Abstract
Macrophages are one of the principal host cell populations in solid tumors. They are capable, due to their plasticity, of acquiring phenotypes that either combat (M1 type) or promote (M2 type) neoplastic growth. These cells, known as tumor-associated macrophages (TAMs), play complex but pivotal roles in the outcome of photodynamic therapy (PDT) of malignant lesions. Among the various parenchymal and stromal cell populations found in tumors, TAMs have been shown to have the greatest capacity for the uptake of systemically administered photosensitizers. Both the tumor-localizing property of photosensitizers and their tumor-localized fluorescence could be partly attributed to the activity of TAMs. Since resident TAMs with accumulated high photosensitizer content will sustain high degrees of PDT damage, this population (predominantly M2 in most tumors) is selectively destroyed, and during the ensuing inflammatory reaction is replaced with newly invading macrophages of M1 phenotype. These macrophages are sentinels responding to DAMP signals from PDT-treated tumor cells and in turn are mobilized to generate a variety of inflammatory/immune mediators and opsonins. They have a critical role in contributing to the therapeutic effect of PDT by mediating disposal of killed cancer cells and by processing/presenting tumor antigens to T lymphocytes. However, TAMs accumulating in the later post-PDT phase can acquire the M2 (healing) phenotype, and could have a role in tumor recurrence by releasing factors that promote angiogenesis and the survival/proliferation of remaining cancer cells. Various therapeutic strategies modulating TAM activity in the PDT response have potential for clinical use for improving PDT-mediated tumor control.
1. Macrophages and their interaction with tumours
Macrophages are the most numerous leukocytes throughout the animal kingdom, with crucial roles in both health and disease.1,2 They are necessary residents in all tissues providing support for tissue homeostasis, repair from cellular senescence and injury, with roles in primary response to infections, resolution of inflammation and wound healing.2,3 With the exception of Langerhans cells in the skin and microglial cells in the brain, tissue-resident macrophages are recruited by differentiation from monocytes that have migrated from peripheral blood.4 A major feature of these cells is highly effective capacity to internalize and digest particles and cell debris (by endocytosis) and engulf pathogens and cancer cells (by phagocytosis). Another crucial function of macrophages is oxygen-dependent and oxygen-independent killing.5 In the former case, the respiratory burst in macrophages results in the production of reactive oxygen and nitrogen species, hydrogen peroxide and chlorine-containing reactive species that destroy the membrane structure of their targets. Oxygen-independent killing involves lysosomes, action of cathepsin and other proteases. An important feature of macrophages is their high plasticity that is governed by a superabundance of surface and other receptors with which they interact with a wide range of growth factors, cytokines, chemokines and other molecules in their microenvironment.2 This interaction, in turn, educates macrophages determining their specific phenotype (reflected in their expression profile) and hence their functional role. Macrophages are characterized by two distinct states of polarized activation, M1 (attacking invaders) and M2 (healing damage).2,3 The engagement of macrophages is central to immune defence, since in addition to being the prominent cellular effector population of innate immunity and the major antigen-presenting cell (APC) population, they direct the Th1- or Th2-like switch of the adaptive immune response.2
As tumors grow they release chemoattractants that mobilize a steady recruitment of monocytes from the peripheral blood, and then these cells relatively quickly differentiate into tumor stromal macrophages, best known as tumor-associated macrophages (TAMs).6 They commonly represent the dominant leukocyte population in solid neoplasms, and in some cases even outnumber the cancer cells themselves. In most tumors, the majority of TAMs have had their function subverted to support tumor progression by adopting an M2 phenotype with an anti-inflammatory character and thereby promote tumor angiogenesis, matrix deposition/remodeling, dead cell and debris/waste removal, immunosuppression and metastasis.3,6 Increased TAM numbers in such tumors correlates with a poor prognosis. In contrast, in some tumor types such as colorectal cancers, TAMs play an overall antitumor role that correlates with a good prognosis. In this case, TAMs are polarized towards the M1 phenotype acting as pro-inflammatory by producing cytokines such as IL-1, IL-6 and IFN-γ, and orchestrating antitumor immune responses.6 A pivotal role of TAMs in securing the effective disposal of dead tumor cells becomes of critical importance in tumors that have been treated by therapies inflicting an immediate trauma at the treated site such as PDT. Following such a tumor-localized insult, extensive efferocytosis of large numbers of rapidly appearing dead cancer cells by TAMs facilitates processing and presentation of tumor antigens to T lymphocytes leading to the development of adaptive antitumor immune response.7,8
2. Role of TAMs in photosensitizer localization
Given the known role of TAMs in entrapping drugs, colloids and other materials that reach tumors,6 it was not surprising that these cells were found to have the highest cellular levels of photosensitizers (with very different chemical structures) amongst all the cell types present in tumors9,10 One of the earliest observations was by Bugelski and co-workers,11 who analyzed autoradiographic distribution of radiolabeled hematophorphyrin derivative (HPD) injected i.p. into tumor-bearing mice. They detected particularly high HPD levels in macrophages scattered throughout the tumor. In their early study, Chan et al12 used flow cytometric analysis and cell sorting to determine the content of the photosensitizer, chloroaluminum sulfonated phthalocyanine in the cellular fraction of a mouse colorectal carcinoma. Upon separating tumor-derived populations of high and low photosensitizer content, they identified macrophages among the cells with a high content. In another early publication,13 Henderson and Bellnier suggested based on their studies that macrophages exhibit extremely high affinity for accumulation of the photosensitizer, Photofrin.
