Abstract
BACKGROUND
Patients with giant cell arteritis (GCA) may experience serious vascular and visual complications. It is unknown, however, to what extent the difficulties of the disease may lead to hospitalization. The goal of this study is to discern whether patients with GCA are at greater risk for all-cause hospitalizations when compared to the general population.
METHODS
This retrospective, population-based cohort study utilized patients with large vessel or visual involvement who were diagnosed with GCA (as defined by the 1990 ACR criteria) between 1/1/1950 and 12/31/2009, and a reference cohort of patients without GCA matched on age, sex, and calendar year. Each patient’s medical record was examined for hospitalizations from 1987 through 2012. For this analysis, follow-up began with the latter of index date or 1/1/1987 and ended at the earlier of death, emigration from Olmsted County, or 12/31/2012. Discharge diagnoses were grouped together using the Clinical Classifications Software (CCS) for ICD-9-CM from Healthcare Cost and Utilization Project (HCUP). Data were analyzed using person-year methods and rate ratios comparing GCA to non-GCA.
RESULTS
The GCA cohort consists of 199 patients with a mean age of 76.2 (79.9% female) and follow-up of 8.2 years. The non-GCA cohort is comprised of 194 patients with a mean age of 75.7 (78.9% female) and follow-up of 8.6 years. The patients with GCA had 816 hospitalizations and the non-GCA patients had 737 hospitalizations. GCA patients proved to be at a marginally greater risk for all causes of hospitalization (Rate Ratio [RR] 1.13, 95% Confidence Interval [CI] 1.02, 1.25); however, the rate of hospitalization for patients with and without GCA decreased significantly from 1987 to 2012.
Two specific discharge categories are of interest. First, transient cerebral ischemia is a greater risk of hospitalization for patients with GCA who had 16 hospitalizations compared to patients without GCA who only had 5 hospitalizations (RR 3.06, CI 1.27, 9.47). Second, patients with GCA (21 hospitalizations) are at greater risk of hospitalization for syncope than patients without GCA (5 hospitalizations) (RR 3.98, CI 1.72, 12.14).
CONCLUSION
In this first ever analysis of all-cause hospitalizations in a population-based cohort, patients with GCA appear to be at a marginally greater risk for hospitalization than patients without GCA, although the rate of hospitalization for GCA patients decreased from 1987 to 2012. Patients with GCA are at increased risk of hospitalization for both transient cerebral ischemia and syncope.
Keywords: giant cell arteritis, hospitalization, health utilization
1.1 INTRODUCTION
Giant cell arteritis (GCA) is the most common form of idiopathic vasculitis. It primarily affects individuals of Northern European ancestry and those age 50 years and older [1–6]. Usually targeting the branches of the external carotid artery, GCA can result in fatigue, headache, tenderness in the temporal region, and loss of vision. GCA affects mainly medium and large sized vessels, especially the aorta and its first order branches, and may result in aortic aneurysm, aortic dissection, and medium and large artery stenosis [7–10].
Despite these associated risks, mortality in patients with GCA does not seem to differ from the general population [9, 11–12]. Nevertheless, vascular and visual complications are serious concerns in the treatment of patients with GCA. These complications and comorbidities such as infection and cardiovascular disease may result in increased need for hospitalization. This study, therefore, aimed to examine for the first time hospitalizations for patients with GCA in order to determine whether there are differences in rates of admission between these patients and the general population.
2.1 MATERIALS AND METHODS
This is a retrospective, population-based cohort study including residents of Olmsted County, Minnesota. This is possible due to the resources of the Rochester Epidemiology Project, a medical records linkage system that contains the complete (inpatient and outpatient) medical records from all health care providers in Olmsted County including the Mayo Clinic and its affiliated hospital, the Olmsted Medical Center, local nursing homes and private practitioners [13, 14].
The incident cohort consists of 245 patients with large vessel or visual involvement who were diagnosed with GCA between January 1, 1950 and December 31, 2009. Each patient fulfills the 1990 American College of Rheumatology criteria for GCA [15]. The reference cohort of 245 patients without GCA was matched by age, sex, and calendar year. Each non-GCA subject was assigned an index date corresponding to the GCA incidence date of the designated patient with GCA.
