Abstract
Background & Aims
Abdominal obesity and increasing body mass index are risk factors for esophageal adenocarcinoma and its main precursor, Barrett’s esophagus; however, there are no known biological mechanisms for these associations or regarding why only some patients with gastroesophageal reflux disease develop Barrett’s esophagus. We evaluated the association between Barrett’s esophagus and multimers of an adipose-associated hormone, adiponectin.
Methods
We conducted a case-control study evaluating the associations between adiponectin (total, high molecular weight, and low/medium molecular weight) and Barrett’s esophagus within the Kaiser Permanente Northern California population. Patients with a new diagnosis of Barrett’s esophagus (cases) were matched to patients with gastroesophageal reflux disease (GERD) without Barrett’s esophagus and to population controls.
Results
Complete serologic and epidemiologic data were available for 284 cases, 294 GERD controls, and 285 population controls. Increasing adiponectin levels were a risk factor for Barrett’s esophagus among patients with gastroesophageal reflux disease (total adiponectin fourth vs. first quartile odds ratio [OR]=1.96; 95% confidence interval (CI) 1.17–3.27; high molecular weight adiponectin OR=1.65; 95% CI 1.00–2.73; low/medium molecular weight adiponectin OR=2.18; 95% CI 1.33–3.56, but not compared with population controls. The associations were significantly stronger among patients reporting frequent GERD symptoms and among smokers (p-values interaction <0.01).
Conclusion
Adiponectin levels are positively associated with the risk of Barrett’s esophagus among patients with GERD and among smokers, but not among population controls without GERD symptoms. Higher adiponectin concentrations may either independently contribute to the aberrant healing of esophageal injury into Barrett’s esophagus or be a marker for other factors.
Keywords: esophageal adenocarcinoma, adiponectin, BMI, Barrett’s esophagus, adipokines
Introduction
The incidence of esophageal adenocarcinoma has increased over 500% in the United States over the last three decades; it accounts for >2% of male cancer deaths.(1, 2) Barrett’s esophagus likely represents a metaplastic healing response to esophageal injury, typically from gastroesophageal reflux disease (GERD);(3) its presence increases the risk for esophageal adenocarcinoma by 30–40 fold.(4) Barrett’s esophagus is associated with obesity, especially abdominal obesity, (5, 6) although the biological links between obesity, Barrett’s esophagus, and cancer are unclear. (5, 7, 8)
Circulating adiponectin, an adiposity-associated hormone, is inversely associated with adiposity and insulin resistance;(9, 10) it may represent a mechanistic link between Barrett’s esophagus and obesity.(11–13) Animal models suggest that adiponectin influences the healing response of the gastrointestinal mucosa.(14) Mice lacking adiponectin, for example, had more ethanol-induced gastric injury than normal mice, while adiponectin administration improved gastric mucosal repair.(14) Adiponectin is also a potentially modifiable factor, given adiponectin agonists and homologs are currently being studied as potential therapeutic agents. (15, 16)
We evaluated the associations between circulating adiponectin subtypes and the risk of Barrett’s esophagus using two control groups: patients diagnosed with GERD and population controls.
Methods
Study Design and Population
We conducted a nested case-control study within Kaiser Permanente Northern California (KPNC).(17) Patients were 18–79 years of age and continuously members for ≥2 years before their index date. The design and analyses were approved by the KPNC institutional review board (September, 2002).
Case Definition
Cases were eligible KPNC members with a new diagnosis of Barrett’s esophagus between October 2002 and September 2005, identified using the International Classification of Disease, Ninth Revision (ICD-9) code 530.2, defined at KPNC as “Barrett’s esophagitis.” A gastroenterologist (DAC) reviewed endoscopy and pathology records. Patients were included if there was a visible length of columnar-type epithelium proximal to the gastro-esophageal junction/gastric folds, and an esophageal biopsy showed specialized intestinal epithelium (18) (after pathologist slide review (GJR)). Patients were excluded if they had only gastric-type or columnar metaplasia without intestinal metaplasia; lacked an esophageal biopsy or had biopsies only of a mildly irregular squamocolumnar junction; had a prior diagnosis of Barrett’s esophagus; or had esophageal cancer (dysplasia was included). The index date was the date of Barrett’s esophagus diagnosis.
GERD Controls
GERD control members had all of the following before entry: a GERD-related ICD-9 code (530.11 [reflux esophagitis] or 530.81 [gastro-esophageal reflux]); a prescription for ≥90 days’ use of a histamine-2 receptor antagonist or a proton pump inhibitor in the previous year; no prior diagnosis of Barrett’s esophagus; and a recent esophagogastroduodenoscopy that did not show esophageal columnar metaplasia of any type.
Population Controls
Population controls were randomly selected from the at-risk (no prior Barrett’s esophagus diagnosis) KPNC membership, using risk set sampling.(19) The index date for controls was the midpoint of each 2–3 month case selection interval. The population and GERD controls were frequency matched to cases by sex, age at index date (5-year age groups), and geographic region (medical facility).
Exposure Measurements
Serum samples were stored at −80°C; adiponectin is stable frozen.(20, 21) Concentrations were assayed in duplicate, using mixed cases and controls, in an experienced adiponectin laboratory (PJH). High molecular weight adiponectin measurements used enzyme-linked immunosorbent assay (ALPCO); total measurements used radioimmunoassay (Millipore). We compared same-sample inter-assay values and adjusted for differences with a conversion factor. The “adiponectin ratio” equals high molecular weight adiponectin/total adiponectin.
All subjects completed: an in-person interview of GERD symptoms and use of medications, tobacco, and alcohol (all for year prior to index date and longer exposures); a validated food frequency questionnaire (Block 1998 full-length);(22) and measurements of height, weight, waist (obtained standing at the iliac crest)/thigh circumferences and serum Helicobacter pylori serum antibody status. Examinations used trained interviewers, most commonly at the subject’s home. GERD assessments used a validated symptom questionnaire (23) for heartburn or acid regurgitation.
