Abstract
Two lick-suppression experiments with rats assessed interference with behavior indicative of conditioned inhibition by a latent inhibition treatment as a function of test context. We asked what effect the test context has, given identical latent inhibition treatment in Phase 1 and identical conditioned inhibition training in Phase 2. In Experiment 1, an AAA vs. AAB context-shift design determined that latent inhibition treatment in Phase 1 attenuated behavior indicative of conditioned inhibition training administered in Phase 2 regardless of the test context, which could reflect a failure to either acquire or express conditioned inhibition. In Experiment 2, an ABA vs. ABB design found that test performance in Contexts A and B reflected the treatments that had been administered in those contexts (i.e., conditioned inhibition was observed in Context B but not A), which could reflect either context specificity of latent inhibition or context specificity of conditioned inhibition. In either case, latent inhibition of conditioned inhibition training in at least some situations was seen to reflect an expression deficit rather than an acquisition deficit. These data, in conjunction with prior reports, suggest that latent inhibition is relatively specific to the context in which it was administered, whereas conditioned inhibition is specific to its training context only when it is the second learned relationship concerning the target cue. These experiments are part of a larger effort to delineate control by the test context of two-phase associative interference as a function of the nature of target training and the nature of interference training.
Keywords: latent inhibition, CS-preexposure effect, conditioned inhibition, context specificity, proactive interference, outcome interference
Seminal studies conducted by Pavlov (1927) and subsequent research by other investigators identified the rules by which paired events become associated. In the simplest case, an animal may learn that one event (e.g., cue X) signals the pending occurrence of a second event (e.g., outcome 1 [O1]). However, life is not always so simple, as in some cases an animal later experiences the cue that had been signaling outcome 1 now predicting another event (e.g., outcome 2 [O2]). Within the associative literature, many studies have examined situations in which a cue has been sequentially paired with more than one outcome, a procedure that often gives rise to what is called associative outcome interference. Specifically, associative outcome interference refers to the decrease in the cue’s potential on a post-training test trial to elicit behavior anticipatory of O1 (i.e., retroactive interference) or O2 (i.e., proactive interference) as a result of the pairing with the nontarget outcome. This seemingly reflects the animal’s possessing conflicting information. Hence, on a test with the cue, the animal must decide whether to respond based on the first or second training experience or some mixture of the two. Given the high frequency with which such situations arise in real life, it is important that we understand the factors that determine which of the two types of training will be expressed at test.
Among the most widely studied types of associative interference situations are extinction (i.e., pairings of the cue with a specific outcome during Phase 1 and unpaired presentations of the target cue during Phase 2) and latent inhibition (i.e., unpaired presentations of the target cue during Phase 1 and pairings of the cue with a specific outcome during Phase 2). As a result of pressure toward translational research, extinction has been by far the most widely studied associative interference situation because it is viewed as modeling exposure therapy for anxiety disorders and drug abuse, and recovery from extinction has been viewed as modeling relapse from exposure therapy (for a review, see Laborda, McConnell, & Miller, 2011).
Although a large number of studies of associative interference have been published, almost all of them have focused exclusively on what happens when one or another specific type of training occurs first (i.e., during Phase 1) and a different specific type of training occurs second (i.e., during Phase 2), rather than seek general principles that might apply independent of the specific nature of the training in Phase 1 and Phase 2 (but see Bouton, 1993). Here too we of course used specific types of training in Phase 1 and Phase 2, but we targeted types of training that have rarely been examined with respect to associative interference. The types of training examined here in and of themselves are of relatively little interest in our opinion. Rather, we examined them as part of a larger plan to look at a wide variety of different types of training in each phase of associative interference situations to collectively determine whether there are general rules that obtain independent of the specific types of training that are administered in each phase.
In search of more general principles that transcend specific associative interference situations, we have been examining diverse interference situations in order to identify commonalities (and differences) across these situations as a function of: (a) the specific nature of Phase 1 training and Phase 2 training (e.g., excitatory conditioning, Pavlovian inhibition training, and simple exposure to the cue, as in extinction and the latent inhibition treatment), (b) whether the target memory at test is what was trained in Phase 1 (i.e., retroactive interference) or in Phase 2 (i.e., proactive interference), and (c) the nature of the ambiguity between training in Phase 1 and Phase 2. Ambiguity between the two training phases can arise from a number of differences such as the nature of the two outcomes or the temporal relationships between the cue and the outcomes (e.g., Escobar & Miller, 2003; Molet, Urcelay, Miguez, & Miller, 2010). The present experiments are a small contribution to the larger project of determining the general rules of associative outcome interference across diverse situations, assuming there are any. Here we examined the test-context specificity of proactive interference, all other things being equal, in the specific case of nonreinforced presentations of the target cue during Phase 1 (i.e., a latent inhibition treatment) serving as the interfering treatment and Pavlovian conditioned inhibition training during Phase 2 serving as the target training that is potentially subject to interference.
In conventional proactive outcome interference situations in which ‘cue-no outcome’ trials provided by a latent inhibition treatment or conditioned inhibition training in Phase 1 constitute the interfering memory, the target association assessed at test is excitatory (i.e., a CS-outcome association, with the outcome usually being an unconditioned stimulus [US]). However, preexposing a cue, as in a latent inhibition treatment, also results in a decrement in expression (or acquisition) of the second-learned association when the second-learned association is inhibitory (i.e., conditioned inhibition training rather than conditioned excitation training; e.g., Friedman, Blaisdell, Escobar, & Miller, 1998; Reiss & Wagner, 1972; Rescorla, 1971). This demonstrates that a form of outcome interference can also occur when the first-learned experience is devoid of an expectancy of any US (such as in a latent inhibition treatment) and the second-learned experience involves a violated expectancy of a specific US (such as in Pavlovian inhibition training).Both latent inhibitors and conditioned inhibitors are retarded in acquiring behavioral control during subsequent excitation training. However, they differ in that a latent inhibitor does not reduce responding to an excitatory CS (a transfer excitor) when it is presented in compound with the excitatory CS any more than does an unfamiliar stimulus, whereas a conditioned inhibitor does (e.g., Lorden, Rickert, & Berry, 1983; Reiss & Wagner, 1972). This observation indicates that a different mechanism underlies conditioned inhibition than the mechanism underlying latent inhibition and that summation tests are a sensitive means of differentiating between information acquired in the two treatments.
In the present research, we examined the nature of the dependency on the test context of interference with Pavlovian conditioned inhibition training (A-US / AX-) by a prior latent inhibition treatment (X-). That is, we were concerned with whether test context influenced this specific type of outcome interference when all other factors are held constant. Prior research in a number of interference situations, although not with a latent inhibition treatment followed by conditioned inhibition training, has generally found that the relative similarity of the test context to each of the training contexts is a critical factor in determining the impact each of the two training experiences will have on responding at test (e.g., Bouton, 1993; Miller & Laborda, 2011). The most widely studied case of context shift effects influencing outcome interference is renewal following extinction (for a review, see Westbrook & Bouton, 2010). In renewal, partial recovery of an extinguished response occurs when testing takes place outside the extinction context (e.g., Bouton & Bolles, 1979; Bouton & King, 1983). Data from outcome interference preparations other than extinction also speak to the dependency of outcome interference on the test context. For instance, a context shift between a latent inhibition treatment in Phase 1 and Pavlovian excitatory conditioning in Phase 2 results in less interference with expression of the CS-US association (i.e., stronger responding) when the CS is tested in the context in which excitation was trained, compared to testing of the CS in the context of the latent inhibition treatment (i.e., ABA renewal vs. an ABB control; Bailey & Westbrook, 2008; Gray, Williams, Fernandez, Ruddle, Good, & Snowden, 2001; Hall & Channel, 1985; Kaplan & Lubow, 2001; Lovibond, Preston, & Mackintosh, 1984; Nakajima, Takahashi, & Blaisdell, 2006; Nelson & Sanjuan, 2006; Rosas & Bouton, 1997; Rudy, 1994; Schiller & Weiner, 2005; Zalstein-Orda & Lubow, 1995) or in a novel context (Bouton & Swartzentruber, 1989; Maes, 2002; Wheeler, Chang, & Miller, 2003; Yap & Richardson, 2005). Moreover, when a latent inhibition treatment is administered in Context A and excitatory conditioning is administered in Context B, a greater decrement in excitatory responding has been observed in Context C relative to Context B than is seen with the same context switch without the initial latent inhibition treatment. This could be viewed as indicating some degree of transfer of latent inhibition beyond the context used for latent inhibition treatment (Swartzentruber & Bouton, 1992). Similarly, Lovibond et al. (1984, Experiment 2) demonstrated that a switch out of the context used for latent inhibition treatment severely reduces, but does not always completely eliminate, the latent inhibition effect.