Data on hospitalizations (admission dates, discharge dates and admission and discharge diagnoses) were retrieved electronically from billing data from Olmsted County medical providers including Mayo Clinic and Olmsted Medical Center and their affiliated hospitals and were available beginning in 1987. Of the original 245 patients with GCA and 245 subjects without GCA, those who died or emigrated from Olmsted County prior to 1987 were excluded (45 GCA and 46 non-GCA) as were those who declined to authorize the use of their medical records for research purposes per Minnesota statute sometime following their initial inclusion in the cohorts (1 GCA and 5 non-GCA). Each patient’s medical record was examined for all-cause hospitalizations beginning in the year 1987 and continuing through 2012. For this analysis, follow-up began with the latter of index date or January 1, 1987 and ended at the earlier of death, migration from Olmsted County, or December 31, 2012.
Primary discharge diagnoses were grouped together using the Clinical Classifications Software (CCS) for ICD-9-CM from the Healthcare Cost and Utilization Project [16]. The CCS groups diagnoses into 18 chapters: infections and parasitic diseases; neoplasms; endocrine, nutritional, and metabolic diseases and immunity disorders; diseases of the blood and blood-forming organs; mental illness; diseases of the nervous system and sense organs; diseases of the circulatory system; diseases of respiratory system; diseases of the digestive system; diseases of the genitourinary system; complications of pregnancy, childbirth and puerperium; diseases of the skin and subcutaneous tissue; diseases of the musculoskeletal system and connective tissue; congenital anomalies; certain conditions originating in the perinatal period; injury and poisonings (which includes fractures); symptoms, signs and ill-defined conditions; and residual codes, unclassified. The CCS also further subdivides diagnoses into 285 mutually exclusive categories. Readmissions were defined as hospital admissions occurring within 30 days of a previous hospital discharge.
Statistical Methods
Descriptive statistics (means percentages, etc.) were used to summarize the data. Data were analyzed using person-year methods and rate ratios comparing GCA to non-GCA. Comparisons of person-year rates were performed using Poisson methods. Poisson regression models with smoothing splines were used to examine trends over time to allow for non-linear effects. Comparisons of length of stay for GCA vs. non-GCA were performed using generalized linear models adjusted for age, sex and calendar year with random intercepts to account for multiple hospitalizations in the same patient. Readmission rates were calculated as the number of readmissions divided by the number of subsequent hospitalizations (not counting the first hospitalization for each patient, as it could not be a readmission by definition). Analyses were performed using SAS version 9.3 (SAS Institute, Cary, NC, USA) and R 3.0.2 (R Foundation for Statistical Computing, Vienna, Austria).
3.1 RESULTS
The GCA cohort consists of 199 patients (79.9% female) with a mean age of 76.2 (standard deviation [SD] 8.3) years at start of follow-up and mean follow-up of 8.2 (SD 5.8) years. The non-GCA cohort is comprised of 194 patients (78.9% female) with a mean age of 75.7 (SD 8.8) years and follow-up of 8.6 (SD 6.6) years. The patients with GCA had 816 hospitalizations during 1641 person-years [py] of follow-up and the subjects without GCA had 737 hospitalizations during 1672 py. The average length of stay was 6 days among the GCA and non-GCA hospitalizations (median 4 days, 25th percentile 2 days, 75th percentile 7 days in both cohorts; p=0.64).
