Abstract
Objective
To identify racial and ethnic differences in mortality and cardiovascular (CV) risk among patients with end-stage renal disease (ESRD) due to lupus nephritis (LN)
Methods
Within the U.S. ESRD registry (1995-2008), we identified individuals aged >17 years with incident ESRD due to systemic lupus erythematosus (SLE). We ascertained demographics, clinical factors, and deaths from registry patient files and CV events (myocardial infarction, heart failure, hemorrhagic and ischemic strokes) from inpatient Medicare claims. We calculated incidence rates [95% confidence intervals (CI)] per 1,000 person-years for study events, stratified by race and ethnicity. We compared probabilities of the events among racial/ethnic groups using cumulative incidence function curves and multivariable-adjusted sub-distribution proportional hazard ratios (HRsd), taking into account the competing events of kidney transplantation and death (for non-fatal CV events).
Results
Of 12,533 patients with LN ESRD, mean age was 40.7 ± 14.9 years; 82% were women and 49% African Americans. The overall mortality rate was 98.1/1,000 persons-years (95%CI 95.3-100.9). In multivariable models, Asian and Hispanic LN ESRD patients had lower mortality than Whites [HRsd 0.70 (95%CI: 0.58-0.84) and 0.79 (95%CI: 0.71-0.88)], whereas African Americans had higher mortality [HRsd 1.27 (95%CI: 1.18-1.36)]. African American patients under age 40 had higher mortality than their White counterparts [HRsd 1.67 (95 %CI:1.44-1.93)]. African Americans were more likely to be admitted for heart failure or hemorrhagic stroke.
Conclusion
Among patients with LN ESRD, Asians and Hispanics experienced lower mortality and CV event risks, while African Americans had higher mortality and CV event risks compared to Whites.
Keywords: End-stage renal disease, lupus nephritis, cardiovascular disease, race, ethnicity, disparities, mortality, survival
Up to 60% of individuals with SLE develop kidney involvement (lupus nephritis, LN) and, among those with LN, up to 30% progress to end-stage renal disease (ESRD) within 10 years, even with aggressive therapy 1-4. We have reported that among all U.S. patients newly presenting with ESRD due to LN from 1995-2006, standardized incidence rates among those in the youngest age group, among African Americans and in the U.S. South, steadily increased5. Unfortunately, the three-year mortality in the LN ESRD population was approximately 27% and this did not decrease between 1995 and 2006 5. In addition, mortality in LN ESRD has been reported to be twice as high as mortality in other causes of ESRD 6.
In the U.S., the incidence rates of both LN and LN-related ESRD are three to five times higher among African Americans compared with Whites, for multifactorial and poorly understood reason 5,7-10. African Americans also develop ESRD at younger ages than do Whites, which has complicated the interpretation of past studies investigating mortality and long-term outcomes among ESRD patients by race11. Despite documented disparities in healthcare for African American compared to White patients with chronic kidney disease, until recently it was thought that African Americans receiving dialysis had improved survival compared to their White counterparts 11,12. However, it is now understood that these past counterintuitive findings were the results of inadequately addressing patient age. In fact, racial disparities in all-cause ESRD mortality are most pronounced in younger age groups. A recent large observational study of individuals with ESRD from any cause in the U.S. reported that African American patients age 50 years and under had increased mortality rates compared to White patients of the same age, after adjustment for age at ESRD onset and the competing and differential risk of kidney transplantation 11. In contrast, Hispanic patients had lower mortality rates than non-Hispanics in an analysis of incident all-cause ESRD patients13. Among all patients with ESRD, the risks of mortality and cardiovascular (CV) events are particularly high in the first year after onset of ESRD; these risks decline in subsequent years14.
While patients with SLE and LN are at high risk of CV disease, they represent a young subgroup of ESRD patients15, 16. Among patients with SLE, African Americans may be at higher CV risk than Whites, although this has not been well or conclusively established17. Among children, we have found that adjusted mortality rates were two times higher for African Americans compared to Whites, and that CV disease and infections were the two leading causes of death 18. Given the lack of studies examining mortality and non-fatal CV events by race and ethnicity in adult patients with LN-related ESRD, the increased CV risks among SLE patients, and the increased prevalence and severity of SLE and LN among non-Whites 5,10, we aimed to investigate racial and ethnic variation in mortality and CV events rates among adult patients with ESRD due to LN.
Methods
Study Population
We used data from the U.S. Renal Data System (USRDS), the national registry of patients with ESRD, to identify all individuals older than 17 years who reached ESRD between January 1, 1995 and December 31, 2008 and whose nephrologist attested on the Medical Evidence Report to the Centers for Medicare and Medicaid Services (CMS) that ESRD was secondary to LN (International Classification of Disease, 9th Revision (ICD-9) code 710.0 for SLE)19. For each patient with incident ESRD, the attending nephrologist is required to complete and submit the Medical Evidence Report form to CMS. The positive predictive value (PPV) of this code on the CMS Medical Evidence Report for the presence of LN on renal biopsy was 100% in a recent validation study20.
While death is reported independent of the type of insurance, studies of non-fatal events require the presence of Medicare claims. Most eligible patients with ESRD qualify for Medicare coverage on day 90 after their reported ESRD date, regardless of their age. Therefore, non-fatal CV events of interest were studied in the subset of patients with Medicare (Parts A+B) as their primary payer on day 90.
Data collection
Exposures
Race and ethnicity are recorded separately in the USRDS database, as they are in the US Census. Race and ethnicity were reported on the Medical Evidence Report and categorized into four race categories: White, African American, Native American and Asian. Ethnicity was classified in two categories: Hispanics and Non-Hispanics. We retained race and ethnicity as separate variables in the primary analyses. We also performed cross-classified analyses of African American or White race and Hispanic or Non-Hispanic ethnicity for the analyses described below.
Other Variables
Covariates at baseline were also ascertained from the Medical Evidence Report and included age at ESRD onset, sex, comorbidities [hypertension, diabetes mellitus, coronary artery disease, peripheral vascular disease, chronic obstructive pulmonary disease , cancer, cerebrovascular accidents , congestive heart failure, smoking history, and intravenous drug abuse] and laboratory measurements [estimated glomerular filtration rate (eGFR), albumin and hemoglobin], body mass index (BMI, kg/m2), employment status (employed, unemployed), medical insurance type prior to ESRD (Medicare, Medicaid, private, no insurance, or other), U.S. region of residence (Northeast, South, Midwest, West or other territories) and initial renal replacement modality (peritoneal dialysis or hemodialysis; pre-emptive kidney transplantation was treated as a competing risk on day 1). Multiple imputation analyses were used to impute missing baseline data for albumin (19.3% missing), BMI (4.2% missing) and eGFR (0.7%). Multiple imputation is a simulation-base technique for handing missing data21. We used SAS 9.3 proc MI, generating 15 imputation sets.
For area-based socioeconomic status (SES), we employed a composite index of seven SES indicators at the zip code level using 2000 U.S. Census data previously developed by Ward using USRDS data22. These include median household income, proportion with income below 200% of the federal poverty level, median home value, median monthly rent, mean education level, proportion of people age ≥25 years who were college graduates, and proportion of employed persons with a professional occupation10.
