Abstract
Studies into the mechanisms in resolution of self-limited inflammation and acute reperfusion injury have uncovered a new genus of pro-resolving lipid mediators coined specialized pro-resolving mediators (SPM) including lipoxins, resolvins, protectins and maresins that are each temporally produced by resolving-exudates with distinct actions for return to homeostasis. SPM evoke potent anti-inflammatory and novel pro-resolving mechanisms as well as enhance microbial clearance. While born in inflammation-resolution, SPM are conserved structures with functions discovered in microbial defense, pain, organ protection and tissue regeneration, wound healing, cancer, reproduction, and neurobiology-cognition. This review covers these SPM mechanisms and other new omega-3 PUFA pathways that open their path for functions in resolution physiology.
Keywords: inflammation, host defense, infection, essential fatty acids, lipid mediator
1. Introduction
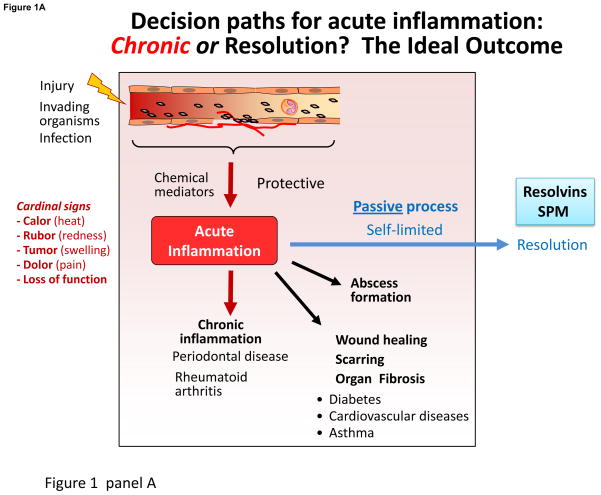
The origins of resolving inflammation trace to the 11th century in the Canon of Medicine [1] as a resolvent promotes the disappearance of inflammation. For historical perspective on resolution, interested readers are directed to ref. [1] for a recent review. Barrier break, trauma and microbial invasion each create the host’s need to neutralize invaders, clear the site, remodel and regenerate tissue (Fig. 1A); the inflammatory response is a terrain where lipid mediators (LM) such as eicosanoids (prostaglandins (PG) and leukotrienes (LT)) [2] and novel pro-resolving mediators uncovered [3, 4] play pivotal roles. The acute inflammatory response is divided into initiation and resolution phases (Fig. 1A).
Figure 1. Lipid mediators in the acute inflammatory response and its outcomes.
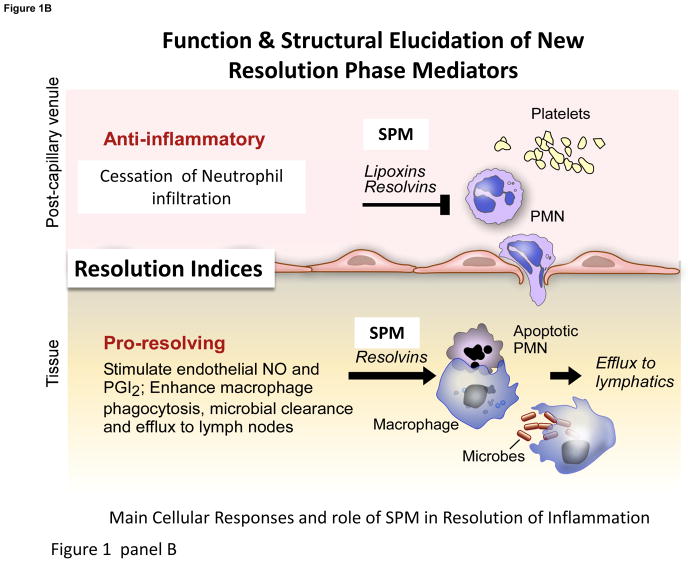
Panel A: LM play pivotal roles in the vascular response and leukocyte trafficking, from initiation to resolution. Eicosanoids are critical in initiating the cardinal signs of inflammation, and the specialized proresolving mediators (SPM), illustrated above, play key roles stimulating resolution (Panel B). Depicted are some roles of resolvins, protectins and maresins, SPM, in leukocyte trafficking, lipid mediator class switching, efferocytosis, resolving exudates to homeostasis and signaling to adaptive immunity via lymphocytes. Failed resolution can lead to enhanced prostaglandin and leukotriene production and chronic inflammation that can lead to fibrosis. SPM (lipoxins, resolvins, protectins and maresins) counterregulate pro-inflammatory chemical mediators, reducing inflammation, and stimulate reepithelialization, wound healing, and tissue regeneration (see text for details).
Leukocyte traffic from circulation forms inflammatory exudates traditionally viewed as a battlefield. The first responders, neutrophils (PMN), swarm like sharks to defend the host along chemotaxic gradients, e.g. LTB4 [5, 6], exiting venules governed by PGE2 and PGI2 and influx to form the exudate. The main events in resolution are cessation of PMN influx and macrophage clearance of debris. But how does the host return the system to homeostasis and what are the roles of chemical mediators and the inflammatory exudate in timely resolution?
Excessive inflammation is widely appreciated as a unifying component in many chronic diseases including vascular diseases, metabolic syndrome, neurological diseases, and many others, and thus a significant public health concern. Since the acute inflammatory response is protective, evolved to permit repair of injured tissues and eliminate invading organisms, it is ideally self-limited and leads to complete resolution enabling return to homeostasis (Fig. 1A). Although resolution of disease is appreciated by clinicians, resolution was considered a passive process [7], passive in that the chemoattractant and other chemical mediators involved in mounting the inflammatory response would just dilute and dissipate [8, 9]. With identification of proresolving mediators, we obtained evidence that resolution of self-limited inflammation is an active programmed response that is “turned on” and not simply a process of diluting chemoattractant gradients. Unexpectedly, n-3 PUFA present in marine oils are precursors of proresolving mediators (Fig. 1A&B).
Since n-3 PUFA EPA and DHA have cardioprotective and anti-inflammatory effects, they were held earlier to simply compete with arachidonic acid for eicosanoid biosynthesis, preventing pro-inflammatory eicosanoids, a process readily discernible in vitro [10]. Utilization of n-3 PUFA by resolving exudates to biosynthesize novel pro-resolving local mediators -- resolvins, protectins and maresins, collectively coined SPM, which potently stimulate cessation of PMN infiltration and enhance macrophage uptake of apoptotic cells, debris and microbes -- has opened a new focus on resolution pathways and innate immune mechanisms to attain homeostasis (Fig. 1B). This review focuses on the role of LM in resolution that now function in other systems including host-defense, nervous, reproductive, and muscle and exercise, giving promise for SPM, their pathways and related products in resolution physiology and pharmacology.
2. Elucidation of Pro-Resolving LM and the Newest Additions RvD3 and MaR1 Pathway
Key to this recent paradigm change is identification of novel families of autacoids that include resolvins, protectins, their aspirin-triggered forms and maresins, providing evidence that resolution is orchestrated by LM (Fig. 2 and Box 1). From this, it’s evident that resolution programs of acute inflammation hold promise and remain largely uncharted [7] (Fig. 1). Challenges ahead are whether we can harness these novel LM that stimulate resolution (i.e. agonists of resolution coined resolvents [1, 11, 12]) and their resolution mechanisms. Dietary n-3 supplements are widely used, but <25% are directed by health care providers [13]. Clinical trials with n-3 PUFA show mixed results [14], suggesting that fatty acids themselves are not highly suitable to consider as drugs, given their many metabolic fates in humans. Hence, it is critical for public health to establish mechanisms that underlie their essential health requirements.
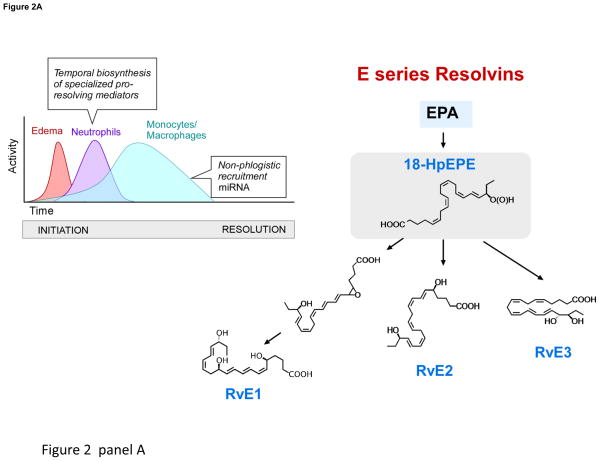
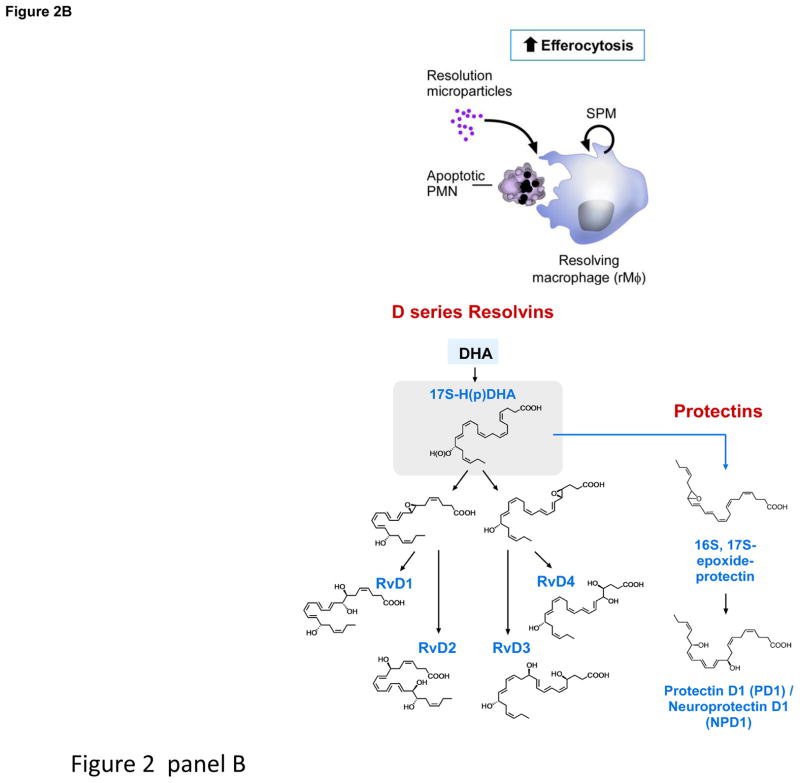
Figure 2. Production of SPM by resolving inflammatory exudates.
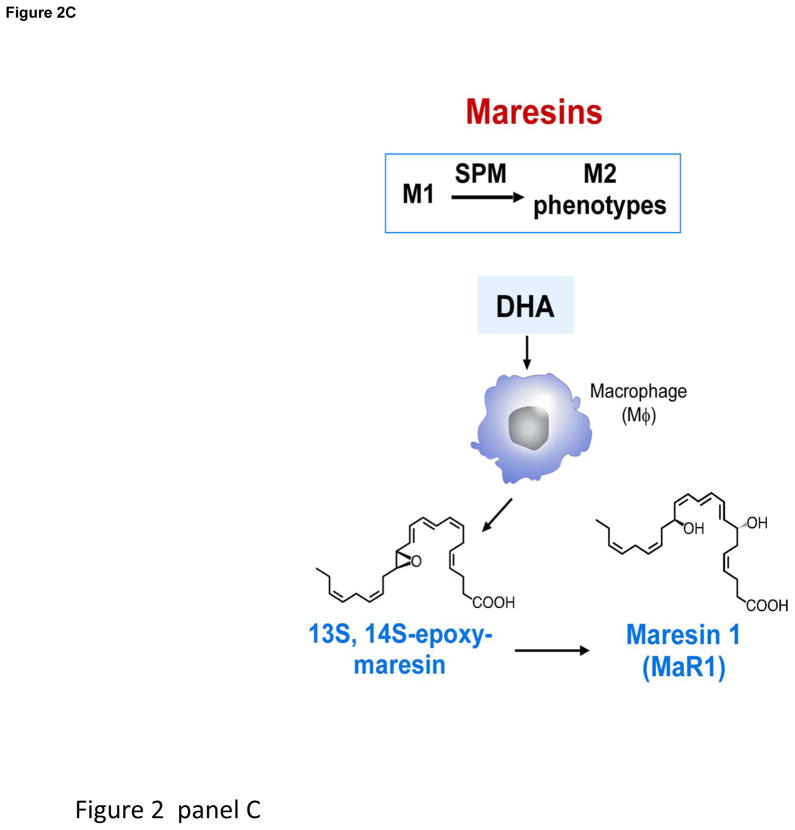
Panel A depicts pus formation, e.g. a purulent exudate beginning with the postcapillary venule and the diapedesis of neutrophils as they are summoned by chemoattractants to leave the vascular circulation to combat invading microbes or foreign objects. The endothelial cell interactions with PMN are a site for E-series resolvin biosynthesis (see text for details). Panel B depicts the time course of self-limited acute inflammatory response, edema, followed by neutrophilic infiltration and nonphlogistic recruitment of monocytes/macrophages from initiation (time 0) to resolution and the uptake of apoptotic PMN by resolving macrophages (rMΦ). Initial biosynthesis of SPM occurs at maximal neutrophilic infiltration through resolution in self-limiting responses. Structures of SPM: D-series resolvins, protectins and (Panel C) maresins. Depicted are resolvins D1–D4, which carry potent actions. 17-HpDHA is also precursor to 16,17-epoxide-protectin intermediate that is converted to protectin D1/neuroprotectin D1 and related protectins such as PDx, 10S,17S-diHDHA. Panel C: Maresins are produced by macrophages via initial lipoxygenation at carbon-14 position by lipoxygenation and insertion of molecular oxygen, producing a 13S,14S-epoxide-maresin intermediate that is enzymatically converted to maresin-1. The stereochemistry of each bioactive SPM is established, and SPM biosynthesis in murine exudates and human tissues confirmed. See ref. [16] for citations of original reports, total organic synthesis and stereochemical assignments and the text for further details.
Box 1. Specialized Proresolving Mediators (SPM): Anti-Phlogistic Pro-Resolving Actions (see Figure 1, Panel B).
SPM Defining Bioactions
Temporal biosynthesis with leukocyte exudate traffic
Cessation of PMN infiltration; Stop signals to limit further PMN recruitment and PMN-mediated tissue damage
Enhance macrophage phagocytosis of apoptotic PMN, cellular debris and bacteria killing
SPM possess broad anti-inflammatory & pro-resolving actions at both transcriptional and translational levels
Counter-regulate & Reduce Cardinal Signs of Inflammation
Prostaglandins (COX-2 expression) and leukotrienes (LTB4, LTC4, LTD4)
PAF formation and actions
Chemokines and cytokines (TNFα, IL-1β, IL-6, IL-8, IL-12, etc.)
NFκB associated gene products
Growth factors (VEGF)
Extracellular ROS
Edema
Activate
Local NO, PGI2 production via endothelial cells
Heme oxygenase (HO-1) induction
Macrophage phagocytosis (bacteria, microbial particles) and efferocytosis (apoptotic cells)
Macrophage IL-10 production
Enhance PMN phagocytosis, phagolysosomal ROS, microbial killing and clearance
IL-1ra production
Adiponectin
Using a systems approach with resolving exudates, we elucidated new essential PUFA-derived SPM pathways [4, 15]. Their biosynthesis and complete stereochemistry of each major resolvin (RvE1, RvD1, RvD2, RvD3 and RvD5) and protectin -- their potent bioactions (reviewed in [16]), now independently confirmed by many worldwide -- are the focus of increasing interest given that SPM control the magnitude and duration of inflammation, infection and tissue injury (Fig. 2, Table 1). The structures (Fig. 2) and nanogram actions of resolvins are extended to many pathologies including vascular [17], airway, dermal, renal, ocular, pain, cancer, fibrosis and wound healing [18]. This is also the case for neuroprotectins/protectins; each has actions throughout the body; vide infra.
Table 1.
