Abstract
Objective
AMH is associated with menopausal timing in several studies. In contrast to prior studies that were restricted to women with regular cycles, our objective was to examine this association in women with either regular or irregular menstrual cycles.
Methods
CARDIA is a longitudinal, population-based study that recruited adults ages 18–30 when it began in 1985–86. AMH was measured in serum stored in 2002–03. Natural menopause was assessed by survey in 2005–06 and 2010–11.
Results
Among 716 premenopausal women, median [25th, 75th] AMH was 0.77 [0.22–2.02] ng/dL at a median age of 42 [39–45] years. Twenty-nine percent of the women (n=207) reported natural menopause during 9 years of follow up. In fully adjusted discrete-time hazard models, a 0.5 ng/dL AMH decrement was associated with higher risk of menopause (p<0.001). Hazard ratios varied with time since AMH measurement. The HR (95% CI) for menopause was 8.1 (2.5–26.1) within 0–3 years and 2.3 (1.7–3.3) and 1.6 (1.3–2.1) for 3–6 and 6–9 years, respectively. When restricted to women with regular menses, results were similar (e.g., HR=6.1; 95% CI: 1.9–20.0 for 0–3 years).
Conclusion
AMH is independently associated with natural menopause. AMH appears most useful in identifying women at risk of menopause in the near future (within 3 years of AMH measurement).
Keywords: Anti-Mullerian Hormone, Menopause, Ovarian Aging, Ovarian Reserve, FSH, CARDIA
1. Introduction
Follicles are the functional units of the ovary that each contain an oocyte and its surrounding granulosa cells. These follicles are necessary for fertility and menstrual cycling and constitute a woman’s “ovarian reserve”[1]. Menopause represents the loss of ovarian reserve and is an inevitable life event for all women who live long enough.[2, 3] The timing of this life event is associated with multiple chronic diseases such as breast cancer (late menopause) and coronary heart disease, stroke, and osteoporosis (early menopause)[4–9]. Thus, biomarkers that can estimate ovarian reserve and the onset of menopause may also have utility in identifying women at low or high risk of common chronic diseases.
Anti-Mullerian hormone (AMH), a dimeric TGF-beta superfamily glycoprotein is produced by the granulosa cells of ovarian follicles in women.[3] AMH has recently emerged as a potentially useful biomarker of ovarian reserve and of the timing of future menopause. Its utility in estimating the onset of menopause has been demonstrated in regularly cycling women from Dutch, Iranian, and US cohorts.[10–12] A particular strength of AMH as a biomarker of ovarian reserve is its relative stability across the menstrual cycle as compared with other ovarian reserve hormones.[13, 14] This makes it especially useful for epidemiologic studies with stored biospecimens from women in various reproductive stages or that that are drawn at a time when the menstrual cycle phase is unclear or unknown.
Our aim in this study was to examine AMH’s association with incident menopause in premenopausal women from CARDIA Women’s Study (CWS). These women were recruited from the general population and inclusion in CWS was not contingent on proven fertility or status in a certain menstrual cycle phase or reproductive aging stage.
1. Materials and Methods
2.1. Study Population
We utilized data from the Coronary Artery Risk Development in Young Adults (CARDIA) study, a longitudinal, epidemiologic investigation of the evolution of cardiovascular risk among young adults [15, 16] and the CARDIA Women’s Study (CWS), an ancillary study to CARDIA. Participants 18–30 years old were recruited from the populations of Birmingham, Chicago, and Minneapolis and through a Kaiser Permanente membership plan (Oakland, California). Baseline examinations were performed in 5,115 participants (51% of eligible persons contacted) in 1985–86, a sample that included 2,788 women. The population was balanced according to age (18–24 or 25–30 years), sex, education (less than high school versus high school or more), and race (black and white) at each CARDIA site. A description of the methodology for recruiting subjects and performing data collection is detailed elsewhere[15, 16]. An institutional review board at each site approved all study procedures; written informed consent was obtained from study participants prior to assessments.
The CWS was designed to examine the associations of androgens, polycystic ovaries, and clinical features of the polycystic ovary syndrome with subclinical atherosclerosis. Women eligible for CWS had to have attended the 2000–01 CARDIA (year 15) examination, have at least one ovary, and not be pregnant. Women with a history of hysterectomy or menstrual cycle irregularity were eligible for participation. The CWS examination occurred in 2002–03 and the examination components that included a blood draw and a trans-vaginal ultrasound are detailed elsewhere [17–19]. Blood draws for CWS were targeted to the follicular phase of the menstrual cycle.
