speed of processing, working memory, attention, problem solving/executive functions, inhibition/impulsivity, verbal learning, verbal memory, visual learning, visual memory, and visuospatial abilities. The meta-analysis indicated that the levels of impairment for the separate areas of dysfunction remained impaired after one year of sobriety. Importantly, the study stressed the need to examine the distinct component processes that contribute to general abilities and intelligence.
Widespread brain damage in alcoholism
Structural abnormalities have been found in widespread brain regions of alcoholics. Relatively smaller volumes have been noted not only in total brain size (Pfefferbaum et al., 1992; Mann et al., 2005; Chen et al., 2012) including the cerebellum (Chanraud et al, 2007), but also in cerebral subdivisions and regional areas (Pfefferbaum et al., 1992; Pfefferbaum et al., 2001b; Makris et al., 2008; Fein et al., 2009; Chen et al., 2012) and numerous subcortical structures (Chanraud et al., 2007; Makris et al., 2008). Smaller white matter volumes likewise have been reported (Harper et al., 1990; Pfefferbaum et al., 1995; Pfefferbaum et al., 2001b; Chen et al., 2012), including the corpus callosum (see Figure 1) (Pfefferbaum et al., 1996; Agartz et al., 1999; Ruiz et al., 2013). Smaller brain volume is accompanied by larger CSF volume (Pfefferbaum et al., 1992; Pfefferbaum et al., 1998; Agartz et al., 1999; Bendszus et al., 2001; Pfefferbaum et al., 2001b; Chen et al., 2012), and researchers have reported larger volumes of the lateral ventricles (Zipursky et al., 1989; Pfefferbaum et al., 1998; Pfefferbaum et al., 2001b; Ruiz et al., 2013) and the third ventricle (Pfefferbaum et al., 2001b).
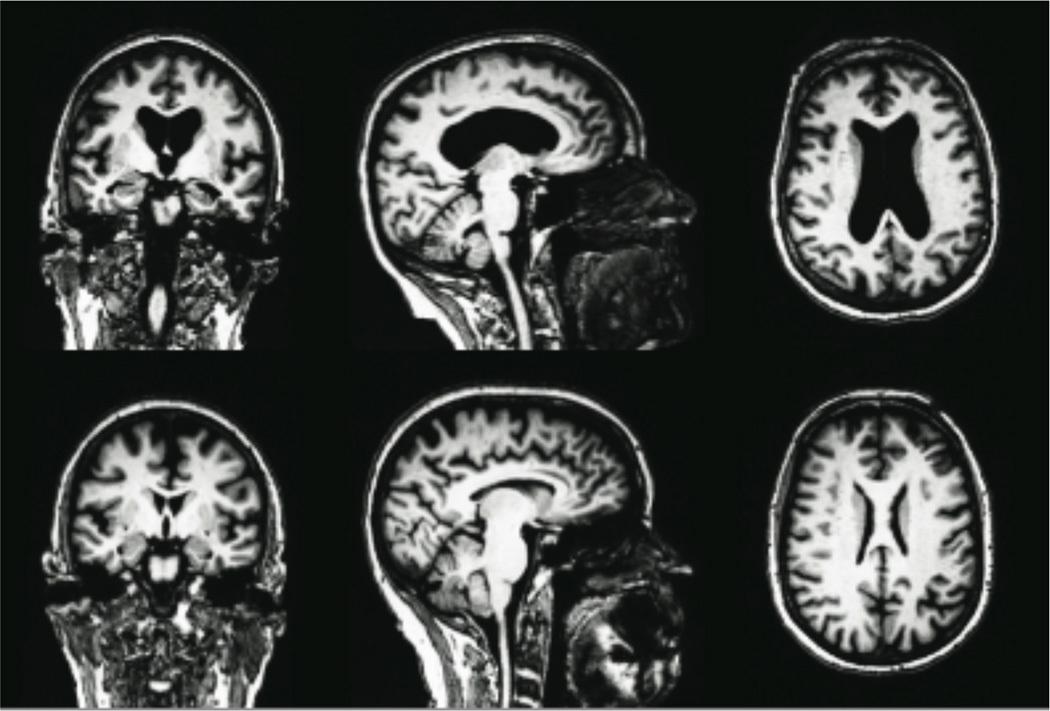
Figure 1.
Severe brain atrophy in a 72-year-old alcoholic woman (top row) compared to an age-matched nonalcoholic control woman (bottom row). Most prominent indications of alcoholism-related brain damage include enlarged ventricles, thinning of the corpus callosum, and hippocampal atrophy.
Structural improvement of gray and white matter tissue has been associated with abstinence. Pfefferbaum et al. (1995) found a trend for increases in anterior cortical gray matter volumes after 20 days of sobriety, and Agartz et al. (2003) reported whole-brain increases in white matter volumes and corresponding decreases in CSF after one month of abstinence. Recovery of white matter microstructure has been demonstrated in longitudinal studies, with improvements in fractional anisotropy (a measure of microscopic abnormalities in axons) in the corpus callosum after one year of abstinence (Alhassoon et al., 2012) and decreases in mean radial diffusivity (a measure related to axonal demyelination) after only one month of sobriety, suggesting recovery of axonal integrity (Gazdzinski et al., 2010b). However, Monnig et al. (2012) reported persistent widespread fractional anisotropy deficits in bilateral parietal regions after a year of abstinence. Another marker for detecting reversal of global alcoholism-related brain damage that remains understudied is cerebral perfusion. The few studies examining perfusion improvements using arterial spin labeling MRI have yielded conflicting results. Mon et al. (2009) reported improvement of frontal and parietal gray matter perfusion after five weeks of abstinence, but Durazzo et al. (2010) were unable to detect longitudinal perfusion increases in parietal gray matter after an average of 35 days of abstinence. Thus, findings associated with recovery of overall brain integrity have been inconsistent.
Frontocerebellar and mesocorticolimbic structures
It is important to note that although abnormalities have been found in widespread brain regions of alcoholics, researchers who have investigated networks of neural structures subserving the specific profiles of neuropsychological domains observed to be impaired (as discussed below) have emphasized the contributions of two distinct but overlapping systems: the frontocerebellar system (Figure 2) and the mesocorticolimbic circuitry (Figure 3) (Sullivan et al., 2003; Fuster, 2008; Harris et al., 2008; Makris et al., 2008; Goldstein and Volkow, 2011). Importantly, both of these extensive systems control functions that could prevent alcoholics from being able to employ good judgment and avoid the negative behaviors and actions associated with addiction.
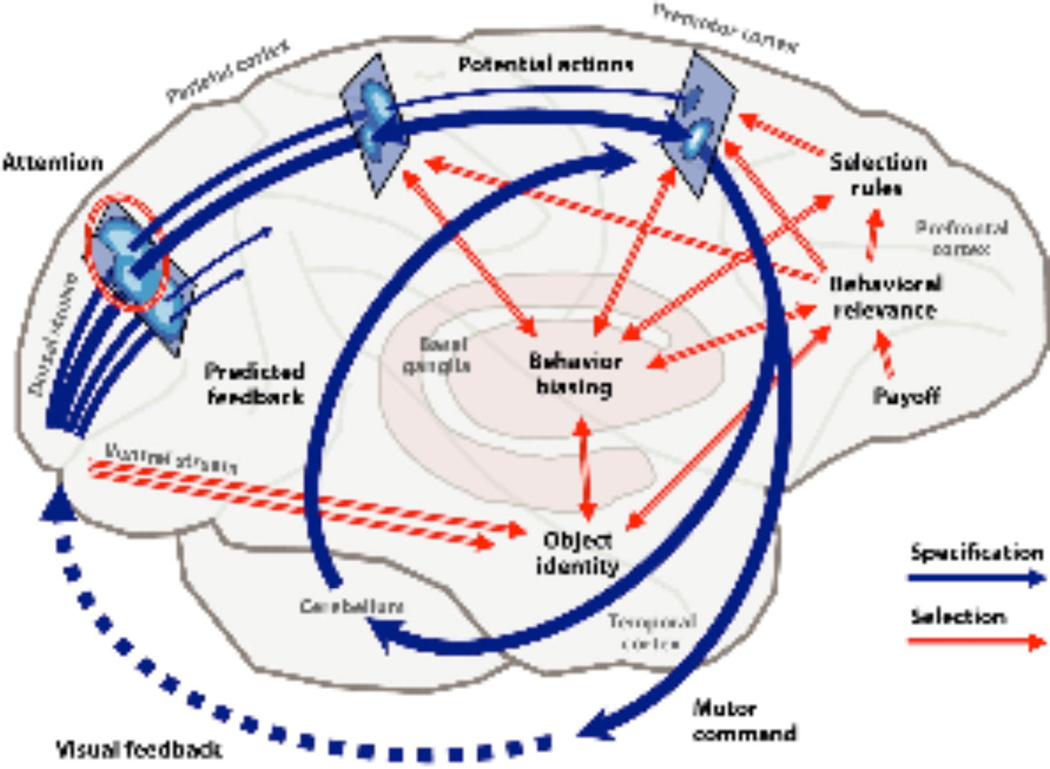
Figure 2.
The frontocerebellar system (Cisek and Kalaska, 2010). This figure illustrates interactions among cortical areas, basal ganglia, and the cerebellum. Reproduced with permission of Annual Reviews.
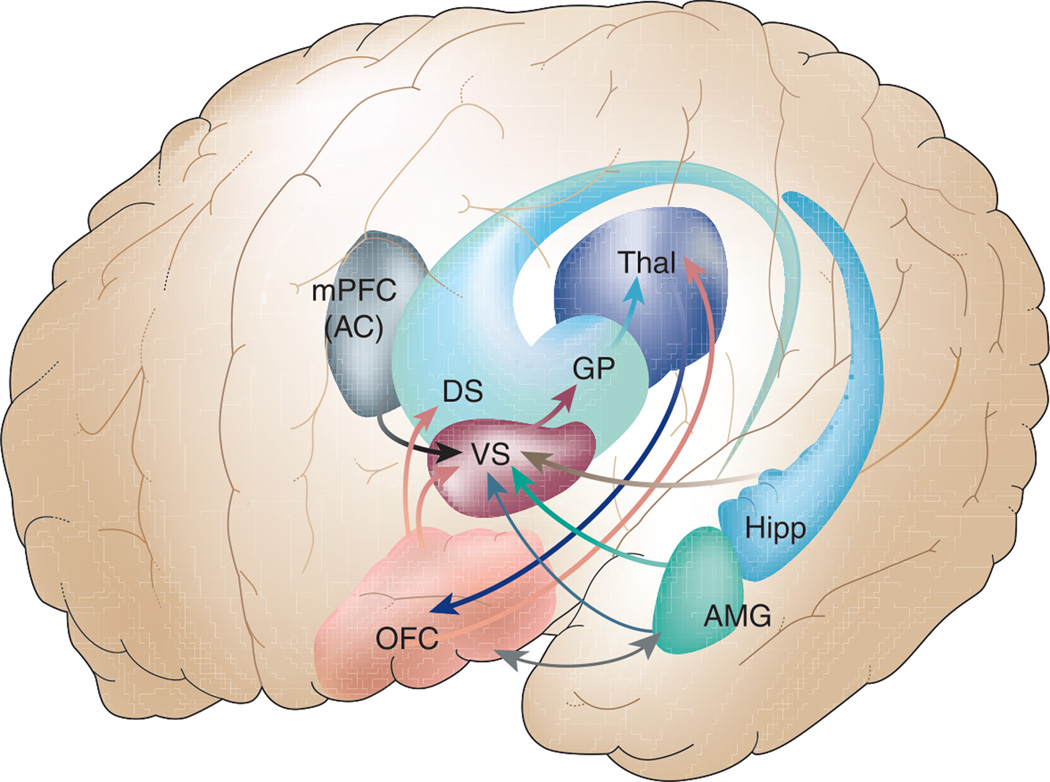
Figure 3.
The mesocorticolimbic circuitry (Salloway et al., 2001). This figure highlights the following structures: orbitofrontal cortex (OFC), medial prefrontal cortex (mPFC), anterior cingulate (AC), amygdala (AMG), hippocampus (Hipp), thalamus (Thal), ventral striatum (VS), dorsal striatum (DS), and globus pallidus (GP). Adapted with permission from The Frontal Lobes and Neuropsychiatric Illness (Copyright 2001), American Psychiatric Association.
The Five Functional Domains
In order to refine our understanding of alcoholism-related impaired, spared, and recovered abilities, we focus on five specific functional domains: (1) memory, (2) executive functions, (3) emotion and psychosocial skills, (4) visuospatial cognition, and (5) psychomotor abilities. Each neuropsychological domain has a unique profile of relative impairment, or lack thereof. For each domain, behavioral deficits are discussed first (summarized in Appendix: Table 1) along with their associated brain abnormalities (Appendix: Table 2), followed by evidence of compensation or recovery.
1. Memory
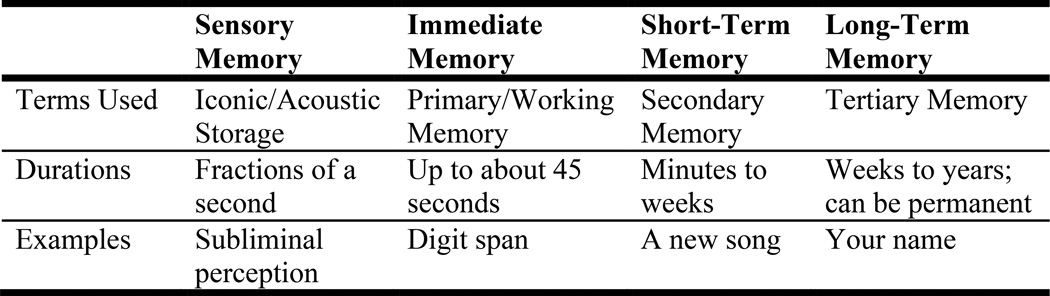
Memory refers to the storage of new and old information for later use. Memories can be stored for very short periods of time — seconds or even milliseconds, during which information is encoded and consolidated — or for long periods of time — weeks, years, or a lifetime. Figure 4 represents a descriptive, atheoretical way of viewing human memory, emphasizing temporal characteristics of the information being stored. Because memory is not unitary, theorists have considered its many different forms, mediated by separate component processes, governed by different principles, and controlled by extensive brain circuitries (Tulving and Craik, 2000). In relation to alcoholism, memory has been explored most frequently with respect to the type of material or event being remembered and the duration of storage. “Working memory,” which stores transitory information in the service of future action despite distractions, is considered a component of executive functions (discussed below).