Statistical Analysis
The study employed standard techniques for unpaired case-control studies, including unconditional logistic regression.(19, 24) Comparisons of proportions used the binomial distribution (Stata version 10.1; Stata Corp, College Station, TX). Continuous adiponectin measures used log-transformed values. Quartiles used distributions among the population controls and gender-specific quartiles for gender stratified analyses.
We evaluated as potential confounders: education (<7, 7–9, 10–11, 12+ years); smoking status (≥20 vs. < 20 lifetime-packs);(25) alcohol (ever vs. never drank alcohol); total daily calories, antioxidants (vitamin A, C and E, carotene and selenium), fat intake, fruits, vegetables, and iron; multivitamins; GERD symptom frequency (<weekly vs. ≥weekly); a comorbidity index;(26, 27) helicobacter pylori serum antibody status; aspirin and nonsteroidal anti-inflammatory medication use; body mass index (BMI), waist circumference, and race. We evaluated for effect modification by race, gender, smoking and GERD symptom frequency using cross-product terms in the crude logistic regression model and stratum-specific odds ratios (ORs).(28)
A potentially confounding variable was retained if it changed the main effect OR by ≥10% for at least two adiponectin variables (total, high molecular weight adiponectin, low/medium molecular weight, and ratio). Final models were therefore adjusted for waist circumference, race, and the main frequency-matched variables (sex and age). The covariates were evaluated separately for both population-based controls and GERD controls; the variables which changed the OR by >10% were similar for both comparison groups, thus the same confounding structure was utilized in the model for both comparisons
Results
Patient Characteristics
Complete data were available for 96.8% (n=923) of all interviewed subjects (Table 1); thirty persons were excluded due to missing values for: waist (n=4), serum availability for adiponectin measurement (n=25), and race/ethnicity (n=1). GERD controls had a lower mean weight (184.7 lbs.) than cases (191.5 lbs.) or population controls (191.8 lbs.). Cases were more likely to have at least weekly GERD symptoms (80.0%) (vs. GERD controls [73.4%] or population controls [28.2%]) and to be non-Hispanic whites (87.4%) (vs. population controls [84.9%] or GERD controls [80.5%]).
Table 1.
Characteristics of Study Groups
| Cases | Population Controls | GERD2 Controls | |
|---|---|---|---|
| No. of subjects, n (%) | 310 (100.0%) | 305 (100.0%) | 308 (100.0%) |
| Age mean (SD1) | 62 (±10.7) | 62 (±10.2) | 62 (±10.7) |
| Age, n (%) | |||
| 20–39 | 7 (2.3%) | 9 (3.0%) | 11 (3.6%) |
| 40–59 | 118 (38.1%) | 102 (33.4%) | 111 (36.0%) |
| 60–79 | 185 (59.7%) | 194 (63.6%) | 186 (60.4%) |
| Race, n (%) | |||
| Non-Hispanic white | 271 (87.4%) | 259 (84.9%) | 248 (80.5%) |
| Black | 4 (1.3%) | 16 (5.2%) | 20 (6.5%) |
| Hispanic | 24 (7.7%) | 12 (3.9%) | 20 (6.5%) |
| Asian | 3 (1.0%) | 8 (2.6%) | 7 (2.3%) |
| Other | 8 (2.6%) | 10 (3.3%) | 13 (4.2%) |
| Sex, n (%) | |||
| male | 227 (73.2%) | 208 (68.2%) | 212 (68.8%) |
| Smoking status (ever smoked), n (%) | 206 (66.5%) | 170 (55.7%) | 183 (59.4%) |
| GERD score3, n (%) | |||
| Any GERD2 symptoms | 289 (93.2%) | 185 (60.7%) | 289 (93.8%) |
| At least weekly | 248 (80.0%) | 86 (28.2%) | 226 (73.4%) |
| Current weight (kg), mean (SD) | 86.9 (±21.4) | 87.0 (±18.8) | 83.8 (±16.8) |
| Waist (cm5), mean (SD1) | 100.7 (±14.9) | 99.1 (±17.5) | 97.2 (±14.3) |
| BMI6, mean (SD1) | 29.4 (±6.1) | 29.4 (±5.8) | 28.8 (±5.2) |
| BMI6, n (%) | |||
| underweight | 5 (1.6%) | 2 (0.7%) | 4 (1.3%) |
| normal | 60 (19.4%) | 68 (22.3%) | 63 (20.5%) |
| overweight | 122 (39.4%) | 116 (38.0%) | 134 (43.5%) |
| obese | 123 (39.7%) | 119 (39.0%) | 107 (34.7%) |
| Adiponectin multimers, mean (SD1) | |||
| Total (μg/ml7) | 12.8 (±7.7) | 12.1 (±6.7) | 11.7 (±6.8) |
| High Molecular Weight (HMW) (μg/ml7) | 3.4 (±2.9) | 3.2 (±2.6) | 3.1 (±2.7) |
| Low + Medium Molecular Weight (μg/ml7) | 4.3 (±2.0) | 4.1 (±1.8) | 3.9 (±1.7) |
| Ratio: HMW/total | 0.40 (±0.1) | 0.40 (±0.1) | 0.40 (±0.1) |
SD= standard deviation
GERD = gastroesophageal reflux disease
GERD score represents patient’s report of frequency of GERD symptoms, which isn’t a criterion for GERD diagnosis. Thus, “any GERD” will not be 100% among GERD control group subjects.
