Abstract
The current study examined sensitivity in detecting emotional faces among children of depressed and nondepressed mothers. A second goal was to examine the potential moderating role of the oxytocin receptor gene (OXTR rs53576), which has been linked to emotion recognition in the past. Participants included 247 children (ages 8-14). Children completed a forced choice emotion identification task. Maternal history of MDD during children’s lives was associated with children’s sensitivity in detecting emotional faces among children homozygous for the OXTR rs53576 G allele, but not among carriers of the A allele. Among G homozygotes, children of depressed mothers exhibited increased sensitivity in detecting sad faces, and reduced sensitivity in detecting happiness, compared to children of nondepressed mothers.
Keywords: maternal depression, oxytocin receptor gene, information-processing, intergenerational transmission, vulnerability
The ability to accurately detect and appropriately respond to facial displays of emotion is a vital component of adaptive interpersonal functioning (Cunningham & Odom, 1986; Hampson, van Anders, & Mullin, 2006; Herba, Landau, Russel, Ecker, & Phillips, 2006). Indeed, deficits in the ability to accurately identify and process facial expressions have been linked to several forms of psychopathology (for reviews, see Edwards, Jackson, & Pattison, 2002; Golarai, Grill-Spector, & Reiss, 2006). Theorists (e.g., Cicchetti, Toth, & Maughan, 2000; Pollak, 2003) have suggested that negative experiences during childhood may contribute to biases in the processing of facial displays of emotion. This research has focused primarily on the role of childhood abuse showing that children with a history of physical abuse exhibit biased attention to angry faces and increased sensitivity in detecting facial displays of anger (for a review, see Pollak, 2003; see also Shackman, Shackman, & Pollak, 2007). These processing biases are specific to angry facial cues and are not observed for other emotional expressions, leading researchers to propose that negative experiences in childhood may contribute to the development of “experience-specific information-processing biases” (e.g., Pollak, 2003). Although it may initially be adaptive for children in abusive situations to develop increased sensitivity to signals of anger, as this may facilitate attempts to avoid the abuse, theorists have suggested that these processing biases may become maladaptive if they develop into relatively trait-like processing styles that are applied rigidly to a broader range of (objectively safer) interpersonal contexts (Cicchetti, Toth, & Maughan, 2000; Pollak, 2003).
To the extent that the information-processing biases observed among abused children resulted from conditioning to the angry expressions of the abuser (cf. Lee, Lim, Lee, Kim, & Choi, 2009; Pischek-Simpson, Boschen, Neumann, & Waters, 2009), which then generalized to facial displays of anger from other individuals, one would predict that consistent exposure to other facial displays of emotion may also contribute to the development of experience-specific information-processing biases. Supporting this theory, there is growing evidence that children of depressed mothers exhibit biased processing specifically of sad facial expressions. For example, studies show that children of mothers with a history of major depressive disorder (MDD) during their lives exhibit attentional biases specifically for sad faces but not for other facial displays of emotion (Gibb, Benas, Grassia, & McGeary, 2009; Joormann, Talbot, & Gotlib, 2007; Kujawa et al., 2011). There is also evidence that children of depressed parents may exhibit increased sensitivity in detecting facial displays of sadness. For example, paralleling the findings observed in relation to childhood physical abuse, a recent study using a morphed faces paradigm showed boys but not girls of parents with a history of MDD identified facial displays of sadness (but not anger) at lower levels of intensity than did children of parents with no history of any psychiatric disorder (Lopez-Duran, Kuhlman, George, & Kovacs, 2013). In contrast, however, another study showed that daughters of mothers with a history of recurrent depression required greater emotional intensity to accurately identify sad facial expressions than did daughters of mothers with no history of any psychiatric disorder (Joormann, Gilbert, & Gotlib, 2010). Although the precise reason for this difference in findings is unclear, Lopez-Duran and colleagues (2013) suggested two possibilities. First, Joormann et al. included an older sample but required that they be lifetime free of any psychopathology, which may have resulted in the inclusion of a particularly resilient group of high-risk girls. Second, whereas Lopez-Duran et al. presented static images at different levels of emotional intensity (morph level) and asked participants to indicate which emotion was being presented, Joormann et al. used dynamically morphing stimuli (from neutral to full emotion) and participants were asked to press a button as soon as they knew the emotion being conveyed, which may have placed a greater influence on reaction times. In the current study, therefore, we focused on statically displayed images and explicitly examined whether the same pattern of findings would be observed in children of depressed mothers generally as well as children of depressed mothers with no personal history of depression themselves.
It should also be noted that not all children of depressed mothers develop depression themselves and not all should be expected to exhibit information-processing biases. Indeed, there is growing interest in identifying specific genetic influences on information-processing biases (e.g., Gibb, Beevers, & McGeary, 2013), which may help to identify which children of depressed mothers are at greatest risk for developing information processing biases (cf. Gibb et al., 2009, 2011). One influence that may be particularly important when seeking to understand the processing of interpersonal cues is oxytocin. Oxytocin, a nine amino-acid neuropeptide synthesized in the hypothalamus, is involved in regulating social behavior such as social recognition, mating, attachment, and caring for offspring (Donaldson & Young, 2008). Studies show that the administration of intranasal oxytocin improves recognition of facial displays of emotion in humans (for a meta-analytic review, see Van IJzendoorn & Bakermans-Kranenburg, 2012). There is evidence that a polymorphism (rs53576) in the oxytocin receptor gene (OXTR) modulates oxytocinergic functioning, with G homozygotes exhibiting greater response to endogenous (Moons, Way, & Taylor, 2014) or exogenous (Marsh et al., 2012) oxytocin than carriers of the A allele. Individuals homozygous for the OXTR rs53576 G allele, compared to carriers of the A allele, exhibit stronger electrophysiological (event-related potential) responses to emotional faces reflecting greater attention to these faces (Peltola et al., 2014) and higher levels of sympathetic arousal and empathic concern while viewing negative social interactions (e.g., Smith et al., 2014). In addition, OXTR rs53576 G homozygotes perform better than carriers of the A allele on the “reading the mind in the eyes” test, which assesses trait empathy and the ability to accurately detect emotional cues (Rodrigues et al., 2009). However, more recent evidence suggests that these seemingly positive effects of being a G carrier might be absent under conditions of adversity, especially those that involve negative early-life experiences (McQuaid et al., 2013). That is, studies show that the OXTR rs53576 G allele interacts with prior environmental exposure to produce poorer outcomes, such as emotion dysregulation (Bradley et al., 2011) and depression risk (McQuaid et al., 2013). Therefore, it is possible that the OXTR rs53576 may interact with other negative environmental conditions (i.e., exposure to a depressed parent) to produce information-processing biases. However, no studies of which we are aware have examined the extent to which the OXTR (rs53576) gene may moderate the association between exposure to maternal depression and sensitivity in detecting facial displays of emotion among children.