SPM Shorten Resolution of Infections, Increase Survival and Outcomes in Murine Infection and Disease Models
| SPM | Increase Survival | Disease | Shorten Resolution | Reference |
|---|---|---|---|---|
| LXA4 | + | Bacterial infections | Walker et al. [71] | |
| 15-epi- LXA4 | + | Lung injury | El Kebir et al. [172] | |
| RvE1 | + | Colitis | + | Arita et al. [173] |
| + | Candida yeast | + | Haas-Stapleton et al. [81] | |
| + | Acid-induced lung injury | + | Levy and Serhan [30] | |
| RvD1, RvD5, PD1 | + | E. coli infection | + | Chiang et al. [32] |
| RvD1 | + | Acute lung injury | + | Wang et al. [174] |
| RvD2 | + | Cecal ligation and puncture sepsis | + | Spite et al. [33] |
| + | Burn wound | + | Bohr et al. [175] |
Within self-limited exudates, RvD3 displays a unique timeframe compared to RvD1 and RvD2, appearing late in resolution, suggesting a key role of RvD3. RvD3’s complete stereochemistry was recently established [11], and confirmed its potent anti-inflammatory and proresolving actions [4]. Macrophage biosynthesis of MaR1 and its potent proresolving and tissue regenerative actions (Fig. 2) are established [12], and involve a 13S,14S-epoxide-maresin intermediate that is also active and stimulates M1 to M2 phenotype-switch [12]. Also, both n-6 docosapentaenoic acid (DPA) and its n-3 form are substrates for SPM [19, 20]. Since n-3 DPA accumulates in certain individuals and is converted to potent n-3 immunoresolvents from each of the SPM families, it is likely n-3 DPA-derived SPM are compensatory in humans since they share resolvin and protectin actions [20].
Maresins are produced by macrophages from docosahexaenoic acid (DHA) and exert potent proresolving and tissue homeostatic actions. Maresin 1 (MaR1; 7R,14S-dihydroxy-docosa-4Z,8E,10E,12Z,16Z,19Z-hexaenoic acid) is the first identified maresin. Recently, we demonstrated formation, stereochemistry, and precursor role of 13,14-epoxy-docosahexaenoic acid, an intermediate in MaR1 biosynthesis. The 14-lipoxygenation of DHA by human macrophage 12-lipoxygenase (hm12-LOX) gave 14-hydro(peroxy)-docosahexaenoic acid (14-HpDHA), as well as several dihydroxy-docosahexaenoic acids, implicating an epoxide intermediate formation by this enzyme (see Table 2 and references within for the role of LOX and the SPM in mouse diseases). Using a stereo-controlled synthesis, enantiomerically pure 13S,14S-epoxy-docosa-4Z,7Z,9E,11E,16Z,19Z-hexaenoic acid (13S,14S-epoxy-DHA) was prepared, and its stereochemistry was confirmed by NMR spectroscopy. When this 13S,14S-epoxide was incubated with human macrophages, it was converted to MaR1. The synthetic 13S,14S-epoxide inhibited leukotriene B4 (LTB4) formation by human leukotriene A4 hydrolase (LTA4H) to a similar extent as LTA4 but was not converted to MaR1 by this enzyme. 13S,14S-epoxy-DHA also reduced arachidonic acid conversion by hm12-LOX and promoted conversion of M1 macrophages to M2 phenotype, which produced more MaR1 from the epoxide than M1. Together, these findings establish the biosynthesis of the 13S,14S-epoxide, its absolute stereochemistry, its precursor role in MaR1 biosynthesis, and its own intrinsic bioactivity. Given its actions and role in MaR1 biosynthesis, this epoxide is now termed 13,14-epoxy-maresin (13,14-eMaR; Fig. 2, Panel C) and exhibits new mechanisms in resolution of inflammation in its ability to inhibit proinflammatory mediator production by LTA4 hydrolase and to block arachidonate conversion by human 12-LOX rather than merely terminating phagocyte involvement.
Table 2.
Anti-inflammatory and tissue-protective roles: 12/15-LOX, 5-LOX and their products
| (A) 12/15-LOX | |||
|---|---|---|---|
| Animal models | Alox15 gene modification | Actions/Phenotypes | References |
| Periodontitis in rabbit | Alox15 transgenic | ↓ PMN-mediated tissue degradation and bone loss | [67] |
| Cornea injury | Alox12/15 deficient mice | ↑ Inflammation ↓ Wound healing ↓ Corneal re-epithelialization ↓ Endogenous LXA4 production ↓ Heme-oxygenase 1 LXA4 rescues exacerbated inflammation and impaired wound healing in Alox15 deficient mice |
[176, 177] |
| Atherosclerosis | Alox12/15 transgenic mice | Delayed atherosclerosis ↓ IL-17, CCL5, PGE2 ↑ PD1 |
[178] |
| Alox12/15 deficient mice | Accelerated atherosclerosis ↑ IL-12p40, CCL5 ↓ LXA4 production in macrophages |
||
| Peritonitis | Alox12/15 deficient mice | ↓ 14-HDHA | [179] |
| Arthritis | Alox12/15 deficient mice | ↑ Inflammatory gene expression ↓ LXA4 in inflamed synovia and macrophages |
[180] |
| Collagen-induced arthritis | COX-2 product PGE2 induces Alox12/15. PGE2 stimulates resolution via LXA4 | [25] | |
| Suture-induced chronic cornea injury | Alox12/15 deficient mice | ↑ Inflammatory neovascularization | [181] |
| Peritonitis | Alox12/15 deficient mice | Resolution deficit caused by eosinophil depletion was rescued by eosinophil restoration or the administration of PD1. Eosinophils from Alox12/15 deficient mice could not rescue the resolution phenotype | [51] |
| Dermal fibrosis | Alox12/15 deficient mice | ↑ TGF-β stimulated MAPK pathway LXA4 counters TGF-β stimulated fibroblast activation |
[182] |
| Endometriosis | Alox12/15 deficient mice | EPA administration decreased the number of lesions in controls but not in Alox12/15 deficient mice. Reduced levels of RvE3 after EPA administration in Alox12/15 deficient mice compared to control | [183] |
| Airway inflammation | Alox12/15 deficient mice | ↓ TLR7-mediated resolution of airway inflammation | [184] |
| Peritonitis | Alox12/15 deficient mice | Low dose inhaled CO treatment reduces PMN infiltration in WT control, but failed to regulate PMN in Alox12/15 deficient mice | [185] |
| (B) 5-LOX deficient mice | ||
|---|---|---|
| Cellular systems | Actions/Phenotypes | References |
| Respiratory Syncytial Virus (RSV)-induced bronchiolitis | ↑ lung pathology Treatment of 5-LO(−/−) macrophages with LXA4 and RvE1, but not LTB4 or LTD4, partially restored expression of alternatively activated Mφ markers |
[186] |
| Lyme arthritis (Borrelia burgdorferi infection) | earlier joint swelling and an inability to resolve arthritis ↓ Macrophage phagocytosis of B. burgdorferi and efferocytosis ↓ PMN uptake of opsonized spirochetes |
[187] |
| Suture-induced cornea injury | ↑ inflammatory neovascularization coincident with increased VEGF-A and FLT4 expression | [181] |
| Mycobacterium tuberculosis infection | ↓ LXA4 levels | [188] |
| Toxoplasma gondii infection | ↓ LXA4 levels ↓ survival ↑ IL-12 and IFN-gamma |
[189] |
Further, PCR mapping of 12-lipoxygenase (12-LOX) mRNA sequence in human macrophages and platelets showed that they are identical. This human 12-LOX mRNA and enzyme are expressed in monocyte-derived cell lineage, and enzyme expression levels increase with maturation to macrophages or dendritic cells. Recombinant human 12-LOX gave essentially equivalent catalytic efficiency (kcat/KM) with arachidonic acid (AA) and DHA as substrates. Lipid mediator metabololipidomics demonstrated that human macrophages produce a novel bioactive product 13,14-dihydroxy-docosahexaenoic acid in addition to MaR1. Co-incubations with human recombinant 12-LOX and soluble epoxide hydrolase (sEH) demonstrated that biosynthesis of 13,14-dihydroxy-docosahexaenoic acid (13,14-diHDHA) involves the 13S,14S-epoxy-maresin intermediate produced from DHA by 12-LOX, followed by conversion via soluble epoxide hydrolase (sEH) [21]. This new 13,14-diHDHA displayed potent anti-inflammatory and pro-resolving actions, and at 1 ng reduced neutrophil infiltration in mouse peritonitis by ~40% and at 10 pM enhanced human macrophage phagocytosis of zymosan by ~90%. However, MaR1 proved more potent than the 13R,14S-diHDHA at enhancing efferocytosis with human macrophages. Taken together, the present findings demonstrate that macrophages produced a novel bioactive product identified in the maresin metabolome as 13R,14S-dihydroxy-docosahexaenoic acid, from DHA via conversion by human 12-LOX followed by sEH. Given its potent bioactions, we coined 13R,14S-diHDHA maresin 2 (MaR2).
3. Aspirin vs. Nonsteroidal Anti-Inflammatory Drugs: Friends or Foes of Resolution?
PG are central to the vascular response, permitting diapedesis of PMN and monocytes to leave postcapillary venules, and their production via COX-1 and COX-2 is critical for initiation and timely resolution (Fig. 1) [22, 23]. PGE2 and PGD2 each evoke pro-inflammatory and anti-inflammatory responses that depend on location. PGE2 enhances LTB4-mediated PMN extravasation and tissue injury that is blocked, for example, by lipoxin A4 (LXA4) and its aspirin-triggered epimer 15-epi-LXA4 [24], illustrating a pro-inflammatory PGE2 function in skin and ability of 15-epi-LXA4 stable analogs to stop PMN infiltration and tissue injury. Temporal LC-MS-MS-based profiling demonstrated the switch from PG and leukotriene production to appearance of lipoxins within inflammatory exudates (Fig. 1). PGE2 or PGD2 added to human PMN increase 15-LOX type I translation from mRNA stores in a cAMP-dependent manner, increasing LX biosynthesis [22].
Inhibition of COX-2 delays resolution [23] because prostaglandins play critical roles in resolution and are initiators of LM class switching (Fig. 1), a process involving utilization of omega-3 for resolvins and other SPM. In mapping resolution, it became clear that initiation of inflammation signals its end [15] and that leukocyte traffic in and out of pus permits prostanoids to signal biosynthesis of resolution mediators (Figure 1 & 2).
Disruption of physiologic LM class switching has deleterious consequences in mouse arthritis [25] and in humans [26]. To pinpoint, critical steps and mechanisms of SPM action within inflammation-resolution, we introduced quantitative indices [27] that are now widely used [28–30]. Resolution indices identified agents that disrupt or delay resolution (resolution interval, Ri), including COX-2 and lipoxygenase inhibitors [27, 28], lidocaine [31]. By contrast, specific SPM, e.g. resolvins, lipoxins and protectins, shorten Ri by limiting PMN recruitment and stimulating macrophage efferocytosis (Fig. 1, Table 1) and bacterial killing [27, 29, 32, 33]; pinpointing the PMN-monocyte sequence needed for tissue repair and regeneration [12]; and glucocorticoids, specific cyclin-dependent kinase inhibitors, statins, annexin peptides and aspirin are resolution friendly [29, 34–36].
Aspirin and NSAIDs inhibit prostanoid biosynthesis, but aspirin is an irreversible inhibitor that acetylates COX, and NSAIDs are considered reversible inhibitors [2]. Aspirin acetylation of COX-2 modifies the catalytic domain, blocking PG-biosynthesis, which is well known, yet remains active producing 15R-HETE from arachidonic acid, 18R-HEPE from EPA and 17R-HDHA from DHA in cells carrying COX-2. These are transformed by leukocytes to aspirin-triggered lipoxins [35], aspirin-triggered resolvins [3, 4] and aspirin-triggered protectins [37]. Each AT-SPM is a potent mediator that stops PMN infiltration and enhances macrophage cleanup, giving a shortened Ri. COX-1 doesn’t produce appreciable aspirin-triggered epimers. NSAIDs that inhibit both COX-1 and COX-2, and selective COX-2 inhibitors prevent their production [3].
Low-dose aspirin triggers 15-epi-LXA4 in skin blisters in humans (75 mg aspirin daily/10 days) to reduce PMN infiltration [28]. In a randomized controlled study of 128 healthy volunteers, low-dose aspirin (8 weeks daily, 81 mg) increased plasma 15-epi-LXA4. Low-dose aspirin thus enhances production of aspirin-triggering mediators in humans while inhibiting thromboxane, giving a net change [38] favorable for pro-resolution, where distinct resolution-phenotypes emerged [39, 40].
4. Microparticles and New Players in Local SPM Biosynthesis
Microparticles (MP) are membrane-derived vesicles produced by a range of cell types and contribute to human pathologies. MP from self-resolving exudates display anti-inflammatory and proresolving capacity [41]. Resolution-MP enhance efferocytosis [41, 42] and carry pro-resolving signals including hydroxy-SPM-precursors esterified in phospholipids [41]. Secretory PLA2 (sPLA2) releases these precursors from MP for conversion by leukocytes [41, 42]. Since nanomedicines are of wide interest [43], we used resolution-MP and their ability to shorten Ri in mouse peritonitis as a basis for biomimicry to construct humanized nanoparticles containing LXA4 analog or AT-RvD1 [41]. These nano-proresolving medicines (NPRM) carrying SPM or SPM-analogs, enhance wound healing of human keratinocytes and are protective in a mouse model of temporomandibular joint disease [41]. There are currently limited treatments for TMJ diseases, and importantly NPRM display reduced nanotoxicity. Along these lines, NPRM containing a novel lipoxin analog (benzo-lipoxin A4, bLXA4) promote hard and soft tissue regeneration in periodontitis in the Hanford miniature pig. NPRM-bLXA4 markedly reduces inflammatory cell infiltrate into chronic periodontal disease sites and increases new bone formation [44]. These findings offer a new therapeutic tissue-engineering approach for the treatment of chronic osteolytic inflammatory diseases.
New microfluidic chambers that permit visualization of cell-cell interactions between leukocyte subpopulations (i.e. human PMN and monocytes) and distinguish phlogistic vs. nonphlogistic leukocyte behavior are ideal to screen SPM individually or incorporated within humanized NPRM [41, 45]. Single cell screening with microfluidic devices permits optimization for enriching MP with SPM and production of NPRM as well as viewing neutrophil-monocyte interactions [45] essential for appreciating signals used in the PMN-monocyte sequence (Figure 1).
Platelet-MP transfers its 12-LOX to mast cells for LX-biosynthesis [46] (see Figure 2, Panel B). Platelet-MP are taken up and enhance LXA4 production, which reduces colitis in mice, suggesting that platelet-derived MP signal immune regulation via LXA4 [46]. MP can also transfer substrate and intermediates to macrophages (MΦ) during efferocytosis enhancing SPM biosynthesis, demonstrated by transfer of deuterium label from precursors to labeled-SPM [42]. Myeloid cells at different stages display agonist- and phenotype-specific LM profiles. For example, human PMN from healthy peripheral blood produce predominantly LTB4, while apoptotic PMN produce PGE2, LXB4 and RvE2 signals for resolution [42].
Both M1 and M2 macrophages display specific markers and pathways specialized to their functions of MΦ subpopulation in inflammation, its resolution and cancer [47]. Human M2 macrophages possess increased Arg1, 15-LOX [48], other markers and specific LM signature profiles. M2 produce SPM with lower LTB4 and PG than M1. Both engulf apoptotic PMN, modulating their LM profiles. In M2, LTB4 is down-regulated and SPM are increased [42], suggesting M1 and M2 subpopulation [47, 48] produce functional LM signatures that can impact both physiologic and pathophysiologic states [42]. Secreted PLA2 group IID is a proresolving PLA2 expressed in CD11c+ dendritic cells and macrophages giving rise to RvD1 and PGJ2 in lymphoid tissue, controlling hypersensitivity [49].
Eosinophils are well appreciated in parasitic infections and allergic responses. In severe asthma, PD1 is present in human exhaled breath condensates [30] and is decreased in human eosinophils from patients with severe asthma [50]. Human eosinophils are a major source of PD1, and at nanomolar concentrations, reduce adhesion molecules (CD11b and L-selectin), eotaxin-1/CCL11 and chemotaxis without affecting degranulation, superoxide generation or cell survival. Impaired PD1 in severe asthmatic patients may contribute to persistence and disease severity (Box 2). Eosinophils also stimulate resolution in mouse peritonitis via SPM initiated by mouse eosinophils [51]. LC-MS-MS-lipidomics identified LXA4, RvD5, 17-HDHA and PD1 eosinophil production [51] and RvE3 (Fig. 2) that limit PMN infiltration and regulate MΦ [50, 52]. Hence, via their ability to produce SPM, eosinophils contribute to resolution. To support this, Arita and colleagues found eosinophil depletion leads to a deficit in resolution rescued by PD1 or eosinophil restoration. Thus, cellular traffic to inflammatory loci has a dynamic impact on LM signatures and specific SPM metabolomes activated within local milieu.
Box 2. General Points on Specialized Pro-resolving Mediators: What’s Established.