2.2. Sample Selection
A total of 1,163 women participated in the CWS and 1,123 of these had serum available for AMH measurement. For the current analyses, participants were excluded if they reported a history of prevalent natural menopause or hysterectomy at baseline or were missing covariates from the CWS baseline examination which occurred between 2002 and 2003. They were also excluded if data on menopause status were missing from both the 2005–06 and 2010–11 examination or if they reported a history of hysterectomy at either examination. After these exclusions, 716 women remained in the final sample.
2.3. Data Collection
2.3.1 Assays
Blood samples were drawn in 2002–03 from the antecubital vein using a protocol that ensured minimal stasis and immediate refrigeration at 4°C. Within one hour of blood draw, serum samples were processed into aliquots and frozen at −70°C until shipped to the ReproSource laboratory (Boston, MA) in 2014 for analyses.
2.3.1.1. AMH
AMH was measured in stored serum samples using Ansh Laboratories (Webster, TX) Ultra-Sensitive AMH ELISA assay. For this assay, the lower limit of detection (LLOD) is 0.02; the lower limit of quantification (LLOQ) is 0.09. The intra-assay CVs ranged from 3–7% and the inter-assay CVs ranged from 5–10% in the Reprosource Laboratory. For the purposes of this analysis, 0.089 was applied to all AMH values ≤0.09. In a pilot project of a random sample of CWS participants (n=129) the Beckman Generation II assay was run in parallel with the Ansh Ultra-Sensitive assay. The results of these assays correlated highly (r =0.99).
2.3.1.2. Estradiol, Progesterone and Follicle Stimulating Hormone (FSH)
Estradiol, progesterone, and FSH were measured in serum with chemiluminescent immunoassays by the ReproSource laboratory using either the Immulite 2000 or Cobas 411e systems.
2.4. Menopause Definition
The primary outcome was self-reported incident natural menopause. Participants completed a self-administered questionnaire during the CARDIA year 20 (2005–06) and/or year 25 examination (2010–11) with the question “Have you gone through menopause or the change of life?” followed by “If yes, how did your periods stop?” with the options of “naturally” “surgically” or “other”. Participants were asked “How old were you when this occurred?”, which was used in analysis as age at menopause. Our definition of natural menopause was also validated against a question regarding the date of last menstrual period. Of the 207 women who reported an incident natural menopause, 202 reported both age and calendar year at which menopause occurred. Ninety-four percent (191/202) reported a date within +/− 1 year of their reported age at menopause.
2.5. Covariates
2.5.1. Reproductive Characteristics
Participants in CWS (2002–03) completed a self-administered questionnaire with the question “Are your menstrual periods regular or irregular. By irregular we mean that you could predict when you period would start at least half the time”. Participants were also queried regarding their current use of hormonal contraceptives.
2.5.2. Sociodemographic, Lifestyle, and Anthropometric Data
Race and birthdate were obtained at the year 0 examination (1985–86). Smoking history was collected at the year 15 examination (2000–01) and women were categorized as current, past, or never smokers. Weight (at the CWS baseline examination) and height (2000–01) were measured according to standardized protocols[20]. Body mass index (BMI) was computed in units of kg/m2. Total physical activity score was calculated using a previously validated algorithm in CARDIA.[21]
2.6 Statistical Methods
To summarize characteristics of women in CWS, we distributed them into two groups depending on whether their baseline AMH levels were below or greater than the median level (0.77 ng/mL). Medians (25th and 75th percentiles) for continuous variables and percentages for categorical variables were computed. The differences in the distributions of sociodemographic and clinical characteristics by AMH category were tested using Wilcoxon rank sum test for continuous variables and the Pearson’s chi-squared test for continuous variables.
For each participant, we computed time from AMH measurement to menopause, loss to follow-up or year 25 examination, whichever came first. We then grouped women according to whether they were above or below the median age (42 years) at AMH measurement and created a four-strata variable (AMH ≤0.77 ng/mL and age ≤42 years; AMH ≤0.77 ng/mL and age >42 years; AMH >0.77 ng/mL and age ≤42 years; and AMH >0.77 ng/mL and age >42 years) which we used to fit a Kaplan-Meier plot. We used the log-rank test to determine whether the incidence of menopause varied by strata of AMH and age at AMH measurement.