In one of the first studies to investigate directly the photosensitizer uptake by macrophages in vitro and in vivo,14 we demonstrated that macrophages had a much greater capacity for Photofrin uptake than SCCVII tumor cells. This was corroborated by showing that the differentiation of human promyelocytic leukemia HL60 cells into macrophages was accompanied by a marked increased of photosensitizer cellular uptake.15 Correspondingly, higher average photosensitizer levels were consistently found in TAMs than in parenchymal cancer cells, throughout a series of different tumor models including spontaneous and various transplantable mouse carcinomas and sarcomas and autochthonous hamster squamous cell carcinomas.16–19 In these studies, the photosensitizer was typically administered to tumor-bearing animals 24 hours before they were sacrificed, and cells that had been dissociated from excised tumors were stained with antibodies raised against leukocyte cell surface markers. Flow cytometry analysis of these samples measured the photosensitizer content (based on their fluorescence) in different cellular populations from the tumor. Generally there was heterogeneity in photosensitizer levels within the different populations because of the influence of factors such as the higher content found when the cells were in close proximity to the nearest blood vessels.20 However, the highest photosensitizer levels were consistently found in the subpopulations of TAMs that had elevated expression of IL-2 receptors (F4/80+CD25+). These cells were also characterized by increased sized and granularity, which is consistent with their identification as activated macrophages.9,19 Based on the above findings it was suggested that the tumor-localized fluorescence of most photosensitizers was due to their high accumulation in activated TAMs.9
There seem to be two mechanisms that can get explain elevated photosensitizer uptake by TAMs. Jori suggested that photosensitizers that remain highly aggregated when delivered systemically are phagocytized in that form by macrophages.21 This was confirmed by the finding that cytochalasin B (a phagocytosis inhibitor) reduced the uptake of Photofrin by peritoneal macrophages in vitro and TAMs of Lewis lung carcinoma in vivo.14,22 The opposite effect of increased photosensitizer uptake by TAMs was obtained by pretreatment of mice with IFN-γ or dodecylglycerol, factors that induce macrophage activation and consequently enhance their phagocytic activity.22 The other suggested mechanism ascribes the elevated photosensitizer accumulation in TAMs to the engagement of their scavenger receptors internalizing the photosensitizer molecules associated with blood lipoproteins.10 Thus it is possible, by using various agents that affect TAM phagocytic activity or by modifying the photosensitizer molecules by adding scavenger receptor ligand, to alter the photosensitizer content in TAMs and consequently affect tumor photosensitizer levels.23,24
3. Macrophage activity in PDT-treated tumours
In vitro studies with peritoneal macrophages revealed that direct PDT treatment of the cells can affect their viability and functional activity. Interestingly, it appears that low PDT doses can in fact have a stimulative effect on macrophage activity in vitro and also in vivo, while the activity will be impaired at higher PDT doses.25–27 Macrophages treated by PDT in vitro were reported to produce TNF-α, and the cytotoxic effect of this cytokine on tumor cells was suggested as a possible mechanism of antitumor effect of PDT.28 Moreover, untreated macrophages co-incubated with PDT-treated tumor cells were also induced to produce TNF-α.29,30 In this case damage-associated molecular patterns (DAMPs) such as heat shock-70 (Hsp70) that is expressed by PDT-treated cells, can serve to trigger signaling pathways in macrophages mediated by Toll-like receptors 2 and 4 (TLR2 and TLR4) that leads to NFκB-based TNF-α generation. The activation of NFκB signaling upregulated also the generation of nitric oxide (NO) in these macrophages.31 Macrophages co-incubated with PDT-treated tumor cells were found to upregulate the production of a number of other potent inflammatory/immune mediators including complement C3, C5, and C9,32 pentraxin proteins,33 the sphingolipids ceramide and sphingosine-1-phosphate,34 as well as the expressions of various receptors including TLR2, TLR4, and C3aR.35,36 These complement and pentraxin proteins can serve as opsonins by binding to dying tumor cells and facilitating their phagocytosis by TAMs.37 In addition to the pattern recognition surface receptors that recognize the DAMPs, macrophages that are in contact with PDT-treated tumor cells also upregulate the intracellular pattern recognition receptor called NOD (nucleotide-binding oligomerization domain)-like receptor pyrin domain-containing 3 (NLRP3).34 The assembly and oligomerization of activated NLRP3 into the multimolecular complex known as the inflammasome, facilitates the production of inflammatory cytokines by these macrophages.38
Critical to understanding the role of macrophages in PDT-treated tumors, is the fact that these lesions become the site of a strong acute inflammatory reaction characterized by a rapid and massive invasion of neutrophils, mast cells and new monocytes.39 Thus, the predominantly M2 tumor-promoting resident TAMs that have accumulated high photosensitizer levels will also sustain high lethal PDT effects and are then replaced by new macrophages differentiated from invading monocytes. These new macrophages are characterized by a M1 phenotype producing IL-1, IL-6, TNF-α, NO, complement proteins and other inflammatory mediators and exhibiting anti-tumor activity.40 The conversion of M2 macrophages into M1 macrophages following TLR activation has been reported by several studies.6 The TAMs isolated from mouse SCCVII tumors 2 hours post-PDT showed almost five-fold greater tumoricidal effect than the TAMs isolated from non-treated tumors.39 In vitro PDT-treated tumor cells were demonstrated to become more susceptible targets for killing by macrophages,41 suggesting that these effector cells recognized PDT-induced (potentially repairable) damage on tumor cells marking them out as their preferential targets.9