Figure 4.
Forms of human memory. Memories are stored for time periods ranging from milliseconds to years. Working memory, a component of executive functions, also is discussed in the section on executive functions. Long-term memory is divided into declarative (explicit) memory and procedural (implicit) memory. Declarative memory can be further subdivided into episodic memory (memory for our experiences) and semantic memory (for facts). Procedural memory is memory of skills and knowing how to do things.
Impairments
Alcoholics have demonstrated some degree of memory impairment, despite evidence that they tend to overestimate their memory capacity (metamemory; Le Berre et al., 2010). The nature and extent of the impairments depend to some degree upon characteristics of the neuropsychological tests (task parameters such as timing of stimulus presentation and type of materials) and on the integrity of other cognitive functions supporting memory. For example, Marinkovic et al. (2009), using the Wechsler Memory Scale, which measures a number of different types of memory including verbal, auditory, visual, short-term, and working memory, found that alcoholics’ reaction times in a deep word-encoding task were negatively correlated both with working memory scores, and performance on a separate test of verbal fluency. These findings suggest that participants with worse executive functioning skills found the encoding task more difficult. The reaction times also showed a tendency to correlate with duration of heavy drinking, indicating that long-term heavy alcohol intake impacted speed of word-encoding. Other investigators used extensive batteries of neuropsychological tests (including the Wechsler Memory Scale, Dementia Rating Scale, Rey-Osterrieth Complex Figures Test, and a word/face recognition test) and found that alcoholics had significantly impaired verbal, nonverbal, and spatial short-term memory (Sullivan et al., 2000b; Sullivan et al., 2002b).
Alcoholics also have been found to perform below normal on tests that evaluate memory typologies or processes such as episodic memory (Le Berre et al., 2010) and free and delayed recall (Pitel et al., 2007; Noël et al., 2012). The tests of episodic memory used by Le Berre et al. (2010) examined free recall, semantic cued recall, and recognition memory for shallow and deeply encoded words; total recall and recognition scores were below those of the controls. Similarly, Pitel et al. (2007) employed a variety of episodic memory tests and found impairments in several components, including encoding and retrieval processes, contextual memory, autonoetic consciousness (the ability to analyze our own thoughts), and learning abilities. Noël et al. (2012) also detected impairments in episodic memory using the California Verbal Learning Test, and suggested that this defect likely reflected executive dysfunction. That is, rather than a retrieval deficit, alcoholics might have difficulties applying strategies in organizing information for later use. Kopera et al. (2012) studied alcoholic patients who presented with deficits in visual episodic memory, and observed that nonverbal domains such as attention, visual memory, and working memory were impaired.
Neuropsychological indications of dysfunctional memory acquisition and processing have been investigated using neuroimaging techniques. In an fMRI study, Akine et al. (2007) employed a long-term false-recognition memory paradigm comparing alcoholics to controls on task performance and brain activation. Although the researchers did not find alcoholics to be impaired on the task, they did find differing patterns of brain activity between the groups. Alcoholics exhibited diminished responses in several small and isolated locations of the brain (right prefrontal cortex, anterior cingulate, right pulvinar, and right ventral striatum). Because the alcoholics in this study were relatively young (mean age in their 30s) and had been abstinent for an average of more than three years, the authors suggested that the abnormal fMRI results, in the absence of observed behavioral deficits, might indicate latent lesions or subclinical pathology.
In addition to abnormal brain activation, structural compromise has been observed in brain regions important for memory functions. Investigators have reported reduced alcoholism-related volumes in the mesocorticolimbic system, including medial temporal-lobe structures (Sullivan et al., 1995; Harding et al., 1997; Agartz et al., 1999; Gazdzinski et al., 2005a; Cardenas et al., 2007; Chanraud et al., 2007; Cardenas et al., 2011; Sameti et al., 2011). White matter abnormalities in memory-associated regions also have been reported. Pfefferbaum et al. (2009), using quantitative fiber tracking derived from DTI, observed defects in microstructural fiber integrity in frontal forceps, internal and external capsules, fornix, and superior cingulate and longitudinal fasciculi. Chanraud et al. (2009) examined white matter integrity in conjunction with measures of verbal episodic memory derived from the Free and Cued Selective Reminding Test. The investigators found that low verbal episodic memory performance in alcoholics was associated with structural compromise of axons in frontal, temporal, hippocampal, and parahippocampal regions.
Compensation and recovery
Although metamemory, short-term memory, episodic memory, and free and delayed recall are impaired in uncomplicated alcoholism, deficits in other memory components have not consistently been identified (Crews et al., 2005). Remote memories, especially those formed before the onset of prolonged heavy drinking, remain relatively preserved compared to recently acquired memories (Mearns and Lees-Haley, 1993). Recovery of some forms of memory functioning has been found to vary in relation to duration of abstinence. On episodic memory tasks, deficits generally have not been found following prolonged abstinence (Reed et al., 1992; Rourke and Grant, 1999; Fein et al., 2006). Pitel et al. (2009) reported that for alcoholics who abstained over a six-month interval, episodic memory performance (as assessed by a selective reminding test) returned to a level of functioning comparable to that of nonalcoholic controls, while this level of recovery was not observed in alcoholic patients who relapsed. Additionally, Pitel et al. (2012a) tested abstinent alcoholics on a face-name learning and recognition-memory task during an fMRI scan. At rest, fMRI activation patterns in the left hippocampal and cerebellar regions were positively synchronized in controls, but negatively synchronized in the alcoholics. During initial learning of the task, the alcoholics did not differ from controls on measures of performance, but the alcoholic group had lower cerebellar activation. During memory-task engagement, both groups exhibited hippocampal-cerebellar fMRI desynchronization. The authors speculated that because there were no significant group differences in recognition-memory performance, the alcoholics’ modulated hippocampal-cerebellar activity was compensatory.
In summary, the consequences of chronic alcoholism for memory functioning are complex and varied (Pitel and Beaunieux, 2013). Uncomplicated alcoholics often experience impairments in working memory, short-term memory, and episodic memory. Some of these deficiencies have been linked to executive dysfunction and damage to associated structures, while others have been connected with damaged mesocorticolimbic regions. Fortunately, long-term memories tend to be preserved, and episodic memory abilities can improve with sobriety.
2. Executive Functions
Executive functions are complex cognitive abilities requiring the synchronization of several sub-processes to achieve conscious and non-conscious goals (Alvarez and Emory, 2006). They control and regulate other abilities and behaviors and involve complicated mental skills that help us to connect past experiences with present actions, plan future behavior, organize, judge, change behavior and strategies, and remember details for our decision-making. Relevant skills include sustained and selective attention, working memory, planning, organization, problem solving, abstraction, and the ability to inhibit inappropriate responses. Intact executive functioning has been associated with integrity of frontal brain circuitry, which involves an overlapping and interconnected network. In alcoholics, the most compromised cortical areas of this network are dorsolateral prefrontal, ventral/orbitofrontal, and anterior cingulate regions. Although theorists differ on whether executive functions are unified with respect to process and component cognitive abilities (Lezak et al., 2012), there is abundant evidence that executive functioning is impaired in uncomplicated alcoholics, and that abnormal frontal brain circuitry is involved.
Impairments
Using tasks adopted from nonhuman animal models (comparative neuropsychology) to test components of executive functioning in alcoholics, Oscar-Berman and colleagues have demonstrated deficits in working memory, problem solving, and susceptibility to interference (see Oscar-Berman and Bardenhagen, 1998, for review). Fernández-Serrano et al. (2010) administered conventional neuropsychological measures of fluency, analogical reasoning, interference, cognitive flexibility, decision-making, self-regulation, and working memory to alcoholics and other drug abusers and found that alcohol abuse was associated with verbal fluency and decision-making deficits. Several groups have reported that alcoholics performed significantly worse than nonalcoholic controls on the Trail Making Test, a measure of visual-conceptual abilities requiring divided attention, mental flexibility, set-shifting proficiency, and motor skills (e.g., Davies et al., 2005; Chanraud et al., 2007; Oscar-Berman et al., 2009; Loeber et al., 2010). Others have reported alcoholism-related impairments on the Wisconsin Card Sorting Test (e.g., Sullivan et al., 1993; Ratti et al., 2002; Oscar-Berman et al., 2009), which measures abstract thinking, cognitive flexibility and shifting, concept identification, hypothesis generation, and sensitivity to feedback. Additional evidence of executive dysfunction in alcoholics has been reported as deficits on the Stroop Color-Word Association test (Ratti et al., 2002) or modifications of the Stroop test (Lusher et al., 2004) that tap into selective attention, perceptual interference, response inhibition, and information-processing speed. Tests of impulsivity have shown alcoholism-related impairments on Go/No-Go tasks (Pandey et al., 2012) that require participants to respond quickly to a target while inhibiting responses when a stop-signal appears. Additional reports of poor executive functioning in alcoholics come from a variety of other tests, including the Letter-Number Sequencing test, which relies on working memory (Chanraud et al., 2007); the Ruff Figural Frequency Test, requiring participants to quickly draw as many unique designs as possible by connecting dots (Oscar-Berman et al., 2009); a delay-discounting decision-making test in which participants choose between a large but delayed reward and a small but more immediate reward (Mitchell et al., 2005); and the Iowa Gambling Test (Fernández-Serrano et al., 2010). The many findings from these combined studies of alcoholics clearly demonstrate executive dysfunction as a characteristic of the disorder. Even though descriptions of the impairments vary across reports, the inconsistencies are plausible because of the multiplicity and complexity of executive functioning components, and because researchers have used diverse tasks to measure them. In any case, executive dysfunction has been found to be associated with low motivation to change drinking behaviors (Le Berre et al., 2012). This creates difficult conditions for recovery from addiction and therefore, likely serves as a cognitive barrier to treatment. Loss of inhibition, poor insight, distractibility, perseverative responding, impaired decision-making, and difficulties with impulse control contribute to increased likelihood of relapse (Crews and Boettiger, 2009; Sullivan et al., 2010).
Goldstein and Volkow (2002) have provided an informative review of neuroimaging findings showing dysfunction of prefrontal cortex in addictions. They suggested that prefrontal abnormalities affect limbic reward regions and lead to dysregulation of executive functions, especially the ability to inhibit maladaptive or disadvantageous behaviors. In alcoholism, abnormalities of task-related and resting-state functional connectivity have been documented in frontal brain circuitry supporting executive functions. In an fMRI study designed to assess reward-guided adaptive decision-making during a visual reinforcement-learning task, Park et al. (2010) found that compared to controls, alcoholics had lower functional connectivity in the right dorsolateral prefrontal cortex and ventral striatum. The abnormalities in frontostriatal activity were associated with decision-making difficulties; however, mean sobriety was only 17 days and may not represent persistent effects. Schulte et al. (2012a) used a modified Stroop task to examine inhibitory control and conflict processing. In controls, posterior cingulate cortex was activated during response switching and deactivated during response repetition, whereas alcoholics showed the opposite pattern, and greater deviations from the normal activity correlated with higher amounts of lifetime alcohol consumption. A functional dissociation of brain network connectivity between the groups further showed that the controls exhibited greater corticocortical connectivity among medial prefrontal, middle cingulate, and posterior cingulate cortices than the alcoholics. In contrast, the alcoholics exhibited greater orbitofrontal-midbrain network connectivity than the controls.
Impairments in executive functioning in alcoholics have been associated with underlying structural abnormalities in frontocerebellar circuitry (Moselhy et al., 2001; Sullivan, 2003; Sullivan et al., 2003; Chanraud et al., 2007; Oscar-Berman and Marinkovic, 2007; Oscar-Berman et al., 2009). Significant volume reductions have been reported in the frontal lobes (Pfefferbaum et al., 1992; Agartz et al., 1999; Kubota et al., 2001; Chanraud et al., 2007; Chen et al., 2012) including reduced gray matter in dorsolateral prefrontal cortex (Chanraud et al., 2007; Makris et al., 2008) and anterior cingulate cortex (Chanraud et al., 2007). Analyses of cortical thickness in alcoholics have demonstrated that the frontal lobes are particularly vulnerable to thinning (Fortier et al., 2011). With DTI scans, researchers have found white matter abnormalities in the cingulum bundle, superior longitudinal fasciculi, and internal and external capsules (Harris et al., 2008; Pfefferbaum et al., 2009; Rosenbloom et al., 2009; Schulte et al., 2012b).
Compensation and recovery
Studies have reported positive associations between executive functioning and maintenance of sobriety. For example, Loeber et al. (2010) reported that improvement of attention and executive functioning, measured by the Trail-Making Test and the Wisconsin Card Sorting Test, occurred within the second of two three-month follow-up sessions subsequent to initial detoxification. Also, Pitel et al. (2009) reported that alcoholic patients who abstained over a six month interval returned to levels of performance comparable to nonalcoholic controls on executive functioning tasks measuring inhibition, flexibility, and updating, while recovery was not observed for relapsers. Additionally, factors such as relapse rates and personality disorders have been associated with different outcomes in recovery of executive functioning. Andó et al. (2012) compared personality traits in recovering alcoholics abstinent for 12 weeks to those abstinent for three years or more. Compared to the long-term abstinent patients, the short-term abstinent patients perceived higher levels of stress and used non-adaptive coping strategies. However, both groups showed evidence of persistent decision-making deficits. The researchers suggested that an adaptive personality profile of stress reduction skills and effective coping strategies likely helps to compensate for decision-making deficits and assists in maintaining long-term abstinence.