Next we used discrete-time hazard regression to determine whether AMH is associated with incident natural menopause before and after adjusting for covariates including age, smoking, race, education level, estradiol, progesterone, FSH, BMI, hormonal contraceptive use, regularity of menses, CARDIA study site, progesterone, and alcohol intake. Since we found a significant association between the Schoenfeld residuals for AMH and time (p < 0.001), suggesting a violation of the proportional hazards assumption, we report results that allow the hazard ratio to change over time. In particular, we report the average hazard ratio in three-year time intervals after AMH collection: 0 to 3 years, 3 to 6 years, and 6 to 9 years. Goodness of fit was assessed with c-statistics, coefficients of determination (r2), and likelihood ratio tests. Analyses were performed using R statistical software (version 3.1.3).
Since AMH was positively skewed, we considered sensitivity analyses in which AMH was either log-transformed or flexibly modeled using restricted cubic splines. The models had similar fit, so we report regression results from models in which AMH was analyzed as an untransformed continuous variable. Because previous AMH studies only included women with regular menses, we conducted an additional sensitivity analysis in which we ran the fully adjusted model in the subset of women with regular menses. A similar analysis in women with irregular menses was precluded by the small number (n=113) of such women in our study population. Next we tested if race modified the association between AMH and the incidence of menopause by fitting a model with the main effects for race, AMH and their interaction term.
3. Results
As compared with the final sample (n= 716), excluded women (n=407) were older (43 vs. 42; p<0.001), more likely to be black (64% versus 46%; p<0.001), and current smokers (27% vs. 19%; p=0.003). Excluded women had lower AMH levels (median 0.56 vs. 0.77 ng/dL; p=0.002), higher FSH levels (7.7 mIU/mL vs. 6.6 mIU/mL; p<0.001) and higher BMIs (29.6 vs. 27.8 ; p<0.001) when compared with those included in the analysis. Most of the excluded women who also had follow-up information at the 2010–11 examination (62% or 190/304) reported a history of surgical menopause.
In the analysis sample (n=716) at baseline (2002–03), the median age was 42 (39–45) years and the median (25th, 75th percentile) AMH level was 0.77 (0.22–2.02) ng/dL. AMH was positively skewed, with 14% of values below detection, and correlated with age at collection (Spearman r = −0.54). Participant characteristics according to median AMH are shown in Table 1. At baseline, women with lower AMH were older, had higher FSH, and were less likely to be black; otherwise, there were no large apparent differences between the two groups.
Table 1.
Demographic Variables by Low or High Anti-Mullerian Hormone (AMH). in 716 Participants in the CARDIA Women’s Study.
| <0.77 ng/dL n=362 |
≥0.77 ng/dL n=354 |
p-value | |
|---|---|---|---|
| Race, % Black* | 148 (41%) | 181 (51%) | 0.007 |
| Age, years | 44 [42, 46] | 40 [37, 43] | <0.001 |
| Smoking, % | 0.31 | ||
| • Never | 211 (58) | 215 (61) | |
| • Past | 86 (24) | 68 (19) | |
| • Current | 65 (18) | 71 (20) | |
| Estradiol, pg/mL | 68 [42,128] | 64 [42, 103] | 0.36 |
| Progesterone, pg/mL | 0.23 [0.19–0.44] | 0.20 [0.19, 0.43 ] | 0.11 |
| FSH, mIU/mL | 7.9 [5.3–12.4] | 5.8 [4.2, 7.5] | <0.001 |
| BMI, kg/m2 | 27 [24–34] | 28 [24–34] | 0.17 |
| Current alcohol use, % | 82 (22) | 77 (22) | 0.91 |
| Total Physical Activity, exercise units | 245 [100–416] | 222 [107–412] | 0.75 |
| Education, years | 15 [13–16] | 15 [13–16] | 0.49 |
| Current Contraceptive Use, % | 60 (17) | 58 (16) | 0.94 |
| Regular Menses, % | 297 (82) | 306 (86) | 0.11 |
Values are medians [25th, 75th percentiles] or n (%).