The present Experiment 2 examined the role of test context in an inhibitory analog of the studies of latent inhibition followed by excitatory conditioning that are described above. The analogy arises from Pavlovian inhibition training being administered in Phase 2 in place of excitatory conditioning of the target cue. In Experiment 2, in which the latent inhibition treatment was administered in Context A during Phase 1 and conditioned inhibition training was administered in Context B during Phase 2, we expected to see behavior reflecting the latent inhibition treatment when the target cue X was tested in Context A (i.e., an ABA procedure) and behavior indicative of the conditioned inhibition treatment when X was tested in Context B (i.e., an ABB procedure). This would parallel what is observed when a latent inhibition treatment in one context is followed by excitatory conditioning in a different context. In Experiment 1, a latent inhibition treatment and subsequent inhibitory conditioning were both administered in the same context (A), and the target cue was tested in either Context A (i.e., an AAA procedure) or a context in which the target cue was not trained (B, i.e., an AAB procedure). Here the results were less certain because prior research with a latent inhibition treatment and conditioned excitation training has found the [first learned] latent inhibition treatment to be context specific (e.g., Hall & Channel 1985; Swartzentruber & Bouton, 1992), but also has found second-learned Pavlovian conditioned inhibition training, following conditioned excitation training, to be context specific (e.g., Nelson, 2002; Sissons & Miller, 2009; Westbrook, Jones, Bailey, & Harris, 2000). If second-learned conditioned inhibition is context specific in this situation (i.e., with the latent inhibition treatment in Phase 1) as is observed when excitatory conditioning is trained in Phase 1, little conditioned inhibition should be observed when testing occurs outside the common context of latent inhibition and conditioned inhibition training. However, without the initial latent inhibition treatment [which makes subsequent conditioned inhibition training second learned], conditioned inhibition training in Context A should readily transfer to Context B.
Most accounts of associative interference were developed on the basis of data from situations in which at least one of the two contradictory associations is excitatory (e.g., Bouton 1994; Laborda & Miller, 2012, Miller & Laborda, 2011). Thus, the dependency of associative interference on the test context when a latent inhibition treatment precedes conditioned inhibition training may not adhere to the rules that have been identified for situations in which one phase of training involves excitatory conditioning of the target cue (i.e., CS-US pairings). Clearly proactive interference with conditioned inhibition (acquisition or expression) sometimes results from a prior latent inhibition treatment (e.g., Friedman et al., 1998), but the degree of context specificity of this interference is currently unclear.
There has been only one previous paper directly relevant to the issues raised here, and it precludes no firm conclusions. Nakajima et al. (2006, Experiment 2) failed to observe any context specificity of interference with conditioned inhibition by latent inhibition. Using rats in a food magazine approach task, Nakajima et al. first preexposed rats to a buzzer in either Context A or B (or no preexposure for a control group). Then all subjects received reinforced trials of a light in Context A, followed by more reinforced presentations of the light interspersed with nonreinforced trials of the light-buzzer compound also in Context A. As a result of this light-US / light+buzzer-noUS procedure, the buzzer presumably became a conditioned inhibitor. The authors compared the rate of acquisition of conditioned inhibition across groups and found preexposure to the buzzer retarded development of conditioned inhibition compared to no preexposure. But they observed no difference in retardation as a function of whether preexposure to the buzzer had occurred in Context A or B.
The lack of context specificity of latent inhibition’s effect on conditioned inhibition observed in Nakajima et al.’s study, however, should be regarded with caution. First, unexpected differences across groups were found during the light-food training phase, though there was convergence by the end of light-food training. Nevertheless, that difference could have interacted later with any effect that latent inhibition may have had on conditioned inhibition. Second, their assessment of conditioned inhibition consisted of comparing responding to the light alone with responding to the light-buzzer compound, which constitutes an unusual test for conditioned inhibition. Notably, there was no summation test for conditioned inhibition using a transfer conditioned excitor compounded with the putative conditioned inhibitor and control condition in which the transfer excitor was compounded with a neutral cue to control for external inhibition. Such a summation test is widely recognized as necessary to certify that a stimulus is a conditioned inhibitor (Rescorla, 1969). Third, in their Experiment 1, simple context-dependency of the latent inhibition treatment’s effect on excitatory conditioning was a small, only observed with a liberal statistical analyses, which suggests that the procedures they used were not particularly sensitive to context shift effects in general. For these reasons we believe that further study is warranted. Furthermore, the intent of present experiments differed somewhat from that of Nakajima’s Experiment 2. Nakajima et al. varied whether conditioned inhibition training occurred in the same or a different context from that of the prior latent inhibition treatment and then assessed the rate of development of conditioned inhibition, whereas within each of our experiments we held constant the context of the latent inhibition treatment and the context of conditioned inhibition training and then we varied the test context.
To maintain comparability with many other studies of associative interference in which two phases of training are followed by a test, our focal interest was in the context specificity of the expression of [proactive outcome] interference in a test phase following the completion of the latent inhibition treatment in Phase 1 and the completion of conditioned inhibition training in Phase 2. Toward this end, we assessed interference in various contexts using [negative] summation tests for conditioned inhibition that were administered following completion of conditioned inhibition training. This contrasts with Nakajima et al. (2006) who administered their test during Phase 2, which presumably assessed differences in the rate of acquisition of stimulus control during Phase 2 (i.e., before conditioned inhibition training was complete). Importantly, in both of the following experiments, all contexts were equated for total exposure and for nonassociative experience in each phase of the study, a feature that was lacking in Nakajima et al.’s (2006) Experiment 2 which otherwise was in several respects conceptually similar to the present Experiment 2. Here, ‘equating nonassociative experience of the contexts’ means administering cue and cue-outcome presentations, but with irrelevant cues substituted for the experimental cues during phases in which the target cue was not trained in a given context, thereby matching contexts with respect to associative history with the outcome. Centrally, both of the present experiments assessed control by the test context of interference with conditioned inhibition by prior latent inhibition treatment; that is, in a situation in which neither type of training (i.e., latent inhibition or conditioned inhibition) involved pairings of the target cue with the outcome. This is what makes the present research novel, save for Nakajima et al.’s Experiment 2, which we view as suggestive but equivocal. In each of our two studies, we gave all four critical groups Phase 1 treatment in the same context and Phase 2 treatment in the same context. Then we varied where testing occurred. This way we were able to examine whether test location per se influenced expression of latent inhibition effects on conditioned inhibition with all other factors held constant. By our so doing, this study served as a [small] part of a larger project concerned with the similarities and differences in the rules of associative interference across diverse interference situations. Additionally, we were interested in whether expression of associative interference can be modified by the specific test context, which speaks to the question of whether this type of interference reflects an absence of the target information or a failure to express the target information.
EXPERIMENT 1
In Experiment 1 (see Table 1), we assessed the influence of test context on latent inhibition’s interference with Pavlovian conditioned inhibition by administering a summation test with a transfer excitor in the context that was used for both the latent inhibition treatment and conditioned inhibition training (A) as well as in a ‘nontraining’ context (B); thus, we examined the effects of an AAB context shift relative to an AAA control. By ‘nontraining’ context, we refer to a context in which events occurred equivalent to those events that occurred during target training, but with irrelevant cues rather than the critical experimental cues. This assured that the nontraining context (B) was matched to the context used for target training (A) with respect to context-US associations. Only a summation test was used because latent inhibition treatments as well as conditioned inhibition training produce retardation of development of excitatory control of behavior by the target cue. Hence, a retardation test, in which the development of excitatory behavioral control by the target cue was assessed, would have been confounded by differences across groups in the presence or absence of the latent inhibition treatment. The procedure for the conditioned inhibition training was borrowed almost exactly from Polack, Laborda, and Miller (2014), who demonstrated that this specific preparation produced a conditioned inhibitor that passed both summation and retardation tests for conditioned inhibition. Our use of these previously established parameters obviated the need for a demonstration that Phase 2 of training in the present research was adequate to produce conditioned inhibition.
Table 1.
Design Summary of Experiment 1
| Groups | Phase 1 Latent Inhibition Training |
Phase 2 Conditioned Inhibition Training |
Phase 3 Excitor Training |
Test |
|---|---|---|---|---|
| AAA-X | (300 X-) A / (300 Y-)B | (21 P+ / 63 PX−) A / (21 Q+ / 63 QY−) B |
(1 R+) B (1 R+) A |
XRA |
| AAA-Con | (300 Z-) A / (300 Y-)B | XRA | ||
| AAB-X | (300 X-) A / (300 Y-)B | XRB | ||
| AAB-Con | (300 Z-) A / (300 Y-)B | XRB | ||
| Sum-Con | (300 X-) A / (300 Y-)B | NRA/B |
Note: The clicks, tone, and white noise served as cues X, Y, Z, and N, counterbalanced within groups. A flashing light and the house light off served as training excitors P and Q, counterbalanced within groups. A SonAlert served as cue R. Footshock served as the US, denoted as +. Nonreinforcement is indicated by −. Numbers refer to number of trial. Sum-Con identifies the group that received neither latent inhibition nor conditioned inhibition treatment with the target cue. Superscript letters A and B denote different contexts. The identities of these two contexts were counterbalanced within groups.
Based on the well-known context specificity of latent inhibition when followed with excitatory training, one might expect latent inhibition to also be context specific when followed by conditioned inhibition training, which would be manifest as reduced conditioned inhibition in the context of the latent inhibition treatment and conditioned inhibition training (A) but not in the context in which the target cue was not trained (B). However, when conditioned inhibition learning is the second-learned information concerning the target cue (X), the context specificity of second-learned information (Bouton, 1993) might be expected to result in weak conditioned inhibition when testing occurs in Context B (i.e., outside the context in which conditioned inhibition training occurred). This would be consistent with the previously reported context specificity of second-learned conditioned inhibition after conditioned excitation training during phase 1 (Nelson 2002, Experiment 2; see also Sissons & Miller, 2009). The critical question in Experiment 1 was the degree of interference with the effects of conditioned inhibition training that would be observed at test in Context B with (i.e., Group AAB-X) and without (i.e., Group AAB-Con) the prior latent inhibition treatment (i.e., X-). A second cue (Y) was subjected to the latent inhibition treatment in Context B during Phase 1 and conditioned inhibition training in Context B during Phase 2 in order to equate the amount of exposure and provide equivalent nonassociative experience and nontarget cue-context pairings in the two contexts (i.e., to equate the two contexts in all respects except for the presentations of the target CS). In Phase 3, a transfer excitor (R) for the subsequent summation test with X was trained in both Contexts A and B. Finally, XR summation tests were conducted in Contexts A and B. Two additional groups, AAA-X and AAA-Con, were treated identically to Groups AAB-X and AAB-Con, respectively, except that they were tested in Context A. Lastly, a fifth group, Sum-Con, was included to assess baseline behavior at test.
