Abstract
The discovery of steroid hormone receptors in brain regions that mediate every aspect of brain function has broadened the definition of “neuroendocrinology” to include the reciprocal communication between the brain and the body via hormonal and neural pathways. The brain is the central organ of stress and adaptation to stress because it perceives and determines what is threatening, as well as the behavioral and physiological responses to the stressor. The adult and developing brain possess remarkable structural and functional plasticity in response to stress, including neuronal replacement, dendritic remodeling, and synapse turnover. Stress causes an imbalance of neural circuitry subserving cognition, decision-making, anxiety and mood that can alter expression of those behaviors and behavioral states. This imbalance, in turn, affects systemic physiology via neuroendocrine, autonomic, immune and metabolic mediators. In the short term, as for increased fearful vigilance and anxiety in a threatening environment, these changes may be adaptive. But, if the danger passes and the behavioral state persists along with the changes in neural circuitry, such maladaptation may need intervention with a combination of pharmacological and behavioral therapies, as is the case for chronic anxiety and depression. There are important sex differences in the brain responses to stressors that are in urgent need of further exploration. Moreover, adverse early-life experience, interacting with alleles of certain genes, produce lasting effects on brain and body over the life-course via epigenetic mechanisms. While prevention is most important, the plasticity of the brain gives hope for therapies that take into consideration brain-body interactions.
Introduction
The fundamental discovery of the communication between hypothalamus and pituitary, by Geoffrey Harris, established the basis for understanding brain-body communication via the neuroendocrine system (Harris 1970). As originally conceived and investigated productively with findings of releasing factors in the hypothalamus for pituitary hormones (e.g., (Guillemin 1978, Schally et al 1973, Vale et al 1981), the field of neuroendocrinology has flourished. At the same time, steroid hormones were shown to bind to intracellular receptors that regulate gene expression in tissues such as liver, or the prostate and uterus in the case of sex hormones (Jensen & Jacobson 1962). The focus of steroid hormone feedback to regulate neuroendocrine function was naturally upon the pituitary and the hypothalamus and this important work continues to uncover essential aspects of neuroendocrine regulation (Meites 1992).
The McEwen laboratory entered this field by serendipitously discovering adrenal steroid, and, later, estrogen receptors, in the hippocampal formation of the rat (Gerlach & McEwen 1972, Loy et al. 1988, McEwen & Plapinger 1970, McEwen et al. 1968, Milner et al. 2001) and we, and others, extended these findings to the infrahuman primate brain, as well as to other regions of the brain involved in cognitive and emotional regulation (Gerlach et al. 1976). This has catalyzed studies that look at actions of hormonal feedback on the brain not only to regulate hypothalamic functions but also to influence neurological, cognitive and emotional functions of the whole brain, with translation to the human brain in relation to aging, mood disorders and the impact of the social environment. This article describes research in our, and other laboratories, that redefined neuroendocrinology as a field that also studies two-way brain body communication via the neuroendocrine, autonomic, immune and metabolic systems. This research has uncovered the remodeling of brain architecture mediated by hormones working together with other cellular mediators. These actions occur via epigenetic mechanisms involving both genomic and non-genomic processes over the life course, and there is ongoing translation of the findings in animal models to the human condition, including the effects of adverse early life experiences and the relationship of socioeconomic status and health through the development of the concept of allostatic load.
Receptors outside of the hypothalamus
By administering 3H corticosterone into adrenalectomized rats, we discovered receptors for adrenal steroids in the hippocampal formation of the rat and, later, the rhesus monkey (Gerlach & McEwen 1972, Gerlach et al 1976, McEwen & Plapinger 1970, McEwen et al 1968). Figure 1. Other work revealed such receptors in the hippocampal equivalent in other species, including birds (Dickens et al 2009, McEwen 1976). In retrospect, these findings broadened the perspective that glucocorticoids provided negative feedback control of the HPA axis to include actions of adrenal steroids on other brain functions such as memory, learning, control of mood and other aspects of behavior (McEwen 2010).
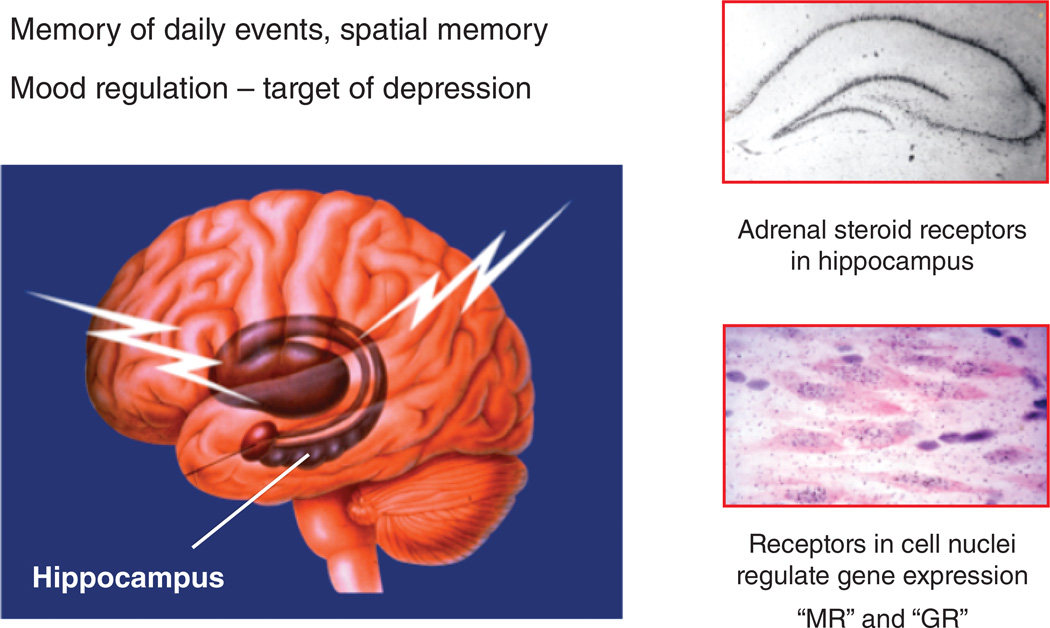
Figure 1.
The hippocampal formation is a target of adrenocortical steroids and is involved in spatial and episodic memory, as well as mood regulation. Both pyramidal neurons in Ammon’s horn and neurons of the dentate express Type I or mineralocorticoid (MR) and Type 2 or glucocorticoid (GR) receptors.
Work by Reul and de Kloet demonstrated that there are two types of adrenal steroid receptors, mineralocorticoid (Type 1 or MR) and glucocorticoid (Type 2 or GR), in hippocampus and other brain regions (Reul & DeKloet 1985). This was further elaborated by immunocytochemical mapping of the receptors (Ahima et al 1991, Ahima & Harlan 1990). Studies in our laboratory, as well as by Diamond and Joëls have shown biphasic effects mediated by MR and GR (Diamond et al 1992, Joels 2006, Pavlides et al 1995). Ultradian fluctuations of glucocorticoids drive GR activation and reactivation, while MR occupancy for nuclear activation is more constant and promotes excitability (Stavreva et al 2009). Moreover, membrane associated GR and MR are linked to the direct stimulation of excitatory amino acid release (Karst et al 2005, Popoli et al 2012).
Much of this information was obtained via studies on the hippocampus, which has then become a gateway into the study of hormone effects on other brain regions involved in cognitive and emotional regulation and other behaviors. The hippocampus is important for episodic and spatial memory and is now also recognized for its role in mood regulation, as will be explained below. For spatial memory (Figure 2), the hippocampus becomes active in London cab drivers during functional MRI imaging when they remember a route from one place to another (Maguire et al 1997) and the hippocampus is also important for food caching behavior in squirrels and birds (Biegler et al 2001, Burger et al 2013, Clayton 2001). The hippocampus is also “the canary in the coal mine” as far as conditions such as ischemia and seizures that cause brain damage as well as brain aging (Sapolsky 1990). And the hippocampus responds to sex hormones with effects on spatial memory and other functions (Sandstrom & Williams 2001), as will be discussed below.
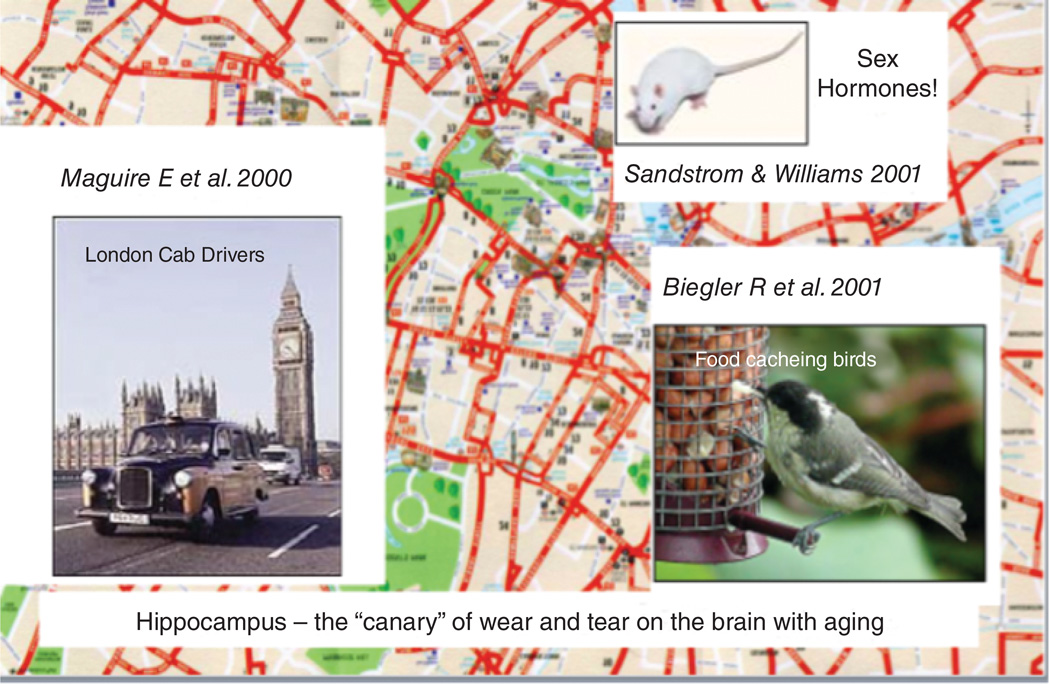
Figure 2.
The hippocampal formation is activated in spatial memory as in the London Cab Driver’s study (Maguire et al 1997), as well as in spatial location of food in food-caching birds and squirrels (see text). The hippocampus is also a target of sex hormones that affect spatial memory (Sandstrom & Williams 2004). The hippocampus is also sensitive to damage by seizures, ischemia and head trauma in which glucocorticoids synergize effects of excitatory amino acid overload (Sapolsky 1990).
Output to multiple interacting mediators and the concept of allostatic load
As originally defined, neuroendocrinology refers to the hypothalamic and pituitary control of neuroendocrine function. The McEwen laboratory has focused upon the return loop of feedback of steroid hormones on the brain to affect molecular, cellular, physiological and behavioral processes throughout the entire brain. This feedback now includes action in brain of metabolic hormones such as insulin, ghrelin, IGF-1 and leptin via specific uptake systems and acting upon receptors residing in hippocampus and other brain regions (for review see (McEwen 2007)). Figure 3. Moreover, given the influence of hormones and autonomic outflow from the brain upon activity of the immune system, the direct and indirect feedback actions of circulating cytokines on the brain must also be considered in a broader definition of neuroendocrinology (Maier & Watkins 1998). Furthermore, the autonomic nervous system itself, both parasympathetic and sympathetic arms, is a partner of neuroendocrine regulation as is here more broadly defined (Sloan et al 1999, Tracey 2002).