Outcomes
We examined all-cause mortality for all incident LN ESRD patients, regardless of Medicare coverage. Deaths were documented on the CMS ESRD Death Notification Form, completion of which is mandatory and enforced by CMS15. We classified the causes of death into three different categories: CV, infectious and other causes (including intra-abdominal, hemorrhagic, neurologic and renal events, as well as missing or not classified). Among patients with Medicare Parts A and B coverage, CV events from inpatient hospital ICD-9 discharge diagnosis codes, including myocardial infarction (MI, ICD-9s: 410.x1), heart failure (ICD-9s: 428, 402.01, 402.11, 402.91, 404.01, 404.11, 404.91, 404.03, 404.13, and 404.93), hemorrhagic stroke (ICD-9s: 430, 431, and 432), and ischemic stroke (ICD-9s: 433.x1, 434. x1, 435, 436), were captured starting >90 days after Medical Evidence Report date of ESRD onset.
Statistical Analyses
To describe the demographics and clinical characteristics of the new onset LN ESRD population at baseline, we employed means and standard deviations for continuous variables and proportions categorical variables. Chi-squared tests were used to compare categorical variables and t-tests were used to compare continuous variables, across categories of race and ethnicity. We corrected for multiple comparisons using the Bonferroni method 23.
We calculated CV event incidence rates (IR) and all-cause mortality rates per 1,000 person-years of follow-up and their corresponding 95% confidence intervals (CI). IRs were compared for statistical differences using 95% CIs. For mortality analyses, subjects were followed from the date of ESRD onset. For CV outcomes, subjects with Medicare parts A and B were followed from >90 days after the date of ESRD onset. Thus, subjects were also excluded from analyses if they died, had a CV event, or loss to follow-up prior to that date. Several data sources are used by USRDS to determine loss to follow-up, including dialysis center and Medicare claims files 24.
We conducted survival analyses for the outcomes of death and non-fatal CV events, which are competing events as individuals who are at high risk for CV events are also at high risk of death. Furthermore, as kidney transplantation rates vary according to race and ethnicity, they are subject to informative or biased censoring5. Thus, we employed survival analyses formally taking non-fatal CV events, kidney transplantation, and death into account as competing risks. (Death was not considered a competing event in the mortality analyses in which it was the outcome). Follow-up started at the date of onset of ESRD for mortality analyses, and from 90 days after the date of ESRD onset for CV events analyses. Individuals were censored when they were lost to follow-up from USRDS. First, we used cumulative incidence function curves to illustrate the probability of all-cause mortality according to race/ethnicity over the study period, with kidney transplantation as a competing event using Gray's Test for equality of cumulative incidence functions 25. Secondly, we employed Fine and Gray sub-distribution proportional hazards (HRsd) models to examine multivariable-adjusted risks of hospitalized CV events by racial and ethnic group, taking the competing risks of death and kidney transplantation into account. In order to examine the effects of adjustment for different sets of potential confounders in a sequential manner (first sociodemographic and baseline clinical values and then baseline CV comorbidities), we fit two different multivariable models to examine the HRsd of race/ethnicity with mortality risk among LN patients26. Model A included sex, age at ESRD onset, calendar year, medical insurance type at ESRD onset, area-level socioeconomic status, BMI, albumin, eGFR, employment status at ESRD onset, U.S. region of residence (Northeast, South, Midwest or West), and initial renal replacement modality. Model B added comorbidities, including smoking, hypertension, diabetes mellitus, coronary artery disease, heart failure, cerebrovascular accidents, peripheral vascular disease, cancer and chronic obstructive pulmonary disease. We also performed age group-stratified analyses among patients 18-39 years, 40-59 years, ≥60 years at ESRD onset and to confirm whether differences between age groups were statistically significant, an additional model was built including interaction terms for each age category and African American race, and testing for multiplicative interactions. In sensitivity analyses, we repeated analyses for models A and B using only data without imputation of baseline laboratory or clinical values.
All p values were calculated with two-sided significance level of 0.05. All statistical analyses were conducted using SAS, version 9.3. Data were obtained from the USRDS through a data use agreement. According to USRDS research regulations, cell sizes of < 11 individuals were suppressed from the results tables. The Partners' Healthcare Institutional Review Board waived human subjects’ approval for this study.
Results
We identified 12,533 patients over age 17 with new-onset LN-associated ESRD who initiated dialysis from 1995 through 2008. Mean age at ESRD onset was 40.7 ±14.9 years; 82% were women, 49% were African American, 44% White, and 84% non-Hispanic (5.0% of Asians, 1.8% of African Americans, 10.7% of Native Americans and 33.8% of Whites were classified as of Hispanic ethnicity.) Baseline characteristics are shown in Table 1. African American patients had lower eGFR, which has been associated with CV risk27, and hemoglobin levels, as well as lower rates of pre-emptive kidney transplantation than did other races. Native American patients had a higher prevalence of hypertension, diabetes mellitus, heart failure, cerebrovascular accidents, peripheral vascular disease and higher mean BMI than other races. Conversely, Asian patients had the lowest BMIs and lower prevalence of these comorbidities. Asian patients also more often received peritoneal dialysis and pre-emptive kidney transplantation, compared to other races.
Table 1.