lb = weight in pounds
cm= height in centimeters
BMI=body mass index categories based on international standards as presented by the ‘World Health Organization Global Database on Body Mass Index’
μg/ml= molecular weight in micro grams per milliliter
Cases vs. GERD Controls
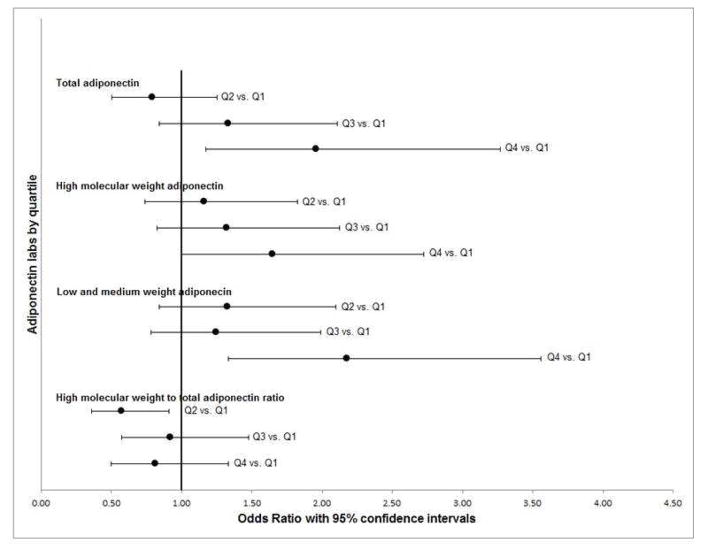
Participants in the fourth (vs. first) quartile of total and low+medium weight adiponectin were twice as likely to have Barrett’s esophagus (total adiponectin OR=1.96; 95% CI 1.17–3.27; low+medium weight OR= 2.18; 95% CI 1.33, 3.56) (Table 2, Figure 1). Similarly, participants in the fourth quartile of high molecular weight adiponectin were more likely to have Barrett’s esophagus (OR=1.65; 95% CI 1.00–2.73) (Table 2, Figure 1). Similar significant associations were seen for continuous measures of total, high molecular weight, and low/medium molecular weight adiponectin (Table 2).
Table 2.
Adiponectin and Barrett’s esophagus: cases vs. population and GERD3 control groups
| Counts | Cases vs. Population Controls | Cases vs. GERD3 Controls | |||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Cases | Population Controls | GERD3 Controls | Adjusted OR1 | 95% CI2 | Adjusted OR1 | 95% CI2 | |
| Total Adiponectin | |||||||
| Quartiles4 (μg/ml6) | |||||||
| <7.35 | 80 | 77 | 81 | ref | ref | ||
| 7.36–10.55 | 63 | 76 | 88 | 0.83 | (0.53, 1.33) | 0.79 | (0.50, 1.25) |
| 10.56–15.41 | 82 | 76 | 75 | 1.13 | (0.72, 1.77) | 1.33 | (0.84, 2.11) |
| >12.46 | 85 | 76 | 64 | 1.36 | (0.83, 2.23) | 1.96 | (1.17, 3.27) |
| Continuous, Log-transformed | 310 | 305 | 308 | 1.36 | (0.97, 1.89) | 1.66 | (1.19, 2.32) |
|
| |||||||
| Adiponectin High MW5 | |||||||
| Quartiles4 (μg/ml6) | |||||||
| <1.46 | 82 | 77 | 89 | ref | ref | ||
| 1.46–2.53 | 74 | 76 | 77 | 0.95 | (0.61, 1.50) | 1.16 | (0.74, 1.83) |
| 2.54–4.00 | 69 | 76 | 66 | 0.92 | (0.58, 1.45) | 1.32 | (0.82, 2.12) |
| >4.02 | 85 | 76 | 76 | 1.32 | (0.80, 2.17) | 1.65 | (1.00, 2.73) |
| Continuous, Log-transformed | 310 | 305 | 308 | 1.20 | (0.96, 1.51) | 1.33 | (1.05, 1.68) |
|
| |||||||
| Adiponectin Low + Medium MW5 | |||||||
| Quartiles4 (μg/ml6) | |||||||
| <2.75 | 72 | 77 | 87 | ref | ref | ||
| 2.75–3.80 | 78 | 76 | 76 | 1.15 | (0.73, 1.81) | 1.33 | (0.84, 2.10) |
| 3.80–5.07 | 69 | 76 | 77 | 1.06 | (0.66, 1.69) | 1.25 | (0.79, 1.99) |
| >5.07 | 91 | 76 | 68 | 1.56 | (0.97, 2.53) | 2.18 | (1.33, 3.56) |
| Continuous, Log-transformed | 310 | 305 | 308 | 1.44 | (0.98, 2.11) | 1.88 | (1.28, 2.78) |
|
| |||||||
| Adiponectin Ratio High MW5/total | |||||||
| Quartiles4 | |||||||
| <0.32 | 86 | 77 | 67 | ref | ref | ||
| 0.32–0.39 | 67 | 76 | 93 | 0.81 | (0.51, 1.27) | 0.58 | (0.36, 0.91) |
| 0.39–0.47 | 82 | 76 | 70 | 1.03 | (0.66, 1.63) | 0.92 | (0.57, 1.48) |
| >0.47 | 75 | 76 | 78 | 0.98 | (0.61, 1.57) | 0.82 | (0.50, 1.34) |
| Continuous, Log-transformed | 310 | 305 | 308 | 1.22 | (0.71, 2.09) | 1.19 | (0.68, 2.08) |
Adjusted OR (odds ratio): adjusts for sex, age, race/ethnicity and waist circumference
CI: confidence interval
GERD: Gastroesophageal reflux disease
Quartiles are based on population control group
MW= molecular weight
μg/ml micro liters per milliliter
Fig. 1. Adiponectin and Barrett’s esophagus: case vs. GERD controls.
Odds ratios (black dots) and 95% confidence intervals (black bars) for adiponectin quartiles adjusted for sex, age, race/ethnicity and waist circumference.