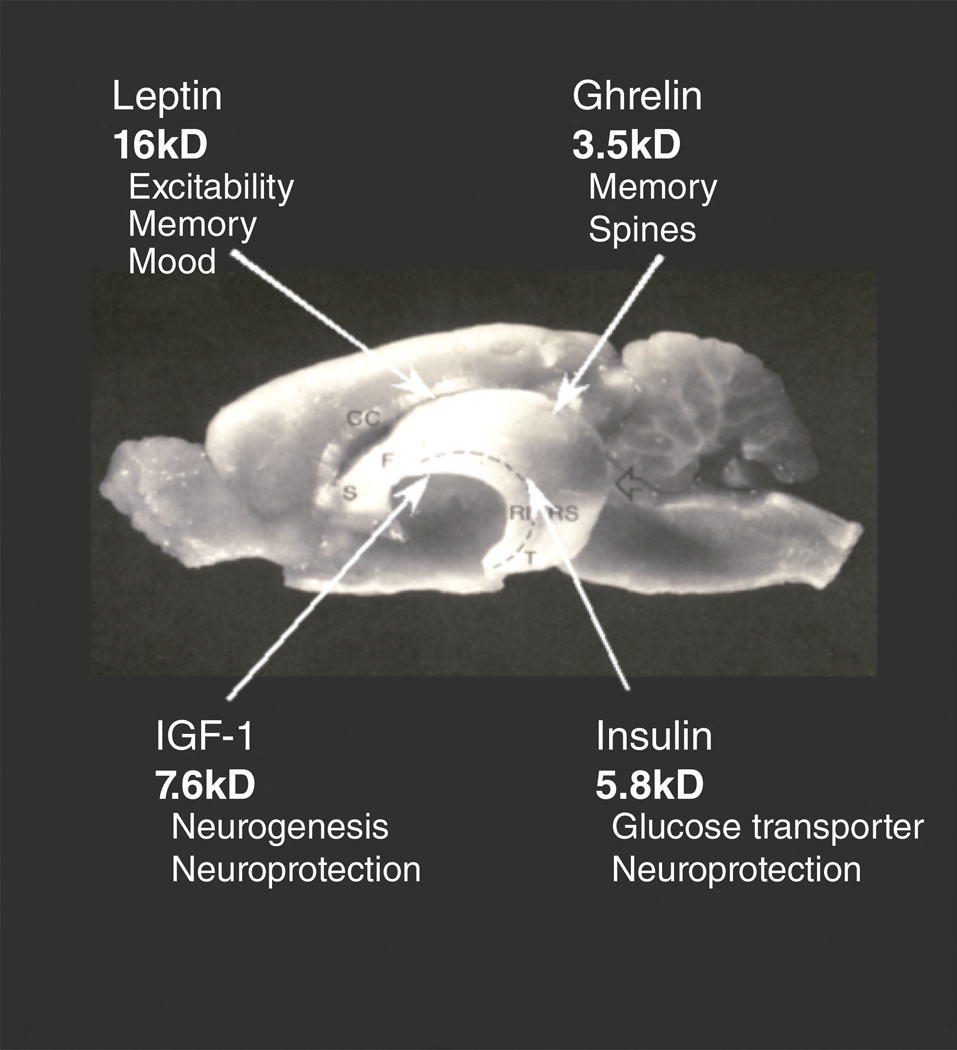
Figure 3.
Four peptide/protein hormones, insulin- like growth factor I (IGF-I), insulin, ghrelin, and leptin, are able to enter the brain and affect structural remodeling or other functions in the hippocampus. A transport process is involved, and specific receptors are expressed in hippocampus, as well as in other brain regions. Molecular sizes are indicated for each hormone in kiloDaltons (kDa): ghrelin, 3.5 kDa; leptin, 16 kDa; insulin, 5.8 kDa; IGF-I, 7.6 kDa. Reprinted from (McEwen 2007).
Within this broader view of neuroendocrinology in relation to brain-body communication, we modified the concept of allostasis (Sterling & Eyer 1988) to refer the active process of maintaining homeostasis via output of hormones and ANS activity and we developed the concept of allostatic load and overload as a means of better understanding the cumulative and potentially damaging, as well as protective, effects of stressors on the brain and body (McEwen 1998, McEwen & Gianaros 2011, McEwen & Stellar 1993, McEwen & Wingfield 2003). Because the mediators of allostasis interact and affect each other’s activity and because each mediator system has biphasic effects in dose and time, the “network of allostasis” is nonlinear (McEwen 2006). When one mediator system changes, the others adjust, and the resulting output can be distorted, as in chronic inflammation or a flat cortisol diurnal rhythm caused by sleep deprivation or depression.
Another important feature of the allostatic load concept is the notion that the mediators that normally help the body and brain adapt to stressors can also become distorted and contribute to cumulative, pathophysiological change such as atherosclerosis or obesity and diabetes (McEwen 1998). Finally, and importantly, the allostatic load concept emphasizes the central role of the brain in response to adaptation to stressors because of its central role in regulating and responding to the broader-defined “neuroendocrine” system (McEwen 1998). Figure 4. Both the physical and social environment contribute experiences that require adaptation of brain architecture and physiological processes (McEwen & Gianaros 2011), as will be discussed later in this review. Quite recently, the epigenetic allostasis concept has introduced another feature of the allostatic load to emphasize the influence of early-life experiences on the development of mood disorders in susceptible individuals and this also points to the key role of MR receptors in the communication with the glutamate system (Nasca et al, 2014), as discussed below.
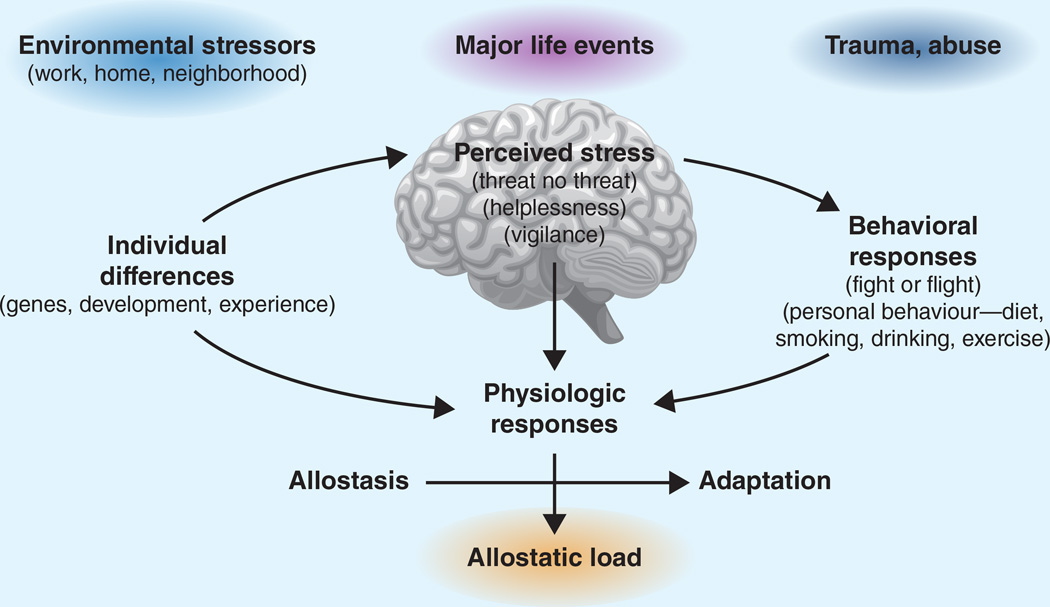
Figure 4.
The Stress Response and Development of Allostatic Load. The perception of stress is influenced by one’s experiences, genetics, and behavior. When the brain perceives an experience as stressful, physiologic and behavioral responses are initiated, leading to allostasis and adaptation. Over time, allostatic load can accumulate, and the overexposure to mediators of neural, endocrine, and immune stress can have adverse effects on various organ systems, leading to disease. Reprinted from (McEwen 1998).
Structural plasticity of brain regions mediating cognition and affect
A remarkable feature of the adult, as well as the developing brain, is its capacity for remodeling of dendrites, turnover of synapses and neurogenesis. We discovered that remodeling of dendrites in the hippocampus in response to chronic stress caused shrinking of dendrites in the CA3 subfield that was also mimicked by chronic glucocorticoid treatment, but also involved mediation by excitatory amino acids and other cellular mediators (McEwen 1999). Figure 5. Similar shrinkage of dendrites was found in medial prefrontal cortex after chronic stress, whereas expansion of dendrites in basolateral amygdala was found under the same conditions (Radley et al 2004, Vyas et al 2002). In hippocampus of hibernating animals, rapid shrinkage of CA3 apical dendrites is seen with onset of hibernation while regrowth of those dendrites occurs within hours of termination of hibernation, suggesting that the cytoskeleton can rapidly depolymerize and repolymerize when needed via a mechanism in which tau phosphorylation is involved (Arendt et al 2003, Magarinos et al 2006).
Figure 5.
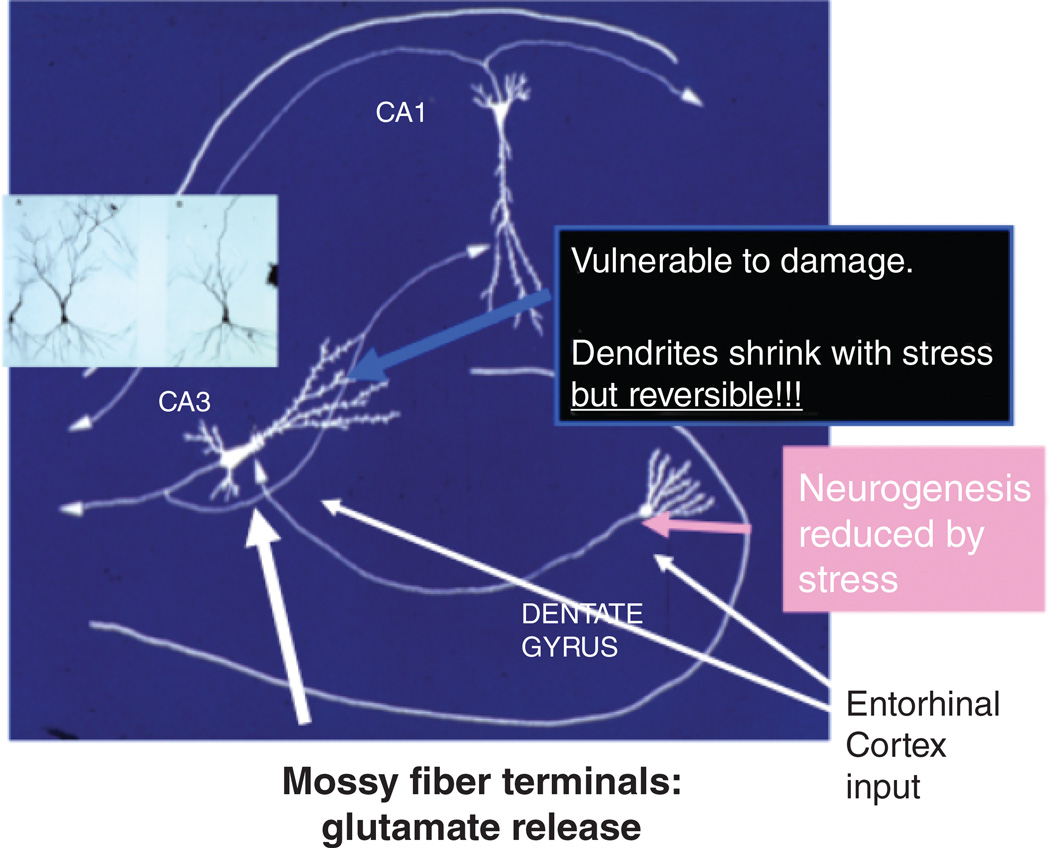
The trisynaptic organization of the hippocampus showing input from the entorhinal cortex to both CA3 and dentate gyrus (DG), with feed forward and feedback connections between these two regions that promotes memory formation in space and time but, at the same time, makes the CA3 vulnerable to seizure-induced excitatory (McEwen 1999). Chronic stress causes apical dendrites of CA3 neurons to debranch and shorten in a reversible manner, and glutamate release by giant mossy fiber terminals is a driving force. Chronic stress also inhibits neurogenesis in DG and can eventually reduce DG neuron number and DG volume.