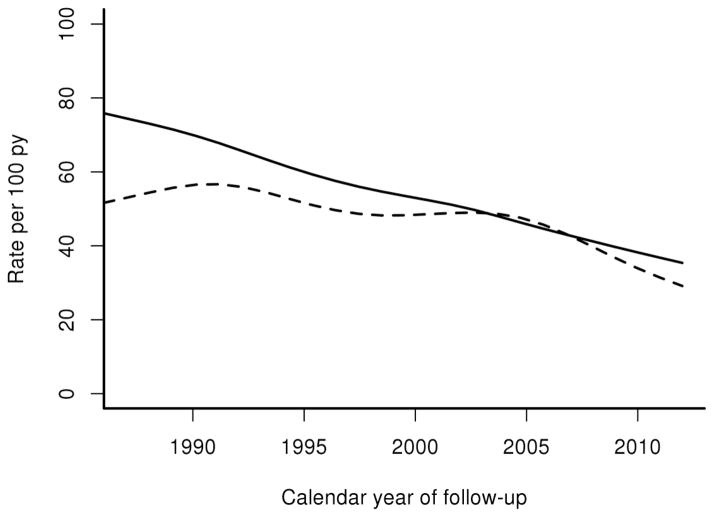
Hospitalization rates were marginally higher in patients with GCA than patients without GCA (Rate Ratio [RR] 1.13, 95% Confidence Interval [CI] 1.02, 1.25) (Table 1). The overall rate of hospitalization decreased significantly for both patients with and without GCA from 1987 to 2012 (Figure 1). However, the rate ratio of hospitalizations for GCA compared to non-GCA remained relatively stable except for a higher rate ratio in the earliest time period (1987–1991; RR 1.26; 95% CI: 1.02, 1.56). Men had higher hospitalization rates than women overall, but the rate ratios of hospitalizations for GCA compared to non-GCA were similar for both men and women. The rates of hospitalization increased with age in both groups, as expected, but there was no statistically significant difference in the rate ratios in GCA compared to non-GCA. Finally, patients with GCA experienced increased overall rates of hospitalization in follow-up years 15–19 (RR 1.78, CI 1.31, 2.45) and 20+ (RR 2.21, CI 1.51, 3.21).
Table 1.
a. Rate of Hospitalization for Patients with Giant Cell Arteritis (GCA) and Patients without GCA
| GCA Rateb | Non-GCA Rateb | Rate Ratio (95% CI) | ||
|---|---|---|---|---|
| Overall | 49.7 | 44.1 | 1.13 (1.02, 1.25) | |
| Sex | Female | 47.2 | 42.5 | 1.11 (0.99, 1.25) |
| Male | 60.7 | 51.0 | 1.19 (0.96, 1.47) | |
| Ages | 50–64 | 15.6 | 16.6 | 0.95 (0.35, 2.42) |
| 65–84 | 45.2 | 40.9 | 1.10 (0.97, 1.26) | |
| 85+ | 58.9 | 52.5 | 1.12 (0.96, 1.30) | |
| Calendar Years | 1987–1991 | 67.1 | 53.1 | 1.26 (1.02, 1.56) |
| 1992–1996 | 55.7 | 49.2 | 1.13 (0.90, 1.42) | |
| 1997–2001 | 47.4 | 40.2 | 1.18 (0.93, 1.50) | |
| 2002–2006 | 46.6 | 50.0 | 0.93 (0.75, 1.16) | |
| 2007–2012 | 39.5 | 33.1 | 1.19 (0.96, 1.48) | |
| Years After GCA Diagnosis | 0–4 | 43.5 | 48.1 | 0.90 (0.77, 1.06) |
| 5–9 | 43.7 | 41.8 | 1.04 (0.85, 1.27) | |
| 10–14 | 55.7 | 43.6 | 1.27 (1.00, 1.61) | |
| 15–19 | 69.6 | 39.0 | 1.78 (1.31, 2.45) | |
| 20+ | 91.6 | 41.6 | 2.21 (1.51, 3.21) |
Statistically significant results are presented in bold font.
Rate of hospitalizations per 100 person-years.
Figure 1.