When component processes of executive functions have not been found to be impaired, there may have been compensatory input through alternate brain pathways. For example, Gilman et al. (2010) found that despite not showing performance deficits during decision-making fMRI tasks, alcoholics recruited a larger frontal brain network than nonalcoholics, especially in the left inferior and right middle frontal gyri. Additionally, Pfefferbaum et al. (2001a) did not identify differences between alcoholics and controls in a spatial working memory fMRI task, but did find that alcoholics used more inferior- and posterior-frontal regions than controls, who activated dorsolateral prefrontal cortex primarily. Similarly, Desmond et al. (2003) reported that alcoholics with sobriety periods from four weeks to two years, while not showing performance impairments (accuracy and reaction time) on a verbal working-memory fMRI task, exhibited greater fMRI activation than a control group in left frontal and right superior cerebellar regions. These findings suggested that increased activation in language and articulatory-control regions may reflect the compensatory recruitment necessary to perform simple decision-making or working-memory tasks.
Recovery of neurocognitive functioning in the executive domain has been associated with improvements in brain neurochemistry. Bartsch et al. (2007) administered an attentional test, requiring prompt detection of three subtly different target stimuli, which also measures aspects of concentration and coordination. They found that performance by alcoholics correlated with increases in choline and N-acetylaspartate levels with six to seven weeks of sobriety, particularly in frontomesial areas. Durazzo et al. (2011) used arterial spin labeling perfusion MRI of frontal and parietal gray matter to study groups of alcoholics with differing durations of abstinence. They found that compared to alcoholics who abstained during a 12-month period, those who resumed drinking showed significantly lower frontal gray matter perfusion; there were no group differences for parietal gray matter.
In summary, impairments in the domain of executive functioning consistently have been shown in alcoholics, but some executive impairments may be reversed with sobriety. Because of the role of frontal brain systems in directing cognition and behavior, this finding has important implications for overcoming addiction. Because many alcoholics report that a central problem is an inability to act in concert with their conscious plans and intentions, especially with respect to abstaining from alcohol, it may be encouraging to note that the brain system involved in these cognitive processes can and does recover with continued abstinence
3. Emotion and Psychosocial Skills
Emotional signals, expressed in voices, faces, and body language, are important aspects of social interactions. Deficiencies in expressing and interpreting these signals can interfere with interpersonal relations, which in turn can lead to increased alcohol consumption as a dysfunctional coping mechanism. Affective and social functions are complex and rely on neural substrates of emotion, intrinsic motivation, decision-making, and sensitivity to reinforcement. Multiple brain networks are involved, most notably mesocorticolimbic and frontoparietal circuitries (Insel and Fernald, 2004; Frith and Frith, 2007; Schulte et al., 2010; Goldstein and Volkow, 2011). These circuitries are involved in many bio-behavioral functions impaired in alcoholics, and breakdown within the networks can initiate drug use or relapse after protracted abstinence (Oscar-Berman and Bowirrat, 2005).
Impairments
Alcoholics exhibit deficits in several aspects of emotional functioning. Research on the perception and decoding of emotional expressions has shown that alcoholics are impaired in perceiving emotions in facial expressions (Philippot et al., 1999; Kornreich et al., 2001; Kornreich et al., 2002; Maurage et al., 2008; Maurage et al., 2009) and in recognizing emotional prosody in speech — a non-linguistic aspect of language that conveys the speaker’s feelings and attitudes (Oscar-Berman et al., 1990a; Monnot et al., 2001; Monnot et al., 2002). For example, Oscar-Berman et al. (1990a) reported that alcoholics had difficulty judging emotional intonations and emotional semantic content of spoken sentences, and Monnot et al. (2001, 2002) found that alcoholics were deficient in the ability to detect emotion and attitude in the voice. These findings indicate a defect in decoding affective prosody. Uekermann et al. (2005) reported that alcoholics were impaired in identifying semantic content when it did not match affective prosody and in matching affective prosody to facial expressions, deficits that may be related to cross-modal difficulties (Oscar-Berman et al., 1990b; Maurage et al., 2007; Maurage et al., 2012a). Research has found that alcoholics responded abnormally to negative emotional stimuli (Clark et al., 2007) and overestimated the intensity of negative facial expressions such as fear, sadness, anger, and threat (Oscar-Berman et al., 1990a; Kornreich et al., 2001; Townshend and Duka, 2003; Clark et al., 2007; Maurage et al., 2009; Kornreich et al., 2013). Using images of morphed facial expressions with varying emotional intensities, Philippot et al. (1999) also reported that alcoholics overestimated the intensity of emotional expressions, and they misinterpreted most of the emotional expressions at all levels of intensity. Moreover, the alcoholics were unaware of their misperceptions.
In an extensive review of studies of social cognition in alcoholics, Uekermann and Daum (2008) summarized behavioral findings of diminished social cognitive functioning in alcoholism such as impairments in facial affect perception (as noted above), humor processing (Uekermann et al., 2007), empathy (Maurage et al., 2007; Uekermann et al., 2007), and theory-of-mind (the ability to ascribe mental states to others) (Uekermann et al., 2007). Kornreich et al. (2011) reported that alcoholics demonstrated impaired reasoning for social contract, precaution, and understanding conditional social rules. The alcoholics also had lower emotional intelligence scores, suggesting that these factors contributed to impaired interpersonal functioning. Further, higher levels of alexithymia, characterized by difficulties identifying, differentiating, and expressing feelings, have been reported in alcoholism (Maurage et al., 2011; Stasiewicz et al., 2012), and positive correlations between alexithymia measures and severity of alcohol use have been reported (Thorberg et al., 2011).
A number of fMRI studies have shown alcoholism-related abnormalities in the prefrontal cortex and the limbic system, which are important in emotion and cognition. For example, one fMRI study examined brain activation patterns in alcoholic and control participants as they were shown photographs of faces with positive, negative, and neutral expressions (Marinkovic et al., 2009). Nonalcoholic participants had strong amygdala and hippocampus activation when viewing emotional (compared with neutral) expressions, while alcoholics had similar responses to both neutral and emotional stimuli, which reflected blunted limbic activation to emotional faces. Another fMRI study showed that alcoholic participants were impaired in recognizing expressions of fearful faces and had lower activation in orbitofrontal and insular cortex than controls when viewing emotional faces (O’Daly et al., 2012). Gilman et al. (2010) found that alcoholics demonstrated greater activation in the inferior frontal gyrus, fusiform gyrus, and amygdala when they passively viewed negative facial expressions, and decreased activation in the precuneus and parahippocampal gyrus when they viewed positive facial expressions. Low activation in the anterior cingulate cortex during decoding of facial expressions of fear, disgust, and sadness also has been reported (Salloum et al., 2007). In a study examining functional brain activity during a task assessing cognitive and affective aspects of empathy, Gizewski et al. (2013) found that alcohol history was associated with low activation in the right anterior insula and deficits in affective empathy. Maurage et al. (2012b) studied psychosocial functioning in the context of a game that simulated interpersonal interactions, and found higher insula and lower frontal activation during social exclusion conditions. In another fMRI study, Maurage et al. (2012a) found that during a face-voice interaction task, alcoholic participants had less activation than controls in the superior temporal sulcus, inferior occipital gyrus, middle frontal gyrus, and superior parietal lobule, as well as poor functional connectivity in frontal regions.
In addition to atypical activation patterns in alcoholics, diminished volumes have been reported for brain structures associated with emotional and social functioning, such as orbitofrontal cortex, rostral anterior cingulate cortex, nucleus accumbens, amygdala, hippocampus, and insula (Makris et al., 2008; Wobrock et al., 2009). These regions, which are part of the “social brain” (Insel and Fernald, 2004) and neural circuitry underlying emotion and reward processing (Schulte et al., 2010), are disproportionately affected in alcoholism (Moselhy et al., 2001; Oscar-Berman and Marinkovic, 2007; Makris et al., 2008). In a study that examined the relationship between DTI-based fiber tracking and fMRI during an emotional Stroop task, Schulte et al. (2012b) found that alcoholics demonstrated poorer Stroop-word performance, suggesting higher emotional interference, and this performance was correlated with lower white-matter integrity in the cingulate and corpus callosum.
Compensation and recovery
Despite persistence of emotional and psychosocial impairments (Kornreich et al., 2001; Kornreich et al., 2002; Foisy et al., 2007; Maurage et al., 2008), recovery of these functions has been reported in relation to relapse rates. For example, Witkiewitz and Aracelliz (2009) found in a longitudinal study that negative affect such as depression and anger contributed to relapse risk. Also, Berking et al. (2011) found that deficits in emotion-regulation skills, especially the ability to tolerate negative emotions, predicted relapse three months after cognitive behavioral treatment for alcoholism. Impairments in decoding of emotional facial expressions are related to interpersonal problems (Kornreich et al., 2002) and may contribute to relapse risk. As such, social and interpersonal skills training for individuals in recovery have focused on the overestimation of intensity of emotions, and misattribution of negative emotions such as anger and fear have been developed as components of cognitive behavioral treatments in relapse prevention (for reviews, see Morgenstern and Longabaugh, 2000; Witkiewitz and Marlatt, 2004). Additionally, social-cognitive and emotional-processing factors may affect the recovery of alcoholic men and alcoholic women differently as gender differences in interpersonal problem solving skills have been reported (Nixon et al., 1992). In any case, abstinence has been associated with increases in positive social relationships (Hibbert and Best, 2011), improved self-efficacy and coping abilities (Rose et al., 2012), and higher rates of help seeking behavior (Dawson et al., 2006).
In summary, alcoholics have been shown to have dysfunctional social and emotional processing abilities. Although those problems continue after abstinence, recovery of emotional and psychosocial functioning is central to positive treatment outcomes, relapse prevention, and maintenance of significant relationships.
4. Visuospatial Cognition
Visuospatial cognition refers to visuoperceptual, visuospatial, and visuoconstruction abilities involving conscious and non-conscious visually guided competencies. These abilities are defined operationally in terms of performance on specific tests. Visuoperceptual skills are reflected in the ability to identify stimuli such as objects, faces, and patterns. Visuospatial skills involve the localization of objects in space, navigation, and conceptualization of distances, areas, and volumes. Visuoconstructive abilities involve organizing elements into correct spatial relationships such as when drawing a house or doing a jigsaw puzzle. Alcoholism-related impairments in visuospatial cognition are among the most frequently reported.
Impairments
Neuropsychological tests consistently have shown that alcoholics suffer from persistent deficits in visuospatial cognition (Beatty et al., 1996; Oscar-Berman and Schendan, 2000; Sullivan et al., 2000b; Sullivan et al., 2002b; Fama et al., 2004; Fein et al., 2006; Sullivan et al., 2010). Early demonstrations of these deficits in alcoholics were apparent as relatively lower scores on Wechsler Adult Intelligence Scale Performance subtests compared to Verbal subtests (reviewed by Ellis and Oscar-Berman, 1989). This discrepancy was largely accounted for by scores on three of the individual Performance subtests, which relied strongly on visuospatial cognitive capacities: Block Design, Object Assembly, and Digit Symbol. Other examples of tests on which alcoholics have performed poorly are the Wechsler Memory Scale Drawing Test (Sullivan et al., 2000b), a letter/symbol cancellation test (Beatty et al., 1996), the Mental Rotation Test (Beatty et al., 1996), the Hidden Figures Test (Sullivan et al., 2000b; Fama et al., 2004), the Rey-Osterrieth Complex Figure Test (Beatty et al., 1996; Sullivan et al., 2000b), and the Gollin Incomplete Pictures Test (Sullivan et al., 2000b; Fama et al., 2004). These tests clearly do not measure homogeneous abilities. Despite the array of subcomponents that have been examined, however, alcoholics’ impairments in visuospatial cognition are evident and endure over time.
In accordance with the neuropsychological deficits observed, brain abnormalities have been identified in a large network of interconnected areas communicating with each other in feedforward and feedback loops to serve visuospatial cognition. Cortical regions throughout the entire cerebrum are involved, as well as the cerebellum and the thalamus (reviewed by Fitzpatrick et al., 2008, 2012). Morphometry studies have shown volume deficits among alcoholics, compared with controls, within the parietal lobes (Fein et al., 2002; Gazdzinski et al., 2005b; Chanraud et al., 2007), and cortical shrinkage in the parietal lobes has been associated with poor performance in spatial processing (Fein et al., 2009). Although parietal cortical volume (measured with conventional MRI) was not a predictor of visuospatial ability in alcoholics, cerebellar hemispheric white matter was such a predictor (Sullivan, 2003). Rosenbloom et al. (2009) examined associations between regional white matter microstructural integrity (measured with DTI) and visuoconstruction accuracy (as measured by the Rey-Osterrieth Complex Figure Test) in alcoholics and healthy controls. For both groups, the investigators found an association between poorer copy accuracy and increased radial diffusivity in the occipital forceps and external capsule. In the alcoholics, poor immediate recall of the Figure was associated with compromised fiber microstructure (lower fractional anisotropy) in inferior cingulate bundles.
Compensation and recovery
Despite the persistence of deficits in visuospatial cognition after years of abstinence, when intact performance levels are observed, they have been attributed to reorganization of brain functioning (Pfefferbaum et al., 2001a). For example, Fama et al. (2004) reported that whereas visuospatial abilities predicted performance on visuoperception tasks by nonalcoholic control participants, for alcoholics it was executive ability, as measured by the Wisconsin Card Sorting Test, that consistently predicted performance on visuoperception tasks. Because completing the Wisconsin Card Sorting Test relies heavily on frontal brain circuitry, the authors suggested that alcoholics likely engage additional frontal systems to perform visuoperceptal tasks at the level of nonalcoholics. In another study, Rosenbloom et al. (2004) compared the cognitive performance of alcoholic and nonalcoholic women across three durations of abstinence (three months, one year, and four years) and found better performance with increased lengths of sobriety for visuospatial scores as measured by the Digit Symbol subtest of the Wechsler Adult Intelligence Scale. After four years of abstinence, the alcoholic women had achieved a performance level that was not significantly different from that of nonalcoholic women.
In summary, a considerable body of research indicates selective deficiencies of multiple facets of visuospatial cognition in association with long-term alcoholism. There is evidence that some of these deficits may recover with abstinence, and that improvements are associated with reorganized brain functioning. In other words, the abilities that appear to have remained intact or recovered through significant lengths of abstinence may be explained by recourse to the compensatory action of recruitment of other brain regions through a dynamic process of reorganization.