-
Mouse resolving exudates: Temporal relationships and in vivo actions
Air pouch, peritonitis, ischemia-reperfusion injury: sterile and bacterial infections
-
Structures of endogenous mediators elucidated: Total organic synthesis
Confirmation of potent actions and complete stereochemical assignments
-
Human cellular biosynthesis:
apoptotic PMN, microparticles, Macrophages M1 and M2;
PMN-interactions with resident endothelial and epithelial cells; PMN-Platelets
-
Human Pathology – Present Yet Diminished SPM: Examples of failed resolution
Asthma [163–165] Localized aggressive periodontitis [166] Adipose [167] Multiple sclerosis patients [106] Alzheimer’s disease brain [89, 96] Rheumatoid arthritis, synovial [168] Breath condensates [30, 169] Scleroderma [133] Cystic fibrosis, nasal polys in cystic fibrosis [170] Ulcerative colitis [171] Conserved structures: fish (trout, zebrafish, anchovy), mouse, rabbit, human
Tissue Regeneration: RvE1, MaR1
Human Blood: RvE1, RvE2 and 17R/S-HDHA, RvD1, RvD2 and 17-epi-RvD1
Human Milk; high levels of SPM: LXA4, RvD1, RvD2
-
Clinical development and human trials: RvE1 mimetics
Ocular indications; Phase III clinical trial
5. SPM Cellular Actions in Disease Models: Receptors and Resolution Mechanisms
SPM are now investigated in animal models of infection (Table 1 and Table 2 for disease models) by investigators worldwide (reviewed in [18]). These include airway, dermatologic, ocular, pain and organ-specific inflammation and tissue injury resulting from collateral damage from excess PMN [18]. The low SPM-doses required to stop ongoing inflammation and promote resolution rely on GPCR receptors via amplifying intracellular signals. Several SPM receptors are identified using library screening, labeled-ligands for specific binding (stereospecific nM Kd) and functional cellular responses. SPM in general do not utilize Ca2+ mobilization in leukocytes for signal transduction but rather activate phosphorylation demonstrated using genetically engineered mice (see Table 3). RvE1 specifically binds to ChemR23 [53] and BLT1 to evoke pro-resolving responses. RvE1 activation of ChemR23 enhances macrophage phagocytosis via phosphoprotein-mediated signaling [54]. RvE1 blocks LTB4 binding and also signals via BLT1 to promote apoptosis of PMN for their clearance by MΦ [55] (LTB4-BLT1 signals PMN survival). PMN RvE1 signaling involves blocking survival signals, an important difference for PMN in the innate response, where they must undergo timely apoptosis and MΦ efferocytosis to achieve homeostasis.
Table 3.
SPM receptors – Genetically modified mice
| Receptor | LM Agonist | Genetic modification | Actions/phenotypes | References |
|---|---|---|---|---|
| ALX | LXA4 RvD1 |
Transgenic mice | Accelerated resolution with LXA4 and RvD1 in peritonitis | [56] |
| Deficient mice | RvD1 action (e.g. PMN trafficking, miR and cytokine regulation) is reduced in peritonitis | [56] | ||
| Deficient mice | RvD1 actions (reduction of PGE2 and LTB4) is abolished in peritonitis | [57] | ||
| Deficient mice | Reduced protection of 15-epi-LXA4 against intimal hyperplasia after carotid ligation | [190] | ||
| Deficient mice | ↑ disease severity (hypothermia and cardiac dysfunction) in sepsis ↓ monocyte recruitment |
[191] | ||
| ChemR23 | RvE1 | Transgenic mice | Potentiated RvE1 actions in: ↓ PMN in peritonitis ↓ bone loss |
[192] |
| Transgenic mice | Heightened RvE1 response in: ↑ PMN phagocytosis of P. gingivalis ↓ PMN influx into the dorsal air pouch |
[193] | ||
| BLT1 | RvE1 (partial) | Deficient mice | RvE1 regulation of PMN infiltration is reduced | [194] |
RvD1 binds and activates human GPR32 and shares human and murine LXA4 receptor (ALX/FPR2) to evoke rapid impedance change with recombinant receptors. Transgenic mice overexpressing human ALX-FPR2 require less RvD1 to stop inflammation [56], and in receptor-deficient mice, RvD1 is apparently without leukocyte actions [57]. Resolution involves specific miR, regulated by SPM receptors [56, 58, 59]. RvD1-GPR32 upregulates miR-208 and IL-10 as well as miR-219, which decreases LTB4 via regulation of 5-lipoxygenase [58].
miR regulation by SPM is an example of long-term SPM-receptor signaling. SPM receptors acutely signal as well. For example, recombinant RvD1-GPR32 blocks histamine receptor (H1)-stimulated increases in intracellular Ca2+ in CHO cells via rapid stimulation of phosphorylation of H1 receptor that stops Ca2+ mobilization [60]. This form of SPM signaling, first documented with conjunctival goblet cells and RvD1, is also functional in salivary glands [61], is likely to be relevant in human PMN, which rapidly stop and change shape on exposure to SPM [45].
In addition to RvD1 and LXA4, ALX/FPR2 is also activated by peptide pro-resolving mediators, e.g. annexin A1, as well as pro-inflammatory peptides, at much higher concentrations [36]. This capacity of ALX/FPR2 involves ligand-biased receptor activation with heterodimerization of ALX with related FPR dictating pro-inflammatory signaling, and ALX homodimer gives pro-resolving signaling [36]. LXA4 also enhances ALX/FPR2 promoter activity, which has a mutation of interest in human disease [62].
RvD3 and RvD5, related to RvD1 (Fig. 2), can also activate human GPR32 [11, 32]. Given the temporal production of these SPM in vivo (Fig. 2), these findings underscore that SPM produced locally at distinct times can impact different target cell types and receptors in a spatial-temporal dependency.
Both EPA and DHA activate GPR120 to signal responses of THP-1 cells [63], which are apparently not activated by resolvins. GPR120 is also a candidate taste receptor [64], requiring fatty acid (μM concentration) to evoke Ca2+ mobilization [65] that appears unrelated to SPM-resolution mechanisms, which are active at picomolar-nanomolar levels [42, 66].
6. Infectious Exudates and Resolution Programs in Host Defense
RvE1 and LXA4 each reduce severity of periodontal disease in rabbits by enhancing P. gingivalis clearance, causative organism in this infection [67, 68]. While anti-inflammatory actions of SPM were established in sterile mouse models [3, 4], the relation between resolution and infection is of interest because of the known eventual immunosuppressive actions of anti-inflammatory drugs [69]. Surprisingly, RvD2 protects mice from cecal ligation-puncture (CLP)-induced sepsis [33], with potent actions enhancing phagocytosis and bacterial killing (Table 1, Box 1). In self-limited live E. coli infections, resolution programs are activated in mice and host PD1, RvD5 and RvD1 are elevated [32]. When added back to mouse phagocytes, human MΦ or PMN, SPM enhance bacterial phagocytosis and killing as well as clearance [32, 33, 70]. Of interest, SPM acting on the host lower antibiotic doses needed to clear infections.
LXA4 is also protective in CLP in rats, reducing bacterial burden and pro-inflammatory mediators via a MΦ NFκB-mediated mechanism reducing systemic inflammation [71]. Aspirin-triggered-LXA4 increases phagocytosis of E. coli in a PI3K-and scavenger receptor-dependent manner, and ALX/FPR2 is upregulated in patients with Crohn’s disease and enhances bacterial clearance [72]. Mycobacterium tuberculosis infections also engage resolution programs via activating LTB4-LXA4 production, regulating host responses in zebrafish, mice and humans [73, 74]. Given importance of rising microbial resistance, activation of resolution programs and SPM-pathways could provide new anti-microbial approaches.
Herpes simplex virus causes ocular infections that lead to stromal keratitis with viral-initiated immunopathology. RvE1 and PD1 are each potent and topically active in this infectious mouse model, reducing pro-inflammatory mediators and stimulating IL-10 [75, 76]. H5N1 virus lethal dissemination activates genes in mice tracked to LX biosynthesis, where sustained inflammation inhibits LX-mediated anti-inflammatory host responses that permit viral dissemination [77]. H1N1 activates host resolution-metabolome increasing PD1 [78]. Host protectins display antiviral activity blocking replication of H5N1 influenza virus. During the time course of H3N2, a low-pathogenicity strain of influenza, anti-inflammatory mediators are produced with infection that correlates with resolution and SPM-related pathway-markers [79]. In children with acute post-streptococcal glomerulonephritis, resolution phase is associated with LXA4 and diminished LTB4 [80]. Pro-resolving actions of SPM also extend to yeast infections, e.g. Candida, where RvE1 enhances yeast killing and clearance [81]. Innate immunodeficiency syndromes are linked to mutations in innate receptors. For example, X-linked lymphoproliferative syndrome type-2 is associated with deficiency in X-linked inhibitor of apoptosis protein (XIAP). Mice deficient in XIAP are highly vulnerable in Candida albicans infection. RvD1 rescued Xiap(−/−) mice from lethal C. albicans infection, suggesting the potential therapeutic value of RvD1 in the treatment of innate immunodeficiency syndromes [82]. These results enforce the notion that treating the host during infection with host-directed pro-resolving molecules could open new opportunities in host-pathogen struggles [32, 70, 83].
7. Tissue Regeneration and Healing
LXA4 stimulates reepithelialization of cornea in a gender-specific fashion [84]. RvE1, RvD1 and RvD2 each stimulate dermal wound healing, reducing neutrophilic infiltration and stimulate reepithelialization of skin wounds when applied to the wound site [85]. Most notably, they reduce the time required for dermal wound closure. D-series resolvins stimulate diabetic wound healing [86, 87].
Given the role of macrophages in wound healing and organ regeneration, the macrophage-derived SPM maresin pathway stimulates tissue regeneration. The maresin pathway and MaR1 (Figure 2) are also produced by planaria Dugesia tigrina, a Platyheminthes used in regeneration studies. RvE1 and MaR1 reduced regeneration time (speed of regrowing head segments). In the presence of inhibitor of MaR1 biosynthesis, regeneration index is reduced and rescued with addition of MaR1. Given the importance of tissue regeneration in trauma and microbial invasion and infection, it is not surprising that SPMs play role(s) in stimulating organ regeneration [12].
8. Neuroinflammation, Pain, and Cognition
Resolvins and protectins are produced in mouse brain tissue [4, 88] and in human brain [89]. These include human microglial cells, where they reduce cytokine expression [4, 88] and their production by trout brain cells indicates SPM are conserved from fish to humans [90]. In mouse ischemic stroke, resolvins, protectins and their aspirin-triggered forms are produced [91], where they are protective, down regulating excess leukocyte infiltration, and reduce local neuronal injury, COX-2 induction, IL-1β and NFκB. Thus, in brain, DHA is precursor to neuroprotective signaling pathways evoked by ischemia-reflow tissue injury. Given its potent actions to reduce neuroinflammation and protect neural cells, we coined this 10,17-dihydroxy-protectin (a docosatriene) neuroprotectin D1 with Bazan and colleagues when biosynthesized and acting in neural tissues and retinal epithelial cells [92].
DHA is enriched in brain, synapses and retina, where its protective role is appreciated, yet its role as a precursor to novel local mediators in resolution and in neuroprotection is still emerging. Bazan and colleagues showed potent protective roles of NPD1 in the nervous system, reducing stress pathways that lead to cell death and increase cell survival, and in several ocular models of important diseases NPD1 targets microglia [4, 93]. Aspirin-triggered DHA metabolome biosynthesizes 17R-NPD1/PD1 that reduces human PMN transmigration, and enhanced MΦ efferocytosis [37] and attenuates cerebral ischemic injury [94]. AT-NPD1 reduces brain edema in the penumbra and subcortical lesion size, infarct volumes are reduced ~60–80%, and improves neurological scores [94]. Hence, new findings focus on unesterified DHA in neural tissues as an important determinant in brain SPM biosynthesis and neuroinflammation [95].
In human Alzheimer’s disease (AD), brain NPD1 is reduced [89], implicating that its loss contributes to neurodegeneration. Indeed, resolution-pathway (SPM-receptors and products) are diminished in brain from AD [96]. LXA4 and RvD1 are reduced in cerebrospinal fluid and hippocampus that correlated to mini-mental state examinations in these patients, which together provide further evidence that failed resolution mechanisms may contribute to human disease and cognitive function [96]. RvD1 added to leukocyte-derived MΦ from AD patients reduces their pro-inflammatory phenotype and enhances their ability to promote phagocytosis of amyloid-beta (Aβ) [97], suggesting that resolvins promote clearance of Aβ deposition to reduce inflammation in AD. Human MΦ from patients with amyotrophic lateral sclerosis, a neuronal degenerative disease, demonstrates that RvD1 reduces their output of pro-inflammatory cytokines and chemokines that contribute to neuroinflammation [98]. RvD1 reduces IL-6 and TNFα by ALS-MΦ and is ~1,000 times more potent than DHA [98]. Hence, resolvins are protective and are likely to play pivotal roles in homeostasis in brain and peripheral tissues, each with selective functions that can impact neuroinflammation, its resolution and cognitive function.
Given the potent actions of resolvins and protectins in neuroinflammation, several groups investigated LX in neurologic systems. LXA4 also displays potent neuroprotection, reducing PMN infiltration, astrocyte activation, TNFα and IL-1β in a rat model of cerebral artery occlusion and ischemia [99], reduces β-amyloid-induced IL-1β and TNFα in cortex and hippocampus of mice [100], is neuroprotective in cerebral ischemia-reperfusion injury in rats [101, 102] and reduces leukotrienes as well as ERK phosphorylation, which limits local neuroinflammation partly mediated by ALX/FPR2 [101]. In experimental stroke, PPAR agonist rosiglitazone is neuroprotective via increasing LXA4 and reducing leukotriene B4 [103]. In rat cerebral ischemia/reperfusion, LXA4 reduces infarct size and improves neurologic scores that are only partially attributed to receptor ALX/FPR2, and induces Nrf2 expression and nuclear translocation as well as heme oxygenase HO-1 [104], suggesting that both receptor-mediated pathways and Nrf2 expression contribute to neuroprotection. LXA4 is produced in brain and is protective via CB1 cannabinoid receptor [105] as an endogenous allosteric enhancer. LXA4 enhances affinity of anandamide, potentiates endocannabinoids and protects from spatial memory loss induced by β-amyloid peptide [105, 106].
Inflammation can evoke pain that may persist, and each SPM displays targeted actions that resolve pain signals. Lipoxins reduce pain in murine models, LXA4 receptor (ALX/FPR2) is on spinal astrocytes, and local spinal LXA4, LXB4 or their metabolically stable analogs reduces inflammation-induced pain [107]. LXs reduce thermal hyperalgesia with as little as 10 μg/kg given i.v. or 0.3 nmol (1 μL/h, 20–24 h) intrathecal (i.t.) [107]. Each SPM dampens pain, having specific targets of action [108] first demonstrated with RvE1 and RvD1 for inflammatory pain involving both central and peripheral sites of action [109]. RvE1 administered i.t. in mice is more potent than morphine or COX-2 inhibitor. RvE1 receptor (ChemR23) is in DRG, where RvE1 regulates pERK-dependent TRPV1inhibition and TNFα-mediated hyperalgesia centrally, and in postsynaptic neurons RvE1 inhibits glutamate and TNFα stimulation of NMDA-R and mechanical allodynia [109].
RvD1 inhibits TRPA1, TRPV3 and TRPV4 channel activation expressed in HEK cells in nanomolar-micromolar range, cultured sensory neurons and keratinocytes as well as displays analgesic properties in pain behavior [110]. AT-RvD1 appears specific for TRPV3 [111], and NPD1/PD1 (0.1–10 ng) blocks spinal LTP, reducing TRPV1-dependent inflammatory pain without affecting basal pain responses [112]. NPD1 also reduces TNFα-dependent pain hypersensitivity [112] and protects against neuropathic pain after nerve trauma in mice [113]. RvD2 inhibits TRPV1 (IC50 0.1 nM) and TRPA1 (IC50 2 nM) in primary sensory neurons. RvE1 selectively blocks TRPV1 (EC50 = 1 nM), and RvD1 inhibits TRPA1 (IC50 9 nM). RvD2, RvE1 and RvD1 (Fig. 2) each differentially regulate TRPV1 and TRPA1 agonist-elicited acute pain and synaptic plasticity in spinal cord [114].
MaR1 inhibits TRPV1 currents in neurons and blocks capsaicin-induced inward current (IC50 0.49 nM), diminishing inflammatory and chemotherapy-evoked neuropathic pain in mice [12]. RvD1 reduces postoperative pain [115], and both AT-RvD1 and 17R-HDHA reduce adjuvant-induced arthritis in rats and associated pain [116], reducing NFκB and COX-2 expression in spinal cord, and within arthritic joints reduce TNFα and IL-1β. In addition to leukocytes and microglia, the currently known SPM-GPC receptors are present on neuronal bodies (DRG), nerve terminals (skin and muscle) and synaptic terminals, where they regulate specific TRP channels. For example, RvE1-ChemR23 (ERV) interaction in DRG regulates TRPV1, but not via direct activation of channels like endocannabinoids [108] or other lipids that act to directly bind TRP channels in micromolar range; rather each SPM activates specific GPCR in pico-nanomolar range to regulate TRP channels involved in pain signaling.