Cases vs. Population Controls
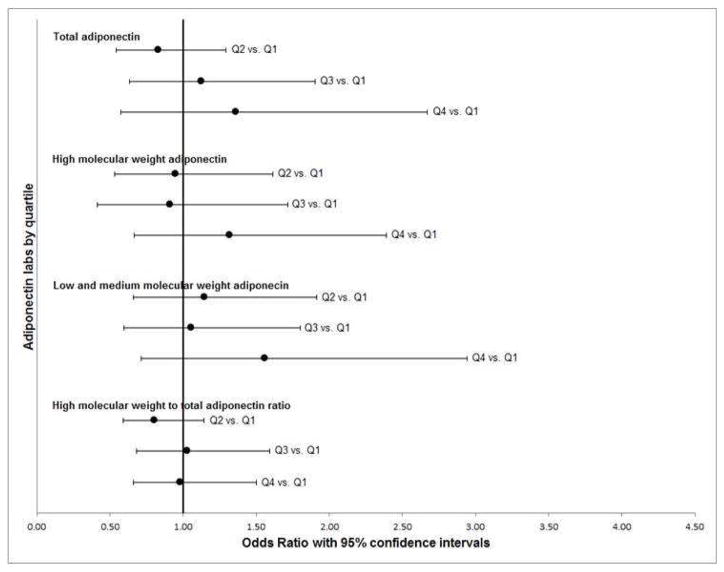
Increasing adiponectin levels were associated with Barrett’s esophagus among population controls with GERD symptoms, but not among population controls overall or population controls with minimal or no GERD symptoms (p-value interaction total adiponectin, P<0.001) (Tables 2&3, Figure 2). Continuous measures of total and high molecular weight adiponectin were positively associated with Barrett’s esophagus among cases/population controls with frequent GERD symptoms (≥weekly), comparable to subjects in the larger physician-defined GERD control group (OR 1.68, total adiponectin; 95% CI 1.01–2.80; OR 1.50, high molecular weight adiponectin; 95% CI 1.04–2.15) (Table 3). Analyses by quartile had overall similar directions, although with wider confidence intervals.
Table 3.
Associations of adiponectin and Barrett’s esophagus: stratified by GERD symptom frequency3 for case vs. population controls
| Less than weekly6 | At least weekly7 | |||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Case | Control9 | Adjusted OR1 | 95% CI2 | Case | Control | Adjusted OR1 | 95% CI2 | |
| Total Adiponectin interaction term Quartiles4 (μg/ml8) | <0.001 | |||||||
| <7.35 | 19 | 51 | ref | 61 | 26 | ref | ||
| 7.36–10.55 | 14 | 51 | 0.67 | (0.29, 1.55) | 49 | 25 | 0.83 | (0.42, 1.64) |
| 10.56–15.41 | 9 | 60 | 0.39 | (0.16, 0.99) | 73 | 15 | 2.11 | (1.00, 4.45) |
| >15.46 | 20 | 56 | 1.24 | (0.52, 2.95) | 65 | 20 | 1.56 | (0.72, 3.37) |
| Continuous, Log-transformed | 62 | 218 | 1.16 | (0.62, 2.20) | 248 | 86 | 1.68 | (1.01, 2.80) |
|
| ||||||||
| Adiponectin High MW5 interaction term Quartiles4 (μg/ml8) | <0.001 | |||||||
| <1.46 | 20 | 48 | ref | 62 | 29 | ref | ||
| 1.46–2.53 | 14 | 53 | 0.55 | (0.24, 1.27) | 60 | 23 | 1.18 | (0.60, 2.32) |
| 2.54–4.00 | 12 | 61 | 0.42 | (0.18, 0.99) | 57 | 15 | 1.72 | (0.82, 3.63) |
| >4.02 | 16 | 56 | 0.84 | (0.35, 2.04) | 69 | 19 | 1.95 | (0.89, 4.29) |
| Continuous, Log-transformed | 62 | 218 | 0.98 | (0.64, 1.51) | 248 | 86 | 1.50 | (1.04, 2.15) |
|
| ||||||||
| Adiponectin Low + Medium MW5 interaction term Quartiles4 (μg/ml8) | <0.001 | |||||||
| <2.75 | 16 | 56 | ref | 56 | 21 | ref | ||
| 2.75–3.80 | 16 | 52 | 1.02 | (0.45, 2.33) | 62 | 24 | 0.91 | (0.45, 1.85) |
| 3.80–5.07 | 12 | 55 | 0.80 | (0.33, 1.93) | 57 | 20 | 1.16 | (0.55, 2.43) |
| >5.07 | 18 | 55 | 1.57 | (0.66, 3.72) | 73 | 21 | 1.33 | (0.62, 2.84) |
| Continuous, Log-transformed | 62 | 218 | 1.52 | (0.72, 3.21) | 248 | 86 | 1.56 | (0.87, 2.80) |
|
| ||||||||
| Adiponectin Ratio High MW5/total interaction term Quartiles4 | <0.001 | |||||||
| <0.32 | 19 | 48 | ref | 67 | 29 | ref | ||
| 0.32–0.39 | 12 | 52 | 0.56 | (0.24, 1.33) | 55 | 24 | 0.95 | (0.48, 1.86) |
| 0.39–0.47 | 16 | 60 | 0.70 | (0.31, 1.58) | 66 | 15 | 1.95 | (0.92, 4.11) |
| >0.47 | 15 | 58 | 0.73 | (0.32, 1.69) | 60 | 18 | 1.49 | (0.70, 3.17) |
| Continuous, Log-transformed | 62 | 218 | 0.66 | (0.26, 1.67) | 248 | 86 | 2.27 | (0.96, 5.41) |
Adjusted OR (odds ratio): adjusts for sex, age, race/ethnicity and waist circumference
CI: confidence interval
GERD: gastroesophageal reflux disease
Quartiles are based on population control group
MW= molecular weight
Reported frequency of GERD symptoms as less than weekly
Reported frequency of GERD symptoms as weekly or more than weekly
μg/ml= microliter per milliliter
Note: Not all populations controls had GERD frequency data
Fig. 2. Adiponectin and Barrett’s esophagus: case vs. population controls.
Odds ratios (black dots) and 95% confidence intervals (black bars) for adiponectin quartiles adjusted for sex, age, race/ethnicity and waist circumference.