5. Psychomotor Abilities
Psychomotor abilities include skills such as quick reaction time, grip strength, fine-finger movement, postural abilities, gait, and balance. Persistent alcoholism-related psychomotor impairments are characteristic of the disorder.
Impairments
Accounts of impairments in reaction time pervade the literature on alcoholism and range from simple tests of psychomotor speed to complex tasks that incorporate higher-level skills. An example of the former was reported by Pfefferbaum et al. (2009) who tested alcoholics and controls for fine-finger movement. The test was timed and required that participants turn a knurled post with the index finger and thumb of each hand separately, and then both hands. The alcoholics were slower than controls on both the unimanual and bimanual conditions. In another study, Kopera et al. (2012) found that alcoholics performed poorly on a simple reaction time task that was part of a larger neuropsychological assessment battery. The task required participants to push a button in response to a stimulus that appeared at random intervals in the same location on a computer screen. In a similar study, Ratti et al. (2002) found that alcoholics were impaired in both simple and choice reaction times. In the simple reaction time task, participants released or pressed a key when a target appeared. In the choice reaction time task, a stimulus could appear in one of three locations, and participants had to press a corresponding key. In both of these studies, the authors suggested that the motor response speeds themselves were not necessarily impaired, but the slower reaction times likely reflected reduced cognitive processing speed. In fact, some researchers have proposed that alcoholics are impaired in cognitive efficiency, that is, the ability to process information both quickly and accurately. In other words, alcoholics demonstrate a speed-accuracy trade-off, such that greater emphasis is placed on responding accurately at the expense of speed, leading to decrements in efficiency (Sullivan et al., 2002a). This conjecture appears confirmed by additional reports of deficits in reaction time, which have ranged from simple target-striking tasks (York and Biederman, 1991) to delays in identifying emotional expressions in facial stimuli (Maurage et al., 2008; Marinkovic et al., 2009; Fein et al., 2010), and performing executive functioning tasks (Pandey et al., 2012).
Although upper limb strength usually remains intact, alcoholics often tend to demonstrate deficits in lower limb competencies, such as posture, gait, and balance (Sullivan et al., 2000b). A number of studies have tested alcoholic and control participants on the Walk-a-Line Ataxia Battery (Sullivan et al., 2000b; Pfefferbaum et al., 2006; Smith and Fein, 2011; Fein and Greenstein, 2013), which consists of three parts, each to be performed with eyes open and then closed. First, participants stand with feet placed heel-to-toe and arms folded across the chest; next, participants stand on one foot; finally, participants walk heel-to-toe. Each of these studies showed impairments in alcoholics; the deficits were persistent and slow to improve (Fein and Greenstein, 2013). Finally, lifetime alcohol consumption, disease duration, and age have been negatively associated with scores on the International Cooperative Ataxia Rating Scale, representing not only poor posture and gait, but also kinetic, speech, and oculomotor abilities (Fitzpatrick et al., 2012).
Neuroimaging has augmented many of these neuropsychological measures in understanding the psychomotor functioning deficits in alcoholics. Rogers et al. (2012) investigated the functional connectivity between cerebellar and cortical brain regions in alcoholic patients. The connectivity analyses focused on cortical regions that exhibited fMRI responses associated with nondominant hand finger tapping in the patients, but not in controls. Functional connectivity in the patients was attenuated in circuits involving (a) premotor areas and the superior cerebellum and (b) prefrontal cortex and the inferior cerebellum. This pattern suggested that structural damage within frontocerebellar circuitry might be responsible for the motor deficits. However, because the alcoholics were abstinent for short durations (average of five days), the results might not reflect persistent effects. Pfefferbaum et al. (2009) also used DTI to assess white matter structural integrity in conjunction with psychomotor measures of balance and response speed. Balance was measured by requiring participants to stand on one foot, and response speed was measured when participants turned a knurled post between the thumb and forefinger, and when participants performed the Digit Symbol subtest of the Wechsler Adult Intelligence Scale. Alcoholics had poorer balance, and they were slower on the speed tests. Poorer performance on all tests correlated with DTI measures of regional white matter degradation in frontal and occipital forceps, internal and external capsules, fornix, and inferior cingulate bundle.
Compensation and recovery
Psychomotor functions, including postural and gait stability, have varying rates of recovery in relation to length of abstinence and gender. Sullivan and colleagues found marginally improved gait and balance in abstinent alcoholic men tested after one month of sobriety and again two to 12 months later (Sullivan et al., 2000a; Sullivan et al., 2000b). However, Rosenbloom et al. (2004) found that alcoholic women tested at 15 weeks of sobriety, and then retested one and four years later, still showed persistent deficits in gait and balance, although there were improvements in psychomotor speed. Smith and Fein (2011) found that alcoholic men and women with multi-year abstinence (1.5 to 22.4 years) showed more normal gait and balance than alcoholics with short-term (6 to 15 weeks) abstinence. For the short-term abstinent alcoholics only, gender comparisons indicated that women performed worse than men on two static balance measures with eyes closed (standing heel-to-toe and standing on left leg).
Parks and colleagues used fMRI to explore brain activation patterns during finger-tapping tasks performed by recently abstinent alcoholics (less than two weeks on average) and control participants (Parks et al., 2003; Parks et al., 2010). In the 2003 study, alcoholics were significantly slower than controls. Although alcoholics’ brain activation patterns were more extensive in motor and premotor cortex and in cerebellar regions compared to the controls, a measure of efficiency of brain activation (tapping rate per unit of brain activation) suggested motor inefficiency and compensatory alterations of corticocerebellar circuits. In the 2010 study, Parks and colleagues were able to equate the groups on self-paced and externally-paced finger-tapping rates, and still found that brain activation patterns for alcoholics differed from controls. Under both conditions, tapping with the nondominant hand was associated with more activation by the alcoholics in frontal, parietal, and temporal brain regions. Additionally, the self-paced tapping activated frontocerebellar networks in the controls, but only precuneus in the alcoholics. Thus, rather than enlisting a frontocerebellar network, as did the controls, the alcoholics recruited a higher-order, “more effortful” or “less automatic,” parietal planning mode not required by the controls. The authors concluded that their findings reflected different neurocognitive strategies and brain circuitries used by alcoholics and controls to complete the task.
In summary, severe deficits in psychomotor abilities demonstrated by alcoholics are associated with functional and structural brain abnormalities. Improvements in these abilities tend to correspond to duration of abstinence, and may differ for men and women. Additionally, to compensate for behavioral inefficiencies, alcoholics may employ brain circuitry that differs from nonalcoholics.
Summary and Conclusions
Alcoholism has heterogeneous origins and manifestations, depending upon variables such as family history, age, gender, and mental or physical health. Consequently, the neuropsychological profiles associated with alcoholism are not uniform among individuals. Within and across research studies, variability among participants is substantial and contributes to the characteristics associated with differential outcomes. Nevertheless, evidence for impairments of neuropsychological processes following years of chronic alcoholism is unequivocal. These impairments are accompanied by structural damage to gray and white matter in the brains of alcoholics, and are measurable on both macrostructural and microstructural levels. The profiles of impairments among alcoholics show that some neuropsychological processes are more permanently disrupted than others. Memory, executive functions, emotion and psychosocial skills, visuospatial cognition, and psychomotor abilities are particularly affected. In accordance with this pattern of deficits, the brain networks that underlie the most impaired functions involve the frontocerebellar system and mesocorticolimbic circuitry.
Over time, usually with abstinence from alcohol, behavioral functions can appear relatively unimpaired. Although it can be difficult to distinguish processes that have been spared, compensated for, or recovered, the brain appears to become reorganized to provide compensation for structural and behavioral deficits. Longitudinal studies have provided clear evidence for recovery of both neuropsychological functioning and brain tissues. Further support for recovery of brain volumes with abstinence has come from cross-sectional studies that have demonstrated positive associations between prolonged abstinence and larger volumes of cortical and subcortical gray matter, and cortical and callosal white matter. Despite substantial evidence for recovery of neuropsychological performance and brain structure with sobriety, some abnormalities persist through abstinence, especially problems with executive functioning, visuospatial cognition, and motor functions.
Because of differences in participant characteristics and methodological variations across studies, aspects of neurobehavioral and brain disruption and repair remain insufficiently documented. However, by relying on a combination of clinical and scientific approaches, including the continued use of longitudinal studies and multiple neuroimaging technologies, future research will help to refine the compensatory roles of healthy brain systems, the degree to which abstinence and treatment facilitate the reversal of brain atrophy and dysfunction, and the importance of individual differences to outcome. Ultimately, an important goal of neuropsychological and neuroimaging research into profiles of impaired, spared, and recovered functions in alcoholism is to inform successful strategies for prevention and treatment. Commendable advances already have been made.
Acknowledgements
The writing of this chapter was supported by NIH grants NIAAA R01-AA07112 and NIAAA K05-AA00219, and funds from the Department of Veterans Affairs (VA) Medical Research Service. We thank Benjamin Thompson for his helpful suggestions.
Appendix
Appendix Table 1.
References to research reviewed in this chapter. The table is organized by neuropsychological domain and assessment tool, and it indicates findings of (a) residual impairments associated with long-term chronic alcoholism, and (b) compensation or recovery of functions with abstinence. Abbreviations: BADS, Behavioral Assessment of the Dysexecutive Syndrome; CANTAB, Cambridge Neuropsychological Test Automated Battery; COWAT, Controlled Oral Word Association Test; ICARS, International Cooperative Ataxia Rating Scale; IQ, Intelligence Quotient; POMS, Profile of Mood States; SILS, Shipley Institute of Living Scale; STM, Short-Term Memory; WAIS, Wechsler Adult Intelligence Scale; WASI, Wechsler Abbreviated Scale of Intelligence; WMS, Wechsler Memory Scale. For descriptions of most tests cited, refer to Lezak et al. (2012) and Strauss et al. (2006).
Appendix Table 2.
References to research reviewed in this chapter. The table is organized by anatomical regions, and it indicates neuroimaging findings based upon brain functional and structural methods. The names of the anatomical regions are listed as given by the researchers in the citations. All impairments are compared to healthy controls. Abbreviations: ADC, apparent diffusion coefficient; FA, fractional anisotropy; MD, mean diffusivity.