In the early phase post-PDT the new M1 macrophages can contribute to PDT-based tumor destruction. By contrast in the later phase post-PDT, the regulatory mechanisms activated to resolve the induced inflammatory process may lead to the establishment of macrophages exhibiting the healing M2 phenotype that can promote the recurrence of treated tumors.7,40 These macrophages release mediators that suppress inflammation and immune response, while promoting angiogenesis of tumor blood vessels and encouraging survival/proliferation of resident cancer cells. These processes are mediated by secreted factors including IL-10, TGF-β, vascular endothelial growth factor (VEGF), cyclooxygenase-2 (COX-2), prostaglandins, matrix metalloproteinases, and the apoptosis inhibitor survivin.40
4. Therapeutic approaches exploiting the role of TAMs in PDT response
Since in most tumors the resident macrophages are considered to be polarized into the M2 phenotype promoting neoplastic growth, these macrophages are regarded to be a valid target as a component of anti-tumor therapy.6,42 It has been shown that PDT can be optimized for selective TAM destruction by attaching the photosensitizer molecule to ligands of the scavenger receptor,43 which is a high capacity route for endocytic internalization of molecules into macrophages in a cell-type specific manner.44 Scavenger-receptor photosensitizer-targeting was proposed to result in a selective accumulation of photosensitizer in tumor macrophages leading to their preferential killing upon photodynamic light exposure.45 A conjugate between the photosensitizer chlorine(e6) and maleylated serum albumin was efficiently taken up by macrophages in vitro and gave highly selective PDT killing.23 However this approach ran into problems when the conjugates were systemically injected into mice. The scavenger-receptor targeted conjugates were very efficiently accumulated in the liver and spleen, organs with high scavenger receptor expression. This drawback was overcome by direct intratumoral injection of the conjugates, when the TAMs residing within the tumor were effectively targeted by the conjugate.46 The strategy of macrophage-targeting by scavenger-receptor targeted conjugates was later utilized to localize photosensitizers to vulnerable atherosclerotic plaques in rabbits, allowing fluorescence diagnosis and PDT; these lesions are particularly rich in macrophages.47,48 Macrophage-targeted photosensitizer delivery for both diagnostic and therapeutic purposes can be facilitated by the nanoparticles technology. The nanoformulations explored for such approach include: biodegradable polymer poly(DL-lactide-co-glycolide) containing photosensitizer bacteriochlorophyll-a49, theranostic nanoparticles prepared by conjugating photosensitizer chlorin e6 to hyaluronic acid50, and positively charged nanoparticles consisting of a calcium phosphate core with shells of carboxymethyl cellulose and poly(ethyleneimine) loaded with photosensitizer 5,10,15,20-tetrakis(3-hydroxyphenyl)-porphyrin (mTHPP)51.
Another strategy focusing on the TAMs in conjunction with PDT is based on exactly the opposite approach of amplifying their activity. This has also proven effective (at least with some tumor models) due to the fact that the PDT treatment selectively kills resident M2 TAMs replacing them with newly invaded M1 macrophages. Highly potent vitamin D3-binding protein-derived macrophage-activating factor (DBPMAF) administered to SCCVII-bearing mice at 0, 4, 8 and 12 days post PDT markedly enhanced the curative effect of PDT.52 Examination of the host mice based on delayed-type contact hypersensitivity response revealed that PDT-induced immunosuppression was greatly reduced by the combined DBPMAF treatment. These results suggested that the adjuvant macrophage activation was responsible for enhanced PDT antitumor immune response resulting in more complete tumor cures.
The same strategy was successfully applied with a variety of somewhat less specific immunoactivating agents that (at least as part of their mechanism) produce macrophage activation. These studies included increased PDT anti-tumor effects obtained by combining PDT with the beta-D-glucan schizophyllan (SPG),53 microbial vaccines such as OK-432,54 Corynebacterium parvum (CP),55 BCG56 and mycobacterial cell wall extracts.57 Many of these immunostimulating agents bind to TLR and other PAMPS that are linked to increased M1 macrophage activation. Therapeutic gain has also been obtained when PDT was combined with cytokines that can affect macrophage activation such as IFN-γ,58 GM-CSF59 as well as with diverse complement-activating agents.60–63
Another macrophage-focused therapeutic strategy for improving the tumor PDT-response is to counteract the activity of mediators produced by M2 TAMs in the healing anti-inflammatory phase after PDT that can function to restore tumor growth.64 This strategy includes blocking the anti-inflammatory cytokines IL-10 or TGF-β,7 neutralizing pro-angiogenic factors like VEGF,65 inhibiting the activity of metalloproteinases or COX-2,64,66 or blocking the activity of anti-apoptotic survivin.67
5. Conclusions
Macrophages are of critical importance to the response of tumors to PDT, due to their multi-factorial role in key processes including photosensitizer uptake/localization, the acute inflammatory reaction, disposal of killed tumor cells, presentation of tumor antigens, and promotion of tumor recurrence. A pivotal benefit of PDT is the selective destruction of resident M2 tumor-promoting TAMs in treated tumors and replacing them with newly invading activated tumor-destroying M1 macrophages. With regards to the actual anti-tumor PDT response, macrophages can be considered a double-edged sword because they can play a role in the events critical for tumor destruction, but also in the events promoting tumor recurrence. A number of strategies effectively exploiting macrophage activity in the anti-tumor PDT response have potential for clinical use for improving PDT-mediated tumor control and establishing an anti-tumor immune response.