Baseline Characteristics of Patients with Lupus Nephritis ESRD in the U.S., 1995-2008
| Total N= 12,533 |
Whites n=5,547 |
African Americans n=6,181 |
Asians n=683 |
Native- Americans n=122 |
p- value* |
Non- Hispanics n=10,502 |
Hispanics n=2,031 |
p-value* | |
|---|---|---|---|---|---|---|---|---|---|
| Demographics | |||||||||
| Female | 10,228 (81.6 %) | 4,334 (78.1%) | 5,228 (84.6%) | 569 (83.3%) | 97 (79.5%) | <0.0001 | 8,565 (81.6%) | 1,663 (82%) | 0.72 |
| Mean age at ESRD onset | 40.78 ± 14.91 | 43.29 ± 16.23 | 39.03 ± 13.41 | 36.58 ± 13.34 | 39.01 ± 13.99 | <0.0001 | 41.64 ± 15.04 | 36.37 ± 13.37 | <0.0001 |
| Laboratory measures | |||||||||
| Hemoglobin (g/dl) | 9.40 ± 1.82 | 9.64 ± 1.82 | 9.14 ± 1.77 | 9.65 ± 1.89 | 9.44 ± 2.00 | <0.0001 | 9.40 ± 1.81 | 9.37 ± 1.86 | 0.54 |
| eGFR (ml/min/1.73m2) (N=12,454) | 8.12 ± 4.89 | 8.74 ± 5.09 | 7.49 ± 4.65 | 8.75 ± 4.85 | 8.50 ± 4.34 | <0.0001 | 8.01 ± 4.76 | 8.69 ± 5.51 | <0.0001 |
| Serum albumin (g/dl) (N=10,123) | 2.92 ± 0.80 | 3.03 ± 0.78 | 2.82 ± 0.80 | 2.91 ± 0.85 | 2.81 ± 0.85 | <0.0001 | 2.92 ± 0.80 | 2.92 ± 0.81 | 0.87 |
| Mean BMI (kg/m2) (N=12,012) | 26.12 ± 6.99 | 25.70 ± 6.66 | 26.77 ± 7.32 | 23.49 ± 5.56 | 26.39 ± 6.70 | <0.0001 | 26.16 ± 7.07 | 25.91 ± 6.52 | 0.15 |
| Comorbidities | |||||||||
| Hypertension | 9,642 (77.0%) | 4,136 (74.5%) | 4,902 (79.3%) | 504 (73.8%) | 100 (82.0%) | <0.0001 | 8,130 (77.4%) | 1,512 (74.4%) | 0.004 |
| Current Smoking | 495 (4.0%) | 269 (4.8%) | 207 (3.3%) | 13 (2.0%) | - | <0.0001 | 459 (4.3%) | 36 (1.7%) | <0.0001 |
| Diabetes Mellitus | 1,123 (9.0%) | 460 (8.2%) | 614 (9.9%) | 36 (5.2%) | 13 (10.6%) | <0.0001 | 959 (9.1%) | 164 (8.0%) | 0.12 |
| Heart Failure | 1,958 (16.0%) | 889 (16.0%) | 970 (15.7%) | 73 (10.7%) | 26 (21.0%) | 0.001 | 1,709 (16.2%) | 249 (12.2%) | <0.0001 |
| Coronary Artery Disease | 845 (7.0%) | 518 (9.3%) | 302 (4.9%) | 16 (2.3%) | - | <0.0001 | 758 (7.2%) | 87 (4.2%) | <0.0001 |
| Cerebrovascular Accident | 649 (5%) | 338 (6.0%) | 284 (4.6%) | 19 (2.8%) | - | <0.0001 | 565 (5.3%) | 84 (4.1%) | 0.02 |
| Peripheral Vascular Disease | 417 (3.0%) | 226 (4.0%) | 173 (2.8%) | - | - | <0.0001 | 361 (3.4%) | 56 (2.7%) | 0.11 |
| Chronic | 295 | 188 | 101 | - | - | <0.000 | 269 | 26 | 0.0005 |
| Obstructive Pulmonary Disease | (2.0%) | (3.4%) | (1.6%) | 1 | (2.5%) | (1.3%) | |||
| Cancer | 189 (1.5%) | 119 (2.1%) | 64 (1.0%) | - | - | <0.0001 | 173 (1.6%) | 16 (0.8%) | 0.004 |
| Medical Insurance | |||||||||
| No Insurance | 1,315 (10.5%) | 531 (9.6%) | 718 (11.6%) | 60 (8.8%) | - | 0.0002 | 987 (9.4%) | 328 (16.1%) | <0.0001 |
| Medicare | 2,057 (16.4%) | 1,175 (21.0%) | 801 (13.0%) | 66 (9.6%) | 15 (12.0%) | <0.0001 | 1,878 (17.8%) | 179 (8.8%) | <0.0001 |
| Medicaid | 4,014 (32.0%) | 1,390 (25.0%) | 2,397 (38.8%) | 172 (25.0%) | 55 (45.0%) | <0.0001 | 3270 (31.1%) | 744 (36.6%) | <0.0001 |
| Private | 3,704 (29.5%) | 1,736 (31.0%) | 1,678 (27.0%) | 254 (37.0%) | 36 (29.0%) | <0.0001 | 3,187 (30.3%) | 517 (25.4%) | <0.0001 |
| Other | 1,443 (11.5%) | 715 (12.9%) | 587 (9.4%) | 131 (19.2%) | - | <0.0001 | 1,180 (11.1%) | 263 (12.9%) | 0.02 |
| Region of Residence | |||||||||
| Northeast | 2,094 (16.7%) | 1,016 (18.3%) | 978 (15.8%) | 93 (13.6%) | - | <0.0001 | 1,801 (17.2%) | 293 (14.4%) | 0.002 |
| West | 2,408 (19.2%) | 1,481 (26.7%) | 492 (8.0%) | 395 (57.8%) | 40 (32.8%) | <0.0001 | 1,642 (15.6%) | 766 (37.7%) | <0.0001 |
| South | 5,460 (43.6%) | 1,814 (32.7%) | 3,501 (56.6%) | 110 (16.1%) | 35 (28.7%) | <0.0001 | 4755 (45.3%) | 705 (34.7%) | <0.0001 |
| Midwest | 2,435 (19.4%) | 1,139 (20.5%) | 1,190 (19.3%) | 67 (9.8%) | 39 (32.0%) | <0.0001 | 2,277 (21.7%) | 158 (7.8%) | <0.0001 |
| Other Territories | 136 (1.1%) | 97 (1.8%) | 20 (0.3%) | 18 (2.6%) | - | <0.0001 | 27 (0.3%) | 109 (5.4%) | <0.0001 |
| Area-level Socioeconomic Status (SES)** | |||||||||
| Lowest Quartile of SES in USRDS | 25% | 24.3% | 27.3% | 5.2% | 49.6% | <0.0001 | 27.2% | 13.2% | <0.0001 |
| Initial ESRD Modality #x00A5; | |||||||||
| Hemodialysis | 10,099 (80.6%) | 4,235 (76.3%) | 5,264 (85.0%) | 505 (74.0%) | 95 (78.0%) | <0.0001 | 8,449 (80.4%) | 1,650 (81.2%) | 0.41 |
| Peritoneal dialysis | 1,376 (11.0%) | 692 (12.5%) | 568 (9.1%) | 102 (15.0%) | 14 (11.0%) | <0.0001 | 1,145 (10.9%) | 231 (11.4%) | 0.53 |
| Pre-emptive transplant | 337 (2.7%) | 252 (4.5%) | 46 (0.7%) | 32 (4.7%) | - | <0.0001 | 293 (2.8%) | 44 (2.2%) | 0.11 |
Chi-squared tests for categorical values and t- tests for continuous variables. With Bonferroni correction for multiple comparisons, threshold for statistical significance= 0.025.
Area-level socioeconomic status: composite index of seven zip code level indicators from 2000 U.S. Census data (Ward MM, Am J Kidney Dis, 2008)
Cell sizes < 11 suppressed per Federal research regulations
#x00A5: 5.7% of patients were classified as uncertain for the initial renal replacement modality in the Medical Evidence Report at USRDS entry.
In comparison to non-Hispanics, Hispanic patients were younger and had lower prevalence of several CV comorbidities including smoking, hypertension, DM, and lower prevalence of peripheral vascular disease. A lower proportion of Hispanic patients received erythropoietin-stimulating agents and more had no medical insurance. There were no differences in terms of clinical laboratories or renal replacement treatment modalities between Hispanics and non-Hispanics.