Direct comparisons between LXA4 and AT-RvD1 in rat mechanical hypersensitivity in inflammation-induced pain indicate that both effectively reduce hypersensitivity and pro-inflammatory mediators from astrocytes [117]. Cognitive decline following major surgery or critical illness is a major public health concern. Cognitive decline results from local increases in pro-inflammatory mediators. Systemic AT-RvD1 prophylaxis improves memory decline in a mouse surgery model and protects from postoperative neuronal dysfunction. AT-RvD1 reduces IL-6 and stimulated endogenous LXA4 [118], implicating positive feed-forward mechanisms in resolution. A human randomized trial of n-3 PUFA dietary intervention in chronic headache found increasing n-3 PUFA associated with reduced headache-pain and increased RvD2, 17-HDHA and 18-HEPE (pro-resolving pathway markers) [119] suggesting local SPM may reduce headache pain in humans as in animal models of pain and behavior.
9. Cross-talk between SPM and nervous systems
In zymosan-initiated acute peritoneal inflammation, vagus nerve regulates local expression of netrin-1, an axonal guidance molecule that activates resolution. Vagotomy reduces local SPM and delays resolution. In netrin-1(+/−) mice, resolvin D1 (RvD1) was less effective in promoting resolution of peritonitis compared with Ntn1(+/+). Netrin-1 and RvD1 display bidirectional activation in that they stimulated each other’s expression and enhanced efferocytosis [120]. These results indicate that the vagus nerve regulates both netrin-1 and SPM, which act in a bidirectional fashion to stimulate resolution, providing evidence for local neuronal control of resolution.
It was reported recently that in the mouse fibromyalgia-like model induced by reserpine, administration of AT-RvD1 and RvD2 significantly reduced mechanical allodynia, thermal sensitization and prevented the depressive-like behavior in reserpine-treated animals. Reserpine triggers a marked decrease of dopamine and serotonin (5-HT) levels. Long-term treatment with RvD2 prevents loss of serotonin in total brain, and AT-RvD1 leads to recovery of dopamine levels in cortex [121]. Therefore, D-series resolvins reduce painful and depressive symptoms associated with fibromyalgia in mice.
10. Reproductive System
In pregnant women, LXA4 is found in circulation at 24 weeks gestation and in lower levels in non-pregnant women [122–124]. Myometrial biopsies from pregnant women show ALX/FPR2 present on myocytes and neutrophils with LXA4 reducing agonist-stimulated IL-6 and IL-8 in myometrium, which suggesting LX may stimulate resolution of local inflammation in both physiologic and pathologic labor in human parturition [122]. LXA4 also modulates estrogen receptor [125] and blocks embryo implantation [126]. In patients and in rat endometrium with ectopic endometriosis, ALX/FPR2 showed higher expression [127]. Since SPM like LXA4 counter-regulate pro-inflammatory cytokines (Box I), LXA4 reverses LPS-induced miscarriages down regulating both uterine and pro-inflammatory mediators from placenta and mast cells in this tissue [123], and may be useful in endometriosis-related infertility [128]. During stress, the mechanism involves inactivation of glucocorticoids inhibiting LX-biosynthesis [123] controlling local inflammatory-homeostasis in pregnancy, suggesting a physiologic role for SPM (Fig. 3A).
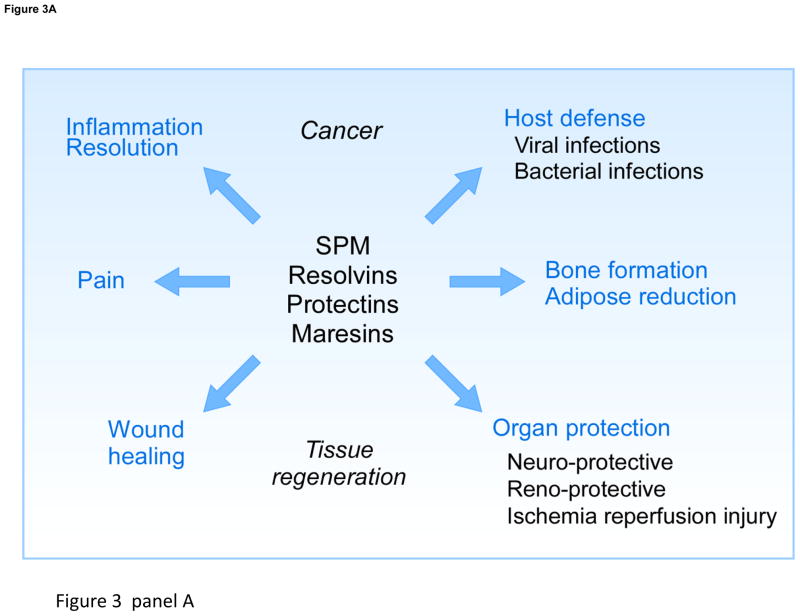
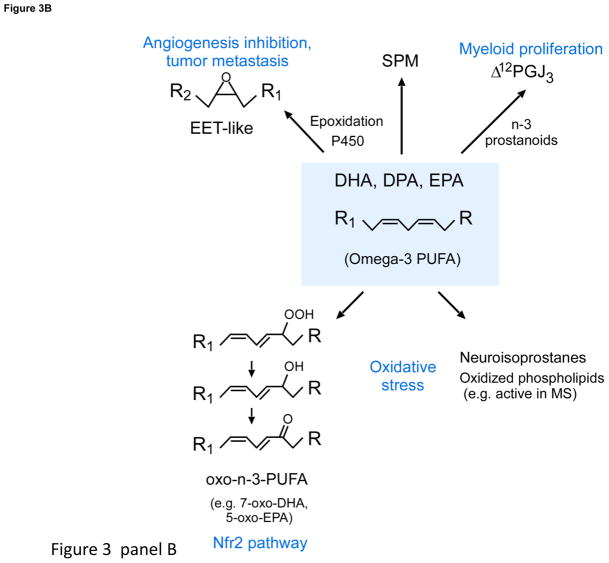
Figure 3. New lipid mediators and biosynthesis routes.
Panel A: SPM actions. Panel B: New routes for n-3 essential fatty acids (DHA, DPA and EPA) conversion via P450 cyclooxygenase, lipoxygenase and oxidative pathways.
In preeclampsia, LX counters pro-inflammatory factors produced in women that stimulate PMN adhesion to vascular endothelial cells [129], and LX appear to regulate implantation which may be useful in contraception. Certainly enzymes that produce SPM are in rat placental tissue and are regulated during gestation [130]. RvE3 in pregnant mice lowers local inflammatory milieu and incidence of preterm birth [131]. D-series resolvins RvD1, RvD2 and protectins (PD1 and 10S,17S-diHDHA, a.k.a. PDx; Cayman Chemical) are present in placenta and are increased with dietary omega-3 [130]. Another strategic location for SPM is in human breast milk [132], where they are orders of magnitude higher levels than inflammatory sites. LXA4, RvD1 and RvE1, identified in milk from mothers during the first month of lactation [132], may each have role(s) in neonatal immunity.
11. Organ Fibrosis
Unresolved inflammation, epithelial and microvascular injury can lead to excessive fibrosis that impairs organ function. In many organs such as lung and kidney, the cause is unknown and can lead to morbidity. Leukotrienes are profibrotic and in humans with scleroderma interstitial lung disease, the relationship between leukotrienes and lipoxins is imbalanced, with LXA4 in bronchoalveolar lavages at levels unable to counter-regulate profibrotic factors [133]. Aspirin-triggered-LX analog reduces bleomycin-induced pulmonary fibrosis [134], and both LXA4 and benzo-LXA4 reduce renal fibrosis [135]. RvE1 and RvD1 protect from renal fibrosis by reducing collagen I and IV, α-SMA and fibronectin [136]. Also, RvD1 reduces pro-inflammatory mediators generated by cigarette smoke exposure and pulmonary toxicants [137] that may reduce COPD-like fibrosis.
12. Cancer Resolution
Unresolved inflammation may link to predisposition to carcinogenesis and tumor invasiveness [3, 138]. RvD1 is chemopreventive in colitis-associated colon carcinogenesis in mice [139, 140]. With D. Panigrahy and colleagues, we found both RvD1 and RvD2 reduce tumor growth in mice in nanogram amounts [141] and may be useful together with cancer chemotherapies.
13. SPM link Innate to Adaptive Immunity
Lymphoid tissue, e.g. mouse spleen, produces RvD1, 17-HDHA, PD1 [142] and LXA4 [143] from endogenous sources, suggesting they’re strategically positioned to act on lymphocytes (Fig. 1). Both 17-HDHA and RvD1 increase human B cell IgM and IgG, a response not shared by PD1. 17-HDHA augments B cell differentiation toward CD27(+)CD38(+) antibody-secreting cell phenotype [142]. PD1 is biosynthesized by human T helper 2-skewed mononuclear cells via 16(17)-epoxy-protectin intermediate (Figs. 2 and 3) and reduces T cell migration, TNFα and INFγ while promoting T cell apoptosis [144]. LXA4, RvE1 and PD1 each upregulate CCR5 expression on leukocytes that bind chemokines, facilitating their clearance and resolution [145]. PD1 reduces CD4+ T cell infiltration into cornea [75], as does RvE1 in Herpes simplex viral infections [76]. RvD1 reduces CD11b+ leukocytes and CD4+ and CD8+ T lymphocytes within the eye in uveitis [146]. RvE1 and RvD1 each regulate T-cell activation in choroid-retina [147]. RvE1 induces apoptosis of activated T cells via 2,3-dioxygenase induction in DC giving a new functional DC-subtype in resolution [148]. RvE1 reduces mouse CD4+ T cells and CD8+ T cells in atopic dermatitis [149].
14. Additional n-3 Pathways and Products
Identification of novel n-3 mediators and ability to profile using LC-MS-MS-based lipidomics [3, 4] opened the possibility for additional pathways that can convert n-3 to bioactive molecules. Recently, Hammock and colleagues found cytochrome P450 epoxygenases convert DHA (Fig. 3B) to EET-like structures that inhibit VEGF and fibroblast growth factor 2-induced angiogenesis. When epoxydocosapentaenoic acids are given to mice with soluble epoxide hydrolase inhibitor, they are blocked from further metabolism, reducing ~70 tumor growth and metastasis [150]. Epoxyeicosatrienoic acids (EETs) evoke an opposite action in micromolar range, increasing tumor progression and angiogenesis [150]. These opposing actions are intriguing, because both the n-3 and n-6 epoxy-products are present in healthy subjects with n-3 supplementation, that display a high degree of inter-individual variability in metabolite phenotyping of these pathways (Fig 3 B) using lipidomics [151].
In addition to epoxidation, p450 monooxygenases can convert PUFA to hydroxy-containing products similar to lipoxygenases, but via a different mechanism [152]. Some of these hydroxy p450-products (i.e. 17-HDHA) are converted in vivo to D-series resolvins [152]. EPA is substrate for prostanoid-like products, some of which exert potent actions; e.g., Δ12-PGJ3 targets leukemia stem cells, activating apoptosis via Michael adducts [153] (Fig. 3B). Human leukocytes can also convert hydroxy-n-3 PUFA to electrophilic α,β unsaturated ketone derivatives that activate Nrf2-dependent gene expression and suppress NF-κB pro-inflammatory-gene expression [154]. Oxidative stress promotes DHA conversion to neuroisoprostanes [155] as well as oxidized phospholipid derivatives that resolve neuroinflammation, uncovered using functional lipidomics [156] active in experimental autoimmune encephalomyelitis and brain lesions in multiple sclerosis.
15. Human studies
Since resolvins and protectins were first identified in mouse exudates, it was essential to establish their biosynthesis by human leukocytes and in human tissues [3, 4, 88]. RvE1 and RvE2 are produced in human blood [53, 70, 157], providing substrate is available. RvD1 and RvD2 are also present in human blood at concentrations commensurate with their known bioactivities [158], and in human milk [132], identifications made possible with LC-MS-MS and availability of SPM [16], thus opening the opportunity for rigorous assessment of their functional roles and potential in human physiology (Table 4, human SPM production).
Table 4.
SPM production in humans
| SPM | Disease/tissues | Formation |
|---|---|---|
| Lipoxins & Aspirin-triggered lipoxins (ATL) | Asthma | Higher urinary ATL levels in aspirin-tolerant asthma than in aspirin-intolerant asthma [164, 195, 196] and regulate NK cell and innate lymphoid cell activation [165, 197] |
| Alzheimer’s disease (AD) | LXA4 levels are reduced in AD brain and CSF [96] | |
| Colitis | Elevated mucosal LXA4 promotes remission in Individuals with ulcerative colitis [171] | |
| Type 2 diabetes | Increased plasma ATL with intake of pioglitazone [198] | |
| Rheumatoid arthritis | LXA4 in synovial fluid from rheumatoid arthritis patients [168] | |
| Localized aggressive periodontitis (LAP) | Less LXA4 in LAP whole blood compared to healthy individuals [166] | |
| Peripheral artery disease | Plasma levels of ATL are lower in patients with symptomatic peripheral artery disease [199] | |
| Adipose tissues | LXA4 identified in human adipocytes from obese patients [200] | |
| Milk | Lipoxins and resolvins at very high levels in the first month of lactation [132] | |
| Resolvins | Synovial fluid | RvD5 present in synovial fluid from rheumatoid arthritis patients [168] |
| Blood (healthy volunteers) | Plasma RvD1 and RvD2 identified with oral omega-3 supplementation [158] | |
| Adipose tissues | RvD1 and RvD2 identified in human adipocytes from obese patients [200] | |
| Human plasma and milk | RvE1 identified in human plasma [157] and milk [132] | |
| Multiple sclerosis | RvD1 was detected and upregulated in serum and cerebrospinal fluid in the highly active group [106] | |
| Human IgA nephropathy | RvE1 identified in patients supplemented with fish oil n-3 [201] | |
| Peripheral blood (plasma and serum), lymphoid organs | RvD1, RvD2 and RvD3, identified in amounts within their bioactive ranges [202] | |
| Protectin | Asthma | PD1 in exhaled breath condensates in asthma exacerbation [169]; decreased PD1 in eosinophils from patients with severe asthma compared to healthy individuals [50] |
| Embryonic stem cells | PD1 produced in embryonic stem cells [203] | |
| Multiple sclerosis | NPD1 was detected in serum and cerebrospinal fluid in the highly active group [106] | |
| Peripheral blood (plasma and serum), lymphoid organs | PD1 identified in amounts within their bioactive ranges. AT-PD1 is significantly increased with EFA and ASA intake [202] | |
| Maresins | Synovial fluid | MaR1 identified in synovial fluid from arthritis patients [168] |
| Peripheral blood (plasma and serum), lymphoid organs | MaR1 identified in amounts within their bioactive ranges [202] |
Muscle inflammation; resistance exercise
In an elegant study, Markworth et al. [26] demonstrated lipid mediator class switching in humans. Using a strenuous resistance exercise protocol, venous blood was collected during the time course post exercise and lipid mediators present in peripheral blood were identified using LC-MS-MS-lipidomics. Initial post-exercise recovery phase demonstrated the presence of prostanoids followed by leukotrienes, EETs and p450-derived eicosanoids, as well as LX, resolvins and protectins. Widely used NSAID for muscle aches and pains, ibuprofen, blocks exercise-induced prostanoids as well as reduces LTB4 and delays and diminishes the appearance of SPM. Resistance-exercise in humans illustrates the initial acute pro-inflammatory mediators and their link to resolution programs [26].
Lipoxin analogs were designed that are topically active [24], orally active, and efficacious in mouse and guinea pig skin inflammation, reducing edema, PMN and eosinophil infiltration and epidermal hyperproliferation [159] in preclinical models that could provide an alternative to using corticosteroids in certain settings. ATL analog is more potent than LTB4 receptor antagonist or glucocorticoid [159]. One setting is pediatric use of steroids. In infantile eczema, topical 15(R/S)-methyl-LXA4 relieved severity and improved quality of life without apparent adverse events in a double-blind trial [160]. In these infants, AT-LXA4 analog was as effective as a steroid mometasone. Also, inhaled LXA4 is safe and efficacious with asthmatic adults [161].
15.1. SPM resolution agonists in clinical development
RvE1, MaR1 and NPD1/PD1 are each in clinical development programs licensed by Brigham and Women’s Hospital/Partners Health Care. RvE1 mimetic is in development for ocular indications1, and NPD1/PD1 for neurodegenerative diseases and hearing loss2, given their broad ability to regulate inflammation without immunosuppression. Results from their pharmacology in animals [18] suggest treatment of inflammation-associated disease may be possible with SPM-agonists, which can reduce the use of inhibitors or antagonists that eventually can turn immunosuppressive [69].