Methods
Subjects
Subjects were 60 male, experimentally naive, Sprague-Dawley descended rats obtained from Harlan Laboratories (Indianapolis, IN). Body weight range was 163–211 g at the beginning of the experiment. Subjects were randomly assigned to one of five groups (ns = 12). The animals were individually housed in standard plastic cages in a vivarium maintained on a 16/8-hr light/dark cycle. Experimental manipulations occurred near the middle portion of the light phase. The animals received free access to Purina Lab Chow pellets, whereas water availability in the home cage was limited to 30 min per day following a progressive deprivation schedule initiated 4 days prior to the start of the study. On experimental days, water in the home cage was provided 1–4 h after the end of each day’s experimental sessions. From the time of arrival in the laboratory until the start of the study, all animals were handled for 30 s, three times per week.
Apparatus
Twenty-four chambers of two distinct types, R and V (12 of each), were used. Each chamber was housed in a separate light- and sound-attenuating environmental isolation chest.
Chamber R was rectangular, measuring 24.0 cm x 9.0 cm x 12.5 cm (l x w x h). The walls and ceiling of Chamber R were clear Plexiglas, and the floor comprised stainless steel cylindrical rods measuring 0.5 cm in diameter, spaced 1.3-cm apart (center to center). The rods were connected by NE-2 bulbs, which permitted the delivery a 0.6–mA, 0.5-s constant current footshock provided by a high voltage AC circuit in series with a 1.0-MΩ resistor. Chamber V was 27-cm long, 29.5-cm high, 21.5-cm wide at the top, and 5.5-cm wide at the bottom. The floor was comprised of two 27-cm long plates, 2-cm wide, with a 1.5-cm gap between the two plates. The ceiling was clear Plexiglas, the front and back walls were black Plexiglas, and the sidewalls and floor were stainless steel. A 0.6-mA, 0.5-s constant-current footshock, produced by a high voltage AC circuit in series with a 1.0-MΩ resistor, could be delivered through the metal walls and floor of the chamber.
Both types of chambers could be equipped with a water-filled lick tube that extended 1-cm into a 5 cm deep cylindrical niche, which was 4.5-cm in diameter, left-right centered, with its bottom 1.75-cm above the floor of the apparatus. In all chambers there was a photobeam detector 1-cm in front of the lick tube that was broken whenever the subject licked the tube. A flashing light (0.25 s on / 0.25 s off) served as a visual cue. The light was produced by either a 25-W bulb (Chamber R) or a 100-W bulb (Chamber V) nominal at 120 VAC, but driven at 80 VAC. The bulbs were mounted on an inside wall of the environmental chest, approximately 30 cm from the center of the experimental chamber. The light intensities inside the animal chambers produced by the flashing light in the two chambers were approximately equal due to the difference in opacities of the walls. A 6S6 bulb (30 V, 6 W) mounted on the interior ceiling of the isolation chest in a corner served as the house light. In the R chambers this bulb was 2/3 blackened, whereas in V chambers the bulb was not masked, which resulted in approximately equivalent illumination in the two chambers due to the difference in opacities of the walls. The house light was normally on; turning it off served as a cue. Three 45-Ω speakers on the interior side and back walls of the isolation chests could deliver a click train (6 Hz), complex tone (500 & 520 Hz presented simultaneously), and white noise, each 8 dB above background. Ventilation fans in each enclosure provided a constant 76-dB background noise. All auditory measurements were on the C-scale. The clicks, tone, and white noise served as cues X, Y, and Z (or N), perfectly counterbalanced within groups. The house light (going off, as it was normally on) and the flashing light, perfectly counterbalanced within groups, served as training excitors P and Q during conditioned inhibition training of X and Y, respectively. Additionally, a SonAlert which could provide a 1900-Hz tone 8 dB above background was mounted on the ceiling of each environmental chest. The SonAlert served as the transfer excitor, R, on the summation test for conditioned inhibition. All audiovisual stimuli were 30-s in duration. The 0.5-s footshock served as the US.
For half of the subjects, Chamber R was Context A and Chamber V was Context B; this was reversed for the other half of the subjects. Chamber assignation was counterbalanced with respect to stimulus assignation using an incomplete Latin square. In each phase of the experiment, all subjects received equal exposure to Contexts A and B. For half of the animals in each group, treatment in Context A occurred on odd numbered days and treatment in Context B occurred on even numbered days; this was reversed for the other half of the animals. Subject assignation to treatment in Context A on odd vs. even numbered days was counterbalanced using an incomplete Latin square with respect to cue designation.
Procedure (see Table 1)
Acclimation
On Days 1 and 2, all subjects were given 30-min of daily exposure to both Contexts A and B. This acclimation was intended to reduce the novelty of the contexts and train the rats to drink from the lick tube. The order of context exposure was counterbalanced within groups and reversed between Day 1 and Day 2. The order of context exposure was perfectly counterbalanced with respect to context, but was counterbalanced using an incomplete Latin square with respect to cue assignment. Sessions within a day were separated by approximately 1 hr. Following Day 2, the lick tubes were removed until Reacclimation.
Phase 1
On Days 3, 5, 7, 9, and 11 (or Days 4, 6, 8, 10, and 12), each rat in Groups AAA-X, AAB-X, and Sum-Con received 60 daily presentations of target cue X during an 120.5-min session in Context A with a mean intertrial interval (ITI) of 90 s (range: 60–120 s), CS onset to CS onset, while subjects in Groups AAA-Con and AAB-Con received similar treatment with nontarget cue Z. Additionally, all subjects received identical treatment on Days 4, 6, 8, 10, and 12 (or Days 3, 5, 7, 9, and 11) in Context B except that cue Y was presented instead of cue X and Z. As previously described, the actual order of X (or Z) and Y sessions across days was counterbalanced within groups.
Phase 2
On Days 13, 15, and 17 (or Days 14, 16, and 18), all subjects experienced an 81-min session in Context A during which 7 reinforced P and 21 nonreinforced PX trials occurred. On Days 14, 16 and 18 (or Days 13, 15, and 17), all subjects experienced an 81-min session in Context B during which 7 reinforced Q and 21 nonreinforced QY trials occurred. On reinforced trials, the US was presented during the last 0.5 s of the 30-s cue presentation. Each session had an average of ITI of 143 s (range: 93–193) from cue onset to cue onset. The actual order of P/PX and Q/QY sessions was counterbalanced within groups as previously described.
Phase 3
On Day 19 (or Day 20), all subjects experienced 60-min sessions in Context A, during which 1 reinforced presentation of R occurred. On Day 20 (or Day 19), all subjects experienced 60-min sessions in Context B, during which 1 reinforced presentation of R occurred. The US was presented during the last 0.5 s of the 30-s presentation of R. On Day 19, the trial started at 20 min into the session, whereas on Day 20 the trial started 40 min into the session. The order of training context was counterbalanced within groups.
Reacclimation
Reacclimation to drinking occurred in both Contexts A and B on both Days 21 and 22 with the lick tubes present. Sessions lasted 60 min per context with about 120 min between sessions. Order of context exposure was counterbalanced within groups. These sessions were intended to restore baseline licking that might have been disrupted by the prior footshocks.
Test
On Day 23, subjects in Conditions AAA and AAB were given a summation test during which the compound XR was presented within an 11-min session. Subjects in Group Sum-Con received presentations of the compound NR instead of XR, in order to assess behavioral control by the transfer excitor (R) in the absence of a conditioned inhibitor. Cue N was intended to control for external inhibition by X of behavioral control by R as well as stimulus generalization decrement going from training on R alone to testing on XR (due to any possible configuring of R with its companion stimulus at test) on behavioral control by R. In the AAB condition, subjects were tested in Context B. In the AAA condition, subjects were tested in Context A. Half of the subjects in Group Sum-Con were tested in Context A and the other half in Context B. There was no basis to expect the data from these two subgroups to differ because cue N had never been presented in either context, cue R had the same history in both contexts, and all other cues (X, Y, P, and Q) had been fully counterbalanced; hence, the data from these two subgroups was pooled. The test compound was presented to each rat when it had finished drinking for 5 cumulative seconds and the time for the subject to drink an additional 5 cumulative seconds was recorded. Thus, all rats were drinking at CS onset. Each animal received 10-min of exposure to the test compound before being removed from the chamber. Subjects taking more than 60 s to complete 5 s of baseline drinking (i.e., before CS onset) were eliminated from the study because such behavior reflected an atypical reluctance to drink.