Rates of death and transplantation are high in this population; as such, outcomes for many patients occurred soon after ESRD onset and the median follow-up time (time from ESRD onset to first noted event) was 2.9 years (range: 0-13.9 years). During follow-up, 28.7% of patients underwent kidney transplantation overall, (35.0% of Whites, 22.4% of African Americans, 36.0% of Asians and 25.4% of Native Americans (p <0.0001), as well as 28.1% of Non-Hispanics and 31.7% of Hispanic patients (p=0.001). Overall, loss to follow-up occurred in 396 of patients (3.1%). By categories, loss to follow-up occurred in 3.8% of Whites, 2.1% of African Americans, 6.3% of Asians and 2.4% of Native Americans (p = 0.006), as well as 2.9% of Non-Hispanics and 4.4% of Hispanic patients (p=0.0002).
The annual mortality rate among all patients with LN ESRD was 98.1/1,000 persons-years (95% CI 83.2, 88.0) (Table 2). All-cause mortality rates were highest among African Americans and Native Americans, and Non-Hispanic patients had higher mortality rates than did Hispanic patients. In race and ethnicity cross-classified analyses, mortality rates were also higher among Non-Hispanic African Americans and Non-Hispanic Whites.
Table 2.
Unadjusted Annual Mortality Rates among Patients with Lupus Nephritis ESRD in the U.S., 1995-2008
| Number of Events | Person-years | Mean Follow-up period years (SD) | Mortality Rate per 1,000 LN ESRD patients per year (95% CI) | |
|---|---|---|---|---|
| All patients n=12,533 | 4,789 | 48,832 | 2.94 (2.75) | 98.1 (95.3, 100.9) |
| Race | ||||
| Whites n= 5,547 | 2,089 | 20,947 | 2.80 (2.75) | 99.7 (95.5, 104.1) |
| African Americans n= 6,181 | 2,505 | 24,739 | 3.03 (2.74) | 101.3 (97.4, 105.3) |
| Asians n= 683 | 151 | 2,711 | 3.38 (2.72) | 55.7 (47.5, 65.3) |
| Native Americans n=122 | 44 | 433 | 3.15 (2.71) | 101.4 (75.5, 136.3) |
| Ethnicity | ||||
| Non-Hispanics n= 10,502 | 4,247 | 40,643 | 2.92 (2.72) | 104.5 (101.4, 107.7) |
| Hispanics n=2,031 | 542 | 8,189 | 3.09 (2.95) | 66.2 (60.9, 72.0) |
| Race/Ethnicity | ||||
| Non-Hispanic African Americans, n=6,070 | 2,465 | 24,330 | 3.04 (2.75) | 101.3 (97.4, 105.4) |
| Hispanic African Americans, n=111 | 40 | 410 | 2.31 (2.22) | 97.6 (71.6, 133.1) |
| Non-Hispanic White, n=3,674 | 1,599 | 13,349 | 2.70 (2.66) | 119.8 (114.1,125.8) |
| Hispanic White, n=1,873 | 490 | 7,599 | 3.12 (3.00) | 64.5 (59.0, 70.5) |
The mean duration of follow-up for all LN ESRD patients was 2.94 (SD 2.75) years. This was longest among Asians and Native Americans (3.38 and 3.15) and those of Hispanic ethnicity (3.09), and shortest among Whites (2.80).
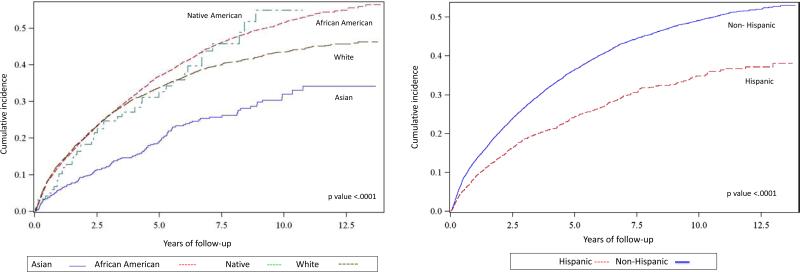
In unadjusted analyses taking the competing risk of loss to follow-up and kidney transplantation into account, both African American and Non-Hispanic LN ESRD patients had higher cumulative incidence rates for all-cause mortality, compared to Asians and Hispanics (Figure 1). The 1-year, 5-year, 10-year patient survival rates were significantly better for Asians (95%, 81%, and 67%) than for Whites (87%, 67%, and 57%), Native Americans (89%, 66%, and 46%), and African Americans (88%, 63%, and 49%) (p <.0001). With respect to ethnicity, 1-year, 5-year and 10-year survival rates were significantly better for Hispanics (92%, 76% and 65%) than for Non-Hispanics (87%, 64%, and 51%) (p <.0001).
Figure 1.
Cumulative Incidence Function Estimates for Mortality by Race and Ethnicity among Patients with Lupus Nephritis ESRD in the U.S., 1995-2008
(Kidney transplantation as competing event)
Multivariable-adjusted HRsd for all-cause mortality by race and ethnicity for LN ESRD patients are presented in Table 3. In model A, adjusting for baseline sociodemographic and clinical values, Asian and Hispanic LN ESRD patients had lower mortality [HRsd 0.65 (95%CI 0.54-0.78) and 0.75 (0.68-0.84)], compared to White LN ESRD patients. In fully-adjusted models (model B), Asian and Hispanic LN ESRD continued to have lower mortality risks than did White LN ESRD patients [HRsd 0.70 (95% CI 0.58-0.84) and 0.79 (95% CI 0.71-0.88)], whereas African American LN ESRD patients experienced higher mortality [HRsd 1.27 (95% CI 1.18-1.36)]. Adjustment for baseline CV comorbidities did not substantially affect the HRsd estimates. In sensitivity analyses of models A and B using only data without imputation of missing baseline laboratory or clinical values, the results were very similar, with lower mortality risks for Asians and Hispanic ( HRsd 0.63 (95% CI 0.53-0.75) and 0.75 (95% CI 0.67-0.83) respectively] and higher risks for African American LN ESRD patients [HRsd 1.18 (95% CI 1.10-1.26)], albeit with larger confidence intervals due to the smaller sample size (n= 10,213).
Table 3.