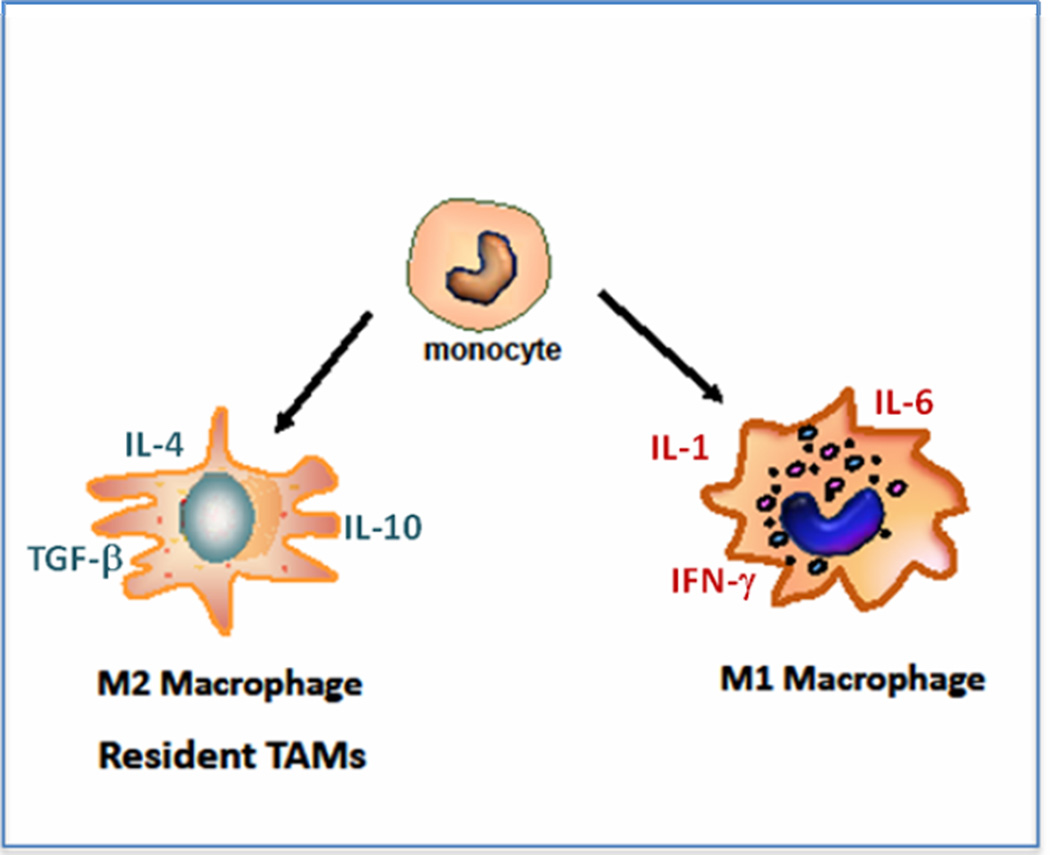
Figure 1. Monocytes can differentiate into two different macrophage phenotypes.
M1 macrophages are designed to attack invaders and producing pro-inflammatory cytokines; M2 are designed to remove debris and stimulate healing and producing anti-inflammatory cytokines. Resident tumor-associated macrophages generally have the M2 phenotype and help the tumor to grow.