Therefore, the primary aim of the current study was to examine sensitivity in detecting facial displays of various emotions (sad, happy, and angry) among two groups of children: those whose mother had a history of MDD during their lives and those whose mothers were lifetime free of any mood disorder. In addition, we examined the potential moderating role of OXTR rs53576 genotype. We predicted that children of depressed mothers would exhibit increased sensitivity in detecting facial displays of sadness, but not happiness or anger, at low levels of emotional intensity (low morph) and that this would be particularly true among children of depressed mothers who were also homozygous for the OXTR rs53576 G allele. Finally, we predicted that these effects would be maintained even after accounting for children’s own experiences with depression.
Method
Participants
Participants in this study were 247 mothers and their children recruited from the community. To qualify for the study, mothers were required to either meet criteria for MDD during the child’s lifetime according to the Diagnostic and Statistical Manual of Mental Disorders – Fourth Edition (DSM-IV; American Psychiatric Association, 2000) (n = 125) or have no lifetime diagnosis of any DSM-IV mood disorder and no current Axis I diagnosis (n = 122). Exclusion criteria for both groups included symptoms of schizophrenia, organic mental disorder, alcohol or substance dependence within the last six months, or history of bipolar disorder. To participate in the study, children had to be between the ages of 8-14 years and only one child per mother could participate. If more than one child in this age range was available, one child was chosen at random to participate. This age range was chosen because studies have suggested that information processing biases develop over the course of childhood and stabilize around age 12 (e.g., Nolen-Hoeksema et al., 1992) and that gender differences in depression begin to emerge around age 13 (e.g., Hankin et al., 1998). For children in our sample, the average age was 10.89 years (SD = 1.91), 51% were female, and 81% were Caucasian. The average age of mothers in our sample was 40.31 years (SD = 6.88, Range = 24-55) and 88% were Caucasian. The median annual family income was $50,001-55,000.
Measures
The Structured Clinical Interview for DSM-IV Axis I Disorders (SCID-I; First, Spitzer, Gibbon, & Williams, 1995) and the Schedule for Affective Disorders and Schizophrenia for School-Age Children – Present and Lifetime Version (K-SADS-PL; Kaufman, Birmaher, Brent, & Rao, 1997) were used to assess for current DSM-IV Axis I disorders in mothers and their children, respectively. Two separate trained interviewers administered the SCID-I and the K-SADS-PL to mothers and children, respectively. The interviewers were graduate students and post-baccalaureate research assistants who were under the supervision of the last author. Mothers’ and children’s responses were integrated to assign diagnoses by using best estimate diagnostic procedures incorporating interview data from parents and children as well as children self-report (questionnaire data). Discrepancies were discussed with a separate interviewer until consensus was achieved. As noted above, 125 mothers met criteria for MDD during their child’s life (25 met criteria for current MDD). In terms of children’s diagnoses, 31 met criteria for a lifetime episode of major or minor depression (25 were children of mothers with a history of MDD themselves), of whom five met criteria for current MDD. To assess inter-rater reliability, a subset of 21 SCID and K-SADS interviews from this project were coded by a second interviewer and kappa coefficients for diagnoses of MDD in mothers and children were excellent (all κs = 1.00).
Children’s symptoms of depression were assessed using the Children’s Depression Inventory (CDI; Kovacs, 1981). The CDI has demonstrated excellent reliability and validity in previous research (e.g., Kovacs, 1981, 1985; Smucker, Craighead, Craighead, & Green, 1986), and in the current study, the CDI exhibited good internal consistency (α = .82).