Results and Discussion
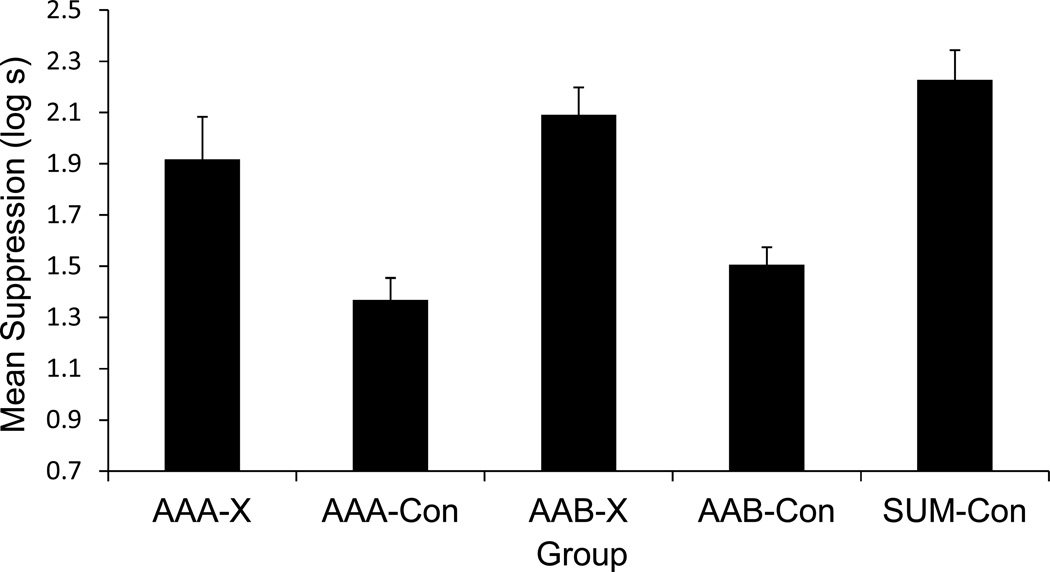
The results of Experiment 1 are depicted in Figure 1. One subject (from Group AAA-X) was eliminated according to the elimination criterion. A second subject (from Group AAB-Con) was eliminated due to an experimenter error.
Figure 1. Results of Experiment 1.
Mean times to complete 5 cumulative seconds of drinking in the presence of the target test compound. Therefore, 0.7 log s is the lowest possible score. Higher scores represent stronger conditioned suppression. Error bars represent standard error of the mean. See text and Table 1 for details.
A one-way ANOVA conducted on baseline drinking (i.e., time to complete 5 cumulative seconds of drinking prior to CS onset) did not detect differences among groups, F(4, 53) = 1.39, p = .24. A similar ANOVA performed on the time to drink 5 cumulative seconds in the presence of the CS revealed differences among the groups, F(4, 53) = 10.91, p < .01, Cohen’s f = 0.83 (95% confidence interval [CI] = 0.51; 1.14), MSE = 0.149.
Planned comparisons using the error term from the ANOVA were conducted to identify the sources of these differences and to test specific hypotheses. As expected, greater conditioned suppression was observed in Group Sum-Con relative to Groups AAA-Con and AAB-Con, F(1, 53) = 29.72, p < .01, Cohen’s f = 0.71 (95% CI = 0.42; 1.00), and F(1, 53) = 20.04, p < .01, Cohen’s f = 0.58 (95% CI = 0.30; 0.86), respectively. This is consistent with X acting as an effective conditioned inhibitor in either test context (given no latent inhibition treatment of X), whereas N never underwent conditioned inhibition training. That is, strong conditioned suppression was expected in Group Sum-Con because the transfer excitor (R) was tested in compound with a neutral cue (N), which should not have impacted conditioned suppression to R (beyond producing some external inhibition and stimulus generalization decrement which should also have been produced by X in Groups AAA-Con and AAB-Con). Group Sum-Con did not reliably differ from Groups AAA-X and AAB-X, F(1, 53) = 3.71, p = .059, and F(1, 53) = 0.75, p = .38, respectively. Strong conditioned suppression was observed in Group AAA-X relative to Group AAA-Con, F(1, 53) = 11.58, p < .01, Cohen’s f = 0.44 (95% CI = 0.31; 0.87). This essentially replicates Friedman et al. (1998) in showing that, when all treatment and testing occurs in a single context, the latent inhibition treatment interferes with subsequent acquisition or expression of conditioned inhibition. Clearly, the inhibitory potential of X in Group AAA-X was attenuated relative to Group AAA-Con due to interference as a result of the latent inhibition treatment of (i.e., nonreinforced exposure to) X in Phase 1. Similarly, the inhibitory potential of X in Group AAB-X was attenuated relative to Group AAB-Con, F(1, 53) = 13.16, p < .01, Cohen’s f = 0.47 (95% CI = 0.30; 0.86). This indicates that latent inhibition of Pavlovian conditioned inhibition transferred to Context B in an AAB paradigm, which was the central question being addressed in this experiment. The following statistics, excluding Group Sum-Con, further support this conclusion.
A 2 (Test Context: A or B) x 2 (Treatment: X or Con) ANOVA detected no interaction, p = .87. An effect of Treatment was detected which represented more suppression to XR when X had been preexposed, F(1, 42) = 25.27, p < .01, Cohen’s f = 0.74 (95% CI = 0.41; 1.07), which indicates that the latent inhibition treatment disrupted negative summation by X during the test. The effect of Test Context was not significant, p = .17. That is, behavioral control by the XR compound in Condition X did not reliably differ regardless of whether testing occurred in the treatment context (A) or outside of it (B).
In summary, when conditioned inhibition was the first-learned relationship concerning X (i.e., no preexposure to cue X during Phase 1; Groups AAA-Con and AAB-Con), behavior indicative of conditioned inhibition was observed not only in the training context but in a context in which the target cue had not been trained. However, when conditioned inhibition was second learned (i.e., following preexposure to X; Groups AAA-X and AAB-X), it was poorly acquired (or at least not expressed) due to the prior latent inhibition treatment when conditioned inhibition was assessed in a context different from that of treatment as well as in the context in which latent inhibition and conditioned inhibition training had occurred. Importantly, the present results could not be due to differences in exposure to or nonassociative experiences in the two test contexts because these were equated across the test contexts.
Although latent inhibition of Pavlovian conditioned inhibition transferred to Context B in an AAB paradigm, the absence of evidence of conditioned inhibition in Context B could have been due to either or both (a) the latent inhibition treatment of Group AAB-X in Phase 1 making the conditioned inhibition training in Phase 2 a second-learned association concerning cue X that did not transfer to Context B or (b) context-nonspecific interference with Phase 2 conditioned inhibition training by the Phase 1 latent inhibition treatment that transferred to Context B. One might argue that the relative lack of conditioned inhibition in Group AAB-X was not likely due to the effect of the latent inhibition treatment transferring into Context B because much prior research has demonstrated that latent inhibition is relatively context specific. For example, Hall and Minor (1984) found latent inhibition to produce a decrement in behavioral control following excitatory fear conditioning when the latent inhibition treatment and fear conditioning were both conducted in the same context relative to different contexts being used for the latent inhibition treatment and fear conditioning (for an analogous result with appetitive conditioning, see Channell & Hall, 1983). However, all of this prior research was conducted with excitatory training in Phase 2 and consequently might not apply to the present experiment in which inhibitory training was administered during Phase 2. Thus, there is no clear basis at present to differentiate between these two accounts of why latent inhibition treatment interfered with behavior indicative of conditioned inhibition training in Group AAB-X. However, the goal of Experiment 1 was to determine whether, after the Phase 1 latent inhibition treatment and Phase 2 Pavlovian conditioned inhibition training both in Context A, interference with behavior indicative of conditioned inhibition would be less pronounced in Context B than Context A. We did not observe this.
Although the present data do not indicate why conditioned inhibition was not evident in Context B, empirically we see that when the latent inhibition treatment and subsequent conditioned inhibition training occurred in the same context, negative summation by the target inhibitor was attenuated in both the training context and a context in which the target cue had not been trained as an inhibitor. That is, the latent inhibition treatment resulted in similarly high levels of proactive interference with conditioned inhibition regardless of whether testing occurred in the treatment context or in a context in which the target cue had not been trained. This conceptually replicates in a situation with an aversive US the findings of Nakajima et al. (2006), which is informative given the previously described concerns with Nakajima et al.’s design and data.
EXPERIMENT 2
Like Experiment 1, Experiment 2 examined interference with Pavlovian conditioned inhibition in Phase 2 by the latent inhibition treatment administered in Phase 1 as a function of the context in which testing occurred. However, in Experiment 2 an ABA (vs. ABB) design rather than an AAB (vs. AAA) design was used (see Table 2). Alternatively stated, here we assessed how test context influenced latent inhibition’s interference with conditioned inhibition, now with latent inhibition treatment and conditioned inhibition training administered in distinctively different contexts, by administering a summation test in each of these two contexts. This allowed us to compare our findings in this situation with conditioned inhibition training in Phase 2 to prior reports of test-context specificity of a latent inhibition treatment on subsequent excitatory conditioning that used analogous context shifts (e.g., Bailey & Westbrook, 2008; Gray et al., 2001; Hall & Channel, 1985; Kaplan & Lubow, 2001; Lovibond et al., 1984; Nakajima et al., 2006; Nelson & Sanjuan, 2006; Rosas & Bouton, 1997; Rudy, 1994; Schiller & Weiner, 2005; Zalstein-Orda & Lubow, 1995). Based on Bouton’s 1993 model, we expected weak conditioned inhibition to be evident in Context A relative to Context B because both nonreinforcement (i.e., latent inhibition treatment in Context A) and a second-learned relationship concerning a cue (here conditioned inhibition treatment in Context B) are posited to be context specific. Critically, behavior indicative of conditioned inhibition in Context B would demonstrate that the conditioned inhibition training had been effective despite the prior latent inhibition treatment of Cue X in Context A. Given this result, we would know that any absence of evidence of conditioned inhibition in Context A was due not to a failure to acquire conditioned inhibition, but a failure to express conditioned inhibition, at least in the present situation.