Smoking
Total adiponectin
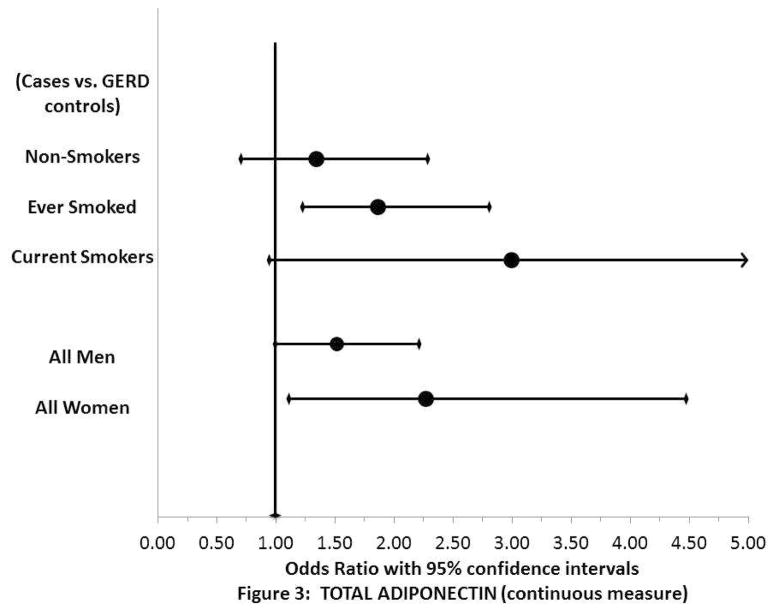
For comparisons with GERD controls, there were positive associations between total, high molecular weight, and low/medium molecular weight adiponectin and Barrett’s esophagus among “ever smokers” (OR=1.83, total adiponectin; 95% CI 1.20–2.78; OR=1.46, high molecular weight adiponectin; 95% CI 1.09–1.97; OR=2.11, low/medium molecular weight adiponectin; 95% CI 1.27–3.49) but not among “never smokers” (Supplementary Table 1, Figure 3). The associations were strongest among current smokers, although with limited power to evaluate current smokers (Supplementary Table 1, Figure 3). Similar positive associations between total, high molecular weight, and medium weight adiponectin and Barrett’s esophagus were also seen in the case vs. population control comparison group for ever smokers, but not never smokers (not shown).
Fig. 3. Adiponectin and Barrett’s esophagus: cases vs. GERD control, stratified by smoking status and sex.
Stratified odds ratios (black dots) and 95% confidence intervals (black bars) for adiponectin log transformed concentrations adjusted for age, race/ethnicity and waist circumference.
Stratifications by sex
The associations between total adiponectin levels and Barrett’s esophagus were somewhat stronger among women than among men, among GERD controls (continuous measure, women: OR=2.27; 95% CI 1.15–4.47; men: OR=1.50; 95% CI 1.02–2.22; p-value interaction=0.10) (Supplementary Table 2). Low/medium molecular weight adiponectin had significant associations or strong trends with the risk of Barrett’s esophagus among both men and women, whereas high molecular weight adiponectin showed significant associations only among women (Supplementary Table 2). For cases vs. population controls, females showed significant associations for total adiponectin, high molecular weight adiponectin and adiponectin ratio, but not for low/medium molecular weight adiponectin (data not shown).
Stratifications by BMI
The nonsignificant trends for associations between adiponectin and Barrett’s esophagus were stronger among persons with a normal BMI (e.g. log-transformed total adiponectin, continuous value OR=2.22, 95% CI 0.98, 5.04), than among overweight (OR=1.32, 95% CI 0.76, 2.30) or obese persons (OR=1.15, 95% CI 0.68, 1.94). Similar patterns were seen for high and low+medium weight adiponectin multimers (Supplementary Table 3).
Influence of proton pump inhibitors
Among the population controls, mean adiponectin levels were similar between PPI users vs. non-users (mean 11.88 vs. 12.99, respectively; p=0.24). A logistic regression model adjusted for sex, age, and BMI demonstrated no significant association between adiponectin levels and PPI use (data not shown).
Evaluation of Assumptions
We evaluated previously reported associations for adiponectin, using the population controls.(29–32) As expected, mean adiponectin levels decreased with rising BMI (p< 0.0001); were lower among men (p<0.001); and increased with age (p<0.0001). Unlike some prior reports, it was not correlated with smoking status (ever vs. never).(32)
If adiponectin mediates the association between waist circumference and Barrett’s esophagus, we would expect the association between adiponectin and Barrett’s esophagus to diminish after controlling for obesity. On the contrary, for cases vs. GERD controls, the association for low/medium molecular weight adiponectin actually strengthened after adjusting for BMI (unadjusted: OR=1.45; 95% CI 1.01–2.08; adjusted: OR=1.55; 95% CI 1.07–2.25) and for both BMI and waist circumference (OR=1.80; 95% CI 1.22–2.65).
Discussion
These results suggest that increasing serum concentrations of total adiponectin, high molecular weight adiponectin and low/medium molecular weight adiponectin are associated with an increased risk of Barrett’s esophagus among people with a physician-assigned GERD diagnosis, population controls with frequent self-reported GERD symptoms and smokers, but not among persons without GERD symptoms and nonsmokers.
The current study adds knowledge regarding the associations between adiponectin and the body’s response to injury. Barrett’s esophagus is thought to result from an aberrant healing response to esophageal injury, most commonly gastroesophageal reflux. A positive association between adiponectin and esophageal healing is biologically plausible; animal models demonstrate it is strongly associated with gastrointestinal mucosal healing after caustic injury.(14) Adiponectin may modify pathways for cell injury and repair, such as the nitric oxide and interleukin pathways.(11, 12) Smoking is also associated with both the risk of esophagitis and the risk of Barrett’s esophagus independent of GERD.(33) Two potential explanations for our findings include: 1) higher adiponectin levels may increase the risk of a metaplastic healing response (i.e. Barrett’s esophagus) in response to GERD-induced injury and smoking; or 2) higher adiponectin levels may increase the risk of esophagitis, which is associated with both smoking and GERD. The second explanation is less likely given that adiponectin was not independently associated with either smoking or GERD symptoms among our population controls (data not shown).