The morphed faces task consisted of a stimulus set of full-color pictures of actors taken from a standardized stimulus set (Matsumoto & Ekman, 1988) displaying a variety of emotions (e.g., sad, happy, angry, neutral). The stimuli consisted of emotional and neutral photographs from each actor, morphed to form a continuum of 10% increments between the two photographs. Each emotion is represented by 4 continua (2 male and 2 female actors), for a total of 12 continua. Eleven morphed images were used from each continuum, representing 10% increments of the two emotions ranging from 100% neutral (0% target emotion) to 100% target emotion (e.g., 90% Neutral, 10% Sad; 80% Neutral, 20% Sad; and so on). The pictures, measuring 8.0(w) × 6.5(h) inches, were presented, one at a time, in random order in 2 blocks and the participant was instructed to indicate which emotion was being presented (sad, happy, angry, neutral) by pressing a corresponding button on a keypad. The image remained on the screen until the participant made a response. Participants completed 264 trials. To measure sensitivity to identification of emotional expressions, we calculated the proportion of times the child correctly identified the target emotion (sad, happy, or angry) per level of morph. We initially attempted to analyze the morphed faces paradigm using the procedure described in the Pollak and Kistler (2002) study. This approach involved fitting separate logistic function models for each emotion continuum to the data for each individual participant, deriving estimates of category shift points and slopes for each individual, and comparing group means on these parameters. However, consistent with other previous studies (e.g., Kee, Horan, Wynn, Mintz, & Green, 2006), it was not possible to fit 15-20% of the participants’ data on each of the emotional continua with these functions, perhaps due to the limited number of trials at each morph level. Also, this approach appears better suited for determining thresholds for categorization between two different emotions (e.g., happy versus sad) rather than examining sensitivity in detecting expressions of emotion at low signal intensity (e.g., low morph). Therefore, consistent with previous research (e.g., Burkhouse, Siegle, & Gibb, 2014; Jenness, Hankin, Young, & Gibb, in press) and to provide an adequate number of trials for the sensitivity analyses, responses were binned into three separate morph conditions for analyses: low (0%, 10%, 20%, and 30%), medium (40%, 50%, 60, and 70%), and high (80%, 90%, and 100%). Only trials in which the child responded with “neutral” or the target emotion were included.1 To reduce the influence of outliers for measures of sensitivity, values were Winsorised at 3 standard deviations above and below the mean.
Genomic DNA was collected and isolated from buccal cells/saliva samples, as previously described (Freeman et al., 1997; Lench, Stanier, & Williamson, 1988). The OXTR SNP genotype (AA, GA, GG) was obtained using the fluorogenic 5' nuclease (Taqman, Applied Biosystems, Foster City, CA) method using reagents (VIC(tm) and FAM(tm) labeled probes and TaqMan® Universal PCR Master Mix without AMPerase® UNG) obtained from Applied Biosystems (ABI). Genotype determination was performed using primers purchased from ABI or Integrated DNA Technologies (Coralville, IA). Genotypes were obtained using an ABI Prism 7900HT Sequence Detection System using both absolute quantification and allelic discrimination modes (Livak, Flood, Marmaro, Giusti, & Deetz, 1995). Within our sample, 107 children were GG carriers (43%), 116 were AG (47%) and 24 children were AA carriers (10%). Regarding mother’s genotype, 111 were GG carriers (45%), 110 were AG (44%), and 26 were AA (11%). Genotyping frequencies did not vary significantly from Hardy Weinberg Equilibrium (child: χ2 = 0.86, p = .36; mother: χ2 = 0.05, p = .83). Consistent with prior research (e.g., Bradley et al., 2011; Marsh et al., 2012; Rodrigues et al., 2009) and to ensure adequate power, genotype was coded as two copies of the G allele (1; n = 107) or at least one copy of the A allele (0; n = 140). Further supporting the grouping and similar to previous research (e.g., Rodrigues et al., 2009), AA and AG carriers did not differ significantly on any of the study variables.
Procedure
Potential participants were recruited from the community through a variety of means (e.g., television, newspaper and bus ads, flyers). Mothers responding to the recruitment advertisements were initially screened over the phone to determine potential eligibility. Upon arrival at the laboratory, mothers were asked to provide informed consent and children were asked to provide assent to be in the study. Next, the child completed the morphed faces paradigm, completed a series of questionnaires, and provided buccal cells for DNA analysis. Following this, the mother was administered the K-SADS-PL by a trained interviewer. After completing the K-SADS-PL with the mother, the same interviewer then administered the K-SADS-PL to the child. While children were being administered the K-SADS-PL, the mother was then administered the SCID-I by a separate interviewer. The Institutional Review Board approved all procedures. Families were compensated a total of $85 for their participation in this part of the study.
Results
A preliminary inspection of the data revealed the presence of missing data (with less than 9% of data missing across all study variables). Given this, we examined whether the data were missing at random, thereby justifying the use of data imputation methods for estimating missing values (Shafer & Graham, 2002). Little’s missing completely at random (MCAR) test, for which the null hypothesis is that the data are MCAR (Little & Rubin, 1987) was non-significant, χ2(275) = 284.05, p = .34, supporting the imputation of missing values. Given these results, maximum likelihood estimates of missing data were created and used in all subsequent analyses (see Shafer & Graham, 2002), giving us an effective sample size of 247 children for all analyses.
As shown in Table 1, there were no significant differences between children of depressed versus nondepressed mothers in terms of age or gender. However, the children of depressed mothers were more likely to come from minority backgrounds and have lower family incomes than children of nondepressed mothers. Neither children’s race nor family income was related to children’s sensitivity in detecting facial displays of emotion (lowest p = .29); therefore, these variables were not included in the following analyses. As a means of investigating potential rGE effects, we examined whether children’s or mother’s OXTR genotype was related to mother’s history of MDD and neither of these analyses was significant (lowest p = .11). We also examined whether children’s OXTR genotype was associated with their MDD history or current symptoms of depression. Neither of these analyses was significant (lowest p = .36).
Table 1.