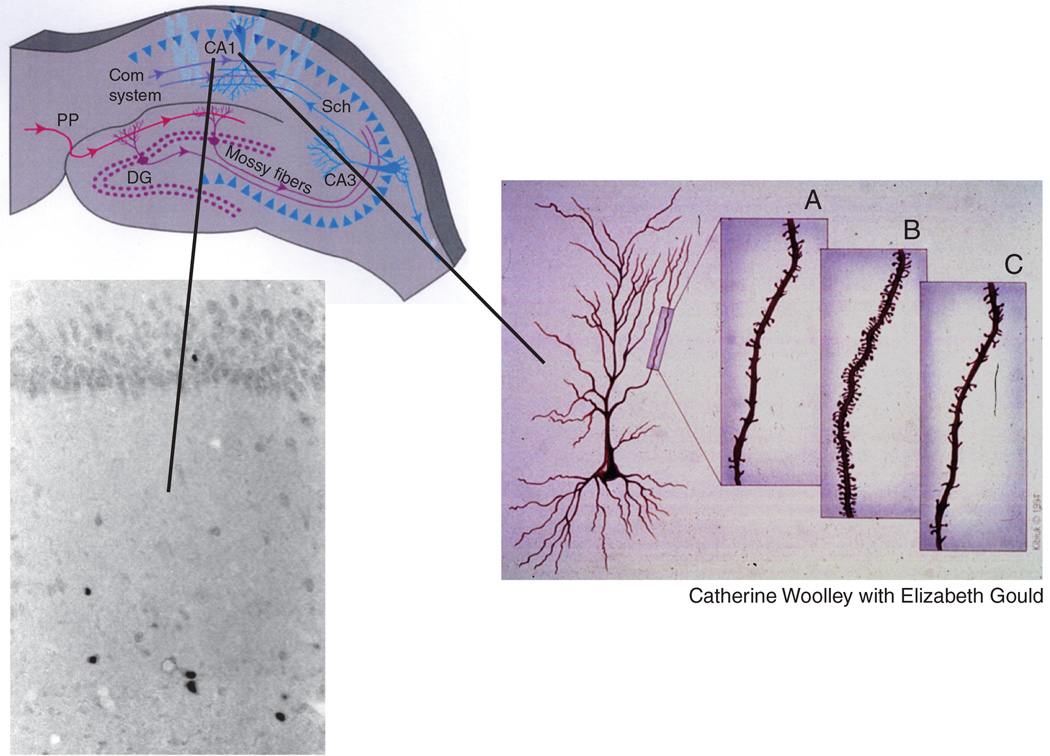
The turnover of spine synapses also occurs in response to stressors and this was shown quite recently in the case of the HPA axis to be dependent on the ultradian pulses of glucocorticoids (Liston et al 2013, Liston & Gan 2011). Chronic stress causes reduced spine density in hippocampus and medial prefrontal cortex, as well as medial amygdala, and increased spine density occurs in basolateral amydala (McEwen & Chattarji 2007). Sex hormones also regulate spine synapse turnover in hippocampus, hypothalamus and prefrontal cortex of female rats and rhesus monkeys by a mechanism involving not only estradiol, but also excitatory amino acids and NMDA receptors (Dumitriu et al 2010, Frankfurt et al 1990, McEwen et al 1995, Woolley 1999). Figure 6.
Figure 6.
Cyclic ovarian function regulates spine synapse turnover in the CA1 regions of the rat hippocampus and it does so via a combination of nuclear and non-nuclear estrogen receptors. The cell nuclear estrogen receptors are found in a subset of inhibitory interneurons whereas the non-nuclear receptors are expressed in presynaptic cholinergic and NPY terminals, in dendrites and mitochondria (Ledoux et al 2009, McEwen & Milner 2007, Nilsen et al 2007).
Structural plasticity also occurs among interneurons involving spine turnover and dendritic remodeling, as well as neurogenesis (Cameron & Dayer 2008, Nacher et al 2013). As first suggested by Altman (Altman 1962), there is turnover and neurogenesis of inhibitory interneurons in the adult cortex occurring at about the same rate as that of granule neurons in the dentate gyrus (Cameron & Dayer 2008). Spine synapse turnover and dendritic remodeling is evident in a class of interneurons that express polysialated neural cell adhesion molecule (PSA-NCAM) and which are widely distributed in the telencephalon of rodents and humans (Nacher et al 2013). Dopamine acting via D2 receptors affects PSA-NCAM expression and some dopamine effects are blocked by the depletion of PSA (Nacher et al 2013).
Neurogenesis in the dentate gyrus of the adult hippocampus was rediscovered by Elizabeth Gould and Heather Cameron based upon earlier work by Altman and Kaplan and findings in the songbird brain by Nottebohm and colleagues (Alvarez-Buylla & Garcia-Verdugo 2002, Cameron & Gould 1994, Gould 2007, Kaplan 2001, Nottebohm 2002). Glucocorticoids and excitatory amino acids are both involved in stress-induced suppression of neurogenesis, which was found not only in rodents, but also in tree shrews and rhesus monkeys (Cameron & Gould 1994, Gould et al 1997, Gould et al 1998). Yet, glucocorticoid levels do not predict the direction of neurogenesis, as shown by studies of male sexual behavior in which increased neurogenesis is found with high glucocorticoid levels; oxytocin appears to play an important role [in glucocorticoid mediated neurogenesis] (Leuner et al 2012, Leuner et al 2010).
Repeated stress in rats has been shown to lead to reduced cell proliferation and neuron number in the dentate gyrus along with reduced dentate gyrus volume (Pham et al 2003). Conversely, physical activity increases neurogenesis and dentate gyrus volume (van Praag et al 1999), as also does living in an enriched environment (Kempermann et al 1997). Hippocampal volume increases in elderly “couch potatoes” who engage in regular, moderate exercise, such as walking (Erickson et al 2011).
Mechanisms of action of glucocorticoids and estrogens
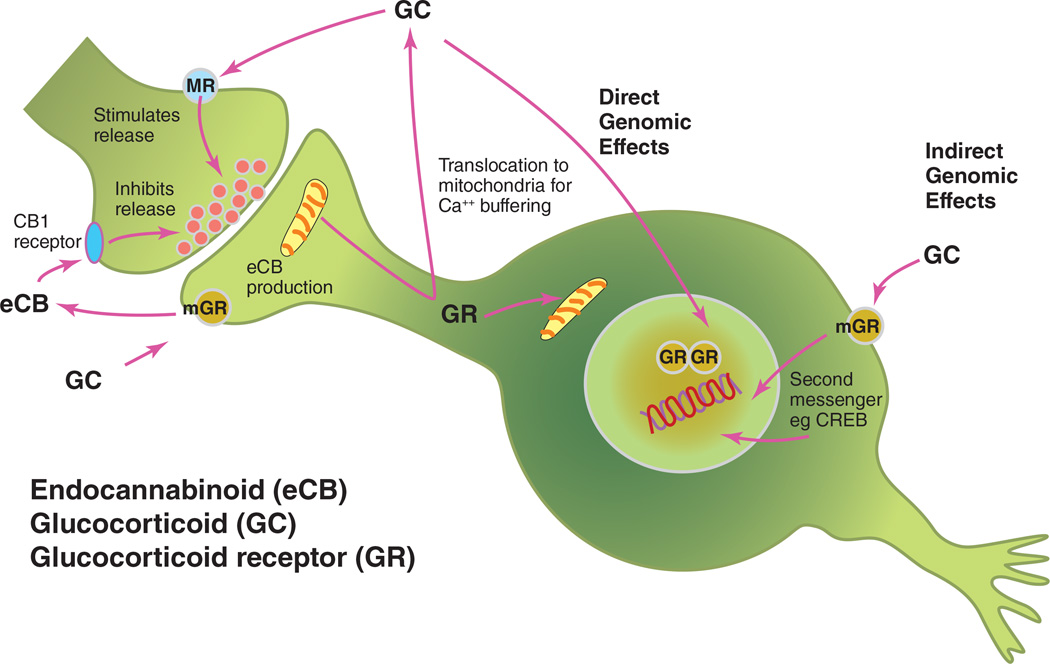
As is now recognized for all steroid hormones, glucocorticoids produce effects on their target cells via both direct and indirect genomic effects, as well as non-genomic actions (Figure 7). Direct actions involve binding of the dimerized GR to the glucocorticoid response element (GRE), whereas indirect genomic actions involve tethering of the GR to other transcription factors such as AP-1, Nf-kB or Stat5 (Ratman et al 2013, Yamamoto 1985). There are also actions of GR on the mitochondrial genome (Du et al 2009a). Non-genomic actions include the stimulation of endocannabinoid production and direct stimulation of glutamate release, as summarized below.
Figure 7.
Glucocorticoids produce both direct and indirect genomic effects, and actions via translocation into mitochondria, as well as direct stimulation of presynaptic glutamate release and other non-genomic actions via signaling pathways that activate endocannabinoid synthesis.
The role of glucocorticoids and estrogens in structural remodeling of the adult brain also involves multiple interacting mediators (McEwen 2010). In the case of stress and adrenal steroids, tissue plasminogen activator (tPA) is involved as a mediator of stress induced changes in medial amygdala and CA1 hippocampal spine density, along with CRH, which is able to stimulate its release (Chen et al 2006a, Pawlak et al 2003, Pawlak et al 2005). Reduced BDNF expression in haploinsufficiency and in the val66met polymorphism is linked to reduced dendritic growth in hippocampus and lack of response to chronic stress (Chen et al 2006b, Magarinos et al 2011), whereas BDNF over-expression is associated with longer dendrites in both hippocampus and amygdala and failure to respond to chronic stress with retraction in hippocampus and elongation in basolateral amygdala (Govindarajan et al 2006). Lipocalin-2 is induced by acute stress and modulates actin dynamics and it down-regulates mushroom spines in hippocampus after 3d restraint stress, while deletion of Lipocalin-2 increases the proportion of mushroom spines along with increased neuronal excitability and anxiety (Mucha et al 2011). In amygdala, 3d restraint stress up-regulates spine density and this effect is lost in lipocalin-2-ko mice (Skrzypiec et al 2013).
Endocannabinoids generated postsynaptically via acute glucocorticoid stimulation inhibit either glutamate or GABA release presynaptically (Hill & McEwen 2010) and this affects not only prefrontal and amygdala control of HPA activity, but also effects of stress on mPFC and basolateral amygdala dendritic branching. Deletion of CB1 receptors exacerbates stress-induced retraction of mPFC dendrites (Hill et al 2011a), whereas deletion of a degradative enzyme, fatty acid amide hydrolase (FAAH), prevents stress induced dendrite expansion in basolateral amygala neurons (Hill et al 2013). Endocannabinoids also play a role in shut-off of HPA function, as well as basal CORT levels after chronic stress and habituation of the CORT response to chronic stress and they appear to do so via the prefrontal cortex and amygdala (Hill et al 2011b).
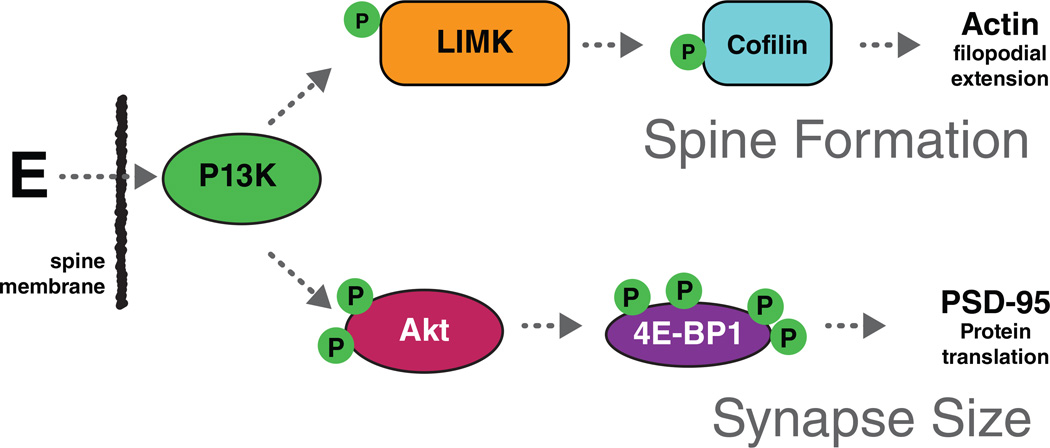
With regard to estrogen actions, there are multiple targets of genomic and non-genomic actions of estradiol. Estradiol stimulates both acetylcholine (Towart et al 2003) and neuropeptide Y release (Ledoux et al 2009) via presynaptic estrogen receptors and it induces actin polymerization and filopodial formation and translation of PSD95 mRNA via PI3kinase (Fig see (Dumitriu et al 2010)). Figure 8. Estradiol stimulated acetylcholine release that inhibit inhibitory interneurons is believed to be responsible for up-regulation of NMDA receptors that are required for estrogen-induced synapse formation (Daniel & Dohanich 2001, Rudick et al 2003, Weiland 1992).
Figure 8.
Non-genomic effects of estrogen. Estrogen initiates a complex set of signal transduction pathways in the hippocampal neuron via several membrane-bound receptors. Above are two examples of estrogen-initiated signal transduction leading to spinogenesis and changes in synapse size. Rapid activation of Akt (protein kinase B) via PI3K is thought to be mediated by ER_. Subsequently, activated Akt initiates translation of PSD-95 by removing the repression of the initiation factor 4E–binding protein1 (4E–BP1). Estradiol-mediated phosphorylation of cofilin has been shown to occur via activation of LIMK. Cofilin is an actin depolymerization factor and it is inactivated by phosphorylation. Therefore, in the presence of estrogen, cofilin repression of actin polymerization is removed, resulting in an increase in filopodial density. The signal transduction pathways illustrated here are an oversimplification of a large body of work done in an in vitro cell line. Reprinted from (Dumitriu et al 2010) by permission.
For both estradiol and glucocorticoids, mitochondria are targets that affect Ca++ sequestration and regulate free radical formation (Brinton 2008, Du et al 2009b). Both ER beta and glucocorticoid receptors translocate to mitochondria where they affect metabolic activity that, at physiological levels, promotes Ca++ sequestration and regulates free radical formation (Rettberg et al 2014) (Moutsatsou et al 2001). At high levels of glucocorticoids, the sequestration mechanism fail and free radicals and oxidative damage takes place (Du et al 2009a).