16. New Pathway in Tissue Regeneration and Host Defense
Upon infection and inflammation, tissue repair and regeneration are essential in reestablishing function. We identified potent molecules present in self-limited infectious murine exudates, regenerating planaria, human milk as well as macrophages that stimulated tissue regeneration in planaria and are pro-resolving [162]. Characterization of their physical properties and isotope tracking indicated that the bioactive structures contained docosahexaenoic acid and sulfido-conjugated (SC) triene double bonds that proved to be 13-glutathionyl,14-hydroxy-docosahexaenoic acid (SCI) and 13-cysteinylglycinyl, 14-hydroxy-docosahexaenoic acid (SCII). These molecules rescued E. coli infection-mediated delay in tissue regeneration in planaria, improving regeneration intervals from ~4.2 to ~3.7 days. Administration of SC protected mice from second organ reflow injury, promoting repair via limiting neutrophil infiltration, upregulating Ki67 and Roof plate-specific spondin 3. At nanomolar potencies these conjugates also resolved E. coli infections by limiting neutrophil infiltration, stimulating bacteria phagocytosis and clearance as well as efferocytosis of apoptotic cells. Together, these findings identify previously undescribed, conserved chemical signals and pathways in planaria, mice and human tissues that enhance host responses to contain infections, stimulate resolution of inflammation and promote the restoration of function.
17. Summation
Heat, redness, swelling, pain and loss of function are the cardinal signs of inflammation, each evoked by mediators, and an emerging body of evidence indicates that they are physiologically counterregulated by SPM. It’s when and where that counts for autacoids, and it’s important to emphasize that the first response of acute inflammation is ubiquitous and mounts throughout the body. Both n-6 and n-3 PUFA play critical and distinct roles in SPM biosynthesis of local mediators that are agonists; namely, immunoresolvents stimulating resolution by cessation of PMN influx and enhancing phagocytosis for clearance and containment, the hallmarks of resolution (Fig. 1). These are the defining SPM functions, with each SPM possessing additional nonredundant roles (Fig. 3) and more likely to be uncovered. At the cellular and molecular level, SPM counterregulate pro-inflammatory chemokines, cytokines and regulate adipokines; regulate miR, transcription and translocation, cell traffic and enhance microbial killing via GPCR-mediated mechanisms (Table 1). SPM are produced in humans (blood, milk and brain) where they can have physiologic actions because they are produced in levels that display potent selective actions in animals [17, 18]. SPM location within lymphoid tissues and actions with lymphocytes place them in strategic positions for communications from innate to adaptive immunity.
These exciting new findings from laboratories worldwide raise many questions on, e.g., whether SPM along with their local actions are also capable of evoking responses at sites distant from their origins. Ample n-3 and n-6 levels in humans are needed to biosynthesize PUFA-derived signals for the inflammatory response that evolved to protect the host. What are optimal levels of n-3 and n-6 PUFA needed to ensure local SPM-biosynthesis and eicosanoids required for timely resolution and homeostasis? Are there age- and gender-related components in SPM-resolution programs? Can we personalize nutrition to optimize local SPM production using LM-metabolipidomic profiling? Is it possible to design therapeutic diets to increase SPM [119], related products [150], and/or use select SPM-receptor agonists to control diseases? Is there a negative side to prolonged supraphysiologic SPM or related n-3-PUFA-derived products? The immediate future will bring evidence to address these fundamental questions now that the SPM structure and spatial temporal resolution-metabolomes are under intensive investigations.
SPM and new PUFA products (Fig. 5) emerge as key regulators in physiologic pathways in unresolved inflammation, obesity, cognitive decline, reproduction, neuroprotection and cancer, beyond PUFA roles in intermediary metabolism and membrane physical dynamics. Identification of SPM metabolomes and the appreciation that exudates drive resolution in part via SPM proresolving actions has set a new terrain in physiology. Moreover, SPM are now proven to have a wide range of actions that open a new vista for resolution physiology and pharmacology, where the mediators and their precursors are vital in supplying chemical signals for catabasis to homeostasis.
Supplementary Material
Highlights.
Biosynthesis of specialized proresolving mediators (SPM)
Functions of resolvins, lipoxins, protectins and maresins
SPM actions in animal models of disease
Acknowledgments
The authors thank Mary Halm Small for help with manuscript preparation and the NIH for support of the author’s research (Grants R01GM038765 and P01GM095467).
Abbreviations
- 17R-HDHA
17R-hydroxy-4Z,7Z,10Z,13Z,15E,19Z-docosahexaenoic acid
- AT
aspirin-triggered
- AT-RvD3
4S,11R,17R-trihydroxydocosa-5Z,7E,9E,13Z,15E,19Z-hexaenoic acid
- GPCR
G protein-coupled receptor
- LC-MS-MS
liquid chromatography-tandem mass spectrometry
- LM
lipid mediator
- LT
leukotriene
- LTB4
leukotriene B4, 5S,12R-dihydroxy-6Z,8E,10E,14Z-eicosatetraenoic acid
- LXA4
lipoxin A4, 5S,6R,15S-trihydroxy-7E,9E,11Z,13E-eicosatetraenoic acid
- LXB4
lipoxin B4, 5S,14R,15S-trihydroxy-6E,8Z,10E,12E-eicosatetraenoic acid
- MaR1
maresin 1, 7R,14S-dihydroxy-docosa-4Z,8E,10E,12Z,16Z,19Z-hexaenoic acid
- MΦ
macrophages
- PD1/NPD1
protectin D1/neuroprotectin D1, 10R,17S-dihydroxy-4Z,7Z,11E,13E,15Z,19Z-docosahexaenoic acid
- PMN
polymorphonuclear neutrophil
- PG
prostaglandin
- PGE2
Prostaglandin E2, 9-oxo-11R,15S-dihydroxy-5Z,13E-prostadienoic acid
- PUFA
polyunsaturated fatty acid
- Rv
resolvin
- RvD1
resolvin D1, 7S, 8R,17S-trihydroxy-4Z, 9E, 11E, 13Z, 15E, 19Z-docosahexaenoic acid)
- RvD2
resolvin D2, 7S, 16R, 17S-trihydroxy-4Z, 8E, 10Z, 12E, 14E,19Z-docosahexaenoic acid
- RvD3
resolvin D3, 4S,11R,17S-trihydroxydocosa-5Z,7E,9E,13Z,15E,19Z-hexaenoic acid
- RvD5
Resolvin D5, 7S,17S-dihydroxy-4Z,8E,10Z,13Z,15E,19Z-docosahexaenoic acid
- RvE1
resolvin E1, 5S,12R,18R-trihydroxy-6Z,8E,10E,14Z,16E-eicosapentaenoic acid
- RvE2
resolvin E2, 5S,18R-trihydroxy-6E,8Z,11Z,14Z,16E-eicosapentaenoic acid
- SPM
specialized pro-resolving mediators
- TRP
transient receptor potential
Footnotes
Auven Therapeutics. RX 10045 – ocular inflammation. http://www.auventx.com/auven/products/rx10045.php
Anida Pharma Inc. Neuroprotectin D1. http://www.anidapharma.com/lead-molecule.html
Competing financial interests: CNS is an inventor on patents [resolvins] assigned to BWH and licensed to Resolvyx Pharmaceuticals. CNS is a scientific founder of Resolvyx Pharmaceuticals and owns equity in the company. CNS’ interests were reviewed and are managed by the Brigham and Women’s Hospital and Partners HealthCare in accordance with their conflict of interest policies.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Serhan CN. The resolution of inflammation: the devil in the flask and in the details. FASEB J. 2011;25:1441–8. doi: 10.1096/fj.11-0502ufm. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Samuelsson B. Role of basic science in the development of new medicines: examples from the eicosanoid field. J Biol Chem. 2012;287:10070–80. doi: 10.1074/jbc.X112.351437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Serhan CN, Clish CB, Brannon J, Colgan SP, Chiang N, Gronert K. Novel functional sets of lipid-derived mediators with antiinflammatory actions generated from omega-3 fatty acids via cyclooxygenase 2-nonsteroidal antiinflammatory drugs and transcellular processing. J Exp Med. 2000;192:1197–204. doi: 10.1084/jem.192.8.1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Serhan CN, Hong S, Gronert K, Colgan SP, Devchand PR, Mirick G, et al. Resolvins: a family of bioactive products of omega-3 fatty acid transformation circuits initiated by aspirin treatment that counter pro-inflammation signals. J Exp Med. 2002;196:1025–37. doi: 10.1084/jem.20020760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Malawista SE, de Boisfleury Chevance A, van Damme J, Serhan CN. Tonic inhibition of chemotaxis in human plasma. Proc Natl Acad Sci U S A. 2008;105:17949–54. doi: 10.1073/pnas.0802572105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lammermann T, Afonso PV, Angermann BR, Wang JM, Kastenmuller W, Parent CA, et al. Neutrophil swarms require LTB4 and integrins at sites of cell death in vivo. Nature. 2013;498:371–5. doi: 10.1038/nature12175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Buckley CD, Gilroy DW, Serhan CN, Stockinger B, Tak PP. The resolution of inflammation. Nat Rev Immunol. 2013;13:59–66. doi: 10.1038/nri3362. [DOI] [PubMed] [Google Scholar]
- 8.Robbins SL, Cotran R. Pathologic Basis of Disease. 2. Philadelphia: W.B. Saunders Co; 1979. [Google Scholar]
- 9.Tauber AI, Chernyak L. Metchnikoff and the Origins of Immunology: From Metaphor to Theory. New York: Oxford University Press; 1991. [Google Scholar]
- 10.Norris PC, Dennis EA. Omega-3 fatty acids cause dramatic changes in TLR4 and purinergic eicosanoid signaling. Proc Natl Acad Sci U S A. 2012;109:8517–22. doi: 10.1073/pnas.1200189109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dalli J, Winkler JW, Colas RA, Arnardottir H, Cheng CYC, Chiang N, et al. Resolvin D3 and aspirin-triggered resolvin D3 are potent immunoresolvents. Chem Biol. 2013;20:188–201. doi: 10.1016/j.chembiol.2012.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dalli J, Zhu M, Vlasenko NA, Deng B, Haeggstrom JZ, Petasis NA, et al. The novel 13S,14S-epoxy-maresin is converted by human macrophages to maresin1 (MaR1), inhibits leukotriene A4 hydrolase (LTA4H), and shifts macrophage phenotype. FASEB J. 2013;27:2573–83. doi: 10.1096/fj.13-227728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bailey RL, Gahche JJ, Miller PE, Thomas PR, Dwyer JT. Why US Adults Use Dietary Supplements. JAMA Intern Med. 2013;173:355–61. doi: 10.1001/jamainternmed.2013.2299. [DOI] [PubMed] [Google Scholar]
- 14.Yates CM, Calder PC. Pharmacology and therapeutics of omega-3 polyunsaturated fatty acids in chronic inflammatory disease. In: Rainger G, editor. Pharmacol Ther. 2013. [DOI] [PubMed] [Google Scholar]
- 15.Serhan CN, Savill J. Resolution of inflammation: the beginning programs the end. Nat Immunol. 2005;6:1191–7. doi: 10.1038/ni1276. [DOI] [PubMed] [Google Scholar]
- 16.Serhan CN, Petasis NA. Resolvins and protectins in inflammation-resolution. Chem Rev. 2011;111:5922–43. doi: 10.1021/cr100396c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Miyahara T, Runge S, Chatterjee A, Chen M, Mottola G, Fitzgerald JM, et al. D-series resolvins attenuate vascular smooth muscle cell activation and neointimal hyperplasia following vascular injury. FASEB J. 2013;27:2220–32. doi: 10.1096/fj.12-225615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Serhan CN, Chiang N. Resolution phase lipid mediators of inflammation: agonists of resolution. Curr Opin Pharmacol. 2013;13:632–40. doi: 10.1016/j.coph.2013.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chiu CY, Gomolka B, Dierkes C, Huang NR, Schroeder M, Purschke M, et al. Omega-6 docosapentaenoic acid-derived resolvins and 17-hydroxydocosahexaenoic acid modulate macrophage function and alleviate experimental colitis. Inflamm Res. 2012;61:967–76. doi: 10.1007/s00011-012-0489-8. [DOI] [PubMed] [Google Scholar]
- 20.Dalli J, Colas RA, Serhan CN. Novel n-3 immunoresolvents: structures and actions. Sci Rep. 2013;3:1940. doi: 10.1038/srep01940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Deng B, Wang CW, Arnardottir HH, Li Y, Cheng CY, Dalli J, et al. Maresin biosynthesis and identification of maresin 2, a new anti-inflammatory and pro-resolving mediator from human macrophages. PLoS One. 2014;9:e102362. doi: 10.1371/journal.pone.0102362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Levy BD, Clish CB, Schmidt B, Gronert K, Serhan CN. Lipid mediator class switching during acute inflammation: signals in resolution. Nat Immunol. 2001;2:612–9. doi: 10.1038/89759. [DOI] [PubMed] [Google Scholar]
- 23.Bandeira-Melo C, Serra MF, Diaz BL, Cordeiro RSB, Silva PMR, Lenzi HL, et al. Cyclooxygenase-2-derived prostaglandin E2 and lipoxin A4 accelerate resolution of allergic edema in Angiostrongylus costaricensis-infected rats: relationship with concurrent eosinophilia. J Immunol. 2000;164:1029–36. doi: 10.4049/jimmunol.164.2.1029. [DOI] [PubMed] [Google Scholar]
- 24.Takano T, Clish CB, Gronert K, Petasis N, Serhan CN. Neutrophil-mediated changes in vascular permeability are inhibited by topical application of aspirin-triggered 15-epi-lipoxin A4 and novel lipoxin B4 stable analogues. J Clin Invest. 1998;101:819–26. doi: 10.1172/JCI1578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chan MM-Y, Moore AR. Resolution of inflammation in murine autoimmune arthritis is disrupted by cyclooxygenase-2 inhibition and restored by prostaglandin E2-mediated lipoxin A4 production. J Immunol. 2010;184:6418–26. doi: 10.4049/jimmunol.0903816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Markworth JF, Vella LD, Lingard BS, Tull DL, Rupasinghe TW, Sinclair AJ, et al. Human inflammatory and resolving lipid mediator responses to resistance exercise and ibuprofen treatment. Am J Physiol Regul Integr Comp Physiol. 2013;305:R1281–R96. doi: 10.1152/ajpregu.00128.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schwab JM, Chiang N, Arita M, Serhan CN. Resolvin E1 and protectin D1 activate inflammation-resolution programmes. Nature. 2007;447:869–74. doi: 10.1038/nature05877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Morris T, Stables M, Hobbs A, de Souza P, Colville-Nash P, Warner T, et al. Effects of low-dose aspirin on acute inflammatory responses in humans. J Immunol. 2009;183:2089–96. doi: 10.4049/jimmunol.0900477. [DOI] [PubMed] [Google Scholar]
- 29.Lucas CD, Dorward DA, Tait MA, Fox S, Marwick JA, Allen KC, et al. Downregulation of Mcl-1 has anti-inflammatory pro-resolution effects and enhances bacterial clearance from the lung. Mucosal Immunol. 2013 doi: 10.1038/mi.2013.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Levy BD, Serhan CN. Resolution of acute inflammation in the lung. Annu Rev Physiol. 2014;76:27.1–6. doi: 10.1146/annurev-physiol-021113-170408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chiang N, Schwab JM, Fredman G, Kasuga K, Gelman S, Serhan CN. Anesthetics impact the resolution of inflammation. PLoS ONE. 2008;3:e1879. doi: 10.1371/journal.pone.0001879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chiang N, Fredman G, Bäckhed F, Oh SF, Vickery TW, Schmidt BA, et al. Infection regulates pro-resolving mediators that lower antibiotic requirements. Nature. 2012;484:524–8. doi: 10.1038/nature11042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Spite M, Norling LV, Summers L, Yang R, Cooper D, Petasis NA, et al. Resolvin D2 is a potent regulator of leukocytes and controls microbial sepsis. Nature. 2009;461:1287–91. doi: 10.1038/nature08541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Birnbaum Y, Ye Y, Lin Y, Freeberg SY, Nishi SP, Martinez JD, et al. Augmentation of myocardial production of 15-epi-lipoxin-A4 by pioglitazone and atorvastatin in the rat. Circulation. 2006;114:929–35. doi: 10.1161/CIRCULATIONAHA.106.629907. [DOI] [PubMed] [Google Scholar]