Demographic Characteristics of Children
| Children of Non-Depressed Mothers |
Children of Depressed Mothers |
t/χ2 | |
|---|---|---|---|
| (n = 122) | (n = 125) | ||
| Sex (% Female) | 52 | 49 | 0.33 |
| Ethnicity (% White) | 90 | 72 | 13.22** |
| Age | 10.94 (1.83) | 10.83 (1.99) | 0.45 |
| Family Income (median) | $65,001 - $70,000 | $45,001 - $50,000 | 5.05** |
| OXTR rs53576 (%GG) | 46 | 41 | 0.65 |
| CDI | 4.89 (5.30) | 7.64 (6.25) | 3.69** |
Note: *p < .001; CDI = Children's Depression Inventory
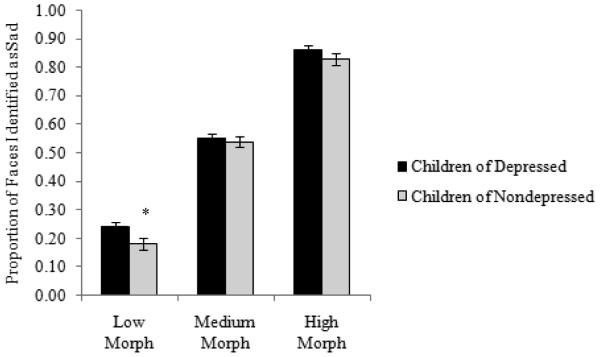
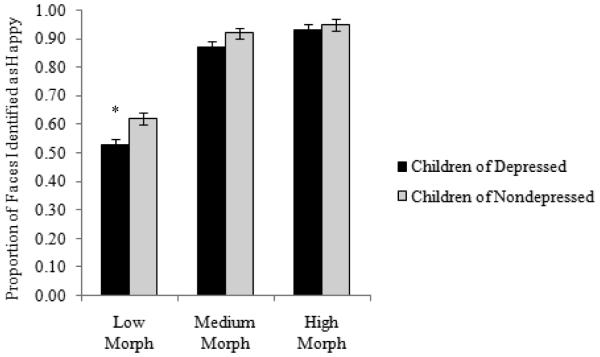
Next, to examine group differences in youths’ sensitivity in detecting facial displays of emotion, we conducted a 2 (Mom MDD history: no, yes) × 2 (Child OXTR rs53576 genotype: AA/AG, GG) × 3 (Target Emotion: angry, happy, sad) × 3 (Morph Level: low, medium, high) repeated measures ANOVA, with proportion of faces correctly identified per level of morph serving as the dependent variable. Results indicated significant main effects for Target Emotion F(2, 242) = 419.94, p < .001, ηp2 = .63, and Morph Level, F(2, 242) = 3902.71, p < .001, ηp2 = .94. The main effect of Mom MDD was non-significant, F(1, 235) = 1.98, p = .16, ηp2 = .01. Examining the main effect of emotion and collapsing across morph levels, youth were better at detecting happy faces (M = .80), compared to angry (M = .58) or sad (M = .56) faces. The main effect of Morph Level revealed, as expected, that there was a general linear increase in the proportion of faces endorsed as angry, happy, and sad from the low (M = .33) to the medium (M = .71) to the high (M = .90) morph levels. Results also indicated significant interactions for Target Emotion × Morph Level, F(2, 242) = 286.46, p < .001, ηp2 = .54, and Mom MDD × Child OXTR rs53576 × Emotion, F(2, 242) = 7.07, p < .001, ηp2 = .03. Importantly, we also found evidence for a significant Mom MDD × Child OXTR rs53576 × Target Emotion × Morph Level interaction, F(4, 240) = 2.79, p = .03, ηp2 = .01. To determine the form of this interaction, we examined the Mom MDD × Target Emotion × Morph Level interaction separately in the two OXTR rs53576 groups (GG vs. AA/AG). Results indicated a significant Mom MDD × Target Emotion × Morph Level interaction among individuals homozygous for the OXTR rs53576 G allele, F(4, 102) = 3.06, p = .02, ηp2 = .03, but not among carriers of the A allele, F(4, 135) = 1.31, p = .27, ηp2 = .01. Among the G homozygotes, the Mom MDD × Target Emotion interaction was significant at low morph levels, F(2, 104) = 9.11, p < .001, ηp2 = .08, but not at medium, F(2, 104) = 1.79, p = .17, ηp2 = .02, or high, F(2, 104) = 2.58, p = .08, ηp2 = .02, morph levels. Examining this further, we found that children homozygous for the OXTR rs53576 G allele who had a depressed mother were significantly better at detecting sad facial expressions (Ms = .24 versus .18; F(1, 105) = 4.95, p = .03, ηp2 = .05) and significantly worse at detecting happy facial expressions (Ms = .53 versus .62; F(1, 105) = 7.43, p < .01, ηp2 = .07) at low levels of signal strength (morph level) than were children of mothers with no history of depression (see Figures 1 and 2). In contrast, the group difference in detecting facial displays of anger was not significant, F(1, 105) = .48, p = .49, ηp2 = .01.
Figure 1.
Proportion of faces identified as sad at low, medium, and high morph levels separated by depression risk status among youth homozygous for the OXTR rs53576 G allele.
A series of analyses was then conducted to test the robustness and specificity of these results. First, as noted above, neither children’s nor mothers’ OXTR genotype was significantly related to mothers’ histories of MDD, suggesting that the effects are not due to rGE with OXTR. Second, to determine whether any of the significant effects were better accounted for by mothers’ OXTR genotype rather than children’s, we re-conducted the analyses focusing on mother genotype. None of the effects for mother OXTR genotype was significant (lowest p = .17). Third, supporting the robustness of the effects observed among children homozygous for the OXTR G allele, they were maintained after excluding children with a lifetime history of major or minor depression and after statistically controlling for the influence of children’s current symptoms of depression using the CDI (highest p = .04) suggesting that the relations are not due simply to current or past depression in children and are observed in children at high risk for depression but with no prior depressive disorders themselves.2 Finally, the effects were also maintained even after excluding dyads in which the mother met criteria for a current MDD (highest p = .04), suggesting that the results were not due simply to the presence of current depression in the mothers.