Table 2.
Design Summary of Experiment 2:
| Groups | Phase 1 Latent Inhibition Training |
Phase 2 Conditioned Inhibition Training |
Phase 3 Excitor Training |
Test |
|---|---|---|---|---|
| ABB-X | (300 X-) A / (300 Y-)B | (21 P+ / 63 PX−) B / (21 Q+ / 63 QY−) A |
(1 R+) B (1 R+) A |
XRB |
| ABB-Con | (300 Z-) A / (300 Y-)B | XRB | ||
| ABA-X | (300 X-) A / (300 Y-)B | XRA | ||
| ABA-Con | (300 Z-) A / (300 Y-)B | XRA | ||
| Sum-Con | (300 X-) A / (300 Y-)B | NRA/B |
Note: The clicks, tone, and white noise served as cues X, Y, Z, and N, counterbalanced within groups. A flashing light and the house light off served as training excitors P and Q, counterbalanced within groups. A SonAlert served as cue R. Footshock served as the US, denoted as +. Nonreinforcement is indicated by −. Numbers refer to number of trials. Sum-Con identifies the group that received neither latent inhibition nor conditioned inhibition training with the target cue. Superscript letters A and B denote different contexts. The identities of these two contexts were counterbalanced within groups.
In Phase 1, subjects received preexposure to target cue X (Groups ABB-X, ABA-X, and Sum-Con) or nontarget cue Z (Groups ABB-Con and ABA-Con) in Context A, and to nontarget cue Y in Context B (in order to equate context exposure and nonassociative experience in the two contexts). During Phase 2, all subjects received Pavlovian conditioned inhibition training with target cue X in Context B, and Pavlovian conditioned inhibition training with nontarget cue Y in Context A (in order to equate the two contexts in all manner except for where target cue training occurred during Phase 1 and Phase 2). In Phase 3, all subjects received excitatory conditioning with cue R, the transfer CS to be used on the summation tests, in both Contexts A and B. Subjects in both the ABB and ABA conditions were tested on X for [negative] summation using transfer excitor R. The test occurred either in Context A (Condition ABA) or B (Condition ABB). Subjects in Group Summation Control (Sum-Con) were tested on N for summation using R as a transfer excitor in Context A or Context B (the two contexts were functionally equivalent for this group), in order to assess baseline behavioral control by R after controlling for any possible generalization decrement or external inhibition produce by N. Planned comparisons between Group Sum-Con and Group ABB-Con allowed us to assess the magnitude of the conditioned inhibition produced with our parameters, in the absence of associative interference or a context shift following the conditioned inhibition training. Planned comparisons between Groups ABB-X and ABB-Con and between Groups ABA-X and ABA-Con allowed us to determine the context specificity of interference with conditioned inhibition produced by the latent inhibition treatment.
Methods
Subjects and Apparatus
Subjects were 60 male, experimentally naive, Sprague-Dawley descended rats obtained from Harlan Laboratories (Indianapolis, IN). Body weight range was 181–209 g at the beginning of the experiment. Subjects were randomly assigned to one of five groups (ns = 12). The animals were maintained and housed as in Experiment 1. Apparatuses were the same as in Experiment 1.
Procedure (see Table 2)
Acclimation
On Days 1 and 2, all subjects were given 30-min of exposure daily to Contexts A and B in a manner identical to that described in Experiment 1.
Phase 1
On Days 3–12, the five groups received exactly the same latent inhibition treatments with X or Z in Context A and Y in Context B as in Experiment 1.
Phase 2
On Days 13–18, all groups received Pavlovian conditioned inhibition training with X in Context B and with Y in Context A. Other than this reversal of contexts for X and Y, conditioned inhibition training was identical to that of Experiment 1.
Phase 3
On Days 19 and 20, all subjects received R-US pairings in Contexts A and B identical to Phase 3 training in Experiment 1.
Reacclimation
Reacclimation to both Contexts A and B occurred on Days 21 and 22 as described in Experiment 1.
Test
On Day 23, subjects in Conditions ABB and ABA were tested on the Compound XR in an 11-min session. The test was conducted as in Experiment 1, except that subjects in the ABB condition were tested in Context B, while the subjects in the ABA condition were tested in Context A. As in Experiment 1, half of the subjects in Group Sum-Con were tested in Context A and the other half in Context B because the elements of the test compound for this group (NR) had the same history in Contexts A and B. The same elimination criterion (i.e., no more than 60 s to complete 5 s of licking prior to CS onset) as was used in Experiment 1 was applied here.
Results and Discussion
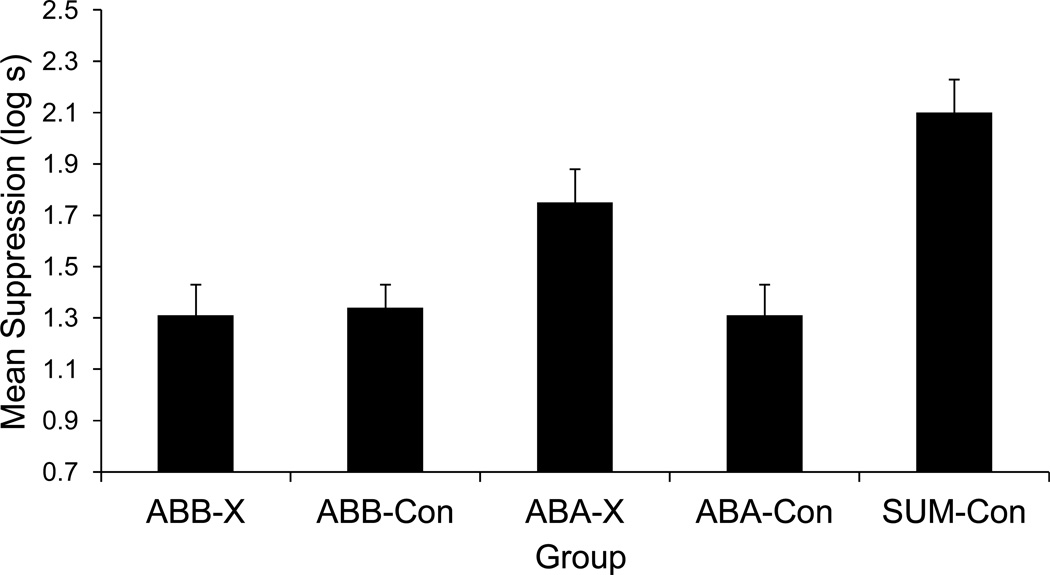
The results of Experiment 2 are depicted in Figure 2. One subject (from Group Sum-Con) was eliminated based on the elimination criterion. Two other subjects (one each in Groups ABA-Con and ABA-X) were eliminated due to experimenter errors.
Figure 2. Results of Experiment 2.
Mean times to complete 5 cumulative seconds of drinking in the presence of the target test compound. Therefore, 0.7 log s is the lowest possible score. Higher scores represent stronger conditioned suppression. Error bars represent standard error of the mean. See text and Table 2 for details.
A one-way analysis of variance (ANOVA) conducted on baseline drinking (i.e., time to complete 5 cumulative seconds of drinking prior to CS onset) did not detect any differences among the groups, F(4, 52) = 0.76, p = .55. A similar ANOVA performed on the times to complete 5 cumulative seconds of drinking in the presence of the CS revealed differences among the groups, F(4, 52) = 8.49, p < .01, Cohen’s f = 0.73 (95% CI = 0.42; 1.04), MSE = 0.164. Additionally, a 2 (Test context: ABA vs. ABB) x 2 (Treatment: X vs. Con) ANOVA revealed a marginal interaction between test context and treatment, F(1, 42) = 4.06, p = .05, Cohen’s f = 0.29 (95% CI = 0.00; 0.57), MSE = 0.160. No other main effects were detected, lowest p > .09.
Planned comparisons were conducted to identify the sources of these differences and to test specific hypotheses. As expected, greater suppression was observed in Group Sum-Con relative to Groups ABB-X, ABB-Con, and ABA-Con, F(1, 52) = 21.87, p < .01, Cohen’s f = 0.61 (95% CI = 0.33; 0.90), F(1, 52) = 19.85, p < .01, Cohen’s f = 0.59 (95% CI = 0.30; 0.87), F(1, 52) = 20.58, p < .01, Cohen’s f = 0.58 (95% CI = 0.31; 0.88), respectively. Stronger suppression was expected in Group Sum-Con because the transfer excitor (R) was tested in compound with N, a neutral cue that was not expected to reduce behavioral control in a summation test other than possibly through external inhibition and/or stimulus generalization decrement. Thus, the putative conditioned inhibitor (X) attenuated suppression to R in Groups ABB-X, ABA-Con, and ABB-Con. This occurred in Group ABB-X (a) because X was tested in the context of conditioned inhibition training, with the test context presumably serving as a positive occasion setter for the expression of the second-learned conditioned inhibition training (Bouton, 1993), and/or (b) because latent inhibition failed to transfer to a context different from where it had been administered. Negative summation would be expected in Groups ABA-Con and ABB-Con because, for these groups, conditioned inhibition training was the first thing learned concerning X (i.e., there was no preexposure to X). There would thus be no ambiguity concerning what X signaled and no basis for this learning to be context specific. Moreover, Group ABA-X exhibited marginally less suppression than Group Sum-Con, F(1, 52) = 4.00, p = .051, Cohen’s f = 0.26 (95% CI = 0.01; 0.52), suggesting that in Group ABA-X some limited amount of conditioned inhibition learning generalized from the context of conditioned inhibition training (B) to the context of the latent inhibition treatment (A). Strong suppression was observed in Group ABA-X relative to Group ABA-Con, F(1, 52) = 6.43, p = .01, Cohen’s f = 0.33 (95% CI = 0.06; 0.60), presumably because (a) in the latent inhibition context (A) the conditioned inhibition potential of X in Group ABA-X was attenuated due to Context A at test facilitating retrieval of the memory of the latent inhibition treatment, and/or (b) the conditioned inhibition acquired in Context B was context specific due to its being second learned (Bouton, 1993). The present data do not permit us to differentiate between these two mechanisms.