Sub-distribution Hazard Ratios (HRsd) with 95% Confidence Intervals for Death among Patients with Lupus Nephritis ESRD in the U.S., 1995-2008 (Kidney transplantation as competing event)
| Model A* | Model B** | |
|---|---|---|
| Race | ||
| Whites | 1.0 (Ref) | 1.0 (Ref) |
| African Americans | 1.22 (1.13-1.31) | 1.27 (1.18-1.36) |
| Asians | 0.65 (0.54- 0.78) | 0.70 (0.58-0.84) |
| Native Americans | 1.13 (0.83-1.55) | 1.13 (0.83-1.55) |
| Ethnicity | ||
| Non-Hispanics | 1.0 (Ref) | 1.0 (Ref) |
| Hispanics | 0.75 (0.68-0.84) | 0.79 (0.71-0.88) |
| Race/Ethnicity | ||
| Non-Hispanic African Americans | 1.0 (Ref) | 1.0 (Ref) |
| Hispanic African Americans | 1.02 (0.73-1.41) | 1.04 (0.75-1.43) |
| Non-Hispanic Whites | 1.0 (Ref) | 1.0 (Ref) |
| Hispanic Whites | 0.70 (0.62-0.79) | 0.73 (0.65-0.82) |
Model A: Adjusted for sex, age at ESRD onset, calendar year, type of medical insurance prior to ESRD, body mass index, estimated glomerula filtration rate, albumin, employment status, area-level socioeconomic status based on seven U.S. Census indicators, U. S. region of residence, and type of initial renal replacement therapy
Model B: Model A + comorbidities (smoking, hypertension, diabetes mellitus, coronary artery disease, heart failure, cerebrovascular accidents, chronic obstructive pulmonary disease, peripheral vascular disease and cancer)
In race and ethnicity cross-classified analyses, Non-Hispanic African American and Hispanic African Americans had similar risk of death in both survival models, whereas Hispanic White LN ESRD patients had a 27% lower risk of death in the fully-adjusted models (model B) compared to their Non-Hispanic Whites counterparts.
In age-stratified analyses, we found that African American LN ESRD patients under age 40 had an almost 70% higher risk of death than their White counterparts, and patients between 40-59 years had a 26% higher risk than their White counterparts (Table 4). Hispanic patients had lower adjusted mortality than non-Hispanics in patients older than 40 and older than 60 years. Interactions were statistically significant, confirming variation in mortality disparities across the age categories (p <0.0001 for African Americans, p=0.03 for Asian and p=0.04 for Native Americans).
Table 4.
Sub-distribution Hazard Ratios (HRsd) with 95% Confidence Intervals for Death, stratified by Age group, among Patients with Lupus Nephritis ESRD in the U.S., 1995-2008 (Kidney transplantation as competing event)
| Age at ESRD Onset | ||||
|---|---|---|---|---|
| 18-39 years | 40-59 years | #x2265; 60 years | p interaction* | |
| Race | ||||
| White | 1.0 (Ref) | 1.0 (Ref) | 1.0 (Ref) | |
| African American | 1.67 (1.44-1.93) | 1.26 (1.12-1.41) | 1.01 (0.86-1.20) | <0.0001 |
| Asian | 0.99 (0.76-1.29) | 0.68 (0.52-0.90) | 0.37 (0.21-0.65) | 0.03 |
| Native American | 1.50 (0.97-2.32) | 1.05 (0.66-1.66) | 0.73 (0.27-1.95) | 0.04 |
| Ethnicity | ||||
| Non-Hispanic | 1.0 (Ref) | 1.0 (Ref) | 1.0 (Ref) | |
| Hispanic | 0.94 (0.79-1.12) | 0.78 (0.65-0.94) | 0.76 (0.60-0.98) | 0.36 |
| Race/Ethnicity | ||||
| Non-Hispanic African Americans | 1.0 (Ref) | 1.0 (Ref) | 1.0 (Ref) | |
| Hispanic African Americans | 0.99 (0.59-1.63) | 1.06 (0.60-1.88) | 1.45 (0.65-3.22) | 0.11 |
| Non-Hispanic Whites | 1.0 (Ref) | 1.0 (Ref) | 1.0 (Ref) | |
| Hispanic Whites | 0.86 (0.69-1.07) | 0.75 (0.61-0.92) | 0.69 (0.52-0.91) | 0.21 |
Models adjusted for sex, age at ESRD onset, calendar year, type of medical insurance prior to ESRD, body mass index, estimated glomerular filtration rate, albumin, employment status, area-level socioeconomic status, U. S. region of residence, type of renal replacement therapy and comorbidities (smoking, hypertension, diabetes mellitus, coronary artery disease, heart failure, cerebrovascular accidents, chronic obstructive pulmonary disease, peripheral vascular disease and cancer).
p interaction= multiplicative interaction from a model additionally including interaction terms for race and each age category.
CV events were the main cause of 32% of deaths, distributed by race as follows: Asians 32%, African Americans 32%, Native Americans 27% and Whites in 33%. Death from CV events was slightly less common in Hispanic (30%) than in non-Hispanics (33%). Overall, infections accounted for 19%, various other causes 20%, and missing 29%.
Non-fatal CV events were studied in the subset of patients with Medicare Parts A and B as their primary payer on day 90 (N= 6,064). Their baseline clinical and sociodemographic characteristics are described in Supplementary Table 1. We found significant differences across racial and ethnic groups. IRs for admission due to non-fatal CV events are presented in Supplementary Table 2. The overall crude annual IR among LN ESRD patients was 87.1 per 1,000 person-years (95% CI 84-3-90.1) for admission due heart failure, 15.1 per 1,000 person-years (95% CI 14.1-16.3) for MI, 14.5 per 1,000 person-years (95% CI 13.5-15.6) for ischemic stroke and 5.2 per 1,000 person-years (95% CI 4.6-5.9) for hemorrhagic stroke. IRs for admission due to heart failure were almost two-fold elevated among African Americans and Native American (100.9 and 117.9) than among Asians (42.9). African Americans and Whites (14.5 and 16.8) had higher crude IRs for admission due to MI than did Asians and Native Americans (9.4 and 9.4) and IRs for admissions for ischemic stroke were twice as high among African Americans and Whites (15.1 and 15.0), than among Asians (6.3). Finally, IRs for admission due hemorrhagic stroke were twice as high among Native Americans (9.3) than among Asians (4.8). In general, IRs for CV events were significantly higher among Non-Hispanic than among Hispanic patients. IR for admission due CV events were similar among Non-Hispanic African Americans and Hispanic African Americans, except that for hemorrhagic stroke which was lower for Hispanic African Americans. Hispanic Whites had lower IRs for admissions due heart failure, myocardial infarction, ischemic and hemorrhagic stroke than did Non-Hispanic Whites (data not shown).
In multivariable-adjusted competing risk analysis, we also found racial variation in the risk of admission for CV events (Table 5). In model A, African American LN ESRD patients had a 34% higher risk of admission for heart failure and 57% higher risk of hemorrhagic stroke. In model B, additionally adjusting for comorbidities, African American LN ESRD patients remained 38% and 66% more likely to be admitted for heart failure and hemorrhagic stroke, respectively, than were White LN ESRD patients. There was a suggestion that Hispanic patients were less likely to be admitted for all non-fatal CV events than Hispanic LN ESRD patients, but these differences were not statistically significant. In race and ethnicity cross-classified analyses, there were no significant differences in risks of admission for non-fatal CV events across groups (data not shown).
Table 5.