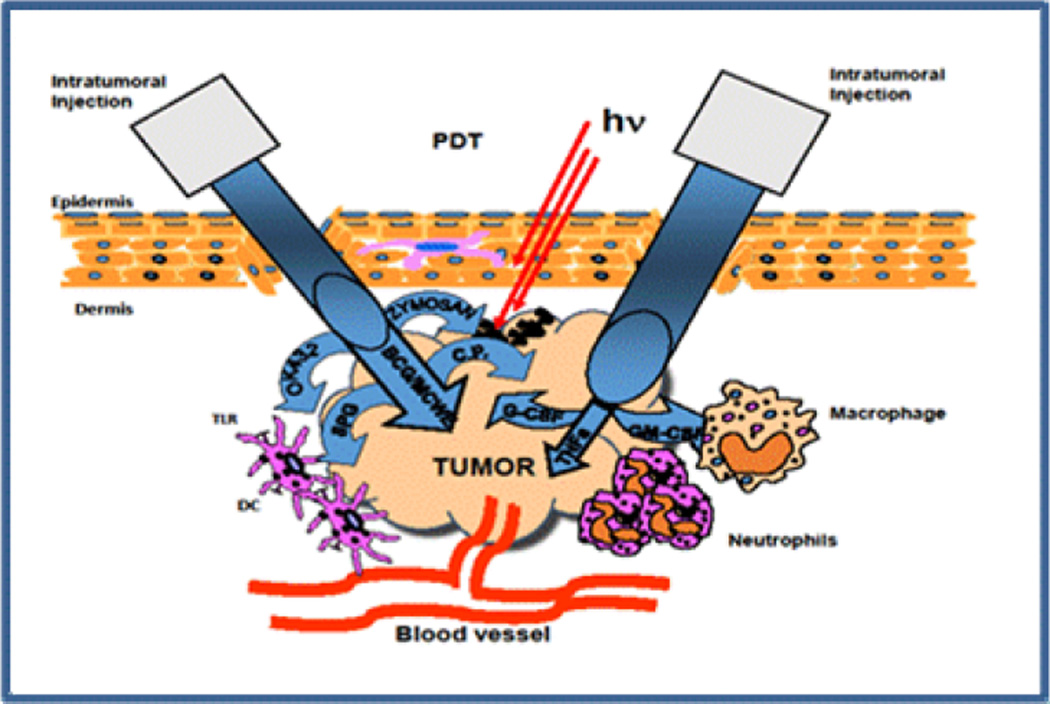
Figure 2. Combination of PDT with immunostimulants.
Intratumoral injection of various Toll-like receptor (TLR) ligands: Bacillus Calmette-Guerin (BCG), Mycobacterial cell-wall extract (MCWE), OK-432, Zymosan, Schizophyllan (SPG) or Corynebacterium parvum (C.P.) effectively activates DCs and enhances the antigen presentation and local inflammation. Injection of various cytokines results in increased infiltration by macrophages (granulocyte-macrophage colony-stimulating factor, GM-CSF), activation of neutrophils (granulocyte colony stimulating factor, G-CSF) and direct destruction of tumor vessels (tumor necrosis factor alpha, TNFα).
Acknowledgements
Research funding for Mladen Korbelik was provided by Canadian Cancer Society (grant # 701132) and US NIH grant R01-CA77475. Michael R Hamblin was supported by US NIH grants R01-CA/AI838801 and R01-AI050875.
References
- 1.Hewald H, Egesten A. The Janus face of macrophages in immunity. J. Innate Immun. 2014;6:713–715. doi: 10.1159/000367718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mills CD, Ley K. M1 and M2 macrophages: the chicken and the egg of immunity. J. Innate Immun. 2014;6:716–726. doi: 10.1159/000364945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Murray PJ, Wynn TA. Protective and pathogenic functions of macrophage subsets. Nat. Rev. Immunol. 2011;11:723–737.B. doi: 10.1038/nri3073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Geissmann F, Manz MG, Jung S, Sieweke MH, Merad M, Ley K. Development of monocytes, macrophages, and dendritic cells. Science. 2010;327:656–661. doi: 10.1126/science.1178331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gwinn MR, Vallyathan V. Respiratory burst: role in signal transduction in alveolar macrophages. J. Toxicol. Environ. Health B. 2006;9:27–39. doi: 10.1080/15287390500196081. [DOI] [PubMed] [Google Scholar]
- 6.Hao N-B, Lü M-H, Fan Y-H, Cao Y-L, Zhang Z-R, Yang S-M. Macrophages in tumor microenvironments and the progression of tumors. Clin. Dev. Immunol. 2012;2012:948098. doi: 10.1155/2012/948098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Korbelik M. PDT-associated host response and its role in the therapy outcome. Lasers Surg. Med. 2006;38:500–508. doi: 10.1002/lsm.20337. [DOI] [PubMed] [Google Scholar]
- 8.Castano AP, Mroz P, Hamblin MR. Photodynamic therapy and anti-tumor immunity. Nat. Rev. Cancer. 2006;6:535–545. doi: 10.1038/nrc1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Korbelik M, Krosl G. Photosensitizer distribution and photosensitized damage of tumor tissue. In: nigsmann HH, Jori G, Young AR, editors. The fundamental bases of phototherapy. Milan: OEMF spa; 1996. pp. 229–245. [Google Scholar]
- 10.Hamblin MR, Newman EL. On the mechanism of tumor-localizing effect in photodynamic therapy. J. Photochem. Photobiol. B. 1994;23:3–8. doi: 10.1016/s1011-1344(94)80018-9. [DOI] [PubMed] [Google Scholar]
- 11.Bugelski PJ, Porter CW, Dougherty TJ. Autoradiographic distribution of hematoporphyrin derivative in normal and tumor tissue of the mouse. Cancer Res. 1981;41:4606–4612. [PubMed] [Google Scholar]
- 12.Chan WS, Marshall JF, Lam GYF, Hart IR. Tissue uptake, distribution, and potency of the photoactivable dye chloraluminum sulfonated phthalocyanine in mice bearing transplantable tumors. Cancer Res. 1988;48:3040–3044. [PubMed] [Google Scholar]
- 13.Henderson BW, Bellnier DA. Tissue localization of photosensitizers and the mechanism of photodynamic tissue destruction. CIBA Found. Symp. 1989;146:112–125. doi: 10.1002/9780470513842.ch8. [DOI] [PubMed] [Google Scholar]
- 14.Korbelik M, Krosl G, Chaplin DJ. Photofrin uptake by murine macrophages. Cancer Res. 1991;51:2251–2255. [PubMed] [Google Scholar]
- 15.Korbelik M, Krosl G, Adomat H, Skov KA. The effect of differentiation on photosensitizer uptake by HL60 cells. Photochem. Photobiol. 1993;58:670–675. doi: 10.1111/j.1751-1097.1993.tb04950.x. [DOI] [PubMed] [Google Scholar]
- 16.Korbelik M, Krosl G, Olive PL, Chaplin DJ. Distribution of Photofrin between tumour cells and tumour associated macrophages. Br. J. Cancer. 1991;64:508–512. doi: 10.1038/bjc.1991.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Korbelik M. Distribution of disulfonated and tetrasulfonated aluminum phthalocyanine between malignant and host cell populations of murine fibrosarcoma. J. Photochem. Photobiol. B. 1993;20:173–181. doi: 10.1016/1011-1344(93)80148-3. [DOI] [PubMed] [Google Scholar]