Sex differences
There are important sex differences in the effects of stress and sex hormones on the hippocampus and prefrontal cortex, extending the seminal work of Harris and Levine (Harris & Levine 1965). Chronic stressors in females do not cause dendrites to shrink in CA3 neurons or in medial prefrontal cortex neurons (Galea et al 1997). In medial prefrontal cortex, neurons that project cortically shrink with chronic stress in males but not in females, whereas neurons that project to the amygdala extend dendrites in females, but not in males, with chronic stress (Shansky et al 2010, Shansky et al 2009). For the females to respond in this way, there must be circulating estrogens (Shansky et al 2009).
That such sex differences exist in a brain region like prefrontal cortex not previously thought to be responsive to sex hormones means that there may be sex difference throughout the brain. Indeed, membrane associated estrogen receptors have been found widely throughout the brain (McEwen & Milner 2007). Studies in men and women of the functional imaging responses of human brain to tests of emotional recognition in which men and women score the same, nevertheless, reveal different patterns of activation across brain regions between the sexes (Derntl et al 2010).
Reversal of sex differences by manipulations during the critical period for sexual differentiation have shown that males treated with an aromatase inhibitor at birth are able to respond to estrogens to induce synapses in the hippocampus, whereas normally males do not respond to estradiol, but do respond to testosterone and dihydrotestosterone for spine synapse induction (Leranth et al 2003, Lewis et al 1995). This is the converse of testosterone treatment of newborn females which defeminizes the ability of ovulation and respond with lordosis (Goy & McEwen 1980). It is important to note that aromatization of testosterone plays a key role in the defeminizing aspects of sexual differentiation postnatally, whereas conversion of testosterone to dihydrotestosterone is involved in masculinizing aspects of brain sexual differentiation that generally occur before birth in the rodent (McEwen et al 1977, Naftolin 1994, Naftolin et al 1971).
Spine synapse induction, that is produced by estradiol in adult female hippocampus and by dihydrotestosterone in male hippocampus, involves somewhat different mechanisms. In the female, as described above, there are multiple mechanisms involving both genomic and non-genomic actions of estradiol and both cholinergic and GABAergic, as well as NMDA receptor mediated activity and estradiol stimulated signaling via PI3kinase (McEwen et al 2001). In the male, where there are genomic androgen receptors in the CA1 region, as well as non-genomic receptors, the cholinergic system does not appear to be involved while androgens upregulate NMDA receptors; this is a topic that needs more in depth investigation (Romeo et al 2005).
Gene expression in an ever-changing brain
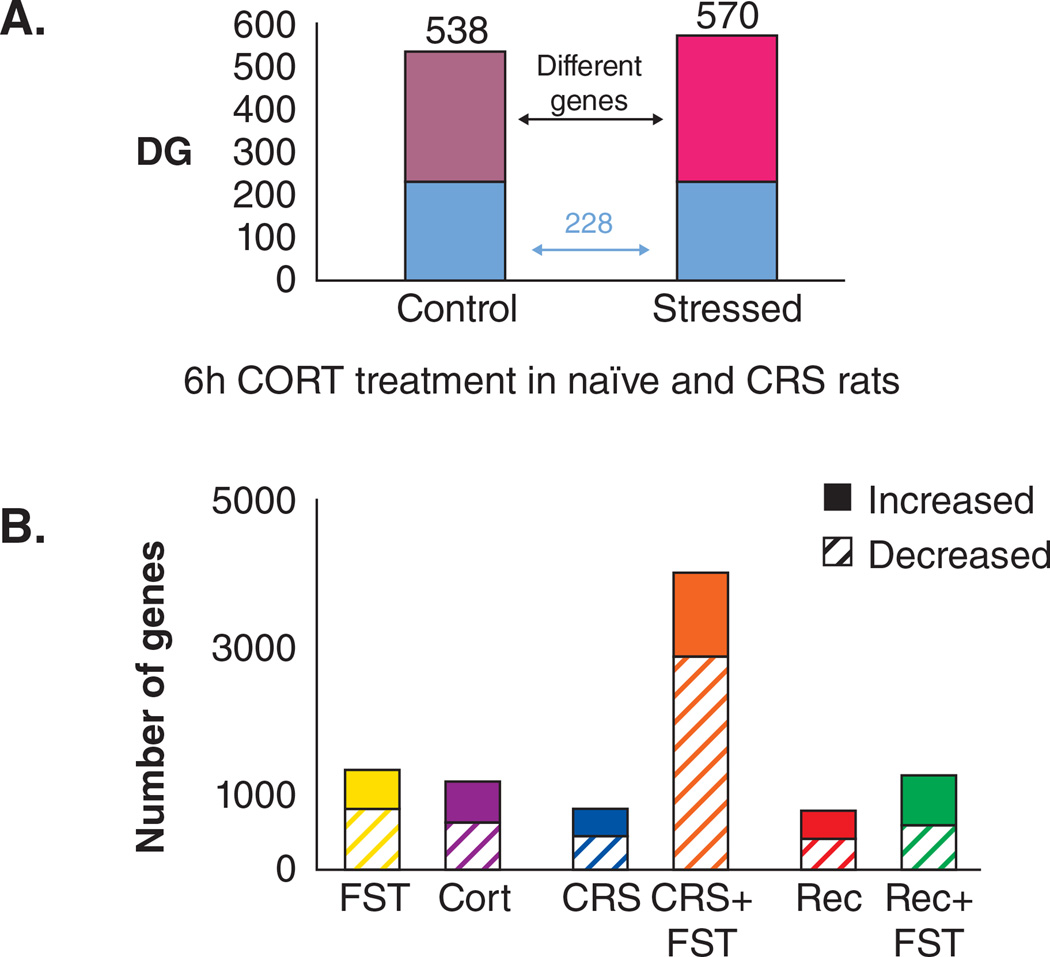
We have found that the expression of genes in the brain is changing continuously with experiences and that novel stressors have different effects upon gene expression in a naïve brain, a chronically stressed brain and a brain recovered from chronic stress. High throughput gene expression profiling of the hippocampal response to an acute forced swim stress revealed a distinct pattern of gene regulation between stress-naïve mice and mice subjected to a forced swim after exposure to 3 weeks of chronic restraint stress (Gray et al 2014). Further, mice allowed three weeks of recovery from chronic stress, which exhibited a normalization of anxiety-like behaviors, still revealed a gene expression profile that was different from the stress naïve state and produced a still different gene expression profile in response to a novel stress. An acute CORT challenge given to either stress naïve or chronically stressed rats also revealed highly different gene expression profiles, depending on the stress history of the animal; while approximately 200 of the genes altered by CORT were the same irrespective of stress history, over 500 were different after a chronic stress exposure. (Datson et al 2013). Figure 9. Together, these studies suggest that while the brain can recover, there remains considerable imprint of past stress experiences which alters future reactivity at the molecular level.
Figure 9.
Effects of stress and acute glucocorticoid treatment on gene expression in hippocampus. A. Naïve and 21d chronically restraint stressed (CRS) rats respond differently to a 6h bolus of corticosterone in which more than half of the genes turned on or turned off are different (Datson et al 2013). B. Naïve mice given acute forced swim stress (FST) show a largely different pattern of gene expression (up and down) from naïve mice given an acute corticosterone bolus. Moreover, mice that are either naïve, or 21d CRS or 21d CRS plus 21d recovery respond, in large, differently to acute FST with respect to gene expression levels. There is a core of genes that always respond to the acute FST. (Gray et al 2014).
Yet a CORT challenge is not equivalent to replicating the complex network of pathways activated during an in vivo stress exposure. Expression profiles resulting from a bolus of corticosterone were found to be highly distinct from that of an acute stressor that elevates CORT (Gray et al 2014). Figure 9. While many of the well-established genes, such as c-Fos and Arc responded the same, there remains a widely unexplored set of new gene targets that have been identified, which are activated by stress, but function outside of traditional CORT or inflammatory signaling pathways.
Epigenetic regulation: search for rapidly acting treatments
Although the action of steroid hormones on cellular processes involves both genomic and non-genomic mechanisms of action, the cumulative actions of the interacting mediators results in changes in gene expression via epigenetic mechanisms involving histone modifications, methylation of cytosine bases on DNA, and the regulatory actions of non-coding RNA’s (Mehler 2008). Regarding histone modifications that either repress gene expression and DNA activity or to enhance such activity, Reul and colleagues have shown that the forced swimming-induced behavioral immobility response requires histone H3 phospho-acetylation and c-Fos induction in distinct dentate granule neurons through recruitment of the NMDA/ERK/MSK 1/2 pathway (Chandramohan et al 2008).
Another histone mark change in hippocampus, and most prominently in the dentate gyrus, is the dramatic induction by an acute restraint stress of trimethylation of lysine 9 on histone H3, which is associated with repression of a number of retrotransposon elements and reduction of the coding and non-coding RNA normally produced by the repressed DNA (Hunter et al 2009). This repression is lost with repeated stress, suggesting the possibility that those retrotransposon elements may impair genomic stability under conditions of chronic stress (Hunter et al 2014).
A current practical application of this is the investigation of rapidly acting antidepressants, because classical antidepressants work slowly and are not effective on every depressed individual. In the course of these studies, we are learning more about epigenetic mechanisms that connect excitatory amino acid function with neural remodeling and stress-related behavior. One novel agent is acetyl-L-carnitine (LAC) that decreases depressive-like behavior within 3 days of treatment in a stress-induced and a genetic model of depression-like behavior while SSRI’s and tricyclic drugs have no effect in that time frame (Nasca et al 2013). Antidepressant effects of LAC have been shown in other animal models that mimic features of the spectrum of depressive disorders in humans (Cuccurazzu et al 2013) and need to be expanded to human treatment resistant depression (Flight 2013, Russo & Charney 2013). The rapid antidepressant action of LAC is mediated by acetylation of the histone H3K27 bound to the promoter gene of the metabotropic glutamate receptor, mGlu2, which inhibits glutamate release to the synapse. Furthermore, a single injection of the HDAC inhibitor, MS-275, mimicked the action of LAC in enhancing mGlu2 receptor expression in FSL rats. Among other mechanisms, LAC also promotes acetylation of the p65, the major component of the NFkB transcription factor, to exert fast antidepressant responses (Cuccurazzu et al 2013). In the course of this work, we become aware that lower mGlu2 expression in hippocampus increases the vulnerability to stress (Nasca et al 2014), as discussed below.
Translation to the human brain
The revelations about how acute and chronic stress affect the brain in animal models has been used by researchers and clinicians to show changes in function within the human brain that result from stress and trauma and correspond to effects seen in the research on animal models (McEwen 2007, McEwen & Gianaros 2011, McEwen & Morrison 2013). These include changes in brain structure and functional activity in depression, PTSD, Cushing’s disease and Type 2 diabetes, as well as effects of jet lag and shift work, chronic life stress, perceived stress and the beneficial effects of physical activity (McEwen & Gianaros 2011, Sheline 2003). For perceived stress, medical students who had high scores on the 10 item perceived stress scale showed impaired functional connectivity by fMRI in a brain circuit involving the prefrontal cortex, as well as impaired performance on a test of mental flexibility (Liston et al 2009); these effects were reversed by a month vacation and we know from animal studies that prefrontal cortical and hippocampal dendrite shrinkage is reversible in young adult animals (Conrad et al 1999, Radley et al 2005). Regarding physical activity, previously sedentary older adults who walk an hour a day for 6 months to a year show enlargement of the hippocampal formation (Erickson et al 2011) and this is likely due, at least in part, to the increased dentate gyrus neurogenesis that is stimulated by exercise and by an enriched environment (Kempermann et al 1997, van Praag et al 1999). It is also noteworthy that hippocampal volume increases with intense learning (Draganski et al 2006), but is also decreased in Cushing’s disease (Starkman et al 1992).
However, there is age-related loss of resilience of the dendrite shrinkage in prefrontal cortex (Bloss et al 2010), as well as age-related memory impairment, which, however, can be reduced by pharmacological intervention (Bloss et al 2008, Pereira et al 2014). These treatments may find their way into treating human mild cognitive impairment and perhaps also dementia.
Another translational application of structural plasticity and the actions of stress mediators is the somewhat surprising role of glucocorticoid elevation at the time of trauma in reducing the risk for post traumatic stress disorder (PTSD) (Rao et al 2012, Schelling et al 2004, Zohar et al 2011). One possibility is that glucocorticoid stimulation of endocannabinoid production may be involved in this protection (Hill & McEwen 2009).
Individual differences, stressful early life events and the life course perspective
What happens early in life determines the trajectory of development for the rest of the individual’s life and biomedical science and medical practice are beginning to recognize this (Halfon et al 2014). The original definition of epigenetics that referred to the emergence of characteristics of an organism with development (Waddington 1942) implied that there was no turning back, but that each stage of development offers possibilities to change the trajectory of brain and body function.