Exploratory analyses were then conducted to determine whether children’s age and/or sex moderated any of the findings. Therefore, the repeated measures ANOVA described above was repeated, but with child age and sex added, including main effects and all interactions (Note. child age was included as a continuous variable). Neither child age nor child sex significantly moderated any of the findings described above (lowest p = .09). Finally, given potential concerns about population stratification, we re-conducted all of the analyses limiting our sample to Caucasians. All of the significant effects were maintained and the pattern of findings was identical.
Discussion
The primary aim of the current study was to examine the impact of maternal depression on children’s sensitivity in detecting facial displays of emotion. Specifically, we examined whether children of mothers with a history of MDD during the children’s lives, compared to children of mothers with no history of any depressive disorder, would exhibit increased sensitivity in detecting facial displays of sadness (i.e., increase recognition at lower signal strength/morph level). Building from research demonstrating the impact of oxytocin on recognition of facial displays of emotion in humans (for a meta-analytic review, see Van IJzendoorn & Bakermans-Kranenburg, 2012), a second aim of this study was to determine whether variation in a gene known to modulate oxytocinergic functioning (OXTR rs53576) would moderate the impact of maternal depression on children’s sensitivity in detecting facial expressions of emotion. In this study, we found no evidence for a main effect of maternal depression on children’s emotion recognition abilities. However, we did find support for the moderation hypothesis showing maternal history of MDD during children’s lives was associated with children’s emotion recognition among children homozygous for the OXTR rs53576 G allele, but not among carriers of the A allele. Specifically, among G homozygotes, children of depressed mothers exhibited increased sensitivity in detecting facial displays of sadness, and reduced sensitivity in detecting happiness, at low emotional signal strength (low morph level). We should also note that these findings were limited to the lowest level of emotion intensity suggesting that the biases were specific to children’s sensitivity in detecting facial displays of emotion rather than an overall ability to recognize emotions at full intensity. Importantly, these findings were maintained after excluding children with a prior diagnosis of MDD and when controlling for children’s current symptoms of depression. This suggests that our findings are not due simply to current or past depression in children and are observed in children at high risk for depression but with no prior depressive disorder.
The current results support the importance of considering genetic influences when examining information processing biases in children of depressed mothers. As previously discussed, not all children of depressed mothers develop depression themselves and not all should be expected to exhibit information-processing biases. Therefore, researchers have highlighted the importance of identifying specific genetic influences on information-processing biases (e.g., Gibb, Beevers, & McGeary, 2013), which may help to identify which children of depressed mothers are at greatest risk for developing information processing biases (cf. Gibb et al., 2009, 2011). Notably, the current study found no evidence for differences in emotion recognition abilities between children of depressed and nondepressed mothers when children’s genotype was not considered. Therefore, these findings highlight the important contribution of genetic influences, particularly the OXTR rs53576 genotype, on emotion recognition abilities among children of depressed mothers.
The current findings extend previous research in a number of important ways. First, the study adds to a growing body of research demonstrating that children of depressed mothers exhibit heightened sensitivity to facial cues of sadness (cf. Lopez-Duran et al., 2012). Perhaps importantly we found that both boys and girls of depressed mothers who carried the OXTR rs53576 GG genotype exhibited increased sensitivity for sad faces (and decreased sensitivity for happy faces), whereas Lopez-Duran et al. found this effect was only observed among boys. Although the exact reason for this difference in findings is unclear, it may be due to differences in sample composition (e.g., Lopez-Duran included both mothers and fathers) and should be explored in future research. Regardless, however, findings on the effects of parental depression extend previous research on the sequelae of childhood abuse to suggest that consistent exposure to different types of affectively salient social signals in one’s environment may alter a child’s processing of those specific signals, leading to the development of preferential processing of, and sensitivity to, specific classes of emotional expression. More specifically, whereas exposure to physical abuse is associated with increased sensitivity specifically to facial displays of anger in children (Pollak, 2003), exposure to maternal depression appears to increase sensitivity to mild signals of sadness. Interestingly, high-risk children in our sample also displayed reduced sensitivity to facial displays of happiness, suggesting that these children required a greater intensity of happiness to be exhibited before they were willing or able to label it as a happy face.
Second, the current findings extend previous research highlighting the role of oxytocin in social cognition (Van IJzendoorn & Bakermans-Kranenburg, 2012) by demonstrating that the processing biases observed among children of depressed mothers are limited to those homozygous for the OXTR rs53576 G allele. Previous research has shown that G homozygotes, compared to carriers of the A allele, exhibit greater response to endogenous (Moons, Way, & Taylor, 2014) and exogenous (Marsh et al., 2012) oxytocin and higher levels of empathy (Rodrigues et al., 2009; Smith et al., 2014). Similar to what has been proposed for survivors of childhood physical abuse, we would expect that heightened sensitivity to subtle cues of sadness would be adaptive for children growing up with a depressed mother so that the child could alter their behavior in these contexts. However, these processing biases likely become maladaptive if they develop into relatively trait-like processing styles that are applied rigidly to a broader range of interpersonal contexts, such as interacting with other peers or other family members. Indeed, researchers have suggested that, in the face of environmental adversity, the more socially attuned individuals (e.g. GG carriers) may be more sensitive to and negatively affected by these experiences (McQuaid et al., 2013). Supporting this hypothesis, there is evidence that OXTR rs53576 G homozygotes are at greater risk than carriers of the A allele for problems with emotion dysregulation (Bradley et al., 2011), depression (McQuaid et al., 2013), and internalizing symptoms more broadly (Hostinar, Cicchetti, & Rogosch, 2014) following childhood abuse. Therefore, it may be that OXTR rs53576 G homozygotes exhibit better functioning in the absence of negative environments in terms of greater social attunement and empathy, but these same traits may place them at greater risk for negative outcomes in the context of maladaptive environments. Future research is needed, however, to determine whether this increased sensitivity to sad stimuli demonstrated among children of depressed mothers and GG carriers in the current study is truly maladaptive in the long run, increasing these children’s risk for depression (cf. Thompson, Hammen, Starr, & Najman, 2014).