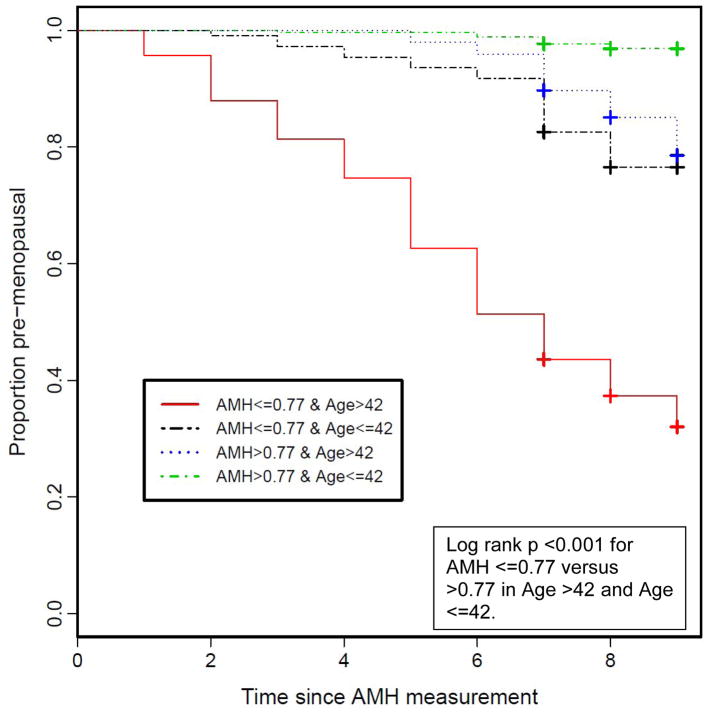
Over nine years of follow up, 207 women reported that they have had natural menopause. In these 207 women, the median (25th, 75th percentile) age at menopause was 50 (48–52) years. Women with lower AMH had a significantly higher incidence of menopause. As shown by the Kaplan-Meier curves, less than 80% of women who were 42 years or younger and with AMH below the median (≤0.77 ng/dL) remained pre-menopausal after 9 years of follow-up and less than 40% of women older than age 42 with low AMH remained premenopausal (Figure 1).
Figure 1.
Kaplan-Meier Plot of Incident Menopause by Low/High Anti-Mullerian Hormone Level in CARDIA Women when measured at ages 34–42 or >42–49 ng/dL (n=716).
In discrete-time hazard models, age at AMH measurement was associated with incident menopause, but age did not modify the hazard ratios. Stated another way, older participants (ages >42 years) at baseline AMH measurement had a higher risk of incident menopause overall, but the hazard for AMH’s relationship with incident menopause was similar in older (>42 years) and younger women (≤42 years).
The relationship between AMH, age at collection, and the probability of menopause is described in more detail in Table 2 where the proportion of subjects who experienced menopause within 6 years is stratified by AMH levels and age at collection. None of the subjects included in the analysis were censored before completing 7 years of follow up, which allowed us to calculate proportions in Table 2 irrespective of censoring events. For women who were 34–39 at AMH collection, only one subject experienced menopause in the subsequent 6 years of follow up, and that subject had an AMH of 0.72 ng/dL. For women who were either ages 40–44 or ages 45–49 at the time of AMH collection, there is an association between decreasing AMH concentration and increasing probability of menopause within 6 years. No women with AMH over 2.0 ng/dL experienced menopause within 6 years. However, if AMH was below the detection limit, 46% of women from ages 40–44 and 72% of women from ages 45–49 experienced menopause within 6 years.
Table 2.
Proportion of 716 CARDIA Women’s Study Participants Reporting Menopause within 6 years of AMH Measurement by Age at AMH measurement and AMH Levels.
| Age at AMH Collection | |||
|---|---|---|---|
|
| |||
| 34 to 39 | 40 to 44 | 45 to 49 | |
| n=208 | n=309 | n=199 | |
| AMH level | |||
| Below Detection* | 0.0% (0/6) | 45.5% (15/33) | 72.0% (46/64) |
| 0.09 to 0.99 ng/dL | 1.9% (1/54) | 9.5% (14/147) | 25.0% (25/102) |
| 1.0 to 1.99 ng/dL | 0.0% (0/46) | 1.7% (1/60) | 5.0% (1/20) |
| 2.0 ng/dL and above | 0.0% (0/102) | 0.0% (0/69) | 0.0% (0/13) |
Values are % (menopause events/total n for age and AMH level group)
We also found that the hazard ratio for a 0.5 ng/dL decrement in AMH changes over time (Table 3). The hazard ratio is highest in the first 3 years after AMH measurement and decreases with longer length of follow-up. In analyses controlling for age, race, and other confounding variables, a 0.5 ng/dL lower AMH level was associated with an 8.1-fold (95% CI: 2.5 to 26.1) increase in the risk of menopause within 3 years from AMH measurement. The adjusted hazard ratio decreases to 2.3 (95% CI: 1.7 to 3.3) and 1.6 (95% CI: 1.3 to 2.1) for the 3 to 6 years and 6 to 9 years after AMH measurement, respectively. This pattern of a lower effect size for AMH with increasing time of follow up remains consistent in both minimally adjusted models (only age and race) and in fully-adjusted models (age, smoking, race, education, estradiol, progesterone, FSH, BMI, current hormonal contraceptive use, examination center, regular menses, and alcohol intake).