Adverse childhood experiences (ACE) produce a lifelong vulnerability to mental and physical health disorders and prevention is paramount (Anda et al 2010, Felitti et al 1998, Shonkoff et al 2009). Where adverse events have happened, it is important to find ways of compensatory remediation. This is an enormous challenge and the newer use of the term “epigenetics”, meaning “above the genome” and referring to the ability to change expression of genetic traits via physical, behavioral and pharmacological intervention, as described above, offers some hope that the brain and body remain dynamic over the lifespan (Bavelier et al 2010, McEwen 2012).
Even without adversity in childhood, individuals with the same genes turn out differently and this is reflected in divergent epigenetic profiles of CpG methylation patters of chromosomes of identical twins as they age (Fraga et al 2005). Cloned mice raised in an enriched environment develop differences in locomotor activity correlated with levels of dentate gyrus neurogenesis (Freund et al 2013). And genetically similar rats screened for anxiety-like behavior early in life show consistent individual anxiety profiles over their life course, and the more anxious rats die 200 days sooner than the less anxious ones (Cavigelli & McClintock 2003, Cavigelli et al 2006). Reduced prefrontal cortical dendrite length and branching is a neuroanatomical feature of elevated anxiety-like behavior in rats (Miller et al 2012).
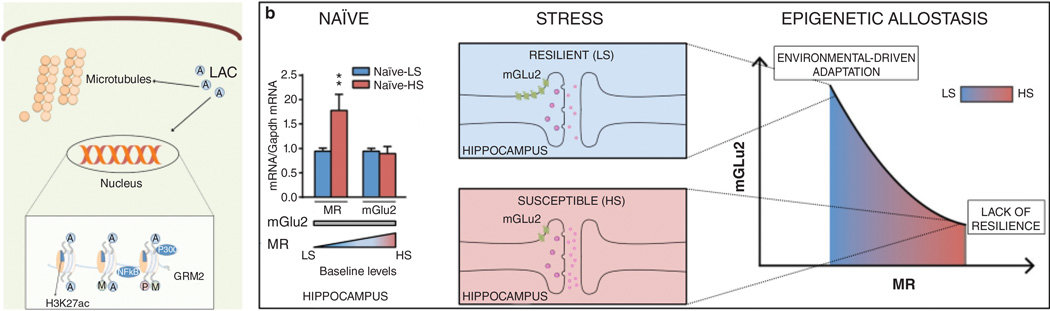
Arising out of the studies of the antidepressant-like actions of LAC is new insight into at least one possible mechanism involving mineralocorticoid receptors in hippocampus by which individual differences arise in susceptibility to stressors for developing anxiety- and depressive-like behaviors. Mice with a mGlu2 knock-out subjected to chronic unpredictable stressors show more signs of coat deterioration, reduced body weight and increased immobility at the forced swim test compared to wilt-type animals subjected to the same regimen of stress (Nasca et al 2014). In wild-type mice, a simple light-dark test revealed a subset with higher anxiety that have elevated hippocampal mineralocorticoid receptors (MR). Those mice with higher MR showed a greater stress-induced reduction in mGlu2 accompanied by more anxiety and depressive-like behaviors; this effect is mediated by a stress-induced reduction of the epigenetic enzyme P300, which regulates acetylation of the histone H3K27 that promotes mGlu2 expression (Nasca et al 2014).
The ability of MR activation to mediate enhanced anxiety and depression-like behavior after acute and chronic stress by down-regulating mGlu2 is consistent with evidence showing a role of MR in anxiety-like behavior (Korte et al 1995) in hippocampus (Bitran et al 1998, Smythe et al 1997) and particularly the ventral hippocampus (McEown & Treit 2011). Despite high baseline cortisol levels, patients with major depression show high functional activity of the MR system along with decreased sensitivity to GR agonists, suggesting an imbalance in the MR/GR ratio (Young et al 2003). Indeed, the MR/GR balance is important not only for emotional regulation but also for cognitive function and HPA regulation (de Kloet 2014).
The epigenetic allostasis model in Figure 10 proposes that early-life epigenetic influences, program each individual to different trajectories of behavioral and physiological responses to later stressful life events, and it remains to be determined whether the higher MR-levels reflect epigenetic influences of maternal care or other experiences early in life. Indeed, the role of consistent and disrupted maternal care, as well as prenatal stress, have have been investigated in animal models (Akers et al 2008, Francis et al 1999, Maccari & Morley-Fletcher 2007, Molet et al 2014, Moriceau & Sullivan 2006, Weinstock 2005) and should be considered.
Figure 10.
Novel mechanisms for rapidly acting medications to treat stress-related disorders. a, The novel antidepressant candidate acetyl-L-carnitine (LAC) may act inside and outside the nucleus to exert fast antidepressant responses: it has been shown that LAC corrects mGlu2 deficits in vulnerable animal models by increasing acetylation of either the histone H3K27 bound to Grm2 promoter gene or the NFkB-p65 member (Nasca et al 2013) b, The use of the light-dark test as a screening method allows identification of clusters of animals with a different baseline susceptibility along with differences in mineralocorticoid receptor (MR) levels in hippocampus. The susceptible mice that are characterized by higher baseline MR levels show reduced hippocampal mGlu2 expression associated with exacerbation of anxious and of depressive-like behaviors after acute and chronic stress, respectively. Conversely, individuals with lower baseline MR levels cope better with stress and show adaptation in mGlu2 receptor expression in hippocampus. The epigenetic allostasis model points to the developmental origins of these individual differences, suggesting that unknown epigenetic influences early in life may lead to alterations in MR hippocampal levels (Nasca et al 2014)
Impact of the social environment
The emerging field of epigenetics, along with the reversible remodeling of brain architecture, has provided a new way of conceptualizing the influence of the social and physical environment on the brain and body. As shown in Figure 4, the brain is the central organ of stress and adaptation because it determines what is threatening and, therefore, stressful. And the brain controls autonomic and neuroendocrine signals that affect the rest of the body to promote adaptation (“allostasis”) and also allostatic load and overload (McEwen 1998, McEwen & Stellar 1993, McEwen & Wingfield 2003). Health behaviors (“lifestyle”), including choice and amount of food intake, smoking, alcohol intake, physical activity, or lack thereof, and social interactions also feed into and contribute to allostasis and allostatic load/overload (McEwen 2006). Finally, the genetic endowment and experiential factors throughout the life course, but especially early in life, influence the trajectory of brain and body function (Danese & McEwen 2012, Halfon et al 2014).
Low socioeconomic status is associated with increased risk for the common diseases of modern life and is associated with increased inflammatory tone and altered white matter structure (Gianaros et al 2013, Seeman et al 2010). Likewise, Type 2 diabetes, which is more common at lower levels of SES, is also associated with altered myelin and impaired cognitive function (Yau et al 2012). On the positive side, meaning and purpose in life appear to have a considerable ability to promote health and ward off cognitive decline, including dementia (Boyle et al 2010, Carlson et al 2009). Likewise, regular physical activity has many benefits for brain and body health (Colcombe et al 2004).
Conclusions
With the discovery of circulating hormone actions throughout the brain on virtually every aspect of brain function, the original definition of “neuroendocrinology” based upon the work of Geoffrey Harris has expanded to encompass many aspects of reciprocal brain-body communication. With the new appreciation of the life-course perspective for human health and disease, along with the emerging field of gene x environment interactions now called “epigenetics” (Halfon et al 2014), the reciprocal communication between the brain and body via hormonal and neural mediators takes a central role in facilitating progress in understanding how the social and physical environment “gets under the skin” to alter trajectories of health and disease. Given the central role of the brain, there is now impetus for interventions that involve policies of government and the private sector, as well as psychosocial interventions at the individual level that produce a “top-down” improvement in the physiological processes that are dysregulated by stress and adversity (Acheson 1998). The emerging recognition of the ability of the brain to change its architecture and function via these top-down interventions involving brain-body communication, where pharmaceutical agents or behavioral interventions that open up “windows of plasticity,” gives hope for redirecting individual trajectories towards better physical and mental health.
Acknowledgements
Research is supported by RO1 MH41256 from NIH, by the Hope for Depression Research Foundation and by the American Foundation for Suicide Prevention to Carla Nasca and NRSA Award #F32 MH102065 to Jason Gray.
Footnotes
Declaration of interest: nothing to declare
References
- Acheson SD. Independent Inquiry into Inequalities in Health Report. London: The Stationary Office; 1998. [Google Scholar]
- Ahima R, Krozowski Z, Harlan R. Type I corticosteroid receptor-like immunoreactivity in the rat CNS: distribution and regulation by corticosteroids. J.Comp.Neurol. 1991;313:522–538. doi: 10.1002/cne.903130312. [DOI] [PubMed] [Google Scholar]
- Ahima RS, Harlan RE. Charting of type II glucocorticoid receptor-like immunoreactivity in the rat central nervous system. Neuroscience. 1990;39:579–604. doi: 10.1016/0306-4522(90)90244-x. [DOI] [PubMed] [Google Scholar]
- Akers KG, Yang Z, DelVecchio DP, Reeb BC, Romeo RD, et al. Social competitiveness and plasticity of neuroendocrine function in old age: influence of neonatal novelty exposure and maternal care reliability. PLoS ONE. 2008;3(7):e2840. doi: 10.1371/journal.pone.0002840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altman J. Are new neurons formed in the brains of adult mammals? Science. 1962;135:1127–1128. doi: 10.1126/science.135.3509.1127. [DOI] [PubMed] [Google Scholar]
- Alvarez-Buylla A, Garcia-Verdugo JM. Neurogenesis in adult subventricular zone. J. Neurosci. 2002;22:629–634. doi: 10.1523/JNEUROSCI.22-03-00629.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anda RF, Butchart A, Felitti VJ, Brown DW. Building a framework for global surveillance of the public health implications of adverse childhood experiences. Am J Prev Med. 2010;39:93–98. doi: 10.1016/j.amepre.2010.03.015. [DOI] [PubMed] [Google Scholar]
- Arendt T, Stieler J, Strijkstra AM, Hut RA, Rudiger J, et al. Reversible paired helical filament-like phosphorylation of tau is an adaptive process associated with neuronal plasticity in hibernating animals. J. Neurosci. 2003;23:6972–6981. doi: 10.1523/JNEUROSCI.23-18-06972.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bavelier D, Levi DM, Li RW, Dan Y, Hensch TK. Removing brakes on adult brain plasticity: from molecular to behavioral interventions. J Neurosci. 2010;30:14964–14971. doi: 10.1523/JNEUROSCI.4812-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biegler R, McGregor A, Krebs JR, Healy SD. A larger hippocampus is associated with longer-lasting spatial memory. Proc. Natl. Acad. Sci. USA. 2001;98:6941–6944. doi: 10.1073/pnas.121034798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bitran D, Shiekh M, Dowd JA, Dugan MM, Renda P. Corticosterone is permissive to the anxiolytic effect that results from the blockade of hippocampal mineralocorticoid receptors. Pharmacology, biochemistry, and behavior. 1998;60:879–887. doi: 10.1016/s0091-3057(98)00071-9. [DOI] [PubMed] [Google Scholar]
- Bloss EB, Hunter RG, Waters EM, Munoz C, Bernard K, McEwen BS. Behavioral and biological effects of chronic S18986, a positive AMPA receptor modulator, during aging. Exp. Neurol. 2008;210:109–117. doi: 10.1016/j.expneurol.2007.10.007. [DOI] [PubMed] [Google Scholar]