- 35.Clària J, Serhan CN. Aspirin triggers previously undescribed bioactive eicosanoids by human endothelial cell-leukocyte interactions. Proc Natl Acad Sci USA. 1995;92:9475–9. doi: 10.1073/pnas.92.21.9475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cooray SN, Gobbetti T, Montero-Melendez T, McArthur S, Thompson D, Clark AJ, et al. Ligand-specific conformational change of the G-protein-coupled receptor ALX/FPR2 determines proresolving functional responses. Proc Natl Acad Sci U S A. 2013;110:18232–7. doi: 10.1073/pnas.1308253110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Serhan CN, Fredman G, Yang R, Karamnov S, Belayev LS, Bazan NG, et al. Novel proresolving aspirin-triggered DHA pathway. Chem Biol. 2011;18:976–87. doi: 10.1016/j.chembiol.2011.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chiang N, Bermudez EA, Ridker PM, Hurwitz S, Serhan CN. Aspirin triggers anti-inflammatory 15-epi-lipoxin A4 and inhibits thromboxane in a randomized human trial. Proc Natl Acad Sci USA. 2004;101:15178–83. doi: 10.1073/pnas.0405445101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Morris T, Stables M, Colville-Nash P, Newson J, Bellingan G, de Souza PM, et al. Dichotomy in duration and severity of acute inflammatory responses in humans arising from differentially expressed proresolution pathways. Proc Natl Acad Sci USA. 2010;107:8842–7. doi: 10.1073/pnas.1000373107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pillai PS, Leeson S, Porter TF, Owens CD, Kim JM, Conte MS, et al. Chemical mediators of inflammation and resolution in post-operative abdominal aortic aneurysm patients. Inflammation. 2012;35:98–113. doi: 10.1007/s10753-011-9294-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Norling LV, Spite M, Yang R, Flower RJ, Perretti M, Serhan CN. Cutting edge: Humanized nano-proresolving medicines mimic inflammation-resolution and enhance wound healing. J Immunol. 2011;186:5543–7. doi: 10.4049/jimmunol.1003865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dalli J, Serhan CN. Specific lipid mediator signatures of human phagocytes: microparticles stimulate macrophage efferocytosis and pro-resolving mediators. Blood. 2012;120:e60–72. doi: 10.1182/blood-2012-04-423525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang J, Byrne JD, Napier ME, DeSimone JM. More effective nanomedicines through particle design. Small. 2011;7:1919–31. doi: 10.1002/smll.201100442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Van Dyke TE, Hasturk H, Kantarci A, Freire MO, Nguyen D, Dalli J, et al. Proresolving Nanomedicines Activate Bone Regeneration in Periodontitis. J Dent Res. 2015;94:148–56. doi: 10.1177/0022034514557331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jones CN, Dalli J, Dimisko L, Wong E, Serhan CN, Irimia D. Microfluidic chambers for monitoring leukocyte trafficking and humanized nano-proresolving medicines interactions. Proc Natl Acad Sci U S A. 2012;109:20560–5. doi: 10.1073/pnas.1210269109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tang K, Liu J, Yang Z, Zhang B, Zhang H, Huang C, et al. Microparticles mediate enzyme transfer from platelets to mast cells: a new pathway for lipoxin A4 biosynthesis. Biochem Biophys Res Commun. 2010;400:432–6. doi: 10.1016/j.bbrc.2010.08.095. [DOI] [PubMed] [Google Scholar]
- 47.Biswas SK, Mantovani A. Macrophage plasticity and interaction with lymphocyte subsets: cancer as a paradigm. Nat Immunol. 2010;11:889–96. doi: 10.1038/ni.1937. [DOI] [PubMed] [Google Scholar]
- 48.Stables MJ, Shah S, Camon EB, Lovering RC, Newson J, Bystrom J, et al. Transcriptomic analyses of murine resolution-phase macrophages. Blood. 2011;118:e192–e208. doi: 10.1182/blood-2011-04-345330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Miki Y, Yamamoto K, Taketomi Y, Sato H, Shimo K, Kobayashi T, et al. Lymphoid tissue phospholipase A2 group IID resolves contact hypersensitivity by driving antiinflammatory lipid mediators. J Exp Med. 2013;210:1217–34. doi: 10.1084/jem.20121887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Miyata J, Fukunaga K, Iwamoto R, Isobe Y, Niimi K, Takamiya R, et al. Dysregulated synthesis of protectin D1 in eosinophils from patients with severe asthma. J Allergy Clin Immunol. 2013;131:353–60. doi: 10.1016/j.jaci.2012.07.048. [DOI] [PubMed] [Google Scholar]
- 51.Yamada T, Tani Y, Nakanishi H, Taguchi R, Arita M, Arai H. Eosinophils promote resolution of acute periotonitis by producing proresolving mediators in mice. FASEB J. 2011;25:561–8. doi: 10.1096/fj.10-170027. [DOI] [PubMed] [Google Scholar]
- 52.Isobe Y, Arita M, Iwamoto R, Urabe D, Todoroki H, Masuda K, et al. Stereochemical assignment and anti-inflammatory properties of the omega-3 lipid mediator resolvin E3. J Biochem. 2013;153:355–60. doi: 10.1093/jb/mvs151. [DOI] [PubMed] [Google Scholar]
- 53.Arita M, Bianchini F, Aliberti J, Sher A, Chiang N, Hong S, et al. Stereochemical assignment, anti-inflammatory properties, and receptor for the omega-3 lipid mediator resolvin E1. J Exp Med. 2005;201:713–22. doi: 10.1084/jem.20042031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ohira T, Arita M, Omori K, Recchiuti A, Van Dyke TE, Serhan CN. Resolvin E1 receptor activation signals phosphorylation and phagocytosis. J Biol Chem. 2010;285:3451–61. doi: 10.1074/jbc.M109.044131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.El Kebir D, Gjorstrup P, Filep JG. Resolvin E1 promotes phagocytosis-induced neutrophil apoptosis and accelerates resolution of pulmonary inflammation. Proc Natl Acad Sci U S A. 2012;109:14983–8. doi: 10.1073/pnas.1206641109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Krishnamoorthy S, Recchiuti A, Chiang N, Fredman G, Serhan CN. Resolvin D1 receptor stereoselectivity and regulation of inflammation and pro-resolving microRNAs. Am J Pathol. 2012;180:2018–27. doi: 10.1016/j.ajpath.2012.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Norling LV, Dalli J, Flower RJ, Serhan CN, Perretti M. Resolvin D1 limits polymorphonuclear leukocytes recruitment to inflammatory loci: receptor dependent actions. Arterioscler Thromb Vasc Biol. 2012;32:1970–8. doi: 10.1161/ATVBAHA.112.249508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Fredman G, Li Y, Dalli J, Chiang N, Serhan CN. Self-limited versus delayed resolution of acute inflammation: temporal regulation of pro-resolving mediators and microRNA. Sci Rep. 2012;2:639. doi: 10.1038/srep00639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Li Y, Dalli J, Chiang N, Baron RM, Quintana C, Serhan CN. Plasticity of leukocytic exudates in resolving acute inflammation is regulated by microRNA and proresolving mediators. Immunity. 2013;39:885–98. doi: 10.1016/j.immuni.2013.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Li D, Hodges RR, Jiao J, Carozza RB, Shatos MA, Chiang N, et al. Resolvin D1 and aspirin-triggered resolvin D1 regulate histamine-stimulated conjunctival goblet cell secretion. Mucosal Immunol. 2013;6:1119–30. doi: 10.1038/mi.2013.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Nelson J, Leigh NJ, Mellas RE, McCall AD, Aguirre A, Baker OJ. The ALX/FPR2 receptor for RvD1 is Expressed and Functional in Salivary Glands. Am J Physiol Cell Physiol. 2013 doi: 10.1152/ajpcell.00284.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Simiele F, Recchiuti A, Mattoscio D, De Luca A, Cianci E, Franchi S, et al. Transcriptional regulation of the human FPR2/ALX gene: evidence of a heritable genetic variant that impairs promoter activity. FASEB J. 2012;26:1323–33. doi: 10.1096/fj.11-198069. [DOI] [PubMed] [Google Scholar]
- 63.Oh DY, Talukdar S, Bae EJ, Imamura T, Morinaga H, Fan W, et al. GPR120 is an omega-3 fatty acid receptor mediating potent anti-inflammatory and insulin-sensitizing effects. Cell. 2010;142:687–98. doi: 10.1016/j.cell.2010.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gilbertson TA, Khan NA. Cell signaling mechanisms of oro-gustatory detection of dietary fat: Advances and challenges. Prog Lipid Res. 2013 doi: 10.1016/j.plipres.2013.11.001. [DOI] [PubMed] [Google Scholar]
- 65.Sun Q, Hirasawa A, Hara T, Kimura I, Adachi T, Awaji T, et al. Structure-activity relationships of GPR120 agonists based on a docking simulation. Mol Pharmacol. 2010;78:804–10. doi: 10.1124/mol.110.066324. [DOI] [PubMed] [Google Scholar]
- 66.Serhan CN. Pro-resolving lipid mediators are leads for resolution physiology. Nature. 2014;510:92–101. doi: 10.1038/nature13479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Serhan CN, Jain A, Marleau S, Clish C, Kantarci A, Behbehani B, et al. Reduced inflammation and tissue damage in transgenic rabbits overexpressing 15-lipoxygenase and endogenous anti-inflammatory lipid mediators. J Immunol. 2003;171:6856–65. doi: 10.4049/jimmunol.171.12.6856. [DOI] [PubMed] [Google Scholar]
- 68.Hasturk H, Kantarci A, Goguet-Surmenian E, Blackwood A, Andry C, Serhan CN, et al. Resolvin E1 regulates inflammation at the cellular and tissue level and restores tissue homeostasis in vivo. J Immunol. 2007;179:7021–9. doi: 10.4049/jimmunol.179.10.7021. [DOI] [PubMed] [Google Scholar]
- 69.Fullerton JN, O’Brien AJ, Gilroy DW. Lipid mediators in immune dysfunction after severe inflammation. Trends Immunol. 2014;35:12–21. doi: 10.1016/j.it.2013.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Oh SF, Pillai PS, Recchiuti A, Yang R, Serhan CN. Pro-resolving actions and stereoselective biosynthesis of 18S E-series resolvins in human leukocytes and murine inflammation. J Clin Invest. 2011;121:569–81. doi: 10.1172/JCI42545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Walker J, Dichter E, Lacorte G, Kerner D, Spur B, Rodriguez A, et al. Lipoxin A4 increases survival by decreasing systemic inflammation and bacterial load in sepsis. Shock. 2011;36:410–6. doi: 10.1097/SHK.0b013e31822798c1. [DOI] [PubMed] [Google Scholar]
- 72.Prescott D, McKay DM. Aspirin-triggered lipoxin enhances macrophage phagocytosis of bacteria while inhibiting inflammatory cytokine production. Am J Physiol Gastrointest Liver Physiol. 2011;301:G487–G97. doi: 10.1152/ajpgi.00042.2011. [DOI] [PubMed] [Google Scholar]
- 73.Divangahi M, Chen M, Gan H, Desjardins D, Hickman TT, Lee DM, et al. Mycobacterium tuberculosis evades macrophage defenses by inhibiting plasma membrane repair. Nat Immunol. 2009;10:899–906. doi: 10.1038/ni.1758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Tobin DM, Roca FJ, Oh SF, McFarland R, Vickery TW, Ray JP, et al. Host genotype-specific therapies can optimize the inflammatory response to mycobacterial infections. Cell. 2012;148:434–46. doi: 10.1016/j.cell.2011.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Rajasagi NK, Reddy PB, Mulik S, Gjorstrup P, Rouse BT. Neuroprotectin D1 reduces the severity of herpes simplex virus-induced corneal immunopathology. Invest Ophthalmol Vis Sci. 2013;54:6269–79. doi: 10.1167/iovs.13-12152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Rajasagi NK, Reddy PBJ, Suryawanshi A, Mulik S, Gjorstrup P, Rouse BT. Controlling herpes simplex virus-induced ocular inflammatory lesions with the lipid-derived mediator resolvin E1. J Immunol. 2011;186:1735–46. doi: 10.4049/jimmunol.1003456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Cilloniz C, Pantin-Jackwood MJ, Ni C, Goodman AG, Peng X, Proll SC, et al. Lethal dissemination of H5N1 influenza virus is associated with dysregulation of inflammation and lipoxin signaling in a mouse model of infection. J Virol. 2010;84:7613–24. doi: 10.1128/JVI.00553-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Morita M, Kuba K, Ichikawa A, Nakayama M, Katahira J, Iwamoto R, et al. The lipid mediator protectin D1 inhibits influenza virus replication and improves severe influenza. Cell. 2013;153:112–25. doi: 10.1016/j.cell.2013.02.027. [DOI] [PubMed] [Google Scholar]
- 79.Tam VC, Quehenberger O, Oshansky CM, Suen R, Armando AM, Treuting PM, et al. Lipidomic profiling of influenza infection identifies mediators that induce and resolve inflammation. Cell. 2013;154:213–27. doi: 10.1016/j.cell.2013.05.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wu S-H, Liao P-Y, Yin P-L, Zhang Y-M, Dong L. Elevated expressions of 15-lipoxygenase and lipoxin A4 in children with acute poststreptococcal glomerulonephritis. Am J Pathol. 2009;174:115–22. doi: 10.2353/ajpath.2009.080671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Haas-Stapleton EH, Lu Y, Hong S, Arita M, Favoreto S, Nigam S, et al. Candida albicans modulates host defense by biosynthesizing the pro-resolving mediator Resolvin E1. PLoS ONE. 2007;2:e1316. doi: 10.1371/journal.pone.0001316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hsieh WC, Chuang YT, Chiang IH, Hsu SC, Miaw SC, Lai MZ. Inability to resolve specific infection generates innate immunodeficiency syndrome in Xiap−/− mice. Blood. 2014;124:2847–57. doi: 10.1182/blood-2014-03-564609. [DOI] [PubMed] [Google Scholar]
- 83.Baillie JK, Digard P. Influenza--time to target the host? N Engl J Med. 2013;369:191–3. doi: 10.1056/NEJMcibr1304414. [DOI] [PubMed] [Google Scholar]
- 84.Wang SB, Hu KM, Seamon KJ, Mani V, Chen Y, Gronert K. Estrogen negatively regulates epithelial wound healing and protective lipid mediator circuits in the cornea. FASEB J. 2012;26:1506–16. doi: 10.1096/fj.11-198036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Menon R. The effect of resolvins on dermal wound healing. New Brunswick, NJ: Rutgers; 2012. [Google Scholar]
- 86.Tang Y, Zhang MJ, Hellmann J, Kosuri M, Bhatnagar A, Spite M. Proresolution Therapy for the Treatment of Delayed Healing of Diabetic Wounds. Diabetes. 2013;62:618–27. doi: 10.2337/db12-0684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Spite M, Claria J, Serhan CN. Resolvins, specialized proresolving lipid mediators, and their potential roles in metabolic diseases. Cell Metab. 2013;19:21–36. doi: 10.1016/j.cmet.2013.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hong S, Gronert K, Devchand P, Moussignac R-L, Serhan CN. Novel docosatrienes and 17S-resolvins generated from docosahexaenoic acid in murine brain, human blood and glial cells: autacoids in anti-inflammation. J Biol Chem. 2003;278:14677–87. doi: 10.1074/jbc.M300218200. [DOI] [PubMed] [Google Scholar]
- 89.Lukiw WJ, Cui JG, Marcheselli VL, Bodker M, Botkjaer A, Gotlinger K, et al. A role for docosahexaenoic acid-derived neuroprotectin D1 in neural cell survival and Alzheimer disease. J Clin Invest. 2005;115:2774–83. doi: 10.1172/JCI25420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hong S, Tjonahen E, Morgan EL, Yu L, Serhan CN, Rowley AF. Rainbow trout (Oncorhynchus mykiss) brain cells biosynthesize novel docosahexaenoic acid-derived resolvins and protectins -- mediator lipidomic analysis. Prostaglandins Other Lipid Mediat. 2005;78:107–16. doi: 10.1016/j.prostaglandins.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 91.Marcheselli VL, Hong S, Lukiw WJ, Hua Tian X, Gronert K, Musto A, et al. Novel docosanoids inhibit brain ischemia-reperfusion-mediated leukocyte infiltration and pro-inflammatory gene expression. J Biol Chem. 2003;278:43807–17. doi: 10.1074/jbc.M305841200. [DOI] [PubMed] [Google Scholar]