| VOLUMETRIC IMPAIRMENTS |
VOLUMETRIC RECOVERY |
|||
|---|---|---|---|---|
|
TOTAL BRAIN VOLUME |
Pfefferbaum et al., 1992; Mann et al., 2005; Chen et al., 2012 |
Gazdzinski et al., 2005a (1 month follow-up); Mann et al., 2005 (5 week follow-up) |
||
| FUNCTIONAL BRAIN ABNORMALITIES | ||||
|
TOTAL GRAY MATTER |
Fein et al., 2009; Chen et al., 2012 | |||
|
Total Cortical Gray Matter |
Pfefferbaum et al., 1992; Pfefferbaum et al., 2001b; Makris et al., 2008 Pfefferbaum et al., 1992; |
|||
| Frontal Lobes |
Pfefferbaum et al., 1998; Gazdzinski et al., 2005b |
|||
| Prefrontal | Pfefferbaum et al., 1992 | Sullivan, 2003 | ||
|
Dorsolateral prefrontal cortex |
Cardenas et al., 2007; Chanraud et al., 2007; Makris et al., 2008 |
Pfefferbaum et al., 2001a (2-Back Task); Akine et al., 2007 (Modified False Recognition Task); Parks et al., 2010 (Finger-Tapping) |
||
|
Ventrolateral prefrontal cortex |
Maurage et al., 2012b (Cyberball Task) | |||
| Orbitofrontal cortex |
Demirakca et al., 2011 (3 month follow-up) |
O’Daly et al., 2012 (Facial Expression Recognition Task) |
||
| Superior frontal gyrus |
Chanraud-Guillermo et al., 2009 (Auditory Language Task); Maurage et al., 2012a (Face- Voice Interaction Task) |
|||
| Middle frontal gyrus |
Pfefferbaum et al., 2001a (2-Back Task); Chanraud-Guillermo et al., 2009 (Auditory Language Task); Maurage et al., 2012a (Face- Voice Interaction Task); Maurage et al., 2012b (Cyberball Task); Pitel et al., 2012a (Face-Name Associative Learning Task) |
|||
| Inferior frontal gyrus |
Pfefferbaum et al., 2001a (2-Back Task); Pitel et al., 2012a (Face-Name Associative Learning Task) |
|||
|
Pre-central gyrus (primary motor cortex) |
Fein et al., 2009 |
Pfefferbaum et al., 2001a (2-Back Task); Parks et al., 2003 (Finger-Tapping Task); Maurage et al., 2012a (Face-Voice Interaction Task); O’Daly et al., 2012 (Facial Expression Recognition Task); Pitel et al., 2012a (Face-Name Associative Learning Task) |
||
| Cingulate Gyrus |
Demirakca et al., 2011 (3 month follow-up) |
Maurage et al., 2012a (Face-Voice Interaction Task) |
||
| Anterior cingulate |
Chanraud et al., 2007; Fein et al., 2009 |
Akine et al., 2007 (Modified False Recognition Task); Salloum et al., 2007 (Emotional Facial Expressions) |
||
|
Middle cingulate gyrus |
Pitel et al., 2012a (Face-Name Associative Learning Task) |
|||
|
Posterior cingulate cortex |
Maurage et al., 2012a (Face-Voice Interaction Task); Schulte et al., 2012a (Stroop Match-to- Sample Task) |
|||
| Parietal Lobes |
Pfefferbaum et al., 1992; Gazdzinski et al. 2005a; Fein et al., 2009 |
|||
| Superior parietal |
Maurage et al., 2012a (Face-Voice Interaction Task); Pitel et al., 2012a (Face-Name Associative Learning Task) |
|||
| Mesial parietal | Fein et al., 2009 | |||
| Lateral parietal | Fein et al., 2009 | |||
| Inferior parietal |
Pfefferbaum et al., 2001a (2-Back Task); Pitel et al., 2012a (Face-Name Associative Learning Task) |
|||
| Supramarginal gyrus | ||||
| Angular gyrus |
Pfefferbaum et al., 2001a (2-Back Task); Pitel et al., 2012a (Face-Name Associative Learning Task) |
|||
|
Post-central gyrus (primary somatosensory cortex) |
Fein et al., 2009 | Parks et al., 2010 (Finger-Tapping) | ||
| Temporo-parietal | Pfefferbaum et al., 1992 | |||
|
Dorsolateral frontal & parietal |
Chanraud et al., 2007 | |||
| Precuneus |
Maurage et al., 2012a (Face-Voice Interaction Task) |
|||
| Insular Cortex |
Chanraud et al 2007; Makris et al., 2008; Fein et al., 2009 |
Demirakca et al., 2011 (3 month follow-up) |
Gizewski et al., 2013 (modified Reading the Mind In The Eyes Test); Maurage et al., 2012b (Cyberball Task); O’Daly et al., 2012 (Facial Expression Recognition Task); Pitel et al., 2012a (Face-Name Associative Learning Task) |
|
| Temporal Lobes |
Gazdzinski 2005b; Cardenas et al., 2007 |
|||
| Superior temporal | Chanraud et al., 2007 |
Maurage et al., 2012a (Face-Voice Interaction Task); Pitel et al., 2012a (Face-Name Associative Learning Task) |
||
|
Inferior temporal (lingual gyrus) |
Chanraud et al., 2007; Fein et al., 2009 |
|||
| Fronto-temporal | Pfefferbaum et al., 1992 | |||
| Middle temporal |
Maurage et al., 2012a (Face-Voice Interaction Task) |
|||
|
Parahippocampal gyrus |
||||
| Fusiform gyrus |
Maurage et al., 2012a (Face-Voice Interaction Task) |
|||
| Occipital Lobes | Fein et al., 2009 | |||
| Parieto-occipital | Pfefferbaum et al., 1992 | |||
| Medial occipital |
Maurage et al., 2012a (Face-Voice Interaction Task) |
|||
| Inferior occipital |
Maurage et al., 2012a (Face-Voice Interaction Task) |
|||
|
Visual association (in occipital lobe) |
Fein et al., 2009 | Pfefferbaum et al., 2001a (2-back task) | ||
|
Primary visual (in occipital lobe) |
Fein et al., 2009 | |||
| Calcarine gyrus |
Pitel et al., 2012a (Face-Name Associative Learning Task) |
|||
| Total Subcortical | Pfefferbaum et al., 1992 | |||
| Thalamus |
Gazdzinski et al., 2005b; Chanraud et al., 2007 |
Akine et al., 2007 (Modified False Recognition Task) |
||
| Ventral striatum |
Akine et al., 2007 (Modified False Recognition Task) |
|||
| Nucleus accumbens | Makris et al., 2008 | |||
| Caudate |
Pitel et al., 2012a (Face-Name Associative Learning Task) |
|||
| Amygdala |
Makris et al., 2008; Wobrock et al., 2009 |
Marinkovic et al., 2009 (Face-Encoding Task); O’Daly et al., 2012 (Facial Expression Recognition Task) |
||
| Hippocampus |
Sullivan et al., 1995; Harding et al., 1997; Agartz et al., 1999 |
Marinkovic et al., 2009 (Face-Encoding Task) | ||
| Midbrain |
Schulte et al., 2012a (Stroop Match-to-Sample Task) |
|||
| Pons | Sullivan, 2003; Chanraud et al., 2007 | |||
| Total Cerebellum | Chanraud et al., 2007 | |||
| Cerebellar other | Chanraud et al., 2007 |
Parks et al., 2003 (Finger-Tapping Task); Chanraud-Guillermo et al., 2009 (Auditory Language Task); Pitel et al., 2012a (Face-Name Associative Learning Task) |
||
|
DIFFUSION IMPAIRMENTS |
DIFFUSION RECOVERY |
|||
|
TOTAL WHITE MATTER (WM) |
Agartz et al., 2003; Demirakca et al., 2011 (3 month follow-up) |
|||
| Total Cerebral WM |
Harper et al., 1990; Pfefferbaum et al., 1992, 1995, 2001b; Chen et al., 2012 |
|||
| Frontal WM |
Gazdzinski et al., 2005b; Chanraud et al., 2007 |
Gazdzinski et al., 2010b (1 month follow-up) |
Gazdzinski et al., 2010b (high MD) |
Gazdzinski et al., 2010b (increased MD after 1 week of abstinence, decreased MD and increased FA after 1 month of abstinence) |
| Frontal forceps |
Pfefferbaum et al., 2009 (low FA, high ADC); Rosenbloom et al., 2009 (low FA) |
|||
| Parietal WM | Gazdzinski et al., 2005b |
Gazdzinski et al., 2010b (high MD) |
Gazdzinski et al., 2010b (increased MD after 1 week of abstinence) |
|
| Temporal WM | Chanraud et al., 2007 |
Gazdzinski et al., 2010b (high MD) |
Gazdzinski et al., 2010b (decreased MD and increased FA after 1 month of abstinence) |
|
| Occipital WM |
Gazdzinski et al., 2010b (increased MD after 1 week of abstinence) |
|||
| Cingulum bundle |
Harris et al., 2008 (low FA); Pfefferbaum et al., 2009 (low FA, high ADC); Rosenbloom et al., 2009 (high ADC) |
|||
| Corpus callosum |
Agartz et al., 1999; Pfefferbaum et al., 1996, 2006; Chanraud et al., 2007; Ruiz et al., 2013 |
Muller-Oehring et al., 2009; Alhassoon et al., 2012 (low FA) |
||
| Internal capsule |
Pfefferbaum et al., 2009 (high ADC) |
|||
| External capsule |
Pfefferbaum et al., 2009 (high ADC); Rosenbloom et al., 2009 (high ADC) |
|||
|
Superior longitudinal fasciculus |
Harris et al., 2008 (low FA); Pfefferbaum et al., 2009 (high ADC); Rosenbloom et al., 2009 (high ADC) |
|||
| Fornix |
Pfefferbaum et al., 2009 (high ADC) |
|||
|
Superior temporal gyrus WM |
Harris et al., 2008 (low FA) |
|||
| Arcuate fasciculus |
Harris et al., 2008 (low FA) |
|||
|
Fronto-temporal junction, limen insula |
Harris et al., 2008 (low FA) |
|||
|
Anterior paracingulate WM |
Chanraud et al., 2007 | |||
| Uncinate fasciculus |
Schulte et al., 2012b (low FA) |
|||
|
TOTAL VENTRICLES OR CSF |
Pfefferbaum et al., 1992; Agartz et al., 1999; Kubota et al., 2001; Bendszus et al., 2001; Mann et al., 2005; Chanraud et al., 2007; Chen et al., 2012 |
Carlen et al., 1978; Gazdzinski et al., 2005a (1 month follow-up); Mann et al., 2005 (5 week follow-up), Demirakca et al., 2011 (3 month follow-up) |
||
| Lateral ventricles |
Pfefferbaum et al., 1992, 1998, 2001b |
Zipursky et al., 1989 (19–28 day follow-up); Wobrock et al., 2009 (6 to 9 month follow-up) |
||
| Third ventricle |
Pfefferbaum et al., 1992, 2001b |
|||
|
Sulcal or extracerebral CSF |
Pfefferbaum et al., 1998, 2001b; Mann et al., 2005 |
Mann et al., 2005 (5 week follow-up) |
||
Contributor Information
Marlene Oscar-Berman, Boston University School of Medicine, L-815, 72 E. Newton St., Boston, MA 02118; oscar@bu.edu; telephone 617-638-4803
Mary M. Valmas, Boston University School of Medicine, L-815, 72 E. Newton St., Boston, MA 02118; mvalmas@bu.edu; telephone 617-638-4803.
Kayle S. Sawyer, Boston University School of Medicine, L-815, 72 E. Newton St., Boston, MA 02118; kslays@bu.edu; telephone 617-638-4803.
Susan Mosher Ruiz, Boston University School of Medicine, L-815, 72 E. Newton St., Boston, MA 02118; smosher@bu.edu; telephone 617-638-4803.
Riya B. Luhar, Boston University School of Medicine, L-815, 72 E. Newton St., Boston, MA 02118; rbluhar@bu.edu; telephone 617-638-4803.
Zoe R. Gravitz, Boston University School of Medicine, L-815, 72 E. Newton St., Boston, MA 02118; zgravitz@bu.edu; telephone 617-638-4803.
References
- Agartz I, Brag S, Franck J, et al. MR volumetry during acute alcohol withdrawal and abstinence: A descriptive study. Alcohol. 2003;38:71–78. doi: 10.1093/alcalc/agg020. [DOI] [PubMed] [Google Scholar]
- Agartz I, Momenan R, Rawlings RR, et al. Hippocampal volume in patients with alcohol dependence. Arch Gen Psychiatry. 1999;56:356–363. doi: 10.1001/archpsyc.56.4.356. [DOI] [PubMed] [Google Scholar]
- Akine Y, Kato M, Muramatsu T, et al. Altered brain activation by a false recognition task in young abstinent patients with alcohol dependence. Alcohol Clin Exp Res. 2007;31:1589–1597. doi: 10.1111/j.1530-0277.2007.00453.x. [DOI] [PubMed] [Google Scholar]
- Alhassoon OM, Sorg SF, Taylor MJ, et al. Callosal white matter microstructural recovery in abstinent alcoholics: A longitudinal diffusion tensor imaging study. Alcohol Clin Exp Res. 2012;36:1–10. doi: 10.1111/j.1530-0277.2012.01808.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez JA, Emory E. Executive function and the frontal lobes: A meta-analytic review. Neuropsychol Rev. 2006;16:17–42. doi: 10.1007/s11065-006-9002-x. [DOI] [PubMed] [Google Scholar]
- Andó B, Must A, Kurgyis E, et al. Personality traits and coping compensate for disadvantageous decision-making in long-term alcohol abstinence. Alcohol Alcohol. 2012;47:18–24. doi: 10.1093/alcalc/agr144. [DOI] [PubMed] [Google Scholar]
- Bartsch AJ, Homola G, Biller A, et al. Manifestations of early brain recovery associated with abstinence from alcoholism. Brain. 2007;130:36–47. doi: 10.1093/brain/awl303. [DOI] [PubMed] [Google Scholar]
- Bates ME, Bowden SC, Barry D. Neurocognitive impairment associated with alcohol use disorders: Implications for treatment. Exp Clin Psychopharmacol. 2002;10:193–212. doi: 10.1037//1064-1297.10.3.193. [DOI] [PubMed] [Google Scholar]
- Beatty WW, Hames KA, Blanco CR, et al. Visuospatial perception, construction and memory in alcoholism. J Stud Alcohol. 1996;57:136–143. doi: 10.15288/jsa.1996.57.136. [DOI] [PubMed] [Google Scholar]
- Beatty WW, Tivis R, Stott HD, et al. Neuropsychological deficits in sober alcoholics: Influences of chronicity and recent alcohol consumption. Alcohol Clin Exp Res. 2000;24:149–154. [PubMed] [Google Scholar]
- Bendszus M, Weijers HG, Wiesbeck G, et al. Sequential MR imaging and proton MR spectroscopy in patients who underwent recent detoxification for chronic alcoholism: Correlation with clinical and neuropsychological data. Am J Neuroradiol. 2001;22:1926–1932. [PMC free article] [PubMed] [Google Scholar]
- Berking M, Margraf M, Ebert D, et al. Deficits in emotion-regulation skills predict alcohol use during and after cognitive behavioral therapy for alcohol dependence. J Consult Clin Psychol. 2011;79:307–318. doi: 10.1037/a0023421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brust JC. Ethanol and cognition: Indirect effects, neurotoxicity and neuroprotection: A review. Int J Environ Res Public Health. 2010;7:1540–1557. doi: 10.3390/ijerph7041540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buhler M, Mann K. Alcohol and the human brain: A systematic review of different neuroimaging methods. Alcohol Clin Exp Res. 2011;35:1771–1793. doi: 10.1111/j.1530-0277.2011.01540.x. [DOI] [PubMed] [Google Scholar]
- Cardenas VA, Durazzo TC, Gazdzinski S, et al. Brain morphology at entry into treatment for alcohol dependence is related to relapse propensity. Biol Psychiatry. 2011;70:561–567. doi: 10.1016/j.biopsych.2011.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardenas VA, Studholme C, Gazdzinski S, et al. Deformation-based morphometry of brain changes in alcohol dependence and abstinence. Neuroimage. 2007;34:879–887. doi: 10.1016/j.neuroimage.2006.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardenas VA, Studholme C, Meyerhoff DJ, et al. Chronic active heavy drinking and family history of problem drinking modulate regional brain tissue volumes. Psychiatry Res. 2005;138:115–130. doi: 10.1016/j.pscychresns.2005.01.002. [DOI] [PubMed] [Google Scholar]
- Carlen PL, Wortzman G, Holgate RC, et al. Reversible cerebral atrophy in recently abstinent chronic alcoholics measured by computed tomography scans. Science. 1978;200:1076–1078. doi: 10.1126/science.653357. [DOI] [PubMed] [Google Scholar]
- Chanraud S, Leroy C, Martelli C, et al. Episodic memory in detoxified alcoholics: Contribution of grey matter microstructure alteration. PLoS One. 2009;4:e6786. doi: 10.1371/journal.pone.0006786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chanraud S, Martelli C, Delain F, et al. Brain morphometry and cognitive performance in detoxified alcohol-dependents with preserved psychosocial functioning. Neuropsychopharmacology. 2007;32:429–438. doi: 10.1038/sj.npp.1301219. [DOI] [PubMed] [Google Scholar]
- Chanraud S, Pitel AL, Muller-Oehring EM, et al. Remapping the brain to compensate for impairment in recovering alcoholics. Cereb Cortex. 2013;23:97–104. doi: 10.1093/cercor/bhr381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chanraud S, Pitel AL, Pfefferbaum A, et al. Disruption of functional connectivity of the default-mode network in alcoholism. Cereb Cortex. 2011;21:2272–2281. doi: 10.1093/cercor/bhq297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chanraud S, Pitel AL, Rohlfing T, et al. Dual tasking and working memory in alcoholism: Relation to frontocerebellar circuitry. Neuropsychopharmacology. 2010;35:1868–1878. doi: 10.1038/npp.2010.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chanraud-Guillermo S, Andoh J, Martelli C, et al. Imaging of language-related brain regions in detoxified alcoholics. Alcohol Clin Exp Res. 2009;33:977–984. doi: 10.1111/j.1530-0277.2009.00918.x. [DOI] [PubMed] [Google Scholar]
- Charness ME. Overview of the chronic neurologic complications of alcohol. 2010:1–12. http://www.uptodate.com.