Hospitalization Rates of Patients with Giant Cell Arteritis (GCA) and Patients without GCA by Calendar
Year
Solid line – patients with GCA
Dashed line – patients without GCA
For four of the eighteen CCS chapters, patients with GCA were hospitalized at a significantly greater rate than patients without GCA (Table 2). These four disease chapters were diseases of the nervous system and sense organs; diseases of the genitourinary system; injury and poisonings and symptoms, signs, and ill-defined conditions and factors influencing health status. Diseases of the nervous system and system and sense organs were the cause of hospitalization for patients with GCA at nearly twice the rate than for patients without GCA (RR 1.71, CI 1.01, 3.04). Female patients with GCA were hospitalized at over twice the rate as female patients without GCA for diseases of the nervous system and sense organs (RR 2.36, CI 1.29, 4.72). Diseases of the genitourinary system were the cause of hospitalization for patients with GCA at nearly twice the rate than for patients without GCA (RR 1.76, CI 1.04, 3.12). An increased rate of hospitalization for diseases of the genitourinary system was also observed for patients with GCA age 85 years and older (15 versus 5 hospitalizations; RR 2.55, CI 1.05, 7.94), and male patients with GCA (12 versus 3 hospitalizations; RR 3.72, CI 1.31, 16.40). Injury and poisonings were also the cause of hospitalization significantly more often for patients with GCA than those without GCA (RR 1.38; 95% CI 1.01, 1.89). Symptoms, signs, and ill-defined conditions were also the cause of hospitalization for patients with GCA at nearly twice the rate than patients without GCA (RR 1.75, CI 1.10, 2.88). Patients with GCA age 85 years and older also were hospitalized at a higher rate than patients 85 and older without GCA for symptoms, signs, and ill-defined conditions (RR 2.29, CI 1.09, 5.57).
Table 2.
a. Rate of Hospitalization for Specific CCS Chapters of Patients with Giant Cell Arteritis (GCA) and Patients without GCA
| CCS Chaptersb | GCA Ratec | Non-GCA Ratec | Rate Ratio (95% CI) |
|---|---|---|---|
| Infections, Parasitic | 1.0 | 1.1 | 0.96 (0.49, 1.87) |
| Neoplasms | 2.3 | 2.2 | 1.05 (0.67, 1.65) |
| Endocrine, Metabolic | 1.9 | 1.4 | 1.37 (0.80, 2.37) |
| Mental | 1.9 | 1.6 | 1.17 (0.70, 1.97) |
| Nervous, Sense Organs | 2.1 | 1.2 | 1.71 (1.01, 3.04) |
| Circulatory | 15.0 | 14.9 | 1.01 (0.84, 1.20) |
| Respiratory | 4.3 | 6.3 | 0.67 (0.50, 0.91) |
| Digestive | 5.4 | 4.3 | 1.24 (0.91, 1.70) |
| Genitourinary | 2.1 | 1.2 | 1.76 (1.04, 3.12) |
| Skin, Subcutaneous | 0.7 | 1.0 | 0.67 (0.30, 1.39) |
| Musculoskeletal, Connective Tissue | 4.1 | 2.9 | 1.44 (1.00, 2.10) |
| Injury, Poisoning | 5.6 | 4.1 | 1.38 (1.01, 1.89) |
| Symptoms and Ill-defined conditions | 2.7 | 1.6 | 1.75 (1.10, 2.88) |
Statistically significant results are presented in bold font.
Results for the following CCS chapters were not shown due to fewer than 10 total hospitalizations in the GCA cases: diseases of the blood and blood forming organs; complications of pregnancy (females only); congenital anomalies; certain conditions originating in the perinatal period; and residual codes/unclassified.
Rate of hospitalizations per 100 person-years.
Patients with GCA had a decreased rate of hospitalization for diseases of the respiratory system when compared to patients without GCA (RR 0.67, CI 0.50, 0.91). There was no difference in the rate ratios of hospitalizations for the respiratory system across age groups or between sexes.
There were several other CCS chapters for which hospitalization rates were increased in specific subgroups of patients, despite no significant increases in the overall rates. Male patients with GCA were hospitalized at nearly four times the rate as male patients without GCA for diseases of the musculoskeletal system and connective tissue (RR 3.72, CI 1.31, 16.40). Endocrine, nutritional, and metabolic diseases and immunity disorders were a cause of increased hospitalization rates for patients with GCA age 85 and older (18 versus 7 hospitalizations; RR 2.23, CI 1.01, 5.81).