More conditioned inhibition (i.e., less conditioned suppression) was observed in Group ABB-X than in to Group ABA-X, F(1, 52) = 8.83, p < .01, Cohen’s f = 0.39 (95% CI = 0.12; 0.66). This is not surprising for two reasons. First, prior research with excitation training following a latent inhibition treatment has found latent inhibition to be context specific (despite its being the first-learned information concerning X; e.g., Bailey & Westbrook, 2008; Bouton & Swartzentruber, 1989; Gray et al., 2001; Hall & Channel, 1985; Kaplan & Lubow, 2001; Lovibond et al., 1984; Maes, 2002; Nakajima et al., 2006; Nelson & Sanjuan, 2006; Rosas & Bouton, 1997; Rudy, 1994; Schiller & Weiner, 2005; Wheeler et al., 2003; Yap & Richardson, 2005; Zalstein-Orda & Lubow, 1995). Second, Group ABA-X was tested in the context of the latent inhibition treatment (A), whereas Group ABB-X was tested in the context in which conditioned inhibition training had occurred (B), which might have allowed Context B to serve as an occasion setter for X acting as a conditioned inhibitor because in this situation conditioned inhibition was second learned (e.g., Nelson 2002, Experiment 2; see also Sissons & Miller, 2009). Despite prior latent inhibition treatment in Context A, conditioned inhibition exhibited by Group ABB-X, which was tested in the context of inhibitory training (B), was sufficiently strong that suppression to XR did not significantly differ from its control ABB-Con, p = .81. This is congruent with the previously mentioned reports of latent inhibition, when followed by excitatory conditioning as opposed to the present inhibitory conditioning) being relatively context specific.
We acknowledge that the present design does not differentiate between the strong conditioned inhibition observed in Group ABB-X arising from the context specificity of latent inhibition, and the occasion setting potential of the context of second learning about X (i.e., the context of conditioned inhibition training). However, unlike Experiment 1, the present data do clearly indicate that lack of evidence of conditioned inhibition in Group ABA-X arose from the latent inhibition treatment preventing expression of conditioned inhibition, not from preventing the acquisition of conditioned inhibition, because conditioned inhibition was observed when testing of Group ABB-X occurred in Context B following the identical treatments received by Group ABA-X. Alternatively stated, the interference with conditioned inhibition produced by latent inhibition treatment observed in Experiment 2 was clearly a failure to express information that was encoded in the subject. This conclusion, however, may be specific to situations like Experiment 2 in which latent inhibition treatment and conditioned inhibition training occur in different contexts.
In summary, empirically speaking, when the latent inhibition treatment was administered in one context and subsequent conditioned inhibition training occurred in a different context, the target cue (X) was a significantly more effective conditioned inhibitor on a summation test in the context of conditioned inhibition training than in the context of latent inhibition treatment. That is, the latent inhibition treatment resulted in more proactive interference with conditioned inhibition when testing occurred in the context of the latent inhibition treatment than in the context of conditioned inhibition training. Moreover, the lack of behavior indicative of conditioned inhibition observed in the context of the latent inhibition treatment was clearly not due to a failure to learn the inhibitory relationship.
General Discussion
Here we conducted two experiments designed to explore the influence of the test context on interference with conditioned inhibition produced by a latent inhibition treatment, as assessed by a summation test. Both of the present experiments found reliable evidence of conditioned inhibition in the absence of the latent inhibition treatment. In Experiment 1, administering the latent inhibition treatment prior to Pavlovian conditioned inhibition training in the same context resulted in the absence of evidence of conditioned inhibition despite the same conditioned inhibition training being effective when there was no prior latent inhibition treatment. In Experiment 2, administering the latent inhibition treatment in one context prior to conditioned inhibition training in a different context resulted in evidence of conditioned inhibition in the context of conditioned inhibition training but not the context of the latent inhibition treatment. Thus, Experiment 2 demonstrated that latent inhibition treatment of a cue later used in conditioned inhibition training makes the cue’s behavioral control susceptible to contextual changes.
In the absence of the latent inhibition treatment, behavior indicative of conditioned inhibition was not context specific in that it transferred from Context A to Context B in Experiment 1 (Group AAB-Con) and from Context B to Context A in Experiment 2 (Group ABA-Con). More interesting is what happened to conditioned inhibition learning when it was preceded by the latent inhibition treatment, particularly as a function of the context used for testing. In Experiment 1, in which the latent inhibition treatment and conditioned inhibition training both occurred in Context A, no evidence of conditioned inhibition was observed in Context A or B despite conditioned inhibition training without the prior latent inhibition treatment producing evidence of conditioned inhibition in both the context of conditioned inhibition training (A) and a different context (B). However, the design of Experiment 1 does not let us determine whether the absence of evidence of conditioned inhibition in Groups AAA-X and AAB-X arose from a failure to learning during conditioned inhibition training of a failure to express conditioned inhibition learning that had occurred.
In Experiment 2, in which the latent inhibition treatment occurred in Context A and conditioned inhibition training occurred in Context B, the target cue (X) acted as a stronger conditioned inhibitor in Context B than in Context A. Thus, in Experiment 2, test behavior was consistent with whatever was previously learned in the test context (A or B). The results of Experiment 2 are in accord with analogous studies that assessed the context specificity of interference by latent inhibition on subsequent excitatory conditioning (e.g., Bailey & Westbrook, 2008; Dexter & Merrill, 1969; Gray et al., 2001; Hall & Channel, 1985; Kaplan & Lubow, 2001; Lovibond et al., 1984; Nakajima et al., 2006; Nelson & Sanjuan, 2006; Rosas & Bouton, 1997; Rudy, 1994; Schiller & Weiner, 2005; Zalstein-Orda & Lubow, 1995). For example, Dexter and Merrill exposed rats to a CS in one of two Skinner boxes used for test (“modified” or “unmodified”), in a holding cage, or they received no exposure. All rats then received conditioning in a dissimilar Gerbrands rat chamber. Finally, the rats were tested in both the modified and unmodified Skinner boxes. Thus, some rats received latent inhibition treatment and testing in the same context. Other rats received latent inhibition treatment and test in two different contexts, and yet other rats received no latent inhibition treatment. They observed maximal latent inhibition effects when the CS was tested in the context of preexposure. Thus, our results are congruent with their observations, although in the present experiments target training was inhibitory rather than excitatory, which adds generality to their findings.
One important conclusion from the present research is that at least in some circumstances (i.e., the ABA design of Experiment 2) latent inhibition of conditioned inhibition is clearly due to a retrieval failure, rather than an acquisition failure as is commonly assumed in attentional accounts of latent inhibition (e.g., Hall & Pearce, 1979). Based on this conclusion and, in the interests of parsimony, seeking a common mechanism for latent inhibition in all situations, one might speculate that latent inhibition generally is a form of context-specific retrieval failure (i.e., a potentially reversible deficit in behavioral control) rather than a failure to encode information concerning the Phase 2 training. This view is consistent with findings of studies in which Phase 2 training consists of excitatory conditioning rather than conditioned inhibition training (e.g., Dexter & Merrill, 1969). More broadly speaking, these observations are concordant with those found when excitatory training, rather than inhibitory training as in the present experiments, occurs during Phase 2, which lends support to the view that there might be a common set of principles that apply across many if not all instances of two-phase associative interference treatment followed by a test.
Based on the present findings as well as prior data concerning the context specificity of latent inhibition followed by excitatory training (e.g., Hall & Channel, 1985) and the context specificity of conditioned inhibition when it is second learned (e.g., Nelson, 2002; Sissons & Miller, 2002), two rules are suggested concerning which of two associations will be expressed when both concern X and at least one association among the two involves the absence of an outcome (i.e., no US). First, inhibitory-like memories that are devoid of a specific US representation such as are established by a latent inhibition treatment are always context specific. Second, inhibitory-like associations involving the absence of an expected US such as are established by conditioned inhibition training appear to be context specific only when they are second learned (i.e., when conditioned inhibition training is preceded by excitatory conditioning or a latent inhibition treatment). However, the test-context specificity of conditioned inhibition trained in one context following a latent inhibition treatment in a different context has not yet been examined in a third context (i.e., an ABC paradigm). The results of such an experiment would be useful in assessing the tentative conclusions stated above.