- Chen CH, Walker J, Momenan R, et al. Relationship between liver function and brain shrinkage in patients with alcohol dependence. Alcohol Clin Exp Res. 2012;36:625–632. doi: 10.1111/j.1530-0277.2011.01662.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cisek P, Kalaska JF. Neural mechanisms for interacting with a world full of action choices. Annu Rev Neurosci. 2010;33:269–298. doi: 10.1146/annurev.neuro.051508.135409. [DOI] [PubMed] [Google Scholar]
- Clark US, Oscar-Berman M, Shagrin B, et al. Alcoholism and judgments of affective stimuli. Neuropsychology. 2007;21:346–362. doi: 10.1037/0894-4105.21.3.346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosgrove KP, Esterlis I, Mason GF, et al. Neuroimaging insights into the role of cortical GABA systems and the influence of nicotine on the recovery from alcohol dependence. Neuropharmacology. 2011;60:1318–1325. doi: 10.1016/j.neuropharm.2011.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crews FT, Boettiger CA. Impulsivity, frontal lobes and risk for addiction. Pharmacol Biochem Behav. 2009;93:237–247. doi: 10.1016/j.pbb.2009.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crews FT, Buckley T, Dodd PR, et al. Alcoholic neurobiology: Changes in dependence and recovery. Alcohol Clin Exp Res. 2005;29:1504–1513. doi: 10.1097/01.alc.0000175013.50644.61. [DOI] [PubMed] [Google Scholar]
- Davies SJ, Pandit SA, Feeney A, et al. Is there cognitive impairment in clinically 'healthy' abstinent alcohol dependence? Alcohol Alcohol. 2005;40:498–503. doi: 10.1093/alcalc/agh203. [DOI] [PubMed] [Google Scholar]
- Dawson DA, Grant BF, Stinson FS, et al. Maturing out of alcohol dependence: The impact of transitional life events. J Stud Alcohol. 2006;67:195–203. doi: 10.15288/jsa.2006.67.195. [DOI] [PubMed] [Google Scholar]
- Demirakca T, Ende G, Kammerer N, et al. Effects of alcoholism and continued abstinence on brain volumes in both genders. Alcohol Clin Exp Res. 2011;35:1678–1685. doi: 10.1111/j.1530-0277.2011.01514.x. [DOI] [PubMed] [Google Scholar]
- Desmond JE, Chen SH, DeRosa E, et al. Increased frontocerebellar activation in alcoholics during verbal working memory: An fMRI study. Neuroimage. 2003;19:1510–1520. doi: 10.1016/s1053-8119(03)00102-2. [DOI] [PubMed] [Google Scholar]
- Devaud LL, Prendergast MA. Introduction to the special issue of Alcohol and Alcoholism on sex/gender differences in responses to alcohol. Alcohol Alcohol. 2009;44:533–534. doi: 10.1093/alcalc/agp046. [DOI] [PubMed] [Google Scholar]
- Di Sclafani V, Finn P, Fein G. Psychiatric comorbidity in long-term abstinent alcoholic individuals. Alcohol Clin Exp Res. 2007;31:795–803. doi: 10.1111/j.1530-0277.2007.00361.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Sclafani V, Finn P, Fein G. Treatment-naive active alcoholics have greater psychiatric comorbidity than normal controls but less than treated abstinent alcoholics. Drug Alcohol Depend. 2008;98:115–122. doi: 10.1016/j.drugalcdep.2008.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durazzo TC, Gazdzinski S, Mon A, et al. Cortical perfusion in alcohol-dependent individuals during short-term abstinence: Relationships to resumption of hazardous drinking after treatment. Alcohol. 2010;44:201–210. doi: 10.1016/j.alcohol.2010.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durazzo TC, Rothlind JC, Gazdzinski S, et al. A comparison of neurocognitive function in nonsmoking and chronically smoking short-term abstinent alcoholics. Alcohol. 2006;39:1–11. doi: 10.1016/j.alcohol.2006.06.006. [DOI] [PubMed] [Google Scholar]
- Durazzo TC, Tosun D, Buckley S, et al. Cortical thickness, surface area, and volume of the brain reward system in alcohol dependence: relationships to relapse and extended abstinence. Alcohol Clin Exp Res. 2011;35:1187–1200. doi: 10.1111/j.1530-0277.2011.01452.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis RJ, Oscar-Berman M. Alcoholism, aging, and functional cerebral asymmetries. Psychol Bull. 1989;106:128–147. doi: 10.1037/0033-2909.106.1.128. [DOI] [PubMed] [Google Scholar]
- Erickson CK, White WL. The neurobiology of addiction recovery. Alcohol Treat Q. 2009;27:338–345. [Google Scholar]
- Fama R, Pfefferbaum A, Sullivan EV. Perceptual learning in detoxified alcoholic men: Contributions from explicit memory, executive function, and age. Alcohol Clin Exp Res. 2004;28:1657–1665. doi: 10.1097/01.alc.0000145690.48510.da. [DOI] [PubMed] [Google Scholar]
- Fama R, Rosenbloom MJ, Nichols BN, et al. Working and episodic memory in HIV infection, alcoholism, and their comorbidity: Baseline and 1-year follow-up examinations. Alcohol Clin Exp Res. 2009;33:1815–1824. doi: 10.1111/j.1530-0277.2009.01020.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fama R, Rosenbloom MJ, Sassoon SA, et al. Differential effect of alcoholism and HIV infection on visuomotor procedural learning and retention. Alcohol Clin Exp Res. 2012;36:1738–1747. doi: 10.1111/j.1530-0277.2012.01790.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fattore L, Altea S, Fratta W. Sex differences in drug addiction: A review of animal and human studies. Women's Health. 2008;4:51–65. doi: 10.2217/17455057.4.1.51. [DOI] [PubMed] [Google Scholar]
- Fein G, Di Sclafani V, Cardenas VA, et al. Cortical gray matter loss in treatment-naive alcohol dependent individuals. Alcohol Clin Exp Res. 2002;26:558–564. [PMC free article] [PubMed] [Google Scholar]
- Fein G, Greenstein D. Gait and balance deficits in chronic alcoholics: No improvement from 10 weeks through 1 year abstinence. Alcohol Clin Exp Res. 2013;37:86–95. doi: 10.1111/j.1530-0277.2012.01851.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fein G, Key K, Szymanski MD. ERP and RT delays in long-term abstinent alcoholics in processing of emotional facial expressions during gender and emotion categorization tasks. Alcohol Clin Exp Res. 2010;34:1127–1139. doi: 10.1111/j.1530-0277.2010.01189.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fein G, Shimotsu R, Chu R, et al. Parietal gray matter volume loss is related to spatial processing deficits in long-term abstinent alcoholic men. Alcohol Clin Exp Res. 2009;33:1806–1814. doi: 10.1111/j.1530-0277.2009.01019.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fein G, Torres J, Price LJ, et al. Cognitive performance in long-term abstinent alcoholic individuals. Alcohol Clin Exp Res. 2006;30:1538–1544. doi: 10.1111/j.1530-0277.2006.00185.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernández-Serrano MJ, Perez-Garcia M, Rio-Valle JS, et al. Neuropsychological consequences of alcohol and drug abuse on different components of executive functions. J Psychopharmacol. 2010;24:1317–1332. doi: 10.1177/0269881109349841. [DOI] [PubMed] [Google Scholar]
- Fitzpatrick LE, Jackson M, Crowe SF. The relationship between alcoholic cerebellar degeneration and cognitive and emotional functioning. Neurosci Biobehav Rev. 2008;32:466–485. doi: 10.1016/j.neubiorev.2007.08.004. [DOI] [PubMed] [Google Scholar]
- Fitzpatrick LE, Jackson M, Crowe SF. Characterization of cerebellar ataxia in chronic alcoholics using the International Cooperative Ataxia Rating Scale (ICARS) Alcohol Clin Exp Res. 2012;35:1943–1951. doi: 10.1111/j.1530-0277.2012.01821.x. [DOI] [PubMed] [Google Scholar]
- Foisy ML, Kornreich C, Fobe A, et al. Impaired emotional facial expression recognition in alcohol dependence: Do these deficits persist with midterm abstinence? Alcohol Clin Exp Res. 2007;31:404–410. doi: 10.1111/j.1530-0277.2006.00321.x. [DOI] [PubMed] [Google Scholar]
- Fortier CB, Leritz EC, Salat DH, et al. Reduced cortical thickness in abstinent alcoholics and association with alcoholic behavior. Alcohol Clin Exp Res. 2011;35:2193–2201. doi: 10.1111/j.1530-0277.2011.01576.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frith CD, Frith U. Social cognition in humans. Curr Biol. 2007;17:R724–R732. doi: 10.1016/j.cub.2007.05.068. [DOI] [PubMed] [Google Scholar]
- Fuster JM. The Prefrontal Cortex. 4th ed. London: Academic Press; 2008. [Google Scholar]
- Gazdzinski S, Durazzo TC, Meyerhoff DJ. Temporal dynamics and determinants of whole brain tissue volume changes during recovery from alcohol dependence. Drug Alcohol Depend. 2005a;78:263–273. doi: 10.1016/j.drugalcdep.2004.11.004. [DOI] [PubMed] [Google Scholar]
- Gazdzinski S, Durazzo TC, Mon A, et al. Body mass index is associated with brain metabolite levels in alcohol dependence: A multimodal magnetic resonance study. Alcohol Clin Exp Res. 2010a;34:2089–2096. doi: 10.1111/j.1530-0277.2010.01305.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gazdzinski S, Durazzo TC, Mon A, et al. Cerebral white matter recovery in abstinent alcoholics: A multimodality magnetic resonance study. Brain. 2010b;133:1043–1053. doi: 10.1093/brain/awp343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gazdzinski S, Durazzo TC, Studholme C, et al. Quantitative brain MRI in alcohol dependence: Preliminary evidence for effects of concurrent chronic cigarette smoking on regional brain volumes. Alcohol Clin Exp Res. 2005b;29:1484–1495. doi: 10.1097/01.alc.0000175018.72488.61. [DOI] [PubMed] [Google Scholar]
- Gilman JM, Davis MB, Hommer DW. Greater activation in left hemisphere language-related regions during simple judgment tasks among substance-dependent patients in treatment for alcoholism. Alcohol Clin Exp Res. 2010;34:331–341. doi: 10.1111/j.1530-0277.2009.01095.x. [DOI] [PubMed] [Google Scholar]
- Gizewski ER, Muller BW, Scherbaum N, et al. The impact of alcohol dependence on social brain function. Addict Biol. 2013;18:109–120. doi: 10.1111/j.1369-1600.2012.00437.x. [DOI] [PubMed] [Google Scholar]
- Goldstein RZ, Volkow ND. Drug Addiction and Its Underlying Neurobiological Basis: Neuroimaging Evidence for the Involvement of the Frontal Cortex. Am J Psychiatry. 2002;159:1642–1652. doi: 10.1176/appi.ajp.159.10.1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein RZ, Volkow ND. Dysfunction of the prefrontal cortex in addiction: Neuroimaging findings and clinical implications. Nat Rev Neurosci. 2011;12:652–669. doi: 10.1038/nrn3119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding AJ, Wong A, Svoboda M, et al. Chronic alcohol consumption does not cause hippocampal neuron loss in humans. Hippocampus. 1997;7:78–87. doi: 10.1002/(SICI)1098-1063(1997)7:1<78::AID-HIPO8>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- Harper CG, Smith NA, Kril JJ. The effects of alcohol on the female brain: A neuropathological study. Alcohol Alcohol. 1990;25:445–448. [PubMed] [Google Scholar]
- Harris GJ, Jaffin SK, Hodge SM, et al. Frontal white matter and cingulum diffusion tensor imaging deficits in alcoholism. Alcohol Clin Exp Res. 2008;32:1001–1013. doi: 10.1111/j.1530-0277.2008.00661.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hibbert LJ, Best DW. Assessing recovery and functioning in former problem drinkers at different stages of their recovery journeys. Drug Alcohol Rev. 2011;30:12–20. doi: 10.1111/j.1465-3362.2010.00190.x. [DOI] [PubMed] [Google Scholar]
- Insel TR, Fernald RD. How the brain processes social information: Searching for the social brain. Annu Rev Neurosci. 2004;27:697–722. doi: 10.1146/annurev.neuro.27.070203.144148. [DOI] [PubMed] [Google Scholar]
- Kim MJ, Kim JK, Yoo BG, et al. Acute Marchiafava-Bignami disease with widespread callosal and cortical lesions. J Korean Med Sci. 2007;22:908–911. doi: 10.3346/jkms.2007.22.5.908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kish GB, Hagen JM, Woody MM, et al. Alcoholics' recovery from cerebral impairment as a function of duration of abstinence. J Clin Psychol. 1980;36:584–589. doi: 10.1002/jclp.6120360234. [DOI] [PubMed] [Google Scholar]
- Kopera M, Wojnar M, Brower K, et al. Cognitive functions in abstinent alcohol-dependent patients. Alcohol. 2012;46:665–671. doi: 10.1016/j.alcohol.2012.04.005. [DOI] [PubMed] [Google Scholar]
- Kornreich C, Blairy S, Philippot P, et al. Deficits in recognition of emotional facial expression are still present in alcoholics after mid- to long-term abstinence. J Stud Alcohol. 2001;62:533–542. doi: 10.15288/jsa.2001.62.533. [DOI] [PubMed] [Google Scholar]
- Kornreich C, Brevers D, Canivet D, et al. Impaired processing of emotion in music, faces and voices supports a generalized emotional decoding deficit in alcoholism. Addiction. 2013;108:80–88. doi: 10.1111/j.1360-0443.2012.03995.x. [DOI] [PubMed] [Google Scholar]
- Kornreich C, Delle-Vigne D, Knittel J, et al. Impaired conditional reasoning in alcoholics: A negative impact on social interactions and risky behaviors? Addiction. 2011;106:951–959. doi: 10.1111/j.1360-0443.2010.03346.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornreich C, Philippot P, Foisy ML, et al. Impaired emotional facial expression recognition is associated with interpersonal problems in alcoholism. Alcohol Alcohol. 2002;37:394–400. doi: 10.1093/alcalc/37.4.394. [DOI] [PubMed] [Google Scholar]