Table 3.
Hazard ratios and 95% confidence intervals for a 0.5 ng/dL decrement in AMH in fully-adjusted models over time since AMH measurement in the full sample (n=716) and after restricting to women with regular menses (n=603). Hazard ratios are reported in 3-year time intervals because the hazard ratios change over time.
| HR [95% CI] by years since AMH collection | |||
|---|---|---|---|
|
| |||
| 0 – 3 years | 3 – 6 years | 6 – 9 years | |
| Unadjusted Model | 23.1 [6.3, 84.2] | 3.1 [2.2, 4.4] | 1.8 [1.5, 2.3] |
| Age, Race Adjusted Model | 14.3 [4.1, 50.3] | 2.4 [1.7, 3.4] | 1.6 [1.3, 1.9] |
| Full Model* | 8.1 [2.5, 26.1] | 2.3 [1.7, 3.3] | 1.6 [1.3, 2.1] |
| Full Model* Limited to those with Regular Menses Only (n=603) | 6.1 [1.9, 20.0] | 2.2 [1.5, 3.1] | 1.8 [1.4, 2.5] |
Full model adjusted for age, race, CARDIA center, smoking (never, past, current), FSH, estradiol, progesterone, BMI, alcohol use, physical activity, education, current contraceptive use, regular menses (unless stratified by regular menses).
In the fully adjusted model, AMH was a significantly associated with time to menopause after controlling for age and the other covariates. Compared to a model without AMH, the C-index increased from 0.84 to 0.89, the generalized r2 increased from 0.23 to 0.30, and AMH was statistically significant by the likelihood ratio test (p < 0.001). In sensitivity analyses limited to women with regular menses, the association between AMH and natural menopause was somewhat attenuated but remained significant (HR=6.1; 95% CI: 1.9 to 20.0) and the pattern was similar to that observed in the analyses that used the full sample of 716 women (Table 3).
4. Discussion
Among women ages 34–49 at baseline, the 9-year hazard of natural menopause was high among women with a low AMH (<0.77 ng/dL). The strength of the association between AMH and incident natural menopause varied with time since AMH measurement and was strongest within the first 3 years since AMH measurement. The risk of menopause was over 6-fold higher for a 0.5 ng/dL decrement in AMH, even when analyses were restricted to women with regular menses and when other known factors associated with menopause were controlled for.
To date, three prospective studies, each with over 9 years of participant follow-up, have demonstrated a strong relationship between AMH and the timing of natural menopause[22–24]. Our study extends the findings of these prior studies to women with more average reproductive histories as prior studies were limited to women with exceptionally healthy reproductive characteristics. For example, all prior studies [22–24] required the presence of menstrual regularity with strict inclusion criteria regarding acceptable lengths of menstrual cycles (e.g., between 22–35 days for the past 3 months [23]). The largest of these studies (Tehrani et al., n=1015 [22]) also required proven fertility (defined as a term pregnancy within one year of stopping pregnancy) for inclusion. In contrast, our findings on AMH level and the incidence of menopause were gleaned from the population-based CARDIA study (a study designed to investigate the reasons for disparity in cardiovascular disease between blacks and whites). The women in CARDIA report histories of infertility [25], menstrual irregularity, and oligomennorhea [26] commonly, thus making our findings generalizable to a broader population of late reproductive age women.