- 18.Korbelik M, Krosl G. Accumulation of benzoporphyrin derivative in malignant and host cell populations of the murine RIF tumor. Cancer Lett. 1995;97:249–254. doi: 10.1016/0304-3835(95)03985-6. [DOI] [PubMed] [Google Scholar]
- 19.Korbelik M, Krosl G. Photofrin accumulation in malignant and host cell populations of various tumors. Br. J. Cancer. 1996;73:506–513. doi: 10.1038/bjc.1996.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Korbelik M, Krosl G. Cellular levels of photosensitizers in tumours: the role of proximity to the blood supply. Br. J. Cancer. 1994;70:604–610. doi: 10.1038/bjc.1994.358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jori G. In vivo transport and pharmacokinetic behaviour of tumor photosensitizers. CIBA Found. Symp. 1989;146:78–86. doi: 10.1002/9780470513842.ch6. [DOI] [PubMed] [Google Scholar]
- 22.Korbelik M, Krosl G, Chaplin DJ. Can PDT be potentiated by immunotherapy? Proc. SPIE. 1991;1616:192–198. [Google Scholar]
- 23.Hamblin MR, Miller JL, Ortel B. Scavenger-receptor targeted photodynamic therapy. Photochem. Photobiol. 2000;72:533–540. doi: 10.1562/0031-8655(2000)072<0533:srtpt>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 24.Hamblin MR, Miller JL, Rizvi I, Ortel B. Degree of substitution of chlorin e6 on charged poly-L-lysine chains affects their cellular uptake, localization and phototoxicity towards macrophages and cancer cells. J. Xray Sci. Technol. 2002;10:139–152. [PubMed] [Google Scholar]
- 25.Marshall JF, Chan W-S, Hart IR. Effect of photodynamic therapy on anti-tumor immune defenses: comparison of the photosensitizers hematoporphyrin derivative and chloro-aluminum sulfonated phthalocyanine. Photochem. Photobiol. 1989;49:627–632. doi: 10.1111/j.1751-1097.1989.tb08434.x. [DOI] [PubMed] [Google Scholar]
- 26.Steubing RW, Yeturu S, Tuccillo A, Sun C-H, Berns MW. Activation of macrophages by Photofrin II during photodynamic therapy. J. Photochem. Photobiol. B. 1991;10:133–145. doi: 10.1016/1011-1344(91)80218-7. [DOI] [PubMed] [Google Scholar]
- 27.Yamamoto N, Hoober JK, Yamamoto N, Yamamoto S. Tumoricidal capacities of macrophages photodynamically activated with hematoporphyrin derivative. Photochem. Photobiol. 1992;56:245–250. doi: 10.1111/j.1751-1097.1992.tb02153.x. [DOI] [PubMed] [Google Scholar]
- 28.Evans S, Matthews W, Perry R, Fraker D, Norton J, Pass HI. Effect of photodynamic therapy on tumor necrosis factor production by murine macrophages. J. Natl. Cancer Inst. 1990;82:34–39. doi: 10.1093/jnci/82.1.34. [DOI] [PubMed] [Google Scholar]
- 29.Korbelik M, Sun J, Cecic I. Photodynamic therapy-induced cell surface expression and release of heat shock proteins: relevance for tumor response. Cancer Res. 2005;65:1018–1026. [PubMed] [Google Scholar]
- 30.Zhou F, Xing D, Chen WR. Regulation of HSP70 on activating macrophages using PDT-induced apoptotic cells. Int. J. Cancer. 2009;125:1380–1389. doi: 10.1002/ijc.24520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Song S, Zhou F, Chen WR, Xing D. PDT-induced HSP70 externalization up-regulates NO production via TLR2 signal pathway in macrophages. FEBS Lett. 2013;587:128–135. doi: 10.1016/j.febslet.2012.11.026. [DOI] [PubMed] [Google Scholar]
- 32.Stott B, Korbelik M. Activation of complement C3, C5, and C9 genes in tumors treated by photodynamic therapy. Cancer Immunol. Immunother. 2007;56:649–658. doi: 10.1007/s00262-006-0221-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Merchant S, Sun J, Korbelik M. Dying cells program their expedient disposal: serum amyloid P component upregulation in vivo and in vitro induced by photodynamic therapy of cancer. Photochem. Photobiol. Sci. 2007;6:1284–1289. doi: 10.1039/b709439f. [DOI] [PubMed] [Google Scholar]
- 34.Korbelik M, Banáth J, Zhang W, Wong F, Bielawski J, Separovic D. Ceramide and sphingosine-1-phosphate/sphingosine act as photodynamic therapy-elicited damage-associated molecular patterns: Release from cells and impact on tumor-associated macrophages. J. Anal. Bioanal. Tech. 2014;S1:009. [Google Scholar]