- 92.Bazan NG, Calandria JM, Serhan CN. Rescue and repair during photoreceptor cell renewal mediated by docosahexaenoic acid-derived neuroprotectin D1. J Lipid Res. 2010;51:2018–31. doi: 10.1194/jlr.R001131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Sheets KG, Jun B, Zhou Y, Zhu M, Petasis NA, Gordon WC, et al. Microglial ramification and redistribution concomitant with the attenuation of choroidal neovascularization by neuroprotectin D1. Mol Vis. 2013;19:1747–59. [PMC free article] [PubMed] [Google Scholar]
- 94.Bazan NG, Eady TN, Khoutorova L, Atkins KD, Hong S, Lu Y, et al. Novel aspirin-triggered neuroprotectin D1 attenuates cerebral ischemic injury after experimental stroke. Exp Neurol. 2012;236:122–30. doi: 10.1016/j.expneurol.2012.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Murphy EJ. A lipid neurochemist’s siren: docosahexaenoic acid and its elusive function in the central nervous system. J Neurochem. 2013;127:299–302. doi: 10.1111/jnc.12439. [DOI] [PubMed] [Google Scholar]
- 96.Wang X, Zhu M, Hjorth E, Cortés-Toro V, Eyjolfsdottir H, Graff C, et al. Resolution of inflammation is altered in Alzheimer’s disease. Alzheimers Dement. 2014 doi: 10.1016/j.jalz.2013.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Mizwicki MT, Liu G, Fiala M, Magpantay L, Sayre J, Siani A, et al. 1alpha,25-Dihydroxyvitamin D3 and Resolvin D1 Retune the Balance between Amyloid-beta Phagocytosis and Inflammation in Alzheimer’s Disease Patients. J Alzheimers Dis. 2013;34:155–70. doi: 10.3233/JAD-121735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Liu G, Fiala M, Mizwicki MT, Sayre J, Magpantay L, Siani A, et al. Neuronal phagocytosis by inflammatory macrophages in ALS spinal cord: inhibition of inflammation by resolvin D1. Am J Neurodegener Dis. 2012;1:60–74. [PMC free article] [PubMed] [Google Scholar]
- 99.Wu Y, Ye XH, Guo PP, Xu SP, Wang J, Yuan SY, et al. Neuroprotective effect of lipoxin A4 methyl ester in a rat model of permanent focal cerebral ischemia. J Mol Neurosci. 2010;42:226–34. doi: 10.1007/s12031-010-9355-8. [DOI] [PubMed] [Google Scholar]
- 100.Wu J, Wang A, Min Z, Xiong Y, Yan Q, Zhang J, et al. Lipoxin A4 inhibits the production of proinflammatory cytokines induced by beta-amyloid in vitro and in vivo. Biochem Biophys Res Commun. 2011;408:382–7. doi: 10.1016/j.bbrc.2011.04.013. [DOI] [PubMed] [Google Scholar]
- 101.Wu L, Miao S, Zou LB, Wu P, Hao H, Tang K, et al. Lipoxin A4 inhibits 5-lipoxygenase translocation and leukotrienes biosynthesis to exert a neuroprotective effect in cerebral ischemia/reperfusion injury. J Mol Neurosci. 2012;48:185–200. doi: 10.1007/s12031-012-9807-4. [DOI] [PubMed] [Google Scholar]
- 102.Ye X-H, Wu Y, Guo P-P, Wang J, Yuan S-Y, Shang Y, et al. Lipoxin A4 analogue protects brain and reduces inflammation in a rat model of focal cerebral ischemia reperfusion. Brain Res. 2010;1323:174–83. doi: 10.1016/j.brainres.2010.01.079. [DOI] [PubMed] [Google Scholar]
- 103.Sobrado M, Pereira MP, Ballesteros I, Hurtado O, Fernández-López D, Pradillo JM, et al. Synthesis of lipoxin A4 by 5-lipoxygenase mediates PPARgamma-dependent, neuroprotective effects of rosiglitazone in experimental stroke. J Neurosci. 2009;29:3875–84. doi: 10.1523/JNEUROSCI.5529-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Wu L, Liu ZJ, Miao S, Zou LB, Cai L, Wu P, et al. Lipoxin A4 ameliorates cerebral ischaemia/reperfusion injury through upregulation of nuclear factor erythroid 2-related factor 2. Neurol Res. 2013;35:968–75. doi: 10.1179/1743132813Y.0000000242. [DOI] [PubMed] [Google Scholar]
- 105.Pamplona FA, Ferreira J, Menezes de Lima O, Jr, Duarte FS, Bento AF, Forner S, et al. Anti-inflammatory lipoxin A4 is an endogenous allosteric enhancer of CB1 cannabinoid receptor. Proc Natl Acad Sci U S A. 2012;109:21134–9. doi: 10.1073/pnas.1202906109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Pruss H, Rosche B, Sullivan AB, Brommer B, Wengert O, Gronert K, et al. Proresolution lipid mediators in multiple sclerosis - differential, disease severity-dependent synthesis - a clinical pilot trial. PLoS One. 2013;8:e55859. doi: 10.1371/journal.pone.0055859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Svensson CI, Zattoni M, Serhan CN. Lipoxins and aspirin-triggered lipoxin stop inflammatory pain processing. J Exp Med. 2007;204:245–52. doi: 10.1084/jem.20061826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Sisignano M, Bennett DL, Geisslinger G, Scholich K. TRP-channels as key integrators of lipid pathways in nociceptive neurons. Prog Lipid Res. 2014;53:93–107. doi: 10.1016/j.plipres.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 109.Xu Z-Z, Zhang L, Liu T, Park J-Y, Berta T, Yang R, et al. Resolvins RvE1 and RvD1 attenuate inflammatory pain via central and peripheral actions. Nat Med. 2010;16:592–7. doi: 10.1038/nm.2123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Bang S, Yoo S, Yang TJ, Cho H, Kim YG, Hwang SW. Resolvin D1 attenuates activation of sensory transient receptor potential channels leading to multiple anti-nociception. Br J Pharmacol. 2010;161:707–20. doi: 10.1111/j.1476-5381.2010.00909.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Bang S, Yoo S, Yang TJ, Cho H, Hwang SW. 17(R)-resolvin D1 specifically inhibits transient receptor potential ion channel vanilloid 3 leading to peripheral antinociception. Br J Pharmacol. 2012;165:683–92. doi: 10.1111/j.1476-5381.2011.01568.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Park CK, Lü N, Xu ZZ, Liu T, Serhan CN, Ji RR. Resolving TRPV1 and TNF-α-mediated spinal cord synaptic plasticity and inflammatory pain with neuroprotectin D1. J Neurosci. 2011;31:15072–85. doi: 10.1523/JNEUROSCI.2443-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Xu ZZ, Liu XJ, Berta T, Park CK, Lu N, Serhan CN, et al. Neuroprotectin/protectin D1 protects against neuropathic pain in mice after nerve trauma. Ann Neurol. 2013;74:490–5. doi: 10.1002/ana.23928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Park CK, Xu ZZ, Liu T, Lu N, Serhan CN, Ji RR. Resolvin D2 is a potent endogenous inhibitor for transient receptor potential subtype V1/A1, inflammatory pain, and spinal cord synaptic plasticity in mice: distinct roles of resolvin D1, D2, and E1. J Neurosci. 2011;31:18433–8. doi: 10.1523/JNEUROSCI.4192-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Huang L, Wang C-F, Serhan CN, Strichartz G. Enduring prevention and transient reduction of post-operative pain by intrathecal resolvin D1. Pain. 2011;152:557–65. doi: 10.1016/j.pain.2010.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Lima-Garcia J, Dutra R, da Silva K, Motta E, Campos M, Calixto J. The precursor of resolvin D series and aspirin-triggered resolvin D1 display anti-hyperalgesic properties in adjuvant-induced arthritis in rats. Br J Pharmacol. 2011;164:278–93. doi: 10.1111/j.1476-5381.2011.01345.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Abdelmoaty S, Wigerblad G, Bas DB, Codeluppi S, Fernandez-Zafra T, El-Awady el S, et al. Spinal Actions of Lipoxin A4 and 17(R)-Resolvin D1 Attenuate Inflammation-Induced Mechanical Hypersensitivity and Spinal TNF Release. PLoS One. 2013;8:e75543. doi: 10.1371/journal.pone.0075543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Terrando N, Gomez-Galan M, Yang T, Carlstrom M, Gustavsson D, Harding RE, et al. Aspirin-triggered resolvin D1 prevents surgery-induced cognitive decline. FASEB J. 2013;27:3564–71. doi: 10.1096/fj.13-230276. [DOI] [PubMed] [Google Scholar]
- 119.Ramsden CE, Faurot KR, Zamora D, Suchindran CM, Macintosh BA, Gaylord S, et al. Targeted alteration of dietary n-3 and n-6 fatty acids for the treatment of chronic headaches: A randomized trial. Pain. 2013;154:2441–51. doi: 10.1016/j.pain.2013.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Mirakaj V, Dalli J, Granja T, Rosenberger P, Serhan CN. Vagus nerve controls resolution and pro-resolving mediators of inflammation. J Exp Med. 2014;211:1037–48. doi: 10.1084/jem.20132103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Klein CP, Sperotto ND, Maciel IS, Leite CE, Souza AH, Campos MM. Effects of D-series resolvins on behavioral and neurochemical changes in a fibromyalgia-like model in mice. Neuropharmacology. 2014;86C:57–66. doi: 10.1016/j.neuropharm.2014.05.043. [DOI] [PubMed] [Google Scholar]
- 122.Maldonado-Pèriz D, Golightly E, Denison FC, Jabbour HN, Norman JE. A role for lipoxin A4 as anti-inflammatory and proresolution mediator in human parturition. FASEB J. 2011;25:569–75. doi: 10.1096/fj.10-170340. [DOI] [PubMed] [Google Scholar]
- 123.Xu Z, Zhao J, Zhang H, Ke T, Xu P, Cai W, et al. Spontaneous miscarriages are explained by the stress/glucocorticoid/lipoxin A4 axis. J Immunol. 2013;190:6051–8. doi: 10.4049/jimmunol.1202807. [DOI] [PubMed] [Google Scholar]
- 124.Macdonald LJ, Boddy SC, Denison FC, Sales KJ, Jabbour HN. A role for lipoxin A4 as an anti-inflammatory mediator in the human endometrium. Reproduction. 2011;142:345–52. doi: 10.1530/REP-11-0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Russell R, Gori I, Pellegrini C, Kumar R, Achtari C, Canny GO. Lipoxin A4 is a novel estrogen receptor modulator. FASEB J. 2011;25:4326–37. doi: 10.1096/fj.11-187658. [DOI] [PubMed] [Google Scholar]
- 126.Xiong J, Zeng P, Cheng X, Miao S, Wu L, Zhou S, et al. Lipoxin A(4) blocks embryo implantation by controlling estrogen receptor alpha activity. Reproduction. 2013;145:411–20. doi: 10.1530/REP-12-0469. [DOI] [PubMed] [Google Scholar]
- 127.Motohashi E, Kawauchi H, Endo H, Kondo H, Kitasato H, Kuramoto H, et al. Regulatory expression of lipoxin A4 receptor in physiologically estrus cycle and pathologically endometriosis. Biomed Pharmacother. 2005;59:330–8. doi: 10.1016/j.biopha.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 128.Canny GO, Lessey BA. The role of lipoxin A4 in endometrial biology and endometriosis. Mucosal Immunol. 2013;6:439–50. doi: 10.1038/mi.2013.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Gil-Villa AM, Norling LV, Serhan CN, Cordero D, Rojas M, Cadavid A. Aspirin triggered-lipoxin A4 reduces the adhesion of human polymorphonuclear neutrophils to endothelial cells initiated by preeclamptic plasma. Prostaglandins Leukot Essent Fatty Acids. 2012;87:127–34. doi: 10.1016/j.plefa.2012.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Jones ML, Mark PJ, Keelan JA, Barden A, Mas E, Mori TA, et al. Maternal dietary omega-3 fatty acid intake increases resolvin and protectin levels in the rat placenta. J Lipid Res. 2013;54:2247–54. doi: 10.1194/jlr.M039842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Yamashita A, Kawana K, Tomio K, Taguchi A, Isobe Y, Iwamoto R, et al. Increased tissue levels of omega-3 polyunsaturated fatty acids prevents pathological preterm birth. Sci Rep. 2013;3:3113. doi: 10.1038/srep03113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Weiss GA, Troxler H, Klinke G, Rogler D, Braegger C, Hersberger M. High levels of anti-inflammatory and pro-resolving lipid mediators lipoxins and resolvins and declining docosahexaenoic acid levels in human milk during the first month of lactation. Lipids Health Dis. 2013;12:89. doi: 10.1186/1476-511X-12-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Kowal-Bielecka O, Kowal K, Distler O, Gay S. Mechanisms of disease: leukotrienes and lipoxins in scleroderma lung disease--insights and potential therapeutic implications. Nat Clin Pract Rheumatol. 2007;3:43–51. doi: 10.1038/ncprheum0375. [DOI] [PubMed] [Google Scholar]
- 134.Martins V, Valença SS, Farias-Filho FA, Molinaro R, Simões RL, Ferreira TP, et al. ATLa, an aspirin-triggered lipoxin A4 synthetic analog, prevents the inflammatory and fibrotic effects of bleomycin-induced pulmonary fibrosis. J Immunol. 2009;182:5374–81. doi: 10.4049/jimmunol.0802259. [DOI] [PubMed] [Google Scholar]
- 135.Borgeson E, Docherty NG, Murphy M, Rodgers K, Ryan A, O’Sullivan TP, et al. Lipoxin A(4) and benzo-lipoxin A(4) attenuate experimental renal fibrosis. FASEB J. 2011;25:2967–79. doi: 10.1096/fj.11-185017. [DOI] [PubMed] [Google Scholar]
- 136.Qu X, Zhang X, Yao J, Song J, Nikolic-Paterson DJ, Li J. Resolvins E1 and D1 inhibit interstitial fibrosis in the obstructed kidney via inhibition of local fibroblast proliferation. J Pathol. 2012;228:506–19. doi: 10.1002/path.4050. [DOI] [PubMed] [Google Scholar]
- 137.Hsiao HM, Sapinoro RE, Thatcher TH, Croasdell A, Levy EP, Fulton RA, et al. A novel anti-inflammatory and pro-resolving role for resolvin D1 in acute cigarette smoke-induced lung inflammation. PLoS One. 2013;8:e58258. doi: 10.1371/journal.pone.0058258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Janakiram NB, Mohammed A, Rao CV. Role of lipoxins, resolvins, and other bioactive lipids in colon and pancreatic cancer. Cancer Metastasis Rev. 2011;30:507–23. doi: 10.1007/s10555-011-9311-2. [DOI] [PubMed] [Google Scholar]
- 139.Lee HN. Role of resolvin D1 in experimentally induced inflammation and colon carcinogenesis. Seoul, South Korea: Seoul National University; 2013. [Google Scholar]
- 140.Lee HN, Na HK, Surh YJ. Resolution of inflammation as a novel chemopreventive strategy. Semin Immunopathol. 2013;35:151–61. doi: 10.1007/s00281-013-0363-y. [DOI] [PubMed] [Google Scholar]
- 141.Panigrahy D. Stimulation of inflammation resolution by resolvins inhibits tumor growth (abstract). 13th International Conference on Bioactive Lipids in Cancer, Inflammation and Related Diseases; San Juan, Puerto Rico. November 3–6, 2013; 2013. [Google Scholar]
- 142.Ramon S, Gao F, Serhan CN, Phipps RP. Specialized proresolving mediators enhance human B cell differentiation to antibody-secreting cells. J Immunol. 2012;189:1036–42. doi: 10.4049/jimmunol.1103483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Hong S, Lu Y, Yang R, Gotlinger KH, Petasis NA, Serhan CN. Resolvin D1, protectin D1, and related docosahexaenoic acid-derived products: analysis via electrospray/low energy tandem mass spectrometry based on spectra and fragmentation mechanisms. J Am Soc Mass Spectrom. 2007;18:128–44. doi: 10.1016/j.jasms.2006.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Ariel A, Li P-L, Wang W, Tang W-X, Fredman G, Hong S, et al. The docosatriene protectin D1 is produced by TH2 skewing and promotes human T cell apoptosis via lipid raft clustering. J Biol Chem. 2005;280:43079–86. doi: 10.1074/jbc.M509796200. [DOI] [PubMed] [Google Scholar]
- 145.Ariel A, Fredman G, Sun Y-P, Kantarci A, Van Dyke TE, Luster AD, et al. Apoptotic neutrophils and T cells sequester chemokines during immune response resolution via modulation of CCR5 expression. Nat Immunol. 2006;7:1209–16. doi: 10.1038/ni1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Settimio R, Clara DF, Franca F, Francesca S, Michele D. Resolvin D1 reduces the immunoinflammatory response of the rat eye following uveitis. Mediators Inflamm. 2012;2012:318621. doi: 10.1155/2012/318621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Tian H, Lu Y, Sherwood AM, Hongqian D, Hong S. Resolvins E1 and D1 in choroid-retinal endothelial cells and leukocytes: biosynthesis and mechanisms of anti-inflammatory actions. Invest Ophthalmol Vis Sci. 2009;50:3613–20. doi: 10.1167/iovs.08-3146. [DOI] [PubMed] [Google Scholar]