The current study benefited from a number of strengths including the use of diagnostic interviews to confirm mothers’ and children’s history of psychopathology and the focus on an at-risk sample. Further, this is the first study to examine how specific genetic influences may moderate the impact of maternal depression on children’s sensitivity to facial cues of emotion. This said, there were also a number of limitations as well, which highlight directions for future research. First, the study utilized a retrospective, cross-sectional design which does not allow us to draw causal conclusions. Additional research is needed to determine whether these differential sensitivities in the identification of sad and happy facial expressions increases risk for diagnoses of depression in children. Second, although our results are consistent with the hypothesis that children’s processing biases result from conditioning experiences to mother’s depressive affect, the impact of maternal depression on children includes both genetic and environmental influences. Because inclusion criteria for this study required that mothers’ MDD episodes be during their child’s life, future research is needed to determine whether similar effects would be observed among children whose mothers experienced MDD before the child was born. If the effects are not limited to children who had direct exposure to their mothers’ depression, it would argue against a pure conditioning model for the emergence of social cognitive processing biases. Third, maternal depression is a fairly heterogeneous phenotype and is associated with a wide range of factors that may impact children’s development (e.g., maladaptive parenting, increased stress within and outside the home, etc.; for reviews, see Goodman, 2007; Gotlib & Colich, 2014). Therefore, although the results are consistent with the hypothesis that variations in children’s sensitivity in detecting facial cues of sadness is the direct result of these children’s greater exposure to facial displays of sadness in their mothers, this cannot be definitively proven with the current design and must be the subject of future research. Fourth, there is always the possibility in any genetic association study of an unmeasured genetic or nongenetic third variable accounting for the associations reported (e.g., population stratification or linkage disequilibrium between measured variant and actual functional variant). Similar to this point, the current study focused exclusively on one genetic risk factor – OXTR – and it is unlikely that the observed genotype effects are unique to OXTR. Importantly, our decision to focus on OXTR was based on evidence of its impact on social cognition and emotion recognition, and maladaptive outcomes in the presence of environmental adversity. However, given the limitations of single candidate gene studies including the fact that they typically only account for a small proportion of variance in psychiatric phenotypes, future studies are needed that examine the influence of multiple genes, ideally using a polygenic approach to examine aggregate levels of genetic influence across genes known to influence social cognition (cf. Gibb et al., 2013). Finally, we should note that although our findings are consistent with prior research examining the influence of OXTR genotype (e.g., Bradley et al., 2011; McQuaid et al., 2013), we did not have access to an independent sample in which to replicate these findings. Therefore, additional research is needed to ensure that they replicate in independent samples.
In summary, the current findings contribute to an emerging body of research suggesting that children exposed to affectively-salient cues in their lives may develop preferential processing for those specific classes of emotional stimuli. Although this may be adaptive in the short term, it may become maladaptive if applied rigidly in broader contexts (e.g., with peers). These effects may be particularly likely among children who are genetically more sensitive to interpersonal cues. These findings highlight one potential pathway through which affective problems may be transmitted through families.
Figure 2.
Proportion of faces identified as happy at low, medium, and high morph levels separated by depression risk status among youth homozygous for the OXTR rs53576 G allele.
Acknowledgments
This project was supported by National Institute of Child Health and Human Development grant HD057066 and National Institute of Mental Health grant MH098060 awarded to B. E. Gibb, and 1S10RR023457-01A1 and Shared equipment grants (ShEEP) from the Medical Research Service of the Department of Veteran Affairs awarded to J. E. McGeary. The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the National Institutes of Health or the Department of Veterans Affairs. We would like to thank Ashley Johnson, Lindsey Stone, Andrea Hanley, Sydney Meadows, Michael Van Wie, and Devra Alper for their help in conducting assessments for this project, and Kayla Beaucage for her help with genotyping.
Footnotes
Although the current study focused solely on emotions rated as neutral or the target emotion, it should be noted that the pattern of findings was identical when including all emotional responses.
The full ANOVA findings were also maintained when including the influence of children’s lifetime history and current symptoms of depression and mothers’ current depression.