Adiponectin’s associations with mucosal healing may differ from its other roles with carcinogenesis. Adiponectin levels have been inversely associated with the risk of prostate, colon, gastric, endometrial and breast cancers.(34–38) Adiponectin inhibits leptin-induced cell growth (39), suggesting that high serum concentrations may protect against cancer. A study by Yildirim et al. found lower levels of adiponectin among patients with esophageal cancer (n=75 cases, 13 with adenocarcinoma).(40) Studies in cancer patients, however, have difficulty excluding changes in adiponectin caused by the cancer, such as alteration of diet, exercise or metabolism. Another study, which looked at the relationship between adiponectin receptor expression in esophageal adenocarcinoma cancer cells,(41) found that greater amounts of visceral fat were associated with greater expression of the adiponectin receptor-2, independent of serum adiponectin levels.(41)
The current results differ from two of three prior smaller studies of Barrett’s esophagus. One found lower levels of low molecular weight serum adiponectin among 112 Barrett’s esophagus patients vs. GERD-control patients (third vs. first tertiles: OR=0.33; 95% CI 0.16–0.69) and no significant associations for total or high molecular weight adiponectin; the study did not include population controls.(42) The second study found lower total adiponectin levels among 177 Barrett’s esophagus cases vs. population controls (third vs. first tertiles: OR= 0.56; 95% CI: 0.32–0.98).(43) In contrast, the third study found a non-significant trend for a positive association between adiponectin and Barrett’s esophagus,(44) and significant positive associations among white males (fourth vs. first quartiles OR= 3.27; 95% CI 1.06–10.05). (44) We cannot fully account for the differences between the prior studies and the current findings, although some groups of patients without GERD in our study did have inverse associations (Table 3) and some of the other studies adjusted for smoking, GERD, and hiatal hernia whereas we evaluated these primarily as sources of interaction. All three prior studies had much smaller sample sizes (decreasing the power to look for interaction), slightly younger populations, and some had fewer smokers in the control groups.(41–44) Similar to the current study, Thompson included only new diagnoses of Barrett’s esophagus, whereas Rubenstein included both prevalent and new diagnoses and reported somewhat lower mean adiponectin levels. (42–44)
The current results run counter to the general inverse associations between adiponectin levels and obesity, and the positive associations between abdominal obesity and Barrett’s esophagus.(5, 45, 46) However, prior positive associations between abdominal obesity and Barrett’s esophagus were mainly observed in comparisons with population based controls; no strong associations were found among patients with GERD.(5) Thus, given the known associations between adiponectin and mucosal healing, our current results represent a potential mechanism whereby only some patients with GERD develop Barrett’s esophagus. The associations between abdominal obesity and Barrett’s esophagus among population controls may not be strongly mediated by adiponectin.
There are several strengths of the current study. First, positive associations between adiponectin and Barrett’s esophagus were found in both population controls with self-reported GERD symptoms and patients with physician-assigned GERD diagnoses. Second, our analyses showed the expected associations between adiponectin levels and sex, age, and BMI; this also makes bias in the laboratory or with patient sampling less likely. Third, we studied a large group of patients with a new diagnosis of Barrett’s esophagus within a community-based population, thereby minimizing selection bias found persons with prevalent Barrett’s esophagus. Fourth, the number of cases is almost twice as large as the largest previously published study (43), providing greater power to evaluate interactions. Fifth, the two control groups allowed a separate evaluation of why only some GERD patients develop Barrett’s esophagus. Sixth, the data were of high quality, with validated questionnaires, detailed anthropometric measurements, direct review of the endoscopy reports and manual pathology slide review.
The analyses have several potential limitations. First, case-control studies cannot establish cause and effect; adiponectin levels may differ without it causing Barrett’s esophagus.(19) We cannot exclude incomplete control of confounding. Second, we did not measure the adiponectin levels at exactly the time the Barrett’s esophagus developed, given that time is unknown. Fourth, low and medium molecular weight adiponectin were calculated through subtraction of measured high molecular weight adiponectin. While the low and medium molecular weight forms of adiponectin together are the predominant forms in the circulation (47) high molecular weight adiponectin appears to be the most bioactive form in terms of regulating glucose homeostasis and insulin sensitivity.(10) The finding of a borderline stronger association between adiponectin and Barrett’s esophagus among women vs. men is interesting, given women are at lower risk than men for this condition. Although adiponectin levels are known to differ between men and women, the interaction term was of marginal statistical significance (p=0.10) and there may be sex-specific differences in its biological activities.
In summary, in a community-based population, there was an association between increasing levels of serum adiponectin multimers and a new diagnosis of Barrett’s esophagus, among patients with GERD. This association was stronger among smokers, and among persons with more frequent GERD. These results suggest a potential for a direct mechanistic role for adiponectin in the process of mucosal healing that may cause Barrett’s esophagus, although is also possible that circulating adiponectin concentrations are a marker for another process, such systemic inflammation related to Barrett’s esophagus. Although the results run counter to the expected direction of obesity’s general associations with Barrett’s esophagus (and with adiponectin levels), they represent one of the first potential biological risk factors identified for why only some patients with GERD and smoking develop Barrett’s esophagus.