Sub-distribution Hazard Ratios and 95% Confidence Intervals for Cardiovascular Events among Patients with Lupus Nephritis ESRD in the U.S., 1995-2008 (Kidney transplantation and death as competing events)
| Heart Failure | Myocardial Infarction | Ischemic Stroke | Hemorrhagic Stroke | |||||
|---|---|---|---|---|---|---|---|---|
| Model A* | Model B** | Model A* | Model B** | Model A* | Model B** | Model A* | Model B** | |
| Race | ||||||||
| White | 1.0 (Ref) | 1.0 (Ref) | 1.0 (Ref) | 1.0 (Ref) | 1.0 (Ref) | 1.0 (Ref) | 1.0 (Ref) | 1.0 (Ref) |
| African American | 1.35 (1.23-1.48) | 1.38 (1.26-1.52) | 1.10 (0.91-1.34) | 1.13 (0.93-1.37) | 1.11 (0.92-1.35) | 1.12 (0.92-1.36) | 1.57 (1.12-2.20) | 1.66 (1.19-2.31) |
| Asian | 0.82 (0.66-1.01) | 0.85 (0.69-1.06) | 1.10 (0.71-1.71) | 1.17 (0.76-1.81) | 0.71 (0.42-1.20) | 0.73 (0.43-1.24) | 1.64 (0.87-3.12) | 1.77 (0.93-3.38) |
| Native American | 1.36 (0.97-1.91) | 1.33 (0.94-1.89) | 0.63 (0.20-1.97) | 0.62 (0.19-1.94) | 0.52 (0.16-1.60) | 0.51 (0.16-1.57) | 2.12 (0.76-5.92) | 2.10 (0.75-5.90) |
| Ethnicity | ||||||||
| Non-Hispanic | 1.0 (Ref) | 1.0 (Ref) | 1.0 (Ref) | 1.0 (Ref) | 1.0 (Ref) | 1.0 (Ref) | 1.0 (Ref) | 1.0 (Ref) |
| Hispanic | 0.93 (0.82-1.06) | 0.97 (0.85-1.11) | 0.77 (0.58-1.04) | 0.79 (0.59-1.06) | 0.85 (0.64-1.14) | 0.86 (0.64-1.16) | 0.92 (0.56-1.50) | 0.97 (0.60-1.58) |
Model A: Adjusted for sex, age at ESRD onset, calendar year, type of medical insurance prior to ESRD, body mass index, estimated glomerular filtration rate, albumin, employment status, area-level socioeconomic status based on seven U.S. Census indicators, U. S. region of residence, and type of renal replacement therapy
Model B: Model A + comorbidities (smoking, hypertension, diabetes mellitus, coronary artery disease, heart failure, cerebrovascular accidents, chronic obstructive pulmonary disease, and peripheral vascular disease and cancer)
Discussion
In a large cohort of >12,000 patients with incident LN ESRD over 14 years, we found substantial racial and ethnic variation in mortality and CV event rates. One, five and 10-year survival rates were better among Asians than Whites and among Hispanics than non-Hispanics. Mortality risks among Asian LN ESRD patients were 30% (95%CI 0.58-0.84) lower and those among Hispanic LN ESRD patients 21% (95%CI 0.71-0.88) lower, compared to those in White patients, even after adjusting for multiple demographic and clinical factors and accounting for the competing risk of kidney transplantation. We also found that African American LN ESRD patients had 27% increased mortality risk in comparison with Whites. That risk was highest among African American LN ESRD patients ages 18-39 years who had a 67% higher risk of death than their White counterparts. African American LN ESRD patients were also more likely to be admitted for heart failure and hemorrhagic stroke than White LN ESRD patients. These results are consistent with a recent study of all incident ESRD patients in the U.S., in which the relationship between race and mortality was found to be modified by patient age: African American patients ages 50 years or younger had significantly higher mortality rates than did Whites of the same age16. We have also found that, within the U.S., Asian patients with LN have 30% lower rates of mortality compared to White patients, after adjusting for multiple possible confounders including age, comorbidities, and BMI. Our study highlights the racial and ethnic variation in outcomes among patients with LN ESRD, and that Hispanic and Asian patients do comparatively well after the development of LN ESRD. We also found that Hispanic White patients had a lower mortality risks than did Non-Hispanic Whites. This survival advantage of Hispanic compared to Non-Hispanic Whites has been observed recently in incident all-cause ESRD patients13.
Past studies of the relationship between African American vs. White race and the risk of CV events among all-cause ESRD patients have not shown a conclusive pattern, and this may also be due to the variation in the ages of the populations compared11, 12. In a population of diabetic veterans with ESRD, the prevalence of MI and HF was reported to be lower among African Americans than Whites28. However, in ARIC (Cardiovascular Health Study, Framingham Heart Study and Framingham Offspring Study), among patients with impaired renal function free of CV events at baseline, African American patients had a significantly higher 10-year probability of developing MI and fatal heart failure than whites29. Wetmore et al. also reported that African American ESRD patients had 45% higher risk than White patients of developing hemorrhagic stroke30. Racial disparities in CV outcomes have not been well studied for SLE or LN patients. In one past study, race and age differences among SLE patients who suffered CV events and CV deaths were investigated in the Nationwide Inpatient Sample database17. African American patients were significantly younger at the time of hospitalization for CV events than were White SLE patients. In the youngest group (<55 years), African American women with SLE were on average 20 years younger than African American women without SLE at the time of death.
In the few past studies that have investigated Hispanic ethnicity and overall mortality and CV events among patients with LN, higher risks among Hispanics than among non-Hispanics have been reported31-33. Although clinical outcomes among Hispanic SLE patients have been reported to be quite poor31, in past U.S. population-based studies, Hispanics have had lower than expected all-cause mortality rates than non-Hispanics34. A recent meta-analysis reported that individuals of Hispanic ethnicity had 18% lower mortality rates than non-Hispanics in the general population. This epidemiological phenomenon, in which U.S. Hispanics have similar or better health outcomes than non-Hispanic Whites, has been called the “Hispanic paradox”35. It has been posited that unhealthy people may emigrate back to their countries of origin at the end of life (“the salmon hypothesis”). In the current study, the rates of loss to follow-up were slightly higher among Asian (6.3%) than White patients (3.8%), and among Hispanic (4.4%) compared to non-Hispanic patients (2.9%).
It is also possible that documented immigrants with LN ESRD have better outcomes than do undocumented immigrants, who were likely excluded from USRDS. However, data from a study of survival among U.S dialysis patients in which membership in Hispanic subgroups (Mexican-American, Puerto Rican, Cuban-American or other) was self-reported reinforce our findings of lower mortality in Hispanic patients with ESRD36. In that study, Mexican Americans and other Hispanic groups had increased survival compared to non-Hispanics36. Another recent study evaluated the differences between Hispanic and non-Hispanic whites with new onset ESRD and confirmed the better survival of Hispanic dialysis patients13. As in our study of LN ESRD, when specifically addressing the possibility of informative censoring from kidney transplantation or through differential loss to follow up, the Hispanic survival benefit was attenuated, but not eliminated.