- Bloss EB, Janssen WG, McEwen BS, Morrison JH. Interactive effects of stress and aging on structural plasticity in the prefrontal cortex. J Neurosci. 2010;30:6726–6731. doi: 10.1523/JNEUROSCI.0759-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyle PA, Buchman AS, Barnes LL, Bennett DA. Effect of a purpose in life on risk of incident Alzheimer disease and mild cognitive impairment in community-dwelling older persons. Arch Gen Psychiatry. 2010;67:304–310. doi: 10.1001/archgenpsychiatry.2009.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinton RD. The healthy cell bias of estrogen action: mitochondrial bioenergetics and neurological implications. Trends Neurosci. 2008;31:529–537. doi: 10.1016/j.tins.2008.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burger DK, Saucier JM, Iwaniuk AN, Saucier DM. Seasonal and sex differences in the hippocampus of a wild rodent. Behav Brain Res. 2013;236:131–138. doi: 10.1016/j.bbr.2012.08.044. [DOI] [PubMed] [Google Scholar]
- Cameron HA, Dayer AG. New interneurons in the adult neocortex: small, sparse, but significant? Biol Psychiatry. 2008;63:650–655. doi: 10.1016/j.biopsych.2007.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron HA, Gould E. Adult neurogenesis is regulated by adrenal steroids in the dentate gyrus. Neuroscience. 1994;61:203–209. doi: 10.1016/0306-4522(94)90224-0. [DOI] [PubMed] [Google Scholar]
- Carlson MC, Erickson KI, Kramer AF, Voss MW, Bolea N, et al. Evidence for neurocognitive plasticity in at-risk older adults: the experience corps program. The journals of gerontology. Series A, Biological sciences and medical sciences. 2009;64:1275–1282. doi: 10.1093/gerona/glp117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavigelli SA, McClintock MK. Fear of novelty in infant rats predicts adult corticosterone dynamics and an early death. Proc. Natl. Acad. Sci. USA. 2003;100:16131–16136. doi: 10.1073/pnas.2535721100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavigelli SA, Yee JR, McClintock MK. Infant temperament predicts life span in female rats that develop spontaneous tumors. Horm. Behav. 2006;50:454–4562. doi: 10.1016/j.yhbeh.2006.06.001. [DOI] [PubMed] [Google Scholar]
- Chandramohan Y, Droste SK, Arthur JS, Reul JM. The forced swimming-induced behavioural immobility response involves histone H3 phospho-acetylation and c-Fos induction in dentate gyrus granule neurons via activation of the N-methyl-D-aspartate/extracellular signal-regulated kinase/mitogen- and stress-activated kinase signalling pathway. Eur J Neurosci. 2008;27:2701–2713. doi: 10.1111/j.1460-9568.2008.06230.x. [DOI] [PubMed] [Google Scholar]
- Chen Y, Fenoglio KA, Dube CM, Grigoriadis DE, Baram TZ. Cellular and molecular mechanisms of hippocampal activation by acute stress are age-dependent. Mol. Psychiat. 2006a:1–12. doi: 10.1038/sj.mp.4001863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z-Y, Jing D, Bath KG, Ieraci A, Khan T, et al. Genetic variant BDNF (Val66Met) polymorphism alters anxiety-related behavior. Science. 2006b;314:140–143. doi: 10.1126/science.1129663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clayton NS. Hippocampal growth and maintenance depend on food-caching experience in juvenile mountain chickadees (Poecile gambeli) Behav. Neurosci. 2001;115:614–625. doi: 10.1037//0735-7044.115.3.614. [DOI] [PubMed] [Google Scholar]
- Colcombe SJ, Kramer AF, McAuley E, Erickson KI, Scalf P. Neurocognitive aging and cardiovascular fitness. J. Mol. Neurosci. 2004;24:9–14. doi: 10.1385/JMN:24:1:009. [DOI] [PubMed] [Google Scholar]
- Conrad CD, Magarinos AM, LeDoux JE, McEwen BS. Repeated restraint stress facilitates fear conditioning independently of causing hippocampal CA3 dendritic atrophy. Behav. Neurosci. 1999;113:902–913. doi: 10.1037//0735-7044.113.5.902. [DOI] [PubMed] [Google Scholar]
- Cuccurazzu B, Bortolotto V, Valente MM, Ubezio F, Koverech A, et al. Upregulation of mGlu2 receptors via NF-kappaB p65 acetylation is involved in the Proneurogenic and antidepressant effects of acetyl-L-carnitine. Neuropsychopharmacology. 2013;38:2220–2230. doi: 10.1038/npp.2013.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danese A, McEwen BS. Adverse childhood experiences, allostasis, allostatic load, and age-related disease. Physiol Behav. 2012;106:29–39. doi: 10.1016/j.physbeh.2011.08.019. [DOI] [PubMed] [Google Scholar]
- Daniel JM, Dohanich GP. Acetylcholine mediates the estrogen-induced increase in NMDA receptor binding in CA1 of the hippocampus and the associated improvement in working memory. J. Neurosci. 2001;21:6949–6956. doi: 10.1523/JNEUROSCI.21-17-06949.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datson NA, van den Oever JM, Korobko OB, Magarinos AM, de Kloet ER, McEwen BS. Previous history of chronic stress changes the transcriptional response to glucocorticoid challenge in the dentate gyrus region of the male rat hippocampus. Endocrinology. 2013;154:3261–3272. doi: 10.1210/en.2012-2233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Kloet ER. From receptor balance to rational glucocorticoid therapy. Endocrinology. 2014;155:2754–2769. doi: 10.1210/en.2014-1048. [DOI] [PubMed] [Google Scholar]
- Derntl B, Finkelmeyer A, Eickhoff S, Kellermann T, Falkenberg DI, et al. Multidimensional assessment of empathic abilities: neural correlates and gender differences. Psychoneuroendocrinology. 2010;35:67–82. doi: 10.1016/j.psyneuen.2009.10.006. [DOI] [PubMed] [Google Scholar]
- Diamond DM, Bennett MC, Fleshner M, Rose GM. Inverted-U relationship between the level of peripheral corticosterone and the magnitude of hippocampal primed burst potentiation. Hippocampus. 1992;2:421–430. doi: 10.1002/hipo.450020409. [DOI] [PubMed] [Google Scholar]
- Dickens M, Romero LM, Cyr NE, Dunn IC, Meddle SL. Chronic stress alters glucocorticoid receptor and mineralocorticoid receptor mRNA expression in the European starling (Sturnus vulgaris) brain. J Neuroendocrinol. 2009;21:832–840. doi: 10.1111/j.1365-2826.2009.01908.x. [DOI] [PubMed] [Google Scholar]
- Draganski B, Gaser C, Kempermann G, Kuhn HG, Winkler J, et al. Temporal and spatial dynamics of brain structure changes during extensive learning. J Neurosci. 2006;26:6314–6317. doi: 10.1523/JNEUROSCI.4628-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du J, McEwen BS, Manji HK. Glucocorticoid receptors modulate mitochondrial function. Commun. & Integr. Biol. 2009a;2:1–3. doi: 10.4161/cib.2.4.8554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du J, Wang Y, Hunter R, Wei Y, Blumenthal R, et al. Dynamic regulation of mitochondrial function by glucocorticoids. Proc. Natl. Acad. Sci. USA. 2009b;106:3543–3548. doi: 10.1073/pnas.0812671106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumitriu D, Rapp PR, McEwen BS, Morrison JH. Estrogen and the aging brain: an elixir for the weary cortical network. Ann N Y Acad Sci. 2010;1204:104–112. doi: 10.1111/j.1749-6632.2010.05529.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson KI, Voss MW, Prakash RS, Basak C, Szabo A, et al. Exercise training increases size of hippocampus and improves memory. Proc Natl Acad Sci U S A. 2011;108:3017–3022. doi: 10.1073/pnas.1015950108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felitti VJ, Anda RF, Nordenberg D, Williamson DF, Spitz AM, et al. Relationship of childhood abuse and household dysfunction to many of the leading causes of death in adults. The adverse childhood experiences (ACE) study. Am.J.Prev.Med. 1998;14:245–258. doi: 10.1016/s0749-3797(98)00017-8. [DOI] [PubMed] [Google Scholar]
- Flight MH. Antidepressant epigenetic action. Nat Rev Neurosci. 2013;14:226. doi: 10.1038/nrn3466. [DOI] [PubMed] [Google Scholar]
- Fraga MF, Ballestar E, Paz MF, Ropero S, Setien F, et al. Epigenetic differences arise during the lifetime of monozygotic twins. Proc. Natl. Acad. Sci. USA. 2005;102:10604–10609. doi: 10.1073/pnas.0500398102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francis D, Diorio J, Liu D, Meaney MJ. Nongenomic transmission across generations of maternal behavior and stress responses in the rat. Science. 1999;286:1155–1158. doi: 10.1126/science.286.5442.1155. [DOI] [PubMed] [Google Scholar]
- Frankfurt M, Gould E, Wolley C, McEwen BS. Gonadal steroids modify dendritic spine density in ventromedial hypothalamic neurons: a golgi study in the adult rat. Neuroendo. 1990;51:530–535. doi: 10.1159/000125387. [DOI] [PubMed] [Google Scholar]
- Freund J, Brandmaier AM, Lewejohann L, Kirste I, Kritzler M, et al. Emergence of individuality in genetically identical mice. Science. 2013;340:756–759. doi: 10.1126/science.1235294. [DOI] [PubMed] [Google Scholar]
- Galea LAM, McEwen BS, Tanapat P, Deak T, Spencer RL, Dhabhar FS. Sex differences in dendritic atrophy of CA3 pyramidal neurons in response to chronic restraint stress. Neuroscience. 1997;81:689–697. doi: 10.1016/s0306-4522(97)00233-9. [DOI] [PubMed] [Google Scholar]
- Gerlach J, McEwen BS. Rat brain binds adrenal steroid hormone: radioautography of hippocampus with corticosterone. Science. 1972;175:1133–1136. doi: 10.1126/science.175.4026.1133. [DOI] [PubMed] [Google Scholar]
- Gerlach J, McEwen BS, Pfaff DW, Moskovitz S, Ferin M, et al. Cells in regions of rhesus monkey brain and pituitary retain radioactive estradiol, corticosterone and cortisol differently. Brain Res. 1976;103:603–612. doi: 10.1016/0006-8993(76)90463-7. [DOI] [PubMed] [Google Scholar]
- Gianaros PJ, Marsland AL, Sheu LK, Erickson KI, Verstynen TD. Inflammatory pathways link socioeconomic inequalities to white matter architecture. Cereb Cortex. 2013;23:2058–2071. doi: 10.1093/cercor/bhs191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould E. How widespread is adult neurogenesis in mammals? Nature Rev. Neuroscience. 2007;8:481–488. doi: 10.1038/nrn2147. [DOI] [PubMed] [Google Scholar]
- Gould E, McEwen BS, Tanapat P, Galea LAM, Fuchs E. Neurogenesis in the dentate gyrus of the adult tree shrew is regulated by psychosocial stress and NMDA receptor activation. J.Neurosci. 1997;17:2492–2498. doi: 10.1523/JNEUROSCI.17-07-02492.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould E, Tanapat P, McEwen BS, Flugge G, Fuchs E. Proliferation of granule cell precursors in the dentate gyrus of adult monkeys is diminished by stress. Proc. Natl. Acad. Sci. USA. 1998;95:3168–3171. doi: 10.1073/pnas.95.6.3168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Govindarajan A, Rao BSS, Nair D, Trinh M, Mawjee N, et al. Transgenic brain-derived neurotrophic factor expression causes both anxiogenic and antidepressant effects. Proc. Natl. Acad. Sci. USA. 2006;103:13208–13213. doi: 10.1073/pnas.0605180103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goy R, McEwen BS. Sexual Differentiation of the Brain. Cambridge: MIT Press; 1980. [Google Scholar]
- Gray JD, Rubin TG, Hunter RG, McEwen BS. Hippocampal gene expression changes underlying stress sensitization and recovery. Mol Psychiatry. 2014;19:1171–1178. doi: 10.1038/mp.2013.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillemin R. Peptides in the brain: the new endocrinology of the neuron. Science. 1978;202:390–402. doi: 10.1126/science.212832. [DOI] [PubMed] [Google Scholar]
- Halfon N, Larson K, Lu M, Tullis E, Russ S. Lifecourse health development: past, present and future. Maternal and child health journal. 2014;18:344–365. doi: 10.1007/s10995-013-1346-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris GW. Effects of the nervous system on the pituitary-adrenal activity. Prog. Brain Res. 1970;32:86–88. [PubMed] [Google Scholar]