References
- American Psychiatric Association . Diagnostic and statistical manual of mental disorders: DSM-IV-TR®. American Psychiatric Pub; 2000. [Google Scholar]
- Belsky J, Jonassaint C, Pluess M, Stanton M, Brummett B, Williams R. Vulnerability genes or plasticity genes? Molecular Psychiatry. 2009;14:746–754. doi: 10.1038/mp.2009.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley B, Westen D, Mercer KB, Binder E, Jovanovic T, Crain D, WIngo A, Heim C. Association between childhood maltreatment and adult emotional dysregulation in a low-income, urban, African American sample: Moderation by oxytocin receptor gene. Development and Psychopathology. 2011;23:439–452. doi: 10.1017/S0954579411000162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burkhouse KL, Siegle GJ, Gibb BE. Pupillary reactivity to emotional stimuli in children of depressed and anxious mothers. Journal of Child Psychology and Psychiatry. 2014;55:1009–1016. doi: 10.1111/jcpp.12225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cicchetti D, Toth SL, Maughan A. An ecological-transactional model of child maltreatment. In: Sameroff AJ, Lewis M, Miller SM, editors. Handbook of developmental psychopathology. 2nd New York; Kluwer Academic/Plenum Publishers: 2000. pp. 689–722. [Google Scholar]
- Costa B, Pini S, Gabelloni P, Abelli M, Lari L, Cardini A, Martini C. Oxytocin receptor polymorphisms and adult attachment style in patients with depression. Psychoneuroendocrinology. 2009;34:1506–1514. doi: 10.1016/j.psyneuen.2009.05.006. [DOI] [PubMed] [Google Scholar]
- Cunningham JG, Odom RD. Differential salience of facial features in children's perception of affective expression. Child Development. 1986;57:136–142. [Google Scholar]
- Donaldson ZR, Young LJ. Oxytocin, vasopressin, and the neurogenetics of sociality. Science. 2008;322:900–904. doi: 10.1126/science.1158668. [DOI] [PubMed] [Google Scholar]
- Edwards J, Jackson HJ, Pattison PE. Emotion recognition via facial expression and affective prosody in schizophrenia: a methodological review. Clinical Psychology Review. 2002;22:789–832. doi: 10.1016/s0272-7358(02)00130-7. [DOI] [PubMed] [Google Scholar]
- Ellis BJ, Boyce WT, Belsky J, Bakermans-Kranenburg MJ, van Ijzendoorn MH. Differential susceptibility to the environment: An evolutionary-neurodevelopment theory. Development and Psychopathology. 2011;23:7–28. doi: 10.1017/S0954579410000611. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for Axis I DSM Disorders – Patient Edition (SCID-I/P) Biometrics Research Department, NY State Psychiatric Institute; New York: 1995. [Google Scholar]
- Freeman B, Powell J, Ball D, Hill L, Graig I, Plomin R. DNA by mail: An inexpensive and noninvasive method for collecting DNA samples from widely dispersed populations. Behavioral Genetics. 1997;27:251–257. doi: 10.1023/a:1025614231190. [DOI] [PubMed] [Google Scholar]
- Gibb BE, Beevers CG, McGeary JE. Toward an integration of cognitive and genetic models of risk for depression. Cognition and Emotion. 2013;27:193–216. doi: 10.1080/02699931.2012.712950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibb BE, Benas JS, Grassia M, McGeary J. Children’s attentional biases and 5-HTTLPR genotype: Potential mechanisms linking mother and child depression. Journal of Clinical Child and Adolescent Psychology. 2009;38(3):415–426. doi: 10.1080/15374410902851705. 415- [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibb BE, Johnson AL, Benas JS, Uhrlass DJ, Knopik VS, McGeary JE. Children’s 5-HTTLPR genotype moderates the link between maternal criticism and attentional biases specifically for facial displays of anger. Cognition and Emotion. 2011;25:1104–1120. doi: 10.1080/02699931.2010.508267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golarai G, Grill-Spector K, Reiss AL. Autism and the Development of Face Processing. Clinical Neuroscience Research. 2006;6:145–160. doi: 10.1016/j.cnr.2006.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman SH. Depression in mothers. Annual Review of Clinical Psychology. 2007;3:107–105. doi: 10.1146/annurev.clinpsy.3.022806.091401. [DOI] [PubMed] [Google Scholar]
- Gotlib IH, Colich NL. Children of parents with depression. In: Gotlib IH, Hammen CL, editors. Handbook of depression. 3rd New York: Guilford: 2014. pp. 240–258. [Google Scholar]
- Hampson E, van Anders SM, Mullin L. A female advantage in the recognition of emotional facial expressions: Test of an evolutionary hypothesis. Evolution and Human Behavior. 2006;27:401–416. [Google Scholar]
- Hankin BL, Abramson LY, Moffit TE, Silva PA, McGee R, Angell KE. Development of depression from preadolescence to young adulthood: Emerging gender differences in a 10-year longitudinal study. Journal of Abnormal Psychology. 1998;107:128–140. doi: 10.1037//0021-843x.107.1.128. [DOI] [PubMed] [Google Scholar]
- Herba CM, Landau S, Russel T, Ecker C, Phillips ML. The development of emotion-processing in children: effects of age, emotion, and intensity. Journal of Child Psychology and Psychiatry. 2006;47:1098–1106. doi: 10.1111/j.1469-7610.2006.01652.x. [DOI] [PubMed] [Google Scholar]
- Hostinar CE, Cicchetti D, Rogosch RA. Oxytocin receptor gene polymorphism, perceived social support, and psychological symptoms in maltreated adolescents. Development and Psychopathology. 2014;26:465–477. doi: 10.1017/S0954579414000066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenness JJ, Hankin BL, Young JF, Gibb BE. Misclassification and identification of emotional facial expressions in depressed youth: A preliminary study. Journal of Clinical Child and Adolescent Psychology. doi: 10.1080/15374416.2014.891226. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joormann J, Gilbert K, Gotlib IH. Emotion identification in girls at high risk for depression. Journal of Child Psychology and Psychiatry. 2010;51:575–582. doi: 10.1111/j.1469-7610.2009.02175.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joormann J, Talbot L, Gotlib IH. Biased processing of emotional information in girls at risk for depression. Journal of Abnormal Psychology. 2007;116:135–143. doi: 10.1037/0021-843X.116.1.135. [DOI] [PubMed] [Google Scholar]