- Kubota M, Nakazaki S, Hirai S, et al. Alcohol consumption and frontal lobe shrinkage: Study of 1432 non-alcoholic subjects. J Neurol Neurosurg Psychiatry. 2001;71:104–106. doi: 10.1136/jnnp.71.1.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Berre AP, Pinon K, Vabret F, et al. Study of metamemory in patients with chronic alcoholism using a feeling-of-knowing episodic memory task. Alcohol Clin Exp Res. 2010;34:1888–1898. doi: 10.1111/j.1530-0277.2010.01277.x. [DOI] [PubMed] [Google Scholar]
- Le Berre AP, Vabret F, Cauvin C, et al. Cognitive barriers to readiness to change in alcohol-cependent patients. Alcohol Clin Exp Res. 2012:1–8. doi: 10.1111/j.1530-0277.2012.01760.x. [DOI] [PubMed] [Google Scholar]
- Leber WR, Parsons OA. Premature aging and alcoholism. Int J Addict. 1982;17:61–88. doi: 10.3109/10826088209054610. [DOI] [PubMed] [Google Scholar]
- Lezak MD, Howieson DB, Bigler ED, et al. Neuropsychological Assessment. 5th. New York: Oxford University Press; 2012. [Google Scholar]
- Loeber S, Duka T, Welzel Marquez H, et al. Effects of repeated withdrawal from alcohol on recovery of cognitive impairment under abstinence and rate of relapse. Alcohol Alcohol. 2010;45:541–547. doi: 10.1093/alcalc/agq065. [DOI] [PubMed] [Google Scholar]
- Lusher J, Chandler C, Ball D. Alcohol dependence and the alcohol Stroop paradigm: Evidence and issues. Drug Alcohol Depend. 2004;75:225–231. doi: 10.1016/j.drugalcdep.2004.03.004. [DOI] [PubMed] [Google Scholar]
- Makris N, Oscar-Berman M, Jaffin SK, et al. Decreased volume of the brain reward system in alcoholism. Biol Psychiatry. 2008;64:192–202. doi: 10.1016/j.biopsych.2008.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann K, Ackermann K, Croissant B, et al. Neuroimaging of gender differences in alcohol dependence: Are women more vulnerable? Alcohol Clin Exp Res. 2005;29:896–901. doi: 10.1097/01.alc.0000164376.69978.6b. [DOI] [PubMed] [Google Scholar]
- Marinkovic K, Oscar-Berman M, Urban T, et al. Alcoholism and dampened temporal limbic activation to emotional faces. Alcohol Clin Exp Res. 2009;33:1880–1892. doi: 10.1111/j.1530-0277.2009.01026.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin PR. Psychiatric comorbidities of alcoholism. In: Pfefferbaum A, Sullivan EV, editors. Alcohol and the Nervous System. New York: Elsevier; 2013. [Google Scholar]
- Maurage P, Campanella S, Philippot P, et al. Impaired emotional facial expression decoding in alcoholism is also present for emotional prosody and body postures. Alcohol Alcohol. 2009;44:476–485. doi: 10.1093/alcalc/agp037. [DOI] [PubMed] [Google Scholar]
- Maurage P, Campanella S, Philippot P, et al. Face processing in chronic alcoholism: A specific deficit for emotional features. Alcohol Clin Exp Res. 2008;32:600–606. doi: 10.1111/j.1530-0277.2007.00611.x. [DOI] [PubMed] [Google Scholar]
- Maurage P, Campanella S, Philippot P, et al. The crossmodal facilitation effect is disrupted in alcoholism: A study with emotional stimuli. Alcohol Alcohol. 2007;42:552–559. doi: 10.1093/alcalc/agm134. [DOI] [PubMed] [Google Scholar]
- Maurage P, Grynberg D, Noel X, et al. Dissociation between affective and cognitive empathy in alcoholism: A specific deficit for the emotional dimension. Alcohol Clin Exp Res. 2011;35:1662–1668. doi: 10.1111/j.1530-0277.2011.01512.x. [DOI] [PubMed] [Google Scholar]
- Maurage P, Joassin F, Pesenti M, et al. The neural network sustaining crossmodal integration is impaired in alcohol-dependence: An fMRI study. Cortex. 2012a doi: 10.1016/j.cortex.2012.04.012. [DOI] [PubMed] [Google Scholar]
- Maurage P, Joassin F, Philippot P, et al. Disrupted regulation of social exclusion in alcohol-dependence: An FMRI study. Neuropsychopharmacology. 2012b;37:2067–2075. doi: 10.1038/npp.2012.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mearns J, Lees-Haley PR. Discriminating neuropsychological sequelae of head injury from alcohol-abuse-induced deficits: A review and analysis. J Clin Psychol. 1993;49:714–720. doi: 10.1002/1097-4679(199309)49:5<714::aid-jclp2270490515>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- Mitchell JM, Fields HL, D'Esposito M, et al. Impulsive responding in alcoholics. Alcohol Clin Exp Res. 2005;29:2158–2169. doi: 10.1097/01.alc.0000191755.63639.4a. [DOI] [PubMed] [Google Scholar]
- Mon A, Durazzo TC, Gazdzinski S, et al. The impact of chronic cigarette smoking on recovery from cortical gray matter perfusion deficits in alcohol dependence: Longitudinal arterial spin labeling MRI. Alcohol Clin Exp Res. 2009;33:1314–1321. doi: 10.1111/j.1530-0277.2009.00960.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monnig MA, Caprihan A, Yeo RA, et al. Diffusion tensor imaging of white matter networks in individuals with current and remitted alcohol use disorders and comorbid conditions. Psychol Addict Behav. 2012 doi: 10.1037/a0027168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monnot M, Lovallo WR, Nixon SJ, et al. Neurological basis of deficits in affective prosody comprehension among alcoholics and fetal alcohol-exposed adults. J Neuropsychiatry Clin Neurosci. 2002;14:321–328. doi: 10.1176/jnp.14.3.321. [DOI] [PubMed] [Google Scholar]
- Monnot M, Nixon S, Lovallo W, et al. Altered emotional perception in alcoholics: Deficits in affective prosody comprehension. Alcohol Clin Exp Res. 2001;25:362–369. [PubMed] [Google Scholar]
- Morgenstern J, Longabaugh R. Cognitive-behavioral treatment for alcohol dependence: A review of evidence for its hypothesized mechanisms of action. Addiction. 2000;95:1475–1490. doi: 10.1046/j.1360-0443.2000.951014753.x. [DOI] [PubMed] [Google Scholar]
- Moselhy HF, Georgiou G, Kahn A. Frontal lobe changes in alcoholism: A review of the literature. Alcohol Alcohol. 2001;36:357–368. doi: 10.1093/alcalc/36.5.357. [DOI] [PubMed] [Google Scholar]
- Muller-Oehring EM, Schulte T, Fama R, et al. Global-local interference is related to callosal compromise in alcoholism: A behavior-DTI association study. Alcohol Clin Exp Res. 2009;33:477–489. doi: 10.1111/j.1530-0277.2008.00858.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nixon K. Alcohol and adult neurogenesis: Roles in neurodegeneration and recovery in chronic alcoholism. Hippocampus. 2006;16:287–295. doi: 10.1002/hipo.20162. [DOI] [PubMed] [Google Scholar]
- Nixon SJ. Sex differences. In: Pfefferbaum A, Sullivan EV, editors. Alcohol and the Nervous System. New York: Elsevier; 2013. [Google Scholar]
- Nixon SJ, Tivis R, Parsons OA. Interpersonal problem-solving in male and female alcoholics. Alcohol Clin Exp Res. 1992;16:684–687. doi: 10.1111/j.1530-0277.1992.tb00661.x. [DOI] [PubMed] [Google Scholar]
- Noël X, Van der Linden M, Brevers D, et al. The contribution of executive functions deficits to impaired episodic memory in individuals with alcoholism. Psychiatry Res. 2012;198:116–122. doi: 10.1016/j.psychres.2011.10.007. [DOI] [PubMed] [Google Scholar]
- O'Daly OG, Trick L, Scaife J, et al. Withdrawal-associated increases and decreases in functional neural connectivity associated with altered emotional regulation in alcoholism. Neuropsychopharmacology. 2012;37:2267–2276. doi: 10.1038/npp.2012.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oscar-Berman M. Function and dysfunction of prefrontal brain circuitry in alcoholic Korsakoff's syndrome. Neuropsychol Rev. 2012;22:154–169. doi: 10.1007/s11065-012-9198-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oscar-Berman M, Bardenhagen F. Nonhuman animal models of memory dysfunction in neurodegenerative disease. In: Troster A, editor. Memory in Neurodegenerative Disease. New York: Cambridge University Press; 1998. pp. 3–20. [Google Scholar]
- Oscar-Berman M, Bowirrat A. Genetic influences in emotional dysfunction and alcoholism-related brain damage. Neuropsychiatr Dis Treat. 2005;1:211–229. [PMC free article] [PubMed] [Google Scholar]
- Oscar-Berman M, Hancock M, Mildworf B, et al. Emotional perception and memory in alcoholism and aging. Alcohol Clin Exp Res. 1990a;14:383–393. doi: 10.1111/j.1530-0277.1990.tb00491.x. [DOI] [PubMed] [Google Scholar]
- Oscar-Berman M, Kirkley SM, Gansler DA, et al. Comparisons of Korsakoff and non-Korsakoff alcoholics on neuropsychological tests of prefrontal brain functioning. Alcohol Clin Exp Res. 2004;28:667–675. doi: 10.1097/01.alc.0000122761.09179.b9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oscar-Berman M, Marinkovic K. Alcohol: Effects on neurobehavioral functions and the brain. Neuropsychol Rev. 2007;17:239–257. doi: 10.1007/s11065-007-9038-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oscar-Berman M, Pulaski JL, Hutner N, et al. Cross-modal functions in alcoholism and aging. Neuropsychologia. 1990b;28:851–869. doi: 10.1016/0028-3932(90)90009-d. [DOI] [PubMed] [Google Scholar]
- Oscar-Berman M, Schendan E. Assymmetries of brain function in alcoholism: Relationship to aging. In: Connor LT, Obler LK, editors. Neurobehavior of Language and Cognition: Studies of Normal Aging and Brain Damage. New York: Kluwer Academic Pub; 2000. pp. 213–240. [Google Scholar]
- Oscar-Berman M, Valmas MM, Sawyer KS, et al. Frontal brain dysfunction in alcoholism with and without antisocial personality disorder. Neuropsychiatr Dis Treat. 2009;5:309–326. doi: 10.2147/ndt.s4882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey AK, Kamarajan C, Tang Y, et al. Neurocognitive deficits in male alcoholics: An ERP/sLORETA analysis of the N2 component in an equal probability Go/NoGo task. Biol Psychol. 2012;89:170–182. doi: 10.1016/j.biopsycho.2011.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park SQ, Kahnt T, Beck A, et al. Prefrontal cortex fails to learn from reward prediction errors in alcohol dependence. J Neurosci. 2010;30:7749–7753. doi: 10.1523/JNEUROSCI.5587-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parks MH, Greenberg DS, Nickel MK, et al. Recruitment of additional brain regions to accomplish simple motor tasks in chronic alcohol-dependent patients. Alcohol Clin Exp Res. 2010;34:1098–1109. doi: 10.1111/j.1530-0277.2010.01186.x. [DOI] [PubMed] [Google Scholar]
- Parks MH, Morgan VL, Pickens DR, et al. Brain fMRI activation associated with self-paced finger tapping in chronic alcohol-dependent patients. Alcohol Clin Exp Res. 2003;27:704–711. doi: 10.1097/01.ALC.0000062759.14944.CF. [DOI] [PubMed] [Google Scholar]
- Petrakis IL, Gonzalez G, Rosenheck R, et al. Comorbidity of alcoholism and psychiatric disorders. Alcohol Res Health. 2002;26:81–89. [Google Scholar]
- Pfefferbaum A, Adalsteinsson E, Sullivan EV. Dysmorphology and microstructural degradation of the corpus callosum: Interaction of age and alcoholism. Neurobiol Aging. 2006;27:994–1009. doi: 10.1016/j.neurobiolaging.2005.05.007. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Desmond JE, Galloway C, et al. Reorganization of frontal systems used by alcoholics for spatial working memory: An fMRI study. Neuroimage. 2001a;14:7–20. doi: 10.1006/nimg.2001.0785. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Lim KO, Desmond JE, et al. Thinning of the corpus callosum in older alcoholic men: A magnetic resonance imaging study. Alcohol Clin Exp Res. 1996;20:752–757. doi: 10.1111/j.1530-0277.1996.tb01682.x. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Lim KO, Zipursky RB, et al. Brain gray and white matter volume loss accelerates with aging in chronic alcoholics: A quantitative MRI study. Alcohol Clin Exp Res. 1992;16:1078–1089. doi: 10.1111/j.1530-0277.1992.tb00702.x. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Rosenbloom M, Deshmukh A, et al. Sex differences in the effects of alcohol on brain structure. Am J Psychiatry. 2001b;158:188–197. doi: 10.1176/appi.ajp.158.2.188. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Rosenbloom M, Rohlfing T, et al. Degradation of association and projection white matter systems in alcoholism detected with quantitative fiber tracking. Biol Psychiatry. 2009;65:680–690. doi: 10.1016/j.biopsych.2008.10.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfefferbaum A, Sullivan EV, Mathalon DH, et al. Longitudinal changes in magnetic resonance imaging brain volumes in abstinent and relapsed alcoholics. Alcohol Clin Exp Res. 1995;19:1177–1191. doi: 10.1111/j.1530-0277.1995.tb01598.x. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Sullivan EV, Rosenbloom MJ, et al. A controlled study of cortical gray matter and ventricular changes in alcoholic men over a 5-year interval. Arch Gen Psychiatry. 1998;55:905–912. doi: 10.1001/archpsyc.55.10.905. [DOI] [PubMed] [Google Scholar]