- Harris GW, Levine S. Sexual differentiation of the brain and its experimental control. J. Physiol. 1965;181:379–400. doi: 10.1113/jphysiol.1965.sp007768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill MN, Hillard CJ, McEwen BS. Alterations in corticolimbic dendritic morphology and emotional behavior in cannabinoid CB1 receptor-deficient mice parallel the effects of chronic stress. Cereb Cortex. 2011a;21:2056–2064. doi: 10.1093/cercor/bhq280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill MN, Kumar SA, Filipski SB, Iverson M, Stuhr KL, et al. Disruption of fatty acid amide hydrolase activity prevents the effects of chronic stress on anxiety and amygdalar microstructure. Mol Psychiatry. 2013;18:1125–1135. doi: 10.1038/mp.2012.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill MN, McEwen BS. Involvement of the endocannabinoid system in the neurobehavioural effects of stress and glucocorticoids. Prog Neuropsychopharmacol Biol Psychiatry. 2010;34:791–797. doi: 10.1016/j.pnpbp.2009.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill MN, McLaughlin RJ, Pan B, Fitzgerald ML, Roberts CJ, et al. Recruitment of prefrontal cortical endocannabinoid signaling by glucocorticoids contributes to termination of the stress response. J Neurosci. 2011b;31:10506–10515. doi: 10.1523/JNEUROSCI.0496-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter RG, Gagnidze K, McEwen BS, Pfaff DW. Stress and the dynamic genome: Steroids, epigenetics, and the transposome. Proc Natl Acad Sci U S A. 2014 doi: 10.1073/pnas.1411260111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter RG, McCarthy KJ, Milne TA, Pfaff DW, McEwen BS. Regulation of hippocampal H3 histone methylation by acute and chronic stress. Proc. Natl. Acad. Sci. USA. 2009;106:20912–20917. doi: 10.1073/pnas.0911143106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen E, Jacobson H. Basic guides to the mechanism of estrogen action. Rec.Prog.Horm.Res. 1962;18:387–408. [Google Scholar]
- Joels M. Corticosteroid effects in the brain: U-shape it. Trends Pharmacol. Sci. 2006;27:244–250. doi: 10.1016/j.tips.2006.03.007. [DOI] [PubMed] [Google Scholar]
- Kaplan MS. Environment complexity stimulates visual cortex neurogenesis: death of a dogma and a research career. Trends Neurosci. 2001;24:617–620. doi: 10.1016/s0166-2236(00)01967-6. [DOI] [PubMed] [Google Scholar]
- Karst H, Berger S, Turiault M, Tronche F, Schutz G, Joels M. Mineralocorticoid receptors are indispensable for nongenomic modulation of hippocampal glutamate transmission by corticosterone. Proc. Natl. Acad. Sci. USA. 2005;102:19204–19207. doi: 10.1073/pnas.0507572102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kempermann G, Kuhn HG, Gage FH. More hippocampal neurons in adult mice living in an enriched environment. Nature. 1997;586:493–495. doi: 10.1038/386493a0. [DOI] [PubMed] [Google Scholar]
- Korte SM, de Boer SF, de Kloet ER, Bohus B. Anxiolytic-like effects of selective mineralocorticoid and glucocorticoid antagonists on fear-enhanced behavior in the elevated plus-maze. Psychoneuroendocrinology. 1995;20:385–394. doi: 10.1016/0306-4530(94)00069-7. [DOI] [PubMed] [Google Scholar]
- Ledoux VA, Smejkalova T, May RM, Cooke BM, Woolley CS. Estradiol facilitates the release of neuropeptide Y to suppress hippocampus-dependent seizures. J. Neurosci. 2009;29:1457–1468. doi: 10.1523/JNEUROSCI.4688-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leranth C, Petnehazy O, MacLusky NJ. Gonadal hormones affect spine synaptic density in the CA1 hippocampal subfield of male rats. J. Neurosci. 2003;23:1588–1592. doi: 10.1523/JNEUROSCI.23-05-01588.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leuner B, Caponiti JM, Gould E. Oxytocin stimulates adult neurogenesis even under conditions of stress and elevated glucocorticoids. Hippocampus. 2012;22:861–868. doi: 10.1002/hipo.20947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leuner B, Glasper ER, Gould E. Sexual experience promotes adult neurogenesis in the hippocampus despite an initial elevation in stress hormones. PLoS One. 2010;5:e11597. doi: 10.1371/journal.pone.0011597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis C, McEwen BS, Frankfurt M. Estrogen-induction of dendritic spines in ventromedial hypothalamus and hippocampus: effects of neonatal aromatase blockade and adult castration. Devel.Brain Res. 1995;87:91–95. doi: 10.1016/0165-3806(95)00052-f. [DOI] [PubMed] [Google Scholar]
- Liston C, Cichon JM, Jeanneteau F, Jia Z, Chao MV, Gan WB. Circadian glucocorticoid oscillations promote learning-dependent synapse formation and maintenance. Nat Neurosci. 2013;16:698–705. doi: 10.1038/nn.3387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liston C, Gan WB. Glucocorticoids are critical regulators of dendritic spine development and plasticity in vivo. Proc Natl Acad Sci U S A. 2011;108:16074–16079. doi: 10.1073/pnas.1110444108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liston C, McEwen BS, Casey BJ. Psychosocial stress reversibly disrupts prefrontal processing and attentional control. Proc. Natl. Acad. Sci. USA. 2009;106:912–917. doi: 10.1073/pnas.0807041106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loy R, Gerlach J, McEwen BS. Autoradiographic localization of estradiol-binding neurons in rat hippocampal formation and entorhinal cortex. Dev.Brain Res. 1988;39:245–251. doi: 10.1016/0165-3806(88)90028-4. [DOI] [PubMed] [Google Scholar]
- Maccari S, Morley-Fletcher S. Effects of prenatal restraint stress on the hypothalamus-pituitary-adrenal axis and related behavioural and neurobiological alterations. Psychoneuroendo. 2007;32:S10–S15. doi: 10.1016/j.psyneuen.2007.06.005. [DOI] [PubMed] [Google Scholar]
- Magarinos AM, Li CJ, Gal Toth J, Bath KG, Jing D, et al. Effect of brain-derived neurotrophic factor haploinsufficiency on stress-induced remodeling of hippocampal neurons. Hippocampus. 2011;21:253–264. doi: 10.1002/hipo.20744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magarinos AM, McEwen BS, Saboureau M, Pevet P. Rapid and reversible changes in intrahippocampal connectivity during the course of hibernation in European hamsters. Proc. Natl. Acad. Sci. USA. 2006;103:18775–18780. doi: 10.1073/pnas.0608785103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire EA, Frackowiak RSJ, Frith CD. Recalling routes around London: Activation of the right hippocampus in taxi drivers. J. Neurosci. 1997;17:7103–7110. doi: 10.1523/JNEUROSCI.17-18-07103.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier SF, Watkins LR. Cytokines for psychologists: Implications of bidirectional immune-to-brain communication for understanding behavior, mood, and cognition. Psychological Rev. 1998;105:83–107. doi: 10.1037/0033-295x.105.1.83. [DOI] [PubMed] [Google Scholar]
- McEown K, Treit D. Mineralocorticoid receptors in the medial prefrontal cortex and hippocampus mediate rats’ unconditioned fear behaviour. Horm Behav. 2011;60:581–588. doi: 10.1016/j.yhbeh.2011.08.007. [DOI] [PubMed] [Google Scholar]
- McEwen B, Akama K, Alves S, Brake WG, Bulloch K, et al. Tracking the estrogen receptor in neurons: implications for estrogen-induced synapse formation. Proc Natl Acad Sci U S A. 2001;98:7093–7100. doi: 10.1073/pnas.121146898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS. Steroid hormone receptors in developing and mature brain tissue. In: Snyder S, McEwen BS, editors. Neurotransmitters, Hormones and Receptors: Novel Approaches. Society of Neuroscience; 1976. pp. 50–66. [Google Scholar]
- McEwen BS. Protective and Damaging Effects of Stress Mediators. New England J.Med. 1998;338:171–179. doi: 10.1056/NEJM199801153380307. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Stress and hippocampal plasticity. Annu.Rev.Neurosci. 1999;22:105–122. doi: 10.1146/annurev.neuro.22.1.105. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Protective and damaging effects of stress mediators: central role of the brain. Dial. in Clin. Neurosci.Stress. 2006;8:367–381. doi: 10.31887/DCNS.2006.8.4/bmcewen. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS. Physiology and neurobiology of stress and adaptation: Central role of the brain. Physiol.Rev. 2007;87:873–904. doi: 10.1152/physrev.00041.2006. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Stress, sex, and neural adaptation to a changing environment: mechanisms of neuronal remodeling. Ann N Y Acad Sci. 2010;(1204 Suppl):E38–E59. doi: 10.1111/j.1749-6632.2010.05568.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS. Brain on stress: how the social environment gets under the skin. Proc Natl Acad Sci U S A. 2012;(109 Suppl 2):17180–17185. doi: 10.1073/pnas.1121254109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS, Chattarji S. Handbook of Neurochemistry and Molecular Neurobiology. New York: Springer-Verlag; 2007. Neuroendocrinology of Stress In; pp. 572–593. [Google Scholar]
- McEwen BS, Gianaros PJ. Stress- and allostasis-induced brain plasticity. Annu Rev Med. 2011;62:431–445. doi: 10.1146/annurev-med-052209-100430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS, Gould E, Orchinik M, Weiland NG, Woolley CS. Oestrogens and the structural and functional plasticity of neurons: implications for memory, ageing and neurodegenerative processes. In: Goode J, editor. Ciba Foundation Symposium #191 The Non-reproductive Actions of Sex Steroids. London: CIBA Foundation; 1995. pp. 52–73. [DOI] [PubMed] [Google Scholar]
- McEwen BS, Lieberburg I, Chaptal C, Krey L. Aromatization: important for sexual differentiation of the neonatal rat brain. Horm.Behav. 1977;9:249–263. doi: 10.1016/0018-506x(77)90060-5. [DOI] [PubMed] [Google Scholar]
- McEwen BS, Milner TA. Hippocampal formation: Shedding light on the influence of sex and stress on the brain. Brain Res. Rev. 2007;55:343–355. doi: 10.1016/j.brainresrev.2007.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS, Morrison JH. The Brain on Stress: Vulnerability and Plasticity of the Prefrontal Cortex over the Life Course. Neuron. 2013;79:16–29. doi: 10.1016/j.neuron.2013.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS, Plapinger L. Association of corticosterone-1,2 3H with macromolecules extracted from brain cell nuclei. Nature. 1970;226:263–264. doi: 10.1038/226263a0. [DOI] [PubMed] [Google Scholar]
- McEwen BS, Stellar E. Stress and the individual. Mechanisms leading to disease. Arch. Intern. Med. 1993;153:2093–2101. [PubMed] [Google Scholar]
- McEwen BS, Weiss J, Schwartz L. Selective retention of corticosterone by limbic structures in rat brain. Nature. 1968;220:911–912. doi: 10.1038/220911a0. [DOI] [PubMed] [Google Scholar]
- McEwen BS, Wingfield JC. The concept of allostasis in biology and biomedicine. Horm. & Behav. 2003;43:2–15. doi: 10.1016/s0018-506x(02)00024-7. [DOI] [PubMed] [Google Scholar]
- Mehler MF. Epigenetic principles and mechanisms underlying nervous system functions in health and disease. Prog. Neurobiol. 2008;86:305–341. doi: 10.1016/j.pneurobio.2008.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meites J. Short history of neuroendocrinology and the International Society of Neuroendocrinology. Neuroendocrinology. 1992;56:1–10. doi: 10.1159/000126201. [DOI] [PubMed] [Google Scholar]
- Miller MM, Morrison JH, McEwen BS. Basal anxiety-like behavior predicts differences in dendritic morphology in the medial prefrontal cortex in two strains of rats. Behav Brain Res. 2012;229:280–288. doi: 10.1016/j.bbr.2012.01.029. [DOI] [PubMed] [Google Scholar]
- Milner TA, McEwen BS, Hayashi S, Li CJ, Reagen L, Alves SE. Ultrastructural evidence that hippocampal alpha estrogen receptors are located at extranuclear sites. J. Comp.Neurol. 2001;429:355–371. [PubMed] [Google Scholar]
- Molet J, Maras PM, Avishai-Eliner S, Baram TZ. Naturalistic rodent models of chronic early-life stress. Developmental psychobiology. 2014;56:1675–1688. doi: 10.1002/dev.21230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriceau S, Sullivan R. Maternal presence serves as a switch between learning fear and attraction in infancy. Nature Neurosci. 2006;8:1004–1006. doi: 10.1038/nn1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moutsatsou P, Psarra A-MG, Tsiapara A, Paraskevakou H, Davaris P, Sekeris CE. Localization of the glucocorticoid receptor in rat brain mitochondria. Arch. Biochem. Biophys. 2001;386:69–78. doi: 10.1006/abbi.2000.2162. [DOI] [PubMed] [Google Scholar]
- Mucha M, Skrzypiec AE, Schiavon E, Attwood BK, Kucerova E, Pawlak R. Lipocalin-2 controls neuronal excitability and anxiety by regulating dendritic spine formation and maturation. Proc Natl Acad Sci U S A. 2011;108:18436–18441. doi: 10.1073/pnas.1107936108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nacher J, Guirado R, Castillo-Gomez E. Structural plasticity of interneurons in the adult brain: role of PSA-NCAM and implications for psychiatric disorders. Neurochemical research. 2013;38:1122–1133. doi: 10.1007/s11064-013-0977-4. [DOI] [PubMed] [Google Scholar]