- Kaufman J, Birmaher B, Brent D, Rao U. Schedule for affective disorders and schizophrenia for school-age children - present and lifetime version (K-SADS-PL): Initial reliability and validity data. Journal of the American Academy of Child & Adolescent Psychiatry. 1997;36:980–988. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- Kee KS, Horan WP, Wynn JK, Mintz J, Green MF. An analysis of categorical perception of facial perception in schizophrenia. Schizophrenia Research. 2006;87:228–237. doi: 10.1016/j.schres.2006.06.001. [DOI] [PubMed] [Google Scholar]
- Kovacs M. Rating scales to assess depression in school-aged children. ActaPaedopsychiatrica. 1981;46:305–315. [PubMed] [Google Scholar]
- Kovacs M. The Children’s Depression Inventory. Psychopharmacology Bulletin. 1985;21:995–998. [PubMed] [Google Scholar]
- Kujawa A, Torpey D, Kim J, Hajcak G, Rose S, Gotlib I, Klein D. Attentional biases for emotional faces in young children of mothers with chronic or recurrent depression. Journal of Abnormal Child Psychology. 2011;39:125–135. doi: 10.1007/s10802-010-9438-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee T-H, Lim S-L, Lee K, Kim H-T, Choi J-S. Conditioning-induced attentional bias for face stimuli measured with the emotional stroop task. Emotion. 2009;9:134–139. doi: 10.1037/a0014590. [DOI] [PubMed] [Google Scholar]
- Lench N, Stanier P, Williamson R. Simple non-invasive method to obtain DNA for gene analysis. Lancet. 1988;1:1356–1358. doi: 10.1016/s0140-6736(88)92178-2. [DOI] [PubMed] [Google Scholar]
- Little RJA, Rubin DB. Statistical analysis with missing data. Wiley; New York: 1987. [Google Scholar]
- Livak KJ, Flood SJ, Marmaro J, Giusti W, Deetz K. Oligonucleotides with fluorescent dyes at opposite ends provide a quenched probe system useful for detecting PCR product and nucleic acid hybridization. PCR Methods Appl. 1995;4:357–362. doi: 10.1101/gr.4.6.357. [DOI] [PubMed] [Google Scholar]
- Lopez-Duran NL, Kuhlman KR, George C, Kovacs M. Facial emotion expression recognition by children at familial risk for depression: high-risk boys are oversensitive to sadness. Journal of Child Psychology and Psychiatry. 2013;54:565–574. doi: 10.1111/jcpp.12005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh AA, Yu HH, Pine DS, Gorodetsky EK, Goldman D, Blair RJR. The influence of oxytocin administration on responses to infant faces and potential moderation by OXTR genotype. Psychopharmacology. 2012;224:469–476. doi: 10.1007/s00213-012-2775-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto D, Ekman P. Japanese and Caucasian facial expressions of emotion. San Francisco State University; San Francisco: 1988. 1988. [Google Scholar]
- McQuaid RJ, McInnis OA, Stead JD, Matheson K, Anisman H. A paradoxical association of the oxytocin receptor gene polymorphism: early-life adversity and vulnerability to depression. Frontiers in Neuroscience. 2013;7:1–7. doi: 10.3389/fnins.2013.00128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moons WG, Way BM, Taylor SE. Oxytocin and vasopressin receptor polymorphisms interact with circulating neuropeptides to predict human emotional reactions to stress. Emotion. 2014 Mar 24; doi: 10.1037/a0035503. Advance online publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolen-Hoeksema S, Girgus JS, Seligman MEP. Predictors and consequences of childhood depressive symptoms: A 5-year longitudinal study. Journal of Abnormal Psychology. 1992;101:405–422. doi: 10.1037//0021-843x.101.3.405. [DOI] [PubMed] [Google Scholar]
- Pischek-Simpson LK, Boschen MJ, Neumann DL, Waters AM. The development of an attentional bias for angry faces following Pavlovian fear conditioning. Behaviour Research and Therapy. 2009;47:322–330. doi: 10.1016/j.brat.2009.01.007. [DOI] [PubMed] [Google Scholar]
- Pollak SD. Experience-dependent affective learning and risk for psychopathology in children. In: King JA, Ferris CF, Lederhendler II, editors. Roots of mental illness in children. Annals of the New York Academy of Sciences; New York: 2003. pp. 102–111. [DOI] [PubMed] [Google Scholar]
- Pollak SD, Kistler DJ. Early experience is associated with the development of categorical representations for facial expressions of emotion. Proceedings of the National Academy of Sciences. 2002;99:9072–9076. doi: 10.1073/pnas.142165999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigues SM, Saslow LR, Garcia N, John OP, Keltner D. Oxytocin receptor genetic variation relates to empathy and stress reactivity in humans. Proceedings of the National Academy of Sciences. 2009;106:21437–21441. doi: 10.1073/pnas.0909579106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafer JL, Graham JW. Missing data: Our view of the state of the art. Psychological Methods. 2002;7:147–177. [PubMed] [Google Scholar]
- Shackman JE, Shackman AJ, Pollak SD. Physical abuse amplifies attention to threat and increases anxiety in children. Emotion. 2007;7:838–852. doi: 10.1037/1528-3542.7.4.838. [DOI] [PubMed] [Google Scholar]
- Smith KE, Porges EC, Norman GJ, Connelly JJ, Decety J. Oxytocin receptor gene variation predicts empathic concern and autonomic arousal while perceiving harm to others. Social Neuroscience. 2014;9:1–9. doi: 10.1080/17470919.2013.863223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smucker MR, Craighead WE, Craighead LW, Green BJ. Normative and reliability data for the Children’s Depression Inventory. Journal of Abnormal Child Psychology. 1986;14:25–39. doi: 10.1007/BF00917219. [DOI] [PubMed] [Google Scholar]
- Thompson SM, Hammen C, Starr LR, Najman JM. Oxytocin receptor gene polymorphism (rs53576) moderates the intergenerational transmission of depression. Psychoneuroendocrinology. 2014;43:11–19. doi: 10.1016/j.psyneuen.2014.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van IJzendoorn MH, Bakermans-Kranenburg MJ. A sniff of trust: Meta-analysis of the effects of intranasal oxytocin administration on face recognition, trust to in-group, and trust to out-group. Psychoneuroendocrinology. 2012;37:438–443. doi: 10.1016/j.psyneuen.2011.07.008. [DOI] [PubMed] [Google Scholar]