- Philippot P, Kornreich C, Blairy S, et al. Alcoholics' deficits in the decoding of emotional facial expression. Alcohol Clin Exp Res. 1999;23:1031–1038. [PubMed] [Google Scholar]
- Pitel AL, Beaunieux H. Component processes of memory. In: Pfefferbaum A, Sullivan EV, editors. Alcohol and the Nervous System. New York: Elsevier; 2013. [Google Scholar]
- Pitel AL, Beaunieux H, Witkowski T, et al. Genuine episodic memory deficits and executive dysfunctions in alcoholic subjects early in abstinence. Alcohol Clin Exp Res. 2007;31:1169–1178. doi: 10.1111/j.1530-0277.2007.00418.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitel AL, Chanraud S, Muller-Oehring EM, et al. Modulation of limbic-cerebellar functional connectivity enables alcoholics to recognize who is who. Brain Struct Funct. 2012a doi: 10.1007/s00429-012-0421-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitel AL, Chanraud S, Rohlfing T, et al. Face-name association learning and brain structural substrates in alcoholism. Alcohol Clin Exp Res. 2012b;36:1171–1179. doi: 10.1111/j.1530-0277.2011.01731.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitel AL, Rivier J, Beaunieux H, et al. Changes in the episodic memory and executive functions of abstinent and relapsed alcoholics over a 6-month period. Alcohol Clin Exp Res. 2009;33:490–498. doi: 10.1111/j.1530-0277.2008.00859.x. [DOI] [PubMed] [Google Scholar]
- Ratti MT, Bo P, Giardini A, et al. Chronic alcoholism and the frontal lobe: Which executive functions are imparied? Acta Neurol Scand. 2002;105:276–281. doi: 10.1034/j.1600-0404.2002.0o315.x. [DOI] [PubMed] [Google Scholar]
- Reed RJ, Grant I, Rourke SB. Long-term abstinent alcoholics have normal memory. Alcohol Clin Exp Res. 1992;16:677–683. doi: 10.1111/j.1530-0277.1992.tb00660.x. [DOI] [PubMed] [Google Scholar]
- Rogers BP, Parks MH, Nickel MK, et al. Reduced fronto-cerebellar functional connectivity in chronic alcoholic patients. Alcohol Clin Exp Res. 2012;36:294–301. doi: 10.1111/j.1530-0277.2011.01614.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose GL, Skelly JM, Badger GJ, et al. Interactive voice response for relapse prevention following cognitive-behavioral therapy for alcohol use disorders: A pilot study. Psychol Serv. 2012;9:174–184. doi: 10.1037/a0027606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenbloom M, Pfefferbaum A. Magnetic resonance imaging of the living brain: Evidence for brain degeneration among alcoholics and recovery with abstinence. Alcohol Res Health. 2008;31:362–376. [PMC free article] [PubMed] [Google Scholar]
- Rosenbloom MJ, Pfefferbaum A, Sullivan EV. Recovery of short-term memory and psychomotor speed but not postural stability with long-term sobriety in alcoholic women. Neuropsychology. 2004;18:589–597. doi: 10.1037/0894-4105.18.3.589. [DOI] [PubMed] [Google Scholar]
- Rosenbloom MJ, Rohlfing T, O'Reilly AW, et al. Improvement in memory and static balance with abstinence in alcoholic men and women: Selective relations with change in brain structure. Psychiatry Res. 2007;155:91–102. doi: 10.1016/j.pscychresns.2006.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenbloom MJ, Sassoon SA, Pfefferbaum A, et al. Contribution of regional white matter integrity to visuospatial construction accuracy, organizational strategy, and memory for a complex figure in abstinent alcoholics. Brain Imaging Behav. 2009;3:379–390. doi: 10.1007/s11682-009-9080-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rourke SB, Grant I. The interactive effects of age and length of abstinence on the recovery of neuropsychological functioning in chronic male alcoholics: A 2-year follow-up study. J Int Neuropsychol Soc. 1999;5:234–246. doi: 10.1017/s1355617799533067. [DOI] [PubMed] [Google Scholar]
- Rourke SB, Grant I. The neurobehavioral correlates of alcoholism. In: Grant I, Adams K, editors. Neuropsychological Assessment of Neuropsychiatric and Neuromedical Disorders. Oxford: Oxford University Press; 2009. pp. 398–454. [Google Scholar]
- Ruiz SM, Oscar-Berman M, Sawyer KS, et al. Drinking history associations with regional white matter volumes in alcoholic men and women. Alcohol Clin Exp Res. 2013;37:110–122. doi: 10.1111/j.1530-0277.2012.01862.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salloum JB, Ramchandani VA, Bodurka J, et al. Blunted rostral anterior cingulate response during a simplified decoding task of negative emotional facial expressions in alcoholic patients. Alcohol Clin Exp Res. 2007;31:1490–1504. doi: 10.1111/j.1530-0277.2007.00447.x. [DOI] [PubMed] [Google Scholar]
- Salloway SMD, Malloy PF, Duffy JD. The Frontal Lobes and Neuropsychiatric Illness. 1st ed. Washington, DC: American Psychiatric Press; 2001. [Google Scholar]
- Sameti M, Smith S, Patenaude B, et al. Subcortical volumes in long-term abstinent alcoholics: Associations with psychiatric comorbidity. Alcohol Clin Exp Res. 2011;35:1067–1080. doi: 10.1111/j.1530-0277.2011.01440.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schottenbauer MA, Hommer D, Weingartner H. Memory deficits among alcoholics: Performance on a selective reminding task. Neuropsychol Dev Cogn B Aging Neuropsychol Cogn. 2007;14:505–516. doi: 10.1080/13825580600681305. [DOI] [PubMed] [Google Scholar]
- Schulte T, Muller-Oehring EM, Pfefferbaum A, et al. Neurocircuitry of emotion and cognition in alcoholism: Contributions from white matter fiber tractography. Dialogues Clin Neurosci. 2010;12:554–560. doi: 10.31887/DCNS.2010.12.4/tschulte. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulte T, Muller-Oehring EM, Sullivan EV, et al. Synchrony of corticostriatal-midbrain activation enables normal inhibitory control and conflict processing in recovering alcoholic men. Biol Psychiatry. 2012a;71:269–278. doi: 10.1016/j.biopsych.2011.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulte T, Muller-Oehring EM, Sullivan EV, et al. White matter fiber compromise contributes differentially to attention and emotion processing impairment in alcoholism, HIV-infection, and their comorbidity. Neuropsychologia. 2012b;50:2812–2822. doi: 10.1016/j.neuropsychologia.2012.07.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulte T, Oberlin BG, Kareken DA, et al. How acute and chronic alcohol consumption affects brain networks: Insights into multimodal neuroimaging. Alcohol Clin Exp Res. 2012c;36:2017–2027. doi: 10.1111/j.1530-0277.2012.01831.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith S, Fein G. Persistent but less severe ataxia in long-term versus short-term abstinent alcoholic men and women: A cross-sectional analysis. Alcohol Clin Exp Res. 2011;35:2184–2192. doi: 10.1111/j.1530-0277.2011.01567.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stasiewicz PR, Bradizza CM, Gudleski GD, et al. The relationship of alexithymia to emotional dysregulation within an alcohol dependent treatment sample. Addict Behav. 2012;37:469–476. doi: 10.1016/j.addbeh.2011.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stavro K, Pelletier J, Potvin S. Widespread and sustained cognitive deficits in alcoholism: A meta-analysis. Addict Biol. 2012 doi: 10.1111/j.1369-1600.2011.00418.x. [DOI] [PubMed] [Google Scholar]
- Strauss E, Sherman EMS, Spreen O. A Compendium of Neuropsychological Tests: Administration, Norms, and Commentary. 3rd ed. New York: Oxford University Press; 2006. [Google Scholar]
- Sullivan EV. Compromised pontocerebellar and cerebellothalamocortical systems: Speculations on their contributions to cognitive and motor impairment in nonamnesic alcoholism. Alcohol Clin Exp Res. 2003;27:1409–1419. doi: 10.1097/01.ALC.0000085586.91726.46. [DOI] [PubMed] [Google Scholar]
- Sullivan EV, Desmond JE, Lim KO, et al. Speed and efficiency but not accuracy or timing deficits of limb movements in alcoholic men and women. Alcohol Clin Exp Res. 2002a;26:705–713. [PubMed] [Google Scholar]
- Sullivan EV, Fama R, Rosenbloom MJ, et al. A profile of neuropsychological deficits in alcoholic women. Neuropsychology. 2002b;16:74–83. doi: 10.1037//0894-4105.16.1.74. [DOI] [PubMed] [Google Scholar]
- Sullivan EV, Harding AJ, Pentney R, et al. Disruption of frontocerebellar circuitry and function in alcoholism. Alcohol Clin Exp Res. 2003;27:301–309. doi: 10.1097/01.ALC.0000052584.05305.98. [DOI] [PubMed] [Google Scholar]
- Sullivan EV, Harris RA, Pfefferbaum A. Alcohol's effects on brain and behavior. Alcohol Res Health. 2010;33:127–143. [PMC free article] [PubMed] [Google Scholar]
- Sullivan EV, Marsh L, Mathalon DH, et al. Anterior hippocampal volume deficits in nonamnesic, aging chronic alcoholics. Alcohol Clin Exp Res. 1995;19:110–122. doi: 10.1111/j.1530-0277.1995.tb01478.x. [DOI] [PubMed] [Google Scholar]
- Sullivan EV, Mathalon DH, Zipursky RB, et al. Factors of the Wisconsin Card Sorting Test as measures of frontal-lobe function in schizzophrenia and in chronic alcoholism. Psychiatry Res. 1993;46:175–199. doi: 10.1016/0165-1781(93)90019-d. [DOI] [PubMed] [Google Scholar]
- Sullivan EV, Pfefferbaum A. Neurocircuitry in alcoholism: A substrate of disruption and repair. Psychopharmacology. 2005;180:583–594. doi: 10.1007/s00213-005-2267-6. [DOI] [PubMed] [Google Scholar]
- Sullivan EV, Rosenbloom MJ, Lim KO, et al. Longitudinal changes in cognition, gait, and balance in abstinent and relapsed alcoholic men: Relationships to changes in brain structure. Neuropsychology. 2000a;14:178–188. [PubMed] [Google Scholar]
- Sullivan EV, Rosenbloom MJ, Pfefferbaum A. Pattern of motor and cognitive deficits in detoxified alcoholic men. Alcohol Clin Exp Res. 2000b;24:611–621. [PubMed] [Google Scholar]
- Thorberg FA, Young RM, Sullivan KA, et al. Alexithymia, craving and attachment in a heavy drinking population. Addict Behav. 2011;36:427–430. doi: 10.1016/j.addbeh.2010.12.016. [DOI] [PubMed] [Google Scholar]
- Tivis R, Beatty WW, Nixon SJ, et al. Patterns of cognitive impairment among alcoholics: Are there subtypes? Alcohol Clin Exp Res. 1995;19:496–500. doi: 10.1111/j.1530-0277.1995.tb01537.x. [DOI] [PubMed] [Google Scholar]
- Townshend JM, Duka T. Mixed emotions: Alcoholics' impairments in the recognition of specific emotional facial expressions. Neuropsychologia. 2003;41:773–782. doi: 10.1016/s0028-3932(02)00284-1. [DOI] [PubMed] [Google Scholar]
- Tulving E, Craik FIM. The Oxford Handbook of Memory, Oxford Handbook Series. New York: Oxford University Press; 2000. [Google Scholar]
- Uekermann J, Channon S, Winkel K, et al. Theory of mind, humour processing and executive functioning in alcoholism. Addiction. 2007;102:232–240. doi: 10.1111/j.1360-0443.2006.01656.x. [DOI] [PubMed] [Google Scholar]
- Uekermann J, Daum I. Social cognition in alcoholism: A link to prefrontal cortex dysfunction? Addiction. 2008;103:726–735. doi: 10.1111/j.1360-0443.2008.02157.x. [DOI] [PubMed] [Google Scholar]
- Uekermann J, Daum I, Schlebusch P, et al. Processing of affective stimuli in alcoholism. Cortex. 2005;41:189–194. doi: 10.1016/s0010-9452(08)70893-1. [DOI] [PubMed] [Google Scholar]
- Vetreno RP, Hall JM, Savage LM. Alcohol-related amnesia and dementia: Animal models have revealed the contributions of different etiological factors on neuropathology, neurochemical dysfunction and cognitive impairment. Neurobiol Learn Mem. 2011;96:596–608. doi: 10.1016/j.nlm.2011.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westlye LT, Walhovd KB, Dale AM, et al. Life-span changes of the human brain white matter: Diffusion tensor imaging (DTI) and volumetry. Cereb Cortex. 2010;20:2055–2068. doi: 10.1093/cercor/bhp280. [DOI] [PubMed] [Google Scholar]
- Witkiewitz K, Aracelliz N. Dynamic association between negative affect and alcohol lapses following alcohol treatment. J Consult Clin Pscychol. 2009;77:633–644. doi: 10.1037/a0015647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witkiewitz K, Marlatt GA. Relapse prevention for alcohol and drug problems: That was Zen, this is Tao. Am Psychol. 2004;59:224–235. doi: 10.1037/0003-066X.59.4.224. [DOI] [PubMed] [Google Scholar]
- Wobrock T, Falkai P, Schneider-Axmann T, et al. Effects of abstinence on brain morphology in alcoholism: A MRI study. Eur Arch Psychiatry Clin Neurosci. 2009;259:143–150. doi: 10.1007/s00406-008-0846-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- York JL, Biederman I. Hand movement speed and accuracy in detoxified alcoholics. Alcohol Clin Exp Res. 1991;15:982–990. doi: 10.1111/j.1530-0277.1991.tb05199.x. [DOI] [PubMed] [Google Scholar]
- Zipursky RB, Lim KC, Pfefferbaum A. MRI study of brain changes with short-term abstinence from alcohol. Alcohol Clin Exp Res. 1989;13:664–666. doi: 10.1111/j.1530-0277.1989.tb00401.x. [DOI] [PubMed] [Google Scholar]