Supplementary Material
Acknowledgments
Grant support: This work was supported by National Institute of Diabetes and Digestive and Kidney Disease grants R56 DK087748 and RO1 DK63616
Abbreviations
- BMI
body mass index
- CI
confidence interval
- GERD
gastroesophageal reflux disease
- ICD-9
International Classification of Disease
- KPNC
Kaiser Permanente Northern California
- OR
odds ratio
Footnotes
Disclosures
None
Author Contributions
Lucy M Almers statistical analysis; analysis and interpretation of data; drafting of manuscript
James E Graham assisted in conducting the study; provided technical support; critical revision of the manuscript for important intellectual content;
Peter J Havel assisted in conducting the study; provided technical support; critical revision of the manuscript for important intellectual content;
Douglas A Corley study concept and design; analysis and interpretation of data; critical revision of the manuscript for important intellectual content; collected data; and assisted in interpreting data and reviewed manuscript; obtained funding
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Blot WJ, McLaughlin JK. The changing epidemiology of esophageal cancer. Semin Oncol. 1999;26:2–8. [PubMed] [Google Scholar]
- 2.Pohl H, Welch HG. The role of overdiagnosis and reclassification in the marked increase of esophageal adenocarcinoma incidence. J Natl Cancer Inst. 2005;97:142–6. doi: 10.1093/jnci/dji024. [DOI] [PubMed] [Google Scholar]
- 3.Reynolds RP. Pathophysiology and investigation of Barrett’s esophagus. Can J Gastroenterol. 1997;11 (Suppl B):41B–44B. [PubMed] [Google Scholar]
- 4.Sikkema M, de Jonge PJ, Steyerberg EW, et al. Risk of esophageal adenocarcinoma and mortality in patients with Barrett’s esophagus: a systematic review and meta-analysis. Clinical gastroenterology and hepatology: the official clinical practice journal of the American Gastroenterological Association. 2010;8:235–44. doi: 10.1016/j.cgh.2009.10.010. quiz e32. [DOI] [PubMed] [Google Scholar]
- 5.Corley DA, Kubo A, Levin TR, et al. Abdominal obesity and body mass index as risk factors for Barrett’s esophagus. Gastroenterology. 2007;133:34–41. doi: 10.1053/j.gastro.2007.04.046. quiz 311. [DOI] [PubMed] [Google Scholar]
- 6.Murray L, Romero Y. Role of obesity in Barrett’s esophagus and cancer. Surg Oncol Clin N Am. 2009;18:439–52. doi: 10.1016/j.soc.2009.03.010. [DOI] [PubMed] [Google Scholar]
- 7.Samanic C, Gridley G, Chow WH, et al. Obesity and cancer risk among white and black United States veterans. Cancer Causes Control. 2004;15:35–43. doi: 10.1023/B:CACO.0000016573.79453.ba. [DOI] [PubMed] [Google Scholar]
- 8.Sharma P, Falk GW, Weston AP, et al. Dysplasia and cancer in a large multicenter cohort of patients with Barrett’s esophagus. Clin Gastroenterol Hepatol. 2006;4:566–72. doi: 10.1016/j.cgh.2006.03.001. [DOI] [PubMed] [Google Scholar]
- 9.Havel PJ. Update on adipocyte hormones: regulation of energy balance and carbohydrate/lipid metabolism. Diabetes. 2004;53 (Suppl 1):S143–51. doi: 10.2337/diabetes.53.2007.s143. [DOI] [PubMed] [Google Scholar]
- 10.Swarbrick MM, Havel PJ. Physiological, pharmacological, and nutritional regulation of circulating adiponectin concentrations in humans. Metabolic syndrome and related disorders. 2008;6:87–102. doi: 10.1089/met.2007.0029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ajuwon KM, Spurlock ME. Adiponectin inhibits LPS-induced NF-kappaB activation and IL-6 production and increases PPARgamma2 expression in adipocytes. Am J Physiol Regul Integr Comp Physiol. 2005;288:R1220–5. doi: 10.1152/ajpregu.00397.2004. [DOI] [PubMed] [Google Scholar]
- 12.Chen H, Montagnani M, Funahashi T, et al. Adiponectin stimulates production of nitric oxide in vascular endothelial cells. J Biol Chem. 2003;278:45021–6. doi: 10.1074/jbc.M307878200. [DOI] [PubMed] [Google Scholar]
- 13.Corley DA. Obesity and the rising incidence of oesophageal and gastric adenocarcinoma: what is the link? Gut. 2007;56:1493–4. doi: 10.1136/gut.2007.124255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yamamoto S, Watabe K, Araki H, et al. Protective role of adiponectin against ethanol-induced gastric injury in mice. Am J Physiol Gastrointest Liver Physiol. 2012 doi: 10.1152/ajpgi.00324.2011. [DOI] [PubMed] [Google Scholar]
- 15.Kupchak BR, Garitaonandia I, Villa NY, et al. Antagonism of human adiponectin receptors and their membrane progesterone receptor paralogs by TNFalpha and a ceramidase inhibitor. Biochemistry. 2009;48:5504–6. doi: 10.1021/bi9006258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Miele M, Costantini S, Colonna G. Structural and Functional Similarities between Osmotin from Nicotiana Tabacum Seeds and Human Adiponectin. PLoS ONE. 2011;6:e16690. doi: 10.1371/journal.pone.0016690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Krieger N. Overcoming the absence of socioeconomic data in medical records: validation and application of a census-based methodology. Am J Public Health. 1992;82:703–10. doi: 10.2105/ajph.82.5.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sharma P, McQuaid K, Dent J, et al. A critical review of the diagnosis and management of Barrett’s esophagus: the AGA Chicago Workshop. Gastroenterology. 2004;127:310–30. doi: 10.1053/j.gastro.2004.04.010. [DOI] [PubMed] [Google Scholar]
- 19.Rothman KJGS. Modern Epidemiology. Philadelphia, PA: Lippencott-Raven; 1998. [Google Scholar]
- 20.Choi KM, Lee J, Lee KW, et al. Serum adiponectin concentrations predict the developments of type 2 diabetes and the metabolic syndrome in elderly Koreans. Clinical endocrinology. 2004;61:75–80. doi: 10.1111/j.1365-2265.2004.02063.x. [DOI] [PubMed] [Google Scholar]
- 21.Pischon T, Hotamisligil GS, Rimm EB. Adiponectin: stability in plasma over 36 hours and within-person variation over 1 year. Clinical chemistry. 2003;49:650–2. doi: 10.1373/49.4.650. [DOI] [PubMed] [Google Scholar]