- 35.Korbelik M. Complement upregulation in photodynamic therapy treated tumors: role of Toll-like receptor pathway and NFκB. Cancer Lett. 2009;281:232–238. doi: 10.1016/j.canlet.2009.02.049. [DOI] [PubMed] [Google Scholar]
- 36.Cecic I, Sun J, Korbelik M. Role of complement anaphylatoxin C3a in photodynamic therapy-elicited engagement of neutrophils and other immune cells. Photochem. Photobiol. 2006;82:558–562. doi: 10.1562/2005-09-09-RA-681. [DOI] [PubMed] [Google Scholar]
- 37.Nauta AJ, Daha MR, van Kooten C, Roos A. Recognition and clearance of apoptotic cells: a role of complement and pentraxins. Trends Immunol. 2003;24:148–154. doi: 10.1016/s1471-4906(03)00030-9. [DOI] [PubMed] [Google Scholar]
- 38.Cassel SL, Sutterwala FS. Sterile inflammatory responses mediated by the NLRP3 inflammasome. Eur. J. Immunol. 2010;40:607–611. doi: 10.1002/eji.200940207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Krosl G, Korbelik M, Dougherty GJ. Induction of immune cell infiltration into murine SCCVII tumour by Photofrin-based photodynamic therapy. Br. J. Cancer. 1995;71:549–555. doi: 10.1038/bjc.1995.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Korbelik M. Tumor-localized insult delivered by photodynamic therapy and the breakdown of tumor immunotolerance. In: Keisari Y, editor. Tumor Ablation. Vol. 5. Dordrecht: Springer; 2013. pp. 121–132. (The Tumor Microenvironment series), ch. 7. [Google Scholar]
- 41.Korbelik M, Krosl G. Enhanced macrophage cytotoxicity against tumor cells treated with photodynamic therapy. Photochem. Photobiol. 1994;60:497–502. doi: 10.1111/j.1751-1097.1994.tb05140.x. [DOI] [PubMed] [Google Scholar]
- 42.Biswas SK, Allavena P, Mantovani A. Tumor-associated macrophages: functional diversity, clinical significance, and open questions. Semin. Immunopathol. 2013;35:585–600. doi: 10.1007/s00281-013-0367-7. [DOI] [PubMed] [Google Scholar]
- 43.Liu Q, Hamblin MR. Macrophage-targeted photodynamic therapy: scavenger receptor expression and activation state. Int. J. Immunopathol. Pharmacol. 2005;18:391–402. doi: 10.1177/039463200501800301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Canton J, Neculai D, Grinstein S. Scavenger receptors in homeostasis and immunity. Nat. Rev. Immunol. 2013;13:621–634. doi: 10.1038/nri3515. [DOI] [PubMed] [Google Scholar]
- 45.Demidova TN, Hamblin MR. Macrophage-targeted photodynamic therapy. Int. J. Immunopathol. Pharmacol. 2004;17:117–126. doi: 10.1177/039463200401700203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Anatelli F, Mroz P, Liu Q, Castano AP, Swietlik E, Hamblin MR. Macrophage-targeted photosensitizer conjugate delivered by intratumoral injection. Mol. Pharm. 2006;3:654–664. doi: 10.1021/mp060024y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tawakol A, Castano AP, Anatelli F, Bashian G, Stern J, Zahra T, Gad F, Chirico S, Ahmadi A, Fischman AJ, Muller JE, Hamblin MR. Photosensitizer delivery to vulnerable atherosclerotic plaque: comparison of macrophage-targeted conjugate versus free chlorin(e6) J. Biomed. Opt. 2006;11:021008. doi: 10.1117/1.2186039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tawakol A, Castano AP, Gad F, Zahra T, Bashian G, Migrino RQ, Ahmadi A, Stern J, Anatelli F, Chirico S, Shirazi A, Syed S, Fischman AJ, Muller JE, Hamblin MR. Intravascular detection of inflamed atherosclerotic plaques using a fluorescent photosensitizer targeted to the scavenger receptor. Photochem. Photobiol. Sci. 2008;7:33–39. doi: 10.1039/b710746c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gomes AJ, Lunardi LO, Marchetti JM, Lunardi CN, Tedesco AC. Photobiological and ultrastructural studies of nanoparticles of poly(lactic-co-glycolic acid)-containing bacteriochlorophyll-a as a photosensitizer useful for PDT treatment. Drug Deliv. 2005;12:159–164. doi: 10.1080/10717540590931846. [DOI] [PubMed] [Google Scholar]
- 50.Kim H, Kim Y, Kim I-H, Kim K, Choi Y. ROS-responsive activable photosensitizing agent for imaging and photodynamic therapy of activated macrophages. Theranostics. 2014;4:1–11. doi: 10.7150/thno.7101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Klesing J, Wiehe A, Gitter B, Gräfe S, Epple M. Positively charged calcium phosphate/polymer nanoparticles for photodynamic therapy. J. Mater. Sci. Mater. Med. 2010;21:997–892. doi: 10.1007/s10856-009-3934-7. [DOI] [PubMed] [Google Scholar]