In addition to the exclusion of undocumented immigrants from the USRDS, other limitations to this study include the lack of data concerning SLE activity and organ involvement and damage. There may be other unmeasured confounding factors, such as dietary intake, physical activity, and family history of CV disease, for which we could not account. The USRDS CMS Medical Evidence Report ICD-9 code for SLE has been demonstrated to have a positive predictive value for SLE of 100%20. However, the sensitivity for capturing SLE as the cause of ESRD was 27% (95% CI 0.12-46%). In this present study, it is thus possible that many cases of LN ESRD were not included as they were misclassified as having glomerulonephritis, hypertension, or other primary cause of ESRD. In a past validation study, agreement for individual disease diagnoses did not differ by sex, race, location of the nephrologist, or whether the biopsy was performed before or after the form was completed, suggesting that the population of LN-associated ESRD patients not included in our study is similar to that included20. Finally, there is also the possibility that patients were misclassified by race or ethnicity, as this classification is in reality far more complicated than represented by the few categories employed in the USRDS database, and genetic ancestry informative markers were not available. Race and ethnicity were documented by attending nephrologists at ESRD onset and no stratified information was available about Hispanic ethnicity subgroups.
The strengths of this study include the U.S.-wide population of LN-associated ESRD cases during a period of 14 years, providing statistical power to detect differences in CV events and mortality and according to race and ethnicity. It is the first non-academic cohort based study to investigate outcomes among LN ESRD patients by race and ethnicity, and provides important clinical outcomes information. Adjustment for multiple comorbidities and renal replacement therapy modality was possible and multiple stratified and sensitivity analyses were performed to fully investigate the reported associations. Given the differential access to kidney transplantation observed in LN ESRD and differences rates of loss to follow-up in the present study, we performed competing risk analyses, accounting for the competing risks of loss to follow-up and kidney transplantation37. In summary, we observed important variation in CV outcomes and mortality by race and ethnicity among LN ESRD patients. Further research should be directed at identifying modifiable factors that could be responsible for the observed variation and developing means to reduce these risks.
Supplementary Material
Significance and Innovative Findings.
We analyzed the United States-wide population of >12,000 patients with incident lupus nephritis (LN) ESRD from 1995-2008, to investigate cardiovascular event and mortality risks according to race and ethnicity. After adjusting for multiple demographic and clinical factors and accounting for the competing risk of kidney transplantation and loss to follow-up, our results illustrate for the first time that Asian (vs. White) and Hispanic (vs. non-Hispanic) LN ESRD patients have lower mortality risks.
We also report striking higher mortality risk and higher risk of admissions for non-fatal cardiovascular events, ESRD.
This is the first non-academic cohort based study to investigate outcomes among LN ESRD patients by race and ethnicity, and provides new and important evidence of outcome disparities in LN ESRD.
Acknowledgements
The authors acknowledge the invaluable assistance of Daniel H. Solomon, MD, MPH, Michael Fischer, MD, MS, and Joanne Foody, MD.
Funding statement
This work was supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health under Award Numbers R01 AR057327 and K24 AR066109. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. JA Gómez-Puerta was supported by Fundación Alfonso Martin Escudero Grant.
Footnotes
Conflict of interest statement
The authors declare no conflicts of interest.
References
- 1.Huong DL, Papo T, Beaufils H, Wechsler B, Bletry O, Baumelou A, Godeau P, Piette JC. Renal involvement in systemic lupus erythematosus. A study of 180 patients from a single center. Medicine (Baltimore) 1999;78(3):148–66. doi: 10.1097/00005792-199905000-00002. [DOI] [PubMed] [Google Scholar]
- 2.Cervera R, Khamashta MA, Font J, Sebastiani GD, Gil A, Lavilla P, Mejia JC, Aydintug AO, Chwalinska-Sadowska H, de Ramon E, Fernandez-Nebro A, Galeazzi M, Valen M, Mathieu A, Houssiau F, Caro N, Alba P, Ramos-Casals M, Ingelmo M, Hughes GR. Morbidity and mortality in systemic lupus erythematosus during a 10-year period: a comparison of early and late manifestations in a cohort of 1,000 patients. Medicine (Baltimore) 2003;82(5):299–308. doi: 10.1097/01.md.0000091181.93122.55. [DOI] [PubMed] [Google Scholar]
- 3.Adler M, Chambers S, Edwards C, Neild G, Isenberg D. An assessment of renal failure in an SLE cohort with special reference to ethnicity, over a 25-year period. Rheumatology (Oxford) 2006;45(9):1144–7. doi: 10.1093/rheumatology/kel039. [DOI] [PubMed] [Google Scholar]
- 4.Siso A, Ramos-Casals M, Bove A, Brito-Zeron P, Soria N, Nardi N, Testi A, Perez-de-Lis M, Diaz- Lagares C, Darnell A, Sentis J, Coca A. Outcomes in biopsy-proven lupus nephritis: evaluation of 190 white patients from a single center. Medicine (Baltimore) 2010;89(5):300–7. doi: 10.1097/MD.0b013e3181f27e8f. [DOI] [PubMed] [Google Scholar]
- 5.Costenbader KH, Desai A, Alarcon GS, Hiraki LT, Shaykevich T, Brookhart MA, Massarotti E, Lu B, Solomon DH, Winkelmayer WC. Trends in the incidence, demographics, and outcomes of end-stage renal disease due to lupus nephritis in the US from 1995 to 2006. Arthritis Rheum. 2011;63(6):1681–8. doi: 10.1002/art.30293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sule S, Fivush B, Neu A, Furth S. Increased risk of death in pediatric and adult patients with ESRD secondary to lupus. Pediatr Nephrol. 2011;26(1):93–8. doi: 10.1007/s00467-010-1640-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ward MM. Changes in the incidence of endstage renal disease due to lupus nephritis in the United States, 1996-2004. J Rheumatol. 2009;36(1):63–7. doi: 10.3899/jrheum.080625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ward MM. Changes in the incidence of end-stage renal disease due to lupus nephritis, 1982-1995. Arch Intern Med. 2000;160(20):3136–40. doi: 10.1001/archinte.160.20.3136. [DOI] [PubMed] [Google Scholar]