Despite no differences in the overall rate ratio for circulatory diagnoses, there were significant differences in hospitalization rates between patients with GCA and patients without GCA in three CCS categories of particular interest in GCA, including transient cerebral ischemia, syncope, and acute myocardial infarction (AMI). Note that syncope is included in the CCS chapter of symptoms, signs and ill-defined conditions. Transient cerebral ischemia was a cause of hospitalization at a significantly greater rate for patients with GCA who had 16 hospitalization compared to 5 hospitalizations in patients without GCA (RR 3.06, CI 1.27, 9.47). Hospitalization rates for syncope were also higher for patients with GCA than patients without GCA (21 versus 5 hospitalizations; RR 3.98, CI 1.72, 12.14). On the other hand, patients with GCA were hospitalized for AMI at half the rate of patients without GCA (14 versus 30 hospitalizations; RR 0.48, CI 0.25, 0.88).
Readmission rates were similar among the GCA subjects with 144 readmissions (22% of 652 subsequent hospitalizations) compared to the non-GCA subjects with 147 readmissions (25% of 578 subsequent hospitalizations; p=0.17).
4.1 DISCUSSION
This first ever study of all-cause hospitalizations for patients with GCA demonstrated that patients with GCA are hospitalized at a higher rate than the general population. Reflecting general trends in declining hospitalization rates over the recent decades, the overall rate of hospitalization decreased for both patients with and without GCA from 1987 to 2012. The rate ratio of hospitalizations for GCA to non-GCA, however, was not significantly different after 1991. Patients with GCA as a whole experienced increased rates of hospitalization for four of the eighteen CCS chapters: diseases of the nervous system and sense organs; diseases of the genitourinary system and injury and poisonings and symptoms, signs and ill-defined conditions. Hospitalizations for diseases of the genitourinary system were increased for male patients with GCA and both diseases of the genitourinary system and symptoms, signs and ill-defined conditions resulted in elevated hospitalization rates for elderly patients with GCA.
It is unclear why patients with GCA experienced increased rates of hospitalization for diseases of the genitourinary system and symptoms, signs and ill-defined conditions, and lower rates of hospitalization for diseases of the respiratory system and for AMI. These issues require further study to elucidate etiologies.
Vascular disease related hospitalization is of particular interest for patients with GCA. In this study, patients with GCA experienced increased rates of hospitalization for transient cerebral ischemia and syncope.
Also of interest, hospitalizations for AMI were observed at half the rate for patients with GCA in comparison the general population. These results may reflect the findings of several recent studies which determined that patients with GCA had lower rates of cardiovascular risk factors than population-based controls at the time of diagnosis [17–19]. These factors include smoking, hypertension, diabetes mellitus, a history of myocardial infarction, and obesity. In addition, Schmidt et al. demonstrated that at diagnosis patients with GCA had less exposure to statins and possessed lower triglyceride levels and higher levels of high density lipoproteins. Rates of AMI in our cohort are not different than the general population [18]. On the other hand, there are several studies which report that patients with GCA are at increased risk for cardiovascular diseases in comparison to the general population [20–22]. We would not expect rates of hospitalization for AMI in patients with GCA in our cohort to be higher; why they were comparatively lower is unclear but may relate to patient or disease related factors, or even coding issues.
We are unaware of any other studies of all-cause hospitalizations for patients with GCA. A study utilizing the Swedish Hospital Discharge Register examined the risk of cancer for patients hospitalized for polymyalgia rheumatica (PMR) and GCA [23]. These patients were at higher risk for cancer, particularly in the first year following a hospitalization for PMR and GCA. However, detailed information about hospitalization rates were not provided in the report; it was commented upon that hospitalizations for PMR and GCA increased with age. Since this Swedish study combined hospitalizations for PMR and GCA, and did not provide detailed information about hospitalization rates but rather focused on occurrence of cancer following hospitalization, it is difficult to compare it to our findings for GCA. Furthermore, as the authors commented, the patients were identified only by hospitalizations for PMR and GCA, thus likely resulting in selection bias wherein only patients with severe or poorly controlled PMR and GCA were followed.