- 148.Vassiliou EK, Kesler OM, Tadros JH, Ganea D. Bone marrow-derived dendritic cells generated in the presence of resolvin E1 induce apoptosis of activated CD4+ T cells. J Immunol. 2008;181:4534–44. doi: 10.4049/jimmunol.181.7.4534. [DOI] [PubMed] [Google Scholar]
- 149.Kim TH, Kim GD, Jin YH, Park YS, Park CS. Omega-3 fatty acid-derived mediator, Resolvin E1, ameliorates 2,4-dinitrofluorobenzene-induced atopic dermatitis in NC/Nga mice. Int Immunopharmacol. 2012;14:384–91. doi: 10.1016/j.intimp.2012.08.005. [DOI] [PubMed] [Google Scholar]
- 150.Zhang G, Panigrahy D, Mahakian LM, Yang J, Liu JY, Stephen Lee KS, et al. Epoxy metabolites of docosahexaenoic acid (DHA) inhibit angiogenesis, tumor growth, and metastasis. Proc Natl Acad Sci U S A. 2013;110:6530–5. doi: 10.1073/pnas.1304321110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Nording ML, Yang J, Georgi K, Hegedus Karbowski C, German JB, Weiss RH, et al. Individual variation in lipidomic profiles of healthy subjects in response to omega-3 Fatty acids. PLoS One. 2013;8:e76575. doi: 10.1371/journal.pone.0076575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Divanovic S, Dalli J, Jorge-Nebert LF, Flick LM, Galvez-Peralta M, Boespflug ND, et al. Contributions of the Three CYP1 Monooxygenases to Pro-Inflammatory and Inflammation-Resolution Lipid Mediator Pathways. J Immunol. 2013;191:3347–57. doi: 10.4049/jimmunol.1300699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Hegde S, Kaushal N, Ravindra KC, Chiaro C, Hafer KT, Gandhi UH, et al. Delta12-prostaglandin J3, an omega-3 fatty acid-derived metabolite, selectively ablates leukemia stem cells in mice. Blood. 2011;118:6909–19. doi: 10.1182/blood-2010-11-317750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Cipollina C, Salvatore SR, Muldoon MF, Freeman BA, Schopfer F. Clinical generation and dietary modulation of anti-inflammatory electrophilic omega-3 fatty acid derivatives (abstract #178). 13th International Conference on Bioactive Lipids in Cancer, Inflammation, and Related Diseases; San Juan, Puerto Rico. November 3–6, 2013; 2013. [Google Scholar]
- 155.Morrow JD, Roberts LJ. The isoprostanes: unique bioactive products of lipid peroxidation. Prog Lipid Res. 1997;36:1–21. doi: 10.1016/s0163-7827(97)00001-5. [DOI] [PubMed] [Google Scholar]
- 156.Ho PP, Kanter JL, Johnson AM, Srinagesh HK, Chang EJ, Purdy TM, et al. Identification of naturally occurring fatty acids of the myelin sheath that resolve neuroinflammation. Sci Transl Med. 2012;4:137ra73. doi: 10.1126/scitranslmed.3003831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Psychogios N, Hau DD, Peng J, Guo AC, Mandal R, Bouatra S, et al. The human serum metabolome. PLoS One. 2011;6:e16957. doi: 10.1371/journal.pone.0016957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Mas E, Croft KD, Zahra P, Barden A, Mori TA. Resolvins D1, D2, and other mediators of self-limited resolution of inflammation in human blood following n-3 fatty acid supplementation. Clin Chem. 2012;58:1476–84. doi: 10.1373/clinchem.2012.190199. [DOI] [PubMed] [Google Scholar]
- 159.Schottelius AJ, Giesen C, Asadullah K, Fierro IM, Colgan SP, Bauman J, et al. An aspirin-triggered lipoxin A4 stable analog displays a unique topical anti-inflammatory profile. J Immunol. 2002;169:7063–70. doi: 10.4049/jimmunol.169.12.7063. [DOI] [PubMed] [Google Scholar]
- 160.Wu SH, Chen XQ, Liu B, Wu HJ, Dong L. Efficacy and Safety of 15(R/S)-Methyl-Lipoxin A(4) in Topical Treatment of Infantile Eczema. Br J Dermatol. 2013;168:172–8. doi: 10.1111/j.1365-2133.2012.11177.x. [DOI] [PubMed] [Google Scholar]
- 161.Christie PE, Spur BW, Lee TH. The effects of lipoxin A4 on airway responses in asthmatic subjects. Am Rev Respir Dis. 1992;145:1281–4. doi: 10.1164/ajrccm/145.6.1281. [DOI] [PubMed] [Google Scholar]
- 162.Dalli J, Chiang N, Serhan CN. Identification of sulfido-conjugated mediators that promote resolution of infection and organ protection. Proc Natl Acad Sci USA. 2014;111:E4753–61. doi: 10.1073/pnas.1415006111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Bhavsar PK, Levy BD, Hew MJ, Pfeffer MA, Kazani S, Israel E, et al. Corticosteroid suppression of lipoxin A4 and leukotriene B4 from alveolar macrophages in severe asthma. Respir Res. 2010;11:71. doi: 10.1186/1465-9921-11-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Yamaguchi H, Higashi N, Mita H, Ono E, Komase Y, Nakagawa T, et al. Urinary concentrations of 15-epimer of lipoxin A(4) are lower in patients with aspirin-intolerant compared with aspirin-tolerant asthma. Clin Exp Allergy. 2011;41:1711–8. doi: 10.1111/j.1365-2222.2011.03839.x. [DOI] [PubMed] [Google Scholar]
- 165.Barnig C, Cernadas M, Dutile S, Liu X, Perrella MA, Kazani S, et al. Lipoxin A4 regulates natural killer cell and type 2 innate lymphoid cell activation in asthma. Sci Transl Med. 2013;5:174ra26. doi: 10.1126/scitranslmed.3004812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Fredman G, Oh SF, Ayilavarapu S, Hasturk H, Serhan CN, Van Dyke TE. Impaired phagocytosis in localized aggressive periodontitis: rescue by resolvin E1. PLoS One. 2011;6:e24422. doi: 10.1371/journal.pone.0024422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Claria J, Nguyen BT, Madenci A, Ozaki CK, Serhan CN. Diversity of lipid mediators in human adipose tissue depots. Am J Physiol Cell Physiol. 2013;304:C1141–C9. doi: 10.1152/ajpcell.00351.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Giera M, Ioan-Facsinay A, Toes R, Gao F, Dalli J, Deelder AM, et al. Lipid and lipid mediator profiling of human synovial fluid in rheumatoid arthritis patients by means of LC-MS/MS. Biochim Biophys Acta. 2012;1821:1415–24. doi: 10.1016/j.bbalip.2012.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Levy BD, Kohli P, Gotlinger K, Haworth O, Hong S, Kazani S, et al. Protectin D1 is generated in asthma and dampens airway inflammation and hyper-responsiveness. J Immunol. 2007;178:496–502. doi: 10.4049/jimmunol.178.1.496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Rozsasi A, Heinemann A, Keck T. Cyclooxygenase 2 and lipoxin A(4) in nasal polyps in cystic fibrosis. Am J Rhinol Allergy. 2011;25:e251–4. doi: 10.2500/ajra.2011.25.3726. [DOI] [PubMed] [Google Scholar]
- 171.Vong L, Ferraz JG, Dufton N, Panaccione R, Beck PL, Sherman PM, et al. Up-regulation of Annexin-A1 and lipoxin A(4) in individuals with ulcerative colitis may promote mucosal homeostasis. PLoS One. 2012;7:e39244. doi: 10.1371/journal.pone.0039244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.El Kebir D, József L, Pan W, Wang L, Petasis NA, Serhan CN, et al. 15-epi-lipoxin A4 inhibits myeloperoxidase signaling and enhances resolution of acute lung injury. Am J Respir Crit Care Med. 2009;180:311–9. doi: 10.1164/rccm.200810-1601OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Arita M, Yoshida M, Hong S, Tjonahen E, Glickman JN, Petasis NA, et al. Resolvin E1, an endogenous lipid mediator derived from omega-3 eicosapentaenoic acid, protects against 2,4,6-trinitrobenzene sulfonic acid-induced colitis. Proc Natl Acad Sci USA. 2005;102:7671–6. doi: 10.1073/pnas.0409271102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Wang B, Gong X, Wan JY, Zhang L, Zhang Z, Li HZ, et al. Resolvin D1 protects mice from LPS-induced acute lung injury. Pulm Pharmacol Ther. 2011;24:434–41. doi: 10.1016/j.pupt.2011.04.001. [DOI] [PubMed] [Google Scholar]
- 175.Bohr S, Patel SJ, Sarin D, Irimia D, Yarmush ML, Berthiaume F. Resolvin D2 prevents secondary thrombosis and necrosis in a mouse burn wound model. Wound Repair Regen. 2013;21:35–43. doi: 10.1111/j.1524-475X.2012.00853.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Gronert K, Maheshwari N, Khan N, Hassan IR, Dunn M, Schwartzman ML. A role for the mouse 12/15-lipoxygenase pathway in promoting epithelial wound healing and host defense. J Biol Chem. 2005;280:15267–78. doi: 10.1074/jbc.M410638200. [DOI] [PubMed] [Google Scholar]
- 177.Biteman B, Hassan IR, Walker E, Leedom AJ, Dunn M, Seta F, et al. Interdependence of lipoxin A4 and heme-oxygenase in counter-regulating inflammation during corneal wound healing. FASEB J. 2007;21:2257–66. doi: 10.1096/fj.06-7918com. [DOI] [PubMed] [Google Scholar]
- 178.Merched A, Ko K, Gotlinger KH, Serhan CN, Chan L. Atherosclerosis: Evidence for impairment of resolution of vascular inflammation governed by specific lipid mediators. FASEB J. 2008;22:3595–606. doi: 10.1096/fj.08-112201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Serhan CN, Yang R, Martinod K, Kasuga K, Pillai PS, Porter TF, et al. Maresins: novel macrophage mediators with potent anti-inflammatory and pro-resolving actions. J Exp Med. 2009;206:15–23. doi: 10.1084/jem.20081880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Kronke G, Katzenbeisser J, Uderhardt S, Zaiss MM, Scholtysek C, Schabbauer G, et al. 12/15-lipoxygenase counteracts inflammation and tissue damage in arthritis. J Immunol. 2009;183:3383–9. doi: 10.4049/jimmunol.0900327. [DOI] [PubMed] [Google Scholar]
- 181.Leedom AJ, Sullivan AB, Dong B, Lau D, Gronert K. Endogenous LXA4 circuits are determinants of pathological angiogenesis in response to chronic injury. Am J Pathol. 2010;176:74–84. doi: 10.2353/ajpath.2010.090678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Kronke G, Reich N, Scholtysek C, Akhmetshina A, Uderhardt S, Zerr P, et al. The 12/15-lipoxygenase pathway counteracts fibroblast activation and experimental fibrosis. Ann Rheum Dis. 2012;71:1081–7. doi: 10.1136/annrheumdis-2011-200745. [DOI] [PubMed] [Google Scholar]
- 183.Tomio K, Kawana K, Taguchi A, Isobe Y, Iwamoto R, Yamashita A, et al. Omega-3 polyunsaturated Fatty acids suppress the cystic lesion formation of peritoneal endometriosis in transgenic mouse models. PLoS One. 2013;8:e73085. doi: 10.1371/journal.pone.0073085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Koltsida O, Karamnov S, Pyrillou K, Vickery T, Chairakaki AD, Tamvakopoulos C, et al. Toll-like receptor 7 stimulates production of specialized pro-resolving lipid mediators and promotes resolution of airway inflammation. EMBO Mol Med. 2013;5:762–75. doi: 10.1002/emmm.201201891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Chiang N, Shinohara M, Dalli J, Mirakaj V, Kibi M, Choi AMK, et al. Inhaled carbon monoxide accelerates resolution of inflammation via unique pro-resolving mediator--heme oxygenase-1 circuits. J Immunol. 2013;190:6378–88. doi: 10.4049/jimmunol.1202969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Shirey KA, Lai W, Pletneva LM, Karp CL, Divanovic S, Blanco JC, et al. Role of the lipoxygenase pathway in RSV-induced alternatively activated macrophages leading to resolution of lung pathology. Mucosal Immunol. 2014;7:549–57. doi: 10.1038/mi.2013.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Blaho VA, Zhang Y, Hughes-Hanks JM, Brown CR. 5-Lipoxygenase-deficient mice infected with Borrelia burgdorferi develop persistent arthritis. J Immunol. 2011;186:3076–84. doi: 10.4049/jimmunol.1003473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Bafica A, Scanga CA, Serhan C, Machado F, White S, Sher A, et al. Host control of Mycobacterium tuberculosis is regulated by 5-lipoxygenase-dependent lipoxin production. J Clin Invest. 2005;115:1601–6. doi: 10.1172/JCI23949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Aliberti J, Serhan C, Sher A. Parasite-induced lipoxin A(4) is an endogenous regulator of IL-12 production and immunopathology in Toxoplasma gondii infection. J Exp Med. 2002;196:1253–62. doi: 10.1084/jem.20021183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Petri MH, Laguna-Fernandez A, Tseng CN, Hedin U, Perretti M, Back M. Aspirin-triggered 15-epi-lipoxin A4 signals through FPR2/ALX in vascular smooth muscle cells and protects against intimal hyperplasia after carotid ligation. Int J Cardiol. 2015;179:370–2. doi: 10.1016/j.ijcard.2014.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Gobbetti T, Coldewey SM, Chen J, McArthur S, le Faouder P, Cenac N, et al. Nonredundant protective properties of FPR2/ALX in polymicrobial murine sepsis. Proc Natl Acad Sci U S A. 2014;111:18685–90. doi: 10.1073/pnas.1410938111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Gao L, Faibish D, Fredman G, Herrera BS, Chiang N, Serhan CN, et al. Resolvin E1 and Chemokine-like Receptor 1 Mediate Bone Preservation. J Immunol. 2013;190:689–94. doi: 10.4049/jimmunol.1103688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Herrera BS, Hasturk H, Kantarci A, Freire MO, Nguyen O, Kansal S, et al. The Impact of Resolvin E1 on Murine Neutrophil Phagocytosis in Type 2 Diabetes. Infect Immun. 2014 doi: 10.1128/IAI.02444-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Arita M, Ohira T, Sun YP, Elangovan S, Chiang N, Serhan CN. Resolvin E1 selectively interacts with leukotriene B4 receptor BLT1 and ChemR23 to regulate inflammation. J Immunol. 2007;178:3912–7. doi: 10.4049/jimmunol.178.6.3912. [DOI] [PubMed] [Google Scholar]
- 195.Levy BD, Bonnans C, Silverman ES, Palmer LJ, Marigowda G, Israel E, et al. Diminished lipoxin biosynthesis in severe asthma. Am J Respir Crit Care Med. 2005;172:824–30. doi: 10.1164/rccm.200410-1413OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Sanak M, Levy BD, Clish CB, Chiang N, Gronert K, Mastalerz L, et al. Aspirin-tolerant asthmatics generate more lipoxins than aspirin-intolerant asthmatics. Eur Respir J. 2000;16:44–9. doi: 10.1034/j.1399-3003.2000.16a08.x. [DOI] [PubMed] [Google Scholar]
- 197.Peebles RS., Jr A new horizon in asthma: inhibiting ILC function. Sci Transl Med. 2013;5:174fs7. doi: 10.1126/scitranslmed.3005881. [DOI] [PubMed] [Google Scholar]
- 198.Gutierrez AD, Sathyanarayana P, Konduru S, Ye Y, Birnbaum Y, Bajaj M. The effect of pioglitazone treatment on 15-epi-lipoxin A4 levels in patients with type 2 diabetes. Atherosclerosis. 2012;223:204–8. doi: 10.1016/j.atherosclerosis.2012.04.016. [DOI] [PubMed] [Google Scholar]
- 199.Ho KJ, Spite M, Owens CD, Lancero H, Kroemer AH, Pande R, et al. Aspirin-triggered lipoxin and resolvin E1 modulate vascular smooth muscle phenotype and correlate with peripheral atherosclerosis. Am J Pathol. 2010;177:2116–23. doi: 10.2353/ajpath.2010.091082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Clària J, Dalli J, Yacoubian S, Gao F, Serhan CN. Resolvin D1 and resolvin D2 govern local inflammatory tone in obese fat. J Immunol. 2012;189:2597–605. doi: 10.4049/jimmunol.1201272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Zivkovic AM, Yang J, Georgi K, Hegedus C, Nording ML, O’Sullivan A, et al. Serum oxylipin profiles in IgA nephropathy patients reflect kidney functional alterations. Metabolomics. 2012;8:1102–13. doi: 10.1007/s11306-012-0417-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Colas RA, Shinohara M, Dalli J, Chiang N, Serhan CN. Identification and signature profiles for pro-resolving and inflammatory lipid mediators in human tissue. Am J Physiol Cell Physiol. 2014;307:C39–54. doi: 10.1152/ajpcell.00024.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Yanes O, Clark J, Wong DM, Patti GG, Sánchez-Ruiz A, Benton HP, et al. Metabolic oxidation regulates embryonic stem cell differentiation. Nat Chem Biol. 2010;6:411–7. doi: 10.1038/nchembio.364. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.