Of the three [22–24] prior 9+ year longitudinal studies of AMH and the timing of menopause, ours is most similar to the Pennsylvania Ovarian Aging Study (POAS) [23] which recruited equal proportions of black and white women (n=401) from a mid-life age range of 35–48 (median age 42 years). In POAS, <10% of participants ages 35–39 had an undetectable AMH. This is similar to the low proportion (3%) of undetectable AMH we observed in our youngest age group. Among the youngest women with undetectable AMH in POAS, the median time to menopause was 9.9 years. Given that the median follow-up of 9.9 years is longer than the maximum length of follow-up for CARDIA, it appears that our finding of only a single menopause event among the youngest CARDIA women should be expected. Longer follow-up and larger sample sizes are needed to determine the long-term clinical significance of low AMH in the youngest women.
CARDIA recruited equal proportions of blacks and whites at its inception in 1985–86. Because of CARDIA’s overall focus on racial differences in disease development between blacks and whites, we believe it is important to assess for racial differences in the relationship between AMH and the timing of menopause. We did not find that race modified the association between AMH and incident menopause. Thus, based on our findings, it appears that the association between AMH and menopause is similar in black and white women. However, our sample size was small and we can only rule out very large differences between the two racial groups. Whether more modest differences between racial groups exist remains an open question. Therefore, larger studies or meta-analyses of existing data are needed.
A major strength of our study is that, AMH, a potential biomarker of menopause, was assessed in a population-based study of women who were unselected for “healthy” reproductive characteristics such as proven fertility and menstrual regularity. A potential limitation in our study is our definition of natural menopause. Our definition was derived from self-reported questions available in CARDIA at multiple in-person examinations (2000–01, 2005–06, and 2010–11). The definition was thus a practical definition based on the questions regarding menopause that were asked at CARDIA examinations and answered by the largest proportion of CARDIA women. This definition, though based on self-reports, was found to be valid when it was tested in a subset of CARDIA women with dates of last menstrual period and has been used in our prior publications[29].
5. Conclusion
AMH is independently associated with incident natural menopause over nine years of follow-up before and after accounting for age, race and other known determinants of menopause. Our findings corroborate those from three previous studies among women with regular menses in which AMH was identified as a potential marker for estimating the onset of natural menopause. Our findings suggest that the relation between AMH and menopause can be generalized to populations of women with more average reproductive histories that include histories of infertility, irregular menses, and oligomennorhea. In summary, AMH holds much promise as a useful marker for estimating the timing of menopause in mid-life women.
Acknowledgments
The Coronary Artery Risk Development in Young Adults Study (CARDIA) is supported by contracts HHSN268201300025C, HHSN268201300026C, HHSN268201300027C, HHSN268201300028C, HHSN268201300029C, and HHSN268200900041C from the National Heart, Lung, and Blood Institute (NHLBI), the Intramural Research Program of the National Institute on Aging (NIA), and an intra-agency agreement between NIA and NHLBI (AG0005). The CARDIA Women’s Study (R01-HL-065611) was supported by the NHLBI. AMH measurement was supported by a National Heart, Lung, and Blood Institute Career Development Award (K23-HL-87114).
Footnotes
The CARDIA study was approved by the Institutional Review Boards of all participating study sites.
Sangeeta Naira – study design, manuscript preparation
James C. Slaughterb – manuscript preparation, statistical analyses
James G. Terrya – manuscript preparation, data management
Duke Appiahc - manuscript writing, analyses
Imo Ebongd - manuscript writing, analyses
Erica Wang e, - manuscript writing, analyses