Strengths of our study include that it utilizes a well-characterized, population-based cohort of patients with GCA, and it includes a comparison cohort of subjects without GCA. Also, the comprehensive resources of the Rochester Epidemiology Project allow complete ascertainment of hospitalizations from all providers in Olmsted County. Usage of CCS is advantageous as it conforms to national data and allows for easy, comparative studies in the future. CCS chapters are disadvantageous, however, due to a lack of granularity of diagnoses and the potential for ambiguity in the classifications of diseases into the chapters. It is possible that some hospitalizations outside Olmsted County may have been missed; however, this is unlikely to be different for patients with and without GCA. As well, this information is captured at subsequent visits to Olmsted County providers.
5.1 CONCLUSIONS
In this first ever analysis of all-cause hospitalizations in a population-based cohort, patients with GCA appear to be at a marginally greater risk for hospitalization than patients without GCA, although the overall rate of hospitalization for GCA patients decreased from 1987 to 2012. Patients with GCA are at increased risk of hospitalization for both transient cerebral ischemia and syncope. We are unaware of any other studies regarding all-cause hospitalizations for patients with GCA and so are unable to compare our findings to previous studies. We hope, however, that this study will inspire similar studies in the future for further analysis of this subject.
Footnotes
The authors do not have any financial conflicts of interest for the work reported herein.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Boesen P, Sørensen SF. Giant cell arteritis, temporal arteritis, and polymyalgia rheumatica in a Danish county: a prospective investigation, 1982–1985. Arthritis Rheum. 1987;30(1):294–299. doi: 10.1002/art.1780300308. [DOI] [PubMed] [Google Scholar]
- 2.Machado EB, Michet CJ, Ballard DJ, Hunder GG, Beard CM, Chu CP, et al. Trends in incidence and clinical presentation of temporal arteritis in Olmsted County, Minnesota, 1950–1985. Arthritis Rheum. 1988;31(6):745–749. doi: 10.1002/art.1780310607. [DOI] [PubMed] [Google Scholar]
- 3.Baldursson O, Steinsson K, Björnsson J, Lie JT. Giant cell arteritis in Iceland: an epidemiologic and histopathologic analysis. Arthritis Rheum. 1994;37(1):1007–12. doi: 10.1002/art.1780370705. [DOI] [PubMed] [Google Scholar]
- 4.Gran JT, Myklebust G. The incidence of polymyalgia rheumatica and temporal arteritis in the county of Aust Agder, south Norway: a prospective study 1987–94. J Rheumatol. 1997;24(1):1739–1743. [PubMed] [Google Scholar]
- 5.Haugeberg G, Paulsen PQ, Bie RB. Temporal arteritis in Vest Agder County in southern Norway: incidence and clinical findings. J Rheumatol. 2000;27(1):2624–2627. [PubMed] [Google Scholar]
- 6.Kermani TA, Schäfer VS, Crowson CS, Hunder GG, Gabriel SE, Matteson EL, et al. Increase in age at onset of giant cell arteritis: a population-based study. Ann Rheum Dis. 2010;69(4):789–781. doi: 10.1136/ard.2009.111005. [DOI] [PubMed] [Google Scholar]
- 7.Evans JM, O’Fallon WM, Hunder GG. Increased incidence of aortic aneurysm and dissection in giant cell (temporal) arteritis: a population-based study. Ann Intern Med. 1995;122(7):502–507. doi: 10.7326/0003-4819-122-7-199504010-00004. [DOI] [PubMed] [Google Scholar]
- 8.Nuenninghoff D, Hunder GG, Christiansen TJH, McClelland R, Matteson EL. Incidence and predictors of large-artery complication (aortic aneurysm, aortic dissection, and/or large-artery stenosis) in patients with giant cell arteritis. a population-based study over 50 years. Arthritis Rheum. 2003;48(12):3522–3531. doi: 10.1002/art.11353. [DOI] [PubMed] [Google Scholar]