- 22.Block G, Thompson FE, Hartman AM, et al. Comparison of two dietary questionnaires validated against multiple dietary records collected during a 1-year period. J Am Diet Assoc. 1992;92:686–93. [PubMed] [Google Scholar]
- 23.Locke GR, Talley NJ, Weaver AL, et al. A new questionnaire for gastroesophageal reflux disease. Mayo Clin Proc. 1994;69:539–47. doi: 10.1016/s0025-6196(12)62245-9. [DOI] [PubMed] [Google Scholar]
- 24.Breslow NEDN. Statistical methods in cancer research. Volume 1: the analysis of case-control studies. Lyon, France: International Agency for Research on Cancer; 1980. [PubMed] [Google Scholar]
- 25.Ferris BG. Epidemiology Standardization Project (American Thoracic Society) Am Rev Respir Dis. 1978;118:1–120. [PubMed] [Google Scholar]
- 26.Zhao Y, Ash AS, Ellis RP, et al. Predicting pharmacy costs and other medical costs using diagnoses and drug claims. Med Care. 2005;43:34–43. [PubMed] [Google Scholar]
- 27.Zhao Y, Ellis RP, Ash AS, et al. Measuring population health risks using inpatient diagnoses and outpatient pharmacy data. Health Serv Res. 2001;36:180–93. [PMC free article] [PubMed] [Google Scholar]
- 28.Hosmer DWLS. Applied logistic regression. New York, NY: Wiley; 2000. [Google Scholar]
- 29.Isobe T, Saitoh S, Takagi S, et al. Influence of gender, age and renal function on plasma adiponectin level: the Tanno and Sobetsu study. Eur J Endocrinol. 2005;153:91–8. doi: 10.1530/eje.1.01930. [DOI] [PubMed] [Google Scholar]
- 30.Kern PA, Di Gregorio GB, Lu T, et al. Adiponectin expression from human adipose tissue: relation to obesity, insulin resistance, and tumor necrosis factor-alpha expression. Diabetes. 2003;52:1779–85. doi: 10.2337/diabetes.52.7.1779. [DOI] [PubMed] [Google Scholar]
- 31.Nishizawa H, Shimomura I, Kishida K, et al. Androgens decrease plasma adiponectin, an insulin-sensitizing adipocyte-derived protein. Diabetes. 2002;51:2734–41. doi: 10.2337/diabetes.51.9.2734. [DOI] [PubMed] [Google Scholar]
- 32.Takefuji S, Yatsuya H, Tamakoshi K, et al. Smoking status and adiponectin in healthy Japanese men and women. Prev Med. 2007;45:471–5. doi: 10.1016/j.ypmed.2007.07.001. [DOI] [PubMed] [Google Scholar]
- 33.Cook MB, Shaheen NJ, Anderson LA, et al. Cigarette smoking increases risk of Barrett’s esophagus: an analysis of the Barrett’s and Esophageal Adenocarcinoma Consortium. Gastroenterology. 2012;142:744–53. doi: 10.1053/j.gastro.2011.12.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Goktas S, Yilmaz MI, Caglar K, et al. Prostate cancer and adiponectin. Urology. 2005;65:1168–72. doi: 10.1016/j.urology.2004.12.053. [DOI] [PubMed] [Google Scholar]
- 35.Ishikawa M, Kitayama J, Kazama S, et al. Plasma adiponectin and gastric cancer. Clin Cancer Res. 2005;11:466–72. [PubMed] [Google Scholar]
- 36.Mantzoros C, Petridou E, Dessypris N, et al. Adiponectin and breast cancer risk. J Clin Endocrinol Metab. 2004;89:1102–7. doi: 10.1210/jc.2003-031804. [DOI] [PubMed] [Google Scholar]
- 37.Petridou E, Mantzoros C, Dessypris N, et al. Plasma adiponectin concentrations in relation to endometrial cancer: a case-control study in Greece. J Clin Endocrinol Metab. 2003;88:993–7. doi: 10.1210/jc.2002-021209. [DOI] [PubMed] [Google Scholar]
- 38.Wei EK, Giovannucci E, Fuchs CS, et al. Low plasma adiponectin levels and risk of colorectal cancer in men: a prospective study. J Natl Cancer Inst. 2005;97:1688–94. doi: 10.1093/jnci/dji376. [DOI] [PubMed] [Google Scholar]
- 39.Ogunwobi OO, Beales IL. Globular adiponectin, acting via adiponectin receptor-1, inhibits leptin-stimulated oesophageal adenocarcinoma cell proliferation. Mol Cell Endocrinol. 2008;285:43–50. doi: 10.1016/j.mce.2008.01.023. [DOI] [PubMed] [Google Scholar]
- 40.Yildirim A, Bilici M, Cayir K, et al. Serum adiponectin levels in patients with esophageal cancer. Jpn J Clin Oncol. 2009;39:92–6. doi: 10.1093/jjco/hyn143. [DOI] [PubMed] [Google Scholar]
- 41.Howard JM, Beddy P, Ennis D, et al. Associations between leptin and adiponectin receptor upregulation, visceral obesity and tumour stage in oesophageal and junctional adenocarcinoma. Br J Surg. 2010;97:1020–7. doi: 10.1002/bjs.7072. [DOI] [PubMed] [Google Scholar]
- 42.Rubenstein JH, Kao JY, Madanick RD, et al. Association of adiponectin multimers with Barrett’s oesophagus. Gut. 2009;58:1583–9. doi: 10.1136/gut.2008.171553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Thompson OM, Beresford SA, Kirk EA, et al. Serum leptin and adiponectin levels and risk of Barrett’s esophagus and intestinal metaplasia of the gastroesophageal junction. Obesity (Silver Spring) 2010;18:2204–11. doi: 10.1038/oby.2009.508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Garcia JM, Splenser AE, Kramer J, et al. Circulating inflammatory cytokines and adipokines are associated with increased risk of Barrett’s esophagus: a case-control study. Clinical gastroenterology and hepatology: the official clinical practice journal of the American Gastroenterological Association. 2014;12:229–238. e3. doi: 10.1016/j.cgh.2013.07.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kramer JR, Fischbach LA, Richardson P, et al. Waist-to-hip ratio, but not body mass index, is associated with an increased risk of Barrett’s esophagus in white men. Clinical gastroenterology and hepatology: the official clinical practice journal of the American Gastroenterological Association. 2013;11:373–381. e1. doi: 10.1016/j.cgh.2012.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kubo A, Cook MB, Shaheen NJ, et al. Sex-specific associations between body mass index, waist circumference and the risk of Barrett’s oesophagus: a pooled analysis from the international BEACON consortium. Gut. 2013;62:1684–91. doi: 10.1136/gutjnl-2012-303753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pajvani UB, Hawkins M, Combs TP, et al. Complex distribution, not absolute amount of adiponectin, correlates with thiazolidinedione-mediated improvement in insulin sensitivity. J Biol Chem. 2004;279:12152–62. doi: 10.1074/jbc.M311113200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.