These conclusions suggest that the memories produced by a latent inhibition treatment and conditioned inhibition training differ appreciably, with the latent inhibition memory being unique in never transferring strongly to a context other than the one in which the latent inhibition treatment was administered (weak transfer has been reported, e.g., Lovibond et al., 1984, Experiment 2). Note that this conclusion assumes that the lack of appreciable conditioned inhibition observed in Group AAB-X of the present Experiment 1 when it was tested in Context B was due to the context specificity of second-learned conditioned inhibition rather than the transfer of latent inhibition to Context B; if it had been due to latent inhibition transferring to Context B, then we might have expected latent inhibition to be evident as reduced conditioned inhibition in Group ABB-X of Experiment 2 when it was tested in Context B, which was not observed. This distinction between the test context specificity of latent inhibition treatment and conditioned inhibition training is not surprising given the differences in the operations that give rise to them. Of course this is a description of the observed behaviors as opposed to an explanation of the underlying mechanisms that differentiate memories formed by a latent inhibition treatment from those formed by Pavlovian conditioned inhibition training.
The present observations suggest that the context of a latent inhibition treatment is a positive occasion setter for latent inhibition of the target cue, but they do not permit us to determine whether the context of conditioned inhibition training (following a latent inhibition treatment) is a positive occasion setter for conditioned inhibition of the target cue. To answer this question, one would need to use an ABC design. Both the Bouton (1993) model, in which second-learned information concerning a cue makes that specific information context specific, and the Rosas, Aguilera, Alvarez, and Abad (2006) model, in which any sort of ambiguity (which results in increased attention to the context) increases context specificity of anything subsequently learned in the context in which the ambiguity was encountered, are in accord with the results of Experiment 2 in predicting positive occasion setting of conditioned inhibition by the context in which conditioned inhibition training occurred. However, Experiment 2 did not assess the alternative possibility of positive occasion setting of latent inhibition by the context in which the latent inhibition treatment had occurred. Moreover, assuming in Experiment 1 that conditioned inhibition was acquired but not expressed in Context A following the latent inhibition treatment, Bouton’s and Rosas’ models both anticipate the present lack of evidence of conditioned inhibition in Context B, again because conditioned inhibition was the second-learned information concerning CS X. But as previously stated, it is unclear in this study whether conditioned inhibition was acquired after the latent inhibition treatment in the same context. To answer the question regarding whether positive occasion setting of conditioned inhibition or negative occasion setting of latent inhibition was at play in the test context specificity observed in Experiment 2, one would need to use an ABC design. We acknowledge the absence of an ABC design as a weakness of the present report. Unfortunately, the need to equate exposure to and experience in all of the contexts in all groups makes this design impractical with the present training procedures.
We emphasize that the central goal of the current report was empirical, that is, we wanted to determine the influence of the test context on interference with conditioned inhibition produced by a latent inhibition treatment. Nevertheless, we have briefly discussed several mechanisms that may have contributed the present observations. Although we are unable to fully differentiate between among these mechanisms based on the current data, our observations do have implications for some accounts of latent inhibition. Let us specifically consider interference models. Most interference models that account for test context specificity of outcome interference are based primarily on extinction data. Following excitatory conditioning in one context and extinction in a second context, what was learned during the extinction treatment appears to be relatively specific to the extinction context (e.g., Bouton & Bolles, 1979; Bouton & King, 1983). Bouton (1994) has suggested that extinction memories are more context specific than are conditioning memories either because the X-no US association is inhibitory-like or because the X-no US association presumed to result from extinction treatment is the second information learned about X (i.e., because ambiguity about the meaning of X arises only when extinction treatment began, causing the extinction context to become an occasion setter for the X-no US relationship). Both of these mechanisms explain the observed test context specificity of extinction learning. However, these two accounts diverge in their predictions for the test context specificity of memory of a latent inhibition treatment in one context (Phase 1) followed by excitatory conditioning in a second context (Phase 2). Many papers have reported that that after a latent inhibition treatment in one context and conditioned excitation in a second context, the excitatory memory generalizes to other test contexts relative to testing back in the latent inhibition context (e.g., Lovibond et al., 1984; Westbrook et al., 2000). Thus, in the case of a latent inhibition treatment, it is the CS-no US memory which is context specific as opposed to the CS-US memory. In contrast, based on existing evidence it appears that it is the second-learned association that is context specific when Pavlovian conditioned inhibition training either precedes or follows conditioned excitation training (Nelson, 2002; Sissons & Miller, 2009). Nelson as well as Sissons and Miller observed that Pavlovian conditioned inhibition training in Phase 2 was specific to the context of conditioned inhibition training when Phase 1 consisted of excitatory conditioning.
Comparing the present observations to the aforementioned outcome interference situations, at least one generality emerges. In Experiment 2, in which latent inhibition and conditioned inhibition treatments were administered in different contexts, the interference effect of latent inhibition on conditioned inhibition was stronger when the test context matched the one in which the latent inhibition treatment had occurred. This indicates that similarity between the test context and the training context influences the expression of the association trained in that context, a result which is identical to the context specificity of the outcome interference effect observed in ABA renewal following both extinction (e.g., Bouton & King, 1983) and counterconditioning (Peck & Bouton, 1990).
We close by noting that most models of associative interference have been devised to explain only a limited number of specific instances of associative interference. For example, Bouton’s (1993) account does well with outcome interference, but fails to speak to cue interference (e.g., X-Outcome followed Y-Outcome; Escobar, Matute, & Miller, 2001; Matute & Pineño, 1998). In contrast, Miller and Escobar (2002) have proposed an account of associative interference that addresses both outcome interference and cue interference. Of course outcome interference and cue interference may possibly arise from different underlying mechanisms. Notably, Miller and Escobar’s account of interference has much in common with the various accounts of so-called retrieval-induced forgetting in humans (e.g., Anderson & Spellman, 1995; Jonker, Seli, & McLoed, 2013), which is clearly a variant of retroactive outcome interference (in the sense that the term has been used in this paper, i.e., X-C training, then X-D training, followed by a test of X-C; see especially use of the ‘extralist paradigm’ in retrieval-induced forgetting, e.g., Bäuml, 2002; Storm, Bjork, Bjork, & Nestojko, 2006); however, retrieval-induced forgetting is seldom related to more traditional associative interference situations (but see Miguez, Mash, Polack, & Miller, 2014; Vadillo, Orgaz, Luque, Cobos, López, & Matute, 2013). Thus, there appears to be a need for researchers to seek commonalities (and differences) across different instances of associative interference rather than simply staying focused on a single instance.
Acknowledgments
This research was supported by National Institute of Mental Health Grant 33881. The authors thank Vincent Deninis, Peter Gerhardstein, Sarah O’Hara, Cody W. Polack, and Deanne Westerman for their comments on an earlier version of this manuscript. Gonzalo Miguez was partially supported by the Fondo de Investigación Científica y Tecnológica (CONICYT-Chile) grant #1130117 and the Programa de Atracción e Inserción de Capital Humano Avanzado (CONICYT-Chile) grant #79140028.