David S. Siscovickf – manuscript writing, study design
Barbara Sternfeldg – manuscript writing, study design
Pamela J. Schreinerc – manuscript writing, analyses, study design
Cora E. Lewish – manuscript writing, study design
Edmond K. Kabagambei – manuscript writing, study design, analyses
Melissa F. Wellonsi – study funding, study design, data analyses, manuscript writing
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kerr JB, Myers M, Anderson RA. The dynamics of the primordial follicle reserve. Reproduction. 2013;146:R205–15. doi: 10.1530/REP-13-0181. [DOI] [PubMed] [Google Scholar]
- 2.Richardson SJ, Senikas V, Nelson JF. Follicular depletion during the menopausal transition: evidence for accelerated loss and ultimate exhaustion. The Journal of clinical endocrinology and metabolism. 1987;65:1231–7. doi: 10.1210/jcem-65-6-1231. [DOI] [PubMed] [Google Scholar]
- 3.Ledger WL. Clinical utility of measurement of anti-mullerian hormone in reproductive endocrinology. The Journal of clinical endocrinology and metabolism. 2010;95:5144–54. doi: 10.1210/jc.2010-0701. [DOI] [PubMed] [Google Scholar]
- 4.Akdeniz N, Akpolat V, Kale A, Erdemoglu M, Kuyumcuoglu U, Celik Y. Risk factors for postmenopausal osteoporosis: anthropometric measurements, age, age at menopause and the time elapsed after menopause onset. Gynecol Endocrinol. 2009;25:125–9. doi: 10.1080/09513590802549817. [DOI] [PubMed] [Google Scholar]
- 5.Jacobsen B, Knutsen S, Fraser G. Age at natural menopause and total mortality and mortality from ischemic heart disease: the Adventist Health Study. Journal of clinical epidemiology. 1999;52:303–7. doi: 10.1016/s0895-4356(98)00170-x. [DOI] [PubMed] [Google Scholar]
- 6.Jacobsen B, Heuch I, Kvåle G. Age at natural menopause and all-cause mortality: a 37-year follow-up of 19,731 Norwegian women. American journal of epidemiology. 2003;157:923–9. doi: 10.1093/aje/kwg066. [DOI] [PubMed] [Google Scholar]
- 7.Hu F, Grodstein F, Hennekens C, Colditz G, Johnson M, Manson J, et al. Age at natural menopause and risk of cardiovascular disease. Archives of internal medicine. 1999;159:1061–6. doi: 10.1001/archinte.159.10.1061. [DOI] [PubMed] [Google Scholar]
- 8.Lacey JV, Jr, Kreimer AR, Buys SS, Marcus PM, Chang SC, Leitzmann MF, et al. Breast cancer epidemiology according to recognized breast cancer risk factors in the Prostate, Lung, Colorectal and Ovarian (PLCO) Cancer Screening Trial Cohort. BMC Cancer. 2009;9:84. doi: 10.1186/1471-2407-9-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lisabeth L, Beiser A, Brown D, Murabito J, Kelly-Hayes M, Wolf P. Age at natural menopause and risk of ischemic stroke: the Framingham heart study. Stroke. 2009;40:1044–9. doi: 10.1161/STROKEAHA.108.542993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Broer SL, Eijkemans MJ, Scheffer GJ, van Rooij IA, de Vet A, Themmen AP, et al. Anti-Mullerian Hormone Predicts Menopause: A Long-Term Follow-Up Study in Normoovulatory Women. J Clin Endocrinol Metab. 2011 doi: 10.1210/jc.2010-2776. [DOI] [PubMed] [Google Scholar]
- 11.Tehrani FR, Solaymani-Dodaran M, Azizi F. A single test of antimullerian hormone in late reproductive-aged women is a good predictor of menopause. Menopause. 2009;16:797–802. doi: 10.1097/gme.0b013e318193e95d. [DOI] [PubMed] [Google Scholar]
- 12.Tehrani FR, Shakeri N, Solaymani-Dodaran M, Azizi F. Predicting age at menopause from serum antimüllerian hormone concentration. Menopause. 2011;18:766–70. doi: 10.1097/gme.0b013e318205e2ac. [DOI] [PubMed] [Google Scholar]
- 13.Kissell KA, Danaher M, Schisterman E, Perkins N, Schliep K, Ahrens K, et al. Serum Anti-Mullerian Hormone Variation Throughout the Menstrual Cycle in Healthy Regularly Menstruating Young Women. Endocrine reviews. 2013;34:OR19–2. [Google Scholar]
- 14.Baird DD, Steiner AZ. Anti-Mullerian hormone: a potential new tool in epidemiologic studies of female fecundability. American journal of epidemiology. 2012;175:245–9. doi: 10.1093/aje/kwr439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cutter GR, Burke GL, Dyer AR, Friedman GD, Hilner JE, Hughes GH, et al. Cardiovascular risk factors in young adults. The CARDIA baseline monograph. Control Clin Trials. 1991;12:1S–77S. doi: 10.1016/0197-2456(91)90002-4. [DOI] [PubMed] [Google Scholar]