- 52.Korbelik M, Naraparaju VR, Yamamoto N. Macrophage directed immunotherapy as adjuvant to photodynamic therapy of cancer. Br. J. Cancer. 1997;75:202–207. doi: 10.1038/bjc.1997.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Krosl G, Korbelik M. Potentiation of photodynamic therapy by immunotherapy: The effect of Schizophyllan (SPG) Cancer Lett. 1994;84:43–49. doi: 10.1016/0304-3835(94)90356-5. [DOI] [PubMed] [Google Scholar]
- 54.Uehara M, Sano K, Wang Z-L, Sekine J, Ikeda H, Inokuchi T. Enhancement of the photodynamic antitumor effect by streptococcal preparation OK-432 in the mouse carcinoma. Cancer Immunol. Immunother. 2000;49:401–409. doi: 10.1007/s002620000134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Myers RC, Lau BH, Kunihira DY, Torrey RR, Woolley JL, Tosk J. Modulation of hematoporphyrin derivative-sensitized phototherapy with corynebacterium parvum in murine transitional cell carcinoma. Urology. 1989;33:230–235. doi: 10.1016/0090-4295(89)90399-3. [DOI] [PubMed] [Google Scholar]
- 56.Korbelik M, Sun J, Posakony JJ. Interaction Between Photodynamic Therapy and BCG Immunotherapy Responsible for the Reduced Recurrence of Treated Mouse Tumors. Photochem. Photobiol. 2001;73:403–409. doi: 10.1562/0031-8655(2001)073<0403:ibptab>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 57.Korbelik M, Cecic I. Enhancement of tumour response to photodynamic therapy by adjuvant mycobacterium cell-wall treatment. J. Photochem. Photobiol., B. 1998;44:151–158. doi: 10.1016/S1011-1344(98)00138-9. [DOI] [PubMed] [Google Scholar]
- 58.Korbelik M, Merchant S, Huang N. Exploitation of immune response-eliciting properties of hypocrellin photosensitizer SL052-based photodynamic therapy for eradication of malignant tumors. Photochem. Photobiol. 2009;85:1418–1424. doi: 10.1111/j.1751-1097.2009.00610.x. [DOI] [PubMed] [Google Scholar]
- 59.Krosl G, Korbelik M, Krosl J, Dougherty GJ. Potentiation of Photodynamic therapy elicited antitumor response by localized treatment with granulocyte-macrophage colony stimulating factor. Cancer Res. 1996;56:3281–3286. [PubMed] [Google Scholar]
- 60.Korbelik M, Dougherty GJ. Complement activation approaches for use in conjunction with PDT for cancer treatment. Proc. SPIE. 2005;5695:17–26. [Google Scholar]
- 61.Korbelik M, Sun J, Cecic I, Serrano K. Adjuvant treatment for complement activation increases the effectiveness of photodynamic therapy of solid tumors. Photochem. Photobiol. Sci. 3004;3:812–816. doi: 10.1039/b315663J. [DOI] [PubMed] [Google Scholar]
- 62.Korbelik M, Cooper PD. Potentiation of photodynamic therapy of cancer by complement: the effect of γ-inulin. Br. J. Cancer. 2007;96:67–72. doi: 10.1038/sj.bjc.6603508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Korbelik M, Cecic I. Complement activation cascade and its regulation: relevance for the response of solid tumors to photodynamic therapy. J. Photochem. Photobiol. B: Biol. 2008;93:53–59. doi: 10.1016/j.jphotobiol.2008.04.005. [DOI] [PubMed] [Google Scholar]
- 64.Gomer CJ, Ferrario A, Luna M, Rucker N, Wong S. Photodynamic therapy: combined modality approaches targeting the tumor microenvironment. Lasers Surg. Med. 2006;38:516–521. doi: 10.1002/lsm.20339. [DOI] [PubMed] [Google Scholar]
- 65.Ferrario A, Gomer CJ. Avastin enhances photodynamic therapy treatment of Kaposi’s sarcoma in a mouse tumor model. J. Environ. Path. Tox. Oncol. 2006;25:251–259. doi: 10.1615/jenvironpatholtoxicoloncol.v25.i1-2.160. [DOI] [PubMed] [Google Scholar]
- 66.Makowski M, Grzela T, Nidrla J, Azarczyk M, Mroz P, Kopee M, Nowis D, Mrowka P, Wasik M, Jakobisiak M, Golab J. Inhibitor of cyclooxigenase-2 indirectly potentiates antitumor effects of photodynamic therapy in mice. Clin. Cancer Res. 2003;9:5417–5422. [PubMed] [Google Scholar]
- 67.Ferrario A, Rucker N, Wong S, Luna M, Gomer CJ, Survivin CJ. a member of the Inhibitor of Apoptosis family, is induced by photodynamic therapy and is a target for improving treatment response. Cancer Res. 2007;67:4989–4995. doi: 10.1158/0008-5472.CAN-06-4785. [DOI] [PubMed] [Google Scholar]