- 9.Demas KL, Costenbader KH. Disparities in lupus care and outcomes. Curr Opin Rheumatol. 2009;21(2):102–9. doi: 10.1097/BOR.0b013e328323daad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Feldman CH, Hiraki LT, Liu J, Fischer MA, Solomon DH, Alarcon GS, Winkelmayer WC, Costenbader KH. Epidemiology and sociodemographics of systemic lupus erythematosus and lupus nephritis among US adults with Medicaid coverage, 2000-2004. Arthritis Rheum. 2013;65(3):753–63. doi: 10.1002/art.37795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kucirka LM, Grams ME, Lessler J, Hall EC, James N, Massie AB, Montgomery RA, Segev DL. Association of race and age with survival among patients undergoing dialysis. JAMA. 2011;306(6):620–6. doi: 10.1001/jama.2011.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Agodoa L, Eggers P. Racial and ethnic disparities in end-stage kidney failure-survival paradoxes in African-Americans. Semin Dial. 2007;20(6):577–85. doi: 10.1111/j.1525-139X.2007.00350.x. [DOI] [PubMed] [Google Scholar]
- 13.Arce CM, Goldstein BA, Mitani AA, Winkelmayer WC. Trends in Relative Mortality Between Hispanic and Non-Hispanic Whites Initiating Dialysis: A Retrospective Study of the US Renal Data System. Am J Kidney Dis. 2013;62(2):312–21. doi: 10.1053/j.ajkd.2013.02.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Collins AJ, Foley RN, Gilbertson DT, Chen SC. The state of chronic kidney disease, ESRD, and morbidity and mortality in the first year of dialysis. Clin J Am Soc Nephrol. 2009;4(Suppl 1):S5–11. doi: 10.2215/CJN.05980809. [DOI] [PubMed] [Google Scholar]
- 15.Foley RN, Murray AM, Li S, Herzog CA, McBean AM, Eggers PW, Collins AJ. Chronic kidney disease and the risk for cardiovascular disease, renal replacement, and death in the United States Medicare population, 1998 to 1999. J Am Soc Nephrol. 2005;16(2):489–95. doi: 10.1681/ASN.2004030203. [DOI] [PubMed] [Google Scholar]
- 16.Hak AE, Karlson EW, Feskanich D, Stampfer MJ, Costenbader KH. Systemic lupus erythematosus and the risk of cardiovascular disease: results from the nurses' health study. Arthritis Rheum. 2009;61(10):1396–402. doi: 10.1002/art.24537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Scalzi LV, Hollenbeak CS, Wang L. Racial disparities in age at time of cardiovascular events and cardiovascular-related death in patients with systemic lupus erythematosus. Arthritis Rheum. 2010;62(9):2767–75. doi: 10.1002/art.27551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hiraki LT, Lu B, Alexander SR, Shaykevich T, Alarcon GS, Solomon DH, Winkelmayer WC, Costenbader KH. End-stage renal disease due to lupus nephritis among children in the US, 1995-2006. Arthritis Rheum. 2011;63(7):1988–97. doi: 10.1002/art.30350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yap DY, Tang CS, Ma MK, Lam MF, Chan TM. Survival analysis and causes of mortality in patients with lupus nephritis. Nephrol Dial Transplant. 2012;27(8):3248–54. doi: 10.1093/ndt/gfs073. [DOI] [PubMed] [Google Scholar]
- 20.Layton JB, Hogan SL, Jennette CE, Kenderes B, Krisher J, Jennette JC, McClellan WM. Discrepancy between Medical Evidence Form 2728 and renal biopsy for glomerular diseases. Clin J Am Soc Nephrol. 2010;5(11):2046–52. doi: 10.2215/CJN.03550410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Montez-Rath ME, Winkelmayer WC, Desai M. Addressing missing data in clinical studies of kidney diseases. Clin J Am Soc Nephrol. 2014;9(7):1328–35. doi: 10.2215/CJN.10141013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ward MM. Socioeconomic status and the incidence of ESRD. Am J Kidney Dis. 2008;51(4):563–72. doi: 10.1053/j.ajkd.2007.11.023. [DOI] [PubMed] [Google Scholar]
- 23.Miller RG. Simultaneous Statistical Infererence. Springer-Verlag; New York: 1981. p. 8. [Google Scholar]
- 24.United States Renal Data System . Researcher's Guide to the USRDS Database. Bethesda, MD: 2008. [Google Scholar]
- 25.Gray R. A class of k-sample tests for comparing the cumulative incidence of a competing risk. The Annals of Statistics. 1988;16:1141–54. [Google Scholar]
- 26.Fine J, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94:496–509. [Google Scholar]
- 27.Matsushita K, van der Velde M, Astor BC, Woodward M, Levey AS, de Jong PE, Coresh J, Gansevoort RT. Association of estimated glomerular filtration rate and albuminuria with all-cause and cardiovascular mortality in general population cohorts: a collaborative meta-analysis. Lancet. 2010;375(9731):2073–81. doi: 10.1016/S0140-6736(10)60674-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Young BA, Maynard C, Boyko EJ. Racial differences in diabetic nephropathy, cardiovascular disease, and mortality in a national population of veterans. Diabetes Care. 2003;26(8):2392–9. doi: 10.2337/diacare.26.8.2392. [DOI] [PubMed] [Google Scholar]
- 29.Weiner DE, Tighiouart H, Amin MG, Stark PC, MacLeod B, Griffith JL, Salem DN, Levey AS, Sarnak MJ. Chronic kidney disease as a risk factor for cardiovascular disease and all-cause mortality: a pooled analysis of community-based studies. J Am Soc Nephrol. 2004;15(5):1307–15. doi: 10.1097/01.asn.0000123691.46138.e2. [DOI] [PubMed] [Google Scholar]
- 30.Wetmore JB, Ellerbeck EF, Mahnken JD, Phadnis M, Rigler SK, Mukhopadhyay P, Spertus JA, Zhou X, Hou Q, Shireman TI. Atrial fibrillation and risk of stroke in dialysis patients. Ann Epidemiol. 2013;23(3):112–8. doi: 10.1016/j.annepidem.2012.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Alarcon GS, McGwin G, Jr., Petri M, Reveille JD, Ramsey-Goldman R, Kimberly RP. Baseline characteristics of a multiethnic lupus cohort: PROFILE. Lupus. 2002;11(2):95–101. doi: 10.1191/0961203302lu155oa. [DOI] [PubMed] [Google Scholar]
- 32.Bastian HM, Roseman JM, McGwin G, Jr., Alarcon GS, Friedman AW, Fessler BJ, Baethge BA, Reveille JD. Systemic lupus erythematosus in three ethnic groups. XII. Risk factors for lupus nephritis after diagnosis. Lupus. 2002;11(3):152–60. doi: 10.1191/0961203302lu158oa. [DOI] [PubMed] [Google Scholar]
- 33.Pistiner M, Wallace DJ, Nessim S, Metzger AL, Klinenberg JR. Lupus erythematosus in the 1980s: a survey of 570 patients. Semin Arthritis Rheum. 1991;21(1):55–64. doi: 10.1016/0049-0172(91)90057-7. [DOI] [PubMed] [Google Scholar]
- 34.Borrell LN, Lancet EA. Race/ethnicity and all-cause mortality in US adults: revisiting the Hispanic paradox. Am J Public Health. 2012;102(5):836–43. doi: 10.2105/AJPH.2011.300345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Crimmins EM, Kim JK, Alley DE, Karlamangla A, Seeman T. Hispanic paradox in biological risk profiles. Am J Public Health. 2007;97(7):1305–10. doi: 10.2105/AJPH.2006.091892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Frankenfield DL, Krishnan SM, Ashby VB, Shearon TH, Rocco MV, Saran R. Differences in mortality among Mexican-American, Puerto Rican, and Cuban-American dialysis patients in the United States. Am J Kidney Dis. 2009;53(4):647–57. doi: 10.1053/j.ajkd.2008.10.049. [DOI] [PubMed] [Google Scholar]
- 37.Devlin A, Waikar SS, Solomon DH, Lu B, Shaykevich T, Alarcon GS, Winkelmayer WC, Costenbader KH. Variation in initial kidney replacement therapy for end-stage renal disease due to lupus nephritis in the United States. Arthritis Care Res (Hoboken) 2011;63(12):1642–53. doi: 10.1002/acr.20607. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.