- 9.Nuenninghoff D, Hunder GG, Christiansen TJH, McClelland R, Matteson EL. Mortality of large-artery complication (aortic aneurysm, aortic dissection, and/or large-artery stenosis) in patients with giant cell arteritis: a population-based study over 50 years. Arthritis Rheum. 2003;48(12):3532–3537. doi: 10.1002/art.11480. [DOI] [PubMed] [Google Scholar]
- 10.Kermani TA, Warrington KJ, Crowson CS, Ytterberg SR, Hunder GG, Gabriel SE, et al. Large-vessel involvement in giant cell arteritis: a population-based cohort study of the incidence-trends and prognosis. Ann Rheum Dis. 2013;72(12):1989–1994. doi: 10.1136/annrheumdis-2012-202408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Matteson EL, Gold KN, Bloch DA, Hunder GG. Long-term survival of patients with giant cell arteritis in the American College of Rheumatology giant cell arteritis classification criteria cohort. Am J Med. 1996;100:193–196. doi: 10.1016/s0002-9343(97)89458-2. [DOI] [PubMed] [Google Scholar]
- 12.Salvarani C, Crowson CS, O’Fallon WM, Hunder GG, Gabriel SE. Reappraisal of the epidemiology of giant cell arteritis in Olmsted County, Minnesota, over a fifty-year period. Arthritis Rheum. 2004;51(2):264–268. doi: 10.1002/art.20227. [DOI] [PubMed] [Google Scholar]
- 13.St Sauver JL, Grossardt BR, Yawn BP, Melton LJ, 3rd, Rocca WA. Use of a medical records linkage system to enumerate a dynamic population over time: the Rochester epidemiology project. Am J Epidemiol. 2011;173(1):1059–1068. doi: 10.1093/aje/kwq482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rocca WA, Yawn BP, StSauver JL, Grossardt BR, Melton LJ., 3rd History of the Rochester epidemiology project: half a century of medical records linkage in US population. Mayo Clin Proc. 2012;87(12):1202–1213. doi: 10.1016/j.mayocp.2012.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hunder GG, Bloch DA, Michel BA, Stevens MB, Arend WP, Calabrese LH, et al. The American College of Rheumatology 1990 criteria for the classification of giant cell arteritis. Arthritis Rheum. 1990;33(1):1122–1128. doi: 10.1002/art.1780330810. [DOI] [PubMed] [Google Scholar]
- 16.Healthcare Cost and Utilization Project. [Accessed November 11, 2014];HCUPnet. http://hcupnet.ahrq.gov/
- 17.Schmidt J, Kermani TA, Muratore F, Crowson CS, Matteson EL, Warrington KJ. Statin use in giant cell arteritis: a retrospective study. J Rheumatol. 2013;40(6):910–915. doi: 10.3899/jrheum.121150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Udayakumar PD, Chandran AK, Crowson CS, Warrington KJ, Matteson EL. Cardiovascular risk and acute coronary syndrome in giant cell arteritis: a population based retrospective cohort study. Arthritis Care Res. 2015 doi: 10.1002/acr.22416. IN PRESS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Matthews JL, Gilbert DN, Farris BK, Siatkowski RM. Prevalence of diabetes mellitus in biopsy-positive giant cell arteritis. J Neuroophthalmol. 2012;32(3):202–6. doi: 10.1097/WNO.0b013e31825103cb. [DOI] [PubMed] [Google Scholar]
- 20.Tomasson G, Peloquin C, Mohammad A, Love TJ, Zhang Y, Choi HK, et al. Risk for cardiovascular disease early and late after a diagnosis of giant-cell arteritis: a cohort study. Ann Intern Med. 2014;160(2):73–80. doi: 10.7326/M12-3046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Uddhammar A, Eriksson AL, Nyström L, Stenling R, Rantapää-Dahlqvist S. Increased mortality due to cardiovascular disease in patients with giant cell arteritis in northern Sweden. J Rheumatol. 2002;29(4):737–42. [PubMed] [Google Scholar]
- 22.Ray JG, Mamdani MM, Geerts WH. Giant cell arteritis and cardiovascular disease in older adults. Heart. 2005;91(3):324–8. doi: 10.1136/hrt.2004.037481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ji J, Liu X, Sundquist K, Sundquist J, Hemminki K. Cancer risk in patients hospitalized with polymyalgia rheumatica and giant cell arteritis: a follow-up study in Sweden. Rheumatology (Oxford) 2010;49(6):1158–63. doi: 10.1093/rheumatology/keq040. [DOI] [PubMed] [Google Scholar]