- 16.Friedman GD, Cutter GR, Donahue RP, Hughes GH, Hulley SB, Jacobs DR, Jr, et al. CARDIA: study design, recruitment, and some characteristics of the examined subjects. Journal of clinical epidemiology. 1988;41:1105–16. doi: 10.1016/0895-4356(88)90080-7. [DOI] [PubMed] [Google Scholar]
- 17.Calderon-Margalit R, Schwartz SM, Wellons MF, Lewis CE, Daviglus ML, Schreiner PJ, et al. Prospective association of serum androgens and sex hormone-binding globulin with subclinical cardiovascular disease in young adult women: the “Coronary Artery Risk Development in Young Adults” women’s study. J Clin Endocrinol Metab. 2010;95:4424–31. doi: 10.1210/jc.2009-2643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wellons MF, Lewis CE, Schwartz SM, Gunderson EP, Schreiner PJ, Sternfeld B, et al. Racial differences in self-reported infertility and risk factors for infertility in a cohort of black and white women: the CARDIA Women’s Study. Fertility and sterility. 2008;90:1640–8. doi: 10.1016/j.fertnstert.2007.09.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang ET, Calderon-Margalit R, Cedars MI, Daviglus ML, Merkin SS, Schreiner PJ, et al. Polycystic ovary syndrome and risk for long-term diabetes and dyslipidemia. Obstet Gynecol. 2011;117:6–13. doi: 10.1097/AOG.0b013e31820209bb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lewis CE, Smith DE, Wallace DD, Williams OD, Bild DE, Jacobs DR., Jr Seven-year trends in body weight and associations with lifestyle and behavioral characteristics in black and white young adults: the CARDIA study. Am J Public Health. 1997;87:635–42. doi: 10.2105/ajph.87.4.635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sidney S, Jacobs DR, Jr, Haskell WL, Armstrong MA, Dimicco A, Oberman A, et al. Comparison of two methods of assessing physical activity in the Coronary Artery Risk Development in Young Adults (CARDIA) Study. American journal of epidemiology. 1991;133:1231–45. doi: 10.1093/oxfordjournals.aje.a115835. [DOI] [PubMed] [Google Scholar]
- 22.Tehrani FR, Solaymani-Dodaran M, Tohidi M, Gohari MR, Azizi F. Modeling age at menopause using serum concentration of anti-mullerian hormone. The Journal of clinical endocrinology and metabolism. 2013;98:729–35. doi: 10.1210/jc.2012-3176. [DOI] [PubMed] [Google Scholar]
- 23.Freeman EW, Sammel MD, Lin H, Gracia CR. Anti-mullerian hormone as a predictor of time to menopause in late reproductive age women. The Journal of clinical endocrinology and metabolism. 2012;97:1673–80. doi: 10.1210/jc.2011-3032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Broer SL, Eijkemans MJ, Scheffer GJ, van Rooij IA, de Vet A, Themmen AP, et al. Anti-mullerian hormone predicts menopause: a long-term follow-up study in normoovulatory women. The Journal of clinical endocrinology and metabolism. 2011;96:2532–9. doi: 10.1210/jc.2010-2776. [DOI] [PubMed] [Google Scholar]
- 25.Wellons MF, Lewis CE, Schwartz SM, Gunderson EP, Schreiner PJ, Sternfeld B, et al. Racial differences in self-reported infertility and risk factors for infertility in a cohort of black and white women: the CARDIA Women’s Study. Fertility and sterility. 2008;90:1640–8. doi: 10.1016/j.fertnstert.2007.09.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang ET, Calderon-Margalit R, Cedars MI, Daviglus ML, Merkin SS, Schreiner PJ, et al. Polycystic ovary syndrome and risk for long-term diabetes and dyslipidemia. Obstetrics and gynecology. 2011;117:6–13. doi: 10.1097/AOG.0b013e31820209bb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bower JK, Schreiner PJ, Sternfeld B, Lewis CE. Black-White differences in hysterectomy prevalence: the CARDIA study. American journal of public health. 2009;99:300–7. doi: 10.2105/AJPH.2008.133702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Richard-Davis G, Wellons M. Racial and ethnic differences in the physiology and clinical symptoms of menopause. Seminars in reproductive medicine. 2013;31:380–6. doi: 10.1055/s-0033-1348897. [DOI] [PubMed] [Google Scholar]
- 29.Wellons MF, Bates GW, Schreiner PJ, Siscovick DS, Sternfeld B, Lewis CE. Antral follicle count predicts natural menopause in a population-based sample: the Coronary Artery Risk Development in Young Adults Women’s Study. Menopause. 2013;20:825–30. doi: 10.1097/GME.0b013e31827f06c2. [DOI] [PMC free article] [PubMed] [Google Scholar]