- Naftolin F. Brain aromatization of androgens. J.Reprod.Med. 1994;39:257–261. [PubMed] [Google Scholar]
- Naftolin F, Ryan KJ, Petro Z. Aromatization of androstenedione by limbic system tissue from human foetuses. J Endocrinol. 1971;51:795–796. doi: 10.1677/joe.0.0510795. [DOI] [PubMed] [Google Scholar]
- Nasca C, Bigio B, Zelli D, Nicoletti F, McEwen BS. Mind the gap: glucocorticoids modulate hippocampal glutamate tone underlying individual differences in stress susceptibility. Mol Psychiatry. 2014 doi: 10.1038/mp.2014.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasca C, Xenos D, Barone Y, Caruso A, Scaccianoce S, et al. L-acetylcarnitine causes rapid antidepressant effects through the epigenetic induction of mGlu2 receptors. Proc Natl Acad Sci U S A. 2013;110:4804–4809. doi: 10.1073/pnas.1216100110. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsen J, Irwin RW, Gallaher TK, Diaz Brinton R. Estradiol in vivo regulation of brain mitochondrial proteome. J. Neurosci. 2007;27:14069–14077. doi: 10.1523/JNEUROSCI.4391-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nottebohm F. Neuronal replacement in adult brain. Brain Res Bull. 2002;57:737–749. doi: 10.1016/s0361-9230(02)00750-5. [DOI] [PubMed] [Google Scholar]
- Pavlides C, Watanabe Y, Magarinos AM, McEwen BS. Opposing role of adrenal steroid Type I and Type II receptors in hippocampal long-term potentiation. Neuroscience. 1995;68:387–394. doi: 10.1016/0306-4522(95)00151-8. [DOI] [PubMed] [Google Scholar]
- Pawlak R, Magarinos AM, Melchor J, McEwen B, Strickland S. Tissue plasminogen activator in the amygdala is critical for stress-induced anxiety-like behavior. Nature Neurosci. 2003;6:168–174. doi: 10.1038/nn998. [DOI] [PubMed] [Google Scholar]
- Pawlak R, Rao BSS, Melchor JP, Chattarji S, McEwen B, Strickland S. Tissue plasminogen activator and plasminogen mediate stress-induced decline of neuronal and cognitive functions in the mouse hippocampus. Proc. Natl. Acad. Sci. USA. 2005;102:18201–18206. doi: 10.1073/pnas.0509232102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira AC, Lambert HK, Grossman YS, Dumitriu D, Waldman R, et al. Glutamatergic regulation prevents hippocampal-dependent age-related cognitive decline through dendritic spine clustering. Proc Natl Acad Sci U S A. 2014;111:18733–18738. doi: 10.1073/pnas.1421285111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pham K, Nacher J, Hof PR, McEwen BS. Repeated, but not acute, restraint stress suppresses proliferation of neural precursor cells and increases PSA-NCAM expression in the adult rat dentate gyrus. J. Neurosci. 2003;17:879–886. doi: 10.1046/j.1460-9568.2003.02513.x. [DOI] [PubMed] [Google Scholar]
- Popoli M, Yan Z, McEwen BS, Sanacora G. The stressed synapse: the impact of stress and glucocorticoids on glutamate transmission. Nat Rev Neurosci. 2012;13:22–37. doi: 10.1038/nrn3138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radley JJ, Rocher AB, Janssen WGM, Hof PR, McEwen BS, Morrison JH. Reversibility of apical dendritic retraction in the rat medial prefrontal cortex following repeated stress. Exper. Neurol. 2005;196:199–203. doi: 10.1016/j.expneurol.2005.07.008. [DOI] [PubMed] [Google Scholar]
- Radley JJ, Sisti HM, Hao J, Rocher AB, McCall T, et al. Chronic behavioral stress induces apical dendritic reorganization in pyramidal neurons of the medial prefrontal cortex. Neuroscience. 2004;125:1–6. doi: 10.1016/j.neuroscience.2004.01.006. [DOI] [PubMed] [Google Scholar]
- Rao RP, Anilkumar S, McEwen BS, Chattarji S. Glucocorticoids protect against the delayed behavioral and cellular effects of acute stress on the amygdala. Biol Psychiatry. 2012;72:466–475. doi: 10.1016/j.biopsych.2012.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratman D, Vanden Berghe W, Dejager L, Libert C, Tavernier J, et al. How glucocorticoid receptors modulate the activity of other transcription factors: a scope beyond tethering. Mol Cell Endocrinol. 2013;380:41–54. doi: 10.1016/j.mce.2012.12.014. [DOI] [PubMed] [Google Scholar]
- Rettberg JR, Yao J, Brinton RD. Estrogen: a master regulator of bioenergetic systems in the brain and body. Front Neuroendocrinol. 2014;35:8–30. doi: 10.1016/j.yfrne.2013.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reul JM, DeKloet ER. Two receptor systems for corticosterone in rat brain: microdistribution and differential occupation. Endocrinology. 1985;117:2505–2511. doi: 10.1210/endo-117-6-2505. [DOI] [PubMed] [Google Scholar]
- Romeo RD, Staub D, Jasnow AM, Karatsoreos IN, Thornton JE, McEwen BS. Dihydrotestosterone increases hippocampal N-methyl-D-aspartate binding but does not affect choline acetyltransferase cell number in the forebrain or choline transporter levels in the CA1 region of adult male rats. Endocrinology. 2005;146:2091–2097. doi: 10.1210/en.2004-0886. [DOI] [PubMed] [Google Scholar]
- Rudick CN, Gibbs RB, Woolley CS. A role for the basal forebrain cholinergic system in estrogen-induced disinhibition of hippocampal pyramidal cells. J. Neurosci. 2003;23:4479–4490. doi: 10.1523/JNEUROSCI.23-11-04479.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo SJ, Charney DS. Next generation antidepressants. Proc Natl Acad Sci U S A. 2013;110:4441–4442. doi: 10.1073/pnas.1301593110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandstrom NJ, Williams CL. Memory retention is modulated by acute estradiol and progesterone replacement. Behav. Neurosci. 2001;115:384–393. [PubMed] [Google Scholar]
- Sandstrom NJ, Williams CL. Spatial memory retention is enhanced by acute and continuous estradiol replacement. Horm. & Behav. 2004;45:128–135. doi: 10.1016/j.yhbeh.2003.09.010. [DOI] [PubMed] [Google Scholar]
- Sapolsky R. Glucocorticoids, hippocampal damage and the glutamatergic synapse. Prog.Brain Res. 1990;86:13–23. doi: 10.1016/s0079-6123(08)63163-5. [DOI] [PubMed] [Google Scholar]
- Schally AV, Arimura A, Kastin AJ. Hypothalamic regulatory hormones. Science. 1973;179:341–350. doi: 10.1126/science.179.4071.341. [DOI] [PubMed] [Google Scholar]
- Schelling G, Roozendaal B, De Quervain DJ-F. Can posttraumatic stress disorder be prevented with glucocorticoids? Ann. N.Y. Acad. Sci. 2004;1032:158–166. doi: 10.1196/annals.1314.013. [DOI] [PubMed] [Google Scholar]
- Seeman T, Epel E, Gruenewald T, Karlamangla A, McEwen BS. Socio-economic differentials in peripheral biology: Cumulative allostatic load. Ann. NY Acad. Sci. 2010;1186:223–239. doi: 10.1111/j.1749-6632.2009.05341.x. [DOI] [PubMed] [Google Scholar]
- Shansky RM, Hamo C, Hof PR, Lou W, McEwen BS, Morrison JH. Estrogen promotes stress sensitivity in a prefrontal cortex-amygdala pathway. Cereb Cortex. 2010;20:2560–2567. doi: 10.1093/cercor/bhq003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shansky RM, Hamo C, Hof PR, McEwen BS, Morrison JH. Stress-induced dendritic remodeling in the prefrontal cortex is circuit specific. Cereb Cortex. 2009;19:2479–2484. doi: 10.1093/cercor/bhp003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheline YI. Neuroimaging studies of mood disorder effects on the brain. Biol. Psychiat. 2003;54:338–352. doi: 10.1016/s0006-3223(03)00347-0. [DOI] [PubMed] [Google Scholar]
- Shonkoff JP, Boyce WT, McEwen BS. Neuroscience, molecular biology, and the childhood roots of health disparities. JAMA. 2009;301:2252–2259. doi: 10.1001/jama.2009.754. [DOI] [PubMed] [Google Scholar]
- Skrzypiec AE, Shah RS, Schiavon E, Baker E, Skene N, et al. Stress-induced lipocalin-2 controls dendritic spine formation and neuronal activity in the amygdala. PLoS One. 2013;8:e61046. doi: 10.1371/journal.pone.0061046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sloan RP, Shapiro PA, Bagiella E, Myers MM, Gorman JM. Cardiac autonomic control buffers blood pressure variability responses to challenge: A psychophysiologic model of coronary artery disease. Psychosomatic Med. 1999;61:58–68. doi: 10.1097/00006842-199901000-00010. [DOI] [PubMed] [Google Scholar]
- Smythe JW, Murphy D, Timothy C, Costall B. Hippocampal mineralocorticoid, but not glucocorticoid, receptors modulate anxiety-like behavior in rats. Pharmacology Biochemistry and Behavior. 1997;56:507–513. doi: 10.1016/s0091-3057(96)00244-4. [DOI] [PubMed] [Google Scholar]
- Starkman MN, Gebarski SS, Berent S, Schteingart DE. Hippocampal formation volume, memory dysfunction, and cortisol levels in partiens with Cushing’s syndrome. Biol Psychiatry. 1992;32:756–765. doi: 10.1016/0006-3223(92)90079-f. [DOI] [PubMed] [Google Scholar]
- Stavreva DA, Wiench M, John S, Conway-Campbell BL, McKenna MA, et al. Ultradian hormone stimulation induces glucocorticoid receptor-mediated pulses of gene transcription. Nat Cell Biol. 2009;11:1093–1102. doi: 10.1038/ncb1922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sterling P, Eyer J. Allostasis: A New Paradigm to Explain Arousal Pathology In. In: Fisher S, Reason J, editors. Handbook of Life Stress, Cognition and Health. New York: John Wiley & Sons; 1988. pp. 629–649. [Google Scholar]
- Towart LA, Alves SE, Znamensky V, Hayashi S, McEwen BS, Milner TA. Subcellular relationships between cholinergic terminals and estrogen receptor-α in the dorsal hippocampus. J. Comp. Neurol. 2003;463:390–401. doi: 10.1002/cne.10753. [DOI] [PubMed] [Google Scholar]
- Tracey KJ. The inflammatory reflex. Nature. 2002;420:853–859. doi: 10.1038/nature01321. [DOI] [PubMed] [Google Scholar]
- Vale W, Spiess J, Rivier C, Rivier J. Characterization of a 41-residue ovine hypothalamic peptide that stimulates secretion of corticotropin and beta-endorphin. Science. 1981;213:1394–1397. doi: 10.1126/science.6267699. [DOI] [PubMed] [Google Scholar]
- van Praag H, Kempermann G, Gage FH. Running increases cell proliferation and neurogenesis in the adult mouse dentate gyrus. Nature Neurosci. 1999;2:266–270. doi: 10.1038/6368. [DOI] [PubMed] [Google Scholar]
- Vyas A, Mitra R, Rao BSS, Chattarji S. Chronic stress induces contrasting patterns of dendritic remodeling in hippocampal and amygdaloid neurons. J Neurosci. 2002;22:6810–6818. doi: 10.1523/JNEUROSCI.22-15-06810.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waddington CH. The epigenotype. Endeavoour. 1942;1:18–20. [Google Scholar]
- Weiland NG. Estradiol selectively regulates agonist binding sites on the N-methyl-D-aspartate receptor complex in the CA1 region of the hippocampus. Endocrinology. 1992;131:662–668. doi: 10.1210/endo.131.2.1353442. [DOI] [PubMed] [Google Scholar]
- Weinstock M. The potential influence of maternal stress hormones on development and mental health of the offspring. Brain, Behav., and Immunity. 2005;19:296–308. doi: 10.1016/j.bbi.2004.09.006. [DOI] [PubMed] [Google Scholar]
- Woolley CS. Effects of estrogen in the CNS. Current Opinion Neurobiol. 1999;9:349–354. doi: 10.1016/s0959-4388(99)80051-8. [DOI] [PubMed] [Google Scholar]
- Yamamoto K. Steroid receptor regulated transcription of specific genes and gene networks. Ann.Rev.Genet. 1985;19:209–252. doi: 10.1146/annurev.ge.19.120185.001233. [DOI] [PubMed] [Google Scholar]
- Yau PL, Castro MG, Tagani A, Tsui WH, Convit A. Obesity and metabolic syndrome and functional and structural brain impairments in adolescence. Pediatrics. 2012;130:e856–e864. doi: 10.1542/peds.2012-0324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young EA, Lopez JF, Murphy-Weinberg V, Watson SJ, Akil H. Mineralocorticoid receptor function in major depression. Arch. Gen. Psychiat. 2003;60:24–28. doi: 10.1001/archpsyc.60.1.24. [DOI] [PubMed] [Google Scholar]
- Zohar J, Yahalom H, Kozlovsky N, Cwikel-Hamzany S, Matar MA, et al. High dose hydrocortisone immediately after trauma may alter the trajectory of PTSD: interplay between clinical and animal studies. Eur Neuropsychopharmacol. 2011;21:796–809. doi: 10.1016/j.euroneuro.2011.06.001. [DOI] [PubMed] [Google Scholar]