References
- Anderson MC, Spellman BA. On the status of inhibitory mechanisms in cognition: Memory retrieval as a model case. Psychological Review. 1995;102:68–100. doi: 10.1037/0033-295x.102.1.68. [DOI] [PubMed] [Google Scholar]
- Bailey GK, Westbrook R. Extinction and latent inhibition of within-event learning are context specific. Journal of Experimental Psychology: Animal Behavior Processes. 2008;34:106–118. doi: 10.1037/0097-7403.34.1.106. [DOI] [PubMed] [Google Scholar]
- Bäuml K-HT. Semantic generation can cause episodic forgetting. Psychological Science. 2002;13:356–360. doi: 10.1111/1467-9280.00464. [DOI] [PubMed] [Google Scholar]
- Bouton ME. Context, time, and memory retrieval in the interference paradigms of Pavlovian learning. Psychological Bulletin. 1993;114:80–99. doi: 10.1037/0033-2909.114.1.80. [DOI] [PubMed] [Google Scholar]
- Bouton ME. Conditioning, remembering, and forgetting. Journal of Experimental Psychology: Animal Behavior Processes. 1994;20:219–231. [PubMed] [Google Scholar]
- Bouton ME, Bolles RC. Contextual control of the extinction of conditioned fear. Learning and Motivation. 1979;10:445–466. [Google Scholar]
- Bouton ME, King DA. Contextual control of the extinction of conditioned fear: Tests for the associative value of the context. Journal of Experimental Psychology: Animal Behavior Processes. 1983;9:248–265. [PubMed] [Google Scholar]
- Bouton ME, Swartzentruber D. Slow reacquisition following extinction: Context, encoding, and retrieval mechanisms. Journal of Experimental Psychology: Animal Behavior Processes. 1989;15:43–53. [Google Scholar]
- Channell S, Hall G. Contextual effects in latent inhibition with an appetitive conditioning procedure. Animal Learning & Behavior. 1983;11:67–74. [Google Scholar]
- Dexter WR, Merrill HK. Role of contextual discrimination in fear conditioning. Journal of Comparative and Physiological Psychology. 1969;69:677–681. doi: 10.1037/h0028210. [DOI] [PubMed] [Google Scholar]
- Escobar M, Matute H, Miller RR. Cues trained apart compete for behavioral control in rats: Convergence with the associative interference literature. Journal of Experimental Psychology: General. 2001;130:97–115. doi: 10.1037/0096-3445.130.1.97. [DOI] [PubMed] [Google Scholar]
- Escobar M, Miller RR. Timing in retroactive interference. Learning & Behavior. 2003;31:257–272. doi: 10.3758/bf03195987. [DOI] [PubMed] [Google Scholar]
- Friedman BX, Blaisdell AP, Escobar M, Miller RR. Comparator mechanisms and conditioned inhibition: Conditioned stimulus preexposure disrupts Pavlovian conditioned inhibition but not explicitly unpaired inhibition. Journal of Experimental Psychology: Animal Behavior Processes. 1998;24:453–466. [PubMed] [Google Scholar]
- Gray NS, Williams J, Fernandez M, Ruddle RA, Good MA, Snowden RJ. Context dependent latent inhibition in adult humans. Quarterly Journal of Experimental Psychology. 2001;54B:233–245. doi: 10.1080/02724990143000027. [DOI] [PubMed] [Google Scholar]
- Hall G, Channell S. Differential effects of contextual change on latent inhibition and on the habituation of an orienting response. Journal of Experimental Psychology: Animal Behavior Processes. 1985;11:470–481. [Google Scholar]
- Hall G, Minor H. A search for context-stimulus associations in latent inhibition. Quarterly Journal of Experimental Psychology. 1984;36B:145–169. [Google Scholar]
- Hall G, Pearce JM. Latent inhibition of a CS during CS-US pairings. Journal of Experimental Psychology: Animal Behavior Processes. 1979;5:31–42. [PubMed] [Google Scholar]
- Hall G, Rodriguez G. Associative and nonassociative processes in latent inhibition: An elaboration of the Pearce-Hall model. In: Lubow R, Winer I, editors. Latent inhibition: Cognition, neuroscience, and applications to schizophrenia. New York, NY: Cambridge University Press; 2010. pp. 114–136. [Google Scholar]
- Jonker TR, Seli P, MacLeod CM. Putting retrieval-induced forgetting in context: An inhibition-free, context-based account. Psychological Review. 2013;120:852. doi: 10.1037/a0034246. [DOI] [PubMed] [Google Scholar]
- Kaplan O, Lubow RE. Context and reminder effects in a visual search analog of latent inhibition. Learning and Motivation. 2001;32:137–153. [Google Scholar]
- Laborda MA, McConnell BL, Miller RR. Behavioral techniques to reduce relapse after exposure therapy: Applications of studies of experimental extinction. In: Schachtman TR, Reilly S, editors. Associative learning and conditioning theory: Human and non-human applications. Oxford, UK: Oxford University Press; 2011. pp. 79–103. [Google Scholar]
- Laborda MA, Miller RR. Reactivated memories compete for expression after Pavlovian extinction. Behavioural Processes. 2012;90:20–27. doi: 10.1016/j.beproc.2012.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorden JF, Rickert EJ, Berry DW. Forebrain monoamines and associative learning: I. Latent inhibition and conditioned inhibition. Behavioural Brain Research. 1983;9:181–199. doi: 10.1016/0166-4328(83)90127-4. [DOI] [PubMed] [Google Scholar]
- Lovibond PF, Preston GC, Mackintosh NJ. Context specificity of conditioning, extinction, and latent inhibition. Journal of Experimental Psychology: Animal Behavior Processes. 1984;10:360–375. [Google Scholar]
- Maes JR. No sex difference in contextual control over the expression of latent inhibition and extinction in Pavlovian fear conditioning in rats. Neurobiology of Learning and Memory. 2002;78:258–278. doi: 10.1006/nlme.2002.4058. [DOI] [PubMed] [Google Scholar]
- Matute H, Pineño O. Stimulus competition in the absence of compound conditioning. Animal Learning & Behavior. 1998;26:3–14. [Google Scholar]
- Miguez G, Mash L, Polack CW, Miller RR. Failure to observe renewal following retrieval-induced forgetting. Behavioural Processes. 2014;103:43–51. doi: 10.1016/j.beproc.2013.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller RR, Escobar M. Associative interference between cues and between outcomes presented together and presented apart: An integration. Behavioural Processes. 2002;57:163–185. doi: 10.1016/s0376-6357(02)00012-8. [DOI] [PubMed] [Google Scholar]
- Miller RR, Laborda MA. Preventing recovery from extinction and relapse: A product of current retrieval cues and memory strengths. Current Directions in Psychological Science. 2011;20:325–329. [Google Scholar]
- Miller RR, Oberling P. Analogies between occasion setting and Pavlovian conditioning. In: Schmajuk NA, Holland PC, editors. Occasion setting: Associative learning and cognition in animals. Washington, DC: American Psychological Association; 1998. pp. 13–35. [Google Scholar]
- Molet M, Urcelay GP, Miguez G, Miller RR. Using context to resolve temporal ambiguity. Journal of Experimental Psychology: Animal Behavior Processes. 2010;36:126–136. doi: 10.1037/a0016055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakajima S, Takanashi K, Blaisdell AP. An assessment of context specificity of the CS-preexposure effect in Pavlovian excitatory and inhibitory conditioning. Behavioural Processess. 2006;73:84–91. doi: 10.1016/j.beproc.2006.03.016. [DOI] [PubMed] [Google Scholar]
- Nelson JB. Context specificity of excitation and inhibition in ambiguous stimuli. Learning and Motivation. 2002;33:284–310. [Google Scholar]
- Nelson J, Sanjuan M. A context-specific latent inhibition effect in a human conditioned suppression task. Quarterly Journal of Experimental Psychology. 2006;59:1003–1020. doi: 10.1080/17470210500417738. [DOI] [PubMed] [Google Scholar]
- Pavlov IP. In: Conditioned reflexes. Anrep GV, editor. London: Oxford University Press; 1927. & Trans. [Google Scholar]
- Peck CA, Bouton ME. Context and performance in aversive-to-appetitive and appetitive-to-aversive transfer. Learning and Motivation. 1990;21:1–31. [Google Scholar]
- Polack CW, Laborda MA, Miller RR. Extinction of a Pavlovian conditioned inhibitor leads to stimulus-specific inhibition. 2014 doi: 10.3758/s13420-019-00396-3. Manuscript submitted for publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rescorla RA. Pavlovian conditioned inhibition. Psychological Bulletin. 1969;72:77–94. [Google Scholar]
- Rescorla RA. Summation and retardation tests of latent inhibition. Journal of Comparative and Physiological Psychology. 1971;75:77–81. doi: 10.1037/h0030694. [DOI] [PubMed] [Google Scholar]
- Rosas JM, Bouton ME. Renewal of a conditioned taste aversion upon return to the conditioning context after extinction in another one. Learning and Motivation. 1997;28:216–229. [Google Scholar]
- Rosas JM, Aguilera JEC, Álvarez MMR, Abad MJF. Revision of retrieval theory of forgetting: What does make information context-specific? International Journal of Psychology & Psychological Therapy. 2006;6:147–166. [Google Scholar]
- Rudy JW. The memory-coherence problem, configural associations, and the hippocampal system. In: Schacter DL, Tulving E, editors. Memory systems. Cambridge, MA US: MIT Press; 1994. pp. 119–146. [Google Scholar]
- Schiller D, Weiner I. Basolateral amygdala lesions in the rat produce an abnormally persistent latent inhibition with weak preexposure but not with context shift. Behavioural Brain Research. 2005;163:115–121. doi: 10.1016/j.bbr.2005.04.005. [DOI] [PubMed] [Google Scholar]
- Sissons HT, Miller RR. Spontaneous recovery of excitation and inhibition. Journal of Experimental Psychology: Animal Behavior Processes. 2009;35:419–426. doi: 10.1037/a0014815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storm BC, Bjork EL, Bjork RA, Nestojko JF. Is retrieval success a necessary condition for retrieval-induced forgetting? Psychonomic Bulletin & Review. 2006;13:1023–1027. doi: 10.3758/bf03213919. [DOI] [PubMed] [Google Scholar]
- Swartzentruber D, Bouton ME. Context sensitivity of conditioned suppression following preexposure to the conditioned stimulus. Animal Learning & Behavior. 1992;20:97–103. [Google Scholar]
- Vadillo MA, Orgaz C, Luque D, Cobos PL, López FJ, Matute H. The role of outcome inhibition in interference between outcomes: a contingency-learning analogue of retrieval-induced forgetting. British Journal of Psychology. 2013;104:167–180. doi: 10.1111/j.2044-8295.2012.02110.x. [DOI] [PubMed] [Google Scholar]
- Westbrook R, Bouton ME. Latent inhibition and extinction: Their signature phenomena and the role of prediction error. In: Lubow RE, Weiner I, editors. Latent inhibition: Cognition, neuroscience and applications to schizophrenia. New York, NY US: Cambridge University Press; 2010. pp. 23–39. [Google Scholar]
- Westbrook R, Jones ML, Bailey GK, Harris JA. Contextual control over conditioned responding in a latent inhibition paradigm. Journal of Experimental Psychology: Animal Behavior Processes. 2000;26:157–173. doi: 10.1037//0097-7403.26.2.157. [DOI] [PubMed] [Google Scholar]
- Wheeler DS, Chang RC, Miller RR. Massive preexposure and preexposure in multiple contexts attenuate the context specificity of latent inhibition. Learning & Behavior. 2003;31:378–386. doi: 10.3758/bf03195998. [DOI] [PubMed] [Google Scholar]
- Yap CL, Richardson R. Latent inhibition in the developing rat: An examination of context-specific effects. Developmental Psychobiology. 2005;47:55–65. doi: 10.1002/dev.20074. [DOI] [PubMed] [Google Scholar]
- Zalstein-Orda N, Lubow RE. Context control of negative transfer induced by preexposure to irrelevant stimuli: Latent inhibition in humans. Learning and Motivation. 1995;26:11–28. [Google Scholar]